The growing use of unsubstantiated complementary and alternative medicine therapies by people in the United States1 along with its increasing coverage by third party payers2 encouraged Congress to create the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health. The centre's mission is “to explore complementary and alternative healing practices in the context of rigorous science; to educate and training CAM researchers; and to disseminate authoritative information to the public and professionals.”3 To complete this mission, NCCAM supports publicly relevant and scientifically rigorous research to identify those complementary and alternative medicine practices that are safe and effective.
The centre's resources, although generous ($68.3m (£46m) for fiscal year 2000), are not sufficient to study all complementary and alternative medicine practices. NCCAM therefore developed criteria to help prioritise the many possible research opportunities (box). As part of the evaluation process, NCCAM seeks advice from its national advisory council, complementary and alternative medicine and conventional clinicians, members of the scientific research community, the public, sister federal agencies, and other stakeholders.
Summary points
Many early clinical trials investigating complementary and alternative medicine have had serious flaws
Clinical investigations of complementary and alternative medicine are made difficult by factors such as use of complex, individualised treatments and lack of standardisation of herbal medicines
Other problems include difficulties in accruing, randomising, and retaining patients and in identifying appropriate placebo interventions
Despite these complexities, rigorously designed clinical trials are possible, including pragmatic studies of complete complementary and alternative medicine systems
Strong commitment is required from the research community to provide information about complementary and alternative medicines to the public and health professionals
Allocation of resources
Staff at the centre are often asked why limited resources are being spent on research that is perceived as replicating previously published work, especially when other western countries have already integrated some of these practices into standard care. Unfortunately, many of the studies have been small, their results variable or inconsistent, and their research designs inadequate. Systematic reviews have found that many clinical trials testing complementary or alternative medicine have major flaws, such as insufficient statistical power, poor controls, inconsistency of treatment or product, and lack of comparisons with other treatments, with placebo, or with both. These reviews typically conclude that larger, well designed studies are necessary before making authoritative recommendations. Specific examples of such reviews include the use of Hypericum perforatum (St John's wort) to treat depression4; Ginkgo biloba to delay cognitive decline in patients with Alzheimer's disease5; Serenoa repens (saw palmetto) to relieve symptoms associated with benign prostatic hyperplasia6; and glucosamine and chondroitin sulphate to treat osteoarthritis.7 NCCAM is currently supporting randomised controlled trials for these four dietary supplements that have been designed with the scientific rigour demanded by experienced scientists and the American public.
One reason for investing so much in research into dietary supplements is that their use is growing rapidly in the United States. Although consultations with complementary and alternative medicine practitioners (acupuncturists, chiropractors, naturopathic physicians, etc) remained stable on a percentage basis from 19938 to 1998,1 use of dietary supplements greatly increased. Billions of dollars are spent on dietary supplements in the United States every year. The Dietary Supplement Health and Education Act, which was passed in 1994, made it easier to obtain these natural products. The act also loosened the federal control over dietary supplements, with the result that most commercially available products are not well characterised or standardised. Another issue is that the optimal dose, schedule, and route of administration of most dietary supplements have not been determined systematically; nor are the frequency and extent of drug reactions and interactions known. NCCAM therefore believes that most dietary supplements are not yet ready for large, expensive trials despite their wide use by patients. At a minimum, preclinical studies, pharmacokinetics testing, and developmental phase I and II trials are necessary before these products can be launched into definitive clinical trials. NCCAM is vigorously encouraging research in these areas through a series of focused initiatives.9
Criteria for prioritising research opportunities
Quantity and quality of available preliminary data to help determine the most appropriate type of research (basic versus clinical research; phase I or II clinical trial versus phase III trial)
Extent of use by the US public (greatest weight given to interventions in wide use)
Public health importance of disease being treated (greatest weight to diseases associated with highest mortality or morbidity or for which conventional medicine has not proved optimal)
Feasibility of conducting the research
Cost of research
Problems with research design
Although many people in the United States self medicate with dietary supplements, many others seek care from practitioners of traditional systems of medicine, including Ayurveda (from India), Kampo (from Japan), traditional Chinese medicine, Native American medicine, and more recently developed systems such as naturopathy and chiropractic.1,10–12 Despite the diverse cultures, geographical locations, and beliefs from which these systems developed, they share several common characteristics such as the use of complex interventions often including botanical medications; individualised diagnosis and treatment of patients; an emphasis on maximising the body's inherent healing ability; and treatment of the “whole” patient by addressing their physical, mental, and spiritual attributes rather than focusing on a specific pathogenic process as emphasised in western biomedicine.
Despite this emphasis on multimodality treatment regimens, most research investigating traditional systems of medicine have examined only one, or perhaps two, interventions taken from a whole treatment system. For instance, there are hundreds of small studies examining the efficacy of acupuncture needling alone for treating asthma, pain, hypertension, or nausea. Yet in real practice, acupuncture needling would be just one of an arsenal of interventions used by a licensed acupuncturist including botanical potions, cupping, dietary changes, exercise therapy (such as Tai Chi or Qi Gong), moxibustion, and Chinese massage. Similarly interventions such as yoga, a single botanical medication, or meditation are just single components of complex systems of medicine. So investigators are faced with either designing a trial of a single intervention that does not accurately reflect true clinical practice or undertaking a multifaceted intervention trial that is complicated to design and implement.
Research design is further confounded by the wide variation in how many forms of complementary and alternative medicine are practised. For instance, there are multiple approaches of chiropractic medicine and acupuncture practised in the United States. Within these approaches the treatment may vary for individual patients presenting with the same conventional diagnosis because practitioners often focus on the symptoms of the disease rather than a primary pathology. Furthermore, the number and length of treatments and the specific treatment used may vary both between individuals and for an individual during the course of treatment. For example, when designing a randomised controlled trial for acupuncture, the investigator is faced with choices concerning the selection of points, the depth of needle insertion, and the frequency and scheduling of treatment. Unless these choices are made in an evidence based fashion, the trial will be compromised.
Difficulties in accruing, randomising, and retaining patients are other potential areas of concern. Some issues common to all clinical trials, such as the use of broad exclusion criteria and inadequate outreach to underserved populations, can limit patient participation and reduce generalisability. We also know that patients with a strong preference for a particular treatment will refuse randomisation.13–15 Moreover, should patients accept randomisation, the easy access of dietary supplements and other complementary interventions in the open market greatly increases the likelihood of “cheating” by the control group. This problem has also been found in trials of dietary and behavioural interventions used in conventional medicine.16
Finding appropriate placebos or shams for treatments such as acupuncture, chiropractic, massage therapy, or complex herbal mixtures is challenging. Complementary and alternative treatments typically involve extended and intensive interactions between the patient and the practitioner, which greatly increase the possibility of a placebo effect.17,18 Double blinding of the interventions may not be possible because the experienced practitioner will know which treatment is sham and which the intervention. The practitioner, in turn, may consciously or unconsciously convey this information to the patient. The variability of practice also affects the choice of a placebo.19 For instance, superficial insertion of acupuncture needles at valid acupuncture points has been used as a control in many acupuncture trials.20,21 Yet, the Japanese school of acupuncture advocates that such superficial needling is effective, and some research supports this view.22
Approaches to good design
Given the complex nature of diagnosis and treatment in traditional systems of medicine, how should we design clinical trials? Approaches vary from that of the typical pharmaceutical drug trial, in which strict, standardised diagnostic criteria are used with a defined and standardised treatment, to the other extreme, in which investigations of a whole system are undertaken in its proper context so that both the diagnosis and treatment may be highly individualised.
In studies of a system of traditional medicine to treat a specific disease the investigators consider the system as a whole, instead of a single core modality. These full spectrum studies can be done without identifying the underlying mechanism of action for each intervention, provided there is a clear, clinically relevant end point. For example, NCCAM is currently supporting a phase II randomised trial comparing three approaches to treating women with temporomandibular disorder: naturopathic medicine, traditional Chinese medicine, and usual conventional care. Patients randomised to receive either naturopathic or Chinese medicine are diagnosed and treated in the traditional manner. The end points for the study include validated measures of temporomandibular disease as well as reassessment of the naturopathic or Chinese medicine diagnosis, with all variables being analysed on an intention to treat basis.
A second approach is to study a specific modality adapted from a traditional system of medicine for treating a specific disease. NCCAM currently supports several such trials, including a double blind randomised controlled trial of acupuncture using traditional Chinese medicine needling points specific for depression. The treatment is compared with acupuncture at points that are used to treat other conditions and a waiting list control. The acupuncture treatments are individualised and based on the Chinese medicine diagnosis. Blinding is maintained by having different practitioners diagnose, treat, and evaluate the patients. Monthly assessment by the diagnosing acupuncturist allows for modifications of the treatment plan as needed. The outcome measures include both standard measures of depression (such as the Hamilton rating scale for depression) and reassessment of the Chinese medicine diagnosis, with all analysis done on an intention to treat basis.
A third approach is a trial of a single intervention, such as a herbal medicine to treat a conventionally diagnosed disease. This is the most common approach currently used to investigate complementary and alternative medicine, and ongoing trials are studying hypericum for depression; acupuncture for symptomatic relief of osteoarthritis; G biloba for preventing dementia; shark cartilage as an adjunctive therapy for non-small cell lung cancer; and glucosamine and chondroitin for osteoarthritis.
All of the above examples are randomised controlled trials. They show that despite increases in complexity and possibly cost, it is possible to design high quality trials investigating complementary and alternative medicine. However, the trials require much more preparation than trials of conventional medicine and individual trial components (blinding, placebo, consistency of intervention even if individualised, etc) often need extensive piloting before the trial.
Although randomised controlled trials are the accepted standard of clinical research, NCCAM values other types of high quality research, including careful observational studies. For many complementary and alternative therapies, there is no reliable information concerning the types of practices used for particular diseases or conditions; the numbers and types of patients who use them; how the practices are delivered (including dose used); how well patients respond to treatment; and relevant side effects. These issues can be investigated in observational studies. In addition, observational studies afford pragmatic ways of answering some types of questions, such as the evaluation of rare adverse events, as well as being a viable research option when randomisation of patients might be considered unethical or unacceptable.
The conduct of high quality research on complementary and alternative medicine requires a commitment by the research community, as well as sustained financial support from governments and industry. This commitment is essential if the public and healthcare providers are to have sufficient information on safety and efficacy to make informed decisions concerning use of complementary and alternative medicine. We envision that compelling data will facilitate meaningful interactions between conventional and complementary practitioners and ultimately lead to the development of interdisciplinary partnerships that incorporate validated complementary practices into patient care.
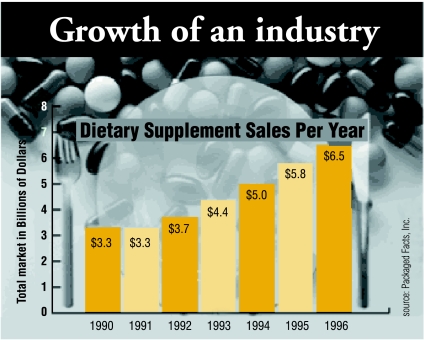
Figure.
National Institutes of Health data show steep growth in expenditure on dietary supplements
Footnotes
Competing interests: None declared.
References
- 1.Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Rompay MV, et al. Trends in alternative medicine use in the United States, 1990-1997. Results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 2.Pelletier KR, Marie A, Krasner M, Haskell WL. Current trends in the integration and reimbursement of complementary and alternative medicine by managed care, insurance carriers, and hospital providers. Am J Health Promot. 1997;12:112–122. doi: 10.4278/0890-1171-12.2.112. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Complementary and Alternative Medicine. Five year strategic plan. http://nccam.nih.gov (accessed 13 December 2000).
- 4.Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. St John's wort for depression—an overview and meta-analysis of randomized clinical trial. BMJ. 1996;313:253–258. doi: 10.1136/bmj.313.7052.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409–1415. doi: 10.1001/archneur.55.11.1409. [DOI] [PubMed] [Google Scholar]
- 6.Wilt TJ, Ishani A, Stark G, MacDonald R, Lau J, Mulrow C. Saw palmetto extracts for treatment of benign prostatic hyperplasia. A systematic review. JAMA. 1998;280:1604–1609. doi: 10.1001/jama.280.18.1604. [DOI] [PubMed] [Google Scholar]
- 7.McAlindon TE, LaValley MP, Gulin JP, Felson DT. Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta-analysis. JAMA. 2000;283:1469–1475. doi: 10.1001/jama.283.11.1469. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Complementary and Alternative Medicine. List of these research initiatives. http://nccam.nih.gov/nccam/fi/concepts (accessed 13 December 2000).
- 10.Paramore LC. Use of alternative therapies: estimates from the 1994 Robert Wood Johnson Foundation National Access to Care Survey. J Pain Symptom Manage. 1997;13:83–89. doi: 10.1016/s0885-3924(96)00299-0. [DOI] [PubMed] [Google Scholar]
- 11.Druss BG, Rosenheck RA. Association between use of unconventional therapies and conventional medical services. JAMA. 1999;282:651–656. doi: 10.1001/jama.282.7.651. [DOI] [PubMed] [Google Scholar]
- 12.Astin JA, Pelletier KR, Marie A, Haskell WL. Complementary and alternative medicine use among elderly persons: one-year analysis of a Blue Shield Medicare supplement. J Gerontol A Biol Sci Med Sci. 2000;55:M4–M9. doi: 10.1093/gerona/55.1.m4. [DOI] [PubMed] [Google Scholar]
- 13.Richardson MA, Post-White J, Singletary SE, Justice B. Recruitment for complementary/alternative medicine trials: who participates after breast cancer. Ann Behav Med. 1998;20:190–198. doi: 10.1007/BF02884960. [DOI] [PubMed] [Google Scholar]
- 14.Ellis PM. Attitudes towards and participation in randomised clinical trials in oncology: a review of the literature. Ann Oncol. 2000;11:939–945. doi: 10.1023/a:1008342222205. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins V, Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br J Cancer. 2000;82:1783–1788. doi: 10.1054/bjoc.2000.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Multiple Risk Factor Intervention Trial Research Group. Multiple risk factor intervention trial. Risk factor changes and mortality results. JAMA. 1982;248:1465–1477. [PubMed] [Google Scholar]
- 17.Roberts AH, Kewman DG, Mercier L, Hovell MF. The power of nonspecific effects in healing: implications for psychosocial and biological treatments. Clin Psych Review. 1993;13:375–391. [Google Scholar]
- 18.Brody H. The placebo response. Recent research and implications for family medicine. J Fam Pract. 2000;49:649–654. [PubMed] [Google Scholar]
- 19.Eskinazi D, Muehsam D. Factors that shape alternative medicine: the role of the alternative medicine research community. Altern Ther Health Med. 2000;6:49–53. [PubMed] [Google Scholar]
- 20.Vincent C, Lewith G. Placebo controls for acupuncture studies. J R Soc Med. 1995;88:199–202. [PMC free article] [PubMed] [Google Scholar]
- 21.Hammerschlag R. Methodological and ethical issues in clinical trials of acupuncture. J Altern Complement Med. 1998;4:159–171. doi: 10.1089/acm.1998.4.159. [DOI] [PubMed] [Google Scholar]
- 22.Birch S, Jamison RN. Controlled trial of Japanese acupuncture for chronic myofascial neck pain: assessment of specific and nonspecific effects of treatment. Clin J Pain. 1998;14:248–255. doi: 10.1097/00002508-199809000-00012. [DOI] [PubMed] [Google Scholar]