Summary
Background:
Andes virus (ANDV) is a zoonotic orthohantavirus leading to hantavirus cardiopulmonary syndrome (HCPS). While most transmission occurs through environmental exposure to rodent feces and urine, rare person-to-person transmission has been documented, mainly for close contact. This study investigates the presence and infectivity of ANDV in body fluids from confirmed ANDV-cases as well as duration of viremia.
Methods:
One-hundred and thirty-one confirmed ANDV-infected cases were enrolled in a prospective study between 2008 and 2022. Clinical samples (buffy-coat, plasma, gingival crevicular fluid (GCF), saliva, nasopharyngeal swabs (NPS), and urine were collected weekly for three weeks together with clinical and epidemiological data. Samples were categorized as acute or convalescent (up to and after 16 days following onset of symptoms). Infectivity of positive fluids was assessed after the culture of samples on Vero E6 cells and use of flow cytometry assays to determine the production of ANDV-N protein.
Findings:
ANDV-RNA was detected in 100% of buffy-coats during acute phase, declining to 95% by day 17, and to 93% between days 23–29. ANDV-RNA in GCF and saliva decreased from 30% and 12%, respectively, during the acute phase to 12% and 11% during convalescent. Successful infectivity assays of RT-qPCR-positive fluids, including GCF, saliva, NPS, and urine, were observed in 42% (18/43) obtained during the acute phase of infection. After re-culture, the capacity to infect Vero E6 cells was maintained in 88·9% (16/18). Severity was associated with the presence of ANDV-RNA in one or more fluids beside blood with a OR of 2·58 (95% IC: 1·42–5·18).
Interpretation:
ANDV-infection is a systemic and viremic infection, that seeds various organs. The presence of infectious particles in body fluids contributes to our understanding of potential mechanisms for person-to-person transmission, supporting the development of preventive strategies. Detection of ANDV-RNA in additional fluids upon hospital admission is a predictor of disease severity.
Keywords: Hantaviruses, Zoonotic Infectious Diseases, viral load, ANDV, transmission
INTRODUCTION
The Orthohantavirus andesense (ANDV) is a zoonotic orthohantavirus that causes a severe form of hantavirus cardiopulmonary syndrome (HCPS) in humans. No specific treatment or vaccine is available for ANDV infection 1. The virus has been primarily detected in Chile and the southern region of Argentina, where seasonal outbreaks occur every year 1,2. The main reservoir is the rodent Oligoryzomys longicaudatus, which is present primarily in rural areas in central and southern of Chile3.
The inhalation of the virus from contaminated environments, specifically those with rodent feces, urine, or saliva, is the main route of infection. Exceptionally for ANDV, close and intimate contact with infected individuals represents an additional risk of infection. ANDV is present in the blood of infected individuals for up to two weeks before the onset of symptoms and during symptomatic disease 4. Following an average incubation period of approximately 18 days 5, human ANDV infection can result in a spectrum of illness. It may manifest as either a subclinical or mild disease, with initial non-specific symptoms including fever, myalgia, and headache, or severe HCPS, in more than 50% of cases, with severe compromise of the lungs and heart, the two main target organs1. Rapid onset of pulmonary edema due to increased vascular permeability, or microvascular leakage, is followed by cardiogenic shock and death in 20–35% of reported cases1,6.
Epidemiological and molecular studies have documented person-to-person transmission of ANDV, particularly when investigating clusters of cases among family members whose index case was infected in environmental risk activities. Nosocomial transmission has also been reported4,7–10. Cases of person-to-person transmission generally represent a low percentage of cases, but occasionally case clusters are observed with a high secondary attack rate 8–10.
In a study in Chile, 476 household contacts of 76 ANDV-confirmed index cases were followed for five weeks. Sixteen of the 476 household contacts were identified as hantavirus infected during prospective of follow up, and risk was ten times higher for sex partners4. However, non-sex partners with close contact such as deep kissing and sharing a bed or room with the index case were also at increased risk. A dramatic ANDV outbreak with 34 cases and 11 deaths occurred in Argentina in 2018–2019, and person-to-person transmission was identified as the most likely source. The outbreak stopped after four generations of infection in humans. The authors postulate that subjects were “super-spreaders” with a high viral load and liver injury. Comparison of genome sequence analysis of the Epuyén/18–19 and Epilink/96 (other genomes associated with person-to-person transmission) showed few genomic mutations, suggesting that the virus maintains specific characteristics that allow it to transition easily from rodents to humans and subsequently spread among humans in close contact situations 10. Finally, a recent report documented two cases of hantavirus transmission from mother to newborn, with detection of ANDV-RNA and viral particles in breast milk in one of these cases 8,11.
It is likely that transmission through direct contact with body fluids is the primary mode of person-to-person transmission. The presence of hantavirus in saliva has been shown in Puumala hantavirus infections by Pettersson et al. 2008, where viral RNA was detected in 10 patients with epidemic nephropathy12. Moreover, ANDV-RNA was detected in semen 278 days after onset of symptoms in an imported case in Switzerland13. These reports suggest that other body fluids can carry infectious ANDV particles, and these fluids can be responsible for person-to-person transmission.
The present study aimed to investigate potential person-to-person sources by examining the presence and infectivity of ANDV particles in gingival crevicular fluid (GCF), saliva, nasopharyngeal secretions (NPS), and urine during acute illness in confirmed cases. Additionally, we documented ANDV circulation in blood and shedding in fluids throughout the acute and convalescent phases.
METHODS:
Study design and participants
Between 2008 and 2022, ANDV-infected cases were invited to participate in a prospective research study conducted in 10 collaborative research hospitals in Chile. Cases were defined as individuals with epidemiological risk factors defined as: residents or visitors of endemic regions of hantavirus within 7–42 days before or, had close contact (sexual contact or sleep in the same room) with a symptomatic ANDV-confirmed patient in the previous six weeks. Clinical inclusion criteria included fever for more than 48 hours, along with symptoms such as headache, myalgia, gastrointestinal symptoms (nausea, vomiting, abdominal pain, diarrhea), and respiratory symptoms such cough, dyspnea, hypoxia with lung infiltrates and a low platelet count (<150,000/mL).
After informed consent, an epidemiological questionnaire was obtained, which collected information on risk factors and symptoms preceding hospital admission. Virologic confirmation by ANDV RT qPCR or IgM testing was completed within 24–48 hours14.
Sample collection and clinical follow-up
Samples were collected after hospital admission and enrollment: day 0, 7, and 14; these two later-in survivors consented to be followed. The samples included peripheral blood (plasma, serum, and buffy-coat (BC)), GCF (collection method see supplementary material), saliva, NPS, and urine (Fig. 1).
Figure 1. Schematic diagram of phases of infection and study sampling.

A) Phases of infection: After onset of symptoms, days of hospitalization can vary from 1 to 5 days. Acute phase of infection was defined as the first 16 days of symptoms (including prodrome), and convalescent days 17 or more. B) Sampling timeline: The first sampling was on hospitalization/enrollment day, the second and third sampling on day 7 and 14 after enrollment. While samples were collected at time points following hospitalization/enrollment (day 0, 7, 14), it is important to acknowledge the variability in the alignment of these sampling days with the onset of symptoms for different subjects. C) Five types of samples were collected: peripheral blood, gingival crevicular fluids (GCF), saliva, nasopharyngeal swabs (NPS), and urine
To analyze the timing of viremia and shedding in relation to the infection’s natural history, we adjusted samples to the onset of symptoms with an acute phase period corresponding to the first 16 days of disease and a convalescent period including days 17 and beyond. The virological results from samples obtained on days 0, 7 and 14 after enrollment, were added to each patient`s timeline. Although sampling occurred on days 0, 7, and 14, for some subjects, day 0 may correspond to day 17 after the first symptoms, resulting in three-time points in the convalescent phase. Vital signs and cardiopulmonary symptoms were registered together with the general laboratory findings.
Clinical severity was classified as mild hantavirus when patients presented fever with or without respiratory symptoms needing supplemental oxygen by mask but not mechanical ventilation, and severe HCPS when vasoactive drugs, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) were required 1. HCPS outcome was categorized as survivors or deceased.
Viral RNA detection
Blood samples were processed to separate plasma, buffy-coat and serum, and these samples as well as GCF, saliva, NPS, and urine samples were stored at −80°C until analysis. To detect viral RNA, the total nucleic acids of all samples were extracted using the automated system MagNAPure® following the manufacturer’s instructions (Roche Diagnostic, Germany). A specific ANDV Real-time RT-qPCR targeting a conserved region of the S segment was employed, using the same assay utilized in diagnostics 14. Positive RT-qPCR was cataloged when the crossing point (Cp) value was less than 40 cycles, being the high LOD (limit of detection) for the assay.
Infectivity Assay
A subset of 43 ANDV RT-qPCR positive fluids obtained during the acute phase of infection were cultured in Vero E6 cells. The viral infection was allowed to proceed for 8 or 48 hours under an atmosphere of 5% CO2 at 37°C. After this incubation, cells were processed for viral immunodetection using anti-ANDV-N protein primary monoclonal antibody. The stained cells were analyzed by flow cytometry (BD FACSCanto II) 15. The negative control fluids used for this assay were obtained from RT-qPCR ANDV-negative subjects enrolled in a previous hantavirus study 16 (extended procedure and figure are in supplementary materials). 15,16,15
Statistical analysis
Descriptive and modeling analysis was performed using R (version 4·3·0). We use information of 122 subjects with demographic data and early phase samples to perform a multivariable logistic regression to associate patientś severity and positive ANDV-RNA fluids adjusted by sex, age, length of hospitalization, and days of symptoms.
For fluids figures Chi-square with Yates correction test was performed using GraphPad Prism version 8·0·0 for Mac, GraphPad Software, San Diego, California USA, www.graphpad.com.
Ethics
Approval for the use of all samples, data, and research protocol design was obtained from the Ethical Review Board of the Facultad de Medicina, Pontificia Universidad Católica de Chile (Code 12–292,16–092 and 200625067) and approved for all the participant hospitals.
Role of the funding source:
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Description of the study population and sample collection
Over a 14-year period, 131 hantavirus-infected subjects, of whom 68% (90) were male, were enrolled at 10 national health centers from five geographical regions, ranging from 33°S to 41°S south latitude. Their median age was 34 years, and 8·2% were 15 years of age or less. Most cases (77%) presented as single cases, while 23% were part of a family or labor-associated cluster. Clusters comprised two to four confirmed secondary cases with close contact with a case. In one-hundred and ten cases (84%) the only possible recognized source was environmental exposure, in 21 cases (16%) also person-to-person exposure was described (Table 1). The most frequent symptoms were fever, headache, general malaise, and myalgias (supplementary Fig 1) and 78% of cases had 2 or more medical evaluation before hospitalization. The median time between onset of symptom and study enrollment at hospital admission was 4·3 days. Of the 129 patients with available medical records, 61 (47%) required mechanical ventilation and 16 (24%) needed ECMO (Table 1). The most remarkable general laboratory findings were low platelet count with a median of 50,000 per mL and a median hematocrit of 44·3% (Table 1). Based on their epidemiological history, three cases in clusters were most likely acquired from person-to-person transmission. Two involved breastfeeding infants aged 9 and 1 month, whose mothers breastfed them during their acute phase infection. The third was a 10-year-old girl who resided outside of the known ANDV risk areas in Chile. Considering the incubation periods, the only plausible source for her infection was her mother, who was in close contact with the girl during the mother’s prodrome.
Table 1.
Study population description.
| Demographic (n=131) | |
|
| |
| Age, median (IQR) | 33.9 (24.3–45.9) |
| Patients <15 years old, n (%) | 11 (8) |
| Male, n (%) | 90 (69) |
|
| |
| Epidemiological presentation | |
|
| |
| Environmental exposure n (%) | 110 (84) |
| Environmental and person-to-person Exposure n (%) | 21(6) |
| Number of clusters, n | 15 |
| Cases in cluster, n (%) | 30 (23) |
| Environmental and person-to-person risk, n (%) | 21(70) |
| Person to person risk, n (%) | 3 (10) |
|
| |
| Clinical Course | |
|
| |
| Patients with ≥ 1 previous medical evaluation before hospital admission, n (%)a | 98 (76) |
| N of previous medical evaluation before hospitalization, median (min- max) a | 3 (1–4) |
| Days between symptom onset and hospitalization mean (min-max) | 4.3 (0–17) |
| Severe cases, n (%)b | 77 (59) |
| Only mechanical ventilation, n (%) | 61 (47) |
| ECMO + mechanical ventilation, n (%)c | 16 (24) |
| Case Fatality Rate, n (%) | 23 (17) |
| Hospital length of stay, median (IQR) | 10 (6.5–15.5) |
| Hospital length of stay among fatality cases, median (IQR) | 2 (2–3.23) |
|
| |
| Initial laboratory values at admission | |
|
| |
| Platelets x1000/mL, median (IQR)d | 50 (38–70) |
| Hematocrit %, median (IQR)e | 44.3(40–48.8) |
patients with available data, n=129
severe cases were those who needed respiratory and cardiac support with mechanical ventilation, ECMO and vaso active drugs.
patients with available data since 2018, n=68
patients with available data, n=125
patients with available data, n=122
Severity and ANDV positive-fluids at hospital admission
Through logistic regression (supplementary table 1), we show that the number of ANDV-positive-fluids and the length of hospitalization are significantly associated with the severity with an OR of 2·58 (95% IC: 1·42–5·18) and 1·01 (95% IC: 1·03–1·19), adjusted by age, sex, and onset of symptoms, respectively. Meanwhile, the association of ANDV-positive fluids (supplementary table 2) and death shows an OR of 6·84 (IC: 2·20–26·07).
Natural history of viral shedding and viremia
The virological results were allocated in the timeline according to the days following onset of symptoms for each case. ANDV RT-qPCR positive samples (Ct<40) were grouped using 5-day intervals until day 29 after the onset of the symptoms. Hantavirus RNA was detected in at least four different fluids, the buffy-coat, plasma, gingival crevicular fluid and saliva, until day 22 (Fig. 2, and supplementary Fig.S1). During the acute period, all buffy-coat samples tested positive, and more than 90% of them were still positive during the convalescent phase. Conversely, in plasma the percentage of positive samples gradually decreased from 80% during the acute phase to negative during the convalescent phase. Among other samples, GCF showed the highest detection percentage, going from 55% during the first five days to 17% in the convalescent phase.
Figure 2. ANDV shedding timeline per fluid.

Each color-line represent a fluid, Y axis is the proportion of subject where ANDV-RNA detected in our cohort and X correspond to sampling time to onset of symptoms as 0–4, 5–10, 11–16, 17–22 and 23–29 days after onset of symptoms (note that only when the total observations were more than 20 the proportion was graphed). Fluids are represented as follows: In red buffy coat; yellow: Plasma; pink GCF: gingival crevicular fluid; green NPS: Nasopharyngeal Swab; blue Saliva; and light blue Urine.
The positivity of ANDV RT-qPCR for all fluids, except for buffy-coat and urine, decreased significantly in the convalescent phase compared to the acute phase, Chi-square test p<0·05 (Table 2, supplementary Fig 3). Only the buffy-coat remained positive for ANDV-RNA detection beyond 23 days after the onset of symptoms. Figure 3 shows results from samples obtained during the first, second and third weeks following onset of symptoms in 16 subjects. There are a predominant number of ANDV-positive fluids during the first week after onset of symptoms, while only a few samples remain ANDV-positive in the third week.
Table 2.
ANDV RNA detection in all samples, distributed by the time of the infection.
| BUFFY COAT | PLASMA | GCF | NPS | SALIVA | URINE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 95%(IC) | N/n | 95%(IC) | N/n | 95%(IC) | N/n | 95%(IC) | N/n | 95%(IC) | N/n | 95%(IC) | N/n | ||
| Viral RNA Detection | Acute phase | 100(100–98) | 154/154 | 54(45-62) | 134/72 | 30(23-38) | 133/40 | 16(11-23) | 131/21 | 12(7–18) | 37/16 | 13(7–24) | 60/8 |
| Convalescent | 96(96–99) | 48/46 | 30(19-45) | 43/13 | 12(5–25) | 42/5 | 2(0–12) | 41/1 | 11(5–23) | 44/5 | 11(0–43) | 9/1 | |
From 2008 to 2012, only one sample at hospitalization was collected.
From 2012 to 2015, days 0.7 and 14 after hospitalization, samples, including urine, were collected.
From 2016 to 2022, urine samples were not collected.
The acute and convalescent phases refer to the days 0–16 and 17 and beyond after the first symptoms, respectively.
Figure 3. Temporal distribution of positive RNA samples in biological fluids from symptom onset in hantavirus infected patients.
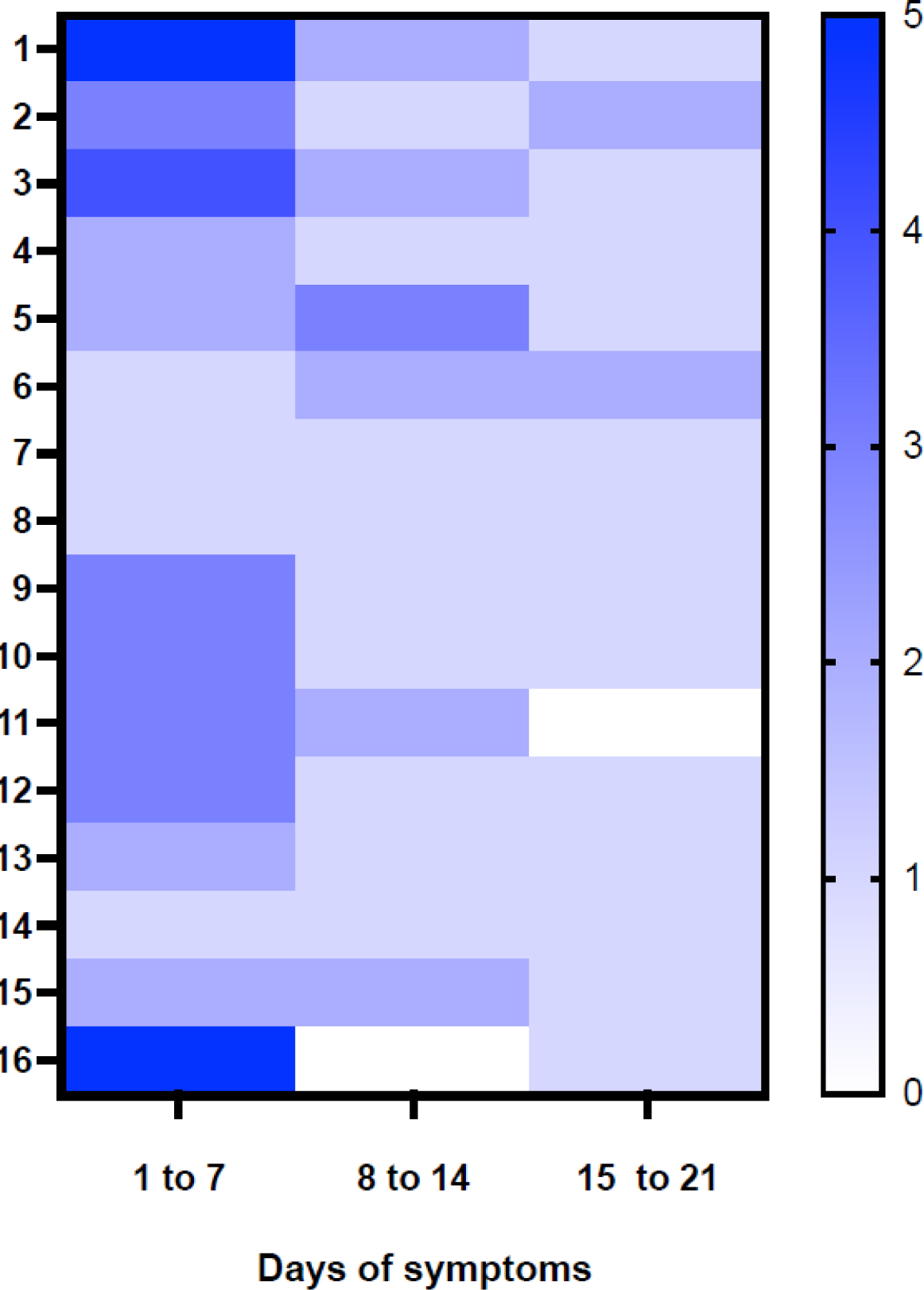
Patients ordered (1–16) on the Y axis according to the minimal to maximal interval of days between first symptoms and enrollment. On the X-axis are intervals of days after the onset of symptoms and represent the first, second, and third sampling. The heatmap score represents the total number of ANDV-positive samples in each visit, 5 (dark blue) is the maximum score (plasma, buffy coat, GCF, saliva and HNF positives) and 0 scores, meaning they are all negative samples (white).
Infectivity assay
To verify infectivity of ANDV-RNA-positive GCF, saliva, NPS and urine sample, a total of 43 samples obtained during the acute phase were cultured in Vero E6 cells. After 8hr post-inoculation and using flow cytometry for detecting infected cells, we identified 42% (18/43) of all the samples with at least 1% of ANDV-infected N-positive cells. For GCF samples 10 of 25 samples (40%) were infective. When we used the 2% cutoff of infection, the positivity decreased for all samples to 14% (6/43). However, when the 18 infective fluids with a cutoff of 1% were re-cultivated for 48hr, the capacity to infect Vero E6 cells was maintained in 88.9% (16/18) (Table 3).
Table 3. ANDV infectivity assay.
Percentage of infection detected by anti-N protein antibody using flow cytometry after inoculation of Vero E6 cells with ANDV-RNA positive samples collected during the acute phase of infection.
| Incubation time | Sample | Number | % Infected cells range | Number of samples >1% infection | Number of samples >2% infection |
|---|---|---|---|---|---|
|
| |||||
| 8 hpi | GCF | 25 | 1–9 | 10 | 3 |
| NPS | 7 | 1–3 | 4 | 1 | |
| Saliva | 10 | 1–13 | 3 | 1 | |
| Urine | 1 | 2 | 1 | 1 | |
| Total | 43 | 18 | 6 | ||
|
| |||||
| 48hpi | GCF | 10 | 0–15 | 8 | 6 |
| NPS | 4 | 1–3 | 4 | 4 | |
| Saliva | 3 | 4–5 | 3 | 3 | |
| Urine | 1 | 9 | 1 | 1 | |
| Total | 18 | 16 | 14 | ||
DISCUSSION.
In this prospective cohort we studied 131 ANDV-infected patients and detected the presence of the virus in different body fluids using RT-qPCR for the S segment of ANDV during the acute and convalescent phases of the infection. Moreover, we were able to detect viral infectivity in oral fluids, particularly in GCF, through virus culture and detection of N-ANDV protein by cytometry assay.
ANDV RNA in buffy-coat was detected in all the tested patients during the acute phase of disease and in a high proportion of them until very late in the convalescent period, reinforcing that buffy-coat sample testing is of extraordinary usefulness for diagnosis of ANDV infection, even if the suspicion of infection is late or retrospective. Other fluids such as plasma, GCF, saliva and NPS were often positive until day 16 after symptomś onset (acute phase) but decreased substantially in the convalescent phase. We also found a correlation between the severity of the illness and the presence of ANDV-RNA in fluids, which can help predict the outcome.
ANDV is the only hantavirus recognized to be transmitted from person-to-person, albeit in a low proportion of cases. Since the first recognition of ANDV associated cases, several observations support person-to-person transmission, among them, family clusters where women and children were the additional cases, the high risk of sexual partners becoming a new ANDV case, absence of environmental exposure in some infected close contacts, and the close contact with fluids such as saliva or infected breast milk as a risk factor4,7,8,10,11,17,18. All support person-to-person transmission. In this report we reinforce the role of the oral fluids likely play in transmission of ANDV disease.
Analyzing the role of different oral fluids in transmission of ANDV, ultrastructural studies found the virus replicating in the human submandibular glands 19. Puumala RNA was also detected in the saliva during the acute phase of illness12. The salivary glands play a role in transmitting other infectious diseases, such as Epstein Barr, cytomegalovirus, rabies and SARS-CoV2. In the case of SARS-CoV-2, the virus has been documented in the acinar and ductal epithelium of the salivary glands and detected in saliva for long periods, even in the absence of any symptoms20,21. In our study, the presence of ANDV RNA in saliva in the acute phase of the disease occurred in 12% of the available samples, with 3 among 10 being infective. Pizarro et al. 2020 described ANDV within the mucus-secreting cells of the acini and the lumen of the entire excretory system in salivary glands tissues of ANDV deceased patients 19. These two findings suggest the feasibility of person-to-person transmission through close contact with these fluids.
It is known that the isolation of hantavirus in cell culture is a laborious task with low efficiency. First, it requires BSL-3 facilities and extensive blind passaging in cell culture to acquire adequate viral titers for further characterization studies22. A surrogate option to detect infectivity could be to measure the viral load before and after cell seeding; however, this option was not chosen since the biological samples used for this study frequently induce cytotoxicity. This is especially true for GCF, which is heavily contaminated with bacteria and cytokines that make it difficult to maintain passages for prolonged periods. In addition, the presence of a low viral load (mean crossing threshold in RT-qPCR of 34) makes it challenging to estimate significant changes in successive passages using the RT-qPCR ANDV technique. Furthermore, accurate estimation of viral load is more difficult in these fluids, which are less uniform in composition than plasma, for example, which is universally used for other viruses loads 23,24.
To address the question if the RNA detected in fluids came from infective viruses, we used flow cytometry to detect and demonstrate infectivity. This technique was validated and published by our group and adapted to biological human samples15. In our infection assays, we employed two different time-point incubation periods. The detection of the N protein at 8 hpi indicates the presence of infective viruses in the inoculum, and detection of N protein after 48hpi is a signal of more viral particle production. The translation process of hantavirus N protein supports this. In an in vitro assay, N protein is detected at 2hpi, mainly in cytoplasmatic granular aggregates, and after 24hpi it can be detected at the Golgi complex. Afterward, the virions assembly begins, and infective viruses are released to infect other cells 15,25. Considering our results on infectivity of fluids in relation to patient histories in our cohort, we note that all patients developed their first symptoms at their homes while being cared by relatives or close friends. Even more, some couples, part of this cohort, slept together, shared kisses and had sexual intercourse during and prior to the febrile prodrome, a described risk factor for acquiring ANDV infection4. As an example of a lack of early recognition of the clinical diagnosis, patients were typically admitted to the hospital approximately four days after the onset of prodromal symptoms. often after two to four outpatient consultations with physical examination and blood sample collection, thereby posing a risk of contagion to healthcare workers. These risk factors have been previously reported in association with additional cases in the hospital environment9. Even more, this time concurs with the most critical period when invasive procedures associated with patient care, such as mechanical ventilation and ECMO, occur shortly after hospital admission.
In fact, within this cohort, 30 cases occurred in clusters, including ten previously reported to be highly suspicious of person-to-person transmission, 8 close household contact and two health care workers9. This information contributes to the understanding of transmission mechanisms and a better recognition of sources of infection that represent a risk for sexual partners, close household contacts, children, and healthcare personnel in reinforcing policies for infection control and prevention strategies.
The presence of the virus in oral fluids during the early stages of infection, often without the individual’s awareness, creates a transmission window for subsequent cases. This risk of transmission is particularly heightened when diagnosis is delayed after the onset of first symptoms. In the prodromal phase of the illness, which is difficult to distinguish from other febrile illnesses based on clinical presentation, all the patients are viremic, as detected by RT-qPCR for ANDV; this typically slowly decreases beyond the first 16 days. This reduction in viral load suggests a potential decline in infectiousness during this period associated with the increase in hantavirus-neutralizing antibodies26. However, it is crucial to know that taking care of patients during this phase still carries risks. One of the limitations of our study was the inability to quantify the viral load during the febrile prodrome before cases are admitted to the hospital, a period when the household contacts are most highly exposed. To actively prevent contagion from a suspected hantavirus patient within a hospital environment, we emphasize adherence to infection control standards, including gown, gloves, and mask until the diagnosis is discarded 27.
To our knowledge, this is the first report documenting the detection of hantavirus in gingival crevicular fluid (GCF). Interestingly, GCF exhibited a high frequency of ANDV RNA detection compared to other fluids. The GCF comprises numerous inflammatory cells, including dendritic cells, and various immunological components, such as cytokines and chemokines 28. Notably, the prevalence of periodontal disease in Chile is estimated to be around 90%29. Taken together, these findings suggest that the presence of ANDV in the GCF may be attributed to the increased recruitment of immune cells to this compartment. Furthermore, it is plausible to consider that the GCF could serve as an important site of infection in susceptible hosts who come into contact with infected secretions from acute hantavirus cases. We also detected ANDV in saliva in a lower proportion than in GCF. However, this difference may be explained by the destruction of viral RNA by enzymes and proteins with antiviral properties highly concentrated in saliva, such as lactoferrins, histatin 5, lysozyme, and mucins28.
Infected respiratory secretions may contribute to the presence of viruses in the mouth, potentially enabling infection transmission during indoor social gatherings. This is reinforced by our detection of infective viruses in nasopharyngeal and oral fluids.
An intriguing aspect to investigate in the virus-host interaction is the role of local inflammation and how it impacts the viral load and infectivity of ANDV. To accomplish this, it is crucial to determine which markers are suitable for assessing the inflammation state in saliva and/or gingival crevicular fluid (GCF) for each patient. These markers could then be correlated with viral load and the risk of transmission.
The results presented in this study help to uncover the potential risk of ANDV infection through biological fluids from symptomatic or acute cases. Understanding the patterns of viral shedding in these body fluids provides critical information that may enhance understanding of person-to-person transmission. In addition, they may play a pivotal role in development of control and prevention measures for healthcare workers and household contacts responsible for caring for individuals with ANDV during the symptomatic phase.
Supplementary Material
Research in context.
Evidence before this study:
This study builds upon previous epidemiological and molecular evidence of ANDV person-to-person transmission in Chile and Argentina. In the previous studies close contact with an infected person, in particular deep kissing, sexual contact, sharing food, drinking with the same straw or breastfeeding during the prodromic phase of the disease, was identified as a high-risk activity for person-to-person transmission. Other studies investigating person-to-person outbreaks rely on next-generation sequencing (NGS) to perform genomic comparisons between of viral RNA epidemiologically related cases.
Added Value of this study:
In this study, we determined the timing of ANDV shedding in clinical samples from a large cohort of cases. Our results suggest that there is a period during the acute phase (up 16 days after onset of symptoms) of infection in which there is significant viral shedding although with variable concentrations and percentages of positivity between compartments. The presence of infectious viral particles in body fluids such as GCF, saliva, and nasopharyngeal secretions suggest that exposure to these fluids may lead to person-to-person transmission. The study also found a correlation between severity and the presence of ANDV-RNA in fluids beside blood.
Implication of all available evidence:
This study contributes to a better understanding of viral shedding in patients during the acute and convalescent phase of the disease. By identifying body fluids with evidence of ANDV presence, we can enhance strategies for infection control for the healthcare personnel and close household contacts, the groups at greatest risk. Also, we expect to provide valuable information for molecular diagnosis from different sample sources.
Funding:
This work was supported by the National Institutes of Health grant N° U01AI055452 and Agencia de Investigación y Desarrollo (ANID), through grant FONDECYT-1161197 and FONDECYT 1211825 to MF, JA and CMV; FONDECYT 1230718 to JA and MF; FONDECYT 1201240 to CV, PV and MF, and CONICYT-Programa de Investigación Asociativa (PIA) ACT1408 to MF and JA; ATE220061 to CV, MF, and PV. NT was supported by Centro Ciencia & Vida (ANID BASAL FB210008), ABV was supported by ANID postdoctoral fellowship (N° 3220310), and CMV was partially supported by ANID doctoral fellowship (N° 21230036), Student PhD in Epidemiology, Facultad de Medicina, Pontificia Universidad Católica de Chile
We thank MINSAL (Viviana Sotomayor, Mauricio Yañez) and local SEREMI (Claudia Dospital, Rita Mansilla, Marco Acuña) for their support and collaboration. And local teams from HHHA:, RN Carolina Palma, RN Vitalia Bahamondes; HBPM:; HHT: RN Carol Burgos,; HBO MT Felipe Vargas, MT Evelyn Mancilla, BQ Jose Gonzalez,; PUC: Catalina Rogers RN; Javiera Pradenas Bs; Jorge Vera-Otarola PhD.
Footnotes
Authors declaration of interests’ statement
All authors of the present study have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing statement
The data is not publicly available due to ethical restriction; as a low incidence infection subject could be easily identify.
REFERENCES
- 1.Vial PA, Ferrés M, Vial C, et al. Hantavirus in humans: a review of clinical aspects and management. Lancet Infect Dis 2023; published online April. [DOI] [PubMed] [Google Scholar]
- 2.Jonsson CB, Figueiredo LTM, Vapalahti O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev 2010; 23: 412–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres-Pérez F, Palma RE, Boric-Bargetto D, et al. A 19 Year Analysis of Small Mammals Associated with Human Hantavirus Cases in Chile. Viruses 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferres M, Vial P, Marco C, et al. Prospective evaluation of household contacts of persons with hantavirus cardiopulmonary syndrome in chile. J Infect Dis 2007; 195: 1563–71. [DOI] [PubMed] [Google Scholar]
- 5.Vial PA, Valdivieso F, Mertz G, et al. Incubation period of hantavirus cardiopulmonary syndrome. Emerg Infect Dis 2006; 12: 1271–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueiredo LTM, Souza WM de, Ferrés M, Enria DA. Hantaviruses and cardiopulmonary syndrome in South America. Virus Res 2014; 187: 43–54. [DOI] [PubMed] [Google Scholar]
- 7.Toro J, Vega JD, Khan AS, et al. An outbreak of hantavirus pulmonary syndrome, Chile, 1997. Emerg Infect Dis 1998; 4: 687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrés M, Mart\’\inez-Valdebenito C, Angulo J, et al. Mother-to-Child Transmission of Andes Virus through Breast Milk, Chile1. Emerg Infect Dis 2020; 26: 1885–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Valdebenito C, Calvo M, Vial C, et al. Person-to-person household and nosocomial transmission of andes hantavirus, Southern Chile, 2011. Emerg Infect Dis 2014; 20: 1629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mart\’\inez VP, Di Paola N, Alonso DO, et al. “Super-Spreaders” and Person-to-Person Transmission of Andes Virus in Argentina. N Engl J Med 2020; 383: 2230–41. [DOI] [PubMed] [Google Scholar]
- 11.Bellomo C, Alonso D, Coelho R, Iglesias A, Periolo N, Mart\’\inez VP. A newborn infected by Andes virus suggests novel routes of hantavirus transmission: a case report. Clin Microbiol Infect 2020; 26: 130–1. [DOI] [PubMed] [Google Scholar]
- 12.Pettersson L, Klingström J, Hardestam J, Lundkvist A, Ahlm C, Evander M. Hantavirus RNA in saliva from patients with hemorrhagic fever with renal syndrome. Emerg Infect Dis. 2008. Mar;14(3):406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuenzli AB, Marschall J, Schefold JC, et al. Hantavirus Cardiopulmonary Syndrome Due to Imported Andes Hantavirus Infection in Switzerland: A Multidisciplinary Challenge, Two Cases and a Literature Review. Clin Infect Dis 2018; 67: 1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vial C, Martinez-Valdebenito C, Rios S, et al. Molecular method for the detection of Andes hantavirus infection: validation for clinical diagnostics. Diagn Microbiol Infect Dis 2016; 84: 36–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barriga GP, Martinez-Valdebenito C, Galeno H, Ferrés M, Lozach P-Y, Tischler ND. A rapid method for infectivity titration of Andes hantavirus using flow cytometry. J Virol Methods 2013; 193: 291–4. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Valdebenito C, Angulo J, Le Corre N, et al. A Single-Nucleotide Polymorphism of αVβ Integrin Is Associated with the Andes Virus Infection Susceptibility. Viruses 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sotomayor P EUV, Aguilera S. X. Epidemiolog\’\ia de la infección humana por hantavirus en Chile. Rev Chilena Infectol 2000; 17: 220–32. [Google Scholar]
- 18.Alonso DO, Pérez-Sautu U, Bellomo Carla M and Prieto K, et al. Person-to-Person Transmission of Andes Virus in Hantavirus Pulmonary Syndrome, Argentina, 2014. Emerg Infect Dis 2020; 26: 756–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pizarro E, Navarrete M, Mendez C, et al. Immunocytochemical and Ultrastructural Evidence Supporting That Andes Hantavirus (ANDV) Is Transmitted Person-to-Person Through the Respiratory and/or Salivary Pathways. Front Microbiol 2020; 10. DOI: 10.3389/FMICB.2019.02992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matuck BF, Dolhnikoff M, Duarte-Neto AN, et al. Salivary glands are a target for SARS-CoV-2: a source for saliva contamination. J Pathol 2021; 254: 239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang N, Pérez P, Kato T, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nature Medicine 2021 27:5 2021; 27: 892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galeno H, Mora J, Villagra E, et al. First human isolate of Hantavirus (Andes virus) in the Americas. Emerg Infect Dis 2002; 8: 657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaniewski E, Dao Ostinelli CH, Chammartin F, et al. Trends in CD4 and viral load testing 2005 to 2018: multicohort study of people living with HIV in Southern Africa. J Int AIDS Soc 2020; 23: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Dummer JS, Estes WR, Meng S, Wright PF, Tang YW. Measurement of Human Cytomegalovirus Loads by Quantitative Real-Time PCR for Monitoring Clinical Intervention in Transplant Recipients. J Clin Microbiol 2003; 41: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knipe DM, Howley PM. Fields’ Virology. Lippincott Williams & Wilkins, 2007. [Google Scholar]
- 26.Padula PJ, Colavecchia SB, Mart\’\inez VP, et al. Genetic diversity, distribution, and serological features of hantavirus infection in five countries in South America. J Clin Microbiol 2000; 38: 3029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministerio de Salud C Guia Clinica de prevención, diagnóstico y tratamiento del sindrome cardiopulmonar por hantavirus. 2013. (January 2024, https://diprece.minsal.cl/wrdprss_minsal/wp-content/uploads/2015/02/Gu%C3%ADa-HANTA-completa.pdf)
- 28.Martinez-Valdebenito C, Andaur C, Angulo J, Henriquez C, Ferrés M, Le Corre N. Characterization of Oral Immunity in Cases and Close Household Contacts Exposed to Andes Orthohantavirus (ANDV). Front Cell Infect Microbiol 2020; 10: 557273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strauss F-J, Espinoza I, Stähli Alexandra M, Cortés R, Morales Alicia J. Dental caries is associated with severe periodontitis in Chilean adults: a cross-sectional study. BMC Oral Health 2019; 19: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is not publicly available due to ethical restriction; as a low incidence infection subject could be easily identify.


