Abstract
Introduction
Indeterminate thyroid cytology diagnoses are associated with intermediate risks of malignancy. Application of molecular testing (MT) to indeterminate specimens provides additional diagnostic and prognostic information. While a positive or suspicious MT result may prompt surgery, a negative MT result is associated with a low probability of cancer or noninvasive follicular thyroid neoplasm with papillary-like nuclear features and approximates that of a benign cytology diagnosis. Furthermore, ThyroSeq v3 MT has a “currently negative” result for findings with the probability of cancer or noninvasive follicular thyroid neoplasm with papillary-like nuclear feature that is slightly greater than that for the negative ThyroSeq v3 MT result but less than 10%, suggesting active surveillance. In this report, we discuss a case of a patient for whom clinical, cytologic, and molecular surveillance led to timely surgery and management.
Clinical details
A 53-year-old man with a thyroid isthmus nodule had a fine-needle aspiration cytology diagnosis of atypia of undetermined significance and a subsequent ThyroSeq v3 MT, which revealed an EIF1AX mutation and a “currently negative” MT result. Surveillance with additional fine-needle aspiration samples demonstrated concerning genomic alterations (fluctuating EIF1AX allelic frequency and a non-V600E BRAF mutation), culminating in the conversion to a positive MT result 3 years later. Resection revealed an encapsulated noninvasive, oncocytic solid subtype of papillary thyroid carcinoma with increased mitotic activity.
Conclusion
The case is notable for clinical, cytologic, and molecular surveillance demonstrating sequential pathologic alterations in an indeterminate thyroid nodule with EIF1AX mutation, leading to timely resection of the neoplasm before invasion manifested.
Keywords: Molecular testing, Indeterminate thyroid nodules, ThyroSeq, Papillary carcinoma, EIF1AX
Introduction
Thyroid nodules are highly prevalent, harbored by up to 67% of the general population, by ultrasonography. However, only a small subgroup (<10%) develops malignancy.1–3 The initial assessment of thyroid nodules includes clinical examination and ultrasonography. If the clinical parameters are of concern, the next step involves fine-needle aspiration (FNA) cytology in the triage of suspicious nodules.4 The Bethesda System for Reporting Thyroid Cytopathology provides a framework to communicate the thyroid FNA diagnostic categories with associated risks of malignancy that are linked to clinical management guidelines.5 Cytologically indeterminate categories, atypia of undetermined significance (AUS; Bethesda III) and follicular neoplasm (FN; Bethesda IV) inherently have intermediate risks of malignancy (~10%−30% and 25%−40%, respectively). However, these risks may not be sufficiently high to recommend surgery. On the other hand, clinical observation may be distressing for patients with concerns that their intermediate risk nodule may represent malignancy. Since repeat FNAs result in a definitive diagnosis in only 40% of cases, other modalities are desired.6 In this regard, molecular testing has made remarkable contributions by providing additional layers of diagnostic and prognostic information for indeterminate FNA specimens.7 If the molecular test result is positive or suspicious, surgery is recommended often with the understanding that the outcome may vary depending on the type of genomic alteration. Conversely, a negative or benign molecular test result brings the probability of cancer or noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) outcome close to that of a benign cytology diagnosis (~3%−4%). Moreover, molecular test panels such as ThyroSeq v3 have an additional “currently negative” category, which is associated with a probability of cancer or NIFTP that is slightly greater than that for the negative category but less than 10%. For cytologically indeterminate thyroid cases with “currently negative” molecular results, active surveillance is suggested.8 In this short communication, we describe a patient with a cytologically indeterminate thyroid nodule with an EIF1AX mutation for which sequential FNA sampling and molecular surveillance data documented the evolution of its disease process.
Case presentation
The authors confirmed with the institutional review board that case reports do not require their approval. Nonetheless, consent was obtained from the patient to release his medical information for the purpose of this case report in an anonymous fashion. A 53-year-old man presented with right-sided neck radicular pain and incidentally was found to have a nodule within the isthmus of the thyroid gland on magnetic resonance imaging in October 2018. Serologic studies were unremarkable with a normal thyroid stimulating hormone level (4.045 μU/mL) and free T4 (1.15 ng/dL). Thyroid ultrasonography showed a 1.8 × 1.6 × 1.0 cm elongated, solid, and hypoechoic isthmus nodule (Fig. 1). The American College of Radiology Thyroid Imaging Reporting and Data System (TI-RADS) category was not provided by our radiologist since the system had not been implemented consistently at that time. FNA diagnosed as AUS demonstrated a nonspecific pattern of epithelial cells with some oncocytic features in loose sheets and microfollicles (Fig. 2A, B). As part of the evaluation of indeterminate thyroid nodules, ancillary molecular testing (ThyroSeq v3, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania) was performed and revealed a low-level, isolated EIF1AX splice-site mutation (p.A113_splice) with an allelic frequency of 11% which was associated with a 5%−10% probability of cancer or NIFTP. The overall ThyroSeq v3 result was reported as “currently negative.” In the absence of highly concerning clinical and radiologic features, he and clinical team elected active surveillance.
Figure 1.

Ultrasonographic thyroid image demonstrating a thyroid isthmus nodule with the largest transverse dimension of 1.6 cm.
Figure 2.
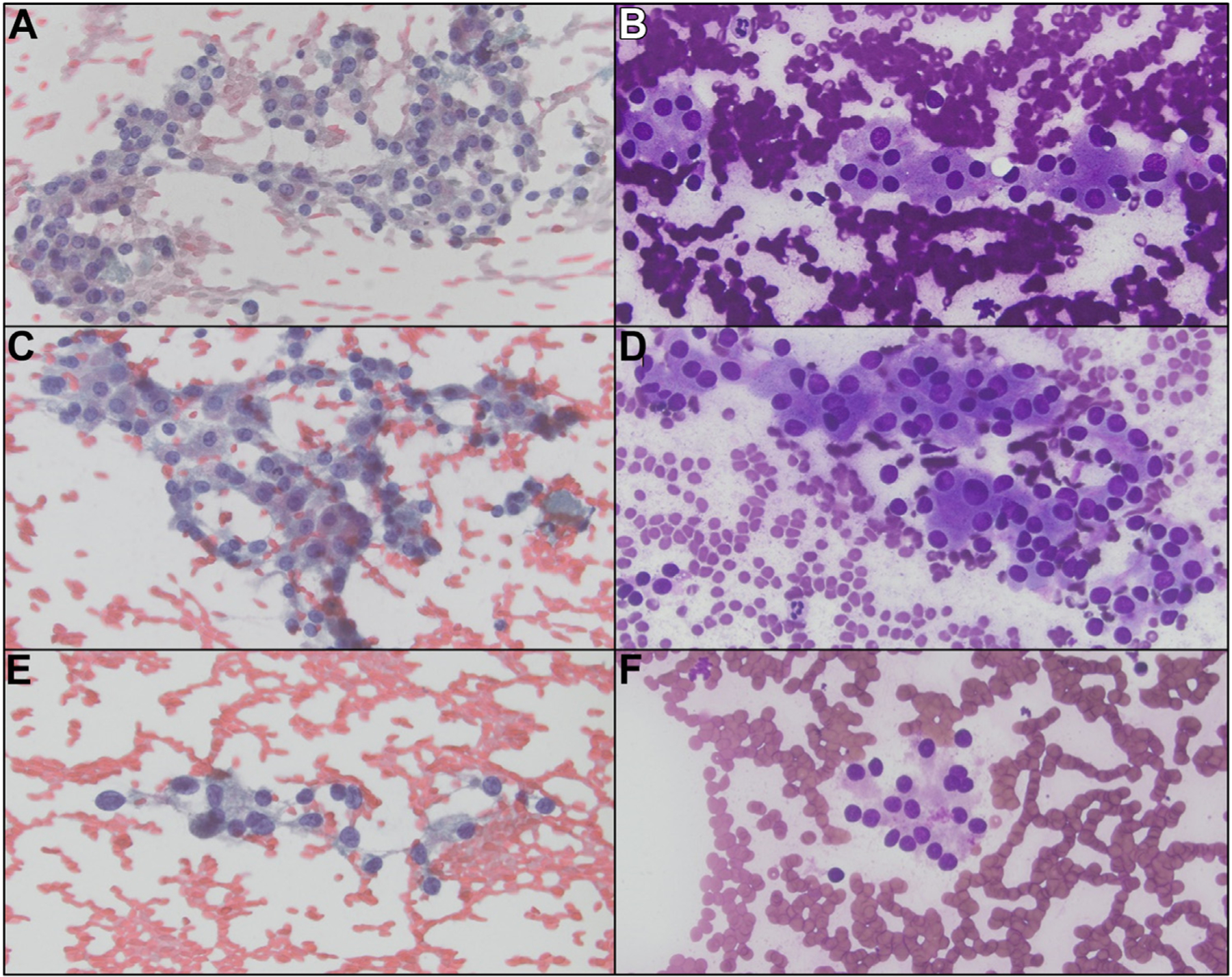
The cytology images from the 3 thyroid FNA specimens that were diagnosed as AUS show similar features. A and B, Epithelial cells with oncocytic features are arranged in a nonspecific pattern of loose sheets and clusters (October 2018). C and D, Similar cell population of oncocytic cells. The overall cellularity of these specimens was not sufficiently high for a follicular neoplasm, oncocytic type diagnosis (December 2019). E and F, 2 years later, a mild increase in nuclear atypia is noted (December 2021). AUS, atypia of undetermined significance; FNA, fine-needle aspiration.
The follow-up thyroid ultrasonography in December 2019 demonstrated slight enlargement of the solid, hypoechoic isthmus thyroid nodule (2.1 × 1.8 × 1.0 cm) with identification of additional small subcentimeter-sized cysts bilaterally. The TI-RADS score was not provided. An ensuing FNA diagnosed as AUS showed mixed architectural patterns with epithelial cells in loose flat sheets and microfollicles and focal oncocytic cell changes (Fig. 2C, D). Molecular testing (ThyroSeq v3) once again showed low-level isolated EIF1AX splice-site mutation (p.A113_splice) with an increased allelic frequency of 25%, compared to the prior FNA, suggesting clonal expansion of neoplastic cells. The overall ThyroSeq v3 result was reported as “currently negative” with an estimated 5%−10% probability of cancer or NIFTP. For the second time, he and clinical team decided to proceed with active surveillance.
Two years later, a thyroid ultrasonography was performed in November 2021 and showed enlargement of the solid isthmus thyroid nodule to 2.7 × 2.2 × 1.8 cm. The TI-RADS category applied to the nodule was TI-RADS 3. A repeat FNA on the thyroid isthmus nodule showed similar cytomorphologic features to those of the 2 prior FNAs and was diagnosed as AUS with demonstration of epithelial cells arranged in microfollicles, crowded clusters, focal oncocytic cell change, and no background colloid; however, increased nuclear atypia was noted (Fig. 2E, F). On this occasion, molecular testing (ThyroSeq v3) on the isthmus thyroid nodule FNA material exhibited not only the EIF1AX splice-site mutation (p.A113_splice, allelic frequency of 12%), but also a concurrent non-V600E BRAF mutation (p.L597Q, allelic frequency of 1%). These results increased the probability of cancer or NIFTP to 50%−60% and the ThyroSeq v3 result was reported as “positive.”
Due to developing clinical concern related to the continued increase in nodule size and the evolving molecular findings, he underwent a right thyroid lobectomy and isthmusectomy in April 2022. Three neoplasms were identified. The first neoplasm (corresponding to the nodule of clinical concern) was located in the isthmus, measured 2.7 cm in greatest dimension, and showed a well-demarcated border with a solid, tan-yellow cut surface (Fig. 3A). Microscopic examination of this isthmus lesion, which correlated to prior FNA samples, exhibited an oncocytic neoplasm enveloped by a fibrous capsule (Fig. 3B) with a predominantly solid growth pattern and focal areas with follicular growth pattern (Fig. 3C). Tumor cell nuclei showed papillary thyroid carcinoma nuclear features, including nuclear enlargement, elongation, crowding, chromatin clearing, irregular nuclear membranes, and grooves with rare pseudoinclusions (Fig. 3D, inset). Focal areas demonstrating increased mitotic activity of greater than 3 per 2 mm2 were noted. The entire capsule was submitted and showed no evidence of invasion. While the neoplasm was noninvasive, the solid growth pattern and the increased mitotic activity precluded the diagnosis of oncocytic NIFTP. Therefore, the diagnosis was encapsulated noninvasive, oncocytic solid subtype papillary thyroid carcinoma with increased mitotic activity, in accordance with the 2022 World Health Organization Classification of Thyroid Neoplasm.9 The second and third neoplasms in the right thyroid lobe (0.4 cm each) were conventional papillary thyroid carcinomas (Fig. 4A, B). Molecular analysis (ThyroSeq v3) performed on the surgical pathology specimen of the thyroid isthmus nodule (2.7 cm) revealed the same EIF1AX splice-site mutation (p.A113_splice, allelic frequency of 64%). The key radiologic, pathologic, and molecular findings of the thyroid isthmus nodule are summarized in Table 1. Due to the presence of 2 additional foci of papillary microcarcinomas, one of which had extrathyroidal extension with margin involvement in association with lymphatic and perineural invasion, completion left thyroid lobectomy was recommended after multidisciplinary discussion. It was performed in June 2022 and revealed thyroid follicular nodular disease with no additional malignancies.
Figure 3.
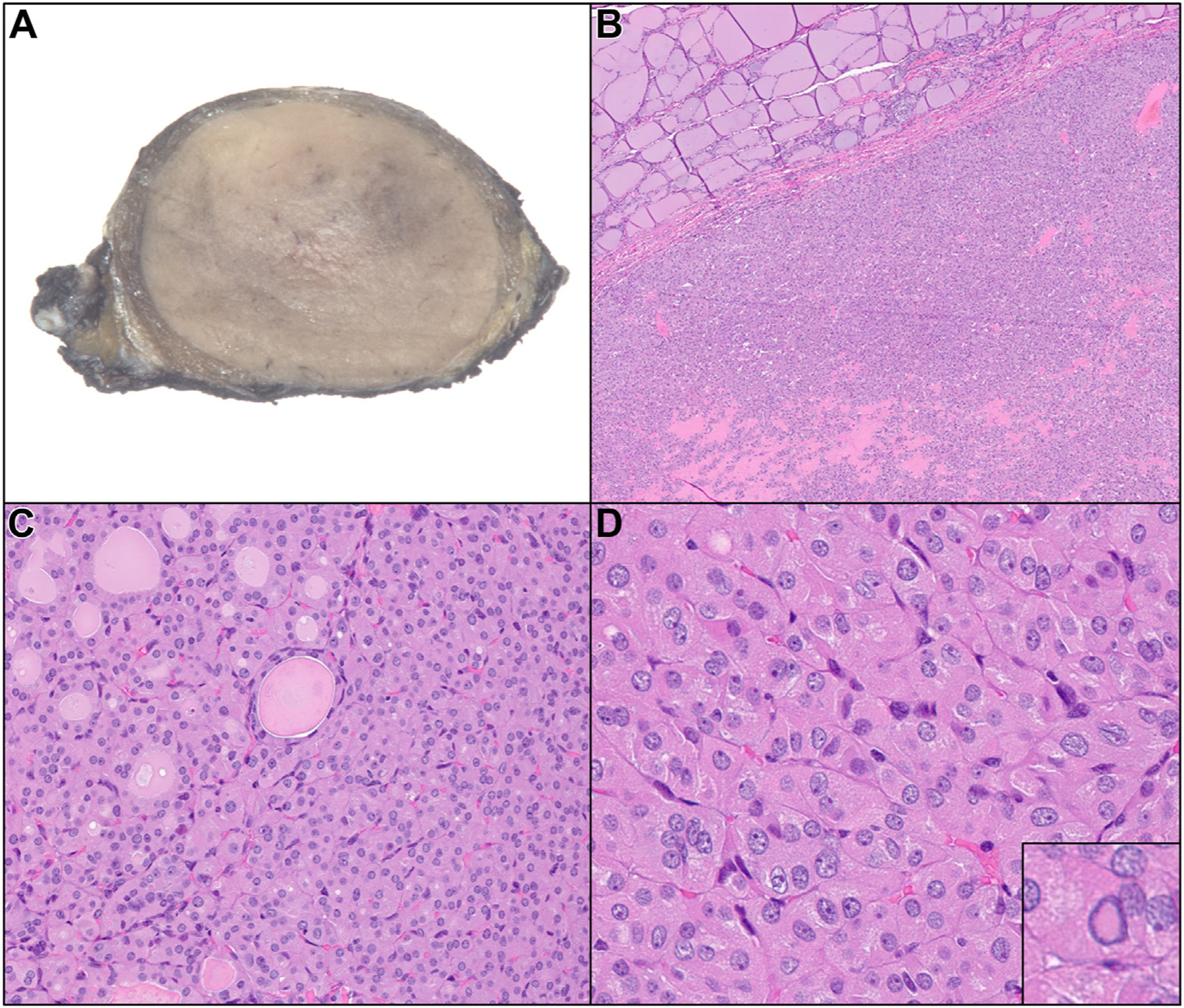
A, Gross image of thyroid isthmus nodule. B, The tumor shows oncocytic features and is enveloped by a fibrous capsule. C, The architecture is predominantly solid with focal areas of follicular growth. D, Tumor nuclei show nuclear features of papillary thyroid carcinoma, including nuclear enlargement, elongation, crowding, chromatin clearing, and irregular nuclear membrane grooves with rare pseudoinclusions (inset).
Figure 4.

A, Gross image of right thyroid lobe nodule (arrow). B, The tumor shows classic histologic features of papillary thyroid carcinoma.
Table 1.
Summary of key radiologic, pathologic, and molecular findings of isthmus nodule.
| Examination date | Ultrasound | Cytopathology/Surgical pathology | ThyroSeq v3 molecular details | ThyroSeq v3 molecular result |
|---|---|---|---|---|
| October 2018 | Thyroid isthmus nodule, solid and hypoechoic, 1.8 cm | FNA: AUS | Low-level, isolated EIF1AX splice-site mutation (p.A113_splice), AF 11% | Currently negative; PCN: Low (5%−10%) |
| December 2019 | Thyroid isthmus nodule, solid and hypoechoic, 2.1 cm | FNA: AUS | Low-level, isolated EIF1AX splice-site mutation (p.A113_splice), AF 25% | Currently negative; PCN: Low (5%−10%) |
| December 2021 | Thyroid isthmus nodule, solid and hypoechoic, 2.7 cm; TR-3 | FNA: AUS | Low-level, isolated EIF1AX splice-site mutation (p.A113_splice), AF 12% Non-V600E BRAF mutation (p.L597Q), AF 1% |
Positive; PCN: Intermediate-high (50%−60%) |
| April 2022 | — | SP: Encapsulated noninvasive, oncocytic solid subtype papillary thyroid carcinoma with increased mitotic activity, 2.7 cm | EIF1AX splice-site mutation (p.A113_splice), AF 64% | — |
Abbreviations: AF, allelic frequency; AUS, atypia of undetermined significance; FNA, fine-needle aspiration; PCN, probability of cancer or noninvasive follicular thyroid neoplasm with papillary-like nuclear features; SP, surgical pathology; TR, thyroid imaging reporting and data system.
Discussion
Cytologically indeterminate results (AUS and FN; Bethesda categories III and IV) occur in 15%−30% of thyroid FNA specimens and carry up to a 40% risk of cancer or NIFTP.5 Practice guidelines recommend molecular diagnostics as a potential next step in management to determine appropriate treatment options.4,10,11 Molecular testing (eg, ThyroSeq – University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania and Sonic Healthcare, Rye Brook, NY, Afirma – Veracyte, Inc., South San Francisco, California, and ThyGeNEXT/ThyraMIR – Interpace Diagnostics, Inc., Parsippany, New Jersey) on thyroid nodule FNA specimens may refine the cytology diagnosis, reduce unnecessary surgeries, and identify low- to high-risk cancers that may warrant surgery or oncologic management.
Since most thyroid cancers are characterized by common occurrence of certain genomic alterations, platforms such as ThyroSeq v3 use next-generation sequencing analysis of 112 thyroid-cancer–related genes to detect single-nucleotide variants, insertions, deletions, gene fusions, gene expression alterations, and copy number alterations to predict the probability of cancer or NIFTP and recommend management.8,12,13 A multicenter prospective, blinded cohort study of 257 indeterminate thyroid cytology nodules (AUS, FN; Bethesda III, IV) using ThyroSeq v3 demonstrated high negative predictive value (97%) and benign call rate (61%), thus potentially obviating the need for diagnostic surgery in many patients with negative ThyroSeq v3 results.14 However, indeterminate nodules with a currently negative ThyroSeq v3 result harbor a low-risk genomic alteration (eg, EIF1AX, PTEN ) that alone is not sufficient for the development of malignancy. The genomic alteration may involve a subpopulation of the lesional cells and most nodules are benign. However, a minority may progress, undergo clonal expansion, acquire additional mutations, and develop malignant phenotypic characteristics. Therefore, these cases are managed differently with closer active surveillance since the probability of cancer or NIFTP is slightly higher.
The current case illustrates the advantages of surveillance and management of an indeterminate thyroid nodule (AUS) with an EIF1AX splice-site mutation. After 3 years of surveillance and monitoring the clinical, cytopathologic, and molecular alterations, the neoplasm was resected. In addition to the molecular alterations, the growth the nodule from 1.8 cm to 2.7 cm and the increase in nuclear atypia from the first to the third FNA specimen (which demonstrated the new non-V600E BRAF mutation) were notable and concerning. At resection, the increased mitotic activity, the high allelic frequency of the EIF1AX splice-site mutation (which may have been due to selective sampling of the most atypical areas), and the presence of a solid pattern were all evidence for biological progression. However, given the active surveillance, this papillary thyroid carcinoma was resected before it manifested invasion.
Some molecular features involving the sequential specimens are interesting and challenging to explain. For example, the appearance of a non-V600E BRAF mutation at a very low allelic frequency (1%) in the third FNA sample and its disappearance in the resection is intriguing. Furthermore, the 64% allelic frequency of the EIF1AX splice-site mutation in the resection specimen suggests the presence of aneuploidy in the papillary carcinoma. The reason for the 64% allelic frequency in the resection specimen compared to the 12% allelic frequency in the previous FNA specimen is uncertain but may be explained by sampling variability with the most atypical (less differentiated) area being tested from the resection specimen.
The EIF1AX gene encodes the eukaryotic translation initiation factor 1A, X-chromosomal (eIF1A) protein and mutations involving this gene are seen in a minority of thyroid neoplasms.15 The analysis of EIF1AX mutation is included in the ThyroSeq v3 panel among other thyroid cancer-related genes. Interestingly, the spectrum of the pathologic outcomes involving the EIF1AX mutation ranges from thyroid follicular nodular disease to anaplastic thyroid carcinoma (Table 2).15,16 Furthermore, the outcome differs depending on whether the EIF1AX mutation is an isolated eventor coexistent with additional mutations. Among the isolated EIF1AX mutations, those with non-splice mutations are associated highly with a benign outcome while those with splice mutations may be associated with malignant outcome. Studies to date are limited in sample size and further investigation of EIF1AX mutations is needed to improve our understanding of complex genomic alterations and outcomes.
Table 2.
| EIF1AX mutation type | Pathologic correlates |
|---|---|
| Isolated nonsplice | FA, TFND |
| Isolated splice | FA, TFND, FC |
| EIF1AX and other mutation(s) | TFND, FA, FC, NIFTP, PTC, AC |
| EIF1AX and RAS mutations only | TFND, FA, NIFTP, PTC |
| EIF1AX, RAS, TERT, and TP53 mutations | AC |
Abbreviations: AC, anaplastic carcinoma; FA, follicular adenoma; FC, follicular carcinoma; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; PTC, papillary thyroid carcinoma; TFND, thyroid follicular nodular disease.
Management recommendations are evolving continuously, and this case illustrates how active surveillance with monitoring of clinical, cytologic and molecular information assisted in determining the appropriate time for surgery. While the current trend in thyroid nodule management emphasizes the prevention of overtreatment, those patients harboring malignancy or indolent neoplasms should have their nodules addressed. Surveillance monitoring by following radiologic imaging studies has been investigated recently.17 Our report adds another dimension with the incorporation of molecular surveillance, which may detect clonal expansion in low-risk nodules.
Conclusion
We herein report, to our knowledge, the first longitudinal case study of a patient with indeterminate thyroid cytology results and EIF1AX mutation, whose clinical and molecular surveillance aided in the decision to resect the neoplasm before histologic invasion manifested.
Footnotes
Conflict of interest disclosures
Y E N and M N own intellectual property related to ThyroSeq and receive royalty; they serve as consultants for Sonic Healthcare USA. All other authors have no conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Russ G, Leboulleux S, Leenhardt L, Hegedüs L. Thyroid incidentalomas: epidemiology, risk stratification with ultrasound and workup. Eur Thyroid J. 2014;3:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS. The Diagnosis and Management of Thyroid Nodules: A Review. JAMA. 2018;319:914–924. [DOI] [PubMed] [Google Scholar]
- 3.Brito JP, Morris JC, Montori VM. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ. 2013;347: f4706. [DOI] [PubMed] [Google Scholar]
- 4.Haugen BR, Alexander EK, Bible KC, et al. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017;27:1341–1346. [DOI] [PubMed] [Google Scholar]
- 6.Allen L, Al Afif A, Rigby MH, et al. The role of repeat fine needle aspiration in managing indeterminate thyroid nodules. J Otolaryngol Head Neck Surg. 2019;48:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino M, Krane JF. Role of Ancillary Techniques in Thyroid Cytology Specimens. Acta Cytol. 2020;64:40–51. [DOI] [PubMed] [Google Scholar]
- 8.Nikiforova MN, Mercurio S, Wald AI, et al. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. 2018;124:1682–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr Pathol. 2022;33:27–63. [DOI] [PubMed] [Google Scholar]
- 10.Haddad RI, Bischoff L, Ball D, et al. Thyroid Carcinoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw JNCCN. 2022;20:925–951. [DOI] [PubMed] [Google Scholar]
- 11.Nishino M, Nikiforova M. Update on Molecular Testing for Cytologically Indeterminate Thyroid Nodules. Arch Pathol Lab Med. 2018; 142:446–457. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab. 2013;98: E1852–E1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steward DL, Carty SE, Sippel RS, et al. Performance of a Multigene Genomic Classifier in Thyroid Nodules With Indeterminate Cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2019;5:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargano SM, Badjatia N, Nikolaus Y, et al. Characterization and Clinical Significance of EIF1AX Mutations and Co-Mutations in Cytologically Indeterminate Thyroid Nodules: A 5-Year Retrospective Analysis. Acta Med Acad. 2021;50:4–12. [DOI] [PubMed] [Google Scholar]
- 16.Karslioglu French E, Nikitski AV, Yip L, Nikiforova MN, Nikiforov YE, Carty SE. Clinicopathological features and outcomes of thyroid nodules with EIF1AX mutations. Endocr Relat Cancer. 2022;29:467–473. [DOI] [PubMed] [Google Scholar]
- 17.Zhu CY, Donangelo I, Gupta D, et al. Outcomes of Indeterminate Thyroid Nodules Managed Nonoperatively after Molecular Testing. J Clin Endocrinol Metab. 2021;106:e1240–e1247. [DOI] [PubMed] [Google Scholar]


