Arguments for a different mechanism of disease
Over the last decade, much has been learned about spontaneous coronary artery dissection (SCAD) as a cause of myocardial infarction in younger women.1 Observations suggest 2 components to an event: a vulnerable coronary wall (consistent with the wider arteriopathy associated with SCAD) and an acute stressor. Yet the mechanisms by which a stressor might provoke disruption remain unknown. Separation of the intima due to endoluminal shear forces? Bleeding into the outer media from abnormalities of vasa vasora? While plausible, these suggestions have been by-and-large speculative.
We offer a collection of observations that support an alternate hypothesis in many cases—that SCAD is a secondary event instigated by stress-induced cardiomyopathy (SICM) (Takotsubo and variants). Specifically, that the extreme and opposing wall motion changes (dyskinesis and hyperkinesis) cause a hinge-point distortion of the coronary arteries resulting in vessel disruption. We suggest the mechanistic term acute mechano-cardiac coronary artery disruption (AMCAD).
-
1.
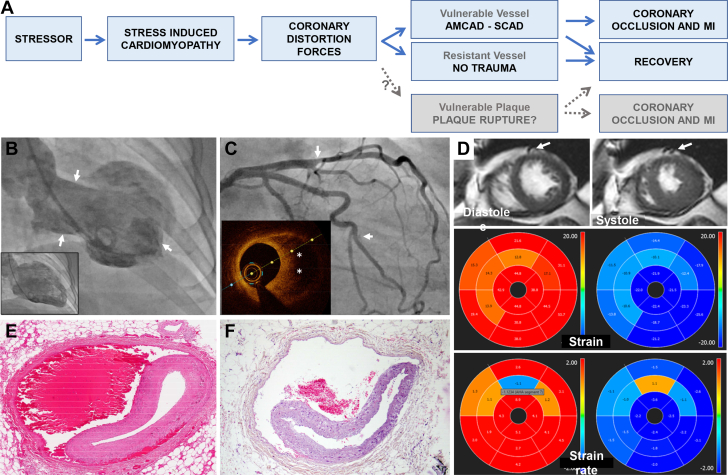
Phenotypic overlap between SICM and SCAD. In both conditions, women comprise 90% of cases and triggering factors of acute emotional or physical stress are frequent. One seeming confounder—that SICM affects individuals on average 15 to 20 years older than SCAD1,2—is supportive of SICM as a single entity, with a phenotypic manifestation that differs according to underlying coronary susceptibility: Patients with vessels resistant to traumatic disruption (older, calcification, atherosclerosis) exhibit SICM in isolation, whereas those with vulnerable vessels (younger, intrinsic susceptibility) manifest as SCAD. Figure 1A illustrates the proposed pathophysiologic pathways, including progression to coronary occlusion causing typical myocardial infarction, or recovery without detectable infarction.
-
2.
Coexistence of SICM and SCAD. Case studies have shown wall motion abnormalities out of proportion to affected territory in coronary dissection, typically an extensive classic apical ballooning pattern beyond the distribution of dissected left anterior descending coronary artery (LAD), suggesting both conditions are present simultaneously.4,5 But these observations in isolation are insufficient to determine which explanation is more likely: Stressor as a cause of both SCAD and SICM, SICM as a consequence of SCAD (distress or transient extensive ischemia) or SICM as the cause of dissection. Consider instead these deeper observations from a case of 2-vessel SCAD with coexistent midventricular ballooning which support SICM as the instigator (Figures 1B to 1D). Wall motion changes are incongruent—hyperkinesis of the apex would be paradoxic for an LAD event. The intersection between abnormal segments localize relatively closely to sites of dissection on angiography. By magnetic resonance imaging, regional dyskinesia is appreciated in American Heart Association segments 7 and 8, with hypokinesia in segments 9 and 12. The proximity of the LAD and diagonal branches to the dyskinetic segment 7 corresponds to the location of the SCAD lesion. The demarcation of directional wall motion abnormalities is seen on feature tracking strain analysis with abnormal peak strain and peak strain rates. These myocardial regions correspond to the level where epicardial coronary branches typically transition to an intramyocardial course, limiting coronary mobility and exacerbating forces on vulnerable coronary vessels adjacent to segments with differing contractility. More so, abnormal contractility is seen in both radial and circumferential strain directions, which may synergistically worsen coronary distortion.
-
3.
Multivessel SCAD. Two- or 3-vessel involvement is observed in up to a quarter of SCAD. Consider which is more likely: Endoluminal shear intimal dissection or spontaneous bleeding in the media occurring in multiple vessels at the same time? Or a primary mechano-cardiac insult causing coronary disruption at multiple hinge-points.
-
4.
Vessel wall disruption. Typical histologic appearance of SCAD affecting the LAD, with bleeding in the outer one-third of the media and separation of the media from the adventitia (Figure 1E). Appearances are near-indistinguishable from traumatic LAD dissection due to blunt chest injury (Figure 1F).3
-
5.
Cervical artery dissections. SCAD is associated with increased risk of dissections in noncoronary arteries. Excessive neck movements can cause carotid and vertebral artery dissections when the normally free artery undergoes torsion or stretch while fixed against a cervical vertebra. Given the arteriopathic association, it is conceivable that SCAD could be caused by mechanical forces in an analogous manner.
Figure 1.
Imaging and Pathologic Evidence for AMCAD
(A) Unifying entity of SICM and pathways to recovery or AMCAD. Greyed boxes for theoretical AMCAD link to plaque rupture, although limited direct observations to support. (B to D) Case example of 74-year-old female admitted with ACS after emotional stress: left ventricle angiogram (B) demonstrating classic appearance of mid-ventricular SICM with hyperkinesis of the apex and base (arrows) and akinesis/dyskinesis in the mid-ventricle in systole. Coronary angiogram (C) showing co-existent 2-vessel SCAD (arrows). Caliber change in mid LAD representing intramural hematoma as identified on optical coherence tomography (inset, asterisk). Contrast staining of short segment intimal dissection plane in branch of ramus. Magnetic resonance imaging short axis, strain and strain rate (D) as described in text. (E) Hematoxylin and eosin cross-section of mid LAD SCAD indicating blood in outer media and separation of media from adventitia in a 52-year-old female with cardiac arrest following myocardial infarction. (F) Near-identical appearance in mid LAD from 58F unexpected death after blunt chest trauma. Image courtesy of Guillaume Gauchotte, with details of case and autopsy findings as previously described.3 ACS = acute coronary syndrome; AMCAD = Acute Mechano-Cardiac Coronary Artery Disruption; LAD = left anterior descending coronary artery; SCAD = spontaneous coronary artery dissection; SICM = stress-induced cardiomyopathy.
What’s next?
It makes sense to direct attention to the strongest candidates for AMCAD: emotional and physical stress-related SCAD, multivessel SCAD, and SCAD without evidence of infarction. Whether the process might even be an instigator of plaque rupture is also worthy of consideration. The occurrence of coronary occlusion downstream in the proposed AMCAD pathway—causing typical myocardial infarction—does make it challenging to distinguish a mechanical extra-coronary instigator from intrinsic coronary mechanisms for both SCAD and non-SCAD causes of acute coronary syndrome. We consider the case for a physical cause of coronary artery disruption to be compelling and here make a call to bring together of fields of SICM, SCAD, brain-heart connection, and biomechanical engineering for novel, kinetic thinking. Perhaps it is time to remove “spontaneous” from the lexicon in some subsets of disease.
Funding support and author disclosures
Dr Tweet is supported by the National Institutes of Health, National Heart, Lung, and Blood Institute K23HL155506 Award. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Tweet M.S., Hayes S.N., Pitta S.R., et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126(5):579–588. doi: 10.1161/CIRCULATIONAHA.112.105718. [DOI] [PubMed] [Google Scholar]
- 2.Templin C., Ghadri J.R., Diekmann J., et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 3.Violon F., Lardenois E., Grafiadis P., Martrille L., Gauchotte G. Fatal left coronary artery dissection due to blunt chest trauma: a case report and literature review. Med Sci Law. 2019;59(4):214–218. doi: 10.1177/0025802419857629. [DOI] [PubMed] [Google Scholar]
- 4.S Y.H., Bohm F. The causal link between spontaneous coronary artery dissection and takotsubo syndrome: a case presented with both conditions. Int J Cardiol. 2016;203:828–831. doi: 10.1016/j.ijcard.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 5.Duran J.M., Naderi S., Vidula M., et al. Spontaneous coronary artery dissection and its association with takotsubo syndrome: novel insights from a tertiary center registry. Catheter Cardiovasc Interv. 2020;95(3):485–491. doi: 10.1002/ccd.28314. [DOI] [PubMed] [Google Scholar]