Abstract
Study objective:
In the early months of the coronavirus disease 2019 (COVID-19) pandemic and before vaccine availability, there were concerns that infected emergency department (ED) health care personnel could present a threat to the delivery of emergency medical care. We examined how the pandemic affected staffing levels and whether COVID-19 positive staff were potentially infectious at work in a cohort of US ED health care personnel in 2020.
Methods:
The COVID-19 Evaluation of Risks in Emergency Departments (Project COVERED) project was a multicenter prospective cohort study of US ED health care personnel conducted from May to December 2020. During surveillance, health care personnel completed weekly electronic surveys and underwent periodic serology and nasal reverse transcription polymerase chain reaction testing for SARS-CoV-2, and investigators captured weekly data on health care facility COVID-19 prevalence and health care personnel staffing. Surveys asked about symptoms, potential exposures, work attendance, personal protective equipment use, and behaviors.
Results:
We enrolled 1,673 health care personnel who completed 29,825 person weeks of surveillance. Eighty-nine (5.3%) health care personnel documented 90 (0.3%; 95% confidence interval [CI] 0.2% to 0.4%) person weeks of missed work related to documented or concerns for COVID-19 infection. Health care personnel experienced symptoms of COVID-19 during 1,256 (4.2%) person weeks and worked at least one shift whereas symptomatic during 1,042 (83.0%) of these periods. Seventy-five (4.5%) participants tested positive for SARS-CoV-2 during the surveillance period, including 43 (57.3%) who indicated they never experienced symptoms; 74 (98.7%; 95% CI 90.7% to 99.9%) infected health care personnel worked at least one shift during the initial period of infection, and 71 (94.7%) continued working until laboratory confirmation of their infection. Physician staffing was not associated with the facility or community COVID-19 levels within any time frame studied (Kendall tau’s 0.02, 0.056, and 0.081 for no shift, one-week time shift, and 2-week time shift, respectively).
Conclusions:
During the first wave of the pandemic, COVID-19 infections in ED health care personnel were infrequent, and the time lost from the workforce was minimal. Health care personnel frequently reported for work while infected with SARS-CoV-2 before laboratory confirmation. The ED staffing levels were poorly correlated with facility and community COVID-19 burden.
INTRODUCTION
Background
The first wave of the coronavirus disease 2019 (COVID-19) pandemic in the spring and summer of 2020 was marked by intense stress and uncertainty for emergency medicine department (ED) health care personnel in the United States.1,2 These ED staff were often the first and sometimes only medical care providers exposed to patients infected with COVID-19.3 At a time prior to COVID-19 vaccine availability, when the communicability of the causative agent, SARS-CoV-2, was unknown and personal protective equipment (PPE) mandates shifted, ED health care personnel had uncertain risk of contracting and transmitting the disease at work. This uncertainty was particularly concerning in light of historical outbreaks of a related virus, SARS-CoV, which carried a significant risk of transmission and mortality in the health care setting.4,5 To quantify these risks, we initiated the nationwide, Centers for Disease Control and Prevention (CDC)-sponsored COVID-19 Evaluation of Risks in Emergency Departments (Project COVERED).6
Importance
Our study provided a unique opportunity to explore the threat infected ED health care personnel pose to the delivery of emergency medical care during the earliest phase of a pandemic. At the facility level, loss of personnel due to widespread illness and work restrictions because of quarantine could disrupt an ED’s capacity to provide care, especially during local COVID-19 surges.7 Perhaps more ominously, infected health care personnel could potentially act as vectors for spreading disease to colleagues and patients.8 This theoretical risk would be especially concerning in cases of asymptomatic infection, which is a hallmark of SARS-CoV-2.9,10 In addition is the problem of “presenteeism,” or the practice of health care personnel continuing to work when experiencing symptoms of illness.11 Our results could provide important lessons for policymakers and administrators in the case of the emergence of a more virulent SARS-CoV-2 strain or an entirely novel pathogen.
Goals of This Investigation
Our study sought to explore the extent of this threat by focusing on the following 3 outcomes: 1) the risk of health care personnel presenting to work while infectious with SARS-COV-2, 2) the effect of COVID-19 symptoms and diagnoses on health care personnel work status and facility burden from missed work, and 3) the association between COVID-19 infection levels and ED staffing, with a particular focus on assessing the potential for impaired staffing during times of increased COVID-19 activity.
MATERIALS AND METHODS
Study Setting and Design
We conducted a multicenter surveillance project at 25 geographically diverse EDs affiliated with 20 US academic emergency medicine residency programs from May through December 2020. The project was reviewed by the CDC and was conducted in compliance with applicable laws and CDC policy.12 This activity was determined to meet the requirements of public health surveillance because it was authorized by a public health authority for assessing risk to health care personnel during the COVID-19 pandemic as defined in 45 CFR 46.102(l)(2).12 Surveillance projects are considered exempt from IRB review, however each site’s IRB was provided a limited evaluation of the project and all agreed with this determination. All participating health care personnel provided informed consent.6
Selection of Participants
A detailed presentation of the methods for the project, including a list of investigative sites, is provided elsewhere.6 To briefly summarize, each site enrolled approximately 80 ED health care personnel volunteers who had not previously been infected with SARS-CoV-2. Each participant was screened with nasal swabs for reverse transcription polymerase chain reaction (RT-PCR) testing and blood samples for SARS-CoV-2 immunoglobulin G (IgG) antibody testing with anyone testing positive being excluded and replaced with a negative-testing health care personnel. These individuals were distributed among 4 groups: 1) physicians and advanced practice providers who were likely to intubate, 2) physicians and advanced practice providers who were unlikely to intubate, 3) nurses, and 4) nonclinical staff. Physicians in groups 1 and 2 could include residents, and nonclinical staff could include any ED personnel with routine clinical contact, including case managers, ED pharmacists, unit clerks, etc.
Measurements
We performed nasal swabs for RT-PCR testing and blood samples for SARS-CoV-2 IgG antibody testing at baseline, week 2, week 4, and every 4 subsequent weeks through week 20. Results were available and reported back to participants within 1 week of testing. Results from testing performed outside the study were also collected. Positive tests were self-reported by participants to their respective facilities with quarantine and return-to-work clearance dictated by local policy. Our primary data element was the person-week survey that individual participants completed each week. These surveys ascertained details on several factors, including COVID-19 symptoms, work status, and adherence to PPE guidelines recommended by the CDC during the surveillance period (surgical or N95 facemask for all patient encounters; eye protection, gown, and gloves additionally for all SARS-CoV-2 patient encounters; and N95 respirator additionally for all SARS-CoV-2 aerosol-generating procedures). Staffing data were externally validated at each site by local principal investigator and included weekly hours worked across all ED positions (provider and ancillary) open during the observation period. Investigators reported prevalence of patients hospitalized with COVID-19 in weekly facility surveys. Community COVID-19 infection incidence was determined from public health reports collated from local health department data for each facility’s health service area.13
Outcomes
SARS-CoV-2 infection was defined as a positive RT-PCR (on either a study test or confirmed from outside) or positive IgG antibody testing.
Analysis
Potential for health care personnel to develop COVID-19 and spread infection.
Using data from weekly surveys, we determined the prevalence of health care personnel who developed COVID-19 since the previous survey and an infectivity window for each. This window represents the period when an infected health care personnel could have been contagious and unknowingly worked, whether symptomatic or asymptomatic. It encompasses the earliest possible date of infection, defined as 3 days before the prior RT-PCR test, through the date of acquisition of the positive test, and ended when the provider was notified of their positive test result.6 From this window, we determined the prevalence of infected health care personnel who worked one or more shifts in the ED during their infectivity window. We also determined whether each infected health care personnel reported masking during this window.
Effect of COVID-19 symptoms and diagnoses on health care personnel work status and facility burden from missed work.
We determined the number of weeks health care personnel (both infected and uninfected) reported symptoms of COVID-19 and the number of weeks that health care personnel did and did not work during these periods. This is a particularly important metric because point-of-care testing with immediate results was not available at the time-to-direct isolation practices.
Association between COVID-19 infection levels and ED staffing.
To assess the facility staffing burdens associated with COVID-19 infections and surges, we ascertained the proportion of reduced staffing events, defined as weeks when participating EDs had staffing levels ≤95% of their numerical average staffing level over the course of the entire study observation period. We tabulated the number and proportion of reduced staffing events while COVID-19 infections (both facility and community levels) were increased above the numerical average infection rates for each facility. To allow for direct comparisons across facilities, we employed relative staffing levels with weekly staffing expressed in terms of the average staffing level for the facility throughout the study. Recognizing that variations in case numbers were likely to have different effects on different facilities, we also employed relative infection levels to allow for a direct comparison of COVID-19 infections across centers.
We created scatterplots illustrating the facility staffing levels as a function of the facility and community COVID-19 infection levels. We used Kendall’s tau to provide a quantitative measure of association for each scatterplot. This statistic looks at ordinal associations using a rank correlation and does not look at linear associations. It can take on values from —1 to 1, with a value of 1 indicating complete agreement (strong positive correlation), a value of —1 indicating inverse relationship (strong negative correlation), and a value near zero indicating little or no relationship. Recognizing that COVID-19 volumes could affect future staffing, we conducted one and 2-week time-shifted scatterplots to examine the association between current infection and future staffing.
We generated sample size and power estimates for the primary outcomes of Project COVERED.6 The material we presented in these analyses is descriptive results. We have adjusted all 95% confidence intervals (CIs) for clustering using intraclass correlation and design effects and completed our statistical analyses using Microsoft Excel v 12.3.6 (Microsoft Corporation) and R v.4.0.4 (R Foundation).14 Throughout our analyses, we regarded missing data as absent and excluded it from our calculated point measures. Specifically, we did not employ imputation or estimation.
RESULTS
The COVERED project enrolled 1,673 health care personnel and collected 29,825 person weeks of observational data from 25 EDs all containing emergency medicine residency programs. The ED annual visits ranged from 33,000 to 247,519 by site (2019 data) (Table 1).Weekly cumulative community COVID-19 infection incidence at sites ranged from 0.9 to 158.1 cases per 100,000 population during the study period. (Table 1, Table E1, available at http://www.annemergmed.com).
Table 1.
Background characteristics, Project COVERED, May-December 2020.
| Sites | Total |
|---|---|
| Emergency departments, n | 25 |
| Affiliation with ED residency, n (%) | 25 (100%) |
| 2019 ED visits, median (IQR) | 73,000 (64,247–94,373) |
| ED beds pre-COVID-19, median (IQR) | 68 (50–82) |
| Community COVID-19 incidence (cases per 100,000), median (IQR) | 12.0 (6.8–19.0) |
| Staffing | Total |
| Health care personnel enrolled, n | 1,673 |
| Number of hours staff physician coverage weekly, median (IQR) | 504 (365–672) |
| Hours worked per week, mean (SD) | |
| MD/APP | 32.5 (9.9) |
| RN | 35.0 (5.0) |
| Clinical Staff | 35.4 (8.1) |
MD, medical doctor; RN, registered nurse; APP, advanced practice providers.
Potential for Health Care Personnel to Develop COVID-19 and Spread SARS-CoV-2
During the surveillance period, 75 (4.5%) participants developed COVID-19 infection, including 31 (3.7%) of 844 physicians/advanced practice providers, 29 (7.0%) of 417 nurses, and 15 (3.7%) of 412 nonclinical personnel. COVID-19 infections were asymptomatic in 43 (57.3%) cases, including 21 (67.7%) of 31 physician/advanced practice providers, 17 (58.6%) of 29 nurses, and 5 (33.3%) of 15 nonclinical health care personnel (Table 2).
Table 2.
Proportion of US ED health care personnel who were infected with COVID-19, Project COVERED, May-December 2020.
| Health Care Personnel | Enrolled (n) | Infected (n, %) | Symptomatic (n, %) | Asymptomatic (n, %) | |||
|---|---|---|---|---|---|---|---|
| Total | 1,673 | 75 | 4.5% | 32 | 43% | 43 | 57% |
| MD/APP | 844 | 31 | 3.7% | 10 | 32% | 21 | 68% |
| Nurses | 417 | 29 | 7.0% | 12 | 41% | 17 | 59% |
| Nonclinical | 412 | 15 | 3.6% | 10 | 67% | 5 | 33% |
Seventy-four (98.7%; 95% CI 90.7% to 99.9%) infected health care personnel reported working in the ED during their infectivity window. Four participants (5.3%) stopped working before their diagnosis because of concerns about possible infection, whereas the remaining 71 (94.7%) continued working until laboratory confirmation of their infection. Cumulatively, symptomatic, active COVID-19-infected health care personnel participated in clinical activities during 19 surveillance weeks (0.06% of all surveillance weeks and 44.9% of the 42.3 weeks that these health care personnel were infected). Only one COVID-19-infected health care personnel (physician) reported failure to use universal masking while caring for non–COVID-19 patients (1.3%; 95% CI 0.1% to 9.3%), whereas 5 COVID-19-infected health care personnel (4 physicians and 1 nurse) reported failing to use appropriate masking while engaged in nonpatient activities.
Effect of COVID-19 Infection Symptoms and Diagnoses on Health Care Personnel Work Patterns and Staffing and Facility Burden From Missed Work
Health care personnel (including infected and uninfected individuals) reported having symptoms consistent with COVID-19 infection in 1,256 (4.3%) of 29,825 weeks, including 1,042 (83.0% of symptomatic weeks) during which they worked one or more shifts and 72 (5.7% of symptomatic weeks) during which they abstained from work. Thirty-two COVID-19-infected health care personnel (42.7%) reported experiencing at least one related symptom before their diagnoses. These health care personnel reported experiencing symptoms while working in the ED in 19 weeks and abstaining from work because of suspected SARS-COV-2 infection in 23 weeks (Table 3).
Table 3.
Person weeks of health care personnel with symptoms consistent with COVID-19 infection and work status, Project COVERED, May-December 2020.
| Work Status | Overall (n, %) | Nontesting wks (n, %) | Testing wks (n, %) | COVID-19-Infected Health Care Personnel (n, %) | ||||
|---|---|---|---|---|---|---|---|---|
| N= 29,825 Person-wks | 1,256 | 4.3% | 876 | 3.0% | 347 | 1.2% | 50 | 2.9% |
| Worked at least 1 shift | 1,042 | 83.0% | 782 | 89.3% | 199 | 57.3% | 19 | 38.0% |
| Abstained | 72 | 5.7% | 26 | 3.0% | 23 | 6.6% | 23 | 46.0% |
Eighty-nine health care personnel reported a total of 90 person weeks (0.3% of all weeks; 95% CI 0.2 to 0.4) of missed work related to COVID-19 and COVID-19 concerns. This included 72 cases in which health care personnel missed work because of concerning symptoms. Thirty-six health care personnel reported missing work based on a positive COVID-19 diagnosis from an outside facility, and 5 health care personnel missed work based on a positive COVID-19 diagnosis from COVERED surveillance testing. No one reported missing work because of an exposure leading to quarantine.
Association Between COVID-19 Infection Levels and ED Staffing
During the surveillance period, the interquartile range for physician-staffing levels at the 25 participating centers varied from 98.7% to 102.0% of normal staffing. Nursing staffing levels exhibited an interquartile range of 98.6% to 103.0% of normal, and nonclinical staffing levels exhibited an interquartile range of 99.6% to 101.0%.
Table 4 shows the frequency of decreased staffing associated with increased facility COVID-19 infection levels. Instances in which staffing fell below 95% of normal and facility cases of COVID-19 increased by 100% occurred in only 7/500 (1.4%) weeks for physicians, 4/490 (0.8%) weeks for nurses, and 2/456 (0.4%) weeks for nonclinical staff. A similar low frequency was seen when staffing was compared to community COVID-19 prevalence, as illustrated in Table E2 (available at http://www.annemergmed.com).
Table 4.
Frequency of reduced staffing events* in association with increased institutional COVID-19 burden among ED health care personnel, Project COVERED, May-December 2020.
| Physicians | Nurses | Nonclinical Personnel | |
|---|---|---|---|
|
| |||
| Reduced staffing events (n, %) | |||
|
| |||
| Relative institutional COVID-19 rate† | N=500 | Total wks Reported n=490 | N=456 |
| Any elevation | 33 (6.6%) | 21 (4.3%) | 14 (3.1%) |
| >10% | 29 (5.8%) | 20 (4.1%) | 11 (2.4%) |
| >20% | 24 (4.8%) | 19 (3.9%) | 8 (1.7%) |
| >50% | 17 (3.4%) | 11 (2.2%) | 5 (1.1%) |
| >100% | 7 (1.4%) | 4 (0.8%) | 2 (0.4%) |
Reduced Staffing Event define as week where participating site reported cumulative hours work less than 95% of baseline staffing levels.
Relative Institutional COVID-19 Rate represents the proportional increase in treated COVID-19 cases relative to each institution’s average weekly number of infections.
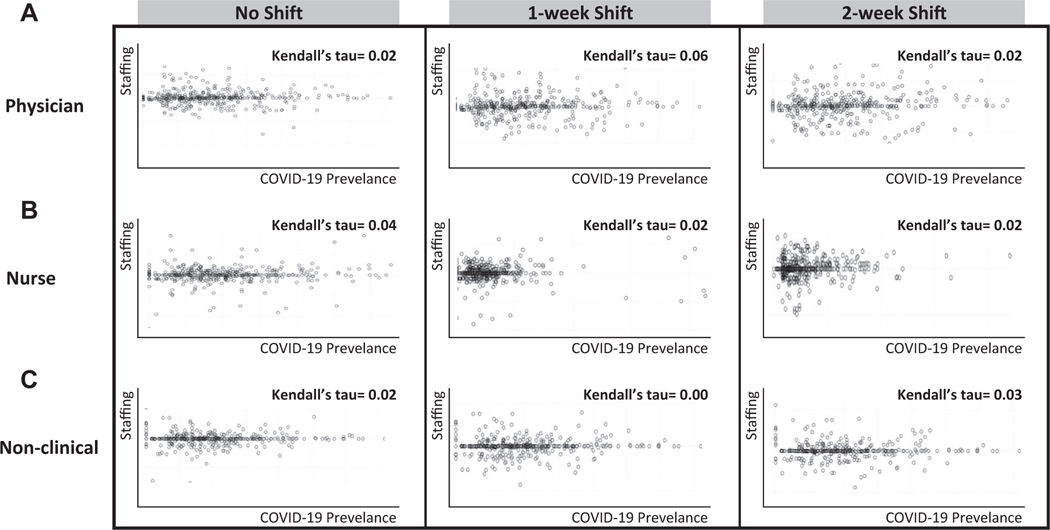
The Figure presents the scatterplots of weekly health care personnel staffing levels and facility COVID-19 volume at the participating centers, including time-shift analysis. The Kendall tau’s for physician staffing were 0.02, 0.056, and 0.081 for no shift, one-week time shift, and 2-week time shift, respectively (Figure, A). Kendall tau’s for nursing staffing and nonclinical staffing show a similar poor correlation (Figure, B, Figure, C). Scatterplots for each type of health care personnel and community COVID-19 levels displayed a similar pattern and can be found in Figure E1 (available at http://www.annemergmed.com).
Figure.
A strong association (positive correlation) between staffing and disease will appear as a clustering that moves from lower left of the graph up to the upper right. A negative relationship (negative correlation) will exhibit clustering that moves from the upper left corner of the graph and extends to the lower right corner. Spherical clustering and clustering that extends horizontally across the graph indicate that there is little relationship between staffing and disease burden.
LIMITATIONS
This study has limitations. Our observations included only academic medical centers in a period that spanned the early segment of the pandemic, missing the effects of the Delta variant surges of late December 2020 and advent of the COVID-19 vaccine. Although this gave us the unique opportunity to characterize a nonimmune population early in the pandemic, these results cannot be generalized to a later phase in the broader ED setting. The ED health care personnel transmission would potentially be more likely with more infectious strains, particularly with constrained PPE supplies, or possibly less as immunity increased from both vaccination and prior infection.15
The study cohort is at risk of selection bias as the volunteer participants may exhibit behavior (diligent PPE use, strict social distancing, etc) that decreases their risk of infection relative to their nonstudy colleagues. Although the main study reported high protocol adherence, data on PPE use and staffing were self-reported, may be subject to recall bias, and may not reflect actual use.6 We based the low risk of SARS-CoV-2 transmission on cumulative time during which infected participants may have interacted with staff and patients. Although this low cumulative infection rate suggests low health care personnel-to-health care personnel and patient-to-health care personnel transmission, a fuller assessment to include health care personnel-to-patient transmission would require extensive monitoring of patients for infection, including follow-up assessments on large numbers of ED patients, molecular epidemiology, and contact tracing measures that were beyond the scope of the main project.
Our analysis likely overestimates the actual time infected health care personnel worked during their infective period.As defined, the infectivity window could take a wide range of values given our testing schedule. For example, our study would calculate an infectivity window of one month or more if the positive test occurred during the period of observation when testing was occurring every 4 weeks. It is not likely a worker would be infectious during this entire period.
It is also important to note that although we have presented our data and associated risks in terms of person weeks of potential exposure and use of PPE among health care personnel, we were not able to assess the effectiveness of PPE in preventing patient and staff exposures. Patients may contract COVID-19 infections despite the appropriate use of PPE, and there may be additional means of transmission that are not accounted for in our analysis.
DISCUSSION
Over a 20-week period of observation, spanning May through December 2020, our study suggests that infected ED health care personnel did not pose a significant threat to the delivery of emergency care. Although we did not determine infection rates across all ED staff at the various facilities, findings in the cohort we followed suggest that a driving factor was the low-provider infection rate. Although the ED is a high-risk environment, the number of COVID-19 infections among ED health care personnel was only 4.5%, a surprise given that this was the earliest part of the pandemic with no COVID-19 vaccine availability or widespread natural immunity. Assuming most infections lasted less than 2 weeks, the total person weeks of potential infection for all infected health care personnel was 148 weeks, representing just 0.5% of the 29,825 person weeks of care provided by the entire cohort.
To our knowledge, our study is the first to report on sickness presenteeism, or working while sick, in an ED health care personnel cohort during the COVID-19 pandemic. Presenteeism presents particular risk as intrastaff and staff-patient spread have been documented to be an important mode of transmission of SARS-CoV-2.16,17 We found that participants (83%) who experienced symptoms consistent with COVID-19 continued to work in the ED. Of those participants ultimately confirmed to have COVID-19 infection, only one did not work at least one shift during their infective period, and almost all (95%) continued to work until receiving laboratory confirmation. In comparison, Linsenmeyer et al,11 found favorably less presenteeism with symptoms (26%) and at COVID-19 diagnosis (50%) in an unselected health care personnel population over an observation period later in the pandemic (December 2020 to September 2021). Although it is possible a component of this difference relates to the ED ethos of always being available for the patient, these results do illustrate the difficulty of navigating presenteeism in the early part of a pandemic, of which our study is reporting. Specifically, there is less understanding of the natural course of disease, in particular, the risk of asymptomatic spread, which may cause workers to be less diligent with self-quarantine in the setting of mild or no symptoms. Additionally, lack of testing with immediate results (our RT-PCR turnaround time was up to 7 days) to direct quarantine measures could increase the rate of presenteeism without strict quarantine during the waiting period. Although we did not include questions about why health care personnel continued to work with symptoms, prior studies, both before and during COVID-19, suggest that it may have as much to do with not wanting to call in sick and burden their fellow health care personnel with added patient care duties as concerns over available paid sick leave.18,19
Although such presenteeism has been associated with SARS-CoV-2 transmission, our findings of low-infection rates suggest transmission risk may have been mitigated by availability and adherence to universal masking.20 Although sites employed strategies to conserve PPE, no site reported running out during the project. Mohr et al6 reported excellent adherence by our entire cohort to CDC-recommended PPE use in clinical and nonclinical activities. When looking specifically at the COVID-19-infected participants, we found only a small percentage (1.3%) reported failing to employ appropriate masking measures. Our findings suggest that the cumulative interaction between infected health care personnel and others resulted in a negligible risk of transmission on the scale of public health emergencies. Previous studies have suggested that health care personnel often rationalize presenteeism on the basis that they know how to prevent transmission by taking appropriate precautions, such as self-masking18; our findings may be unique in lending support to this belief. However, these results are predicated on strict masking adherence and cannot be generalized to periods when masking requirements had been lifted, and COVID-19 vaccination became available, there was increased acquired immunity from natural infection, and new SARS-CoV-2 variants emerged. These findings are particularly important for ED and hospital administration, especially in the setting of future pandemics. Leadership must adopt open communication and supportive staffing policies to address presentism and adopt strict masking policies when conditions of high transmissibility exist.
Similarly, COVID-19 symptoms and infections within the community and facilities had a very small effect on staffing, and facilities were generally able to maintain consistent levels. Reduced staffing during COVID-19 surges (with facility COVID-19 cases increasing above baseline levels by ≥50%) was rare, occurring in only a small fraction of weeks (3.4% for physicians, 2.2% for nurses, and 1.1% for nonclinical providers). The scatter plots illustrate random patterns of staffing with respect to COVID-19 burden and reveal poor associations between any staffing levels and COVID-19 levels, including facility and community COVID-19 burdens and time-shifted staffing. Specifically, participating centers did not increase or decrease future staffing in anticipation or as a consequence of COVID-19 surges or declines.
There are several potential reasons staffing did not vary.Centers may have made staffing changes before the initiation of the study. Although we did not collect prestudy data on our centers, an observational study of 136 EDs by Pines et al21 demonstrated declines in physician and advanced practice provider hours of 15% and 20% in the period preceding our study’s enrollment. Our observation period did not include the Delta and Omicron waves, which were accompanied by headlines of hospital understaffing.22 By contrast, the episodic surges seen during our study period were relatively mild by comparison and may not have required sites to adjust staffing.23 It is possible that the sites included in this study may have made different staffing decisions during those phases of the pandemic. We did not collect qualitative measures of staffing on facility surveys as part of the main project, and we are unable to discern whether the administrators at participating EDs considered their facilities adequately staffed during the survey period. Staffing decisions are multifactorial, and it is possible that sites maintained understaffed or overstaffed conditions relative to SARS-COV-2 volume during this timeframe.
Overall, our results demonstrate the cumulative incidence of COVID-19 among a cohort of ED health care personnel was low during the early stages of the pandemic. ED health care personnel who were ultimately diagnosed with COVID-19 exhibited a high rate of presenteeism. ED staffing did not fluctuate greatly during the study and showed no association with prevailing local COVID-19 levels.
Supplementary Material
Editor’s Capsule Summary.
What is already known on this topic
Asymptomatic health care workers with coronavirus disease 2019 (COVID-19) may infect others while on duty.
What question this study addressed
What was the prevalence of asymptomatic infection among emergency department (ED) staff during the first phase of the pandemic?
What this study adds to our knowledge
In this prospective surveillance study of 1,673 health care workers in 25 EDs, 4.5% of individuals tested positive for COVID-19 during weekly monitoring. Fifty-seven percent were asymptomatic, ie, working while sick.
How this is relevant to clinical practice
These historical data from the first phase of the pandemic are unlikely to apply to current COVID-19 variants and a widely vaccinated population; however, they may assist planning for future pandemics.
Funding and support:
By Annals’ policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist. Funded by a cooperative agreement from the Centers for Disease Control and Prevention (CDC) (U01CK000480) and the Institute for Clinical and Translational Science at the University of Iowa through a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health (UL1TR002537).
Footnotes
All authors attest to meeting the four ICMJE.org authorship criteria:(1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The Project COVERED Emergency Department Network includes the following: Monica Bahamon, MPH, Jestin N. Carlson MD, MSc, Makini Chisolm-Straker, MD, MPH, Brian Driver, MD, Brett Faine, Pharm D, MS, Brian M. Fuller, MD, James Galbraith, MD, John P. Haran, MD, PhD, Amanda Higgins, MS, Jeremiah Hinson, MD, Stacey House, MD, PhD, Ahamed H. Idris, MD, Efrat Kean, MD, Elizabeth Krebs, MD, MSc, Michael C. Kurz, MD, MS, Lilly Lee SM, MD, Stephen Y. Liang, MD, MPHS, Stephen C. Lim, MD, Juan Carlos Montoy, MD, PhD, Robert M. Rodriguez, MD, Gregory Moran, MD, Utsav Nandi, MD, MSCI, Kavitha Pathmarajah, MPH, James H. Paxton MD, Yesenia Perez, BA, Lynne D. Richardson, MD, Richard Rothman, MD, PhD, Walter A. Schrading MD, Jessica Shuck, BA, Patricia Slev, MD, Howard A. Smithline, MD, Michelle St. Romain, MD, Kimberly Souffront, PhD, FNP-BC, RN, Mark T. Steele, MD, Amy Stubbs, MD, Morgan B. Swanson, Josh Tiao, MD, Jesus R. Torres, MD, MPH, Stacy A. Trent MD MPH, Lisandra Uribe, BS, Arvind Venkat, MD, Gregory Volturo, MD, and James Willey, MD.
Data sharing statement:
The deidentified data upon which this manuscript is based has been posted on Open Science Framework (osf.io) and can be accessed from the following doi: https://osf.io/dr2kj/.
REFERENCES
- 1.Hoogenboom WS, Pham A, Anand H, et al. Clinical characteristics of the first and second COVID-19 waves in the Bronx, New York: a retrospective cohort study. Lancet Reg Health Am. 2021: 100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez RM, Medak AJ, Baumann BM, et al. Academic emergency medicine physicians’ anxiety levels, stressors, and potential stress mitigation measures during the acceleration phase of the COVID-19 pandemic. Acad Emerg Med. 2020;27:700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mutambudzi M, Niedzwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK biobank participants. Occup Environ Med. 2021;78:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varia M, Wilson S, Sarwal S, et al. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ. 2003;169:285–292. [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds MG, Anh BH, Thu VH, et al. Factors associated with nosocomial SARS-CoV transmission among healthcare workers in Hanoi, Vietnam, 2003. BMC Public Health. 2006; 6:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohr NM, Krishnadasan A, Harland KK, et al. Emergency department personnel patient care-related COVID-19 risk. PLoS One. 2022;17: e0271597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jo HJ, Kim JS, Choe PG, et al. Work restrictions for healthcare personnel with potential in-hospital exposure to SARS-CoV-2: experience at a tertiary hospital. J Korean Med Sci. 2021;36:e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belingheri M, Paladino ME, Riva MA. Beyond the assistance: additional exposure situations to COVID-19 for healthcare workers. J Hosp Infect. 2020;105:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohr NM, Harland KK, Krishnadasan A, et al. Diagnosed and undiagnosed COVID-19 in US emergency department health care personnel: a cross-sectional analysis. Ann Emerg Med. 2021;78:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang D, Xu H, Rebaza A, et al. Protecting healthcare workers from subclinical coronavirus infection. Lancet Respir Med. 2020;8:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linsenmeyer K, Mohr D, Gupta K, et al. Sickness presenteeism in healthcare workers during the coronavirus disease 2019 (COVID-19) pandemic: an observational cohort study. Infect Control Hosp Epidemiol. 2023;44:1693–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
- 13.Makuc DM, Haglund B, Ingram DD, et al. Health service areas for the United States. Vital Health Stat 2. 1991;112:1–102. [PubMed] [Google Scholar]
- 14.R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ [Google Scholar]
- 15.Mallapaty S. COVID-19: how Omicron overtook Delta in three charts. Nature. Published March 4, 2022. 10.1038/d41586-022-00632-3 [DOI] [PubMed] [Google Scholar]
- 16.Schneider S, Piening B, Nouri-Pasovsky PA, et al. SARS-Coronavirus-2 cases in healthcare workers may not regularly originate from patient care: lessons from a university hospital on the underestimated risk of the healthcare worker to healthcare worker transmission. Antimicrob Resist Infect Control. 2020;9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Hu B, Chang H, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansen V, Aronsson G, Marklund S. Positive and negative reasons for sickness presenteeism in Norway and Sweden: a cross-sectional survey. BMJ Open. 2014;4:e004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniels S, Wei H, Han Y, et al. Risk factors associated with respiratory infectious disease-related presenteeism: a rapid review. BMC Public Health. 2021;21:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tori K, Dinh TTH, Mather C. Healthcare professional presenteeism during a COVID-19 outbreak in an Australian rural healthcare environment: a case analysis. Int J Environ Res Public Health. 2021;18:8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pines JM, Zocchi MS, Black BS, et al. The effect of the COVID-19 pandemic on the economics of United States emergency care. Ann Emerg Med. 2021;78:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamond D. First, they ran short of PPE, then ventilators. Now, the shortage is hospital staff. Washington Post. Published December 30, 2021. Accessed July 5, 2023. https://www.washingtonpost.com/health/2021/12/30/hospitals-staffing-shortages-omicron/ [Google Scholar]
- 23.Centers for Disease Control and Prevention. CDC COVID data tracker. Accessed January 17, 2023. https://covid.cdc.gov/covid-data-tracker/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The deidentified data upon which this manuscript is based has been posted on Open Science Framework (osf.io) and can be accessed from the following doi: https://osf.io/dr2kj/.