Abstract
Apicomplexa is a phylum of protist parasites, notable for causing life-threatening diseases including malaria, toxoplasmosis, cryptosporidiosis, and babesiosis. Apicomplexan pathogenesis is generally a function of lytic replication, dissemination, persistence, host cell modification, and immune subversion. Decades of research have revealed essential roles for apicomplexan protein kinases in establishing infections and promoting pathogenesis. Protein kinases modify their substrates by phosphorylating serine, threonine, or tyrosine residues, resulting in rapid functional changes in the target protein. Post-translational modification by phosphorylation can activate or inhibit a substrate, alter its localization, or promote interactions with other proteins or ligands. Deciphering direct kinase substrates is crucial to understand mechanisms of kinase signaling, yet can be challenging due to the transient nature of kinase phosphorylation and potential for downstream indirect phosphorylation events. However, with recent advances in proteomic approaches, our understanding of kinase function in Apicomplexa has improved dramatically. Here, we discuss methods that have been used to identify kinase substrates in apicomplexan parasites, classifying them into three main categories: i) kinase interactome, ii) indirect phosphoproteomics and iii) direct labeling. We briefly discuss each approach, including their advantages and limitations, and highlight representative examples from the Apicomplexa literature. Finally, we conclude each main category by introducing prospective approaches from other fields that would benefit kinase substrate identification in Apicomplexa.
Keywords: Apicomplexa, Kinase, Protein Phosphorylation, Phosphoproteomics
1. Introduction
Phosphorylation is one of the most common and well-studied post-translational modifications (PTM) of proteins, influencing every aspect of cellular growth, metabolism, and homeostasis [1]. Protein kinases act by catalyzing the transfer of a gamma phosphate group from adenosine triphosphate (ATP) onto a residue of a protein substrate (usually serine, threonine, or tyrosine) [2, 3]. Other molecules, such as lipids, carbohydrates, and nucleotides can also be phosphorylated by specialized kinases [4, 5], but our focus here is to discuss protein kinase phosphorylation of protein substrates. The apicomplexan kinome (i.e. the complete set of kinases) is considered one of the smallest among eukaryotes [6]. A kinome search of the current catalog of 128 sequenced apicomplexan genomes (VEuPathDB Release 67; PFAM: PF00069 or PF14531) revealed that apicomplexan kinomes range from 40 genes (Theileria orientalis) to 181 genes (Besnoitia besnoiti). These kinomes include both putative active kinases and putative inactive kinases (pseudokinases). For example, the Toxoplasma gondii (T. gondii) kinome consists of 108 kinases and 51 pseudokinases [7]. Parasite kinases have been shown to be essential for a variety of processes including such as lytic growth, development, egress, motility, host modulation, and pathogenesis, as nicely reviewed previously [6, 8-11]. While several kinase clades are conserved between apicomplexans and humans, apicomplexans also possess many kinase clades that are only partially conserved or absent in humans [12], making them especially attractive targets for therapeutic development.
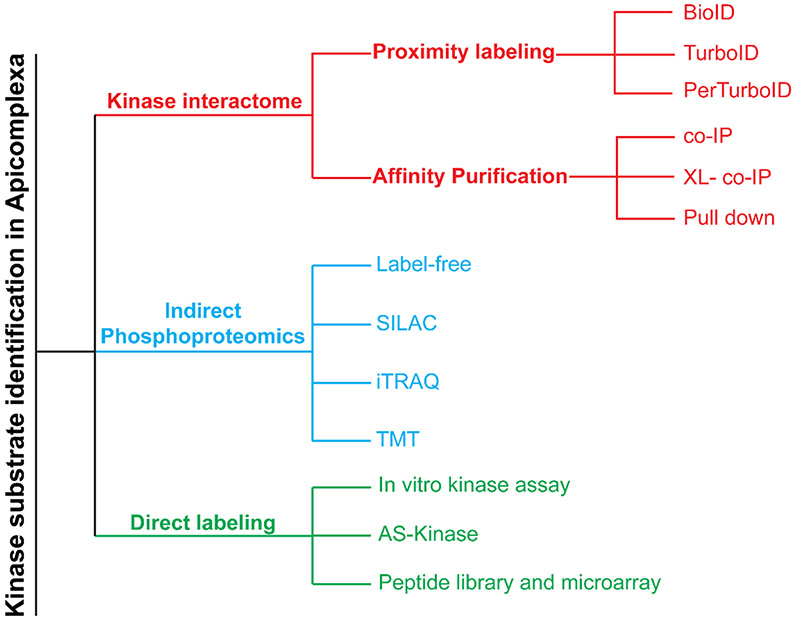
Recent advances in proteomic and phosphoproteomic fields have contributed to a better understanding of the diverse roles kinases fulfill in apicomplexan parasites [13, 14]. To date, kinase substrate identification in Apicomplexa can be categorized by three general proteomic approaches: i) kinase interactome, ii) indirect phosphoproteomics and iii) direct labeling. These approaches can be combined to identify kinase protein substrates and map specific phosphorylation motifs. Here, we organized our discussion around these three proteomic approaches for kinases substrate identification in Apicomplexa (Figure 1), highlighting their main advantages and limitations (Table 1). We briefly discuss each method and highlight studies that have implemented the method for kinase substrate discovery in Apicomplexa (Figure 2). Finally, we provide an outlook of complementary or alternative approaches that have been used to successfully identify kinase substrates in other organisms that could be adapted for apicomplexan research.
Figure 1 –
Proteomic approaches for apicomplexan kinase substrate identification Approaches used for kinase substrate identification in Apicomplexa can be classified in three general categories: i) Kinase interactome, ii) Indirect phosphoproteomics, and iii) Direct labeling.
Table 1.
Advantages and limitations of proteomic approaches used to identify kinase substrates in Apicomplexa.
| Approach | Advantages | Limitations | |
|---|---|---|---|
| Kinase interactome | BioID | Allows full protein solubilization; Provides a snapshot of protein complexes in live cells; Transient interactions are more likely to be labeled | Slow tagging kinetics; Reduced Activity below 37°C (BioID2 has improved efficiency and temperature range); False positive non-interacting neighboring proteins |
| TurboID | Fast labeling kinetics; Active between 20-37°C; Transient interactions are more likely to be labeled | Higher background labeling than BioID; Less control of labeling window; False positive non-interacting neighboring proteins | |
| co-IP | Interacting proteins are captured in the native conformation; More likely to find direct interactors | False positive interactions created post lysis; False negatives created by low affinity or transient interactions | |
| XL-co-IP | Stabilizes weak or transient interactions | False negatives from solubility issues or peptic digest issues | |
| Quantitative phosphopro | Label-free | Simple and fast workflow; Lower cost when compared to label-based approaches | Samples cannot be combined and analyzed simultaneously; Increased post-acquisition data analysis time; semi-quantitative |
| SILAC | Limited sample multiplexing; Quantitative | Unintended interconversion of amino acids; Some cell lines may not be stable in SILAC media | |
| iTRAQ | Allows sample multiplexing; Labelling at peptide level allows flexibility in experiment design; Quantitative | Reduced peptide identification efficiency when compared to label-free | |
| TMT | Allows sample multiplexing; Labelling at peptide level allows flexibility in experiment design; High precision in phosphopeptide quantification | Reduced peptide identification efficiency when compared to label-free | |
| Direct labeling | In vitro kinase assay | Gold standard for kinase substrate validation | Use of radioactive ATP; Low throughput |
| AS kinase | Unbiased labeling of direct targets; Thiophosphate group is resistant to dephosphorylation by phosphatases | Not all kinases can be mutated to use ATPγS | |
| Peptide library and Microarray | Straightforward screening of kinase substrates; Identifies motif preference of the target kinase | Complex folded protein motifs are not represented by linear synthetic peptides |
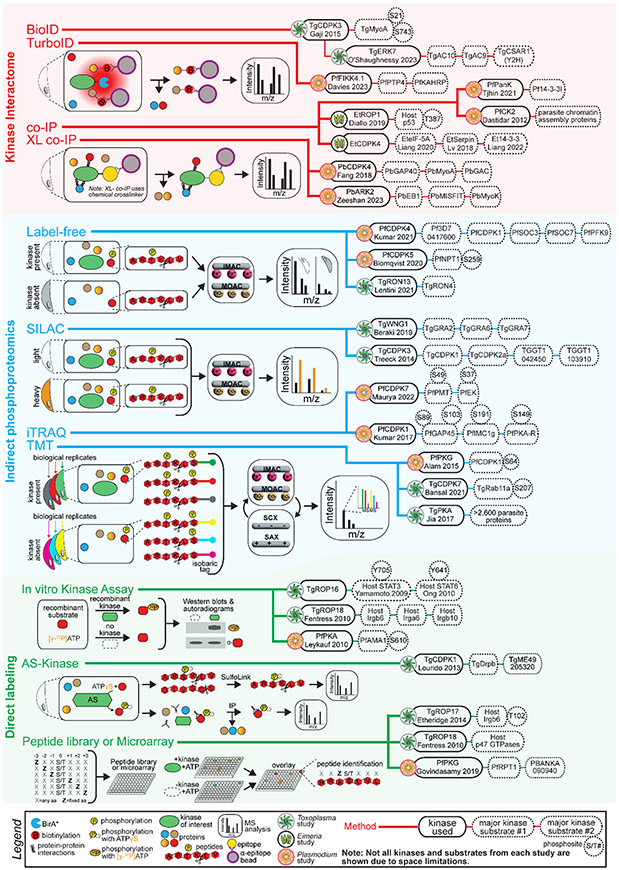
Figure 2 –
Overview of kinase substrate identification in Apicomplexa
Representative kinase substrate discovery in Apicomplexa organized by category and approach. The approaches can be classified in three general categories: i) Kinase interactome (BioID, TurboID, PerTurboID, co-IP and XL-co-IP) highlighted in red; ii) Indirect phosphoproteomics (Label-free, SILAC, iTRAQ and TMT) highlighted in blue; iii) Direct labeling (in vitro kinase assay, AS-kinase and Peptide library and microarray) highlighted in green. The kinase of interest and putative interactors/substrates identified in each study is addressed.
2. Kinase interactome
Protein kinases physically interact with proteins or protein complexes that they phosphorylate since close contact is required for the phosphate transfer. Perhaps the most straightforward approach to identifying potential kinase substrates is to determine which proteins kinases specifically interact with (i.e. kinase interactome). Two general proteomic approaches have been used to define apicomplexan kinase interactomes: proximity labeling and affinity purification [15]. Each has their own advantages and limitations but can also be combined to enhance confidence in the enriched hits.
2.1. Proximity labeling
2.1.1. BioID
Proximity-dependent biotinylation using promiscuous biotin ligases is an interactome profiling tool applied in live cells for mapping protein-protein interactions [16]. Protein complexes have pivotal roles in cellular functions such as signal transduction and cellular growth [17]. Therefore, mapping protein complexes provides a better understanding of the dynamic aspects of cellular functions. Proximity-dependent biotin identification (BioID) is an approach for interactome profiling that uses a highly promiscuous biotin ligase (BirA*) fused to a protein of interest (POI; bait) to biotinylate proteins in close proximity (prey) [18]. It is important to include negative controls to establish the baseline biotinylated proteome, such as the parental cell line (no BirA*) treated with biotin. Additional controls such as fusion of BirA* to an irrelevant target such as GFP (non-specific control), or fusion of BirA* to a protein in the same compartment as the protein of interest (spatial control), are useful for data analysis to identify proteins specifically biotinylated and enriched by the POI-BirA* fusion. Following several hours of biotin treatment (usually overnight), biotinylated proteins are pulled-down using streptavidin- or neutravidin-coated beads, eluted, proteolytically digested into peptides, and identified by liquid chromatography tandem mass spectrometry (LC-MS/MS) [18]. There are several advantages to BioID. First, stable and transient kinase interactors can potentially get labelled for capture. Second, proteins can be fully solubilized after lysis with no need to maintain protein complexes. Third, BioID provides a snapshot of protein complexes in living cells, making it less susceptible to false-positive or false-negative results that may occur post-lysis under native conditions used with co-immunoprecipitation (co-IP). Limitations of this approach include the relatively slow labeling kinetics of BirA*, caused by its low catalytic activity, potential for false positive interactions caused by nearby bystander proteins getting labeled, and inherent exclusion of endogenously-biotinylated proteins [18]. A smaller version of BioID called BioID2 has an increased temperature range and increased affinity for biotin so that less is needed [19]. BioID2 has been used to map proteins of the apical annuli of T. gondii [20], suggesting that the approach could be adapted for apicomplexan kinase interactome analysis as well.
In Apicomplexa, BioID has been used to identify putative TgCDPK3 substrates in T. gondii [21]. TgCDPK3 is a calcium-dependent protein kinase (CDPK) that controls microneme secretion and host cell egress [22-25]. A total of fourteen proteins were exclusively captured and detected from biotin-treated TgCDPK3-BirA* parasites compared to the negative control, including the essential glideosome protein TgMyoA. TgCDPK3 phosphorylation of a TgMyoA peptide array was confirmed using an in vitro kinase assay [21]. Mutational analysis of TgMyoA demonstrated that phosphorylation of serine S21 and S743 residues by TgCDPK3 is important for parasite motility and egress [21]. In a separate phosphoproteomics study, TgMyoA was less phosphorylated at serine S20 and S21 residues in TgCDPK3 mutant parasites when compared to wild-type (WT) control [26], further indicating that TgMyoA is regulated by TgCDPK3 in vivo (discussed further in section 3.2 SILAC). Additional roles for TgCDPK3 have also been identified, indicating that it may regulate multiple substrates. Following egress, TgCDPK3 is able to sense extracellular stress to promote extracellular survival by phosphorylating TgeEF2 to repress translation [27]. In this study, TgCDPK3 was shown to interact with TgeEF2 in the cytosol under stress conditions using co-IP with immunoblotting and also immunofluorescence microscopy [27]. Therefore, it is important to consider that each parasite kinase may regulate multiple protein substrates in distinct signaling pathways.
BioID was also used to identify putative interactors and potential substrates of TgERK7 in T. gondii [28]. TgERK7 is a mitogen activated protein kinase (MAPK) [29] that localizes to the parasite apical cap and is required for maturation of the apical complex [30]. The TgERK7 BioID approach identified a number of apical cap proteins including TgAC9 and TgAC10 [28], which are known to recruit TgERK7 to the apical cap [31, 32]. To validate these hits and further explore direct TgERK7 protein interactions, a yeast-two-hybrid system was implemented using TgERK7 as bait to capture protein products coexpressed in yeast from a T. gondii cDNA library [28]. A total of 21 candidate interactors of TgERK7 were identified by yeast-two-hybrid screening, 8 of which were also identified through BioID, including TgAC9 and TgAC10, suggesting a high confidence overlap of candidates in both methods [28]. Interestingly, yeast-two-hybrid screening also identified TgCSAR1, an E3 ubiquitin ligase, which was investigated as a downstream substrate of TgERK7. TgCSAR1 was found to be essential for functional maturation of the apical complex and regulated by TgERK7 [28].
In P. falciparum, BioID was used to gain insights into how PfCRK4, a cyclin-dependent kinase related kinase (CRK), regulates S-phase in blood stage parasites [33]. Cyclin-dependent kinases (CDKs) are activated by cyclins to precisely control cell cycle progression in eukaryotes [34]. Until cyclin-dependent activation has been established, CDKs are typically referred to as CDK-related kinases (CRK)s. Previous studies demonstrated that loss of PfCRK4 suppresses DNA synthesis [35, 36], likely by phosphorylating effector proteins involved in origin of replication firing [36]. Using BioID, PfCRK4 was shown to potentially interact with 44 proteins, including four proteins known to be hypophosphorylated (PF3D7_0624600, PF3D7_0209800, PF3D7_1357400, and PF3D7_0303500; PfSPB) in the absence of PfCRK4 [33]. Additional experiments revealed that PfCRK4 regulates both microtubule rearrangement and DNA replication during schizogony [33]. Interestingly, PfCRK4 clades closely with TgCrk6 and TgCrk4 by phylogenetic analyses, which also regulate replication in T. gondii [37] (discussed further in section 3.1 Label-free). In summary, BioID is useful for defining kinase substrate interactions in apicomplexan parasites. However, due to lengthy labeling times, it is suboptimal for characterizing rapid kinase substrate interactions.
2.1.2. TurboID
To enhance the efficiency and speed of BioID, Branon and colleagues developed TurboID by mutating 15 amino acids of E.coli BirA [38]. TurboID fusions permit kinetic interactome analyses at minute to sub-minute resolution. TurboID is also active at broad temperature range (20-37°C), allowing its use in a variety of organisms [38]. Since TurboID is more efficient than BioID, it is important to minimize available biotin to reduce background labeling.
TurboID has been used in various fields for identification of kinase substrates, including apicomplexan parasites. Using TurboID, Davies et al. [39] first defined the dynamic interactome of FIKK4.1 at two timepoints in the P. falciparum asexual blood stage. FIKK4.1 is a kinase belonging to the FIKK family and is involved in the modulation of the infected red blood cell (iRBC) rigidity and efficient translocation of PfEMP1 to the iRBC surface [40]. PfEMP1 is a key virulence factor in P. falciparum responsible for the cytoadhesion of the iRBC to the host vascular endothelium system [41]. Using FIKK4.1-TurboID, 101 parasite proteins were enriched over the WT controls [39]. The majority of these proteins are predicted to be exported and include likely direct targets of FIKK4.1 that regulate PfEMP1 presentation on the iRBC surface such as PfEMP1-trafficking protein 4 (PTP4) and knob-associated histidine-rich protein (KAHRP) [40, 42, 43]. To evaluate the local protein environment and interactors of PTP4 and KAHRP, and how it changes after FIKK4.1 deletion, PTP4-TurboID and KAHRP-TurboID fusions were generated in FIKK4.1 conditional knockout (KO) parasites, respectively. This approach, named PerTurboID, revealed interactors of PTP4 and KAHRP, based on the enrichment and detection biotinylated peptides, that are modulated by the presence of FIKK4.1 [39]. These examples demonstrate the power and adaptability of proximity labeling for revealing kinase substrate interactions in Apicomplexa.
2.2. Affinity purification
2.2.1. Co-immunoprecipitation
Immunoprecipitation (IP) and co-immunoprecipitation (co-IP) utilize antibodies for affinity purification of target proteins and protein complexes from complex mixtures, respectively. Co-IP coupled with peptide identification by LC-MS/MS is a common and straightforward approach to define protein interactomes [44]. In a typical co-IP experiment, native cell lysate is incubated with beads coated with a target-specific antibody to capture a target protein complex, unbound material is removed by washing, then captured proteins are trypsin digested for peptide identification by LC-MS/MS [44]. In comparison to proximity labeling, co-IP requires that protein interactors stably interact with the bait protein of interest throughout lysis and washing steps, increasing the chances of finding direct interactors [44, 45]. The main limitations of this approach stem from false positive interactions that can occur post lysis or from off target antibody capture, and false negative results from low affinity or transient protein-protein interactions, or weak antibody interactions, that can be lost during washing steps [46]. Thus, it is important to include negative controls such as antibody isotype control beads or cells lacking the target of target-specific antibody beads to establish a baseline for non-specific binding.
Co-IP with LC-MS/MS has been used to define the interactomes of several kinases in Apicomplexa. For example, this approach has been used to define signaling pathways that regulate Plasmodium blood stages. P. falciparum casein kinase 2 (PfCK2), consisting of two regulatory subunits (β1 and β2) and a catalytic subunit (α), is required for asexual proliferation and sexual development of blood-stage parasites [47-49]. PfCK2 is thought to function by regulating several other kinases [50]. Interactome analysis by co-IP with LC-MS/MS of PfCK2β1 and PfCK2β2 regulatory subunits revealed a large number of components from parasite chromatin assembly pathway, suggesting the involvement of PfCK2 in chromatin dynamics. In support, recombinant PfCK2α was capable of phosphorylating nucleosome assembly proteins, histones, and two members of the Alba family proteins using in vitro kinase assays [48], which is a common direct-labeling approach.
In T. gondii, co-IP with LC-MS/MS analysis was used to identify putative substrates of TgARK1. Aurora kinases (ARK) are a conserved family of serine/threonine kinases that regulate chromosomal segregation and cytoskeletal remodeling during cell division [51]. TgARK1 regulates daughter budding during endodyogeny by organizing the mitotic spindle as part of the chromosomal passenger complex (CPC) [52]. The interactome of TgARK1 suggested that this kinase may phosphorylate cytoskeleton-associated proteins, nuclear proteins, and other known cell cycle regulators [52]. Interestingly, the interactomes of WT and kinase dead TgARK1 were similar but with some unique hits to each. The use of enzyme dead mutants for co-IP is known as substrate trapping, since dead enzymes tend to interact with their substrates longer than usual [52].
Kinase interactome analysis can also be used to define kinase complexes or upstream kinase regulators. For instance, co-IP was used to define the pantothenate kinase (PanK) complex in P. falciparum and T. gondii [53]. PanK catalyzes the first step in the conversion of pantothenate to coenzyme A [54], an essential cofactor in most organisms [55]. Co-IP with LC-MS/MS analysis revealed that PfPanK1 and PfPanK2 associate and form a heteromeric pantothenate kinase complex with Pf14-3-3I in the intraerythrocytic stage of P. falciparum [53]. In T. gondii, TgPank1 and TgPank2 also associate to form a PanK complex to phosphorylate pantothenate, but Tg14-3-3I was not detected by TgPank1 or TgPank2 co-IP by immunoblotting [53]. Unlike other eukaryotic PanK complexes that are homodimeric, this work suggested that apicomplexans encode multiple PanK genes to generate heteromeric PanK complexes. A similar strategy was used define the AMP Kinase (AMPK) complex in T. gondii [56]. AMPK is a serine/threonine kinase composed of one catalytic subunit (AMPKα) and two regulatory subunits (AMPKβ and AMPKγ), that regulates cellular metabolism by monitoring the levels of AMP and ATP in the cell [57]. AMPKβ acts as the structural core of the AMPK complex by binding both AMPKα and AMPKγ. AMPKγ senses AMP to phosphorylate and activate AMPKα, which phosphorylates a number of different protein substrates important for cellular metabolism [58]. T. gondii encodes orthologs of AMPK, including one putative catalytic subunit (TGGT1_233905, TgAMPKα), and two putative regulatory subunits (TGGT1_268960, TgAMPKβ; TGGT1_239870, TgAMPKγ) [56]. To define the AMPK composition and interactions in T. gondii, putative TgAMPK subunits were tagged with Ty or HA, then captured by co-IP and analyzed by LC-MS/MS. When using TgAMPKα, TgAMPKβ, or TgAMPKγ as bait, each identified other AMPK subunits as top hits, strongly suggesting that these three proteins form the TgAMPK complex [56]. Potential regulators or substrates were also found to associate with the TgAMPK complex, including the kinases TgCDPK6, TgCDPK2a, TgPKG, TgCDPK1 and TgCDPK3 [56]. Follow-up experiments demonstrated that TgAMPKγ depletion drastically reduces TgAMPKα phosphorylation, which is expected to inactivate TgAMPKα and prevent downstream signaling. To test this possibility, the TgAMPKγ-dependent phosphoproteome was defined using indirect phosphoproteomics [56]. In total, 170 proteins were differently phosphorylated upon TgAMPKγ depletion, many of which that are known or expected to regulate cellular metabolism T. gondii [56]. Thus, these studies nicely represent the use of multiple approaches to identify mechanisms of kinase function in Apicomplexa.
Kinase interactome analyses using co-IP have also been performed in Eimeria tenella, the major causative agent of avian coccidiosis in chickens. Co-IP of EtCDPK4, a kinase important for invasion of host cells [59], and subsequent LC-MS/MS analysis identified 91 putative interactors of EtCDPK4 not found in the negative control [60]. To narrow the list of potential EtCDPK4 substrates, recombinant HIS-tagged EtCDPK4 was immobilized to Co2+ resin, then incubated with sporulated oocyst proteins to pull down interacting proteins. A total of 8 proteins pulled down with rHIS-EtCDPK4 as detected by LC-MS/MS, with EteIF-5A also found by co-IP. EteIF-5A and EtCDPK4 were confirmed to interact by bimolecular fluorescence complementation and GST pulldown. EteIF-5A colocalizes with EtCDPK4 in the cytoplasm of sporozoites and trophozoites, suggesting that both proteins may work together for invasion [60]. Studies from the same group also determined that EtCDPK4 interacts with a serine protease inhibitor (EtSerpin) [61] and Et14-3-3 [62]. These interactions may contribute to EtCDPK4’s role in host cell invasion but has not yet been demonstrated. In a separate study, co-IP with LC-MS/MS protein analysis revealed putative interactors of EtROP1 kinase in E. tenella [63]. Among the 29 putative interactors of EtROP1 was the host protein p53. An in vitro kinase assay confirmed that EtROP1 kinase phosphorylates host p53 at threonine T387 [63]. Additional experiments showed that phosphorylation of host p53 by EtROP1 causes inhibition of host cell apoptosis during intracellular development of E. tenella and G0/G1 host cell cycle arrest [63]. These studies highlight the utility of co-IP to identify stable interactors of target kinases but may miss transient kinase interactions in the absence of protein crosslinkers.
2.2.2. Crosslinking co-immunoprecipitation
Due to the transient nature of kinase substrate interactions, it can be beneficial to crosslink proteins in whole cells prior to lysis for co-IP interactome analysis. Chemical crosslinking co-IP (XL co-IP) has been used to stabilize and capture weak or transient interactions in a protein complex prior to LC-MS/MS analysis [64-66]. There are many chemical crosslinkers available, but formaldehyde has been extensively used because of its many advantages [65-67].
Formaldehyde is a very small molecule with a short spacer arm ranging from 2.3-2.7 Å [67], but formaldehyde crosslinks may actually be the dimerization product of two formaldehyde-modified amino acids (usually lysine or arginine), effectively doubling the crosslinking diameter [68]. Formaldehyde strongly stabilizes protein-protein interactions through covalent bonds between proteins and other molecules, which allows the samples to be washed in more stringent conditions for removal of non-specific binders compared to native co-IP conditions. Chemical crosslinking has a few limitations that should be considered when using this method for proteomic analysis. Chemical crosslinking can decrease protein solubility so it is important to optimize lysis conditions to insure that the target cross-linked protein complex is retained in the soluble fraction that will be used for co-IP [67]. Also, crosslinking proteins is dependent on the number and the position of the certain residues like lysine and arginine, therefore, there is a risk false negative results for real interactors that lack the necessary residues for crosslinking [66]. Protein crosslinking can also interfere with tryptic digestion of XL-co-IP samples, which may prevent non-digested peptides from being identified by LC-MS/MS [69]. Despite these limitations, XL-co-IP has a better chance of capturing transient kinase interactions than traditional co-IP.
In Apicomplexa, XL-co-IP and LC-MS/MS analysis has been used to identify PbCDPK4 and PbCDPK1 interactomes in P. berghei [70]. In malaria parasites, CDPK4 is an essential regulator of cell cycle progression during male gametogenesis, and subsequent transmission to the mosquito vector [70-73], while CDPK1 is involved in phosphorylation of multiple inner membrane complex proteins, suggesting a role in inner membrane complex (IMC) assembly or stability [74]. Furthermore, CDPK4 and CDPK1 functionally interact and can complement each other, working synergistically with protein kinase G (PKG), an essential kinase that controls key calcium signaling events in malaria parasites [75], to promote ookinete gliding and control asexual growth [70]. Interactome analysis of XL-co-IP PbCDPK4-3HA tagged parasites identified 19 interactors enriched over the WT control, including PbGAP40, PbMyoA, and PbGAC [70]. These proteins are associated with IMC biogenesis or parasite motility [76, 77], strongly suggesting that PbCDPK4 regulates the molecular machinery responsible for parasite motility, host cell invasion, and egress. To validate the putative interaction between CDPK4 and GAP40, reciprocal XL-co-IP of GAP40-3HA parasites identified CDPK4 among the 20 interactors, supporting the model that CDPK4 and GAP40 are interactors [70]. Indirect phosphoproteomics-based approaches have also been utilized to characterize CDPK4 function in Plasmodium sexual blood stages (further discussed in section 3.1 Label-free).
To define the interactome of PbPKG, XL-co-IP was used to capture PbPKG-3HA complexes and identify co-purifying proteins by LC-MS/MS [78]. The top hit was Important for Calcium Mobilization 1 (PbICM1), which was validated by reciprocal XL-co-IP and LC-MS/MS. Similar results were obtained in P. falciparum using standard co-IP and LC-MS/MS and further confirmed using a chemoproteomic approach [78]. P. falciparum protein extracts were incubated with kinobeads (sepharose beads conjugated broad spectrum kinase inhibitors), washed, and treated with ML10 (PKG inhibitor) to displace PfPKG and its interactors from the kinobeads. Based on LC-MS/MS of the kinobead assay, PfPKG and PfICM1 were both displaced by ML10, providing additional evidence of interaction [78]. Furthermore, indirect phosphoproteomic analysis identified PKG-dependent phosphosites on ICM1 in P. falciparum and P. berghei, suggesting that it is likely a substrate of PKG in multiple species of Plasmodium. Functionally, ICM1 was required for PKG-dependent Ca2+ mobilization in Plasmodium. This study nicely represents how kinase interactome approaches can identify candidate substrates to define mechanisms of kinase function in apicomplexan parasites.
In a recent study, XL-co-IP and LC-MS/MS analysis was applied to identify the aurora related kinase 2 (ARK2) interactome on the sexual stages of P. berghei parasites [79]. To better understand the function of PbARK2, interactome analysis was performed using XL-co-IP followed by LC-MS/MS analysis in PbARK2-GFP P. berghei gametocytes, identifying a network of microtubule associated proteins, such as PbEB1, PbMISFIT and PbMyoK [79]. These data suggests that PbARK2 may interact with these proteins to form a complex that is important for the spindle organization and chromosome segregation during cell division of the malaria parasite. This hypothesis is further supported by the fact that reciprocal XL-co-IP of EB1-GFP parasites also captured and identified ARK2 [79].
2.2.3. Kinase interactome outlook
Proximity labeling (BioID, TurboID, PerTurboID) and affinity purification (co-IP, XL-co-IP) strategies have been useful in defining apicomplexan kinase interactomes as potential substrates. However, other useful proximity labeling and affinity purification techniques could be adapted to define apicomplexan kinase interactomes. Such techniques include ascorbate peroxidase (APEX) or APEX2 proximity labeling, high throughput kinase pull down, and kinobead based assays.
APEX is a proximity labeling approach used to biotinylate proteins in close proximity to an APEX-tagged bait protein in live cells for proteomic analysis [80]. In the presence of hydrogen peroxide, APEX catalyzes the oxidation of biotin-phenol to reactive biotin-phenoxyl radicals, labeling nearby proteins for streptavidin capture and identification by LC-MS/MS [80]. APEX has a limited sensitivity when expressed at low levels, possibly due to protein misfolding or stability. A new mutant version of APEX with improved sensitivity was generated, named APEX2 [81]. This new version has been successfully applied to identify MAPKs interactome in cardiomyocytes [82], and secreted T. gondii effectors that modulates host cell immune response [83]. Therefore, APEX or APEX2 are feasible alternatives to BioID or TurboID for kinase proximity labeling in Apicomplexa.
Similar to co-IP, pull down assays are useful for investigating protein-protein interactions [84, 85]. Unlike co-IP, which relies on antibody capture, pull down assays rely on affinity tagged proteins (bait) bound to immobilized affinity ligands (affinity support) to capture interactors (prey). Captured prey can be detected by SDS-PAGE and analyzed by LC-MS/MS [84, 85]. This approach identified an interaction network of kinases involved in human diseases using a library of affinity tagged kinases as bait [86], and therefore may be applied to identify kinase interactors in Apicomplexa.
A newer approach known as kinobead competition and correlation analysis (kiCCA) has been developed for profiling kinase interactomes in cancer cell lines [87]. This high throughput method is based on the incubation of cell and tissue lysates with kinobeads, for enrichment of kinase along with its interactor partners [88, 89], and ATP-competitive kinase inhibitors, to displace the kinase-interactor complex from the kinobead [90, 91]. However, instead of using a single selective kinase inhibitor, a panel of multiple kinase inhibitors probes [88, 92, 93] is applied in the kiCCA approach for displacement of hundreds of kinase-associated protein complex that are further analyzed through LC-MS/MS [87]. Then, the intensity values for the kinases are analyzed using Pearson correlation, where the highest Pearson r value represents the most likely kinase interactor. This approach identified thousands of protein-protein interactions in different cancer cell lines [87]. Adaptation of kiCCA for use in apicomplexan parasites would facilitate high throughput multiplexed kinase interactome discovery.
3. Indirect phosphoproteomics
In addition to kinase interactome analysis, kinase substrates can be identified from indirect phosphoproteomics-based approaches and direct labeling approaches. The phosphoproteome is the subset of the total proteome that contains phosphorylated proteins [94]. Indirect phosphoproteomics based approaches can be used to measure kinase-dependent changes in the phosphoproteome to identify candidate kinase substrates. The approach is considered indirect because it cannot distinguish between primary and secondary phosphorylation events. A general strategy to identify putative kinase substrates through phosphoproteomics is to perturb the target kinase activity or expression in vivo using chemical or genetic approaches, then quantify changes in the phosphoproteome compared to the wild-type kinase control to assign specific kinase-dependent phosphosites. Usually, a bottom-up phosphoproteomics workflow strategy is applied, involving protein extraction from biological samples, protein digestion with a protease (usually trypsin), label-free or label-based quantitative strategies, phosphopeptide enrichment and fractionation, and LC-MS/MS analysis [95].
Strategies have been developed to improve accuracy and precision in protein and peptide quantification by mass spectrometry, including protein phosphorylation [96]. Among the label-based strategies, stable isotope labeling by amino acids in cell culture (SILAC), isobaric tags for relative and absolute quantification (iTRAQ), and tandem mass tag (TMT) has been extensively used in quantitative phosphoproteomics. Following the label-free or label-based strategy, the enrichment of the phosphorylated serine, threonine, or tyrosine by affinity approaches is a key step in quantitative phosphoproteomics analysis [97, 98]. Immobilized metal affinity chromatography (IMAC) [99] and metal oxide affinity chromatography (MOAC) have been extensively used in recent years for purifying phosphopeptides [100]. In IMAC, negatively charged phosphopeptides bind to positively charged metal ions such as Fe3+ or Zr4+, that can be immobilized using magnetic beads or silica resins to retain the phosphopeptides. In MOAC, titanium dioxide (TiO2) is usually used to bind phosphopeptides in metal oxide matrixes. Furthermore, fractionation of phosphopeptides based on charge attraction or repulsion using strong cation exchange chromatography (SCX) [101], or strong anion exchange chromatography (SAX) [102], prior to or after the phosphopeptide enrichment step, can also be used to enhance phosphopeptide discovery. In the following section, we discuss the current quantitative phosphoproteomic strategies that have been used to identify putative kinase substrates in apicomplexan parasites [13, 75, 103].
3.1. Label-free
Label-free phosphoproteomics is useful for quantifying phosphoproteomes without the use of isotopic labels based on normalization methods. In this approach, proteins are extracted from cells or tissues (test vs control), enzymatically digested, enriched for phosphopeptide discovery and analyzed by LC-MS/MS. This method is popular in due to flexible experimental design, straightforward analysis with simple and fast workflow, and low cost when compared to labeling methods [104]. However, samples in label-free approach cannot be combined and analyzed simultaneously, which increases acquisition runtime to accommodate individual samples. Robust data acquisition and analysis software, such as MaxLFQ integrated in MaXQuant [105], and fast workflow have made the label-free an attractive approach for quantitative phosphoproteomics in apicomplexan parasites [72, 106].
Label-free phosphoproteomics has been used to study kinase signaling in P. falciparum. Label-free phosphoproteomics with IMAC enrichment identified 58 phosphorylation sites on 50 proteins that were significantly reduced after PfCDPK5 knockdown in P. falciparum schizonts [106]. PfCDPK5 is a key regulator of parasite egress since blood-stage parasites lacking PfCDPK5 develop into mature schizonts but become reversibly trapped within host cells [107]. Among the hits, novel putative transporter (NPT) PfNPT1 serine S259 residue was significantly less phosphorylated after PfCDPK5 knockdown, suggesting that PfNPT1 is a potential PfCDPK5 substrate in P. falciparum schizont. This interaction was further confirmed using an in vitro kinase assay, which is a direct labeling approach, and both co-localize at the plasma membrane [106]. Phosphorylation of PfNPT1 by PfCDPK5 may regulate parasite egress but warrants further testing. A similar approach was used to characterize the signaling network controlled by PfCDPK4 in P. falciparum gametocytes, a protein critical for male gametogenesis [72]. A total of 193 phosphosites in 170 proteins were quantified in WT parasites, but were not detected in parasites lacking PfCDPK4. Among the putative substrates of PfCDPK4, synthetic peptides from an uncharacterized protein (PF3D7_0417600), PfCDPK1, PfSOC3, PfSOC7, and PfPFK9 were directly phosphorylated by recombinant PfCDPK4 using an in intro kinase assay [72]. These PfCDPK4 substrates have important and diverse roles in Plasmodium gametocytogenesis. In P. berghei, PbSOC3 is involved in parasite exflagellation [73], while PfSOC7 is involved in DNA synthesis in P. falciparum [108]. A recent study found that PfPFK9 regulates glycolysis, representing a key source of energy for flagellar motion in male gametes [109]. In P. falciparum, PfCDPK1 is involved in parasite exflagellation [110], indicating that crosstalk between PfCDPK1 and PfCDPK4 may occur. These data support a model in which CDPK4 regulates Plasmodium gametocyte biology by phosphorylating multiple substrates.
The role of kinases in T. gondii has also been investigated using label-free phosphoproteomics. RON13 is a rhoptry-resident kinase and a key virulence factor in T. gondii that plays a crucial role in host cell invasion by phosphorylating components of the RON complex, stabilizing it at the moving junction [111]. Comparative phosphoproteomics of WT and TgRON13-KD parasites revealed a total of 63 phosphosites in 42 proteins as highly probable targets of TgRON13. TgRON4 plays a critical role in moving junction formation and was differently phosphorylated in parasites lacking TgRON13. Further analysis through in vitro kinase assays confirmed that TgRON13 directly phosphorylates TgRON4. These data suggest that phosphorylation of TgRON4 and other members of the RON complex, such as TgRON2/4/5/8 [112], is a crucial requirement for proper assembly of the moving junction to ensure host cell invasion [111]. Using a similar approach, substrates of TgCrk4 were identified T. gondii [113]. Ten Crks and seven atypical cyclins have been described in T. gondii, and several of them are required for cell cycle progression [37]. Specifically, TgCrk1, TgCrk2, TgCrk4 and TgCrk6 were found to be required or essential for T. gondii replication [37]. Label-free phosphoproteome analysis of TgCrk4-arrested tachyzoites identified a total of 1112 up- and 883 downregulated phosphosites from multiple proteins, including nucleic acid binding proteins, kinases, and IMC proteins [113]. The TgCrk4 label-free phosphoproteome analysis led to the identification of its substrates, including TgORC4, TgCdc20, TgGCP2 and TgPP2ACA [113]. Further analysis through co-IP and LC-MS/MS demonstrated that TgCrk4 and TgCyc4 form a complex to regulate the G2 phase in T. gondii, and this complex interacts with TgiRD1, a protein that controls DNA and centrosome reduplication in tachyzoites [113]. Taken together, label-free technologies have been successfully used to define kinase-dependent phosphoproteomes in multiple apicomplexan parasites.
3.2. SILAC
Label-based approaches allow for improved quantification of proteomic and phosphoproteomic approaches. Stable isotope labeling with amino acids in cell culture (SILAC) is a straightforward metabolic labeling approach that allows the relative and absolute sample quantification by LC-MS/MS [114]. One cell population grows in culture media containing “light” (normal) amino acids, while the other grows in culture media containing “heavy” (isotopes) amino acids. After a certain period of cell divisions or passages, “heavy” amino acids such as arginine or lysine (13C instead of 12C, or 15N instead of 14N) are incorporated into peptides leading to a known mass shift when compared to the normal “light” amino acids. Samples from both populations are then mixed in a 1:1 ratio and analyzed by LC-MS/MS for protein identification and quantification [114, 115]. Each peptide appears as a pair in the mass spectra, and as the light and heavy amino acids are chemically identical, except by their mass, the ratio of peak intensity between the light and heavy peptides reflects the relative protein abundance between the two cell populations. One of the main advantages of using SILAC in quantitative phosphoproteomics is its application at more upstream level (i.e. labeling in cell culture), when compared to chemical labeling (i.e. labeling after protein digestion), reducing false positive hits due to experimental errors in downstream steps [115]. SILAC also demands attention regarding the cell culture and the type of amino acid used for the metabolic labeling, as arginine can be metabolic converted to proline via the arginase pathway in some cell lines, compromising the data acquisition and quantification accuracy [116]. Furthermore, limitations regarding the cell line also need to be considered, as some cell lines do not divide in culture or may not be stable in SILAC media.
SILAC has been successfully applied in quantitative phosphoproteomics to identify downstream targets of kinases in apicomplexan parasites by comparing the changes in phosphorylation signaling events between WT and kinase perturbed parasites [26, 117]. SILAC-based quantitative phosphoproteomics was used to identify TgWNG1 substrates in T. gondii, a kinase that localizes to the parasitophorous vacuole (PV) lumen and is critical for the PV intravacuolar network biogenesis through phosphorylation of resident proteins [117]. A total of 10 proteins had downregulated phosphosites in TgWNG1 KO parasites when compared to WT parasites, including TgGRA2, TgGRA6, and TgGRA7. The TgGRA2 and TgGRA6 proteins are key players in the intravacuolar network morphology [118]. In vitro kinase assay using recombinant TgWNG1 confirmed that TgGRA2, TgGRA6, and TgGRA7 are direct substrates of TgWNG1 [117].
Treeck and colleagues used SILAC-based quantitative phosphoproteomics and SCX/IMAC for phosphopeptide enrichment, to identify phosphoregulatory events dependent on TgCDPK3 [26]. As described earlier, TgCDPK3 is an essential kinase that controls microneme secretion and host cell egress in T. gondii [23-25]. By comparing WT and TgCDPK3-KO parasites, TgCDPK3-dependent phosphosites were identified in TgCDPK1, TgCDPK2a, a putative calmodulin (TGGT1_042450) and a putative calcium-transporting ATPase (TGGT1_103910), providing strong evidence that TgCDPK3 controls calcium-regulated processes [26]. The only member of the glideosome with significant TgCDPK3-dependent phosphorylation was TgMyoA as discussed earlier (section 2.1.1 BioID). Taken together, SILAC has been shown to facilitate quantitative phosphoproteomics in multiple apicomplexan species.
3.3. iTRAQ
Isobaric tag for relative and absolute quantification (iTRAQ) is a technique developed by AB SCIEX company that uses isobaric reagents for protein identification and quantification by LC-MS/MS [119]. The isobaric tag is composed of a reporter group, a balance group, and an amine-reactive group. The reporter group contains a specific mass for peptide quantification. The balance group contains a mass to make all tags isobaric. The amine-reactive group covalently attaches the tag to the peptide which upon fragmentation, releases low mass reporter ions. The iTRAQ approach is based on protein labeling by isobaric tags in the N-terminus and side chain amine groups, where peptides can be distinguished based on the reporter ion mass and quantified based on the reporter ion intensity. One of the main advantages of iTRAQ is sample multiplexing, allowing high-throughput quantitative proteomics [119]. Furthermore, as iTRAQ is used at peptide level, it can be applied to all biological samples which allow flexibility in the experimental design using well-established labeling kits. When compared to label-free methods, iTRAQ may lead to more variations in protein sample caused by sample manipulation during the protein digestion process when compared to label-free or SILAC methods. Furthermore, the number of identified proteins and reproducibility may be lower in iTRAQ approach when compared to label-free methods [120], as well as more expensive and time consuming.
The use of iTRAQ and phosphopeptide enrichment with TiO2 combined with LC-MS/MS successfully identified potential PfCDPK1 and PfCDPK7 substrates in P. falciparum [74, 121]. Conditional knockdown of PfCDPK1 using an FKBP destabilization domain system and iTRAQ identified 79 phosphosites from 64 proteins were downregulated after PfCDPK1 knockdown, including PfGAP45 and PfIMC1g [74]. In vitro kinase assay using recombinant PfCDPK1 further confirmed that GAP45, PfIMC1g and PfPKA-R are direct substrates of PfCDPK1, which may represent mechanisms by which PfCDPK1 controls invasion of host erythrocytes [74].
iTRAQ was also used to identify potential PfCDPK7 substrates [121], a critical kinase for P. falciparum asexual development [122]. iTRAQ quantitative phosphoproteomics in PfCDPK7-KO parasites revealed that 82 phosphosites from 68 proteins were hypophosphorylated, including phosphoethanolamine-N-methyltransferase on serine S49 residue and ethanolamine kinase on serine S37 residue, indicating a potential role of PfCDPK7 regulating phosphatidylcholine synthesis in Plasmodium [121].
3.4. TMT
Tandem mass tags (TMTs) are isobaric chemical tags that were first introduced by Thompson et al. [123] for identification and quantification of peptides by LC-MS/MS. TMTs general structure is composed of an amine reactive group, a cleavable unique mass reporter and a fragmentation-vulnerable spacer arm. Upon fragmentation in LC-MS/MS, each tag generates a low molecular mass ion reporter that is used for relative peptide quantification, as the intensity of the ion reporter represents the relative abundance of the tagged molecule. This method allows high-throughput analysis by multiplexing up to 18 samples in a single LC-MS/MS run, reducing instrument time, cost per sample, and experiment variability. Furthermore, as the chemical tags are isobaric and chemically identical, this ensures that identical peptides labeled with different tags will have the same elution profile and the same ionization condition in the LC-MS/MS run, reducing data distortion. Systematic comparison between different quantitative phosphoproteomic approaches have shown that TMT labeling have the highest precision in phosphopeptide quantification when compared to label-free and SILAC methods [124]. Despite the greater advantages, TMT labeling cannot be applied in vivo and is usually more expensive than other quantitative proteomic methods, such as label-free.
This approach has been increasingly used in large-scale phosphoproteomic profiling of complex peptide mixtures [124], and has also allowed the identification of putative kinase substrates in apicomplexan parasites [22, 40, 103, 125-128]. By using TMT and phosphopeptide enrichment with IMAC/TiO2, Alam et al. [103] identified PfPKG substrates in Plasmodium schizonts by comparing WT and PfPKGT618Q parasites treated with compound 2, a selective PKG inhibitor [129]. PfPKGT618Q parasites harbors a substitution of threonine gate-keeper with a bulkier glutamine residue, reducing PKG sensitivity to compound 2 by 2,000-fold [130]. Therefore, by comparing the phosphoproteomes of WT and PfPKGT618Q parasites treated with compound 2, PKG-dependent phosphosites can be assessed between the two samples. Phosphoproteomics identified 107 phosphorylation sites on 69 proteins that were dependent on PfPKG activity, including the serine S64 residue on PfCDPK1. An in vitro kinase assay using recombinant PfPKG and a catalytic inactive version of PfCDPK1 showed that PfPKG directly phosphorylates S64 of PfCDPK1, and this phosphorylation is important for maintaining PfCDPK1 as a high molecular weight complex [103]. Similarly, TMT-based phosphoproteomics has been used to define Ca2+- and cGMP-dependent phosphoproteomes in T. gondii at sub-minute resolution [13, 131].
TMT-based phosphoproteomics has also been employed to investigate the function of several kinases in T. gondii. For instance, loss of TgCDPK7 modulated the abundance of 301 phosphosites on 215 proteins based on TMT-based phosphoproteomics [125]. Interestingly, TgRab11a possessed TgCDPK7-dependent phosphorylation, a protein previously shown to be involved in vesicular trafficking [132]. An in vitro kinase assay demonstrated that TgRab11a S207 is directly phosphorylated by TgCDPK7. Furthermore, this phosphosite was shown to be important for its localization in a punctate endosome-like compartment [125].
TMT-based phosphoproteomics was also applied to gain insights into the pathways controlled by TgPKA, a cAMP-dependent kinase, in T. gondii [133]. A total of 191 proteins had TgPKA-dependent phosphosites, including proteins thought to regulate cell cycle, as well as 83 proteins with no functional annotation [133]. Interestingly, inhibition of TgPKA increased phosphorylation of phosphodiesterase 2 (TgPDE2), which degrades cAMP, suggesting a feedback loop exists between TgPKA and cAMP levels [133]. TgPDE2 has been shown to regulate several steps of the lytic cycle, likely by modulating TgPKA activity through cAMP regulation [134, 135]. Further analysis showed that TgPKAc1 acts as a balancing regulator of cGMP metabolism to control T. gondii egress by acting as a repressor of PKG-mediated signaling and downstream Ca2+ signaling [133, 136]. In addition to cAMP regulation, TgPKA may also be regulated by kinase phosphorylation. A recent study investigated the role of Store Potentiating/Activating Regulatory Kinase (SPARK) in regulating other kinases in T. gondii [137]. SPARK is an ortholog of the phosphoinositide-dependent protein kinase 1, a protein that in metazoans regulates the second messenger signaling through the regulation of AGC kinases [138]. In T. gondii, recent studies revealed that TgSPARK has a key role in host cell invasion and egress by regulating the calcium release from intracellular stores [139]. In Plasmodium, an ortholog of SPARK called 3-phosphoinositide–dependent protein kinase-1 (PfPDK1) plays a key role in host cell invasion and parasite proliferation by regulating the PfPKA pathway [140], suggesting that TgSPARK may also regulate TgPKA in T. gondii. TMT-based proteomics and phosphoproteomics revealed 138 phosphopeptides were downregulated after TgSPARK knockdown compared to untreated controls [137]. Enrichment analysis of the TgSPARK-dependent phosphopeptides revealed that TgSPARK may regulate AGC kinases (including TgPKA and TgPKG), phosphatases, ubiquitin ligases, nucleic acid binding proteins, and transporters [137]. Co-IP and TurboID interactome analyses also revealed further interactors and consequently the pathways that TgSPARK might be regulating in T. gondii [137]. To gain further insights into how TgSPARK regulates AGC kinases in T. gondii, the TgPKG-, TgPKAc1-, and TgPKAc3-dependent phosphoproteomes were also analyzed using TMT-based phosphoproteomics [137]. By comparing these kinase-dependent phosphoproteomes, a model was proposed that TgSPARK regulates the stability and activity TgPKG and TgPKA [137].
3.5. Indirect phosphoproteomics outlook
Large-scale analysis of protein phosphorylation through indirect phosphoproteomics is a powerful tool for identifying apicomplexan kinase substrates. As the name suggests, the main limitation of indirect phosphoproteomics is discerning direct kinase targets from indirect kinase targets. New technologies have been developed that can help discern direct targets in phosphoproteomic data.
Kinase assay linked with phosphoproteomics (KALIP) is an approach that combines in vitro kinase reaction with kinase-dependent phosphoproteomics [141]. For this approach, it is important that the kinase and substrates are derived from the same cell type. First, an in vitro kinase assay is performed using a purified kinase of interest and dephosphorylated peptides derived from cell lysates as substrates. The resulting phosphopeptides are enriched using polymer based metal ion affinity capture (PolyMac) [142] and identified by LC-MS/MS. Second, the in vivo kinase of interest-dependent phosphoproteome (kinase+ vs kinase−) is defined by indirect phosphoproteomics analysis. Phosphopeptides enriched in both sets of experiments are more likely to be direct substrates of the kinase of interest. This approach successfully identified 64 candidate substrates of spleen tyrosine kinase in B cells and 23 in breast cancer cells [141]. Thus, KALIP represents a new approach that should be readily adaptable for kinase substrate discovery in Apicomplexa.
4. Direct labeling
Kinase interactome and phosphoproteomics can reveal candidate kinase substrates, especially when combined. The third main approach to identifying kinase substrates is to screen for proteins that are directly labeled (phosphorylated) by a kinase of interest.
4.1. In vitro kinase assay
The in vitro kinase assay is the simplest and most straightforward direct labeling approach to identify kinase substrates or phosphorylation motifs [143, 144]. Substrate phosphorylation demands a direct interaction between the kinase and the substrate protein or protein complex [145-147]. In a typical in vitro kinase assay, the recombinant purified kinase and potential substrate are incubated with radio-labeled ATP (γ-32P), and the transfer of the γ-32P from ATP to the substrate can be visualized through autoradiogram or quantified using a scintillation counter. Despite the in vitro kinase assay being considered a gold standard for kinase substrate identification, the use of radio-labeled ATP requires proper handling of the samples and further correct disposal of radioactive material. Furthermore, artificial labeling of substrates may occur by using high concentration of purified kinase/substrate in the reaction [148-150].
Several apicomplexan kinases have evolved to phosphorylate host proteins to promote parasite survival and virulence. For instance, certain rhoptry (ROP) kinases/pseudokinases are injected into host cells during invasion and modify host proteins to inactivate immune defenses and modulate host cell transcription [11, 151-154]. TgROP16 is a polymorphic tyrosine kinase and a key virulence factor that plays a crucial role in host cell immunity evasion by phosphorylating host STATs [155-158]. TgROP16 traffics to the host nucleus and directly phosphorylates host STAT3 at tyrosine Tyr705 residue [155] and STAT6 at tyrosine Tyr641 residue [156], as confirmed using in vitro kinase assays. Phosphorylation of STAT3/6 by TgROP16 plays a key role in host immune response evasion by suppressing proinflammatory cytokines [154] and controlling parasite replication by induction of arginase-1 in the host [158].
In vitro kinase assays also provided crucial findings regarding the substrates of T. gondii ROP18 kinase. TgROP18 is a serine/threonine kinase injected in the host cell during invasion that forms a complex with TgROP5, a pseudokinase that controls T. gondii virulence by regulating TgROP18 activity [159]. In virulent T. gondii type I strains, both TgROP5 and TgROP18 interacts and decorates the parasitophorous vacuole membrane to phosphorylate immunity-related GTPases (IRGs) [160, 161], important effectors induced by IFN-γ that restrict T. gondii growth [162-167]. In vitro kinase assays and immunoprecipitation experiments showed that TgROP18 binds to and directly phosphorylates IRGs family members, such as Irgb6, Irga6 and Irgb10, but not when TgROP18 was mutated to a kinase-dead version [160]. This data confirmed that TgROP18 is a key virulence factor by blocking the accumulation of IRGs family members on the parasitophorous vacuole membrane and subsequent parasite clearance. In a different study, a western blot using anti-pT102 and anti-pT108 antibodies for Irga6 in parallel with in vitro kinase assay demonstrated that TgROP18 directly phosphorylates Irga6, confirming the crucial of TgROP18 in host immunity evasion [161].
In vitro kinase assays have been used in a number of Plasmodium kinase studies. For instance, these assays have been used to validate the target Plasmodium PKA, which plays a key role in merozoite invasion in human erythrocytes [168, 169]. In vitro kinase assays confirmed that PfPKA directly phosphorylates S610 in the cytoplasmic tail of PfAMA1, an event that is essential for red blood cells invasion [169]. Overall, direct-labeling approaches through in vitro kinase assays have been extremely useful to identify putative kinase substrates in Apicomplexa, revealing important aspects of parasite biology [170-179].
4.2. Analog-sensitive kinases
Eukaryotic protein kinases are highly conserved in their catalytic site structure and basically all use the same phosphorylation mechanism to transfer the γ-phosphate of ATP to a substrate [180]. Allen and colleagues developed an innovative method that uses a genetically engineered analog-sensitive (AS) kinase and atypical ATP analogs to identify kinase substrates [181]. AS kinases are genetically engineered to harbor a mutation in the ATP-binding pocket creating a new active site that allows the AS kinase to accept bulky side chains of ATP analogs. This creates a “lock and key” system that allows only AS kinase to use ATP analogs to phosphorylate substrates, creating an unbiased labeling of direct targets of the kinase on a global scale. A variety of kinase substrate identification methods using AS kinases and ATP analogs have been described [182], including the use of an ATP analog that carries a γ-thiophosphate group (ATP-γ-S). The thiophosphate group works as a unique phosphate mimetic tag that is resistant to dephosphorylation by serine/threonine phosphatases [183, 184] and can be covalent captured by iodoacetyl-agarose beads (Sulfolink) or affinity purified using specific antibodies to reveal direct targets of kinases by LC-MS/MS [181, 185-187]. One disadvantage of this approach is that it is not universally applicable, as not all kinases can be mutated to efficiently use ATP-γ-S, limiting its applicability [185]. Furthermore, as thiophosphate group is resistant to phosphatases activity, its application in vivo can be problematic caused by cell toxicity.
The AS kinase approach has been used successfully to identify TgCDPK1 substrates in T. gondii by comparing TgCDPK1 WT and conditional knockout parasites [188]. Two proteins were found to be exclusively thiophosphorylated in TgCDPK1 WT parasites: dynamin-related protein B and a phosphorylated repeat protein (TGME49_205320, formerly TGME49_005320), suggesting that both substrates are phosphorylated in a TgCDPK1-dependent manner [188]. This approach was further developed to identify additional targets of TgCDPK1 by thiophosphorylation [131]. In total, 104 proteins were identified as direct substrates of TgCDPK1 including TgHOOK which regulates microneme secretion and host cell invasion, partially recapitulating the function of TgCDPK1 [131].
4.3. Peptide library and microarray
Protein kinase assays using peptide and microarray libraries allows a straightforward screening of putative kinase substrates by incubating the purified kinase of interest with a library of putative substrates [189, 190]. Different library types have been developed over the last decades for large-scale analysis of kinase substrates and can be summarized in two general groups: knowledge-based libraries and random libraries [189]. The knowledge-based libraries are generated using natural occurring proteins and allows the identification of the phospho-acceptor residue by scanning overlapping peptides or by use of sequences that only surrounds the potential phospho-acceptor residue [189, 191]. Random libraries consist of arbitrarily generated single or mixed peptides by combinatorial approaches and are particularly convenient for use with orphan kinases which the orthologs are not known [189, 192]. Peptide libraries can be immobilized on a solid support to build high-density peptide microarrays that allows substrate screening in a high throughput manner, identifying protein phosphorylation and motif preference of a target kinase [193]. Using small amounts of reagents, the kinase of interest is incubated with the arrayed peptide library and phosphorylated peptides scanned by distinct detection methods such as radiolabeling, immunoblotting, and fluorescence [194]. Despite the many advantages for kinase substrate discovery, there are important limitations regarding this approach that need to be considered. The use of concentrated purified kinase for the in vitro reaction may led to artificial labeling of substrates, which may reduce reaction specificity [148-150]. Furthermore, as the kinase-substrate reaction occurs in vitro, some important regulatory mechanisms that occurs physiologically may be lost [149, 150]. Therefore, is critical to confirm that the putative substrate found in vitro is physiologically relevant through in vivo studies [195].
Positional scanning peptide library [196, 197] approaches have been extensively used in Apicomplexa to determine kinase substrates and its preferred consensus motif. For TgROP18 in T. gondii, an optimal substrate phosphorylation motif was determined, and shows a broad sequence selectivity that can be generalized as (X)-(X, not E)-(X)-(E)-(H)-(T)-(R/mixed, not P and not negatively charged)-(Ar)-(Ar)-(Ar), where the phospho-acceptor is underlined, X denotes any amino acid residue, and Ar denotes an aromatic residue [198]. This approach was also used to explore the peptide preference of TgCDPK1, which showed a strong preference for serine over threonine, for arginine at the −3 position, and for hydrophobic residues at the −5 position [188]. In T. gondii, TgROP17 is a serine/threonine kinase that accumulates in the external face of the parasitophorous vacuole membrane (PVM) and forms a complex with TgROP5 to phosphorylates IRGs, therefore, developing a crucial role in parasite pathogenesis [199]. Positional scanning peptide library analysis showed that TgROP17 has a strong preference for threonine over serine as phospho-acceptor, with a slight preference for hydrophobic amino acid surrounding the phosphorylation site [199]. Furthermore, an in vitro kinase assay using recombinant Irgb6 and Irga6 showed that TgROP17 prefers Irgb6 as substrate, and phosphorylates Irgb6 at threonine Thr102 residue, as demonstrated by semiquantitative LC-MC/MS [199]
In Plasmodium spp., the positional-scanning peptide library approach identified the PfPKG substrate specificity, that showed a strong preference for basic residues, in particular, Arg, at the −5 and −3 positions relative to the phosphorylation site, basic residues at −2 position and hydrophobic residues at the +1 position [200]. Furthermore, PfPKG generally deselect acidic residues at both upstream and downstream of the phospho-acceptor residue and display a strong preference for threonine over serine [200]. Further analysis by in vitro kinase assay showed that PfPKG directly phosphorylates RPT1, a subunit of the 19S proteasome and PBANKA_090940, a hypothetical protein [200]. In Plasmodium, PfPK7 is an “orphan” kinase with no human homologs [201]. Using peptide microarray library, Merckx and colleagues identified the PfPK7 consensus peptide substrate sequence as (r-R-R/K-K/R-S/T-P-K/R-K-R).
4.4. Direct labeling outlook
We have provided examples of how direct labeling assays have been successfully used to identify kinases substrates in Apicomplexa [188, 198, 200, 202]. Additional direct labeling approaches could also prove useful for identifying kinase substrates in Apicomplexa. For instance, the kinase-catalyzed biotinylation with inactivated lysates for discovery of substrates (K-BILDS) has been used to identify kinase substrates in cell lysates [203]. In this method, kinases in lysates are inactivated with FSBA, a covalent inhibitor that reacts with lysine in the kinase active site and inhibits the kinase activity irreversibly [204]. Next, FSBA is removed, and exogenous active kinase of interest is added with ATP-biotin in the lysates, biotinylated proteins are enriched with streptavidin resin, separated by SDS-PAGE and analyzed by LC-MS/MS [203]. As proof of concept, this approach was applied for discovery of PKA substrates in HeLa cells lysates, identifying 279 candidate substrates, from which 56 was previously known PKA substrates [203]. The fact that this approach can be used to basically all kinases [205] and the putative substrates can be analyzed by high-throughput LC-MS/MS, makes this an promising approach for kinase substrate identification in Apicomplexa.
Another useful direct labeling approach is called Kinase-interacting substrate screening (KISS) [206]. In this approach, cell or tissue lysates containing affinity beads coated with a GST-tagged catalytic fragment of the kinase of interest are loaded through a glutathione-sepharose affinity column. Kinase-bound proteins are incubated with ATP/Mg2+ (phosphorylated samples) or without ATP/Mg2+ (non-phosphorylated samples) in a reaction mixture to promote on-bead phosphorylation, followed by tryptic digestion. Subsequently, phosphopeptides are enriched using titanium oxide column and analyzed by LC-MS/MS [206].
Using KISS, Amano and colleagues [206] identified 356 phosphorylation sites in 140 proteins as candidate substrates of Rho-associated kinase, including previously known and new candidates. Furthermore, the KISS method was also applied to other kinases, efficiently identifying its substrates and phosphorylation sites, confirming it broad range applicability. This approach may represent a new interesting and broad applicable method for kinase substrate discovery in apicomplexan parasites.
5. Conclusions
Protein phosphorylation is one of the most well studied post-translational modifications, and controls many aspects of cell biology [1]. Advances in proteomic technologies have facilitated apicomplexan kinase substrate identification. Here, we have summarized and provided examples of the three categories of proteomic approaches for apicomplexan kinase substrate discovery and validation: i) kinase interactome, ii) indirect phosphoproteomics, and iii) direct labeling. New technologies emerge each year that make kinase substrate identification even more robust, efficient, and feasible. Each approach has advantages and limitations that affect kinase substrate identification. Several studies have combined two or more of these approaches to enhance confidence in apicomplexan kinase substrate identification [73, 78, 131]. Kinase substrate screens can generate dozens of candidate substrates, so prioritization is key. For instance, fitness screens can help determine which candidate substrates are essential or likely to as important downstream mediators of the kinase of interest [207-209]. It is also important to validate the putative kinase substrates identified using in vitro kinase assays of purified kinases and substrates. Once direct substrates are confirmed, mutational analysis of the phosphorylation site through genetic complementation is useful for determining its biological significance in vivo [21, 103, 106, 121, 125]. Identifying the critical post-translational modifications from each essential apicomplexan kinase will reveal regulatory features of essential substrates that could be targeted for novel therapeutic intervention.
Highlights.
Multiple mass spectrometry-based proteomic approaches are currently available for protein kinase substrate discovery in Apicomplexa
Kinase interactome analysis using proximity labeling, co-immunoprecipitation, or pull down can reveal candidate kinase substrates and potential regulators
Indirect phosphoproteomics using label free or label-based technologies can reveal kinase-dependent phosphorylation sites
Direct labeling approaches such as analog sensitive kinases can identify direct kinase substrates
Acknowledgments
We regret that we were unable to discuss all pertinent examples of apicomplexan kinase substrate discovery from the literature in the interest of space, balance, and flow. This work was supported by NIH NIGMS 5P20GM134973 to KMB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors have declared that no competing interests exist.
CRediT author statement
Gabriel Cabral: Conceptualization, Writing – Original Draft, Writing – Review and Editing, Visualization. William J. Moss: Visualization, Writing – Review and Editing. Kevin M. Brown: Conceptualization, Writing - Review and Editing, Supervision, Funding acquisition.
Declarations of interest:
none
References
- [1].Cohen P, Protein kinases--the major drug targets of the twenty-first century?, Nat Rev Drug Discov 1(4) (2002) 309–15. [DOI] [PubMed] [Google Scholar]
- [2].Burnett G, Kennedy PE, The enzymatic phosphorylation of proteins, J Biol Chem 211(2) (1954) 969–80. [PubMed] [Google Scholar]
- [3].Anderson NG, Maller JL, Tonks NK, Sturgill TW, Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase, Nature 343(6259) (1990) 651–3. [DOI] [PubMed] [Google Scholar]
- [4].Karataeva NA, Nevinsky GA, Enzymes phosphorylating lipids and polysaccharides, Biochemistry (Mosc) 72(4) (2007) 367–79. [DOI] [PubMed] [Google Scholar]
- [5].Van Rompay AR, Johansson M, Karlsson A, Phosphorylation of nucleosides and nucleoside analogs by mammalian nucleoside monophosphate kinases, Pharmacol Ther 87(2–3) (2000) 189–98. [DOI] [PubMed] [Google Scholar]
- [6].Miranda-Saavedra D, Gabaldón T, Barton GJ, Langsley G, Doerig C, The kinomes of apicomplexan parasites, Microbes Infect 14(10) (2012) 796–810. [DOI] [PubMed] [Google Scholar]
- [7].Peixoto L, Chen F, Harb OS, Davis PH, Beiting DP, Brownback CS, Ouloguem D, Roos DS, Integrative genomic approaches highlight a family of parasite-specific kinases that regulate host responses, Cell Host Microbe 8(2) (2010) 208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gaji RY, Sharp AK, Brown AM, Protein kinases in Toxoplasma gondii, Int J Parasitol 51(6) (2021) 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hakimi MA, Olias P, Sibley LD, Toxoplasma Effectors Targeting Host Signaling and Transcription, Clin Microbiol Rev 30(3) (2017) 615–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boothroyd JC, Dubremetz JF, Kiss and spit: the dual roles of Toxoplasma rhoptries, Nat Rev Microbiol 6(1) (2008) 79–88. [DOI] [PubMed] [Google Scholar]
- [11].Boothroyd JC, Have it your way: how polymorphic, injected kinases and pseudokinases enable Toxoplasma to subvert host defenses, PLoS Pathog 9(4) (2013) e1003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Talevich E, Mirza A, Kannan N, Structural and evolutionary divergence of eukaryotic protein kinases in Apicomplexa, BMC Evol Biol 11 (2011) 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Herneisen AL, Li ZH, Chan AW, Moreno SNJ, Lourido S, Temporal and thermal profiling of the Toxoplasma proteome implicates parasite Protein Phosphatase 1 in the regulation of Ca(2+)-responsive pathways, Elife 11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Montano H, Anandkrishnan R, Carruthers VB, Gaji RY, TgTKL4 Is a Novel Kinase That Plays an Important Role in Toxoplasma Morphology and Fitness, mSphere 8(2) (2023) e0064922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kimmel J, Kehrer J, Frischknecht F, Spielmann T, Proximity-dependent biotinylation approaches to study apicomplexan biology, Mol Microbiol 117(3) (2022) 553–568. [DOI] [PubMed] [Google Scholar]
- [16].Choi-Rhee E, Schulman H, Cronan JE, Promiscuous protein biotinylation by Escherichia coli biotin protein ligase, Protein Sci 13(11) (2004) 3043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Arkin MR, Whitty A, The road less traveled: modulating signal transduction enzymes by inhibiting their protein-protein interactions, Curr Opin Chem Biol 13(3) (2009) 284–90. [DOI] [PubMed] [Google Scholar]
- [18].Roux KJ, Kim DI, Raida M, Burke B, A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells, J Cell Biol 196(6) (2012) 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim DI, Jensen SC, Noble KA, Kc B, Roux KH, Motamedchaboki K, Roux KJ, An improved smaller biotin ligase for BioID proximity labeling, Mol Biol Cell 27(8) (2016) 1188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Engelberg K, Chen CT, Bechtel T, Sánchez Guzmán V, Drozda AA, Chavan S, Weerapana E, Gubbels MJ, The apical annuli of Toxoplasma gondii are composed of coiled-coil and signalling proteins embedded in the inner membrane complex sutures, Cell Microbiol 22(1) (2020) e13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gaji RY, Johnson DE, Treeck M, Wang M, Hudmon A, Arrizabalaga G, Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during Toxoplasma gondii egress, PLoS Pathog 11(11) (2015) e1005268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nofal SD, Dominicus C, Broncel M, Katris NJ, Flynn HR, Arrizabalaga G, Botté CY, Invergo BM, Treeck M, A positive feedback loop mediates crosstalk between calcium, cyclic nucleotide and lipid signalling in calcium-induced Toxoplasma gondii egress, PLoS Pathog 18(10) (2022) e1010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McCoy JM, Whitehead L, van Dooren GG, Tonkin CJ, TgCDPK3 regulates calcium-dependent egress of Toxoplasma gondii from host cells, PLoS Pathog 8(12) (2012) e1003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lourido S, Tang K, Sibley LD, Distinct signalling pathways control Toxoplasma egress and host-cell invasion, Embo j 31(24) (2012) 4524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Garrison E, Treeck M, Ehret E, Butz H, Garbuz T, Oswald PB, Settles M, Boothroyd J, Arrizabalaga G, A forward genetic screen reveals that calcium-dependent protein kinase 3 regulates egress in Toxoplasma, PLoS Pathog 8(11) (2012) e1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Treeck M, Sanders JL, Gaji RY, LaFavers KA, Child MA, Arrizabalaga G, Elias JE, Boothroyd JC, The calcium-dependent protein kinase 3 of toxoplasma influences basal calcium levels and functions beyond egress as revealed by quantitative phosphoproteome analysis, PLoS Pathog 10(6) (2014) e1004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lis A, Baptista CG, Dahlgren K, Corvi MM, Blader IJ, Identification of Toxoplasma calcium-dependent protein kinase 3 as a stress-activated elongation factor 2 kinase, mSphere 8(4) (2023) e0015623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].O'Shaughnessy WJ, Hu X, Henriquez SA, Reese ML, Toxoplasma ERK7 protects the apical complex from premature degradation, J Cell Biol 222(6) (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].O'Shaughnessy WJ, Dewangan PS, Paiz EA, Reese ML, Not your Mother's MAPKs: Apicomplexan MAPK function in daughter cell budding, PLoS Pathog 18(10) (2022) e1010849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].O'Shaughnessy WJ, Hu X, Beraki T, McDougal M, Reese ML, Loss of a conserved MAPK causes catastrophic failure in assembly of a specialized cilium-like structure in Toxoplasma gondii, Mol Biol Cell 31(9) (2020) 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Back PS, O'Shaughnessy WJ, Moon AS, Dewangan PS, Hu X, Sha J, Wohlschlegel JA, Bradley PJ, Reese ML, Ancient MAPK ERK7 is regulated by an unusual inhibitory scaffold required for Toxoplasma apical complex biogenesis, Proc Natl Acad Sci U S A 117(22) (2020) 12164–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Back PS, O'Shaughnessy WJ, Moon AS, Dewangan PS, Reese ML, Bradley PJ, Multivalent Interactions Drive the Toxoplasma AC9:AC10:ERK7 Complex To Concentrate ERK7 in the Apical Cap, mBio 13(1) (2021) e0286421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Machado M, Klaus S, Klaschka D, Guizetti J, Ganter M, Plasmodium falciparum CRK4 links early mitotic events to the onset of S-phase during schizogony, mBio 14(4) (2023) e0077923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Malumbres M, Cyclin-dependent kinases, Genome Biol 15(6) (2014) 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Simon CS, Funaya C, Bauer J, Voβ Y, Machado M, Penning A, Klaschka D, Cyrklaff M, Kim J, Ganter M, Guizetti J, An extended DNA-free intranuclear compartment organizes centrosome microtubules in malaria parasites, Life Sci Alliance 4(11) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ganter M, Goldberg JM, Dvorin JD, Paulo JA, King JG, Tripathi AK, Paul AS, Yang J, Coppens I, Jiang RH, Elsworth B, Baker DA, Dinglasan RR, Gygi PS, Duraisingh MT, Plasmodium falciparum CRK4 directs continuous rounds of DNA replication during schizogony, Nat Microbiol 2 (2017) 17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Alvarez CA, Suvorova ES, Checkpoints of apicomplexan cell division identified in Toxoplasma gondii, PLoS Pathog 13(7) (2017) e1006483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Branon TC, Bosch JA, Sanchez AD, Udeshi ND, Svinkina T, Carr SA, Feldman JL, Perrimon N, Ting AY, Efficient proximity labeling in living cells and organisms with TurboID, Nat Biotechnol 36(9) (2018) 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Davies H, Belda H, Broncel M, Dalimot J, Treeck M, PerTurboID, a targeted in situ method reveals the impact of kinase deletion on its local protein environment in the cytoadhesion complex of malaria-causing parasites, Elife 12 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Davies H, Belda H, Broncel M, Ye X, Bisson C, Introini V, Dorin-Semblat D, Semblat PJ, Tibúrcio M, Gamain B, Kaforou M, Treeck M, An exported kinase family mediates species-specific erythrocyte remodelling and virulence in human malaria, Nat Microbiol 5(6) (2020) 848–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Leech JH, Barnwell JW, Miller LH, Howard RJ, Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes, J Exp Med 159(6) (1984) 1567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rug M, Prescott SW, Fernandez KM, Cooke BM, Cowman AF, The role of KAHRP domains in knob formation and cytoadherence of P falciparum-infected human erythrocytes, Blood 108(1) (2006) 370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Maier AG, Rug M, O'Neill MT, Brown M, Chakravorty S, Szestak T, Chesson J, Wu Y, Hughes K, Coppel RL, Newbold C, Beeson JG, Craig A, Crabb BS, Cowman AF, Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes, Cell 134(1) (2008) 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Free RB, Hazelwood LA, Sibley DR, Identifying novel protein-protein interactions using co-immunoprecipitation and mass spectroscopy, Curr Protoc Neurosci Chapter 5 (2009) Unit 5.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Husain A, Begum NA, Kobayashi M, Honjo T, Native Co-immunoprecipitation Assay to Identify Interacting Partners of Chromatin-associated Proteins in Mammalian Cells, Bio Protoc 10(23) (2020) e3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].ten Have S, Boulon S, Ahmad Y, Lamond AI, Mass spectrometry-based immunoprecipitation proteomics - the user's guide, Proteomics 11(6) (2011) 1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Holland Z, Prudent R, Reiser JB, Cochet C, Doerig C, Functional analysis of protein kinase CK2 of the human malaria parasite Plasmodium falciparum, Eukaryot Cell 8(3) (2009) 388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dastidar EG, Dayer G, Holland ZM, Dorin-Semblat D, Claes A, Chêne A, Sharma A, Hamelin R, Moniatte M, Lopez-Rubio JJ, Scherf A, Doerig C, Involvement of Plasmodium falciparum protein kinase CK2 in the chromatin assembly pathway, BMC Biol 10 (2012) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hitz E, Grüninger O, Passecker A, Wyss M, Scheurer C, Wittlin S, Beck PH, Brancucci NMB, Voss TS, The catalytic subunit of Plasmodium falciparum casein kinase 2 is essential for gametocytogenesis, Commun Biol 4(1) (2021) 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pease BN, Huttlin EL, Jedrychowski PM, Talevich E, Harmon J, Dillman T, Kannan N, Doerig C, Chakrabarti R, Gygi PS, Chakrabarti D, Global analysis of protein expression and phosphorylation of three stages of Plasmodium falciparum intraerythrocytic development, J Proteome Res 12(9) (2013) 4028–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Willems E, Dedobbeleer M, Digregorio M, Lombard A, Lumapat PN, Rogister B, The functional diversity of Aurora kinases: a comprehensive review, Cell Div 13 (2018) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Berry L, Chen CT, Francia ME, Guerin A, Graindorge A, Saliou JM, Grandmougin M, Wein S, Bechara C, Morlon-Guyot J, Bordat Y, Gubbels MJ, Lebrun M, Dubremetz JF, Daher W, Toxoplasma gondii chromosomal passenger complex is essential for the organization of a functional mitotic spindle: a prerequisite for productive endodyogeny, Cell Mol Life Sci 75(23) (2018) 4417–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tjhin ET, Howieson VM, Spry C, van Dooren GG, Saliba KJ, A novel heteromeric pantothenate kinase complex in apicomplexan parasites, PLoS Pathog 17(7) (2021) e1009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Spry C, Kirk K, Saliba KJ, Coenzyme A biosynthesis: an antimicrobial drug target, FEMS Microbiol Rev 32(1) (2008) 56–106. [DOI] [PubMed] [Google Scholar]
- [55].Leonardi R, Zhang YM, Rock CO, Jackowski S, Coenzyme A: back in action, Prog Lipid Res 44(2–3) (2005) 125–53. [DOI] [PubMed] [Google Scholar]
- [56].Li Y, Niu Z, Yang J, Yang X, Chen Y, Li Y, Liang X, Zhang J, Fan F, Wu P, Peng C, Shen B, Rapid metabolic reprogramming mediated by the AMP-activated protein kinase during the lytic cycle of Toxoplasma gondii, Nat Commun 14(1) (2023) 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Long YC, Zierath JR, AMP-activated protein kinase signaling in metabolic regulation, J Clin Invest 116(7) (2006) 1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yan Y, Zhou XE, Xu HE, Melcher K, Structure and Physiological Regulation of AMPK, Int J Mol Sci 19(11) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang Z, Huang B, Dong H, Zhao Q, Zhu S, Xia W, Xu S, Xie Y, Cui X, Tang M, Men Q, Yang Z, Li C, Zhu X, Han H, Molecular Characterization and Functional Analysis of a Novel Calcium-Dependent Protein Kinase 4 from Eimeria tenella, PLoS One 11(12) (2016) e0168132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Liang S, Dong H, Zhu S, Zhao Q, Huang B, Yu Y, Wang Q, Wang H, Yu S, Han H, Eimeria tenella Translation Initiation Factor eIF-5A That Interacts With Calcium-Dependent Protein Kinase 4 Is Involved in Host Cell Invasion, Front Cell Infect Microbiol 10 (2020) 602049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lv L, Huang B, Zhao Q, Zhao Z, Dong H, Zhu S, Chen T, Yan M, Han H, Identification of an interaction between calcium-dependent protein kinase 4 (EtCDPK4) and serine protease inhibitor (EtSerpin) in Eimeria tenella, Parasit Vectors 11(1) (2018) 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Liang S, Zhao Q, Ye Y, Zhu S, Dong H, Yu Y, Huang B, Han H, Characteristics analyses of Eimeria tenella 14-3-3 protein and verification of its interaction with calcium-dependent protein kinase 4, Eur J Protistol 85 (2022) 125895. [DOI] [PubMed] [Google Scholar]
- [63].Diallo MA, Sausset A, Gnahoui-David A, Ribeiro ESA, Brionne A, Le Vern Y, Bussière FI, Tottey J, Lacroix-Lamandé S, Laurent F, Silvestre A, Eimeria tenella ROP kinase EtROP1 induces G0/G1 cell cycle arrest and inhibits host cell apoptosis, Cell Microbiol 21(7) (2019) e13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Back JW, de Jong L, Muijsers AO, de Koster CG, Chemical cross-linking and mass spectrometry for protein structural modeling, J Mol Biol 331(2) (2003) 303–13. [DOI] [PubMed] [Google Scholar]
- [65].Sinz A, Chemical cross-linking and mass spectrometry for mapping three-dimensional structures of proteins and protein complexes, J Mass Spectrom 38(12) (2003) 1225–37. [DOI] [PubMed] [Google Scholar]
- [66].Vasilescu J, Guo X, Kast J, Identification of protein-protein interactions using in vivo cross-linking and mass spectrometry, Proteomics 4(12) (2004) 3845–54. [DOI] [PubMed] [Google Scholar]
- [67].Sutherland BW, Toews J, Kast J, Utility of formaldehyde cross-linking and mass spectrometry in the study of protein-protein interactions, J Mass Spectrom 43(6) (2008) 699–715. [DOI] [PubMed] [Google Scholar]
- [68].Tayri-Wilk T, Slavin M, Zamel J, Blass A, Cohen S, Motzik A, Sun X, Shalev DE, Ram O, Kalisman N, Mass spectrometry reveals the chemistry of formaldehyde cross-linking in structured proteins, Nat Commun 11(1) (2020) 3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sinz A, Chemical cross-linking and mass spectrometry to map three-dimensional protein structures and protein-protein interactions, Mass Spectrom Rev 25(4) (2006) 663–82. [DOI] [PubMed] [Google Scholar]
- [70].Fang H, Gomes AR, Klages N, Pino P, Maco B, Walker EM, Zenonos ZA, Angrisano F, Baum J, Doerig C, Baker DA, Billker O, Brochet M, Epistasis studies reveal redundancy among calcium-dependent protein kinases in motility and invasion of malaria parasites, Nat Commun 9(1) (2018) 4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V, Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite, Cell 117(4) (2004) 503–14. [DOI] [PubMed] [Google Scholar]
- [72].Kumar S, Haile MT, Hoopmann MR, Tran LT, Michaels SA, Morrone SR, Ojo KK, Reynolds LM, Kusebauch U, Vaughan AM, Moritz RL, Kappe SHI, Swearingen KE, Plasmodium falciparum Calcium-Dependent Protein Kinase 4 is Critical for Male Gametogenesis and Transmission to the Mosquito Vector, mBio 12(6) (2021) e0257521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fang H, Klages N, Baechler B, Hillner E, Yu L, Pardo M, Choudhary J, Brochet M, Multiple short windows of calcium-dependent protein kinase 4 activity coordinate distinct cell cycle events during Plasmodium gametogenesis, Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kumar S, Kumar M, Ekka R, Dvorin JD, Paul AS, Madugundu AK, Gilberger T, Gowda H, Duraisingh MT, Keshava Prasad TS, Sharma P, PfCDPK1 mediated signaling in erythrocytic stages of Plasmodium falciparum, Nat Commun 8(1) (2017) 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Brochet M, Collins MO, Smith TK, Thompson E, Sebastian S, Volkmann K, Schwach F, Chappell L, Gomes AR, Berriman M, Rayner JC, Baker DA, Choudhary J, Billker O, Phosphoinositide metabolism links cGMP-dependent protein kinase G to essential Ca2+ signals at key decision points in the life cycle of malaria parasites, PLoS Biol 12(3) (2014) e1001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Frénal K, Polonais V, Marq JB, Stratmann R, Limenitakis J, Soldati-Favre D, Functional dissection of the apicomplexan glideosome molecular architecture, Cell Host Microbe 8(4) (2010) 343–57. [DOI] [PubMed] [Google Scholar]
- [77].Jacot D, Tosetti N, Pires I, Stock J, Graindorge A, Hung YF, Han H, Tewari R, Kursula I, Soldati-Favre D, An Apicomplexan Actin-Binding Protein Serves as a Connector and Lipid Sensor to Coordinate Motility and Invasion, Cell Host Microbe 20(6) (2016) 731–743. [DOI] [PubMed] [Google Scholar]
- [78].Balestra AC, Koussis K, Klages N, Howell SA, Flynn HR, Bantscheff M, Pasquarello C, Perrin AJ, Brusini L, Arboit P, Sanz O, Castaño PL, Withers-Martinez C, Hainard A, Ghidelli-Disse S, Snijders PA, Baker DA, Blackman MJ, Brochet M, Ca(2+) signals critical for egress and gametogenesis in malaria parasites depend on a multipass membrane protein that interacts with PKG, Sci Adv 7(13) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zeeshan M, Rea E, Abel S, Vukušić K, Markus R, Brady D, Eze A, Rashpa R, Balestra AC, Bottrill AR, Brochet M, Guttery DS, Tolić IM, Holder AA, Le Roch KG, Tromer EC, Tewari R, Plasmodium ARK2 and EB1 drive unconventional spindle dynamics, during chromosome segregation in sexual transmission stages, Nat Commun 14(1) (2023) 5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Rhee HW, Zou P, Udeshi ND, Martell JD, Mootha VK, Carr SA, Ting AY, Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging, Science 339(6125) (2013) 1328–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lam SS, Martell JD, Kamer KJ, Deerinck TJ, Ellisman MH, Mootha VK, Ting AY, Directed evolution of APEX2 for electron microscopy and proximity labeling, Nat Methods 12(1) (2015) 51–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Dumont AA, Dumont L, Berthiaume J, Auger-Messier M, p38α MAPK proximity assay reveals a regulatory mechanism of alternative splicing in cardiomyocytes, Biochim Biophys Acta Mol Cell Res 1866(12) (2019) 118557. [DOI] [PubMed] [Google Scholar]
- [83].Rosenberg A, Sibley LD, Toxoplasma gondii secreted effectors co-opt host repressor complexes to inhibit necroptosis, Cell Host Microbe 29(7) (2021) 1186–1198.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Junttila MR, Saarinen S, Schmidt T, Kast J, Westermarck J, Single-step Strep-tag purification for the isolation and identification of protein complexes from mammalian cells, Proteomics 5(5) (2005) 1199–203. [DOI] [PubMed] [Google Scholar]
- [85].Schmidt TG, Skerra A, The Strep-tag system for one-step purification and high-affinity detection or capturing of proteins, Nat Protoc 2(6) (2007) 1528–35. [DOI] [PubMed] [Google Scholar]
- [86].Buljan M, Ciuffa R, van Drogen A, Vichalkovski A, Mehnert M, Rosenberger G, Lee S, Varjosalo M, Pernas LE, Spegg V, Snijder B, Aebersold R, Gstaiger M, Kinase Interaction Network Expands Functional and Disease Roles of Human Kinases, Mol Cell 79(3) (2020) 504–520.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Golkowski M, Lius A, Sapre T, Lau HT, Moreno T, Maly DJ, Ong SE, Multiplexed kinase interactome profiling quantifies cellular network activity and plasticity, Mol Cell 83(5) (2023) 803–818.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bantscheff M, Eberhard D, Abraham Y, Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau C, Reader V, Sweetman G, Bauer A, Bouwmeester T, Hopf C, Kruse U, Neubauer G, Ramsden N, Rick J, Kuster B, Drewes G, Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors, Nat Biotechnol 25(9) (2007) 1035–44. [DOI] [PubMed] [Google Scholar]
- [89].Daub H, Olsen JV, Bairlein M, Gnad F, Oppermann FS, Körner R, Greff Z, Kéri G, Stemmann O, Mann M, Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle, Mol Cell 31(3) (2008) 438–48. [DOI] [PubMed] [Google Scholar]
- [90].Golkowski M, Vidadala VN, Lau HT, Shoemaker A, Shimizu-Albergine M, Beavo J, Maly DJ, Ong SE, Kinobead/LC-MS Phosphokinome Profiling Enables Rapid Analyses of Kinase-Dependent Cell Signaling Networks, J Proteome Res 19(3) (2020) 1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ong SE, Schenone M, Margolin AA, Li X, Do K, Doud MK, Mani DR, Kuai L, Wang X, Wood JL, Tolliday NJ, Koehler AN, Marcaurelle LA, Golub TR, Gould RJ, Schreiber SL, Carr SA, Identifying the proteins to which small-molecule probes and drugs bind in cells, Proc Natl Acad Sci U S A 106(12) (2009) 4617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Klaeger S, Heinzlmeir S, Wilhelm M, Polzer H, Vick B, Koenig PA, Reinecke M, Ruprecht B, Petzoldt S, Meng C, Zecha J, Reiter K, Qiao H, Helm D, Koch H, Schoof M, Canevari G, Casale E, Depaolini SR, Feuchtinger A, Wu Z, Schmidt T, Rueckert L, Becker W, Huenges J, Garz AK, Gohlke BO, Zolg DP, Kayser G, Vooder T, Preissner R, Hahne H, Tõnisson N, Kramer K, Götze K, Bassermann F, Schlegl J, Ehrlich HC, Aiche S, Walch A, Greif PA, Schneider S, Felder ER, Ruland J, Médard G, Jeremias I, Spiekermann K, Kuster B, The target landscape of clinical kinase drugs, Science 358(6367) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Golkowski M, Vidadala RS, Lombard CK, Suh HW, Maly DJ, Ong SE, Kinobead and Single-Shot LC-MS Profiling Identifies Selective PKD Inhibitors, J Proteome Res 16(3) (2017) 1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ramazi S, Zahiri J, Posttranslational modifications in proteins: resources, tools and prediction methods, Database (Oxford) 2021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Dupree EJ, Jayathirtha M, Yorkey H, Mihasan M, Petre BA, Darie CC, A Critical Review of Bottom-Up Proteomics: The Good, the Bad, and the Future of this Field, Proteomes 8(3) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bantscheff M, Schirle M, Sweetman G, Rick J, Kuster B, Quantitative mass spectrometry in proteomics: a critical review, Anal Bioanal Chem 389(4) (2007) 1017–31. [DOI] [PubMed] [Google Scholar]
- [97].Sefton BM, Measurement of stoichiometry of protein phosphorylation by biosynthetic labeling, Methods Enzymol 201 (1991) 245–51. [DOI] [PubMed] [Google Scholar]
- [98].Cooper JA, Estimation of phosphorylation stoichiometry by separation of phosphorylated isoforms, Methods Enzymol 201 (1991) 251–61. [DOI] [PubMed] [Google Scholar]
- [99].Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, Ross MM, Shabanowitz J, Hunt DF, White FM, Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae, Nat Biotechnol 20(3) (2002) 301–5. [DOI] [PubMed] [Google Scholar]
- [100].Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jørgensen TJ, Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns, Mol Cell Proteomics 4(7) (2005) 873–86 [DOI] [PubMed] [Google Scholar]
- [101].Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villén J, Li J, Cohn MA, Cantley LC, Gygi PS, Large-scale characterization of HeLa cell nuclear phosphoproteins, Proc Natl Acad Sci U S A 101(33) (2004) 12130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Dong M, Ye M, Cheng K, Song C, Pan Y, Wang C, Bian Y, Zou H, Depletion of acidic phosphopeptides by SAX to improve the coverage for the detection of basophilic kinase substrates, J Proteome Res 11(9) (2012) 4673–81. [DOI] [PubMed] [Google Scholar]
- [103].Alam MM, Solyakov L, Bottrill AR, Flueck C, Siddiqui FA, Singh S, Mistry S, Viskaduraki M, Lee K, Hopp CS, Chitnis CE, Doerig C, Moon RW, Green JL, Holder AA, Baker DA, Tobin AB, Phosphoproteomics reveals malaria parasite Protein Kinase G as a signalling hub regulating egress and invasion, Nat Commun 6 (2015) 7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Neilson KA, Ali NA, Muralidharan S, Mirzaei M, Mariani M, Assadourian G, Lee A, van Sluyter SC, Haynes PA, Less label, more free: approaches in label-free quantitative mass spectrometry, Proteomics 11(4) (2011) 535–53. [DOI] [PubMed] [Google Scholar]
- [105].Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M, Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ, Mol Cell Proteomics 13(9) (2014) 2513–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Blomqvist K, Helmel M, Wang C, Absalon S, Labunska T, Rudlaff RM, Adapa S, Jiang R, Steen H, Dvorin JD, Influence of Plasmodium falciparum Calcium-Dependent Protein Kinase 5 (PfCDPK5) on the Late Schizont Stage Phosphoproteome, mSphere 5(1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Dvorin JD, Martyn DC, Patel SD, Grimley JS, Collins CR, Hopp CS, Bright AT, Westenberger S, Winzeler E, Blackman MJ, Baker DA, Wandless TJ, Duraisingh MT, A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes, Science 328(5980) (2010) 910–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chakrabarti D, Schuster SM, Chakrabarti R, Cloning and characterization of subunit genes of ribonucleotide reductase, a cell-cycle-regulated enzyme, from Plasmodium falciparum, Proc Natl Acad Sci U S A 90(24) (1993) 12020–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Talman AM, Prieto JH, Marques S, Ubaida-Mohien C, Lawniczak M, Wass MN, Xu T, Frank R, Ecker A, Stanway RS, Krishna S, Sternberg MJ, Christophides GK, Graham DR, Dinglasan RR, Yates JR 3rd, Sinden RE, Proteomic analysis of the Plasmodium male gamete reveals the key role for glycolysis in flagellar motility, Malar J 13 (2014) 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bansal A, Molina-Cruz A, Brzostowski J, Liu P, Luo Y, Gunalan K, Li Y, Ribeiro JMC, Miller LH, PfCDPK1 is critical for malaria parasite gametogenesis and mosquito infection, Proc Natl Acad Sci U S A 115(4) (2018) 774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lentini G, Ben Chaabene R, Vadas O, Ramakrishnan C, Mukherjee B, Mehta V, Lunghi M, Grossmann J, Maco B, Visentin R, Hehl AB, Korkhov VM, Soldati-Favre D, Structural insights into an atypical secretory pathway kinase crucial for Toxoplasma gondii invasion, Nat Commun 12(1) (2021) 3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Guérin A, Corrales RM, Parker ML, Lamarque MH, Jacot D, El Hajj H, Soldati-Favre D, Boulanger MJ, Lebrun M, Efficient invasion by Toxoplasma depends on the subversion of host protein networks, Nat Microbiol 2(10) (2017) 1358–1366. [DOI] [PubMed] [Google Scholar]
- [113].Hawkins LM, Wang C, Chaput D, Batra M, Marsilia C, Awshah D, Suvorova ES, The Crk4-Cyc4 complex regulates G(2)/M transition in Toxoplasma gondii, Embo j (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M, Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics, Mol Cell Proteomics 1(5) (2002) 376–86. [DOI] [PubMed] [Google Scholar]
- [115].Mann M, Functional and quantitative proteomics using SILAC, Nat Rev Mol Cell Biol 7(12) (2006) 952–8. [DOI] [PubMed] [Google Scholar]
- [116].Ong SE, Kratchmarova I, Mann M, Properties of 13C-substituted arginine in stable isotope labeling by amino acids in cell culture (SILAC), J Proteome Res 2(2) (2003) 173–81. [DOI] [PubMed] [Google Scholar]
- [117].Beraki T, Hu X, Broncel M, Young JC, O'Shaughnessy WJ, Borek D, Treeck M, Reese ML, Divergent kinase regulates membrane ultrastructure of the Toxoplasma parasitophorous vacuole, Proc Natl Acad Sci U S A 116(13) (2019) 6361–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lopez J, Bittame A, Massera C, Vasseur V, Effantin G, Valat A, Buaillon C, Allart S, Fox BA, Rommereim LM, Bzik DJ, Schoehn G, Weissenhorn W, Dubremetz JF, Gagnon J, Mercier C, Cesbron-Delauw MF, Blanchard N, Intravacuolar Membranes Regulate CD8 T Cell Recognition of Membrane-Bound Toxoplasma gondii Protective Antigen, Cell Rep 13(10) (2015) 2273–86. [DOI] [PubMed] [Google Scholar]
- [119].Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ, Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents, Mol Cell Proteomics 3(12) (2004) 1154–69. [DOI] [PubMed] [Google Scholar]
- [120].Patel VJ, Thalassinos K, Slade SE, Connolly JB, Crombie A, Murrell JC, Scrivens JH, A comparison of labeling and label-free mass spectrometry-based proteomics approaches, J Proteome Res 8(7) (2009) 3752–9. [DOI] [PubMed] [Google Scholar]
- [121].Maurya R, Tripathi A, Kumar M, Antil N, Yamaryo-Botté Y, Kumar P, Bansal P, Doerig C, Botté CY, Prasad TSK, Sharma P, PI4-kinase and PfCDPK7 signaling regulate phospholipid biosynthesis in Plasmodium falciparum, EMBO Rep 23(2) (2022) e54022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kumar P, Tripathi A, Ranjan R, Halbert J, Gilberger T, Doerig C, Sharma P, Regulation of Plasmodium falciparum development by calcium-dependent protein kinase 7 (PfCDPK7), J Biol Chem 289(29) (2014) 20386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Thompson A, Schäfer J, Kuhn K, Kienle S, Schwarz J, Schmidt G, Neumann T, Johnstone R, Mohammed AK, Hamon C, Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS, Anal Chem 75(8) (2003) 1895–904. [DOI] [PubMed] [Google Scholar]
- [124].Hogrebe A, von Stechow L, Bekker-Jensen DB, Weinert BT, Kelstrup CD, Olsen JV, Benchmarking common quantification strategies for large-scale phosphoproteomics, Nat Commun 9(1) (2018) 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Bansal P, Antil N, Kumar M, Yamaryo-Botté Y, Rawat RS, Pinto S, Datta KK, Katris NJ, Botté CY, Prasad TSK, Sharma P, Protein kinase TgCDPK7 regulates vesicular trafficking and phospholipid synthesis in Toxoplasma gondii, PLoS Pathog 17(2) (2021) e1009325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Pease BN, Huttlin EL, Jedrychowski PM, Dorin-Semblat D, Sebastiani D, Segarra DT, Roberts BF, Chakrabarti R, Doerig C, Gygi PS, Chakrabarti D, Characterization of Plasmodium falciparum Atypical Kinase PfPK7(−) Dependent Phosphoproteome, J Proteome Res 17(6) (2018) 2112–2123. [DOI] [PubMed] [Google Scholar]
- [127].Invergo BM, Brochet M, Yu L, Choudhary J, Beltrao P, Billker O, Sub-minute Phosphoregulation of Cell Cycle Systems during Plasmodium Gamete Formation, Cell Rep 21(7) (2017) 2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Patel A, Perrin AJ, Flynn HR, Bisson C, Withers-Martinez C, Treeck M, Flueck C, Nicastro G, Martin SR, Ramos A, Gilberger TW, Snijders AP, Blackman MJ, Baker DA, Cyclic AMP signalling controls key components of malaria parasite host cell invasion machinery, PLoS Biol 17(5) (2019) e3000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Donald RG, Zhong T, Wiersma H, Nare B, Yao D, Lee A, Allocco J, Liberator PA, Anticoccidial kinase inhibitors: identification of protein kinase targets secondary to cGMP-dependent protein kinase, Mol Biochem Parasitol 149(1) (2006) 86–98. [DOI] [PubMed] [Google Scholar]
- [130].McRobert L, Taylor CJ, Deng W, Fivelman QL, Cummings RM, Polley SD, Billker O, Baker DA, Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase, PLoS Biol 6(6) (2008) e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Chan AW, Broncel M, Yifrach E, Haseley N, Chakladar S, Andree E, Herneisen AL, Shortt E, Treeck M, Lourido S, Analysis of CDPK1 targets identifies a trafficking adaptor complex that regulates microneme exocytosis in Toxoplasma, eLife Sciences Publications, Ltd, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Agop-Nersesian C, Naissant B, Ben Rached F, Rauch M, Kretzschmar A, Thiberge S, Menard R, Ferguson DJ, Meissner M, Langsley G, Rab11A-controlled assembly of the inner membrane complex is required for completion of apicomplexan cytokinesis, PLoS Pathog 5(1) (2009) e1000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Jia Y, Marq JB, Bisio H, Jacot D, Mueller C, Yu L, Choudhary J, Brochet M, Soldati-Favre D, Crosstalk between PKA and PKG controls pH-dependent host cell egress of Toxoplasma gondii, Embo j 36(21) (2017) 3250–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Moss WJ, Patterson CE, Jochmans AK, Brown KM, Functional Analysis of the Expanded Phosphodiesterase Gene Family in Toxoplasma gondii Tachyzoites, mSphere 7(1) (2022) e0079321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Moss WJ, Brusini L, Kuehnel R, Brochet M, Brown KM, Apicomplexan phosphodiesterases in cyclic nucleotide turnover: conservation, function, and therapeutic potential, mBio 15(2) (2024) e0305623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Uboldi AD, Wilde ML, McRae EA, Stewart RJ, Dagley LF, Yang L, Katris NJ, Hapuarachchi SV, Coffey MJ, Lehane AM, Botte CY, Waller RF, Webb AI, McConville MJ, Tonkin CJ, Protein kinase A negatively regulates Ca2+ signalling in Toxoplasma gondii, PLoS Biol 16(9) (2018) e2005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Herneisen AL, Peters ML, Smith TA, Lourido S, SPARK regulates AGC kinases central to the Toxoplasma gondii asexual cycle, eLife 13 (2024) RP93877. [Google Scholar]
- [138].Mora A, Komander D, van Aalten DM, Alessi DR, PDK1, the master regulator of AGC kinase signal transduction, Semin Cell Dev Biol 15(2) (2004) 161–70. [DOI] [PubMed] [Google Scholar]
- [139].Smith TA, Lopez-Perez GS, Herneisen AL, Shortt E, Lourido S, Screening the Toxoplasma kinome with high-throughput tagging identifies a regulator of invasion and egress, Nat Microbiol 7(6) (2022) 868–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Hitz E, Wiedemar N, Passecker A, Graça BAS, Scheurer C, Wittlin S, Brancucci NMB, Vakonakis I, Mäser P, Voss TS, The 3-phosphoinositide-dependent protein kinase 1 is an essential upstream activator of protein kinase A in malaria parasites, PLoS Biol 19(12) (2021) e3001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Xue L, Wang WH, Iliuk A, Hu L, Galan JA, Yu S, Hans M, Geahlen RL, Tao WA, Sensitive kinase assay linked with phosphoproteomics for identifying direct kinase substrates, Proc Natl Acad Sci U S A 109(15) (2012) 5615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Iliuk AB, Martin VA, Alicie BM, Geahlen RL, Tao WA, In-depth analyses of kinase-dependent tyrosine phosphoproteomes based on metal ion-functionalized soluble nanopolymers, Mol Cell Proteomics 9(10) (2010) 2162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Boyle WJ, van der Geer P, Hunter T, Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates, Methods Enzymol 201 (1991) 110–49. [DOI] [PubMed] [Google Scholar]
- [144].Wettenhall RE, Aebersold RH, Hood LE, Solid-phase sequencing of 32P-labeled phosphopeptides at picomole and subpicomole levels, Methods Enzymol 201 (1991) 186–99. [DOI] [PubMed] [Google Scholar]
- [145].Sharifpoor S, Nguyen Ba AN, Youn JY, van Dyk D, Friesen H, Douglas AC, Kurat CF, Chong YT, Founk K, Moses AM, Andrews BJ, A quantitative literature-curated gold standard for kinase-substrate pairs, Genome Biol 12(4) (2011) R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Ubersax JA, Ferrell JE Jr., Mechanisms of specificity in protein phosphorylation, Nat Rev Mol Cell Biol 8(7) (2007) 530–41. [DOI] [PubMed] [Google Scholar]
- [147].Good MC, Zalatan JG, Lim WA, Scaffold proteins: hubs for controlling the flow of cellular information, Science 332(6030) (2011) 680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].DeMarco AG, Hall MC, Phosphoproteomic Approaches for Identifying Phosphatase and Kinase Substrates, Molecules 28(9) (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Xue L, Tao WA, Current technologies to identify protein kinase substrates in high throughput, Front Biol (Beijing) 8(2) (2013) 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Mok J, Im H, Snyder M, Global identification of protein kinase substrates by protein microarray analysis, Nat Protoc 4(12) (2009) 1820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Behnke MS, Khan A, Wootton JC, Dubey PJ, Tang K, Sibley LD, Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases, Proc Natl Acad Sci U S A 108(23) (2011) 9631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, Ajioka JW, Boothroyd JC, Polymorphic secreted kinases are key virulence factors in toxoplasmosis, Science 314(5806) (2006) 1780–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, Hajj HE, Jerome M, Behnke MS, White M, Wootton JC, Sibley LD, A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii, Science 314(5806) (2006) 1776–80. [DOI] [PubMed] [Google Scholar]
- [154].Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC, Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue, Nature 445(7125) (2007) 324–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Yamamoto M, Standley DM, Takashima S, Saiga H, Okuyama M, Kayama H, Kubo E, Ito H, Takaura M, Matsuda T, Soldati-Favre D, Takeda K, A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3, J Exp Med 206(12) (2009) 2747–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Ong YC, Reese ML, Boothroyd JC, Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6, J Biol Chem 285(37) (2010) 28731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Ong YC, Boyle JP, Boothroyd JC, Strain-dependent host transcriptional responses to Toxoplasma infection are largely conserved in mammalian and avian hosts, PLoS One 6(10) (2011) e26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Butcher BA, Fox BA, Rommereim LM, Kim SG, Maurer KJ, Yarovinsky F, Herbert DR, Bzik DJ, Denkers EY, Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control, PLoS Pathog 7(9) (2011) e1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Behnke MS, Fentress SJ, Mashayekhi M, Li LX, Taylor GA, Sibley LD, The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18, PLoS Pathog 8(11) (2012) e1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Fentress SJ, Behnke MS, Dunay IR, Mashayekhi M, Rommereim LM, Fox BA, Bzik DJ, Taylor GA, Turk BE, Lichti CF, Townsend RR, Qiu W, Hui R, Beatty WL, Sibley LD, Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence, Cell Host Microbe 8(6) (2010) 484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Steinfeldt T, Könen-Waisman S, Tong L, Pawlowski N, Lamkemeyer T, Sibley LD, Hunn JP, Howard JC, Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii, PLoS Biol 8(12) (2010) e1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Khaminets A, Hunn JP, Könen-Waisman S, Zhao YO, Preukschat D, Coers J, Boyle JP, Ong YC, Boothroyd JC, Reichmann G, Howard JC, Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole, Cell Microbiol 12(7) (2010) 939–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC, Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases, PLoS Pathog 1(3) (2005) e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Taylor GA, Feng CG, Sher A, Control of IFN-gamma-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases), Microbes Infect 9(14–15) (2007) 1644–51. [DOI] [PubMed] [Google Scholar]
- [165].Howard JC, Hunn JP, Steinfeldt T, The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii, Curr Opin Microbiol 14(4) (2011) 414–21. [DOI] [PubMed] [Google Scholar]
- [166].Zhao YO, Khaminets A, Hunn JP, Howard JC, Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death, PLoS Pathog 5(2) (2009) e1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, Yap GS, Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages, J Exp Med 203(9) (2006) 2063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Wilde ML, Triglia T, Marapana D, Thompson JK, Kouzmitchev AA, Bullen HE, Gilson PR, Cowman AF, Tonkin CJ, Protein Kinase A Is Essential for Invasion of Plasmodium falciparum into Human Erythrocytes, mBio 10(5) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Leykauf K, Treeck M, Gilson PR, Nebl T, Braulke T, Cowman AF, Gilberger TW, Crabb BS, Protein kinase a dependent phosphorylation of apical membrane antigen 1 plays an important role in erythrocyte invasion by the malaria parasite, PLoS Pathog 6(6) (2010) e1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Vaid A, Thomas DC, Sharma P, Role of Ca2+/calmodulin-PfPKB signaling pathway in erythrocyte invasion by Plasmodium falciparum, J Biol Chem 283(9) (2008) 5589–97. [DOI] [PubMed] [Google Scholar]
- [171].Tewari R, Dorin D, Moon R, Doerig C, Billker O, An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite, Mol Microbiol 58(5) (2005) 1253–63. [DOI] [PubMed] [Google Scholar]
- [172].Ridzuan MA, Moon RW, Knuepfer E, Black S, Holder AA, Green JL, Subcellular location, phosphorylation and assembly into the motor complex of GAP45 during Plasmodium falciparum schizont development, PLoS One 7(3) (2012) e33845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Green JL, Rees-Channer RR, Howell SA, Martin SR, Knuepfer E, Taylor HM, Grainger M, Holder AA, The motor complex of Plasmodium falciparum: phosphorylation by a calcium-dependent protein kinase, J Biol Chem 283(45) (2008) 30980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Zhang M, Mishra S, Sakthivel R, Rojas M, Ranjan R, Sullivan WJ Jr., Fontoura BM, Ménard R, Dever TE, Nussenzweig V, PK4, a eukaryotic initiation factor 2α(eIF2α) kinase, is essential for the development of the erythrocytic cycle of Plasmodium, Proc Natl Acad Sci U S A 109(10) (2012) 3956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Philip N, Haystead TA, Characterization of a UBC13 kinase in Plasmodium falciparum, Proc Natl Acad Sci U S A 104(19) (2007) 7845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Tham WH, Lim NT, Weiss GE, Lopaticki S, Ansell BR, Bird M, Lucet I, Dorin-Semblat D, Doerig C, Gilson PR, Crabb BS, Cowman AF, Plasmodium falciparum Adhesins Play an Essential Role in Signalling and Activation of Invasion into Human Erythrocytes, PLoS Pathog 11(12) (2015) e1005343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Dorin D, Le Roch K, Sallicandro P, Alano P, Parzy D, Poullet P, Meijer L, Doerig C, Pfnek-1, a NIMA-related kinase from the human malaria parasite Plasmodium falciparum Biochemical properties and possible involvement in MAPK regulation, Eur J Biochem 268(9) (2001) 2600–8. [DOI] [PubMed] [Google Scholar]
- [178].Lye YM, Chan M, Sim TS, Pfnek3: an atypical activator of a MAP kinase in Plasmodium falciparum, FEBS Lett 580(26) (2006) 6083–92. [DOI] [PubMed] [Google Scholar]
- [179].Fennell C, Babbitt S, Russo I, Wilkes J, Ranford-Cartwright L, Goldberg DE, Doerig C, PfeIK1, a eukaryotic initiation factor 2alpha kinase of the human malaria parasite Plasmodium falciparum, regulates stress-response to amino-acid starvation, Malar J 8 (2009) 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Hanks SK, Hunter T, Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification, Faseb j 9(8) (1995) 576–96. [PubMed] [Google Scholar]
- [181].Allen JJ, Lazerwith SE, Shokat KM, Bio-orthogonal affinity purification of direct kinase substrates, J Am Chem Soc 127(15) (2005) 5288–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Koch A, Hauf S, Strategies for the identification of kinase substrates using analog-sensitive kinases, Eur J Cell Biol 89(2-3) (2010) 184–93. [DOI] [PubMed] [Google Scholar]
- [183].Elphick LM, Lee SE, Gouverneur V, Mann DJ, Using chemical genetics and ATP analogues to dissect protein kinase function, ACS Chem Biol 2(5) (2007) 299–314. [DOI] [PubMed] [Google Scholar]
- [184].Eckstein F, Nucleoside phosphorothioates, Annu Rev Biochem 54 (1985) 367–402. [DOI] [PubMed] [Google Scholar]
- [185].Allen JJ, Li M, Brinkworth CS, Paulson JL, Wang D, Hübner A, Chou WH, Davis RJ, Burlingame AL, Messing RO, Katayama CD, Hedrick SM, Shokat KM, A semisynthetic epitope for kinase substrates, Nat Methods 4(6) (2007) 511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Blethrow JD, Glavy JS, Morgan DO, Shokat KM, Covalent capture of kinase-specific phosphopeptides reveals Cdk1-cyclin B substrates, Proc Natl Acad Sci U S A 105(5) (2008) 1442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Chi Y, Welcker M, Hizli AA, Posakony JJ, Aebersold R, Clurman BE, Identification of CDK2 substrates in human cell lysates, Genome Biol 9(10) (2008) R149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Lourido S, Jeschke GR, Turk BE, Sibley LD, Exploiting the unique ATP-binding pocket of toxoplasma calcium-dependent protein kinase 1 to identify its substrates, ACS Chem Biol 8(6) (2013) 1155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Schutkowski M, Reineke U, Reimer U, Peptide arrays for kinase profiling, Chembiochem 6(3) (2005) 513–21. [DOI] [PubMed] [Google Scholar]
- [190].Songyang Z, Carraway KL 3rd, Eck MJ, Harrison SC, Feldman RA, Mohammadi M, Schlessinger J, Hubbard SR, Smith DP, Eng C et al. , Catalytic specificity of protein-tyrosine kinases is critical for selective signalling, Nature 373(6514) (1995) 536–9. [DOI] [PubMed] [Google Scholar]
- [191].Szallasi Z, Denning MF, Chang EY, Rivera J, Yuspa SH, Lehel C, Olah Z, Anderson WB, Blumberg PM, Development of a rapid approach to identification of tyrosine phosphorylation sites: application to PKC delta phosphorylated upon activation of the high affinity receptor for IgE in rat basophilic leukemia cells, Biochem Biophys Res Commun 214(3) (1995) 888–94. [DOI] [PubMed] [Google Scholar]
- [192].Haubrich BA, Swinney DC, Enzyme Activity Assays for Protein Kinases: Strategies to Identify Active Substrates, Curr Drug Discov Technol 13(1) (2016) 2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Ptacek J, Devgan G, Michaud G, Zhu H, Zhu X, Fasolo J, Guo H, Jona G, Breitkreutz A, Sopko R, McCartney RR, Schmidt MC, Rachidi N, Lee SJ, Mah AS, Meng L, Stark MJ, Stern DF, De Virgilio C, Tyers M, Andrews B, Gerstein M, Schweitzer B, Predki PF, Snyder M, Global analysis of protein phosphorylation in yeast, Nature 438(7068) (2005) 679–84. [DOI] [PubMed] [Google Scholar]
- [194].Kim M, Shin DS, Kim J, Lee YS, Substrate screening of protein kinases: detection methods and combinatorial peptide libraries, Biopolymers 94(6) (2010) 753–62. [DOI] [PubMed] [Google Scholar]
- [195].Delom F, Chevet E, Phosphoprotein analysis: from proteins to proteomes, Proteome Sci 4 (2006) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Hutti JE, Jarrell ET, Chang JD, Abbott DW, Storz P, Toker A, Cantley LC, Turk BE, A rapid method for determining protein kinase phosphorylation specificity, Nat Methods 1(1) (2004) 27–9. [DOI] [PubMed] [Google Scholar]
- [197].Turk BE, Hutti JE, Cantley LC, Determining protein kinase substrate specificity by parallel solution-phase assay of large numbers of peptide substrates, Nat Protoc 1(1) (2006) 375–9. [DOI] [PubMed] [Google Scholar]
- [198].Lim D, Gold DA, Julien L, Rosowski EE, Niedelman W, Yaffe MB, Saeij PJ, Structure of the Toxoplasma gondii ROP18 kinase domain reveals a second ligand binding pocket required for acute virulence, J Biol Chem 288(48) (2013) 34968–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Etheridge RD, Alaganan A, Tang K, Lou HJ, Turk BE, Sibley LD, The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice, Cell Host Microbe 15(5) (2014) 537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Govindasamy K, Khan R, Snyder M, Lou HJ, Du P, Kudyba HM, Muralidharan V, Turk BE, Bhanot P, Plasmodium falciparum Cyclic GMP-Dependent Protein Kinase Interacts with a Subunit of the Parasite Proteasome, Infect Immun 87(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Ward P, Equinet L, Packer J, Doerig C, Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote, BMC Genomics 5 (2004) 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Merckx A, Echalier A, Langford K, Sicard A, Langsley G, Joore J, Doerig C, Noble M, Endicott J, Structures of P. falciparum protein kinase 7 identify an activation motif and leads for inhibitor design, Structure 16(2) (2008) 228–38. [DOI] [PubMed] [Google Scholar]
- [203].Embogama DM, Pflum MK, K-BILDS: A Kinase Substrate Discovery Tool, Chembiochem 18(1) (2017) 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Knight JD, Tian R, Lee RE, Wang F, Beauvais A, Zou H, Megeney LA, Gingras AC, Pawson T, Figeys D, Kothary R, A novel whole-cell lysate kinase assay identifies substrates of the p38 MAPK in differentiating myoblasts, Skelet Muscle 2 (2012) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Senevirathne C, Embogama DM, Anthony TA, Fouda AE, Pflum MK, The generality of kinase-catalyzed biotinylation, Bioorg Med Chem 24(1) (2016) 12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Amano M, Hamaguchi T, Shohag MH, Kozawa K, Kato K, Zhang X, Yura Y, Matsuura Y, Kataoka C, Nishioka T, Kaibuchi K, Kinase-interacting substrate screening is a novel method to identify kinase substrates, J Cell Biol 209(6) (2015) 895–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Sidik SM, Huet D, Ganesan SM, Huynh MH, Wang T, Nasamu AS, Thiru P, Saeij JPJ, Carruthers VB, Niles JC, Lourido S, A Genome-wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes, Cell 166(6) (2016) 1423–1435.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Bushell E, Gomes AR, Sanderson T, Anar B, Girling G, Herd C, Metcalf T, Modrzynska K, Schwach F, Martin RE, Mather MW, McFadden GI, Parts L, Rutledge GG, Vaidya AB, Wengelnik K, Rayner JC, Billker O, Functional Profiling of a Plasmodium Genome Reveals an Abundance of Essential Genes, Cell 170(2) (2017) 260–272.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Zhang M, Wang C, Otto TD, Oberstaller J, Liao X, Adapa SR, Udenze K, Bronner IF, Casandra D, Mayho M, Brown J, Li S, Swanson J, Rayner JC, Jiang RHY, Adams JH, Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis, Science 360(6388) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]