Abstract
Background:
People with HIV (PHW) are at greater risk of depression than the general population. Insight into the time-to-treatment-response and predictors of response to psychotherapy may improve implementation in primary care.
Methods:
We assessed depression treatment response among 80 participants in a trial of cognitive-behavioral therapy for adherence and depression (CBT-AD) for PWH with MDD and suboptimal antiretroviral therapy (ART) adherence. Participants self-reported depressive symptoms (CES–D) at each therapy session. Clinicians assessed participants’ depression (HAM–D), along with potential predictors of response, every four months for one year. Latent class analyses examined classes of responders for the active and the post-treatment phases. Regression analyses identified predictors of class membership for each phase.
Results:
During the active treatment phase (CES–D) we identified an early response (at session 2 and with continued trajectory of improvement) and a non-response group. There were also two classes during post-treatment (HAM-D): early responders (4-month) and late responders (12-month). Distress aversion was associated with lower likelihood of early response to CBT-AD (aOR = 0.74, 95%CI[0.56–0.90], p = .009), and social support was associated with increased likelihood of early response (aOR = 2.24, 95%CI[1.07–5.46], p = .045)
Limitations:
Self-reported depression during the treatment phase may have resulted from social desirability bias.
Conclusions:
Most participants responded to CBT-AD early during treatment (89 %) and had sustained improvements in depression by 4 months (80 %). Distress aversion was a risk factor for late response, and social support was protective. Future research is needed to assess the optimal dose of CBT-AD in resource limited settings.
Keywords: Intervention, Depression, Cognitive-behavioral therapy (CBT), Task-sharing, Task-shifting, Randomized controlled trial, Global mental health, Treatment response
1. Introduction
The treatment of major depressive disorder (MDD) comorbid with chronic medical conditions, such as HIV, is key to ensuring maximum benefits to quality of life and health outcomes (Bengtson et al., 2015; Lofgren et al., 2020). Though the prevalence of MDD in general populations is estimated to occur in 10 % of individuals, rates in persons with HIV infection are higher (Bernard et al., 2017). Some authors have suggested that, in people with HIV (PWH), MDD is twice as prevalent as in the general population (Ciesla and Roberts, 2001). In high HIV-prevalence settings, which includes many regions of sub-Saharan Africa and South Africa especially, MDD comorbid with HIV may exert several significant negative effects on key HIV-related health outcomes (Pence et al., 2018). These include poor care engagement, poor antiretroviral therapy (ART) adherence, and faster progression to disability and death (Alciati et al., 2007; Bangsberg et al., 2001; Kulisewa et al., 2019). However, interventions to improve MDD alone do not appear to effectively improve ART adherence, and changes in ART adherence among PWH with MDD appear to be related to integrated treatments targeting both MDD and adherence (Mendez et al., 2021).
Effective treatments for MDD exist, and these include psychotropic treatments, psychotherapies, or combinations of both. Of the psychotherapies, the greatest evidence-base exists for cognitive-behavioral therapy (CBT) (Cuijpers et al., 2021, 2013). CBT is a short-term, manualized, and collaborative treatment, which lends itself to task-shared delivery and has been adapted for different contexts. With the relative absence of prescribers in low- and middle-income countries (LMICs), frequent medication stock-outs (unavailability), side-effects, and drug-drug interactions (where persons are taking medications for other chronic illness, such as ART), CBT is an effective and appealing option. However, in LMICs including South Africa (a high-middle-income country), health systems are under-resourced, resulting in a wide mental health treatment gap (Lund et al., 2010). Some suggest that up to 75 % of persons living with a mental disorder will not receive treatment (Eaton et al., 2011; Seedat et al., 2008). In addition, there are wide disparities between services provided for mental health care in the public and private health care systems (Ruffieux et al., 2021).
Approaches to address the resource limitations, specifically in the provision of mental health care, include task-sharing. This model has now been widely adopted and trialed in multiple LMIC and low-resource settings, and despite some challenges, will likely be a cornerstone of comprehensive healthcare provision in the future (Bruckner et al., 2011; Patel, 2009). This is especially true for primary health care clinics, where the majority of patients are seen. Many of the services provided in these clinics are nurse-, peer, or community health worker-led (Singla et al., 2017), with a paucity of doctors and clinical psychologists available in clinic settings. Given the scarcity of available resources, we need to improve understanding of who will respond to psycho-therapy, the time to response, as well as predictors of response. This may allow health systems to tailor therapies, reduce duration or supply additional sessions, triage cases, and monitor non-responders.
The majority of effective psychotherapies for MDD are CBT-based, delivered individually, and typically 8–12 sessions long (Cuijpers et al., 2021). The dose-response relationship in psychotherapy was first examined by Howard et al. (1986); these early findings suggested a curvilinear relationship between number of sessions and effectiveness of psychotherapy, as well as that most gains were accrued within the first 8 sessions. Although some patients may respond to treatment quickly, it is uncommon to observe a meaningful response to psychotherapy in <4 sessions (Robinson et al., 2020). Most patients receiving psychotherapy for common mental disorders, such as MDD, tend to fall within either an early response or gradual response group (Robinson et al., 2020). With respect to psychotherapy for MDD specifically, one meta-analysis found a curvilinear relationship between the number of sessions and effect size, such that 12–16 session interventions were associated with the largest effect sizes on MDD treatment compared to 4–6 session or 7–10 session interventions, and there were diminishing returns for 18–24 session interventions (Cuijpers et al., 2013). However, in LMICs and other resource limited contexts, longer duration therapies represent a substantial challenge.
CBT-AD, an evidence-based, 8–10 session treatment for depression that simultaneously improves adherence to chronic medication, has been adapted for and assessed in multiple contexts (Safren et al., 2021, 2016, 2012, 2009, 2007a, 2007b; Simoni et al., 2013). Studies that examined the effectiveness and/or efficacy of CBT-AD have been conducted in the United States, at the U.S.-Mexico border, and in South Africa. CBT-AD has been shown to be effective at reducing both MDD and key chronic disease outcomes in HIV, including improved ART adherence and viral load over a 12-month period among PWH in South Africa (Safren et al., 2021). The CBT-AD trial among PWH in South Africa included eight sessions, with optional booster sessions, administered in a task-shared model by a nurse who was trained and supervised by a clinical psychologist (Safren et al., 2021). In South Africa, nurses, and even mental health nurses are not trained to administer more than basic counseling.
Although the 8-session CBT-AD intervention has been shown to be effective for both depression and HIV outcomes, the approach can be considered resource-intense in terms bringing it to scale given its 1:1 delivery and the number of sessions. Scale-up of CBT-AD will require significant investment in recruitment, training, supervision, and wide-spread program buy-in from public health, governmental, and non-profit officials. Given the resources that will be needed to implement CBT-AD in primary care clinics in South Africa and the long waitlist of patients who could greatly benefit from the treatment, it would be beneficial to understand which individuals should be prioritized for treatment and/or which patients might need longer (e.g., boosted) treatment packages. PWH with MDD are a vulnerable group and many individuals are likely to benefit from psychotherapy. Understanding the characteristics of early vs late responders to CBT-AD may help interventionists tailor the therapy in terms of content, duration, extension, and referral to other modalities of care within a highly resource-constrained environment. Therefore, the aim of this secondary data analysis is to explore the response to treatment over time within a larger treatment trial (Safren et al., 2021), in order to explore (a) whether treatment response varies and whether clusters of individuals are present with respect to treatment response, and (b) predictors of early vs late treatment response.
2. Methods
This is a secondary analysis of data from a randomized clinical trial of task-shared cognitive-behavioral therapy for adherence and depression (CBT- AD) in HIV, delivered by study-employed mental health nurses in South African primary-care (Joska et al., 2020; Safren et al., 2021). In the parent study, a total of 161 participants with MDD and virally uncontrolled HIV were recruited from primary care clinics providing HIV care in Khayelitsha, South Africa and randomized into one of two arms. Arm 1 was task-shared, nurse-delivered cognitive- CBT-AD (80 participants); arm 2 was enhanced treatment as usual (eTAU; 81 participants). The Hamilton Depression Rating Scale (HAM–D) was administered at baseline, 4, 8 and 12 months by blinded assessors. Primary outcomes were blinded HAM-D scores at 4 months, and weekly adherence via real-time monitoring (Wisepill). Secondary outcomes were adherence and depression over 4-, 8-, and 12-month follow-ups, proportion of participants with undetectable viremia, and continuous CD4 cell counts at 12-months. In addition to HAM-D ratings, participants completed the Center for Epidemiology Studies- Depression (CES–D) scale (Radloff, 1977) prior to each session of CBT-AD. This allowed the team to monitor change in self-reported depression on a session-by-session basis.
CBT-AD has been adapted for use in this setting, including training, supervision and task-sharing (Andersen et al., 2018; Everitt-Penhale et al., 2019). Formative work suggested that certain components were less feasible and therefore were not included in the treatment package (Andersen et al., 2023). This included “cognitive restructuring”, which focuses on identifying and challenging distorted or maladaptive thoughts. Elements retained in the treatment included Life-Steps for adherence (LS; would add ref. here), introduction to CBT and psycho-education (one session), behavioral activation (two sessions), problem-solving (two sessions), relaxation training (one session), and relapse prevention (one session). LS was integrated into modules for depression and HIV. While the nurse-therapist could structure sessions and modules around individual patient needs, it was most common to provide LS, psycho-education and behavioral activation from sessions one to three, followed by the other modules. Up to nine monthly booster sessions were offered.
The main study was approved by the Human Research Ethics Committee at the University of Cape Town (014/2014), and by the Institutional Review Boards of MGH and Miami University. The study was conducted in accordance with the Declaration of Helsinki, revised 1989. All participants provided informed consent to participate, and for their anonymised medical record data to be used in analyses.
2.1. Measures
HAM–D. Depression was assessed using the GRID version of the 17-item Hamilton Depression Rating Scale (HAM–D) (Williams et al., 2008). The HAM-D was administered by nurse-interventionists who worked as the study clinicians at baseline and was administered by a trained independent and blinded assessor at the 4-, 8-, and 12-month follow-ups. The HAM-D measures various symptoms of depression, and a cutoff of 17 was used to indicate depression remission.
CES–D. The Center for Epidemiologic Studies Depression Scale (CES–D) was used as a measure of self-reported depressive symptoms. The CES-D is a 20-item scale (Radloff, 1977) that has been widely used to measure depression and validated for use among PWH in South Africa (e.g., (Myer et al., 2008). The CES-D was collected at each major time point, as well as each individual treatment session prior to beginning that session. We used a score of 20 as the cut-off for MDD, based on findings that this score provided better sensitivity and specificity among community populations than does the cutoff of 16 often used in research (Henry et al., 2018; Vilagut et al., 2016).
Predictors. We assessed the following constructs, which were included as predictors of treatment response. Sex, age, and income were assessed via self-report. Viral load and ART regimen were assessed via chart extraction from participants’ medical records when available, or via blood draw by study nurses when not available. Viral load was log-transformed for analyses. Food insecurity was measured using the total score of the 9-item Household Food Insecurity and Access Scale (HFIAS) (Coates et al., 2007). We also used the 29-item Behavioral Activation Depression Scale (BADS; (Kanter et al., 2007) and the 22-item Ruminative Response Scale (RRS) (Nolen-Hoeksema and Morrow, 1991) to measure specific aspects of depression. We assessed substance use with the WHO-ASSIST (Humeniuk and World Health Organization, 2010), and alcohol use with the AUDIT (Saunders et al., 1993). Lastly, we measured distress aversion and behavioral avoidance using subscales of the Multidimensional Experiential Avoidance Questionnaire (MEAQ) (Gámez et al., 2011 ).
2.2. Statistical analysis
All analyses were conducted using R, version 4.0.3 (R Development Core Team, 2016), using the lcmm R package (Proust-Lima et al., 2022). Depression ratings on the CES-D were dichotomized using a cutoff of 20 (Henry et al., 2018), such that scores of 20 or higher were indicative of clinically significant self-report MDD, whereas scores of 19 or lower were not. A small minority of included participants (n = 9) scored <20 on the CES–D. Similarly, we utilized a cutoff of 17 on the clinician-rated HAM-D to indicate clinically significant MDD (Davies et al., 2020).
To investigate treatment response to CBT-AD, several analytic approaches were used. Firstly, we utilized latent class analysis (LCA) to examine whether there were different classes of depression treatment response among the 80 participants who received the CBT-AD intervention. We examined response on the CES-D (i.e., achieving a score < 20) during the active treatment phase (when participants completed the CES-D at each of the 8 treatment sessions) and on the HAM-D (i.e., achieving a clinician-rated score < 17), which was conducted at each major assessment visit (baseline, 4, 8 and 12 months). This approach allowed us to explore whether there were one or more groups of responders, as well as the time to treatment response, during both the weekly active treatment phase and for the post-treatment phase. Examination of the visual plots of the latent class trajectories of the CES-D allowed us to identify the specific treatment session at which the self-reported improvement occurred.
Secondly, we grouped the participants according to their response class for the active (self-reported CES–D) and post-treatment (clinician-rated HAM–D) analyses, and we conducted logistic regressions to explore predictors of response class. The following demographic and baseline clinical variables were examined as predictors: age, gender, income, log-transformed viral load at baseline, ART treatment regimen, baseline depression levels, food insecurity, substance use, baseline total score on the Behavioral Activation Depression Scale, baseline total score on the Ruminative Response Scale, alcohol use, the behavioral avoidance subscale score of the MEAQ, and the distress aversion subscale score of the MEAQ. Additionally, when assessing predictors of response class utilizing the HAM-D score over the follow-up period, we also examined whether response class on the CES-D during the active treatment phase was a predictor of response class on the HAM–D.
3. Results
Participant demographic data and descriptive statistics of variables are presented in Table 1, and Safren and colleagues present the results of the randomized controlled parent trial elsewhere (Safren et al., 2021). Briefly, participants were mostly isiXhosa-speaking Black (99.4 %) women (69.9 %), with an average monthly income of R2,187.72 (SD = 2693.41; approximately M = $125, SD = $154 per month at the time of the study) and a mean age of 39.46 (SD = 9.23) years. At baseline, participants had high viral load (log transformed mean = 4.13, SD = 0.98; and raw viral load mean = 81,374.79, SD = 180,357.57), problematic levels of alcohol and substance use (add some measure or score that reflects this), and food insecurity (add mean, SD). Additionally, the descriptive statistics for depression, measured by both the CES-D and the HAM–D, are presented in Table 2. Across time, participants in the CBT-AD condition had lower mean scores on the CES-D and HAM-D compared to participants in the treatment as usual control group, which is reported elsewhere (Safren et al., 2021).
Table 1.
Baseline demographics and descriptive statistics.
| Variable | M (SD) [Range] | Variable | N (%) |
|---|---|---|---|
|
| |||
| Age | 39.46 (9.23) [18–69] | Gender | |
| Monthly Income (ZAR) | 2187.72 (2693.41) [0–18,000] | Man | 48 (29.4 %) |
| Log viral load | 4.13 (0.98) [0–6.09] | Woman | 114 (69.9 %) |
| Food insecurity | 12.57 (7.40) [0–27] | Transgender man | 1 (0.6 %) |
| Substance use | 21.05 (23.05) [0–125] | Race | |
| Alcohol use | 10.37 (11.24) [0–37] | Black | 160 (99.4 %) |
| BADS | 62.26 (21.98) [16–120] | Coloured | 1 (0.6 %) |
| RRS | 56.91 (12.03) [23–88] | ART treatment regimen at baseline | |
| MEAQ behavioral avoidance | 60.37 (7.20) [28–66] | ||
| MEAQ distress aversion | 71.91 (7.60) [40–78] | Re-initiated on first line | 82 (50.9 %) |
| Second line | 79 (49.1 %) | ||
Table 2.
Descriptive statistics of depression over time by condition.
| CES-D | ||
|---|---|---|
|
| ||
| CBT-AD | TAU | |
|
|
|
|
| Time | M (SD) [range] | M (SD) [range] |
|
| ||
| Baseline | 33.61 (11.90) [8–60] | 36.86 (10.98) [10–57] |
| Session 1 / Randomization | 21.06 (14.13) [0–53] | – |
| Session 2 | 14.21 (11.01) [0–48] | – |
| Session 3 | 13.08 (12.53) [0–47] | – |
| Session 4 | 10.89 (11.67) [0–54] | – |
| Session 5 | 10.48 (11.96) [0–50] | – |
| Session 6 | 9.42 (11.30) [0–47] | – |
| Session 7 | 6.73 (9.38) [0–41] | – |
| Session 8 | 6.46 (10.50) [0–49] | – |
| 4-month follow-up | 7.45 (11.85) [0–49] | 19.45 (16.45) [0–53] |
| 8-month follow-up | 7.92 (12.22) [0–44] | 17.53 (18.06) [0–60] |
| 12-month follow-up | 7.34 (11.65) [0–44] | 14.61 (15.02) [0–59] |
|
| ||
| HAM-D | ||
|
| ||
| CBT-AD | TAU | |
|
|
|
|
| Time | M (SD) [range] | M (SD) [range] |
|
| ||
| Baseline | 23.75 (6.91) [8–37] | 25.74 (6.29) [13–38] |
| 4-month follow-up | 8.00 (7.86) [0–32] | 14.69 (9.03) [1–34] |
| 8-month follow-up | 8.95 (7.82) [0–31] | 13.79 (9.12) [1–32] |
| 12-month follow-up | 7.69 (6.97) [0–30] | 13.03 (9.02) [1–32] |
Among participants in the CBT-AD condition, the latent class analysis during the active treatment phase suggested the presence of two classes of treatment response: early treatment response and non-response. Compared to the 1-class (BIC = 4911.871), 3-class (BIC = 4911.210), and 4-class (BIC = 4923.329) solutions, the 2-class solution had the best model fit (BIC = 4901.829). Most participants who received the CBT-AD intervention were considered early responders (89.47 %), whereas few were non-responders (10.53 %). Fig. 1 presents the predicted trajectories of self-reported depression (CES–D) for each of the two latent classes during the active treatment phase. Inspection of the predicted values revealed one class (non-responders) without a predicted CES-D score below the threshold for clinically significant depression symptoms, and one class that achieved a predicted CES-D score below the threshold at the second session (early responders).
Fig. 1.
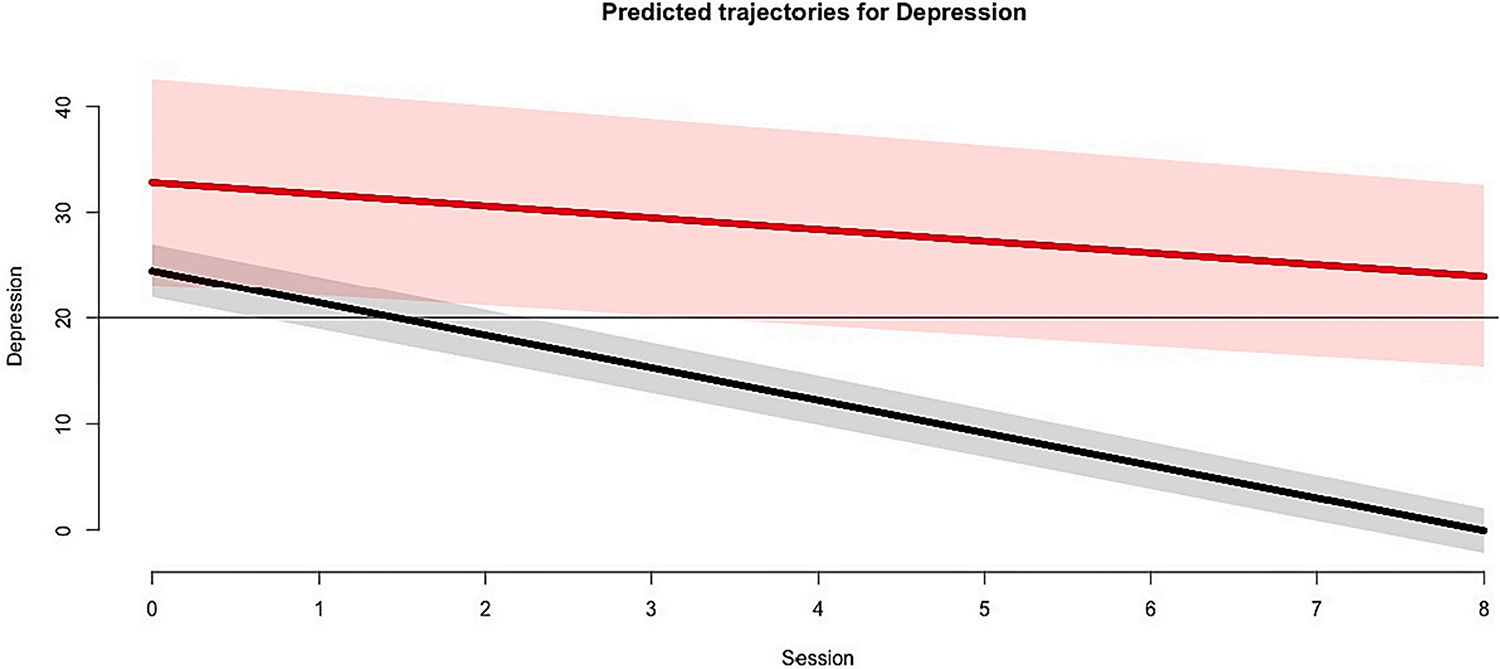
Predicted trajectories of depression by latent class during the active treatment phase, as measured by the CES-D.
Note. Solid lines represented predicted values, and corresponding shaded areas represent the 95 % confidence intervals associated with the prediction. The horizontal line represents the cut-off score of the CES-D utilized in the present study, which was added to assist with the interpretation of this figure.
In the latent class analysis that used the clinician-assessed depression (HAM–D) during the posttreatment phase, results also suggested the presence of two classes. Similarly, compared to the 1-class (BIC = 1962.058), 3-class (BIC = 1978.396), and 4-class (BIC = 1995.924) solutions, the 2-class solution had the best model fit (BIC = 1961.157) and the best discernment between classes. Fig. 2 presents the predicted trajectories of depression for each of the two latent classes over the course of the study. The majority of participants were classified as early responders (80 %), with some were classified as late responders (20 %). In Fig. 2, one class (early responders) has a predicted HAM-D score below the threshold for clinically significant symptoms of depression at the 4-month follow-up, which occurred acutely after treatment completion. The other class (late responders) achieves a predicted HAM-D score below the threshold of clinical significance at the 12-month follow-up.
Fig. 2.
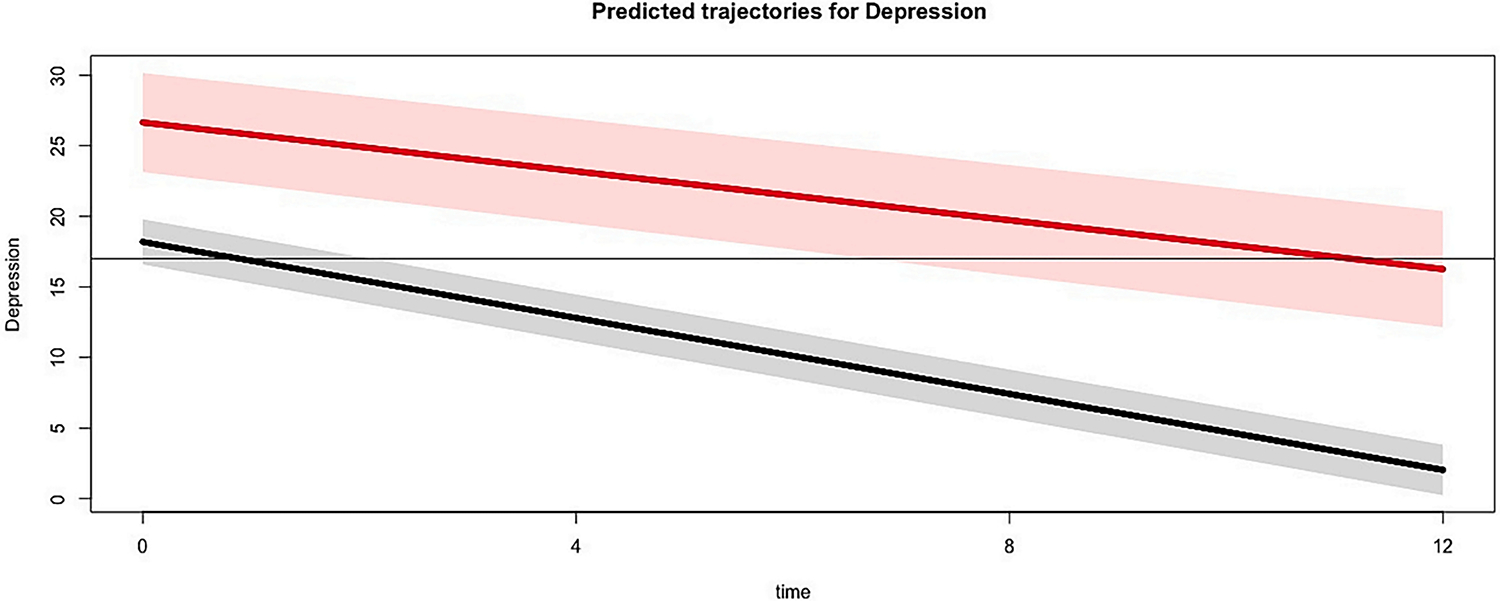
Predicted trajectories of depression by latent class during the entire study period, as measured by the HAM-D.
Note. Solid lines represented predicted values, and corresponding shaded areas represent the 95 % confidence intervals associated with the prediction. The horizontal line represents the cut-off score of the HAM-D utilized in the present study, which was added to assist with the interpretation of this figure.
In a multivariate model assessing predictors of class membership, none of the factors significantly (p’s > 0.05) predicted treatment response during the active treatment phase (measured by the CES–D; Table 3). However, in a multivariate model examining predictors of treatment response during the posttreatment phase (measured by the HAM–D), there were two significant predictors (Table 4). Individuals with greater distress aversion at baseline were 26 % (aOR = 0.74, 95 % CI [0.56–0.90], p = .009) less likely to be classified as an early treatment responder over the follow-up period, when controlling for baseline levels of depression. However, those with greater social support at baseline were 124 % more likely to be classified as an early treatment responder (aOR = 2.24, 95 % CI [1.07–5.46], p = .045).
Table 3.
Logistic regression of mean-centered baseline levels of predictors of treatment response class on the CES—D.
| Predictors | Odds ratios | CI | p |
|---|---|---|---|
|
| |||
| (Intercept) | 5.52 | 0.70–72.65 | 0.134 |
| Gender | 5.84 | 0.39–136.34 | 0.219 |
| Age | 0.94 | 0.80–1.06 | 0.347 |
| Income | 1.00 | 1.00–1.00 | 0.975 |
| Log viral load | 2.41 | 0.48–16.53 | 0.305 |
| ART regimen | 4.42 | 0.46–88.94 | 0.248 |
| Baseline CES-D score | 0.93 | 0.82–1.03 | 0.170 |
| Food insecurity | 0.98 | 0.82–1.18 | 0.842 |
| Substance use | 1.00 | 0.94–1.08 | 0.970 |
| BADS | 1.03 | 0.97–1.12 | 0.339 |
| RRS | 1.00 | 0.88–1.14 | 0.993 |
| Alcohol use | 0.94 | 0.80–1.08 | 0.378 |
| MEAQ Behavioral avoidance | 1.09 | 0.92–1.30 | 0.284 |
| MEAQ Distress aversion | 0.83 | 0.59–1.04 | 0.193 |
| Social support | 0.75 | 0.19–2.14 | 0.625 |
| Baseline HAM-D score | 1.11 | 0.92–1.40 | 0.293 |
| Observations | 76 | ||
| R2 Tjur | 0.248 | ||
Note: acronyms are: antiretroviral therapy (ART), Center for Epidemiologic Studies Depression Scale (CES—D), Behavioral Activation Depression Scale (BADS), Ruminative Response Scale (RRS), Multidimensional Experiential Avoidance Questionnaire (MEAQ), Hamilton Depression Rating Scale (HAM—D). Significant p-values are bolded.
Table 4.
Logistic regression of mean-centered baseline levels of predictors of treatment response class on the HAM—D.
| Predictors | Odds ratios | CI | p |
|---|---|---|---|
|
| |||
| (Intercept) | 8.68 | 1.49–81.55 | 0.030 |
| Gender | 1.86 | 0.17–21.01 | 0.603 |
| Age | 0.97 | 0.87–1.08 | 0.612 |
| Income | 1.00 | 1.00–1.00 | 0.407 |
| Log viral load | 0.48 | 0.15–1.38 | 0.192 |
| ART regimen | 1.00 | 0.14–7.24 | 0.998 |
| Baseline HAM-D score | 0.81 | 0.68–0.94 | 0.011 |
| Food insecurity | 1.06 | 0.93–1.23 | 0.362 |
| Substance use | 0.98 | 0.93–1.04 | 0.515 |
| BADS | 1.01 | 0.96–1.06 | 0.777 |
| RRS | 0.97 | 0.87–1.08 | 0.589 |
| Alcohol use | 1.07 | 0.96–1.21 | 0.251 |
| MEAQ Behavioral avoidance | 1.14 | 0.97–1.35 | 0.101 |
| MEAQ Distress aversion | 0.74 | 0.56–0.90 | 0.009 |
| Social support | 2.24 | 1.07–5.46 | 0.045 |
| Observations | 76 | ||
| R2 Tjur | 0.443 | ||
Bold values represent the significant p values (p<.05).
4. Discussion
This study examined treatment response trajectories among a sample of PWH in South Africa who received CBT-AD, both during the acute treatment phase (via the self-reported CES–D) and during the 1-year posttreatment follow up period (via the clinician-administered HAM–D). Findings suggest the presence of two classes of responders in the acute phase of CBT-AD treatment: the majority were self-reported responders (89 %), and a minority were non-responders (11 %). Treatment response for responders was observed at the second CBT-AD session. From the 4-month HAM-D assessment, 80 % of participants were in an early response group and had depression scores below the cut-off for clinical significance at the 4-month follow-up visit, directly after the acute treatment phase. The other 20 % of participants were classified as late responders and did not achieve depression remission until the 12-month follow-up. There were no predictors of class membership during the acute treatment phase. However, during the posttreatment phase, greater distress aversion was associated with a lower likelihood of being in the early responder class, and higher social support was associated with an increased likelihood of responding to CBT-AD.
The majority of participants receiving CBT-AD (89 %) reported improved mood symptoms by the second session, with a mean CES-D score of 14.21. This rapid improvement in the severity of self-reported depression symptoms might have several explanations. Firstly, early treatment benefits could be attributable to the first session content, which included life-steps adherence counseling, rapport building, psychoeducation and a brief introduction to the treatment plan. Participants might have experienced relief at understanding the nature of their depression and learning that depression can be treated, which in turn may have been associated with decreased distress and hopelessness. Secondly, a certain amount of improvement might be ascribed to trial participation on its own—the security of being seen weekly, having a space to talk about problems, and interacting with empathetic therapist (Browne et al., 2021). For most participants, the trial was their first experience engaging in therapy. It must be noted that the reduction in CES-D score from an average of 33.61 at baseline to an average of 14.21 at session two does not indicate remission. As can be seen in Table 2 and Fig. 1, there was a continued trajectory of improvement with each subsequent session, such that by the end of session 8, the mean CES-D score was 6.45. This pattern indicates that participants received benefit from all 8 sessions.
Whilst others note that most individuals generally attend only one session of psychotherapy when they present for treatment (Dryden, 2018), session attendance in our study was high, with 86 % (n = 69) of participants in the CBT-AD arm attending all eight sessions. The non-responding group (11 %) are a key minority for whom more intensive, or multi-modal approaches might be required. This might include facilitated pharmaco-therapy (where uptake was poor), or intensified psycho-therapy or even brief hospitalization.
When we examined predictors of treatment response during the acute phase (e.g., demographic variables, HIV disease factors, measures of income and food insecurity, psychological constructs), no significant relationships emerged. This finding might be due to the fact that self-reported treatment response represents more of an initial trial participation reflecting hope for future remission, rather than a clinically meaningful symptom reduction at session two. Also, if small effects were present, they might have been not detected due to sample size and little variation as the majority of participants being classified as responders (89 %). It is not clear whether interrupting treatment after two sessions is sufficient to produce sustained remission in early responders: more complex implementation trial designs, with step-up rules may be required.
Two classes of treatment response also emerged during the post-treatment phase, when clinicians assessed participant depression using the HAM–D. One group responded (80 %) at the 4-month post-treatment assessment, while the other group remitted by 12 months (20 %). Note that booster sessions were available after the acute treatment phase. Although remission at 12 months is longer than some extant research on the natural length of depressive episodes (e.g., Richards, 2011; Spijker, 2002), these studies have been conducted among people who do not have HIV and are living in high-income countries. Persons with HIV with poorly controlled viral loads might have underlying biological neural correlates of depression and apathy, although these were not measured in this study. Improved control of HIV is a component of the CBT-AD therapy, with adherence being a focus. In addition, PWH might also experience increased stressors associated with living with HIV in a resource-constrained setting, such as medical illness, bereavement, and stigma. These perpetuating factors may result in delays to treatment remission (see for example Patel et al., 2016). We did, however, observe that late responders remitted by 12 months, suggesting that CBT-AD effects may take up to a year or more to emerge and that booster sessions may be particularly helpful for certain patients (Safren et al., 2007a, 2007b).
In the posttreatment analyses that were based on the clinician-rated assessment, greater distress aversion at baseline was associated with a lower likelihood of being classified as an early responder to treatment. Previous research has shown a link between low distress tolerance and poorer management of HIV (O’Cleirigh et al., 2007). High distress aversion, a form of distress intolerance, may also be linked to poorer coping strategies, such as rumination or “thinking too much” (Magidson et al., 2020; Andersen et al., 2023), that are more entrenched and difficult to challenge (Lass and Winer, 2020). For patients with high distress aversion, the integration of distress tolerance strategies may help improve treatment response.
In addition to distress aversion, higher levels of social support at baseline were associated with an increased likelihood of responding to CBT-AD after treatment concluded. Social support is a protective factor against depression in PWH (Li et al., 2009) and is related to improved ART adherence (Gonzalez et al., 2004). It is possible that PWH with higher social support experience less HIV-related stigma and discrimination; these are depression-perpetuating factors. In addition, in ecological systems theory (Bronfenbrenner, 2005), social support may facilitate the development of a microsystem in which PWH with depression are able to uptake skills learned in CBT via sharing acquired knowledge with others. Engaging with others around their new skills may reinforce their use.
What can be concluded from our findings in terms of treatment response and its predictors? Firstly, that many PWH with major depression report feeling better after only one session of CBT-AD, and this may be due to the experience of being in treatment, and indeed in a trial. The effects of therapeutic alliance, feeling heard, being supported, and knowing that skills to manage depression will be part of the treatment cannot be under-estimated. Secondly, these early benefits do not represent full treatment response: to increase the likelihood of remission and sustained improvement, a full course of treatment, with all 8 sessions and associated content, should be delivered. The effectiveness of delivering fewer sessions needs to be explored among participants in a closely-observed stepped-care trial design. Thirdly, some people take much longer to respond: up to 12 months. We found that distress aversion (negative) and social support (positive) were related factors. Patients who are highly averse to distress may need additional therapeutic approaches. Similarly, those with poor social support may require additional support from health systems structures—perhaps through adherence clubs, social clubs, or facilitating treatment partners.
The findings of the present study should be interpreted in the context of several limitations. First, while we noted that depressive symptoms declined rapidly during early treatment, we cannot recommend reducing the dose of CBT-AD at this time. Future research should conduct additional randomized controlled trials and/or stepped-care trials to determine an optimal number of sessions of CBT-AD. It is possible that some individuals will remit with a minimal of problem-solving and information, but we cannot say now who those people are. Relatedly, the present study was a secondary analysis, and the trial was not designed to examine the optimal dosage of CBT-AD. We note that the 8-session intervention is already shorted than is typically offered in high-resource settings. Clinician-administered ratings of depression were only available every 4 months, and it is possible the more frequent independent assessments of depression for those receiving CBT-AD may have resulted in a different pattern of results. This study occurred among PWH in South Africa who met diagnostic assessment for depression and had failed first-line ART. As such, the generalizability of these findings to other PWH in other settings may be limited. Future research may include implementation designs to account for differing doses of therapy, with options to step-up if lower doses fail; and to consider expanding to populations with other chronic conditions.
Acknowledgments
Funding for this project came from a National Institute of Mental Health grant R01MH103770. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. Some of the author time, and resources for statistical consultation were also supported by grant 1P30MH116867.
The authors also wish to thank and acknowledge the inputs of Michelle Jacobo, PhD, Rosana Smith-Alvarez, Noelle Mendez, Norik Kirakosian, Nicola De Kock, MA, Hlombekazi Sybil Majokweni, Jade Witten, Patricia Yoliswa Mtingeni, Nokuphumla Nofeliti, Thulani Njengele, Andiswa Gidana, Tandiwe Mngxuma, Nomvula Mdwaba, Neliswa Kotelo, Zimkhitha Ndinga, and Carla Freeman. We would also like to acknowledge the City of Cape Town Department of Health for their support and for granting us access to their clinics. Thank you also to the staff, patients, and Community Advisory Boards at the respective clinics for their time, effort, and support.
Declaration of competing interest
The study was funded by a National Institutes of Mental Health grant (1RO1MH103770-01A1). The authors report no competing conflicts of interest.
AS was supported by an NIMH grant award: K23MH131438. SS received royalties from Oxford University Press, Guilford Publications and Springer/Humana Press.
Footnotes
CRediT authorship contribution statement
The parent study was conceptualized by SS, JJ, LSA and CC. The paper concept was developed by JJ, AS, CC and JSL. Data were obtained and analysed by JSL and JJ. The paper was written by JJ and JSL. The paper was deeply reviewed and edited by LSA, AS, CC and SS.
References
- Alciati A, Gallo L, Monforte AD, Brambilla F, Mellado C, 2007. Major depression-related immunological changes and combination antiretroviral therapy in HIV-seropositive patients. Hum. Psychopharmacol. Clin. Exp. 22, 33–40. 10.1002/hup.813. [DOI] [PubMed] [Google Scholar]
- Andersen LS, Magidson JF, O’Cleirigh C, Remmert JE, Kagee A, Leaver M, Stein DJ, Safren SA, Joska J, 2018. A pilot study of a nurse-delivered cognitive behavioral therapy intervention (Ziphamandla) for adherence and depression in HIV in South Africa. J. Health Psychol. 23, 776–787. 10.1177/1359105316643375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen LS, Stanton AM, Magidson JF, Joska JA, O’Cleirigh C, Lee JS, Kagee A, Witten JA, Safren SA, 2023. Cognitive and behavioral contributions to depression severity, quality of life, and functioning among people living with HIV in South Africa. Behav. Ther. 54, 91–100. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Perry S, Charlebois ED, Clark RA, Robertson M, Zolopa AR, Moss A, 2001. Non-adherence to highly active antiretro-viral therapy predicts progression to AIDS. AIDS 15, 1181–1183. [DOI] [PubMed] [Google Scholar]
- Bengtson A, Pence BW, O’Donnell J, Thielman N, Heine A, Zinski A, Modi R, McGuinness T, Gaynes B, 2015. Improvements in depression and changes in quality of life among HIV-infected adults. AIDS Care 27, 47–53. 10.1080/09540121.2014.946386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Dabis F, Rekeneire N. de, 2017. Prevalence and factors associated with depression in people living with HIV in sub-Saharan Africa: a systematic review and meta-analysis. PLoS One 12, e0181960. 10.1371/journal.pone.0181960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U, 2005. Ecological systems theory (1992). In: Making Human Beings Human: Bioecological Perspectives on Human Development. Sage Publications Ltd, Thousand Oaks, CA, pp. 106–173. [Google Scholar]
- Browne J, Cather C, Mueser KT, 2021. Common Factors in Psychotherapy [WWW Document]. Oxford Research Encyclopedia of Psychology. 10.1093/acrefore/9780190236557.013.79. [DOI] [Google Scholar]
- Bruckner TA, Scheffler RM, Shen G, Yoon J, Chisholm D, Morris J, Fulton BD, Dal Poz MR, Saxena S, 2011. The mental health workforce gap in low- and middle-income countries: a needs-based approach. Bull. World Health Organ. 89, 184–194. 10.2471/BLT.10.082784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE, 2001. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. AJP 158, 725–730. 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Coates J, Swindale A, Bilinsky P, 2007. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: indicator Guide: Version 3. Washington, DC: Food and Nutrition Technical Assistance Project, Academy for Educational Development. 10.1037/e576842013-001. [DOI] [Google Scholar]
- Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS, 2013. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can. J. Psychiatr. 58, 376–385. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Quero S, Noma H, Ciharova M, Miguel C, Karyotaki E, Cipriani A, Cristea IA, Furukawa TA, 2021. Psychotherapies for depression: a network meta-analysis covering efficacy, acceptability and long-term outcomes of all main treatment types. World Psychiatry 20, 283–293. 10.1002/wps.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T, Garman EC, Lund C, Schneider M, 2020. Adaptation and validation of a structured version of the Hamilton Depression Rating Scale for use by non-clinicians in South Africa (AFFIRM-HDRS). J. Eval. Clin. Pract. 26, 1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden W, 2018. The modal number of therapy sessions internationally is ‘one’, and the majority of people who attend for one session are satisfied. In: Single-Session Therapy (SST). Routledge, pp. 22–24. [Google Scholar]
- Eaton J, McCay L, Semrau M, Chatterjee S, Baingana F, Araya R, Ntulo C, Thornicroft G, Saxena S, 2011. Scale up of services for mental health in low-income and middle-income countries. Lancet 378, 1592–1603. 10.1016/S0140-6736(11)60891-X. [DOI] [PubMed] [Google Scholar]
- Everitt-Penhale B, Kagee A, Magidson JF, Joska J, Safren SA, O’Cleirigh C, Witten J, Lee JS, Andersen LS, 2019. ‘I went back to being myself’: acceptability of a culturally adapted task-shifted cognitive-behavioural therapy (CBT) treatment for depression (Ziphamandla) for South African HIV care settings. Psychol. Health Med. 24, 680–690. 10.1080/13548506.2019.1566624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gámez W, Chmielewski M, Kotov R, Ruggero C, Watson D, 2011. Development of a measure of experiential avoidance: the Multidimensional Experiential Avoidance Questionnaire. Psychol. Assess. 23, 692. [DOI] [PubMed] [Google Scholar]
- Gonzalez JS, Penedo FJ, Antoni MH, Durán RE, McPherson-Baker S, Ironson G, Isabel Fernandez M, Klimas NG, Fletcher MA, Schneiderman N, 2004. Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychol. 23, 413. [DOI] [PubMed] [Google Scholar]
- Henry SK, Grant MM, Cropsey KL, 2018. Determining the optimal clinical cutoff on the CES-D for depression in a community corrections sample. J. Affect. Disord. 234, 270–275. 10.1016/j.jad.2018.02.071. [DOI] [PubMed] [Google Scholar]
- Howard KI, Kopta SM, Krause MS, Orlinsky DE, 1986. The dose–effect relationship in psychotherapy. Am. Psychol. 41, 159. [PubMed] [Google Scholar]
- Humeniuk R, World Health Organization, 2010. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): Manual for Use in Primary Care. World Health Organization, Geneva. [Google Scholar]
- Joska JA, Andersen LS, Smith-Alvarez R, Magidson J, Lee JS, O’Cleirigh C, Safren SA, 2020. Nurse-delivered cognitive behavioral therapy for adherence and depression among people living with HIV (the Ziphamandla study): protocol for a randomized controlled trial. JMIR Res. Prot. 9, e14200 10.2196/14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter JW, Mulick PS, Busch AM, Berlin KS, Martell CR, 2007. The Behavioral Activation for Depression Scale (BADS): psychometric properties and factor structure. J. Psychopathol. Behav. Assess. 29, 191. [Google Scholar]
- Kulisewa K, Stockton MA, Hosseinipour MC, Gaynes BN, Mphonda S, Udedi MM, Pence BW, 2019. The role of depression screening and treatment in achieving the UNAIDS 90–90–90 goals in sub-Saharan Africa. AIDS Behav. 23, 153–161. 10.1007/s10461-019-02593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass ANS, Winer ES, 2020. Distress tolerance and symptoms of depression: a review and integration of literatures. Clin. Psychol. Sci. Pract. 27, e12336 10.1111/cpsp.12336. [DOI] [Google Scholar]
- Li L, Lee S-J, Thammawijaya P, Jiraphongsa C, Rotheram-Borus MJ, 2009. Stigma, social support, and depression among people living with HIV in Thailand. AIDS Care. 10.1080/09540120802614358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgren SM, Bond DJ, Nakasujja N, Boulware DR, 2020. Burden of depression in outpatient HIV-infected adults in sub-Saharan Africa; systematic review and Meta-analysis. AIDS Behav. 24, 1752–1764. 10.1007/s10461-019-02706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund C, Kleintjes S, Kakuma R, Flisher AJ, the MHaPP Research Programme Consortium, 2010. Public sector mental health systems in South Africa: inter-provincial comparisons and policy implications. Soc. Psychiat. Epidemiol. 45, 393–404. 10.1007/s00127-009-0078-5. [DOI] [PubMed] [Google Scholar]
- Magidson JF, Andersen LS, Satinsky EN, Myers B, Kagee A, Anvari M, Joska JA, 2020. “Too much boredom isn’t a good thing”: Adapting behavioral activation for substance use in a resource-limited South African HIV care setting. Psychotherapy (Chic) 57 (1), 107–118. 10.1037/pst0000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez NA, Mayo D, Safren SA, 2021. Interventions addressing depression and HIV-related outcomes in people with HIV. Curr. HIV/AIDS Rep. 18, 377–390. 10.1007/s11904-021-00559-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer L, Smit J, Roux LL, Parker S, Stein DJ, Seedat S, 2008. Common mental disorders among HIV-infected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care STDs 22, 147–158. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J, 1991. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta earthquake. J. Pers. Soc. Psychol. 61, 115. [DOI] [PubMed] [Google Scholar]
- O’Cleirigh C, Ironson G, Smits JAJ, 2007. Does distress tolerance moderate the impact of major life events on psychosocial variables and behaviors important in the management of HIV? Behav. Ther. 38, 314–323. 10.1016/j.beth.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, 2009. The future of psychiatry in low- and middle-income countries. Psychol. Med. 39, 1759–1762. 10.1017/S0033291709005224. [DOI] [PubMed] [Google Scholar]
- Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T, Ferrari AJ, Hyman S, Laxminarayan R, Levin C, Lund C, Medina Mora ME, Petersen I, Scott J, Shidhaye R, Vijayakumar L, Thornicroft G, Whiteford H, 2016. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet 387, 1672–1685. 10.1016/S0140-6736(15)00390-6. [DOI] [PubMed] [Google Scholar]
- Pence BW, Mills JC, Bengtson AM, Gaynes BN, Breger TL, Cook RL, Moore RD, Grelotti DJ, O’Cleirigh C, Mugavero MJ, 2018. Association of increased chronicity of depression with HIV appointment attendance, treatment failure, and mortality among HIV-infected adults in the United States. JAMA Psychiatry 75, 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust-Lima C, Philipps V, Diakite A, Liquet B, Proust MC, 2022. Package ‘lcmm.’ CRAN R. [Google Scholar]
- R Development Core Team, 2016. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Radloff LS, 1977. The CES-D scale a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401. [Google Scholar]
- Richards D, 2011. Prevalence and clinical course of depression: a review. Clin. Psychol. Rev. 31, 1117–1125. 10.1016/j.cpr.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Robinson L, Delgadillo J, Kellett S, 2020. The dose-response effect in routinely delivered psychological therapies: a systematic review. Psychother. Res. 30, 79–96. 10.1080/10503307.2019.1566676. [DOI] [PubMed] [Google Scholar]
- Ruffieux Y, Efthimiou O, Heuvel LLV den, Joska., Cornell M., Seedat S., Mouton JP., Prozesky H., Lund., Maxwell N., Tlali M., Orrell C., Davies M-A., Maartens G, Haas AD., 2021. The treatment gap for mental disorders in adults enrolled in HIV treatment programmes in South Africa: a cohort study using linked electronic health records. Epidemiol. Psychiatr. Sci. 30, e37 10.1017/S2045796021000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Gonzalez JS, Soroudi N, 2007a. Coping with Chronic Illness: Cognitive Behavioral Therapy for Adherence and Depression, Client Workbook. Oxford University Press, NY. [Google Scholar]
- Safren SA, Gonzalez JS, Soroudi N, 2007b. Coping with Chronic Illness: Cognitive Behavioral Therapy for Adherence and Depression, Therapist Guide. Oxford University Press, NY. [Google Scholar]
- Safren SA, O’Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, Mayer KH, 2009. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 28, 1–10. 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH, 2012. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: a randomized controlled trial. J. Consult. Clin. Psychol. 80, 404–415. 10.1037/a0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Bedoya CA, O’Cleirigh C, Biello KB, Pinkston MM, Stein MD, Traeger L, Kojic E, Robbins GK, Lerner JA, 2016. Cognitive behavioural therapy for adherence and depression in patients with HIV: a three-arm randomised controlled trial. Lancet HIV 3, e529–e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh C, Andersen LS, Magidson JF, Lee JS, Bainter SA, Musinguzi N, Simoni J, Kagee A, Joska JA, 2021. Treating depression and improving adherence in HIV care with task-shared cognitive behavioural therapy in Khayelitsha, South Africa: a randomized controlled trial. J. Int. AIDS Soc. 24, e25823 10.1002/jia2.25823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M, 1993. Development of the alcohol use disorders identification test (AUDIT). WHO collaborative project on early detection of persons with harmful alcohol consumption-II. In: ADDICTION-ABINGDON- 88, pp. 791–804. [DOI] [PubMed] [Google Scholar]
- Seedat S, Stein DJ, Herman A, Kessler R, Sonnega J, Heeringa S, Williams S, Williams D, 2008. Twelve-month treatment of psychiatric disorders in the South African Stress and Health study (World Mental Health survey initiative). Soc. Psychiatry Psychiatr. Epidemiol. 43, 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni JM, Wiebe JS, Sauceda JA, Huh D, Sanchez G, Longoria V, Bedoya CA, Safren SA, 2013. A preliminary RCT of CBT-AD for adherence and depression among HIV-positive Latinos on the US-Mexico border: the Nuevo Dia study. AIDS Behav. 17, 2816–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla DR, Kohrt BA, Murray LK, Anand A, Chorpita BF, Patel V, 2017. Psychological treatments for the world: lessons from low- and middle-income countries. Annu. Rev. Clin. Psychol. 13, 149–181. 10.1146/annurev-clinpsy-032816-045217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker J, 2002. Chronic Depression: Determinants and Consequences of Chronic Major Depression in the General Population. Utrecht University. [Google Scholar]
- Vilagut G, Forero CG, Barbaglia G, Alonso J, 2016. Screening for depression in the general population with the Center for Epidemiologic Studies Depression (CES-D): a systematic review with meta-analysis. PLoS One 11, e0155431. 10.1371/journal.pone.0155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JB, Kobak KA, Bech P, Engelhardt N, Evans K, Lipsitz J, Olin J, Pearson J, Kalali A, 2008. The GRID-HAMD: standardization of the Hamilton depression rating scale. Int. Clin. Psychopharmacol. 23, 120–129. [DOI] [PubMed] [Google Scholar]


