Abstract
Early visual cortex (V1–V3) is believed to be critical for normal visual awareness by providing the necessary feedforward input. However, it remains unclear whether visual awareness can occur without further involvement of early visual cortex, such as re-entrant feedback. It has been challenging to determine the importance of feedback activity to these areas because of the difficulties in dissociating this activity from the initial feedforward activity. Here, we applied single-pulse transcranial magnetic stimulation (TMS) over the left posterior parietal cortex to elicit phosphenes in the absence of direct visual input to early visual cortex. Immediate neural activity after the TMS pulse was assessed using the event-related optical signal (EROS), which can measure activity under the TMS coil without artifacts. Our results show that: 1) The activity in posterior parietal cortex 50 ms after TMS was related to phosphene awareness, and 2) Activity related to awareness was observed in a small portion of V1 140 ms after TMS, but in contrast (3) Activity in V2 was a more robust correlate of awareness. Together, these results are consistent with interactive models proposing that sustained and recurrent loops of activity between cortical areas are necessary for visual awareness to emerge. In addition, we observed phosphene-related activations of the anteromedial cuneus and lateral occipital cortex, suggesting a functional network subserving awareness comprising these regions, the parietal cortex and early visual cortex.
Keywords: Visual awareness, Phosphenes, Early visual cortex, Single pulse TMS, Event-related optical signal (EROS)
1. Introduction
It is widely believed that early visual cortex, and in particular primary visual cortex (V1), plays a critical role in visual awareness. However, it is still debated whether this role is purely due to its anatomical position as the gateway of visual information into the cortex, or if it is also involved in ongoing generation of visual awareness. These two possibilities have been difficult to assess, in part because it is difficult to dissociate visual input from continued processes.
During normal visual processing, information from the eye enters the cortex primarily through V1. After this, the visual pathway comprises a hierarchy of feedforward and feedback projections from V1 to extrastriate cortex and higher cognitive areas (de Graaf et al., 2012; Hurme et al., 2019; Lamme and Roelfsema, 2000; Wokke et al., 2013), as well as local connections within a region (Lamme et al., 1998; Roebuck et al., 2014). Disruptions to the visual pathway caused by lesion or trauma to V1 lead to a profound loss of conscious visual awareness in the corresponding visual field, a condition known as hemianopia (Cavézian et al., 2010; Holmes, 1918; Teuber et al., 1960). Traditionally, hemianopia is considered the strongest evidence that V1 may be necessary for visual awareness. However, recently this necessity has been challenged, with some reports of awareness in patients without V1 (Ffytche and Zeki, 2011; Mazzi et al., 2014). Similarly, phosphene awareness has been produced by stimulating V2 while ensuring indirect stimulation in V1, implicating V2 as a critical correlate of visual awareness (Salminen-Vaparanta et al., 2012). Others have suggested that the correlates of awareness are downstream of even V2, beginning in V3 and later (Tse et al., 2005).
Regardless of which regions are necessary for awareness, it is unclear what specific role these areas play in visual awareness. Some have put forward what Tong (2003) refers to as hierarchical models (Crick and Koch, 1995; Rees et al., 2002), which argue that the primary reason hemianopics lack awareness in their blind field is that damage to V1 disrupts the input of information into the rest of visual cortex. A similar argument can be extended to other regions of occipital cortex. Others have put forward what Tong (2003) refers to as interactive models in which awareness depends on recurrent activity into V1 or other early visual areas (Breitmeyer, 2007; Di Lollo et al., 2000; Enns and Di Lollo, 2000; Fahrenfort et al., 2007, 2008; Lamme, 2000, 2006; Lamme and Roelfsema, 2000; Pascual-Leone and Walsh, 2001; Silvanto et al., 2005; Silvanto and Pascual-Leone, 2008; Tapia and Beck, 2014; Tong, 2003).
Although data from hemianopics provide the strongest evidence of the necessity of early visual cortex for normal visual awareness, it is not a good case study for distinguishing hierarchical from interactive models of awareness. Having lost portions of V1, hemianopics have, in theory, lost both the input functions and any recurrent activity into early visual cortex. Thus, the absence of visual awareness in hemianopia patients cannot be attributed to either the lack of sufficient input to anterior regions or feedback.
The majority of evidence supporting the involvement of feedback activity in visual awareness come from the timing of so-called interference of processes related to awareness in the intact brain. In particular, it has been argued that the timing of the interference reflects disruptions of feedback processes (Breitmeyer, 2007; Di Lollo et al., 2000; Enns and Di Lollo, 2000; Fahrenfort et al., 2007, 2008; Lamme, 2000, 2006; Lamme and Roelfsema, 2000; Pascual-Leone and Walsh, 2001; Silvanto, 2008; Silvanto et al., 2005a,b; Tapia and Beck, 2014). Specifically, these studies included interference of ongoing signals by either a visual mask (Breitmeyer, 2007; Di Lollo et al., 2000; Enns and Di Lollo, 2000; Fahrenfort et al., 2007, 2008) or transcranial magnetic stimulation (TMS) (Pascual-Leone and Walsh, 2001; Silvanto et al., 2005a,b; Silvanto et al., 2008) occurring at varying delays. Typically, these studies found maximum disruption of target awareness when visual masks or TMS pulses occurred at precise delays relative to the targets. Such delays are usually larger than the widely assumed retino-cortical transmission time (60 ms; Foxe and Simpson, 2002; Railo and Koivisto, 2012), which led to the idea that these interferences must be disrupting feedback signals, and thus supports the role of feedback in visual awareness. However, the retino-cortical transmission time and the suppression latency vary widely based on stimulus characteristics (White and Jeffreys, 1982; Musselwhite and Jeffreys, 1985; Hansen et al., 2016; Gebodh et al., 2017; de Graaf et al., 2014; Paulus et al., 1999). Such inferences are fraught with assumptions about the timing of visual signals in different cortical regions, which may or may not hold true across all studies (Center et al., 2019), since the retino-cortical transmission time and TMS suppression latency vary widely based on stimulus characteristics (White and Jeffreys, 1982; Musselwhite and Jeffreys, 1985; Hansen et al., 2016; Gebodh et al., 2017; de Graaf et al., 2014; Paulus et al., 1999). The suppression posed by visual masks or TMS may also interfere with feedforward activity or ongoing horizontal recurrent in the early visual cortex (Center et al., 2019). Thus, we are in need of new approaches to investigate the role of feedback activity in isolation.
In the current study, we sought to assess whether the involvement of early visual cortex in awareness is due purely to its role as a feedforward gateway into the cortex for visual information, or if it is also involved with later feedback processing stages as well. To this end, we bypassed early visual cortex as the region of visual input by administering single-pulse TMS over the posterior parietal cortex. Previous studies have shown that TMS to posterior parietal cortex can evoke brief and transient flashes of light in the visual field, referred to as phosphenes (Bagattini et al., 2015; Fried et al., 2011; Marzi et al., 2009; Mazzi et al., 2014, 2017a; Parks et al., 2015; Tapia et al., 2014; Bonfanti et al., 2024). Moreover, this posterior parietal stimulation results in subsequent activity in occipital cortex suggestive of feedback (Parks et al., 2015). By administering TMS to posterior parietal cortex, we were able to sidestep the normal visual pathway and eliminated early visual cortex’s role as an input gateway, while still generating conscious visual awareness. Using posterior parietal cortex as the input location also sets aside the need for assumptions about the timing of visual signals from the eyes to the brain, a timing that is necessary to distinguish between feedforward and feedback processing (Center et al., 2019). We accomplish this by stimulating the cortex directly, and not relying on the retinocortical pathway to deliver visual information to the brain.
To capture the cortical activity giving rise to visual awareness without loss of localization power due to electric field volume conduction, and without artifacts due to the application of the TMS pulse, in the current study we used the event-related optical signal (EROS; Gratton et al., 1995;2006; Gratton and Fabiani, 1998, 2003). EROS measures changes in the amount of near infrared (NIR) light scattering in active neural tissue, compared to a baseline. Specifically, light scattering is reduced when cells depolarize, and this reduces both the photons time-of-flight and light intensity, measurements that can then be used to infer neural activity. EROS is sensitive enough to capture neural activity in the millisecond range, similar to the temporal resolution of event-related brain potentials (ERPs). Because NIR light attenuates quickly from the point of input, EROS has a high spatial resolution, similar to functional magnetic resonance imaging (fMRI), albeit limited to outer cortical layers. Unlike EEG and fMRI, optical imaging is not impacted by the electrical and magnetic artifacts produced by TMS (Parks et al., 2012, 2015; Tse et al., 2018), because optical fibers are made of glass and therefore are not impacted by these artifacts. Moreover, previous work from our lab demonstrated that cortical activations caused by TMS to posterior parietal cortex result in early visual cortex activity that can be reliably measured by EROS (Parks et al., 2015).
Based on these previous studies, we recorded EROS from bilateral occipital cortex and left posterior parietal cortex while administering single-pulse TMS over the left posterior parietal cortex. The onset of the TMS pulse was time-locked to the optical recording. By combining TMS and EROS, we were able to investigate temporally and spatially the role of ongoing feedback to early visual cortex during visual awareness. We hypothesized that activity in early visual cortex, as well as activity under the TMS coil, would be related to awareness; that is, activity there will differ as a function of whether or not the participant experiences phosphenes. We note that we are investigating the role of feedback activity as a component of visual awareness rather than as a generator of awareness.
2. Methods
2.1. Participants
Seventy-seven participants were tested during an initial evaluation session, of whom 12 (8 females, mean age = 21.75, SD = 2.8) passed all criteria (see TMS procedures below) and completed all experimental sessions (this low ratio of participants passing the TMS criteria is typical of experiments requiring the elicitation of visual phosphenes, Center et al., 2019). Participants were recruited from the University of Illinois at Urbana-Champaign community through an online newsletter and were screened for TMS suitability based on the guidelines listed in Rossi et al. (2009). All participants reported having no history of neurological or psychiatric disorder and had normal or corrected-to-normal vision. All participants provided written informed consent and were compensated $15 per hour for their time. The protocol was approved by the Institutional Review Board of the University of Illinois.
2.2. Transcranial magnetic stimulation (TMS) pilot session
Single-pulse TMS was delivered using a Magstim 220 Rapid bi-phasic stimulator with a 70-mm figure-8 coil (Magstim Company Limited, Whitland, UK). During the initial evaluation session, participants wore a cap containing the International 10–20 EEG locations for electrodes Cz, Oz, O1, O2, Pz, P3, and P4 (Herwig et al., 2003). With the TMS handle oriented rostro-lateral from the inion, the center of the wand was placed against the scalp over O1, corresponding to the left visual cortex. While viewing a mid-grey uniform background, participants fixated on the central target and a single TMS pulse was delivered at 65% of the maximum stimulator output (MSO). After each pulse, participants were instructed to give a verbal yes/no response regarding the detection of a phosphene. With each “no” response, the coil was moved to a slightly different location and/or angle within 2 cm of O1, until reliable phosphenes were reported. The procedure was repeated over O2 and P3. Participants who reliably reported having experienced phosphenes over P3 were asked to return for the subsequent experimental sessions. Phosphenes were deemed reliable if 1) they occurred in the contralateral visual field to the site of stimulation, 2) were perceived with eyes both open and closed, and 3) their location followed the eyes’ gaze (Kammer, 1999). The session lasted approximately 30–60 min. Fourteen participants reported seeing no phosphenes and 34 reported seeing only occipital phosphenes. Of the remaining 29 who reported seeing parietal phosphenes, 12 agreed to return for the subsequent MRI and TMS-EROS experimental sessions.
2.3. Structural MRI scanning
A high-resolution structural 3D MPRAGE (192 slices, 0.9x0.9 × 0.9 mm voxel size; TR, 1.9 s; TE, 2.32 ms, flip angle, 9°) in the coronal plane was acquired for each participant passing the TMS criteria (N = 12) with a 3T Siemens Trio scanner and 64-channel head coil. Structural images were used for localization and co-registration of both participants’ TMS stimulation site and EROS optical source and detector scalp locations.
2.4. Event-related optical signal (EROS) recordings
EROS data were acquired using four synchronized frequency-domain Imagent oximeters (ISS Inc., Champaign, IL). Thirteen diodes, emitting near-infrared light at 830 nm, coupled with 400-μm silica fibers, were used as sources. Eleven sources were held in place orthogonally over the occipital cortex using a custom-made soft foam helmet (Fig. 1). A custom-made thin rubber patch was affixed to the helmet under the location of the TMS coil and held the remaining two sources. Twenty 3-mm fiber-optic bundles connected to photomultiplier tubes (detectors) were used to capture the light exiting the head. Sixteen detectors were mounted orthogonally to the scalp on the helmet to record occipital activity, and four were mounted parallel to the scalp on the rubber patch to accommodate the TMS coil and record parietal activity from beneath the coil. A prism was attached to each of the patch-mounted detectors to enable light capture under the TMS coil, allowing for it to be positioned very close to the head surface (Parks et al., 2012, Fig. 1(A) and (B)) Sources were modulated at 110 MHz and detectors at 110.003125 MHz, creating a cross-correlation (heterodyning) frequency of 3125 Hz.
Fig. 1.
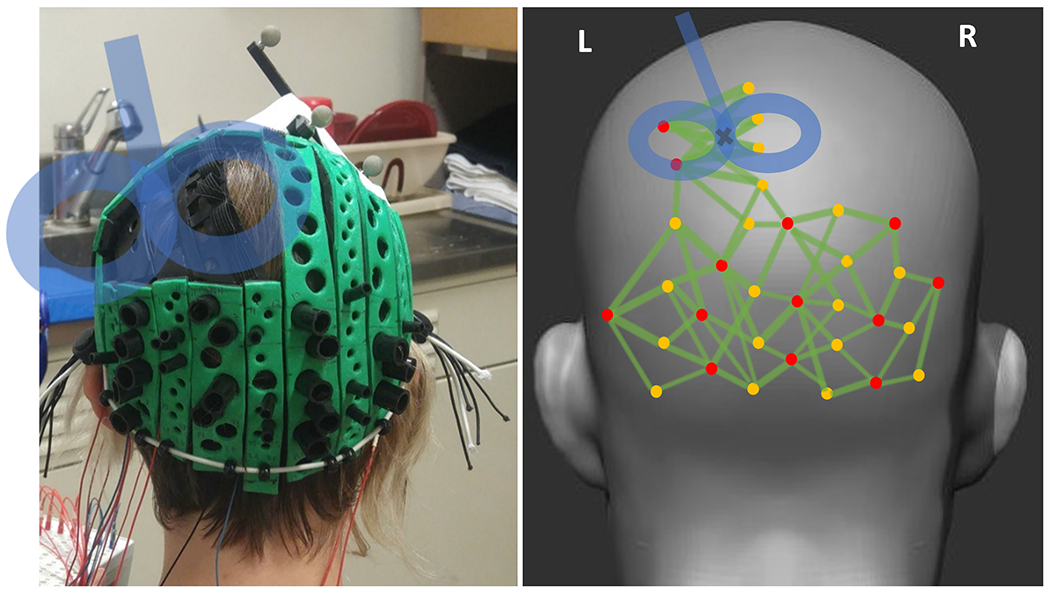
TMS-EROS helmet before fibers were inserted. A custom-made thin rubber patch was affixed to the helmet over left parietal cortex. Sixteen detectors (large black holders in the left image and yellow dots in the right image) and eleven sources (small black holders in the left image and red dots in the right image) were used to record from the occipital cortex. Four detectors (yellow) and two sources (red) were mounted on the rubber patch parallel to the surface, to allow for the TMS coil to be sufficiently close to the scalp while recording parietal activity underneath the coil. The right figure shows the channels (green lines) formed by pairs of detectors (yellow) and sources(red).
A montage of 160 source-detector pairs (channels) was used to capture the relative phase delay data at a sampling rate of 78.125 Hz, equal to intervals of 12.8 ms. We will refer to the phase delay data as activity, as it reflects the average post-TMS increase of time (delay) in picoseconds needed for photons to travel from the sources to the detectors, via the cortex, when compared to the average pre-TMS baseline (Gratton et al., 2006; Gratton et al., 2000). The montages and their relative position with respect to the head and the location of the TMS coil in a representative participant are shown in Fig. 1.
To avoid crosstalk between the optical channels, sources were positioned so that no two sources were simultaneously active within the same cortical hemisphere, nor within the range of any one detector (75 mm) at the same time (Mathewson et al., 2014). Sources were also time multiplexed so that each detector could pick up 8 different sources at different times within a multiplexing cycle. Channel lengths shorter than 25 mm (measuring shallow depths) were excluded due to their low likelihood of reaching the cortex; channels longer than 50 mm (measuring greater depths) were excluded due to the low number of photons detected, and therefore their high likelihood of having an S/N too low to yield useful data (Gratton et al., 2006).
Immediately after the EROS session, source and detector locations were digitized using a Polhemus 3Space Fastrak (Colchester, VT) for five of the 12 participants. These locations were taken for each participant and were co-registered to the participant’s structural MR images using the nasion and preauricular points as fiducials (Whalen et al., 2008). Because of the experiment time limit set by the IRB protocol, the remaining seven participants did not complete the digitization. Instead, for them, we used a lab template obtained from other participants The co-registered source and detector locations were MNI-transformed to allow registration across all participants (Chiarelli et al., 2015).
2.5. TMS/EROS experimental session
A mid-grey uniform background with a dark blue central fixation cross was presented on a 17-inch cathode ray tube (CRT) monitor (Gateway VX 920, 60 Hz, 1280 x 1024 resolution). The visual display was generated using MATLAB and Psychophysics Toolbox (Brainard and Vision, 1997). Participants were seated approximately 90 cm from the monitor in a dark room with their head situated on a head and chin rest. Disposable ear plugs were worn for protection against the auditory noise created by each TMS pulse (Rossi et al., 2009).
We applied Brainsight neuronavigation software (Rogue Research, Montreal, Canada) and a Polaris Vicra 3D optical infrared camera digitizer (NDI, Waterloo, Canada) to guide the manual placement of the TMS coil and stimulation and to ensure the consistency of the location and angle of the coil throughout the session. Specifically, the coil was placed over the posterior parietal cortex near the P3 EEG electrode location and held by the experimenter throughout the session. The neuronavigator was used to confirm that the coil’s location and angle did not move from block to block. The camera was uncovered at the end of each block to make the measurements, and then covered again before the next experimental block began. The wavelength (635 nm) of the Polaris Vicra interfered with the fast optical image recording (described later) and thus it was covered with a dark absorbing fabric during recording.
Participants’ individual phosphene thresholds (PT) were measured using the method of constant stimulation (MOCS) whereby 64 self-administered pulses ranging from 57 to 78% MSO, in steps of 3%, were administered in a randomized order (Mazzi et al., 2017b). After each pulse, participants responded yes or no to having experienced a phosphene. Results were fitted to a psychometric function calculated using Palamedes Toolbox (www.palamedestoolbox.org) and the TMS intensity yielding 50% yes-no responses was used for the remainder of the experiment.
After PT was determined, participants completed 240–600 trials (Mean = 318.08 SD = 134.47) divided into blocks of 40 trials. The variation in trial numbers was due to a limited experiment session time set by the IRB protocol. Subjects who required more time for the experimental setup completed fewer trials as a result. Trials began with a random delay (800–1400 ms) followed by a single-TMS pulse. Participants were instructed to fixate on a central target throughout the entire trial and report phosphene perception via keypress (arrow keys on keyboard) using a graded scale: 1 (right arrow) = “yes”, 2 (up arrow) = “maybe yes”, 3 (down arrow) = “maybe no”, 4 (left arrow) = “no”. Twenty catch trials were administered throughout the session (4 trials every 3 blocks) to gauge false-positive responses. During the catch trials, the stimulator was surreptitiously lowered 20 units below the threshold intensity, an intensity that should not elicit phosphene percepts.
2.6. Regions of interest
We were primarily interested in three regions of interest (ROIs): one placed directly under the coil, which was defined as the two sources and four detectors that were mounted to the rubber patch under the coil; and two in occipital areas, V1 and V2, which were defined using BA17 and BA18 from an NiFTI file in MNI space, with Brodmann areas marked by appropriate numbers (separately for the left and right hemisphere, Maldjian et al., 2003). In addition, we conducted post-hoc analyses on two additional ROIs showing differences between the phosphene-present and phosphene-absent conditions (See Table 1 for coordinates): the anteromedial cuneus and the lateral occipital cortex (including V2 and V3). These ROIs were not corrected for multiple comparisons and thus were only reported for exploratory purposes.
Table 1.
ROI coordinates used in this study. The coordinates of V1, V2, Lateral Occipital lobe are determined with a probabilistic atlas (Maldjian et al., 2003).
| ROI | X | Y | Z |
|---|---|---|---|
| Anteromedial Cuneus | [−20, 20] | [−100, −70] | [15, 45] |
| Left Posterior Parietal Cortex | [−55, −35] | [−70, −50] | [50, 70] |
2.7. Preprocessing and data analysis
The relative phase delay of modulated light measured by the detectors served as our dependent variable. Data were preprocessed using in-house MATLAB package P-POD (Pre-Processing of Optical Data). Recordings were corrected for phase wrapping, pulse artifacts (Gratton and Corballis, 1995), and amplitudes were normalized (within each block of trials). No band-pass filter was used in the analyses. The data were 3-D reconstructed using an approach based on the physics of light diffusion in highly scattering media described by (Feng et al., 1995) adapted for phase delay data. A model assuming homogenous absorption and scattering characteristics of the tissue was used for the 3D reconstruction. The signal was segmented into 1000 ms epochs spanning 500 ms prior and 500 ms after the TMS pulse. The average phase delay during the first 230 ms of the epoch was used for baseline correction. In addition, a 6-mm Gaussian spatial filter was applied for spatial smoothing.
Statistical analyses for each ROI were performed by first calculating a bootstrapped distribution of the grand average values using 1000 samples, each formed by an average of 12 individual subject values, obtained by randomly sampling the individual subject distribution with replacement. The 1000 bootstrapped values were rank-ordered, and the probability of the true grand average value was obtained by comparing its value with this “bootstrapped” distribution. A z-score corresponding to this probability was also computed. This bootstrapping procedure allowed us to avoid statistical assumptions that may not be valid given our smaller sample size (N = 11 or 12, depending on the ROI (i.e., for the parietal ROI, one subject was removed because of insufficient light detected). For statistical purposes, responses were grouped into two trial types: 1) phosphene-present trials containing the “yes” responses and 2) phosphene-absent trials containing the “no” responses. The trials with “maybe yes” and “maybe no” responses were excluded to maximize the subjective difference between the percept for phosphene-present and phosphene-absent trials. Statistical parametric maps were orthogonally projected in the coronal and axial plane in MNI space. Differences in peak voxel z scores were considered statistically significant at p < 0.05 after a Bonferroni correction for multiple comparisons (i.e. number of voxels per ROI).
3. Results
On average, during the experimental session, participants reported “clearly seeing” phosphenes (i.e., responded “yes”) on 33.3% (number of trials: Mean = 148.42 SD = 136.76) of the trials and “clearly not seeing” phosphenes (i.e., responded “no”) on 38.2% (number of trials: Mean = 169.67 SD = 103.71) of the trials. Phosphene-present reports varied from 9% to 84% across subjects (Fig. 2). The response latency for phosphene-absent conditions (Mean = 1.19 s SD = 0.62 s) was longer than that for phosphene-present conditions (Mean = 1.13 s SD = 0.57 s), in line with previous research (Mazzi C et al., 2014; Bagattini, C et al., 2015; Bonfanti et al., 2024). The response latency for the “maybe” conditions (Mean = 1.51 s SD = 0.30 s) was longer than when subjects were more confident about their responses (Mean = 1.02 s SD = 0.31 s). Maps of the EROS activity at various latencies are presented in Figs. 3–5. Fig. 3 shows group-level bootstrap Z score activity for phosphene-present versus phosphene-absent trials on a coronal Y = −60 slice. Fig. 4 shows group-level bootstrap Z score activity for phosphene-present versus phosphene-absent trials on axial (Z = 0, 5, 10, 15) slices, with V1 and V2 identified by a probabilistic atlas (Maldjian et al., 2003). Fig. 5 shows the coronal surface projection of the group-level bootstrap Z score activity for phosphene-present versus phosphene-absent trials in a 243 ms time window after the TMS pulse.
Fig. 2.
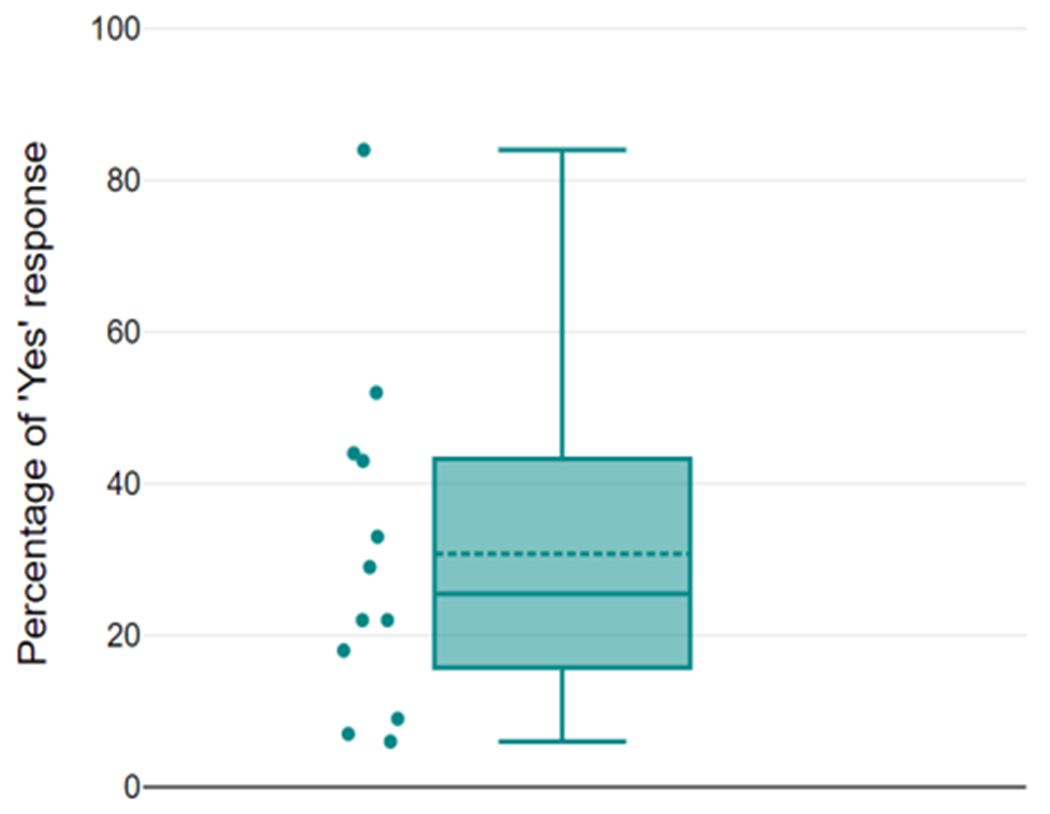
Distribution of each participant’s detection rate (N = 12). Individual points plotted on the left. In the boxplot, the upper and lower whiskers refer to the maximum and minimum score. The upper and lower boundaries of the box refer to the upper and lower quartiles, respectively, and the horizontal solid line through the box is the median (25.5).The horizontal dotted line is the mean (30.75).
Fig. 3.
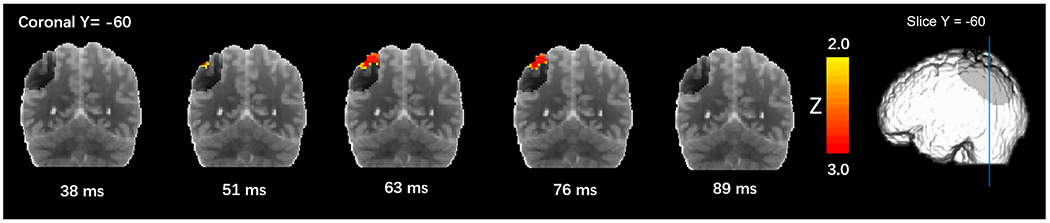
Coronal slice (Y = −60) showing group-level bootstrap Z score activity for phosphene-present versus phosphene-absent trials. Darker grey shading in each slice indicates the regions accessible to measurement, given the placement of sources and detectors.
Fig. 5.
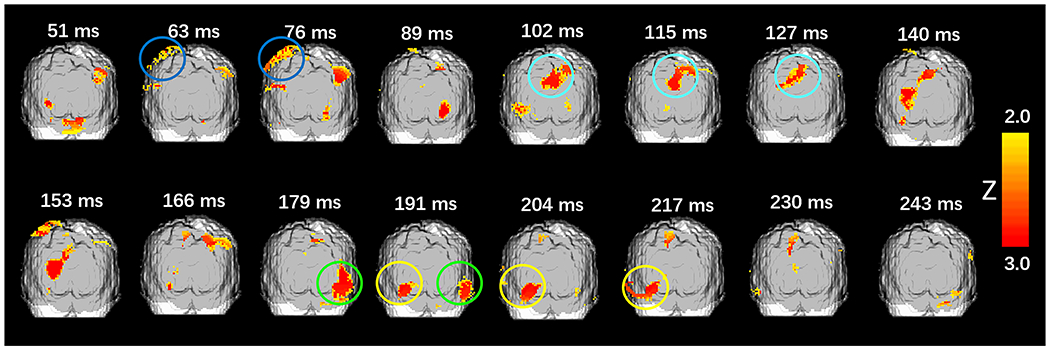
Coronal surface projections of group-level bootstrap Z score activity after TMS. Regions marked by circles: Posterior Parietal Cortex (Dark Blue); Anteromedial cuneus (Cyan); Lateral Occipital Cortex (Green, right and yellow, left). Darker grey shading in each slice indicates the regions accessible to measurement, given the placement of sources and detectors.
Fig. 4.
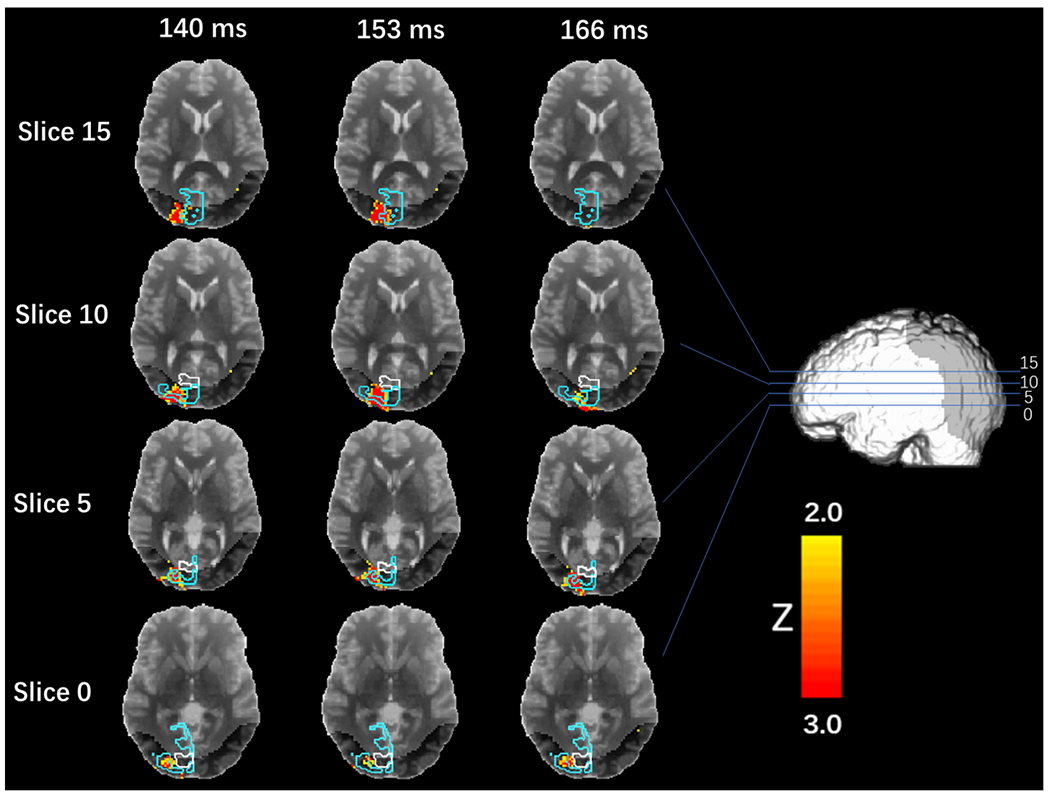
Axial slices (Z = 0, 5, 10, 15) showing group-level bootstrap Z score activity for phosphene-present versus phosphene-absent trials. ROIs: BA17 V1 (white) and BA18 V2 (Blue). Darker grey shading in each slice indicates the regions accessible to measurement, given the placement of sources and detectors.
3.1. Parietal ROI: comparison between phosphene-present versus phosphene-absent trials
We first examined the optical signal in the left posterior parietal cortex, the ROI directly underneath the TMS coil, and assessed by the optical imaging patch. For one participant, there was insufficient light reaching the detector under the patch and thus the patch data for this subject were removed from the parietal ROI analysis. This participant’s data from the remaining montage, however, were acceptable and so they were included in the analyses described below. For the parietal patch data (N = 11), a significant difference in activity between phosphene-present versus phosphene-absent trials was observed at 51 ms (Z = 3.070, Zcrit = 2.24), 63 ms (Z = 3.070, Zcrit = 2.24) and 76 ms (Z = 3.070, Zcrit = 2.24) after the TMS pulse (Fig. 3). These data suggest that greater and more sustained neural activity at the site of stimulation is related to phosphene awareness (see Table 2).
Table 2.
Z scores for phosphene-present versus phosphene-absent tests. p <.05 corrected for multiple correction.
| Slice | Region | 140 ms | 153 ms | 166 ms |
|---|---|---|---|---|
| Axial 0 | V1 | 2.048 | 1.873 | **2.891 |
| V2 | 2.987 | 2.987 | 2.987 | |
| Axial 5 | V1 | **2.987 | **2.987 | **2.987 |
| V2 | **3.143 | **3.143 | **3.143 | |
| Axial 10 | V1 | 1.760 | **2.648 | 1.792 |
| V2 | **3.143 | **3.143 | **3.070 | |
| Axial 15 | V2 | **3.143 | **3.143 | 2.087 |
p < 0.05
3.2. Occipital ROI: comparison of phosphene-present versus phosphene-absent trials
We next analyzed data from the occipital channels as a function of phosphene presence versus absence. Analysis of this contrast indicated a significant difference in activations in ipsilateral occipital cortex beginning at 140 ms (Z = 3.143, Zcrit = 3.012) after the TMS pulse (see Fig. 4). To further localize the effect, we plotted different axial slice projections within BA17 (V1) and BA18 (V2) ROIs with white and cyan outlines, respectively. The majority of voxels showing a significant phosphene-present versus -absent difference fell in V2 (cyan) with so few voxels in V1 (white) and given the limitations of registration, we cannot confidentially conclude V1 was activated. The two-tailed z test indicated a similar result (see Table 2).
In addition to these planned analyses, we also observed significant activation in the anteromedial region of the cuneus, occurring temporally between the effect in the parietal cortex and primary visual cortex at 102 ms (Z = 3.070), 115 ms (Z = 3.070) and127 ms (Z = 3.070) after the TMS pulse, respectively (Fig. 5, cyan). In addition, we observed a bilateral activation of lateral occipital cortex (Fig. 5, circled in green and yellow in a later temporal window at 179 ms (Right LO: Z = 3.143), 191 ms (Left LO: Z = 3.070, Right LO: Z = 3.070), 204 ms (Left LO: Z = 3.143), and 217 ms (Left LO: Z = 3.143). These findings were not predicted, so their validity needs to be confirmed in future research.
4. Discussion
The aim of the current study was to assess the role of early visual cortex during visual awareness; that is, is early visual cortex’s role in awareness confined to its role as the input to subsequent areas (hierarchical models of awareness) or must it receive feedback from anterior regions (interactive models of awareness)? We applied single-pulse TMS to left posterior parietal cortex to induce a phosphene and measured the underlying cortical activity using fast optical imaging. The combination of TMS and EROS allowed us to bypass direct visual input to the occipital cortex, and to record directly under the TMS coil without artifacts, making it possible to bypass initial feedforward activity from early visual cortex and assess the role of feedback to early visual cortex in isolation. TMS intensity was set at each participant’s individual phosphene threshold, allowing us to compare trials in which participants reported a phosphene to those in which they did not. Under these conditions, we found that activity in both parietal cortex and V2 was associated with awareness. Specifically, activity underneath the coil in the left posterior parietal cortex was related to phosphene perception 51–76 ms following the TMS pulse, while activity in V2 (BA18) was related to phosphene perception in a later time window, between 140 and 166 ms after the pulse. Differential activity in posterior parietal areas as a function of awareness is in line with previous evidence showing that the intraparietal sulcus can be an independent generator of conscious experience (Bagattini et al., 2015). Moreover, these results support previous findings (Parks et al., 2015) showing that TMS pulses delivered to the posterior parietal cortex can induce activity in occipital cortex, and further suggest that the strength of the occipital activity predicts awareness. The role of this activity in awareness could not be determined in the Parks et al. (2015) study because the phosphene-present and phosphene absent trials came from different stimulation sites and thus it is unclear whether the amount of activity in occipital cortex is related to phosphene experiences.
In addition to parietal activity, we also observed significant activations in early visual cortex in a later time window (140–166 ms post-TMS). Although there were some active voxels that appear to be localized to V1, the vast majority were contained within V2. Even for these few voxels, their assignment to V1 is not definitive because of the limitation in spatial resolution – approximately 5 mm – of the optical imaging technique and the uncertainty of co-registration. Because activity originated in parietal cortex rather than in V1, the early visual cortex activity must be a result of trans-synaptic stimulation, which was either orthodromic and/or antidromic in nature. Whether or not the activity reaches early visual cortex via normal feedback projections (orthodromic) or traveled backwards on feedforward connections (antidromic), the late activation associated with phosphene perception is consistent with interactive models (Tong, 2003) in which awareness depends on later recurrent activity.
Interestingly, in contrast to interactive models of awareness that specifically predict feedback to V1 (Breitmeyer, 2007; Di Lollo et al., 2000; Enns and Di Lollo, 2000; Fahrenfort et al., 2007, 2008; Lamme, 2000, 2006; Lamme and Roelfsema, 2000; Pascual-Leone and Walsh, 2001; Silvanto et al., 2005; Silvanto and Pascual-Leone, 2008; Tapia and Beck, 2014; Tong, 2003), the feedback activity we observed was more obvious in V2. However, EROS has limited penetration (on the order of 30 mm), and thus it may not be able to probe the most peripheral/deepest regions of V1. Moreover, our localization of the activity is approximate, and our atlas is probabilistic. Despite these limitations, we did observe some activity that could be localized to V1, at least with a probabilistic atlas. Even if one questions whether the effect includes V1, a null effect cannot definitively rule out the possibility that activity in V1 is related to phosphene perception. In short, our data suggest that the stimulation of parietal cortex results in feedback activity to early visual cortex that is related to awareness but leave open the question as to which early visual areas play a role in awareness.
It should also be noted that neither the visual masking nor TMS-disruption, cited in support of interactive models of awareness, can unequivocally tie their results to V1. As mentioned earlier, visual masking studies (Breitmeyer, 2007; Di Lollo et al., 2000; Enns and Di Lollo, 2000; Fahrenfort et al., 2007, 2008) use delayed masking to infer feedback to V1. Even if such masking reflects a disruption of feedback processes, this conclusion is not without controversy (Center et al., 2019), the relative timing of signals to V1 versus V2, estimated to be 14 ms in humans (Hagler, 2014), would be extremely difficult to resolve behaviorally. In other words, it is possible that the critical source of re-entrant activity responsible for visual masking could be V2. TMS suppression studies also cannot guarantee a V1 source. (Pascual-Leone and Walsh, 2001; Silvanto et al., 2005; Silvanto et al., 2008; Silvanto et al., 2005). First, due to anatomical variations across individuals, V1 is not always accessible to TMS from the scalp (Stokes et al., 2005; Stensaas et al., 1974). Second, due to TMS-suppression protocols that include small changes in coil position in order to empirically maximize suppression, coupled with the spread of the electrical field induced by TMS (Jalinous, 1991; Miranda et al., 2003), TMS-suppression has been localized, when assessed, to V1, V2, and even V3 (Center et al., 2019; Kammer et al., 2005; McKeefry et al., 2009; Thielscher et al., 2010; Salminen-Vaparanta et al., 2012). Another possibility is that the TMS suppression targeted at V1 may feedforward to V2, which, in turn, interrupts the recurrence and causes the absence of perception. In other words, even if TMS initially stimulated V1, the critical interference may still arise in V2.
Putting limitation in measuring V1 activity aside, our observation of activation of V2 rather than V1 associated with awareness is consistent with reports of awareness in patients without V1 (Ffytche and Zeki, 2011; Mazzi et al., 2014, 2019a). As we did here, Mazzi et al. (2014) applied single-pulse TMS over the posterior parietal cortex of two hemianopic patients. Despite complete destruction of either left or right V1, both patients showed similar sensitivity to phosphenes in their blind visual field as healthy participants, suggesting that feedback to V1 is not necessary for phosphene awareness. It is possible that feedback to V2, as was shown here, or other visual areas played a role in phosphene perception. Watanabe et al. (2011) argued that earlier fMRI reports of V1 activity associated with awareness (Polonsky et al., 2000; Tong and Engel, 2001; Maier et al., 2008) stem from an attention confound rather than a critical role in awareness.
As mentioned, our limited effect in V1, like that of Watanabe et al. (2011), does not rule out the possibility that more sensitive methods may reveal that activity in V1 is related to awareness. The results with hemianopic patients, however, do suggest that V1 activity is not strictly necessary. Instead, taking all the data together, it might make more sense to consider the entire early visual cortex (V1–V3) as critically involved in awareness, with activity in a subset of these areas being sufficient (along with frontoparietal activity) for awareness.
In exploratory analyses we also observed a significant activation of the anteromedial region of the cuneus at an intermediate latency between the parietal (under the TMS coil) and occipital regions. Interestingly, a previous MEG study investigating the cortical response to visual stimulation localized early visual activity simultaneously to this anteromedial region of the cuneus and V1 50 ms after stimulus onset and before the signal transfers to extrastriate cortices (Vanni et al., 2001). These authors argue that this temporally positions the cuneus to potentially interact with V1 and thereby modulate the activity arriving in V2 (Vanni et al., 2001). Recent tractography, however, shows extensive connections within the cuneus, including between this anteromedial region and regions that contain V1 and V2 (Palejwala et al., 2021), leaving open the possibility of a direct pathway from the anteromedial cuneus to V2.
This is not the first time this anteromedial region of the cuneus has been implicated in awareness. Mathewson et al. (2014) located the source of the posterior alpha EEG signal that predicted awareness in a metacontrast paradigm to a similar region. However, in their case the activity was not only oscillatory, but it preceded the target stimulus.
In a later temporal window from 179 ms to 217 ms, we observed a bilateral activation of lateral occipital (LO) cortex (Fig. 4). Previous MEG and low-resolution electromagnetic tomography (LORETA) studies have linked a late LO activity in 200–300 ms range with the visual awareness negativity (VAN) component of the ERP (Koivisto and Revonsuo, 2010; Liu et al., 2012). Among the candidate electrophysiological correlates of visual awareness, including P1, VAN and late positivity (LP), VAN most consistently emerges across different manipulations of visual awareness (Koivisto and Revonsuo, 2010; Wilenius-Emet et al., 2004; Tagliabue et al., 2016; Mazzi et al., 2019b, 2020). In our case, the LO activation starts earlier (179ms) than the VAN timing measured relative to visual stimulus. However, such timing is reasonable given that TMS over the parietal cortex bypasses retina-cortical transmission delay (Samaha et al., 2017). We note that, unlike the ipsilateral effect in the early visual cortex and parietal cortex, the effect in LO is robust in both hemispheres. Such symmetry is also reported by Koivisto and Revonsuo (2010) when targets appeared on the right visual field, which is consistent with the phosphene location in our study. The bilateral activation is also not surprising given that the receptive fields of neurons in LO are both large and bilateral, indicating that both the right and left LO must receive input from both the ipsilateral and contralateral hemispheres (Decramer et al., 2019). Given the late timing of this activity, however, it is unclear whether it is the result of feedforward activation from V2 or feedback from the parietal cortex.
It is important to note that our contrastive design does not allow us to definitively distinguish those processes directly contributing to consciousness (NCC proper: neural correlates of consciousness proper) from the pre-requisites (enabling consciousness but not directly contributing to the content of experience) and the consequences of consciousness (Aru et al., 2012; De Graaf et al., 2012). The consequences of awareness are particularly difficult to eliminate in designs such as ours where we must rely on participants overt responses to understand their states of consciousness. Researchers have utilized no-report paradigms to minimize the contribution of report-related activity or other task-related activity such as selective attention, expectation, self-monitoring, unconscious stimulus processing, or task planning (Tsuchiya, et al., 2015; Koch et al., 2016). All such processes could impact activity in our paradigm. However, the timing of our activity may provide some clues.
First, given the early onset (<140ms) compared to the average response time in the phosphene-present condition (1.13 s) and the localization of our observed activity to the posterior parietal (50 ms) and early visual cortex (140 ms), we believe it is less likely that this activity is response-related.
Second, as noted above, the bilateral activation of the LOC at 180 ms after stimulation most likely corresponds to the VAN ERP component. Across a variety of paradigms, the VAN has emerged as the most likely candidate for NCC proper (i.e. reflects the emergence of awareness; Mazzi et al., 2020; Koivisto and Revonsuo, 2008a; Koivisto et al., 2009; Koivisto and Revonsuo, 2007). If we consider the activation in LOC as indicative of the NCC, then the preceding activation in PPC (~60 ms), cuneus (~100 ms), and early visual cortex (~140 ms) may serve as the prerequisite for visual awareness. Such prerequisites may reflect, for example, a sufficient level of excitation in the parietal-occipital network to enable the processes necessary to generate awareness. Indeed, the excitability of these regions fluctuates periodically, as marked by prestimulus neural oscillations, and those fluctuations have, in turn, impacted the likelihood of awareness (Mathewson et al., 2014; Samaha et al., 2017; Benwell C et al., 2017; Tafuro A et al., 2023; Dugué et al., 2011; Romei et al., 2008).
Although we ran 77 participants, because of individual differences in the ease of parietal phosphene generation, our final sample (N = 12) was relatively small. Of the 77 subjects participating in the pilot session, only 29 were able to reliably report seeing parietal phosphenes. Out of these, 12 subjects agreed to return for subsequent experimental sessions. Similar difficulties have been encountered with other studies on parietal phosphenes (Bagattini et al., 2015; Marzi et al., 2009; Fried et al., 2011). Despite this limitation, we observed statistically reliable effects in occipital cortex that were associated with awareness. Our study addresses the critical research question of the importance of feedback activity in visual awareness, which requires a clear identification of feedback activity. This has been challenging for previous studies due to the difficulties of distinguishing feedback from feedforward activity because they overlap spatially and are temporally difficult to disambiguate. Our approach of eliciting parietal phosphene enabled us to examine feedback activity in isolation, since visual input from V1 was absent. This approach relies on the few people who can consistently detect parietal phosphene, in a way similar to working with patients.
In conclusion, this study adds to a growing body of work investigating interactions between frontoparietal cortex and occipitotemporal cortex in giving rise to awareness (Baars, 2005; Beck et al., 2001; Crick and Koch, 2003; Dehaene et al., 2006; Dehaene and Changeux, 2011; Driver and Vuilleumier, 2001; Tononi and Koch, 2008). We observed that activity in both posterior parietal cortex and extrastriate cortex (V2) is related to the presence of experienced phosphenes. Importantly in this case, activity originated in posterior parietal cortex and was observed approximately 140–166 ms later in V2 cortex, consistent with theories of awareness that state that feedback to early visual cortex is necessary for awareness (Breitmeyer, 2007; Di Lollo et al., 2000; Enns and Di Lollo, 2000; Fahrenfort et al., 2007, 2008; Lamme, 2000, 2006; Lamme and Roelfsema, 2000; Pascual-Leone and Walsh, 2001; Silvanto et al., 2005; Silvanto and Pascual-Leone, 2008; Tapia and Beck, 2014; Tong, 2003). Of course, our data are also consistent with any interactive theory that posits that awareness is the result of an interaction among areas whether or not those interactions include any particular gatekeeper of awareness (Mazzi et al., 2017b; Mazzi et al., 2019a; Silvanto, 2015). Importantly, by combining both TMS and EROS in the same experiment we are able to observe those interactions in both a spatially and temporally resolved way.
Acknowledgments
Drs. Kathy Low, Ed Maclin, Brian Metzger, and Daniel Bowie helped with the experimental setup or data analysis.
Funding sources
This work was supported in part by the National Eye Institute of the National Institutes of Health (Grant R01EY022605-01) to Diane M Beck, Gabriele Gratton, and Monica Fabiani. We have no conflict of interest to declare.
Footnotes
CRediT authorship contribution statement
Ramisha S. Knight: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Conceptualization, Visualization, Writing – review & editing. Tao Chen: Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. Evan G. Center: Conceptualization, Resources, Writing – review & editing. Gabriele Gratton: Conceptualization, Formal analysis, Methodology, Software, Supervision, Writing – review & editing, Funding acquisition. Monica Fabiani: Funding acquisition, Methodology, Resources, Software, Supervision, Writing – review & editing, Conceptualization. Silvia Savazzi: Conceptualization, Writing – review & editing. Chiara Mazzi: Conceptualization, Writing – review & editing. Diane M. Beck: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Software, Supervision, Writing – original draft, Writing – review & editing.
Data availability
Data will be made available on request.
References
- Aru J, Bachmann T, Singer W, Melloni L, 2012. Distilling the neural correlates of consciousness. Neurosci. Biobehav. Rev 36 (2), 737–746. 10.1016/j.neubiorev.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Baars BJ, 2005. Global workspace theory of consciousness: toward a cognitive neuroscience of human experience. Prog. Brain Res 150, 45–53. 10.1016/S0079-6123(05)50004-9. [DOI] [PubMed] [Google Scholar]
- Bagattini C, Mazzi C, Savazzi S, 2015. Waves of awareness for occipital and parietal phosphenes perception. Neuropsychologia 70, 114–125. 10.1016/j-neuropsychologia.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Beck D, Rees G, Frith C, et al. 2001. Neural correlates of change detection and change blindness. Nat. Neurosci 4, 645–650. 10.1038/88477. [DOI] [PubMed] [Google Scholar]
- Benwell C, Tagliabue C, Veniero D, Cecere R, Savazzi S, Thut G, 2017. Prestimulus EEG power predicts conscious awareness but not objective visual performance. eNeuro 4 (6), 1–17. 10.1523/ENEURO.0182-17.2017 e0182-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti D, Mazzi C, Savazzi S, 2024. Mapping the routes of perception: hemispheric asymmetries in signal propagation dynamics. Psychophysiology, e14529. 10.1111/psyp.14529. [DOI] [PubMed] [Google Scholar]
- Brainard DH, Vision S, 1997. The psychophysics toolbox. Spatial Vis. 10 (4), 433–436. 10.1163/156856897x00357. [DOI] [PubMed] [Google Scholar]
- Breitmeyer B, 2007. Visual masking: past accomplishments, present status, future developments. Adv. Cognit. Psychol 3 (1–2), 9–20. 10.2478/V10053-008-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavézian C, Gaudry I, Coubard O, Doucet G, Peyrin C, Marendaz C, Obadia M, Gout O, Chokron S, 2010. Specific impairments in visual processing following lesion side in hemianopic patients. Cortex 46 (9), 1123–1131. 10.1016/j.cortex.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Center EG, Knight R, Fabiani M, Gratton G, Beck DM, 2019. Examining the role of feedback in TMS-induced visual suppression: a cautionary tale. Conscious. Cognit 75 (July), 102805 10.1016/j.concog.2019.102805. [DOI] [PubMed] [Google Scholar]
- Chiarelli AM, Maclin EL, Low KA, Fabiani M, Gratton G, 2015. Comparison of procedures for co-registering scalp-recording locations to anatomical magnetic resonance images. J. Biomed. Opt 20 (1), 016009 10.1117/l.jbo.20.1.016009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F, Koch C, 1995. Are we aware of the neural activity in visual cortex? Nature 375 (6527), 121–123. 10.1038/375121a0. [DOI] [PubMed] [Google Scholar]
- Crick F, Koch C, 2003. A framework for consciousness. Nature Neurosci. 6 (2), 119–126. [DOI] [PubMed] [Google Scholar]
- Decramer T, Premereur E, Uytterhoeven M, Van Paesschen W, van Loon J, Janssen P, Theys T, 2019. Single-cell selectivity and functional architecture of human lateral occipital complex. PLoS Biol. 17 (9), e3000280 10.1371/journal.pbio.3000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, 2011. Experimental and theoretical approaches to conscious processing. Neuron 70 (2), 200–227. 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C, 2006. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cognit. Sci 10 (5), 204–211. 10.1016/j.tics.2006.03.007 [DOI] [PubMed] [Google Scholar]
- De Graaf TA, Goebel R, Sack AT, 2012. Feedforward and quick recurrent processes in early visual cortex revealed by TMS? Neuroimage 61 (3), 651–659. 10.1016/j.neuroimage.2011.10.020. [DOI] [PubMed] [Google Scholar]
- De Graaf TA, Hsieh PJ, Sack AT, 2012. The ‘correlates’ in neural correlates of consciousness. Neurosci. Biobehav. Rev 36 (1), 191–197. 10.1016/j.neubiorev.2011.05.012. [DOI] [PubMed] [Google Scholar]
- de Graaf TA, Koivisto M, Jacobs C, Sack AT, 2014. The chronometry of visual perception: review of occipital TMS masking studies. Neurosci. Biobehav. Rev 45, 295–304. 10.1016/j.neubiorev.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Enns JT, Rensink RA, 2000. Competition for consciousness among visual events: the psychophysics of reentrant visual processes. J. Exp. Psychol. Gen 129 (4), 481–507. 10.1037/0096-3445.129.4.481. [DOI] [PubMed] [Google Scholar]
- Driver J, Vuilleumier P, 2001. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition 79 (1–2), 39–88. 10.1016/S0010-0277(00)00124-4. [DOI] [PubMed] [Google Scholar]
- Dugué L, Marque P, VanRullen R, 2011. The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. J. Neurosci 31 (33), 11889–11893. 10.1523/JNEUROSCI.1161-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns JT, Di Lollo V, 2000. What’s new in visual masking? Trends Cognit. Sci 4 (9), 345–352. 10.1016/S1364-6613(00)01520-5. [DOI] [PubMed] [Google Scholar]
- Fahrenfort JJ, Lamme VAF, Scholte HS, 2008. The spatiotemporal profile of cortical processing leading up to visual perception. J. Vis 8 (1), 1–12. 10.1167/8.1.12. [DOI] [PubMed] [Google Scholar]
- Fahrenfort JJ, Scholte HS, Lamme VAF, 2007. Masking disrupts reentrant processing in human visual cortex. J. Cognit. Neurosci 19 (9), 1488–1497. 10.1162/jocn.2007.19.9.1488. [DOI] [PubMed] [Google Scholar]
- Feng S, Zeng FA, Chance B, 1995. Photon migration in the presence of a single defect: a perturbation analysis. Appl. Opt 34 (19), 3826–3837. 10.1364/A0.34.003826. [DOI] [PubMed] [Google Scholar]
- Ffytche DH, Zeki S, 2011. The primary visual cortex, and feedback to it, are not necessary for conscious vision. Brain 134 (1), 247–257. 10.1093/brain/awq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, 2002. Flow of activation from V1 to frontal cortex in humans: a framework for defining” early” visual processing. Exp. Brain Res 142, 139–150. [DOI] [PubMed] [Google Scholar]
- Fried PJ, Elkin-Frankston S, Rushmore RJ, Hilgetag CC, Valero-Cabre A, 2011. Characterization of visual percepts evoked by noninvasive stimulation of the human posterior parietal cortex. PLoS One 6 (11), e27204. 10.1371/journal.pone.0027204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebodh N, Vanegas MI, Kelly SP, 2017. Effects of stimulus size and contrast on the initial primary visual cortical response in humans. Brain Topogr. 30, 450–460. 10.1007/s10548-016-0530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Brumback CR, Gordon BA, Pearson MA, Low KA, Fabiani M, 2006. Effects of measurement method, wavelength, and source-detector distance on the fast optical signal. Neuroimage 32 (4), 1576–1590. 10.1016/j.neuroimage.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Gratton G, Corballis PM, Cho E, Fabiani M, Hood DC, 1995. Shades of gray matter: noninvasive optical images of human brain responses during visual stimulation. Psychophysiology 32 (5), 505–509. 10.1111/j.1469-8986.1995.tb02102.x. [DOI] [PubMed] [Google Scholar]
- Gratton G, Fabiani M, 1998. Dynamic brain imaging: event-related optical signal (EROS). Review 5 (4), 535–563. 10.3758/BF03208834. [DOI] [Google Scholar]
- Gratton G, Fabiani M, 2003. The event-related optical signal (EROS) in visual cortex: replicability, consistency, localization, and resolution. Psychophysiology 40 (4), 561–571. 10.1111/1469-8986.00058. [DOI] [PubMed] [Google Scholar]
- Gratton G, Sarno A, Maclin E, Corballis PM, Fabiani M, 2000. Toward noninvasive 3-D imaging of the time course of cortical activity: investigation of the depth of the event-related optical signal. Neuroimage 11 (5), 491–504. 10.1006/nimg.2000.0565. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, 2014. Visual field asymmetries in visual evoked responses. J. Vis 14 (14), 13. 10.1167/14.14.13, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen BC, Haun AM, Johnson AP, Ellemberg D, 2016. On the differentiation of foveal and peripheral early visual evoked potentials. Brain Topogr. 29, 506–514. 10.1007/s10548-016-0475-5. [DOI] [PubMed] [Google Scholar]
- Herwig U, Satrapi P, Schönfeldt-Lecuona C, 2003. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 16 (2), 95–99. 10.1023/B:BRAT.0000006333.93597.9d. [DOI] [PubMed] [Google Scholar]
- Holmes G, 1918. Disturbances of vision by cerebral lesions. Br. J. Ophthalmol 2 (7), 353–384. 10.1136/bjo.2.7.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurme M, Koivisto M, Revonsuo A, Railo H, 2019. V1 activity during feedforward and early feedback processing is necessary for both conscious and unconscious motion perception. Neuroimage 185 (October 2018), 313–321. 10.1016/j.neuroimage.2018.10.058. [DOI] [PubMed] [Google Scholar]
- Jalinous R, 1991. Technical and practical aspects of magnetic nerve stimulation. J. Clin. Neurophysiol.: official publication of the American Electroencephalographic Society 8 (1), 10–25. 10.1097/00004691-199101000-00004. [DOI] [PubMed] [Google Scholar]
- Kammer T, 1999. Phosphenes and transient scotomas induced by magnetic stimulation of the occipital lobe: their topographic relationship. Neuropsychologia 37 (2), 191–198. 10.1016/S0028-3932(98)00093-1. [DOI] [PubMed] [Google Scholar]
- Kammer T, Puls K, Erb M, Grodd W, 2005. Transcranial magnetic stimulation in the visual system. II. Characterization of induced phosphenes and scotomas. Exp. Brain Res 160, 129–140. 10.1007/s00221-004-1992-0. [DOI] [PubMed] [Google Scholar]
- Koch C, Massimini M, Boly M, Tononi G, 2016. Neural correlates of consciousness: progress and problems. Nat. Rev. Neurosci 17 (5), 307–321. 10.1038/nrn.2016.22. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Kainulainen P, Revonsuo A, 2009. The relationship between awareness and attention: evidence from ERP responses. Neuropsychologia 47 (13), 2891–2899. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Revonsuo A, 2007. Electrophysiological correlates of visual consciousness and selective attention. Neuroreport 18 (8), 753–756. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Revonsuo A, 2008. The role of selective attention in visual awareness of stimulus features: electrophysiological studies. Cognit. Affect Behav. Neurosci 8 (2), 195–210. 10.3758/CABN.8.2.195. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Revonsuo A, 2010. Event-related brain potential correlates of visual awareness. Neurosci. Biobehav. Rev 34 (6), 922–934. 10.1016/j.neubiorev.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Lamme VAF, 2000. Neural mechanisms of visual awareness: a linking proposition. Brain Mind 1 (3), 385–406. 10.1023/A:1011569019782. [DOI] [Google Scholar]
- Lamme VAF, 2006. Towards a true neural stance on consciousness. Trends Cognit. Sci 10 (11), 494–501. 10.1016/j.tics.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Lamme VAF, Roelfsema PR, 2000. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 23 (11), 571–579. 10.1016/S0166-2236(00)01657-X. [DOI] [PubMed] [Google Scholar]
- Lamme VAF, Supèr H, Spekreijse H, 1998. Feedforward, horizontal, and feedback processing in the visual cortex. Curr. Opin. Neurobiol 8 (4), 529–535. 10.1016/S0959-4388(98)80042-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Paradis A-L, Yahia-Cherif L, Tallon-Baudry C, 2012. Activity in the lateral occipital cortex between 200 and 300 ms distinguishes between physically identical seen and unseen stimuli. Front. Hum. Neurosci 6. https://www.frontiersin.org/articles/10.3389/fnhum.2012.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Wilke M, Aura C, et al. 2008. Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat. Neurosci 11, 1193–1200. 10.1038/nn.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH, 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19 (3), 1233–1239. 10.1016/S1053-8119(03)00169-l. [DOI] [PubMed] [Google Scholar]
- Marzi CA, Mancini F, Savazzi S, 2009. Interhemispheric transfer of phosphenes generated by occipital versus parietal transcranial magnetic stimulation. Exp. Brain Res 192 (3), 431–441. 10.1007/s00221-008-1496-4. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Beck DM, Ro T, Maclin EL, Low KA, Fabiani M, Gratton G, 2014. Dynamics of alpha control: preparatory suppression of posterior alpha oscillations by frontal modulators revealed with combined EEG and event-related optical signal. J. Cognit. Neurosci 26 (10), 2400–2415. 10.1162/jocn_a_00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzi C, Mancini F, Savazzi S, 2014. Can IPS reach visual awareness without V1? Evidence from TMS in healthy subjects and hemianopic patients. Neuropsychologia 64, 134–144. 10.1016/j.neuropsychologia.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Mazzi C, Mazzeo G, Savazzi S, 2017a. Markers of TMS-evoked visual conscious experience in a patient with altitudinal hemianopia. Conscious. Cognit 54, 143–154. 10.1016/j.concog.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Mazzi C, Savazzi S, Abrahamyan A, Ruzzoli M, 2017b. Reliability of TMS phosphene threshold estimation: toward a standardized protocol. Brain Stimul. 10 (3), 609–617. 10.1016/j.brs.2017.01.582. [DOI] [PubMed] [Google Scholar]
- Mazzi C, Savazzi S, Silvanto J, 2019a. On the “blindness” of blindsight: what is the evidence for phenomenal awareness in the absence of primary visual cortex (V1)? Neuropsychologia 128, 103–108. 10.1016/j.neuropsychologia.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Mazzi C, Tagliabue CF, Mazzeo G, Savazzi S, 2019b. Reliability in reporting perceptual experience: behaviour and electrophysiology in hemianopic patients. Neuropsychologia 128, 119–126. 10.1016/j.neuropsychologia.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzi C, Mazzeo G, Savazzi S, 2020. Late positivity does not meet the criteria to be considered a proper neural correlate of perceptual awareness. Front. Syst. Neurosci 14, 36. 10.3389/fnsys.2020.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeefry DJ, Gouws A, Burton MP, Morland AB, 2009. The noninvasive dissection of the human visual cortex: using FMRI and TMS to study the organization of the visual brain. Neuroscientist 15 (5), 489–506. 10.1177/1073858409334424. [DOI] [PubMed] [Google Scholar]
- Miranda PC, Hallett M, Basser PJ, 2003. The electric field induced in the brain by magnetic stimulation: a 3-D finite-element analysis of the effect of tissue heterogeneity and anisotropy. IEEE Transactions on Biomedical Engineering 50 (9), 1074–1085. [DOI] [PubMed] [Google Scholar]
- Musselwhite MJ, Jeffreys DA, 1985. The influence of spatial frequency on the reaction times and evoked potentials recorded to grating pattern stimuli. Vis. Res 25 (11), 1545–1555. 10.1016/0042-6989(85)90125-7. [DOI] [PubMed] [Google Scholar]
- Palejwala AH, Dadario NB, Young IM, O’Connor K, Briggs RG, Conner AK, et al. 2021. Anatomy and white matter connections of the lingual gyrus and cuneus. World neurosurgery 151, e426–e437. 10.1016/j.wneu.2021.04.050. [DOI] [PubMed] [Google Scholar]
- Parks NA, Maclin EL, Low KA, Beck DM, Fabiani M, Gratton G, 2012. Examining cortical dynamics and connectivity with simultaneous single-pulse transcranial magnetic stimulation and fast optical imaging. Neuroimage 59 (3), 2504–2510. 10.1016/j.neuroimage.2011.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks NA, Mazzi C, Tapia E, Savazzi S, Fabiani M, Gratton G, Beck DM, 2015. The influence of posterior parietal cortex on extrastriate visual activity: a concurrent TMS and fast optical imaging study. Neuropsychologia 78, 153–158. 10.1016/j.neuropsychologia.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, 2001. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science 292 (5516), 510–512. 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- Paulus W, Korinth S, Wischer S, Tergau F, 1999. Differential inhibition of chromatic and achromatic perception by transcranial magnetic stimulation of the human visual cortex. Neuroreport 10 (6), 1245–1248. 10.1097/00001756-199904260-00017. [DOI] [PubMed] [Google Scholar]
- Polonsky A, Blake R, Braun J, et al. 2000. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nat. Neurosci 3, 1153–1159. 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- Railo H, Koivisto M, 2012. Two means of suppressing visual awareness: A direct comparison of visual masking and transcranial magnetic stimulation. Cortex 48 (3), 333–343. [DOI] [PubMed] [Google Scholar]
- Rees G, Kreiman G, Koch C, 2002. Neural correlates of consciousness in humans. Nat. Rev. Neurosci 3 (4), 261–270. 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- Roebuck H, Bourke P, Guo K, 2014. Role of lateral and feedback connections in primary visual cortex in the processing of spatiotemporal regularity—a TMS study. Neuroscience 263, 231–239. 10.1016/j.neuroscience.2014.01.027. [DOI] [PubMed] [Google Scholar]
- Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, Thut G, 2008. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cerebr. Cortex 18 (9), 2010–2018. 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol 120 (12), 2008–2039. 10.1016/j.dinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen-Vaparanta N, Koivisto M, Noreika V, Vanni S, Revonsuo A, 2012. Neuronavigated transcranial magnetic stimulation suggests that area V2 is necessary for visual awareness. Neuropsychologia 50 (7), 1621–1627. 10.1016/j.neuropsychologia.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Samaha J, Gosseries O, Postle BR, 2017. Distinct oscillatory frequencies underlie excitability of human occipital and parietal cortex. J. Neurosci 37 (11), 2824–2833. 10.1523/JNEUROSCI.3413-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, 2008. A re-evaluation of blindsight and the role of striate cortex (V1) in visual awareness. Neuropsychologia 46 (12), 2869–2871. 10.1016/j.neuropsychologia.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Silvanto J, 2015. Why is “blindsight” blind? A new perspective on primary visual cortex, recurrent activity and visual awareness. Consciousness and Cognition 32, 15–32. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Cattaneo Z, Battelli L, Pascual-Leone A, 2008. Baseline cortical excitability determines whether TMS disrupts or facilitates behavior.J. Neurophysiol 99 (5), 2725–2730. 10.1152/jn.01392.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Cowey A, Lavie N, Walsh V, 2005a. Striate cortex (V1) activity gates awareness of motion. Nat. Neurosci 8 (2), 143–144. 10.1038/nn1379. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V, 2005b. Double dissociation of V1 and V5/MT activity in visual awareness. Cerebr. Cortex 15 (11), 1736–1741. 10.1093/cercor/bhi050. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A, 2008. State-dependency of transcranial magnetic stimulation. Brain Topogr. 21 (1), 1–10. 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensaas SS, Eddington DK, Dobelle WH, 1974. The topography and variability of the primary visual cortex in man. J. Neurosurg 40 (6), 747–755. [DOI] [PubMed] [Google Scholar]
- Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB, Mattingley JB, 2005. Simple metric for scaling motor threshold based on scalp-cortex distance: application to studies using transcranial magnetic stimulation. J. Neurophysiol 94 (6), 4520–4527. [DOI] [PubMed] [Google Scholar]
- Tafuro A, Mazzi C, Savazzi S, 2023. The spectral dynamics of Visual Awareness: an interplay of different frequencies? Eur. J. Neurosci 57 (12), 2136–2148. 10.1111/ejn.15988. [DOI] [PubMed] [Google Scholar]
- Tagliabue CF, Mazzi C, Bagattini C, Savazzi S, 2016. Early local activity in temporal areas reflects graded content of visual perception. Front. Psychol 7, 572. 10.3389/fpsyg.2016.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia E, Beck DM, 2014. Probing feedforward and feedback contributions to awareness with visual masking and transcranial magnetic stimulation. Front. Psychol 5 (October), 1–14. 10.3389/fpsyg.2014.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia E, Mazzi C, Savazzi S, Beck DM, 2014. Phosphene-guided transcranial magnetic stimulation of occipital but not parietal cortex suppresses stimulus visibility. Exp. Brain Res 232 (6), 1989–1997. 10.1007/s00221-014-3888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber H-L, Battersby WS, Bender MB, 1960. Visual field defects after penetrating missile wounds of the brain. In: Visual Field Defects after Penetrating Missile Wounds of the Brain. Harvard University Press. 10.4159/harvard.9780674593121. [DOI] [Google Scholar]
- Thielscher A, Reichenbach A, Uğurbil K, Uludağ K, 2010. The cortical site of visual suppression by transcranial magnetic stimulation. Cerebr. Cortex 20 (2), 328–338. 10.1093/cercor/bhp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong F, Engel S, 2001. Interocular rivalry revealed in the human cortical blind-spot representation. Nature 411, 195–199. 10.1038/35075583. [DOI] [PubMed] [Google Scholar]
- Tong F, 2003. Primary visual cortex and visual awareness. Nat. Rev. Neurosci 4 (3), 219–229. 10.1038/nrn1055. [DOI] [PubMed] [Google Scholar]
- Tononi G, Koch C, 2008. The neural correlates of consciousness: an update. Ann. N. Y. Acad. Sci 1124 (1), 239–261. 10.1196/annals.1440.004. [DOI] [PubMed] [Google Scholar]
- Tse CY, Yip LY, Lui TKY, Xiao XZ, Wang Y, Chu WCW, Parks NA, Chan SSM, Neggers SFW, 2018. Establishing the functional connectivity of the frontotemporal network in pre-attentive change detection with Transcranial Magnetic Stimulation and event-related optical signal. Neuroimage 179 (June), 403–413. 10.1016/j.neuroimage.2018.06.053. [DOI] [PubMed] [Google Scholar]
- Tse PU, Martinez-Conde S, Schlegel AA, Macknik SL, 2005. Visibility, visual awareness, and visual masking of simple unattended targets are confined to areas in the occipital cortex beyond human V1/V2. Proc. Natl. Acad. Sci. USA 102 (47), 17178–17183. 10.1073/pnas.0508010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya N, Wilke M, Frässle S, Lamme VA, 2015. No-report paradigms: extracting the true neural correlates of consciousness. Trends Cognit. Sci 19 (12), 757–770. 10.1016/j.tics.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Vanni S, Tanskanen T, Seppä M, Uutela K, Hari R, 2001. Coinciding early activation of the human primary visual cortex and anteromedial cuneus. Proc. Natl. Acad. Sci. USA 98 (5), 2776–2780. 10.1073/pnas.041600898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Cheng K, Murayama Y, Ueno K, Asamizuya T, Tanaka K, Logothetis N, 2011. Attention but not awareness modulates the BOLD signal in the human V1 during binocular suppression. Science 334 (6057), 829–831. https://www.science.org/doi/10.1126/science.1203161. [DOI] [PubMed] [Google Scholar]
- Whalen C, Maclin EL, Fabiani M, Gratton G, 2008. Validation of a method for coregistering scalp recording locations with 3D structural MR images. Hum. Brain Mapp 29 (11), 1288–1301. 10.1002/hbm.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MM, Jeffreys DA, 1982. Pattern-evoked potentials and Bloch’s law. Vis. Res 22 (8), 897–903. 10.1016/0042-6989(82)90026-8. [DOI] [PubMed] [Google Scholar]
- Wilenius-Emet M, Revonsuo A, Ojanen V, 2004. An electrophysiological correlate of human visual awareness. Neurosci. Lett 354 (1), 38–41. 10.lOl6/j.neulet.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Wokke ME, Vandenbroucke ARE, Scholte HS, Lamme VAF, 2013. Confuse your illusion: feedback to early visual cortex contributes to perceptual completion. Psychol. Sci 24 (1), 63–71. 10.1177/0956797612449175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


