Keywords: β-agonist, diabetes, nephropathy
Abstract
We have previously shown that the long-acting β2-adrenergic receptor (β2-AR) agonist formoterol induced recovery from acute kidney injury in mice. To determine whether formoterol protected against diabetic nephropathy, the most common cause of end-stage kidney disease (ESKD), we used a high-fat diet (HFD), a murine type 2 diabetes model, and streptozotocin, a murine type 1 diabetes model. Following formoterol treatment, there was a marked recovery from and reversal of diabetic nephropathy in HFD mice compared with those treated with vehicle alone at the ultrastructural, histological, and functional levels. Similar results were seen after formoterol treatment in mice receiving streptozotocin. To investigate effects in humans, we performed a competing risk regression analysis with death as a competing risk to examine the association between Veterans with chronic kidney disease (CKD) and chronic obstructive pulmonary disease (COPD), who use β2-AR agonists, and Veterans with CKD but no COPD, and progression to ESKD in a large national cohort of Veterans with stage 4 CKD between 2011 and 2013. Veterans were followed until 2016 or death. ESKD was defined as the initiation of dialysis and/or receipt of kidney transplant. We found that COPD was associated with a 25.6% reduction in progression from stage 4 CKD to ESKD compared with no COPD after adjusting for age, diabetes, sex, race-ethnicity, comorbidities, and medication use. Sensitivity analysis showed a 33.2% reduction in ESKD in Veterans with COPD taking long-acting formoterol and a 20.8% reduction in ESKD in Veterans taking other β2-AR agonists compared with those with no COPD. These data indicate that β2-AR agonists, especially formoterol, could be a treatment for diabetic nephropathy and perhaps other forms of CKD.
NEW & NOTEWORTHY Diabetic nephropathy is the most common cause of ESKD. Formoterol, a long-acting β2-adrenergic receptor (β2-AR) agonist, reversed diabetic nephropathy in murine models of type 1 and 2 diabetes. In humans, there was an association with protection from progression of CKD in patients with COPD, by means of β2-AR agonist intake, compared with those without COPD. These data indicate that β2-AR agonists, especially formoterol, could be a new treatment for diabetic nephropathy and other forms of CKD.
INTRODUCTION
Glomerular function is highly dependent on specialized cells known as podocytes, which are critical components of glomeruli. Diseases affecting podocytes and the glomerulus, such as diabetic nephropathy and glomerulonephritides, are the leading causes of chronic kidney disease (CKD) and end-stage kidney disease (ESKD) (1), and there are currently no specific therapies that restore injury-induced loss of podocyte structure and function (2). We recently showed using mouse models of podocyte injury that formoterol, a long-acting β2-adrenergic receptor (β2-AR) agonist given 4 h following acute kidney injury (AKI), when glomerular dysfunction is already established, restored glomerular structure, significantly reduced proteinuria, and accelerated recovery of glomerular function (3). We and others showed that the mechanism behind this rescue was increased mitochondrial biogenesis (3–5). This study investigated whether β2-AR agonist use can improve CKD, specifically diabetic nephropathy, in mice and whether there is an association with improved outcomes in humans.
METHODS
Animals
Six-week-old male C57BL/6 mice were obtained from Charles River Laboratories. All animals were housed in the Animal Facility at the Medical University of South Carolina (MUSC) with a stable environment maintained at 22 ± 1°C with a 12:12-h light-dark cycle. The control group was fed a low-fat diet (LFD, D12450Ji) with 10 kcal% fat (matching sucrose to D12492) and the experimental group was fed a high-fat diet (HFD, D12492i) with 60 kcal% fat purchased from Research Diets (New Brunswick, NJ). All animal experiments were conducted per the protocol approved by the MUSC Institutional Animal Care and Use Committee (IACUC) and per the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Protocol No. IACUC-2021-01272). Six-week-old mice were fed the LFD or HFD for 12 wk. Body weight, blood glucose, and urine albumin were measured to confirm the presence of diabetes and proteinuria. Control (vehicle alone) or formoterol (1 mg/kg body wt) was administered (intraperitoneally) for 14 days following the LFD or HFD. During the vehicle or formoterol treatment, the mice continued receiving their respective LFD or HFD. Detailed experimental plans for drug administration and urine collection are provided in the schematic diagram presented in Fig. 1A. For the type 1 model, 6-wk-old male C57BL/6 mice were treated with streptozotocin or vehicle. After 8 wk, control (vehicle alone) or formoterol (1 mg/kg body wt) was administered (intraperitoneally) to the streptozotocin-treated mice for 14 days as described in the schematic diagram presented in Fig. 3A. Urine samples were spun at 4,000 g for 5 min and then frozen at −80°C for subsequent analysis. Similarly, serum samples were obtained from the mice and kept at 37°C for 1 h and then centrifuged at 10,000 g for 30 min at 4°C.
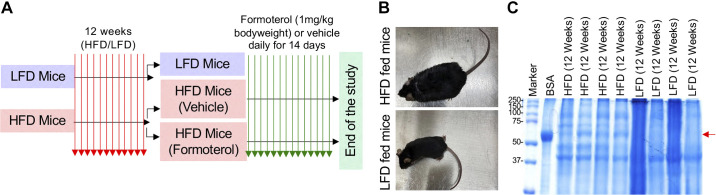
Figure 1.
High-fat diet (HFD) mice develop significant weight gain and albuminuria. A: the schematic of the experimental plan to investigate the role of formoterol in recovery from diabetic renal disease is shown. B: HFD compared with low-fat diet (LFD) mice after 12 wk showed a significant weight gain. Representative mice from both groups are shown. C: HFD mice had albuminuria by SDS-PAGE of urine samples, whereas LFD mice had no albuminuria. The red arrow indicates albumin on the gel. Bovine serum albumin (BSA) was run on lane 2; as a positive control. n = 4 for both groups in this experiment with each lane representing a mouse. The experiments were repeated twice with similar results.
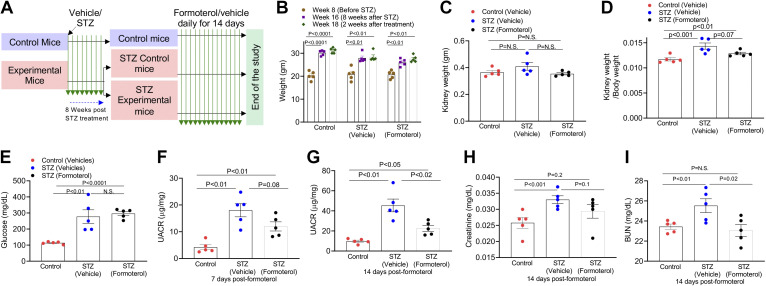
Figure 3.
Formoterol accelerates the recovery from streptozotocin (STZ)-induced diabetic renal injury. A: schematic of the experimental plan to evaluate the efficacy of formoterol treatment in a type I diabetes mouse model. B: STZ-treated animals had significant increases in weight after 8 wk, which did not change after treatment for an additional 2 wk with formoterol or vehicle. C: kidney weights were not statistically different among the three groups. D: kidney/body weight was significantly higher in STZ-treated mice compared with control mice. E: 4-h fasting glucose levels were found to be significantly higher in STZ-treated mice compared with controls. F and G: measurement of urine albumin/creatinine ratios by ELISA showed a reduction in albuminuria at 14 days in STZ mice treated with formoterol compared with mice treated with vehicle alone. H: serum creatinine was increased in STZ-treated mice compared with control mice. I: blood urea nitrogen (BUN) levels were increased in STZ-treated mice compared with control mice, and formoterol decreased BUN in these mice compared with mice treated with vehicle alone. These experiments were repeated twice with similar results. For A–I, n = 5 for each group with each dot representing a mouse. ns, nonsignificant.
Humans
We used administrative data from the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW) to assemble a national retrospective cohort of Veterans 65 yr or older with stage 4 CKD from 2011 to 2013. Incident stage 4 CKD was defined as having at least two consecutive outpatient estimated glomerular filtration rate (eGFR) values between 15 and 29 mL/min/1.73 m2, 90 or more days apart, but no greater than 1 year. The date of the second eGFR value was considered the time of cohort entry (i.e., baseline). We used the 2009 CKD Epidemiology Collaboration (CKD-EPI) equation to determine eGFR. Veterans were followed until death or December 31, 2016. The median follow-up time was 3 years. Moreover, our Veterans Affairs (VA) data were linked with the United States Renal Data System (USRDS) and the Centers for Medicare & Medicaid Services (CMS) to reliably obtain our outcome of interest, ESKD, and minimize the proportion of Veterans with missing race-ethnicity information. Non-Veterans and those enrolled in the USRDS before cohort entry were excluded. We defined ESKD as the initiation of dialysis and/or receipt of kidney transplant as determined by entry into the USRDS registry. The date of death during follow-up was obtained from the Master VHA Vital Status File, which contains mortality data from VA and non-VA sources [i.e., the Beneficiary Identification Records Locator Subsystem (BIRLS) Death File, Medical SAS Inpatient Datasets, Social Security Administration (SSA) Death File, and the Medicare Vital Status File]. Both ESKD and death were coded as binary variables. Our exposure of interest, COPD, was defined as having two or more of the following inpatient or outpatient International Classification of Diseases, Ninth Revision (ICD-9) diagnoses (491.xx, 492.xx, 493.2, and 496.xx) and being prescribed a β-agonist at least once (i.e., VA drug class RE102) in the 2 years before cohort entry. ICD-9 code 491.xx specifies chronic bronchitis, 492.xx specifies emphysema, 493.2 specifies chronic obstructive asthma, and 496.xx specifies chronic airway obstruction, all diseases likely to require β2-AR agonists as treatment. Veterans with COPD but no known prescription for β-agonists were considered nonexposed in our analyses. The following VA drug classes were used to identify Veterans taking angiotensin-converting enzyme (ACE) inhibitors (CV800) and/or diuretics (CV701–CV704 and CV709) at least once in last 2 years before cohort entry. All pharmacy information was obtained from the Pharmacy Managerial Cost Accounting National Data Extract (PHA MCA NDE). We defined Veterans with type 2 diabetes as having two or more of the following inpatient or outpatient ICD-9 diagnoses (250.xx, 357.2, 362.0x, and 366.41) during the 2 years before cohort entry. Similarly, we used inpatient or outpatient ICD-9 codes in the 2 years before cohort entry to identify select Elixhauser comorbidities like congestive heart failure (CHF: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4–425.9, and 428.x), complicated and uncomplicated hypertension (HTN: 401.x and 402.x-405.x), and liver disease (070.22, 070.23, 070.32, 070.33, 070.44, 070.54, 070.6, 070.9, 456.0–456.2, 570.x, 571.x, 572.2–572.8, 573.3, 573.4, 573.8, 573.9, and V42.7) as well as to calculate the weighted sum of all 30 Elixhauser comorbidities resulting in a continuous score measure of comorbidity burden.
Because death impeded on our ability to observe our outcome of interest, we used a competing risk regression, specifically the Fine-Gray model to assess the relationship between COPD, by means of β-agonist intake, and ESKD progression. All-cause death was considered as our competing event. In the model, we adjusted for age, diabetes, sex, race-ethnicity, the Elixhauser comorbidity burden score, diabetes, CHF, HTN, liver disease, ACE inhibitors, and diuretic use. Subjects were considered censored if they reached the end of the study without having either the event of interest (ESKD) or the competing event (death). COPD, sex, diabetes, CHF, HTN, liver disease, ACE inhibitors, and diuretic use were coded as binary variables. Age and the Elixhauser comorbidity burden score were considered as continuous variables, whereas race-ethnicity was categorized into four groups: non-Hispanic White (NHW), non-Hispanic Black (NHB), Hispanic, and others (i.e., Asian, American Indian or Alaska Native, or Native Hawaiian or other Pacific Islander). SAS Enterprise Guide 8.2 (SAS Institute, Cary, NC) was used to assemble the national retrospective cohort in the VA Informatics and Computing Infrastructure (VINCI). All analyses were performed using RStudio version 4.0.5 (2021-03-31). A P value of <0.05 or a confidence interval (CI) that does not cross 1 was considered statistically significant. The retrospective study was approved by the appropriate Institutional Review Boards (IRBs) at the Medical University of South Carolina and the Ralph H. Johnson Veterans Affairs Medical Center (VAMC).
Urine and Serum Analysis
For each urine sample, 10 μL were diluted twofold with water and mixed with 2× sample buffer and analyzed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Coomassie blue staining. Urine albumin/creatinine ratios (UACRs) were obtained using an enzyme-linked immunosorbent assay, the Albuwell kit (Ethos Biosciences, Philadelphia, PA) and a creatinine companion kit (TECO Diagnostics), whereas serum creatinine was measured using the QuantiChrom creatinine assay kit (DICT-500; BioAssay Systems, Hayward, CA), and the results were analyzed by an unpaired two-tailed t test (GraphPad Prism 7). For glucose measurements, we used a ReliOn Confirm glucometer with Micro Plus blood glucose test strips. All glucose values were obtained after an overnight fast in a chow-free cage.
Histological and Ultrastructural Analysis
Kidneys were isolated following perfusion of the mice with Hank’s buffered salt solution for 3 min. The kidneys were excised, decapsulated, transected, fixed for 12 h in 4% paraformaldehyde, rinsed, stored in 70% ethanol, and submitted to the MUSC Histology Core for paraffin embedding using a Shandon Histocentre 3 machine (Thermo Scientific). Five-micrometer sections were made from the blocks using a HM340E microtome (Microm). For hematoxylin and eosin staining, the sections were deparaffinized by soaking in xylene for 10 min, rehydrated through a gradation of ethanol beginning with 100% and ending in 95%, rinsed with water and stained in hematoxylin 7211 solution (Cat. No. 41810-472, VWR) for 5 min, rinsed again with water and dipped in 1% acid alcohol for 5 s, rinsed in water, dipped for 10 s in 1% ammonia alcohol, rinsed in water, stained with eosin Y with phloxine (Cat. No. 84000-104, VWR) for 10 s, dehydrated through a gradient of ethanol beginning with 95% and ending in 100%, and cleared with two changes of xylene, and a coverslip was then placed. For periodic acid-Schiff (PAS) staining, sections were deparaffinized as described earlier, oxidized by staining in 1% periodic acid solution for 10 min, rinsed in water, stained with Schiff reagent for 15 min, rinsed in hot water until a pink color developed, stained in Ehrlich’s hematoxylin (Cat. No. 41810-472, VWR) for 2 min, rinsed again in water, differentiated with 1% acid alcohol, rinsed once more with water, stained in 1% ammonia alcohol until the tissue turned blue, and dehydrated as described earlier and a coverslip placed. For Masson’s trichrome staining, the sections were deparaffinized described earlier incubated in Bouin’s solution (saturated picric acid 75 mL, formalin 25 mL, and glacial acetic acid 5 mL) for 1 h at 56°C, rinsed in water until the water was clear, stained in Weigert’s iron hematoxylin working solution (hematoxylin 1 g, 100 mL 95% alcohol, 4 mL 29% ferric chloride, 95 mL distilled water, and 1 mL hydrochloric acid) for 10 min, rinsed in water, stained in 1% Biebrich scarlet-1% acid Fuchsin solution for 3 min and rinsed with water, differentiated in phosphomolybdic-phosphotungstic acid (2.5 g phosphomolybdic, 2.5 g phosphotungstic, and 100 mL distilled water) for 10 min and rinsed in water, stained in aniline blue solution (2.5 g aniline, 2 mL glacial acetic acid, and 100 mL distilled water) for 5 min and rinsed in water, differentiated in 1% glacial acetic acid for 3 min, rinsed in water, and dehydrated as described earlier and a coverslip placed. Representative images were collected on an inverted Zeiss Axiovert-200-M confocal microscope (Carl Zeiss, Oberkochen, Germany) at the MUSC Cell & Molecular Imaging Facility and the Vectra Polaris Automated Slide Scanner, MUSC Hollings Cancer Center. To calculate percent fibrosis, the area of blue staining on the Masson’s trichrome slides was determined per total area using ImageJ. Kidney samples were fixed in 2% glutaraldehyde and 2% paraformaldehyde, stored overnight, and submitted to the MUSC Electron Microscopy Core for transmission electron microscopy (TEM). TEM sections were used to calculate the number of slit diaphragms per micrometers of glomerular basement membrane. The observers were blinded to the experimental group.
Western Blot Analysis, Antibodies, and Reagents
Kidney tissues were lysed in radioimmunoprecipitation assay (RIPA) buffer, and protein estimation was performed using the bicinchoninic acid (BCA) method. Protein lysate samples of 10 mg were used for Western blot analysis. Images were collected and densitometric analysis was performed using a LI-COR imaging station (LI-COR, Lincoln, NE). Primary antibodies against peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) (Invitrogen), oxidative phosphorylation proteins (cocktail of complex I–V) (Invitrogen), and GAPDH (Santa Cruz) were used for immunoblotting.
RESULTS
Given our previous data showing that formoterol protected renal function in mice with AKI (3), we wanted to test whether formoterol was also protective in CKD. Diabetic nephropathy is the leading cause of ESKD (6). Type 2 diabetes is a much more common cause of diabetic nephropathy than type 1 diabetes due to its relative prevalence, especially in the Veteran population; therefore, we first used a HFD, which is a well-described murine model of type 2 diabetes. Six-week-old C57BL/6J mice were fed for 12 wk with either a LFD (10% of total calories from fat) or a HFD (60% of total calories from fat), which leads to diabetic nephropathy. After 12 wk on the HFD, mice gained significant weight and were albuminuric, similar to human type 2 diabetic nephropathy (Fig. 1). Following 14 days of treatment with formoterol at 1 mg/kg, there was a marked recovery from diabetic injury in the HFD mice treated with formoterol compared with mice treated with vehicle alone. Although the HFD led to a significant increase in body weight compared with the LFD, formoterol did not appear to have an effect on body weight (Fig. 2A). As expected, overnight fasting glucose levels were significantly higher in mice receiving the HFD, though again formoterol did not appear to have an effect on glucose levels (Fig. 2B). After 7 and 14 days of formoterol treatment, albuminuria was significantly reduced in HFD mice treated with formoterol compared with HFD mice treated with vehicle alone (Fig. 2, C and D). HFD mice had a significant increase in kidney and glomerular size compared with LFD mice, something also seen in human diabetic nephropathy, and treatment with formoterol reduced the kidney and glomerular sizes back to those seen in LFD mice (Fig. 2, E–H). HFD mice had more kidney fibrosis than LFD mice and the fibrosis largely resolved in the HFD mice treated with formoterol compared with vehicle alone (Fig. 2, I and J). Serum creatinine also increased in HFD mice compared with LFD mice, indicating worsening kidney function, and decreased with formoterol treatment (Fig. 2K). Electron microscopy (EM) was performed, which showed podocyte foot process effacement in HFD mice but not in LFD or formoterol-treated HFD mice. We previously showed that the mechanism of action of formoterol in recovery from renal injury was via mitochondrial biogenesis (3). Mitochondria were rounded in HFD mice, suggesting injury, as opposed to the normal oblong shape seen in LFD and formoterol-treated HFD mice (Fig. 2L). Finally, we looked at mitochondrial biogenesis by measuring electron transport chain complex proteins via Western blot. All electron transport chain complex proteins showed a decrease in HFD mice compared with LFD mice and a subsequent increase following treatment with formoterol, although only complex II was statistically significant (Fig. 2, M and N). Similar results were seen at the ultrastructural, histological, and functional levels following pancreatic islet cell injury with streptozotocin, a murine model of type 1 diabetes (7), and subsequent treatment with formoterol or vehicle alone (Figs. 3 and 4). Electron transport chain complex I, IV, and V proteins were significantly increased in streptozotocin + formoterol-treated kidneys compared with control kidneys, and complex I and IV proteins were significantly increased in streptozotocin + formoterol-treated kidneys compared with streptozotocin + vehicle-treated kidneys (P < 0.05) (Fig. 4, G and H). A trend toward an increase in PGC-1α, a master regulator of mitochondrial biogenesis in podocytes (8), was also seen in streptozotocin + formoterol-treated kidneys compared with control kidneys (P = 0.06).
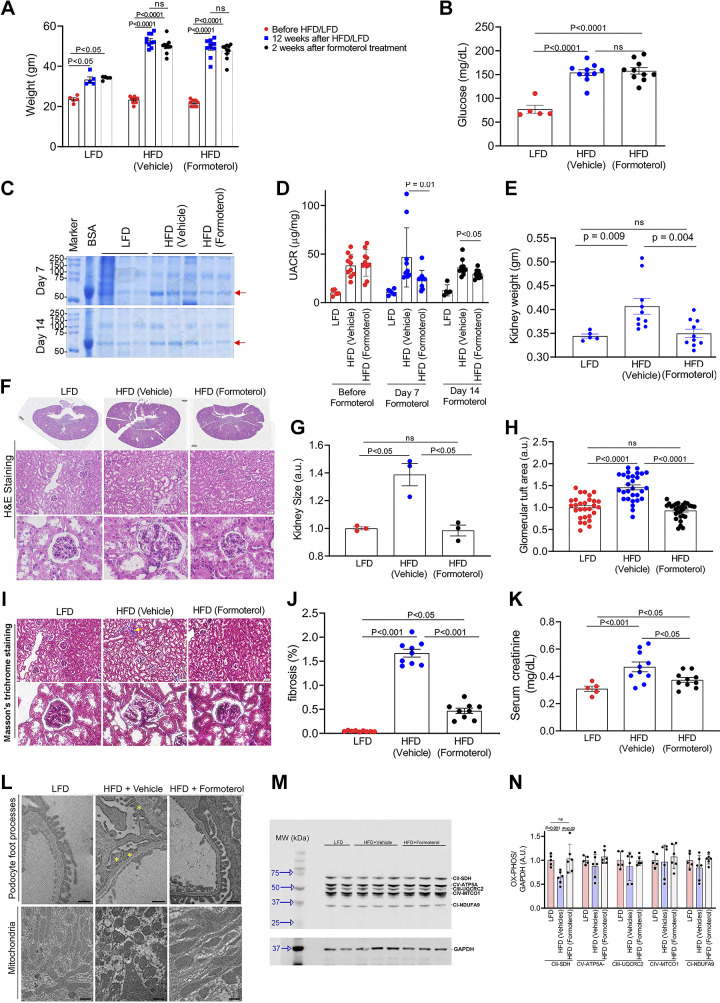
Figure 2.
Formoterol treatment results in recovery from high-fat diet (HFD)-induced diabetic renal injury. A: HFD induced a significant increase in body weight compared with a low-fat diet (LFD). B: overnight fasting glucose levels were found to be significantly higher in mice that received the HFD compared with the LFD. C: SDS-PAGE of urine samples after 7 and 14 days of formoterol treatment showed a decrease in albumin excretion in HFD mice treated with formoterol compared with HFD mice treated with vehicle alone. Red arrow points to albumin. BSA, bovine serum albumin. D: measurement of urine albumin to creatinine ratio (UACR) by ELISA showed a significant reduction in albuminuria at 7 and 14 days in HFD mice treated with formoterol compared with HFD mice treated with vehicle alone. E: HFD mice had a significantly increased kidney weight compared with LFD mice, and this was reversed with formoterol treatment. F: histology, hematoxylin and eosin (H&E) staining, of kidney sections in LFD, HFD, and HFD + formoterol mice. Bar in the full kidney sections = 800 μm. Bar in middle row = 50 μm. G and H: quantitative analysis of kidney size and glomerular tuft area showed that both were increased in HFD mice compared with LFD mice and that formoterol treatment decreased these parameters back to what was seen in LFD mice. I: histology and Masson’s trichrome staining of kidney sections in LFD, HFD, and HFD + formoterol mice examining fibrosis. Yellow arrow points to fibrosis in blue. Bar in top row = 50 μm. J: quantitative analysis showing increased fibrosis in kidneys of HFD vs. LFD mice, which was largely reversed with formoterol. K: serum creatinine increased in HFD mice compared with LFD mice and decreased with formoterol treatment. L: representative EM sections show podocyte foot process effacement (yellow *) in HFD mice but not in LFD or HFD + formoterol mice. Mitochondria were rounded in HFD mice as opposed to oblong in LFD and HFD + formoterol mice. Bar = 800 nm. M: markers of mitochondrial biogenesis in kidney lysates were evaluated by Western blotting using OXPHOS (cocktail antibodies of mitochondrial electron transport chain complex I to V) and GAPDH antibodies. GAPDH is a loading control. N: quantitative analysis showing that complex II was significantly decreased in HFD mice compared with LFD mice, and levels were restored in HFD + formoterol mice. ns, nonsignificant. All data are presented as means ± SE. These experiments were repeated twice with similar results. For F, I, and L, representative sections are shown. For C, G, M, and N, representative experiments are shown with each dot representing a mouse. For A, B, D, E, J, and K, data from all the experiments were aggregated with each dot representing a mouse. For glomerular tuft area in H, three measurements from each mouse were used. EM, electron microscopy.
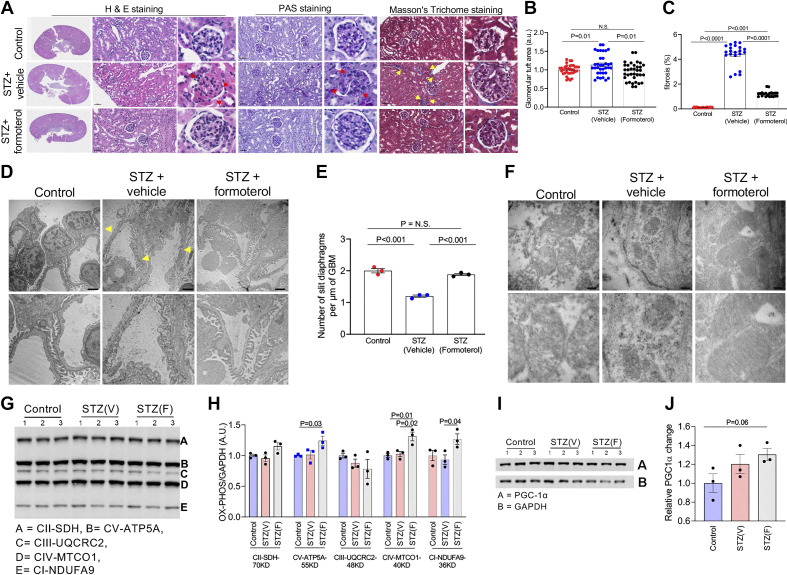
Figure 4.
Formoterol treatment resulted in recovery from streptozotocin (STZ)-induced diabetic renal injury. A: representative images from H&E, PAS, and Masson’s trichrome staining of sections from paraffin-embedded renal tissue. Histological analysis showed that formoterol treatment reduced fibrosis (yellow arrows) in STZ-treated mice compared with mice treated with vehicle alone. STZ mice had nodular lesions typical of diabetic nephropathy (red arrows). Formoterol treatment reversed these changes. Scale bar = 50 μm. B: quantitative analysis of the glomerular tuft area demonstrated a significant reduction in formoterol-treated mice compared with mice treated with vehicle alone. C: formoterol treatment in STZ-treated mice mice showed a significant reduction in fibrosis compared with mice treated with vehicle alone. All data are presented as means ± SE. D: representative TEM images of podocyte foot processes in control, STZ + vehicle, and STZ + formoterol-treated mice. Foot processes appeared effaced and GBM thickened in STZ-treated mice (yellow arrow). Scale bar = 2 μm. E: quantitative analysis of electron micrographs showed significant reduction in the number of slit diaphragms per micrometers in STZ-treated mice, which was reversed with formoterol. F: representative EM images of mitochondria whose shape and size were distorted following STZ and restored with formoterol. Scale bar = 200 nm. G: markers of mitochondrial biogenesis in kidney lysates were evaluated by Western blotting using OXPHOS (cocktail antibodies of mitochondrial electron transport chain complex I to V) and GAPDH antibodies. H: densitometric analysis of immunoblots showed significant increases in complexes V-ATP5A, IV-MTCO1, and I-NDUFA9 proteins in STZ + formoterol-treated kidneys compared with control kidneys and in IV-MTCO1 and I-NDUFAS proteins in STZ + formoterol treated kidneys compared with STZ + vehicle- treated kidneys (P < 0.05). All data are presented as means ± SE. I and J: a trend toward increased PGC-1α expression was found in STZ animals treated with formoterol. These experiments were repeated twice with similar results. For A, D, and F, representative sections are shown. For E and G–J, representative experiments are shown with each dot representing a mouse. For glomerular tuft area in B, three sections from each mouse were used. For fibrosis measurements in C, three sections from each mouse were used. EM, electron microscopy; H&E, hematoxylin and eosin; ns, nonsignificant; PAS, periodic acid-Schiff.
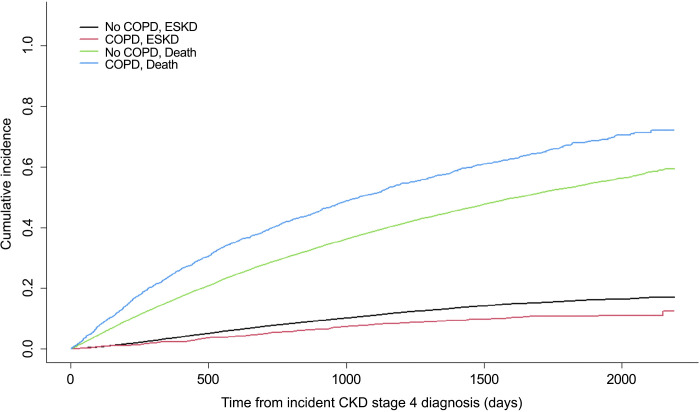
To determine whether a similar protective effect of β2-AR agonists might occur in humans, we designed a retrospective cohort study to evaluate the association between β2-AR agonists and their effect on progression of kidney disease. Such an analysis can be performed since COPD is highly prevalent in Veterans, affecting almost 15% of VHA users (9), and long-acting β2-AR agonists, including formoterol, and its derivatives have historically been used as first-line therapy in COPD (10, 11). We identified a total of 24,133 Veterans aged 65 years or over with incident stage 4 CKD from 2011 to 2013 (Table 1). Given that the rate of progression from stage 4 CKD to ESKD is faster than the progression from earlier CKD stages, this allowed for a reasonable follow-up time to perform our analysis of ESKD progression in our stage 4 CKD cohort. About 5.6% of Veterans had COPD and were receiving β2-AR agonists before cohort entry, of which 40.4% were taking formoterol. A majority of Veterans were taking short-acting β2-AR agonists, especially those with COPD (58.2% vs. 13%). A higher proportion of Veterans with COPD were using long-acting β2-AR agonists alone (9% vs. 1.2%) or short- and long-acting β2-AR agonists (32.8% vs. 3.2%) compared with those without COPD. A quarter (25.5%) of our total cohort had diabetes. Over half (52.7%) of Veterans with COPD had diabetes compared with 23.9% of Veterans without COPD. The median age of the cohort was 80 years old, and the cohort was predominantly male. The proportion of males was slightly lower among those with COPD than without COPD (96.9% vs. 98.3%). Veterans with COPD tended to be sicker and use ACE inhibitors and diuretics more frequently than their non-COPD counterparts. Irrespective of COPD status, most of the Veterans were NHW, followed by NHB, Hispanic, and other. An unadjusted cumulative incidence plot of ESKD and death by COPD status from the time of incident stage 4 CKD diagnosis indicates that Veterans with COPD had a lower incidence of ESKD but a higher incidence of death compared with those without COPD (Fig. 5). After adjusting for age, sex, race-ethnicity, the Elixhauser comorbidity burden score, diabetes, CHF, HTN, liver disease, ACE inhibitors, and diuretic use, we found COPD to be associated with protection against ESKD progression (Table 2). Specifically, there was a 25.6% reduction in the rate of ESKD in Veterans with COPD than without [hazard ratio (HR): 0.74; 95% CI: 0.62–0.89]. The effect of COPD was slightly greater against ESKD progression in the unadjusted model (HR: 0.67; 95% CI: 0.57–0.80). An increased Elixhauser comorbidity burden score revealed a 2% reduction in the rate of ESKD (HR: 0.98; 95% CI: 0.98–0.99). This is likely due to Veterans with more comorbidities dying before reaching ESKD. Similarly, the rate of ESKD was lower in Veterans with CHF or HTN than without CHF or HTN, but these effects were not statistically significant. Using appropriate interaction terms, we found no evidence of interaction between COPD and diabetes (P = 0.84), meaning that the effect of COPD on ESKD progression was similar in both diabetic and nondiabetic Veterans in our study population. Diabetes was significantly associated with a 48% increase in the rate of ESKD (HR: 1.48; 95% CI: 1.34–1.64), whereas liver disease was not (P = 0.08). ACE inhibitors as expected slowed ESKD progression (HR: 0.91; 95% CI: 0.85–0.97). As for diuretics, the rate of ESKD among Veterans who used diuretics compared with nonusers was not statistically significant (P = 0.46). Given the small proportion of female Veterans in our retrospective cohort (1.8%), we also performed a sensitivity analysis by excluding them from our model. No significant changes in effect (both in magnitude and direction) were noted neither for COPD nor for any of the covariates in the model after doing so. These rates were similar to published findings based on an analysis examining the progression of Veterans from CKD stage 3 to 4 who received care in the VHA Veterans Integrated Services Network 7 (VISN 7) (12).
Table 1.
Descriptive characteristics of national retrospective cohort of older Veterans (aged 65 yr or older) with incident stage 4 CKD from 2011 to 2013 stratified by COPD status
| COPD | No COPD | Total | |
|---|---|---|---|
| n = 1,348 | n = 22,785 | n = 24,133 | |
| Formoterol, % | 40.4 | 4.2 | 6.2 |
| Type of β2-AR agonist, % | |||
| Long | 9 | 1.2 | 1.7 |
| Short | 58.2 | 13 | 15.5 |
| Both | 32.8 | 3.2 | 4.8 |
| Demographics | |||
| Median age (IQR) | 79 (71–84) | 80 (71–86) | 80 (71–86) |
| Male, % | 96.9 | 98.3 | 98.2 |
| Race-ethnicity, % | |||
| NHW | 79.6 | 81.2 | 81.1 |
| NHB | 12.5 | 13.4 | 13.3 |
| Hispanic | 4.3 | 3.3 | 3.3 |
| Other | 3.6 | 2.1 | 2.3 |
| Comorbidity burden | |||
| Mean Elixhauser comorbidity burden score (SD) | 15.9 (8.7) | 4.2 (7.1) | 4.8 (7.7) |
| Diabetes, % | 52.7 | 23.9 | 25.5 |
| CHF, % | 54.2 | 11.7 | 14 |
| HTN, % | 93.7 | 40.2 | 43.2 |
| Liver disease, % | 2.9 | 1.1 | 1.2 |
| Other medication use | |||
| ACE inhibitors | 61.7 | 52.4 | 53 |
| Diuretics | 83.5 | 64.5 | 65.5 |
ACE, angiotensin-converting enzyme; β2-AR, β2-adrenergic receptor; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HTN, hypertension; IQR, interquartile range; NHB, non-Hispanic Black; NHW, non-Hispanic White; SD, standard deviation.
Figure 5.
Unadjusted cumulative incidence plot of ESKD and death among a retrospective cohort of older Veterans (aged 65 yr or over) by COPD status from time of incident stage 4 CKD diagnosis. CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESKD, end-stage kidney disease.
Table 2.
Competing risk regression between COPD and ESKD progression in older Veterans (aged 65 yr or older) with incident stage 4 CKD from 2011 to 2013
| HR | 95% CI | P Value | |
|---|---|---|---|
| Main effect | |||
| COPD | 0.74 | 0.62–0.89 | <0.01 |
| Covariates | |||
| Age | 0.92 | 0.91–0.92 | <0.0001 |
| Male (vs. female) | 2.30 | 1.59–3.31 | <0.0001 |
| Race-ethnicity | |||
| NHW | 1.00 | ||
| NHB | 1.79 | 1.65–1.94 | <0.0001 |
| Hispanic | 1.69 | 1.46–1.96 | <0.0001 |
| Other | 1.43 | 1.18–1.73 | <0.001 |
| Elixhauser comorbidity burden score |
0.98 | 0.98–0.99 | <0.0001 |
| Diabetes | 1.48 | 1.34–1.64 | <0.0001 |
| CHF | 0.91 | 0.80–1.04 | 0.16 |
| HTN | 0.94 | 0.84–1.04 | 0.24 |
| Liver disease | 1.26 | 0.97–1.62 | 0.079 |
| ACE inhibitors | 0.91 | 0.85–0.97 | <0.01 |
| Diuretics | 1.03 | 0.95–1.11 | 0.46 |
HR, hazard ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; NHW, non-Hispanic White; NHB, non-Hispanic Black; CHF, congestive heart failure; HTN, hypertension; ACE, angiotensin-converting enzyme; ESKD, end-stage kidney disease.
A sensitivity analysis was performed to determine the independent effects of long-acting formoterol, other β2-AR agonists, and the use of neither formoterol nor other β2-AR agonists on ESKD progression in Veterans with incident CKD stage 4. Only inpatient and outpatient ICD-9 diagnostic codes (491.xx, 492.xx, 493.2, and 496.xx) were used to define COPD. Subjects with two or more of the previously listed ICD-9 diagnoses in the 2 years before cohort entry were considered to have COPD. β2-AR agonist intake was based on having a β2-AR agonist prescription (i.e., VA drug class RE102) in the 2 years before cohort entry. A Fine-Gray competing risk regression was performed, and our main exposure variable was categorized into four groups: no COPD, COPD and formoterol, COPD and other β2-AR agonists, and COPD and no β2-AR agonists. Compared with the no COPD group, we observed an increasingly protective effect against ESKD progression when going from no β2-AR agonist intake to β2-AR agonist use other than formoterol to formoterol use in subjects with COPD after adjusting for demographics, comorbidities, and medications. Specifically, there was a 33.2% reduction in the rate of ESKD in Veterans with COPD taking formoterol (HR: 0.67; 95% CI: 0.50–0.89) and a 20.8% reduction in the rate of ESKD in Veterans with COPD taking β2-AR agonists other than formoterol (HR: 0.79; 95% CI: 0.64–0.99) when compared with Veterans with no COPD (Table 3). The effect of COPD and formoterol was greater against ESKD progression in the unadjusted model (HR: 0.58; 95% CI: 0.44–0.77). A high proportion of patients with COPD did use ACE inhibitors (61.7%), which are known to reduce ESKD progression; however, after adjusting for ACE inhibitor use, we still observed a lower risk of ESKD progression in those who had COPD compared with those who did not have COPD (Tables 1–3).
Table 3.
Sensitivity analysis of a competing risk regression between COPD (defined using ICD-9 diagnoses only) and ESKD progression in older Veterans (aged 65 yr or older) with incident stage 4 CKD from 2011 to 2013
| HR | 95% CI | P Value | |
|---|---|---|---|
| Main effect | |||
| No COPD | 1.00 | ||
| COPD and formoterol | 0.67 | 0.50–0.89 | <0.01 |
| COPD and other β2-AR agonist | 0.79 | 0.64–0.99 | 0.039 |
| COPD and no β2-AR agonist | 0.99 | 0.79–1.23 | 0.91 |
| Covariates | |||
| Age | 0.92 | 0.91–0.92 | <0.0001 |
| Male (vs. female) | 2.30 | 1.59–3.31 | <0.0001 |
| Race-ethnicity | |||
| NHW | 1.00 | ||
| NHB | 1.79 | 1.65–1.94 | <0.0001 |
| Hispanic | 1.69 | 1.46–1.96 | <0.0001 |
| Other | 1.43 | 1.18–1.73 | <0.001 |
| Elixhauser comorbidity burden score | 0.98 | 0.98–0.99 | <0.0001 |
| Diabetes | 1.48 | 1.34–1.64 | <0.0001 |
| CHF | 0.91 | 0.80–1.04 | 0.16 |
| HTN | 0.94 | 0.84–1.04 | 0.24 |
| Liver disease | 1.26 | 0.97–1.62 | 0.079 |
| ACE inhibitors | 0.91 | 0.85–0.97 | <0.01 |
| Diuretics | 1.03 | 0.95–1.11 | 0.46 |
HR, hazard ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; β2-AR, β2-adrenergic receptor; NHW, non-Hispanic White; NHB, non-Hispanic Black; CHF, congestive heart failure; HTN, hypertension; ACE, angiotensin-converting enzyme; ESKD, end-stage kidney disease; CKD, chronic kidney disease.
DISCUSSION
We report several principal findings, all of which are important for the treatment of diabetic nephropathy. First, our animal data confirm the strong effect of β2-AR agonists, specifically formoterol, on diabetic nephropathy. The HFD model is a good model of type 2 diabetes mellitus and mice on the HFD experienced weight gain, increased overnight fasting glucose levels, and albuminuria, all things seen in human diabetic nephropathy. Mice on the HFD also had increased glomerular and kidney sizes, something that is also seen in human diabetic nephropathy. Histologically, there was increased fibrosis and podocyte effacement along with signs of mitochondrial injury at the ultrastructural level. Formoterol over 7 and 14 days largely reversed these injury phenotypes. Currently available therapies, such as ACE or SGLT2 inhibitors, slow the progression of diabetic nephropathy but do not reverse it (2). Interestingly, formoterol did not appear to have an effect on body weight or overnight fasting glucose levels, implying that formoterol is acting through a different pathway. With respect to how formoterol may be acting, β2-AR agonists are known modulators of oxidative metabolism and energy expenditure (8, 13). If mitochondria play a central role in podocyte/glomerular structure and function, it would be expected that patients with mutations in genes that directly or indirectly affect mitochondrial function would exhibit glomerular injury phenotypes. Indeed, many patients with mitochondrial mutations, including mitochondrially encoded tRNA-Leu 1 (MT-TL1) (14), coenzyme Q2 (COQ2) (15), coenzyme Q6 (COQ6) (16), and prenyl diphosphate synthase subunit 1 (PDSS1) (17), present with glomerular injury phenotypes. Mice lacking genes such as mammalian target of rapamycin complex 1 (mTORC1) (18), Rho associated coiled-coil containing protein kinase 1 (ROCK1) (19), and autophagy related 5 (ATG5) (20), which indirectly affect mitochondrial function, also show a glomerular injury phenotype. Mice with podocyte-specific knockout of prenyl diphosphate synthase subunit 2 (Pdss2), which results in mitochondrial Co-Q deficiency, exhibit proteinuria and podocyte foot process effacement (21). Deletions in mitochondrial DNA have been noted previously in ∼60% of patients with primary focal segmental glomerulosclerosis (FSGS) (22, 23). Indeed, we have previously shown that the mechanism of action of formoterol in the recovery from AKI was via mitochondrial biogenesis (3). In this study, we showed by EM that mitochondrial injury was reversed in diabetic mouse models by formoterol. Streptozotocin in the diabetic type 1 mouse model and HFD in the diabetic type II mouse model significantly decreased levels of electron transport chain proteins, and formoterol rescued this. The contribution of mitochondrial oxidative stress to mitochondrial dysfunction and CKD is well established in patients with diabetes, where it is mediated by the overproduction of reactive oxygen species (ROS), a common pathway in the pathogenesis of diabetic microvascular complications (24). Others have also recently shown that mitochondrial energetics and dynamics proteins were increased in the proximal tubules of db/db diabetic mice treated with formoterol (25).
Second, our large retrospective cohort of 24,133 Veterans with incident stage 4 CKD showed a significant 25.6% decrease in rate of ESKD progression in Veterans with COPD who were treated with β-agonists compared with Veterans without COPD or with COPD but with no known β-agonist intake. Diabetic nephropathy is the most common cause of ESKD, accounting for 44% of all ESKD (6), so much of the total reduction in progression of CKD in patients with COPD occurred in patients with diabetic COPD. A recent cohort study also showed that long-term use of β2-AR agonists may exert a protective effect against diabetic vascular complications (26). Given that the global prevalence of stage 4 CKD is 0.4% (27), there is a substantial number of potential candidates for this therapy if a prospective randomized double-blind placebo-controlled clinical trial confirmed that β2-AR agonists reduce the risk of progression from stage 4 CKD to ESKD by 25.6%. The number of ESKD cases prevented would rise if β2-AR agonists are shown to reduce the progression of stage 4 CKD by more than 25.6% or if β2-AR agonists are shown to be effective at an earlier stage of CKD. Similarly, if formoterol is used as the β2-AR agonist and is more effective than a generic β2-AR agonist, as our human data suggest, then the number of preventable cases would also increase.
A limitation of our study is that the dose of formoterol that the mice were treated with was ∼50 times higher than is prescribed for patients (28). We used this dose as it was the same dose that has been used in multiple previous studies by different groups (3); however, it was reassuring that the protective effect of β2-AR agonists that we saw in mice was also associated with protection in our human retrospective cohort. The next step will be to rigorously test the hypothesis that β2-AR agonists are protective in diabetic nephropathy by performing a randomized double-blind placebo-controlled prospective study.
In summary, we have identified a potential treatment for diabetic nephropathy, which is the leading cause of ESKD. Importantly, no current therapies exist that reverse diabetic nephropathy as formoterol does in the HFD- and streptozotocin-treated mice. β2-AR agonists, such as formoterol, have been successfully used by clinicians for decades and are relatively safe and inexpensive (29). There are possible cardiovascular risks of systemic administration of formoterol in humans including increased heart rate and decreased blood pressure, especially at the doses we gave to the mice. β-ARs are coupled via stimulatory G proteins to adenylyl cyclase. This enzyme produces the second messenger cAMP that subsequently activates protein kinase A in cells (30). A recent study from the Schnellmann laboratory used biodegradable and biocompatible polymeric nanoparticles containing formoterol that targeted the kidney, thereby decreasing the effective dose, and was able to show renal protection following ischemia and reperfusion injury in mice (5). This approach could lessen cardiovascular effects while restoring kidney function and could be used to treat diabetic nephropathy should a prospective randomized trial confirm our findings. Finally, it is possible that formoterol has off-target effects. To test this, we are planning similar studies using β2-AR knockout mice.
DATA AVAILABILITY
Data will be made available upon request.
GRANTS
This work was supported in part by Veterans Affairs Clinical Science Research and Development Merit Award CX002391, Biomedical Laboratory Research and Development Merit Award BX000820, Veterans Affairs Health Services Research and Development HX001229, the Charleston Health Equity and Rural Outreach Innovation Center, and Dialysis Clinic Incorporated.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.A., D.M., K.J.H., and J.H.L. conceived and designed research; E.A., D.M., A.S., X.Z., Y.S., and Y.D. performed experiments; E.A., D.M., O.P., M.-A.C., B.J.W., K.J.H., and J.H.L. analyzed data; E.A., D.M., A.S., B.W., K.L., A.I.K., O.P., B.J.W., K.J.H., and J.H.L. interpreted results of experiments; E.A., D.M., and J.H.L. prepared figures; E.A., D.M., K.J.H., and J.H.L. drafted manuscript; E.A., D.M., A.S., X.Z., B.W., K.L., A.I.K., O.P., M.-A.C., B.J.W., K.J.H., and J.H.L. edited and revised manuscript; B.J.W., K.J.H., and J.H.L. approved final version of manuscript.
ACKNOWLEDGMENTS
Lisa Lipschutz is gratefully acknowledged for reading and reviewing the manuscript. Support for VA/CMS data was provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development Service, and Veterans Affairs Information Resource Center (Project Nos. SDR 02-237 and 98-004).
REFERENCES
- 1. Phillips J, Chen JHC, Ooi E, Prunster J, Lim WH. Global epidemiology, health outcomes, and treatment options for patients with type 2 diabetes and kidney failure. Front Clin Diabetes Healthc 2: 731574, 2021. doi: 10.3389/fcdhc.2021.731574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamazaki T, Mimura I, Tanaka T, Nangaku M. Treatment of diabetic kidney disease: current and future. Diabetes Metab J 45: 11–26, 2021. doi: 10.4093/dmj.2020.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arif E, Solanki AK, Srivastava P, Rahman B, Fitzgibbon WR, Deng P, Budisavljevic MN, Baicu CF, Zile MR, Megyesi J, Janech MG, Kwon SH, Collier J, Schnellmann RG, Nihalani D. Mitochondrial biogenesis induced by the β2-adrenergic receptor agonist formoterol accelerates podocyte recovery from glomerular injury. Kidney Int 96: 656–673, 2019. doi: 10.1016/j.kint.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, Schnellmann RG. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol 25: 1157–1162, 2014. doi: 10.1681/ASN.2013090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vallorz EL, Janda J, Mansour HM, Schnellmann RG. Kidney targeting of formoterol containing polymeric nanoparticles improves recovery from ischemia reperfusion-induced acute kidney injury in mice. Kidney Int 102: 1073–1089, 2022. doi: 10.1016/j.kint.2022.05.032. [DOI] [PubMed] [Google Scholar]
- 6. Burrows NR, Hora I, Geiss LS, Gregg EW, Albright A. Incidence of end-stage renal disease attributed to diabetes among persons with diagnosed diabetes - United States and Puerto Rico, 2000-2014. MMWR Morb Mortal Wkly Rep 66: 1165–1170, 2017. doi: 10.15585/mmwr.mm6643a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc 1: e78, 2021. doi: 10.1002/cpz1.78. [DOI] [PubMed] [Google Scholar]
- 8. Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, Schnellmann RG. The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther 342: 106–118, 2012. doi: 10.1124/jpet.112.191528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roman J, Perez RL. COPD in VA hospitals. Clin Cornerstone 5: 37–44, 2003. doi: 10.1016/s1098-3597(03)90007-x. [DOI] [PubMed] [Google Scholar]
- 10. Kim D, Glaum M, Lockey R. Evaluation of combination long-acting beta-2 agonists and inhaled glucocorticosteroids for treatment of asthma. Expert Opin Drug Metab Toxicol 5: 933–940, 2009. doi: 10.1517/17425250903127226. [DOI] [PubMed] [Google Scholar]
- 11. Rodrigo GJ, Price D, Anzueto A, Singh D, Altman P, Bader G, Patalano F, Fogel R, Kostikas K. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 12: 907–922, 2017. doi: 10.2147/COPD.S130482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arora P, Jalal K, Gupta A, Carter RL, Lohr JW. Progression of kidney disease in elderly stage 3 and 4 chronic kidney disease patients. Int Urol Nephrol 49: 1033–1040, 2017. doi: 10.1007/s11255-017-1543-9. [DOI] [PubMed] [Google Scholar]
- 13. Pearen MA, Myers SA, Raichur S, Ryall JG, Lynch GS, Muscat GE. The orphan nuclear receptor, NOR-1, a target of beta-adrenergic signaling, regulates gene expression that controls oxidative metabolism in skeletal muscle. Endocrinology 149: 2853–2865, 2008. doi: 10.1210/en.2007-1202. [DOI] [PubMed] [Google Scholar]
- 14. Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348: 651–653, 1990. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 15. Quinzii C, Naini A, Salviati L, Trevisson E, Navas P, Dimauro S, Hirano M. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am J Hum Genet 78: 345–349, 2006. doi: 10.1086/500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heeringa SF, Chernin G, Chaki M, Zhou W, Sloan AJ, Ji Z, , et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest 121: 2013–2024, 2011. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mollet J, Giurgea I, Schlemmer D, Dallner G, Chretien D, Delahodde A, Bacq D, de Lonlay P, Munnich A, Rötig A. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J Clin Invest 117: 765–772, 2007. doi: 10.1172/JCI29089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121: 2197–2209, 2011. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab 15: 186–200, 2012. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benoit G, Machuca E, Antignac C. Hereditary nephrotic syndrome: a systematic approach for genetic testing and a review of associated podocyte gene mutations. Pediatr Nephrol 25: 1621–1632, 2010. doi: 10.1007/s00467-010-1495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hagiwara M, Yamagata K, Capaldi RA, Koyama A. Mitochondrial dysfunction in focal segmental glomerulosclerosis of puromycin aminonucleoside nephrosis. Kidney Int 69: 1146–1152, 2006. doi: 10.1038/sj.ki.5000207. [DOI] [PubMed] [Google Scholar]
- 23. Yamagata K, Muro K, Usui J, Hagiwara M, Kai H, Arakawa Y, Shimizu Y, Tomida C, Hirayama K, Kobayashi M, Koyama A. Mitochondrial DNA mutations in focal segmental glomerulosclerosis lesions. J Am Soc Nephrol 13: 1816–1823, 2002. doi: 10.1097/01.asn.0000019772.17954.f8. [DOI] [PubMed] [Google Scholar]
- 24. Galvan DL, Green NH, Danesh FR. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int 92: 1051–1057, 2017. doi: 10.1016/j.kint.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cleveland KH, Brosius FC 3rd, Schnellmann RG. Regulation of mitochondrial dynamics and energetics in the diabetic renal proximal tubule by the β2-adrenergic receptor agonist formoterol. Am J Physiol Renal Physiol 319: F773–F779, 2020. doi: 10.1152/ajprenal.00427.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee HJ, Lee H, Oh SH, Park S, Jung KY, Kim H, Kwon SH, Jeon JS, Han DC, Noh H. Association between beta2-adrenergic receptor agonists and the risk of vascular complications in diabetic patients: a population-based cohort study. J Clin Med 8: 1145, 2019. doi: 10.3390/jcm8081145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hill NR, Fatoba ST, Oke JL, Hirst JA, O'Callaghan CA, Lasserson DS, Hobbs FD. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One 11: e0158765, 2016. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7: 27–31, 2016. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Faulds D, Hollingshead LM, Goa KL. Formoterol. A review of its pharmacological properties and therapeutic potential in reversible obstructive airways disease. Drugs 42: 115–137, 1991. doi: 10.2165/00003495-199142010-00007. [DOI] [PubMed] [Google Scholar]
- 30. Cazzola M, Page CP, Rogliani P, Matera MG. β2-agonist therapy in lung disease. Am J Respir Crit Care Med 187: 690–696, 2013. doi: 10.1164/rccm.201209-1739PP. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request.