Abstract
Background
This review has been updated by a new team. The new review title is: Chawanpaiboon S, Laopaiboon M, Lumbiganon P, Sangkomkamhang US, Dowswell T. Terbutaline pump maintenance therapy after threatened preterm labour for reducing adverse neonatal outcomes. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No.: CD010800. DOI: 10.1002/14651858.CD010800.pub2.
Women with preterm labor that is arrested with tocolytic therapy are at increased risk of recurrent preterm labor. Terbutaline pump maintenance therapy has been given to such women to decrease the risk of recurrent preterm labor, preterm birth, and its consequences.
Objectives
To determine the effectiveness and safety of terbutaline pump maintenance therapy after threatened preterm labor in preventing preterm birth and its complications.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 April 2007). We updated the search in January 2010 and added the results to the awaiting classification section.
Selection criteria
Randomized trials comparing terbutaline pump maintenance therapy with alternative therapy, placebo, or no therapy after threatened preterm labor.
Data collection and analysis
Two review authors independently assessed the studies for inclusion and then extracted data from eligible studies.
Main results
We included two studies. Terbutaline pump maintenance therapy did not appear to offer any advantages over the saline placebo pump or oral terbutaline maintenance therapy in preventing preterm births by prolonging pregnancy or its complications among women with arrested preterm labor. The weighted mean difference (WMD) for gestational age at birth was ‐0.14 weeks (95% confidence interval (CI) ‐1.66 to 1.38) for terbutaline pump therapy compared with saline placebo pump for both trials combined and 1.40 weeks (95% CI ‐1.13 to 3.93) for terbutaline pump versus oral terbutaline therapy for the first trial. The second trial reported a relative risk (RR) of 1.17 (95% CI 0.79 to 1.73) of preterm birth (less than 37 completed weeks) and a RR of 0.97 (95% CI 0.51 to 1.84) of very preterm birth (less than 34 completed weeks) for terbutaline pump compared with saline placebo pump. Terbutaline pump therapy also did not result in a higher rate of therapy continuation or a lower rate of infant complications. No data were reported on long‐term infant outcomes, costs, or maternal assessment of therapy.
Authors' conclusions
Terbutaline pump maintenance therapy has not been shown to decrease the risk of preterm birth by prolonging pregnancy. Furthermore, the lack of information on the safety of the therapy, as well as its substantial expense, argues against its role in the management of arrested preterm labor. Future use should only be in the context of well‐conducted, adequately powered randomized controlled trials.
[Note: The two citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Keywords: Female; Humans; Pregnancy; Drug Administration Schedule; Infusion Pumps; Obstetric Labor, Premature; Obstetric Labor, Premature/prevention & control; Randomized Controlled Trials as Topic; Terbutaline; Terbutaline/therapeutic use; Tocolytic Agents; Tocolytic Agents/therapeutic use
Plain language summary
Terbutaline pump maintenance therapy after threatened preterm labor for preventing preterm birth
This review has been updated by a new team. The new review title is: Chawanpaiboon S, Laopaiboon M, Lumbiganon P, Sangkomkamhang US, Dowswell T. Terbutaline pump maintenance therapy after threatened preterm labour for reducing adverse neonatal outcomes. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No.: CD010800. DOI: 10.1002/14651858.CD010800.pub2.
Terbutaline pumps may not be able to prevent a woman who had preterm labor stopped with drugs going back into labor too soon.
Women who go into very early labor (before 34 weeks) and have their contractions stopped by intravenous drugs are at high risk of going back into preterm labor. Terbutaline is a drug that can relax the uterus and possibly stop contractions. Taken orally, though, it does not seem to prevent contractions returning. Another option is to use a small portable pump that feeds a continuous dose of terbutaline under the skin. The review found there are not enough large trials to show whether this is safe or effective. Some evidence suggests that terbutaline by pump does not prevent the return of preterm labor.
Background
This review has been updated by a new review team. Readers are referred to the new review for updated results (Chawanpaiboon 2014).
Preterm birth is a major cause of perinatal mortality and morbidity (Escobedo 1988; NIH 1994). Additionally, women who give birth to preterm babies are at increased risk of psychological distress (Meyer 1995). Consequently, women who experience threatened preterm labor at 34 weeks of gestation or earlier are often treated with tocolytic therapy to arrest uterine contractions and stop the progress of active labor. Tocolytics are often used in the short term to allow for administration of corticosteroids to accelerate fetal lung maturity. Because women whose labor is initially arrested with tocolytics are at high risk of recurrent preterm labor, many centers have advocated maintenance tocolytic therapy to reduce this risk. However, the effectiveness of these therapies in decreasing the risk of preterm birth or low birthweight or increasing the interval until birth is unproven (Sanchez‐Ramos 1999).
Agents of various classes have been investigated, and previous systematic reviews have shown that neither oral terbutaline nor oral magnesium sulfate is effective maintenance tocolytic therapy (Crowther 1998; Macones 1995). Moreover, these therapies may have serious adverse effects such as sudden death, pulmonary edema, cardiac arrhythmias, and hepatitis (Crowther 1998; Hudgens 1993; Perry 1995; Quinn 1994). Possible reasons cited for the lack of benefit from oral terbutaline maintenance therapy include desensitization of beta‐adrenergic receptors in the myometrium, inconsistent drug levels, and lack of compliance (Lam 1998).
In an attempt to overcome these problems, some health care providers have used a portable pump similar to an insulin pump to deliver a subcutaneous infusion of terbutaline at a low basal delivery rate with boluses as needed for increased uterine activity. Terbutaline was chosen for this purpose because it is more easily absorbed subcutaneously than other agents and is less costly. Pump therapy has several reported advantages over oral therapy: lower total daily medication dose, causing fewer side effects and less tachyphylaxis; faster onset of action, allowing control of contractions before cervical change; and improved patient compliance. The terbutaline pump also allows women to remain at home for therapy, as compared with prolonged intravenous tocolysis. This allows women to avoid sleep disruption, possibly reducing stress, and increasing her sense of control. Proponents of the therapy believe that these advantages will lead to greater prolongation of pregnancy compared with alternative or no therapy. However, reported adverse effects such as maternal death, chest pain, pulmonary edema, and tachycardia have prompted the US Food and Drug Administration to issue a warning letter to health providers regarding the use of terbutaline pump therapy for preterm labor.
Objectives
To determine the effectiveness and safety of terbutaline pump maintenance therapy after threatened preterm labor in preventing preterm birth and its complications.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomized trials with available data that compared outcomes between women given terbutaline pump maintenance therapy and controls given alternative therapy, placebo, or no therapy after successful arrest of threatened preterm labor. Trial eligibility criteria included random allocation to treatment and comparison groups and reasonable measures to ensure allocation concealment.
Types of participants
Pregnant women with intact membranes and either singleton or multiple gestations who had at least one episode of threatened preterm labor that was stopped with initial tocolytic therapy before delivery.
Types of interventions
Terbutaline pump maintenance therapy compared with alternate drug therapy, placebo, or no therapy.
Types of outcome measures
Infant
Gestational age
Preterm birth (less than 37 completed weeks)
Very preterm birth (less than 34 completed weeks)
Extremely preterm birth (less than 28 completed weeks)
Perinatal mortality
Birthweight
Respiratory distress syndrome
Periventricular hemorrhage
Need for mechanical ventilation
Neurological sequelae (general intelligence, hearing, vision, cerebral palsy, and disability)
Maternal
Cardiovascular complications (death, cardiac arrest, myocardial infarction, arrhythmia, hypotension)
Other serious complications (pulmonary edema, hepatitis)
Side effects (chest pain, palpitations, shortness of breath, hyperglycemia, hypokalemia)
Maternal compliance with therapy
Maternal assessment of therapy
Healthcare system
Rehospitalization for threatened preterm labor
Costs of treatment
Admission to neonatal intensive care unit
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (30 April 2007). We updated this in January 2010 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group's Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
monthly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness search of a further 36 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the 'Search strategies for identification of studies' section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are given a code (or codes) depending on the topic. The codes are linked to review topics. The Trials Search Co‐ordinator searches the register for each review using these codes rather than keywords.
We did not apply any language restrictions.
We searched reference lists of identified studies and existing reviews, and we consulted with experts in the field.
Data collection and analysis
We developed an inclusion checklist and data extraction sheet before evaluating identified trials. We evaluated trials for inclusion and study quality using standard criteria without consideration of their results (Clarke 2002; Moher 2001). Two review authors (Lynley Cook and Maria Gallo) each assessed the studies independently for inclusion and study quality. Discrepancies were resolved by consensus. Review authors were not blinded to authorship.
Each review author extracted data independently. A third review author (Kavita Nanda) reviewed the abstracted data for accuracy and consistency. Discrepancies in data abstraction were resolved by discussion and consensus. We sought additional information from the authors when necessary; however, we received no responses. We calculated weighted mean differences with 95% confidence intervals (CIs) for continuous outcomes such as gestational age at delivery and birthweight. We calculated relative risks with 95% CIs for the dichotomous outcomes of preterm birth, very preterm birth, respiratory distress syndrome, neonatal intensive care unit stay, and early discontinuation from therapy. Data were entered into the Cochrane Collaboration Review Manager software (RevMan 2000). The small number of studies precluded stratification by study quality. We examined outcomes separately in subgroup analyses by types of therapies:
women treated with terbutaline pump maintenance therapy compared with saline placebo pump maintenance therapy;
women treated with terbutaline pump maintenance therapy compared with oral terbutaline maintenance therapy.
Results
Description of studies
We identified four randomized controlled trials that appeared to meet the inclusion criteria; however, the investigators of the two unpublished trials did not respond to our requests for information (Morales 1990; Morrison 1993), and their trials could not be included. (Two reports from an updated search on 31 January 2010 were added to Studies awaiting classification.)
The published trial by Guinn and colleagues (Guinn 1998) recruited 52 women with singleton gestations from 22 to less than 34 weeks of gestation with intact membranes who experienced suspected preterm labor that was arrested with intravenous magnesium sulfate therapy for at least 24 hours before study entry. The study was conducted at the University of Alabama, Alabama, USA. The authors calculated sample size a priori, and determined that a total sample size of 48 women was needed to have 80% power to detect a clinically significant difference of a 14‐day delay in the interval from treatment to delivery between the two therapies. Guinn 1998 reported a strict case definition of preterm labor. Women had to experience more than four uterine contractions per hour and at least one of the following: 80% or greater effacement, one centimeter or greater dilation, or documented cervical change. Terbutaline pump maintenance therapy was compared with the placebo, saline placebo pump maintenance therapy. Both therapies used portable subcutaneous pumps (model 404‐S; MiniMed Technologies, Sylmar, CA). Terbutaline therapy consisted of the continuous administration of 1 mg terbutaline/ml saline at the rate of 0.05 ml/hour with five daily bolus injections programmed at regular intervals. The women could also self‐administer boluses of 0.25 ml twice daily for increased uterine activity. Saline placebo pump therapy was identical to terbutaline pump therapy except that saline replaced the terbutaline. Women were followed up on a weekly basis during maintenance therapy, and nursing support was constantly available. In the event of recurrent preterm labor before 34 weeks of gestation, pump therapy was discontinued, and another course of intravenous magnesium therapy was given unless there was advanced cervical dilation or ruptured membranes. Pump maintenance therapy resumed if labor was arrested. Both pump therapies continued until either delivery or 36 weeks of gestation was reached.
Wenstrom and colleagues' published trial (Wenstrom 1997) recruited 42 women with singleton or multiple gestations from 20 to less than 35 weeks of gestation who had suspected preterm labor that was arrested with intravenous magnesium sulfate therapy for at least 48 hours before study entry. The authors do not mention sample size calculations. The study was conducted at the University of Iowa, Iowa, USA. Preterm labor was defined only as regular, persistent uterine contractions that produced observed cervical change. Three maintenance therapies were compared: terbutaline pump, saline placebo pump and oral terbutaline. Terbutaline pump and saline pump therapies used the same portable pump model as in the Guinn 1998 trial. The terbutaline pump group received 1 mg terbutaline/ml saline initially administered at rate of 0.05 ml/hour with 0.25 ml bolus injections every six hours. While the basal rate was kept constant, the bolus dose was adjusted to the women's unique contraction pattern. The saline placebo pump group received therapy identical to the terbutaline pump group except that saline replaced the terbutaline. The oral terbutaline group received five to 10 mg terbutaline orally every two to six hours, titrated to maintain maternal heart rate at 90 beats per minute or higher and to prevent uterine contractions from occurring at a rate of more than four per hour. If recurrent preterm labor occurred despite maximization of therapy, the treatment arm assignment was determined and therapy was changed. Women receiving oral terbutaline or saline placebo therapy were switched to terbutaline pump, and women receiving terbutaline pump were switched to oral terbutaline. Women who had recurrent preterm labor that was not resolved after switching therapies were treated with intravenous tocolysis and aggressive management. Women were followed up on a weekly or biweekly basis according to the department's standard protocol for outpatient management of preterm labor. No gestational age was specified for the discontinuation of therapy.
Risk of bias in included studies
Treatment allocation in the Guinn 1998 trial was based on computer‐generated randomization with stratification by cervical dilation (< 3 cm versus >= 3 cm) and previous indomethacin use. Only the research pharmacist had knowledge of treatment assignment; the pharmacist prepared the pump solutions, which were clear, odorless, and indistinguishable. The authors reported only one case of loss to follow up (in the terbutaline arm).
The pharmacist in the Wenstrom 1997 trial randomized participants using a random‐number table and prepared all solutions. Again, the pump solutions were identical in appearance and only the pharmacist knew the pump assignments; however, the oral therapy was not blinded. Over 90% of eligible women in this trial declined participation. The study authors attributed the low participation rate to patient concerns about the pump and its needle or the use of placebo. Physician reluctance to the trial was not addressed. The treatment arm was broken for women with recurrent preterm labor that could not be resolved with the assigned therapy. The assignment code was broken for nine of the 15 women in the terbutaline pump arm; one was switched to oral terbutaline, five received intravenous tocolytic therapy, and three delivered. The code was broken for eight of the 12 women in the saline placebo pump arm; three were switched to terbutaline pump, three received intravenous tocolytics, and two delivered. Two of the 15 women in the oral terbutaline group received intravenous tocolytics. All cases were analyzed on an intention‐to‐treat basis. The authors did not describe any cases of loss to follow up.
We wrote to the authors of both studies to query for additional unreported outcomes. We also queried whether the numbers following the estimates in the tables, which were unlabeled, represented standard deviations. Since we did not receive a response from the authors, we assumed that they represented standard deviations.
Effects of interventions
Terbutaline pump versus saline placebo pump
Terbutaline pump maintenance therapy was not associated with a reduction in preterm birth or its complications in either trial. For the first infant outcome of gestational age at delivery, no significant difference in the mean gestational age at delivery between terbutaline pump and the saline placebo pump was found; the weighted mean difference (WMD) for gestational age at birth was ‐0.14 weeks (95% confidence interval (CI) ‐1.66 to 1.38) for both trials combined. The gestational age at delivery also did not differ by treatment when measured by the dichotomous outcomes, preterm (less than 37 completed weeks) or very preterm (less than 34 completed weeks) birth. The Guinn 1998 trial found a relative risk (RR) of 1.17 (95% CI 0.79 to 1.73) of preterm birth and a RR of 0.97 (95% CI 0.51 to 1.84) of very preterm birth for terbutaline pump compared with saline placebo pump therapy. Preterm and very preterm birth were not reported outcomes in the Wenstrom 1997 trial.
Birthweight was not significantly different between treatments. The WMD for birthweight was 107.90 g (95% CI ‐216.24 to 432.04) for terbutaline pump versus saline placebo pump therapy for both trials combined. No perinatal deaths occurred in either study, and the Guinn 1998 study reported that no cases of intraventricular hemorrhage occurred. Respiratory distress syndrome (RDS) did not appear to differ by treatment received. Both trials combined showed a summary RR of 0.82 (95% CI 0.23 to 2.93) of RDS for terbutaline pump versus saline placebo pump. The Guinn 1998 study found a RR of 0.94 (95% CI 0.51 to 1.73) for neonatal intensive care unit (NICU) stay greater than 24 hours. In the Wenstrom 1997 study no significant differences were reported in length of neonatal hospital stay between the terbutaline pump and saline placebo pump groups. Wenstrom 1997 also found no differences between these groups for Apgar scores at one and five minutes. No data were available from either study on need for mechanical ventilation or long‐term neurological sequelae.
Neither trial reported any serious complications of therapy. Terbutaline pump therapy did not result in a higher rate of therapy continuation. For both trials combined, the RR of early discontinuation from assigned therapy was 1.15 (95% CI 0.68 to 1.95) for terbutaline pump compared with the saline placebo pump. The only cases of early discontinuation reported in the Wenstrom 1997 trial were those resulting from recurrent preterm labor that required the treatment assignment be broken and therapy switched. Thus, the discontinuation rate is based on our assumption that these constituted all cases of early discontinuation. In contrast, the Guinn 1998 trial described both discontinuations resulting from physician decisions (n = 9) as well as those resulting from patient choices (n = 11). No data were presented regarding costs.
Terbutaline pump versus oral terbutaline
Only the Wenstrom 1997 trial included a comparison of terbutaline pump with oral terbutaline. In this study, the WMD for gestational age at birth was 1.40 weeks (95% CI ‐1.13 to 3.93) for terbutaline pump compared with oral terbutaline therapy. Birthweight was not significantly different; the WMD for birthweight was 484.00 g (95% CI ‐25.00 to 993.00) for terbutaline pump versus oral terbutaline therapy.
The relative risk of RDS in the Wenstrom 1997 trial was 1.00 (95% CI 0.16 to 6.20) for the terbutaline pump compared with oral terbutaline. Wenstrom 1997 also found no statistically significant differences between the terbutaline pump and oral terbutaline pump arms in terms of Apgar scores at one and five minutes or length of neonatal hospital stay. No data were available on need for mechanical ventilation or long‐term neurological sequelae. Wenstrom 1997 found no difference in early discontinuation with the terbutaline pump compared with oral terbutaline (RR 3.00, 95% CI 0.72 to 12.55).
Discussion
Terbutaline pump maintenance therapy does not appear to offer any advantages over the placebo, saline placebo pump, or oral terbutaline maintenance therapy in preventing preterm births by prolonging pregnancy among women with arrested preterm labor. Neither trial found terbutaline pump therapy to be efficacious in increasing gestational age at birth or in reducing preterm birth and its complications.
Both trials had small sample sizes. The Guinn 1998 trial only enrolled 52 women; however, the sample size was calculated to have sufficient power to detect a clinically significant difference (two weeks) between the two therapies in the interval from initiation of therapy to delivery. The Wenstrom 1997 trial enrolled only 42 women despite an enrollment period of four years and four months. Over 90% of eligible women declined participation in the study. Although the authors state that no apparent differences existed between those who agreed to participate and those who declined, the possibility of differences cannot be eliminated. Both trials restricted enrollment to women with arrested preterm labor and, thus, at high risk for preterm delivery. The ability to detect an association between terbutaline pump therapy and preterm birth, most likely, was strengthened by the exclusion of women with lower risk of preterm birth.
Terbutaline pump therapy did not have a higher rate of continuation than the other therapies. Furthermore, the safety of terbutaline pump maintenance therapy was not assessed adequately. Neither trial was designed to have adequate statistical power to evaluate major adverse events that occur at an infrequent rate. With an average cost of $200/day compared with oral therapy or no therapy (Adkins 1993), terbutaline pump therapy imposes an additional expense without proven benefits.
Authors' conclusions
Implications for practice.
Terbutaline pump maintenance therapy has not been shown to decrease the risk of preterm birth by prolonging pregnancy. Furthermore, the lack of information on the safety of the therapy, as well as its substantial expense, argues against its use in the management of arrested preterm labor. Terbutaline pump maintenance therapy should not be used outside well‐designed randomized controlled trials.
Implications for research.
Researchers who consider that the terbutaline pump might be an effective maintenance therapy should conduct adequately powered well‐designed randomized controlled trials.
[Note: The two citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
What's new
| Date | Event | Description |
|---|---|---|
| 25 March 2014 | Amended | This review is no longer being updated. A new team have produced a new protocol and published a review on the same topic. Text has been added to the Abstract, Plain language summary, Background, and Published notes sections to indicate that this review has been updated by a new team. Readers are referred to the new review (Chawanpaiboon 2014) for the most up‐to‐date results. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 29 January 2013 | Amended | Contact details updated. |
| 31 January 2010 | Amended | Seach updated. Two reports added to Studies awaiting classification. |
| 12 May 2009 | Amended | Contact details updated. |
| 20 September 2008 | Amended | Converted to new review format. |
| 30 April 2007 | New search has been performed | Search updated. No new trials identified. |
Notes
This review has been updated by a new review team. Readers are referred to the new review for updated results (Chawanpaiboon 2014).
Acknowledgements
None.
Data and analyses
Comparison 1. Terbutaline pump versus saline pump.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal intensive care unit admission > 24 hours | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.51, 1.73] |
| 2 Gestational age at delivery (in weeks) | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐1.66, 1.38] |
| 3 Preterm birth (< 37 weeks) | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.79, 1.73] |
| 4 Very preterm birth (< 34 weeks) | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.51, 1.84] |
| 5 Early discontinuation | 2 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.68, 1.95] |
| 6 Respiratory distress syndrome | 2 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.23, 2.93] |
| 7 Birthweight | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | 107.90 [‐216.24, 432.04] |
1.1. Analysis.
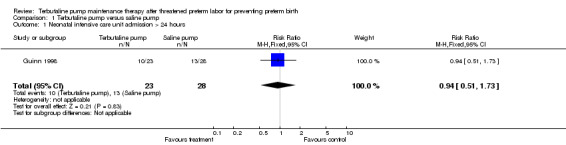
Comparison 1 Terbutaline pump versus saline pump, Outcome 1 Neonatal intensive care unit admission > 24 hours.
1.2. Analysis.
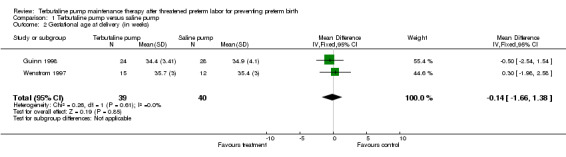
Comparison 1 Terbutaline pump versus saline pump, Outcome 2 Gestational age at delivery (in weeks).
1.3. Analysis.
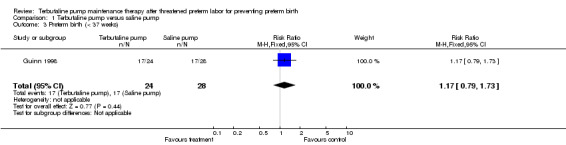
Comparison 1 Terbutaline pump versus saline pump, Outcome 3 Preterm birth (< 37 weeks).
1.4. Analysis.
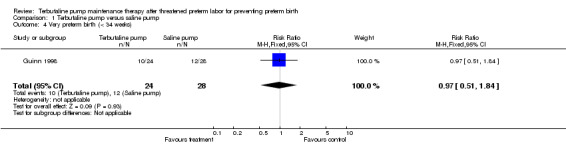
Comparison 1 Terbutaline pump versus saline pump, Outcome 4 Very preterm birth (< 34 weeks).
1.5. Analysis.
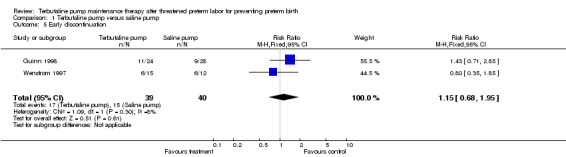
Comparison 1 Terbutaline pump versus saline pump, Outcome 5 Early discontinuation.
1.6. Analysis.
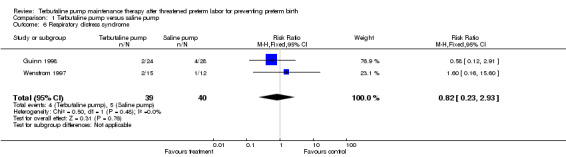
Comparison 1 Terbutaline pump versus saline pump, Outcome 6 Respiratory distress syndrome.
1.7. Analysis.
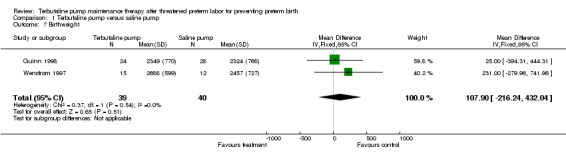
Comparison 1 Terbutaline pump versus saline pump, Outcome 7 Birthweight.
Comparison 2. Terbutaline pump versus oral terbutaline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birthweight | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 484.0 [‐25.00, 993.00] |
| 2 Gestational age at delivery (in weeks) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐1.13, 3.93] |
| 3 Early discontinuation | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.72, 12.55] |
| 4 Respiratory distress syndrome | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.20] |
2.1. Analysis.
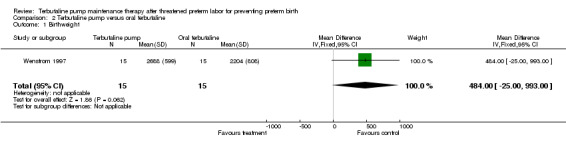
Comparison 2 Terbutaline pump versus oral terbutaline, Outcome 1 Birthweight.
2.2. Analysis.
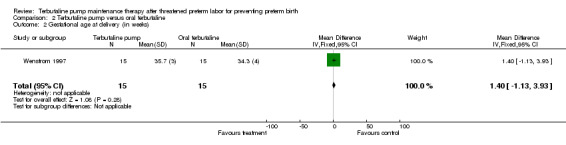
Comparison 2 Terbutaline pump versus oral terbutaline, Outcome 2 Gestational age at delivery (in weeks).
2.3. Analysis.
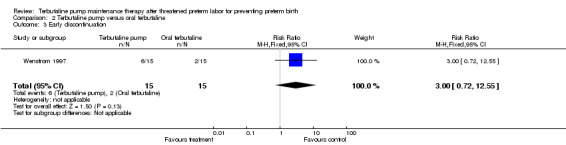
Comparison 2 Terbutaline pump versus oral terbutaline, Outcome 3 Early discontinuation.
2.4. Analysis.
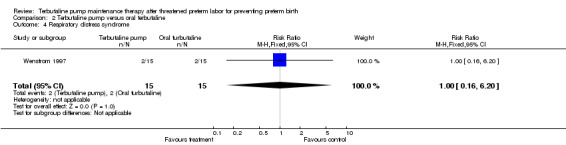
Comparison 2 Terbutaline pump versus oral terbutaline, Outcome 4 Respiratory distress syndrome.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Guinn 1998.
| Methods | Computer‐generated randomization with stratification by cervical dilation ( <3 cm versus >= 3 cm) and previous indomethacin use. Research pharmacist prepared identical pump solutions. Participant, investigator and outcome assessor were masked. | |
| Participants | 52 women with singleton gestations and intact membranes from 22 to 33 6/7 weeks' gestation with preterm labor, defined as > 4 uterine contractions/hour and at least one of: >= 1 cm cervical dilation; >= 80% cervical effacement; documented cervical change. Only women who received magnesium sulfate therapy and had arrested labor (i.e. < 4 contractions/hour for >= 24 hours) were eligible. Exclusions included contraindications to tocolysis, persistent maternal tachycardia, history of cardiac arrythmia, history of pulmonary edema, uncontrolled diabetes, suspected chorioamnionitis. | |
| Interventions | (1) Terbutaline pump group (n = 24) received 1 mg terbutaline/1 mL saline at 0.05 mL/hour with bolus injections 5 times daily. Participant could self‐administer 0.25 mL twice daily for increased uterine activity. (2) Saline pump group (n = 28) received identical regimen to terbutaline pump group except terbutaline replaced with saline. Both groups received intensive education. | |
| Outcomes | Primary outcome: mean interval time from initiation of maintenance therapy to delivery. Secondary outcomes: episodes of recurrent preterm labor; delivery < 37 weeks, delivery < 34 weeks; gestational age at delivery; mean birthweight; neonatal intensive care stay > 24 hours; respiratory distress syndrome; intraventricular hemorrhage; discontinuations from therapy. | |
| Notes | Sample size designed to have 80% power to detect a 2‐week difference in time to delivery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Wenstrom 1997.
| Methods | Randomization by random‐number table. Pharmacist prepared identical solutions. Participant, investigator, and outcome assessor masked to the 2 pump therapies but not oral therapy. | |
| Participants | 42 women >= 20 to < 35 weeks' gestation with preterm labor, defined as with regular, persistent uterine contractions with cervical change. Only women who received intravenous magnesium sulfate therapy and, if needed, oral indomethacin and had arrested labor were eligible. Exclusions included contraindications to betamimetics, contraindications to tocolysis; cervical dilation > 4 cm. | |
| Interventions | (1) Terbutaline pump group (n = 15) received 1 mg terbutaline/1 mL saline at 0.05 mL/hour with 0.25 bolus injections every 6 hours. Bolus dose adjusted to women's contraction pattern. (2) Saline pump group (n = 12) received identical regimen to terbutaline pump group except terbutaline replaced with saline. (3) Oral terbutaline group (n = 15) received 5‐10 mg terbutaline orally every 2‐6 hours titrated to keep maternal heart rate >= 90 bpm and to keep uterine contractions to <= 4/hour. All groups placed on bedrest. | |
| Outcomes | Mean gestational age at delivery; mean birthweight; drug boluses required; net gain in weeks; median Apgar scores; length of infant hospital stay; antenatal complications; neonatal complications. | |
| Notes | Over 90% of eligible women declined participation. If recurrent preterm labor occurred despite maximization of therapy, code was broken and therapy was changed (9 women in terbutaline group; 8 women in saline group). Study Table 1 reports 8 mothers with twins, but neonatal complications reported using denominator of number of participants per group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
BPM: beats per minute
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Morales 1990 | Study investigator did not respond to inquiries for study information. |
| Morrison 1993 | Study investigator did not respond to inquiries for study information. |
Contributions of authors
Kavita Nanda and David Grimes developed the idea. Kavita Nanda and Lynley Cook drafted the protocol, which was reviewed by David Grimes. Lynley Cook and Maria Gallo reviewed the studies and abstracted the data. Maria Gallo and Kavita Nanda drafted the review, which was approved by Lynley Cook and David Grimes. Kavita Nanda revised the review, which was approved by all other review authors.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Guinn 1998 {published data only}
- Guinn DA, Goepfert AR, Owen J, Wenstrom KD, Hauth JC. Terbutaline pump maintenance therapy for prevention of preterm delivery: a double‐blind trial. American Journal of Obstetrics and Gynecology 1998 Oct;179(4):874‐8. [DOI] [PubMed] [Google Scholar]
Wenstrom 1997 {published data only}
- Wenstrom KD, Weiner CP, Merrill D, Niebyl J. A placebo‐controlled randomized trial of the terbutaline pump for prevention of preterm delivery. American Journal of Perinatology 1997 Feb;14(2):87‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Morales 1990 {unpublished data only}
- Morales WJ, Marko BH. Adjusted oral terbutaline vs subcutaneous terbutaline pump therapy in the management of preterm labor. Proceedings of the 10th Annual Meeting of the Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:149.
Morrison 1993 {unpublished data only}
- Morrison JC. Terbutaline administered by subcutaneous programmable infusion pump versus oral terbutaline in those women with recurrent preterm labor. Personal communication 1993.
References to studies awaiting assessment
Elliott 1999 {published data only}
- Elliott J, Lam, Morrison J. Terbutaline [letter]. American Journal of Obstetrics and Gynecology 1999;180(6 Pt 1):1593‐5. [DOI] [PubMed] [Google Scholar]
Wenstrom 1995 {published data only}
- Wenstrom K, Weiner C, Merrill D, Niebyl J. A placebo‐controlled trial of the terbutaline (T) pump for prevention of preterm delivery [abstract]. American Journal of Obstetrics and Gynecology 1995;172:416. [DOI] [PubMed] [Google Scholar]
Additional references
Adkins 1993
- Adkins RT. Reply: Terbutaline pump treatment of premature labor [letter]. Southern Medical Journal 1993;86:1076. [DOI] [PubMed] [Google Scholar]
Clarke 2002
- Clarke M, Oxman AD. Cochrane Reviewers’ Handbook 4.1.5 [updated April 2002]. In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software. Updated quarterly.
Crowther 1998
- Crowther CA, Moore V. Magnesium maintenance therapy for preventing preterm birth after threatened preterm labour. Cochrane Database of Systematic Reviews 1998, Issue 1. [Google Scholar]
Escobedo 1988
- Escobedo MB. Follow‐up of prematurely born infants. Clinical Obstetrics and Gynecology 1988;31(3):662‐7. [DOI] [PubMed] [Google Scholar]
Hudgens 1993
- Hudgens DR, Conradi SE. Sudden death associated with terbutaline sulfate administration. American Journal of Obstetrics and Gynecology 1993;169(1):120‐1. [DOI] [PubMed] [Google Scholar]
Lam 1998
- Lam F, Elliott J, Jones JS, Katz M, Knuppel RA, Morrison J, et al. Clinical issues surrounding the use of terbutaline sulfate for preterm labor. Obstetrical & Gynecological Survey 1998;53(11 Suppl):S85‐S95. [DOI] [PubMed] [Google Scholar]
Macones 1995
- Macones GA, Berlin M, Berlin JA. Efficacy of oral beta‐agonist maintenance therapy in preterm labor: a meta‐analysis. Obstetrics & Gynecology 1995;85(2):313‐7. [DOI] [PubMed] [Google Scholar]
Meyer 1995
- Meyer EC, Garcia Coll CT, Seifer R, Ramos A, Kilis E, Oh W. Psychological distress in mothers of preterm infants. Journal of Developmental and Behavioral Pediatrics 1995;16(6):412‐7. [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [PubMed] [Google Scholar]
NIH 1994
- National Institutes of Health. Report of the Consensus Development Conference on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. NIH publication No. 95‐3784. Washington, 1994 Nov.
Perry 1995
- Perry KG Jr, Morrison JC, Rust OA, Sullivan CA, Martin RW, Naef RW 3rd. Incidence of adverse cardiopulmonary effects with low‐dose continuous terbutaline infusion. American Journal of Obstetrics and Gynecology 1995;173(4):1273‐7. [DOI] [PubMed] [Google Scholar]
Quinn 1994
- Quinn PG, Sherman BW, Tavill AS, Gibas AL. Terbutaline hepatitis in pregnancy: report of two cases and literature review. American Journal of Gastroenterology 1994;89(5):781‐4. [PubMed] [Google Scholar]
RevMan 2000 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.1 for Windows. Oxford, England: The Cochrane Collaboration, 2000.
Sanchez‐Ramos 1999
- Sanchez‐Ramos L, Kaunitz AM, Gaudier FL, Delke I. Efficacy of maintenance therapy after acute tocolysis: a meta‐analysis. American Journal of Obstetrics and Gynecology 1999;181(2):484‐90. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chawanpaiboon 2014
- Chawanpaiboon S, Laopaiboon M, Lumbiganon P, Sangkomkamhang US, Dowswell T. Terbutaline pump maintenance therapy after threatened preterm labour for reducing adverse neonatal outcomes. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD010800.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


