Abstract
Demand for anal cancer screening is expected to rise following the recent publication of the ANCHOR trial, which showed that treatment of HSIL significantly reduces the rate of progression to anal cancer. While screening for HPV-associated squamous lesions in the cervix is well-established and effective, this is less true for other sites in the lower anogenital tract. Current anal cancer screening and prevention rely on high-resolution anoscopy (HRA) with biopsies. This procedure has a steep learning curve for providers and may cause patient discomfort. Scattering-based light-sheet microscopy (sLSM) is a novel imaging modality with the potential to mitigate these challenges through real-time, microscopic visualization of disease-susceptible tissue. Here, we report a proof-of-principle study that establishes feasibility of dysplasia detection using an sLSM device. We imaged 110 anal biopsy specimens collected prospectively at our institution’s dysplasia clinic (including 30 nondysplastic, 40 LSIL and 40 HSIL specimens) and found that these optical images are highly interpretable and accurately recapitulate histopathologic features traditionally used for the diagnosis of HPV-associated squamous dysplasia. A reader study to assess diagnostic accuracy suggests that sLSM images are noninferior to H&E for the detection of anal dysplasia (sLSM accuracy = 0.87, H&E accuracy = 0.80; p = 0.066). Given these results, we believe that sLSM technology holds great potential to enhance the efficacy of anal cancer screening by allowing accurate sampling of diagnostic tissue at the time of anoscopy. While the current imaging study was performed on ex vivo biopsy specimens, we are currently developing a handheld device for in vivo imaging that will provide immediate microscopic guidance to HRA providers.
INTRODUCTION
The majority of anal cancers are human papillomavirus (HPV)-associated squamous cell carcinomas that disproportionately affect high-risk populations, with the incidence of anal squamous cell carcinoma at 131 per 100,000 person-years among human immunodeficiency virus (HIV)-positive men-who-have-sex-with-men (MSM).1 Furthermore, the incidence of anal squamous carcinoma has been increasing at a rate of 2.7% per year between 2001 and 2015, and within this period, both incidence of higher stage disease and anal cancer mortality increased.2 These increases, at least in part, likely reflect the natural progression of a communicable disease that is inadequately screened and treated. Previously, there were no standard anal cancer screening guidelines due to a lack of prospective data. However, in late 2022, the ANCHOR Trial was published.3 The ANCHOR trial was a Phase III trial at 25 sites within the United States that randomized 4459 HIV-positive patients, at least 35 years of age, with biopsy-proven anal high-grade squamous intraepithelial lesion (HSIL), 1:1 to either a treatment arm or an active monitoring arm. The trial was concluded early when accumulating data showed the rate of progression to anal cancer was significantly lower (57%; 95% CI, 6 to 80; P = 0.03 by log-rank test) in the treatment group than in the active monitoring group. Following publication of the ANCHOR trial results, the International Anal Neoplasia Society published anal cancer screening guidelines to support implementation of anal cancer screening and treatment as standard of care.4
However, there are challenges associated with anal cancer screening. While cytology is an established component of cervical cancer screening, anal cytology is known to underestimate anal lesions.5–8 At present, abnormal anal cancer screening tests are evaluated further with high-resolution anoscopy (HRA) and biopsies.9 This procedure has a steep learning curve and there is a shortage of experienced providers nationwide. Additionally, anal biopsies, especially repeated biopsies for screening over time, can be associated with patient discomfort and morbidity.10,11
In vivo microscopy (IVM) is the acquisition of microscopic images in real time during clinical procedures. It does not require tissue removal from patients, or the time and resources required for subsequent traditional histopathology tissue processing.12–14 In the setting of anal cancer screening, IVM has the potential to accurately identify diagnostic tissue at the time of anoscopy for the acquisition of high-yield biopsies, thereby reducing number of required biopsies and associated complications. IVM may also be used as an educational tool for real-time feedback for HRA practitioners in training. Furthermore, large volume scanning of the anal canal using IVM technology has the potential to augment and possibly replace cytology as a primary screening tool. Finally, IVM may be utilized in “see-and-treat” protocols in low-resource settings where lack of pathology services causes delay in diagnosis and treatment.
IVM technologies, such as optical coherence tomography (OCT) and reflectance confocal microscopy (RCM), have been extensively explored for microscopic evaluation of tissue in the fields of ophthalmology, dermatology, cardiology, and gastroenterology. However, both OCT and RCM have technological limitations that are suboptimal for the evaluation of HPV-related squamous dysplasia of the lower anogenital tract. While RCM generates high-quality images with cellular resolution (1–2 μm lateral resolution; 5 μm axial resolution), the field of view (FOV) is limited to 500–700 μm, which poses challenges in imaging large regions of disease-susceptible tissue.15–17 More critically, RCM generates en face images when cross-sectional images are required for the evaluation of squamous maturation, a critical diagnostic parameter of HPV-associated squamous dysplasia. Although OCT generates cross-sectional images, its resolution is around 10–20 μm, which is suitable for evaluation of architectural features but not cellular morphology.18,19
Scattering-based light sheet microscopy (sLSM) is a promising optical imaging modality given its combined features of cross-sectional imaging, cellular resolution (1–2 μm lateral resolution; 5 μm axial resolution), large field of view (1.5–3 mm), and imaging depth sufficient to visualize a typical full-thickness squamous epithelium (100–200 μm).20,21 sLSM uses a thin illumination light sheet to create optical sections. Although exogenous fluorescence agents are often needed in LSM used for basic life science applications, this is not required in sLSM; images may be generated based on endogenous light-scattering properties of tissue. Given that LSM has been primarily used for basic life science research, it was necessary for our group to optimize the optical parameters of our sLSM device for human tissue imaging.22–24 We then conducted a pilot study by imaging formalin-fixed human tissue, the results of which have been previously reported in abstract form (platform presentation at the 112th Annual Meeting of the United-States-and-Canadian-Academy-of-Pathology (Meeting Abstract: 1529)). Briefly, tissue remnants from our pathology laboratory, ready to be discarded after clinical evaluation was completed, were obtained for sLSM imaging. Ten sections of normal anal mucosa and transformation zone, 2 sections of low-grade squamous intraepithelial lesion (LSIL), and 2 sections of HSIL were imaged and a total of 2974 sLSM images were generated (Supplemental Figure 1). The study showed that sLSM image resolution was sufficient to evaluate cellular features such as nuclear size variation and degree of crowding, as well as cell membrane and cytoplasm features. The imaging depth was also sufficient for the evaluation of epithelial maturation. The composite findings in the sLSM images allowed accurate distinction of normal epithelium, LSIL, and HSIL in fixed tissue. With these results in hand, we proceeded with a prospective clinical study to image fresh biopsy tissue with the intent to establish proof-of-principle that our sLSM device can accurately recapitulate diagnostic features of squamous dysplasia and can become an effective tool for anal cancer screening.
MATERIALS AND METHODS
Bench sLSM setup:
The schematic of the bench sLSM setup used for imaging anal biopsied is shown in Figure 1A. In the illumination path (left half of Figure 1A), light from a red LED (GH CSSRM4.24, OSRAM; wavelength = 657 nm) was used as the light source. Use of an LED provided advantages over using a coherent light source commonly used for LSM such as a laser: low cost, and reduction in speckle noise and shadow artifacts.25 However, poor coupling efficiency between the LED and the illumination slit reduced the illumination power on the sample, which in turn reduced the imaging speed. The LED light was filtered by a slit (width = 3 μm) and formed a light sheet illumination with the thickness of 5.0 μm and width of 1.5 mm over a depth range of 195 μm. In the detection path (right half of Figure 1A), scattered light from the tissue was captured by a water-immersion objective lens (N10XW-PF, Nikon; NA = 0.3; magnification = 10) and re-focused by the tube lens (focal length = 85 mm) on a monochromatic CMOS sensor (acA4024–29um, Basler; pixel size = 2.0 μm; 3,840 × 2,160 pixels). The bench sLSM setup provided a lateral resolution of 1.4 μm and axial resolution of 5.2 μm. A photo of the bench sLSM setup is shown in Figure 1B. The specimen was placed on a rotational stage and two linear stages to coincide the angle and location of the specimen with the sLSM imaging area. A motorized linear stage was used to translate the specimen automatically and acquire sLSM images from multiple locations of the specimen. The illumination power on the sample was 9.8 μW. The bench sLSM setup was housed inside an enclosure with black acrylic sheets (enclosure dimension = 58 × 33 × 28 cm3). The enclosure and laptop used for data acquisition were placed on a cart with wheels for easy transport between exam rooms. The material cost of the bench sLSM microscope was approximately $5,000.
Figure 1:

A) Diagram of sLSM imaging setup, B) Photo of sLSM imaging setup used for imaging anal biopsy specimens, with additional labels, C) Photo of specimen holder for fresh anal biopsy specimens, D) Screen capture of sLSM imaging software while imaging fresh biopsies in the anoscopy clinic.
sLSM, scattering-based light sheet microscopy
Study Design:
All study participants were adult patients of M.J.K. undergoing HRA with biopsies for diagnostic and/or treatment purposes as part of the Stanford PEACH (Prevention and Education of Anogenital Cancers and HPV-associated diseases) Program. No additional biopsies were obtained for the sole purpose of this study. All participants gave written informed consent after receiving an explanation of the aims, risks, and benefits of the study. This study was conducted with University of Arizona institutional review board approval. M.J.K. obtained anal biopsies per routine clinic protocols. Fresh biopsy specimens were temporarily placed in 3–5% acetic acid. The biopsy specimens were oriented within an optically clear specimen holder (Figure 1C; CoverWell, Grace Bio-Labs) on the imaging stage, with mucosal surface, a sheet of transparent plastic film (Fluorinated ethylene propylene; thickness = 250 μm) and a generous layer of optically clear ophthalmic gel (GenTeal Tears Lubricant Gel for Severe Dry Eye Relief, Alcon) facing the objective lenses. Then, serial cross-sectional images at 0.1 mm intervals were acquired automatically by the custom image acquisition code developed in LabVIEW (National Instruments) (Figure 1D). Specimens were then placed in labeled containers of formalin and sent for routine histopathology. Final histology was compared with sLSM images to identify distinctive features of normal epithelium, LSIL, and HSIL. Relevant clinical and demographic information, including age, gender, high-risk HPV status, HIV or other immunocompromised status, and smoking history were collected from the electronic medical record.
Diagnostic Accuracy Reader Study:
A training set comprised of a digital slide show presentation with side-by-side sLSM and H&E images of non-dysplastic, LSIL, and HSIL cases (with corresponding descriptions of diagnostic features) was sent out to all participants. Eleven pathologists (including 7 GYN pathologists, 2 GI pathologists, 1 GYN/GI pathologist and 1 pediatric pathologist) participated in the study. Experience levels ranged from pathology fellows (2) to attending pathologists at academic institutions (early career (2), mid-career (3), and late career (4)). All participating GYN and GI pathologists regularly evaluate HPV-associated squamous intraepithelial lesions (SIL) of the lower anogenital tract. Pediatric pathologists do not regularly evaluate SIL; however, one was included in the study to preliminarily assess the possibility of performance discrepancies for pathologists not specialized in the evaluation of SIL. After reviewing the training set, participants completed 2 separate quizzes composed of 1) 15 sLSM images and 2) 15 H&E images (from the same cases but presented in a different order). Each quiz contained 5 non-dysplastic, 5 LSIL, and 5 HSIL cases. Quiz images were selected for best diagnostic features and best morphologic match between sLSM and H&E from the same case. Answer choices were limited to: 1) Non-dysplastic, 2) LSIL and 3) HSIL, and scored for accuracy based on the final pathologic diagnosis based on the H&E.
Statistical Analysis:
We conducted a repeated-measures multivariate analysis of diagnostic accuracy between H&E and sLSM using a linear mixed effects model with imaging methods (H&E, sLSM) and gold standard diagnostic categories (non-dysplastic, LSIL, HSIL) as fixed effects and the readers (11 pathologists) as random effects. Comparative analysis using paired t-test was also performed. The analysis was performed using the Matlab Statistics and Machine Learning Toolbox. We also conducted separate comparisons for each gold standard diagnostic category.
RESULTS
Patient population and biopsy tissue characteristics:
A total of 110 anal biopsies from 31 patients underwent sLSM imaging. Average patient age was 51.8 years. Twenty-one (67.7%) patients were male, 24 (77.4%) were high-risk HPV-positive, 14 (45.2%) were current or former smokers, and 11 (35.5%) were HIV-positive (Table 1). Anal biopsies averaged 0.34 cm in greatest dimension and approximately two minutes were required to image each specimen. A total of 3904 sLSM images of fresh anal biopsy tissue were acquired (all images reviewed by E.Y and B.L). Final pathology reports revealed 30 (27.2%) non-dysplastic, 40 (36.4%) LSIL, and 40 (36.4%) HSIL, diagnoses (Table 2).
Table 1.
Patient Characteristics
| Characteristic | Number (%) |
|---|---|
| Total Number of Patients | 31 |
| Mean Age [range] (years) | 51.8 [21 – 75] |
| Gender | |
| Cisgender male | 21 (67.7) |
| Cisgender female | 9 (29.0) |
| Transgender male | 0 (0.0) |
| Transgender female | 1 (3.2) |
| Nonbinary | 0 (0.0) |
| History of Receptive Anal Sex | |
| Reported | 21 (67.7) |
| Not reported | 10 (32.3) |
| HPV vaccination status | |
| HPV vaccination series complete | 11 (35.5) |
| HPV vaccination series in progress | 5 (16.1) |
| No HPV vaccination | 15 (48.4) |
| HIV status | |
| HIV positive | 11 (35.5) |
| HIV negative | 19 (61.3) |
| HIV not tested | 1 (3.2) |
| Other immunocompromised state * | 3 (9.7) |
| Smoking status | |
| Current smoker | 4 (12.9) |
| Former smoker | 10 (32.3) |
| Never smoker | 17 (54.8) |
Includes two patients status post solid organ transplantation and one patient on immunosuppressing medication for an autoimmune condition
HPV, human papilloma virus; HIV, human immunodeficiency virus
Table 2.
Biopsy Characteristics
| Characteristic | Number (%) |
|---|---|
| Total Number of Biopsies | 110 |
| Mean biopsy size [range] (cm) | 0.34 [0.1 – 0.7] |
| Final histopathology | |
| Negative for dysplasia | 30 (27.3) |
| LSIL | 40 (36.4) |
| HSIL | 40 (36.4) |
LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion;
sLSM image characteristics of non-dysplastic tissue:
On sLSM imaging of fresh anal tissue, normal squamous mucosa of the anal canal (Figure 2A) is characterized by hyper-reflective round nuclei with uniform size and shape that are densely arranged at the base and become sparser in the superficial layers. Dermal papillae are well-visualized, with bright basal nuclei lining the epithelial edge (Figure 2A bottom left corner marked by arrow). In the superficial layers of some well-differentiated squamous epithelium, faint polygonal cellular borders may be visible in contrast to the hypo-reflective (dark) cytoplasm (Supplemental Figure 2). When present, the most superficial keratinized layer is visualized as an intensely hyper-reflective (bright) band at the top of the epithelial surface. The intense surface signal is caused by the difference in refractive indices between the tissue and its surrounding medium (remnant of low-concentration acetic acid). The trend of intense surface signals has been also observed in reflectance confocal microscopy of human skin.26 The epithelium of anal transition zone (Figure 2C) similarly contains round nuclei with uniform size, shape and distribution, though with a less pronounced gradient of nuclear density from base to superficial layers (i.e., nuclear density remains relatively high even in the superficial layers). Cytoplasmic borders are generally indistinct. Within the colorectal zone (Figure 2E), colonic glands appear as large round or tubular structures with hyper-reflective nuclei outlining the gland (arrows; Figure 2E). Centrally, the mucinous cytoplasm is hypo-reflective (“C” label, Figure 2E); however, a mildly hyper-reflective signal is seen at the inner edge of cytoplasm, which appears to delineate the central luminal space of the gland (“L” label, figure 2E).
Figure 2:

A) Normal squamous zone epithelium, sLSM image. Arrows demarcate dermal papillae B) Normal squamous zone epithelium, H&E, scale bar = 50 μm, Arrows demarcate dermal papillae C) Anal transition zone epithelium, sLSM image, D) Anal transition zone epithelium, H&E, scale bar = 50 μm, E) Colorectal zone epithelium, sLSM image Arrows mark gland nuclei. “C” = cytoplasm. “L” = gland lumen. F) Colorectal zone epithelium, H&E, scale bar = 50 μm. Arrows mark gland nuclei. “C” = cytoplasm. “L” = gland lumen.
sLSM, scattering-based light sheet microscopy; H&E, hematoxylin and eosin
On occasion, a denuded area of the biopsy specimen was imaged, or the biopsy specimen was inadvertently imaged with the epithelial surface facing down. In these cases, the stroma was directly imaged (Supplemental Figure 3). On sLSM imaging, stroma demonstrates hyper-reflective elongated nuclei arranged haphazardly within connective tissue. The collagenous stromal tissue appears as hyper-reflective strands, while the edematous regions appear as intervening hypo-reflective spaces. In cases where the specimen was found upside down, the specimen was re-mounted for re-imaging.
sLSM image characteristics of LSIL:
LSIL (Figure 3) is characterized by enlarged, variably sized hyper-reflective nuclei present at the superficial epithelial layers. The contrast between the hyper-reflective cytoplasmic borders (example of cytoplasmic border marked by arrows; Figure 3A) and hypo-reflective cytoplasm is intense compared to non-dysplastic tissue, and it creates an appearance resembling the koilocytic changes seen on H&E-stained slides of LSIL. Some cells appear to be devoid of nuclei likely because they were not present within the plane of the imaged optical section, creating a moth-eaten appearance within the epithelium. Squamous maturation is apparent from the low nuclear-to-cytoplasmic ratio of the cells in the superficial layers.
Figure 3:
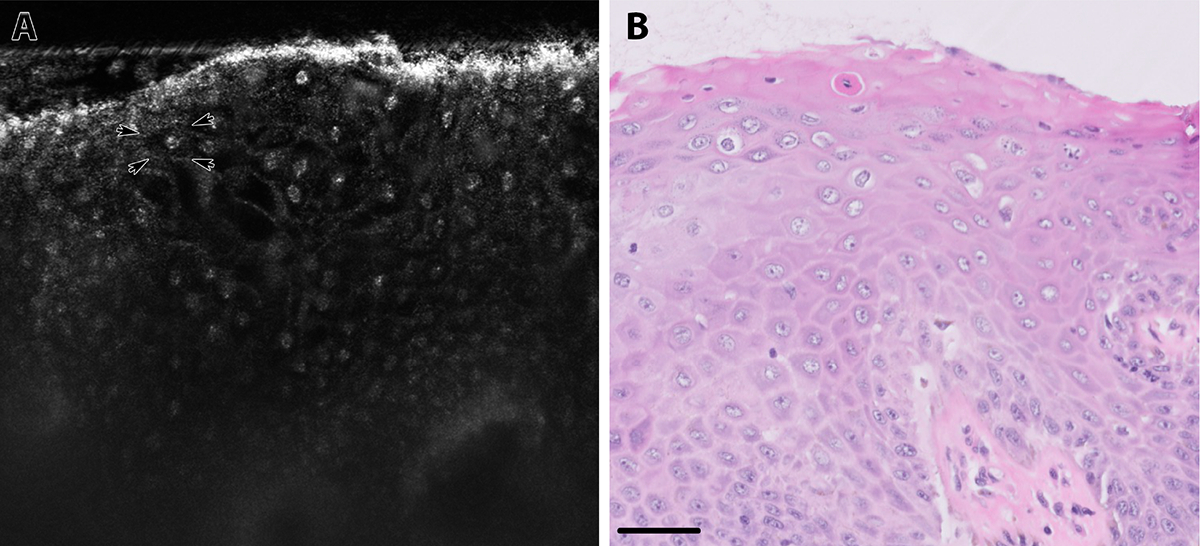
A) LSIL, sLSM image. Arrows mark cytoplasmic border. B) LSIL, H&E image, scale bar = 50 μm
LSIL, low-grade squamous intraepithelial lesion; sLSM, scattering-based light sheet microscopy; H&E, hematoxylin and eosin
sLSM image characteristics of HSIL:
HSIL (Figure 4) is characterized by enlarged, unevenly sized, and markedly crowded hyper-reflective nuclei spanning the full thickness of the epithelium. Cytoplasmic borders are indistinct. Compared to anal transformation zone (Figure 2C, which also lacks overt squamous maturation and may demonstrate some morphologic overlap with HSIL), the nuclear density is clearly greater in HSIL, and HSIL nuclei are more hyper-reflective. A colorectal gland is visible as a hyper-reflective round structure deep to dysplastic squamous epithelium; however, at this depth, nuclear details are lost (Figure 4A, marked by arrows).
Figure 4:

A) HSIL, sLSM image, arrows mark deep colorectal gland B) HSIL, H&E image, scale bar = 50 μm, arrows mark deep colorectal gland
HSIL, high-grade squamous intraepithelial lesion; sLSM, scattering-based light sheet microscopy; H&E, hematoxylin and eosin
Diagnostic Accuracy Reader Study:
11 pathologists (including 7 GYN pathologists, 2 GI pathologists, 1 GYN/GI pathologist and 1 pediatric pathologist) participated in the diagnostic accuracy reader study (Quiz results are shown in Supplemental Tables 1A–1B). Multivariate analysis showed that imaging methods and diagnostic categories had no significant marginal effects on accuracy (p = 0.072, p = 0.122, respectively). Our findings from the comparative analysis indicated that there was no statistically significant difference in overall diagnostic accuracy, with accuracy values of 0.80 for H&E and 0.87 for sLSM (p = 0.066). When stratified by diagnostic categories, the diagnostic accuracy for LSIL was significantly higher for sLSM (0.65 for H&E versus 0.87 for sLSM, p = 0.020). No statistically significant difference was observed in the diagnostic accuracy for HSIL, with values of 0.85 for H&E and 0.91 for sLSM (p = 0.400). The diagnostic accuracy for non-dysplastic images was significantly higher for H&E (0.89 for H&E versus 0.82 for sLSM, p = 0.021).
DISCUSSION
In this study, we demonstrate that sLSM images accurately recapitulate morphologic features seen on traditional H&E sections. We found that key diagnostic features, such as koilocytic changes in LSIL and full-thickness atypia in HSIL, are readily appreciated in a visual format (cross-sectional images at cellular resolution) that is already familiar to pathologists. We are currently developing a handheld device for in vivo imaging, which would allow real-time acquisition of high-quality microscopic images. With the guidance of such a device, we believe that anoscopists will be able to increase the diagnostic yield of their biopsies and reduce the total number of biopsies, leading to enhanced efficacy and lowered morbidity of anal cancer screening.
An additional advantage of the sLSM device is its potentially low cost. sLSM uses inexpensive, low- magnification objective lenses, and consumer-grade optoelectrical components such as LEDs and CMOS sensors that have become available at low cost for building consumer electronics. The benchtop microscope utilized in this study was built with a material cost of $5,000, and a hand-held device is currently under development with an initial material cost of $2,000. While it is premature to predict the market price of clinically viable sLSM devices, a lower cost device has greater potential for widening access to anal cancer screening. A high-priority use case of our device is deployment in low-resource settings in which pathology laboratory infrastructure is not available. In such settings where see-and-treat approaches would prevent delays in treatment, a low-cost sLSM device would allow real-time microscopic visualization and identification of tissue that require treatment. We envision a scenario in which images from our low-cost device are remotely interpreted by pathologists around the world, providing diagnoses within minutes rather than weeks to months; this is a significant step towards democratizing pathology expertise and medical care.
One fundamental limitation of sLSM is that the scattering signal is not specific to one particular sub-cellular component or molecule, as is the case with fluorescence-based LSM with exogenous contrast agents. However, through comparative analysis between paired sLSM and H&E images, we found that the size, shape, and arrangement of the scatters could be used to identify certain cellular and sub-cellular components in sLSM images. In all tissue types we imaged, we found that squamous epithelial cell nuclei generated distinctive, bright round scattering signals similar to scattering-based microscopy of cervical and esophageal tissues.27,28 Nuclear features discernible on sLSM images (e.g., size, shape, density, and brightness) closely correlated with those seen in the corresponding H&E sections and allowed identification of squamous intraepithelial lesions based on diagnostic features analogous to those utilized in traditional histopathology. For example, diagnostic features of HSIL on H&E include enlarged hyperchromatic nuclei without epithelial maturation, which correlates with enlarged, bright, densely arranged nuclei on the sLSM image (Figure 4). Cytoplasmic features are appreciated on sLSM as well. Most notably, the perinuclear halo of a koilocyte (hallmark morphologic feature of LSIL) is accurately recapitulated on the sLSM image by hyper-reflective enlarged nuclei surrounded by a contrasting hypo-reflective cytoplasm. The accuracy with which sLSM emulates H&E morphologic features is underscored by how readily pathologists were able to adapt to this novel imaging modality and demonstrate high performance on the reader study. In contrast to traditional histopathology, imaging depth is a major limitation for sLSM. While the imaging depth was sufficient to evaluate epithelial maturation, degradation of image quality was notable at the base of the epithelium. Deep structures such as colorectal glands are recognizable as bright, round and tubular structures, but no cellular detail is visible at that depth. Given the above, without any significant modifications to the parameters of our sLSM device, evaluation of stromal invasion (i.e., detection of squamous cell carcinoma) will be challenging and invasive cancer detection may require deferral to digital anorectal examination (DARE) and tissue biopsy.
sLSM imaging faces diagnostic challenges analogous to those faced in traditional H&E evaluation. For example, anal intraepithelial neoplasia 2 (AIN2) is a poorly reproducible diagnostic category that requires p16 immunohistochemical stain for confirmation.29 A major challenge is that there is significant morphologic overlap between reactive squamous metaplasia in the anal transition zone and AIN2, in that both entities feature epithelium with mild-to-moderate nuclear enlargement/atypia in the setting of minimal epithelial maturation. We observed that sLSM images also recapitulate this morphologic overlap (Supplemental Figure 4 – A) AIN2 vs. B) anal transition zone). While one may argue that AIN2 demonstrates slightly more nuclear size variation and brightness, judgement calls on such subtle differences are not reproducible. In our usual pathology practice, we utilize the p16 stain to ensure we are not overcalling reactive squamous metaplasia as AIN2/HSIL; however, such immunohistochemical analysis is not possible on sLSM images, and this is a situation in which a tissue biopsy would be required. A future goal is to train an artificial intelligence algorithm to recognize features of squamous dysplasia in sLSM images for diagnostic assistance; it may be that AI will eventually be able to recognize subtle or novel features that are undetectable to the human eye and will obviate the need for p16. Even if AI cannot emulate and surpass the diagnostic/prognostic performance of traditional H&E and immunohistochemical stains, it may serve as a practical triaging tool for anoscopists practicing without access to a pathologist.
We noted some challenges handling small biopsy specimens, as they needed to be manipulated gently to not compromise tissue integrity. Tissue samples that did not sit steadily on the stage, samples that were contorted/twisted, and samples with rough surfaces yielded images with blurry areas. However, given that we acquired an average of 35 sLSM images per specimen (analogous to 35 level sections on H&E), occasional foci of poorly focused tissue did not hinder lesion detection by sLSM. Furthermore, these tissue handling challenges are unique to ex vivo imaging of delicate, small biopsy tissue, and are largely expected to be resolved in the setting of in vivo imaging.
Results from our diagnostic accuracy reader study suggest noninferiority of sLSM images compared to H&E images for the detection of dysplasia. In fact, when stratified by diagnostic category, diagnostic accuracy for LSIL was superior on sLSM. Several factors likely contributed to these findings. First, the diagnostic reproducibility of LSIL is notoriously low on traditional histopathological evaluation and our H&E accuracy is in keeping with what is reported in literature.30,31 Second, the morphologic features of LSIL seen on sLSM images were more accentuated. In particular, the hypo-reflective perinuclear cytoplasm in contrast to the hyper-reflective nucleus and cytoplasmic borders created a “koilocytic” appearance much more readily appreciated sLSM compared to the corresponding H&E. Conversely, the diagnostic accuracy for non-dysplastic images was significantly higher for H&E. A non-dysplastic case that had the lowest diagnostic accuracy on sLSM (case 4, accuracy = 0.545) was characterized by hyperkeratosis (bright signal at the superficial epithelial layer) and reactive enlargement of nuclei which led to an overcall as LSIL (2 pathologists) or HSIL (3 pathologists). In a discussion with one of the reader study participants, the findings were not definitive for dysplasia and the option for an indeterminate diagnostic category may have prevented an overcall. The addition of an indeterminate category is an important consideration when moving forward with clinical deployment, as it may represent the threshold for tissue acquisition for ancillary studies (e.g., p16 immunohistochemical stain or HPV in situ hybridization). Finally, diagnostic accuracy for HSIL was high for both sLSM and H&E, a critical feature for any diagnostic modality targeted for dysplasia detection and screening. All study participants were GYN/GI pathologists who regularly evaluate HPV-associated squamous intraepithelial lesions (SIL) of the lower anogenital tract, except for one pediatric pathologist who was included to preliminary assess the possibility of performance discrepancy in pathologists not specialized in the evaluation of SIL. Although it is premature to draw conclusions based on this single participant, we did not observe any significant difference in performance (Accuracy = 0.8 on both sLSM and H&E images). A limitation of this reader study is the small sample size. Although a larger test set was considered, a smaller test set was chosen to encourage participation from a group of busy pathologists. Nonetheless, these preliminary findings of diagnostic accuracy are promising and provide support to move forward with the development of a handheld IVM device using sLSM technology.
In vivo microscopy (IVM) offers exciting opportunities for pathologists to expand their practice to patients’ bedsides while simultaneously streamlining and reducing morbidity associated with diagnostic procedures. In fields such as gastroenterology and dermatology, clinicians are already working to incorporate IVM into their practices. As experts in microscopic image interpretation, pathologists’ input is expected to improve diagnostic accuracy while reducing the clinician’s burden to adapt to novel technology. While not explicitly designed for this purpose, our current study, conducted within the Stanford PEACH Program, demonstrates the feasibility of pathologists providing diagnostic input in real-time while working with clinicians and their staff in a busy outpatient clinic setting. Admittedly, pathology is currently an overburdened specialty and we acknowledge the increased workload associated with this proposed model. As a field, we must capitalize on the advances made by digital pathology and integrated computational analysis strategies to improve pathologist efficiency and accuracy. Deployment of optical imaging solutions is no exception, and we remain hopeful that pathologist-trained AI algorithms will dramatically enhance patient care without adding strain to the current system.
Supplementary Material
Funding Statement
This study was supported by the National Institute of Biomedical Imaging and Bioengineering (R21EB030079).
The University of Arizona has a technology-licensing agreement with ArgosMD on the portable confocal microscopy technology. Dongkyun Kang has the rights to receive royalties as a result of this licensing agreement. Dongkyun Kang serves as a scientific advisor to ArgosMD. No other authors have conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics Approval / Consent to Participate
This study was conducted with University of Arizona institutional review board approval (protocol 64839). All patient participants gave written informed consent after receiving an explanation of the aims, risks, and benefits of the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Silverberg MJ, Lau B, Justice AC, Engels E, Gill MJ, Goedert JJ, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012. Apr;54(7):1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deshmukh AA, Suk R, Shiels MS, Sonawane K, Nyitray AG, Liu Y, et al. Recent Trends in Squamous Cell Carcinoma of the Anus Incidence and Mortality in the United States, 2001–2015. J Natl Cancer Inst. 2020. Aug 1;112(8):829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palefsky JM, Lee JY, Jay N, Goldstone SE, Darragh TM, Dunlevy HA, et al. Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer. N Engl J Med. 2022. Jun 16;386(24):2273–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stier EA, Clarke MA, Deshmukh AA, Wentzensen N, Liu Y, Poynten IM, et al. International Anal Neoplasia Society’s consensus guidelines for anal cancer screening. Int J Cancer [Internet]. 2024. Jan 31; Available from: 10.1002/ijc.34850 [DOI] [PubMed] [Google Scholar]
- 5.Palefsky JM, Holly EA, Hogeboom CJ, Berry JM, Jay N, Darragh TM. Anal cytology as a screening tool for anal squamous intraepithelial lesions. J Acquir Immune Defic Syndr Hum Retrovirol. 1997. Apr 15;14(5):415–22. [DOI] [PubMed] [Google Scholar]
- 6.Bean SM, Chhieng DC. Anal-rectal cytology: a review. Diagn Cytopathol. 2010. Jul;38(7):538–46. [DOI] [PubMed] [Google Scholar]
- 7.Zhao C, Domfeh AB, Austin RM. Histopathologic outcomes and clinical correlations for high-risk patients screened with anal cytology. Acta Cytol. 2012;56(1):62–7. [DOI] [PubMed] [Google Scholar]
- 8.Betancourt EM, Wahbah MM, Been LC, Chiao EY, Citron DR, Laucirica R. Anal cytology as a predictor of anal intraepithelial neoplasia in HIV-positive men and women. Diagn Cytopathol. 2013. Aug;41(8):697–702. [DOI] [PubMed] [Google Scholar]
- 9.Park IU, Palefsky JM. Evaluation and Management of Anal Intraepithelial Neoplasia in HIV-Negative and HIV-Positive Men Who Have Sex with Men. Curr Infect Dis Rep. 2010. Mar;12(2):126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camus M, Lesage AC, Fléjou JF, Hoyeau N, Atienza P, Etienney I. Which lesions should be biopsied during high-resolution anoscopy? Prospective descriptive study of simple morphological criteria. J Low Genit Tract Dis. 2015. Apr;19(2):156–60. [DOI] [PubMed] [Google Scholar]
- 11.Wasserman P, Rubin DS, Turett G. Review: Anal Intraepithelial Neoplasia in HIV-Infected Men Who Have Sex with Men: Is Screening and Treatment Justified. AIDS Patient Care STDS. 2017. Jun;31(6):245–53. [DOI] [PubMed] [Google Scholar]
- 12.Hariri LP. In vivo microscopy: will the microscope move from our desk into the patient. Arch Pathol Lab Med. 2015. Jun;139(6):719–20. [DOI] [PubMed] [Google Scholar]
- 13.Bishop KW, Maitland KC, Rajadhyaksha M, Liu JTC. In vivo microscopy as an adjunctive tool to guide detection, diagnosis, and treatment. J Biomed Opt. 2022. Apr;27(4):040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnamurthy S, Brown JQ, Iftimia N, Levenson RM, Rajadhyaksha M. Ex Vivo Microscopy: A Promising Next-Generation Digital Microscopy Tool for Surgical Pathology Practice. Arch Pathol Lab Med. 2019. Sep;143(9):1058–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cinotti E, Gergelé L, Perrot JL, Dominé A, Labeille B, Borelli P, et al. Quantification of capillary blood cell flow using reflectance confocal microscopy. Skin Res Technol. 2014. Aug;20(3):373–8. [DOI] [PubMed] [Google Scholar]
- 16.Meyer LE, Otberg N, Sterry W, Lademann J. In vivo confocal scanning laser microscopy: comparison of the reflectance and fluorescence mode by imaging human skin. J Biomed Opt. 2006;11(4):044012. [DOI] [PubMed] [Google Scholar]
- 17.Peterson G, Zanoni DK, Ardigo M, Migliacci JC, Patel SG, Rajadhyaksha M. Feasibility of a Video-Mosaicking Approach to Extend the Field-of-View For Reflectance Confocal Microscopy in the Oral Cavity In Vivo. Lasers Surg Med. [published online: May 08, 2019]. 10.1002/lsm.23090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welzel J Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001. Feb;7(1):1–9. [DOI] [PubMed] [Google Scholar]
- 19.Leitgeb RA. En face optical coherence tomography: a technology review [Invited]. Biomed Opt Express. 2019. May 1;10(5):2177–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Power RM, Huisken J. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat Methods. 2017. Mar 31;14(4):360–73. [DOI] [PubMed] [Google Scholar]
- 21.Chen BC, Legant WR, Wang K, Shao L, Milkie DE, Davidson MW, et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science. 2014. Oct 24;346(6208):1257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z, Downie H, Rozbicki E, Dupuy LX, MacDonald MP. Light Sheet Tomography (LST) for in situ imaging of plant roots. Opt Express. 2013. Jul 15;21(14):16239–47. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen CD, O’Neal PK, Kulkarni N, Yang E, Kang D. Scattering-Based Light-Sheet Microscopy for Rapid Cellular Imaging of Fresh Tissue. Lasers Surg Med. 2021. Aug;53(6):872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J, Kulkarni N, Dobo E, Khan MJ, Yang E, Kang D. Investigation of different wavelengths for scattering-based light sheet microscopy. Biomed Opt Express. 2022. Jul 1;13(7):3882–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J, Liang B, Dobo E, Khan MJ, Yang E, Kang D. Speckle and shadow artifacts reduction in scattering-based light sheet microscopy. In: Biophotonics Congress: Optics in the Life Sciences 2023 (OMA, NTM, BODA, OMP, BRAIN). Washington, D.C.: Optica Publishing Group; 2023. p. DTu2A.3. [Google Scholar]
- 26.Scope A, Benvenuto-Andrade C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007. Oct;57(4):644–658. [DOI] [PubMed] [Google Scholar]
- 27.Sung KB, Richards-Kortum R, Follen M, Malpica A, Liang C, Descour M. Fiber optic confocal reflectance microscopy: a new real-time technique to view nuclear morphology in cervical squamous epithelium in vivo. Opt Express. 2003;11(24):3171–81. [DOI] [PubMed] [Google Scholar]
- 28.Kang D, Schlachter SC, Carruth RW, Kim M, Wu T, Tabatabaei N, Vacas-Jacques P, Shishkov M, Woods K, Sauk JS, Leung J, Nishioka NS, Tearney GJ. Comprehensive confocal endomicroscopy of the esophagus in vivo. Endosc Int Open. 2014;2(3):E135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darragh TM, Colgan TJ, Thomas Cox J, Heller DS, Henry MR, Luff RD, et al. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol. 2013. Jan;32(1):76–115. [DOI] [PubMed] [Google Scholar]
- 30.Stoler MH, Schiffman M. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001. Mar 21;285(11):1500–5. [DOI] [PubMed] [Google Scholar]
- 31.Mills AM, Coppock JD, Willis BC, Stoler MH. HPV E6/E7 mRNA In Situ Hybridization in the Diagnosis of Cervical Low-grade Squamous Intraepithelial Lesions (LSIL). Am J Surg Pathol. 2018. Feb;42(2):192–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


