Abstract
As a key sensor of double-stranded DNA (dsDNA), cyclic GMP-AMP synthase (cGAS) detects cytosolic dsDNA and initiates the synthesis of 2′3′ cyclic GMP-AMP (cGAMP) that activates the stimulator of interferon genes (STING). This finally promotes the production of type I interferons (IFN-I) that is crucial for bridging innate and adaptive immunity. Recent evidence show that several antitumor therapies, including radiotherapy (RT), chemotherapy, targeted therapies and immunotherapies, activate the cGAS-STING pathway to provoke the antitumor immunity. In the last decade, the development of STING agonists has been a major focus in both basic research and the pharmaceutical industry. However, up to now, none of STING agonists have been approved for clinical use. Considering the broad expression of STING in whole body and the direct lethal effect of STING agonists on immune cells in the draining lymph node (dLN), research on the optimal way to activate STING in tumor microenvironment (TME) appears to be a promising direction. Moreover, besides enhancing IFN-I signaling, the cGAS-STING pathway also plays roles in senescence, autophagy, apoptosis, mitotic arrest, and DNA repair, contributing to tumor development and metastasis. In this review, we summarize the recent advances on cGAS-STING pathway’s response to antitumor therapies and the strategies involving this pathway for tumor treatment.
Keywords: cGAS, STING agonist, IFN-I, tumor
Introduction
Despite cancer remaining a significant contributor to global mortality [1], the past few decades have witnessed new milestones in clinical antitumor therapies which substantially increase tumor survival. Notably, the emergence of immune checkpoint blocking therapy (ICB) and chimeric antigen receptor (CAR)-T cell therapy have ushered in a new era in the field of clinical cancer treatment. Unfortunately, immunotherapies yield tumor remission for only a small fraction of patients. Therefore, it is imperative to increase the response rate of ICB therapy and resolve the bottleneck issue of CAR-T therapy in solid tumor treatment [2, 3].
The activation of innate immune sensing serves as a crucial mechanism for inducing adaptive antitumor immune responses inside the tumor microenvironment (TME); therefore, the therapeutic effect heavily relies on innate immune sensing pathways. Mechanistically, innate immune sensing, generally initiating in antigen-presenting cells (APCs) or macrophages, facilitating antigen processing and presentation to prime tumor-specific CD8+ T cells by promoting the production of type I interferons (IFN-I) and other proinflammatory cytokines [4], [5], [6], [7], [8], [9]. Moreover, innate immune sensing could also recruit APCs and CD8+ T cells to the TME and activate CD8+ T cells or natural killer (NK) cells to eliminate tumor cells. Activation of innate immune sensing pathways has consistently been linked to better prognosis and improved overall survival in cancer patients receiving conventional therapies or immunotherapies [10], [11], [12]. Up to now, several tactics have been devised with the objective of activating intrinsic sensing pathways, and certain approaches have demonstrated the potential to enhance the efficacy of conventional anticancer treatments. However, innate immune sensing pathways are intended to be the first line of defense against pathogen invasion and thus are broadly expressed across the whole body. This not only predicts how toxicity is induced, but also highlights the significance of localizing the innate immune sensing activation in TME.
Innate immune sensing is mediated by pattern-recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) releasing from treated tumor tissues to trigger the innate and adaptive antitumor immunity [9, 13], [14], [15]. In the past decade, cytoplasmic double-stranded DNA (dsDNA) sensing pathway via cyclic GMP-AMP synthase (cGAS) stimulator of interferon genes (STING) has received more and more attention in basic research and clinical practice. Numerous studies have uncovered the critical role of cGAS-STING in traditional antitumor therapies and immunotherapy [16], [17], [18], [19], [20]. The cGAS, as a cytoplasmic dsDNA sensor, recognizes various exogenous dsDNA and also self-derived dsDNA to resist pathogen infection and reject cancer, also contributes to autoimmune disorders [21], [22], [23], [24], [25], [26], [27], [28]. Following its recognition of dsDNA, cGAS assembles into a dimer or oligomer ladder to synthesize cyclic GMP-AMP (cGAMP), which binds to STING in the endoplasmic reticulum (ER), and translocates to endoplasmic reticulum (ER)-Golgi intermediate compartment (ERGIC) and Golgi [29]; furthermore, activated STING will activate downstream signaling. During STING transport activation, armadillo-like helical domain-containing protein 3 (ARMH3) recruits phosphatidylinositol 4-kinase beta (PI4KB) to synthesize phosphatidylinositol 4-phosphate (PI4P), which directs STING Golgi-to-endosome trafficking, and helps STING activate more stably and efficiently [30]. This will lead to production of pro-inflammatory cytokines, especially IFN-I and interleukin (IL)-1β, to initiate inflammatory responses and activate CD8+ T cells [4], [5], [6], [7, 31], [32], [33]. In the classical framework of tumor immunology, radiotherapy (RT) and immunotherapy activate innate immune sensing pathways, particularly the cGAS-STING pathway, controlling tumors through immune activation beyond the direct killing effect [15, 16, 33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]. Nevertheless, recent works provided evidence supporting that cGAS-STING could also trigger the initiation of autophagy and suppress the RT-mediated repair of DNA damage to diminish the RT-induced antitumor immunity [47, 48]. Additionally, persistent activation of cGAS-STING signaling leads to a pro-tumor effect by reshaping an immunosuppressive TME, thus helping tumor evade immunosurveillance and promoting metastasis [49, 50]. Therefore, the cGAS-STING pathway exhibits dual functionality in the TME: one to be hijacked by tumor cells to resist therapeutic interventions and facilitate tumor progression, the other to provoke anti-tumor immunity by innate immune sensing. Here, we review the advances of cGAS-STING mediated dsDNA sensing in tumor study and emphasize the significance of local activation of cGAS-STING signaling in TME.
Regulatory mechanisms of the cGAS-STING pathway
As one of the primary cytosolic DNA sensors, cGAS exhibits a particular ability to detect cytosolic dsDNA [51] or RNA-DNA hybrids [52] in a sequence independent manner and catalyzes the conversion of GTP and ATP to 2′3′-cGAMP. The activation of STING by 2′3′-cGAMP triggers an inflammatory response via the TANK-binding kinase 1 (TBK1)-Interferon Regulatory Factor 3 (IRF3) pathway, ultimately leading to the production of IFN-I [5, 23]. Importantly, the IFN-I further bridges the innate and adaptive immune response by promoting the maturation, activation and migration of DC, T cells and NK cells, while also impeding regulatory T (Treg) cells and inhibitory macrophage differentiation [4, 6, 53]. Intriguingly, both cGAS and STING are IFN-stimulated genes (ISGs), indicating a positive feedback loop in this pathway. Persisting cytosolic dsDNA will thereafter augment IFN-I response by boosting local cGAS production and subsequent cGAS activation via positive feedback [54]. In parallel, STING also activates I kappa B kinase (IKK) to mediate the induction of Nuclear factor Kappa-B (NF-κB)-driven pro-inflammatory cytokines production, such as tumor necrosis factor-α (TNF-α), IL-1β and IL-6 [8, 9, 55].
Mutual regulation of cGAS-STING and cell death pathways
The cGAS-STING pathway not only enhances the production of proinflammatory cytokines, but it also plays a crucial role in initiating cell death through many mechanisms. Intact cGAS-STING pathway is activated by treatment to regulate tumor cell growth, senescence and immune surveillance [55]. Therefore, in multiple cancers, cancer cells often exhibit abnormalities in the cGAS-STING pathway as a result of epigenetic hypermethylation [56, 57]. Additionally, cell death pathways can also be hijacked by tumor cells or pathogens to suppress the activation of cGAS-STING pathway and diminish the immune response [58, 59] (Figure 1).
Figure 1:
cGAS-STING and cell death pathways. cGAS recognizes cytosolic dsDNA released from the nucleus, mitochondria or exogenous pathogens, and then catalyzes the conversion of GTP and ATP to cGAMP. cGAMP initiates an inflammatory response via the SITNG-TBK1-IRF3-IFN and IKK-NF-κB pathways. cGAS-STING also involves in regulating cell death pathways. Chronic activation of STING increases the mitochondrial apoptotic pathway by enhancing the interaction of IRF-3 and Bax. STING also activates pyroptosis by initiating potassium efflux and upregulating and maintaining NLRP3. On the contrary, activated CASP9/3/7/1 will suppress the activation of cGAS-STING pathway and avoid to awaking the antitumor immunity. Moreover, STING also regulates necroptosis through ZBP1-RIPK3-MLKL axis. Furthermore, STING also activate autophagy independent of TBK1 and IFN by translocating to ERGIC and Golgi, which leads to LC3 lipidation and autophagosome formation. cGAS, GMP-AMP synthase; dsDNA, double-stranded DNA; cGAMP, cyclic GMP-AMP; SITNG, stimulator of interferon genes; TBK1, TANK-binding kinase 1; IRF3, Interferon Regulatory Factor 3; IFN, interferons; IKK, I kappa B kinase; NF-κB, nuclear factor Kappa-B; CASP, caspase; ZBP1, Z-DNA binding protein 1; MLKL, mixed-linage kinase domain like; ERGIC, endoplasmic reticulum-Golgi intermediate compartment.
Persistent stimulation of cGAS-STING signaling can directly induce cell death in a variety of machineries. Importantly, STING-mediated IRF-3 activation enhances the interaction between IRF-3 and the pro-apoptotic protein Bcl2-associated X gene (Bax) and thereby triggers the mitochondrial apoptotic pathway [60]. In addition, IFN-I could upregulate apoptosis inducers, such as TNF-related apoptosis-inducing ligand (TRAIL), initiating cell death [61], [62], [63]. Furthermore, STING can also activate autophagy through a mechanism independent of TBK1 and IFN-I [47, 64], which also involves in negatively regulating the activation of cGAS-STING pathway and cell death. Upon binding cGAMP, STING translocates to the ERGIC and Golgi resulting in the lipidation of microtubule-associated protein 1 light chain 3 (MAP1LC3, hereafter referred to as LC3) and the fomation of autophagosomes, to defend against viral infection or degradation of damaged organelles [47]. Moreover, the cGAS-STING pathway activates receptor-interacting protein kinase 3 (RIPK3) to initiate necroptosis, which is coordinated by the actions of with IFN-I and TNF-α [65]. Meanwhile, cGAS-STING also regulates necroptosis by maintaining the expression of the key necroptosis executor mixed-linage kinase domain like (MLKL) [66], or through the Z-DNA binding protein 1 (ZBP1)-RIPK3 axis [65, 67], [68], [69]. Recent studies have also shown that STING could facilitate the activation of pyroptosis by initiating potassium efflux and upregulating and maintaining expression of NLR family pyrin domain containing 3 (NLRP3) [70, 71].
Caspase 9 (CASP9) is critical to initiate intrinsic apoptosis. In 2014, Richard A. Flavell and Benjamin T. Kile groups separately reported that viral infection or stress-induced cell apoptosis could trigger mitochondrial membrane permeabilization, thereby resulting in mitochondrial DNA (mtDNA) release into the cytosol and activation of CASP9 signaling. However, the activation of CASP9/3/7 further suppresses the mtDNA-mediated cGAS-STING activation [72, 73]. It was later discovered that CASP3 can cleave cGAS and other key factors to limit cGAS-mediated IFN-I production [74]. However, tumor cells seem to utilize a different mechanism to hijack apoptosis and suppress tumor-intrinsic cGAS-STING activation. We previously reported that blocking CASP9 signaling with Emricasan or Q-VD-OPh could facilitate mtDNA mediated cGAS-STING activation after radiation treatment; however, blocking caspases does not further raise cGAS-STING axis protein expression level, indicating that cleavage of cGAS may not be the major mechanism of caspase mediated cGAS-STING inhibition in tumor cells [58]. Furthermore, Jiang et al. demonstrated that the activation of key enzymes in pyroptosis, caspase-1, -4, -5, and -11 cleaved cGAS, reduces cGAMP levels, and inhibits IFN-I production [75]; Rathinam et al. reported that gasdermin D (GSDMD) forms membrane pores after inflammasome activation depletes cytosolic potassium that is essential for activation of cGAS-STING signaling [76]. However, unlike pyroptosis, MLKL mediated necroptosis seems to trigger cGAS-STING activation by facilitating the release of mtDNA into the cytosol [77]. Considering the critical role of caspases in regulating the cell death, the regulatory network between caspases and cGAS-STING signaling still needs to be further validated. Thus, cGAS-STING bridges the innate immune sensing pathways and the cell death pathways. However, tumor could hijack the cell death pathways to regulate the activation of cGAS-STING pathway and influence antitumor immunity.
Additionally, some other variables affecting the activation of the cGAS-STING pathway have also been recently identified. These factors are related to cytoplasmic accumulation of mitochondrial DNA. DNA methyltransferase 3 alpha (DNMT3A) and TET methylcytosine dioxygenase 2 (TET2) maintain mtDNA integrity by regulating the expression of transcription factor A mitochondria (TFAM), and deletion or mutation of both causes damage to mtDNA integrity and activation of cGAS signaling [78]. Oxidized DNA in the mitochondria is repaired by 8-oxoguanine DNA glycosylase (OGG1), or cleaved into fragments by endonuclease flap endonuclease (FEN1). The fragment of mtDNA leaks into cytosol via mitochondrial permeability transition pore (mPTP)- and voltage dependent anion channel (VDAC)-dependent channels to initiate cGAS-STING activation [79]. Instability in the mitochondrial genome also promotes the accumulation of Z-type DNA, upregulation of ZBP1 expression, and nucleates a cytosolic complex containing cGAS, RIPK1, and RIPK3 to sustain IFN-I signaling [80]. In addition, the loss of fumarate hydratase (FH1) also leads to the release of mtDNA into the cytoplasm through mitochondrial derived vesicles (MDVs), promoting the activation of the cGAS-STING pathway [81]; however, whether the apoptosis pathway is involved in the cGAS-STING activation in FH1 deficient cells remains unclear.
Posttranslational modifications of cGAS-STING
Posttranslational modifications (PTMs), including phosphorylation, ubiquitination, SUMOylation and others, can also directly regulate cGAS-STING signaling (Figure 2). The E3 ubiquitin ligase membrane associated ring-CH-type finger 8 (MARCH8) ubiquitinates cGAS at Lys411, inhibiting the binding of cGAS to DNA, thereby reducing the production of cGAMP and IFN-I [82]. Moreover, ovarian-tumor-domain-containing deubiquitinase 3 (OTUD3) binds to cGAS and targets Lys279 to deubiquitinate K48-linked ubiquitination, thereby enhancing the stability of the cGAS protein and its DNA-binding ability [83]. Similarly, Death-associated protein kinase 3 (DAPK3) facilitates the K63-linked ubiquitination and stabilizes the interaction of STING and TBK1 [84]. Tripartite motif 10 (TRIM10) binds to STING, catalyzing the polyubiquitination of STING at K289 and K370 sites at K27- and K29-, thereby promoting the Golgi transport and activation of STING [85]. On the contrary, myb-like SWIRM and MPN domains 1 (MYSM1) and ubiquitin specific peptidase 35 (USP35) suppress the activation of STING by cleaving STING K63-linked ubiquitination [86, 87]. Besides, phosphorylation, SUMOylation, methylation, glutamylation, acetylation and palmitoylation can also regulate the activities of cGAS-STING. It has been reported that the epidermal growth factor receptor (EGFR) and Akt (protein kinase B, PKB) axis inhibits cGAS activity by phosphorylating S291 or S305 residues [88]. Phosphorylation also involves in regulating cGAS sensing genomic DNA. As a cytosolic dsDNA sensor, it is not surprising that cGAS recognizes cytosolic DNA, including micronuclei DNA in cytoplasm. However, how genomic DNA avoids activating cGAS during nuclear envelope disappearing in mitosis has attracted more attention. In 2019, the Funabiki lab reported that nucleosome DNA traps more cGAS during mitotic envelope breakdown; unlike naked dsDNA, nucleosome DNA inhibits cGAS activation through H2A-B binding to cGAS [89]. However, during mitotic arrest, cGAS is slightly activated and promotes the aggregation of IRF3 to initiate cell apoptosis [89]. Later, multiple studies uncovered the structure of nucleosome-cGAS and revealed that nucleosome traps the cGAS in H2A-B to block dsDNA binding and maintains cGAS in a monomeric state [90], [91], [92], [93], [94]. Besides, cGAS could also be hyperphosphorylated by mitotic kinases, including Aurora kinase B and DNA-dependent protein kinase (DNA-PK), to suppress genomic DNA recognition [95, 96]. Notably, TBK1 is also involved in regulating STING by through phosphorylation. TBK1 phosphorylates the Ser366 residues of STING in a conserved pLxIS motif that is also necessary for STING activity [97, 98].
Figure 2:
Regulation of cGAS-STING signaling pathway. Different PTMs of cGAS-STING, including ubiquitylation, palmitoylation, acetylation, can directly trigger the function of cGAS-STING. DNA-PK, as a DNA sensor, has multiple roles in regulating the cGAS-STING pathway. In addition to direct regulation, DNA-PK can promote cytoplasmic translocation of PARP1, and inhibits cGAS-DNA-binding ability through PARylating cGAS. TREX1 and DNAse1L3 degrade the dsDNA, and ENPP1 hydrolyzes extracellular cGAMP, thereby all inhibit the activation of downstream STING. Nucleosome DNA can also inhibit the cGAS activation through H2A-B binding to the cGAS and maintain cGAS in a monomeric state. PTMs, posttranslational modifications; cGAS, GMP-AMP synthase; SITNG, stimulator of interferon genes; DNA-PK, DNA-dependent protein kinase; PARP1, polymerase 1; TREX1, 3′ repair exonuclease 1; ENPP1, ecto-nucleotide pyrophosphatase phosphodiesterase 1.
SUMOylation is also vital to the activation of cGAS. Viral infection first facilitates the SUMOylation of both cGAS and STING by tripartite motif protein 38 (TRIM38), increasing the stability of cGAS and STING. However, in the late phase of viral infection, cGAS and STING are deSUMOylated by Sentrin/SUMO-specific protease 2 (SENP2) [99] and SENP7 [100], leading to their degradation. Similarly, glutaminylation is also reversibly regulated by multiple enzymes to balance the activation of cGAS. Tubulin tyrosine ligase-like 6 (TTLL6) and TTLL4 inhibit the activity of cGAS by glutaminylation, whereas cytosolic carboxypeptidase 6 (CCP6) and CCP5 remove the glutaminylation of cGAS, jointly regulating the activation of cGAS [101]. Acetylation of cGAS in different domains has different effects on its activity. Acetylation of the C-terminal catalytic domain (CCD) of cGAS is regulated by multiple PTMs to influence its enzymatic activity; for example, aspirin could also promote the acetylation of the C-terminal of cGAS and inhibit its function [24]. Meanwhile, acetylation of N-terminal unstructured domain (NUD) by lysine acetyltransferase 5 (KAT5) enhances the binding ability of cGAS to DNA [24, 102]. Besides, other kinds of PTMs also involve in cGAS regulation. Protein arginine methyltransferase 5 (PRMT5) was reported to methylate cGAS, thereby blocking its DNA-binding ability [103]. More recently, Yin et al. also found that palmitoyltransferase ZDHHC18 negatively regulates the function of cGAS through palmitoylation [104]. In contrast, palmitoylation of STING at Cys residues (Cys88/91) promotes its activation [105].
The DNA repair system is not only involve in DNA generation, but also participates in regulating the cGAS pathway. Recently, it has been found that DNA virus infection facilitates the activation of DNA-PK, which promotes cytoplasmic translocation of poly (ADP-ribose) polymerase 1 (PARP1). PARP1 thereafter inhibits cGAS-DNA-binding ability through PARylating cGAS [106]. However, the role of DNA-PK in regulating cGAS-STING signaling is complicated; DNA-PK, itself as a DNA sensor [107], has been reported to trigger cGAS-STING activation, but in some case also inhibits cGAS-STING [96, 108]. Besides, the degradation of cGAMP also represents a form of negative regulation that suppresses cGAS-STING signaling. Ecto-nucleotide pyrophosphatase phosphodiesterase 1 (ENPP1) hydrolyzes extracellular cGAMP and thus prevents extensive spread, thereby inhibiting the activation of downstream STING [109].
Metalloimmunotherapy and cellular localization regulates the function of cGAS-STING pahtway
The activity of cGAS-STING pathway can also be regulated by metal ions. Mg2+ is revealed to be a cofactor for cGAS enzyme [110]; later, the Jiang lab reported that Mn2+ releases into the cytosol and promotes the cGAS-STING activation during virus infection [111]; furthermore, they also validated that Mn2+ activates the cGAS-STING in a dsDNA-independent manner to potentiate the immunotherapies [112, 113]. Moreover, the Chen lab revealed that binding of dsDNA also induces a cGAS liquid phase separation, which is critical to cGAS synthesizing cGAMP, while zinc ions promote DNA-induced phase separation to activate the cGAS [114]. Notably, stimulation with cGAMP or Mn2+ also promotes the STING to form a liquid–liquid phase-separation (LLPS) and a gel-like transition; however, condensation of ER-resident STING could form a puzzle-like structure to limit the overactivation of STING-TBK1 signaling [115]. Additionally, potassium is also required for the full activation of cGAS-STING pathway; therefore, inflammasome pathway could trigger the cytosolic potassium efflux to block the activation of cGAS-STING signaling [70, 76].
In addition to directly regulating the function of cGAS or STING, the activation of cGAS-STING pathway could also be regulated by adjusting the formation of cytosolic dsDNA [116]. DNA 3′ repair exonuclease 1 (TREX1) is a 3′-5′ single strand exonuclease with Mg2+ as a cofactor that is reported to degrade dsDNA and DNA-RNA hybrid in cases where it exhibits helicase activity [117, 118]. It is reported that TREX1 can degrade damaged cytosolic DNA, inhibiting the activation of cGAS [119, 120]. However, the activated cGAS-dsDNA complex could resist TREX1-mediated degradation by forming the phase separation [121]. Moreover, it is found that p53 induces degradation of TREX1, resulting in cytosolic dsDNA accumulation, then engages the cGAS/STING cytosolic DNA sensing pathway for tumor suppression [122]. Besides TREX1, DNase1L3 is also reported to degrade the serum vascular dsDNA and prevent the self-DNA mediated autoimmune disease [123], [124], [125]. However, the role of other nucleases is also under evaluation.
In addition to molecules that regulate the release of mtDNA, the amino terminal (N terminal) of cGAS also has an influence on the DNA reactivity of the catalytic domain. The activity of the human cGAS catalytic domain was inhibited by the N-terminal. In contrast, the N-terminal of mouse cGAS promotes its own DNA reactivity [126]. And there are other molecules that affect the stability of STING. Adaptor protein complex 1 (AP-1) sorted phosphorylated STING into clathrin-coated transport vesicles for delivery to the endolysosomal system, leading to degradation of STING, and thereby inhibiting long-term activation of STING [127]. STING has also recently been reported to act as a proton channel to mediate the outflow of hydrogen ions in Golgi, resulting in an increase in the pH value of the Golgi apparatus, induction of LC3B lipidation and autophagy formation, and ultimately degradation of STING [128].
cGAS-STING, a double-edged sword for cancer treatments
Unlike host immune cells, tumor cells seem to hijack plenty of pathways to suppress intrinsic cGAS-STING activation. Even though some previous studies reported that antitumor treatments could induce the slight increase of tumor-derived IFN-I and provoke the antitumor effect in a tumor-intrinsic cGAS-STING dependent manner [46, 84, 129], [130], [131]. However, considering the opposite role of chronic and acute production of IFN-I in antitumor immunity, the treatment slight increasing of IFN-I could blur the clinical outcome [16]. Therefore, it is still under debate whether tumor-intrinsic cGAS-STING signaling provokes antitumor immunity by increasing IFN-I or other cell stress related pathways. Unexpectedly, chromosomal instability (CIN) can contribute to the aggregation of cytosolic dsDNA and activate cGAS in tumor cells [49, 132, 133]; similarly, the aggregation of cytosolic dsDNA is also vital to provoking antitumor immunity in DNA mismatch repair deficiency (dMMR) tumor models responding to ICB [130]. Tumor cells with CIN typically undergo chromosome missegregation during cell division, resulting in chromosomes fragments are enveloped by primitive nuclear membranes to form micronuclei. Micronuclear membranes readily decompose and release dsDNA contained therein [134] and trigger the cGAS-STING pathway [135]. However, the impact of tumor-intrinsic cGAS-STING pathway varies across different tumor types. Clinical data showed that low expression of STING in tumor tissues is associated with poor prognosis of gastric cancer [136]. Multiple studies add proofs of the positive role of tumor-intrinsic cGAS-STING in strengthening antitumor immunity [18, 137, 138]. However, other groups have also showed that upregulated cGAS-STING is negatively correlated with immune cell infiltration in some tumors and also correlates with poor prognosis [139], thereby promotes the tumor metastasis [49]. In summary, the cGAS-STING pathway exhibits dual roles in cancer, acting both as a suppressor and as a promoter of oncogenic processes. Therefore, identifying specific biomarkers to delineate the role of the cGAS-STING pathway in the TME warrants further investigation.
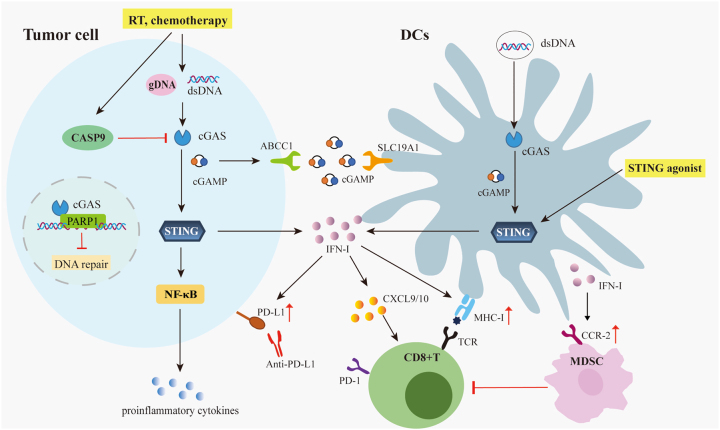
Activation of the cGAS-STING pathway in the TME can promote infiltration of immune cells and antitumor immunity. Tumor cells or tumor cell-derived vesicles, including exosomes and macrovesicles, can be captured by phagocytes and antigen presenting cells. The phagocytosed dsDNA is released into the cytosol of immune cells and activates the cGAS-STING signaling [140, 141]. This will further enhance the secretion of IFN-I, the presentation of tumor antigens, and the recruitment of tumor-specific CD8+ T cells to exert anti-tumor effects [16, 18]. In addition, IFN-I also upregulates the expression of multiple chemokines such as C-X-C motif chemokine ligand 9 (CXCL9) and CXCL10, which promote the infiltration of APCs and effector T (Teff) cells [32, 142, 143]. Interestingly, tumor-derived cGAMP is reportedly secreted into the TME, possibly via ATP binding cassette subfamily C member 1 (ABCC1) transporter, and enters immune cells via the folate transporter solute carrier family 19 member 1 (SLC19A1) [144, 145]. Delaying dead clearance by blocking MerTK will enhance the extracellular ATP that could trigger the macrophage capture tumor-derived cGAMP to enhance antitumor immunity [138]. Tumor-derived cGAMP also triggers STING-mediated interferon responses in non-tumor cells to activate an NK cell mediated antitumor effect [137]. However, activation of the cGAS-STING pathway in the TME does not always indicate a better prognosis. The Ge lab reported that DNA damage could promote cGAS translocation into the nucleus and inhibit homologous recombination by preventing the PARP1-Timeless complex formation [146]. Moreover, CIN chronically activates STING-dependent noncanonical NF-κB signaling to drive secretion of proinflammatory cytokines and metastasis formation, thereby contributing to CIN maintenance [49]. CIN tumors can also activate cell survival signals and form a metastasis-promoting tumor microenvironment through NF-κB-IL-6-STAT3 pathway and endoplasmic reticulum stress [147, 148]. Known for its role in RNA editing, APOBEC3A also promotes CIN and triggers the formation of micronuclei to activate cGAS-STING in tumor cells; this helps tumors to develop and metastasize [149, 150]. Another study showed that cGAS anchored to the mitochondrial outer membrane can promote dynamin-related protein 1 (DRP1) oligomerization, inhibiting mitochondrial ROS accumulation and ferroptosis, and further promoting tumor progression [151]. Therefore, it is important to avoid promoting the pro-tumorigenesis functions of cGAS-STING while endeavoring to promote antitumor immunity.
Over the past decade, immunotherapy has heralded a new era in cancer treatment. This inspiration has led researchers to progressively uncover the role of the immune responses in traditional antitumor therapies, such as radiotherapy, chemotherapy, and targeted therapy. Besides directly killing tumor cells, traditional antitumor therapies also activate innate immune sensing pathways, especially the cGAS-STING pathway, to provoke anti-tumor immunity in the TME [7, 15, 34], [35], [36], [37], [38]. However, the antitumor immunity induced by RT and chemotherapy often proves insufficient to maintain long-term antitumor effects; relapse remains one of the major challenges in clinical cancer treatment [152]. Therefore, identifying ways to further enhance therapy-induced antitumor immunity remains a crucial area of research to synergize with traditional cancer therapy and immunotherapy [153].
Radiation
The traditional dogma was that RT functions only by inducing DNA double strand breaks (DSB) to kill tumor cells [154]. The current radioimmunology theory highlights the vital role of antitumor immunity induced by RT. Irradiated tumor cells can activate the cGAS-STING pathway in DCs, which then produce IFN-I to provoke the adaptive antitumor immunity [16, 140, 155, 156]. Meanwhile, several studies also revealed that in certain settings, tumor-intrinsic cGAS could also be activated by RT to contribute to awaking the function of CD8+ T cells [108, 129, 140, 156, 157]. However, the slight increase in irradiated tumor-derived IFN-I suggests that tumor infiltrated immune cells should be the major cGAS-STING acting cells [16]. Generally, irradiated tumor cells fail to fully activate endogenous cGAS-STING signaling. We previously identified that RT-induced intrinsic apoptosis signaling is hijacked by tumor cells to suppress cGAS activation. Knocking out intrinsic apoptosis signaling switches tumor cells to be the major source of IFN-I in tumor tissue and inhibits tumor relapse after radiation [58]. Tumor-intrinsic TREX1 and autophagy were also reported to limit cGAS-STING activation after RT [129, 140]. Notably, given the high heterogeneity of tumors, they can hijack various negative cGAS-STING regulators to suppress the endogenous innate immune sensing to avoid strengthening the antitumor immunity. Therefore, it is valuable to map the network of tumor-intrinsic innate immune sensing negative regulators, which is critical to develop the new strategies to synergize with RT and immunotherapy. On the other hand, RT-induced cGAS-STING activation not only provokes the anti-tumor immune response, but also induces immunosuppressive factors to limit excessive activation of the immune response. For example, RT-triggered STING activation promotes innate immunosuppression by recruiting monocyte-derived suppressor cells (MDSC) into the TME, thus playing a pro-tumor role [158]. Meanwhile, in contrast to the antitumor effect of the canonical NF-κB pathway, the activation of the non-canonical NF-κB pathway in DCs inhibits the production of radiation-induced, STING-mediated IFN-I and inhibited the therapeutic effect of radiotherapy [159].
Generally, high dose of RT is effective in exterminating tumor lesions; however, when a tumor is adjacent to vital organs, such high doses and subsequent adaptive irradiation may not fully control tumor cells growth. Therefore, tumor relapse and the extremely rare abscopal effect remain the two major challenges of RT in clinic. Previous studies have revealed that blocking T cell infiltration after RT does not reduce the antitumor effect of RT, indicating the pre-existing CD8+ T cells are vital to RT [160, 161]. Consistently, a more recent study reported that RT-induced neutrophil NETosis blocks CD8+ T cell infiltration into the tumor area [162]. Meanwhile, combining RT with ICB can enhance therapeutic effects, yet it still fails to completely eradicate metastases [153, 163]. Our research demonstrated that irradiating CASP9-deficient tumors significantly trigger the activation of cGAS-STING to provoke CD8+ T cell mediated antitumor effect, and the CASP9-deficient tumor disappeared after RT without relapse. However, irradiated CASP9-deficient tumors failed to induce systemic antitumor immunity to reject the distal nonirradiated tumor tissue; combining with ICB induced an abscopal effect but still failed to totally reject the distal nonirradiated tumors [58]. Moreover, previous studies have reported that DCs activated by local radiation mediates the activation of CD8+ T cells and contribute to tumor control by RT [156]. It is suggested the crosstalk between TME and peripheral immunity seems to be interfered after RT. To date, further research is needed to elucidate the mechanisms by which radiotherapy seldom induces an abscopal response. Considering the technological advancements in RT delivery, it is now more feasible to specifically irradiate tumor tissues without damaging adjacent healthy tissues. Therefore, there is an urgent need to refine current radioimmunology theories and develop novel strategies that effectively harness systemic antitumor immunity.
Chemotherapy
Chemotherapy functions to inhibit tumor cells proliferation and directly kill the tumor cells. Similar to RT, plenty of chemotherapeutic drugs, including anthracyclines, oxaliplatin, doxorubicin, and teniposide, topotecan, 6-thio-dG and others, can induce DNA DSBs and activate the cGAS-STING pathway to provoke the anti-tumor immunity [19, 32, 43, 164], [165], [166], [167]. Following DNA damage, tumor cells engage the DNA damage response (DDR) mechanisms to maintain genomic integrity [168, 169]. One of the earliest events in the DDR is recruitment of PARP1 to various kinds of DNA lesions [170, 171]. Drugs that target DDR proteins, including PARP inhibitors, and checkpoint kinase 1 (CHK1) inhibitors, have been utilized in anti-tumor therapies. These targeted therapeutics have been shown to amplify DNA damage in tumor cells, further activate the cGAS-STING pathway, regulate the immune microenvironment, and improve the efficacy of anti-PD-1 therapy when used in combination [45, 46, 120, 172]. Ataxia telangiectasia mutated (ATM) is also a component of the DDR; thus, inhibition of ATM can further promote the activation of cGAS-STING signaling, paving the road for synergizing with ICB therapy [173, 174]. Notably, the Liu lab recently revealed that the chemotherapy paclitaxel diminishes CXCL13+ CD8+ T cells to limit the synergistic effect of chemotherapy and ICB [175], indicating potential risks in using chemotherapies to awaken anti-tumor immunity.
Immunotherapy
Immunotherapies function by awakening the antitumor immunity; therefore, immunotherapies show a great potential to synergize with RT and other antitumor therapies. ICB therapies, as the most famous immunotherapies, block immunosuppressive receptors on the cell surface, including programmed cell death-1 (PD-1)/programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and specifically provoke adaptive immunity. Therefore, the effectiveness of immunotherapies depends on the adaptive antitumor immunity, most notably the recognition of tumor antigens and generation of tumor-specific cytotoxic T cells (CTLs). The Chen lab first reported that cGAS-deficient mice show less tumor antigen-specific CD8+ T cells infiltration and respond worse to PD-L1 blockade, suggesting that cGAS is essential for the antitumor effects of immune checkpoint blockade [20]. In addition, Fu lab demonstrated that the anti-tumor effect of CD47 blockade also requires DC-intrinsic, STING-mediated T cell activation [41, 176]. In some cases, endogenous tumor STING signaling can also contribute to the response to ICB therapy. The Greenberg lab revealed that irradiated B16-STING knockout cells, as a vaccine, reduced tumor sensitivity to anti-CTLA4 treatment [108]. Notably, considering the role of cGAS in DNA damage repair and mitotic arrest, the mechanism of how tumor-intrinsic factors influence to ICB therapy is still under-evaluated. On the other hand, cGAS-STING generally functions through IFN-I; however, the Zitvogel lab also observed that IFNαR1-deficient mice respond better to anti-PD1 therapy [177], indicating that an IFN-I-independent role of cGAS-STING pathway may also play a role in the effectiveness of immunotherapies.
Notably, although radiotherapy and chemotherapy can activate the tumor-intrinsic cGAS-STING pathway in some certain tumor cell lines or experimental setting, the activation is often not strong enough to fully awaken antitumor immunity, and the host cGAS-STING signaling still seems to play the major role post antitumor treatment. This is mainly caused by the complicated negative regulation network in tumor cells. Some factors, such as caspases, TREX1 and autophagy, induced by antitumor therapies to initiate cell death, can also be hijacked by tumor cells to evade immunosurveillance [129, 140, 178], [179], [180]. Interestingly, interfering with the major negative regulators successfully switch tumor cells to become the major IFN-I source in the TME and play a vital role in enhancing antitumor immunity [32, 58]. These findings point out a new direction for novel antitumor strategies development (Figure 3).
Figure 3:
Antitumor therapies awake the cGAS-STING signaling to accelerate the antitumor immunity. Features of the tumor microenvironment like chromatin instability and growth stress can cause cytoplasmic or extracellular accumulation of dsDNA to activate the cGAS-STING pathway. Similarly, anti-tumor therapies such as radiotherapy and chemotherapy can also cause accumulation of genomic or mitochondria-derived dsDNA in the cytosol. Tumor-derived cGAMP can also secrete into the TME, and enter immune cells via the folate transporter SLC19A1. However, DNA damage recruits the cGAS translocating to nuclear and inhibits the homologous recombination by interfering the PARP1-timeless complex formation. On the other hand, antitumor therapies also upregulate negative regulators of cGAS-STING, as CASP9 signaling, to suppress the activation of the intrinsic cGAS-STING pathway. Therefore, targeting such negative regulators or delivery of STIGN agonists could specifically activate the cGAS-STING signaling in the TME. This will help to upregulate the expression of Th1 chemokines (CXCL9 and CXCL10) and antigen presenting molecules (MHCI), recruit CD8+T cells, and activate innate immune cells. However, the increase of IFN secretion may also enhance the infiltration of MDSC and upregulate PD-L1 and other immunosuppressive factors to exert an inhibitory effect. dsDNA, double-stranded DNA; cGAS, GMP-AMP synthase; SITNG, stimulator of interferon genes; cGAMP, cyclic GMP-AMP; CASP, caspase; TME, tumor microenvironment; MDSC, monocyte-derived suppressor cells.
cGAS-STING based agonists in cancer therapies
Unlike other innate immune sensing platforms, the cGAS-STING pathway is broadly expressed and can be activated by small molecular cGAMP and its analogues; therefore, multiple STING agonists were developed and validated in pre-clinical models and clinical practice. However, considering the widespread expression of STING, high-dose local treatment or systemic administration of STING agonists might induce immune cells’ death to diminish the enhanced antitumor immunity; therefore, how to specifically activate STING in TME should be further considered as critical next-generation STING agonist-based strategies.
STING agonist
Cytosolic dsDNA triggers cGAS to synthesize cGAMP, a cyclic dinucleotide (CDN), to bind and activate STING. Besides cGAMP, plenty of natural CDNs have been reported in other organisms [181], [182], [183], indicating the conserved role of this axis. Antitumor modulation of CDNs was revealed that c-di-GMP could inhibit tumor cell proliferation [184]. The intratumoral injection of cGAMP has been reported to limit tumor growth and promote the CD8+ T cell infiltration, and this ability could be further synergized with ICB or RT [16, 20, 185]. Beyond naturally derived CDNs, synthetic CDNs with better properties have been developed, including ADU-S100 and MK-1454, which have entered phase II clinical trials for the treatment of head and neck tumors in combination with pembrolizumab [186]. Especially, MK-1454 was developed by Merck as a STING agonist for various tumors with an enzymatic cascade system [187] (Table 1).
Table 1:
STING agonists.
| STING agonist | Agents | Targets | Phase | Clinical trial ID/PMID | |
|---|---|---|---|---|---|
| Direct | ADU-S100(MIW815) | ADU-S100 | STING | Phase 2 | NCT03937141 |
| ADU-S100+/−Ipilimumab | STING+/−CTLA-4 | Phase 1 | NCT02675439 | ||
| ADU-S100 + PDR001 | STING + PD-1 | Phase 1 | NCT03172936 | ||
| E7766 | E7766 | STING | Phase 1 | NCT04109092 | |
| E7766 | STING | Phase 1 | NCT04144140 | ||
| IMSA101 (GB492) | IMSA101+/−PULSAR-ICI | STING+/−PD-1 | Phase 2 | NCT05846659 | |
| NCT05846646 | |||||
| GSK3745417 | GSK3745417 | STING | Phase 1 | NCT03843359 | |
| MK-1454 | MK-1454 | STING | Phase 1 | NCT03010176 | |
| MK-1454+/−pembrolizumab | STING + PD-1 | Phase 2 | NCT04220866 | ||
| MK2118 | MK-2118+/− pembrolizumab | STING + PD-1 | Phase 1 | NCT03249792 | |
| BMS-986301 | BMS-986301 | STING | Phase 1 | NCT03956680 | |
| SB 11285 | SB 11285 | STING | Phase 1 | NCT04096638 | |
| SNX281 | SNX281+/−pembrolizumab | STING+/−PD-1 | Phase 1 | NCT04609579 | |
| TAK-500 | TAK-500+/−pembrolizumab | STING+/−PD-1 | Phase 1 | NCT05070247 | |
| c-di-GMP | c-di-GMP | STING | Pre-clinical | PMID: 15721270 | |
| MSA-2 | MSA-2 | STING | Pre-clinical | PMID: 32820094 | |
| SR-717 | SR-717 | STING | Pre-clinical | PMID: 32820126 | |
| SYNB1891 | SYNB1891+/−Atezolizumab | STING+/−PD-L1 | Phase 1 | NCT04167137 | |
| DMXAA (ASA404) | DMXAA | STING | Phase 3 | NCT00738387 | |
| CMA (Cridanimod) | CMA + progestin | STING | Phase 2 | PMID: 23604073 | |
| α-Mangostin | α-Mangostin | STING | Pre-clinical | PMID: 30079976 | |
| CRD3874-SI | CRD3874-SI | STING | Phase 1 | NCT06021626 | |
| Compound 12b | Compound 12b | STING | Pre-clinical | PMID: 31927317 | |
| C53 | C53 | STING | Pre-clinical | PMID: 35388221 | |
| G10 | G10 | STING | Pre-clinical | PMID: 32911484 | |
| C11 | C11 | STING | Pre-clinical | PMID: 29263267 | |
| Indirect | Emricasan | Emricasan + anti-PD-L1 | Caspases + PD-L1 | Pre-clinical | PMID: 32231300 |
| prexasertib | prexasertib | CHK1 | Phase 2 | PMID: 30777870 | |
| Olaparib | Olaparib | PARP1 | Phase 2 | PMID: 30540933 | |
| Rucaparib | Rucaparib | PARP1 | Phase 2 | PMID: 30589644 | |
| Talazoparib | Talazoparib | PARP1 | Phase 2 | PMID: 33495297 | |
| Topotecan | Topotecan | Topoisomerase I | Phase 2 | PMID: 28069806 | |
| MM-398 | MM-398 | Topoisomerase I | Phase 1 | PMID: 29267866 | |
| Doxorubicin | Doxorubicin | Topoisomerase II | Phase 2 | PMID: 25344738 | |
| Teniposide | Teniposide | Topoisomerase II | Phase 2 | PMID: 31408442 | |
| Cisplatin | Cisplatin | DNA replication | Phase 2 | PMID: 33423764 PMID: 29658856 | |
| 6-thio-dG | 6-thio-dG | Telomerase | Phase 2 | PMID: 32619407 NCT05208944 | |
| AZD1390 | AZD1390 | ATM | Phase 1 | PMID: 33290271 NCT03215381 | |
Dimethyloxoxanthenyl acetic acid (DMXAA) is the first non-CDN STING agonist, but DMXAA and its analogous 10-carboxymethyl-9-acridanone (CMA), failes in clinical trials. Later, it is revealed that they can only activate mouse STING, but not human STING [188], [189], [190], [191]. The natural compound α-mangostin is a bioflavonoid-like molecule with antiviral activity and can activate hSTING [192]. A series of oxoacridinyl acetic acid derivatives with the same skeleton as CMA were also synthesized, which could be used as hSTING agonists, but their antitumor effects are still being evaluated. Thereby, several groups aim to develop systemically available STING agonists that can circumvent these issues. Amidobenzimidazoles (ABZI), a novel STING agonist designed for patients through systemic administration, has significantly enhanced binding affinity using the 4-carbon butane linker (di-ABZI) for dimerization; however, its safety remains to be considered and the hemodynamics of patients using these drugs need to be closely monitored [193, 194]. Furthermore, another non-nucleotide cGAMP mimetic small-molecule STING agonist, SR-717, was developed for oral administration [195]. SR-717 promotes the activation of CD8+ T cells, dendritic cells and limited tumor growth, but failed to synergize with ICB in pre-clinical models [195]. SR-717 also induces the expression of clinically relevant targets, including PD-L1, in a STING-dependent manner [195]. MSA-2, another orally available non-nucleotide STING agonist with antitumor activity, is also moderately or poorly synergistic with PD-1 blockade; combinations of MSA-2 and anti-PD-1 antibody were superior in inhibiting tumor growth and prolonging survival over monotherapy [196]. Notably, most STING agonists only trigger the activation of host-derived, but not tumor-intrinsic, STING signaling. STING-deficient mice fail to increase IFN-I levels and resisted STING agonist treatment, even in tumor models that have an intact cGAS-STING axis [195, 197]. This indicates that tumor cells could hijack some negative regulators to suppress STING signaling. Therefore, in future studies, it will be worth to continue to explore the activation and inhibition mechanisms of the cGAS-STING pathway in the TME, as well as the mechanism of action on tumors, to develop better drugs targeting this pathway, reduce drug toxicity and improve bioavailability. Meanwhile, the mechanism of combined action of STING agonists and existing immunotherapy methods must be actively explored to find the best combined treatment strategy for different tumors.
Although STING agonists have shown promising clinical benefit, most of them can only be delivered by intratumoral administration, in case of their poor cell membrane permeability, low bioavailability, or high toxicity [197], [198], [199]. Systemic administration of STING agonists also induced the occurrence of Breg (regulatory B cells), secreted IL-35, and inhibited the NK cell-mediated anti-tumor response [200]. Notably, MSA-2 and SR-717 were available as STING agonists by oral administration, but toxicity could not be ignored when systemically administered in mice [195, 196]. Therefore, preferred activation of STING in the TME is critical for clinical benefit. The design of systemically administered STING agonists will be crucial, since activation of STING in normal tissues may induce an inflammatory response, leading to side effects. For example, diABZI, administered by intratracheal injection, induces a neutrophil response in the bronchoalveolar space, leading to STING-dependent acute respiratory distress syndrome (ARDS) [194]. However, MSA-2 takes advantage of the weak acidity of the TME to synergize with ICB [196]. At present, there are some strategies for STING agonist targeted activation. For example, incorporation of cGAMP into non-infectious enveloped virus-like particles (VLPs) can selectively activate STING in APC and reduce the production of Treg [201]. In addition, bacterial-derived cyclic dimeric adenosine monophosphate (CDA) encapsulated in nanoscale coordination polymer to form Zinc cyclic di-AMP nanoparticles, intravenous injection of ZnCDA can enhance tumor accumulation by destroying endothelial cells in the tumor vasculature, with priority targeting tumor-associated macrophages, and then enhance anti-tumor immune response [202]. The effective penetration of STING agonists into the tumor can also be achieved by delivering STING agonists by tumor-penetrating PEG-lipid nanodiscs [203]. However, these strategies still activate STING pathways in immune cells in the tumor microenvironment, and cannot avoid the death of immune cells caused by overactivation of STING. Therefore, novel strategies should be considered to achieve the specific activation of STING in tumor tissues when STING agonists are used alone or in combination with other tumor immunotherapies.
STING agonist combined with radiation, chemotherapy and targeted therapy
Although traditional antitumor therapies can partially activate cGAS-STING in the TME, combining these therapies with STING agonists can strengthen anti-tumor immunity. Fu lab previously demonstrated that cGAMP could synergize with RT [16]. STING agonist, diABZI, could also significantly promote apoptosis in irradiated non‐small cell lung cancer (NSCLC) cells, and enhance radiosensitivity of NSCLC cells [204]. ADU-S100 has the similar effect of enhancing radiotherapy sensitivity in esophageal cancer models [205]. Besides RT, STING agonists could also synergize with chemotherapy. Compared with carboplatin single treatment, carboplatin combined with STING agonist significantly prolonged survival of mice with high-grade serious ovarian cancer [206]; furthermore, carboplatin combined with STING agonist and anti-PD-1 antibody had better therapeutic effects [206]. In addition, cGAMP could also synergize with AKT 1/2 inhibitor to induce extensive apoptosis of tumor endothelial cells and reject spontaneous tumors [207].
STING agonist combined with CAR-T therapy
CAR-T cells recognize a specific antigen on the surface of tumor cells through their engineered single-chain variable fragment domain [208, 209]. CAR-T cell therapy has shown substantial activity against human CD19 or B-cell maturation antigen (BCMA)-expressing B cell malignancies [210, 211], but has been less successful in solid tumors [212], mainly due to the suboptimal migration, impaired function mediated by the immunosuppressive TME and CAR-T cell exhaustion. The search for ways to enhance adoptive T cells in solid tumors continues. Studies have shown that, compared with CAR-T alone, the combination of STING agonist c-di-GMP and CAR-T cells can significantly activate host APCs and lymphocyte responses, further enhancing the tumor treatment effect, and prolonging survival in mice [213]; however, considering the role of STING in cell death, it is still under debate whether STING agonist-based immunotherapy could synergize with other cancer therapies in clinical. In addition, CAR-T cells generated from Th/cytotoxic T (Tc) 17 cells given with the STING agonists DMXAA or cGAMP also greatly enhanced tumor control, allowing for more CAR-T cell infiltration in the TME [214].
STING agonist combined with ICB treatment
Although immune checkpoint inhibitors (ICIs) has become a pillar of cancer therapy, the actual clinical application is also limited by low response rate. For example, cancer patients’ overall response rate to ICIs is about 20–30 %, which seems to be associated with a pre-existing pro-inflammatory TME with higher immune cell infiltration or PD-L1 expression. Indeed, “immune desert” or “cold” tumors appear to be less responsive to ICIs [215], [216], [217]. Given the critical role of the cGAS-STING pathway in activating the TME immune response, the antitumor potential of combination therapy with STING agonists and ICIs is currently being actively evaluated.
STING agonists hold the promise to switch “cold tumors” to “hot tumors” and promote T cell infiltration, increase the IFN-I mediated expression of antigen presenting molecules, and improve the sensitivity of tumor cells to NK and CTL immune killing. On the other hand, activated cGAS-STING is accompanied by up-regulation of PD-L1 expression [218], and anti-PD-1/PD-L1 treatment could neutralize the immunosuppressive effect of STING agonists. STING agonists combined with ICB therapy have achieved remarkable results in many clinically refractory tumor models, including c-di-GMP and low dose of ADU-S100 [219], [220], [221]. In anti-CTLA-4 treatment, the combination of STING agonists can also reduce the activation threshold of T cells and amplify the tumor-specific immune response [222].
Notably, as mentioned above, activation of STING signaling leads to cell death of immune cells, especially for T cells and DCs. Consistently, high dose of ADU-S100 i.t. injection could regress tumors in a CD8+ T cells independent manner and induce immune cell death in tumor draining lymph nodes; however, low-dose ADU-S100 treatment could increase the cytotoxicity of CD8+ T cells and synergize with ICB [197]. This indicates that adjustment of STING agonist dose based on the activity is essential to avoid induction of the immune cell death and synergize with other antitumor therapies.
STING agonist: cancer vaccine adjuvant
Cancer vaccines are generally made up of cancer cells, parts of cells, or pure antigens (tumor-associated antigens, TAAs). Due to central and peripheral tolerance, the immunogenicity of TAAs is always very weak [223, 224]. Appropriate adjuvants are essential to overcome tolerance and enhance tumor-specific immunity. Therefore, adjuvant substances have attracted great attention from cancer vaccinologists. Considering the role of STING in innate immune sensing, STING agonists could be an ideal cancer vaccine adjuvant.
The first STING agonist-based cancer vaccine, STINGVAX, which consisted of CDNs and granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting cancer cells, has in vivo antitumor efficacy in several established cancer models, and can cure established tumors that resist PD-1 blockade [225]. Compared with CDN-free GM-CSF secreting cancer cell vaccines, STINGVAX treatment enhanced the IFNγ response and recruited more CD8+ T cells in TME, overall enhancing antitumor efficacy [225]. Meanwhile, after STINGVAX treatment, the expression of PD-L1 increased, enhancing the efficacy of anti-PD-1 therapy, and all established tumors in the CT26 model subsided after combination therapy [225]. In subsequent studies, the feasibility of using different STING agonists as cancer vaccine adjuvants has also been repeatedly verified [226, 227], indicating the promising function of STING agonists as adjuvants for tumor vaccines.
Concluding remarks
The cGAS-STING pathway, as a crucial intrinsic sensing platform, has garnered significant attention in academic research and plays a pivotal role in many anticancer therapeutic approaches. At now, there have been several STING agonists that have been produced for the purpose of treating tumors, exhibiting positive clinical therapeutic outcomes. Simultaneously, the STING agonists also synergize with immunotherapies, such as CAR-T and ICB. Nevertheless, STING activation can lead to the overexpression of immune checkpoints and induce immune cell death at higher concentrations, potentially resulting in pro-tumor consequences. Hence, effectively and specifically stimulating the cGAS-STING pathway in TME holds promise as a therapeutic strategy for tumor treatment, especially when combined with cancer immunotherapy in future endeavors.
Footnotes
Ethical approval: Not applicable.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state not conflict of interest.
Research funding: This work was supported by National Key Research and Development Program of China 2023YFC3404600 and National Natural Science Foundation of China grant (82371848).
Contributor Information
Sirui Li, Email: lsr519@ad.unc.edu.
Chuanhui Han, Email: chuanhui.han@bjmu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca – Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5:915–9. doi: 10.1158/2159-8290.cd-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013;34:67–73. doi: 10.1016/j.it.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun LJ, Wu JX, Du FH, Chen X, Chen ZJJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–91. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8(+) T cell responses through CD8 alpha(+) dendritic cells. J Exp Med. 2011;208:2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–8. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Han C, Fu YX. Targeting innate sensing in the tumor microenvironment to improve immunotherapy. Cell Mol Immunol. 2020;17:13–26. doi: 10.1038/s41423-019-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrales L, Matson V, Flood B, Spranger S, Gajewski TF. Innate immune signaling and regulation in cancer immunotherapy. Cell Res. 2017;27:96–108. doi: 10.1038/cr.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 12.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365–79. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Zheng W, Yang K, Harris KG, Ni K, Xue L, et al. Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J Exp Med. 2020;217:e20192282. doi: 10.1084/jem.20192282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Liu Z, Zhang A, Han C, Shen A, Jiang L, et al. NQO1 targeting prodrug triggers innate sensing to overcome checkpoint blockade resistance. Nat Commun. 2019;10:3251. doi: 10.1038/s41467-019-11238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Han C, Dong C, Shen A, Hsu E, Ren Z, et al. Hypofractionated EGFR tyrosine kinase inhibitor limits tumor relapse through triggering innate and adaptive immunity. Sci Immunol. 2019;4:eaav6473. doi: 10.1126/sciimmunol.aav6473. [DOI] [PubMed] [Google Scholar]
- 16.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–92. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–42. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Chen J, Hu J, Zhang H, Xu F, He W, et al. cGAS/STING axis mediates a topoisomerase II inhibitor-induced tumor immunogenicity. J Clin Invest. 2019;129:4850–62. doi: 10.1172/jci127471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Hu S, Chen X, Shi H, Chen C, Sun L, et al. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc Natl Acad Sci USA. 2017;114:1637–42. doi: 10.1073/pnas.1621363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng ZL, Dai T, He XL, Zhang ZK, Xie F, Wang S, et al. The interactions between cGAS-STING pathway and pathogens. Signal Transduct Targeted Ther. 2020;5:501–21. doi: 10.1038/s41392-020-0198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov. 2020;10:26–39. doi: 10.1158/2159-8290.cd-19-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan DS, Jiang W, Hao JW. Research advances in how the cGAS-STING pathway controls the cellular inflammatory response. Front Immunol. 2020;11:615. doi: 10.3389/fimmu.2020.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai J, Huang YJ, He X, Zhao M, Wang X, Liu ZS, et al. Acetylation blocks cGAS activity and inhibits self-DNA-induced autoimmunity. Cell. 2019;176:1447–60 e14. doi: 10.1016/j.cell.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21:548–69. doi: 10.1038/s41577-021-00524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Tang Z, An R, Ye L, Zhong B. USP29 maintains the stability of cGAS and promotes cellular antiviral responses and autoimmunity. Cell Res. 2020;30:914–27. doi: 10.1038/s41422-020-0341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao D, Li T, Li XD, Chen X, Li QZ, Wight-Carter M, et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc Natl Acad Sci USA. 2015;112:E5699–705. doi: 10.1073/pnas.1516465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao N, Wei J, Xu S, Du H, Huang M, Zhang S, et al. cGAS activation causes lupus-like autoimmune disorders in a TREX1 mutant mouse model. J Autoimmun. 2019;100:84–94. doi: 10.1016/j.jaut.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–9. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 30.Fang R, Jiang Q, Jia X, Jiang Z. ARMH3-mediated recruitment of PI4KB directs Golgi-to-endosome trafficking and activation of the antiviral effector STING. Immunity. 2023;56:500–15.e6. doi: 10.1016/j.immuni.2023.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Wu JX, Sun LJ, Chen X, Du FH, Shi HP, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–30. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han C, Zhang A, Liu Z, Moore C, Fu YX. Small molecular drugs reshape tumor microenvironment to synergize with immunotherapy. Oncogene. 2021;40:885–98. doi: 10.1038/s41388-020-01575-7. [DOI] [PubMed] [Google Scholar]
- 33.Han C, Godfrey V, Liu Z, Han Y, Liu L, Peng H, et al. The AIM2 and NLRP3 inflammasomes trigger IL-1-mediated antitumor effects during radiation. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abc6998. [DOI] [PubMed] [Google Scholar]
- 34.Lee YJ, Auh SL, Wang YG, Burnette B, Wang Y, Meng YR, et al. Therapeutic effects of ablative radiation on local tumor require CD8(+) T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–96. doi: 10.1158/0008-5472.can-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn J, Xia TL, Konno H, Konno K, Ruiz P, Barber GN. Inflammation-driven carcinogenesis is mediated through STING. Nat Commun. 2014;5:5166. doi: 10.1038/ncomms6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–70. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 39.Park S, Jiang ZJ, Mortenson ED, Deng LF, Radkevich-Brown O, Yang XM, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–70. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Lu L, Lu J, Wang X, Yang C, Jin J, et al. cGAS-STING-mediated DNA sensing maintains CD8(+) T cell stemness and promotes antitumor T cell therapy. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aay9013. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–15. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heidegger S, Wintges A, Stritzke F, Bek S, Steiger K, Koenig PA, et al. RIG-I activation is critical for responsiveness to checkpoint blockade. Sci Immunol. 2019;4:eaau8943. doi: 10.1126/sciimmunol.aau8943. [DOI] [PubMed] [Google Scholar]
- 43.Mender I, Zhang A, Ren Z, Han C, Deng Y, Siteni S, et al. Telomere stress potentiates STING-dependent anti-tumor immunity. Cancer Cell. 2020;38:400–11 e6. doi: 10.1016/j.ccell.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segovia M, Russo S, Jeldres M, Mahmoud YD, Perez V, Duhalde M, et al. Targeting TMEM176B enhances antitumor immunity and augments the efficacy of immune checkpoint blockers by unleashing inflammasome activation. Cancer Cell. 2019;35:767–81 e6. doi: 10.1016/j.ccell.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim C, Wang XD, Yu Y. PARP1 inhibitors trigger innate immunity via PARP1 trapping-induced DNA damage response. Elife. 2020;9:e60637. doi: 10.7554/elife.60637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chabanon RM, Muirhead G, Krastev DB, Adam J, Morel D, Garrido M, et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Invest. 2019;129:1211–28. doi: 10.1172/jci123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gui X, Yang H, Li T, Tan XJ, Shi PQ, Li MH, et al. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567:262–6. doi: 10.1038/s41586-019-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamazaki T, Kirchmair A, Sato A, Buque A, Rybstein M, Petroni G, et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat Immunol. 2020;21:1160–71. doi: 10.1038/s41590-020-0751-0. [DOI] [PubMed] [Google Scholar]
- 49.Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–72. doi: 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Z, Jia S, Shao C, Shi Y. Irradiation induces cancer lung metastasis through activation of the cGAS-STING-CCL5 pathway in mesenchymal stromal cells. Cell Death Dis. 2020;11:326. doi: 10.1038/s41419-020-2546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Civril F, Deimling T, de Oliveira Mann CC, Ablasser A, Moldt M, Witte G, et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498:332–7. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crossley MP, Song C, Bocek MJ, Choi JH, Kousorous J, Sathirachinda A, et al. R-loop-derived cytoplasmic RNA-DNA hybrids activate an immune response. Nature. 2023;613:187–94. doi: 10.1038/s41586-022-05545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15:405–14. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 54.Ma F, Li B, Liu SY, Iyer SS, Yu Y, Wu A, et al. Positive feedback regulation of type I IFN production by the IFN-inducible DNA sensor cGAS. J Immunol. 2015;194:1545–54. doi: 10.4049/jimmunol.1402066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Bai XC, Chen ZJ. Structures and mechanisms in the cGAS-STING innate immunity pathway. Immunity. 2020;53:43–53. doi: 10.1016/j.immuni.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Xia T, Konno H, Ahn J, Barber GN. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep. 2016;14:282–97. doi: 10.1016/j.celrep.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Falahat R, Berglund A, Putney RM, Perez-Villarroel P, Aoyama S, Pilon-Thomas S, et al. Epigenetic reprogramming of tumor cell-intrinsic STING function sculpts antigenicity and T cell recognition of melanoma. Proc Natl Acad Sci USA. 2021;118:e2013598118. doi: 10.1073/pnas.2013598118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han C, Liu Z, Zhang Y, Shen A, Dong C, Zhang A, et al. Tumor cells suppress radiation-induced immunity by hijacking caspase 9 signaling. Nat Immunol. 2020;21:546–54. doi: 10.1038/s41590-020-0641-5. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez-Ruiz ME, Buque A, Hensler M, Chen J, Bloy N, Petroni G, et al. Apoptotic caspases inhibit abscopal responses to radiation and identify a new prognostic biomarker for breast cancer patients. OncoImmunology. 2019;8:e1655964. doi: 10.1080/2162402x.2019.1655964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chattopadhyay S, Marques JT, Yamashita M, Peters KL, Smith K, Desai A, et al. Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J. 2010;29:1762–73. doi: 10.1038/emboj.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu Q, Man SM, Karki R, Malireddi RKS, Kanneganti TD. Detrimental type I interferon signaling dominates protective AIM2 inflammasome responses during francisella novicida infection. Cell Rep. 2018;22:3168–74. doi: 10.1016/j.celrep.2018.02.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herzer K, Hofmann TG, Teufel A, Schimanski CC, Moehler M, Kanzler S, et al. IFN-alpha-induced apoptosis in hepatocellular carcinoma involves promyelocytic leukemia protein and TRAIL independently of p53. Cancer Res. 2009;69:855–62. doi: 10.1158/0008-5472.can-08-2831. [DOI] [PubMed] [Google Scholar]
- 63.Kotredes KP, Gamero AM. Interferons as inducers of apoptosis in malignant cells. J Interferon Cytokine Res. 2013;33:162–70. doi: 10.1089/jir.2012.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu D, Wu H, Wang C, Li Y, Tian H, Siraj S, et al. STING directly activates autophagy to tune the innate immune response. Cell Death Differ. 2019;26:1735–49. doi: 10.1038/s41418-018-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brault M, Olsen TM, Martinez J, Stetson DB, Oberst A. Intracellular nucleic acid sensing triggers necroptosis through synergistic type I IFN and TNF signaling. J Immunol. 2018;200:2748–56. doi: 10.4049/jimmunol.1701492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarhan J, Liu BC, Muendlein HI, Weindel CG, Smirnova I, Tang AY, et al. Constitutive interferon signaling maintains critical threshold of MLKL expression to license necroptosis. Cell Death Differ. 2019;26:332–47. doi: 10.1038/s41418-018-0122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X, Wu J, Liu Q, Li X, Li S, Chen J, et al. mtDNA-STING pathway promotes necroptosis-dependent enterocyte injury in intestinal ischemia reperfusion. Cell Death Dis. 2020;11:1050. doi: 10.1038/s41419-020-03239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schock SN, Chandra NV, Sun Y, Irie T, Kitagawa Y, Gotoh B, et al. Induction of necroptotic cell death by viral activation of the RIG-I or STING pathway. Cell Death Differ. 2017;24:615–25. doi: 10.1038/cdd.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen D, Tong J, Yang L, Wei L, Stolz DB, Yu J, et al. PUMA amplifies necroptosis signaling by activating cytosolic DNA sensors. Proc Natl Acad Sci USA. 2018;115:3930–5. doi: 10.1073/pnas.1717190115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gaidt MM, Ebert TS, Chauhan D, Ramshorn K, Pinci F, Zuber S, et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. 2017;171:1110–24. doi: 10.1016/j.cell.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang W, Hu D, Wu C, Feng Y, Li A, Liu W, et al. STING promotes NLRP3 localization in ER and facilitates NLRP3 deubiquitination to activate the inflammasome upon HSV-1 infection. PLoS Pathog. 2020;16:e1008335. doi: 10.1371/journal.ppat.1008335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rongvaux A, Jackson R, Harman CC, Li T, West AP, de Zoete MR, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–77. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–62. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ning XH, Wang YT, Jing M, Sha MY, Lv MZ, Gao PF, et al. Apoptotic caspases suppress type I interferon production via the cleavage of cGAS, MAVS, and IRF3. Mol Cell. 2019;74:19–31. doi: 10.1016/j.molcel.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Wang YT, Ning XH, Gao PF, Wu SX, Sha MY, Lv MZ, et al. Inflammasome activation triggers caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity. 2017;46:393–404. doi: 10.1016/j.immuni.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 76.Banerjee I, Behl B, Mendonca M, Shrivastava G, Russo AJ, Menoret A, et al. Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity. 2018;49:413–26. doi: 10.1016/j.immuni.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Y, Wu M, Cao D, Yang C, Jin J, Wu L, et al. ZBP1-MLKL necroptotic signaling potentiates radiation-induced antitumor immunity via intratumoral STING pathway activation. Sci Adv. 2021;7:eabf6290. doi: 10.1126/sciadv.abf6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cobo I, Tanaka TN, Chandra Mangalhara K, Lana A, Yeang C, Han C, et al. DNA methyltransferase 3 alpha and TET methylcytosine dioxygenase 2 restrain mitochondrial DNA-mediated interferon signaling in macrophages. Immunity. 2022;55:1386–401.e10. doi: 10.1016/j.immuni.2022.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xian H, Watari K, Sanchez-Lopez E, Offenberger J, Onyuru J, Sampath H, et al. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity. 2022;55:1370–85.e8. doi: 10.1016/j.immuni.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lei Y, VanPortfliet JJ, Chen YF, Bryant JD, Li Y, Fails D, et al. Cooperative sensing of mitochondrial DNA by ZBP1 and cGAS promotes cardiotoxicity. Cell. 2023;186:3013–32.e22. doi: 10.1016/j.cell.2023.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zecchini V, Paupe V, Herranz-Montoya I, Janssen J, Wortel IMN, Morris JL, et al. Fumarate induces vesicular release of mtDNA to drive innate immunity. Nature. 2023;615:499–506. doi: 10.1038/s41586-023-05770-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang X, Shi C, Li H, Shen S, Su C, Yin H. MARCH8 attenuates cGAS-mediated innate immune responses through ubiquitylation. Sci Signal. 2022;15:eabk3067. doi: 10.1126/scisignal.abk3067. [DOI] [PubMed] [Google Scholar]
- 83.Cai X, Zhou Z, Zhu J, Liu X, Ouyang G, Wang J, et al. Opposing effects of deubiquitinase OTUD3 in innate immunity against RNA and DNA viruses. Cell Rep. 2022;39:110920. doi: 10.1016/j.celrep.2022.110920. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi M, Lio CJ, Campeau A, Steger M, Ay F, Mann M, et al. The tumor suppressor kinase DAPK3 drives tumor-intrinsic immunity through the STING-IFN-beta pathway. Nat Immunol. 2021;22:485–96. doi: 10.1038/s41590-021-00896-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kong L, Sui C, Chen T, Zhang L, Zhao W, Zheng Y, et al. The ubiquitin E3 ligase TRIM10 promotes STING aggregation and activation in the Golgi apparatus. Cell Rep. 2023;42:112306. doi: 10.1016/j.celrep.2023.112306. [DOI] [PubMed] [Google Scholar]
- 86.Tian M, Liu W, Zhang Q, Huang Y, Li W, Wang W, et al. MYSM1 represses innate immunity and autoimmunity through suppressing the cGAS-STING pathway. Cell Rep. 2020;33:108297. doi: 10.1016/j.celrep.2020.108297. [DOI] [PubMed] [Google Scholar]
- 87.Zhang J, Chen Y, Chen X, Zhang W, Zhao L, Weng L, et al. Deubiquitinase USP35 restrains STING-mediated interferon signaling in ovarian cancer. Cell Death Differ. 2021;28:139–55. doi: 10.1038/s41418-020-0588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seo GJ, Yang A, Tan B, Kim S, Liang QM, Choi Y, et al. Akt kinase-mediated checkpoint of cGAS DNA sensing pathway. Cell Rep. 2015;13:440–9. doi: 10.1016/j.celrep.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zierhut C, Yamaguchi N, Paredes M, Luo JD, Carroll T, Funabiki H. The cytoplasmic DNA sensor cGAS promotes mitotic cell death. Cell. 2019;178:302–15. doi: 10.1016/j.cell.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pathare GR, Decout A, Gluck S, Cavadini S, Makasheva K, Hovius R, et al. Structural mechanism of cGAS inhibition by the nucleosome. Nature. 2020;587:668–72. doi: 10.1038/s41586-020-2750-6. [DOI] [PubMed] [Google Scholar]
- 91.Michalski S, de Oliveira Mann CC, Stafford CA, Witte G, Bartho J, Lammens K, et al. Structural basis for sequestration and autoinhibition of cGAS by chromatin. Nature. 2020;587:678–82. doi: 10.1038/s41586-020-2748-0. [DOI] [PubMed] [Google Scholar]
- 92.Zhao B, Xu P, Rowlett CM, Jing T, Shinde O, Lei Y, et al. The molecular basis of tight nuclear tethering and inactivation of cGAS. Nature. 2020;587:673–7. doi: 10.1038/s41586-020-2749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kujirai T, Zierhut C, Takizawa Y, Kim R, Negishi L, Uruma N, et al. Structural basis for the inhibition of cGAS by nucleosomes. Science. 2020;370:455–8. doi: 10.1126/science.abd0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao D, Han X, Fan X, Xu RM, Zhang X. Structural basis for nucleosome-mediated inhibition of cGAS activity. Cell Res. 2020;30:1088–97. doi: 10.1038/s41422-020-00422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peitzmeier SM, Silberholz J, Gardner IH, Weinand J, Acevedo K. Time to first onset of chest binding-related symptoms in transgender youth. Pediatrics. 2021;147:e20200728. doi: 10.1542/peds.2020-0728. [DOI] [PubMed] [Google Scholar]
- 96.Sun X, Liu T, Zhao J, Xia H, Xie J, Guo Y, et al. DNA-PK deficiency potentiates cGAS-mediated antiviral innate immunity. Nat Commun. 2020;11:6182. doi: 10.1038/s41467-020-19941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang C, Shang G, Gui X, Zhang X, Bai XC, Chen ZJ. Structural basis of STING binding with and phosphorylation by TBK1. Nature. 2019;567:394–8. doi: 10.1038/s41586-019-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu S, Cai X, Wu J, Cong Q, Chen X, Li T, et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347:aaa2630. doi: 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]
- 99.Hu MM, Yang Q, Xie XQ, Liao CY, Lin H, Liu TT, et al. Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity. 2016;45:555–69. doi: 10.1016/j.immuni.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 100.Cui Y, Yu HS, Zheng X, Peng R, Wang Q, Zhou Y, et al. SENP7 potentiates cGAS activation by relieving SUMO-mediated inhibition of cytosolic DNA sensing. PLoS Pathog. 2017;13:e1006156. doi: 10.1371/journal.ppat.1006156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xia PY, Ye BQ, Wang S, Zhu XX, Du Y, Xiong Z, et al. Glutamylation of the DNA sensor cGAS regulates its binding and synthase activity in antiviral immunity. Nat Immunol. 2016;17:369–78. doi: 10.1038/ni.3356. [DOI] [PubMed] [Google Scholar]
- 102.Song ZM, Lin H, Yi XM, Guo W, Hu MM, Shu HB. KAT5 acetylates cGAS to promote innate immune response to DNA virus. P Natl Acad Sci USA. 2020;117:21568–75. doi: 10.1073/pnas.1922330117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma DP, Yang M, Wang QS, Sun CY, Shi HB, Jing WQ, et al. Arginine methyltransferase PRMT5 negatively regulates cGAS-mediated antiviral immune response. Sci Adv. 2021;7:eabc1834. doi: 10.1126/sciadv.abc1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi C, Yang X, Liu Y, Li H, Chu H, Li G, et al. ZDHHC18 negatively regulates cGAS-mediated innate immunity through palmitoylation. EMBO J. 2022;41:e109272. doi: 10.15252/embj.2021109272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mukai K, Konno H, Akiba T, Uemura T, Waguri S, Kobayashi T, et al. Activation of STING requires palmitoylation at the Golgi. Nat Commun. 2016;7:11932. doi: 10.1038/ncomms11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang F, Zhao M, Chang B, Zhou Y, Wu X, Ma M, et al. Cytoplasmic PARP1 links the genome instability to the inhibition of antiviral immunity through PARylating cGAS. Mol Cell. 2022;82:2032–49. doi: 10.1016/j.molcel.2022.03.034. [DOI] [PubMed] [Google Scholar]
- 107.Ferguson BJ, Mansur DS, Peters NE, Ren H, Smith GL. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. Elife. 2012;1:e00047. doi: 10.7554/elife.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548:466–70. doi: 10.1038/nature23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li L, Yin Q, Kuss P, Maliga Z, Millan JL, Wu H, et al. Hydrolysis of 2′3′-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat Chem Biol. 2014;10:1043–8. doi: 10.1038/nchembio.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lohofener J, Steinke N, Kay-Fedorov P, Baruch P, Nikulin A, Tishchenko S, et al. The activation mechanism of 2′-5′-oligoadenylate synthetase gives new insights into OAS/cGAS triggers of innate immunity. Structure. 2015;23:851–62. doi: 10.1016/j.str.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 111.Wang C, Guan Y, Lv M, Zhang R, Guo Z, Wei X, et al. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity. 2018;48:675–87. doi: 10.1016/j.immuni.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 112.Zhao Z, Ma Z, Wang B, Guan Y, Su XD, Jiang Z. Mn(2+) directly activates cGAS and structural analysis suggests Mn(2+) induces a noncanonical catalytic synthesis of 2′3′-cGAMP. Cell Rep. 2020;32:108053. doi: 10.1016/j.celrep.2020.108053. [DOI] [PubMed] [Google Scholar]
- 113.Lv M, Chen M, Zhang R, Zhang W, Wang C, Zhang Y, et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30:966–79. doi: 10.1038/s41422-020-00395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Du M, Chen ZJ. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 2018;361:704–9. doi: 10.1126/science.aat1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu X, Zhang L, Shen J, Zhai Y, Jiang Q, Yi M, et al. The STING phase-separator suppresses innate immune signalling. Nat Cell Biol. 2021;23:330–40. doi: 10.1038/s41556-021-00659-0. [DOI] [PubMed] [Google Scholar]
- 116.Andreeva L, Hiller B, Kostrewa D, Lässig C, de Oliveira Mann CC, Jan Drexler D, et al. cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein-DNA ladders. Nature. 2017;549:394–8. doi: 10.1038/nature23890. [DOI] [PubMed] [Google Scholar]
- 117.Grieves JL, Fye JM, Harvey S, Grayson JM, Hollis T, Perrino FW. Exonuclease TREX1 degrades double-stranded DNA to prevent spontaneous lupus-like inflammatory disease. Proc Natl Acad Sci USA. 2015;112:5117–22. doi: 10.1073/pnas.1423804112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yuan F, Dutta T, Wang L, Song L, Gu L, Qian L, et al. Human DNA exonuclease TREX1 is also an exoribonuclease that acts on single-stranded RNA. J Biol Chem. 2015;290:13344–53. doi: 10.1074/jbc.m115.653915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–98. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pantelidou C, Sonzogni O, Taveira MD, Mehta AK, Kothari A, Wang D, et al. PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 2019;9:722–37. doi: 10.1158/2159-8290.cd-18-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou W, Mohr L, Maciejowski J, Kranzusch PJ. cGAS phase separation inhibits TREX1-mediated DNA degradation and enhances cytosolic DNA sensing. Mol Cell. 2021;81:739–55. doi: 10.1016/j.molcel.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ghosh M, Saha S, Li J, Montrose DC, Martinez LA. p53 engages the cGAS/STING cytosolic DNA sensing pathway for tumor suppression. Mol Cell. 2023;83:266–80.e6. doi: 10.1016/j.molcel.2022.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao Q, Yang C, Wang J, Li Y, Yang P. Serum level of DNase1l3 in patients with dermatomyositis/polymyositis, systemic lupus erythematosus and rheumatoid arthritis, and its association with disease activity. Clin Exp Med. 2017;17:459–65. doi: 10.1007/s10238-016-0448-8. [DOI] [PubMed] [Google Scholar]
- 124.Sisirak V, Sally B, D’Agati V, Martinez-Ortiz W, Ozcakar ZB, David J, et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell. 2016;166:88–101. doi: 10.1016/j.cell.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Soni C, Perez OA, Voss WN, Pucella JN, Serpas L, Mehl J, et al. Plasmacytoid dendritic cells and type I interferon promote extrafollicular B cell responses to extracellular self-DNA. Immunity. 2020;52:1022–38. doi: 10.1016/j.immuni.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mosallanejad K, Kennedy SN, Bahleda KM, Slavik KM, Zhou W, Govande AA, et al. Species-specific self-DNA detection mechanisms by mammalian cyclic GMP-AMP synthases. Sci Immunol. 2023;8:eabp9765. doi: 10.1126/sciimmunol.abp9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Y, Xu P, Rivara S, Liu C, Ricci J, Ren X, et al. Clathrin-associated AP-1 controls termination of STING signalling. Nature. 2022;610:761–7. doi: 10.1038/s41586-022-05354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu B, Carlson RJ, Pires IS, Gentili M, Feng E, Hellier Q, et al. Human STING is a proton channel. Science. 2023;381:508–14. doi: 10.1126/science.adf8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu C, Guan J, Lu S, Jin Q, Rousseau B, Lu T, et al. DNA sensing in mismatch repair-deficient tumor cells is essential for anti-tumor immunity. Cancer Cell. 2021;39:96–108. doi: 10.1016/j.ccell.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tian J, Zhang D, Kurbatov V, Wang Q, Wang Y, Fang D, et al. 5-Fluorouracil efficacy requires anti-tumor immunity triggered by cancer-cell-intrinsic STING. EMBO J. 2021;40:e106065. doi: 10.15252/embj.2020106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, et al. DNA breaks and chromosome pulverization from errors in mitosis. Nature. 2012;482:53–8. doi: 10.1038/nature10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang W, Liu W, Jia L, Chen D, Chang I, Lake M, et al. Targeting KDM4A epigenetically activates tumor-cell-intrinsic immunity by inducing DNA replication stress. Mol Cell. 2021;81:2148–65. doi: 10.1016/j.molcel.2021.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang CZ, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, et al. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522:179–84. doi: 10.1038/nature14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mackenzie KJ, Carroll P, Martin CA, Murina O, Fluteau A, Simpson DJ, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548:461–5. doi: 10.1038/nature23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Song S, Peng P, Tang Z, Zhao J, Wu W, Li H, et al. Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci Rep. 2017;7:39858. doi: 10.1038/srep39858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marcus A, Mao AJ, Lensink-Vasan M, Wang L, Vance RE, Raulet DH. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity. 2018;49:754–63. doi: 10.1016/j.immuni.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou Y, Fei M, Zhang G, Liang WC, Lin W, Wu Y, et al. Blockade of the phagocytic receptor MerTK on tumor-associated macrophages enhances P2X7R-dependent STING activation by tumor-derived cGAMP. Immunity. 2020;52:357–73. doi: 10.1016/j.immuni.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 139.An X, Zhu Y, Zheng T, Wang G, Zhang M, Li J, et al. An analysis of the expression and association with immune cell infiltration of the cGAS/STING pathway in pan-cancer. Mol Ther Nucleic Acids. 2019;14:80–9. doi: 10.1016/j.omtn.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Diamond JM, Vanpouille-Box C, Spada S, Rudqvist NP, Chapman JR, Ueberheide BM, et al. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol Res. 2018;6:910–20. doi: 10.1158/2326-6066.cir-17-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang H, Tang K, Zhang Y, Ma R, Ma J, Li Y, et al. Cell-free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling. Cancer Immunol Res. 2015;3:196–205. doi: 10.1158/2326-6066.cir-14-0177. [DOI] [PubMed] [Google Scholar]
- 142.Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–53. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liang Y, Hannan R, Fu YX. Type I IFN activating type I dendritic cells for antitumor immunity. Clin Cancer Res. 2021;27:3818–24. doi: 10.1158/1078-0432.ccr-20-2564. [DOI] [PubMed] [Google Scholar]
- 144.Maltbaek JH, Cambier S, Snyder JM, Stetson DB. ABCC1 transporter exports the immunostimulatory cyclic dinucleotide cGAMP. Immunity. 2022;55:1799–812.e4. doi: 10.1016/j.immuni.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ritchie C, Cordova AF, Hess GT, Bassik MC, Li L. SLC19A1 is an importer of the immunotransmitter cGAMP. Mol Cell. 2019;75:372–81. doi: 10.1016/j.molcel.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu H, Zhang H, Wu X, Ma D, Wu J, Wang L, et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature. 2018;563:131–6. doi: 10.1038/s41586-018-0629-6. [DOI] [PubMed] [Google Scholar]
- 147.Li J, Hubisz MJ, Earlie EM, Duran MA, Hong C, Varela AA, et al. Non-cell-autonomous cancer progression from chromosomal instability. Nature. 2023;620:1080–8. doi: 10.1038/s41586-023-06464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hong C, Schubert M, Tijhuis AE, Requesens M, Roorda M, van den Brink A, et al. cGAS-STING drives the IL-6-dependent survival of chromosomally instable cancers. Nature. 2022;607:366–73. doi: 10.1038/s41586-022-04847-2. [DOI] [PubMed] [Google Scholar]
- 149.Bakhoum SF. APOBEC3A drives STING-dependent metastasis. Nat Can (Ott) 2021;2:1293–5. doi: 10.1038/s43018-021-00286-6. [DOI] [PubMed] [Google Scholar]
- 150.Wormann SM, Zhang A, Thege FI, Cowan RW, Rupani DN, Wang R, et al. APOBEC3A drives deaminase domain-independent chromosomal instability to promote pancreatic cancer metastasis. Nat Can (Ott) 2021;2:1338–56. doi: 10.1038/s43018-021-00268-8. [DOI] [PubMed] [Google Scholar]
- 151.Qiu S, Zhong X, Meng X, Li S, Qian X, Lu H, et al. Mitochondria-localized cGAS suppresses ferroptosis to promote cancer progression. Cell Res. 2023;33:299–311. doi: 10.1038/s41422-023-00788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment – tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–75. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 153.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/jci67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cohen-Jonathan E, Bernhard EJ, McKenna WG. How does radiation kill cells? Curr Opin Chem Biol. 1999;3:77–83. doi: 10.1016/s1367-5931(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 155.Fang C, Mo F, Liu L, Du J, Luo M, Men K, et al. Oxidized mitochondrial DNA sensing by STING signaling promotes the antitumor effect of an irradiated immunogenic cancer cell vaccine. Cell Mol Immunol. 2021;18:2211–23. doi: 10.1038/s41423-020-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8(+) T cells via dendritic cell activation. J Immunol. 2012;189:558–66. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 157.Schadt L, Sparano C, Schweiger NA, Silina K, Cecconi V, Lucchiari G, et al. Cancer-cell-intrinsic cGAS expression mediates tumor immunogenicity. Cell Rep. 2019;29:1236–48. doi: 10.1016/j.celrep.2019.09.065. [DOI] [PubMed] [Google Scholar]
- 158.Liang H, Deng LF, Hou YZ, Meng XJ, Huang XN, Rao EY, et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat Commun. 2017;8:1736. doi: 10.1038/s41467-017-01566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hou Y, Liang H, Rao E, Zheng W, Huang X, Deng L, et al. Non-canonical NF-kappaB antagonizes STING sensor-mediated DNA sensing in radiotherapy. Immunity. 2018;49:490–503. doi: 10.1016/j.immuni.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen HY, Xu L, Li LF, Liu XX, Gao JX, Bai YR. Inhibiting the CD8(+) T cell infiltration in the tumor microenvironment after radiotherapy is an important mechanism of radioresistance. Sci Rep. 2018;8:11934. doi: 10.1038/s41598-018-30417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Crittenden MR, Zebertavage L, Kramer G, Bambina S, Friedman D, Troesch V, et al. Tumor cure by radiation therapy and checkpoint inhibitors depends on pre-existing immunity. Sci Rep. 2018;8:7012. doi: 10.1038/s41598-018-25482-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shinde-Jadhav S, Mansure JJ, Rayes RF, Marcq G, Ayoub M, Skowronski R, et al. Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nat Commun. 2021;12:2776. doi: 10.1038/s41467-021-23086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McBride S, Sherman E, Tsai CJ, Baxi S, Aghalar J, Eng J, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2021;39:30–7. doi: 10.1200/jco.20.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–9. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 165.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 166.Erdal E, Haider S, Rehwinkel J, Harris AL, McHugh PJ. A prosurvival DNA damage-induced cytoplasmic interferon response is mediated by end resection factors and is limited by Trex1. Gene Dev. 2017;31:353–69. doi: 10.1101/gad.289769.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kitai Y, Kawasaki T, Sueyoshi T, Kobiyama K, Ishii KJ, Zou J, et al. DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. J Immunol. 2017;198:1649–59. doi: 10.4049/jimmunol.1601694. [DOI] [PubMed] [Google Scholar]
- 168.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28:739–45. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 169.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pascal JM. The comings and goings of PARP-1 in response to DNA damage. DNA Repair. 2018;71:177–82. doi: 10.1016/j.dnarep.2018.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chaudhuri AR, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18:610–21. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sen T, Rodriguez BL, Chen LM, Della Corte CM, Morikawa N, Fujimoto J, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019;9:646–61. doi: 10.1158/2159-8290.cd-18-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang L, Yang L, Wang C, Zhao W, Ju Z, Zhang W, et al. Inhibition of the ATM/Chk2 axis promotes cGAS/STING signaling in ARID1A-deficient tumors. J Clin Invest. 2020;130:5951–66. doi: 10.1172/jci130445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hu M, Zhou M, Bao X, Pan D, Jiao M, Liu X, et al. ATM inhibition enhances cancer immunotherapy by promoting mtDNA leakage and cGAS/STING activation. J Clin Invest. 2021;131:e139333. doi: 10.1172/jci139333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang Y, Chen H, Mo H, Hu X, Gao R, Zhao Y, et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell. 2021;39:1578–93. doi: 10.1016/j.ccell.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 176.Xu MM, Pu Y, Han D, Shi Y, Cao X, Liang H, et al. Dendritic cells but not macrophages sense tumor mitochondrial DNA for cross-priming through signal regulatory protein alpha signaling. Immunity. 2017;47:363–73. doi: 10.1016/j.immuni.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jacquelot N, Yamazaki T, Roberti MP, Duong CPM, Andrews MC, Verlingue L, et al. Sustained type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res. 2019;29:846–61. doi: 10.1038/s41422-019-0224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Buque A, Rodriguez-Ruiz ME, Fucikova J, Galluzzi L. Apoptotic caspases cut down the immunogenicity of radiation. OncoImmunology. 2019;8:e1655364. doi: 10.1080/2162402x.2019.1655364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 180.Chen M, Meng Q, Qin Y, Liang P, Tan P, He L, et al. TRIM14 inhibits cGAS degradation mediated by selective autophagy receptor p62 to promote innate immune responses. Mol Cell. 2016;64:105–19. doi: 10.1016/j.molcel.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 181.Witte G, Hartung S, Buttner K, Hopfner KP. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol Cell. 2008;30:167–78. doi: 10.1016/j.molcel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 182.Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–5. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McWhirter SM, Barbalat R, Monroe KM, Fontana MF, Hyodo M, Joncker NT, et al. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J Exp Med. 2009;206:1899–911. doi: 10.1084/jem.20082874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Karaolis DKR, Cheng KR, Lipsky M, Elnabawi A, Catalano J, Hyodo M, et al. 3′,5′-Cyclic diguanylic acid (c-di-GMP) inhibits basal and growth factor-stimulated human colon cancer cell proliferation. Biochem Bioph Res Co. 2005;329:40–5. doi: 10.1016/j.bbrc.2005.01.093. [DOI] [PubMed] [Google Scholar]
- 185.Demaria O, De Gassart A, Coso S, Gestermann N, Di Domizio J, Flatz L, et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci USA. 2015;112:15408–13. doi: 10.1073/pnas.1512832112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Harrington KJ, Brody J, Ingham M, Strauss J, Cemerski S, Wang M, et al. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann Oncol. 2018;29:viii712. doi: 10.1093/annonc/mdy424.015. [DOI] [Google Scholar]
- 187.McIntosh JA, Liu Z, Andresen BM, Marzijarani NS, Moore JC, Marshall NM, et al. A kinase-cGAS cascade to synthesize a therapeutic STING activator. Nature. 2022;603:439–44. doi: 10.1038/s41586-022-04422-9. [DOI] [PubMed] [Google Scholar]
- 188.Woon ST, Reddy CB, Drummond CJ, Schooltink MA, Baguley BC, Kieda C, et al. A comparison of the ability of DMXAA and xanthenone analogues to activate NF-kappaB in murine and human cell lines. Oncol Res. 2005;15:351–64. doi: 10.3727/096504005776449743. [DOI] [PubMed] [Google Scholar]
- 189.Roberts ZJ, Goutagny N, Perera PY, Kato H, Kumar H, Kawai T, et al. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204:1559–69. doi: 10.1084/jem.20061845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Conlon J, Burdette DL, Sharma S, Bhat N, Thompson M, Jiang Z, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol. 2013;190:5216–25. doi: 10.4049/jimmunol.1300097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cavlar T, Deimling T, Ablasser A, Hopfner KP, Hornung V. Species-specific detection of the antiviral small-molecule compound CMA by STING. EMBO J. 2013;32:1440–50. doi: 10.1038/emboj.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang Y, Sun Z, Pei J, Luo Q, Zeng X, Li Q, et al. Identification of alpha-mangostin as an agonist of human STING. ChemMedChem. 2018;13:2057–64. doi: 10.1002/cmdc.201800481. [DOI] [PubMed] [Google Scholar]
- 193.Ramanjulu JM, Pesiridis GS, Yang JS, Concha N, Singhaus R, Zhang SY, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564:439–43. doi: 10.1038/s41586-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 194.Messaoud-Nacer Y, Culerier E, Rose S, Maillet I, Rouxel N, Briault S, et al. STING agonist diABZI induces PANoptosis and DNA mediated acute respiratory distress syndrome (ARDS) Cell Death Dis. 2022;13:269. doi: 10.1038/s41419-022-04664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chin EN, Yu C, Vartabedian VF, Jia Y, Kumar M, Gamo AM, et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science. 2020;369:993–9. doi: 10.1126/science.abb4255. [DOI] [PubMed] [Google Scholar]
- 196.Pan BS, Perera SA, Piesvaux JA, Presland JP, Schroeder GK, Cumming JN, et al. An orally available non-nucleotide STING agonist with antitumor activity. Science. 2020;369:eaba6098. doi: 10.1126/science.aba6098. [DOI] [PubMed] [Google Scholar]
- 197.Sivick KE, Desbien AL, Glickman LH, Reiner GL, Corrales L, Surh NH, et al. Magnitude of therapeutic STING activation determines CD8(+) T cell-mediated anti-tumor immunity. Cell Rep. 2018;25:3074–85. doi: 10.1016/j.celrep.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 198.Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AKR, Sevimli S, et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat Nanotechnol. 2019;14:269–78. doi: 10.1038/s41565-018-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Koshy ST, Cheung AS, Gu L, Graveline AR, Mooney DJ. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv Biosyst. 2017;1:1600013. doi: 10.1002/adbi.201600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li S, Mirlekar B, Johnson BM, Brickey WJ, Wrobel JA, Yang N, et al. STING-induced regulatory B cells compromise NK function in cancer immunity. Nature. 2022;610:373–80. doi: 10.1038/s41586-022-05254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jneid B, Bochnakian A, Hoffmann C, Delisle F, Djacoto E, Sirven P, et al. Selective STING stimulation in dendritic cells primes antitumor T cell responses. Sci Immunol. 2023;8:eabn6612. doi: 10.1126/sciimmunol.abn6612. [DOI] [PubMed] [Google Scholar]
- 202.Yang K, Han W, Jiang X, Piffko A, Bugno J, Han C, et al. Zinc cyclic di-AMP nanoparticles target and suppress tumours via endothelial STING activation and tumour-associated macrophage reinvigoration. Nat Nanotechnol. 2022;17:1322–31. doi: 10.1038/s41565-022-01225-x. [DOI] [PubMed] [Google Scholar]
- 203.Dane EL, Belessiotis-Richards A, Backlund C, Wang J, Hidaka K, Milling LE, et al. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat Mater. 2022;21:710–20. doi: 10.1038/s41563-022-01251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xue A, Shang Y, Jiao P, Zhang S, Zhu C, He X, et al. Increased activation of cGAS-STING pathway enhances radiosensitivity of non-small cell lung cancer cells. Thorac Cancer. 2022;13:1361–8. doi: 10.1111/1759-7714.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zaidi AH, Kelly RJ, Gorbunova A, Omstead AN, Salvitti MS, Zheng P, et al. Intratumoral immunotherapy with STING agonist, ADU-S100, induces CD8+ T-cell mediated anti-tumor immunity in an esophageal adenocarcinoma model. Oncotarget. 2021;12:292–303. doi: 10.18632/oncotarget.27886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ghaffari A, Peterson N, Khalaj K, Vitkin N, Robinson A, Francis JA, et al. STING agonist therapy in combination with PD-1 immune checkpoint blockade enhances response to carboplatin chemotherapy in high-grade serous ovarian cancer. Br J Cancer. 2018;119:440–9. doi: 10.1038/s41416-018-0188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jeong SH, Yang MJ, Choi S, Kim J, Koh GY. Refractoriness of STING therapy is relieved by AKT inhibitor through effective vascular disruption in tumour. Nat Commun. 2021;12:4405. doi: 10.1038/s41467-021-24603-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma-subunit or zeta-subunit of the immunoglobulin and T-cell receptors. P Natl Acad Sci USA. 1993;90:720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–99. doi: 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 210.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44. doi: 10.1056/nejmoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48. doi: 10.1056/nejmoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tchou J, Zhao YB, Levine BL, Zhang PJ, Davis MM, Melenhorst JJ, et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol Res. 2017;5:1152–61. doi: 10.1158/2326-6066.cir-17-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Smith TT, Moffett HF, Stephan SB, Opel CF, Dumigan AG, Jiang XY, et al. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J Clin Invest. 2017;127:2176–91. doi: 10.1172/jci87624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xu N, Palmer DC, Robeson AC, Shou P, Bommiasamy H, Laurie SJ, et al. STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J Exp Med. 2021;218:e20200844. doi: 10.1084/jem.20200844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ngiow SF, Young A, Jacquelot N, Yamazaki T, Enot D, Zitvogel L, et al. A threshold level of intratumor CD8(+) T-cell PD1 expression dictates therapeutic response to anti-PD1. Cancer Res. 2015;75:3800–11. doi: 10.1158/0008-5472.can-15-1082. [DOI] [PubMed] [Google Scholar]
- 216.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–9. doi: 10.1158/1078-0432.ccr-08-1608. [DOI] [PubMed] [Google Scholar]
- 217.Tang H, Wang Y, Chlewicki LK, Zhang Y, Guo J, Liang W, et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016;29:285–96. doi: 10.1016/j.ccell.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19:1189–201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Foote JB, Kok M, Leatherman JM, Armstrong TD, Marcinkowski BC, Ojalvo LS, et al. A STING agonist given with OX40 receptor and PD-L1 modulators primes immunity and reduces tumor growth in Tolerized mice. Cancer Immunol Res. 2017;5:468–79. doi: 10.1158/2326-6066.cir-16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Moore E, Clavijo PE, Davis R, Cash H, Van Waes C, Kim Y, et al. Established T cell-inflamed tumors rejected after adaptive resistance was reversed by combination STING activation and PD-1 pathway blockade. Cancer Immunol Res. 2016;4:1061–71. doi: 10.1158/2326-6066.cir-16-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ager CR, Reilley MJ, Nicholas C, Bartkowiak T, Jaiswal AR, Curran MA. Intratumoral STING activation with T-cell checkpoint modulation generates systemic antitumor immunity. Cancer Immunol Res. 2017;5:676–84. doi: 10.1158/2326-6066.cir-17-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ok CY, Young KH. Checkpoint inhibitors in hematological malignancies. J Hematol Oncol. 2017;10:103. doi: 10.1186/s13045-017-0474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dubensky TW, Reed SG. Adjuvants for cancer vaccines. Semin Immunol. 2010;22:155–61. doi: 10.1016/j.smim.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 224.Yi M, Qin S, Zhao W, Yu S, Chu Q, Wu K. The role of neoantigen in immune checkpoint blockade therapy. Exp Hematol Oncol. 2018;7:28. doi: 10.1186/s40164-018-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7:283ra52. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang Z, Celis E. STING activator c-di-GMP enhances the anti-tumor effects of peptide vaccines in melanoma-bearing mice. Cancer Immunol Immunother. 2015;64:1057–66. doi: 10.1007/s00262-015-1713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kinkead HL, Hopkins A, Lutz E, Wu AA, Yarchoan M, Cruz K, et al. Combining STING-based neoantigen-targeted vaccine with checkpoint modulators enhances antitumor immunity in murine pancreatic cancer. JCI Insight. 2018;3 doi: 10.1172/jci.insight.122857. [DOI] [PMC free article] [PubMed] [Google Scholar]