Abstract
Background
Abrocitinib, an oral, once-daily, Janus kinase 1-selective inhibitor, is efficacious in moderate-to-severe atopic dermatitis with a manageable long-term safety profile.
Objective
We aimed to provide updated integrated long-term safety results for abrocitinib from available data accrued up to a maximum of almost 4 years in patients with moderate-to-severe atopic dermatitis from the JADE clinical development program.
Methods
Analysis included 3802 patients (exposure: 5213.9 patient-years) from the phase II monotherapy study (NCT02780167) and the phase III studies JADE MONO-1 (NCT03349060), JADE MONO-2 (NCT03575871), JADE TEEN (NCT03796676), JADE COMPARE (NCT03720470), JADE DARE (NCT04345367; 200 mg only), JADE REGIMEN (NCT03627767), and JADE EXTEND (NCT03422822; data cutoff 25 September, 2021). Data from patients receiving one or more doses of abrocitinib 200 mg or 100 mg were pooled in a consistent-dose cohort (patients were allocated to receive the same abrocitinib dose throughout exposure in the qualifying parent study and/or long-term study) or a variable-dose cohort (patients received open-label abrocitinib 200 mg; responders were randomized to abrocitinib 200 mg, 100 mg, or placebo, and could then receive abrocitinib 200 mg plus topical corticosteroids as rescue therapy). Incidence rates of adverse events of special interest were assessed. Cox regression analysis of risk factors for herpes zoster and serious infections was performed.
Results
Overall, this safety analysis of long-term data up to a maximum of ~ 4 years of abrocitinib exposure does not indicate any changes from the previously reported risk profile. The most frequent serious infections (per Medical Dictionary for Regulatory Activities preferred term) with consistent-dose abrocitinib 200 mg and 100 mg were herpes zoster (0.5% and 0.2%), pneumonia (0.2% with either dose), and herpes simplex (0.1% with either dose). Risk factors for herpes zoster were a history of herpes zoster, abrocitinib 200-mg dose, age ≥ 65 years, absolute lymphocyte count < 1 × 103/mm3 before the event, and residing in Asia. For serious infections, > 100 kg body weight was a risk factor. Incidence rate/100 patient-years (95% confidence interval) with the consistent abrocitinib 200-mg and 100-mg dose combined was higher in older (aged ≥ 65 years) patients versus younger (aged 18 to < 65 years) patients for serious adverse events (17.6 [11.7‒25.4] vs 6.7 [5.8‒7.8]), malignancy excluding non-melanoma skin cancer (2.4 [0.6‒6.0] vs 0.1 [0.0‒0.4]), non-melanoma skin cancer (2.4 [0.6‒6.1] vs 0.2 [0.1‒0.4]), lymphopenia (3.5 [1.3‒7.6] vs 0.1 [0.0‒0.3]), and venous thromboembolism (1.7 [0.4‒5.1] vs 0.1 [0.0‒0.3]). Incident rate/100 patient-years (95% confidence interval) of non-melanoma skin cancer with the consistent abrocitinib 200-mg and 100-mg dose combined was higher in current/former smokers (0.9 [0.4‒1.6]) vs never-smokers (0.0 [0.0‒0.1]).
Conclusions
This safety update showed a consistent profile for abrocitinib with no new safety signals and continues to support that abrocitinib has a manageable long-term safety profile in patients with moderate-to-severe atopic dermatitis. Risk of specific adverse events was higher in certain patient populations, especially those aged ≥ 65 years. [Video abstract available.]
Clinical Trial Registration
NCT02780167; study start date: April, 2016; primary completion date: March, 2017; study completion date: April, 2017. NCT03349060; study start date: 7 December, 2017; study completion date: 26 March, 2019. NCT03575871; study start date: 29 June, 2018; study completion date: 13 August, 2019. NCT03720470; study start date: 29 October, 2018; primary completion date: 27 December, 2019; study completion date: 6 March, 2020. NCT03796676; study start date: 18 February, 2019; study completion date: 8 April, 2020. NCT03627767; study start date: 11 June, 2018; primary completion date: 2 September, 2020; study completion date: 7 October, 2020. NCT04345367; study start date: 11 June, 2020; primary completion date: 16 December, 2020; study completion date: 13 July, 2021. NCT03422822; study start date: 8 March, 2018; study completion date: ongoing (estimated completion date: 31 January, 2026).
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-024-00869-w.
Plain Language Summary
Abrocitinib is an approved treatment for people with moderate or severe atopic dermatitis, also known as AD or atopic eczema. Abrocitinib is a tablet that is taken by mouth once a day. This safety analysis looked at the side effects of treatment in a large group of adults and adolescents with moderate or severe AD who took abrocitinib up to a maximum of almost 4 years. This analysis also looked at which people were more likely to have certain side effects after taking abrocitinib. The results from this analysis were similar to those of previous safety analyses with abrocitinib, with no new side effects. Infections such as shingles, pneumonia, or herpes simplex can occur during treatment with abrocitinib. Shingles was more likely to occur in people who previously had shingles before taking abrocitinib, or who took the higher dose of abrocitinib (200 mg), or were 65 years of age or older, or had certain blood test results, or lived in Asia. People who are 65 years of age or older and took abrocitinib were more likely to develop some types of cancer, have certain abnormal blood test results, or develop blood clots in the veins than people with AD who were younger and took abrocitinib. Current or former smokers with AD who took abrocitinib were more likely to develop skin cancer (but not melanoma) than people with AD who took abrocitinib but have never smoked. This analysis further shows that abrocitinib had manageable safety in patients with moderate-to-severe AD.
Video abstract: Integrated safety update of abrocitinib in 3802 patients with moderate-to-severe atopic dermatitis: data from more than 5200 patient-years with up to 4 years of exposure (MP4 63720 KB)
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-024-00869-w.
| Digital Features for this article can be found at 10.6084/m9.figshare.25734210 |
Key Points
| This updated safety analysis of abrocitinib with longer exposure up to a maximum of ~ 4 years shows a consistent safety profile with no new safety signals. Risk of specific adverse events was higher in certain patient populations, especially those aged ≥ 65 years and current or former smokers. |
| Patients aged ≥ 65 years have a higher rate of serious adverse events, a greater likelihood of developing low platelet and absolute lymphocyte counts, a trend toward an increased risk for serious infections, non-melanoma skin cancer, malignancy excluding non-melanoma skin cancer, major adverse cardiovascular events, venous thromboembolism, pulmonary embolism, and an increased risk of herpes zoster infection; current or former smokers have a higher rate of non-melanoma skin cancer than those who never smoked. |
| Risk factors for herpes zoster infection were history of herpes zoster, abrocitinib 200 mg dose, age ≥ 65 years, absolute lymphocyte count < 1 × 103/mm3 before the event, and residing in Asia compared with other regions of the world. |
Introduction
Abrocitinib is an oral, once-daily, selective Janus kinase (JAK) 1 inhibitor approved for the treatment of adults and adolescents with moderate-to-severe atopic dermatitis (AD) [1–3]. In clinical trials, abrocitinib 200 mg or 100 mg once daily was efficacious as monotherapy [4–7] or in combination with medicated topical therapies [7–9], with a rapid reduction in the severity of signs and symptoms of AD, such as itch, and improvement in quality of life in patients with moderate-to-severe AD [5–9].
Abrocitinib has a manageable safety profile over the long term [10], with many common and serious risks quantified with moderate precision. Common adverse events (AEs) include headache, nausea, and acne. Additional AEs were hematologic laboratory changes and infections, including herpes-related infections. Malignancies, venous thromboembolism (VTE), and cardiovascular events are other potential risks [10] and periodic integrated safety analyses of long-term data provide greater precision for estimates of rare risks and identification of potentially new safety signals.
A comprehensive analysis of the long-term safety of abrocitinib was previously performed using integrated data up to April 2020 from 2856 patients with moderate-to-severe AD with a cumulative abrocitinib exposure of up to 1614 patient-years (PY) [10]. An additional data cutoff in July 2020, inclusive of adolescent patients, was also previously conducted [11]. Here, we report a further update on the abrocitinib integrated long-term safety with data accrued for more than a year since the previous report, including data from additional randomized controlled trials and longer observation in patients from the long-term extension study (JADE EXTEND [NCT03422822]; up to 25 September, 2021). This data set comprised 3802 patients with more than 5200 PY of cumulative exposure to abrocitinib, including those who received treatment for up to almost 4 years.
Methods
Long-Term Safety Population
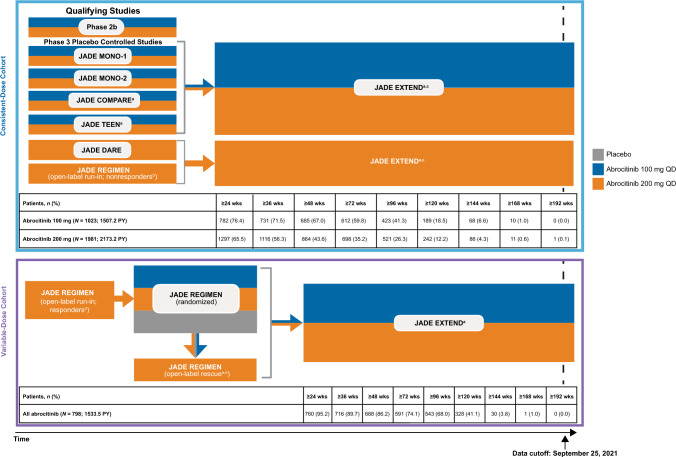
This analysis includes all patients who enrolled into qualifying parent studies in the JADE clinical program and met the individual study inclusion/exclusion criteria [4–9, 12]. Data cutoff for the JADE EXTEND long-term extension study was 25 September, 2021 (this study is ongoing and data may change). Adverse events of special interest were assessed, providing additional insights into long-latency events. Using a new pooling strategy, data from patients who received one or more doses of abrocitinib 200 mg or 100 mg in the JADE clinical trial program were pooled for analysis in two separate cohorts: (1) consistent-dose cohort or (2) variable-dose cohort (Fig. 1).
Fig. 1.
Study design and cohorts. JADE EXTEND is ongoing. QD once daily. aPatients in JADE COMPARE, JADE TEEN, and the open-label rescue period of JADE REGIMEN received abrocitinib in combination with topical corticosteroids, and in JADE EXTEND, patients were permitted to use medicated or non-medicated topical treatments throughout the study. bNon-responders were patients who did not achieve an Investigator’s Global Assessment (IGA) score of clear/almost clear with a ≥ 2-grade improvement from baseline and a ≥ 75% improvement from baseline in Eczema Area and Severity Index (EASI) after 12 weeks of treatment with abrocitinib 200 mg. cIncludes patients who received their first dose of abrocitinib (100 mg or 200 mg) in JADE EXTEND after receiving placebo in a phase III placebo-controlled trial (MONO-1, MONO-2, COMPARE, TEEN) or dupilumab in JADE COMPARE or JADE DARE, as well as patients who first received abrocitinib in a qualifying phase III trial. dPatients in the open-label run-in period who responded (IGA score of clear/almost clear with a ≥ 2-grade improvement from baseline and a ≥ 75% improvement from baseline in EASI) after 12 weeks of treatment with abrocitinib 200 mg were randomly allocated to treatment with abrocitinib 200 mg, abrocitinib 100 mg, or placebo. ePatients who experienced flare (≥ 50% loss of week 12 EASI improvement and new IGA score ≥ 2) during maintenance entered the open-label rescue period (abrocitinib 200 mg plus topical medicated treatment)
The consistent-dose cohort included patients who received the same abrocitinib dose during the entire exposure time in the phase IIb study (NCT02780167) or qualifying phase III studies and/or the long-term extension study JADE EXTEND. Qualifying studies were JADE MONO-1 (NCT03349060), JADE MONO-2 (NCT03575871), JADE TEEN (NCT03796676), JADE COMPARE (NCT03720470), JADE DARE (NCT04345367; 200 mg only), and JADE REGIMEN (NCT03627767; included only those patients from the open-label run-in phase who received abrocitinib 200 mg and did not subsequently enter the randomized double-blind phase). Patients in this cohort may have received their first dose of abrocitinib in JADE EXTEND if they previously received placebo in the placebo-controlled qualifying studies and/or dupilumab in JADE COMPARE or JADE DARE. Patients enrolled in the phase IIb monotherapy study did not enroll in JADE EXTEND but were included in the consistent-dose cohort if they had received either abrocitinib 200 mg or 100 mg.
The variable-dose cohort included patients who could have received different doses of abrocitinib (100 mg and 200 mg) throughout exposure in the parent study JADE REGIMEN and enrolled in JADE EXTEND. Patients in this cohort completed the 12-week open-label period of JADE REGIMEN (abrocitinib 200 mg only) and entered the randomized phase as responders during which they received abrocitinib 200 mg, abrocitinib 100 mg, or placebo. Some patients subsequently entered the JADE REGIMEN rescue phase (abrocitinib 200 mg) and/or JADE EXTEND (abrocitinib 200 mg or 100 mg).
Statistical Analysis
Incidence rates (IRs; number of unique patients with events per 100 PY) and 95% confidence intervals (CIs) were calculated for AEs of special interest, including serious AEs (SAEs); serious infections; malignancies; cardiovascular events, including major adverse cardiovascular events (MACE) and VTE; deaths; and laboratory measures. Serious infections were defined as SAEs that required parenteral antimicrobial therapy or hospitalization for treatment or met other criteria that required the infection to be reported as an SAE. Per protocol, all serious infections that met the SAE criteria required discontinuation from the study. Adjudication committees (external experts blinded to treatment and independent from the study sponsor) were established to review opportunistic infections, malignancies, and cardiovascular events (including VTE).
Adverse events of special interest, including MACE, VTE, and malignancies, were analyzed by baseline disease severity, sex, smoking status, and race. Smoking duration in current or past smokers was not assessed in the abrocitinib clinical studies; thus patients were classified only as ‘current/former smoker’ or ‘never smoker.’ Incidence rates for AEs of special interest were also analyzed by age in patients aged ≥ 65 years and 18 to < 65 years.
A Cox proportional hazards regression analysis was performed to evaluate risk factors for herpes zoster (HZ) and serious infections. Risk factors evaluated for HZ and serious infections included abrocitinib dose, age, baseline disease severity, sex, ethnicity, baseline body weight, baseline body mass index, geographic region, prior systemic therapy, baseline estimated glomerular filtration rate, confirmed absolute lymphocyte count (ALC), and medical history of HZ. In this analysis, a significance level of 0.05 was used to evaluate the comparisons, and the corresponding factors with a p value of < 0.05 were considered potential risk factors. The risk period was defined as the smallest of the last dose date plus 28 days, date of death, or the data cutoff date in JADE EXTEND.
Results
Abrocitinib Exposure, Baseline Demographics, and Disease Characteristics
This analysis included data from 3802 patients representing a total of 5213.9 PY of abrocitinib exposure, including those who received abrocitinib for up to almost 4 years. The consistent-dose cohort comprised 3004 patients; of those, 1981 received abrocitinib 200 mg, representing 2173.2 PY of exposure, and 1023 received abrocitinib 100 mg, representing 1507.2 PY of exposure. In this cohort, 1549 (51.6%), 944 (31.4%), 431 (14.3%), and 154 (5.1%) patients received abrocitinib for ≥ 48, ≥ 96, ≥ 120, and ≥ 144 weeks, respectively. Among these patients, 864 (43.6%) and 521 (26.3%) received abrocitinib 200 mg for ≥ 48 and ≥ 96 weeks, respectively, and 685 (67.0%) and 423 (41.3%) received the 100-mg dose over the same period. The duration of treatment in the consistent-dose cohort, across treatment arms, ranged from 1 to 1369 days. The variable-dose cohort comprised 798 patients, representing 1533.5 PY of exposure. In this cohort, 688 (86.2%), 543 (68.0%), 328 (41.1%), and 30 (3.8%) patients received abrocitinib for ≥ 48, ≥ 96, ≥ 120, and ≥ 144 weeks, respectively. The duration of treatment ranged from 89 to 1181 days.
The median age was 31.0 and 29.0 years in the abrocitinib 100-mg and 200-mg groups, respectively, in the consistent-dose cohort and 29.0 years in the variable-dose cohort (Table 1). In the consistent-dose (n = 3004) and variable-dose (n = 798) cohorts, 16.3% and 18.2% of patients were adolescents (aged 12 to < 18 years), and 4.9% and 3.8% were ≥ 65 years of age, respectively. Patient demographics and baseline disease characteristics were largely comparable between the consistent-dose and variable-dose cohorts (Table 1).
Table 1.
Demographics and disease characteristics
| Consistent-dose cohort Abrocitinib 100 mg (N = 1023; 1507.2 PY) |
Consistent-dose cohort Abrocitinib 200 mg (N = 1981; 2173.2 PY) |
Variable-dose cohort All abrocitinib (N = 798; 1533.5 PY) |
|
|---|---|---|---|
| Age, years, median (Q1‒Q3) | 31.0 (21.0‒44.0) | 29.0 (21.0‒43.0) | 29.0 (20.0‒41.0) |
| Female, n (%) | 464 (45.4) | 920 (46.4) | 359 (45.0) |
| Race, n (%) | |||
| White | 718 (70.2) | 1359 (68.6) | 621 (77.8) |
| Black or African American | 59 (5.8) | 139 (7.0) | 33 (4.1) |
| Asian | 228 (22.3) | 423 (21.4) | 124 (15.5) |
| Othera | 18 (1.8) | 60 (3.0) | 20 (2.5) |
| Investigator’s Global Assessment, % | |||
| Moderate | 63.0 | 58.8 | 63.7 |
| Severe | 37.0 | 41.2 | 36.3 |
| Prior systemic therapy, n (%) | 431 (42.1) | 957 (48.3) | 475 (59.5) |
| Non-biologic agents | 382 (37.3) | 863 (43.6) | 431 (54.0) |
| Corticosteroids | 297 (29.0) | 699 (35.3) | 350 (43.9) |
| Cyclosporine | 126 (12.3) | 261 (13.2) | 117 (14.7) |
| Other non-biologic agents | 85 (8.3) | 175 (8.8) | 79 (9.9) |
| Biologic agents | 49 (4.8) | 94 (4.7) | 44 (5.5) |
| Dupilumab | 28 (2.7) | 51 (2.6) | 32 (4.0) |
| Other biologic agents | 22 (2.2) | 48 (2.4) | 15 (1.9) |
PY patient-years, Q quartile
aOther includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, other, multi-racial, and not reported
In the consistent-dose cohort, 42.1% and 48.3% patients in the abrocitinib 100-mg and abrocitinib 200-mg groups, respectively, had prior use of systemic therapies. In the variable-dose cohort, 59.5% of patients had prior use of systemic therapies (Table 1).
Serious AEs, AEs Leading to Study Discontinuation, and Deaths
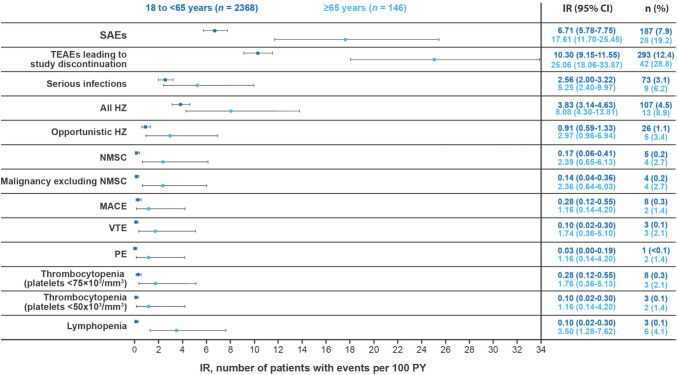
The IRs for SAEs in the consistent-dose and variable-dose cohorts are shown in Table 2. Incidence rates for AEs leading to study discontinuation were highest with abrocitinib 200 mg in the consistent-dose cohort (Table 2). Incidence rates for SAEs and treatment-emergent AEs leading to study discontinuation were higher in older (aged ≥ 65 years) patients than in younger (aged 18 to < 65 years) patients (Fig. 2).
Table 2.
Serious adverse events, adverse events leading to study discontinuation, and deaths
| Consistent-dose cohort Abrocitinib 100 mg (N = 1023) |
Consistent-dose cohort Abrocitinib 200 mg (N = 1981) |
Variable-dose cohort All abrocitinib (N = 798) |
|
|---|---|---|---|
| Serious adverse events | |||
| n (%) | 93 (9.1) | 157 (7.9) | 61 (7.6) |
| IR (95% CI) | 6.20 (5.00‒7.59) | 7.21 (6.13‒8.43) | 4.00 (3.06‒5.13) |
| Adverse events leading to study discontinuation | |||
| n (%) | 123 (12.0) | 258 (13.0) | 91 (11.4) |
| IR (95% CI) | 8.00 (6.65‒9.54) | 11.63 (10.26‒13.14) | 5.88 (4.73‒7.22) |
| Deaths | |||
| n (%) | 2 (0.2)a | 5 (0.3)b | 0 |
| IR (95% CI) | 0.13 (0.02‒0.46) | 0.22 (0.07‒0.52) | 0 (0.00‒0.24) |
CI confidence interval, COVID-19 coronavirus disease 2019, IR incidence rate
aCauses of death: sudden death (n = 1; previously reported in abrocitinib published safety analysis [10]), COVID-19 (n = 1)
bCauses of death: septic shock (n = 1), COVID-19 (n = 2; one of which was previously reported in the abrocitinib published safety analysis [10]), cardiac failure (n = 1), intracranial hemorrhage/cardiorespiratory arrest (n = 1)
Fig. 2.
Incidence rates (IRs) of adverse events of special interest in patients ≥ 65 years of age and 18 to < 65 years of age. For IR calculations, exposure for patients with events was counted to the time of their first event; confidence intervals (CIs) for IR assume that the actual count of cases arises from a Poisson distribution. HZ herpes zoster, MACE major adverse cardiovascular event, NMSC non-melanoma skin cancer, PE pulmonary embolism, PY patient-years, SAE serious adverse event, TEAE treatment-emergent adverse event, VTE venous thromboembolism
Five deaths occurred in the abrocitinib 200-mg group: two due to coronavirus disease 2019 (COVID-19) [one of these deaths was reported in the previously published safety analysis [10]], one due to septic shock, one due to cardiac failure, and one due to intracranial hemorrhage/cardiorespiratory arrest. Two deaths occurred in the abrocitinib 100-mg group: one due to sudden death (previously reported in the published safety analysis [10]) and one due to COVID-19. All deaths except the death from septic shock (deemed related to the study drug and concomitant drug triamcinolone) were considered unrelated to the study drug by the investigators (Table 1 of the Electronic Supplementary Material [ESM]). Two additional deaths occurred after the risk period for calculating the IR for deaths; both were due to malignancies (one due to gastric adenocarcinoma [previously reported and considered unrelated to treatment [10]] and one due to squamous cell carcinoma of the lung [event considered unrelated to treatment by the study investigator]). No deaths occurred in the variable-dose cohort.
AEs of Special Interest
Infections
Incidence rates for infections in the consistent-dose and variable-dose cohorts are shown in Table 3. Given the largely overlapping CIs, a dose-dependent response cannot be concluded for serious infections in the consistent-dose cohort. The most frequent serious infections with abrocitinib 200 mg and 100 mg were those related to HZ, herpes simplex (HS), and pneumonia. The data for all HZ and all HS infections suggest a dose-dependent response, with non-overlapping CIs (Table 3).
Table 3.
Adverse events of special interest: infections
| Consistent-dose cohort Abrocitinib 100 mg (N = 1023) |
Consistent-dose cohort Abrocitinib 200 mg (N = 1981) |
Variable-dose cohort All abrocitinib (N = 798) |
|
|---|---|---|---|
| Serious infections | |||
| n (%) | 34 (3.3) | 60 (3.0) | 29 (3.6) |
| IR (95% CI) | 2.20 (1.53‒3.08) | 2.69 (2.05‒3.46) | 1.87 (1.25‒2.69) |
| All herpes zoster (CMQ) | |||
| n (%) | 32 (3.1) | 102 (5.1) | 48 (6.0) |
| IR (95% CI) | 2.11 (1.44‒2.98) | 4.70 (3.84‒5.71) | 3.18 (2.34‒4.21) |
| Serious herpes zoster (CMQ)a | |||
| n (%) | 2 (0.2)b | 10 (0.5)b | 1 (0.1)c |
| IR (95% CI) | 0.13 (0.02‒0.46) | 0.45 (0.21‒0.82) | 0.06 (0.00‒0.36) |
| All herpes simplex (CMQ) | |||
| n (%) | 75 (7.3) | 161 (8.1) | 83 (10.4) |
| IR (95% CI) | 5.14 (4.04‒6.44) | 7.71 (6.56‒8.99) | 5.74 (4.57‒7.11) |
| Serious herpes simplex (CMQ) | |||
| n (%) | 2 (0.2)b | 7 (0.4)b | 2 (0.3)d |
| IR (95% CI) | 0.13 (0.02‒0.46) | 0.31 (0.13‒0.64) | 0.13 (0.02‒0.46) |
| All eczema herpeticum | |||
| n (%) | 24 (2.3) | 19 (1.0) | 11 (1.4) |
| IR (95% CI) | 1.56 (1.00‒2.32) | 0.85 (0.51‒1.32) | 0.71 (0.35‒1.27) |
| Serious eczema herpeticum | |||
| n (%) | 6 (0.6) | 3 (0.2) | 2 (0.3) |
| IR (95% CI) | 0.39 (0.14‒0.84) | 0.13 (0.03‒0.39) | 0.13 (0.02‒0.46) |
| All pneumonia (CMQ) | |||
| n (%) | 11 (1.1) | 21 (1.1) | 17 (2.1) |
| IR (95% CI) | 0.71 (0.36‒1.27) | 0.94 (0.58‒1.43) | 1.10 (0.64‒1.76) |
| Serious pneumonia (CMQ) | |||
| n (%) | 4 (0.4)b | 11 (0.6)b | 6 (0.8) |
| IR (95% CI) | 0.26 (0.07‒0.66) | 0.49 (0.24‒0.88) | 0.39 (0.14‒0.84) |
| Adjudicated tuberculosis | |||
| n (%) | 0 | 1 (0.1) | 0 |
| IR (95% CI) | 0 (0‒0.24) | 0.04 (0‒0.25)e | 0 (0‒0.24) |
| Adjudicated herpes zoster | |||
| n (%) | 9 (0.9) | 26 (1.3) | 8 (1.0) |
| IR (95% CI) | 0.58 (0.27‒1.10) | 1.17 (0.76‒1.71) | 0.52 (0.22‒1.02) |
| All COVID-19-related events (SMQ) | |||
| n (%) | 47 (4.6) | 103 (5.2) | 76 (9.5) |
| IR (95% CI) | 3.08 (2.26‒4.10) | 4.69 (3.83‒5.69) | 5.04 (3.97‒6.30) |
| Serious COVID-19-related events (SMQ) | |||
| n (%) | 3 (0.3) | 11 (0.6) | 8 (1.0) |
| IR (95% CI) | 0.19 (0.04‒0.56) | 0.49 (0.24‒0.88) | 0.51 (0.22‒1.01) |
CI confidence interval, CMQ customized MedDRA query, COVID-19 coronavirus disease 2019, IR incidence rate, MedDRA Medical Dictionary for Regulatory Activities, SMQ standardized MedDRA query
aReported as the number of patients with serious herpes zoster (first occurrence only); the case of disseminated serious herpes zoster was also not included as part of the calculated incidence rate because it was categorized as a separate event outside of the CMQ
bPer MedDRA preferred term only, infection rates for serious herpes zoster were 0.2% and 0.5% with consistent-dose abrocitinib 100 mg and 200 mg, respectively; 0.1% with either dose for serious herpes simplex; and 0.2% with either dose for serious pneumonia
cOne case of herpes zoster meningitis
dBoth were cases of ophthalmic herpes simplex
eOne serious adverse event of disseminated tuberculosis
Most HZ-related events were cutaneous and single dermatome. Serious HZ-related events, regardless of first or recurrent event, included disseminated varicella zoster virus infection (n = 1), HZ (n = 12), and ophthalmic HZ (n = 1). Events of HZ that were adjudicated as opportunistic were mostly non-serious multi-dermatomal cutaneous infections, including two extracutaneous events, one each in the consistent-dose (serious disseminated varicella zoster virus) and variable-dose (serious HZ meningitis) cohorts. Serious HS-related events included HS (n = 3), eczema herpeticum (EH; n = 9), ophthalmic HS (n = 3), oral herpes (n = 1), herpes dermatitis (n = 1), and HS pharyngitis (n = 1). Serious pneumonia-related events included atypical pneumonia (n = 1), pneumonia (n = 6), pneumonia bacterial (n = 1), pneumonia pneumococcal (n = 1), and COVID-19 pneumonia (n = 6) (Table 2 of the ESM).
Based on analyses of IRs by each age group, the incidence of serious infections, all HZ infections, and opportunistic HZ infections tended to be higher in older (aged ≥ 65 years) patients versus younger (18 to < 65 years) patients (Fig. 2). One patient in the consistent-dose cohort in the 200-mg group developed disseminated tuberculosis (considered an opportunistic infection) on day 828 of abrocitinib exposure, which was confirmed by adjudication. This patient (Asian female individual; 70 years of age) had a negative result for both a tuberculosis blood test (QuantiFERON®-TB Gold In-tube assay) and on a chest X-ray at screening. She had a history of type 2 diabetes mellitus. There were no cases of tuberculosis in the 100-mg group.
The data for this update, accrued between July 2020 and September 2021, overlapped with the global COVID-19 pandemic. In the consistent-dose cohort, 150 of 3004 patients (5.0%) had a COVID-19-related event. Of these 150 patients, < 10% had a serious COVID-19 event (11 in the abrocitinib 200-mg group and three in the abrocitinib 100-mg group); no meaningful dose response was observed. Most patients with a serious COVID-19 infection had known or potential risk factors for COVID-19 hospitalization, such as asthma, hypertension, obesity, and type 2 diabetes [13]. Three were fatal events (Table 1 of the ESM).
Cardiovascular Safety
Incidence rates for cardiovascular events in the consistent-dose and variable-dose cohorts are shown in Table 4. In the consistent-dose cohort, IRs for MACE (defined as cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke) and deep vein thrombosis (DVT) showed no trend toward a dose response (Table 4). There was a trend toward a dose response in the IR point estimates for both VTE and pulmonary embolism (PE) events observed in the consistent-dose cohort (Table 4). Precision for MACE and VTE estimates was limited by the width of the CI relative to the point estimate in each treatment group. Major adverse cardiovascular events occurred in five patients each in the abrocitinib 100-mg group and 200-mg group. Major adverse cardiovascular events in the 100-mg group were acute myocardial infarction (n = 1), sudden death (n = 1; previously reported [10]), cerebrovascular accident (n = 1), ischemic stroke (n = 1), and non-serious retinal vein occlusion adjudicated as a neurovascular event (n = 1) and deemed by the investigator to be related to study drug (Table 3 of the ESM). Major adverse cardiovascular events in the 200-mg group were cardiorespiratory arrest and intracranial hemorrhage (n = 1), myocardial infarction (n = 2; previously reported [10]), non-serious retinal vascular disorder adjudicated as a neurovascular event (n = 1) that was deemed unrelated to study drug and related to ‘disease retina’, and cardiac failure (n = 1). Analysis of MACE by baseline disease severity, sex, smoking status, and race did not show any notable differences (Table 4 of the ESM). All cases had identified relevant medical history and/or risk factors, except for the one case of retinal vein occlusion noted above, which occurred in a 39-year-old Asian female individual receiving 100 mg of abrocitinib who had no relevant history or specifically identified risk factors.
Table 4.
Adverse events of special interest: cardiovascular and malignancy
| Consistent-dose cohorta Abrocitinib 100 mg (N = 1023; 1507.2 PY) |
Consistent-dose cohorta Abrocitinib 200 mg (N = 1981; 2173.2 PY) |
Variable-dose cohorta All abrocitinib (N = 798; 1533.5 PY) |
July 24, 2020, data cut All abrocitinib (N = 3128) |
|
|---|---|---|---|---|
| Major adverse cardiovascular events | ||||
| n (%) | 5 (0.5) | 5 (0.3) | 2 (0.3) | 4 (0.1) |
| IR (95% CI) | 0.32 (0.10‒0.75) | 0.22 (0.07‒0.52) | 0.13 (0.02‒0.46) | 0.19 (0.05‒0.47) |
| Venous thromboembolism | ||||
| n (%) | 2 (0.2) | 5 (0.3) | 2 (0.3) | 6 (0.2) |
| IR (95% CI) | 0.13 (0.02‒0.46) | 0.22 (0.07‒0.52) | 0.13 (0.02‒0.46) | 0.28 (0.10‒0.60) |
| Deep vein thrombosis | ||||
| n (%) | 2 (0.2) | 2 (0.1) | 1 (0.1) | 3 (0.1) |
| IR (95% CI) | 0.13 (0.02‒0.46) | 0.09 (0.01‒0.32) | 0.06 (0.00‒0.36) | 0.14 (0.03‒0.41) |
| Pulmonary embolism | ||||
| n (%) | 1 (0.1) | 3 (0.2) | 1 (0.1) | 3 (0.1) |
| IR (95% CI) | 0.06 (0.00‒0.36) | 0.13 (0.03‒0.39) | 0.06 (0.00‒0.36) | 0.14 (0.03‒0.41) |
| Malignancies (excluding non-melanoma skin cancers) | ||||
| n (%) | 2 (0.2) | 6 (0.3) | 2 (0.3) | 3 (0.1) |
| IR (95% CI) | 0.13 (0.02‒0.46) | 0.27 (0.10‒0.58)b | 0.13 (0.02-0.46) | 0.14 (0.03-0.41) |
| Non-melanoma skin cancer | ||||
| n (%) | 6 (0.6) | 3 (0.2) | 4 (0.5) | 7 (0.2) |
| IR (95% CI) | 0.39 (0.14‒0.84) | 0.13 (0.03‒0.39) | 0.26 (0.07‒0.66) | 0.32 (0.13‒0.67) |
CI confidence interval, IR incidence rate, PY patient-years
aData as of 25 September, 2021 cutoff
bIR = 0.22 events per 100 PY (95% CI 0.07‒0.52) excluding one event of adenocarcinoma, which occurred early in the treatment period with symptoms being present prior to taking study treatment
In the abrocitinib 100-mg group, one case of PE (along with DVT in the same patient) occurred in a 74-year-old White male individual with a history of hypertension and cardiac ablation (Table 3 of the ESM). An additional DVT was reported in a 65-year-old White male individual with a history of hypertension receiving abrocitinib 100 mg who had a clot around a peripherally inserted central catheter line. In the abrocitinib 200-mg group in the consistent-dose cohort, PE events occurred in three patients (all previously reported [10]); DVT occurred in two patients, one in a 44-year old White female individual (event occurred within a week of arthroscopic surgery) and another in a 59-year old White male individual with a history of sleep apnea, hypertension, history of pulmonary mass, and lower leg edema since 2018.
The analysis of VTE events by baseline severity, sex, and race did not identify meaningful differences (Table 4 of the ESM). In current/former smokers, the IRs (95% CI) per 100 PY for both VTE (0.4 [0.1‒1.0]) and the subset of PE events (0.2 [0.0‒0.7]) with abrocitinib 200 mg and 100 mg combined were slightly higher with overlapping CIs than those who never smoked (VTE: 0.1 [0.0‒0.3]; PE: 0.1 [0.0‒0.3]). The IRs for MACE, VTE, and the subset of PE events were generally higher in older (aged ≥ 65 years) patients versus younger (aged 18 to < 65 years) patients (Fig. 2).
Malignancies
Incidence rates for malignancies for the consistent-dose and variable-dose cohorts are shown in Table 4. In the consistent-dose cohort, there was a trend toward a dose response in the IR point estimates for malignancies excluding non-melanoma skin cancer (NMSC). However, the precision for these estimates was limited, as evidenced by the width of the CI relative to the point estimate in each treatment group. In addition, there was one event in the abrocitinib 200-mg group of gastric adenocarcinoma, which occurred early in the treatment period; symptoms were present prior to taking study medication. There was no trend toward a dose response in the IRs for NMSC (Table 4).
There were five new cases of malignancy (excluding NMSC) in the consistent-dose cohort that were not previously reported in the integrated safety analysis (Table 5 of the ESM) [10]. In the abrocitinib 100-mg group, a 53-year-old White female individual had breast cancer onset on day 639 of abrocitinib exposure. Four cases occurred in the abrocitinib 200-mg group: a White male individual aged 60 years at the time of diagnosis with lymphoma (onset on day 748), a 37-year-old White female individual with breast cancer (onset on day 546), a 58-year-old White female individual with an intraductal proliferative breast lesion (onset on day 419), and a 71-year-old White male individual with squamous cell carcinoma of the lung (onset on day 426).
There was an increased risk observed for NMSC and malignancy excluding NMSC in older (≥ 65 years) patients versus younger (18 to < 65 years) patients (Fig. 2). There were no meaningful differences in IRs for NMSC and malignancies (excluding NMSC) in the subgroups by baseline disease severity, sex, and race (Table 4 of the ESM). All cases of NMSC occurred in current or former smokers. Incidence rates (95% CI) per 100 PY with abrocitinib 200 mg and 100 mg combined were higher for NMSC in current/former smokers (0.9 [0.4‒1.6]) than those who never smoked (0.0 [0.0‒0.1]). The IR (95% CI) per 100 PY of malignancies excluding NMSC was also numerically higher (with overlapping CIs) in current/former smokers (0.4 [0.1‒1.0]) than in those who never smoked (0.2 [0.0‒0.4]).
Laboratory Abnormalities
In the consistent-dose cohort, five patients (two were previously reported) in the abrocitinib 200-mg group had confirmed platelet counts of < 50 × 103/mm3. Of the three cases that were not previously reported, two patients had low values noted in the first 4 weeks, and one case occurred beginning on day 613 after the patient had discontinued the study drug (abrocitinib 200 mg) on day 597 because of a HZ infection neurological (from day 572 to day 612) and HS (from day 572 to day > 666). This patient had thrombocytopenia from day 613 to day > 666, and the study drug was never restarted. There were no events of confirmed thrombocytopenia (platelet counts < 50 × 103/mm3) in the abrocitinib 100-mg group. No hemorrhages occurred due to thrombocytopenia.
There were no meaningful differences in the IRs for thrombocytopenia by baseline disease severity, sex, or race (Table 4 of the ESM). Lymphopenia (ALC < 0.5 × 103/mm3) occurred in nine patients (four were previously reported [10], all in the 200-mg group); two events occurred within approximately the first 4 weeks of treatment. There were no meaningful differences in the rate of lymphopenia by baseline disease severity or sex (Table 4 of the ESM). The IRs for thrombocytopenia and lymphopenia were generally higher in older (≥ 65 years of age) patients than in younger (18 to < 65 years of age) patients (Fig. 2).
On day 483 of abrocitinib exposure, one patient (33-year-old Black/African American female individual) in the consistent-dose cohort 200-mg group experienced a non-serious event of asymptomatic rhabdomyolysis defined as two sequential elevations of creatine kinase levels >10 times the upper limit of normal. Sodium, potassium, and creatine values remained mostly within reference ranges. The study drug was permanently discontinued on day 486 of exposure.
Retinal Detachment
A total of four patients had events of retinal detachment in the consistent-dose cohort and all were deemed unrelated to the study drug. Two patients in the 100-mg group (both female; 17 years of age and 62 years of age) had bilateral or partial retinal detachment, and two patients in the abrocitinib 200-mg group (a female individual, 48 years of age; and a male individual, 19 years of age) had retinal detachment in the right or left eye. All patients had a medical history of cataracts, myopia, or retinal degeneration. There were no signals for ophthalmic AEs or conjunctivitis.
Risk Factors for AEs
A Cox regression analysis showed that potential risk factors for treatment-emergent HZ infections were a higher abrocitinib dose; age ≥ 65 years; ALC of < 1 × 103/mm3 prior to the event; residing in Asia; and a prior medical history of HZ (Table 5). A Cox regression analysis showed that abrocitinib dose, age, and confirmed ALC of < 1 × 103/mm3 prior to the event were not significant risk factors for serious infection, although IRs were numerically higher in older versus younger patients, as previously noted. Only weight > 100 kg was identified as a significant risk factor for serious infections.
Table 5.
Risk factors for herpes zoster from a Cox regression model in the consistent-dose cohort
| Risk factor | Hazard ratio (95% CI) | P value |
|---|---|---|
| Study treatment | ||
| Abrocitinib 100 mg QD vs 200 mg QD | 0.46 (0.30‒0.70) | 0.0003 |
| Age | 0.0029 | |
| ≥ 65 vs 18 to < 65 years | 3.07 (1.59‒5.93) | |
| ≥ 65 vs <18 years | 3.83 (1.43‒10.27) | |
| 18 to < 65 vs < 18 years | 1.25 (0.58‒2.70) | |
| Geographic region | 0.0007 | |
| Asia vs Eastern Europe/Russia | 2.40 (1.41‒4.06) | |
| Asia vs USA/Canada/Australia | 2.24 (1.29‒3.91) | |
| Latin America vs USA/Canada/Australia | 2.88 (0.74‒11.25) | |
| Western Europe vs USA/Canada/Australia | 2.06 (1.25‒3.38) | |
| Confirmed ALC <1.0 (103/mm3) prior to event | 2.04 (1.30‒3.21) | 0.0020 |
| Prior medical history of herpes zoster | ||
| Yes vs no | 3.53 (1.81‒6.87) | 0.0002 |
ALC absolute lymphocyte count, CI confidence interval, eGFR estimated glomerular filtration rate, QD once daily
Hazard ratio and its associated CI were estimated from a Cox regression model, including fixed effects of treatment, study (parent), categorical variables of baseline age, sex, ethnicity, baseline body weight, body mass index, geographic region, baseline disease severity, prior systemic therapy and baseline eGFR, time-dependent variable of confirmed absolute lymphocyte count prior to the event (≥ 1.0 or < 1.0 × 103/mm3), and medical history
Comparisons with P values < 0.05 were included in this table
Comparisons with P values > 0.05 were: baseline disease severity (severe vs moderate), sex (female vs male), ethnicity (Hispanic or Latino vs not Hispanic or Latino), baseline body weight (≤ 100 vs > 100 kg), body mass index, prior systemic therapy (no vs yes), and baseline eGFR
For MACE, VTE, and malignancies, there was an insufficient number of events to conduct a formal risk analysis. A risk factor analysis was not performed for HS or COVID-19. Baseline characteristics, including cardiovascular and malignancy risk factors as well as smoking history, are shown in Table 6 of the ESM.
Discussion
This updated analysis expands on the previously published integrated analysis [10] of abrocitinib safety data in patients with moderate-to-severe AD and reports results from a larger cohort of patients, with a longer follow-up and greater PY of exposure. The previous analysis (data as of 22 April, 2020 cutoff) included 606 patients with ≥ 48 weeks of exposure and a total of 1614 PY of exposure [10]. The current analysis, with new data as of 25 September, 2021, includes 1487 patients with ≥ 96 weeks of exposure and provides an increase in exposure to 5213.9 PY, with maximal exposure to abrocitinib of almost 4 years. Although a formal comparative analysis with the previous abrocitinib integrated safety data or other JAK inhibitors was not performed, descriptive information is discussed here for context. Overall, this update reveals a consistent safety profile for abrocitinib with no new safety signals, despite longer exposure and a larger number of treated patients, and refines the risk estimates.
Initial reports with non-selective JAK inhibitors in patients with rheumatoid arthritis show an increased risk for serious infections [14]. In this analysis, IRs (95% CI) per 100 PY of serious infections with abrocitinib (200 mg: 2.7 [2.0‒3.5]; 100 mg: 2.2 [1.5‒3.1]) were similar to those reported in the previous integrated safety analysis (200 mg: 2.3 [1.5‒3.5]; 100 mg: 2.6 [1.6‒4.2]) [10]. Patient age, abrocitinib dose, and confirmed ALC prior to the event were not significant risk factors for serious infections. However, body weight of >100 kg was a risk factor for serious infections. This is in line with other studies that showed obesity increased the risk of serious infections in the general population [15–17]. A UK population-based cohort showed that IRs for serious infections ranged from 8.0 to 13.5/1000 PY (or 0.8–1.4/100 PY) in patients with AD compared with 6.9/1000 PY (or 0.7/100 PY) in those without AD [18].
Herpes zoster infections are common in patients treated with JAK inhibitors [19]. Janus kinase-dependent immune functions are implicated in various steps of the life cycle of Varicella zoster, the HZ virus, and inhibition of these JAK-dependent pathways may increase the susceptibility to HZ infections [14, 19]. The IR of HZ in the general population (North America, Europe, and Asia-Pacific) is 0.3‒0.5 events/100 PY [20]. The crude IRs of HZ in an adult population of patients with AD ranged from 5.3 to 7.8/1000 PY (or 0.5‒0.8/100 PY) in patients with mild-to-severe AD compared with 4.7/1000 PY (or 0.5/100 PY) in patients without AD [18]. In this analysis, IRs (95% CI) per 100 PY of HZ (200 mg: 4.7 [3.8‒5.7]; 100 mg: 2.1 [1.4‒3.0]) were dose dependent and similar to those reported in the previous integrated safety analysis (200 mg: 4.3 [3.2‒5.8]; 100 mg: 2.0 [1.1‒3.5]) [10]; most events were mild or moderate. Consistent with a previous analysis of integrated safety [10], the risk factors for HZ infection were a higher abrocitinib dose (200 mg vs 100 mg) and older age (≥ 65 years vs 18 to < 65 years and < 18 years). In this current analysis, confirmed ALC of < 1 × 103/mm3 prior to the event; residing in Asia; and a prior medical history of HZ were also found to be risk factors for HZ. Although not significant in the current Cox regression analysis, severe AD at baseline was previously identified as a risk factor for HZ infection [10].
Pneumonia was among the most frequent serious infections in patients receiving abrocitinib. Pneumonia-related events in this analysis (200 mg: 0.9 events/100 PY [95% CI 0.6‒1.4]; 100 mg: 0.7 events/100 PY [95% CI 0.4‒1.3]) were generally consistent with those in the previous integrated safety analysis (≤ 0.2%) [10].
The entire update period, July 2020 through September 2021, was conducted during the global COVID-19 pandemic. Data from the consistent-dose cohort did not suggest a dose-dependent response for COVID-19-related events or for serious COVID-19-related events. Of the 150 patients who had a COVID-19-related event in the consistent-dose cohort, fewer than 10% of these events were serious.
Incidence rates (95% CI) per 100 PY for HS in this analysis showed a dose-dependent response (200 mg: 7.7 [6.6‒9.0]; 100 mg: 5.1 [4.0‒6.4]) and the trend is similar to the previous integrated safety analysis (200 mg: 11.8 [9.8‒14.2]; 100 mg: 8.7 [6.6‒11.4]) [10]. Most HS events were non-serious.
Atopic dermatitis disrupts skin barrier function, and patients with AD tend to develop HS infection, which may develop into EH in some patients [21, 22]. Furthermore, mutations in interferon pathway-related genes such as interferon-gamma and interferon-gamma receptor 1 were shown to increase the risk of EH in patients with AD [23, 24]. The IRs (95% CI) per 100 PY of EH in the current analysis were 0.8 (0.5‒1.3) for abrocitinib 200 mg and 1.6 (1.0‒2.3) for abrocitinib 100 mg, and in the previous analysis were 0.8 (0.3‒1.5) for abrocitinib 200 mg and 2.3 (1.3‒3.9) for abrocitinib 100 mg [10]. The potential inverse dose relationship for the incidence of EH that was observed in both analyses might be related to greater improvement in both skin lesions and the skin barrier with abrocitinib 200 mg versus 100 mg, which potentially limits HS dissemination [5, 6].
Cardiovascular events are associated with uncontrolled AD, independent of genetic risk, and may be due to the complex relationship with immune-mediated inflammation in AD [25–27]. A modest absolute increase in the risk of cardiovascular outcomes was reported in patients with AD relative to the general population [26, 28]. In a US cohort of 8197 patients who were ≥ 12 years of age and had moderate-to-severe AD, the IRs (95% CI) per 100 PY of MACE, VTE, DVT, and PE were 0.3 (0.2‒0.3), 0.20 (0.15–0.25), 0.2 (0.1–0.2), and 0.1 (0.0–0.1), respectively [29]. In a Danish cohort of patients who were ≥ 15 years of age and had severe AD, the IR ratio of MACE was 1.2 (0.9‒1.5) [30]. The IRs for MACE do not suggest a dose-dependent response (200 mg: 0.2 [95% CI 0.1‒0.5]; 100 mg: 0.3 [0.1‒0.8]). Although, a trend toward a dose response was observed for the IR point estimates of VTE, the CIs largely overlap (200 mg: 0.2 [0.1‒0.5]; 100 mg: 0.1 [0.0‒0.4]). The IRs for both MACE and VTE were similar to those observed in the previous integrated safety analysis (MACE: 0.2 [0.0–0.5]; VTE: 0.3 [0.1–0.7], all in the 200-mg group) [10]. The IR (95% CI) per 100 PY for PE (200 mg: 0.1 [0.0‒0.4]; 100 mg: 0.1 [0.0‒0.4]) was also similar to that in the previous analysis (0.2/100 PY) [10]. Patients with cardiovascular conditions such as acute myocardial infarction, serious arrhythmias, cardiomyopathy, major congenital heart disease, and Fridericia-corrected Q wave interval abnormalities were excluded from the JADE clinical trial program. Patients at risk for VTE or with a history of VTE were not excluded from the JADE clinical trials, except in the JADE DARE study. Baseline characteristics, including cardiovascular risk factors for these patients, are provided in Table 6 of the ESM. No meaningful differences were found in the IRs of MACE and VTE by baseline disease severity, sex, or smoking status; however, the IRs of MACE and VTE were numerically higher (with non-overlapping 95% CIs for VTE) in older patients than in younger patients. Higher risks for cardiovascular events were also identified in patients with active rheumatoid arthritis who were ≥ 50 years of age, had inadequate response to methotrexate, had one or more additional cardiovascular risk factors, and were treated with tofacitinib compared with those treated with a tumor necrosis factor inhibitor [31]. The risk for MACE in patients with rheumatoid arthritis is higher than in the general population [32]. Compared with patients with AD, patients with rheumatoid arthritis tend to be older and have higher body mass index [33]. Therefore, a higher incidence of MACE in patients with rheumatoid arthritis treated with JAK inhibitors could be related in part to the specific patient profile [31].
Patients with chronic inflammatory conditions, including AD, have an increased risk of malignancies [34–36]. Previous studies have identified a significant association between AD and NMSC, but no association with most other cancers [37–39]. In a cohort of patients with AD in the UK, the IR (95% CI) per 100 PY for malignancy (excluding NMSC) was 0.4 (0.4‒0.4) in the overall population and 0.3 (0.3‒0.4) in those with AD [40]. The IR (95% CI) per 100 PY for NMSC was 0.1 (0.1‒0.1) in the overall population and 0.1 (0.1‒0.1) in those with AD [40]. In a cohort of 7050 adult patients in the USA with moderate-to-severe AD, the IR (95% CI) per 100 PY of NMSC was 0.5 (0.4–0.6) [41]. Patients with malignancies or a history of malignancies, with the exception of adequately treated or excised non-metastatic basal cell or squamous cell cancer of the skin, or cervical carcinoma in situ, were excluded from the JADE clinical trial program. Baseline characteristics including risk factors for malignancies in these patients are provided in Table 6 of the ESM. In the current analysis, a trend toward a dose response was observed for the IR point estimates of malignancies (excluding NMSC) but the CIs largely overlap (200 mg: 0.3 [0.1‒0.6]; 100 mg: 0.1 [0.0‒0.5]). The IRs for NMSC do not suggest a dose-dependent response. All events of NMSC occurred in current/former smokers, and IRs for malignancy (excluding NMSC) were numerically higher in current/former smokers than in those who never smoked. These results are in line with previous reports showing an increased risk of squamous cell carcinoma of the skin in smokers versus non-smokers [42, 43]. No meaningful differences in IRs for NMSC or malignancies (excluding NMSC) were identified according to baseline disease severity, sex, or race. The IRs of NMSC and malignancy (excluding NMSC) were higher in older versus younger patients.
In the previously reported integrated safety analysis, there was a dose-dependent transient decrease in platelet counts in patients treated with abrocitinib versus placebo, with maximum effects observed at 4 weeks [10]. In the current analysis, no meaningful differences in the IR for thrombocytopenia (platelet count < 50 × 103/mm3) were found by baseline disease severity, sex, or race, and no hemorrhage events due to thrombocytopenia occurred. Incidence rates for thrombocytopenia were numerically higher in older (≥ 65 years of age) compared with younger patients (18 to < 65 and < 18 years of age).
In the previous integrated safety analysis, four patients, all in the abrocitinib 200-mg group and ≥ 65 years of age, had an event of lymphopenia [10]. Similarly in the current analysis, all cases of lymphopenia occurred in the 200-mg group, and higher rates were seen in older (≥ 65 years of age) versus younger patients. There were no meaningful differences in the rate of lymphopenia by baseline disease severity or sex. In this analysis, there were four patients with events of retinal detachment; not reported in the previous integrated safety analysis [10]. These events were deemed unrelated to the study drug by the study investigators and patients had a prior medical history of eye disorders.
This analysis comprised patients treated with abrocitinib with longer exposure periods than the previous safety update; 5.1% and 3.8% of patients in the consistent-dose cohort and variable-dose cohort, respectively, had exposure to abrocitinib for ≥ 144 weeks, 0.7% and 0.1% had exposure for ≥ 168 weeks, and < 0.1% (n = 1) and 0% for ≥ 192 weeks. Less than 0.1% (n = 3) of patients in the consistent-dose and 0% of patients in the variable-dose cohort had exposure for ≥ 182 weeks (~ 3.5 years). It should be noted that the exposure period was determined by the number of dosing days, and the maximum exposure time of ~ 4 years was calculated from the upper value of the treatment duration (as of the data cutoff date) which ranged from 1 to 1369 days in the consistent-dose cohort and 89–1181 days in variable-dose cohort. This JADE EXTEND long-term extension study is ongoing and future analyses with later data cutoffs will include more patients with longer exposure times. The relationship of any AE to study drug was determined by the investigators based on their clinical judgment at the time of development of the clinical program. Any potential dose–response relationships observed in this analysis are generally limited by the precision of the IR estimates.
Conclusions
Abrocitinib had a manageable long-term safety profile in this large population of patients with moderate-to-severe AD who require systemic therapy. This larger and longer safety analysis was generally consistent with the previously reported safety profile of abrocitinib. Patients ≥ 65 years of age had a higher rate of serious AEs and study discontinuations due to AEs, a greater likelihood of developing low platelet and ALC values, a trend toward an increased risk for serious infections, NMSC, malignancy excluding NMSC, MACE, VTE, and PE, and an increased risk of HZ infection. The risk for HZ infection was also greater in patients who received a higher dose of abrocitinib, had a confirmed ALC of < 1 × 103/mm3 prior to the event, had a prior medical history of HZ, or were residing in Asia. The risk for HS was also greater in patients who received a higher dose of abrocitinib with a potential inverse dose relationship for EH. There was no clear dose-dependent response observed for serious infections, MACE, VTE, NMSC, malignancies (excluding NMSC), and COVID-19 events due to overlapping CIs. All events of NMSC occurred in current/former smokers, and IRs for malignancy (excluding NMSC) were numerically higher in current/former smokers than in those who never smoked.
Both patient selection and dose selection remain important considerations for physicians who prescribe abrocitinib for moderate-to-severe AD. Risk minimization measures as described in the abrocitinib product labels should be followed to maintain an appropriate risk–benefit relationship, and appropriate patient selection and monitoring as per the label are recommended.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing support under the guidance of the authors was provided by Kristine De La Torre, PhD, and Amanda Mabhula, PhD, of ApotheCom (San Francisco, CA, USA), and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022; 10.7326/M22-1460).
Declarations
Funding
This study was funded by Pfizer, Inc. The open access fee was funded by Pfizer Inc.
Conflict of interest
Eric L. Simpson has received grants from Pfizer Inc., Eli Lilly and Company, Kyowa Kirin, LEO Pharma, Merck, and Regeneron Pharmaceuticals, and personal fees from Pfizer Inc., Bausch Health (Valeant), Dermira, Eli Lilly and Company, Galderma, LEO Pharma, Menlo Therapeutics, Novartis, Regeneron Pharmaceuticals, and Sanofi Genzyme. Jonathan I. Silverberg served as an investigator for Celgene, Eli Lilly and Company, F. Hoffmann-La Roche, Menlo Therapeutics, Realm Therapeutics, Regeneron Pharmaceuticals, and Sanofi Genzyme; as a consultant for Pfizer Inc., AbbVie, Anacor, AnaptysBio, Arena Pharmaceuticals, Dermavant, Dermira, Eli Lilly and Company, Galderma, GlaxoSmithKline, Glenmark, Incyte, Kiniksa Pharmaceuticals, LEO Pharma, Menlo Therapeutics, Novartis, Realm Therapeutics, Regeneron Pharmaceuticals, and Sanofi Genzyme; and as a speaker for Regeneron Pharmaceuticals and Sanofi Genzyme. Audrey Nosbaum is a consultant for Pfizer Inc., AbbVie, Eli Lilly and Company, Galderma, LEO Pharma, Novartis, and Sanofi Genzyme; a speaker for AbbVie, Regeneron Pharmaceuticals, and Sanofi Genzyme; an investigator for AbbVie, Eli Lilly and Company, Incyte, LEO Pharma, Novartis, and Sanofi Genzyme; and is on advisory boards for Pfizer Inc., AbbVie, LEO Pharma, and Sanofi Genzyme. Kevin Winthrop reports research grants from Pfizer Inc. and Bristol Myers Squibb, and consulting fees from Pfizer Inc., AbbVie, AstraZeneca, Bristol Myers Squibb, Eli Lilly and Company, Galapagos, Gilead, GlaxoSmithKline, Novartis, Regeneron Pharmaceuticals, Roche, and Union Chimique Belge (UCB). Emma Guttman-Yassky has served as an advisory board member for Pfizer Inc., Asana BioSciences (honorarium), Celgene, Dermira, Galderma, Glenmark, MedImmune, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Stiefel/GlaxoSmithKline, and Vitae; a consultant for Pfizer Inc., AbbVie, Almirall (honorarium), Anacor, Asana BioSciences, Celgene, Dermira, Eli Lilly and Company, Galderma, Glenmark, Kiowa Kirin, LEO Pharma, MedImmune, Mitsubishi Tanabe, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Stiefel/GlaxoSmithKline, and Vitae; and an investigator for Celgene, Eli Lilly and Company (grants to institution), LEO Pharma, MedImmune, and Regeneron Pharmaceuticals. Karin M. Hoffmeister has no disclosures to report. Alexander Egeberg has received research funding from Pfizer Inc., AbbVie, Almirall, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly and Company, Novartis, Janssen, the Danish National Psoriasis Foundation, the Simon Spies Foundation, and the Kongelig Hofbuntmager Aage Bangs Foundation; and honoraria as consultant and/or speaker from Pfizer Inc., AbbVie, Amgen, Almirall, Bristol Myers Squibb, Boehringer Ingelheim, Dermavant, Eli Lilly and Company, Galápagos NV, Galderma, Horizon Therapeutics, Janssen, LEO Pharma, McNeil Consumer Healthcare, Mylan, Novartis, Samsung Bioepis Co., Ltd., Sun Pharmaceutical, UCB, UNION Therapeutics, and Zuellig Pharma Ltd. He is a current employee of LEO Pharma. Hernan Valdez, Haiyun Fan, Saleem A. Farooqui, Gary Chan, Justine Alderfer, William Romero, and Kanti Chittuluru are employees and shareholders of Pfizer Inc.
Ethics approval
All study documents and procedures were approved by the appropriate institutional review boards/ethics committees at each study site. The studies were conducted in compliance with the ethical principles from the Declaration of Helsinki and all International Council for Harmonisation Good Clinical Practice Guidelines. All local regulatory requirements were followed.
Consent to participate
Informed consent was obtained from all participants of the individual studies included in this updated integrated safety analysis.
Consent for publication
Not applicable.
Availability of data and material
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Code availability
Not applicable.
Author contributions
ELS, JIS, AN, KW, EGY, KMH, AE, HV, SAF, GC, JA, WR, and KC contributed to the conceptualization and design of the analysis. HF performed or supported the data analysis. All authors interpreted the data, provided critical feedback on the manuscript, approved the final manuscript for submission, and are accountable for the accuracy and integrity of the article.
Footnotes
Affiliation at the time of this analysis: Alexander Egeberg, Hernan Valdez, William Romero, Kanti Chittuluru.
References
- 1.Cibinqo 100 mg film-coated tablets. Summary of product characteristics. Kent: Pfizer, Limited; 2021. Updated March 10, 2023.
- 2.European Medicines Agency. Cibinqo® (abrocitinib). Summary of product characteristics. Pfizer Europe MA EEIG; 2024.
- 3.Cibinqo (abrocitinib) tablets, for oral use. Prescribing information. New York (NY): Pfizer Inc.; 2023.
- 4.Gooderham MJ, Forman SB, Bissonnette R, Beebe JS, Zhang W, Banfield C, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155(12):1371–1379. doi: 10.1001/jamadermatol.2019.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–873. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266. doi: 10.1016/s0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]
- 7.Blauvelt A, Silverberg JI, Lynde CW, Bieber T, Eisman S, Zdybski J, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J Am Acad Dermatol. 2022;86(1):104–112. doi: 10.1016/j.jaad.2021.05.075. [DOI] [PubMed] [Google Scholar]
- 8.Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101–1112. doi: 10.1056/NEJMoa2019380. [DOI] [PubMed] [Google Scholar]
- 9.Eichenfield LF, Flohr C, Sidbury R, Siegfried E, Szalai Z, Galus R, et al. Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: the JADE TEEN randomized clinical trial. JAMA Dermatol. 2021;157(10):1165–1173. doi: 10.1001/jamadermatol.2021.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson EL, Silverberg JI, Nosbaum A, Winthrop KL, Guttman-Yassky E, Hoffmeister KM, et al. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am J Clin Dermatol. 2021;22(5):693–707. doi: 10.1007/s40257-021-00618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cork MJ, Deleuran M, Geng B, Silverberg JI, Simpson EL, Stein Gold LF, et al. Abrocitinib treatment in patients with moderate-to-severe atopic dermatitis: safety of abrocitinib stratified by age. In: European Academy of Dermatology and Venereology (EADV) 30th Congress; 29 Sep–2 Oct 2021; Berlin.
- 12.Reich K, Thyssen JP, Blauvelt A, Eyerich K, Soong W, Rice ZP, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet. 2022;400(10348):273–282. doi: 10.1016/s0140-6736(22)01199-0. [DOI] [PubMed] [Google Scholar]
- 13.Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunzini F, McInnes I, Siebert S. JAK inhibitors and infections risk: focus on herpes zoster. Ther Adv Musculoskelet Dis. 2020;12:1759720X20936059. 10.1177/1759720X20936059. [DOI] [PMC free article] [PubMed]
- 15.Ghilotti F, Bellocco R, Ye W, Adami HO, Trolle LY. Obesity and risk of infections: results from men and women in the Swedish National March Cohort. Int J Epidemiol. 2019;48(6):1783–1794. doi: 10.1093/ije/dyz129. [DOI] [PubMed] [Google Scholar]
- 16.Harpsøe MC, Nielsen NM, Friis-Møller N, Andersson M, Wohlfahrt J, Linneberg A, et al. Body mass index and risk of infections among women in the Danish national birth cohort. Am J Epidemiol. 2016;183(11):1008–1017. doi: 10.1093/aje/kwv300. [DOI] [PubMed] [Google Scholar]
- 17.Phung DT, Wang Z, Rutherford S, Huang C, Chu C. Body mass index and risk of pneumonia: a systematic review and meta-analysis. Obes Rev. 2013;14(10):839–857. doi: 10.1111/obr.12055. [DOI] [PubMed] [Google Scholar]
- 18.Wan J, Shin DB, Syed MN, Abuabara K, Lemeshow AR, Gelfand JM. Risk of herpesvirus, serious and opportunistic infections in atopic dermatitis: a population-based cohort study. Br J Dermatol. 2022;186(4):664–672. doi: 10.1111/bjd.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke B, Yates M, Adas M, Bechman K, Galloway J. The safety of JAK-1 inhibitors. Rheumatology (Oxford). 2021;60(Suppl. 2):ii24–ii30. 10.1093/rheumatology/keaa895. [DOI] [PMC free article] [PubMed]
- 20.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49(2):198–205. doi: 10.1067/s0190-9622(03)00896-x. [DOI] [PubMed] [Google Scholar]
- 22.Damour A, Garcia M, Seneschal J, Lévêque N, Bodet C. Eczema herpeticum: clinical and pathophysiological aspects. Clin Rev Allergy Immunol. 2020;59(1):1–18. doi: 10.1007/s12016-019-08768-3. [DOI] [PubMed] [Google Scholar]
- 23.Gao L, Bin L, Rafaels NM, Huang L, Potee J, Ruczinski I, et al. Targeted deep sequencing identifies rare loss-of-function variants in IFNGR1 for risk of atopic dermatitis complicated by eczema herpeticum. J Allergy Clin Immunol. 2015;136(6):1591–1600. doi: 10.1016/j.jaci.2015.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung DY, Gao PS, Grigoryev DN, Rafaels NM, Streib JE, Howell MD, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127(4):965–73.e1–5. 10.1016/j.jaci.2011.02.010. [DOI] [PMC free article] [PubMed]
- 25.Ungar B, Pavel AB, Robson PM, Kaufman A, Pruzan A, Brunner P, et al. A preliminary (18)F-FDG-PET/MRI study shows increased vascular inflammation in moderate-to-severe atopic dermatitis. J Allergy Clin Immunol Pract. 2020;8(10):3500–3506. doi: 10.1016/j.jaip.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ascott A, Mulick A, Yu AM, Prieto-Merino D, Schmidt M, Abuabara K, et al. Atopic eczema and major cardiovascular outcomes: a systematic review and meta-analysis of population-based studies. J Allergy Clin Immunol. 2019;143(5):1821–1829. doi: 10.1016/j.jaci.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Standl M, Tesch F, Baurecht H, Rodriguez E, Muller-Nurasyid M, Gieger C, et al. Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137(5):1074–1081. doi: 10.1016/j.jid.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 28.Silverwood RJ, Forbes HJ, Abuabara K, Ascott A, Schmidt M, Schmidt SAJ, et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ. 2018;361:k1786. doi: 10.1136/bmj.k1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedderson MM, Asgari MM, Xu F, Quesenberry CP, Sridhar S, Geier J, et al. Rates of cardiovascular events among patients with moderate-to-severe atopic dermatitis in an integrated health care system: a retrospective cohort study. PLoS ONE. 2022;17(11):e0277469. doi: 10.1371/journal.pone.0277469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen YMF, Egeberg A, Gislason GH, Hansen PR, Skov L, Thyssen JP. Risk of myocardial infarction, ischemic stroke, and cardiovascular death in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138(1):310–2.e3. doi: 10.1016/j.jaci.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 32.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71(9):1524–1529. doi: 10.1136/annrheumdis-2011-200726. [DOI] [PubMed] [Google Scholar]
- 33.Montez-Rath ME, Lubwama R, Kapphahn K, Ling AY, LoCasale R, Robinson L, et al. Characterizing real world safety profile of oral Janus kinase inhibitors among adult atopic dermatitis patients: evidence transporting from the rheumatoid arthritis population. Curr Med Res Opin. 2022;38(8):1431–1437. doi: 10.1080/03007995.2022.2088715. [DOI] [PubMed] [Google Scholar]
- 34.Hagströmer L, Ye W, Nyrén O, Emtestam L. Incidence of cancer among patients with atopic dermatitis. Arch Dermatol. 2005;141(9):1123–1127. doi: 10.1001/archderm.141.9.1123. [DOI] [PubMed] [Google Scholar]
- 35.Olesen AB, Engholm G, Storm HH, Thestrup-Pedersen K. The risk of cancer among patients previously hospitalized for atopic dermatitis. J Invest Dermatol. 2005;125(3):445–449. doi: 10.1111/j.0022-202X.2005.23839.x. [DOI] [PubMed] [Google Scholar]
- 36.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Bierbrier R, Drucker AM, Chan AW. Noncutaneous and cutaneous cancer risk in patients with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156(2):158–171. doi: 10.1001/jamadermatol.2019.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruff S, Egeberg A, Andersen YMF, Gislason G, Skov L, Thyssen JP. Prevalence of cancer in adult patients with atopic dermatitis: a nationwide study. Acta Derm Venereol. 2017;97(9):1127–1129. doi: 10.2340/00015555-2703. [DOI] [PubMed] [Google Scholar]
- 39.Mansfield KE, Schmidt SAJ, Darvalics B, Mulick A, Abuabara K, Wong AYS, et al. Association between atopic eczema and cancer in England and Denmark. JAMA Dermatol. 2020;156(10):1086–1097. doi: 10.1001/jamadermatol.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arana A, Wentworth CE, Fernández-Vidaurre C, Schlienger RG, Conde E, Arellano FM. Incidence of cancer in the general population and in patients with or without atopic dermatitis in the U.K. Br J Dermatol. 2010;163(5):1036–43. 10.1111/j.1365-2133.2010.09887.x. [DOI] [PubMed]
- 41.Hedderson MM, Asgari MM, Xu F, Quesenberry CP, Sridhar S, Geier J, et al. Rates of malignancies among patients with moderate to severe atopic dermatitis: a retrospective cohort study. BMJ Open. 2023;13(3):e071172. doi: 10.1136/bmjopen-2022-071172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leonardi-Bee J, Ellison T, Bath-Hextall F. Smoking and the risk of nonmelanoma skin cancer: systematic review and meta-analysis. Arch Dermatol. 2012;148(8):939–946. doi: 10.1001/archdermatol.2012.1374. [DOI] [PubMed] [Google Scholar]
- 43.Rollison DE, Iannacone MR, Messina JL, Glass LF, Giuliano AR, Roetzheim RG, et al. Case-control study of smoking and non-melanoma skin cancer. Cancer Causes Control. 2012;23(2):245–254. doi: 10.1007/s10552-011-9872-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.