Abstract
Spinal cord injury (SCI) is a complex, multifaceted, progressive, and yet incurable complication that can cause irreversible damage to the individual, family, and society. In recent years strategies for the management and rehabilitation of SCI besides axonal regeneration, remyelination, and neuronal plasticity of the injured spinal cord have significantly improved. Although most of the current research and therapeutic advances have been made in animal models, so far, no specific and complete treatment has been reported for SCI in humans. The failure to treat this complication has been due to the inherent neurological complexity and the structural, cellular, molecular, and biochemical characteristics of spinal cord injury. In this review, in addition to elucidating the causes of spinal cord injury from a molecular and pathophysiological perspective, the complexity and drawbacks of neural regeneration that lead to the failure in SCI treatment are described. Also, recent advances and cutting-edge strategies in most areas of SCI treatment are presented.
Key Words: Combination therapy, Neural tissue engineering, Neuron, SCI, Spinal cord injury
Introduction
Spinal cord injury (SCI) is defined as impairment in the brainstem/cortex and spinal neurons between the ascending and descending neural pathways, leading to a loss in sensory-motor functions.1
The spinal cord is a tubular network comprised of nervous tissue which prolongs from the brainstem and keeps distally before narrowing at the lower thoracic or upper lumbar zone as the conus medullaris. The surrounding cerebrospinal fluid (CSF) protects the spinal cord. The spinal cord length in humans is about 45 cm in males and 43 cm in females. The spinal cord covers the cervical, thoracic, lumbar, sacral, and coccygeal segments by a total of 31 nerve root segments. The gray and white matter organization of the spinal cord can be seen in cross-sectional view.2
SCI is one of the most severe conditions in orthopedic/spine surgery. The defeat of functional recovery after SCI is primarily caused by the axons' poor regenerative ability, formation of scar tissue through an inhibitory environment, and demyelination around the lesion site that delays axonal repair.3,4
Spinal cord disorders may include: infection (e.g. meningitis), vascular injuries, developmental anomalies, tumors/malignancies, herniation, syringomyelia, transverse myelitis, degenerative conditions, and traumatic injuries (compression, hemisection, and complete section). Traumatic injuries are the most common, accounting for nearly 90% of all spinal cord injuries and frequent consequence of traffic accidents, violence, falls, and sports injuries. They can have devastating effects on the patient's life. Lower thoracic damage leads to paraplegia, while lesions in the cervical area lead to quadriplegia.5-7
About 265,000 people in the United States live with SCI, and 12,000 new cases occur each year by accidents, sports, or falls. The estimated annual cost for SCI
(including the medical costs and lost productivity) totals $20 billion. Enormous emotional, social, and economic costs demand for treatment approaches.8,9
Over the past decade, the age and sex standardized incidence of traumatic SCI (TSCI) has remained stable at 26.5 cases per million population (68.3% males, and mean age 59.2 years). The incidence has been age-related and associated with a female-to-male ratio. Furthermost, TSCIs have been cervically involved (52.1%) and motor vehicle accidents and work related injuries have been the most common reasons for injury (29.9% and 29.8%, respectively). Gender and cause of trauma or hospitalization were not related with an inflated risk of death. The mortality rate was higher for cervical lesions, increased with age, and remained consistently higher in older subjects.10-12 Patients with SCI are at increased risk of cardiac events, thromboses, extreme hypertension, respiratory depression, osteoporosis, and neuropathic pains as well as anxiety and depression.13,14
As a part of the central nervous system (CNS), the spinal cord links the brain to the peripheral nervous system. The spinal cord mainly includes neurons and several types of glia such as the astrocytes, microglia, and oligodendrocytes as well as a small population of endogenous stem cells, oligodendrocyte progenitor cells, and ependymal cells.15
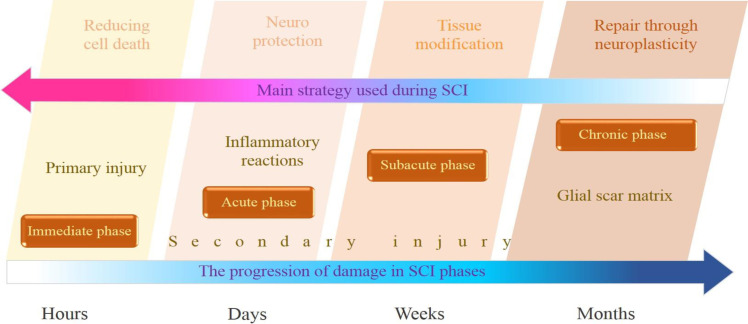
Glial cells are the numerous considerable cells in the nervous system, playing important roles in maintaining the blood-brain barrier, neuronal survival, synapse formation, strength, and turnover. Star-shape astrocytes help to keep the resilience of the chemical environment in the spinal cord. Oligodendrocytes myelinate the axons in the central nervous system, enable rapid transmission of nerve impulses, and contribute to axonal integrity.16 Microglia are the immune cells in the CNS. Experimental SCI models have proven the activation and proliferation of microglia in the injured spinal cord.17 Injury progression in SCI can be organized by the natural history of the disease, comprised of immediate (hours), acute (days), subacute (weeks to months), and chronic (>6 months) stages. This progress is mostly in line with the main approaches that can be used in this timeframe such as reduced cell death (immediate), enhanced neuroprotection (acute), tissue remodeling (subacute), and repair via neuroplasticity (chronic).18 Figure 1 indicates the injury progression of SCI [Figure 1].
Figure 1.
Injury progression in spinal cord injury can be organized into immediate, acute, subacute, and chronic phases, including the main strategies used in SCI during this timeframe. Each phase presents unique challenges and opportunities for recovery
Different types and stages of SCI are detected by using various tools such as radiographic investigation, magnetic resonance imaging (MRI), electrophysiological evaluations, and biomarkers. Biomarkers in SCI conditions are secreted into the serum or cerebrospinal fluid (CSF).19,20 MRI is the most helpful imaging modality for spinal cord lesion. It allows the imaging of gray and white matter surrounding CSF, as well as the traumatic, ischemic, and hemorrhagic lesions.21 The prognosis of spinal cord injury is primarily determined by the extent of initial neurological damage. Neurological recovery is not significantly influenced by factors such as the fracture type, use of steroids, or signal changes observed on MRI scans.22
Knowledge of the causes of spinal cord injury and associated factors is critical in the development of successful prevention programs.
Main body
Pathophysiology of SCI
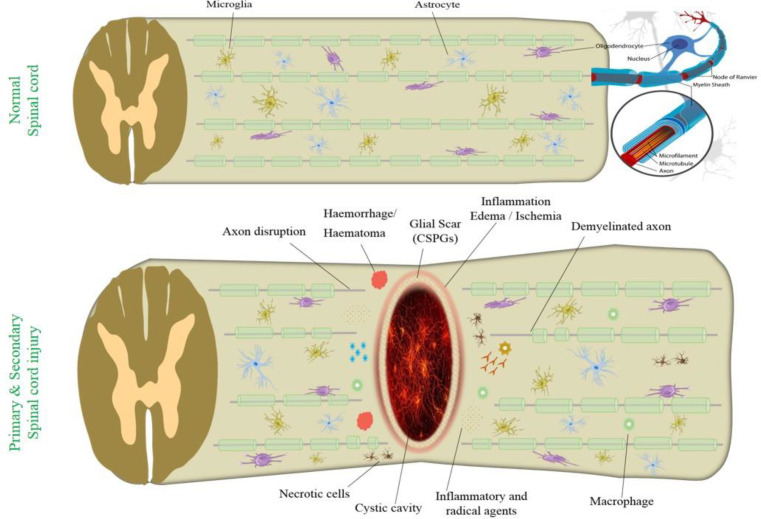
The pathophysiology of SCI consists of a two-phase process: the primary mechanical injury and the subsequent cascade of auto-destructive damages. Mechanical trauma quickly causes axonal damage, neural cell death, demyelination, blood-spinal barrier disruption, and extracellular matrix (ECM) degeneration, leading to a cascade of secondary injury, which extends the further inflammatory reaction at the lesion site, finally causing a cystic cavity formation.23 The primary mechanical injury of the spinal cord is followed by a secondary phase of injury with edema, ischemia, vascular dysfunction, excitotoxicity, inflammation, electrolyte shifts, free radical production, and postponed apoptotic cell death.24,25
Increased autophagy activity was detected in neurons, astrocytes, and oligodendrocytes at the lesion zone. However, it has been shown that programmed cell death plays important roles in the pathological process of SCI.26 The sequential events involved in the pathophysiology of SCI, highlighting both primary and secondary injury mechanisms are illustrated in [Figure 2].
Figure 2.
Pathophysiology of primary and secondary injury during spinal cord injury. Primary and secondary injury mechanisms lead to inflammation, hemorrhage, demyelination, axon disruption, apoptosis, and necrosis. CSPGs (chondroitin-sulfate proteoglycans)
Various pathological processes occur in the secondary phase of SCI injury, including augmented cell permeability, apoptotic signaling, ischemia, inflammation, vascular damage, demyelination, edema, lipid peroxidation, free radical formation, Wallerian degeneration, fibroglial scar, and formation of cyst that endures for several weeks following the primary injury phase. Hemorrhage is caused by disruption of blood vessels followed by an invasion of neutrophils, monocytes, T and B lymphocytic cells, and macrophages into the spinal tissues [Figure 2]. This condition is due to the release of inflammatory cytokines such as interleukin (IL)-1a, IL-1b, IL-6, and tumor necrosis factor (TNF)-α 6–12 hours after injury. Attack of inflammatory cytokines and immune cells promotes neuronal inflammation.27,28
The regenerative capacity of the CNS is confined in functional recovery during the chronic phase of SCI. After spinal cord injury, innate neural stem cells differentiate into astrocytes instead of neurons, leading to the formation of glial scars. IL-6 and IL-6 receptor expression are greatly increased during the acute phase after SCI, and IL-6 can be a potent inducer of neural stem cell differentiation into astrocytes.17 Regeneration after spinal cord injury is confined by the glial scar and inhibitory cell signaling pathways that promote scar formation over intrinsic neuronal regrowth.29
Repair and regeneration occur in response to injury, whereas the scar tissue formation complicates these processes. During the subacute phase of SCI, the fibrous scar composed of fibroblasts and the glial scar composed of astrocytes are formed. These scars secrete numerous chemical inhibitors that also physically block axonal regeneration.30
Despite the benefits of glial scar in injury prevention, it hinders neuronal regrowth. The ECM produced by the scar corresponding cells contains axonal growth inhibitors such as chondroitin-sulfate, proteoglycans, collagen, and fibronectin. This is one reason for limited neuronal regeneration after SCI.31
ECM proteins, including collagen are degraded by proteinase enzymes which can be referred to as matrix metalloproteinases (MMP).32 The activity of various proteinases in progression and treatment of SCI are still unclear. Improving the efficacy of neurological recovery after SCI has always been challenging. Effective therapy for SCI would decrease the damage of features and extend local glial scars to stimulate axonal regeneration; it also mitigates secondary effects such as inflammation, apoptosis, and necrosis.33 Understanding these complex processes is crucial for developing effective therapeutic strategies aimed at minimizing the secondary injury and promoting neuroprotection and regeneration in patients with spinal cord injuries.
Therapeutic Strategies of SCI
Currently, there is no ultimate cure for SCI. Although extensive research has been performed on spinal cord injuries, no effective treatment has yet been found to restore motor and sensory function. Nonetheless, significant advances in managing and caring for patients with SCI have significantly reduced their mortality rate. Current treatments that are considered to improve SCI outcomes include medicinal therapy, surgery, and rehabilitation.34
The spinal cord exhibits limited self-repair capacity after injury. Pharmacological treatments such as neuroprotective and anti-inflammatory agents are recommended during the primary stages of injury and inflammation. Decompressive surgery can remove discs or bone rudiments to protect cells and tissue from further damage.35
Pharmacological Therapies
There is a lot of debate regarding pharmacological management and potential effects of corticosteroid use. In particular, the efficacy of steroids such as methylprednisolone as a scavenger for free radicals that reduces the inflammatory response has been controversial and is still intensely debated for dosage and time to administration after spinal cord injury.36
Excitatory neurotransmitters in the spinal cord can be directly influenced through NmethylDaspartate (NMDA) receptors. For instance, blocking the NMDA receptors in animal models has a preserving effect on trauma and ischemia due to the secondary injury. Furthermore, NMDA antagonists can diminish edema and enhance neurological functions.37 The motivation of NMDA and non-NMDA receptors may play an influential role in excitotoxic damage after injury. NMDA receptor antagonist such as magnesium decrease inflammatory and toxicity effects. Magnesium ions can diminish edema and vascular permeability and also reduce lipid peroxidation in SCI.38,39
Following SCI, elevated levels of systemic inflammation markers including the C-reactive protein (CRP) and IL-6, have been demonstrated. Non-steroidal anti-inflammatory drugs (NSAIDs) reduce inflammation by suppressing prostaglandins that deliver through the cyclo-oxygenase (COX) enzyme pathway. This enzyme plays a key role during the inflammatory early phase of SCI. Ibuprofen may have safety and efficacy for treatment of various outcomes in chronic SCI in human.40
Lately, investigations on the impact of NSAIDs in SCI animal models have noted improvement in controlling secondary damage, contributing to fiber sprouting and functional recovery. Administration of RhoA-inhibiting NSAIDs after traumatic SCI is associated with axonal myelination in white matter tracts. Nonetheless, naproxen, a non-RhoA-inhibiting NSAID, has revealed no such effect on locomotor function.41,42
Ion channel antagonists such as calcium channel blockers like nimodipine, methylprednisolone, dextran, and sodium channel blockers can improve the functional results and neuroprotection of the white matter. Blocking the potassium channels is also a possible therapeutic target for treating SCI. For example, in axonal regeneration, 4‑aminopyridine (4‑AP) has exhibited positive effects.37 Infusion of high-dose methylprednisolone for 24-hour in adult patients within eight hours of acute SCI has been identified as a treatment option.43
SCI pain is a complex multifactorial object which significantly influences the health and quality of life of the affected patients. The use of non-opioid and non-invasive pharmacologic medications such as tramadol, lidocaine, cannabinoids, topiramate, selective serotonin, norepinephrine reuptake inhibitors, pregabalin, gabapentin, and tricyclic antidepressants in the treatment of chronic SCI-related pains has been documented. Further investigations are needed to elucidate the place of calcitonin, lithium, and marijuana.44
Surgical Therapy
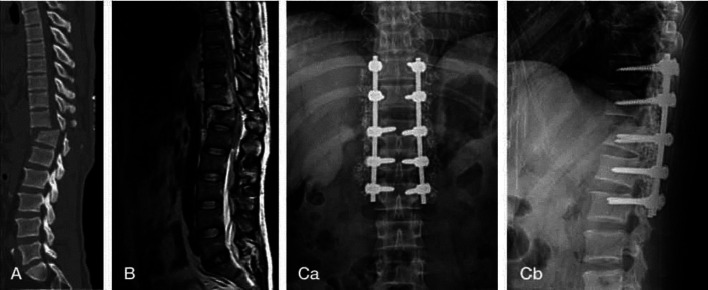
Current therapies for SCI include pharmacological treatments and surgical interventions to prevent further damage. Spinal decompression surgery is the most important surgical intervention within the first 24 hours after the injury that improves six-month outcomes, regardless of the level of SCI.45 The impact of surgical timing in neurological recovery in SCI is still a subject of discussion.Several studies support the advantages of emergency (<8 hours) decompression for patients with traumatic SCI in terms of neurological recovery.46 Conversely, a meta-analysis has shown no significant beneficial effects for early decompression surgery (within 24 h) in patients with thoracolumbar and thoracic SCI.47 In the latest pooled analysis, decompression surgery within 24 h of acute SCI has been correlated with amended sensorimotor recovery. The first 24-36 hours after injury represents a critical time frame for achieving optimal neurological recovery with decompressive surgery after acute SCI. 48 [Figure 3].
Figure 3.
Surgery on compression fractures in T12-L1of the spine of 45-year-old man who become complete paraplegia following crushing injury. Steroid administration was done, and operation was done within 24 hours. Shows no neurologic recovery (ASIA impairment scale A to A). (A) CT: Fx. & D/L at T12-L1 (B) MRI T2: No significant signal intensity changes observed (Ca,b) Open reduction & interal fixation, T10-11-L1-2. Figure based on data originally published in. 22 Fx: Fracture; D/L: Dislocation; ASIA: american spinal injury association scale
Surgical repair of nerve injury is an urgent medical need and a major clinical challenge. Autologous nerve grafting remains the gold standard for filling the extensive gaps in transected nerves. For nerve transection injuries, direct suture without grafting is the gold standard therapy for peripheral nerves with a short gap (<5mm). Unified repair with fibrin glue is the alternative choice to reduce the recovery time. For nerve conduction recovery, fiber alignment and axonal regeneration have no differences between suturing and sutureless repair using fibrin glue.49 Larger defects need autologous sensory nerve grafting. In long-gap injuries (>3 cm), autografts indicate notable recovery results. Bridging the nerve gaps with acellular nerve allografts is a feasible therapy, while operation for lower limb repair has caused the poorest results. Allografts have been found advantageous for reducing pain and restoring a functional level of sensation in chronic patients.50 A combination of surgical intervention with other methods can be improved in SCI treatment.
Surgical intervention combined with weight-bearing locomotor training in 320 patients with clinically complete SCI at one month post-injury has been reported to promote neurological recovery.51 For chronic SCI treatment, epidural spinal cord stimulation (eSCS) has been admitted as a recent approach.52 Implanted spinal neuromodulation approaches are operated in chronic pain treatment. Surgeons implant electrodes around the dorsal root ganglion or spinal cord and a pulse generator unit under the skin. Electrical stimulation reduces the intensity of pain. SCS is associated with difficulties such as infection, need for re-implantation, and electrode failure or migration. There are minor significant evidence about SCS benefits on pain intensity compared to placebo stimulation.53
The field of spinal surgery is slowly developing in various directions, such as bioelectrical impedance signals, imaging signals, force signals, etc. Nonetheless, current techniques still have some drawbacks. The anatomy of the spinal system is complex and has unique features. Also, various surgical methods, instruments, paths, surgical speeds, and other elements eventually affect tissue recovery after the operation. Ultimately, no relevant insight technology applicable to clinical practice is currently available. Future perspectives in spinal cord research should focus on learning about precise methods, so that valuable data is obtained intraoperatively through tissue condition.54,55
Acupuncture, Exercise and Massage Therapy
Acupuncture is a simple and inexpensive method needling through the skin into deeper tissues at precise sites (acupuncture points) for therapy or to prevent disease. A growing number of clinical researches have revealed that acupuncture and electro-acupuncture can effectively progress recovery of sensory and motor functions in patients with various types of CNS injuries including SCI.56 By inhibition of inflammation, oxidation, and apoptosis, acupuncture can be effective in neuroprotection for SCI-induced neurologic deficiencies. Furthermore, acupuncture stimulates axonal sprouting and nerve regeneration by activating numerous cell signaling pathways such as the Rho/Rho kinase (ROCK), Notch, and Wnt.57 In traditional Chinese medicine, Governor Vascular Electro-Acupuncture (GV-EA) combines acupuncture with modern electrical stimulation. It can protect the microenvironment of damaged neurons, decrease inflammation, and stimulate myelination by promoting the levels of endogenous neurotrophic factors.58
Patients suffering from neurologic traumas such as spinal cord injury have reduced activity and risk for secondary health conditions that alter the body compositions such as decreased bone density, reduced lean body mass, and increased fat mass indices.59 Moderate-to-intense exercise required for fitness improves the cardiac health in patients with SCI, given the international harmonization of exercise guidelines.60 Exercise therapy after spinal cord injury has various effects ranging from prevention of apoptosis, circuit formation, axonal sprouting, shifts in chloride homeostasis, to many further changes that may associate with neuronal repair and functional recovery.3
Recent testimony suggests that cycling exercises with functional electrical stimulation may improve the health of lower-body muscles and power output in SCI patients.61
Massage therapy and relaxation are both active treatments that provide potential clinical benefits to reduce pain and fatigue in adults with chronic SCI.62 Though massage therapy is recommended for neuropathic pain treatment but there is no convincing evidence proving its efficacy in SCI-related pain.63 Hydrotherapy offers a valuable rehabilitation tool for patients with SCI, promoting movement facilitation, physical and cardiovascular exercise, and overall body relaxation.64
Low-level laser (LLL) therapy as a non-invasive method has been suggested to regulate inflammatory processes, resulting in influential progress in neurological symptoms after SCI, neuroprotection, and restoration of motor function.65-67
Pharmacological and surgical interventions as well as physiotherapy have shown limited nerve regeneration and functional recovery in comparison with stem cells medication in the treatment of SCI.68
Cell Therapy
Cell therapy is a favorable treatment for SCI. It probably harmonizes multiple mechanisms such as immunomodulation and neuroprotection by trophic factor release, as well as axon and myelin regeneration to promote functional recovery after SCI.69 The principal ideal of cell transplantation therapy is the regeneration of glial cells and neurons that survive cell death after SCI. Cell therapy has been an effective therapeutic strategy for SCI by promoting motor functions in several animal models. The transplanted cells have multiple actions: promoting axonal elongation and lessening retrograde axonal degeneration by secreting neurotrophic factors; reconstructing neural circuits by forming synapses between host and graft-derived neurons; and prompting regeneration and plasticity in the injured spinal cord by enhancing remyelination of damaged axons.70 Issues about cells number and type employed, as well as the safety of methods, stay to be addressed.71
Regenerative cell therapy for SCI uses various cell sources including the embryonic stem cells (ESCs) and ESC-derived oligodendrocyte progenitor cells (OPCs),72 hematopoietic stem cell (HSC),73 mesenchymal stem cell (MSC),74 neural stem cells (NSCs),69 induced pluripotent stem cells (iPSCs),75 glial cells,76 olfactory ensheathing cells (OECs),77 schwann cells (SCs),78 and stem cell derived extracellular vesicles (EVs).79
Stem cells can repair dysfunctional or damaged tissues by administering therapeutic agents.80 As a topic of growing clinical research, stem cell-based therapy is promising for SCI to have multiple targets and reactivity profits. To date, most data from Phase I and Phase III clinical trials demonstrate that injection of stem cells into the spinal cord is safe with minimal side effects.81
The progress in mesenchymal stem cell (MSC) therapy has practically advanced in SCI animal models. Cell therapy with intravenous infusion of MSCs improves the functional outcomes in SCI. This therapy proves the practicability, safety, and functional gains of transplanted MSCs in SCI patients. The potential mechanisms include repair of blood-brain/spinal cord barrier, induction of axonal sprouting, remyelination, immunomodulation, neuroprotection, anti-inflammatory properties, neuro-nutrients release, and promoting vascular regeneration.82,83 NSCs and MSCs can be engineered, cloned, and transplanted. Cell therapies for SCI have been performed using various methods from direct injection itto the epicenter of spinal cord lesion to intravenous administration. Many registered trials design to deliver cells intrathecally, but nearly one third of the studies delivered the cells to the spinal cord parenchyma or around the injury site. The frequencies of cell therapy routs have been reported as: intrathecal (35%), intramedullary (33%)(including 76% cellular injection and 24% cellular biomaterials), multiple routes (14%), intravenous (10%), and undetermined (8%).84 Adipose-derived mesenchymal stem cells (ADMSCs) have a superior potential therapeutic strategy for SCI. Also, transplantation of higher densities (≥106) of stem cells has shown better therapeutic effects. Moreover, intralesional stem cell transplantation in the subacute phase has been reported to be the optimal route and timing.85
Transplantation of MSCs as an immunomodulatory approach has been shown in recent years. MSCs in the secondary phase of SCI can regulate neuroinflammation via macrophages, astrocytes, and T lymphocytes and form a microenvironment that supports tissue repair and regeneration.86 MSC therapy's effectiveness in SCI results in the secretion of soluble factors and alteration of ECM microenvironment that supplies neural protection. MSCs secrete neurotrophic factors such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), vascular endothelial growth factors (VEGFs), transforming growth factor beta (TGF-β), and hepatocyte growth factor (HGF). These factors have beneficial outcomes on axonal elongation, glial and neuronal survival, and angiogenesis.70
Axonal regeneration may be promoted by grafted stem/progenitor cells through cellular scaffolds. Due to improving the efficacy of cell transplantation strategies for SCI, combinatorial approaches suggested using a polymer scaffold to fill the lesion cavity. 87 However, traditional cell transplantation approaches retain some drawbacks such as stem cell survival rate and unsatisfying homing, which cause their random distribution from the injection site to surrounding tissues.
Tissue Engineering Strategies
The pharmacological strategy for SCI offers dissatisfactory outcomes; furthermore, the clinical approach of employing nerve autografts for injury suffers from a tissue deficiency. As an alternative, tissue engineering is a hopeful strategy for regenerating the spinal cord and peripheral nerves. 88
Biomaterial scaffolds can restrain the viability of stem cells and promote their therapeutic results in lesion sites. 89 Biomaterials as a filler or mechanical stabilizer reconstitute ECM and supply a condition for transduction with host cells in SCI injury sites. They can bridge the cavities to guide axonal sprouting across the gap. 90 Several biomaterial architectures including fibers,91 channels,92 conduits,93 scaffolds,94 and magnetic microgels95 have been investigated to guide neural regeneration and nerve extension.
Among novel strategies for SCI treatment, biomaterials such as hydrogels can supply trophic and physical support to the injured spinal cord and also can regulate the localized delivery of the immunomodulatory components. 96
Brain and spinal cord have the weakest resistance in human tissues. Mechanically mismatch between the graft and the spinal cord soft tissue may result in fibrotic tissue or cysts, inhibiting the tissue repair. Also, inflexible biomaterials fails to adapt to the shape of the lesion appropriately. The mechanical characteristics of the biomaterial scaffold affect the cells phenotype and function, and plays an significant role in axonal growth and elongation necessary for effective integration into the surrounding tissue. 97
Neuronal cultures on soft constructs have shown increased branching and neurite progress; conversely, stiffer constructs enhance astrocyte growth. Rigid gels induce elongated primary dendrites but shorter axons in spinal cord neurons. After CNS injuries, tissue softening might associate with the failure of neuronal regrowth. 97 In particular, in order to guide the axons across the injury, the microarchitecture of the engineered scaffold must mimic the native ECM with aligned or parallel channels, pores, or conduits. 88,98
Recently, there has been progress in the treatment of SCI with the use of both cell-laden and cell-free scaffolds. Scaffold-based tissue engineering through guiding and sustaining neuronal regeneration is going to be extensively used for SCI treatment. Nonetheless, improving the ability of axonal regeneration following SCI remains a challenge. 99
The cell type, size, morphology, and distribution may differ wildly between different parts of the CNS. Aging, development, and pathology may alter these compositions. The gray matter is particularly stiffer than the white matter, however both become hardened with increasing pressure. The white matter stands isotropic under tension and crosswise isotropic under compression, while gray matter remains anisotropic and regionally heterogeneous. 100
SCI Treatment depends on the knowledge of mechanisms that impacts axon regeneration and environmental drawbacks to design beneficial therapies. 101
Currently, natural, synthetic, and combined materials are utilized to manufacture biomaterial scaffolds based on their different characteristics for SCI restoration. Natural materials are widely used for their benefits, such as low toxicity, suitable biocompatibility, biodegradability, cell-cell interactions, and many biological functions. Collagen, hyaluronic acid, chitosan, fibrin, agarose, gelatin, alginate, and self-assembling peptides are the familiar natural materials used in SCI repair. Synthetic materials used as scaffolds in neural regeneration have a variety of benefits, comprising low inflammatory reaction, customized physicochemical and biomechanical properties, defined biodegradability, low toxicity, and controllable porosity and pore size. Synthetic polymers can be blended for use as a new style of biomaterial with unique features. Various biocompatible polymers such as polycaprolactone (PCL), polylactic acid (PLA), poly lactic-co-glycolic acid (PLGA), polyhydroxybutyrate (PHB), polyethylene glycol (PEG), poly(2-hydroxyethyl methacrylate) (pHEMA), and poly(N-(2-hydroxypropyl)methacrylamide (PHPMA) have been employed in the restoration and treatment of SCI. 91-93,102-104
Graphene and graphene-based composites can maximize electrical signal stimulation due to their good conductivity, so, they are used in spinal cord tissue engineering to facilitate nerve regeneration. In addition, they can be operated as carriers for cells, trophic factors, and drugs. They may also have cytotoxic properties, raise oxidative stress, and impede pulmonary vessels. 105
Other investigations have demonstrated that injectable ECM-mimetic hydrogels help or improve axon regeneration via ECM remodeling. They can be directly injected into targeted sites or tissues through minimally invasive methods to escape secondary damage. These hydrogels have shown mechanical and conductive properties comparable to natural spinal cord tissues and have been suggested as ideal biomaterials for traumatic SCI treatment. 106 Self-healing injectable hybrid hydrogel can closely mimic natural ECM at the injury spot to restore axons and cells. They have been fabricated from various materials (e.g. laminin and chitosan) with conjugates and encapsulated exogenic factors capable of constant released to promote neurite outgrowth and simplify functional recovery. 107
Hydrogels derived from native neural tissue materials are promising for neural tissue engineering as they retain native biochemical cues. Decellularized nerve tissue matrix has shown remarkable features in promoting neural tissue regeneration. Injectable hydrogels composed of decellularized rat nerves have reported to support viability and metabolic activity of Schwann cells and astrocyte spreading. Also, ECM containing scaffolds are promising drug delivery vehicles for neural injury combination therapy approach. 108
A comparative analysis showed that decellularized spinal cord matrix (DSCM-gel) engaged an ECM-like structure and demonstrated more increased porosity than decellularized peripheral nerve matrix hydrogel (DNM-gel). NSCs have more potential viability, proliferation, and migration in 3D cell culture, enabling differentiation into neurons and synaptic formation. Moreover, DSCM-gel in complete transected SCI bridges the lesion site that is proper for axonal regeneration. 109
Electroconductive and magnetic components can be incorporated into scaffolds. Using of electromagnetic and electroconductive materials may allow cell proliferation, differentiation, and migration and guide neurite growth. Anisogel is an injectable hybrid magneto-responsive hydrogel with oriented fibers that induce unidirectional growth of nerve cells in the direction of an external low magnetic field by spontaneous electrical activity with calcium signals spreading along the anisotropis axis of the material. 110 Graphene crosslinked collagen as a cryogel is porous and electroconductive, regulating astrocyte sensitivity and improving the ratio of M2/M1 polarization macrophages. 111 Electrospinning micro/nano fibers have been increasingly used in SCI therapy due to their large specific surface area, complex porous structure, and biocompatibility. Multichannel fibers simulate nerve bundles and guide axon growth. Nanofibrous constructs can be used as carriers loaded with drugs, growth factors, and cells. Fibers with conductive polymers are capable of electrical stimulation of nerve function. 112 For instance electrospun PCL/ Polysialic acid (PSA (nanofiber hybrid mats encapsulated with methylprednisolone has been developed for SCI treatment. 99
An ideal scaffold for SCI application should satisfy many biochemical and biomechanical properties such as biocompatibility, proliferative and differentiative effects, noncytotoxicity, nonantigenicity, flexibility, degradability, sufficient biomechanics, conductivity, and suitable porosity and pore size with good integration into the host tissues. In addition, scaffolds in nerve tissue engineering need to supply an anisotropic network corresponding to the native ECM to grant cell orientation and conducting channels or pores for axon growth and reconnection. 88,113
In vitro and in vivo research regarding spinal cord and anisotropic peripheral nerve scaffold results have promoted knowledge of nerve regeneration. However, the reaction of cells at genetic and molecular levels to these topographical cues needs to be clarified. There still needs to be clinically useful scaffolds. This deficiency is because the nerve tissue has inherent complexities concerning the sensitivity, vascularization, exogenous biochemical molecules, and electrical incitement. 88
Advanced Three-dimentional Bioprinting for SCI repair
Three dimensional (3D) bioprinting has been developed as a beneficial method for fabricating precise complex living neural architecture with spatial allocations of many types of cells for SCI repair. 114 The strategy of combining and printing cells and affiliated cytokines at the same time has slowly improved neural regeneration. 3D bioprinting technology has a specific potential for personalizing the regeneration of the nervous system and SCI. 115
Hydrogels utilized as bio-ink can be assembled with synthetic and natural biopolymers. Natural biopolymers including collagen, chitosan, hyaluronic acid, gelatin, alginate, agarose, fibrin, and synthetic polymers such as PCL, PLA, Poly-glycolic acid (PGA), PLGA, and PEG are broadly operated in neural tissue engineering. 116
Different cell types have been used for 3D bioprinting nerve constructs to promote neural regeneration. These cell types include Schwann cells, neural stem cells (NSCs), iPSCs, olfactory ensheathing cells (OECs), Human fibroblasts, oligodendrocyte precursors, and mesenchymal stem cells (MSCs). Printed viable cells can be contained in the bioprinting medium (bio-ink) or implanted into the construct. 117 Stem cells or NSC-laden 3D bioprinting still encounter major challenges including minimal cell-material interaction, poor cell viability, and unmanageable printing process. 114
While bioprinting has numerous benefits, it still meets multiple challenges. To date, only a few distinct cell types and scaffold models have been examined in spinal cord bioprinting. Common drawbacks of bioprinting technology include high viscosity, low cell density, poor mechanical properties, and small nozzle dimensions and flow rates that limit the collected bioink volume per drop. Bioprinting strategy for spinal cord tissue engineering has not yet overcome the limitations around immunosuppression, inflammation, and vascularization, providing many areas for development. 115
Up to date, summary of SCI studies shows that rats were the furthermost species employed for animal models of spinal cord injury and complete transection was the generally operated injury pattern. In most studies, immediate intervention after injury has been leaded, and eight weeks has been the standard final time point for result investigation. A broad spectrum of natural and synthetic biomaterials with various structures including scaffolds, hydrogels, and particles have been utilized as a part of the tissue engineering approach for SCI.
Alterations in biomaterials due to more functionality and combination with cells and biomolecules can effectively fabricate microenvironments to repair SCI in preclinical animal models. 118
Recent advances in the clinical management of patients with SCI have greatly improved their prognosis, survival rate, and quality of life. Also, substantial progress has happened in basic science research around cellular and molecular events of SCI, facilitating the development of pharmacologic agents, stem-cell based therapy, and tissue engineering. Despite these efforts, there is still no definitive therapy to restore the function of silent axons and SCI nerve regeneration. These challenges have led to an increased focus on alternative therapeutic approaches. 119-121
All innovative therapeutic strategies target secondary damage of spinal cord injury, while there are no therapeutic strategies for neurological alterations caused by primary injury. 41 Suppression of the secondary neurodegeneration damage processes is a potential approach for SCI treatment. Current treatments seem ineffective due to the complexity of generating and the outcomes of spinal cord injuries; hence, it is better to look at new treatments. Modern strategies may be considered a complement to previous treatments. Multiple approaches are needed to achieve satisfactory outcomes.
Emerging strategies and future direction for SCI treatment Biochemical strategies
Glial reaction at the injured area form glial scars are primarily comprised of activated astrocytes and express considerable chondroitin-sulfate proteoglycans (CSPGs), which cause axon growth cone collapse and avert of axons from sprouting across the injury site. 122 Failure of axonal regrowth is a significant barrier to treating adult CNS injuries, and proteoglycans are strong inhibitory cues. Furthermore, CS and keratan sulfate (KS) chains are linked covalently to some proteoglycans. The proteoglycan degrading enzymes promote functional recovery after SCI, such as approaches operating KS knockout in mice and KS-degrading enzyme, keratanase II (K-II) and chondroitinase. 17
CSPGs in glial scarring are known as powerful inhibitors of neuronal regeneration. Chondroitinase ABC (ChABC), a bacterial lyase enzyme, degrades the glycosaminoglycan (GAG) side chains of CSPGs and stimulates spinal cord tissue regeneration. However, the weakness of ChABC is its thermal instability and failing all activities at 37°C within a few hours. Various groups of copolymers assembled and stabilized ChABC at physiological temperature has been reported, which is a profitable pathway toward supported neural tissue regeneration. 123
Removal of CSPGs with ChABC from the defect site has resulted in enhanced sensory recovery, axon sprouting, and recovery of neural functional properties after SCI. 124 The efficacy of ChABC as a treatment for SCI has proved in pre-clinical models. 125 ChABC for SCI treatment has not been applicable in clinical trials until now. However, this lysis enzyme was evaluated in phase III clinical trial in Japan as an alternative to surgery for lumbar disc herniation. This indicates the beneficial use of ChABC for treating SCI in humans. 101 This enzyme therapy strategy may be helpful for the clinical treatment following spinal cord injury combining chondroitinase with other strategies known to promote neural recovery. 126
Cellular strategies
Cell therapy seems potential for repairing the human spinal cord. However, current clinical approaches are challenging, including systemic or direct delivery of transplanted cells into neural tissue. Moreover, confirming efficacy studies, meeting strict regulatory standards and establishing tolerable long-term budgets are critical challenges that remain for cell therapy trials in this field. 84,127 MSCs have emerged as promising carriers with various advantages. 128 Paracrine effects of MSCs, small molecules, drug delivery, and employing neurotrophic factors are remarkable strategies to overwhelm graft rejection. 15 Alleviated neuropathic pain after remyelination and induced practical recovery and neuronal regeneration have been accomplished with peripheral nerve-derived stem cell spheroids. 129
The glial and neural cells are mechanosensitive; therefore, engineered systems hold to design and prepare mechanotransduction platforms. Mechanotransduction proves that neural regeneration is a new strategy for designing a future therapy. 130 Organ on-a-chip, organoids, and assembloids as human multicellular 3D models of the nervous system and spinal cord have recently been presented and developed for research and transplanted into animals. 131 Cell reprogramming technologies have been manipulated to generate fresh neurons after SCI. Astrocytes, fibroblasts, and NG2 glia have been reprogrammed into neurons. Also, astrocytes with NG2 glial cells are activated and organized nascent astrocytes. Therefore, reprogramming astrocytes into neurons is a prospective approach for repairing the injured spinal cords. 132
Molecular strategies
Growth factors influence the morphology of axons along the nervous system development and promote axonal sprouting and regeneration after injury. Furthermore, growth factors modulate the survival of neurons, neurite outgrowth, synaptic plasticity, facilitating myelination of regenerated axons, neurotransmission, and neuroprotective properties after SCI. Neurotrophic growth factors have been candidate medicines for SCI treatment through multiple roles in functional recovery after SCI. The role of various growth factors in spinal cord injury has recently been evaluated, particularly in animal models using brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), nerve growth factor (NGF), neurotrophin 3 (NT3) and neurotrophin 4/5 (NT-4/5), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF or FGF2), and insulin-like growth factor-1 (IGF-1). Growth factors are applied alone or cocktail and highlighted with a hydrogel releasing strategy besides this approach may overcome some barriers. For instance, overexpression of NT-3 and IGF-1 may promote motor function and reduce the occurrence of spasm after SCI. The presence of growth factors provides positive effects on axon regrowth. Delivery of growth factors to the injured spinal cord has been tested for many growth factors, delivery timing, and methods. However, more studies involving specific growth factors therapeutically for SCI are needed. 133-137
Granulocyte colony-stimulating factor (G-CSF) is a growth factor/cytokine exerts relevant CNS activities in particular after lesions, activates the differentiation of granulocyte colonies, and stimulates the migration of bone marrow-derived stem cells into the injured spinal cord. G-CSF seems to have the potential for antiapoptotic, anti-inflammatory, angiogenesis, myelin-protective, and axon-regenerative activities in acute, subacute, and chronic CNS lesions. Preclinical and clinical information suggests that G-CSF is safe and helpful for treating acute and chronic traumatic spinal cord injuries. 138 Using G-CSF for incomplete chronic spinal cord injuries is related to effective motor, sensory, and functional improvement. 139
Biomolecules are candidates for SCI treatment by modulating immune responses. Blocking the IL-7 receptor promotes the formation of M2 phenotype macrophages (anti-inflammatory and prohealing) and enhances recovery processes after experimental spinal cord injury in mice. Administration of IL-4 and IL-10 chemokines which stimulate macrophages to accept an M2-like phenotype in mouse models, has improved recovery after SCI. Using a chemokine, such as fractalkine, can recruits tending to repair monocytes and increase the regeneration of practical nerve faults in rats. 90
Determining specific mechanisms resulting from secondary injury is crucial to minimize tissue damage and improving neurological functions. Macrophage inflammatory protein 1-alpha (MIP-1-alpha) or Chemokine (C-C motif) ligand 3 (CCL3) can play a significant role in the CNS inflammation with affected in the recruitment of inflammatory agents. CCL3 is a potential target to regulate the inflammatory reactions and secondary damage after SCI. Regulating the inflammatory cascade in secondary damage after SCI by controlling proinflammatory cytokines and chemokines besides heterogeneity of neutrophils offers new therapeutic opportunities. 140,141
The mTOR pathway has an influential role in CNS juries. Chinese herbal medicine can improve the microenvironment and stimulate neural repair after SCI by impeding the SCI-activated mTOR pathway. 142
Evidence suggest that non-coding RNAs (ncRNAs), mostly microRNAs (miRNAs) and circular RNAs (circRNAs), are involved in the pathophysiology of nervous system disorders. Modulating circRNAs may promote angiogenesis, suppress inflammation, inhibit apoptosis, and regulate autophagy in acute CNS injuries.
Although circRNAs are associated with diverse biological processes and functions in diagnosing and treating neuronal processes, their functions in the SCI still need to be elucidated. Throughout sequencing and bioinformatics analyses, a novel circRNA, CircPlek, was identified that increased expression after SCI. These suggested factors decrease the inflammatory response at the spinal cord lesion and elevate functional motor recovery. 112,143 Exosomes as carriers of miRNAs indicated significant potential in the SCI treatment. The miRNA-modified exosome impacts were superior to exosomes alone in improving motor function scores in animal models with SCI. 144,145
The repair mechanism in spinal cord injury by astrocytes and non-astrocytes is organized by the concurrent expression of key genes. Analysis of 19 expression modules using 5216 differentially expressed genes found that miR-494, XIST, and other genes were individually expressed in SCI patients and positively regulated in dysfunctional modules shown to play a role. These genes have been identified as driving genes for SCI. 146
Next-generation transcript sequencing, single-cell RNA sequencing, SCI transcriptomics, and genomic-targeting techniques developments present a visionary for a better understanding of obstacles that inhibit or promote axon regeneration and functional recovery. 147
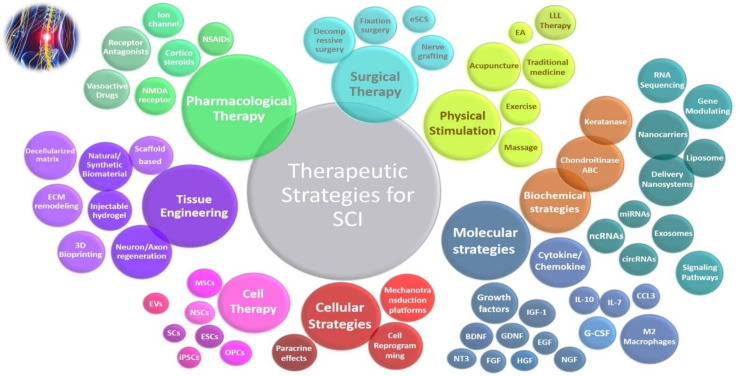
Novel strategies for spinal cord regeneration presented by delivery nanosystems such as metal-based nanocarriers (e.g. gold, maghemite), polymer-based nanocarriers (e.g. PLGA, polymer-nanoparticles, PLA, Chitosan), and liposome-based nanosystems (e.g. cationic liposomes with vitamin, liposome covered by a polymer) as well as other nanocarriers (e.g. nanogel-based nanovector, drug-loaded nanovesicles, drug loaded microsphere, encapsulated nanoparticles) can improve recovery time by targeting the localization, altering the signaling pathways, and cellular uptake. 148 Drug-loaded nanomotors technology.149 Local injection of nanoparticles into spinal cord injury can decrease the cavity sizes and may improve motor functional recovery in SCI in animal model. 150 Use of machine learning and artificial intelligence (AI) in spinal cord injury research and care. 151, 152 A summary of therapeutic strategies for SCI is shown in [Figure 4].
Figure 4.
Visual representation of therapeutic strategies for spinal cord injury: Larger circles denote higher importance or titles, with those closer to the center representing regular treatments. Cases located further from the central circle indicate recent treatments. Overlapping or closed circles symbolize related approaches and comprehensive coverage of therapeutic strategies
Combination therapy
Combination therapy has the potential to compile a variety of therapies such as combining neural stem cells, endothelial progenitor cells, and biomimetic hydrogel matrix therapy for induction of angiogenesis and neurogenesis in SCI model. 153 Exosomes secreted from stem cells evolve a research topic for their application as therapeutic agents. 154 Combination of electroconductive hydrogels with BMSC-exosomes have been established for the synergistic treatment of SCI. Exosome-loaded in electroconductive hydrogels can modulate M2 microglia polarization through the NF-κB pathway and synergistically improve differentiation of neural stem cells to neuron and oligodendrocyte whereas impeding astrocyte differentiation, even increasing axon extension via the PTEN/PI3K/AKT/mTOR pathway. 155 Motor function training can improve the expression levels of endogenous NT-3, NGF, and IGF-1 following the spinal cord injury, as well as inhibiting cell apoptosis that results in a more acceptable recovery of motor function. 134 Combination of MSCs transplantation and acupuncture may be a novel and effective strategy for treatment of SCI 57. Electrical stimulation of the proximal nerve in the transection site and surgical repair accelerates sensory and motor nerve regeneration. 156 Combining adult stem cell transplantation with electro-acupuncture (EA) seems to be a more promising strategy. 58 Epidural electrical stimulation of the spinal cord has shown motor function recovery. Multisite epidural electrical stimulation combined with gene therapy by triple genes (VEGF, GDNF, and NCAM) established a limited functional improvement for the treatment of SCI in rat models. 157
Multimodal treatment strategy of SCI including neural stem cell transplantation and magnetic or electrical stimulation combined with rehabilitation exercise, can improve nerve repair and regeneration. 158 Cell transplantation therapies for SCI should be combined with growth factors to satisfy tissue engineering, while environmental modification strategies using chondroitinase ABC, trophic support, and ultimately rehabilitation may promote neural plasticity and increase the efficacy of spinal cord injury regeneration. 159 Combination therapies are proposed as superior to promoting neuroprotection and neuroregeneration after SCI.
Clinical trial in combination therapy of SCI
Extensive research endeavors have resulted in clinical trials for several promising treatment alternatives, although currently no therapy can consistently restore the lost spinal cord functions. 160 Clinical trials play a crucial role in advancing the treatment options for SCI and improving the quality of life for individuals affected by this debilitating condition. These trials are essential for testing the safety and efficacy of new therapies, interventions, and medical devices specifically designed to address the complex challenges associated with SCI. A summary of clinical trials for combination therapy of spinal cord injury presented in [Table 1].
Table 1.
Summary of clinical trials for combination therapy of spinal cord injury
| NCT Number | Status | Conditions | Study Type | Phase | Interventions | Population | Study Design / Primary Purpose | Outcome Measures | Date(Study Start / Last Update) | Locations | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrollment /Age | Sex | |||||||||||
| NCT00291317 | Completed | Spinal Cord Injury | InterventionalClinical Trial | N/A | Device: RT 300-PFES Cycle |
6 / 4 Years to 21 Years (Child, Adult) | All | Single Group Assignment / Treatment |
Change in Pediatric Quality of Life/ Change in Bone Mineral Density | 2006 / 2012 | United States | |
| NCT01143597 | Completed | Spinal Cord Injury | InterventionalClinical Trial | N/A | Exercise: Arm and hand training |
48/ 16 Years to 70 Years (Child, Adult, Older Adult) | All | RandomizedParallel Assignment / Treatment |
Cortical motorexcitability via transcranial magnetic stimulation (TMS) |
2007 / 2013 | United States | |
| NCT01471613 | Completed | Spinal Cord Injury | InterventionalClinical Trial | I,II | Procedure: Conventional Treatment Drug: Lithium Carbonate Tablet, PlaceboBiological: Cord Blood Cell |
16 / 18 Years to 65 Years (Adult, Older Adult) |
All | Randomized,Parallel Assignment / Treatment |
Change from Baseline in AIS Motor and sensory scores Walking Functional assessment Locomotion Pain | 2011 / 2014 | China | |
| NCT01232907 | Completed | Spinal Cord Injury (SCI) | InterventionalClinical Trial | II | Drug: L-Carnitine |
2 / 19 Years to 65 Years (Adult, Older Adult) |
All | Randomized,Single Group Assignment / Treatment |
Fatigue, Pain, Depression will be completed by subjects biweekly | 2010 / 2014 | Canada | |
| NCT02098135 | Completed | -Stroke -Spinal Cord Injury |
InterventionalClinical Trial | N/A | Device: ArmeoSenso |
14 / 18 Years and older (Adult, Older Adult) | All | Single Group Assignment / Treatment |
Training Improvement in the Arm function | 2013 / 2015 | Switzerland | |
| NCT01484184 | Completed | Spinal Cord Injury | InterventionalClinical Trial | I, II | Drug: SPINALON (buspirone + levodopa + cardidopa) |
50/ 18 Years to 65 Years (Adult, Older Adult) | All | Randomized, Factorial Assignment /Treatment | Pharmacokinetics Blood pressure Respiration rate Oxygen saturation Change in hematology and biochemistry laboratory Parameters Occurrence of rhythmic leg EMGs | 2013 / 2015 | Canada | |
| NCT00634426 | Completed | - Metastatic Epidural - Spinal Cord Compression |
Observational | Procedure: Surgical excision of the metastatic processRadiation: Radiotherapy of the metastatic spine process |
163 / 18 Years and older (Adult, Older Adult) |
All | CohortProspective | Change in spineassociated pain intensity Neurological outcomes Survival Adverse events | 2008 / 2015 | United States, Canada | ||
| NCT01621113 | Completed | Spinal Cord Injury | InterventionalClinical Trial | II | Drug: Dalfampridine, Placebo |
27 / 18 Years to 70 Years (Adult, Older Adult) |
All | Randomized,Parallel Assignment / Treatment |
Change in Walk Test Change in Spinal Cord Injury Functional Ambulation Index (SCI-FAI) Change in Lower-Extremity Motor Scores | 2012 / 2017 | United States | |
| NCT01435798 | Completed | -Central Neuropathic Pain- Allodynia- Spinal Cord Injury |
InterventionalClinical Trial | II | Drug: Dextromethorphan |
26 / 18 Years to 70 Years (Adult, Older Adult) |
All | Randomized,Crossover Assignment /Treatment | Mean Pain Intensity (Percent Change From Baseline) Satisfaction | 2003 / 2017 | United States | |
| NCT02218203 | Completed | -Central Neuropathic Pain- Allodynia- Spinal Cord Injury |
InterventionalClinical Trial | II | Drug: DextromethorphanDrug: LidocaineDrug: Placebo (Dextromethorphan)Drug: Placebo (Lidocaine) |
26 / 18 Years to 70 Years (Adult, Older Adult) |
All | Randomized,Factorial Assignment /Treatment | Percent Change in Peak Pain Intensity | 2003 / 2018 | United States | |
| NCT01225055 | Completed | - Spinal Cord Injury- Bone Loss-Osteoporosis | InterventionalClinical Trial | II | Drug: Teriparatide Device: Vibration |
60 / 21 Years and older (Adult, Older Adult) | All | Randomized,Parallel Assignment / Treatment |
Bone Mineral Density (Hip, Lumbar Spine, Femoral Neck)C-terminal Telopeptide Bone-specific Alkaline PhosphataseAmino-terminal Propeptide of Type Collagen | 2010 / 2018 | United States | |
| Table 1. Continued | ||||||||||||
| NCT03112941 | Completed | Spinal Cord Injuries | InterventionalClinical Trial | N/A | Procedure: Control groupOther: hyperbaric oxygen group |
164 / 18 Years and older (Adult, Older Adult) | All | Randomized,Parallel Assignment / Treatment |
Modified Barthel index American Spinal Injury Association impairment scale grading | 2012 / 2018 | ||
| NCT01621984 | Completed | Neuromuscular Disease |
InterventionalClinical Trial | I | Other: Therapeutic Riding/ Hippotherapy Intervention |
60 / 4 Years to 18 Years(Child, Adult) |
All | Single Group Assignment /Treatment | Improvement in Gross Motor Function and PerformanceDecrease of spasticity Improvement in Quality of life | 2012 / 2018 | Greece | |
| NCT03810963 | Completed | Spinal Cord Injuries | InterventionalClinical Trial | N/A | Combination Product: HIIT-FES Cycling combined with Nutritional CounselingOther: Nutritional Counseling Only |
15 / 21 Years to 65 Years(Adult, Older Adult) |
All | Non- Randomized,Parallel Assignment / Treatment |
Body Fat Percentage Arterial health via flow mediated dilation Blood glucose testing Pre- post- intervention three day dietary recall | 2017 / 2019 | United States | |
| NCT03457714 | Completed | - Spinal Cord Injuries- Depression- Anxiety | Observational | Behavioral: Guided ICBT for persons with Spinal Cord InjuryOther: Survey |
20 / 18 Years and older (Adult, Older Adult) | All | Cohort Prospective |
Change in depression Change in anxiety Pain interference Quality of life | 2017 / 2019 | Canada | ||
| NCT04034108 | Completed | Spinal Cord Injuries | InterventionalClinical Trial | N/A | Procedure: Surgical intervention combined with weight-supported ambulation training |
339 / 4 Years to 76 Years(Child, Adult, Older Adult) |
All | Single Group Assignment /Treatment | Change of Kunming Locomotor Scale (KLS) Change of American Spinal Injury Association Impairment Scale (AIS) Magnetic Resonance Imaging | 2000 / 2019 | China | |
| NCT02495545 | Completed | Spinal Cord Injury | InterventionalClinical Trial | N/A | Procedure: CSFD and elevation of MAPProcedure: Maintenance of MAP |
15 / 18 Years to 75 Years(Adult, Older Adult) |
All | Randomized,Parallel Assignment /Treatment |
Change in ISNCSCI Motor Score ISNCSCI Sensory ScoresISNCSCI Upper & Lower Extremity Motor Score Spinal Cord Independence Measure Pain level per patient report | 2015 / 2019 | United States | |
| NCT04624607 | Completed | - Spinal Cord Injuries- Paraplegia, Spinal- Tetraplegia/ Tetraparesis | InterventionalClinical Trial | N/A | Device: Transspinal transcortical paired-associative stimiulation combined with robotic gait training |
14 / 18 Years to 70 Years(Adult, Older Adult) |
All | Randomized,Crossover Assignment / Treatment | Plasticity of cortical and corticospinal neural circuits Plasticity of spinal neural circuits Sensorimotor leg motor function Walking function |
2018 / 2020 | United States | |
| NCT04566809 | Completed | Spinal Cord Injury at C5-C7 Level | InterventionalClinical Trial | N/A | Other: FES+CBA |
16 / 18 Years to 75 Years(Adult, Older Adult) |
All | Randomized,Crossover Assignment /Health Services Research |
Performance test: Bimanual Ability Test Spinal Cord Independence Measure (SCIM) Grasping Strength (GS) | 2016 / 2020 | Italy | |
| NCT03690726 | Completed |
- Spinal Cord Injury- Rehabilitation- Transcranial Magnetic Stimulation-Neurorehabilitation |
InterventionalClinical Trial | N/A | Device: Repetitive transcranial magnetic stimulationOther: Sham stimulation |
19 / 18 Years to 80 Years(Adult, Older Adult) |
All | Randomized,Parallel Assignment /Treatment |
Walking test Lower limb maximal muscle strength Timed up and go test Quantitative Sensory Testing Pressure algometry Self-reported pain |
2019 / 2020 | Denmark | |
| Table 1. Continued | ||||||||||||
| NCT03179475 | Completed | Chronic Pain | InterventionalClinical Trial | IV | Drug: Oxycodone Naloxone Combination |
1 / 18 Years to 65 Years(Adult, Older Adult) |
Single Group Assignment /Treatment | Change in management of pain related to SCI Change from Baseline of autonomic function Change from Baseline of quality of life.Change from Baseline in depressive symptoms Change from baseline of opioid side effects | 2019 / 2021 | Canada | ||
| NCT04670406 | Completed |
- Spinal Cord Injuries- Psychological Distress |
InterventionalClinical Trial | N/A | Behavioral: acceptance and commitment therapy (ACT) combined with psychoeducation |
10 / 18 Years and older(Adult, Older Adult) | All | Single Group Assignment /Treatment | Engagement in Meaningful Activities Survey Action and Acceptance Questionnaire(SCI-QOL) Resilience Short form Mindful Attention Awareness Scale |
2021 / 2021 | United States | |
| NCT04790149 | Completed | Spinal Cord Injuries | InterventionalClinical Trial | N/A | Other: Conventional RehabilitationOther: NEUROM |
56 / 25 Years to 40 Years (Adult) |
All | Randomized,Parallel Assignment /Treatment |
ASIA Vividness of motor imagery questionnaire (VMIQ) Assessment of movement attempt and execution | 2017 / 2021 | Lebanon | |
| NCT02830074 | Completed |
- Spinal Cord Injury- Sleep-disordered Breathing- Spinal Cord Disease- Multiple Sclerosis |
InterventionalClinical Trial | N/A | Behavioral: Best practices PAP + patient Education +ongoing Support and TrainingBehavioral: Sleep Education |
73 / 18 Years and older(Adult, Older Adult) | All | Randomized,Parallel Assignment /Treatment |
Subjective Sleep Quality Was Measured by The (PSQI) Quality of Life Respiratory Function Depressive Symptom Severity Fatigue Symptoms |
2017 / 2021 | United States | |
| NCT04697472 | Completed | Chronic Spinal Cord Injury |
InterventionalClinical Trial | N/A | Device: LIFT System |
65 / 22 Years to 75 Years(Adult, Older Adult) |
All | Sequential Assignment /Treatment | Incidence of serious adverse events (SAEs) Change in upper extremity strength and function Superiority of combined FTP and ARC Therapy with LIFT vs. FTP alone |
2021 / 2022 | United States Canada |
|
| NCT04171375 | Completed | - Spinal Cord Injuries- Stroke- Paralysis | InterventionalClinical Trial | N/A | Device: Transspinal Electrical Stimulation (tsES) |
2 / 19 Years to 55 Years (Adult) |
All | Single Group Assignment /Treatment | ASIA Impairment ScaleNeuromuscular Recovery Scale Spinal Cord Independence Measure Walking test |
2019 / 2022 | Hong Kong | |
| NCT03240601 | Completed | -Transcutaneous Spinal Stimulation- Walking- Spasticity- Spinal Cord Injuries | InterventionalClinical Trial | N/A | Device: Transcutaneous spinal stimulation |
18 / 18 Years to Years(Adult, Older Adult) |
All | Randomized,Parallel Assignment / Treatment |
Change in Walking FunctionChange in Spasticity -MuscleChange in Spasticity - Spinal Cord Assessment Stimulation Tolerability |
2017 / 2022 | United States | |
N/A: Not Applicable; Interventional (Clinical Trial); SCI: spinal cord injury; International Standards for Classification of Spinal Cord Injury Motor Score (ISNCSCI, formerly ASIA) Spinal Cord Injury-Quality of Life (SCI-QOL); ASIA: American Spinal Injury Association Scale; PSQI: Pittsburgh Sleep Quality Index
By involving the participants who have experienced spinal cord injuries, clinical trials provide valuable insights into the potential benefits and risks of experimental treatments. They also contribute to the development of evidence-based guidelines and protocols that guide healthcare professionals in delivering optimal care to SCI patients. Through rigorous scientific and data analysis, clinical trials pave the way for innovative breakthroughs, fostering hope for improved functional recovery, enhanced mobility, and ultimately, a better future for patients with SCI.
Conclusion
According to the review of research and studies and considering the complications of spinal cord injuries, treatments with only one management method seem inefficient. Several factors are involved in the failure of healing after SCI, containing chronic local inflammation, biochemical cues, and the release of anti-regenerative factors. All of the discussed treatments in this article possess inherent limitations. Prescriptions, surgeries, and other mentioned management are based on previous experiences with patient or animal samples that only represent a division of the current capabilities for SCI treatment as an intricate disease. Future research aims to develop new and prospective treatments that build upon these current interventions, taking into account the specific conditions and severity of spinal cord lesions in individual patients. Generally, such investigations are conducted following drug administration or initial surgery, once the patient has attained a stable state, thereby enabling the implementation of novel strategies. As an alternative, depending on the type and cause of the spinal cord lesion, given the diverse and numerous complications
associated with SCI, prospective treatments should possess the ability to anticipate a wide range of forthcoming issues before accomplishment and subsequently offer tailored solutions based on the unique conditions of each patient. Currently, it appears that a more promising approach lies in the integration of both established and novel strategies within a combined treatment paradigm. It is often more effective to employ a combination of approaches simultaneously or for a period as an alternative treatment. By adopting this perspective, it may be possible to overcome the limitations inherent to each individual treatment modality by incorporating alternative approaches, ultimately leading to improved outcomes in the management of SCI.
Acknowledgment
We would like to express our gratitude to Professor Mohammad Hosein Ebrahimzadeh, for his insightful recommendations and valuable guidance, which have significantly improved the quality of this manuscript.The authors would like to appreciate the support from the Clinical Research Development Unit, Ghaem Hospital, Mashhad University of Medical Sciences, Mashhad, IRAN.
Authors Contribution:
Davood Yari: Conceptualization, design, data collection, writing the original draft, and final approval.
Arezoo Saberi: Data acquisition, visualization.
Zahra Salmasi: Data collection, investigation.
Seyed Alireza Ghoreishi: Investigation, data interpretation.
Leila Etemad: Data collection, investigation.
Jebrail Movaffagh: Supervision, data interpretation, and final approval.
Babak Ganjeifar: Supervision, data interpretation, validation, and final approval.
Conflict of interest:
None
Funding:
None
References
- 1.Bajjig A, Cayetanot F, Taylor JA, Bodineau L, Vivodtzev I. Serotonin 1A Receptor Pharmacotherapy and Neuroplasticity in Spinal Cord Injury. Pharmaceuticals (Basel). 2022;15:4. doi: 10.3390/ph15040460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adigun OO, Reddy V, Varacallo M. Anatomy, Back, Spinal Cord. StatPearls; 2022. [PubMed] [Google Scholar]
- 3.Bilchak JN, Caron G, Cote MP. Exercise-Induced Plasticity in Signaling Pathways Involved in Motor Recovery after Spinal Cord Injury. Int J Mol Sci. 2021;22:9. doi: 10.3390/ijms22094858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebrahimpour A, Razi M, Mortazavi SJ, et al. Job satisfaction, Career Burnout, and Work-Related Well-Being Prevalence among Orthopedic Surgeons: A Nationwide Study. Arch Bone Jt Surg. 2023;11(4):293–300. doi: 10.22038/ABJS.2022.66683.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan YS, Lui F. InStatPearls [Internet] StatPearls Publishing: 2022. Neuroanatomy, Spinal Cord. [PubMed] [Google Scholar]
- 6.Ebrahimzadeh MH, Makhmalbaf H, Soltani-Moghaddas SH, Mazloumi SM. The spinal cord injury quality-of-life-23 questionnaire, Iranian validation study. J Res Med Sci. 2014;19(4):349–54. [PMC free article] [PubMed] [Google Scholar]
- 7.Ebrahimzadeh MH, Shojaei BS, Golhasani-Keshtan F, Soltani-Moghaddas SH, Fattahi AS, Mazloumi SM. Quality of life and the related factors in spouses of veterans with chronic spinal cord injury. Health Qual Life Outcomes. 2013;11:48 . doi: 10.1186/1477-7525-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neural Injury and Repair Research Group. [Accessed, 2021]. Available at: https://neurosciences.ucsd.edu/research/interest-groups/neural-injury-repair.html.
- 9.Ebrahimzadeh MH, Soltani-Moghaddas SH, Birjandinejad A, Omidi-Kashani F, Bozorgnia S. Quality of life among veterans with chronic spinal cord injury and related variables. Arch Trauma Res. 2014;3(2):e17917. doi: 10.5812/atr.17917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbiellini Amidei C, Salmaso L, Bellio S, Saia M. Epidemiology of traumatic spinal cord injury: a large population-based study. Spinal Cord. 2022;60(9):812–819. doi: 10.1038/s41393-022-00795-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khadour FA, Khadour YA, Meng L, Lixin C, Xu T. Epidemiological features of traumatic spinal cord injury in Wuhan, China. J Orthop Surg Res. 2023;18(1):72 . doi: 10.1186/s13018-023-03554-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebrahimzadeh MH, Golhasani-Keshtan F, Shojaee BS. Correlation between health-related quality of life in veterans with chronic spinal cord injury and their caregiving spouses. Arch Trauma Res. 2014;3(4):e16720. doi: 10.5812/atr.16720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdelrahman S, Ireland A, Winter EM, Purcell M, Coupaud S. Osteoporosis after spinal cord injury: aetiology, effects and therapeutic approaches. J Musculoskelet Neuronal Interact. 2021;21(1):26–50. [PMC free article] [PubMed] [Google Scholar]
- 14.Ebrahimzadeh MH, Shojaee BS, Golhasani-Keshtan F, Moharari F, Kachooei AR, Fattahi AS. Depression, anxiety and quality of life in caregiver spouses of veterans with chronic spinal cord injury. Iran J Psychiatry. 2014;9(3):133–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Lv B, Zhang X, Yuan J, et al. Biomaterial-supported MSC transplantation enhances cell-cell communication for spinal cord injury. Stem Cell Res Ther. 2021;12(1):36 . doi: 10.1186/s13287-020-02090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domingues HS, Portugal CC, Socodato R, Relvas JB. Corrigendum: Oligodendrocyte, Astrocyte and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front Cell Dev Biol. 2016;4:79. doi: 10.3389/fcell.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchida K, Nakamura M, Ozawa H, Katoh S, Toyama Y. Neuroprotection and regeneration of the spinal cord. 1st ed. Springer Tokyo; 2014. [Google Scholar]
- 18.Lescaudron L, Rossignol J, Dunbar GL. Stem Cells and Neurodegenerative Diseases. 1st ed. Routledge Taylor & Francis Group; 2014. [Google Scholar]
- 19.Faridaalee G, Keyghobadi Khajeh F. Serum and Cerebrospinal Fluid Levels of S-100beta Is A Biomarker for Spinal Cord Injury; a Systematic Review and Meta-Analysis. Arch Acad Emerg Med. 2019;7(1):e19. [PMC free article] [PubMed] [Google Scholar]
- 20.Ganjeifar B, Mehrad-Majd H, Barforooshi AG, Baharvahdat H, Zabihyan S, Moradi A. Diagnostic Value of Computed Tomography Angiography in Confirmation of Brain Death. World Neurosurg. 2023:178:e275–e281. doi: 10.1016/j.wneu.2023.07.042. [DOI] [PubMed] [Google Scholar]
- 21.Molinaro F, La Zazzera PL, Ferraris M, Morbidoni G, Zaca D, Rinaldis A, Carpanese F, Cioffi A, Naddeo F, Boccaccini L, Bergui M. Chapter 4 - MRI as an imaging tool for in vivo noninvasive morphological and (partially) functional examination of injured spinal cord. In: Perale G, editor. Spinal Cord Injury (SCI) Repair Strategies. 1st ed. WP Publishing; 2020. [Google Scholar]
- 22.Seo JH, Kim HJ, Lee KY, Wang L, Park JW. The Prognostic Factors of Neurologic Recovery in Spinal Cord Injury. J Korean Soc Spine Surg. 2015;22(1):1–7. [Google Scholar]
- 23.Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med. 2004;10(12):580–3. doi: 10.1016/j.molmed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25(5) doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 25.Seblani M, Decherchi P, Brezun JM. Edema after CNS Trauma: A Focus on Spinal Cord Injury. Int J Mol Sci. 2023;24 doi: 10.3390/ijms24087159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Z, Yuan S, Shi L, et al. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021;54(3):e12992. doi: 10.1111/cpr.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anjum A, Yazid MD, Fauzi Daud M, et al. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int J Mol Sci. 2020;21:20. doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu X, Xu W, Ren Y, et al. Spinal cord injury: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):245. doi: 10.1038/s41392-023-01477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clifford T, Finkel Z, Rodriguez B, Joseph A, Cai L. Current Advancements in Spinal Cord Injury Research-Glial Scar Formation and Neural Regeneration. Cells. 2023;12:6. doi: 10.3390/cells12060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson MA, Burda JE, Ren Y, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carelli S, Giallongo T, Rey F, et al. Neuroprotection, Recovery of Function and Endogenous Neurogenesis in Traumatic Spinal Cord Injury Following Transplantation of Activated Adipose Tissue. Cells. 2019;8:4. doi: 10.3390/cells8040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yari D, Ehsanbakhsh Z, Validad MH, Langroudi FH. Association of TIMP-1 and COL4A4 Gene Polymorphisms with Keratoconus in an Iranian Population. J Ophthalmic Vis Res. 2020;15(3):299–307. doi: 10.18502/jovr.v15i3.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q, Shi B, Ding J, et al. Polymer scaffolds facilitate spinal cord injury repair. Acta Biomater. 2019;88:57–77. doi: 10.1016/j.actbio.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 34.Yousefifard M, Vazirizadeh-Mahabadi MH, Haghani L, et al. Early General Hypothermia Improves Motor Function after Spinal Cord Injury in Rats; a Systematic Review and Meta-Analysis. Arch Acad Emerg Med. 2020;8(1):e80. [PMC free article] [PubMed] [Google Scholar]
- 35.Venkatesh K, Ghosh SK, Mullick M, Manivasagam G, Sen D. Spinal cord injury: pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019;377(2):125–151. doi: 10.1007/s00441-019-03039-1. [DOI] [PubMed] [Google Scholar]
- 36.Lee BJ, Jeong JH. Review: Steroid Use in Patients with Acute Spinal Cord Injury and Guideline Update. Korean J Neurotrauma. 2022;18(1):22–30. doi: 10.13004/kjnt.2022.18.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Al Mamun A, Yuan Y, et al. Acute spinal cord injury: Pathophysiology and pharmacological intervention (Review) Mol Med Rep. 2021;23:6. doi: 10.3892/mmr.2021.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Gu R, Zhu Q, Liu J. Changes of Spinal Edema and Expression of Aquaporin 4 in Methylprednisolone-treated Rats with Spinal Cord Injury. Ann Clin Lab Sci. 2018;48(4):453–459. [PubMed] [Google Scholar]
- 39.Roohbakhsh A, Etemad L, Karimi G. Resolvin D1: A key endogenous inhibitor of neuroinflammation. Biofactors. 2022;48(5):1005–1026. doi: 10.1002/biof.1891. [DOI] [PubMed] [Google Scholar]
- 40.Park A, Anderson D, Battaglino RA, Nguyen N, Morse LR. Ibuprofen use is associated with reduced C-reactive protein and interleukin-6 levels in chronic spinal cord injury. J Spinal Cord Med. 2022;45(1):117–125. doi: 10.1080/10790268.2020.1773029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayta E, Elden H. Acute spinal cord injury: A review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J Chem Neuroanat. 2018;87:25–31. doi: 10.1016/j.jchemneu.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Roohbakhsh A, Moshiri M, Salehi Kakhki A, Iranshahy M, Amin F, Etemad L. Thymoquinone abrogates methamphetamine-induced striatal neurotoxicity and hyperlocomotor activity in mice. Res Pharm Sci. 2021;16(4):391–399. doi: 10.4103/1735-5362.319577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fehlings MG, Wilson JR, Tetreault LA, et al. A Clinical Practice Guideline for the Management of Patients with Acute Spinal Cord Injury: Recommendations on the Use of Methylprednisolone Sodium Succinate. Global Spine J. 2017;7(3 Suppl):203S–211S. doi: 10.1177/2192568217703085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kupfer M, Formal CS. Non-opioid pharmacologic treatment of chronic spinal cord injury-related pain. J Spinal Cord Med. 2022;45(2):163–172. doi: 10.1080/10790268.2020.1730109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yousefifard M, Hashemi B, Forouzanfar MM, Khatamian Oskooi R, Madani Neishaboori A, Jalili Khoshnoud R. Ultra-early Spinal Decompression Surgery Can Improve Neurological Outcome of Complete Cervical Spinal Cord Injury; a Systematic Review and Meta-analysis. Arch Acad Emerg Med. 2022;10(1):e11. doi: 10.22037/aaem.v10i1.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma Y, Zhu Y, Zhang B, Wu Y, Liu X, Zhu Q. The Impact of Urgent (<8 Hours) Decompression on Neurologic Recovery in Traumatic Spinal Cord Injury: A Meta-Analysis. World Neurosurg. 2020;140:e185–e194. doi: 10.1016/j.wneu.2020.04.230. [DOI] [PubMed] [Google Scholar]
- 47.Ter Wengel PV, Martin E, De Witt Hamer PC, et al. Impact of Early (<24 h) Surgical Decompression on Neurological Recovery in Thoracic Spinal Cord Injury: A Meta-Analysis. J Neurotrauma. 2019;36(18):2609–2617. doi: 10.1089/neu.2018.6277. [DOI] [PubMed] [Google Scholar]
- 48.Badhiwala JH, Wilson JR, Witiw CD, et al. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. 2021;20(2):117–126. doi: 10.1016/S1474-4422(20)30406-3. [DOI] [PubMed] [Google Scholar]
- 49.Gu X, Ding F, Yang Y, Liu J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93(2):204–30. doi: 10.1016/j.pneurobio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Leckenby JI, Furrer C, Haug L, Juon Personeni B, Vogelin E. A Retrospective Case Series Reporting the Outcomes of Avance Nerve Allografts in the Treatment of Peripheral Nerve Injuries. Plast Reconstr Surg. 2020;145(2):368e–381e. doi: 10.1097/PRS.0000000000006485. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Xie JX, Niu F, et al. Surgical intervention combined with weight-bearing walking training improves neurological recoveries in 320 patients with clinically complete spinal cord injury: a prospective self-controlled study. Neural Regen Res. 2021;16(5):820–829. doi: 10.4103/1673-5374.297080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mansour NM, Pino IP, Freeman D, et al. Advances in Epidural Spinal Cord Stimulation to Restore Function after Spinal Cord Injury: History and Systematic Review. J Neurotrauma. 2022;39(15-16):1015–1029. doi: 10.1089/neu.2022.0007. [DOI] [PubMed] [Google Scholar]
- 53.O'Connell NE, Ferraro MC, Gibson W, et al. Implanted spinal neuromodulation interventions for chronic pain in adults. Cochrane Database Syst Rev. 2021;12:CD013756. doi: 10.1002/14651858.CD013756.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qu H, Zhao Y. Advances in tissue state recognition in spinal surgery: a review. Front Med. 2021;15(4):575–584. doi: 10.1007/s11684-020-0816-3. [DOI] [PubMed] [Google Scholar]
- 55.Shahpari O, Mortazavi J, Ebrahimzadeh MH, Bagheri F, Mousavian A. Role of Hip Arthroscopy in the Treatment of Avascular Necrosis of the Hip: A Systematic Review. Arch Bone Jt Surg. 2022;10(6):480–489. doi: 10.22038/ABJS.2021.58534.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiong F, Fu C, Zhang Q, et al. The Effect of Different Acupuncture Therapies on Neurological Recovery in Spinal Cord Injury: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Alternat Med. 2019;2019:2371084. doi: 10.1155/2019/2371084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang H, Guo Y, Zhao Y, et al. Effects and Mechanisms of Acupuncture Combined with Mesenchymal Stem Cell Transplantation on Neural Recovery after Spinal Cord Injury: Progress and Prospects. Neural Plast. 2020;2020:8890655. doi: 10.1155/2020/8890655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng YS, Ding Y, Xu HY, et al. Electro-acupuncture and its combination with adult stem cell transplantation for spinal cord injury treatment: A summary of current laboratory findings and a review of literature. CNS Neurosci Ther. 2022;28(5):635–647. doi: 10.1111/cns.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Scheer JW, Totosy de Zepetnek JO, Blauwet C, et al. Assessment of body composition in spinal cord injury: A scoping review. PLoS One. 2021;16(5):e0251142. doi: 10.1371/journal.pone.0251142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ginis KAM, van der Scheer JW, Latimer-Cheung AE, et al. Correction: Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord. 2018;56(11):1114 . doi: 10.1038/s41393-018-0194-8. [DOI] [PubMed] [Google Scholar]
- 61.van der Scheer JW, Goosey-Tolfrey VL, Valentino SE, Davis GM, Ho CH. Functional electrical stimulation cycling exercise after spinal cord injury: a systematic review of health and fitness-related outcomes. J Neuroeng Rehabil. 2021;18(1):99 . doi: 10.1186/s12984-021-00882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lovas J, Tran Y, Middleton J, Bartrop R, Moore N, Craig A. Managing pain and fatigue in people with spinal cord injury: a randomized controlled trial feasibility study examining the efficacy of massage therapy. Spinal Cord. 2017;55(2):162–166. doi: 10.1038/sc.2016.156. [DOI] [PubMed] [Google Scholar]
- 63.Franz S, Schulz B, Wang H, et al. Management of pain in individuals with spinal cord injury: Guideline of the German-Speaking Medical Society for Spinal Cord Injury. Ger Med Sci. 2019:17:Doc05. doi: 10.3205/000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palladino L, Ruotolo I, Berardi A, Carlizza A, Galeoto G. Efficacy of aquatic therapy in people with spinal cord injury: a systematic review and meta-analysis. Spinal Cord. 2023;61(6):317–322. doi: 10.1038/s41393-023-00892-4. [DOI] [PubMed] [Google Scholar]
- 65.Vafaei-Nezhad S, Pour Hassan M, Noroozian M, et al. A Review of Low-Level Laser Therapy for Spinal Cord Injury: Challenges and Safety. J Lasers Med Sci. 2020;11(4):363–368. doi: 10.34172/jlms.2020.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J, Kim EH, Lee K, et al. Low-Level Laser Irradiation Improves Motor Recovery after Contusive Spinal Cord Injury in Rats. Tissue Eng Regen Med. 2017;14(1):57–64. doi: 10.1007/s13770-016-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tehrani MR, Nazary-Moghadam S, Zeinalzadeh A, Moradi A, Mehrad-Majd H, Sahebalam M. Efficacy of low-level laser therapy on pain, disability, pressure pain threshold, and range of motion in patients with myofascial neck pain syndrome: a systematic review and meta-analysis of randomized controlled trials. Lasers Med Sci. 2022;37(9):3333–3341. doi: 10.1007/s10103-022-03626-9. [DOI] [PubMed] [Google Scholar]
- 68.Farid MF, Y SA, Rizk H. Stem cell treatment trials of spinal cord injuries in animals. Auton Neurosci. 2021;238:102932. doi: 10.1016/j.autneu.2021.102932. [DOI] [PubMed] [Google Scholar]
- 69.Huang L, Fu C, Xiong F, He C, Wei Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transplant. 2021;30:963689721989266. doi: 10.1177/0963689721989266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nori S, Nakamura M, Okano H. Plasticity and regeneration in the injured spinal cord after cell transplantation therapy. Prog Brain Res. 2017;231:33–56. doi: 10.1016/bs.pbr.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 71.Chhabra HS, Sarda K. Clinical translation of stem cell based interventions for spinal cord injury - Are we there yet? Adv Drug Deliv Rev. 2017;120:41–49. doi: 10.1016/j.addr.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 72.Lowry LE, Herzig MC, Christy BA, et al. Neglected No More: Emerging Cellular Therapies in Traumatic Injury. Stem Cell Rev Rep. 2021;17(4):1194–1214. doi: 10.1007/s12015-020-10086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takami T, Shimokawa N, Parthiban J, Zileli M, Ali S. Pharmacologic and Regenerative Cell Therapy for Spinal Cord Injury: WFNS Spine Committee Recommendations. Neurospine. 2020;17(4):785–796. doi: 10.14245/ns.2040408.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan S, Mafi P, Mafi R, Khan W. A Systematic Review of Mesenchymal Stem Cells in Spinal Cord Injury, Intervertebral Disc Repair and Spinal Fusion. Curr Stem Cell Res Ther. 2018;13(4):316–323. doi: 10.2174/1574888X11666170907120030. [DOI] [PubMed] [Google Scholar]
- 75.Kong D, Feng B, Amponsah AE, et al. hiPSC-derived NSCs effectively promote the functional recovery of acute spinal cord injury in mice. Stem Cell Res Ther. 2021;12(1):172 . doi: 10.1186/s13287-021-02217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng W, Li Q, Zhao C, Da Y, Zhang HL, Chen Z. Differentiation of Glial Cells From hiPSCs: Potential Applications in Neurological Diseases and Cell Replacement Therapy. Front Cell Neurosci. 2018;12:239. doi: 10.3389/fncel.2018.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X, Kuang N, Chen Y, et al. Transplantation of olfactory ensheathing cells promotes the therapeutic effect of neural stem cells on spinal cord injury by inhibiting necrioptosis. Aging (Albany NY). 2021;13(6):9056–9070. doi: 10.18632/aging.202758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monje PV, Deng L, Xu XM. Human Schwann Cell Transplantation for Spinal Cord Injury: Prospects and Challenges in Translational Medicine. Front Cell Neurosci. 2021;15:690894. doi: 10.3389/fncel.2021.690894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beatriz M, Vilaca R, Lopes C. Exosomes: Innocent Bystanders or Critical Culprits in Neurodegenerative Diseases. Front Cell Dev Biol. 2021;9:635104. doi: 10.3389/fcell.2021.635104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pishavar E, Oroojalian F, Salmasi Z, Hashemi E, Hashemi M. Recent advances of dendrimer in targeted delivery of drugs and genes to stem cells as cellular vehicles. Biotechnol Prog. 2021;37(4):e3174. doi: 10.1002/btpr.3174. [DOI] [PubMed] [Google Scholar]
- 81.Upadhyayula PS, Martin JR, Rennert RC, Ciacci JD. Review of operative considerations in spinal cord stem cell therapy. World J Stem Cells. 2021;13(2):168–176. doi: 10.4252/wjsc.v13.i2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Honmou O, Yamashita T, Morita T, et al. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin Neurol Neurosurg. 2021;203:106565. doi: 10.1016/j.clineuro.2021.106565. [DOI] [PubMed] [Google Scholar]
- 83.Xia Y, Zhu J, Yang R, Wang H, Li Y, Fu C. Mesenchymal stem cells in the treatment of spinal cord injury: Mechanisms, current advances and future challenges. Front Immunol. 2023;14:1141601. doi: 10.3389/fimmu.2023.1141601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bartlett RD, Burley S, Ip M, Phillips JB, Choi D. Cell Therapies for Spinal Cord Injury: Trends and Challenges of Current Clinical Trials. Neurosurgery. 2020;87(4):E456–E472. doi: 10.1093/neuros/nyaa149. [DOI] [PubMed] [Google Scholar]
- 85.Shang Z, Wang R, Li D, et al. Spinal Cord Injury: A Systematic Review and Network Meta-Analysis of Therapeutic Strategies Based on 15 Types of Stem Cells in Animal Models. Front Pharmacol. 2022;13:819861. doi: 10.3389/fphar.2022.819861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pang QM, Chen SY, Fu SP, et al. Regulatory Role of Mesenchymal Stem Cells on Secondary Inflammation in Spinal Cord Injury. J Inflamm Res. 2022;15:573–593. doi: 10.2147/JIR.S349572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim BG, Hwang DH, Lee SI, Kim EJ, Kim SU. Stem cell-based cell therapy for spinal cord injury. Cell Transplant. 2007;16(4):355–64. doi: 10.3727/000000007783464885. [DOI] [PubMed] [Google Scholar]
- 88.Xue W, Shi W, Kong Y, Kuss M, Duan B. Anisotropic scaffolds for peripheral nerve and spinal cord regeneration. Bioact Mater. 2021;6(11):4141–4160. doi: 10.1016/j.bioactmat.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.saberi A, Khodaverdi E, Kamali H, et al. Fabrication and Characterization of Biomimetic Electrospun Cartilage Decellularized Matrix (CDM)/Chitosan Nanofiber Hybrid for Tissue Engineering Applications: Box-Behnken Design for Optimization. Journal of Polymers and the Environment. 2023:1–20. [Google Scholar]
- 90.Ashammakhi N, Kim HJ, Ehsanipour A, et al. Regenerative Therapies for Spinal Cord Injury. Tissue Eng Part B Rev. 2019;25(6):471–491. doi: 10.1089/ten.teb.2019.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elkhenany H, Bonilla P, Giraldo E, et al. A Hyaluronic Acid Demilune Scaffold and Polypyrrole-Coated Fibers Carrying Embedded Human Neural Precursor Cells and Curcumin for Surface Capping of Spinal Cord Injuries. Biomedicines. 2021;9:12. doi: 10.3390/biomedicines9121928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu S, Sun X, Wang T, et al. Nano-fibrous and ladder-like multi-channel nerve conduits: Degradation and modification by gelatin. Mater Sci Eng C Mater Biol Appl. 2018;83:130–142. doi: 10.1016/j.msec.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 93.Li R, Liu H, Huang H, et al. Chitosan conduit combined with hyaluronic acid prevent sciatic nerve scar in a rat model of peripheral nerve crush injury. Mol Med Rep. 2018;17(3):4360–4368. doi: 10.3892/mmr.2018.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinez-Ramos C, Doblado LR, Mocholi EL, et al. Biohybrids for spinal cord injury repair. J Tissue Eng Regen Med. 2019;13(3):509–521. doi: 10.1002/term.2816. [DOI] [PubMed] [Google Scholar]
- 95.Licht C, Rose JC, Anarkoli AO, et al. Synthetic 3D PEG-Anisogel Tailored with Fibronectin Fragments Induce Aligned Nerve Extension. Biomacromolecules. 2019;20(11):4075–4087. doi: 10.1021/acs.biomac.9b00891. [DOI] [PubMed] [Google Scholar]
- 96.Walsh CM, Wychowaniec JK, Brougham DF, Dooley D. Functional hydrogels as therapeutic tools for spinal cord injury: New perspectives on immunopharmacological interventions. Pharmacol Ther. 2022;234:108043. doi: 10.1016/j.pharmthera.2021.108043. [DOI] [PubMed] [Google Scholar]
- 97.Kubinová Š. Chapter 7 - Soft and rigid scaffolds for spinal cord injury regeneration. In: Perale G, editor. Spinal Cord Injury (SCI) Repair Strategies. Woodhead Publishing; 2020. [Google Scholar]
- 98.Yari D, Movaffagh J, Ebrahimzadeh MH, Saberi A, Qujeq D, Moradi A. Biomimetic ECM-Based Hybrid Scaffold for Cartilage Tissue Engineering Applications. Journal of Polymers and the Environment. 2024:1–9. [Google Scholar]
- 99.Zhang S, Wang XJ, Li WS, et al. Polycaprolactone/polysialic acid hybrid, multifunctional nanofiber scaffolds for treatment of spinal cord injury. Acta Biomater. 2018;77:15–27. doi: 10.1016/j.actbio.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 100.Koser DE, Moeendarbary E, Hanne J, Kuerten S, Franze K. CNS cell distribution and axon orientation determine local spinal cord mechanical properties. Biophys J. 2015;108(9):2137–47. doi: 10.1016/j.bpj.2015.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu J, Jin LQ, Selzer ME. Inhibition of central axon regeneration: perspective from chondroitin sulfate proteoglycans in lamprey spinal cord injury. Neural Regen Res. 2022;17(9):1955–1956. doi: 10.4103/1673-5374.335144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu S, Xie YY, Wang B. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen Res. 2019;14(8):1352–1363. doi: 10.4103/1673-5374.253512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xiang W, Cao H, Tao H, et al. Applications of chitosan-based biomaterials: From preparation to spinal cord injury neuroprosthetic treatment. Int J Biol Macromol. 2023;230:123447. doi: 10.1016/j.ijbiomac.2023.123447. [DOI] [PubMed] [Google Scholar]
- 104.Feng C, Deng L, Yong YY, et al. The Application of Biomaterials in Spinal Cord Injury. Int J Mol Sci. 2023;24:1. doi: 10.3390/ijms24010816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang SX, Lu YB, Wang XX, et al. Graphene and graphene-based materials in axonal repair of spinal cord injury. Neural Regen Res. 2022;17(10):2117–2125. doi: 10.4103/1673-5374.335822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luo Y, Fan L, Liu C, et al. An injectable, self-healing, electroconductive extracellular matrix-based hydrogel for enhancing tissue repair after traumatic spinal cord injury. Bioact Mater. 2022:7:98–111. doi: 10.1016/j.bioactmat.2021.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luo J, Shi X, Li L, et al. An injectable and self-healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioact Mater. 2021;6(12):4816–4829. doi: 10.1016/j.bioactmat.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bousalis D, McCrary MW, Vaughn N, et al. Decellularized peripheral nerve as an injectable delivery vehicle for neural applications. J Biomed Mater Res A. 2022;110(3):595–611. doi: 10.1002/jbm.a.37312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu Y, Zhou J, Liu C, et al. Understanding the role of tissue-specific decellularized spinal cord matrix hydrogel for neural stem/progenitor cell microenvironment reconstruction and spinal cord injury. Biomaterials. 2021;268:120596. doi: 10.1016/j.biomaterials.2020.120596. [DOI] [PubMed] [Google Scholar]
- 110.Omidinia-Anarkoli A, Boesveld S, Tuvshindorj U, Rose JC, Haraszti T, De Laporte L. An Injectable Hybrid Hydrogel with Oriented Short Fibers Induces Unidirectional Growth of Functional Nerve Cells. Small. 2017;13:36. doi: 10.1002/smll.201702207. [DOI] [PubMed] [Google Scholar]
- 111.Agarwal G, Roy A, Kumar H, Srivastava A. Graphene-collagen cryogel controls neuroinflammation and fosters accelerated axonal regeneration in spinal cord injury. Biomater Adv. 2022;139:212971. doi: 10.1016/j.bioadv.2022.212971. [DOI] [PubMed] [Google Scholar]
- 112.Zhang L, Li Z, Mao L, Wang H. Circular RNA in Acute Central Nervous System Injuries: A New Target for Therapeutic Intervention. Front Mol Neurosci. 2022;15:816182. doi: 10.3389/fnmol.2022.816182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yari D, Ebrahimzadeh MH, Movaffagh J, et al. Biochemical Aspects of Scaffolds for Cartilage Tissue Engineering; from Basic Science to Regenerative Medicine. Arch Bone Jt Surg. 2022;10(3):229–244. doi: 10.22038/ABJS.2022.55549.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu X, Hao M, Chen Z, et al. 3D bioprinted neural tissue constructs for spinal cord injury repair. Biomaterials. 2021;272:120771. doi: 10.1016/j.biomaterials.2021.120771. [DOI] [PubMed] [Google Scholar]
- 115.Yuan TY, Zhang J, Yu T, Wu JP, Liu QY. 3D Bioprinting for Spinal Cord Injury Repair. Front Bioeng Biotechnol. 2022;10:847344. doi: 10.3389/fbioe.2022.847344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bedir T, Ulag S, Ustundag CB, Gunduz O. 3D bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater Sci Eng C Mater Biol Appl. 2020;110:110741. doi: 10.1016/j.msec.2020.110741. [DOI] [PubMed] [Google Scholar]
- 117.Yu X, Zhang T, Li Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers (Basel). 2020;12:8. doi: 10.3390/polym12081637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li JJ, Liu H, Zhu Y, et al. Animal Models for Treating Spinal Cord Injury Using Biomaterials-Based Tissue Engineering Strategies. Tissue Eng Part B Rev. 2022;28(1):79–100. doi: 10.1089/ten.TEB.2020.0267. [DOI] [PubMed] [Google Scholar]
- 119.Choi EH, Gattas S, Brown NJ, et al. Epidural electrical stimulation for spinal cord injury. Neural Regen Res. 2021;16(12):2367–2375. doi: 10.4103/1673-5374.313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Golhasani-Keshtan F, Ebrahimzadeh MH, Fattahi AS, Soltani-Moghaddas SH, Omidi-kashani F. Validation and cross-cultural adaptation of the Persian version of Craig Handicap Assessment and Reporting Technique (CHART) short form. Disabil Rehabil. 2013;35(22):1909–14. doi: 10.3109/09638288.2013.768710. [DOI] [PubMed] [Google Scholar]
- 121.Rajabi-Mashhadi MT, Mashhadinejad H, Ebrahimzadeh MH, Golhasani-Keshtan F, Ebrahimi H, Zarei Z. The Zarit Caregiver Burden Interview Short Form (ZBI-12) in spouses of Veterans with Chronic Spinal Cord Injury, Validity and Reliability of the Persian Version. Arch Bone Jt Surg. 2015;3(1):56–63. [PMC free article] [PubMed] [Google Scholar]
- 122.Melrose J, Hayes AJ, Bix G. The CNS/PNS Extracellular Matrix Provides Instructive Guidance Cues to Neural Cells and Neuroregulatory Proteins in Neural Development and Repair. Int J Mol Sci. 2021;22:11. doi: 10.3390/ijms22115583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kosuri S, Borca CH, Mugnier H, et al. Machine-Assisted Discovery of Chondroitinase ABC Complexes toward Sustained Neural Regeneration. Adv Healthc Mater. 2022;11(10):e2102101. doi: 10.1002/adhm.202102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hayes AJ, Melrose J. Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity. Biomolecules. 2020;10:9. doi: 10.3390/biom10091244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Muir E, De Winter F, Verhaagen J, Fawcett J. Recent advances in the therapeutic uses of chondroitinase ABC. Exp Neurol. 2019;321:113032. doi: 10.1016/j.expneurol.2019.113032. [DOI] [PubMed] [Google Scholar]
- 126.Jevans B, James ND, Burnside E, et al. Combined treatment with enteric neural stem cells and chondroitinase ABC reduces spinal cord lesion pathology. Stem Cell Res Ther. 2021;12(1):10. doi: 10.1186/s13287-020-02031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Woods W, Evans D, Mogas Barcons A, Tzerakis N, Adams C, Maitreyi Chari D. Stem cell sprays for neurological injuries: a perspective. Emerg Top Life Sci. 2021 Oct;5(4):519–522. doi: 10.1042/ETLS20210113. [DOI] [PubMed] [Google Scholar]
- 128.Azimifar MA, Salmasi Z, Doosti A, Babaei N, Hashemi M. Evaluation of the efficiency of modified PAMAM dendrimer with low molecular weight protamine peptide to deliver IL-12 plasmid into stem cells as cancer therapy vehicles. Biotechnol Prog. 2021;37(4):e3175. doi: 10.1002/btpr.3175. [DOI] [PubMed] [Google Scholar]
- 129.Lee HL, Yeum CE, Lee H, et al. Peripheral Nerve-Derived Stem Cell Spheroids Induce Functional Recovery and Repair after Spinal Cord Injury in Rodents. Int J Mol Sci. 2021;22:8. doi: 10.3390/ijms22084141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marinval N, Chew SY. Mechanotransduction assays for neural regeneration strategies: A focus on glial cells. APL Bioeng. 2021;5(2):021505. doi: 10.1063/5.0037814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pașca SP, Arlotta P, Bateup HS, et al. A nomenclature consensus for nervous system organoids and assembloids. Nature. 2022;609(7929):907–910. doi: 10.1038/s41586-022-05219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fan B, Wei Z, Feng S. Progression in translational research on spinal cord injury based on microenvironment imbalance. Bone Res. 2022;10(1):35. doi: 10.1038/s41413-022-00199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Awad BI, Carmody MA, Steinmetz MP. Potential role of growth factors in the management of spinal cord injury. World Neurosurg. 2015;83(1):120–31. doi: 10.1016/j.wneu.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 134.Talifu Z, Qin C, Xin Z, et al. The Overexpression of Insulin-Like Growth Factor-1 and Neurotrophin-3 Promote Functional Recovery and Alleviate Spasticity after Spinal Cord Injury. Front Neurosci. 2022;16:863793. doi: 10.3389/fnins.2022.863793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cooke P, Janowitz H, Dougherty SE. Neuronal Redevelopment and the Regeneration of Neuromodulatory Axons in the Adult Mammalian Central Nervous System. Front Cell Neurosci. 2022;16:872501. doi: 10.3389/fncel.2022.872501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gu Y, Wen G, Zhao H, Qi H, Yang Y, Hu T. Delivery of FGF10 by implantable porous gelatin microspheres for treatment of spinal cord injury. Mol Med Rep. 2023;28:1. doi: 10.3892/mmr.2023.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moshiri M, Hosseiniyan SM, Moallem SA, et al. The effects of vitamin B (12) on the brain damages caused by methamphetamine in mice. Iran J Basic Med Sci. 2018;21(4):434–438. doi: 10.22038/IJBMS.2018.23362.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aschauer-Wallner S, Leis S, Bogdahn U, Johannesen S, Couillard-Despres S, Aigner L. Granulocyte colony-stimulating factor in traumatic spinal cord injury. Drug Discov Today. 2021;26(7):1642–1655. doi: 10.1016/j.drudis.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 139.Derakhshanrad N, Saberi H, Yekaninejad MS, Joghataei MT, Sheikhrezaei A. Granulocyte-colony stimulating factor administration for neurological improvement in patients with postrehabilitation chronic incomplete traumatic spinal cord injuries: a double-blind randomized controlled clinical trial. J Neurosurg Spine. 2018;29(1):97–107. doi: 10.3171/2017.11.SPINE17769. [DOI] [PubMed] [Google Scholar]
- 140.Pelisch N, Rosas Almanza J, Stehlik KE, Aperi BV, Kroner A. CCL3 contributes to secondary damage after spinal cord injury. J Neuroinflammation. 2020;17(1):362. doi: 10.1186/s12974-020-02037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim HN, McCrea MR, Li S. Advances in molecular therapies for targeting pathophysiology in spinal cord injury. Expert Opin Ther Targets. 2023;27(3):171–187. doi: 10.1080/14728222.2023.2194532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ding Y, Chen Q. mTOR pathway: A potential therapeutic target for spinal cord injury. Biomed Pharmacother. 2022;145:112430. doi: 10.1016/j.biopha.2021.112430. [DOI] [PubMed] [Google Scholar]
- 143.Wang W, He D, Chen J, et al. Circular RNA Plek promotes fibrogenic activation by regulating the miR-135b-5p/TGF-betaR1 axis after spinal cord injury. Aging (Albany NY). 2021;13(9):13211–13224. doi: 10.18632/aging.203002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hu M, Cao Z, Jiang D. The Effect of miRNA-Modified Exosomes in Animal Models of Spinal Cord Injury: A meta-Analysis. Front Bioeng Biotechnol. 2021;9:819651. doi: 10.3389/fbioe.2021.819651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shen Y, Cai J. The Importance of Using Exosome-Loaded miRNA for the Treatment of Spinal Cord Injury. Mol Neurobiol. 2023;60(2):447–459. doi: 10.1007/s12035-022-03088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu XY, Guo JW, Kou JQ, Sun YL, Zheng XJ. Repair mechanism of astrocytes and non-astrocytes in spinal cord injury. World J Clin Cases. 2020;8(5):854–863. doi: 10.12998/wjcc.v8.i5.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tran AP, Warren PM, Silver J. New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 2022;387(3):319–336. doi: 10.1007/s00441-021-03477-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Costachescu B, Niculescu AG, Dabija MG, Teleanu RI, Grumezescu AM, Eva L. Novel Strategies for Spinal Cord Regeneration. Int J Mol Sci. 2022;23:9. doi: 10.3390/ijms23094552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bao T, Li N, Chen H, et al. Drug-Loaded Zwitterion-Based Nanomotors for the Treatment of Spinal Cord Injury. ACS Appl Mater Interfaces. 2023;15(27):32762–32771. doi: 10.1021/acsami.3c05866. [DOI] [PubMed] [Google Scholar]
- 150.Behroozi Z, Rahimi B, Hamblin MR, Nasirinezhad F, Janzadeh A, Ramezani F. Injection of Cerium Oxide Nanoparticles to Treat Spinal Cord Injury in Rats. J Neuropathol Exp Neurol. 2022 doi: 10.1093/jnen/nlac026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murphy C, Thomas FP. Generative AI in spinal cord injury research and care: Opportunities and challenges ahead. J Spinal Cord Med. 2023;46(3):341–342. doi: 10.1080/10790268.2023.2198926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Khan O, Badhiwala JH, Grasso G, Fehlings MG. Use of Machine Learning and Artificial Intelligence to Drive Personalized Medicine Approaches for Spine Care. World Neurosurg. 2020;140:512–518. doi: 10.1016/j.wneu.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 153.Marrotte EJ, Johnson K, Schweller RM, et al. Induction of Neurogenesis and Angiogenesis in a Rat Hemisection Spinal Cord Injury Model With Combined Neural Stem Cell, Endothelial Progenitor Cell, and Biomimetic Hydrogel Matrix Therapy. Crit Care Explor. 2021;3(6):e0436. doi: 10.1097/CCE.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ebrahimian M, Hashemi M, Etemad L, Salmasi Z. Thymoquinone-loaded mesenchymal stem cell-derived exosome as an efficient nano-system against breast cancer cells. Iran J Basic Med Sci. 2022;25(6):723–731. doi: 10.22038/IJBMS.2022.64092.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fan L, Liu C, Chen X, et al. Exosomes-Loaded Electroconductive Hydrogel Synergistically Promotes Tissue Repair after Spinal Cord Injury via Immunoregulation and Enhancement of Myelinated Axon Growth. Adv Sci (Weinh). 2022;9(13):e2105586. doi: 10.1002/advs.202105586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Senger JB, Chan AWM, Chan KM, et al. Conditioning Electrical Stimulation Is Superior to Postoperative Electrical Stimulation in Enhanced Regeneration and Functional Recovery Following Nerve Graft Repair. Neurorehabil Neural Repair. 2020;34(4):299–308. doi: 10.1177/1545968320905801. [DOI] [PubMed] [Google Scholar]
- 157.Fadeev FO, Bashirov FV, Markosyan VA, et al. Combination of epidural electrical stimulation with ex vivo triple gene therapy for spinal cord injury: a proof of principle study. Neural Regen Res. 2021;16(3):550–560. doi: 10.4103/1673-5374.293150. [DOI] [PMC free article] [PubMed] [Google Scholar]