Abstract
Objective:
Viral load suppression (VLS) is critical in reducing morbidity and mortality associated with HIV as well as minimizing the likelihood of HIV transmission to uninfected individuals. The objective of this study was to identify factors associated with VLS among people living with HIV (PLWH) on antiretroviral (ARVs) therapy in a population-based survey to inform HIV program strategies in Nigeria.
Design and methods:
Adult participants,15–64 years old, from the 2018 Nigeria HIV/AIDS Indicator and Impact Survey (NAIIS) who self-reported to be a PLWH or had detectable ARVs were analyzed to examine factors associated with VLS. NAIIS measured HIV prevalence, viral load, HIV incidence, HIV drug resistance, ARV, and hepatitis B among PLWH.
Results:
Of 1,322 participants, 949 (71.8%) were women and 1,305 (98.7%) had detectable ARVs. The median age was 31 (interquartile range [IQR]: 39 – 48) years. Weighted prevalence of VLS was 80.6%. Compared to participants with detectable ARVs, those with undetected ARVs in their blood specimens had lower odds of VLS (adjusted odds ratio [aOR] = 0.24; 95% CI, 0.08–0.64). Those with hepatitis B infection (aOR = 0.29; 95% CI, 0.20–0.58) and non-nucleoside reverse transcriptase inhibitor-based regimen (aOR = 0.34; 95% CI, 0.12–1.01) were also associated with lower odds of VLS. However, older individuals, (45–54 vs. 15–24 years) had increased odds of VLS (aOR = 2.81; 95% CI, 1.14 – 6.90).
Conclusion:
Young individuals and those with undetectable ARVs were less likely to be virally suppressed. Targeted interventions focusing on young individuals and improved adherence to medication are needed to achieve the 95-95-95 goals, critical to HIV epidemic control. The study also confirms the superiority of protease inhibitor-based regimen for ARV treatment.
Keywords: Viral suppression, people living with HIV, Antiretroviral, Survey
Introduction
In view of ending the HIV epidemic by 2030, the UNAIDS 95-95-95 targets recommend diagnosing 95% of people living with HIV (PLWH), linking 95% of those diagnosed to care and treatment, and achieving viral suppression in 95% of those linked in care and treatment [1]. Optimal viral load suppression (VLS) is critical in reducing morbidity and mortality associated with HIV and its associated opportunistic infections as well as minimizing the likelihood of HIV transmission to uninfected individuals [2–5]. Despite these benefits, challenges still exist and some patients receiving antiretroviral (ARV) therapy fail to achieve optimal viral suppression [6–8]. To achieve these UNAIDS targets, unbiased population-based data on the determinants of VLS are needed to inform targeted interventions.
Results from the 2018 Nigeria HIV/AIDS Indicator and Impact Survey (NAIIS) estimated the HIV prevalence among adults (15–49) years at 1.3%, about half of previous model based estimates [9]. NAIIS data showed that among adults aged 15–64 years, 96.4% self-reported being on ARV treatment or had detectable ARVs and 80.9% were virally suppressed. While impressive progress has been made following large-scale surge efforts in states with the highest prevalence of HIV and undiagnosed cases of HIV, more needs to be done to meet the UNAIDS targets and move Nigeria closer to epidemic control. The number of HIV-associated deaths and number of new infections decreased by 35% and 17%, respectively, from 2010 to 2019 [10] which is encouraging. Nevertheless, based on the strong relationship between VL and transmission probability [3]; targeted assessment and intervention to increase VLS is vital to sustain progressive decline in the number of new infections.
Effective monitoring of VLS should parallel high VL testing services for PLWH. Innovative interventions implemented in Nigeria such as deployment of surge members to community, improving patient contact information, and prevention of consumables stock outs facilitate VL coverage [11]. For example, community ARV therapy teams collected blood sample for VL testing in addition to facilitating the dispensing of ARV treatment. These interventions lead to improvement of viral load coverage to 88% at the beginning of 2020 [12].
The objective of this study was to identify factors associated with VLS among PLWH on ARV therapy to inform HIV program strategies in Nigeria focused on ending the HIV epidemic.
METHODS
Survey design
The NAIIS is a nationally representative, cross-sectional household (HH) survey that was conducted in 2018. NAIIS used a two-stage cluster-based sampling design, selecting enumeration areas followed by HH as previously described [9]. The survey measured HIV prevalence, VLS, HIV incidence, HIV drug resistance (DR), ARV detection, and hepatitis B among PLWH. Laboratory specific details, blood collection, testing, and specimen management for all population-based HIV impact assessment (PHIA) surveys are described in the manuscript by Patel et al. [13]. The NAIIS survey followed similar approach, as the PHIA surveys, where participants were offered HIV testing and counseling in their homes using the national HIV rapid testing algorithm. Additionally, all HH positive participants also received their CD4 cell counts (Pima CD4; Abbott, Abbott Park, Illinois) results. All HIV-positive specimens from HH, underwent confirmatory testing using the Geenius™ HIV 1/2 Supplemental Assay (Bio-Rad, Hercules, California, United States). Positive specimens on Geenius™ HIV 1/2 were considered final HIV positive test result for the survey. Additionally, all confirmed HIV-positive specimens were tested for plasma HIV-1 RNA using the Roche COBAS® AmpliPrep/COBAS® TaqMan® (CAP/CTM) HIV-1 Test, version 2.0 (Roche Diagnostics, Pleasanton, CA, USA). In few cases when plasma specimen was not available, dried blood spot (DBS) VL was performed. DBS specimens were tested for the presence of ARVs; efavirenz (EFV), nevirapine (NVP), lopinavir (LPV), and atazanavir (ATV), using the qualitative high-performance liquid chromatography and tandem mass spectrometry (LC/MS-MS) assay [14]. Additional information on laboratory tests is available in the 2018 NAIIS Technical Report [9].
Eligibility
All HIV-positive adults aged 15 to 64 years who participated in the NAIIS, provided consent for biomarker testing, and either self-reported to be on ARV therapy or had detectable ARVs in their blood specimens were included in this analysis.
Variables and definitions
VLS was defined as plasma HIV-1 RNA < 1,000 cp/mL as per WHO guidelines [15]. CD4 cell count was categorized as <500 and ≥ 500 cells/mm3. Age was categorized as 15–24, 25–34, 35–44, 45–54, and 55–64 years; education as no education, primary, secondary, tertiary, and other; marital status as never married, married or living together, divorced/separated, widowed; residence as urban or rural; ARVs as detected or not detected. Asset-based wealth index was used to categorize the wealth index scores into quintiles (lowest, second, middle, and highest) [16].
Statistical analysis
The weighted prevalence and 95% confidence intervals (CI) for VLS was computed as a proportion of individuals sampled. We compared participant covariate characteristics by VLS using the Rao-Scott chi-square tests. We used bivariate and multivariable logistic regression to compute odds ratios (OR) for factors associated with VLS. Logistic regression models accounted for survey weights, stratification, and clustering in the sample design. We considered sociodemographic and other characteristics in bivariate analysis. Variables associated with the outcome at a significance level of ≤0.05 in bivariate analysis and variables that had been reported as biologically plausible determinants of VLS were considered in multivariable analysis. Missing data ranged from 0.08% to 1% and therefore complete case analyses was performed. All analyses were conducted in SAS 9.4 (Carey, NC).
Ethical considerations
The Nigeria National Health Research Ethics Committee, University of Maryland, Baltimore Institutional Review Board, and the United States Centers for Disease Control and Prevention Institutional Review Board approved NAIIS. Consent was obtained from participants who were 18 years or older and parental/guardian permission was obtained for minors prior to obtaining individual assent.
Results:
Of 2,739 adults, aged 15 to 64 years, who tested HIV-positive during the survey, 1,417 (51.7%) were either newly diagnosed or had no information on self-reported use of ARV therapy and no detectable ARVs and therefore excluded from this analysis (Figure 1). The final analyses included 1,322 participants of whom, 743 (56.2%) self-reported to be a PLWH and 579 (43.7%) did not self-report as a PLWH but tested HIV positive and had detectable ARVs in their blood specimens. The median age was 31 (interquartile range (IQR): 39 – 48) years. Of the study sample, 949 (71.8%) were women and 922 (69.7%) were 35 years or older. Seven hundred and sixty (57.5%) lived in urban areas and 811 (61.3%) were married or living together. Over half, 747 (56.5%) had CD4 count ≥ 500 cells/mm3 and 99 (7.5%) had hepatitis B infection (Table 1).
Figure 1.
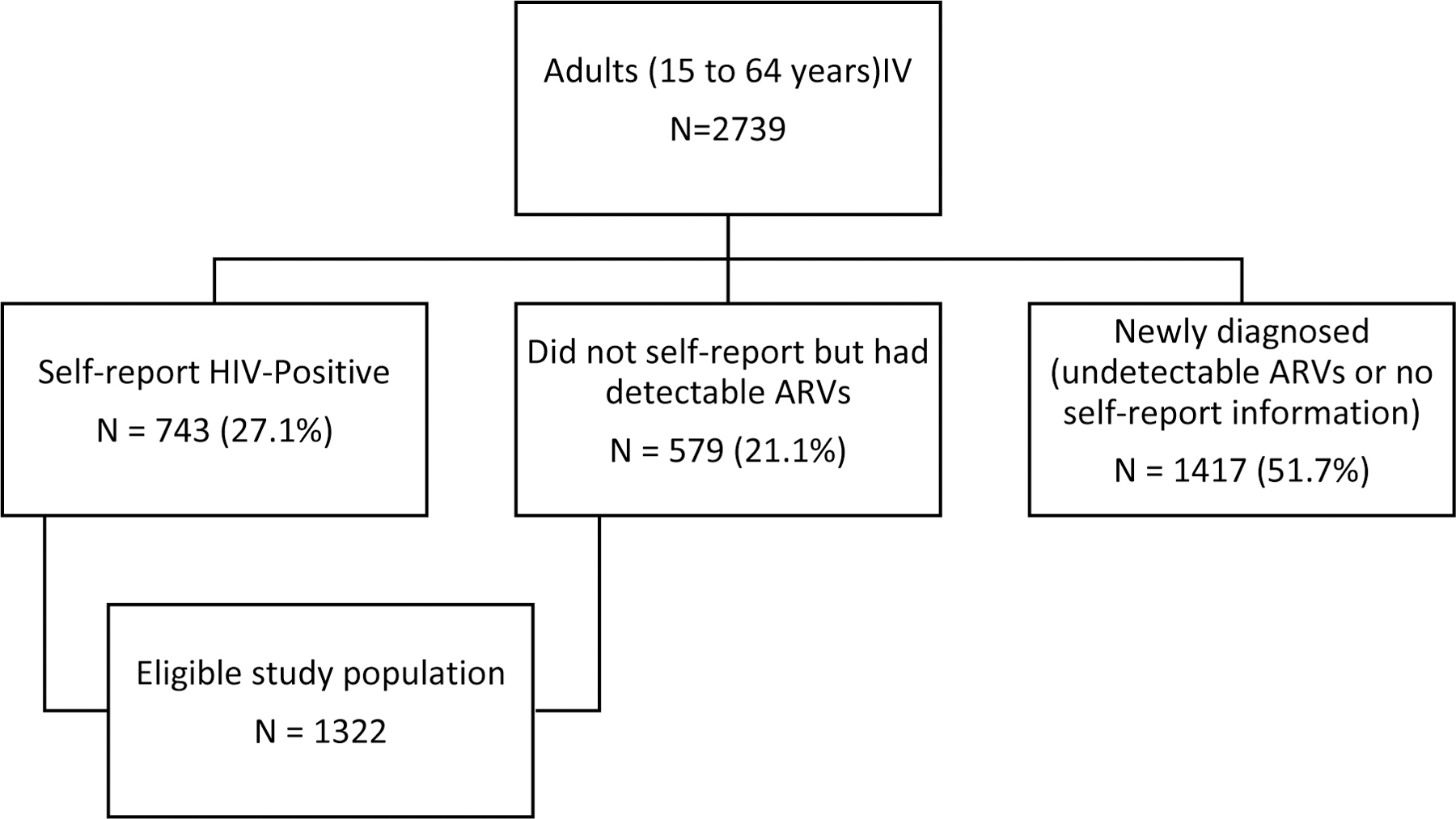
Description of enrolled study population
Table 1.
Percentage distribution of persons ages 15–64 years who tested HIV positive and are on ARV treatment in the NAIIS survey by viral load status and selected demographic characteristics, NAIIS 2018
| Characteristics | Suppressed n = 1066 |
Not suppressed n = 256 |
P-value | ||
|---|---|---|---|---|---|
| n | Weighted % (95% CI) |
n | Weighted % (95% CI) |
||
| Gender | |||||
| Male | 297 | 31.08 (27.8–34.36) | 76 | 34.57 (26.96–42.18) | 0.42 |
| Female | 769 | 68.92 (65.64–72.2) | 180 | 65.43 (57.82–73.04) | |
| Age | |||||
| 15–24 | 66 | 7.64 (5.26–10.02) | 24 | 9.61 (5.25–13.96) | 0.05 |
| 25–34 | 240 | 20.02 (17.02–23.03) | 70 | 27.93 (21.18–34.67) | |
| 35–44 | 355 | 33.6 (30.14–37.05) | 92 | 35.12 (27.71–42.54) | |
| 45–54 | 271 | 27.05 (23.64–30.46) | 47 | 16.68 (11.62–21.74) | |
| 55–64 | 134 | 11.69 (9.48–13.9) | 23 | 10.66 (4.93–16.38) | |
| Education | |||||
| No education | 208 | 16.9 (14–19.8) | 43 | 15.56 (10.73–20.39) | 0.75 |
| Primary | 265 | 23.57 (20.5–26.64) | 58 | 22.8 (16.44–29.15) | |
| Secondary | 393 | 40.43 (36.5–44.37) | 106 | 42.43 (34.87–49.99) | |
| Tertiary | 178 | 16.27 (13.31–19.24) | 40 | 15.34 (9.48–21.2) | |
| Others | 19 | 2.04 (0.66–3.43) | 9 | 3.87 (0–7.81) | |
| Missing | 3 | N/A | 0 | N/A | |
| Marital Status | . | ||||
| Never married | 104 | 11.4 (8.62–14.19) | 47 | 18.24 (12.7–23.77) | 0.09 |
| Married or living together | 671 | 61.02 (56.97–65.06) | 140 | 56.48 (49.01–63.94) | |
| Divorced or separated | 94 | 8.46 (6.29–10.62) | 25 | 8.49 (4.85–12.14) | |
| Widowed | 196 | 19.07 (16.02–22.12) | 43 | 16.17 (11.03–21.3) | |
| Missing | 1 | N/A | 1 | N/A | |
| Type of Union | |||||
| In polygynous union | 169 | 15.2 (12.07–18.32) | 29 | 10.3 (5.58–15.02) | 0.26 |
| Not in polygynous union | 499 | 45.45 (41.29–49.6) | 110 | 46.05 (38.25–53.85) | |
| Not currently in union | 394 | 38.93 (34.89–42.97) | 115 | 42.9 (35.46–50.34) | |
| Missing | 4 | N/A | 2 | N/A | |
| Place of residence | |||||
| Urban | 462 | 48.86 (44.03–53.69) | 100 | 44.43 (36.54–52.32) | 0.31 |
| Rural | 604 | 51.14 (46.31–55.97) | 156 | 55.57 (47.68–63.46) | |
| Geopolitical zone | |||||
| Northwest | 69 | 11.70 (8.46–14.95) | 15 | 10.52 (4.98–16.05) | 0.33 |
| Northeast | 192 | 13.36 (10.26–16.45) | 64 | 17.59 (12.68–22.5) | |
| Northcentral | 385 | 28.49 (24.9–32.08) | 67 | 20.93 (15.78–26.08) | |
| Southeast | 138 | 12.29 (9.81–14.78) | 35 | 13.37 (8.93–17.82) | |
| South-south | 173 | 17.87 (14.85–20.89) | 53 | 21.83 (15.93–27.72) | |
| Southwest | 109 | 16.29 (13.33–19.25) | 22 | 15.76 (8.69–22.83) | |
| Wealth quintile | |||||
| Lowest | 119 | 9.83 (7.52–12.14) | 37 | 12.73 (8–17.46) | 0.53 |
| Second | 204 | 16.99 (14.11–19.87) | 40 | 13.51 (8.89–18.12) | |
| Middle | 266 | 23.76 (20.15–27.36) | 71 | 27.17 (20.33–34.01) | |
| Fourth | 275 | 28.01 (24.14–31.87) | 65 | 25.83 (19.17–32.49) | |
| Highest | 202 | 21.42 (17.85–24.99) | 43 | 20.76 (13.83–27.69) | |
| Making decisions about health care | |||||
| Self | 216 | 20.67 (17.73–23.62) | 49 | 24.12 (16.71–31.53) | 0.28 |
| Spouse/partner | 189 | 16.36 (13.51–19.22) | 34 | 12.71 (7.45–17.97) | |
| Jointly | 258 | 23.33 (20.03–26.63) | 56 | 19.15 (13.84–24.46) | |
| Missing | 403 | N/A | 117 | N/A | |
| Hepatitis B infection | |||||
| Positive | 68 | 5.61 (4.02–7.19) | 31 | 14.39 (8.27–20.51) | 0.0003 |
| Negative | 996 | 93.77 (91.81–95.74) | 225 | 85.61 (79.49–91.73) | |
| Missing | 2 | N/A | 0 | N/A | |
| CD4 count, cells/uL | |||||
| < 500 | 376 | 35.7 (32.08–39.33) | 176 | 67.99 (60.59–75.38) | <.0001 |
| ≥ 500 | 676 | 62.55 (58.87–66.24) | 71 | 28.93 (21.61–36.25) | |
| Missing | 14 | N/A | 9 | N/A | |
| ARVs in blood specimen | |||||
| Detectable | 1050 | 97.99 (96.69–99.28) | 237 | 91.87 (88.19–95.55) | 0.0002 |
| On ART but not detected in blood | 16 | 2.01 (0.72–3.31) | 19 | 8.13 (4.45–11.81) | |
| Regimen type | |||||
| PI | 59 | 6.9 (4.8–9.0) | 9 | 3.1 (0.5–5.7) | 0.06 |
| NNRTI | 991 | 93.1 (91.0–95.2) | 228 | 96.9 (94.3–99.5) | |
Abbreviations: CI, Confidence intervals; ART, antiretroviral therapy; ARV, antiretroviral; PI, Protease inhibitors; NNRTI, Non-Nuclease Reverse Transcriptase Inhibitors; NAIIS, Nigeria HIV/AIDS Indicator and Impact Survey
Characteristics of and ARV response
Overall, of 1,322 individuals included in the final analysis, 1,305 (98.7%) had detectable ARVs in the blood samples of which most (819, 62.7%) had detectable EFV, followed by NVP (417; 31.9%). Weighted prevalence of VLS was 80.6% (n=1066). The median CD4 count was 354 (IQR 545 to 765 cells/mm3).
Factors associated with VLS
Compared to participants who were 15–24 years, those who were 45–54 years had increased odds of VLS (aOR = 2.81; 95% CI, 1.14 – 6.90) (Table 2). Having higher CD4 cell count was associated with increased odds of VLS (CD4 count, ≥ 500 vs. < 500 (aOR = 5.00; 95% CI, 3.25 – 7.68). Compared to participants with detectable ARVs, those who self-report that they were on ARV therapy but not detected in their blood specimens had lower odds of VLS (aOR = 0.24; 95% CI, 0.08–0.64). Having hepatitis B infection was associated with lower odds of VLS (aOR = 0.29; 95% CI, 0.20–0.58). Patients on non-nucleoside reverse transcriptase inhibitor-based regimen had lower odds of viral suppression (aOR = 0.34; 95% CI, 0.12–1.01) compared to protease inhibitor-based regimen, although result was not statistically significant.
Table 2.
Factors associated with viral suppression, among persons ages 15–64 years who tested HIV positive and are on ART in the NAIIS survey, NAIIS 2018.
| Characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | aOR | 95% CI | P-value | |
| Gender | ||||||
| Male | Ref | Ref | ||||
| Female | 1.17 | (0.80–1.72) | 0.42 | 1.18 | (0.72–1.93) | 0.52 |
| Age | ||||||
| 15–24 | Ref | Ref | ||||
| 25–34 | 0.9 | (0.46–1.79) | 0.768 | 1.12 | (0.72–2.56) | 0.79 |
| 35–44 | 1.2 | (0.63–2.30) | 0.574 | 1.75 | (0.77–3.96) | 0.18 |
| 45–54 | 2.04 | (1.03–4.03) | 0.04 | 2.81 | (1.14–6.90) | 0.02 |
| 55–64 | 1.38 | (0.60–3.15) | 0.444 | 1.93 | (0.62–6.00) | 0.26 |
| Education | ||||||
| No education | Ref | |||||
| Primary | 0.95 | (0.58–1.56) | 0.845 | |||
| Secondary | 0.88 | (0.57–1.36) | 0.557 | |||
| Tertiary | 0.98 | (0.55–1.74) | 0.937 | |||
| Others | 0.49 | (0.13–1.79) | 0.279 | |||
| Marital Status | ||||||
| Never married | Ref | Ref | ||||
| Married or living together | 1.73 | (1.06–2.82) | 0.029 | 1.27 | (0.70–2.31) | 0.43 |
| Divorced or separated | 1.59 | (0.82–3.11) | 0.172 | 1.58 | (0.71–3.53) | 0.26 |
| Widowed | 1.89 | (1.08–3.30) | 0.026 | 1.15 | (0.56–2.37) | 0.71 |
| Type of Union | ||||||
| In polygynous union | Ref | |||||
| Not in polygynous union | 0.67 | (0.37–1.20) | 0.179 | |||
| Not currently in union | 0.61 | (0.34–1.10) | 0.104 | |||
| Place of residence | ||||||
| Urban | Ref | |||||
| Rural | 0.84 | (0.59–1.18) | 0.306 | |||
| Wealth quintile | ||||||
| Lowest | Ref | |||||
| Second | 1.63 | (0.92–2.87) | 0.091 | |||
| Middle | 1.13 | (0.66–1.95) | 0.653 | |||
| Fourth | 1.4 | (0.82–2.39) | 0.212 | |||
| Highest | 1.34 | (0.74–2.42) | 0.34 | |||
| Hepatitis B infection | ||||||
| Negative | Ref | Ref | ||||
| Positive | 0.36 | (0.20–0.64) | 0.001 | 0.29 | (0.20–0.58) | < 0.01 |
| CD4 count– cells/mm3 | ||||||
| < 500 | Ref | Ref | ||||
| ≥ 500 | 4.12 | (2.80–6.06) | 0 | 5 | (3.25–7.68) | < 0.01 |
| ARVs in blood specimen | ||||||
| Detectable | Ref | Ref | ||||
| On ART but not detected | 0.23 | (0.10–0.54) | 0.001 | 0.24 | (0.08–0.67) | 0.007 |
| Regimen type | ||||||
| PI | ||||||
| NNRTI | 0.23 | (0.10–0.54) | 0.001 | 0.34 | (0.12–1.01) | 0.05 |
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; CI, Confidence intervals; ART, antiretroviral therapy; ARV, antiretroviral; PI, Protease inhibitors; NNRTI, Non-Nuclease Reverse Transcriptase Inhibitors; NAIIS, Nigeria HIV/AIDS Indicator and Impact Survey
Discussion
In this 2018 nationally representative population-based survey in Nigeria, 80.6% of participants on ARV treatment achieved VLS and 98.7% had ARVs detected in their specimens although the levels of ARVs was not measured. Although duration on ARV treatment was unknown, these data are encouraging, and it may indicate high level of medication adherence among Nigerian PLWH although the prevalence of viral suppression needs to be higher to attain epidemic control. While higher CD4 count, not being co-infected with hepatitis B infection, and older individuals were associated with increased odds of VLS, not having detectable ARVs was associated with lower odds of VLS.
The proportion of VLS from this survey is similar to the findings from other studies involving routinely collected data from HIV care and treatment centers [17–19]. Although there is variation in defining VLS, duration on treatment, and inclusion criteria, the range of VLS falls within what was found in the current survey. Consistent with other studies, younger individuals including adolescent and young adults are less likely to achieve VLS [20–22]. The period of mental maturation among young individuals is often associated with poor mental health and limited social support [23–25]. Prior research showed that young individual’s psychosocial well-being could potentially affects VLS and retention in HIV care as well as supporting proper transition from pediatric into adult HIV care [26–28]. Addressing psychological challenges, supporting proper transitioning from pediatric to adult HIV care services and continued support to young individuals have been shown to improve HIV treatment outcomes including VLS [29,30]. ARV detection is a measure of medication adherence, an important component in achieving VLS [31]. Those with undetectable ARV were less likely to achieve VLS. The benefits of ARV treatment adherence among PLWH are substantial. Besides improvement of immune function, VLS and subsequent reduction of HIV transmission, ARV adherence is associated with good health related quality of life [32]. Different adherence interventions have been studied and adopted extensively [33], however, no one size fits all. Identification of individuals failing treatment and subsequent performance of adherence barrier analysis and implementation of interventions targeted to the identified barriers will likely improve adherence and VLS. Barrier analysis have shown to improve HIV treatment outcomes including retention in care [34]. The improved immune system indicated by higher CD4 counts in those adherent to their ARVs may explain the lower prevalence of Hep B associated with those individuals in our study.
Majority of survey participants were on non-nucleoside reverse transcriptase inhibitors (NNRTI) based regimen with 31% being on nevirapine. We noted patients on NNRTI tended to have lower odds of viral suppression. With the phasing out of nevirapine and the introduction of Dolutegravir based regimen, it is hoped that VLS will be improved.
Our study has several strengths and limitations. We used nationally representative data enabling inferences to the target population of all PLWH in Nigeria. Although some information was self-reported, collection of blood sample and testing for ARVs enabled us to identify PLWH participants who were on ARV treatment but did not self-report. The inclusion of individuals who did not self-report if they were PLWH in our analyses, limit our ability to understand their duration on ARV treatment. If most of these participants were on treatment for less than six months, our estimate on viral suppression may be underestimated. Given the cross-sectional nature of the study, inferences should be interpreted as associational and not causal.
Conclusions
As scaling up of ART coverage continues, efforts to maximize the benefits of treatment such as viral suppression should be prioritized. Young individuals and those with undetectable ARV lag-behind VLS goals. Targeted interventions to identify PLWH who do not know their status, link them to care, and improve medication adherence are needed to achieve 95-95-95 goals and reaching the target of ending HIV epidemic. In addition, our study underscores the need to develop an affordable method to monitoring levels of ARVs in treated persons to streamline targeted adherence counseling. While findings from this study are consistent with VLS observed within the HIV clinical settings among people who are on ART in Nigeria, they also underscore the need for population-based surveys to periodically assess the population-based achievement of epidemic control. Reliance solely on clinical data would overestimate population level VLS and could hinder achievement of epidemic control. The high percentage of individuals who did not report being PLWH and yet had ARVs in their blood shows that stigma associated with HIV and its treatment is still entrenched in these populations.
Acknowledgements
The NAIIS Group includes Principal Investigators: Isaac Adewole (Federal Ministry of Health), Sani Aliyu (National Agency for the Control of AIDS), Mahesh Swaminathan (CDC Nigeria), Megan Bronson (CDC Atlanta), Manhattan Charurat (University of Maryland, Baltimore); Co-Investigators: Evelyn Ngige, Sunday Aboje, Charles Nzelu, Emanuel Meribole, Chike Ihekweazu, Chukuma Anyaike, Kayode Ogungbemi, Mukhtar Muhammad, Gregory Ashefor, Ibrahim Dalhatu, Ibrahim Jahun, Victor Sebastian, Ahmed Mukhtar, Tapdiyel Jelpe, Orji Bassey, McPaul Okoye, Aminu Yakubu, Bharat Parekh, Hetal Patel, Andrew Voetsch, Daniel B. Williams, Kristin Brown, Stephen McCracken, Anne McIntyre, Nibretie Workneh, Bryan Morris, Rex Gadama Mpazanje, Wondimagegnehu Alemu, Erasmus Morah, Gatien Ekanmian, Gambo Aliyu, Alash’le Abimiku, Bola Gobir, Mercy Niyang, Isiramen Olajide, Baffa Ibrahim, Stephen Ohakanu, Chinedu Agbakwuru, Ryan Leo, Geoffrey Greenwell, Adedayo Adeyemi, Bamgboye Afolabi, Ekanem, Mustapha Jamda, Annie Chen, Otse Ogorry, Aminu Suleiman, Kolapo Usman, Ojor R. Ayemoba, Adebobola Bashorun; Collaborating Institutions: Federal Ministry of Health (FMOH), National Agency for the Control of AIDS (NACA), National Population Commission (NPopC), National Bureau of Statistics (NBS), the U.S. Centers for Disease Control and Prevention (CDC) Nigeria, CDC Atlanta, The Global Funds to Fight AIDS, Tuberculosis, and Malaria, University of Maryland Baltimore (UMB), ICF International, African Field Epidemiology Network, University of Washington, the Joint United Nations Programme on HIV and AIDS (UNAIDS), the World Health Organization (WHO), and the United Nations Children’s Fund (UNICEF).
Funding Statement
This project is supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the cooperative agreement #U2GGH002108 to the University of Maryland, Baltimore and by the Global Funds to Fight AIDS, Tuberculosis, and Malaria through the National Agency for the Control of AIDS, Nigeria, under the contract # NGA-H-NACA to the University of Maryland, Baltimore.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
References
- 1.UNAIDS. Understanding Fast-Track: Accelerating Action to End the AIDS Epidemic by 2030. 2015.https://www.unaids.org/en/resources/documents/2015/201506_JC2743_Understanding_FastTrack (accessed 17 Dec2020).
- 2.Coker M, Etiebet M-A, Chang H, Awwal G, Jumare J, Maiyaki Musa B, et al. Socio-Demographic and Adherence Factors Associated with Viral Load Suppression in HIV-Infected Adults Initiating Therapy in Northern Nigeria: A Randomized Controlled Trial of a Peer Support Intervention. Curr HIV Res 2015; 13. [DOI] [PubMed] [Google Scholar]
- 3.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med 2000; 342:921–929. [DOI] [PubMed] [Google Scholar]
- 4.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–1404. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chime OH, Ndibuagu EO, Orji CJ. Rates and predictors of adherence and retention for antiretroviral therapy among HIV-positive adults in Enugu, Nigeria. Malawi Med J 2019; 31:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okoronkwo I, Okeke U, Chinweuba A, Iheanacho P. Nonadherence factors and sociodemographic characteristics of HIV-infected adults receiving antiretroviral therapy in Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria. ISRN AIDS 2013; 2013:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bijker R, Jiamsakul A, Kityo C, Kiertiburanakul S, Siwale M, Phanuphak P, et al. Adherence to antiretroviral therapy for HIV in sub-Saharan Africa and Asia: a comparative analysis of two regional cohorts. J Int AIDS Soc 2017; 20:21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Federal Ministry of Health Nigeria. Nigeria HIV/AIDS Indicator and Impact Survey (NAIIS) 2018: Technical Report. Abuja, Nigeria:; 2019. www.health.gov.ng (accessed 16 Dec2020). [Google Scholar]
- 10.UNAIDS. AIDSinfo. 2020.https://aidsinfo.unaids.org/ (accessed 16 Sep2020).
- 11.Data for Implementation (Data.FI). Improving Viral Load Testing Coverage across USAID-Supported States in Nigeria. 2020.https://datafi.thepalladiumgroup.com/news-and-resources/data-for-action/ (accessed 30 Aug2021).
- 12.UNAIDS. Putting people at the centre brings good results in Nigeria. 2021.https://www.unaids.org/en/resources/presscentre/featurestories/2021/march/20210305_people-at-the-centre-nigeria (accessed 30 Aug2021).
- 13.Patel HK, Duong YT, Birhanu S, Dobbs T, Lupoli K, Moore C, et al. A comprehensive approach to assuring quality of laboratory testing in HIV surveys: lessons learned from the population-based HIV impact assessment project. J Acquir Immune Defic Syndr 2021; 87:S17–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koal T, Burhenne H, Römling R, Svoboda M, Resch K, Kaever V. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2005; 19:2995–3001. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Consolidated guidelines on HIV testing services. WHO. 2019.https://www.who.int/publications/i/item/978-92-4-155058-1 (accessed 16 Dec2020). [PubMed] [Google Scholar]
- 16.Foreit KG, Schreiner M. Comparing Alternative Measures of Poverty: Assets-Based Wealth Index vs. Expenditures-Based Poverty Score — MEASURE Evaluation. 2011.https://www.measureevaluation.org/resources/publications/wp-11-123 (accessed 16 Dec2020).
- 17.Stafford KA, Odafe SF, Lo J, Ibrahim R, Ehoche A, Niyang M, et al. Evaluation of the clinical outcomes of the Test and Treat strategy to implement Treat All in Nigeria: results from the Nigeria Multi-Center ART Study. PLoS One 2019; 14:e0218555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badejo O, Noestlinger C, Jolayemi T, Adeola J, Okonkwo P, Van Belle S, et al. Multilevel modelling and multiple group analysis of disparities in continuity of care and viral suppression among adolescents and youths living with HIV in Nigeria. BMJ Glob Heal 2020; 5:3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agaba PA, Meloni ST, Sule HM, Agbaji OO, Sagay AS, Okonkwo P, et al. Treatment outcomes among older human immunodeficiency virus-infected adults in Nigeria. Open Forum Infect Dis 2017; 4:ofx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsondai PR, Sohn AH, Phiri S, Sikombe K, Sawry S, Chimbetete C, et al. Characterizing the double-sided cascade of care for adolescents living with HIV transitioning to adulthood across Southern Africa. J Int AIDS Soc 2020; 23:e25447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS 2014; 28:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in Southern Africa. J Acquir Immune Defic Syndr 2009; 51:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyes ME, Cluver LD, Meinck F, Casale M, Newnham E. Mental health in South African adolescents living with HIV: correlates of internalising and externalising symptoms. AIDS Care 2019; 31:95–104. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y, Li X, Lou C, Sonenstein FL, Kalamar A, Jejeebhoy S, et al. The association between social support and mental health among vulnerable adolescents in five cities: findings from the study of the well-being of adolescents in vulnerable environments. J Adolesc Heal 2014; 55:S31–S38. [DOI] [PubMed] [Google Scholar]
- 25.Laurenzi CA, Skeen S, Gordon S, Akin-Olugbade O, Abrahams N, Bradshaw M, et al. Preventing mental health conditions in adolescents living with HIV: an urgent need for evidence. J Int AIDS Soc 2020; 23. doi: 10.1002/jia2.25556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dow DE, Turner EL, Shayo AM, Mmbaga B, Cunningham CK, O’Donnell K. Evaluating mental health difficulties and associated outcomes among HIV-positive adolescents in Tanzania. AIDS Care 2016; 28:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Wyk BE, Davids LAC. Challenges to HIV treatment adherence amongst adolescents in a low socio-economic setting in Cape Town. South Afr J HIV Med 2019; 20:1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mutumba M, Musiime V, Lepkwoski JM, Harper GW, Snow RC, Resnicow K, et al. Examining the relationship between psychological distress and adherence to anti-retroviral therapy among Ugandan adolescents living with HIV. AIDS Care 2016; 28:807–815. [DOI] [PubMed] [Google Scholar]
- 29.P R, T M, D P, R P, V T. Linkage to and retention in care following healthcare transition from pediatric to adult HIV care. AIDS Care 2016; 28:561–565. [DOI] [PubMed] [Google Scholar]
- 30.Griffith D, Jin L, Childs J, Posada R, Jao J, Agwu A. Outcomes of a comprehensive retention strategy for youth with HIV after transfer to adult care in the United States. Pediatr Infect Dis J 2019; 38:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castillo-Mancilla JR, Haberer JE. Adherence Measurements in HIV: New Advancements in Pharmacologic Methods and Real-Time Monitoring. Curr HIV/AIDS Rep 2018; 15:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onu DU. Treatment adherence mediates the relationship between HIV-related stigma and health-related quality of life. AIDS Care 2021; 33:1335–1339. [DOI] [PubMed] [Google Scholar]
- 33.Haberer JE, Sabin L, Amico KR, Orrell C, Galárraga O, Tsai AC, et al. Improving antiretroviral therapy adherence in resource-limited settings at scale: a discussion of interventions and recommendations. J Int AIDS Soc 2017; 20:21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makokha V, Ramadhani H, Amadi E, Ng’eno C, Ndaga A, Wandina D, et al. Structured support through a return to care package to reduce lost to follow-up among patients on antiretroviral therapy in rural Western Kenya [Abstract]. AIDS 2020; 23:937–938. [Google Scholar]


