Abstract
Background
Percutaneous coronary intervention (PCI) is a standard treatment for coronary heart disease (CHD). Restenosis, defined as a 50% reduction in luminal diameter at six months after PCI, indicates a need for revascularisation. Restenosis has proven to be a major drawback to PCI. Tong‐xin‐luo is one of the prophylactic strategies for cardiovascular events in patients after PCI that is widely used in China, but its efficacy and safety have not been systematically evaluated.
Objectives
To systematically assess the efficacy and safety of Tong‐xin‐luo capsules in preventing cardiovascular events after PCI in patients with CHD.
Search methods
We searched the Cochrane Central Register of Controlled Trials in The Cochrane Library, MEDLINE (OVID), EMBASE (OVID), WanFang, Chinese Biomedical Database, Chinese Medical Current Contents, and China National Knowledge Infrastructure from their inception to June 2014. We also searched other resources, including ongoing trials and research registries. We applied no language restrictions.
Selection criteria
Randomised controlled trials of participants with CHD after PCI were included. Participants in the intervention group received Tong‐xin‐luo capsules for at least three months.
Data collection and analysis
Two review authors independently extracted data and assessed the risk of bias. Any disagreements were resolved by discussion with a third review author. The primary outcomes included occurrence of angiographic restenosis and adverse events; the secondary outcomes included myocardial infarction, heart failure, angina, all cause mortality, mortality due to any cardiovascular event, use of revascularisation, patient acceptability, quality of life and cost‐effectiveness. Dichotomous data were measured with risk ratios (RRs) with 95% confidence intervals (CIs).
Main results
Sixteen studies involving 1063 participants were identified. The risk of bias for fifteen studies was high and along with imprecision and possible publication bias, this lowered our confidence in the results. There was low quality evidence that Tong‐xi‐luo reduced the rates of angiographic restenosis (RR 0.16, 95% CI 0.07 to 0.34), myocardial infarction (RR 0.32, 95% CI 0.16 to 0.66), heart failure (RR 0.26, 95% CI 0.11 to 0.62), and use of revascularisation (RR 0.26, 95% CI 0.15 to 0.45). There was very low quality evidence for the effect of Tong‐xin‐luo on all‐cause mortality (RR 0.38, 95% CI 0.06 to 2.56), angina (RR 0.24, 95% CI 0.17 to 0.34) and death due to any cardiovascular event (RR 0.31, 95% CI 0.08 to 1.12). Adverse events were seldom reported, and included gastrointestinal reactions and nausea.
Authors' conclusions
The addition of Tong‐xin‐luo to conventional Western medicine may possibly prevent restenosis and recurrence of cardiovascular events in patients with CHD after PCI. However, the data are limited by publication bias and high risk of bias for included studies. Further high‐quality trials are required to evaluate the potential effects of this intervention.
Keywords: Humans; Percutaneous Coronary Intervention; Angina Pectoris; Angina Pectoris/prevention & control; Capsules; Cause of Death; Coronary Disease; Coronary Disease/drug therapy; Coronary Restenosis; Coronary Restenosis/prevention & control; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Heart Failure; Heart Failure/prevention & control; Myocardial Infarction; Myocardial Infarction/prevention & control; Randomized Controlled Trials as Topic; Secondary Prevention; Secondary Prevention/methods; Treatment Outcome
Plain language summary
Tong‐xin‐luo capsule for patients with coronary heart disease after percutaneous coronary intervention
Review question: What is the efficacy and safety of Tong‐xin‐luo capsules in preventing cardiovascular events after percutaneous coronary intervention, a procedure involving placing a stent to open up the heart's blood vessels, in patients with coronary heart disease?
Background: Coronary heart disease is a major cause of mortality globally. Percutaneous coronary intervention is regarded as a standard treatment for coronary heart disease to improve symptoms of heart‐related chest pain. However, a major drawback of percutaneous coronary intervention is the need for a repeat procedure due to symptoms related to recurring narrowing of the heart's blood vessels. Previous studies have indicated that Tong‐xin‐luo capsule, a Chinese herbal medicine product, might be effective in preventing recurrence of narrowing of a blood vessel after percutaneous coronary intervention.
Study characteristics: We included sixteen randomised controlled trials (1063 participants) comparing Tong‐xin‐luo capsules plus conventional treatment with placebo plus/or conventional treatment (literature search date: though June 2014). All studies were undertaken in China. The sample size was from 50 to 178 and the duration of follow‐up ranged from three months to two years.
Key results: We found that Tong‐xin‐luo may possibly reduce the risk of narrowing of a blood vessel detected by angiography, cardiovascular events (including myocardial infarction, angina and heart failure) and use of repeat procedure. Adverse events were seldom reported.
Quality of evidence: Because of high risk of bias for fifteen studies, imprecision and possible publication bias, the quality of evidence was low or very low for all study outcomes.
Summary of findings
for the main comparison.
| Tong‐xin‐luo compared with placebo/no treatment for patients with CHD after PCI | ||||||
|
Patient or population: participants with CHD after PCI Settings: China Intervention: Tong‐xin‐luo capsules Comparison: placebo/no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment | Tong‐xin‐luo | |||||
|
Occurrence of angiographic restenosis [follow‐up: 6 months] |
134 per 1000 | 21 per 1000 (9 to 46) | RR 0.16 (0.07 to 0.34) | 595 (6) | ⊕⊕⊝⊝ low | Quality of evidence downgraded two levels due to study limitations (lack of blinding in all studies, high risk of reporting bias in one study). |
| Adverse event | NA | NA | NA | NA | NA | Adverse events were not evaluated as very limited information was reported from original studies. |
|
Occurence of myocardial infarction [follow‐up: 3‐48 months] |
Myocardial infarction | RR 0.32 (0.16 to 0.66) | 1306 (13) | ⊕⊕⊝⊝ low | Quality of evidence downgraded two levels due to study limitations (lack of blinding in all studies except Wang 2010, high risk of reporting bias in two studies). | |
| 22 per 1000 | 7 per 1000 (3 to 21) | |||||
| Acute myocardial infarction | ||||||
| 53 per 1000 | 16 per 1000 (6 to 44) | |||||
|
Occurrence of angina [follow‐up: 3‐48 months] |
211 per 1000 | 51 per 1000 (36 to 72) | RR 0.24 (0.17 to 0.34) | 1210 (11) | ⊕⊝⊝⊝ very low | Quality of evidence downgraded two levels due to study limitations (lack of blinding in all studies except Wang 2010, high risk of reporting bias in one study), and one additional level due to publication bias. |
|
Occurence of heart failure [follow‐up: 3‐48 months] |
88 per 1000 | 23 per 1000 (10 to 54) | RR 0.26 (0.11 to 0.62) | 529 (4) | ⊕⊕⊝⊝ low | Quality of evidence downgraded two levels due to study limitations (lack of blinding in all studies, high risk of reporting bias in one study). |
|
All‐cause mortality [follow‐up: 3‐48 months] |
12 per 1000 | 5 per 1000 (1 to 31) | RR 0.38 (0.06 to 2.56) | [419] (4) | ⊕⊝⊝⊝ very low | Quality of evidence downgraded two levels due to study limitations (lack of blinding in all studies except Wang 2010, high risk of reporting bias in one study), and one additional level due to imprecision of the estimate. |
|
Mortality due to any cardiovascular event [follow‐up: 3‐48 months] |
34 per 1000 | 11 per 1000 (3 to 39) | RR 0.31 (0.08 to 1.12) | 552 (5) | ⊕⊝⊝⊝ very low | Quality of evidence downgraded two levels due to study limitations (lack of blinding in all studies, high risk of reporting bias in one study), and one additional level due to imprecision of the estimate. |
|
Use of revascularisation [follow‐up: 3‐48 months] |
166 per 1000 | 43 per 1000 (25 to 75) | RR 0.26 (0.15 to 0.45) | 806 (8) | ⊕⊕⊝⊝ low | Quality of evidence downgraded two levels due to study limitations (lack of blinding in all studies, high risk of reporting bias in two studies). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CHD: coronary heart disease; PCI: percutaneous coronary intervention; CI: confidence interval; RR: risk ratio; NA: not available. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Cardiovascular diseases (CVDs), which mainly refers to a group of disorders of the heart and blood vessels, are a major cause of death and disability globally (Lonn 2010; Lozano 2012). According to the fact sheet published by the World Health Organization (WHO) in September 2011, CVDs caused about 17.3 million deaths in 2008 and are responsible for 30% of all deaths worldwide (WHO 2011). Moreover, CVDs are expected to account for 46.6% of all deaths by 2020 and 80% of CVD deaths will occur in developing countries (WHO 2002; WHS 2010). Among all deaths attributed to CVDs, about half were due to coronary heart disease (CHD; Go 2014). CHD is a condition in which plaque builds up inside the coronary arteries and can decrease oxygen supply to the heart muscle (NHLBI 2011). According to previous studies, about one in six deaths in the United States were due to CHD in 2010 (Go 2014) and one in six men and one in seven women in the UK died from CHD in 2008 (Bhattarai 2012). Percutaneous coronary intervention (PCI), a non‐surgical procedure that involves placing a stent to open up the blood vessels obstructed by plaque, is an effective treatment for CHD (Grech 2003;Singh 2011). However, restenosis has proven to be the major drawback to PCI and frequently causes a return of symptoms and the need for repeated coronary intervention (Kang 2012; Richard 2002; Testa 2009).
Restenosis is a recurrence causing significant narrowing in the treated vessel, which is generally considered as a wound healing response to trauma of the vascular wall (Agema 2001; Odell 2006). Clinically, restenosis is defined as recurrent angina pectoris that indicates a need for revascularisation of the treated vessel (Cutlip 2007). It is angiographically defined as a 50% reduction in luminal diameter at six months after PCI. So far, the mechanism of restenosis has not been fully clarified. It is thought that restenosis is due to the combined effect of early elastic recoil in vessels, mural thrombus formation, neointimal proliferation, extracellular matrix formation, and chronic geometric arterial changes (Dangas 1996). According to the study by Giglioli et al, more than 40% of patients have restenosis detected within six months after an angioplasty (Giglioli 2009). This frequently results in renewed symptoms such as angina that hamper the long‐term efficacy of PCI (Fattori 2003; Rajaqopal 2003). There are many strategies aimed at preventing cardiovascular events in patients after PCI, which include using antiplatelet drugs, thrombin inhibitors, and Tong‐xin‐luo. However, some of these interventions have adverse effects (Dörffler 2005; Serruys 1995; Rosanio 1999).
Description of the intervention
Tong‐xin‐luo capsule is a drug that has been studied since 1995 based on the theory of Chinese herbal medicine.The recommended dose range is 1.14 g to 1.52 g in three to four capsules (0.38 g in each capsule) and administered three to four times a day, with a total dose of between 3.5 g and 6.0 g per day. The treatment is given as a four‐week course (Wu 2001; Zhou 2011). Currently, Tong‐xin‐luo capsule is widely used for treating cardiac‐cerebral vascular diseases in China. Its effect on prevention and treatment of restenosis among CHD patients after PCI has become an important topic in both Chinese and Western medical research areas. Previous studies indicated that Tong‐xin‐luo can effectively relieve angina, reduce the frequency of acute attacks of angina, and prevent restenosis of stents (Wang 2003; Zhang 2004; Zhou 2007 a; Zhu 2004). Potential interactions with other drugs are unclear. Related adverse events have been reported which include gastrointestinal tract symptoms, gingival bleeding, and increased partial thromboplastin time (Nong 2000).
How the intervention might work
The main composition of Tong‐xin‐luo capsules is complex and includes Hirudo, Radix Ginseng, Scorpio, Eupolyphaga Seu Steleophage, Scolopendra, Periostracum Cicadae, Lignum Dalbergiae Odoriferae, Radix Paeoniae Rubra, and Borneolum Syntheticum (Zhou 2011). Previous studies have shown the following.
Hirudin‐like materials, which are produced by Scorpio, Hirudo, Eupolyphaga Seu Steleophage, and Scolopendra (Huntington 2003; Yu 2011; Zhou 1997), can stop blood clotting by reducing the secretion of extracellular matrix (Paul 1994) and suppressing the aggregation of platelets and inflammatory cells (Chen 2007; Modi 1995).
Radix Ginseng has cardiokinetic function as well as inhibiting the platelet adhesion reaction and thrombosis formation (Pei 1986; Xu 1988).
Radix Paeoniae Rubra dilates coronary arteries, causes thrombolysis, improves myocardial ischemias, inhibits proliferation of vascular smooth muscle cells, and prevents onset of endothelial injury (Lu 2006; Ruan 2003; Wang 2008).
Periostracum Cicadae is an anticonvulsive and significantly slows heart rate (Zheng 1998).
Scolopendra extraction could increase blood vessel perfusion flow and strengthen cardiac contractility (Chen 1985).
Eupolyphaga Seu Steleophage could improve tolerance to hypoxia and increases cardiac output (Nong 1989).
Why it is important to do this review
Due to incomplete evidence on the benefits and possible adverse effects of Tong‐xin‐luo capsule, a systematic review on the therapeutic efficacy and safety of Tong‐xin‐luo capsule use is needed. The results of our review may shed new light on the prevention of cardiovascular events for patients with coronary heart disease after PCI and may also provide new treatment methods for cardiovascular disease with reduced expense and side effects.
Objectives
To systematically assess the efficacy and safety of Tong‐xin‐luo capsules in preventing cardiovascular events after PCI in patients with CHD.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs). Single‐arm trials, animal studies and reviews were excluded.
Types of participants
Participants were male or female of any age or ethnic origin with CHD after PCI. Participants were eligible regardless of recruitment through clinical or community settings.
Types of interventions
Tong‐xin‐luo capsule was regarded as the intervention in our study. Tong‐xin‐luo capsule used alone after PCI and with a treatment duration of no less than three months was compared with at least one valid comparator, including no intervention, placebo, or an effective Western medicine intervention supported by reasonable clinical evidence.
Types of outcome measures
We considered the following outcome measures.
Primary outcomes
Occurrence of angiographic restenosis
Adverse events
Secondary outcomes
Occurrence of myocardial infarction (MI), heart failure, or angina
All cause mortality
Mortality (death) due to any cardiovascular event
Use of revascularisation, including PCI and coronary artery bypass graft surgery
Patient acceptability
Quality of life
Cost‐effectiveness
Search methods for identification of studies
A comprehensive search strategy was formulated in an attempt to identify all relevant studies regardless of publication status. Electronic databases were searched and handsearching of journals was carried out.
Electronic searches
We searched the following main electronic databases, with no restriction in publication language.
Cochrane Central Register of Controlled Trials (CENTRAL Issue 5, 2014; The Cochrane Library).
MEDLINE (OVID, 1950 to June week 1 2014).
EMBASE (OVID, 1947 to week 23 2014).
WanFang (1998 to August week 2 2014).
China National Knowledge Infrastructure (CNKI; 1993 to April week 2 2013).
Chinese Biomedical Database (CBM; 1978 to April week 2 2013).
Chinese Medical Current Contents (CMCC; 1994 to April week 2 2013).
The search strategies used can be found in Appendix 1. No language restrictions were applied.
Searching other resources
We searched the following clinical trial registries for ongoing and unpublished trials from 1994 to June 2013.
Current Controlled Trials (http://www.controlled‐trials.com).
WHO International Clinical Trials Registry Platform (ICTRP; http://apps.who.int/trialsearch/).
ClinicalTrials.gov (www.clinicaltrials.gov).
Chinese Clinical Trial Register (www.chictr.org).
China Master's Theses Full‐text Databases (CMFD; http://oversea.cnki.net/kns55/brief/result.aspx?dbPrefix=CMFD).
China Doctoral Dissertation Full‐text Database (CDFD; http://oversea.cnki.net/kns55/brief/result.aspx?dbPrefix=CDFD).
China Proceedings of Conference Full‐text Database (CPCD).
The search strategies used can be found in Appendix 1.
Handsearching
Handsearching was not performed as the journals we would have planed to search manually (including China Journal of Chinese Materia Medica, Chinese Journal of Basic Medicine in Traditional Chinese Medicine, and Chinese Journal of Integrated Traditional and Western Medicine) were included in WanFang database.
Data collection and analysis
Two authors independently carried out selection of studies, data extraction, and quality assessment. Data analysis was conducted using Review Manager Version 5.1 (RevMan 2008) according to the statistical guidelines referenced in the current version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two authors (XF, ZY) independently scanned the titles and abstracts of every record retrieved for eligibility assessment. The full‐texts of any literature with unclear information in the title or abstracts, and potential eligible studies were retrieved for clarification. All studies selected by the two authors were cross checked. Any disagreement was resolved by discussion with a third author (CM).
Data extraction and management
Two authors (XF, YQ) independently extracted data using a standard form pre‐designed for this review. The data extracted were as follows: study characteristics (authors, location, title, etc.), patient characteristics (number of patients, age, gender, race, baseline disease severity, etc.), intervention, control, methodological information (randomisation, allocation concealment, blinding, loss to follow‐up, selective outcome reporting),outcomes. The authors of the original studies were consulted for unclear or missing information where necessary. Any disagreement were resolved by discussion.
Assessment of risk of bias in included studies
The risk of bias in selected studies was independently assessed by two authors (YQ, VC), under the guidance of the Cochrane Heart Group and using the tool described in the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011). Any disagreement was resolved by a third author (CM). In order to confirm and validate the methods of allocation concealment and the randomisation procedure, the original authors of all trials were contacted. Risk of bias assessments are presented in tables on the following domains (Higgins 2011).
Sequence generation.
Allocation concealment.
Blinding.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
The risk of bias for each domain was categorized as:
Low risk of bias;
High risk of bias;
Unclear risk of bias.
Measures of treatment effect
Dichotomous data (occurrence of angiographic restenosis, adverse events, myocardial infarction, heart failure, angina, all cause mortality, mortality due to any cardiovascular event, use of revascularisation) were analysed using risk ratios (RRs) with 95% confidence intervals (CIs). It has been shown that RR is more intuitive (Boissel 1999) than the odds ratio (OR) and that OR tend to be interpreted as RR by clinicians (Deeks 2000), which leads to an overestimate of the effect. We planned to analyse continuous outcomes using weighted mean differences (with 95% CI). Standardized mean differences would have been employed if different measurement scales were used. Skewed data and non‐quantitative data would have been presented descriptively.
Unit of analysis issues
We did not include any cluster randomised trials or cross‐over studies in this review. All included studies are two‐arm trials and no control group was used more than once in the meta‐analysis. When assessing event data, we avoided counting more than one outcome event for any participant. Where we were unclear about the data (for example, where a study reported numbers of myocardial infarctions, but did not report the number of people who experienced a myocardial infarctions) we asked authors for further information.
Dealing with missing data
When there were missing data, we contacted the original authors of the study to obtain the relevant missing data. If missing data could not be obtained, an imputation method was used. We used sensitivity analysis to assess the impact on the overall treatment effects of inclusion of trials which did not report an intention‐to‐treat analysis, had high rates of participant attrition, or had other missing data.
Assessment of heterogeneity
The clinical and methodological heterogeneity were evaluated by considering the variability in important participant factors among trials and trial factors (randomisation concealment, blinding of outcome assessment, losses to follow‐up, treatment type, co‐interventions), respectively. Statistical heterogeneity was tested using the Chi2 test (significance level 0.1; Lau 1997) and I2 statistic (0% to 40%: minimal heterogeneity; 30% to 60%: may represent moderate heterogeneity; 50% to 100%: substantial heterogeneity). We planned to explain the source of heterogeneity by subgroup analysis and meta‐regression.
Assessment of reporting biases
We used funnel plots to assess potential publication bias (Egger 1997) for the meta‐analyses with the outcomes MI and angina, as these analyses included 10 or more studies (Higgins 2011; Sterne 2001).
Data synthesis
Each outcome was combined and calculated using the statistical software RevMan 5.1 (RevMan 2008), according to the statistical guidelines referenced in the current version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). All the meta‐analyses were performed with a fixed‐effect model using the Mantel‐Haenszel method as no significant heterogeneity was found (I2 < 50% or P > 0.1).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses based on the following factors. However, subgroup analyses were not carried out because: there were insufficient data in the original studies allow us to classify the included studies into different subgroups according the pre‐specified factors.
Patient characteristic (age, sex).
Types of treatment (Western medicine alone, Western medicine plus Tong‐xin‐luo).
Follow‐up period.
Type of stent.
Sensitivity analysis
We planned to perform sensitivity analysis to explore the source of heterogeneity as follows, however there were insufficient numbers of studies.
Quality components, including full‐text publications versus abstracts, preliminary results versus final results, published versus unpublished data.
Risk of bias (by omitting studies that were judged to be at high risk of bias).
Results
Description of studies
For a detailed description of studies, see Characteristics of included studies.
Results of the search
As shown in the flow chart (Figure 1), our search in electronic bibliographic databases yielded 2168 citations, of which 59 were classified as potentially relevant and were subjected to full text assessment. Sixteen studies with 20 citations met the defined inclusion criteria.
1.
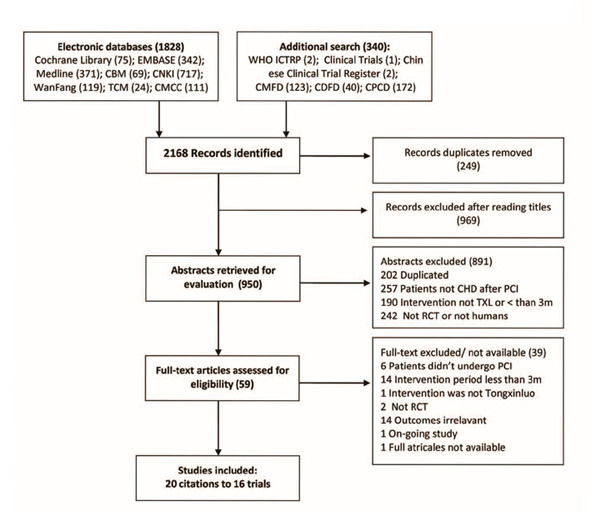
Flow chart of study selection
Included studies
Design of included studies
All the included studies were RCTs of single centre, parallel design, with a control group; one study used a randomised, double‐blind, placebo‐controlled design (Wang 2010). All included studies were conducted in China, and were performed in hospital settings.
Participants in included studies
A total of 1603 participants were included in the 16 trials, and the number of participants in the studies ranged from 50 (Di 2012) to 178 (Zhang 2009a). The age of participants ranged from 30 to 78 years. All participants showed objective symptoms of CHD and had received a successful PCI. The duration of follow‐up ranged from three months (Dai 2011) to two years (Chen 2011) in studies where length of follow‐up was provided.
Interventions in included studies
All included trials compared Tong‐xin‐luo capsule plus conventional treatment with the same conventional treatment alone except one study (Wang 2010), which compared Tong‐xin‐luo capsule plus conventional treatment with placebo plus the same conventional treatment. Participants all received aspirin, clopidogrel, and low molecular weight heparin after PCI as background therapy. Eight studies reported the use of statins as routine treatment, of which four studies prescribed atorvastatin, one prescribed pravastatin, and three studies did not report which class of statin was prescribed. Other treatments including beta‐blockers, nitrates, and angiotension conversion enzyme inhibitors were selectively applied according to illness status of participants. Tong‐xin‐luo capsule (three to four capsules, three times per day) was initiated on the day of PCI. In one study (Wu 2010), participants in the treatment group began to take Tong‐xin‐luo seven days before PCI. The treatment duration and time of follow ranged from three months (Yang 2009a) to two years (Zhang 2009a).
Outcome measures of included studies
Six studies (Chen 2011; Dai 2011; Liang 2010; Wu 2010; Xiao 2007; Zhou 2007b) reported on the incidence of restenosis.
Three studies (Di 2012; Huang 2010; Zhang 2009a) mentioned occurrence of adverse events.
Five studies (Dong 2012; Huang 2010; Liang 2010; Wang 2009a; Wu 2010) reported on the incidence of acute myocardial infarction (AMI), eight studies (Di 2012; Li 2004; Wang 2010; Xiao 2007; Yang 2009a; Zhang 2009a; Zheng 2010; Zhou 2007b) reported on the incidence of myocardial infarction (MI).
Eleven studies (Chen 2011; Di 2012; Li 2004; Liang 2010; Wang 2009a; Wang 2010; Wu 2010; Xiao 2007; Yao 2006; Zhang 2009a; Zhou 2007b) assessed occurrence of angina.
Four studies (Chen 2011; Xiao 2007; Yang 2009a; Zhang 2009a) measured occurrence of heart failure.
Four studies (Di 2012; Wang 2010; Yang 2009a; Zhang 2009a) reported on all‐cause mortality; five studies (Dong 2012; Huang 2010; Xiao 2007; Yang 2009a; Zhang 2009a) had data available for mortality due to any cardiovascular event.
Eight studies (Di 2012; Dong 2012; Huang 2010; Wu 2010; Yang 2009a; Zhang 2009a; Zheng 2010; Zhou 2007b) assessed the use of revascularisation, including PCI and coronary artery bypass graft surgery.
No studies reported mortality (death) due to restenosis after PCI. None of the studies mentioned patient acceptability, quality of life or cost‐effectiveness.
Regarding study outcomes, all studies reported the number of patients who experienced related event, except one study (Li 2004) which reported the number of events in each group.
Excluded studies
In total, thirty eight articles were excluded on the basis of their full‐text versions for the following reasons (Characteristics of excluded studies): six studies (Chang 2004; Qu 2011; Su 2000; Xiao 2004; Zhang 2008; Zhang 2009b ) included patients who did not receive coronary stenting; one trial (Jiang 2008) did not use Tong‐xin‐luo as an intervention; the intervention period of Tong‐xin‐luo in 14 articles (Chen 2007; Fan 2008; Fu 2012; Han 2009; Han 2011; Liu 2009; Lu 2012; Wang 2009c; Xu 2007; Zhang 2007; Zhang 2006; Zhang 2005; Zhao 2010; Zhao 2011) was less than three months; two studies (Gao 2007; Xu 2008) were not RCTs; 14 studies (Guo 2012; Kuang 2011; Li 2012; Li 2008; Ma 2009; Tao 2012; Tu 2006; Wang 2007; Wang 2012; Wang 2009b; Yang 2009b; Yang 2010; Yang 2012; Zhang 2010) didn't repot relevant outcomes; and for one study (Hou 2008) only an abstract was published with no data available. We found one on‐going study (Han 2012; Characteristics of ongoing studies).
Risk of bias in included studies
All included studies, except for the study by Wang et al (Wang 2010), were considered to have a high risk of bias as at least one domain of methodology was judged at high risk of bias. We tried to contact the study authors to clarify domains of unclear risk of bias but received no reply. Detailed information for the assessment of risk of bias for each included study is described in risk of bias tables under Characteristics of included studies, and authors' judgements about each risk of bias item are presented in Figure 2.
2.
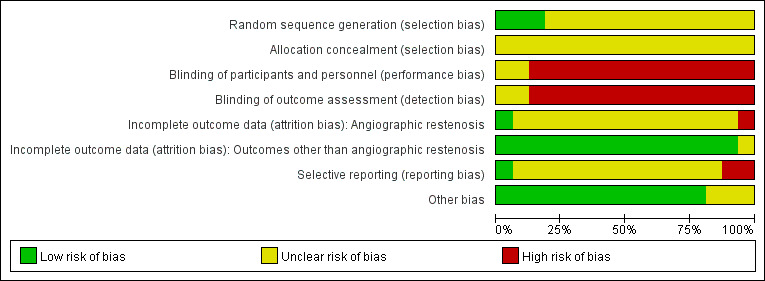
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All the included studies mentioned randomisation. However, only three studies (Chen 2011; Yang 2009a; Zhang 2009a) reported allocation sequence generated by using random number tables or drawing of lots. None of the others described the method of randomisation in details. None of the included studies mentioned allocation concealment.
Blinding
Only one study (Wang 2010) mentioned blinding, but no detailed information about the blinding method was provided. We considered other studies to be at high risk of bias because blinding was not reported and placebo was not used. Patients, doctors and other key study personnel were highly likely to know the allocation.
Incomplete outcome data
One study (Yang 2009a) reported one withdrawal before randomisation. None of the studies mentioned losses to follow‐up after randomisation, except that more than half of participants in one study (Xiao 2007) did not undergo coronary angiography, so the occurrence of angiographic restenosis was not reported. There was no loss to follow‐up in terms of the other outcomes in this study. None of the other studies reported withdrawals or loss to follow‐up. None of the studies mentioned intention‐to‐treat analysis (ITT).
Selective reporting
No study protocols were available for the included studies. Therefore, most studies were considered to have an unclear risk of bias. Two studies that did not report all the outcomes listed in the methods section were judged as having high risk of bias (Yang 2009a; Zhou 2007b).
Other potential sources of bias
The included studies may have been influenced by the small study effect as none of the included studies mentioned sample size calculation and most of them were small (ranged from 50 to 178 patients). However, baseline information including sex, age, comorbidity, types of coronary pathological changes, number of stents implanted and other risk factors appeared to be balanced between Tong‐xin‐luo groups and control groups in 13 studies (P > 0.05), and the usage of Tong‐xin‐luo capsule and conventional treatment were similar, so we considered these 13 trials to be of low risk of bias from other potential sources. The other three studies (Liang 2010; Zheng 2010; Zhou 2007b) were judged as having an unclear risk of bias, because sufficient information was not provided on baseline characteristics.
Effects of interventions
See: Table 1
Primary outcome measures
1. Occurrence of angiographic restenosis
Six trials (Chen 2011; Dai 2011; Liang 2010; Wu 2010; Xiao 2007; Zhou 2007b) involving 595 participants had relevant data on angiographic restenosis investigated at 6 month follow‐up comparingTong‐xin‐luo plus conventional treatment with conventional treatment alone. Tong‐xin‐luo plus conventional treatment was better at reducing the risk of occurrence of angiographic restenosis (6/305 vs. 41/290, RR 0.16, 95% CI 0.07 to 0.34, P < 0.00001; see Analysis 1.1).
1.1. Analysis.
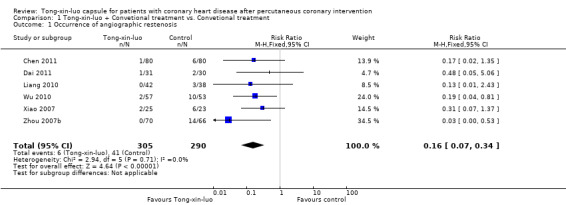
Comparison 1 Tong‐xin‐luo + Convetional treatment vs. Convetional treatment, Outcome 1 Occurrence of angiographic restenosis.
2. Adverse events
Meta‐analysis for the outcome of adverse events was not performed due to insufficient data. In one study there was one case of gastrointestinal reaction relieved without medication in the treatment group and one case of gastrointestinal reaction relieved after symptomatic treatment in the control group (Di 2012). Three patients had mild nausea and mild pain in the upper left abdomen at the beginning of Tong‐xin‐luo treatment in one study (Zhang 2009a); the symptoms disappeared once the patients started taking the medication between meals. No severe adverse events were reported in these two studies. One study reported no adverse events in the treatment group (Huang 2010).
Secondary outcome measures
1. Occurrence of MI
Five trials (Dong 2012; Huang 2010; Liang 2010; Wang 2009a; Wu 2010) with 453 participants reported on the outcome of AMI, and eight trials (Di 2012; Li 2004; Wang 2009a; Xiao 2007; Yang 2009a; Zhang 2009a; Zheng 2010; Zhou 2007b) with 853 patients reported MI. The pooled estimate of these 13 studies showed that the addition of Tong‐xin‐luo to conventional treatment reduced the risk of MI compared to conventional treatment alone (7/659 vs. 27/647, RR 0.32, 95% CI 0.16 to 0.66, P 0.002; see Analysis 1.2).
1.2. Analysis.
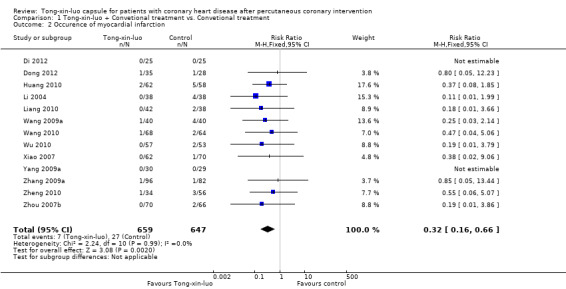
Comparison 1 Tong‐xin‐luo + Convetional treatment vs. Convetional treatment, Outcome 2 Occurence of myocardial infarction.
2. Occurrence of heart failure
Four studies (Chen 2011; Xiao 2007; Yang 2009a; Zhang 2009a) of 529 participants investigated occurrence of heart failure. The pooled results showed that the combined treatment of Tong‐xin‐luo and conventional treatment reduced the risk of heart failure (6/268 vs. 23/261 RR 0.26, 95% CI 0.11‐0.62, P 0.003; See Analysis 1.3).
1.3. Analysis.
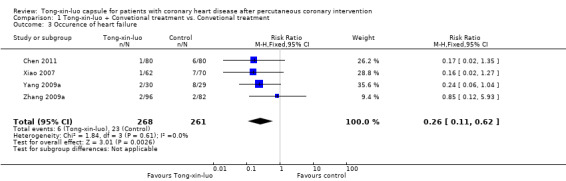
Comparison 1 Tong‐xin‐luo + Convetional treatment vs. Convetional treatment, Outcome 3 Occurence of heart failure.
3. Occurrence of angina
A total of 11 RCTs involving 1210 participants (Chen 2011; Di 2012; Li 2004; Liang 2010; Wang 2009a; Wang 2010; Wu 2010; Xiao 2007; Yao 2006; Zhang 2009a; Zhou 2007b) provided data on occurrence of angina. Pooled results demonstrated a reduced incidence of angina in the Tong‐xin‐luo group (33/616 vs. 137/594, RR 0.24, 95% CI 0.17‐0.34, P 0.00001; See Analysis 1.4).
1.4. Analysis.
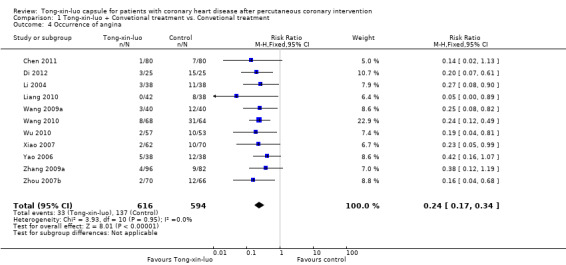
Comparison 1 Tong‐xin‐luo + Convetional treatment vs. Convetional treatment, Outcome 4 Occurrence of angina.
4. All‐cause mortality and mortality due to any cardiovascular event
Four studies of 419 participants reported all‐cause mortality. Two studies (Di 2012; Wang 2010) reported no deaths in either treatment or control groups, so the estimate was pooled from the two other studies (Yang 2009a; Zhang 2009a). There was no evidence of a difference between the Tong‐xin‐luo group and the control group (1/219 vs. 3/200, RR 0.38, 95% CI 0.06‐2.56, P 0.32; see Analysis 1.5)
1.5. Analysis.
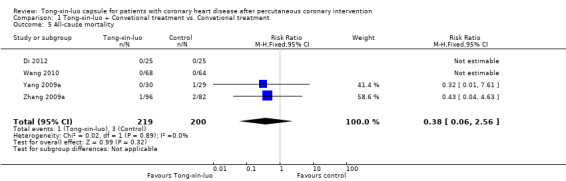
Comparison 1 Tong‐xin‐luo + Convetional treatment vs. Convetional treatment, Outcome 5 All‐cause mortality.
Five studies (Dong 2012; Huang 2010; Xiao 2007; Yang 2009a; Zhang 2009a) consisting of 532 participants reported outcomes of mortality due to cardiovascular events, and there was some evidence that Tong‐xin‐luo plus conventional treatment reduced the risk of death due to cardiovascular events in comparison to conventional treatment alone, though the test did not reach statistic significance (1/285 vs. 7/267, RR 0.31, 95% CI 0.08‐1.12, P 0.07; see Analysis 1.6).
1.6. Analysis.
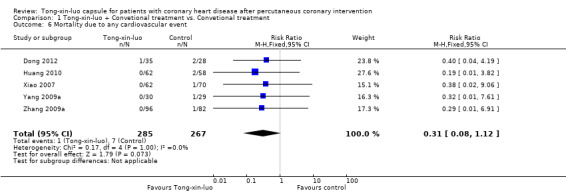
Comparison 1 Tong‐xin‐luo + Convetional treatment vs. Convetional treatment, Outcome 6 Mortality due to any cardiovascular event.
5. Use of revascularisation
Pooled analysis of eight studies (Di 2012; Dong 2012; Huang 2010; Wu 2010; Yang 2009a; Zhang 2009a; Zheng 2010; Zhou 2007b) including 806 participants showed a significant effect in favour of the addition of Tong‐xin‐luo to conventional treatment (15/409 vs. 58/397, RR 0.26, 95% CI 0.15 to 0.45, P < 0.00001; see Analysis 1.7).
1.7. Analysis.
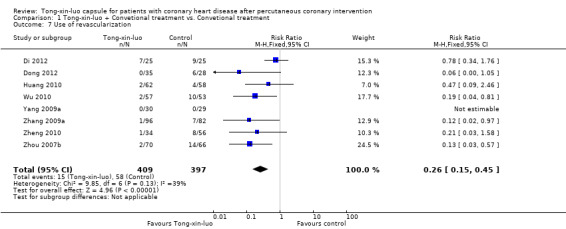
Comparison 1 Tong‐xin‐luo + Convetional treatment vs. Convetional treatment, Outcome 7 Use of revascularization.
6. Patient acceptability, quality of life or cost‐effectiveness
Meta‐analyses were not done as none of the included studies reported these outcomes.
Sensitivity analysis
Sensitivity analyses by study quality were not performed because most of the included studies were considered to have high risk of bias. There was an insufficient number of studies for meta‐analysis after removing studies at high risk of bias.
Publication bias
Funnel plots were used to assess potential publication bias for the outcomes of MI and angina as more than 10 trials were included for each of them. A symmetrical funnel plot of MI indicate no risk of publication bias (see Figure 3), but an asymmetrical funnel plot of angina suggested that high risk of publication bias existed (see Figure 4).
3.
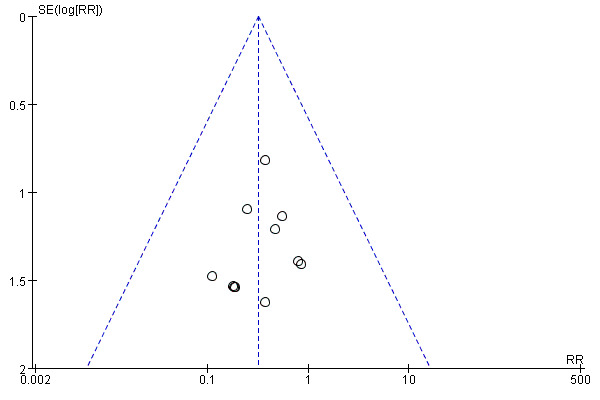
Funnel plot of comparison: 1 Tong‐xin‐luo + Conventional treatment vs. Conventional treatment, Outcome: 1.2 Occurence of myocardial infarction.
4.
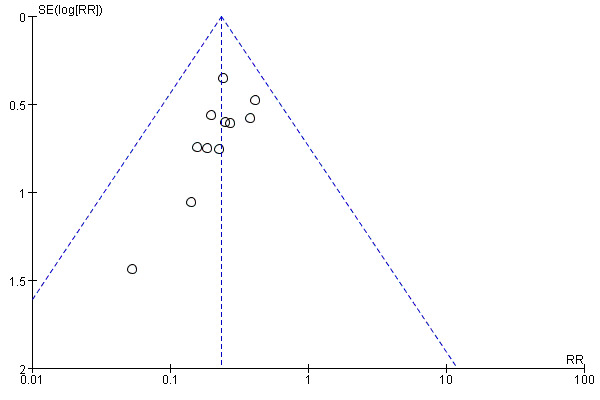
Funnel plot of comparison: 1 Tong‐xin‐luo + Conventional treatment vs. Conventional treatment, Outcome: 1.4 Occurrence of angina.
Discussion
Summary of main results
This systematic review included 16 RCTs which evaluated the effects of Tong‐xin‐luo in addition to conventional treatment. The meta‐analysis demonstrated that Tong‐xin‐luo capsule in combination with conventional Western medicine was significantly more effective in preventing angiographic restenosis, cardiovascular events (MI, angina and heart failure), and revascularisation, when compared with conventional Western medicine alone. There was some evidence that the addition of Tong‐xin‐luo to conventional treatment reduced the risk of all‐cause mortality or mortality due to any cardiovascular event though the estimates failed to achieve statistical significance. This might be due to small sample size. The statistical heterogeneity was low in all meta‐analyses. No severe adverse events were observed in the patients treated with Tong‐xin‐luo.
The effect of Tong‐xin‐luo was generally not obvious in included trials, but the pooled estimates from meta‐analyses were significant when the sample size increased. However, the study findings should be interpreted with caution because most of the included studies were at high risk of bias.
Overall completeness and applicability of evidence
Sixteen RCTs involving 1063 patients were included in this review. This review examined the efficacy of Tong‐xin‐luo in patients with CHD after PCI. Although the age and gender of participants in the included trials were representative of CHD patients who undergo successful PCI operations, the sample size of the included trials was generally small. Furthermore, all participants were recruited from Chinese populations. This may limit the generalizability of the study results to patients of other ethnicities.
Quality of the evidence
Because of the limitations of the included studies, including imprecision and publication bias, the quality of evidence was considered low or very low for most study outcomes of this systematic review. Most publications provided very limited information about trial design and methodology. Only three studies described the methods of randomisation. Methods of allocation concealment or blinding were not mentioned in most of the included studies. Lack of blinding may have lead to an unbalanced prescription of other treatments such as statins, which may have influenced the results. As most of the included studies generally did not provide sufficient information for us to assess whether the prescription of other medicines were consistent and balanced, the potential influence is unclear. In addition, no included studies mentioned sample size calculation and most of the included studies consisted of small numbers of participants. As smaller studies are usually less rigorous than large scale studies and are more likely to show a large treatment effect (Pereira 2012), the treatment effects of Tong‐xin‐luo need to be evaluated by future large‐scale RCTs. Additonal potential problems in the design and conduct of traditional Chinese medicine research (Wang 2007) should also be noted. Thus, although this review shows a potentially protective effect of Tong‐xin‐luo, the results should be interpreted with caution. Future research is very likely to change the estimates.
Potential biases in the review process
We attempted to minimise bias in the review process. One of the major challenges to conducting reviews across different cultural medical disciplines is the language barrier and resulting insufficient literature search (Wu 2013). A comprehensive search without language restrictions was conducted to identify all relevant studies. Study selection, assessment of risk of bias and data extraction were independently carried out by two review authors. The review was strictly conducted according to the protocol, however, potential biases might still exist. Asymmetrical funnel plots of angina suggest that publication bias may be present; this might lead to overestimates of effect size of Tong‐xin‐luo in reducing the risk of angina. In addition, the inclusion criteria of participants in different trials varied from each other, with some trials only including patients with AMI, while others only included patients with angina; this suggests that there may be some clinical heterogeneity among included studies. Lastly, the overall analysis did not consider the varying trial durations and drug usage as part of routine treatment. However, no significant heterogeneity was identified in the meta‐analysis, which suggests the impact of trial duration and drug usage on the treatment effect was low.
Agreements and disagreements with other studies or reviews
We did not identify any other systematic reviews with a focus on Tong‐xin‐luo capsule for restenosis after PCI in patients with CHD. Two systematic reviews on the efficacy of Tong‐xin‐luo were identified. One included 18 studies and assessed the effects of Tong‐xin‐luo in patients with unstable angina pectoris (Wu 2006). This study's results suggested that Tong‐xin‐luo reduces the risk of AMI, angina and need for revascularisation, but the poor quality of included studies was of concern. The other review included only two trials assessed the efficacy for acute cerebral infarction (Zhuo 2008) and was unable to determine whether Tong‐xin‐luo has a favourable effect or not in acute ischaemic stroke.
Authors' conclusions
Implications for practice.
This systematic review indicated that Tong‐xin‐luo may possibly reduce incidence of angiographic restenosis, revascularisation, and CVDs, without causing severe adverse effects, in patients with CHD after PCI. However, these results should be considered as hypothesis generating rather than hypothesis testing due to the small sample size, high risk of bias in the included studies, publication bias and low quality of evidence.
Implications for research.
This systematic review has important implications for future research. Firstly, further studies of high quality are needed. Improvement of methodological quality should be achieved, including use of placebo and clear descriptions of randomisation and allocation concealment. Secondly, future studies should report quality of life, patient acceptability and cost. Morever, most of the included studies were small. More studies with large sample sizes are needed to verify the protective effect of Tong‐xin‐luo. Lastly, cost‐effectiveness studies of Tong‐xin‐luo are also needed as such evidence is lacking.
Acknowledgements
We would like to thank the members of the Cochrane Heart Group for their advice during the development of the protocol.
Appendices
Appendix 1. Search strategies
Cochrane Library
#1 Tongxinluo #2 Tong Xin Luo #3 Tong‐xin‐luo #4 TXL #5 Tongxinluo* #6 Enter terms for search#1 or #2 or #3 or #4 or #5
MEDLINE
1. exp Cardiovascular Diseases/ 2. cardio*.tw. 3. cardia*.tw. 4. heart*.tw. 5. coronary*.tw. 6. angina*.tw. 7. ventric*.tw. 8. myocard*.tw. 9. pericard*.tw. 10. isch?em*.tw. 11. emboli*.tw. 12. arrhythmi*.tw. 13. thrombo*.tw. 14. atrial fibrillat*.tw. 15. tachycardi*.tw. 16. endocardi*.tw. 17. (sick adj sinus).tw. 18. exp Stroke/ 19. (stroke or stokes).tw. 20. cerebrovasc*.tw. 21. cerebral vascular.tw. 22. apoplexy.tw. 23. (brain adj2 accident*).tw. 24. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 25. exp Hypertension/ 26. hypertensi*.tw. 27. peripheral arter* disease*.tw. 28. ((high or increased or elevated) adj2 blood pressure).tw. 29. exp Hyperlipidemias/ 30. hyperlipid*.tw. 31. hyperlip?emia*.tw. 32. hypercholesterol*.tw. 33. hypercholester?emia*.tw. 34. hyperlipoprotein?emia*.tw. 35. hypertriglycerid?emia*.tw. 36 or/1‐35 37. tongxinluo.tw. 38. Drugs, Chinese Herbal/ 39. tong xin luo.tw. 40. (chinese adj3 (medic* or drug* or capsul*)).tw. 41. Medicine, Chinese Traditional/ 42 or/37‐41 43. randomized controlled trial.pt. 44. controlled clinical trial.pt. 45. randomized.ab. 46. placebo.ab. 47. drug therapy.fs. 48. randomly.ab. 49. trial.ab. 50. groups.ab. 51. 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 57. exp animals/ not humans.sh. 58. 51 not 57 59 36 and 42 and 58
EMBASE
#1 Tongxinluo.tw. #2 Tong xin luo.tw. #3 Tong‐xin‐luo.tw. #4 TXL.tw. #5 tongxinluo.mp. #6 tongxinluo$.mp. #7 #1 or #2 or #3 or #4 or #5 or #6
Wangfang
(通心络 or 通新络 or 通欣络 or 通心洛 or 痛心络) and (介入治疗 or 介入手术 or 经皮冠状动脉 or 冠状动脉介入 or 冠脉介入 or 冠脉成型 or 冠脉成形 or 冠状动脉成形 or 冠状动脉成型 or 经皮经管冠状动脉 or 经皮经管冠脉 or 经皮腔内冠状动脉 or 经皮腔内冠脉 or 支架 or PCI)
CMCC
((通心络 OR 通新络 OR 通欣络 OR 通心洛 OR 痛心络/fld=关键词) OR (通心络 OR 通新络 OR 通欣络 OR 通心洛 OR 痛心络/fld=摘要) OR (通心络 OR 通新络 OR 通欣络 OR 通心洛 OR 痛心络) OR (通心络 OR 通新络 OR 通欣络 OR 通心洛 OR 痛心络/fld=题名) ) AND ( (介入治疗 OR 介入手术 OR 经皮冠状动脉 OR 冠状动脉介入OR 冠脉介入 OR 冠脉成型 OR冠脉成形 OR 冠状动脉成形 OR 冠状动脉成型OR 经皮经管冠状动脉 OR经皮经管冠脉 OR 经皮腔内冠状动脉 OR经皮腔内冠脉 OR 支架 OR PCI/fld=题名) OR (介入治疗 OR 介入手术 OR 经皮冠状动脉 OR 冠状动脉介入OR 冠脉介入 OR 冠脉成型 OR冠脉成形 OR 冠状动脉成形 OR 冠状动脉成型OR 经皮经管冠状动脉 OR经皮经管冠脉 OR 经皮腔内冠状动脉 OR经皮腔内冠脉 OR 支架 OR PCI/fld=关键词) OR (介入治疗 OR 介入手术 OR 经皮冠状动脉 OR 冠状动脉介入OR 冠脉介入 OR 冠脉成型 OR冠脉成形 OR 冠状动脉成形 OR 冠状动脉成型OR 经皮经管冠状动脉 OR经皮经管冠脉 OR 经皮腔内冠状动脉 OR经皮腔内冠脉 OR 支架 OR PCI/fld=摘要) OR (介入治疗 OR 介入手术 OR 经皮冠状动脉 OR 冠状动脉介入OR 冠脉介入 OR 冠脉成型 OR冠脉成形 OR 冠状动脉成形 OR 冠状动脉成型OR 经皮经管冠状动脉 OR经皮经管冠脉 OR 经皮腔内冠状动脉 OR经皮腔内冠脉 OR 支架 OR PCI) )
TCM
#1 WXTIC=通心络 OR 通新络 OR 通欣络 OR 通心洛 OR 痛心络
#2 WXMES=通心络 OR 通新络 OR 通欣络 OR 通心洛 OR 痛心络
#3 WXKW=通心络 OR 通新络 OR 通欣络 OR 通心洛 OR 痛心络
#4 %通心络 OR 通新络 OR 通欣络 OR 通心洛 OR 痛心络%
#5 #1 OR #2 OR #3 OR #4
#6 %介入治疗 or 介入手术 or 经皮冠状动脉 or 冠状动脉介入 or 冠脉介入 or 冠脉成型 or 冠脉成形 or 冠状动脉成形 or 冠状动脉成型 or 经皮经管冠状动脉 or 经皮经管冠脉 or 经皮腔内冠状动脉 or 经皮腔内冠脉 or 支架 or PCI%
#7 WXTIC=%介入治疗 or 介入手术 or 经皮冠状动脉 or 冠状动脉介入 or 冠脉介入 or 冠脉成型 or 冠脉成形 or 冠状动脉成形 or 冠状动脉成型 or 经皮经管冠状动脉 or 经皮经管冠脉 or 经皮腔内冠状动脉 or 经皮腔内冠脉 or 支架 or PCI%
#8 WXMES=介入治疗 or 介入手术 or 经皮冠状动脉 or 冠状动脉介入 or 冠脉介入 or 冠脉成型 or 冠脉成形 or 冠状动脉成形 or 冠状动脉成型 or 经皮经管冠状动脉 or 经皮经管冠脉 or 经皮腔内冠状动脉 or 经皮腔内冠脉 or 支架 or PCI
#9 WXKW=%介入治疗 or 介入手术 or 经皮冠状动脉 or 冠状动脉介入 or 冠脉介入 or 冠脉成型 or 冠脉成形 or 冠状动脉成形 or 冠状动脉成型 or 经皮经管冠状动脉 or 经皮经管冠脉 or 经皮腔内冠状动脉 or 经皮腔内冠脉 or 支架 or PCI%
#10 #6 OR #7 OR #8 OR #9
#11 #5 and #10
CNKI
(SU='通心络' OR TI='通心络' OR KY='通心络' OR AB='通心络' OR FT='通心络' OR SU='通新络' OR TI='通新络' OR KY='通新络' OR AB='通新络' OR FT='通新络' OR SU='通欣络' OR TI='通欣络' OR KY='通欣络' OR AB='通欣络' OR FT='通欣络' OR SU='痛心络' OR TI='痛心络' OR KY='痛心络' OR AB='痛心络' OR FT='痛心络' OR SU='通心洛' OR TI='通心洛' OR KY='通心洛' OR AB='通心洛' OR FT='通心洛') and (SU='介入治疗' OR AB='介入治疗' OR KY='介入治疗' OR FT='介入治疗' OR SU='介入手术' OR AB='介入手术' OR KY='介入手术' OR FT='介入手术' OR SU='经皮冠状动脉' OR AB='经皮冠状动脉' OR KY='经皮冠状动脉' OR FT='经皮冠状动脉' OR SU='冠状动脉介入' OR AB='冠状动脉介入' OR KY='冠状动脉介入' OR FT='冠状动脉介入' OR SU='冠脉介入' OR AB='冠脉介入' OR KY='冠脉介入' OR FT='冠脉介入' OR SU='冠脉成型' OR AB='冠脉成型' OR KY='冠脉成型' OR FT='冠脉成型' OR SU='冠脉成形' OR AB='冠脉成形' OR KY='冠脉成形' OR FT='冠脉成形' OR SU='冠状动脉成形' OR AB='冠状动脉成形' OR KY='冠状动脉成形' OR FT='冠状动脉成形' OR SU='冠状动脉成型' OR AB='冠状动脉成型' OR KY='冠状动脉成型' OR FT='冠状动脉成型' OR SU='经皮经管冠状动脉' OR AB='经皮经管冠状动脉' OR KY='经皮经管冠状动脉' OR FT='经皮经管冠状动脉' OR SU='经皮经管冠脉' OR AB='经皮经管冠脉' OR KY='经皮经管冠脉' OR FT='经皮经管冠脉' OR SU='经皮腔内冠状动脉' OR AB='经皮腔内冠状动脉' OR KY='经皮腔内冠状动脉' OR FT='经皮腔内冠状动脉' OR SU='经皮腔内冠脉' OR AB='经皮腔内冠脉' OR KY='经皮腔内冠脉' OR FT='经皮腔内冠脉' OR SU='支架' OR AB='支架' OR KY='支架' OR FT='支架' OR SU=' PCI ' OR AB=' PCI ' OR KY=' PCI ' OR FT=' PCI ' ) and (SU='临床试验' or SU='随机对照试验' or AB='随机' or AB='随机对照' or AB='临床试验' or AB='临床研究' or AB='临床观察' or AB='临床效果' or AB='临床分析' or AB='临床疗效' or AB='临床比较' or AB='对照研究' or AB='对照试验' or AB='对照治疗' or AB='对照观察' or AB='对照分析' or AB='对比研究' or AB='对比观察' or AB='对比分析' or AB='分组研究' or AB='比较研究' or AB='多中心研究' or AB='疗效观察' or AB='疗效评价' or AB='疗效分析' or AB='疗效比较' or AB='治疗研究' or AB='治疗比较' or AB='效果比较' or AB='盲法' or AB='双盲' or AB='单盲' or AB='安慰剂' OR FT='随机' )
Search items for clinical trial registers
Tongxinluo, Tong Xin Luo, Tong‐xin‐luo, TXL, Tongxinluo, 通心络 , 通新络 , 通欣络, 通心洛 , 痛心络.
Data and analyses
Comparison 1. Tong‐xin‐luo + Convetional treatment vs. Convetional treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Occurrence of angiographic restenosis | 6 | 595 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.07, 0.34] |
| 2 Occurence of myocardial infarction | 13 | 1306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.16, 0.66] |
| 3 Occurence of heart failure | 4 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.11, 0.62] |
| 4 Occurrence of angina | 11 | 1210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.17, 0.34] |
| 5 All‐cause mortality | 4 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.06, 2.56] |
| 6 Mortality due to any cardiovascular event | 5 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.08, 1.12] |
| 7 Use of revascularization | 8 | 806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.15, 0.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2011.
| Methods | Study design: randomised controlled trial.
Method of randomisation: random number table.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 6 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Patients were all diagnosed as having ACS based on coronary arteriography, and in accordance with the diagnostic criteria formulated by the European Society of Cardiology in September 2000. Tong‐xin‐luo group: 80 patients (male/female: 48/32; age: 71.5 ± 5.6 years). Control group: 80 patients (male/female: 46/34; age: 70.7 ± 5.8 years). |
|
| Interventions | Control: conventional treatment (pre‐operation: aspirin 100 mg qd, clopidogrel 75 mg qd; post‐operation: aspirin, clopidogrel, nitroglycerin, low molecular weight heparin, ACEI, atorvastatin). Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (4 capsules tid; 0.26 g/capsule). Duration of intervention: 6 months. |
|
| Outcomes | Occurrence of angiographic restenosis; Occurrence of heart failure and angina. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, random number table was used. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Low risk | Same numbers of participants randomly assigned and analysed. No missing outcome data. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | Same numbers of participants randomly assigned and analysed. No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | Baseline characteristics (sex, age, type of angina, disease severity, disease duration, comorbidities, blood glucose, blood pressure, serum lipoid, etc.) were comparable between the two groups (P > 0.05). The study appeared to be free of other sources of bias. |
Dai 2011.
| Methods | Study design: randomised controlled trial.
Method of randomisation: no description.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 6 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Included participants were 45 to 74 years old, all diagnosed as CHD based on coronary arteriography, and successfully underwent PCI operation. Tong‐xin‐luo group: 31 participants (male/female: 19/12; age: 61.3 ± 7.23 years). Control group: 30 participants (male/female: 17/13; age: 63.5 ± 6.1 years). |
|
| Interventions | Control: conventional treatment (aspirin 100 mg qd, clopidogrel 75 mg qd, atorvastatin 20 mg qn; other symptomatic treatment was applied according to illness state). Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules. Duration of intervention: 6 months. |
|
| Outcomes | Occurrence of angiographic restenosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | No missing outcome data. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Unclear risk | Outcomes were not reported. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | Baseline information showed no statistical differences in sex, age, disease duration and type of PCI operation between two groups (P > 0.05). The study appeared to be free of other sources of bias. |
Di 2012.
| Methods | Study design: randomised controlled trial.
Method of randomisation: no description.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 6‐12 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Included participants were over 18 years of age, with evidence of coronary arteriography showing stenosis of more than 70% deduction in small vessels. Tong‐xin‐luo group: 25 participants (male/female: 18/7; age: 64.60 ± 8.69 years). Control group: 25 participants (male/female: 16/9; age: 63.04 ± 7.84 years). |
|
| Interventions | Control: conventional treatment (aspirin 100 mg qd, clopidogrel 75 mg qd, atorvastatin 10‐20 mg qd). Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (3 capsules tid; 0.38 g/capsule). Duration of intervention: 6‐12 months. Co‐intervention: none. |
|
| Outcomes | Occurrence of myocardial infarction and angina; All cause mortality; Use of revascularisation, including PCI and coronary artery bypass graft surgery. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | The outcome was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | Baseline information between groups showed no statistical differences in sex, age, smoking history, history of disease, types of CHD, levels of blood glucose and levels of blood lipoid (P > 0.05). The study appeared to be free of other sources of bias. |
Dong 2012.
| Methods | Study design: randomised controlled trial.
Method of randomisation: no description.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 6 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Participants were those who underwent emergency PCI within 6 hours of AMI. Tong‐xin‐luo group: 35 participants (male/female: 21/14; age: 61.20 ± 11.47 years). Control group: 28 participants (male/female: 15/13; age: 58.80 ± 8.96 years). |
|
| Interventions | Control group: conventional treatment (low molecular weight heparin 5000 IU subcutaneous injection bid for 3 days; aspirin 100 mg qd, clopidogrel 75 mg qd, ACEI, nitroglycerin, etc). Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (4 capsules tid). Duration of intervention: 12 months. |
|
| Outcomes | Occurrence of myocardial infarction; Mortality (death) due to any cardiovascular event. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | The outcome was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but study reported same outcomes as described in the methods. |
| Other bias | Low risk | Baseline information between groups showed no statistical differences in sex, age, smoking, comorbidities, number of stents and length of lesion between two groups (P > 0.05). The study appears to be free of other sources of bias. |
Huang 2010.
| Methods | Study design: randomised controlled trial.
Method of randomisation: no description.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 6 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Participants were diagnosed as acute ST segment elevation myocardial infarction based on the Acute Myocardial Infarction Diagnosis and Treatment Guidelines formulated by the Chinese Society of Cardiology of the Chinese Medical Doctors Associations in 2001, and received PCI within 12 hours after occurrence of AMI. There were 62 patients in the Tong‐xin‐luo group, and 58 in the control group. male/female: 68/52; age: 58.3 ± 12.6 years. |
|
| Interventions | Control group: conventional treatment (low molecular weight heparin, aspirin 100 mg qd, clopidogrel 75 mg qd). Tong‐xin‐luo group: conventional treatment + Tong‐xin‐luo capsules (4 capsules, tid). Duration of intervention: 6 months. |
|
| Outcomes | Occurrence of myocardial infarction; Mortality (death) due to any cardiovascular event; Use of revascularisation. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | The outcome was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | Same numbers of participants randomly assigned and analysed. No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | Baseline information showed no statistical differences in sex, age, smoking, drinking habits, comorbidities, Killip's cardiac functional classification, number and distributions of coronary pathological changes, blood pressure, levels of blood glucose and levels of blood lipoid between the two groups (P > 0.05). The study appeared to be free of other sources of bias. |
Li 2004.
| Methods | Study design: randomised controlled trial.
Method of randomisation: no description.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 6 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Participants were those who underwent PTCA and stenting successfully. Tong‐xin‐luo group: 38 participants (age: 56.6 ± 11.2 years). Control group: 38 participants (age: 54.9 ± 11.8 years). |
|
| Interventions | Conventional treatment:
• Pre‐operation (from 3 days before operation): aspirin 300 mg qd, clopidogrel 250 mg bid. • Intra‐operation: heparin 10000 IU, then 1000 IU/hour during operation. • Post‐operation: aspirin 300 mg qd, then reduced to 100 mg qd after 2 weeks; clopidogrel 250 mg bid, reduced to 250 mg qd after 4 weeks and applied for 3 months. ACEI, nitroglycerin and statin, were selectively applied. Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (4 capsules, tid). Control: conventional treatment. Duration of intervention: 6 months. Co‐intervention: none. |
|
| Outcomes | Occurrence of myocardial infarction and angina. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | The outcome was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | Baseline information between groups showed no statistical differences in age, number of stents implanted, types of CHD and distributions of coronary pathological changes between the two groups (P > 0.05). The study appeared to be free of other sources of bias. |
Liang 2010.
| Methods | Study design: randomised controlled trial.
Method of randomisation: no description.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 6 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Participants were patients with AMI, with evidence of coronary arteriography showing stenosis ≥ 70%, who successfully received stent implantations. There were 42 patients in Tong‐xin‐luo group, and 38 in control group. male/female: 52/28; mean age: 55 years (ranging from 40 to 70). |
|
| Interventions | Control group: conventional treatment (low molecular weight heparin, aspirin, clopidogrel, beta‐blockers, nitrates, statin). Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (0.52 g, tid). Duration of intervention: 6 months. Co‐intervention: none. |
|
| Outcomes | Occurrence of angiographic restenosis; Occurrence of myocardial infarction and angina. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | Whether all participants were brought back for angiography was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | Insufficient information to assess whether potential risk of other bias existed. |
Wang 2009a.
| Methods | Study design: randomised controlled trial. Method of randomisation: no description. Concealment of allocation: no description. Exclusions post randomisation: none. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. | |
| Participants | Participants were those who underwent PCI successfully (residual stenosis < 20%), aging from 35 to 74. Tong‐xin‐luo group: 40 participants (male/female: 28/12; age: 54.2 ± 10.2 years). Control group: 40 participants (male/female: 26/14; age: 52.5 ± 11 years). |
|
| Interventions | Control group: Conventional treatment (low molecular weight heparin 6000 IU subcutaneous injection bid for 5 days; aspirin 100 mg qd, clopidogrel 75 mg qd). Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (3 capsules, tid; 0.38 g/capsule). Duration of intervention: 6 months. Co‐intervention: none. |
|
| Outcomes | Occurrence of myocardial infarction and angina. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | The outcome was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | Baseline information showed no statistical differences in sex, age, smoking, comorbidities, length of lesion and distributions of coronary pathological changes between the two groups (P > 0.05). The study appeared to be free of other sources of bias. |
Wang 2010.
| Methods | Study design: randomised, double‐blind, parallel, placebo‐controlled trial.
Method of randomisation: no description.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 12 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Participants were diagnosed as having type II diabetes mellitus based on oral glucose tolerance test, and had evidence of coronary arteriography showing stenosis of no less than 70% reduction in small vessels with length of lesions no less than 3 mm. Tong‐xin‐luo group: 68 participants (male/female: 43/25; age: 65.2 ± 9.9 years). Control group: 64 participants (male/female: 45/19; age: 65.1 ± 10.4 years). |
|
| Interventions | Control group: conventional treatment (aspirin 300 mg qd, and reduced to 100 mg qd after a month; clopidogrel 75 mg qd; atorvastatin 20 mg qn) + placebo. Tong‐xin‐luo group: conventional treatment + Tong‐xin‐luo capsules (3 capsules, tid; 0.38 g/capsule). Duration of intervention: 12 months. |
|
| Outcomes | Occurrence of myocardial infarction and angina; All cause mortality. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | The outcome was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | Baseline information showed no statistical differences in sex, age, smoking, comorbidities, number and length of stents implanted, number and distributions of coronary pathological changes and level of serum creatinine between the two groups (P > 0.05). The study appeared to be free of other sources of bias. |
Wu 2010.
| Methods | Study design: randomised controlled trial.
Method of randomisation: no description.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 6 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Participants were patients with unstable angina based on the Unstable Angina Diagnosis and Treatment Guidelines formulated by the Chinese Society of Cardiology of the Chinese Medical Doctors Associations in 2007, and underwent PCI successfully. Tong‐xin‐luo group: 57 patients (male/female: 32/25; age: 71.4 ± 4.5 years). Control group: 53 patients (male/female: 31/22; age: 69.8 ± 4.3 years). |
|
| Interventions | Control: conventional treatment:
• Pre‐operation: aspirin 100 mg qd, clopidogrel 75 mg qd for more than 7 days.
• Post‐operation: aspirin, clopidogrel, nitroglycerin, low molecular weight heparin, ACEI, etc. Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (4 capsules, tid; 0.38 g/capsule) Duration of intervention: 6 months. Co‐intervention: none. |
|
| Outcomes | Occurrence of angiographic restenosis; Occurrence of myocardial infarction, heart failure and angina; Use of revascularisation, including PCI and coronary artery bypass graft surgery. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | Whether all participants were brought back for angiography was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | Baseline information showed no statistical differences in sex, age, comorbidities, number and distributions of coronary pathological changes, blood pressure, levels of blood glucose and levels of blood lipoid between the two groups (P > 0.05). The study appeared to be free of other sources of bias. |
Xiao 2007.
| Methods | Study design: randomised controlled trial.
Method of randomisation: no description.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 6 months. Losses to follow‐up: 37 of 62 participants in Tong‐xin‐luo group and 47 of 70 participants in control group did not undergo coronary arteriography, the occurrence of angiography restenosis for these participants was lost to follow‐up. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Participants were those who successfully received bare‐metal coronary stents. Tong‐xin‐luo group: 62 participants (male/female: 42/20; age: 53 ± 12 years). Control group: 70 participants (male/female: 49/21; age: 55 ± 10 years). |
|
| Interventions | Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (4 capsules, tid). Control group: Conventional treatment. Duration of intervention: 6 months. Co‐intervention: none. |
|
| Outcomes | Occurrence of angiographic restenosis; Occurrence of myocardial infarction, heart failure, and angina; Mortality (death) due to any cardiovascular event. |
|
| Notes | There were no losses to follow‐up in the outcomes measured, except angiographic restenosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | High risk | 37 of 62 participants in Tong‐xin‐luo group and 47 of 70 participants in control group did not undergo coronary arteriography. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | Baseline information showed no statistical differences in sex, age, comorbidities, types of coronary pathological changes and average number of stents between the two groups (P > 0.05). The study appeared to be free of other sources of bias. |
Yang 2009a.
| Methods | Study design: randomised controlled trial.
Method of randomisation: random number table.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 3 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Participants were diagnosed as having AMI based on the Acute Myocardial Infarction Diagnosis and Treatment Guidelines formulated by the Chinese Society of Cardiology of the Chinese Medical Doctors Associations in 2001, who successfully underwent PCI. Tong‐xin‐luo group: 30 participants (male/female: 22/8; age mean: 58 years). Control group: 29 participants (male/female: 25/4; age mean: 56 years ). |
|
| Interventions | Control group: conventional treatment:
• Pre‐operation: aspirin 300 mg qd, clopidogrel 600 mg qd.
• Intra‐operation: heparin 1000 IU/kg, then 1000 IU/hour during operation.
• Post‐operation: low molecular weight heparin 5000 IU subcutaneous injection bid for 7 days; aspirin 300 mg qd. Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (3 capsules, tid). Duration of intervention: more than 24 months. Co‐intervention: none. |
|
| Outcomes | Occurrence of myocardial infarction and heart failure; Mortality (death) due to any cardiovascular event; All cause mortality; Use of revascularisation. |
|
| Notes | Withdrawal of one participant before randomisation was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, random number table was used as randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | The outcome was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | Same numbers of participants randomly assigned and analysed. No missing outcome data. |
| Selective reporting (reporting bias) | High risk | The study protocol is not available, but some outcomes of interest described in the methods were not reported. |
| Other bias | Low risk | Baseline information showed no statistical differences in sex, age, comorbidities, numbers of coronary pathological changes and classification of the New York Heart Function Assessment between the two groups (P > 0.05). The study appeared to be free of other sources of bias. |
Yao 2006.
| Methods | Study design: randomised controlled trial.
Method of randomisation: no description.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 6 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Participants were patients with CHD, with evidence of coronary arteriography showing stenosis ≥ 70%, who successfully received stenting. Tong‐xin‐luo group: 38 participants (age: 56.6 ± 10.9 years). Control group: 38 participants (age: 50.1 ± 12.4 years ). |
|
| Interventions | Control group: conventional treatment:
• Pre‐operation(3 days before operation): aspirin 300 mg qd, clopidogrel 75 mg qd, simvastatin 20 mg qd.
• Intra‐operation: heparin 10000 IU/kg, then 1000 IU/hour during operation.
• Post‐operation: low molecular weight heparin 600 ml subcutaneous injection bid for 5 days; aspirin 300 mg qd, clopidogrel 75 mg qd. Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (4 capsules, tid). Duration of intervention: 6 months. Co‐intervention: none. |
|
| Outcomes | Occurrence of angina. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | The outcome was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | Baseline information between groups showed no statistical differences in sex, age, length of lesions, distributions of coronary pathological changes and severity of stenosis between the two groups (P > 0.05). The study appears to be free of other sources of bias. |
Zhang 2009a.
| Methods | Study design: randomised controlled trial.
Method of randomisation: drawing of lots.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 3‐48 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Participants were diagnosed as having AMI based on the diagnostic criteria formulated by the European Society of Cardiology in September, 2000, and underwent PCI successfully. Tong‐xin‐luo group: 96 participants (male/female: 70/26; age: 60 ± 5 years). Control group: 82 participants (male/female: 57/25; age: 58 ± 8 years). |
|
| Interventions | Conventional treatment:
• Pre‐operation: aspirin 100‐300 mg, clopidogrel 600 mg.
• Post‐operation:aspirin 100 mg qd; clopidogrel 75 mg qd for 9‐12 months, other symptomatic treatment (ACEI, nitroglycerin, etc.) was applied according to illness state. Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (3 capsules, tid). Duration of intervention: more than 24 months. Co‐intervention: none. |
|
| Outcomes | Occurrence of myocardial infarction, angina and heart failure; Mortality (death) due to any cardiovascular event; All cause mortality; Use of revascularisation. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, drawing of lots was used as randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | The outcome was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | Same numbers of participants randomly assigned and analysed. No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | Baseline information between groups showed no statistical differences in sex, age, comorbidities, smoking, length of lesions, number of stents, types of stents and number of coronary pathological changes between the two groups (P > 0.05). The study appeared to be free of other sources of bias. |
Zheng 2010.
| Methods | Study design: randomised controlled trial.
Method of randomisation: no description.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 6 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Participants were patients diagnosed as having ACS who underwent emergency PCI 12‐24 hours after admission. Tong‐xin‐luo group: 34 participants (male/female: 28/6; age: 63 ± 10 years). Control group: 56 participants (male/female: 46/10; age: 61 ± 11 years). |
|
| Interventions | Control group: conventional treatment (low molecular weight heparin, aspirin, clopidogrel, beta‐blockers, nitrates, statin, etc.). Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (3 capsules, tid; 0.38 g/capsule). Duration of intervention: 6 months. Co‐intervention: none. |
|
| Outcomes | Occurrence of myocardial infarction; Use of revascularisation. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description about blinding. No placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description about blinding. No placebo. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | The outcome was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | Same numbers of participants randomly assigned and analysed. No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | Insufficient information to assess whether potential risk of other bias exists. |
Zhou 2007b.
| Methods | Study design: randomised controlled trial.
Method of randomisation: no description.
Concealment of allocation: no description.
Exclusions post randomisation: none. Length of follow‐up: 6 months. Losses to follow‐up: none. Intention‐to‐treat analysis: not mentioned. |
|
| Participants | Participants were patients diagnosed as having CHD based on the World Health Organization diagnostic criteria, who successfully received stent implantations. There were 70 patients in the Tong‐xin‐luo group, and 66 in the control group. male/female: 86/50; mean age: 56.8 years (ranging from 30 to 76). |
|
| Interventions | Control group: conventional treatment (low molecular weight heparin, aspirin, clopidogrel, beta‐blockers, nitrates, statin, etc.). Tong‐xin‐luo group: conventional treatment plus Tong‐xin‐luo capsules (3 capsules, tid). Duration of intervention: 6 months. Co‐intervention: none. |
|
| Outcomes | Occurrence of myocardial infarction and angina; Use of revascularisation, including PCI and coronary artery bypass graft surgery. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but detailed information on the randomisation method was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No description about the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description about blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description about blinding. |
| Incomplete outcome data (attrition bias) Angiographic restenosis | Unclear risk | Whether all participants were brought back for angiography was not reported. |
| Incomplete outcome data (attrition bias) Outcomes other than angiographic restenosis | Low risk | Same numbers of participants randomly assigned and analysed. No missing outcome data. |
| Selective reporting (reporting bias) | High risk | The study protocol is not available, but some outcomes of interest described in the methods were not reported in results. |
| Other bias | Unclear risk | Insufficient information to assess whether potential risk of other bias exists. |
Abbreviations
ACS: acute coronary syndrome qd: daily ACEI: angiotensin‐converting‐enzyme inhibitor CHD: coronary heart disease qn: every night PCI: percutaneous coronary intervention tid: three times daily bid: twice daily AMI: acute myocardial infarction PCTA: percutaneous transluminal coronary angioplasty
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chang 2004 | Patients did not undergo PCI. |
| Chen 2007 | Intervention period was less than 3 months. |
| Fan 2008 | Intervention period was less than 3 months. |
| Fu 2012 | Intervention period was less than 3 months. |
| Gao 2007 | Not RCT. |
| Guo 2012 | Did not report outcomes listed in inclusion criteria. |
| Han 2009 | Intervention period was less than 3 months. |
| Han 2011 | Intervention period was less than 3 months. |
| Hou 2008 | No full‐text or data available. |
| Jiang 2008 | Intervention was not Tong‐xin‐luo. |
| Kuang 2011 | Did not report outcomes listed in inclusion criteria. |
| Li 2008 | Did not report outcomes listed in inclusion criteria; randomisation not mentioned. |
| Li 2012 | Did not report outcomes listed in inclusion criteria. |
| Liu 2009 | Intervention period was less than 3 months. |
| Lu 2012 | Intervention period was less than 3 months. |
| Ma 2009 | Did not report outcomes listed in inclusion criteria. |
| Qu 2011 | Patients did not undergo PCI. |
| Su 2000 | Patients did not undergo PCI. |
| Tao 2012 | Did not report outcomes listed in inclusion criteria. |
| Tu 2006 | Did not report outcomes listed in inclusion criteria. |
| Wang 2007 | Did not report outcomes listed in inclusion criteria. |
| Wang 2009b | Did not report outcomes listed in inclusion criteria. |
| Wang 2009c | Intervention period was less than 3 months. |
| Wang 2012 | Did not report outcomes listed in inclusion criteria. |
| Xiao 2004 | Patients did not undergo PCI. |
| Xu 2007 | Intervention period was less than 3 months. |
| Xu 2008 | Not RCT, a review. |
| Yang 2009b | Did not report outcomes listed in inclusion criteria. |
| Yang 2010 | Did not report outcomes listed in inclusion criteria; randomisation not mentioned. |
| Yang 2012 | Did not report outcomes listed in inclusion criteria. |
| Zhang 2005 | Intervention period was less than 3 months. |
| Zhang 2006 | Intervention period was less than 3 months. |
| Zhang 2007 | Intervention period was less than 3 months. |
| Zhang 2008 | Patients did not undergo PCI. |
| Zhang 2009b | Patients did not undergo PCI. |
| Zhang 2010 | Didn’t report outcomes listed in inclusion criteria. |
| Zhao 2010 | Intervention period was less than 3 months. |
| Zhao 2011 | Intervention period was less than 3 months. |
Abbreviations
PCI: percutaneous coronary intervention RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Deng 2013.
| Methods | RCT |
| Participants | Participants were patients diagnosed as having CHD based on the World Health Organization diagnostic criteria, who successfully received stent implantations. There were 31 patients in Tong‐xin‐luo group, and 30 in control group. male/female: 41/20; mean age: ranging from 41 to 78. |
| Interventions | Tong‐xin‐luo |
| Outcomes | The minimum diameter of the target vessel, degree of stenosis; The occurrence of restenosis. |
| Notes |
Abbreviations
RCT: randomised controlled trial CHD: coronary heart disease
Characteristics of ongoing studies [ordered by study ID]
Han 2012.
| Trial name or title | Tongxinluo Improve High on Clopidogrel Platelet Reactivityn Patients With Coronary Heart Disease |
| Methods | Randomised double‐blind trial |
| Participants | ACS (including unstable angina pectoris, non‐ST‐segment elevation myocardial infarction and ST‐elevation myocardial infarction; at least one coronary stent; age between18 and 75; high on‐treatment platelet reactivity defined as an ADP‐induced platelet aggregation (by VerifyNow, PRU ≥ 236) at 24 hours after clopidogrel loading (300 ‐ 600 mg) or 24 hours after PCI. |
| Interventions | Tong‐xin‐luo |
| Outcomes | Platelet aggregation rate; Inflammation Marker (hsCRP, CD62P‐CD41); BT, FIB and aPTT; Major ischemic event (including MI, ischemic stroke and all‐cause mortality); Bleeding event; Adverse drug reaction and withdrawal rate; Angina recurrence; Traditional Chinese medicine angina symptoms scores; Intra‐stent thrombosis. |
| Starting date | November 1, 2012 |
| Contact information | Yaling Han, MD Tel: +86‐24‐23922184 E‐mail: 13998847715@qq.com |
| Notes |
Abbreviations
ACS: acute coronary syndrome PRU: P2Y12 reactivity unit PCI: percutaneous coronary intervention aPTT: activated partial thromboplastin time MI: miocardial infarction
Differences between protocol and review
In the protocol, we planned to perform subgroup analyses based on patient characteristics, type of treatment, follow‐up period and type of stent. However, in this review, type of stent was not reported in most studies and it was difficult to separate participants with different baseline characteristics from original trials, so subgroup analyses based on patient characteristics or type of stent were not performed. Intervention of treatment groups in all included trials was Tong‐xin‐luo plus conventional treatment, so there was no obvious difference in types of treatment. Also, the follow‐up period varied among studies; for most outcomes, limited studies provided outcome data according to time of follow‐up, which prevented us from analysing outcome data according to the follow‐up period.
In addition, we planed to perform sensitivity analyses by excluding studies with high risk of bias. However, such sensitivity analyses were not performed because most of the included studies were considered to have high risk of bias. There were insufficient numbers of studies for meta‐analysis after removing studies with high risk of bias.
Lastly, because it is very difficult to ascribe CV death due to restenosis, we did not pool the mortality due to restenosis after PCI, which was a primary outcome in the study protocol. In addition, we did not identify any study that reported this outcome.
Contributions of authors
Review design: Chen Mao and Jin‐Ling Tang
Protocol drafting: Chen Mao, Yafang Huang, Zu‐Yao Yang and Jin‐Qiu Yuan
Study search and selection: Xiao‐Hong Fu, Zu‐Yao Yang and Jin‐Qiu Yuan
Data extraction: Xiao‐Hong Fu and Jin‐Qiu Yuan
Data entering: Xiao‐Hong Fu, Yafang Huang, Zu‐Yao Yang, Jin‐Qiu Yuan and Ying Qin
Quality assessment: Vincent CH Chung, Ying Qin and Chen Mao
Date analysis: Chen Mao, Wilson Wai San Tam and Jin‐Ling Tang
Results interpretation: Chen Mao, Xiao‐Hong Fu, Yafang Huang, Jin‐Qiu Yuan, Vincent CH Chung, Joey SW Kwong, Wei Xie and Jin‐Ling Tang
Review drafting: Chen Mao, Xiao‐Hong Fu, Jin‐Qiu Yuan, Yafang Huang, Zu‐Yao Yang and Jin‐Ling Tang
Sources of support
Internal sources
No sources of support supplied
External sources
The Grant from the Chinese Medicine Department of Hospital Authority ( MD09948 ), Hong Kong.
Declarations of interest
Nothing to declare.
New
References
References to studies included in this review
Chen 2011 {published data only}
- Chen ZQ. Effect of Tongxinluo capsule on platelet activity and function of vascular endothelium functions as well as prognosis at different stage in patients with acute coronary syndrome after undergoing percutaneous coronary intervention [通心络胶囊对急性冠状动脉综合征患者介入治疗不同时期血小板活化和血管内皮功能以及预后的影响]. South China Journal of Cardiovascular Diseases 2011;suppl:84. [Google Scholar]
- Chen ZQ, Hong L, Wang H, Yin Q. Effect of Tongxinluo capsule on platelet activities and vascular endothelium functions as well as prognosis in patients with acute coronary syndrome undergoing percutaneous coronary intervention [通心络胶囊对急性冠状动脉综合征患者介入治疗后血小板活化和血管内皮功能及预后的影响]. Chinese Journal of Integrated Traditional and Western Medicine 2011;31(04):487‐91. [PubMed] [Google Scholar]
- Chen ZQ, Hong L, Wang H, Yin Q, Lu LX, Lai YL. Effect of Tongxinluo capsule on platelet activities and vascular endothelium functions at different stages in patients with acute coronary syndrome undergoing percutaneous coronary intervention [通心络胶囊对急性冠状动脉综合征患者介入治疗后血小板活化血管内皮功能的影响]. Chinese Pharmaceutical Journal 2012;47(4):311‐5. [Google Scholar]
Dai 2011 {published data only}
- Dai GF, Yang SJ. Clinical observation for the effect of Tong‐xin‐luo capsules in preventing restenosis in patients with coronary heart disease after percutaneous coronary intervention [通心络胶囊干预冠心病PCI 术后再狭窄临床观察]. Guangming Journal of Chinese Medicine 2011;26(9):1823‐4. [Google Scholar]
Di 2012 {published data only}
- Di J. Research on the Effect of Tongxinluo capsule on the Instent Restenosis of Small Vessels and Long Affection in Coronary Artery [通心络胶囊对冠状动脉小血管长病变支架术后再狭窄的影响研究][Master's thesis]. Nanjing: Nanjing University of Chinese Medicine, 2012. [Google Scholar]
Dong 2012 {published data only}
- Dong SQ, Dong YW. Influence of Tongxinluo on inflammatory and cytokinesendothelial function in patients with acute myocardial infarction after percutaneous coronary intervention [通心络胶囊对急性心肌梗死患者经皮冠状动脉介入治疗术后炎症因子及血管内皮功能的影响]. Chinese Journal of Clinicians (electronic edition) 2012;6(7):1865‐7. [Google Scholar]
Huang 2010 {published data only}
- Huang B. Influence of Tongxinluo on inflammation reaction and endothelial function in patients with acute myocardial infarction after percutaneous coronary intervention [通心络胶囊对急性心肌梗死介入术后炎性反应和血管内皮功能的影响]. Journal of New Chinese Medicine 2010;42(9):22‐4. [Google Scholar]
Li 2004 {published data only}
- Li YW. Research on the Effects of Tongxinluo Capsules on Local Cerebral Blood Flow in Patients with Cerebral Infarction [通心络胶囊对植入冠状动脉支架预后影响的研究]. Journal of Bethune Military Medical College 2004;2(2):75‐7. [Google Scholar]
Liang 2010 {published data only}
- Liang YM, Wang ZJ, Su XY. The effect of Tongxinluo capsules on coronary restennosis of patients with acute myocardial infarction after percutaneous coronary intervention [通心络胶囊对心肌梗死冠状动脉介入治疗患者术后再狭窄的疗效评价]. Chinese Journal of TCM WM Critical Care 2010;17(003):175‐6. [Google Scholar]
Wang 2009a {published data only}
- Wang YH, Shi HN. Effect of Tongxinluo capsules to prevent restenosis in patients after percutaneous intervention [通心络胶囊防治冠脉支架术后再狭窄疗效观察]. Modern Journal of Integrated Traditional Chinese and Western Medicine 2009;18(1):39. [Google Scholar]
Wang 2010 {published data only}
- Wang XD, Lou B. Effect of Tongxinluo Capsule on Restenosis after Coronary Artery Stent Implantation in Patients with Type‐2 Diabetes Mellitus [通心络对2型糖尿病冠状动脉小血管长病变支架术后再狭窄的影响]. Journal of Nanjing TCM University 2010;26(006):464‐7. [Google Scholar]
Wu 2010 {published data only}
- Wu ZY, Yang DC, Hou B, Chen YG. Effects of Tongxinluo Capsules on interventional therapy of patients with unstable angina. China Medical Herald, 2010, 20: 012. [通心络胶囊在不稳定型心绞痛患者介入治疗中的作用]. China Medical Herald 2010;7(20):17‐20. [Google Scholar]
Xiao 2007 {published data only}
- Xiao HB, Zhang DD, Gu J. Effects of tongxinluo on C‐reactive protein and clinical prognosis in patients after coronary stenting [通心络对冠心病支架术后C 反应蛋白及预后的影响]. Cardiac Intervention 2007;16(8):520‐2. [Google Scholar]
Yang 2009a {published data only}
- Yang W. The protective effects of Tongxinluo capsule on myocardium after reperfusion in patients with acute myocardial infarction [通心络胶囊对急性心肌梗塞急诊PCI患者心肌保护的临床研究][Master's thesis]. Shaanxi: Shaanxi University of Chinese Medicine, 2009. [Google Scholar]
- Yang W, Wang S, Xu XH, Zhang CF. NA [通心络胶囊对急性心肌梗死急诊PCI术后左室重构的影响]. Chinese Community Doctors 2012;30:8. [Google Scholar]
- Yang W, Xu XH, Wang S, Zhang CF. [通心络胶囊对急性心肌梗死急诊PCI术后患者血清Ⅲ型前胶原氨基端肽及左室重构的影响]. Chinese Journal of Difficult and Complicated Cases 2012;11(6):452‐3. [Google Scholar]
Yao 2006 {published data only}
- Yao FH, Liu N, Ge GY. Clinical study of reconstriction after PCI operation in patients with coronary disease intervened by Tongxinluo capsule [通心络胶囊干预冠心病患者PCI术后再狭窄的临床研究]. Chinese Journal of Difficult and Complicated Cases 2006;5(3):191‐2. [Google Scholar]
Zhang 2009a {published data only}
- Zhang XP, Zhang Y. Long‐term effect of Tongxinluo in patients with acute myocardial infarction after coronary stenting [通心络对急性心肌梗死患者置入支架后远期疗效的影响]. Chinese Remedies & Clinics 2009;9(3):243‐4. [Google Scholar]
Zheng 2010 {published data only}
- Zheng H, Teng ZH, Ma LQ. The effect of Tongxinluo capsule on level of serum CRP and blood lipid in patients with ACS post PCI [通心络胶囊对急性冠状动脉综合征介入术后患者C‐反应蛋白、血脂的影响]. Chinese Journal of Difficult and Complicated Cases 2010;9(7):487‐8. [Google Scholar]
Zhou 2007b {published data only}
- Zhou J, Guo JT. Clinical Study of Tongxinluo on Preventing Re‐stenosis of Stents in CHD [通心络预防冠心病患者支架术后再狭窄的临床研究]. Hebei Medicine 2007;13(10):1188‐91. [Google Scholar]
References to studies excluded from this review
Chang 2004 {published data only}
- Chang SF, Zhao M. Clinical observation for Tongxinluo in the treatment of 68 cases of unstable angina pectoris [通心络胶囊治疗不稳定型心绞痛 68 例临床观察]. Modern Journal of Integrated Traditional Chinese and Western Medicine 2004;13(2):189. [Google Scholar]
Chen 2007 {published data only}
- Chen ZQ, Lai YY, Hong L, Wang H, Qiu Y, Yin QL, et al. The effects of Tongxinluo capsule on platelet activity and vascularendothelium function in patients with ACS after PCI [通心络对急性冠状动脉综合征患者介入术后血小板活化及血管内皮功能的影响]. Chinese Journal of Geriatric Heart Brain and Vessel Diseases 2007;9(7):460‐3. [Google Scholar]
Fan 2008 {published data only}
- Fan SM. Treatment Effects of Tongxinluo capsule on acute myocardial infarction after coronary stent [通心络胶囊在急性心肌梗死支架术后的临床应用研究]. Qinghai Medical Journal 2008;38(1):8‐10. [Google Scholar]
Fu 2012 {published data only}
- Fu DH, Song XL, Feng CH. Clinical observation of Tongxinluo capsules in treating patients with angina after PCI [通心络胶囊对冠心病介入术后心绞痛的疗效观察]. Journal of Guiyang College of Traditional Chinese Medicine 2012;34(3):171‐2. [Google Scholar]
Gao 2007 {published data only}
- Gao CY, Yang L, Zhang J, Wu YL. The long‐term efficacy of Tongxinluo in patients after percutaneous coronary intervention [通心络胶囊对经皮冠状动脉介入患者长期预后的影响]. Hebei Medical Journal 2007;29(10):1067‐8. [Google Scholar]
Guo 2012 {published data only}
- Guo YH, Wang Y. Evaluation of the therapeutic effect of Tongxinluo for acute myocardial infarction using 99Tcm‐MIBI SPECT imaging [99Tcm‐MIBI心肌灌注断层显像评价通心络对急性心肌梗死后心肌无再流的影响]. Chinese Journal of Nucleau Medicine and Molecular Imaging 2012;32(2):127‐30. [Google Scholar]
Han 2009 {published data only}
- Han XT. Study of the changes of myeloperoxidase concentration in plasma and the interventional effect of Tongxinluo capsule on patients with unstable angina [不稳定型心绞痛患者介入治疗前后血浆髓过氧化物酶水平变化及通心络胶囊的干预作用]. Journal of Emergency in Traditional Chinese Medicine 2009;18(5):730‐1. [Google Scholar]
Han 2011 {published data only}
- Han XT, Cui XJ, Li YR, Chu FY. The effect of Tongxinluo on blood lipid, hs‐CRP and MMP‐9 in patients with unstable angina pectoris after PCI [通心络胶囊对不稳定型心绞痛患者PCI术后血脂、血浆高敏C反应蛋白、基质金属蛋白酶一9的影响]. Journal of Emergency in Traditional Chinese Medicine 2011;20(6):873‐4. [Google Scholar]
Hou 2008 {published data only}
- Hou AJ, Liu MY, Zhao HY, Zhang M, Yuan L, Jin YZ, et al. The effect of Tongxinluo on restenosis in patients with ACS [通心络对急性冠状动脉综合征患者支架术后再狭窄的影响]. Chinese Journal of Cardiology 2008;36:221‐2. [Google Scholar]
Jiang 2008 {published data only}
- Jiang XG, Yang YB, Qi HW, Yang M. Research of prevention effect of traditional Chinese medicine on restenosis after percutaneous coronary intervention [冠心病介入治疗后再狭窄的中医药防治研究]. Heilongjiang Journal of Traditional Chinese Medicine 2008;37(4):58‐9. [Google Scholar]
Kuang 2011 {published data only}
- Kuang B, Yang Y. Protection of Tongxinluo capsule against myocardial injury in patients after elective percutaneous coronary stenting posting [通心络胶囊对择期冠脉支架置入术患者的心肌保护作用]. Modern Hospital 2011;11(5):21‐3. [Google Scholar]
Li 2008 {published data only}
- Li MJ. Inflammatory reaction and change of vascular endothelial function after percutaneous coronary intervention and the effect of Tongxinluo [冠心病介入术后炎症反应和血管内皮功能变化及通心络的干预作用]. Journal of Chinese Physician 2008;10(10):1392‐4. [Google Scholar]
Li 2012 {published data only}
- Li H. The effect of drug intervention on vascular inflammatory reaction and change of vascular endothelial function after percutaneous coronary intervention [冠状动脉介入术后血管炎症反应及内皮功能改变的药物干预分析]. China Practical Medical 2012;07(13):159‐60. [Google Scholar]
Liu 2009 {published data only}
- Liu SJ. The effect of Tongxinluo on perioperative CRP patients with ACS [通心络胶囊对急性冠脉综合征患者围手术期C反应蛋白影响的研究]. Journal of New Chinese Medicine 2009;41(7):41‐2. [Google Scholar]
Lu 2012 {published data only}
- Lu Y, Zhao XJ, Liao XC. The effect of Tongxinluo capsules on the change of hemorheology in patients with CHD after PCI [通心络胶囊对冠心病患者PCI 术后血液流变学的影响]. Inner Mongol Journal of Traditional Chinese Medicine 2012;14(4):1‐2. [Google Scholar]
Ma 2009 {published data only}
- Ma QL, Zhang SD, Ning YG, Pu XQ, Yu LG, Zheng SF, et al. Effect of Tongxinluo on endothelial function and hypersensitiveC‐reactive protein in acute coronary syndrome patients undergoing percutaneous coronary intervention [通心络对急性冠脉综合征介入治疗术患者内皮功能和高敏C反应蛋白的影响]. Journal of Central South University (Medical Sciences) 2009;34(06):550‐4. [PubMed] [Google Scholar]
Qu 2011 {published data only}
- Qu JQ, Ma JE, Bai RL. Clinical observation of Tongxinluo in treating patients with CHD [通心络胶囊治疗冠心病心绞痛疗效观察]. Chinese and Foreign Medical Research 2011;9(12):22. [Google Scholar]
Su 2000 {published data only}
- Su XY, Huang JX, Zhao ZJ, Zhao L, Hu YL, Su HR. Clinical observation of 96 cases of variant angina pectoris treated with Tongxinluo [通心络治疗变异型心绞痛96例临床观察]. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Cricitical Care 2000;7(2):119. [Google Scholar]
Tao 2012 {published data only}
- Tao WG, Zhu KY. Curative effect of Tongxinluo in treatment of angina after percutaneous coronary intervention (PCI) operation [通心络治疗PCI 术后心绞痛的疗效观察]. Medical Innovation of China 2012;9(5):9‐10. [Google Scholar]
Tu 2006 {published data only}
- Tu YP, Lei MJ, Zeng LF, Gong AB, Wei WR, Wang LL, et al. Influence of Tongxinluo on C‐reactive Protein in patients with diabetes after percutaneous coronary intervention [通心络胶囊对糖尿病患者冠状动脉介入术后C反应蛋白的影响]. Chinese Remedies & Clinics 2006;6(3):229‐30. [Google Scholar]
Wang 2007 {published data only}
- Wang G. Clinical study Oil Lvedv and Lvef after blood flow reconstruction interfered by Tongxinluo capsule in patients with AMI after percutaneous coronary intervention [通心络胶囊对急性心肌梗死介入治疗后左室舒张末容积和射血分数的影响]. Chinese Journal of Primary Medicine and Pharmacy 2007;14(4):570‐1. [Google Scholar]
Wang 2009b {published data only}
- Wang WY, Liu YB, Sun Y. Effect of Tongxinluo on inflammatory factor in patients with ACS [通心络胶囊对急性冠脉综合症患者介入治疗后炎症因子的影响]. Prevention and Treatment of Cardio‐cerebral‐vascular disease 2009;9(4):308‐9. [Google Scholar]
Wang 2009c {published data only}
- Wang X, Li J. Clinical observation for Tongxinluo in the treatment of CHD [通心络胶囊治疗冠心病的临床疗效观察]. Chinese Journal of Modern Drug Application 2009;22(3):105‐6. [Google Scholar]
Wang 2012 {published data only}
- Wang L, Liu H, Hu YC. The effect of drug intervention on vascular inflammatory reaction and vascular endothelial function after percutaneous coronary intervention [冠状动脉介入术后血管炎症反应及内皮功能改变的药物干预探讨]. China Clinical Practical Medicine 2012;7(18):169‐70. [Google Scholar]
Xiao 2004 {published data only}
- Xiao YQ, Li WK. The effect of Tongxinluo in treating patients with unstable angina pectoris [通心络治疗不稳定型心绞痛的临床观察]. Acta medicinae Sinica 2004;17(2):152‐3. [Google Scholar]
Xu 2007 {published data only}
- Xu Z, Li X. Influence of Tongxinluo on inflammatory factor of acute coronary syndrome patients after interventional therapy and its early heart complication [通心络对急性冠脉综合征患者介入治疗术后炎性因子及早期心脏并发症的影响]. Modern Journal of Integrated Traditional Chinese Western Medicine 2007;16(2):156‐7. [Google Scholar]
Xu 2008 {published data only}
- Xu LD, Liu LY. The prevention of restenosis after percutaneous coronary intervention [冠状动脉支架内再狭窄的防治]. Practical Cardio‐cerebral Pulmonary Vascular Disease 2008;16(4):73‐6. [Google Scholar]
Yang 2009b {published data only}
- Yang GK. Inflammatory reaction and change of vascular endothelial function after percutaneous coronary intervention and the effect of drug intervention [冠心病介入术后血管炎症反应和内皮功能变化及药物干预作用]. Sichuan Medical Journal 2009;30(11):1788‐90. [Google Scholar]
Yang 2010 {published data only}
- Yang JX, Li MJ, Yang XH. Effect of Tongxinluo on Change of Vascular Inflammation and EndothelialFunction after Percutaneous Coronary Intervention [冠状动脉介入术后血管炎症反应和内皮功能改变及药物干预作用]. Journal of Liaoning University of TCM 2010;12(2):94‐6. [Google Scholar]
Yang 2012 {published data only}
- Yang W, Xu XH, Wang S, Zhang CF. The effect of Tongxinluo on serum PⅢNP and ventricular remodeling in patients with AMI after PCI [通心络胶囊对急性心肌梗死急诊PCI术后患者血清 Ⅲ型前胶原氨基端肽及左室重构的影响]. Chinese Journal of Difficult and Complicated Cases 2012;11(6):452‐3. [Google Scholar]
Zhang 2005 {published data only}
- Zhang W. Effect of Tongxinluo Capsule on Coagulant and Fibrinolysis System andAngiotension II in Patients with CHD after Intracoronary Stenting [通心络胶囊对冠脉内支架置入术后患者凝血纤溶系统和血管紧张素II的影响]. Hebei Medicine 2005;12(1):37‐9. [Google Scholar]
Zhang 2006 {published data only}
- Zhang JW, Yang DC. The protective effects of Tongxinluo on myocardium after reperfusion in PCI patients with acute myocardium infarction [通心络对急性心肌梗死急诊PCI患者再灌注损伤的保护性研究]. Medicine Industry Information 2006;3(21):8‐10. [Google Scholar]
Zhang 2007 {published data only}
- Zhang HW. Clinical analysis of integrated traditional and western medicine in treating 62 cases of CHD [中西医结合治疗冠心病62例临床分析]. Theory and Practice of Chinese Medicine 2007;17(7):722‐3. [Google Scholar]
Zhang 2008 {published data only}
- Zhang LJ, Li HT, Guo JT, Zhou J. The Effect of Tongxinluo Capsules on the Function of BloodVessel Endothelium(BVE)on the Patientswith Coronary Heart Disease [通心络胶囊对冠状动脉粥样硬化性心脏病人血管内皮功能的影响]. Hebei Medicine 2008;14(7):761‐7. [Google Scholar]
Zhang 2009b {published data only}
- Zhang TX, Zhou JZ. The effect of Tongxinluo on inflammatory cytokins in patients with ACS [通心络胶囊对急性冠脉综合症患者炎症因子的影响]. China Journal of Chinese Materia Medica 2009;34(14):1857‐8. [Google Scholar]
Zhang 2010 {published data only}
- Zhang HT, Jia ZH, Zhang J, Ye ZK, Yang WX, Tian YQ, et al. No‐reflow protection and long‐term efficacy for acute myocardialinfarction with Tongxinluo: a randomized double‐blind placebo—controlled multicenter clinical trial. Chinese Medical Journal 2010;123(20):2858‐64. [PubMed] [Google Scholar]
Zhao 2010 {published data only}
- Zhao H, Tian JH, Hao XY, Ma L. Effects of Tongxinluo on Inflammatory factors in patients with coronary heart disease after percutaneous coronary intervention [通心络对冠心病病人PCI 术后炎症因子的影响]. Acta Academiae Mediciane Qindao Universitatis 2010;46(4):349‐351. [Google Scholar]
Zhao 2011 {published data only}
- Zhao X, Song ZJ, Hou LX. Clinical observation of Tongxinluo in treating patiens after percutaneous coronary intervention [通心络治疗支架置入术后患者的临床分析]. Medical Information 2011;24(7):173. [Google Scholar]
References to studies awaiting assessment
Deng 2013 {published data only}
- Deng X. Effect of Tongxinlou capsule on restenosis after PCI in patients with CHD [通心络胶囊干预冠心病PCI术后再狭窄临床观察]. China Medicine and Pharmacy 2013;3(12):67‐8. [Google Scholar]
References to ongoing studies
Han 2012 {published data only}
- NCT01721590. Tongxinluo improve high on Clopidogrel platelet reactivity in patients with coronary heart disease. http://clinicaltrials.gov/show/NCT01721590 (accessed November 2012).
Additional references
Agema 2001
- Agema WRP, Jukema JW, Pimstone SN, Kastelein JJP. Genetic aspects of restenosis after percutaneous coronary interventions; towards more tailored therapy. European Heart Journal 2001;22(22):2058‐74. [DOI] [PubMed] [Google Scholar]
Bhattarai 2012
- Bhattarai N, Charlton J, Rudisill C, Gulliford MC. Coding, recording and incidence of different forms of coronary heart disease in primary care. PLoS ONE 2012;7(1):e29776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Chen 1985
- Chen CY. Effect of Scolopendra extraction in the experiment of rabbit heart. Chinese Medicine Pharmacology and Clinical Practice 1985;1(1):124‐5. [Google Scholar]
Chen 2007
- Chen ZQ, Lai YY, Hong L, Wang H, Qiu Y, Yin QL, et al. The effects of Tongxinluo capsule on platelet activity and vascular endothelium function in patients with ACS after PCI. Chinese Journal of Geriatric Heart Brain and Vessel Diseases 2007;9(7):460‐3. [Google Scholar]
Cutlip 2007
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, Es GA. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115(17):2344‐51. [DOI] [PubMed] [Google Scholar]
Dangas 1996
- Dangas G, Fuster V. Management of restenosis after coronary intervention. American Heart Journal 1996;132(2 Pt 1):428‐36. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analyses of binary data. Abstracts of 8th International Cochrane Colloquium. Cape Town: The Cochrane Collaboration, Oct 25‐28th, 2000.
Dörffler 2005
- Dörffler‐Melly J, Koopman MM, Prins MH, Büller HR. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD002071.pub2] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder CSO. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;13(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fattori 2003
- Fattori R, Piva T. Drug‐eluting stents in vascular intervention. Lancet 2003;361(9353):247‐9. [DOI] [PubMed] [Google Scholar]
Giglioli 2009
- Giglioli C, Valente S, Margheri M, Comeglio M, Chiostri M, Romano SM, et al. An angiographic evaluation of restenosis rate at a six‐month follow‐up of patients with ST‐elevation myocardial infarction submitted to primary percutaneous coronary intervention. Internation Journal of Cardiology 2009;131(3):362‐9. [DOI] [PubMed] [Google Scholar]
Go 2014
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart Disease and Stroke Statistics ‐ 2014 Update. A report from the American Health Association. Circulation 2014;129(3):e28‐e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grech 2003
- Grech ED. ABC of interventional cardiology: percutaneous coronary intervention II: the procedure. BMJ 2003;326(24):1137‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.0.2 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Huntington 2003
- Huntington JA, Baglin TB. Targeting thrombin – rational drug design from natural mechanisms. Trends in Pharmacological Sciences 2003;24(11):589‐95. [DOI] [PubMed] [Google Scholar]
Kang 2012
- Kang Y, Lao H, Yu X, Chen J, Zhong S. Progress in genetic and epigenetic research on in‐stent restenosis after percutaneous coronary interventions. Chinese Journal of Medical Genetics 2012;29(1):38‐42. [DOI] [PubMed] [Google Scholar]
Lau 1997
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Annals of Internal Medicine 1997;127(9):820‐6. [DOI] [PubMed] [Google Scholar]
Lonn 2010
- Lonn E, Bosch J, Teo KK, Pais P, Xavier D, Yusuf S. The polypill in the prevention of cardiovascular diseases: key concepts, current status, challenges, and future directions. Circulation 2010;122(20):2078‐88. [DOI] [PubMed] [Google Scholar]
Lozano 2012
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010. Lancet 2012;380(9859):2095‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2006
- Lu XY, Shi DZ, Xu H. Clinical study on effect of Xiongshao capsule on restenosis after percutaneous coronary intervention. Zhongguo Zhong Xi Yi Jie He Za Zhi 2006;26(1):13‐7. [PubMed] [Google Scholar]
Modi 1995
- Modi NB, Baughman SA, Paasch BD. Pharmacokinetics and pharmacodynamics of TP‐9201, a GPIIbIIIa antagonist, in rats and dogs. Journal of Cardiovascular Pharmacology 1995;25(6):888. [DOI] [PubMed] [Google Scholar]
NHLBI 2011
- National Heart Lung and Blood Institute (NHLBI). Health Topic: Coronary heart disease. Available from http://www.nhlbi.nih.gov/health/health‐topics/topics/cad/ 2011.
Nong 1989
- Nong ZJ, Shen YY, Dong HC. The effect of Eupolyphaga Sinensis Walk alkaloids on rabbits cardial pump function. Journal of Anhui Traditional Chinese Medical College 1989;8(3):84‐6. [Google Scholar]
Nong 2000
- Nong MZ. Research on combined treatment of unstable angina pectoris with TCM and WM. Zhong Yi Han Shou Tong Xun 2000;19(4):51‐2. [Google Scholar]
Odell 2006
- Odell A, Grip L, Hallberg LR. Restenosis after percutaneous coronary intervention (PCI): experiences from the patients' perspective. European Journal of Cardiovascular Nursing 2006;5(2):150‐7. [DOI] [PubMed] [Google Scholar]
Paul 1994
- Johnson PH. Hirudin: Clinical potential of a thrombin inhibitor. Annual Review of Medicine 1994;45:165‐77. [DOI] [PubMed] [Google Scholar]
Pei 1986
- Pei YQ, Wen SR, Wang XY, Huo SP, Wei MC. Study of cardiac stimulation of Ginsenoside of upper part over grand. Journal of Beijing Medical University 1986;18(2):140‐1. [Google Scholar]
Pereira 2012
- Pereira TV, Horwitz RI, Ioannidis JP. Empirical evaluation of very large treatment effects of medical interventions. JAMA 2012;308(14):1676‐84. [DOI] [PubMed] [Google Scholar]
Rajaqopal 2003
- Rajaqopal V, Rockson SG. A review of mechanisms and management. American Journal of Medicine 2003;115(7):547‐53. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Richard 2002
- Holubkov R, Laskey WK, Haviland A, Slater JC, Bourassa MG, Vlachos HA, et al. Angina 1 year after percutaneous coronary intervention: a report from the NHLBI Dynamic Registry. American Heart Journal 2002 Nov;144(5):826‐33. [DOI] [PubMed] [Google Scholar]
Rosanio 1999
- Rosanio S, Tocchi M, Patterson C, Runge MS. Prevention of restenosis after percutaneous coronary interventions: the medical approach. Journal of Thrombosis and Haemostasis 1999;82 Suppl 1:164‐70. [PubMed] [Google Scholar]
Ruan 2003
- Ruan JL, Zhao ZX, Zeng QZ, Qian ZM. Recent advances in study of component and pharmacological roles of Radix Paeoniae Rubra. Chinese Pharmacological Bulletin 2003;19(9):965‐70. [Google Scholar]
Serruys 1995
- Serruys PW, Herrman JP, Simon R, Rutsch W, Bode C, Laarman GJ, et al. A comparison of hirudin with heparin in the prevention of restenosis after coronary angioplasty. The New England Journal of Medicine 1995;333(12):757‐63. [DOI] [PubMed] [Google Scholar]
Singh 2011
- Singh IM, Holmes DR Jr. Myocardial revascularization by percutaneous coronary intervention: past, present, and the future. Current Problems in Cardiology 2011;36(10):375‐401. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54(10):1046‐55. [DOI] [PubMed] [Google Scholar]
Testa 2009
- Testa L, Gaal WJ, Biondi Zoccai GG, Agostoni P, Latini RA, Bedogni F, et al. Myocardial infarction after percutaneous coronary intervention: a meta‐analysis of troponin elevation applying the new universal definition. QJM 2009;102(6):369‐78. [DOI] [PubMed] [Google Scholar]
Wang 2003
- Wang HJ, Huang YW, Sun J, Zhu CH, Zhang L. Effect of Tongxinluo capsule on function of vascular endotheliumin patients with unstable angina pectoris. Chinese Journal of Integrated Traditional Chinese and Western Medicine 2003;23(8):587‐9. [PubMed] [Google Scholar]
Wang 2007
- Wang G, Mao B, Xiong ZY, Fan T, Chen XD, Wang L, et al. The quality of reporting of randomized controlled trials of traditional Chinese medicine: a survey of 13 randomly selected journals from mainland China. Clinical Therapeutics 2007;29(7):1456‐67. [DOI] [PubMed] [Google Scholar]
Wang 2008
- Wang CH, Wang R, Cheng XM, He YQ, Wang ZT, Wu C, et al. Comparative pharmacokinetic study of paeoniflorin after oral administration of decoction of Radix Paeoniae Rubra and Radix Paeoniae Alba in rats. Journal of Ethnopharmacology 2008;117(3):467‐72. [DOI] [PubMed] [Google Scholar]
WHO 2002
- World Health Organization. Life course perspectives on coronary heart disease, stroke and diabetes. Department of Noncommunicable Disease Prevention and Health Promotion. Noncommunicable Disease and Mental Health Cluster 2002.
WHO 2011
- World Health Organization. Health topic: cardiovascular diseases (CVDs). Available from http://www.who.int/mediacentre/factsheets/fs317/en/index.html 2011.
WHS 2010
- World Health Statistics. Cause‐specific mortality and morbidity. Available at: http://www.who.int/whosis/whostat/EN_WHS09_Table2.pdf 2010.
Wu 2001
- Wu YL. Luobing theory of Chinese medicine and cardio‐cerebral antipathy. 1st Edition. Beijing: Chinese Science and Technical Publish House, 2001. [Google Scholar]
Wu 2006
- Wu T, Harrison RA, Chen XY, Ni J, Zhou L, Qiao J, et al. Tongxinluo (Tong xin luo or Tong‐xin‐luo) capsule for unstable angina pectoris. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004474.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2013
- Wu XY, Tang JL, Mao C, Yuan JQ, Qin Y, Chung VC. Systematic reviews and meta‐analyses of traditional Chinese medicine must search Chinese databases to reduce language bias. Evidence Based Complementary and Alternative Medicine 2013:812179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 1988
- Xu YJ. Effect of inhibition platelet adhesion reaction of Radix Ginseng. Journal of Pharmacology 1988;23(5):284‐5. [Google Scholar]
Yu 2011
- Yu M, Huang YN, Mo W. Progress and prospect of Hirudin and Its derivants research. Pharmaceutical Biotechnology 2011;18(4):351‐4. [Google Scholar]
Zhang 2004
- Zhang Q, Yang LH, Qiao P, Cui TX. Effects of tongxinluo capsule in the treatment of 68 patients with unstable angina pectoris. Chinese Journal of Primary Medicine and Pharmacy 2004;11(9):1080‐1. [Google Scholar]
Zheng 1998
- Zheng HZ, Dong ZH, Se J. Periostracum Cicadae. Modern Study of Traditional Chinese Medicine. 1st Edition. Beijing: Xue Yuan Press, 1998:5137‐45. [Google Scholar]
Zhou 1997
- Zhou JK, Liu C. Thrombin inhibitor—hirudin. Chinese New Drugs Journal 1997;6(3):186‐7. [Google Scholar]
Zhou 2007 a
- Zhou J, Guo JT. Clinical study of Tongxinluo on preventing re‐stenosis of stents in CHD. Hebei Medicine 2007;13(10):1188‐91. [Google Scholar]
Zhou 2011
- Zhou ZR, Tang HQ, Li JH, Yang LL, Hu H. Tongxinluo capsule for coronary heart disease: a systematic review. Chinese Journal Of Evidence‐Based Medicine 2011;11(9):1078‐83. [Google Scholar]
Zhu 2004
- Zhu QL. Study on Tongxinluo capsule in preventing and treating coronary restenosis after PTCA. Journal of Shandong University of Traditional Chinese Medicine 2004:CNKI:CDMD:2.2004.102498.
Zhuo 2008
- Zhuo Q, Yang X, Wu T, Liu G, Zhou L. Tongxinluo capsule for acute stroke. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004584.pub2] [DOI] [PubMed] [Google Scholar]


