Abstract
Background
Gastric cancer with peritoneal dissemination (PD) has a dismal prognosis, and current treatments have shown little efficacy. CLDN18.2-targeted therapies have shown promising efficacy against gastric cancers that express high levels of CLDN18. Because of the limited information regarding CLDN18.2 status in PD, we analyzed PD-positive gastric cancers for CLDN18 status in both primary and PD, along with HER2 and PD-L1 combined positive score (CPS).
Methods
Immunohistochemical analyses were performed on 84 gastric cancer cases using paired primary and PD tissue samples.
Results
At 40% cut-off, CLDN18 was positive in 57% (48/84) primary tumors and in 44% (37/84) PDs. At 75% cut-off, 28.6% (24/84) primary tumors and 20.2% (17/84) PDs were CLDN18-positive. The concordance rate between primary tumors and PD was 79.8% at 40% cut-off and 75% at 75% cut-off. When comparing biopsy and surgical specimens, the concordance rates were 87.5% at 40% cut-off and 81.3% at 75% cut-off. Within a tumor, the superficial area tended to have a higher CLDN18-positive rate than the invasive front (P = 0.001). Although HER2 -positivity was only 11.9% in this cohort, CLDN18 positivity in HER2-negative tumors (n = 74) was relatively high: 60.8% at 40% cut-off and 28.4% at 75% cut-off. Among double-negative (HER2 − and PD-L1 CPS < 1) tumors, CLDN18 positivity was 67.6% at 40% cut-off and 26.5% at 75% cut-off.
Conclusions
CLDN18 expression is generally maintained in PD and is relatively high even in double-negative tumors, making it a promising therapeutic target for PD-positive gastric cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10120-024-01505-6.
Keywords: Gastric cancer, Claudin-18, Peritoneal dissemination, Molecular targeted therapy, Immunohistochemistry
Introduction
Gastric cancer is the fourth leading cause of cancer-related deaths worldwide [1]. The morbidity and mortality rates are declining because of the decreasing prevalence of Helicobacter pylori infection, widespread H.pylori eradication therapy, and early detection through endoscopy. However, the prognosis of patients with advanced gastric cancer, especially those with peritoneal dissemination (PD), is still poor [2]. Chemotherapy, anti-human epidermal growth factor receptor 2 (HER2) antibodies (such as trastuzumab), anti-VEGFR2 antibodies (such as ramucirumab), and immune checkpoint inhibitors are standard therapies for unresectable or recurrent gastric cancer. PD-positive gastric cancers typically exhibit a diffuse-type histology and this subtype is HER2-negative in most cases. PD-L1expression is also relatively low in the diffuse-type subtype when compared to the microsatellite instability and Epstein-Barr virus-positive subtypes. Therefore, new treatment strategies for PD-positive gastric cancers are urgently required.
Recently, zolbetuximab, which targets the tight junction molecule claudin-18 isoform 2 (CLDN18.2), was developed. Zolbetuximab is a monoclonal immunoglobulin G1 (IgG1) antibody that binds CLDN18.2 and induces antibody- and complement-dependent cellular cytotoxicity. Randomized phase 2 (FAST, NCT01630083) and phase 3 trials (SPOTLIGHT, NCT03504397, and GLOW, NCT03653507) have demonstrated the clinical efficacy of zolbetuximab in combination with chemotherapy in patients with HER2-negative unresectable/recurrent gastric cancer showing high expression of CLDN18.2 [3–5]. In these clinical trials, CLDN18.2 expression was assessed using immunohistochemistry with an anti-CLDN18 antibody (clone 43-14A), and the proportion of neoplastic cells with moderate (2 +) or strong (3 +) expression was evaluated at different cut-off values. In the FAST trial, a cut-off value of 40% was used [5], whereas a cut-off value of 75% was used in the SPOTLIGHT and GLOW trials [3, 4]. Although the clone 43-14A antibody reacts with both CLDN18.1 and CLDN18.2, the antibody was used for the detection of CLDN18.2 in these clinical trials because CLDN18 expressed in the stomach is exclusively CLDN18.2, not CLDN18.1 [6, 7]. Therefore, this antibody was used to analyze CLDN18.2 expression in the present study.
Earlier studies have suggested that CLDN18.2 expression decreases during cancer progression, but information regarding the concordance between primary and metastatic lesions is still limited [8, 9]. Rohde et al. reported that CLDN18.2 expression status in primary gastric cancers was frequently maintained in regional lymph node metastases [10]. Regarding PD, a recent study analyzed CLDN18 expression using effusion cell block samples, demonstrating a high concordance (83.7%) between primary tumor tissue and effusion cells [11]. However, no study has evaluated CLDN18 expression using tissue samples, not suspended cells in ascites, that was resected from PD. Because of its clinical importance and the lack of data, we examined the expression of CLDN18 in both primary tumors and PD to determine the applicability of zolbetuximab in patients with PD-positive gastric cancer. We also focused on intratumoral heterogeneity by comparing the CLDN18 status among different tumor areas (surface vs. center vs. invasive front) and different sample types (biopsy vs. surgical resection). In addition, the correlation between the CLDN18 status and other biomarkers, including HER2 and PD-L1, was clarified.
Materials and methods
Tissue samples
This retrospective study included 84 gastric cancer cases with tissue samples from both primary and PD, collected from the pathological archive at The University of Tokyo Hospital between April 2000 and December 2021. For primary lesions, the tissue types included 78 biopsies, 16 surgical specimens, and six autopsy cases, and PD tissues includes 87 surgical specimens and six autopsy cases (Fig. 1). In nine cases, both pre- and post-chemotherapy samples were available. Clinicopathological data, including age, sex, tumor location, tumor size, T stage, lymphatic invasion, and venous invasion were collected from pathological records. Gastric cancer histology was classified according to the Lauren classification [12].
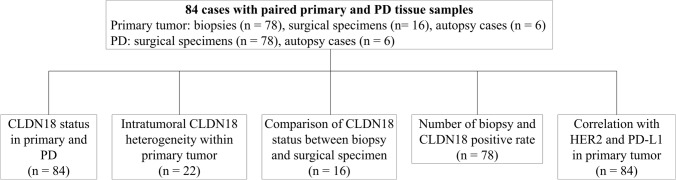
Fig. 1.
Summary of specimens used in each analysis. A total of 84 cases with gastric cancer with PD were included in the study, and five main analyses were performed. The numbers in parentheses indicate the number of cases studied
Follow-up data were collected from the medical record. Four patients who were revealed to have gastric cancer for the first time at autopsy were excluded from survival analyses. Overall survival (OS) time was defined as period from the date of diagnosis to the date of death of any cause or last follow-up. Because patients with PD could not usually receive curative resection, recurrence free survival analysis was not performed.
This study was performed according to the Declaration of Helsinki, and the study protocol was approved by the institutional review board (approval number G3521).
Immunohistochemistry
Sections of 3-μm thickness were prepared from formalin-fixed paraffin-embedded tissue blocks. Immunohistochemistry was performed using a Ventana Benchmark Ultra automated immunostainer (Ventana Medical Systems, Tucson, AZ, USA), according to the manufacturer’s instructions. Immunohistochemistry of CLDN18 was performed using the mouse monoclonal antibody clone 43-14A (Ventana Medical Systems).
Membrane expression of CLDN18 was evaluated based on the staining intensity and proportion. The staining intensity was classified as 0 (no staining), 1 + (weak staining), 2 + (moderate staining), and 3 + (strong staining). In this study, CLDN18 positivity was defined as moderate (2 +) or strong (3 +) CLDN18 staining using two cut-off values: ≥ 40% (FAST trial eligibility criterion) and ≥ 75% (SPOTLIGHT and GLOW trial eligibility criteria). In specimens resected by surgery or autopsy, the primary tumor area was subdivided into three areas (superficial, central, and invasive front), and CLDN18 expression was evaluated separately in each area and in the whole tumor. CLDN18 expression in normal gastric epithelium served as an internal positive control. Immunohistochemical evaluations were performed independently by two observers (H.O. and H.A.) who were blinded to the clinicopathological data. In case of disagreement, the slides were re-evaluated by the two observers to reach a final decision.
HER2 status was evaluated based on the American Society of Clinical Oncology guidelines [13]. In brief, immunohistochemistry of HER2 was performed using a rabbit monoclonal anti-HER2 antibody (clone 4B5, prediluted, Roche, Basel, Switzerland) and evaluated at a score of 0, 1 + , 2 + , or 3 + . For cases with a score of 2 + , dual-color ISH (DISH) for HER2 was performed using the Ventana INFORM HER2 DualColor ISH Kit (Roche) to assess HER2 amplification. Cases with immunohistochemistry 3 + or 2 + with HER2 amplification were considered HER2-positive.
Immunohistochemistry for PD-L1 was performed using a rabbit monoclonal anti-PD-L1 antibody (clone SP263, prediluted; Roche). PD-L1 expression was evaluated using the combined positive score (CPS) [14]. The PD-L1 CPS was defined as the number of PD-L1-expressing cells (tumor cells, lymphocytes, and macrophages) divided by the total number of tumor cells, multiplied by 100. PD-L1 expression was classified as CPS < 1, 1 ≤ CPS < 5, and CPS ≥ 5 based on the criterion of the preceding clinical trial for nivolumab treatment for patients with gastric cancer [15]. In this study, HER2 and PD-L1 were evaluated only in the primary tumors.
Statistical analyses
All statistical analyses were performed using the JMP Pro 17 software (SAS Institute Inc., Cary, NC, USA). Continuous variables were analyzed using a paired Student’s t-test, and categorical data were analyzed using Fisher’s exact test. The Kaplan–Meier method was used to draw survival curves and estimate OS. OS was compared between CLDN18-positive and -negative groups by Wilcoxon test. The Cox proportional hazards model was used to calculate the hazard ratio (HR) and 95% confidence interval (CI). Statistical significance was set at P < 0.05.
Results
CLDN18 status in primary and its clinicopathological correlation
Figure 2 shows representative images of each CLDN18 immunohistochemistry score. Among primary tumors (n = 84), 48 (57.1%) showed ≥ 40% and 24 (28.6%) showed ≥ 75% CLDN18 expression (Table 1). There was no significant correlation between the CLDN18 status and sex, age, or tumor site. Histologically, 66 primary tumors (78.6%) were of the diffuse type and 18 (21.4%) were of the intestinal type. There was no significant difference in CLDN18 status between intestinal- and diffuse-type histology.
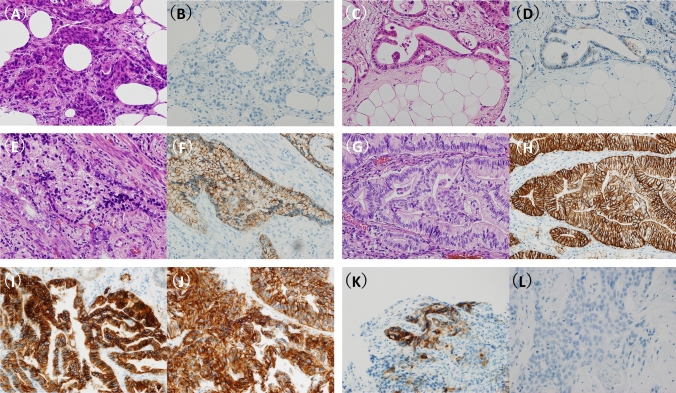
Fig. 2.
Representative images of CLDN18 immunohistochemistry. Pair images (H&E staining and CLDN18 immunohistochemistry) of four categories in CLDN18 intensity: 0, no expression (A and B); 1 + , weak expression (C and D); 2 + , moderate expression (E and F); and 3 + , strong expression (G and H). CLDN18 immunostainings of a representative case with CLDN18-positive in both the primary (I) and peritoneal dissemination (J). CLDN18 immunostainings of a case with discordant results that showed CLDN18-positive in the primary tumor (K) but negative in the peritoneal dissemination (L)
Table 1.
CLDN18 status at two cut-off values and clinicopathological characteristics in primary tumors and peritoneal disseminations
| n | CLDN18 in primary tumors | CLDN18 in peritoneal disseminations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40% cut-off | 75% cut-off | 40% cut-off | 75% cut-off | ||||||||||
| − | + | P-value* | − | + | P-value* | − | + | P-value* | − | + | P-value* | ||
| Total | 84 | 36 | 48 | 60 | 24 | 47 | 37 | 67 | 17 | ||||
| Sex | 0.35 | 0.62 | 0.82 | 0.78 | |||||||||
| Male | 55 | 26 | 29 | 38 | 17 | 30 | 25 | 43 | 12 | ||||
| Female | 29 | 10 | 19 | 22 | 7 | 17 | 12 | 24 | 5 | ||||
| Age | 0.27 | 0.48 | 0.13 | 0.60 | |||||||||
| < 65 | 44 | 16 | 28 | 33 | 11 | 21 | 23 | 34 | 10 | ||||
| ≥ 65 | 40 | 20 | 20 | 27 | 13 | 26 | 14 | 33 | 7 | ||||
| Tumor site | 0.53 | 0.09 | 0.06 | 0.08 | |||||||||
| U | 17 | 7 | 10 | 9 | 8 | 7 | 10 | 10 | 7 | ||||
| M | 51 | 20 | 31 | 37 | 14 | 27 | 24 | 43 | 8 | ||||
| L | 16 | 9 | 7 | 14 | 2 | 13 | 3 | 14 | 2 | ||||
| Histologic type | 0.11 | 0.77 | 1.0 | 1.0 | |||||||||
| intestinal | 18 | 11 | 7 | 12 | 6 | 10 | 8 | 15 | 3 | ||||
| diffuse | 66 | 25 | 41 | 48 | 18 | 37 | 29 | 52 | 14 | ||||
CLDN Claudin
*Fisher’s exact test
In overall survival analysis, no significant difference was observed between CLDN18-positive and –negative groups at either 40% or 75% cut-offs (Supplementary Fig. 1). Comparing CLDN18 status in PD before and after chemotherapy in nine patients, CLDN18 expression was positive in four (44.4%) (40% cut-off) and two (22.2%) (75% cut-off) patients before chemotherapy, whereas it was positive in five (55.6%) (40% cut-off) and four (44.4%) (75% cut-off) patients after chemotherapy. The concordance rate of CLDN18 status before and after chemotherapy was 88.9% (8/9) at 40% cut-off and 66.6% (6/9) at 75% cut-off.
Comparison of CLDN18 expression between primary and PD
In PD (n = 84), 37 (44.0%) showed ≥ 40% and 17 (20.2%) showed ≥ 75% CLDN18 expression. The relationship between primary tumors and PD is shown in Fig. 3 and Tables 2. Although a positive correlation existed between CLDN18 expression in primary tumors and PD, CLDN18 expression in PD was significantly lower than that in primary tumors (P < 0.001). The concordance rate between primary tumors and PD was 79.8% (67/84) at the 40% cut-off and 75% (63/84) at the 75% cut-off.
Fig. 3.
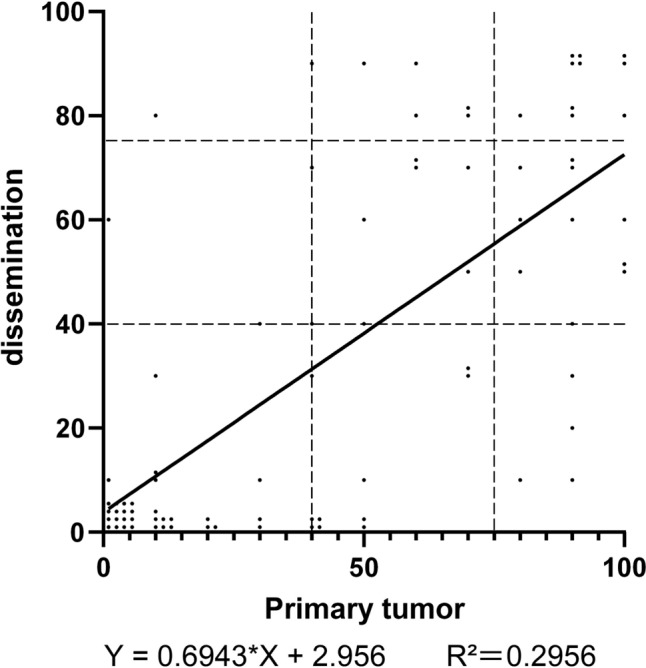
Comparison of CLDN18 expression between primary tumors and peritoneal dissemination. The percentage of CLDN18-positive tumor cells in the primary tumor is shown on the X-axis, and the percentage of peritoneal dissemination is shown on the Y-axis. The line represents a linear regression curve
Table 2.
CLDN18 expression in primary tumors and peritoneal disseminations
| (A) | |||
|---|---|---|---|
| 40% cut-off | CLDN18 in peritoneal disseminations | ||
| − | + | ||
|
CLDN18 in primary tumors |
− | 33 (39.3%) | 3 (3.6%) |
| + | 14 (16.7%) | 34 (40.5%) | |
| concordance rate 79.8% | P* < 0.001 | ||
| (B) | |||
|---|---|---|---|
| 75% cut-off | CLDN18 in peritoneal disseminations | ||
| − | + | ||
|
CLDN18 in primary tumors |
− | 53 (63.1%) | 7 (8.3%) |
| + | 14 (16.7%) | 10 (11.9%) | |
| concordance rate 75.0% | P* = 0.005 | ||
CLDN Claudin
*Fisher’s exact test
Intratumoral CLDN18 heterogeneity
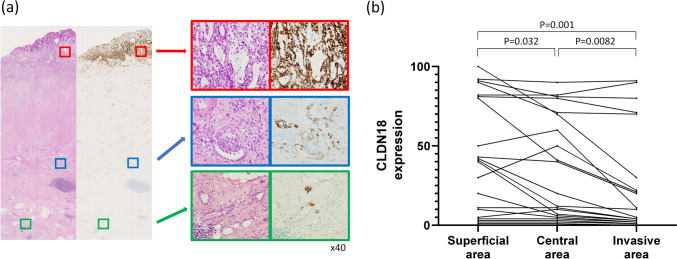
The intratumoral heterogeneity of CLDN18 expression was examined in 22 primary tumors that were surgically resected or autopsied. CLDN18 expression was evaluated separately in the superficial, central, and invasive frontal areas (Fig. 4a). The positivity rate of CLDN18 was highest in the superficial areas, lower in the central areas, and lowest in the invasive front areas (superficial vs. central, P = 0.032; central vs. invasive front, P = 0.008; superficial vs. invasive front, P = 0.001) (Fig. 4b).
Fig. 4.
Intratumoral CLDN18 heterogeneity. a A representative case exhibiting heterogeneous CLDN18 expression. Loupe view of H&E and CLDN18 immunostaining (left). At higher power magnification, on the right, diffuse CLDN18 expression in the superficial area (upper pictures in the red box), partial positive staining in the center (middle pictures in the blue box), and almost negative staining in the invasive front (lower pictures in the green box) were noted. b The Percentage of CLDN18-positive neoplastic cells in the superficial, central, and invasive front areas. CLDN18 expression tended to decrease from the superficial to deep areas
Comparison between biopsies and surgical specimens
CLDN18 expression was compared in 16 cases in which both biopsy and surgical specimens of the primary tumor were available (Supplementary Fig. 2). No significant difference were elucidated between the biopsy and resected specimens (P = 0.44), with the concordance rate between the biopsy and resection specimens being 87.5% (14/16) at a 40% cut-off and 81.3% (13/16) at a 75% cut-off.
Number of biopsies and CLDN18 positive rate
To determine whether the positivity of CLDN18 depends on the number of tissue fragments obtained by biopsy, we examined the relationship between the number of fragments containing tumor tissue and CLDN18 positivity in 78 cases for which biopsy samples were available. In this cohort, the number of biopsied fragments, including tumor tissue, was one in 11 cases, two in 21 cases, three in 20 cases, four in 14 cases, five in eight cases, and six in four cases, with an average of 3.0 fragments per biopsy. At a cut-off of 40%, the CLDN18-positive ratio was 65.4% (34/52) of the ≤ 3 biopsies group and 46.2% (12/26) of the ≥ 4 biopsies group. At a cut-off of 75%, it was 32.7% (17/52) of the ≤ 3 biopsies group and 26.9% (7/26) of the ≥ 4 biopsies group. Therefore, no significant difference was observed between the number of biopsied fragments and CLDN18 positive ratio.
Correlation with HER2 and PD-L1
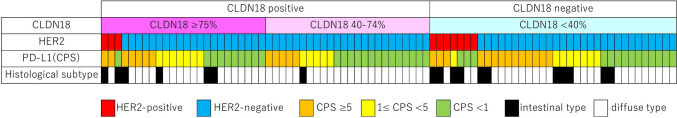
The expression profiles of HER2 and PD-L1 (CPS) as well as CLDN18 status are shown in Fig. 5. HER2-test revealed that 11.9% (10/84) of the primary tumors were HER2-positive, including 33.3% (6/18) of intestinal-type and 6.7% (4/66) of diffuse-type histology. PD-L1 CPS was ≥ 5 in 31.0% (26/84), 1–5 in 23.8 (20/84), and < 1 in 45.2% (38/84) cases.
Fig. 5.
Correlation between CLDN18 status and HER2, PD-L1 CPS, and Lauren histological classification. Although CLDN18 status was not significantly correlated with HER2 and PD-L1, a significant proportion of HER2-negative or PD-L1 CPS < 1 cases were CLDN18-positive
No significant correlations were noted between CLDN18 and HER2, or between CLDN18 and PD-L1 CPS in the primary tumors. CLDN18-positivity in HER2-negative tumors (n = 74) was 60.8% (45/74) at 40% cut-off and 28.4% (21/74) at 75% cut-off. Among double-negative (that is, HER2 − and PD-L1 CPS < 1) tumors, CLDN18 positivity was 67.6% at 40% cut-off and 26.5% at 75% cut-off.
Discussion
The present study demonstrated that CLDN18 positivity in PD-positive gastric cancers was 54.8% at 40% cut-off and 28.6% at 75% cut-off in primary tumors, and the frequency was slightly lower in disseminated lesions, showing positivity in 44% at 40% cut-off and 20.2% at 75% cut-off. In addition, we showed intratumoral CLDN18 heterogeneity with a tendency to decrease from the superficial to the deep side of the primary tumor, which is in line with earlier publications [8, 16]. Because CLDN18 is a tight junction protein, the loss of CLDN18 may induce epithelial-mesenchymal transition and promote cancer cell dissemination into the peritoneal cavity. Interestingly, studies have reported that CLDN18 expression is relatively preserved in lymph node metastases [10, 17], suggesting that the significance of the loss of CLDN18 expression is not as high as that in PD. Although there was a tendency for reduced CLDN18 expression in PD, the concordance rate of CLDN18 status between primary and PD tumors was more than 75%, suggesting that zolbetuximab may be effective for patients with PD, even when tested for CLDN18 status in primary tumors. However, because a small subset of cases showed highly discordant CLDN18 expression between primary tumors and PD, it is preferable to test CLDN18 expression in PD as well as in primary tumors when considering the indication of zolbetuximab.
Regarding biomarker testing, studies have repeatedly reported the significance of the types of specimens tested (that is, biopsy vs. surgical resection) and the number of biopsy fragments, especially in the setting of HER2-testing because of the heterogeneous pattern of HER2 expression [18–22]. Based on our observations, the CLDN18 expression pattern is also usually heterogeneous, similar to HER2. In our analyses, no significant correlation was noted between CLDN18 positivity and the type of specimen (biopsies vs. surgical specimens) or the number of biopsy fragments. The reason for this finding is probably the higher CLDN18 positivity in the superficial area from which biopsy is taken. However, in several cases, there was a mismatch in CLDN18 positivity between the biopsy and surgical specimens, such as CLDN18-negative biopsy and CLDN18-positive paired surgical specimens. Owing to the limited number of specimens analyzed in this study, further studies are required to determine the optimal biopsy procedure for assessing CLDN18 expression in gastric cancer.
Earlier studies, as well as our current study, demonstrated that there was no significant difference in CLDN18 expression between HER2-positive and -negative cases [17, 23, 24]. Among HER2-negative cases (n = 74) in our cohort, CLDN18 was positive in 45 cases (60.8%) at the 40% cut-off and 21 cases (28.4%) at the 75% cut-off. As HER2 is more frequently positive in the intestinal type than in the diffuse type [25], gastric cancer with PD, which is predominantly the diffuse type, is usually not a target of anti-HER2 drugs. In contrast, CLDN18 expression was frequently observed in the diffuse and intestinal types. Thus, zolbetuximab is a promising drug for patients with HER2-negative gastric cancer for whom anti-HER2 drugs are not indicated.
In a previous study, CLDN18 expression was significantly lower in gastric cancer with PD than those without PD [26]. This is consistent with our result that CLDN18-positive ratio in this study was lower than those in the FAST and SPOTLIGHT studies. Also, PD-L1 expression has been reported to be lower in diffuse-type than intestinal-type [15, 27] and in distant metastasis than primary tumor [28, 29]. Considering the relatively low PD-L1 expression and moderate CLDN18 positivity regardless of PD-L1 status, CLDN18 is a promising therapeutic target for patients with PD-positive gastric cancer who exhibit low PD-L1 expression. Among double-negative (HER2 − and PD-L1 CPS < 1) tumors, CLDN18-positivity was 67.6% at 40% cut-off and 26.5% at 75% cut-off. Therefore, zolbetuximab is expected to partially meet the unmet need for the treatment of PD-positive gastric cancer.
The present study had several limitations. First, the study was retrospectively designed in a single institution, and the number of cases was relatively small because PD is infrequently surgically resected in routine clinical practice for gastric cancer treatment. However, the present study is the first to examine CLDN18 expression in resected PD tissues. Although a recent study examined CLDN18 expression in ascites cytology specimens, our study is the first to evaluate PD nodules because the pharmacokinetics, including the presence of blood supply, are notably different between floating cells in ascites and lesions that form disseminated nodules in the peritoneum. This is an important point because zolbetuximab is administered intravenously and primarily targets peritoneal invasive cancer tissues with blood supply, and therefore information concerning CLDN18 status in the resected PD tissue samples, not just tumor cells floating in ascites, is needed. Second, we used clone SP263 to detect PD-L1 expression, which is different from companion diagnostic PD-L1 testing for gastric cancer, the 22C3 pharmDx assay (Dako, Santa Clara, CA, USA) or the 28–8 pharmDx assay (Dako) [14, 30]. However, previous studies have reported that 22C3 and SP263 antibodies are highly concordant in PD-L1 scoring. Thus, SP263 is considered equivalent to these companion diagnostic methods [31–33]. Finally, HER2 and PD-L1 were evaluated only in the primary tumors in this study. Although the information is still limited, the concordance ratio between primary and PD tissues was reported as 73.1% in HER2 status [34]. Concerning PD-L1 status, the concordance ratio between primary and distant metastasis (metastatic sites are not specified) was reported as 69.4% [35]. From these observations, it appears that the concordance rates between primary and metastatic sites for the three markers seem around 70%.
In conclusion, to the best of our knowledge, this is the first study to compare CLDN18 expression between primary tumors and PDs. Although CLDN18 expression tended to be mildly lower in deeper than in superficial areas, and also in disseminated lesions, the concordance rate of CLDN18 positivity between primary tumors and PDs was more than 75%. Our findings suggest that zolbetuximab is a promising treatment option for patients with PD-positive gastric cancer.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Kei Sakuma and Minato Murata for their technical assistance. This work was financially supported by the Japan Society for the Promotion of Science (KAKENHI, grant numbers 21K06882 [T. Ushiku] and 23K06458 [H. Abe]).
Author contributions
T. Ushiku designed the study. H. Ogawa, K. Yagi, and Y. Seto provided clinical information. H. Ogawa and H. Abe performed the immunohistochemistry and analyzed the data. H. Ogawa, H. Abe, and T. Ushiku wrote the manuscript, which was reviewed and approved by all the authors.
Funding
Open Access funding provided by The University of Tokyo.
Declarations
Conflict of interest
The authors declare no conflicts of interest related to this study.
Ethical approval
This study was performed according to the Declaration of Helsinki, and the study protocol was approved by the institutional review board (approval number G3521). The requirement to obtain written informed consent was waived because this was a retrospective study using existing pathology slides and formalin-fixed, paraffin-embedded blocks. Instead, we used an opt-out approach to provide participants with an informed choice about participation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haruki Ogawa and Hiroyuki Abe equally contributed to this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Inoue M. Changing epidemiology of Helicobacter pylori in Japan. Gastric Cancer. 2017;20:3–7. doi: 10.1007/s10120-016-0658-5. [DOI] [PubMed] [Google Scholar]
- 3.Shitara K, Lordick F, Bang Y-J, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. The Lancet. 2023 doi: 10.1016/S0140-6736(23)00620-7. [DOI] [PubMed] [Google Scholar]
- 4.Shah MA, Shitara K, Ajani JA, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023 doi: 10.1038/s41591-023-02465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin U, Türeci Manikhas G, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32:609–619. doi: 10.1016/j.annonc.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Niimi T, Nagashima K, Ward JM, et al. claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol. 2001;21:7380–7390. doi: 10.1128/mcb.21.21.7380-7390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14:7624–7634. doi: 10.1158/1078-0432.CCR-08-1547. [DOI] [PubMed] [Google Scholar]
- 8.Oshima T, Shan J, Okugawa T, et al. Down-regulation of claudin-18 is associated with the proliferative and invasive potential of gastric cancer at the invasive front. PLoS One. 2013 doi: 10.1371/journal.pone.0074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jun KH, Kim JH, Jung JH, Choi HJ, Chin HM. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int J Surg. 2014;12:156–162. doi: 10.1016/j.ijsu.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Rohde C, Yamaguchi R, Mukhina S, Sahin U, Itoh K, Türeci Ö. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2019;49:870–876. doi: 10.1093/jjco/hyz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai J, Zheng H, Jin J, Cheng Y, Xu H. Claudin18.2 expression and clinicopathological features in cytology effusion specimens from gastric adenocarcinoma: a comparative study with tissue specimens. Cancer Cytopathol. 2023;131:365–372. doi: 10.1002/cncy.22688. [DOI] [PubMed] [Google Scholar]
- 12.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 13.Bartley AN, Washington MK, Colasacco C, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the college of american pathologists, American society for clinical pathology, and the American society of clinical oncology. J Clin Oncol. 2017;35:446–464. doi: 10.1200/JCO.2016.69.4836. [DOI] [PubMed] [Google Scholar]
- 14.Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143:330–337. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 15.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. The Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda Y, Semba S, Ueda J, et al. Gastric and intestinal claudin expression at the invasive front of gastric carcinoma. Cancer Sci. 2007;98:1014–1019. doi: 10.1111/j.1349-7006.2007.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coati I, Lotz G, Fanelli GN, et al. Claudin-18 expression in oesophagogastric adenocarcinomas: a tissue microarray study of 523 molecularly profiled cases. Br J Cancer. 2019;121:257–263. doi: 10.1038/s41416-019-0508-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida H, Yamamoto N, Taniguchi H, et al. Comparison of HER2 status between surgically resected specimens and matched biopsy specimens of gastric intestinal-type adenocarcinoma. Virchows Arch. 2014;465:145–154. doi: 10.1007/s00428-014-1597-3. [DOI] [PubMed] [Google Scholar]
- 19.Okines AFC, Thompson LC, Cunningham D, et al. Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann Oncol. 2013;24:1253–1261. doi: 10.1093/annonc/mds622. [DOI] [PubMed] [Google Scholar]
- 20.Xu C, Liu Y, Ge X, et al. Tumor containing fragment number influences immunohistochemistry positive rate of HER2 in biopsy specimens of gastric cancer. Diagn Pathol. 2017;12:41. doi: 10.1186/s13000-017-0616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn S, Ahn S, Van Vrancken M, et al. Ideal number of biopsy tumor fragments for predicting HER2 status in gastric carcinoma resection specimens. Oncotarget. 2015;6(35):38372–38380. doi: 10.18632/oncotarget.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tominaga N, Gotoda T, Hara M, et al. Five biopsy specimens from the proximal part of the tumor reliably determine HER2 protein expression status in gastric cancer. Gastric Cancer. 2016;19:553–560. doi: 10.1007/s10120-015-0502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellino A, Brignola S, Riello E, et al. Association of cldn18 protein expression with clinicopathological features and prognosis in advanced gastric and gastroesophageal junction adenocarcinomas. J Pers Med. 2021 doi: 10.3390/jpm11111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubota Y, Kawazoe A, Mishima S, et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open. 2023 doi: 10.1016/j.esmoop.2022.100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Cutsem E, Bang YJ, Feng-yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476–484. doi: 10.1007/s10120-014-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SR, Shin K, Park JM, Lee HH, Song KY, Lee SH, et al. Clinical significance of CLDN18.2 expression in metastatic diffuse-type gastric cancer. J Gastric Cancer. 2020;20:408–20. doi: 10.5230/jgc.2020.20.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. The Lancet. 2018;392:123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 28.Xing X, Guo J, Wen X, Ding G, Li B, Dong B, et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology. 2018 doi: 10.1080/2162402X.2017.1356144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He PX, Ma ZL, Han H, Zhang XY, Niu SH, Du LN, et al. Expression of programmed death ligand 1 (PD-L1) is associated with metastasis and differentiation in gastric cancer. Life Sci. 2020 doi: 10.1016/j.lfs.2019.117247. [DOI] [PubMed] [Google Scholar]
- 30.Fashoyin-Aje L, Donoghue M, Chen H, et al. FDA approval summary: pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist. 2019;24:103–109. doi: 10.1634/theoncologist.2018-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12:208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 32.Adam J, Le Stang N, Rouquette I, et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol. 2018;29:953–958. doi: 10.1093/annonc/mdy014. [DOI] [PubMed] [Google Scholar]
- 33.Park Y, Koh J, Na HY, et al. PD-L1 testing in gastric cancer by the combined positive score of the 22C3 PharmDx and SP263 assay with clinically relevant cut-offs. Cancer Res Treat. 2020;52:661–670. doi: 10.4143/crt.2019.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito T, Nakanishi H, Mochizuki Y, Ito S, Ito Y, Misawa K, et al. Preferential HER2 expression in liver metastases and EGFR expression in peritoneal metastases in patients with advanced gastric cancer. Gastric Cancer. 2015;18:711–719. doi: 10.1007/s10120-014-0417-4. [DOI] [PubMed] [Google Scholar]
- 35.Liu DHW, Grabsch HI, Gloor B, Langer R, Dislich B. Programmed death-ligand 1 (PD-L1) expression in primary gastric adenocarcinoma and matched metastases. J Cancer Res Clin Oncol. 2023;149:13345–13352. doi: 10.1007/s00432-023-05142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.