Abstract
Objective:
Bipolar disorder and disruptive mood dysregulation disorder (DMDD) are clinically and pathophysiologically distinct, yet irritability can be a clinical feature of both illnesses. The authors examine whether the neural mechanisms mediating irritability differ between bipolar disorder and DMDD, using a face emotion labeling paradigm because such labeling is deficient in both patient groups. The authors hypothesized that during face emotion labeling, irritability would be associated with dysfunctional activation in the amygdala and other temporal and prefrontal regions in both disorders, but that the nature of these associations would differ between DMDD and bipolar disorder.
Method:
During functional MRI acquisition, 71 youths (25 with DMDD, 24 with bipolar disorder, and 22 healthy youths) performed a labeling task with happy, fearful, and angry faces of varying emotional intensity.
Results:
Participants with DMDD and bipolar disorder showed similar levels of irritability and did not differ from each other or from healthy youths in face emotion labeling accuracy. Irritability correlated with amygdala activity across all intensities for all emotions in the DMDD group; such correlation was present in the bipolar disorder group only for fearful faces. In the ventral visual stream, associations between neural activity and irritability were found more consistently in the DMDD group than in the bipolar disorder group, especially in response to ambiguous angry faces.
Conclusions:
These results suggest diagnostic specificity in the neural correlates of irritability, a symptom of both DMDD and bipolar disorder. Such evidence of distinct neural correlates suggests the need to evaluate different approaches to treating irritability in the two disorders.
The nosologic implications of irritability have received considerable attention in the child psychiatry literature in recent years, especially with regard to the diagnosis of bipolar disorder in youths. Indeed, the diagnosis of disruptive mood dysregulation disorder (DMDD) was introduced in DSM-5 in part to provide an appropriate diagnosis, distinct from bipolar disorder, for children with severe, nonepisodic irritability. By definition, the irritability seen in youths with DMDD is severe and relatively invariant over time. In contrast, some youths with bipolar disorder may have irritability while euthymic (i.e., trait irritability), and irritability may increase markedly during manic or depressive episodes (i.e., state-related irritability). Thus, while the clinical presentation of irritability differs between DMDD and bipolar disorder, the symptom is important in both disorders. However, it is unknown whether the neural mechanisms mediating irritability differ between DMDD and bipolar disorder; the question has potential treatment implications. In this study, we used a face emotion labeling paradigm to compare brain activation associated with irritability in DMDD and bipolar disorder.
Face emotion labeling deficits have been shown in both DMDD and bipolar disorder (1–5), particularly in response to less intense, ambiguous facial expressions (6, 7). Indeed, one behavioral study found that irritability symptoms mediate the association between bipolar disorder and face emotion labeling deficits (5). In addition, evidence suggests that DMDD and bipolar disorder may have distinct brain profiles when processing emotional faces, as youths with DMDD show less amygdala activation (greater deactivation) compared with youths with bipolar disorder (8), as well as functional differences in other temporal, parietal, occipital, and prefrontal regions (9, 10) associated with face processing (11) and implicated in bipolar disorder (12).
Our study addresses several gaps in the literature. First, whereas previous research has provided evidence that bipolar disorder and DMDD are pathophysiologically distinct (8–10), this study is the first to examine whether irritability is subserved by different neural mechanisms in these two patient groups. Second, although several functional MRI (fMRI) studies in DMDD and bipolar disorder have focused on face emotion processing (e.g., 8, 13), this is the first to use a face emotion labeling scanning paradigm per se. It is important to scan face emotion labeling, as opposed to other aspects of face processing, because this is where behavioral deficits have been found in bipolar disorder and DMDD (1–6). Moreover, although previous work examined related constructs, such as trait aggression (14), this study is the first, to our knowledge, to identify brain mechanisms of the irritability dimension. Also of note, previous studies on bipolar disorder, DMDD, and other disorders have identified ambiguous faces as important in eliciting group differences in emotion labeling processes (6, 7), but they did not examine nonlinear patterns across emotional face intensity. As modeling nonlinear relationships may be necessary to fully capture pathophysiology (15), this study models both linear and nonlinear patterns in brain activation across emotional face intensity.
Thus, this study compares neural correlates of irritability severity in DMDD, bipolar disorder, and healthy development. Youths with bipolar disorder, youths with DMDD, and healthy comparison youths performed a labeling task with happy, fearful, and angry faces of varying emotional intensity during fMRI acquisition. We hypothesized that activation elicited by emotional face labeling in the amygdala, as well as in other temporal and prefrontal regions previously identified as pathophysiologically distinct in DMDD and bipolar disorder (8–10), would be associated with irritability, but that this association would differ between DMDD and bipolar disorder, because the clinical presentation of irritability differs in these two disorders.
METHOD
Participants
Data from 71 youths (ages 9–21 years) were included; 25 had DMDD, 24 had bipolar disorder, and 22 were healthy comparison youths. Of a total 95 participants who completed the scan, eight were excluded because of excessive motion (average motion per time point >0.25 mm, one youth with bipolar disorder, seven youths with DMDD), 14 because of insufficient data in one of the conditions (<62 time points per condition, corresponding to 8–10 trials, after motion censoring and removal of incorrect responses; three healthy youths, three youths with bipolar disorder, eight youths with DMDD), and one youth with DMDD for poor signal-to-noise ratio (<100). Excluded and included participants with DMDD did not differ significantly in gender, likelihood of having an anxiety disorder, anxiety severity, medication status, IQ, or irritability. However, excluded participants tended to be younger (t=2.29, df=39, p=0.03) and were more likely to have comorbid attention deficit hyperactivity disorder (ADHD)(χ2=6.72, df=1, p=0.01). In the final sample, the DMDD, bipolar disorder, and healthy groups did not differ significantly in age, and the DMDD and bipolar disorder groups did not differ in likelihood of having ADHD or any other comorbid diagnosis (Table 1). Inclusion in the DMDD or bipolar disorder group required a lifetime history of the disorder, as diagnosed using the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) (16) in youths under age 18 (N=58) or the Structured Clinical Interview for DSM-III-R (17) in youths over age 18 (N=13), with the DMDD supplement, and clinical consensus. Exclusion criteria were conditions for which MRI is contraindicated (including orthodontic braces), history of neurological disorders, and an IQ below 80. Participants were recruited through advertisements, and they received monetary compensation. Participants over age 18 and parents of minor participants gave written informed consent after receiving a complete description of the study; minors gave written assent. Procedures were approved by the Institutional Review Board of the National Institute of Mental Health.
TABLE 1.
Demographic and Clinical Characteristics of Participants in a Study of the Neural Correlates of Irritability in Disruptive Mood Dysregulation Disorder (DMDD) and Bipolar Disorder
| Characteristica | Healthy Group (N=22) | Bipolar Disorder Group (N=24) | DMDD Group (N=25) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % | N | % | N | % | |
|
| ||||||
| Female | 10 | 45.5 | 8 | 33.3 | 10 | 40.0 |
|
| ||||||
| Mean | SD | Mean | SD | Mean | SD | |
|
| ||||||
| Age (years) | 15.3 | 2.8 | 16.5 | 2.7 | 15.3 | 2.4 |
| Task accuracy (%) | 73.5 | 10.3 | 70.7 | 11.3 | 73.7 | 11.9 |
| IQ | 113.1 | 10.0 | 111.1 | 9.8 | 111.2 | 12.0 |
| Irritability score | 0.9 | 1.3 | 4.1 | 3.3 | 4.9 | 2.4 |
| Anxiety score | 6.4 | 5.1 | 21.2 | 11.8 | 18.7 | 9.6 |
| Global functioning score | 49.0 | 10.9 | 56.6 | 9.8 | ||
| Depression score (child) | 27.6 | 6.7 | 24.8 | 4.5 | ||
| Depression score (adult) | 7.7 | 3.9 | 6.5 | 3.0 | ||
| Young Mania Rating Scale score | 6.5 | 6.2 | ||||
|
| ||||||
| Mood state at time of scan | N | % | N | % | ||
|
| ||||||
| Depressed | 1 | 4.2 | 0 | 0.0 | ||
| Manic | 0 | 0.0 | ||||
| Hypomanic | 5 | 20.8 | ||||
| Mixed | 0 | 0.0 | ||||
| Euthymic | 18 | 75.0 | ||||
| Bipolar type | ||||||
| I | 15 | 62.5 | ||||
| II | 9 | 37.5 | ||||
| Psychotropic medications | 22 | 91.7 | 15 | 60.0 | ||
| Antidepressants | 10 | 41.7 | 10 | 40.0 | ||
| Stimulants | 7 | 29.2 | 12 | 48.0 | ||
| Nonstimulant anti-ADHD drugs | 8 | 33.3 | 7 | 28.0 | ||
| Antiepilepticsb | 14 | 58.3 | 4 | 16.0 | ||
| Atypical antipsychoticsb | 21 | 87.5 | 4 | 16.0 | ||
|
| ||||||
| Number of medications | Mean | SD | Mean | SD | ||
|
| ||||||
| Antidepressants | 0.5 | 0.7 | 0.4 | 0.5 | ||
| Stimulants | 0.3 | 0.6 | 0.5 | 0.5 | ||
| Nonstimulant anti-ADHD drugs | 0.4 | 0.6 | 0.4 | 0.6 | ||
| Antiepilepticsb | 0.8 | 0.9 | 0.2 | 0.4 | ||
| Atypical antipsychoticsb | 1.1 | 0.6 | 0.2 | 0.4 | ||
| Total psychotropic drugsb | 3.2 | 1.4 | 1.6 | 1.5 | ||
|
| ||||||
| Lifetime comorbidity | N | % | N | % | ||
|
| ||||||
| Major depressive disorder | 0 | 0.0 | 3 | 12.0 | ||
| Oppositional defiant disorder | 2 | 8.3 | 0 | 0.0 | ||
| Anxiety disorders | 12 | 50.0 | 12 | 48.0 | ||
| Separation anxiety disorder | 2 | 8.3 | 1 | 4.0 | ||
| Simple/specific phobia | 2 | 8.3 | 3 | 12.0 | ||
| Social anxiety disorder | 2 | 8.3 | 2 | 8.0 | ||
| Generalized anxiety disorder | 7 | 29.2 | 11 | 44.0 | ||
| Obsessive-compulsive disorder | 2 | 8.3 | 0 | 0.0 | ||
| Posttraumatic stress disorder | 1 | 4.2 | 0 | 0.0 | ||
| ADHD | 10 | 41.2 | 14 | 56.0 | ||
The irritability score is the mean of parent- and child-report ratings on the Affective Reactivity Index. The anxiety score is the mean of parent- and child-report ratings on the Screen for Child Anxiety-Related Disorders. The global functioning score is the score on the Children’s Global Assessment Scale or the Global Assessment of Functioning. The child depression score is the score on the Children’s Depression Rating Scale (administered to youths under 18), with the irritability items excluded, and the adult depression score is the score on the Structured Interview Guide for the Hamilton Depression Rating Scale–Seasonal Affective Disorders (administered to youths over 18—seven youths with bipolar disorder, four with DMDD). Missing data for healthy group: anxiety ratings, N=2; for the bipolar disorder group: global functioning ratings, N=5; for the DMDD group: anxiety ratings, N=1; global functioning ratings, N=3. ADHD=attention deficit hyperactivity disorder.
Significant difference between the DMDD and bipolar disorder groups (p<0.05); otherwise no differences between these two groups.
Face Emotion Labeling Task
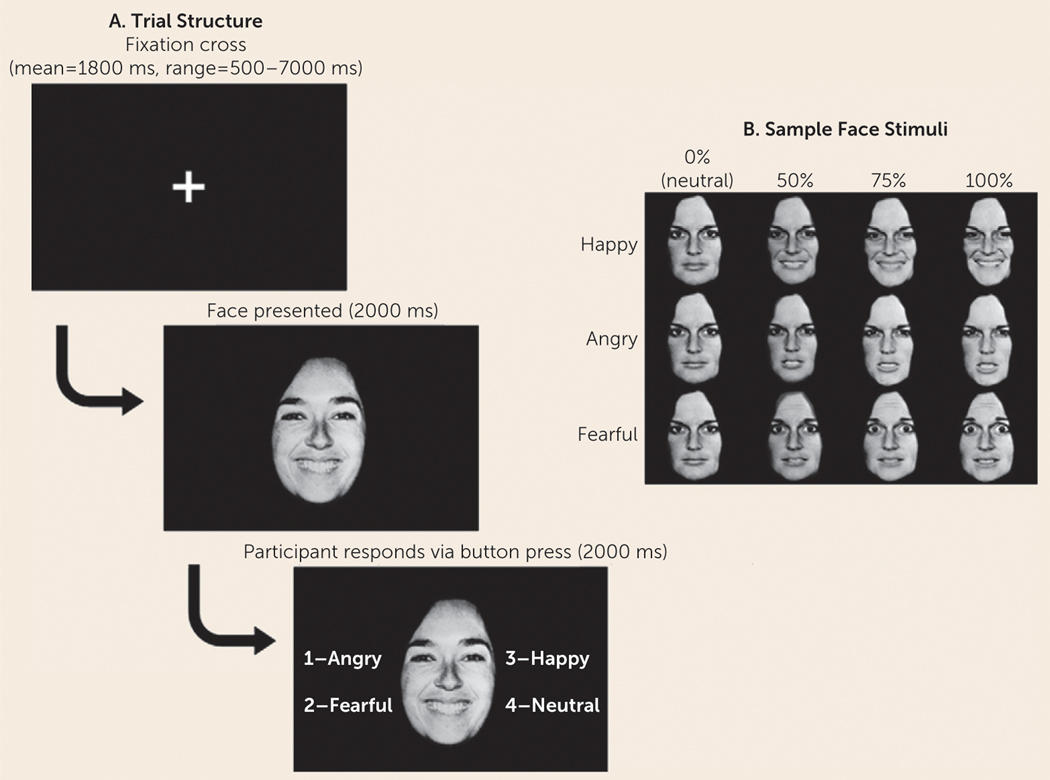
Participants performed a jittered, event-related task during fMRI acquisition in which they labeled the emotion on angry, fearful, and happy faces morphed with neutral faces to create 0%(i.e., neutral), 50%, 75%, and 100% intensity faces presented for 4000 ms total (2000 ms of face only and 2000 ms of face with options to label the emotion on the face) (Figure 1). Across four 8.5-minute runs, there were 28 trials per emotion intensity condition, except for neutral faces, of which there were 84 trials (28 trials×3). Details on the task are provided in the data supplement that accompanies the online edition of this article.
FIGURE 1. Schematic of the Face Emotion Labeling Taska.
a Panel A shows screenshots from a sample trial. Fixation cross timing varies across trials, and each set of timings was unique to each participant. Panel B presents an example of stimuli from one actor. Emotion faces were morphed with neutral to create varying intensities of emotion.
Irritability Measure
The Affective Reactivity Index (18) was used to operationalize irritability symptoms as a continuous measure. To include information from multiple informants, parent and child reports were averaged. The Affective Reactivity Index score consisted of the sum of six items, such as “gets angry easily” and “often loses his/her temper,” rated on a 0–2 scale, based on the past 6 months. The Affective Reactivity Index shows excellent internal consistency in both clinical and nonclinical samples (Cronbach’s alpha values >0.88) (18).
Behavioral Data Analysis
To examine whether accuracy in identifying face emotion differs by diagnostic group, emotion, and intensity level, an analysis of variance was conducted with diagnosis (healthy youths, DMDD, bipolar disorder) as a between-subject factor and emotion (angry, fearful, happy) and intensity (0%, 50%, 75%, 100%) as within-subject factors. To investigate significant interactions, false-discovery-rate-corrected post hoc comparisons were performed.
fMRI Data Analysis
Parameters for MRI data acquisition and preprocessing steps are provided in the online data supplement.
Individual-level models.
For each participant, correct trials were categorized by emotion (happy, fearful, angry) and intensity (0%, 50%, 75%, 100%). The resulting event types (i.e., conditions) were modeled as regressors convolved with AFNI’s BLOCK basis function over 4000 ms of face presentation for each trial. Incorrect trials were modeled with a nuisance regressor. Motion parameters (estimated in the x, y, z, roll, pitch, yaw directions) and fourth-degree polynomials modeling low-frequency drift, based on run durations of 508 seconds, were included in the baseline model. To further address excessive head motion, time point pairs with >1 mm frame-wise displacement were censored. Beta coefficients were estimated for each voxel and each regressor. The beta images, which represented estimated activation in each condition for each participant, were then used in group-level analyses.
Group-level models.
AFNI’s 3dLME was utilized to create a whole brain linear mixed-effects model with diagnostic group (healthy, DMDD, bipolar disorder) as a between-subject factor, irritability score as a quantitative variable, and emotion (fearful, happy, angry) and intensity (0%, 50%, 75%, 100%) weighted linearly, quadratically, and cubically as within-subject factors. To identify brain regions where the association between irritability and activation when labeling emotions of varying degrees of intensity varied by diagnostic group, we examined diagnostic group-by-irritability-by-emotion-by-intensity interactions, with intensity modeled linearly, quadratically, and cubically (all interactions specified within the same model). The cluster extent threshold was set to k≥39 (609 mm3) with a height threshold of p<0.005, equivalent to a whole-brain-corrected false positive probability of p<0.05, as calculated by 3dClustSim, using blur estimates averaged across participants. Activation maps were masked to include only areas of the brain for which 90% of participants had valid data. To characterize significant diagnostic group-by-irritability-by-emotion-by-intensity interactions, false-discovery-rate-corrected post hoc analyses were performed in SPSS (IBM, Armonk, N.Y.) using values extracted and averaged from the clusters. These post hoc analyses tested whether irritability was associated with the brain response modeled cubically across intensity levels for each emotion and diagnostic group separately.
RESULTS
Participants’ demographic and clinical characteristics are summarized in Table 1.
Behavior
The bipolar disorder and DMDD groups did not differ significantly in mean irritability scores, and both the bipolar disorder and DMDD groups had greater irritability scores than the healthy group (p values, 0.001; omnibus F=16.0, df=2, 68, p,0.001). The bipolar disorder and DMDD groups were highly overlapping in their irritability distributions (see Figure S1 in the online data supplement). The diagnostic group-by-irritability-by-emotion-by-intensity interaction did not significantly predict accuracy in face labeling, nor were there any other group differences in face labeling accuracy, nor any associations between irritability and accuracy. (See Table S1 in the data supplement for results of a parallel analysis with all scanned participants.)
Brain Function
Whole brain analyses indicate significant four-way interactions among diagnostic group, irritability, emotion, and intensity modeled cubically in multiple clusters, including the amygdala and multiple temporal, parietal, occipital, and prefrontal cortical areas (superior temporal sulcus, temporo-parietal and temporo-parietal-occipital junctions, temporal pole, postcentral gyrus, lingual gyrus, and lateral prefrontal cortex) (Table 2). In these regions, the strength and direction of the association between irritability severity and brain response varied depending on diagnostic group as well as stimulus qualities (face emotion and intensity of emotion). Here, wereport details from clusters identified in the highest-order interaction (diagnostic group by irritability by emotion by intensity, modeled cubically), that is, brain areas for which the cubic shape of the brain response across intensity levels differed significantly depending on irritability level, diagnostic group, and emotion; lower-order quadratic and linear results are reported in Table 2.
TABLE 2.
Additional Whole Brain Results in a Study of the Neural Correlates of Irritability in Disruptive Mood Dysregulation Disorder and Bipolar Disordera
| k | F (df=4, 715) | x | y | z | BA | Region |
|---|---|---|---|---|---|---|
| Diagnostic group by irritability by emotion by intensity (modeled cubically) | ||||||
| 300 | 12.19 | 34 | −46 | 24 | 13 | Posterior superior temporal sulcus/temporo-parietal junction |
| 128 | 6.99 | –46 | –64 | 11 | 37 | Temporo-parietal-occipital junction |
| 84 | 11.28 | 31 | 19 | –21 | 38 | Temporal pole |
| 75 | 14.50 | –61 | –26 | 11 | 22, 41 | Superior temporal sulcus |
| 63 | 9.33 | −19 | −4 | −11 | n/a | Amygdala |
| 47 | 8.70 | −19 | −54 | 6 | 30 | Lingual gyrus |
| 45 | 6.90 | 41 | 26 | 9 | 46 | Lateral prefrontal cortex |
| 40 | 6.59 | 34 | −44 | 59 | 40 | Postcentral gyrus |
| Diagnostic group by irritability by emotion by intensity (modeled quadratically) | ||||||
| 2,760 | 24.40 | −4 | −36 | 61 | 7, 18, 6 | Precuneus, cingulate, lingual gyrus |
| 790 | 24.17 | −46 | −71 | −4 | 13, 22 | Posterior superior temporal sulcus, supramarginal |
| gyrus | ||||||
| 663 | 12.77 | 64 | −26 | 19 | 41, 13 | Superior temporal gyrus/Rolandic operculum |
| 190 | 14.98 | 21 | −69 | 24 | 31 | Cuneus, calcarine gyrus |
| 182 | 8.57 | 6 | 19 | 6 | Caudate nucleus, putamen | |
| 178 | 9.75 | −1 | −16 | 14 | Thalamus | |
| 174 | 20.63 | 21 | −49 | −51 | Cerebellum | |
| 170 | 12.92 | −26 | 1 | −11 | Amygdala/hippocampus | |
| 131 | 16.94 | 36 | −54 | −16 | 37 | Fusiform, inferior temporal gyrus |
| 130 | 9.51 | −51 | −14 | 44 | 4, 3 | Postcentral gyrus |
| 96 | 13.20 | −31 | −61 | 14 | 19 | Middle temporal gyrus |
| 81 | 6.97 | 46 | −66 | 11 | 37 | Temporo-occipital junction |
| 74 | 23.53 | 46 | −54 | −39 | Cerebellum | |
| 67 | 8.20 | −19 | −79 | 21 | 18 | Middle/superior occipital gyrus |
| 66 | 6.30 | −11 | −71 | 24 | 18 | Calcarine gyrus, cuneus |
| 62 | 6.78 | 31 | −19 | 36 | 4 | Precentral gyrus |
| 53 | 8.15 | −24 | −26 | 39 | Postcentral gyrus | |
| 52 | 8.65 | −1 | −66 | −31 | Cerebellar vermis | |
| 45 | 7.16 | −16 | −71 | −16 | Cerebellum | |
| 41 | 8.18 | −9 | 21 | −16 | 25 | Superior orbital gyrus |
| Diagnostic group by irritability by emotion by intensity (modeled linearly) | ||||||
| 54 | 11.10 | 31 | 19 | −19 | 47 | Temporal pole |
| 53 | 9.06 | 1 | 59 | 6 | 10 | Mid orbital gyrus |
| 46 | 10.01 | 14 | 49 | 14 | 10 | Medial prefrontal cortex |
BA=Brodmann’s area. Clusters significant at a whole-brain-corrected false positive probability threshold of p<0.05. See Figure 2 and Figures S2–S5 in the online data supplement for brain images and plots of clusters significant in the diagnostic group-by-irritability-by-emotion-by-intensity interaction, modeled cubically, with post hoc analyses significant after correction.
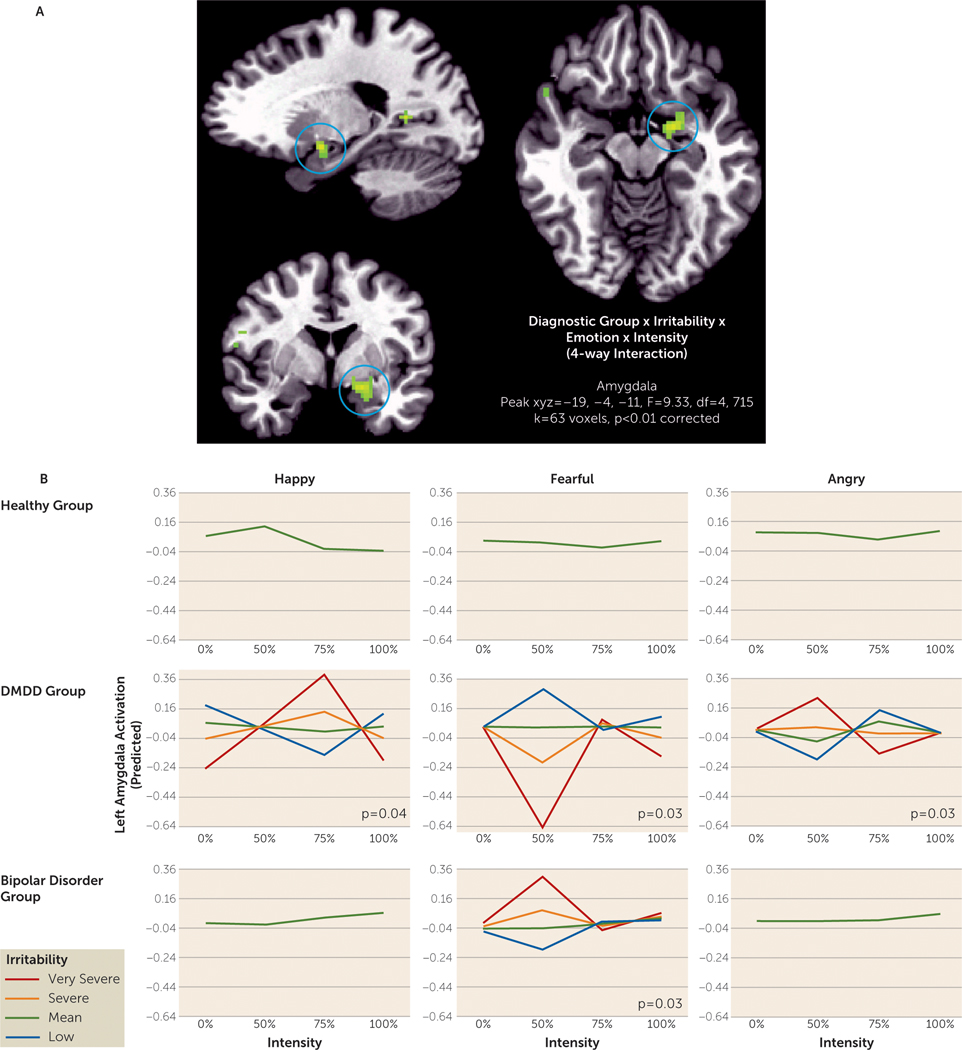
In the amygdala, false-discovery-rate-corrected post hoc analyses indicate that the diagnostic group-by-irritability-by-emotion-by-intensity interaction was driven by exaggerated responses to middle-intensity (50%, 75%) faces in youths with greater levels of irritability, although the specific pattern (i.e., greater or less activation) depended on emotion and diagnostic group (peak coordinates, −19, −4, −11; F=9.33, df=4, 715, k=63 voxels, p<0.05 whole brain corrected) (Figure 2). Irritability was significantly associated with change in brain response, modeled cubically across intensities, for all emotions in the DMDD group (happy, p=0.04; fearful, p=0.03; angry, p=0.03), but only for fearful faces in the bipolar disorder group (p=0.03). In particular, whereas in youths in the bipolar disorder group greater levels of irritability were associated with greater activation in response to middle-intensity fearful faces, youths in the DMDD group showed the opposite pattern—less activation with increasing irritability to the same stimuli. Irritability did not significantly predict changes in the cubic shape of brain response to intensity levels for happy or angry faces in youths with bipolar disorder, or for any emotion in healthy youths.
FIGURE 2. Variable Association Between Irritability and Amygdala Response, Depending on Diagnostic Group and Stimulus Characteristics (Face Emotion and Intensity of Emotion)a.
a Panel A shows brain images presented in sagittal, axial, and coronal sections, in radiological orientation (right=left) with the threshold set at a whole-brain-corrected false probability rate of p<0.05. The graphs in panel B show the predicted left amygdala cluster activation based on selected levels of irritability to illustrate significant group-by-irritability-by-emotion-by-intensity interaction identified at the whole brain level, with intensity modeled cubically. Irritability was used as a continuous variable in the analyses, but for illustrative purposes, selected irritability levels are shown in the plots (low=0 [∼1 SD below the mean], mean=3.4, severe=6.4 [∼1 SD above the mean], very severe=12 [maximum of scale]). The plots were created using SPSS’s GLM graphing module. False-discovery-rate-corrected post hoc analyses examined the association between irritability and intensity levels modeled cubically for each emotion of each group. Plots for nonsignificant post hoc analyses (p values >0.05 not shown) display amygdala response across intensity levels at the mean irritability level. DMDD=disruptive mood dysregulation disorder.
Intemporal, parietal, andoccipitalareas (temporo-parietal-occipital junction, temporal pole, superior temporal sulcus, lingual gyrus) identified as significant in the diagnostic group-by-irritability-by-emotion-by-intensity contrast with intensity modeled cubically, false-discovery-rate-corrected post hoc analyses indicate that the interactions were driven by significant associations between irritability severity and brain response across intensity levels of negative emotion faces in the DMDD group only (see Figures S2–S5 in the online data supplement). Within the DMDD group, youths with greater irritability exhibited exaggerated brain responses to middle-intensity fearful faces or to fearful and angry faces, depending on the brain region. Regions in the temporo-parietal-occipital junction and temporal pole in the bipolar disorder group showed associations between irritability and response to intensities of fearful faces that were in the opposite direction of those in the DMDD group, but these were marginally significant after false discovery rate correction.
Post hoc analyses for other temporal and parietal (posterior superior temporal sulcus/temporo-parietal junction, postcentral gyrus) and lateral prefrontal cortex clusters identified in the diagnostic group-by-irritability-by-emotion-by-intensity contrast, with intensity modeled cubically, were marginally significant or not significant after false discovery rate correction and thus are not discussed further.
Additional Analyses
Additional analyses were performed to address potential effects of medication, mood state, anxiety, global functioning, and age on the amygdala cluster identified in the diagnostic group-by-irritability-by-emotion-by-intensity cubic whole brain analysis (Figure 2). These additional analyses indicated that the results were not primarily driven by these factors. Moreover, follow-up analyses on the main result in the amygdala were performed with irritability and diagnostic group separately to contrast with the results from the primary analysis that included both in the statistical model. Neither irritability nor diagnostic group separately identified the full extent of amygdala dysfunction related to irritability (see the online data supplement).
DISCUSSION
We demonstrated different brain activation patterns associated with irritability in DMDD compared with bipolar disorder when labeling emotional faces. Using a whole brain analysis, we observed divergent alterations in amygdala activation related to irritability in youths with DMDD and youths with bipolar disorder. In temporo-occipital regions that are important in face processing (11), the DMDD group in particular showed associations between irritability and activation in response to ambiguous angry faces. Our findings extend previous work by using a novel paradigm and comparing the neural correlates of irritability, operationalized dimensionally, between bipolar disorder and DMDD.
This study has implications for our understanding of pathophysiological differences between DMDD and bipolar disorder. Eighteen of 24 youths with bipolar disorder in this study were euthymic when scanned, and the level of irritability was similar between the bipolar disorder and DMDD groups at the time of scanning. Thus, cross-sectionally in our samples, the irritability of the DMDD group could not be distinguished from that of the bipolar disorder group. However, previous research has shown that longitudinally, the pattern of irritability differs markedly between DMDD and bipolar disorder. Specifically, the defining feature of DMDD is persistent and severe irritability, whereas in bipolar disorder, the extent of irritability during euthymia can differ among individuals, and the degree of irritability across mood states can differ within an individual. In addition, the clinical course between bipolar disorder and DMDD differs in that youths with bipolar disorder have manic and depressive episodes, whereas such an outcome is unusual in patients with DMDD (19). Our results indicate that these differences in clinical presentation and outcome are associated with different brain mechanisms mediating irritability. As with many psychiatric diagnoses, the diagnostic distinction between DMDD and bipolar disorder sometimes relies on retrospective recall, which can be fallible; this finding raises the possibility that brain imaging might eventually aid in the differential diagnosis.
Our results are largely consistent with previous findings suggesting that DMDD and bipolar disorder can be differentiated by brain response to emotional faces (8–10). Of note, divergent alterations in neural responses associated with irritability in DMDD compared with bipolar disorder were apparent when subjects correctly labeled subtle (50%–75% intensity) faces, not the overt (100% intensity) faces often used in face tasks. This may indicate that subtle, ambiguous social stimuli are necessary to capture differences in neural correlates, possibly because these faces are more difficult to identify correctly; consistent with this, treatment approaches drawing on the present findings, discussed below, focus on training responses to ambiguous faces. Overall, our findings suggest that even though both disorders feature irritability symptoms, DMDD and bipolar disorder are in fact distinct categories. Additionally, our finding of different neural correlates of irritability across diagnoses suggests that treatments may have to differ as well.
Although we showed overall pathophysiological differences between youths with DMDD and bipolar disorder, consistent with previous work (8–10), we failed to replicate findings of hypoactivation in the left amygdala to neutral faces the DMDD group (8). This discrepancy may be due to key differences in the scanning paradigms between this study and previous work. Specifically, the psychological process probed in the paradigm used here (emotion labeling) differs from that of Brotman et al. (8) (rating subjective fear). The present study focused on emotion labeling because behavioral deficits have been consistently found with emotion labeling in DMDD and bipolar disorder (1–5).
Our findings suggest that including information about the larger diagnostic context (i.e., DMDD versus bipolar disorder) may be essential to identify unique pathophysiological mechanisms of irritability symptoms. Indeed, an analysis that included only diagnosis showed no group differences, and another that examined the neural correlates of irritability irrespective of diagnosis found an association between irritability and response to the intensity of happy but not fearful or angry faces (see the online data supplement). Thus, these results suggest that in order to best capture the pathophysiology of irritability, it is necessary to consider both symptom measures and diagnosis.
Our results have potential treatment implications. The adverse effects of many new treatments (e.g., novel medications, brain stimulation) limit their use in children. Hence, it is particularly important to test noninvasive computer-based cognitive training techniques in children. Such techniques are developed by identifying perturbed psychological functions in a disorder, characterizing their associated neural correlates, and designing training regimens to alter symptoms and their associated neurocognitive correlates simultaneously. For example, attention bias modification treatment for anxiety disorders gained traction when investigators identified alterations in the engagement of threat circuitry in anxious patients (20, 21); the present results delineate a parallel path for novel treatments in irritability.
Specifically, in this and previous studies, irritable youths showed face emotion labeling deficits (1–7) and associated perturbations in underlying neural circuitry (8–10). These deficits motivated the creation of cognitive training protocols designed to ameliorate the specific tendency of irritable youths to interpret rapidly presented ambiguous faces as angry (22). In these training protocols, youths receive positive feedback for rating ambiguous faces on the happy-angry continuum as happy rather than angry. Such training shifts the patient’s judgment and was found to be associated with decreased irritability and anger in a double-blind controlled trial in youths at high risk for criminal offending (23) and in an open trial of youths with DMDD (22). These clinical findings in irritable youths are consistent with the DMDD-related dysfunction we found in the present study. In response to ambiguous faces, associations between irritability and neural activity differed between the DMDD and bipolar disorder groups in the amygdala, superior temporal sulcus, frontal pole, and lingual gyrus, all components of the ventral visual processing stream. In these regions, we found associations between neural activity in response to ambiguous angry faces and irritability in the DMDD group but not the bipolar disorder group. This suggests that training designed to normalize a tendency to interpret ambiguous faces as angry might be effective in DMDD, consistent with the existing data, but not in bipolar disorder. However, because of the lack of behavioral differences in this particular study, this conclusion should be considered tentative; further research will be needed to confirm it.
This study has several limitations. First, although it included data from three diagnostic groups, there were relatively few participants (Ns ranging from 22 to 25) in each group. These sample sizes are comparable to those of other fMRI studies on pediatric bipolar disorder (mean=19, SD=6.8) in a recent meta-analysis (24), with Ns ranging from 10 to 32 participants. However, the results will need to be replicated with larger samples, which would also afford better coverage across the entire irritability dimension.
Second, psychotropic medication usage was very high, particularly in the bipolar disorder group, which could potentially affect results. Of note, when covarying number of medications, excluding individuals on each class of medications or including only the medication-free participants in the DMDD group (N=10; all but three youths in the bipolar disorder group were on medications), the results still stood (see the online data supplement). This decreases the likelihood that the results were primarily driven by medication usage, although definitive evidence would have to be drawn from a sample of medication-naive individuals with bipolar disorder and DMDD, which, given the high rates of medication usage, would be a challenge to recruit.
Third, among participants included in the imaging analyses, the groups did not differ significantly in emotion labeling accuracy, nor was accuracy related to irritability, unlike in previous behavioral studies (1–6). However, in order to obtain a robust estimate of brain activation, we included only those youths who had at least 62 time points in each condition after removing incorrect trials and censoring. In contrast, previous behavioral studies did not exclude participants for low accuracy. Indeed, when we included participants who had been excluded for insufficient data in each of the conditions (in part for poor performance), we found impaired emotion labeling ability in DMDD, consistent with previous studies (1–6).
CONCLUSIONS
This study lays the foundation for future studies by examining irritability across a range of clinical presentations. The results support different neural correlates of irritability in DMDD and bipolar disorder and have implications for treatment. Future studies investigating other transdiagnostic symptom dimensions, such as anxiety or depression symptoms, could use this integrated approach to better identify mechanisms of symptoms.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of NIMH, conducted under NIH Clinical Study Protocols 02-M-0021 (ClinicalTrials.gov identifier: NCT00025935) and 00-M-0198 (ClinicalTrials.gov identifier: NCT00006177).
The authors gratefully acknowledge the NIH Functional Magnetic Resonance Imaging Facility for technical support and the Emotion and Development Branch, including Dr. Kenneth Towbin, Elizabeth Harkins, and Derek Hsu, for assistance with recruitment, data collection, and processing.
The authors report no financial relationships with commercial interests.
REFERENCES
- 1.Guyer AE, McClure EB, Adler AD, et al. : Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry 2007; 48:863–871 [DOI] [PubMed] [Google Scholar]
- 2.Brotman MA, Skup M, Rich BA, et al. : Risk for bipolar disorder is associated with face-processing deficits across emotions. J Am Acad Child Adolesc Psychiatry 2008; 47:1455–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClure EB, Treland JE, Snow J, et al. : Deficits in social cognition and responseflexibility in pediatric bipolar disorder. Am JPsychiatry 2005; 162:1644–1651 [DOI] [PubMed] [Google Scholar]
- 4.Brotman MA, Guyer AE, Lawson ES, et al. : Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am J Psychiatry 2008; 165:385–389 [DOI] [PubMed] [Google Scholar]
- 5.Shankman SA, Katz AC, Passarotti AM, et al. : Deficits in emotion recognition in pediatric bipolar disorder: the mediating effects of irritability. J Affect Disord 2013; 144:134–140 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Rich BA, Grimley ME, Schmajuk M, et al. : Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev Psychopathol 2008; 20:529–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace GL, Case LK, Harms MB, et al. : Diminished sensitivity to sad facial expressions in high functioning autism spectrum disorders is associated with symptomatology and adaptive functioning. J Autism Dev Disord 2011; 41:1475–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brotman MA, Rich BA, Guyer AE, et al. : Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry 2010; 167:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas LA, Brotman MA, Bones BL, et al. : Neuralcircuitry of masked emotional face processing in youth with bipolar disorder, severe mood dysregulation, and healthy volunteers. Dev Cogn Neurosci 2014; 8:110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas LA, Brotman MA, Muhrer EJ, et al. : Parametric modulation of neural activity by emotion in youth with bipolar disorder, youth with severe mood dysregulation, and healthy volunteers. Arch Gen Psychiatry 2012; 69:1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flack TR, Andrews TJ, Hymers M, et al. : Responses in the right posterior superior temporal sulcus show a feature-based response to facial expression. Cortex 2015; 69:14–23 [DOI] [PubMed] [Google Scholar]
- 12.Pavuluri MN, O’Connor MM, Harral E, et al. : Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry 2007; 62:158–167 [DOI] [PubMed] [Google Scholar]
- 13.Perlman SB, Fournier JC, Bebko G, et al. : Emotional face processing in pediatric bipolar disorder: evidence for functional impairments in the fusiform gyrus. J Am Acad Child Adolesc Psychiatry 2013; 52: 1314–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawliczek CM, Derntl B, Kellermann T, et al. : Anger under control: neural correlates of frustration as a function of trait aggression. PLoS One 2013; 8:e78503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuthbert BN: The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry 2014; 13:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman J, Birmaher B, Brent D, et al. : Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988 [DOI] [PubMed] [Google Scholar]
- 17.Spitzer RL, Williams JB, Gibbon M, et al. : The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629 [DOI] [PubMed] [Google Scholar]
- 18.Stringaris A, Goodman R, Ferdinando S, et al. : The Affective Reactivity Index: a concise irritability scale for clinical and research settings. J Child Psychol Psychiatry 2012; 53:1109–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stringaris A, Baroni A, Haimm C, et al. : Pediatric bipolar disorder versus severe mood dysregulation: risk for manic episodes on follow-up. J Am Acad Child Adolesc Psychiatry 2010; 49:397–405 [PMC free article] [PubMed] [Google Scholar]
- 20.Monk CS, Telzer EH, Mogg K, et al. : Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry 2008; 65:568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browning M, Holmes EA, Murphy SE, et al. : Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biol Psychiatry 2010; 67:919–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoddard J, Sharif-Askary B, Harkins EA, et al. : An open pilot study of training hostile interpretation bias to treat disruptive mood dysregulation disorder. J Child Adolesc Psychopharmacol (in press) [DOI] [PMC free article] [PubMed]
- 23.Penton-Voak IS, Thomas J, Gage SH, et al. : Increasing recognition of happiness in ambiguous facial expressions reduces anger and aggressive behavior. Psychol Sci 2013; 24:688–697 [DOI] [PubMed] [Google Scholar]
- 24.Lee MS, Anumagalla P, Talluri P, et al. : Meta-analyses of developing brain function in high-risk and emerged bipolar disorder. Front Psychiatry 2014; 5:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.