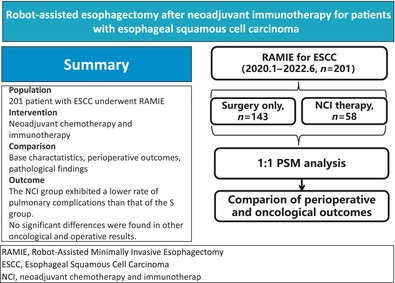
Abstract
Background
To determine the safety and efficacy of robot‐assisted minimally invasive esophagectomy (RAMIE) for locally advanced esophageal squamous cell carcinoma (ESCC) after neoadjuvant chemoimmunotherapy (NCI).
Methods
Data from patients who underwent RAMIE between January 2020 and June 2022 were retrospectively analyzed. The oncological and operative outcomes of the NCI and surgery‐only (S) groups were compared by both unmatched and 1:1 propensity score‐matched (PSM) analysis.
Results
A total of 201 patients with ESCC who underwent three‐incision RAMIE were included in this study (143 patients in the S group and 58 patients in the NCI group). Of the 58 patients who underwent NCI, a pathologically complete response (pCR) (ypT0N0) was identified in 14 (24.1%) patients. The patients in the NCI group were younger than those in the S group (p = 0.017), and had more advanced cT (p < 0.001) and cN stage diseases (p = 0.002). After 1:1 PSM of the confounders, 55 patients were allocated to each of the NCI and S groups. No significant differences were found in oncological and operative results, including surgical blood loss, operative time, and lymph node harvest (all p > 0.05). However, the NCI group exhibited a lower rate of pulmonary complications than the S group (3.6% vs. 14.5%, p = 0.047). No significant difference between the groups was found for other complications (all p > 0.05).
Conclusion
These findings indicate that NCI could result in a high pCR rate without increased complications in locally advanced ESCC. RAMIE is safe and feasible in patients with ESCC after NCI.
Keywords: esophagus, immunotherapy, neoadjuvant therapy, robotic surgical system, squamous cell carcinoma
Neoadjuvant chemoimmutherapy can result in a high pathologically complete response rate without increased complications in locally advanced esophageal squamous cell carcinoma. Robotic‐assisted esophagectomy is safe and feasible in patients with esophageal squamous cell carcinoma after neoadjuvant chemoimmutherapy.
INTRODUCTION
Esophageal cancer is one of the most common malignant tumors, ranking seventh in incidence and sixth in cause of death with an estimated 604 100 new cases and 544 076 deaths, respectively, in 2021. 1 Esophageal cancers can be divided into esophageal squamous cell carcinomas (ESCCs) and adenocarcinomas. ESCC is the main pathological type of ESCC found in East Asia, including China.
In recent decades, the treatment mode of ESCC has changed from single treatment to multidisciplinary comprehensive treatment, significantly improving the survival of patients with esophageal cancer. In the CROSS study, the 5‐ and 10‐year overall survival rates were 61.0% and 46%, respectively, which were significantly higher than those in the surgery‐only (S) group (30% and 23%, respectively). 2 , 3 , 4 In the NEOCRTEC5010 study, the median overall survival time was 100.1 months, whereas the median survival time of the S group was only 66.5 months. 5 Neoadjuvant chemoradiotherapy (NCR) followed by surgery remains the standard treatment for locally advanced ESCC. 6
Recurrence and metastasis are critical factors affecting the long‐term survival of patients with ESCC after neoadjuvant therapy. In the CROSS study, the local recurrence rate was 8%, the local recurrence and distant metastasis rate was 13%, and the distant metastasis rate alone was 27%. 3 In the NEOCRTEC5010 study, the recurrence rate was 33.7% after a median followup of 38.4 months, with a local recurrence rate of 9.8% and a distant metastasis rate of 19.6%. 7 Therefore, a new treatment mode should be explored to improve the survival of patients with ESCC, and to reduce recurrence and metastasis. 8
Neoadjuvant chemotherapy plus immunotherapy (NCI) is a novel treatment modality. Some phase II studies have shown that NCI is safe and efficient for the treatment of esophageal cancer. 9 , 10 , 11 A meta‐analysis including 27 clinical trials and 815 patients found that the pathologically complete response (pCR) rate was 31.4% and the incidence rate of treatment‐related adverse reactions was 26.9%. 12 These results suggest that NCI may be a promising treatment model for ESCC; however, it still requires further verification by long‐term follow‐up and prospective randomized controlled trials. 13 , 14
Esophagectomy combined with lymph node dissection is the primary surgical treatment for ESCC. Technical innovations in esophageal surgery include the transition from open to minimally invasive procedures, especially the introduction and application of da Vinci robotic surgery. At present, the rate of minimally invasive resection in some centers is as high as 80–90%, which can reduce the occurrence of postoperative complications, improve quality of life, and further improve patient survival. 15 , 16 , 17 , 18 , 19 , 20 Our previous study confirmed that robot‐assisted minimally invasive esophagectomy (RAMIE) showed significant advantages over traditional endoscopic surgery by increasing upper mediastinal lymph node harvest and reducing the incidence of recurrent laryngeal nerve paralysis. 21 Whether RAMIE is safe and feasible for patients with ESCC who undergo NCI has not yet been reported. Through a retrospective study of large cases, the present study combined RAMIE with NCI, discussed its clinical application, and compared the impact of NCI on operative and oncological results.
PATIENTS AND METHODS
Patients and surgical procedure
This retrospective study was approved by the Institutional Review Board of the Tianjin Medical University Cancer Institute and Hospital (approval number: bc2021157). Patients who underwent RAMIE between January 2020 and June 2022 at our cancer center were selected. The inclusion criteria in the present study were as follows: ESCC pathological type and patients who underwent McKeown robotic surgery. The exclusion criteria included conversion to open or endoscopic surgery, the Ivor–Lewis procedure, non‐SCC pathology, and preoperative chemotherapy or chemoradiotherapy.
Chemotherapy consisted of platinum combined with paclitaxel, docetaxel, or fluorouracil. Different immune checkpoint inhibitors inhibiting programmed cell death protein 1 (PD‐1) were included as immunotherapy agents combined with chemotherapeutic medications in the NCI group. The preoperative evaluation and surgical procedures were detailed in our previous study. 21
Data collection
Demographic data, neoadjuvant treatment details, including NCI protocols and chemotherapy regimens, surgical details (operative time and blood loss), length of postoperative hospital stay, postoperative morbidity and mortality, and pathological data were collected. All major complications were evaluated based on the Esophagectomy Complications Consensus Group criteria. 22 All patients were staged using the American Joint Committee on Cancer 8th edition Tumor‐Node‐Metastasis Staging System. 23
Statistical analysis
All data are shown as mean ± standard deviation for continuous variables and as frequencies (%) for categorical variables. A propensity score‐matched (PSM) approach was used to assemble a well‐balanced cohort using available explanatory factors. The logistic regression model was used to estimate the propensity scores. Thus, PSM analysis (NCI group:S group in a 1:1 match) was conducted in a blinded manner and a caliper distance of 0.1, without replacement to adjust for identifiable factors which may have affected the results. Unpaired Student's t‐test or Wilcoxon rank‐sum test was used for continuous variables. The chi‐squared test or Fisher's exact test was used to analyze categorical variables. A two‐sided p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 25 (IBM SPSS Statistics, Windows, version 25.0).
RESULTS
A total of 417 patients underwent McKeown RAMIE at our cancer center during the study period. After excluding 13 cases of conversion to open or exploration surgery owing to tumor invasion (n = 8), pleural adhesion (n = 3), thoracic hemorrhage (n = 1), and malignant arrhythmia (n = 1), 27 non‐SCC pathological types, and 47 patients who underwent preoperative chemotherapy/radiotherapy, 330 patients were selected. During the study period between January 2020 and June 2022, 143 patients underwent surgery only and 58 underwent NCI followed by surgery. The study flowchart is shown in Figure 1.
FIGURE 1.
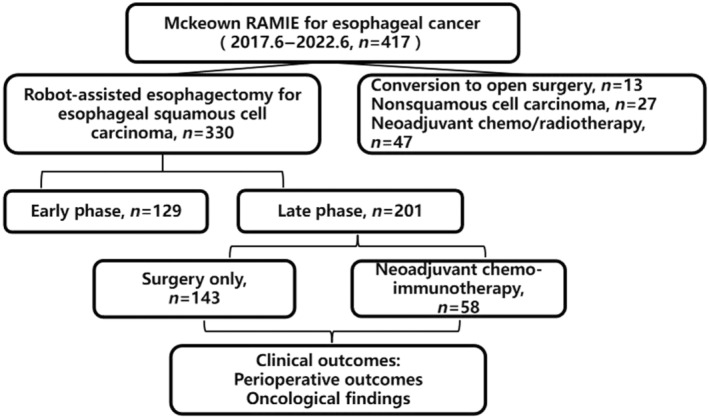
The study flowchart. RAMIE, robot‐assisted minimally invasive esophagectomy.
Patient demographics
Patient demographic characteristics are listed in Table 1. The mean age of the 201 patients was 62.2 ± 7.9 years. The majority of patients were male (178/201, 88.6%) with a cT3 (130/201, 64.7%) stage tumor. The clinical stage N0 was found in 69 cases (34.3%), N1 in 70 cases (34.8%), N2 in 58 cases (28.9%), and N3 in four cases (2.0%). After 1:1 PSM analysis, a total of 110 patients with locally advanced ESCC (55 patients in each group) were obtained. The baseline characteristics, including age, sex, morbidity, and tumor location, cT and cN stage were not significantly different between the NCI and S groups.
TABLE 1.
Demographic variables of ESCC patients with RAMIE.
| Variables | All patients, n = 201 (%) | Before matching | p | After matching | p | ||
|---|---|---|---|---|---|---|---|
| NCI, n = 58 (%) | Surgery only, n = 143 (%) | NCI, n = 55 (%) | Surgery only, n = 55 (%) | ||||
| Age, years, mean ± SD | 62.2 ± 7.9 | 61.0 ± 7.4 | 63.8 ± 8.0 | 0.017 | 60.8 ± 6.9 | 62.7 ± 5.7 | 0.111 |
| Sex ratio (M:F) | 178:23 | 49:9 | 129:14 | 0.405 | 46:9 | 50:5 | 0.392 |
| Smoking (n, %) | 137 (68.2) | 40 (69.0) | 97 (67.8) | 0.876 | 39 (70.9) | 36 (65.5) | 0.683 |
| Drinking (n, %) | 135 (67.2) | 41 (70.7) | 94 (65.7) | 0.498 | 40 (72.7) | 36 (65.5) | 0.536 |
| Comorbidity | |||||||
| Hypertention | 53 (26.4) | 15 (25.9) | 38 (26.6) | 0.917 | 15 (27.3) | 14 (25.5) | 0.829 |
| Diabetes | 23 (11.4) | 7 (12.1) | 16 (11.2) | 0.859 | 6 (10.9) | 6 (10.9) | 1.000 |
| Heart disease | 10 (5.0) | 1 (1.7) | 9 (6.3) | 0.287 | 1 (1.8) | 4 (7.3) | 3.363 |
| Tumor location | 0.093 | 0.354 | |||||
| 20–25 cm | 17 (8.5) | 3 (5.2) | 14 (9.8) | 2 (3.6) | 4 (7.3) | ||
| >25 and ≤30 cm | 64 (31.8) | 25 (43.1) | 39 (27.3) | 25 (45.5) | 18 (32.7) | ||
| >30 cm | 120 (59.7) | 30 (51.7) | 90 (62.9) | 28 (50.9) | 33 (50.9) | ||
| cT stage | <0.001 | 0.251 | |||||
| T1 | 42 (20.9) | 2 (3.4) | 40 (28.0) | 0 | 0 | ||
| T2 | 18 (9.0) | 0 | 18 (12.6) | 0 | 3 (5.5) | ||
| T3 | 130 (64.7) | 50 (86.2) | 80 (55.9) | 49 (89.1) | 48 (87.3) | ||
| T4 | 11 (5.5) | 6 (10.3) | 5 (3.5) | 6 (10.9) | 4 (7.3) | ||
| cN stage | 0.002 | 0.689 | |||||
| N0 | 69 (34.3) | 9 (15.5) | 60 (42.0) | 8 (14.5) | 6 (10.9) | ||
| N1 | 70 (34.8) | 26 (44.8) | 44 (30.8) | 25 (45.5) | 30 (54.5) | ||
| N2 | 58 (28.9) | 22 (37.9) | 36 (25.2) | 21 (38.2) | 19 (34.5) | ||
| N3 | 4 (2.0) | 1 (1.7) | 3 (2.1) | 1 (1.8) | 0 | ||
Abbreviations: ESCC, esophageal squamous cell carcinoma; F, female; M, male; NCI, neoadjuvant chemoimmunotherapy; RAMIE, robot‐assisted minimally invasive esophagectomy; SD, standard deviation.
NCI
During the study period, 58 patients underwent NCI followed by surgery. Patient characteristics are listed in Table 1. The mean age of the 58 patients was 61.0 years. The majority of patients were male (49/58, 84.5%) with a cT3 (50/58, 86.2%) stage tumor. The patients in the NCI group were younger (p = 0.017) than those in the S group, and more of them had advanced cT (p < 0.001) and cN stage diseases (p = 0.002).
Forty‐nine (84.5%) patients received pembrolizumab, seven (12.1%) received camrelizumab, one (1.7%) received sintilimab, and one (1.7%) received toripalimab. Most patients (54/58, 93.1%) completed three cycles of NCI therapy, two (3.4%) completed two cycles, and two (3.4%) completed four cycles. Paclitaxel/docetaxel + cisplatin/nedaplatin (TP/DP, n = 57) or docetaxel + fluorouracil + cisplatin (DCF, n = 1) were administered as neoadjuvant chemotherapy in the NCI group. The representative enhanced CT images before and after NCI are shown in Figure 2.
FIGURE 2.
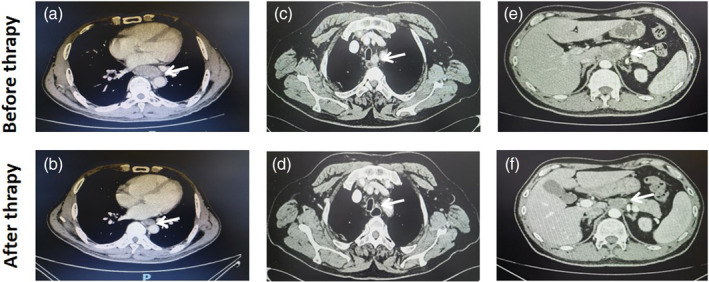
The representative enhanced computed tomography images before and after neoadjuvant chemotherapy and immunotherapy. (a, b) Case 1 had cancer in the lower esophagus with cT3 stage disease before therapy, and pathological diagnosis subsequently confirmed no residual cancer (ypT0). (c, d) Case 2 had lymph node metastasis around the left recurrent laryngeal nerve (cN+), and pathological diagnosis confirmed the node to be metastatic (ypN+). (e, f) Case 3 had bulky lymph node metastasis around the gastric left artery (cN+), and pathological diagnosis confirmed no residual cancer in the nodes (ypN−).
Surgical and pathological outcomes
No differences between the NCI and S groups were found in blood loss and operation time (all p > 0.05). Furthermore, no significant differences were found between the two groups in other perioperative parameters, including hospital stay after surgery, ICU stay, and postoperative complications (all p > 0.05). However, the rate of pulmonary complications in the NCI group was lower than that in the S group (3.1% vs. 12.2%, p = 0.039 before matching; 3.6% vs. 14.5%, p = 0.047 after matching; Table 2). The lymph node dissections, including the total, thoracic, and recurrent laryngeal nerves, were similar between the NCI and S groups (all p > 0.05; Table 3). The pCR rate (ypT0N0) was identified in 14 patients (14/58, 24.1%) and complete response of primary tumor was found in 23 patients (23/58, 39.7%). In addition, there was a significant difference between the NCI and S groups in terms of pT (p < 0.001) and pN (p = 0.026) stage after PSM analysis.
TABLE 2.
Perioperative outcomes of ESCC patients after RAMIE.
| Variables | All patients n = 201 (%) | Before matching | p | After matching | p | ||
|---|---|---|---|---|---|---|---|
| NCI, n = 58 (%) | Surgery only, n = 143 (%) | NCI, n = 55 (%) | Surgery only, n = 55 (%) | ||||
| Operation time, min, mean ± SD | 303.4 ± 55.9 | 303.4 ± 59.2 | 293.8 ± 52.9 | 0.248 | 304.4 ± 61.4 | 308.7 ± 62.7 | 0.720 |
| Surgical blood loss, mL, mean ± SD | 163.6 ± 109.9 | 153.3 ± 135.8 | 153.5 ± 87.2 | 0.992 | 152.9 ± 144.0 | 162.9 ± 96.1 | 0.669 |
| Hospital stay, days, mean ± SD | 15.1 ± 10.0 | 14.2 ± 8.6 | 14.5 ± 12.0 | 0.812 | 14.5 ± 9.1 | 15.1 ± 11.1 | 0.750 |
| Intensive care unit stay, n (%) | 14 (7.0) | 1 (1.7) | 13 (9.1) | 0.069 | 1 (1.8) | 6 (10.9) | 0.113 |
| Mortality | 1 (0.5) | 0 | 1 (0.7) | 1.000 | 0 | 1 (1.8) | 1.000 |
| Overall complications | 43 (21.4) | 12 (20.7) | 31 (21.7) | 0.565 | 11 (20.0) | 16 (29.1) | 0.171 |
| RLN paralysis | 10 (5.0) | 4 (6.9) | 6 (4.2) | 0.728 | 3 (5.5) | 1 (1.8) | 0.618 |
| Pulmonary complication | 19 (9.5) | 2 (3.4) | 17 (11.9) | 0.039 | 2 (3.6) | 8 (14.5) | 0.047 |
| Anastomotic leakages | 11 (5.5) | 2 (3.4) | 9 (6.3) | 0.508 | 2 (3.6) | 4 (7.2) | 0.679 |
| Surgical site infections | 2 (1.0) | 1 (1.7) | 1 (0.7) | 0.532 | 1 (1.8) | 1 (1.8) | 0.532 |
| Chylothorax | 5 (2.5) | 2 (3.4) | 3 (2.1) | 1.000 | 2 (3.6) | 2 (3.6) | 1.000 |
| Cardiovascular complications | 1 (0.5) | 0 | 1 (0.7) | 1.000 | 0 | 0 | 1.000 |
| VTE | 1 (0.5) | 0 | 1 (0.7) | 1.000 | 0 | 0 | 1.000 |
| Others | 5 (2.5) | 3 (5.2) | 2 (1.4) | 0.181 | 3 (5.5) | 2 (3.6) | 0.181 |
Abbreviations: ESCC, esophageal squamous cell carcinoma; NCI, neoadjuvant chemoimmunotherapy; RAMIE, robot‐assisted minimally invasive esophagectomy; RLN, recurrent laryngeal nerve; SD, standard deviation; VTE, venous thromboem.
TABLE 3.
Pathological outcomes of ESCC patients after RAMIE.
| Variables | All patients n = 201 (%) | Before matching | p | After matching | p | ||
|---|---|---|---|---|---|---|---|
| NCI, n = 58 (%) | Surgery only, n = 143 (%) | NCI, n = 55 (%) | Surgery only, n = 55 (%) | ||||
| Number of resected lymph node | |||||||
| Total, mean ± SD | 31.5 ± 13.3 | 35.0 ± 12.0 | 33.7 ± 14.9 | 0.512 | 35.0 ± 12.1 | 36.5 ± 15.1 | 0.569 |
| Thoracic, mean ± SD | 19.6 ± 10.0 | 21.1 ± 8.3 | 21.5 ± 10.6 | 0.788 | 21.3 ± 8.7 | 22.8 ± 12.2 | 0.473 |
| RRLN lymph node, mean ± SD | 3.4 ± 2.9 | 3.4 ± 2.5 | 4.0 ± 3.4 | 0.160 | 3.5 ± 2.6 | 3.8 ± 3.1 | 0.530 |
| Dissection rate, n (%) | 191 (95.0) | 54 (93.1) | 137 (95.8) | 0.328 | 51 (92.7) | 52 (94.5) | 0.552 |
| Metastasis rate, n (%) | 42 (20.9) | 11 (19.0) | 31 (21.7) | 0.712 | 10 (18.2) | 22 (32.7) | 0.125 |
| LRLN lymph node | 3.7 ± 3.4 | 4.3 ± 3.7 | 4.1 ± 3.7 | 0.653 | 4.4 ± 3.8 | 4.3 ± 4.2 | 0.962 |
| Dissection rate, n (%) | 169 (84.1) | 51 (87.9) | 118 (82.5) | 0.387 | 48 (87.3) | 44 (80) | 0.440 |
| Metastasis rate, n (%) | 27 (13.4) | 7 (12.1) | 20 (15.5) | 0.501 | 7 (12.7) | 14 (25.5) | 0.144 |
| pT stage | <0.001 | <0.001 | |||||
| pT0 | 23 (11.4) | 23 (39.7) | 0 | 23 (41.8) | 0 | ||
| pT1 | 58 (28.9) | 13 (22.4) | 45 (31.5) | 13 (23.6) | 0 | ||
| pT2 | 25 (12.4) | 7 (12.1) | 18 (12.9) | 7 (12.7) | 2 (3.6) | ||
| pT3 | 95 (47.3) | 15 (25.9) | 80 (55.9) | 12 (21.8) | 53 (96.4) | ||
| pN stage | 0.691 | 0.026 | |||||
| pN0 | 92 (45.8) | 27 (46.6) | 65 (45.5) | 26 (47.3) | 12 (21.8) | ||
| pN1 | 55 (27.4) | 16 (27.6) | 39 (27.3) | 16 (29.1) | 18 (32.7) | ||
| pN2 | 41 (20.4) | 13 (22.2) | 28 (19.6) | 11 (20.0) | 20 (36.4) | ||
| pN3 | 13 (6.5) | 2 (3.4) | 11 (7.7) | 2 (3.6) | 5 (9.1) | ||
| pCR (ypT0N0) | / | 14 (24.1) | / | / | / | / | / |
Abbreviations: ESCC, esophageal squamous cell carcinoma; LRLN, left recurrent laryngeal nerve; NCI, neoadjuvant chemoimmunotherapy; pCR, pathological complete response; RAMIE, robot‐assisted minimally invasive esophagectomy; RRLN, right recurrent laryngeal nerve; SD, standard deviation.
DISCUSSION
This retrospective case–control study provides evidence for the combination of anti‐PD‐1 antibody with chemotherapy for locally advanced ESCC, which resulted in a pCR rates of 24.1% for primary tumor and lymph node and 39.1% for primary tumor, the short‐term results of patients who underwent NCI were similar to those for patients who underwent surgery alone in regards to perioperative and oncological results. Robotic‐assisted esophagectomy is safe and feasible in patients with ESCC after NCI.
Neoadjuvant therapy for ESCC is currently being explored. A prospective randomized controlled study compared the short‐term results of NCR to those of patients who underwent neoadjuvant chemotherapy (NC). The study found that the pCR rate of the NCR group was 35.7% and that of the NC group was only 3.8%. 6 The JCOG1109 study presented in 2022 ASCO‐GI reported that the pCR in the neoadjuvant CF + RT group was 36.7%, in the neoadjuvant DCF group it was 18.6%, and in the neoadjuvant CF group it was 2.2%. The 3‐year overall survival rates in the three groups were 68.3%, 72.1%, and 62.6%, respectively. Survival in the neoadjuvant DCF group was better than that in the neoadjuvant CF group, and no significant difference was found between the neoadjuvant CF and CF + RT groups. Therefore, a higher pCR in the NCR group did not translate into a higher survival rate.
A few recent studies have found that when compared with chemotherapy alone, immunotherapy plus chemotherapy can significantly prolong the overall survival and progression‐free survival of patients with advanced ESCC. 24 , 25 , 26 Immunotherapy combined with chemotherapy has become the first‐line treatment for advanced ESCC. The application of immunotherapy as neoadjuvant therapy for ESCC is also being explored and updated. A recent retrospective study found that when compared with NC, NCI for patients with locally advanced ESCC provided an advantage in pathological response (21.7% vs. 4.5%), and could improve 1‐ and 2‐year disease‐free survival with good safety and feasibility. 27 However, in two recent retrospective studies NCR and NCI achieved comparable pathological responses. 11 , 28
In the present study, 58 patients with ESCC, including 56 patients with cT3‐4 disease, received NCI and 23 patients (39.7%) had complete remission of the primary tumor (ypT0) after NCI. Nine patients (15.5%) had cN0 disease and 37 patients (46.6%, p = 0.001) had ypN0 disease after neoadjuvant therapy. The overall pCR rate of the 58 patients in this study was 24.1%. We found that the pCR rates reported in different studies were not completely the same. 9 , 10 , 11 The main reason for this finding is likely related to the clinical stage of the selected patients, different immune checkpoint inhibitors, and different chemotherapy schemes, all of which require further study and optimization.
RAMIE after NCI has not yet been reported. A total of 201 patients with ESCC who underwent robotic surgery and lymph node dissection were included in the present study, of which 58 underwent NCI and 143 underwent surgery alone. All the patients underwent RAMIE after the learning curve. To further study the impact of NCI on the perioperative and oncological results of RAMIE, we compared and analyzed the clinical data of 55 patients in the NCI group and S group during the same period after PSM analysis. The results showed that the cT and cN stages of patients in the NCI group were more advanced than those in the S group before PSM analysis, but the pT and pN stage after NCI was significantly better than that in the S group after PSM analysis.
Operation times and bleeding volumes are typically used to measure the success of the operation. This study found no significant differences between the NCI and S groups in operation times and blood loss. In our experience, local fibrosis and edema caused by NCR influence surgical outcomes and increase surgical difficulty to a certain extent. However, we found that NCI had no influence on the operation and even decreased the surgical difficulty for tumor regression. Radical lymph node dissection is the primary quality control standard during esophagectomy for esophageal cancer. Standardized surgical procedures and lymph node dissection intensities were adopted for all patients in the present study. The results showed no significant differences between the NCI and S groups in the number of lymph node dissections (35.0 vs. 33.9, p > 0.05).
Bilateral recurrent laryngeal nerve lymph node dissection is the most technically challenging part of transthoracic esophagectomy and usually leads to recurrent laryngeal nerve injury. The present study further analyzed the upper mediastinal lymph node dissection rate and the number of lymph node dissections in the NCI and S groups. The mean numbers of lymph node dissections in the left recurrent laryngeal nerve of the NCI and S groups were 4.4 and 4.3, respectively, and the lymph node dissection rates were 87.0% and 80.0%, respectively, without significant differences. These results showed that the lymph node dissection intensity in the NCI group achieved satisfactory results when compared with the S group, and did not increase operation time and blood loss.
In addition to surgical and oncological results, postoperative complications and mortality are important indicators for measuring the safety of surgery. Whether NCI increases the incidence of postoperative complications and mortality compared to surgery only has not been reported. Our results showed that the overall incidences of complications in the NCI and S groups were 20.0% and 29.1% after PSM analysis, respectively. No deaths were reported in the NCI group and one death was reported in the S group, with no significant difference. In addition, we found that the incidence of pneumonia in the NCI group was significantly lower than that in the surgery‐only group (3.6% and 14.5%, respectively). Correspondingly, the proportion of patient re‐entry into the ICU of the NCI group was also lower than that of the S group before PSM analysis (1.7% vs. 9.1%, respectively). This result may be related to the strict screening of patients in the NCI group that were involved in the clinical trials. The overall physical condition of patients in the NCI group was better than that in the S group, reflecting that NCI did not increase the incidence of perioperative complications.
LIMITATIONS
As a retrospective study, selection bias must be considered, although PSM analysis was used to reduce the imbalance between the two groups. PSM analysis cannot eliminate some inherent differences between these two groups and this may be driving some of the superiority in the clinical outcomes, including less pneumonia in the NCI group. This should be considered carefully. In addition, the number of included cases is small, and there were no follow‐up and survival data. Furthermore, several different immunotherapy regimens were used in this study, based on oncologist preference, and these various protocols require further evaluation in future clinical studies. Adverse reactions during NCI are important indicators of the safety of neoadjuvant therapy. Because this was a retrospective study, relevant data were not obtained from some patients.
CONCLUSION
The present study confirmed that NCI as a new treatment modality can achieve a relatively high pCR rate. In addition, RAMIE after NCI for ESCC did not increase surgical difficulty or the incidence of perioperative complications and death. Radical lymph node dissection and satisfactory oncological results were obtained. RAMIE is safe and feasible in patients with ESCC after NCI.
AUTHOR CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: H.J. and X.D. Acquisition of data: F.G., F.Z., and X.Z. Analysis and interpretation of the data: F.G., F.Z., and X.Z. Drafting of the manuscript: F.G., F.Z., and X.Z. Critical revision of the manuscript for important intellectual content: X.D. and H.J. Statistical analysis: F.Z. and X.Z. Obtained funding: H.J. and X.D. Administrative, technical and material support: H.J. and X.D. Study supervision: H.J. and X.D.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest to declare.
STATEMENT OF INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Guo F, Zhang X, Zhao F, Jiang H, Duan X. Neoadjuvant chemoimmunotherapy followed by robot esophagectomy has no effect on short‐term results compared with surgery alone. Thorac Cancer. 2024;15(18):1446–1453. 10.1111/1759-7714.15334
Feng Guo, Xu Zhang, and Fangdong Zhao contributed equally to this work.
Contributor Information
Hongjing Jiang, Email: jianghongjing@tmu.edu.cn.
Xiaofeng Duan, Email: xduan@tmu.edu.cn.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 2. Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long‐term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. [DOI] [PubMed] [Google Scholar]
- 3. Eyck BM, van Lanschot JJB, Hulshof M, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten‐year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. 2021;39(18):1995–2004. [DOI] [PubMed] [Google Scholar]
- 4. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. [DOI] [PubMed] [Google Scholar]
- 5. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open‐label clinical trial. J Clin Oncol. 2018;36(27):2796–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang H, Tang H, Fang Y, Tan L, Yin J, Shen Y, et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg. 2021;156(5):444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu S, Wen J, Yang H, Li Q, Chen Y, Zhu C, et al. Recurrence patterns after neoadjuvant chemoradiotherapy compared with surgery alone in oesophageal squamous cell carcinoma: results from the multicenter phase III trial NEOCRTEC5010. Eur J Cancer. 2020;138:113–121. [DOI] [PubMed] [Google Scholar]
- 8. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE‐1). Eur J Cancer. 2021;144:232–241. [DOI] [PubMed] [Google Scholar]
- 9. Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H, et al. Multicenter, single‐arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10(3):e004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hong ZN, Gao L, Weng K, Huang Z, Han W, Kang M. Safety and feasibility of esophagectomy following combined immunotherapy and chemotherapy for locally advanced esophageal squamous cell carcinoma: a propensity score matching analysis. Front Immunol. 2022;13:836338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng J, Guo M, Yang Y, Liu Y, Hu W, Shang Q, et al. Perioperative outcomes of minimally invasive esophagectomy after neoadjuvant immunotherapy for patients with locally advanced esophageal squamous cell carcinoma. Front Immunol. 2022;13:848881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ge F, Huo Z, Cai X, Hu Q, Chen W, Lin G, et al. Evaluation of clinical and safety outcomes of neoadjuvant immunotherapy combined with chemotherapy for patients with resectable esophageal cancer: a systematic review and meta‐analysis. JAMA Netw Open. 2022;5(11):e2239778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shang X, Zhao G, Liang F, Zhang C, Zhang W, Liu L, et al. Safety and effectiveness of pembrolizumab combined with paclitaxel and cisplatin as neoadjuvant therapy followed by surgery for locally advanced resectable (stage III) esophageal squamous cell carcinoma: a study protocol for a prospective, single‐arm, single‐center, open‐label, phase‐II trial (Keystone‐001). Ann Transl Med. 2022;10(4):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shang X, Zhang W, Zhao G, Liang F, Zhang C, Yue J, et al. Pembrolizumab combined with neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy followed by surgery for locally advanced oesophageal squamous cell carcinoma: protocol for a multicentre, prospective, randomized‐controlled, phase III clinical study (Keystone‐002). Front Oncol. 2022;12:831345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Straatman J, van der Wielen N, Cuesta MA, Daams F, Roig Garcia J, Bonavina L, et al. Minimally invasive versus open esophageal resection: three‐year follow‐up of the previously reported randomized controlled trial: the TIME trial. Ann Surg. 2017;266(2):232–236. [DOI] [PubMed] [Google Scholar]
- 16. Weksler B, Sullivan JL. Survival after esophagectomy: a propensity‐matched study of different surgical approaches. Ann Thorac Surg. 2017;104(4):1138–1146. [DOI] [PubMed] [Google Scholar]
- 17. Yamashita K, Watanabe M, Mine S, Toihata T, Fukudome I, Okamura A, et al. Minimally invasive esophagectomy attenuates the postoperative inflammatory response and improves survival compared with open esophagectomy in patients with esophageal cancer: a propensity score matched analysis. Surg Endosc. 2018;32(11):4443–4450. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Dong D, Cao Y, Huang M, Li J, Zhang J, et al. Robotic versus conventional minimally invasive esophagectomy for esophageal cancer: a meta‐analysis. Ann Surg. 2023;278(1):39–50. [DOI] [PubMed] [Google Scholar]
- 19. Merboth F, Nebelung H, Wotschel N, Liebscher H, Eckert F, von Renesse J, et al. Robotic esophagectomy compared with open esophagectomy reduces sarcopenia within the first postoperative year: a propensity score‐matched analysis. J Thorac Oncol. 2023;18(2):232–244. [DOI] [PubMed] [Google Scholar]
- 20. van der Horst S, Weijs TJ, Braunius WW, Mook S, Mohammed NH, Brosens L, et al. Safety and feasibility of robot‐assisted minimally invasive esophagectomy (RAMIE) with three‐field lymphadenectomy and neoadjuvant chemoradiotherapy in patients with resectable esophageal cancer and cervical lymph node metastasis. Ann Surg Oncol. 2023;30(5):2743–2752. [DOI] [PubMed] [Google Scholar]
- 21. Duan X, Yue J, Chen C, Gong L, Ma Z, Shang X, et al. Lymph node dissection around left recurrent laryngeal nerve: robot‐assisted vs. video‐assisted McKeown esophagectomy for esophageal squamous cell carcinoma. Surg Endosc. 2021;35(11):6108–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D'Journo XB, et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg. 2015;262(2):286–294. [DOI] [PubMed] [Google Scholar]
- 23. Rice TW, Ishwaran H, Hofstetter WL, Kelsen DP, Apperson‐Hansen C, Blackstone EH, et al. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29(8):897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first‐line treatment of advanced oesophageal cancer (KEYNOTE‐590): a randomised, placebo‐controlled, phase 3 study. Lancet. 2021;398(10302):759–771. [DOI] [PubMed] [Google Scholar]
- 25. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT‐15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022;377:e068714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Song Y, Zhang B, Xin D, Kou X, Tan Z, Zhang S, et al. First‐line serplulimab or placebo plus chemotherapy in PD‐L1‐positive esophageal squamous cell carcinoma: a randomized, double‐blind phase 3 trial. Nat Med. 2023;29(2):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jing SW, Zhai C, Zhang W, He M, Liu QY, Yao JF, et al. Comparison of neoadjuvant immunotherapy plus chemotherapy versus chemotherapy alone for patients with locally advanced esophageal squamous cell carcinoma: a propensity score matching. Front Immunol. 2022;13:970534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu L, Wei XF, Li CJ, Yang ZY, Yu YK, Li HM, et al. Pathologic responses and surgical outcomes after neoadjuvant immunochemotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. Front Immunol. 2022;13:1052542. [DOI] [PMC free article] [PubMed] [Google Scholar]


