Abstract
Objective:
Implementation of evidence-based care processes (EBP) into the emergency department (ED) is challenging and there are only a few studies of real-world use of theory-based implementation frameworks. We report final implementation results and sustainability of an EBP geriatric screening program in the ED using the Consolidated Framework for Implementation Research (CFIR).
Methods:
The EBP involved nurses screening older patients for delirium (Delirium Triage Screen), fall risk (4-Stage Balance Test), and vulnerability (Identification of Seniors at Risk score) with subsequent appropriate referrals to physicians, therapy specialists, or social workers. The proportions of screened adults ≥65 years old were tracked monthly. Outcomes are reported January 2021–December 2022. Barriers encountered were classified according to CFIR. Implementation strategies were classified according to the CFIR-Expert Recommendations for Implementing Change (ERIC).
Results:
Implementation strategies increased geriatric screening from 5% to 68%. This did not meet our prespecified goal of 80%. Change was sustained through several COVID-19 waves. Inner setting barriers included culture and implementation climate. Initially, the ED was treated as a single inner setting, but we found different cultures and uptake between ED units, including night versus day shifts. Characteristics of individuals barriers included high levels of staff turnover in both clinical and administrative roles and very low self-efficacy from stress and staff turnover. Initial attempts with individualized audit and feedback were not successful in improving self-efficacy and may have caused moral injury. Adjusting feedback to a team/unit level approach with unitwide stretch goals worked better. Identifying early adopters and conducting on-shift education increased uptake. Lessons learned regarding ED culture, implementation in interconnected health systems, and rapid cycle process improvement are reported.
Conclusions:
The pandemic exacerbated barriers to implementation in the ED. Cognizance of a large ED as a sum of smaller units and using the CFIR model resulted in improvements.
INTRODUCTION
Each year, one-fifth of older adults in the United States receive health care in the emergency department (ED).1 The geriatric ED guidelines were derived to improve care for the many older patients in the ED.2 These guidelines recommend integrating validated screening tools for geriatric syndromes into the ED visit to improve the recognition and management of delirium, fall risk, and home health needs during the ED visit. Despite the guidelines and further research suggesting that geriatric screening improves care quality and resource utilization, very few EDs have successfully integrated geriatric screening.3 While work is being done in this area, there is currently little guidance on how to implement geriatric screening into the already complex ED work flow and culture.4 We report on the processes and outcomes of implementation of geriatric screening in a large academic ED.
Implementing a new process into any medical setting is difficult, but implementation into the fast paced, high acuity, continuously active ED setting is especially challenging. ED patient care activities are continuous and concurrent, so any new process initially requires a higher cognitive load for staff, can increase interruptions, and detracts from time spent on other types of patient care.5–7 Additionally, patients in the ED are the most heterogenous of any medical setting, so new processes often are not applicable to all patients. For instance, screening all older adults for fall risk may not apply to patients in nursing homes who already have fall risk precautions in place or to patients who are non-ambulatory. Some Emergency Medicine physicians can also perceive evidence-based medicine protocols as overriding their clinical judgment.8 Interviews of ED nurses revealed that they perceived geriatric screening for delirium as a very low priority activity due to the competing demands, lack of time, and heavy workload in the ED.9 Competing priorities is also an issue for emergency physicians and residents, who list time pressures, extra workload, and lack of routine as barriers to geriatric screening.10 Given these known barriers, we recognized that a pragmatic, comprehensive approach would be needed to implement geriatric screening into the ED.
Implementation science is a rigorous approach to quality improvement (QI) that focuses on improving local performance (adherence to evidence-based care) in a manner that produces generalizable knowledge about the process and outcomes. Implementation science uses frameworks and/or models to guide planning, execution, and outcome reporting.11 Our ED initially used an informal QI approach to begin geriatric screening for delirium, fall risk, and transition of care needs (the Identification of Seniors at Risk score) in 2018. Despite a 2-h nurse training program and administrative support, screening levels remained very low (5%–10%). The geriatric ED leadership team (consisting of the medical director for the geriatric ED, nurse educators, and nursing leadership) acknowledged that a different approach was needed. We decided to use the Consolidated Framework for Implementation Research (CFIR), an implementation science framework with a strong implementation focus and high-construct flexibility allowing us to apply it to the ED setting.12
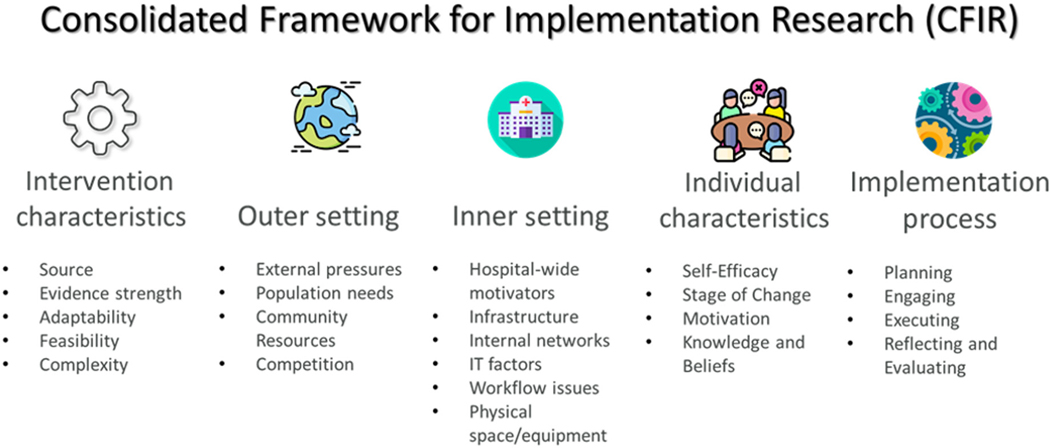
CFIR guides implementation efforts by organizing barriers and facilitators into intervention characteristics, outer setting, inner setting, individual characteristics, and implementation strategy (Figure 1). CFIR has previously been applied to the ED setting for the implementation of care pathways for syncope and asthma.13,14 For our geriatric ED, implementation planning began in 2020 with a survey of ED nurses based on CFIR and initial implementation strategies chosen according to the CFIR-Expert Recommendations for Implementation Change (CFIR-ERIC) tool.15 This raised geriatric screening rates from 5% to 42%, which was still not at goal of 80%. With successive challenges to EDs from external forces, such as COVID-19 waves and staffing barriers, implementation strategies had to be reassessed and adapted. While some strategies specifically addressed COVID-19–related barriers, most also addressed general barriers. We report lessons learned from implementation in a stressed ED system.
FIGURE 1.
The Consolidated Framework for Implementation Research (CFIR) includes five domains with multiple subdomains.
METHODS
Study type
Implementation study of a geriatric screening evidence-based care processes (EBP). Data collected for this study were collected as part of normal QI and operational processes and therefore was exempt from institutional review board review.
Setting
The setting is a 106-bed academic, tertiary referral hospital ED that cares for approximately 82,000 patients a year of which 20% are ≥65 years old. The ED includes a 15-bed oncology unit with oncology/emergency dual-trained nurses and a 20-bed ED observation unit. The ED was accredited as a Level I geriatric ED in 2018.16 One of the 66 EM physicians is geriatric-fellowship trained and one nurse educator is geriatric emergency nurse certified. There are 180 ED nurses. Prior to COVID-19, geriatric training for nurses initially involved a 2-h training session designed after the topics covered by the Emergency Nurses Association geriatric training program.
Evidence-based practice
The geriatric EBP protocol includes standardized delirium screening with the Delirium Triage Screen, fall risk evaluation with the 4-Stage Balance Test, and a protocol for assessment of function and functional decline with the Identification of Seniors at Risk tool (ISAR; see Data S1 and S2 for the assessments).17–19 The screening is done by the bedside nurse and takes 90 sec.20 The ED physician team reviews the results and orders any relevant consultations. Case managers are available during daytime hours for comprehensive home assessment, social workers are available 24/7 for elder abuse assessment or other assistance. Physical and occupational therapists are available during daytime hours Monday–Saturday. Geriatric consultation from the inpatient internal medicine geriatric consult service is available Monday– Friday during daytime hours. Comprehensive geriatric assessment, when needed, is typically done out of the ED observation unit to allow for care coordination between all these teams.21
Targeted population
The target population is all adults ≥65 years old in the ED. Exclusions for delirium screening and the ISAR screening include when the patient is unable to answer questions due to mental status changes or unable to answer questions due to medical treatment (such as intubation). Exclusions for balance testing include when the patient is unable to stand due to medical treatment or a medical condition, unable to follow commands, uncooperative with exam, or non-ambulatory at baseline.
Implementation support
The geriatric ED team consisted of two physicians; the ED nurse manager; three ED nurse educators; an ED resident; and champions from geriatrics, physical therapy, pharmacy, and case management. The ED group met biweekly to discuss data and current strategies, with the champions contacted as needed. A Tableau dashboard (Tableau Software) was built for quick data visualization. With the initial rollout in 2018, the bioinformatics team built the assessments into the hospital electronic health record (EHR; Epic Systems Corp.). The team had grant funding for implementation activities that covered some of the lead physician’s time, data analysis, and implementation supplies. There was no funding for increased staff involvement. Formal nursing education about the screening program was built into nurse onboarding and annual training by the ED nurse educators. Education to the ED physicians and advanced practice providers was provided formally in residency conference sessions and via email updates and presentations at monthly faculty meetings.
Nurses were contacted by group emails and individual audit emails and intermittently during daily shift change huddles and shift education. The nurses and/or physicians on the implementation team rounded in the ED at least twice weekly to check on progress, perform on-shift education/training, and troubleshoot any barriers to screening. The EHR had a column allowing quick visualization of older adults who had been screened and who still needed screening, allowing the rounder to easily find the appropriate nurses.
Implementation strategies
CFIR was used to design implementation strategies, guide data collection, and guide data interpretation. The design of the initial implementation strategies were derived from the previously reported baseline ED nurse survey.15 Additional strategies needed and initial strategy adaptations were chosen by the implementation team by consensus without a formal investigation given the rapidity of changing situations. Changes to the implementation strategies were tracked in meeting notes are reported using FRAME-IS (Framework for Reporting Adaptations and Modifications to Evidence-based Implementation Strategies; Data S3).22 FRAME-IS allows for standardized reporting of modifications, such as changing audit and feedback from monthly to weekly. Breaks in implementation team meetings and activities are reported in relation to hospital COVID volumes (Data S4).
The initial plan for the implementation strategy was to use Lean Six Sigma, a QI strategy of rapid cycle process improvement (define, measure, analyze, improve, and control) informed by data. A team charter, run charts, process mapping, and biweekly implementation meetings were accomplished according to define and measure phases of Lean Six Sigma. The analyze and improve phases were more difficult; because of staff shortages we could not adapt data reports easily, control when changes were rolled out, or adjust staffing or staff productivity.
Implementation outputs
The relevant process measure reported for this study is the proportion of older adults who received the geriatric screening or a documented exclusion to screening, extracted from the EHR retrospectively. The proportion of older adults receiving geriatric screening was reported monthly from January 2021 to December 2022. We also developed audit and feedback reports to individual nurses, residents, and attending physicians from these reports. Changes to implementation strategies were noted in the implementation team’s meeting notes and emails and retrospectively collated and aligned with CFIR categories by the study team.
Data analysis
The proportion of older adults screened was analyzed using run charts of monthly data and trendlines. As prior studies suggest that patient burden and competing priorities are barriers to ED screening, we recorded total ED volume, total encounters of patients ≥65 years, total COVID encounters, and boarding hours.8–10 Boarding hours are defined as each hour a patient admitted to the hospital is waiting in the ED to move to a hospital bed. Our health system reports this starting at Hour 4 after both the admission order and the admission service are placed. For example, a patient admitted at 07:00 who gets moved to a hospital bed at 10:00 will contribute no boarding hours. A patient admitted at 07:00 who gets moved to a hospital bed at 15:00 will contribute 4 boarding hours to the total report. ED boarding is reported as average boarding hours per day per month. ED boarding is a significant burden on ED staff as well as an adequate substitution for hospital capacity problems.23 We used line graphs over time to assess the impact of boarding and COVID-19 volumes on our process measure.
RESULTS
Implementation results
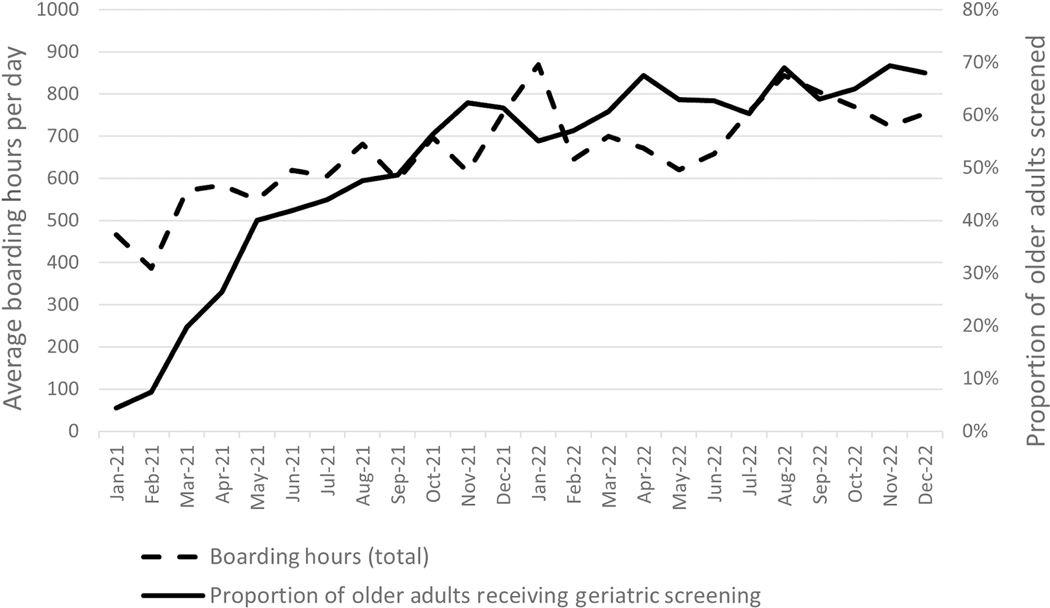
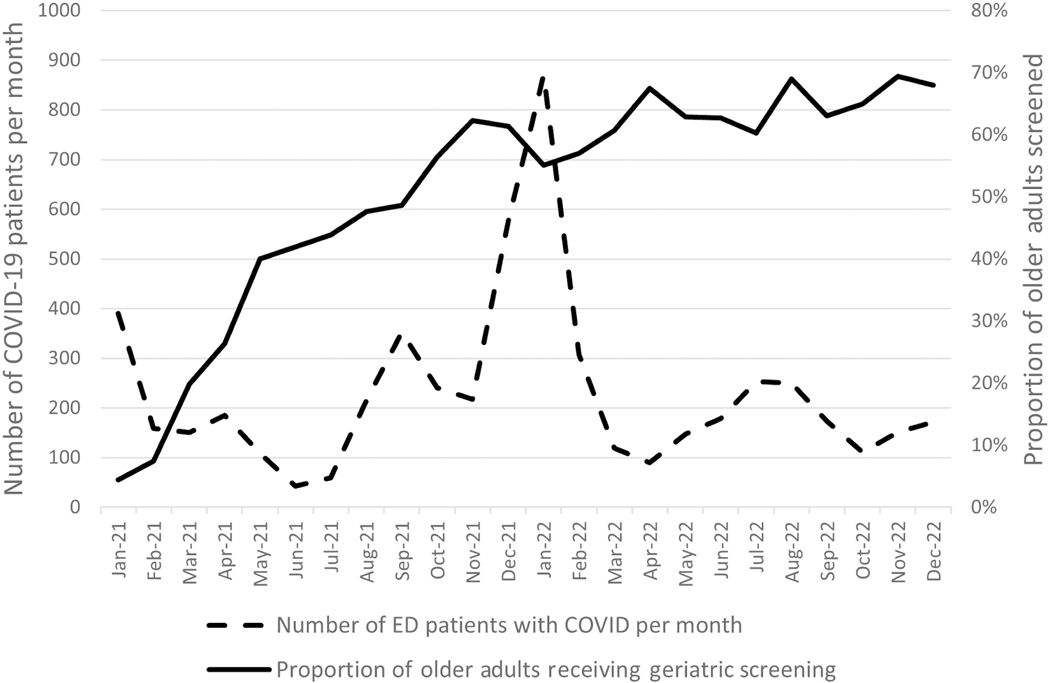
The implementation activities increased the geriatric screening rate from 5% to 62% within the first 8 months and 68% by the end of 24 months. In general, the rate improved from month to month except during two periods of high ED capacity as represented by average ED boarding hours (Figure 2). The first time period, December 2021 through January 2022, corresponded to high-capacity hospitals and boarding hours over 800 h per day during the winter COVID wave of 2021–2022 (Figure 3).24,25 The second, August 2022 through October 2022, corresponded to an increase in boarding over 800 h per day due to the triple pandemic of influenza virus, respiratory syncytial virus, and COVID-19. An ED with 800 h per day of boarding is the equivalent of having 33.3 beds filled with inpatients for 24 h, or, assuming a 1:5 nurse to patient ratios, 800 h per day of boarding is the equivalent of six to seven nurses dedicated to taking care of boarding inpatients per shift. During some high-capacity times, the hospital responded by clustering some boarding patients and assigning hospital inpatient nurses to the ED, but no more than three inpatient nurses were available during any given shift. This helped but did not alleviate the entire burden on ED nurses.
FIGURE 2.
Average daily hours of boarding inpatients in the ED per month and corresponding levels of geriatric screening during the study time frame.
FIGURE 3.
COVID-19 surge in December 2021–January 2022 resulted in temporarily pausing implementation activities and a drop in screening rates from 62% to 55%.
Implementation strategies
The initial implementation strategies of promote adaptability, alter incentive/allowance structures, and identify champions were chosen due to an initial survey of nurses and the barriers they identified.15 Individual audit and feedback was also chosen to track progress and promote ownership of the process. These were combined with increased on-shift education and educational emails. This combination improved screening from 5%–7% in January/February 2021 to 40% in May 2021. Improvement then slowed. Informal discussions with non-champion nurses were performed by several implementation team members. The implementation team noted high stress levels among nurses and were told by staff that the individual audit and feedback reports were considered unhelpful and were increasing feelings of moral injury among staff. In simple terms, when nurses already felt understaffed and overwhelmed, telling them they were not doing enough did not make them feel better or help them do better.
The next strategies chosen were inform local opinion leaders, involve executive boards, and audit and feedback by unit. The ED is divided into many smaller units—an oncology section, two traditional ED units, an observation unit, a mental health unit, and an arrivals unit (an area that consists of fast track or quick care rooms, the waiting room, and triage bays). The implementation team chose to focus efforts on the oncology unit because a Pareto chart demonstrated that it was one of the two lowest performing units, and the other low-performing unit did not clinically evaluate many older adults.26 Investigations found that the oncology unit had no nurse manager or nursing leadership to encourage the program and the nurses were feeling overwhelmed and understaffed. Tensions between the night and day shift teams were also discovered, with day shift nurses feeling like night shift nurses left many tasks undone. To address these issues, we worked with the newly hired nursing management team and nursing leadership, obtained more equipment for the unit, and allowed them to pick their own incentives (Table 1). Night shift champions were identified. One equipment request was more computers on wheels to care for patients boarded in hallways and chairs. These interventions improved adherence in this unit from 32% to 65%.
TABLE 1.
Barriers to implementation of geriatric screening in the ED and the strategies used by the implementation team to address them.
| CFIR domain | Initially identified barriers | Barriers encountered during Implementation | Strategies used |
|---|---|---|---|
| Intervention characteristics | |||
| Adaptability | Stakeholders do not believe that the intervention can be sufficiently adapted, tailored, or reinvented to meet local needs. | Shortage of hospital technology staff delayed IT interventions to address adaptability. | • Promote adaptabilitya: Added exclusions to screening to adapt the intervention to critically ill or nonambulatory patients. |
| COVID-19 specific: Intervention could not be done remotely, had to be in person, and had to be using PPE for COVID patients. | • Promote adaptabilitya: Some aspects were changed to telephone assessment, such as case manager assessment, but screening had to be in person for fall safety. | ||
| Complexity | Even 90 sec was considered too much to add on to clinical care when there were staffing shortages and/or high patient volumes. | • Paused implementation when ED staff were overwhelmed, October 2020-January 2021 and December 2021-January 2022. • Create new clinical teams: National Guard units were sent to assist the hospital and ED but were not able to do nursing assessments or the geriatric screening. |
|
| Outer setting | |||
| Peer pressure | There is little pressure to implement the intervention because other key peer or competing organizations have not already implemented the intervention nor is the organization doing this in a bid for a competitive edge. | COVID-19 specific: Reporting measures and national accreditations were even less meaningful with daily COVID patients coming to the ED. We had triage tents doing COVID screening and were focused on daily reports comparing COVID levels and crowding between hospitals than quality measures. | • Paused implementation when ED staff were overwhelmed, October 2020-January 2021 and December 2021-January 2022. |
| External policy and incentives | Lack of knowledge of external guidelines and accreditation policies. | • Inform local opinion leadersa: Educated senior nurses on external accreditation needs. | |
| Patient needs/resources | COVID-19 specific: Staff felt that the hospital and the community were not providing the resources to meet patient needs, leading to moral injury. | • Create new clinical teams: National Guard units were sent to assist the hospital and ED but were not able to do nursing assessments or the geriatric screening. • Change physical structure and equipment: We reevaluated how the intervention could help nurses address patient needs. The team provided additional equipment to assist in the care of delirious patients. We also changed the EHR to have a symbol to show on the trackboard when screening was not yet complete. |
|
| Inner setting | |||
| Culture | Cultural norms, values, and basic assumptions of the organization hinder implementation. | ED staff felt undervalued, which decreased motivation. | • Identify and prepare championsa: Day and night shift nurse champions for each unit were identified. Also, supporting champions from nurse assistants and unit clerks were helpful. • Involve executive boards: Nurse leadership had eroded with staff turnover. New leadership in the pods and restarting daily huddles improved the messaging that this was important to leadership. |
| Tension for change | Nurses do not see the current situation as intolerable or do not believe they need to implement the intervention. | COVID-19 specific: COVID led to frequent changes and an increased cognitive load, limiting the ability to consider change in other areas of clinical care. | • Involve executive boards: Messaging about the importance from nurse leadership. |
| Relative priority | Stakeholders perceive that implementation of the intervention takes a backseat to other initiatives or activities. | COVID-19 specific: Changes in organization, protocols and care pathways for different COVID waves and challenges made normal care processes as lower priority. | • Increase demand: Developed a template to pull the screening results into the physician notes and trained physician team to ask if screening not completed. |
| Organizational incentives and rewards | There are no tangible (e.g., goal-sharing awards, performance reviews, promotions, salary raises) or less tangible (e.g., increased stature or respect) incentives in place for implementing the intervention. | Nurses felt that even their regular pay was not incentive enough for working in the ED when patient volumes were high and/or staffing was low. Staff were motivated by different aspects of the program. We had to change incentives. |
• Alter incentive/allowance structuresa: Nurses chose their prices, incentives, and stretch goals. |
| Implementation climate | Tension about workload between night/day nurses and different nursing units. | • Identify and prepare championsa: Unit-specific night team champions helped to improve screening on nights and reduce the tension of patients left unscreened in the morning. | |
| Goals and feedback | COVID-19 specific: Lack of nursing leadership led to less feedback on goals. | • Alter incentive/allowance structuresa: Nurses chose their prices, incentives, and stretch goals. • Audit and provide feedbacka: Unitwide goals were more appreciated than individual feedback, which was felt to be punitive. Individual audit and feedback restarted May 2022. |
|
| Available resources | COVID-19 specific: Staffing resources decreased. Temporary shortages in supplies due to supply chain disruptions did not affect this program directly but added to the general stress of care. | • Conduct educational meetings: Rapid education of travelers and float nurses. • Conduct local needs assessment: Each unit was asked what they needed to make screening happen. Oncology pod needed more computers on wheels to assess patients in chairs and hallways. |
|
| Characteristics of individuals | |||
| Self-efficacy | Nurses did not have confidence in their capabilities to execute courses of action to achieve implementation goals. | Staff turnover led to loss of many senior nurses and intervention champions. Staff shortages and overtime hours limited time for education. At times, staff morale was very low, with high patient burdens, mandatory overtime, and lack of support. |
• [No matching CFIR-ERIC strategy]: Letting the nurses know when we were pausing the implementation pressure demonstrated solidarity. • Identify early adopters: Praising early adopters in email feedback and sharing tips from them. • Conduct educational outreach visits: Rapid on shift education to providers and nurses was provided. Identified many people who never read the educational emails. |
| Individual stage of change | Nurses were not enthusiastic about using the intervention in a sustained way. | • Make training dynamic: Training decreased from a 2-h didactic session to lots of huddle updates and on-shift training. This made it seem more clinically practical. • Increase demand: Educated residents and gave individual resident feedback on how well their patients were getting screened. This pulling demonstrated that the information was important to clinical care. This idea came from the Lean Six Sigma approach. |
|
| Process of implementation | |||
| Process | Lean Six Sigma, a form of rapid cycle process improvement. | Rapid changes could not be done due to lack of staff to make EHR modifications or data analyses. | • Purposely reexamine the implementation: Lean Six Sigma was not adhered to strictly, as rapid process improvement could not be used. Also variability in boarding, staffing, and COVID levels made lean analysis ineffective. |
| Opinion leaders and champions | Staff turnover led to loss of many senior staff and opinion leaders as well as project champions. | • Identify and prepare championsa: Team had to frequently reengage new nursing leadership and opinion leaders. | |
| Engaging | Engaging nurses had to be done on shift with quick questions as overtime and harder shifts led to minimal staff showing up for engagement events. | • Alter incentive/allowance structuresa: Nurses chose their prices, incentives, and stretch goals. • Conduct educational outreach visits: Rapid on-shift education to providers and nurses was provided. |
|
| Audit and feedback | Initial plan was for individual audit and feedback. | Individual feedback was ignored or demoralizing for many. | • Audit and provide feedback: Unitwide goals were more appreciated than individual feedback, which was felt to be punitive. Individual audit and feedback started May 2022. • Audit and provide feedback: Feedback to providers was also given to faculty and residents, with monthly acknowledgement for those reaching >80% screening for their patients. • Remind clinicians: Built in a reminder nudge into the EHR trackboard. |
Note: Some strategies targeted multiple barriers and so are listed multiple times.
Abbreviations: CFIR, Consolidated Framework for Implementation Research; EHR, electronic health records; ERIC, Expert Recommendations for Implementation Change.
Denotes an initial strategy chosen by the implementation team using the CFIR-ERIC tool.
One adherence obstacle we were not able to overcome was the number of patients evaluated in the arrival zone (i.e., waiting room, triage chairs, or hallway chairs). This became common due to the high rates of ED boarding. Often these patients did not get a dedicated assigned nurse and care was not standardized. For example, in December 2022, a total of 117 older adults (9.1%) were seen out of the waiting room, triage, or hallway chairs and 51 (4%) never had a nurse assigned. Overcrowding led to care being provided in suboptimal spaces.
Lessons learned about ED implementation
Intervention characteristics
The intervention involved documentation in the EHR, which was linked to other spaces in the health system. The health system has two EDs and multiple urgent care facilities, but implementation was only at one ED site. Any changes to that site’s EHR would affect the other ED units and potentially documentation at health system urgent cares as well. For this reason, a hard stop reminder in the chart to perform or review the screening prior to discharge was not possible. We were able to make some EHR changes, but they could not be pushed out to all providers and had to be individually “wrenched” into the views for each staff member. The lesson is that implementation in units or single sites within large health systems can be complicated by interconnected EHRs.
Process
We adjusted our process of implementation (i.e., Lean Six Sigma) because rapid cycle process improvement was not possible during the pandemic. IT staffing shortages resulted in delays in EHR changes. There were many external factors impacting nurse screening, such as staff turnover of both staff nurses and travel nurses, and changes in capacity, which influenced our ability to engage staff and interpret monthly changes. Additionally, all implementation activities were halted when the hospital was over capacity and the implementation team had increased clinical duties. Patient volumes were very high and the Ohio National Guard was deployed to help the hospital between January 2022 and March 2022. Requested changes to the EHR and data reports also took many months to be completed, so “rapid” changes in process were not possible. The lesson learned is that Lean Six Sigma and rapid cycle process improvement are not possible in low-resource and low-staff settings or when staff are already functioning at their highest capacity.
Inner setting
Inner setting barriers included culture and implementation climate. Initially, the ED was treated as one inner setting but we found different cultures and uptake between nursing units. Uptake in the ED observation unit was the highest. This was anticipated as the observation unit was our pilot unit. Uptake in the mental health and arrival units was low. Adjusting feedback to a team/unit level approach with unitwide stretch goals was successful. Prizes for reaching stretch goals included white noise machines with ceiling light projectors to help patients with delirium and personal staff rewards system points through the hospital. Relative priority was also a barrier when nurses were short staffed and prioritizing time sensitive medical interventions over less critical action items such as fall risk screening. The lesson learned is that a large ED is not a single setting, but a combination of smaller units each with its own culture, including day/night variations.
Characteristics of Individuals
Barriers included high levels of staff turnover in both front-line and administrative roles and very low self-efficacy from stress and staff turnover. In 2021 we had 21 traveler nurses in the ED (12% of total ED nurses) and were using many critical care float pool nurses. In 2022 staffing shortages continued and the ED hired 57 travel nurses (32% of ED nurses). Float and travel nurses required rapid individual on-shift education as group rollouts were not possible with new staff joining at irregular intervals. Additionally, our initial program champions were all in nurse leadership and management, which also led some to feel that the program was not in touch with what was happening “in the trenches.” Recruiting and training clinical registered nurses as advocates and champions for each unit and day/night shift improved self-efficacy. The lesson is that an unwanted side effect of QI processes can be increased stress on staff. Including bedside nurses in the implementation team from the beginning could have improved implementation.
DISCUSSION
Implementation of EBP in the ED is difficult, and our team found that ED overcrowding (as measured by inpatient boarding), understaffing, and staff stress all contributed to decreased ability to adopt, adapt, and sustain quality measures. While we were not able to reach our goal of >80% compliance, the team did improve compliance to >65%, which has remained stable. This could indicate routinization of the process and culture change.
Our success rate is better than previous studies of geriatric screening in the ED. A prepandemic study of implementation of geriatric screening in a small Dutch ED (26,000 annual visits) reached 59% compliance.27 Another study in a mid-sized ED (67,000 annual visits) that added technical staff assistants to perform the geriatric screening improved fall risk screening to 67% and cognition screening to 38%.28 The most recently reported results come from a consortium of Veteran’s Affairs EDs in the United States. This group used a boot camp and collaborative educational approach in 50 EDs and found compliance ranged from 11% to 24% for delirium screening, ~2% for fall risk screening, and from 31% to 58% for the ISAR.29 These studies together with our own suggest that high compliance with geriatric screening is difficult to achieve across ED settings: large, small, Veterans Affairs, academic, or community EDs. Interestingly, the implementation science approach used in this study resulted in the highest reported compliance to date, despite the disadvantages of not adding additional staff and COVID-19 pandemic barriers.
Implementation was complicated by staffing turnover and shortages, ED crowding, and a lower relative priority for the staff and health system at large as it dealt with COVID-19 and other respiratory viral surges across our community. These barriers could have caused us to adapt the intervention, such as dropping some screening or documentation burdens. However, we chose instead to adapt our implementation strategies. The essential intervention (fidelity to core elements) remained unchanged. Using FRAME-IS language, the training of personnel was adapted and implementation strategies was adjusted (sometimes ad hoc) due to changing systems and staffing.22 Intervention fidelity was possible because the intervention was fast and had been previously piloted to address barriers. Even so, we still discovered necessary EHR adaptions to make it easier for the physicians to document, increased equipment needs (such as computers on wheels), and debated changing the timing of the intervention within patient flow through the ED.
One barrier that we could not find an implementation strategy for was ED crowding and boarding. We found that when average daily boarding increased month to month, there was a visual trend with decreasing geriatric screening rates. Other ED studies have found an association between boarding and compliance with quality metrics. A 2018 systematic review of consequences of ED crowding found that crowding was associated with decreased guideline-recommended therapies and lower likelihood of adherence to sepsis measures.30 While increased workload may be one reason why compliance to guideline measures is difficult in the ED, in regard to geriatric screening there are likely additional barriers than workload. ED staff may be less likely to perform geriatric screening when stress and crowding is high because many feel confident in their ability to detect and manage delirium without screening tools. In one study of seven EDs in Canada, the team prospectively evaluated ED patients for delirium and found that staff were very self-confident in their ability to recognize delirium (8/10 confidence) but in practice missed half of the patients with CAM-ICU–positive delirium.31 ED nurses have also reported that delirium screening is a low priority in the ED and that knowing who was delirious was less important than other clinical tasks.9 The combination of high self-confidence and low prioritization makes convincing ED staff to use formal tools difficult and likely contributes to the low uptake seen in many studies. Additionally, relative priority could be lower because we lack compelling evidence for delirium interventions.32,33
In this study, low prioritization of this task over others in the ED was targeted with several implementation strategies: increase demand, alter incentive/allowance structures, and assess for readiness and identify barriers and facilitators.15 Despite this, the screening remained lower priority for many ED nurses. Given the acuity of patients in the ED and high-priority competing demands, a national discussion is needed about the goal rates of compliance for different quality measures in the ED. Should antibiotics for an unstable patient with sepsis come before delirium screening for a stable patient? Probably so. If high levels of compliance are necessary for the highest quality of patient care, then EDs need to reevaluate their current staffing levels to reduce workload on ED nurses. Nurse staffing in the ED is typically run very lean, even in comparison to inpatient staffing. Ratios as high as 16 beds per nurse have been reported, with one study from New Jersey finding ED staffing of eight to 11 patients per nurse in comparison to inpatient staffing of four to five patients per nurse.34,35 ED nurses are expected to care for more patients and more critical patients than inpatient teams, which means each shift nurses are prioritizing care needs of their patients. Relative prioritization will likely be a barrier for any QI endeavor in the ED. Until staffing levels are improved to where nurses no longer have to prioritize care, the true goal of an ED screening program should not be 100%.
One method of improving prioritization of geriatric screening for this study that we did not use during implementation was a hard stop or “best practice advisory” in the EHR. These have had mixed success in other QI programs.36–38 In addition to these mixed data and general feelings among nurses and physicians that interruptions in care are not well liked, this option was infeasible for this study due to the shared EHR. The single health system has two EDs and multiple urgent cares, and the shared EHR would have required that the hard stops be placed before any ED discharge, including where implementation was not occurring.
LIMITATIONS
Limitations to the generalizability of this study include that having external funding and external impetus from the geriatric ED accreditation program likely improved our team’s abilities and allowed us to employ different strategies, such as alter incentives. Other programs may not have funding for prizes and stretch goals. Another limitation is that this program was pilot tested and refined prior to the start of implementation, which other EDs may not be able to do. However, we feel our discoveries about culture change, moral injury from audit and feedback, and difficulties from ED crowding are not affected by these limitations to generalizability of our process. Finally, at this time we do not know the impact of this improved screening on patient outcomes. A sister arm of the study is evaluating the effectiveness of geriatric screening on patient centered outcomes, and that study is still ongoing.
CONCLUSIONS
The implementation team found Consolidated Framework for Implementation Research to be useful in planning for implementation and tracking strategies. Despite not reaching our predefined goal, we learned lessons about ED culture and the impact on quality improvement, the usefulness of audit and feedback when staff are under stress, and how larger health systems may be limited in electronic health record changes if they are rolling out quality improvement procedures in only one area or one ED. As research on geriatric screening continues to suggest their utility in the care of older adults with acute illness, we suspect many more EDs will be creating geriatric screening protocols. We hope these lessons will help future EDs planning geriatric screening or other quality improvement in the ED.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank John Brown who assisted with the EHR changes. We are also grateful to all the ED nurses and nonnurse ED staff members who were champions for the program, from unit clerks and nursing assistants through physical therapists and case managers.
Funding information
LTS was funded by NIA K23AG061284. KMH was funded by NIA K76 AG074941. The funders had no role in the study design, interpretation of data, or writing of the report.
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1.U.S. Department of Health and Human Services, Administration on Community Living. 2021 Profile of Older Americans. ed. 2022. Accessed October 20, 2022. https://acl.gov/sites/default/files/Profile%20of%20OA/2021%20Profile%20of%20OA/2021ProfileOlderAmericans_508.pdf
- 2.American College of Emergency Physicians, American Geriatrics Society, Emergency Nurses Association, Society for Academic Emergency Medicine, Geriatric Emergency Department Guidelines Task Force. Geriatric emergency department guidelines. Ann Emerg Med. 2014;63(5):e7–e25. doi: 10.1016/j.annemergmed.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 3.Kennedy M, Lesser A, Israni J, et al. Reach and adoption of a geriatric emergency department accreditation program in the United States. Ann Emerg Med. 2022;79(4):367–373. doi: 10.1016/j.annemergmed.2021.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaughan CP, Burningham Z, Kelleher JL, et al. A cluster randomized trial of two implementation strategies to deliver audit and feedback in the EQUIPPED medication safety program. Acad Emerg Med. 2023;30:340–348. doi: 10.1111/acem.14697 [DOI] [PubMed] [Google Scholar]
- 5.Ruskin KJ, Stiegler MP, Rosenbaum SH. Quality and Safety in Anesthesia and Perioperative Care. Oxford University Press; 2016. [Google Scholar]
- 6.Chisholm CD, Collison EK, Nelson DR, Cordell WH. Emergency department workplace interruptions: are emergency physicians “interrupt-driven” and “multitasking”? Acad Emerg Med. 2000;7(11):1239–1243. doi: 10.1111/j.1553-2712.2000.tb00469.x [DOI] [PubMed] [Google Scholar]
- 7.Mobeen A, Shafiq M, Aziz MH, Mohsin MJ. Impact of workflow interruptions on baseline activities of the doctors working in the emergency department. BMJ Open Qual. 2022;11(3):e001813. doi: 10.1136/bmjoq-2022-001813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabbour M, Newton AS, Johnson D, Curran JA. Defining barriers and enablers for clinical pathway implementation in complex clinical settings. Implement Sci. 2018;13(1):139. doi: 10.1186/s13012-018-0832-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eagles D, Cheung WJ, Avlijas T, et al. Barriers and facilitators to nursing delirium screening in older emergency patients: a qualitative study using the theoretical domains framework. Age Ageing. 2022;51(1):afab256. doi: 10.1093/ageing/afab256 [DOI] [PubMed] [Google Scholar]
- 10.van der Burgh R, Wijnen N, Visscher M, de Groot B, Lucke J. The feasibility and acceptability of frailty screening tools in the emergency department and the additional value of clinical judgment for frailty detection. Eur J Emerg Med. 2022;29(4):301–303. doi: 10.1097/MEJ.0000000000000910 [DOI] [PubMed] [Google Scholar]
- 11.Pinnock H, Barwick M, Carpenter CR, et al. Standards for reporting implementation studies (StaRI) statement. BMJ. 2017;356:i6795. doi: 10.1136/bmj.i6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabak RG, Khoong EC, Chambers DA, Brownson RC. Bridging research and practice: models for dissemination and implementation research. Am J Prev Med. 2012;43(3):337–350. doi: 10.1016/j.amepre.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Smyth SS, Clouser JM, McMullen CA, Gupta V, Williams MV. Planning implementation success of syncope clinical practice guidelines in the emergency department using CFIR framework. Medicina (Kaunas). 2021;57(6):570. doi: 10.3390/medicina57060570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shade L, Ludden T, Dolor RJ, et al. Using the consolidated framework for implementation research (CFIR) to evaluate implementation effectiveness of a facilitated approach to an asthma shared decision making intervention. J Asthma. 2021;58(4):554–563. doi: 10.1080/02770903.2019.1702200 [DOI] [PubMed] [Google Scholar]
- 15.Southerland LT, Hunold KM, Van Fossen J, et al. An implementation science approach to geriatric screening in an emergency department. J Am Geriatr Soc. 2022;70(1):178–187. doi: 10.1111/jgs.17481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American College of Emergency Physicians. Geriatric Emergency Department Accreditation Program. Accessed October 20, 2022. https://www.acep.org/geda/#sm.0000h0eb9k15z2emhtnd9t8m2cloj
- 17.Han JH, Wilson A, Vasilevskis EE, et al. Diagnosing delirium in older emergency department patients: validity and reliability of the delirium triage screen and the brief confusion assessment method. Ann Emerg Med. 2013;62(5):457–465. doi: 10.1016/j.annemergmed.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens JA. The STEADI tool kit: a fall prevention resource for health care providers. IHS Prim Care Provid. 2013;39(9):162–166. [PMC free article] [PubMed] [Google Scholar]
- 19.McCusker J, Bellavance F, Cardin S, Trepanier S. Screening for geriatric problems in the emergency department: reliability and validity. Identification of seniors at risk (ISAR) steering committee. Acad Emerg Med. 1998;5(9):883–893. [DOI] [PubMed] [Google Scholar]
- 20.Elder NMBK, Gregory ME, Gulker P, Southerland LT. Are geriatric screening tools too time consuming for the emergency department? A workflow time study. J Geriatr Emerg Med. 2021;2(6). Accessed October 20, 2022. https://gedcollaborative.com/wp-content/uploads/2021/05/Are-Geriatric-Screening-Tools-Too-Time-Consuming-for-the-Emergency-Department-A-Workflow-Time-Study.pdf [Google Scholar]
- 21.Southerland LT, Vargas AJ, Nagaraj L, Gure TR, Caterino JM. An emergency department observation unit is a feasible setting for multidisciplinary geriatric assessments in compliance with the geriatric emergency department guidelines. Acad Emerg Med. 2018;25(1):76–82. doi: 10.1111/acem.13328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller CJ, Barnett ML, Baumann AA, Gutner CA, Wiltsey-Stirman S. The FRAME-IS: a framework for documenting modifications to implementation strategies in healthcare. Implement Sci. 2021;16(1):36. doi: 10.1186/s13012-021-01105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moskop JC, Sklar DP, Geiderman JM, Schears RM, Bookman KJ. Emergency department crowding, part 1–concept, causes, and moral consequences. Ann Emerg Med. 2009;53(5):605–611. doi: 10.1016/j.annemergmed.2008.09.019 [DOI] [PubMed] [Google Scholar]
- 24.Griffin G, Krizo J, Mangira C, Simon EL. The impact of COVID-19 on emergency department boarding and in-hospital mortality. Am J Emerg Med. 2023;67:5–9. doi: 10.1016/j.ajem.2023.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilaru AS, Scheulen JJ, Harbertson CA, Gonzales R, Mondal A, Agarwal AK. Boarding in US academic emergency departments during the COVID-19 pandemic. Ann Emerg Med. 2023;S0196–0644(22)01328–2. doi: 10.1016/j.annemergmed.2022.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hessing T. Pareto Chart (Pareto Diagram). Accessed October 20, 2022. https://sixsigmastudyguide.com/pareto-chart/
- 27.Blomaard LC, de Groot B, Lucke JA, et al. Implementation of the acutely presenting older patient (APOP) screening program in routine emergency department care: a before-after study. Z Gerontol Geriatr. 2021;54(2):113–121. doi: 10.1007/s00391-020-01837-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hullick C, Conway J, Higgins I, et al. An assistant workforce to improve screening rates and quality of care for older patients in the emergency department: findings of a pre- post, mixed methods study. BMC Geriatr. 2018;18(1):126. doi: 10.1186/s12877-018-0811-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang U, Runels T, Han L, et al. Dissemination and implementation of age-friendly care and geriatric emergency department accreditation at veterans affairs hospitals. Acad Emerg Med. 2023;30:270–277. doi: 10.1111/acem.14665 [DOI] [PubMed] [Google Scholar]
- 30.Morley C, Unwin M, Peterson GM, Stankovich J, Kinsman L. Emergency department crowding: a systematic review of causes, consequences and solutions. PloS One. 2018;13(8):e0203316. doi: 10.1371/journal.pone.0203316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JS, Tong T, Chignell M, et al. Prevalence, management and outcomes of unrecognized delirium in a National Sample of 1,493 older emergency department patients: how many were sent home and what happened to them? Age Ageing. 2022;51(2):afab214. doi: 10.1093/ageing/afab214 [DOI] [PubMed] [Google Scholar]
- 32.Shih RD, Carpenter CR, Tolia V, Binder EF, Ouslander JG. Balancing vision with pragmatism: the geriatric emergency department guidelines-realistic expectations from emergency medicine and geriatric medicine. J Am Geriatr Soc. 2022;70(5):1368–1373. doi: 10.1111/jgs.17745 [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Chen H, Hibino S, et al. Can we improve delirium prevention and treatment in the emergency department? A systematic review. J Am Geriatr Soc. 2022;70(6):1838–1849. doi: 10.1111/jgs.17740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wise S, Fry M, Duffield C, Roche M, Buchanan J. Ratios and nurse staffing: the vexed case of emergency departments. Australas Emerg Nurs J. 2015;18(1):49–55. doi: 10.1016/j.aenj.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 35.de Cordova PB, Jones T, Riman KA, Rogowski J, McHugh MD. Staffing trends in magnet and non-magnet hospitals after state legislation. J Nurs Care Qual. 2020;35(4):323–328. doi: 10.1097/NCQ.0000000000000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szlosek D, Haydar SA, Williams RJ, et al. The impact of an electronic best practice advisory on brain computed tomography ordering in an academic emergency department. Am J Emerg Med. 2017;35(11):1776–1777. doi: 10.1016/j.ajem.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 37.Ford JS, Chechi T, Toosi K, et al. Universal screening for hepatitis C virus in the ED using a best practice advisory. West J Emerg Med. 2021;22(3):719–725. doi: 10.5811/westjem.2021.1.49667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valentino K, Campos GJ, Acker KA, Dolan P. Abnormal vital sign recognition and provider notification in the pediatric emergency department. J Pediatr Health Care. 2020;34(6):522–534. doi: 10.1016/j.pedhc.2020.05.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.