Abstract
Background:
The regulation of p21 in the pyroptosis of cartilage cells still needs to be further clarified. We aimed to explore the regulation of p21 on the pyroptosis of cartilage cells and to reveal the improvement of osteoarthritis.
Methods:
Chondrocytes were collected and isolated from patients with osteoarthritis (average age 58.64 ± 4.32) in Xuzhou Third People’s Hospital, China in 2019, and healthy volunteers (average age 58.23 ± 3.91) were enrolled as the control group. mRNA expression levels of p21 and pyroptosis-related proteins (NLRP3, ASC, total caspase1 and cleaved-Caspase1) were detected by Western blot and real-time PCR. Cell activity, total number of cells and number of dead cells were calculated with CCK-8, MTT. And the regulation of p21 on the pyroptosis of cartilage cells was verified with overexpression and knockdown of p21 in cartilage cells.
Results:
In cartilage cells of patients with osteoarthritis, the transcription and translation levels of pyrolysis-related genes (NLRP3, cleaved-caspase 1, and ASC) significantly increased (P<0.01). p21 expression was up-regulated and positively correlated with the changing trend of pyrolysis-related proteins (P<0.01). Overexpressing p21 genes in normal cartilage cells significantly increased the expression of pyrolysis-related proteins (P<0.01).
Conclusion:
The pyroptosis of cartilage cells is causally related to the process of osteoarthritis, and can be regulated by transcription factor p21, which is a potential therapeutic target for osteoarthritis.
Keywords: Osteoarthritis, p21, Pyroptosis, Cartilage cells
Introduction
As a chronic degenerative joint disease, osteoarthritis can affect almost all large and small joints in the body, with multiple complex pathogenic factors, which further leads to joint damage. The symptoms of osteoarthritis include pain, short morning stiffness, and a feeling of stumbling while walking. The characteristics of end-stage osteoarthritis are physical instability and disability, which have a significant negative impact on quality of life (1). However, there is currently a lack of effective and curable treatment options for osteoarthritis. It poses significant challenges to the clinical treatment of osteoarthritis due to the constantly changing pathophysiological processes and various pathogenic factors.
Inflammatory factors are upregulated during osteoarthritis mainly through signal transduction, including nuclear factor κB (NF-κB), mitogen-activated protein kinase, and other inflammations and stress-induced pathways (2). Osteoarthritis is still considered to be a kind of all-articular diseases associated with pathological changes in all involved tissues. The pathology changes of osteoarthritis include progressive loss and destruction of cartilage, subchondral sclerosis, osteophyte formation, varying degrees of synovial inflammation, degeneration of ligaments and meniscus, and hypertrophy of the entire articular capsule, which may be closely related to pain in osteoarthritis. Articular cartilage is a highly specialized tissue, the survival of cartilage cells playing an important role in the maintenance of cartilage matrix and the integrity of joints. In patients with osteoarthritis, the decrease in the number of cartilage cells is a key evidence of cartilage cell death as an important factor in the progression of osteoarthritis (3).
Pyroptosis is a proinflammatory cell death triggered by inflammatory corpuscles, which will induce cell rupture and release cell contents, similar to necrosis, associated with development, homeostasis, and senescence of cells (4,5). Therefore, it is of great significance to determine the role of pyroptosis of cartilage cells in osteoarthritis and to explore its regulatory mechanism for the treatment of osteoarthritis. Transcription factor p21 is an important regulator involved in a variety of physiological and pathological processes. There has no clear study on the pyroptosis of cells, especially cartilage cells.
We aimed to explore the pyroptosis levels of cartilage cells in patients with osteoarthritis, and to verify the regulation of transcription factor p21 on the pyroptosis of cartilage cells.
Methods
Primary culture of human cartilage cells
Chondrocytes in tissue specimens were collected from 28 patients who underwent the surgery of joint replacement for osteoarthritis and 10 patients with traumatic amputation. Their average age was 58.64 ± 4.32 yr, with 26 males and 12 females. A control group consisting of 10 age-matched healthy volunteers (average age 58.23 ± 3.91, 7 males and 3 females) were also enrolled. All the participants were admitted to Xuzhou Third People’s Hospital (Xuzhou, China) from January 2019 to January 2020, with signed informed consent.
This study has been approved by the Ethics Committee of Xuzhou Third People’s Hospital (Approval No. 2019-03-005-K01).
Human normal cartilage cells and osteoarthritis cartilage cells were isolated and cultured according to the methods of primary culture in previous studies. Specific methods are outlined below: Cartilage tissues were immediately put into aseptic PBS solution after collecting on ice-bath, and quickly transferred to a super clean table. Then, the processed cartilage tissues were added into a new petri dish, and PBS containing double anti-penic streptomycin was added to remove the synovium, fat and ligaments on cartilage tissues, followed by three times of washing. The above cartilage tissues were cut into fragments about 1 mm3 with an ophthalmic scissors, with 2 mL trypsin added, and digested at 37 °C for 30min. The digestion suspension was centrifuged in the centrifugal machine at 1000 rpm for 5 min, then the supernatant was discarded, and 2 mL collagenase was added after washing with PBS. After digestion overnight, the suspension was filtered in a 70-μm cell filter, and then centrifuged again at 1000 rpm for 5 min. After repeating the centrifugation once, the precipitate was resuspended by adding DMEM-F12 medium containing 10% FBS and 1% double resistance to penicillin streptomycin, and inoculated in petri dishes after being beaten and mixed, and put into constant-temperature incubator for incubation. The solution was changed every 2 days, and the cells were digested and passed with trypsin when 80% ~ 90% of the cells were covered with the plate bottom. The cells of the 3rd ~ 5th generation were collected for subsequent experiments.
RNA extraction and qPCR amplification
Total RNA was extracted from cells with TRIzol reagent (Invitrogen, USA). Total RNA was extracted from cartilage cells with TRIzol reagent. p21 and the levels of pyroptosis-related factors (ASC and NLRP3) were detected by SYBR Green method and ABI 7500fast real-time PCR system (Applied Biosystems, USA) with high-volume cDNA reverse transcription kit (Applied Biosystems, Cargo No.: 4368814) according to the instructions. As internal control, the primer sequences of GAPDH are shown in Table 1. All experiments were repeated three times.
Table 1:
Primer sequences of GAPDH
| Gene | Primer Sequence |
|---|---|
| p21 | F: 5′- GAGGGCAAGTACGAGTGGCAA3′ |
| R: 5′- CTGCGCATTGCTCCGCTAACC3 | |
| NLRP3 | F: 5′- CAACCTCACGTCACACTGCT3′ |
| R: 5′- TTTCAGACAACCCCAGGTTC-3′ | |
| ASC | F: 5′- CTGACGGATAGAGCAGTACCA3′ |
| R: 5′- CAGGATGATTTGGTGGGATT3′ | |
| GAPDH | F: 5′- CCACCCATGGCAAATTCCATGGCA3′ |
| R: 5′- TCTAGACGGCAGGTCAGGTCC3′ |
Western blot analysis
The steps of protein extraction and western blot analysis were performed with reference to literature. Antibody: p21 (1:1000 diluted, ab109520, abcam, USA), NLRP3 (1:1000 diluted, ab263899, abcam, USA), total caspase 1 (1:1000 diluted, ab179515, abcam, USA), cleaved-caspase 1 (1:1000 diluted, Asp297, CST, USA), ASC (1:1000 diluted, ab151700, abcam, USA), GAPDH (1:1000 diluted, ab8245, abcam, USA). The related detection was performed with polyclonal goat anti-rabbit antibody (Cell Signaling Technology) and Western blotting detection system (Odyssey).
Cellular transfection
Cartilage cells were inoculated into 6-well plates with a density of 2×106 cells/holes. Before transfection, the serum-free medium was cultured for 24 h. According to the instructions, 100 nM pcDNA3.1p21 plasmids were transfected with Lipofect 2000 transfection reagent, with the pcDNA3.1 vectors, which were taken as the control group. p21 knockdown siRNA and its negative control (Negative control, NC) were purchased from Santa cruz biotechnology (Cargo No.: sc-29428, USA), with NC sequences CUACAACAGCCACAACGUCdTdT. The transfection operation was conducted according to the product specification. After transfection for 6h, the transfected medium was replaced with the conventional medium. After transfection for 48h, the cells were harvested and used in vitro experiments.
Cell proliferation and cell count
Cartilage cells were inoculated at 96-well plates after transfection for 48h, CCK-8 reagent or trypan blue reagent (10μL/holes) was added to each hole, and then incubated continuously for 4 h. After that, the optical density values (OD 450 nm) and the number of cells were measured by ELIASA or cell counter. All experiments were repeated three times.
Statistical analysis
All the results were expressed as mean ± standard errors (S.E.M). The statistical comparison was carried out by one-way ANOVA between the two groups, and P <0.05 means that the difference was statistically significant. Data analysis was performed with GraphPad Prism 7.0 software. Correlation analysis was conducted with regression analysis, correlation coefficient was expressed with r2.
Results
Pyroptosis levels of cartilage cells in patients with osteoarthritis increased significantly
The differences in the expression of Nlrp3 and Asc in cartilage cells between the two groups are shown in Fig. 1. The pyrolysis levels of cartilage cells in patients with osteoarthritis significantly increased, suggesting that cartilage damage during osteoarthritis was significantly associated with the pyrolysis upregulation of cartilage cells (P<0.01).
Fig. 1:
Changing expression of pyroptosis-related proteins of cartilage cells in patients with osteoarthritis
Note: A–B: mRNA levels of pyroptosis-related genes, Nlrp3 and Asc, of cartilage cells in each group was detected by real-time method, n=5, **P<0.01
p21 expression of cartilage cells in patients with osteoarthritis was positively correlated with the pyroptosis levels of cartilage cells
p21 expression levels in normal cartilage cells and osteoarthritis cartilage cells were shown in Fig. 2A. mRNA expression of cartilage cells in patients with osteoarthritis was significantly up-regulated (P<0.01). A correlation analysis of p21 expression with the levels of pyroptosis-related factors was performed, with the results shown in Fig. 2B–E. p21 was positively correlated with the mRNA and protein levels of NLRP3, c-caspase 1, and ASC, namely, p21 was positively correlated with the pyroptosis of cartilage cells, suggesting that p21 can be an important regulatory factor to mediate the pyroptosis of cartilage cells.
Fig. 2:
Trends in expression of transcription factor p21 positively correlated with the pyroptosis of cartilage cells
Note: A: Expression levels of p21 in normal cartilage cells and osteoarticular cartilage cells were detected with real-time PCR method, n=5, ** P<0.01; B–E: Correlation between p21 and the pyroptosis-related factors of cartilage cells was analyzed by regression curve, with the correlation degree presented by r2, n=10
Overexpression of transcription factor p21 promoted the pyroptosis of cartilage cells
As shown in Fig. 3 A–C, after transfection, p21 overexpression vectors significantly increased the mRNA and protein levels of p21 in cartilage cells, and the feasibility of transfection efficiency was verified. p21 overexpression significantly increased the mRNA and protein levels of NLRP3, c-caspase 1, and ASC in cartilage cells. Cell activity and number of cartilage cells significantly decreased (Fig. 3D,E), while cell death was significantly upregulated in the overexpression group (Fig. 3F).
Fig. 3:
p21 overexpression significantly increased pyroptosis levels of cartilage cells
Note: A, B: Transfection efficiency of p21 overexpression vectors were verified with Western blot, real-time PCR method, n=5, ***P<0.001; C–E: Levels of NLRP3, caspase 1, and ASC in cartilage cells of each group were detected by Western blot method, n=5, ***P<0.001; G, H: Levels of Nlrp3 and Asc in cartilage cells of each group were detected by Nlrp3, of the pyroptosis of cartilage cellsrelated genes in cartilage cells of each group were detected with real-time method, n=5, ***P<0.001; I–K: Cell activity, cell number and number of dead cells were detected with CCK-8 and trypan blue staining, n=5, ***P<0.001
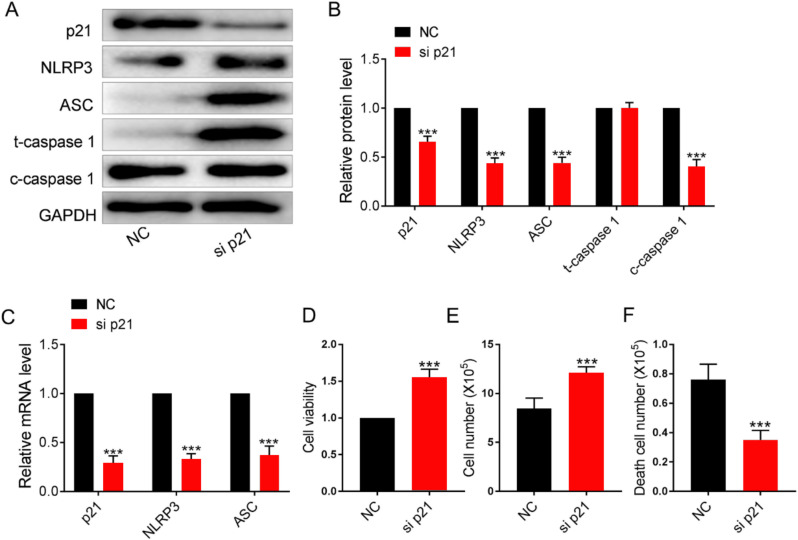
Knockdown of transcription factor p21 inhibited the pyroptosis levels of cartilage cells in patients with osteoarthritis
As shown in Fig. 4A–C, the transfection of si p21 significantly decreased p21 expression level, significantly inhibited the pyroptosis-related factors of cartilage cells, enhanced cell activity and quantity (Fig. 4D,E), and reduced the number of dead cells (Fig. 4F).
Fig. 4:
Knockdown of transcription factor p21 inhibited the pyroptosis levels of osteoarthritis cartilage cells
Note: A,B: The transfection efficiency of p21 knockdown was checked with Western blot, namely, real-time PCR method, n=5, ***P<0.001; C–E: Levels of NLRP3, caspase 1, and ASC in cartilage cells of each group were detected by Western blot method, n=5, ***P<0.001; G,H: mRNA levels of the pyroptosis of Nlrp3 and Asc in cartilage cells of each group were detected with real-time PCR method, n=5, ***P<0.001; I–K: Cell activity, cell number and number of dead cells were detected with CCK-8 and trypan blue staining, n=5, ***P<0.001
Discussion
Metabolic disorders, aging, infectious joint diseases and damages are major risk factors for osteoarthritis, which can induce cartilage tissues to generate various types of DAMPs or PAMPs, including adipokines, microcrystals, and uric acid, exacerbating the incidence of osteoarthritis (6,7). Transcription factor Cbfa1 can promote osteo-blast differentiation and chondrocyte maturation, while Cbf β in cartilage cells can mediate cartilage cell differentiation by regulating downstream Runx3 (8,9). Articular cartilage cells are used as therapeutic targets for repairing joint degeneration (10–14). Cartilage degeneration may be caused by the decrease of cartilage cells in articular cartilage tissues of the elderly, and cannot properly regenerate and remodel the cartilage (15). However, the apoptosis of cartilage cells is contradictory in the pathogenesis of osteoarthritis, with the apoptosis level varying from less than 1% to about 20% (16,17). It suggested that in addition to the apoptosis of cartilage cells, there may be other procedural death processes.
Inflammatory corpuscles consist of NLRP3, ASC, and caspase 1 (18), suggesting that the pyroptosis of cartilage cells may be closely related to the pathological changes associated with osteoarthritis. In addition, pain in osteoarthritis is associated with the severity of inflammation, especially in cartilage tissues, indicating that the occurrence of the pyroptosis of cartilage cells further promotes inflammatory factors, mediating pain in osteoarthritis. So far, a small number of studies have focused on the role of inflammatory corpuscle NLRP3 in the pathogenesis of osteoarthritis. A lot of evidence suggested that NLRP3 is a potential marker for other diseases, such as atherosclerosis, gout, and colorectal cancer. Therefore, the study on the relationship between NLRP3 and osteoarthritis has important significance.
Cartilage degeneration is the most common pathological change in osteoarthritis. Patients have metabolic disorders, aging, infectious joint diseases and damages, and so on in cartilage tissues. The joint tissues of them can generate poly-type DAMPs or PAMPs, including adipokines, microcrystals, and uric acid. Metabolic disorders are associated with the pathogenesis of osteoarthritis. Obesity is an important risk factor for the occurrence and progression of osteoarthritis. Adipokines is a kind of metabolic factors associated with obesity, which has been shown to induce cartilage degeneration. Leptin, a adipokine, adversely affects the proliferation of cartilage cells by increasing IL-1β and MMPs. In addition, obese people have lower circulating 25-hydroxyvitamin D, which is positively related to the development and deterioration of knee osteoarthritis, including cartilage cell death and increased pain. Adipose tissue macrophages (ATMs) exhibit proinflammatory M1 phenotypes in obese conditions, and showed M2 dominance under normal conditions. Proinflammatory ATMs produce tumor-promoting cell factors, inlcuding TNF-α, IL-1β, IL-6, IL-8, IL-18 and IL-32, which can be a source of local and systemic pro-inflammatory mediators.
Factors such as oxidized low density lipoprotein and cholesterol can activate inflammatory corpuscle NLRP3. Obesity promotes the pyroptosis of cartilage cells by promoting the release of inflammatory corpuscle NLRP3, which may be associated with osteoarthritis progression. Besides, obese people also have elevated levels of lipopolysaccharide in their blood, which activates caspase-4/5/11. Caspase causes inflammatory chondrocyte death at systemic and tissue levels through atypical inflammatory corpuscles, resulting in the increase of inflammatory cartilage cell death and cartilage loss. Microcrystals, including basic calcium phosphate, calcium pyrophosphate and uric acid, are considered as a “danger signal”. BCP crystals have been found in knee and hip joints of patients with osteoarthritis, which can drive osteoarthritic cartilage lesions associated with pyrotosis by activating NLRP3 and releasing IL-1β from macrophages.
Past reports indicate that IL-36, a member of the IL-1 cell factor subfamily, plays a key role in inflammatory disease by stimulating the expression of inflammatory mediators in synovial fibroblasts and articular chondrocytes. Aging is also an important risk factor for many chronic diseases, as older people are more vulnerable due to reduced immune and metabolic activity. There is a highly critical event during aging, the first is the accumulation of moisture and activation of inflammatory corpuscle NLRP3, which are stimulated by endogenous by-products and then the byproducts were recognized by PRRs in macrophages, triggering chronic inflammation. In turn, chronic inflammation plays an important role in the progression of metabolic disorders, such as obesity, gouty osteoarthritis, and atherosclerosis. Under the stimulus of DAMPS, macrophages around cartilage undergo necrosis after DAMPs or PAMPs activate caspase-1 and release inflammatory corpuscles, which increases the release of cartilage surface IL-1β and IL-18, leading to increased concentration of proinflammatory cell factors in cartilage cells, further promoting pyroptosis. These cell factors stimulate cartilage cells to secrete enzymes that cause cartilage degradation, such as MMP13 and ADAMTS5, resulting in cartilage destruction (19).
Studies on the role of the pyroptosis of cartilage cells in the pathogenesis of osteoarthritis are incomplete, lack of strong evidence. p21 is considered as a cell cycle inhibitor, which can block the cell cycle by inhibiting cyclin D. Meanwhile, transcription factor p21 have many physiological functions that involved in many transcriptional, post-transcriptional and post-translational pathway regulatory (20), suggesting that p21 can be a potential target for the treatment of multiple diseases. Previous studies have shown that, both BRAC1a and BRAC1b activate p21 transcriptionally in both p53-dependent and non-dependent ways, and can also regulate p21 expression through phosphorylation of SMADs. Moreover, the activation of mitogen-activated protein kinase (MAPK) kinase pathway contributes to the regulation of TGF-β by p21. Furthermore, in cancer cells, High levels of c-myc protein inhibit the binding of TGF-β to SMAD, replacing the activator from the p21 promoter and thus activated on p21. Ligase ubiquitination and proteasomal degradation are two mechanisms that negatively regulate p53 and p21. MKRN1 is an E3 ubiquitin ligase, which increases the degradation of p53 through ubiquitination and reduces the transcription of p53 on p21. In addition, MKRN1 can directly bind to p21 and degrade p21 in a p53-independent manner.
A study has reported p21 regulation on programmed cell death, namely, apoptosis. p21 can regulate apoptosis in many types of cells. For example, it has been reported that p21 overexpression in breast cancer cell lines reduces cell sensitivity to infrared (IR)-induced apoptosis. Further experimental studies show that p21 can protect cells from IR-induced apoptosis by inhibiting CDKs. Additionally, p21 plays an important role in cell cycle progression, expression of DNA repair and apoptosis-regulated genes, including E2F families, NF-κB, c-myc, STAT, and p300/CPB. A number of studies have shown that, p21 plays a key role in tumorigenesis and promotion. The inhibition of apoptosis is the most famous oncogenic function of p21. The radiation inhibits p21 expression, reducing tumorigenesis. C-myc is an important factor involved in apoptosis, cell cycle regulation and differentiation. Studies have reported that c-myc can inhibit p21. There are many mechanisms for c-myc inhibiting p21 functions, such as the interaction of c-myc with transcription factors such as MIS-1 inhibits p21 transcription, which blocks p21 functions, leading to c-myc induction of apoptosis. In addition, the interaction of p21 with tumor necrosis factor can inhibit anti-apoptotic proteins, such as Bcl-2, C-FLIP, Bcl-XL and XIAP, and promote the induction of apoptosis. p21 can enhance the anti-apoptotic effect of these transcription factors by inducing P300/CPB and other transcriptional coactivators. In some recent studies, it has been observed that p21 is cut by caspase 3, which can lead to cancer cell apoptosis (21,22). Despite, the regulation of p21 on the pyroptosis process, especially in the pyrolysis of cartilage cells during osteoarticular process is not clear currently.
Conclusion
We focused on the pyroptosis level of cartilage cells in patients with osteoarthritis. We observed and found that the pyroptosis levels of cartilage cells was significantly upregulated in osteoarthritis. Meanwhile, transcription factor p21 was significantly upregulated in osteoarthritis cartilage cells and regulated the pyroptosis of cartilage cells. In this study, the role and regulatory factors of the pyroptosis of cartilage cells in the process of osteoarthritis were identified, which provided a new research basis and potential target for clinical treatment and pain relief of osteoarthritis.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This study was funded by:
The project of introducing clinical medical experts in Xuzhou City (Xuzhou City Government Project, Municipal Finance Bureau, Municipal Health Commission, Project Number: 2018TD012). Project start and end time: 2018 to 2023. Team name: Professor Guoyong Yin, Jiangsu Provincial People’s Hospital. Research direction: comprehensive treatment of osteoporotic fractures with surgical treatment, the development and application of new technologies for osteoarthritis treatment. Total funding: 5 million yuan (2018, project leader: Xiao Ouyang).
Jiangsu University 2018 Clinical Medicine Science and Technology Development Fund Project (Natural Science), Science and Technology Project of Jiangsu University, Project Number: JLY20180116). Project start and end time: 2018 to 2020. Project name: femoral condyle after anterior cruciate ligament rupture MRI measurement of the fossa and its relevance. Total funding: 20,000 yuan (2018, project leader: Xiao Ouyang).
Jiangsu University 2018 Clinical Medicine Science and Technology Development Fund Project (teaching reform category), Science and Technology Project of Jiangsu University, Project Number: JLY20180279. Project start and end time: 2018 to 2020. Project name: 3D printing technology in orthopedics clinical teaching. The total amount of funding is 20,000 yuan (2018, project leader: Xiao Ouyang).
Xuzhou City Special Funds for Science and Technology Innovation in 2019 (Science and Technology Project of Xuzhou Science and Technology Bureau, Project Number: KC19188). Project start and end time: 2019 to 2021. Project name: the application of digital design and 3D printing technology in the distal tibia crushing internal fixation in surgical treatment of fractures. Total funding: 80,000 yuan (2019, project leader: Xiao Ouyang).
The 2019 Educational Research Project of Nanjing Medical University (Nanjing Medical University Educational Research Project, Project Number: 2019ZC037). The start and end time of the project: 2019 to 2021. The name of the project: the research of clinical intern tutor system to enhance the innovation ability of undergraduate students. The total funding is 20,000 yuan (in 2019, project leader: Xiao Ouyang).
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Charlier E, Deroyer C, Ciregia F, et al. (2019). Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem Pharmacol, 165: 49–65. [DOI] [PubMed] [Google Scholar]
- 2.Rim YA, Nam Y, Ju JH. (2020). The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int J Mol Sci, 21: 2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo P, Gao F, Niu D, et al. (2019). The Role of Autophagy in Chondrocyte Metabolism and Osteoarthritis: A Comprehensive Research Review. Biomed Res Int, 2019: 5171602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi J, Gao W, Shao F. (2017). Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci, 42: 245–254. [DOI] [PubMed] [Google Scholar]
- 5.Ovacs SB, Miao EA. (2017). Gasdermins: Effectors of Pyroptosis. Trends Cell Biol, 27: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgiev T, Angelov AK. (2019). Modifiable risk factors in knee osteoarthritis: treatment implications. Rheumatol Int, 39: 1145–1157. [DOI] [PubMed] [Google Scholar]
- 7.An S, Hu H, Li Y, Hu Y. (2020). Pyroptosis Plays a Role in Osteoarthritis. Aging Dis, 11: 1146–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park NR, Lim KE, Han MS, et al. (2016). Core Binding Factor β Plays a Critical Role During Chondrocyte Differentiation. J Cell Physiol, 231: 162–171. [DOI] [PubMed] [Google Scholar]
- 9.Rabie AB, Tang GH, Hägg U. (2004). Cbfa1 couples chondrocytes maturation and endochondral ossification in rat mandibular condylar cartilage. Arch Oral Biol, 49: 109–118. [DOI] [PubMed] [Google Scholar]
- 10.Hoemann CD, Tran-Khanh N, Chevrier A, et al. (2015). Chondroinduction Is the Main Cartilage Repair Response to Microfracture and Microfracture With BST-CarGel: Results as Shown by ICRS-II Histological Scoring and a Novel Zonal Collagen Type Scoring Method of Human Clinical Biopsy Specimens. Am J Sports Med, 43: 2469–2480. [DOI] [PubMed] [Google Scholar]
- 11.Lee P, Tran K, Zhou G, et al. (2015). Guided differentiation of bone marrow stromal cells on co-cultured cartilage and bone scaffolds. Soft Matter, 11: 7648–7655. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Yang X, Tang S, et al. (2015). Repair of massively defected hemi-joints using demineralized osteoarticular allografts with protected cartilage. J Mater Sci Mater Med, 26: 227. [DOI] [PubMed] [Google Scholar]
- 13.McMahon LA, O’Brien FJ, Prendergast PJ. (2008). Biomechanics and mechanobiology in osteochondral tissues. Regen Med, 3: 743–759. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Teguh D, Wu JP, et al. (2015). Protein kinase C delta null mice exhibit structural alterations in articular surface, intra-articular and subchondral compartments. Arthritis Res Ther, 17: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams CS, Horton WE., Jr (1998). Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat Rec, 250: 418–425. [DOI] [PubMed] [Google Scholar]
- 16.Aigner T, Hemmel M, Neureiter D, et al. (2001). Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum, 44: 1304–1312. [DOI] [PubMed] [Google Scholar]
- 17.Héraud F, Héraud A, Harmand MF, et al. (2000). Apoptosis in normal and osteoarthritic human articular cartilage. Ann Rheum Dis, 59: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi J, Zhao Y, Wang Y, et al. (2014). Inflammatory caspases are innate immune receptors for intracellular LPS. Nature, 514: 187–192. [DOI] [PubMed] [Google Scholar]
- 19.Eckelman BP, Salvesen GS. (2006). The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem, 281: 3254–3260. [DOI] [PubMed] [Google Scholar]
- 20.Jung YS, Qian Y, Chen X. (2010). Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal, 22: 1003–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karimian A, Ahmadi Y, Yousefi B. (2016). Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst), 42: 63–71. [DOI] [PubMed] [Google Scholar]
- 22.Soria G, Gottifredi V. (2010). PCNA-coupled p21 degradation after DNA damage: The exception that confirms the rule? DNA Repair (Amst), 9: 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]