Abstract
Mortality from measles virus (MV) infection is caused mostly by secondary infections associated with a pronounced immunosuppression. Dendritic cells (DCs) represent a major target of MV and could be involved in immunosuppression. In this study, human monocyte-derived DCs were used to demonstrate that DC apoptosis in MV-infected DC–T-cell cocultures is Fas mediated, whereas apoptotic T cells could not be rescued by blocking the Fas pathway. Two novel consequences of DC apoptosis after MV infection were demonstrated. (i) Fas-mediated apoptosis of DCs facilitates MV release, while CD40 activation enhances MV replication in DCs. Indeed, detailed studies of infectious MV release and intracellular MV nucleoprotein (NP) showed that inhibition of CD40-CD40L ligand interaction blocks NP synthesis. We conclude that the CD40 ligand expressed by activated T cells first enhances MV replication in DCs, and then Fas ligand produced by activated T cells induces Fas-mediated apoptosis of DCs, thus facilitating MV release. (ii) Not only MV-infected DCs but also bystander uninfected DCs undergo a maturation process confirmed by CD1a, CD40, CD80, CD86, CD83, and major histocompatibility complex type II labeling. The bystander maturation effect results from contact and/or engulfment of MV-induced apoptotic DCs by uninfected DCs. A model is proposed to explain how both a specific immune response and immunosuppression can simultaneously occur after MV infection through Fas-mediated apoptosis and CD40 activation of DCs.
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that capture and process antigens, playing a critical role in antigen presentation and subsequently effector T-cell differentiation (3). Immature CD83-negative DCs residing in peripheral tissues act as immune sentinels by their ability to collect information about invading pathogens. Activation of immature DCs directly by a pathogen or indirectly by a pathogen-induced cytokine, such as interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), or granulocyte-macrophage colony-stimulating factor (GM-CSF), results in their migration to T-cell areas of lymph nodes and the up-regulation of their stimulatory capacity. There, mature CD83+ DCs act as effective inducers of primary responses of antigen-specific naive T cells. The ability of mature DCs to stimulate naive T cells has been attributed to a variety of factors such as the high expression of major histocompatibility complex class II molecules (MHC-II), CD80, CD86, CD40, and diverse adhesion molecules which favor T-cell receptor engagement and costimulation (8, 10, 22). When the mature DCs have reached secondary lymphoid organs, they interact with T cells, receiving signals that induce their terminal differentiation into mature effector DCs. CD40-CD40 ligand (CD40L) interaction between DCs and T cells provides survival signal to DCs (26), is essential for optimal IL-12 production (11, 24), and renders DCs able to prime CD8+ cytotoxic responses (4, 32, 35). As DCs are able to initiate immune response, regulation of their survival may be a mechanism aimed at controlling the initiation and termination of the immune response. Several studies suggest that DCs represent a major target of measles virus (MV) and could be involved in MV-induced immunosuppression, the major cause of the high morbidity and mortality rate associated with measles. Langerhans cells, CD34+ progenitor-derived DCs, and monocyte-derived DCs are susceptible to infection with both MV vaccine and wild-type strains in vitro (18, 19, 34). After MV infection, immature DCs undergo a maturation process similarly to TNF-α or lipopolysaccharide (LPS) activation (34, 36), but they do not behave as mature effector DCs. Indeed, contrary to uninfected DCs, MV-infected DCs block T-cell proliferation whether T cells are syngeneic and activated or allogeneic and naive (18, 19). We have recently reported that CD40L-dependent terminal differentiation of DCs is impaired by MV infection as demonstrated by down-regulation of CD25, CD69, CD71, CD40, CD80, CD86, and CD83, inhibition of IL-12 and induction of IL-10 mRNA synthesis, and inhibition of CD40L-dependent CD8+ T-cell proliferation (36). Furthermore, in MV-infected DC-T cell cocultures, both intensive MV replication and massive apoptosis of DCs and T cells were observed. In vivo, a reduction in the numbers of DCs was observed in human immunodeficiency virus-positive patients (30), but MV is the only virus that has been directly implicated in DC apoptosis. However, the mechanisms and consequences of DC apoptosis induced by MV have not been elucidated.
In this study, we used monocyte-derived DCs to demonstrate that DC apoptosis in MV-infected DC-T cell cocultures is Fas mediated. Two consequences of DC apoptosis observed after MV infection were documented. First, in addition to virus budding and cell lysis, Fas-mediated apoptosis of MV-infected DCs participates in the release of infectious MV particles, while CD40 activation of DCs boosts MV replication. Second, apoptotic MV-infected DCs induce bystander maturation of uninfected DCs, a phenomenon that may be involved in the initiation of an MV-specific response. A model is proposed to explain how both specific immune response and immunosuppression can simultaneously occur after MV infection through Fas-mediated apoptosis and CD40 activation of DCs.
MATERIALS AND METHODS
Reagents.
CD1a-phycoerythrin (PE) (BL6), CD3-PE (UCHT1), CD80-fluorescein isothiocyanate (FITC) (monoclonal antibody [MAb] 104), CD83-PE (HB15a), CD86-FITC (HA5.2B7), CD95/Fas-FITC (UB2), and anti-HLA-DR-FITC (B8.12.2) antibodies were purchased from Immunotech (Marseille, France), CD40-PE (LOB7/6) and CD86 (BU63) antibodies were from Serotec Ltd. (Oxford, England), and CD80-PE (L307.4) was from Becton Dickinson Immunochemistry Systems (San Jose, Calif.). An immunoglobulin G1 (IgG1)-FITC–IgG2a-PE irrelevant antibody cocktail (Immunotech) was used as isotype controls. Mouse IgG1, IgG2a, and IgG2b (Sigma Chemical Co., St. Louis, Mo.) were used for isotype controls. FITC-conjugated, affinity-isolated F(ab′)2 fraction of a sheep anti-mouse Ig antibody (Silenus, Hawthorn, Victoria, Australia) was used for indirect immunofluorescence labeling procedures. Anti-human CD40L used at 10 μg/ml (MAb LL2), human recombinant GM-CSF (hrGM-CSF), and hrIL-4 were generously provided by the Schering-Plough Laboratory for Immunological Research (Dardilly, France).
Patients.
The role of CD40-CD40L interaction can be addressed with cells originate from patient suffering from X-linked immunodeficiency hyper-IgM syndrome, a genetic immunodeficiency that has been attributed to mutations in the CD40L gene (15). Two patients suffering from X-linked hyper-IgM syndrome were included in this study. Mutations in the CD40L gene were characterized and led to the absence of CD40L expression. Informed consent was obtained from each patient family for this study.
Cells.
Monocyte-derived DCs were generated in vitro as previously described (18). After 6 days of culture in the presence of hrGM-CSF (50 ng/ml) and hrIL-4 (500 U/ml), more than 95% of the cells were DCs as assessed by CD1a labeling. Cultures of DCs were performed in 24-well flat-bottomed microtiter plates (Falcon), in a total volume of 1 ml, in RPMI 1640 (Life Technologies) supplemented with 10 mM HEPES (Life Technologies), 2 mM l-glutamine (Life Technologies), gentamicin (40 μg/ml; Life Technologies), and 10% fetal calf serum (Boehringer Mannheim, Meylan, France). Peripheral blood lymphocytes (PBL) or T cells were activated with a combination of phorbal myristate acetate (PMA; 10 ng/ml Sigma) and ionomycin (1 μg/ml; Sigma) for 6 to 12 h. After activation, cells were washed three times. DCs alone were cultured at 106 cells/ml. In PBL or T-cell cocultures, 0.5 × 106 DCs/ml were cultured together with 0.5 × 106 activated PBL or T cells/ml. In the murine fibroblast cocultures, 106 DCs/ml were cultured in the presence of 2 × 105 irradiated (7,000 rads) fibroblastic CD40L- or CD32-transfected L cells (both kindly provided by Schering-Plough Laboratory for Immunological Research) per ml.
MV infection and detection.
DCs were infected at day 6 with Vero cell-derived MV Hallé (the Hallé strain is classified with the vaccine MV strain Edmonston [33]) (1 PFU/cell) pulsed with MV neutralized by 254-nm UV rays for 30 min (UVMV) (1 PFU/cell), or mock infected. After 3 h of incubation at 37°C, the DCs were washed three times to be free of unattached virus and then put in culture. For PFU measurement, virus contents were quantified by limiting dilution from 10 to 10 until 10−10 on confluent Vero cells. A single plaque in the Vero cell confluent culture represents one PFU generated by an individual infectious virus. For nucleoprotein (NP) staining, after 15 min of permeabilization with 0.33% saponin (Sigma), cells were stained with anti-NP viral protein MAb (clone 25; kindly provided by F. Wild) followed by incubation with PE-labeled anti-mouse Ig (Immunotech). This NP staining clearly shows separate positive and negative subpopulations, thus demonstrating that sensitivity of this antibody is sufficient to detect all infected DCs 3 days after infection.
Phenotypic analysis.
All immunostainings were performed in 1% bovine serum albumin and 3% human serum–phosphate-buffered saline. Direct immunostaining was performed with 2 μg of FITC-conjugated or PE-conjugated antibodies per ml. Indirect immunostaining was performed with 2 μg of the first mouse MAb per ml and revealed with 2 μg of the FITC-conjugated, affinity-isolated F(ab′)2 fraction of a sheep anti-mouse Ig antibody per ml.
RNase protection assays.
RNA was extracted from 107 uninfected or MV-infected monocyte-derived DCs, using RNA NOW-TC reagent (Biogentex, Seabrook, Tex.). The RNase protection was performed with 4 μg of RNA with the RiboQuant multiprobe RNase assay system (Pharmingen, San Diego, Calif.) as specified by the manufacturer. In brief, RNA was hybridized overnight with the in vitro-translated 32P-labeled probe (hAPO-3 kit; Pharmingen). Following hybridization, samples were treated with RNases A and T1 plus proteinase K, phenol chloroform extracted, and ethanol precipitated. The protected fragments were resolved by electrophoresis on a 5% acrylamide-urea gel and exposed on a PhosphorImager screen (Molecular Dynamics, Inc., Sunnyvale, Calif.) for 12 h to quantify the intensity of the bands.
CFSE staining.
As previously described, CFSE (5-carboxyfluorescein diacetate-succinimidyl; Molecular Probes, Inc., Eugene, Oreg.) was diluted to 5 mM in dimethyl sulfoxide, aliquoted, and stored at −20°C until used. Cells were resuspended at 10 × 106/ml in RPMI 1640. The CFSE stock solution was added to the suspension at a final dilution of 1/200. After 10 min of incubation at 37°C, with inversion every 3 min, cells were washed twice in the same medium and then put in culture. CFSE labeling decreased at each cellular division, but as DCs do not divide, they remain highly positive for CFSE (40).
Apoptotic death detection, blocking, and induction.
An ApopTag in situ apoptosis detection kit (S7110-KIT, Oncor, Gaithersburg, Md.) was used to detect apoptotic cells by fluorescence-activated cell sorting (FACS) detection of digoxigenin-labeled genomic DNA. DiOC6(3) (3,3′-diexyloxacarbocyanine)- (41) propidium iodide (PI) double staining was performed to detect apoptotic cells by flow cytometry. Cells were incubated 15 min at 37°C with 40 nM DiOC6 (Molecular Probes) in culture medium to evaluate mitochondrial transmembrane potential (ΔΨm). As ΔΨm decreases with cell commitment to apoptosis, DiOC6(3) stained living cells but not apoptotic cells. PI (0.5 μg/ml) was added before FACS analysis of the cells. ZB4 anti-Fas blocking antibody (Immunotech) was used at 500 ng/ml, while the CH11 anti-Fas agonistic antibody (Immunotech) was used at 1 μg/ml.
RESULTS
DC apoptosis is Fas mediated in MV-infected DC-T cell cocultures.
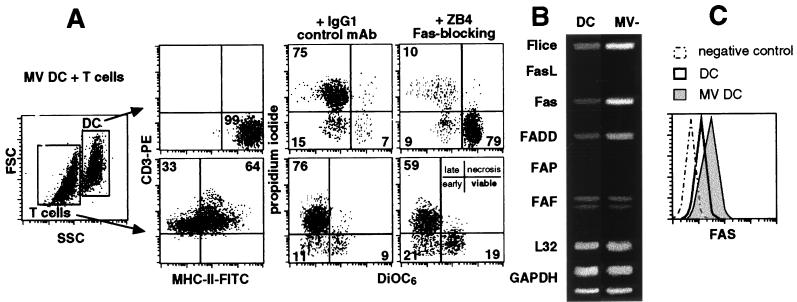
We previously showed that most DCs and T cells were dead in MV DC-T cell cocultures at day 5 (18). To determine whether DCs and/or T cells died from Fas-dependent apoptosis, ZB4 anti-Fas blocking antibody or a related isotype control antibody was added to the MV-infected DC-T cell cocultures. DiOC6-PI double stainings were performed at day 5 to analyze the percentage of DiOC6-negative, PI-positive apoptotic cells (Fig. 1A). As assessed by MHC-II–CD3 double staining, DCs were gated in SSChigh/FSChigh, whereas T cells were gated in SSClow/FSClow. At day 5 of culture, DCs (7% viable) and T cells (9% viable) were dead by apoptosis, in contrast to the control cocultures of uninfected DCs or UVMV-pulsed DCs with T cells (95% of DCs and 82% of T cells were viable [data not shown]). When Fas-mediated apoptosis was blocked, up to 72% of the DCs (79% viable) but only 10% of the T cells (19% viable) could be rescued from apoptosis. After 24 h of culture, an RNase protection assay was performed on uninfected or MV-infected DCs to quantify the amount of six mRNAs which encode proteins involved in Fas-dependent apoptosis (Fig. 1B). Flice/caspase-8, Fas, and FADD/adapter protein mRNAs were up-regulated 11-, 15-, and 4-fold, respectively, by MV infection. Fas ligand (FasL) and FAP mRNAs were not detected in DCs or MV-infected DCs, while the amount of FAF mRNA was not modified by MV infection. FACS analysis (Fig. 1C) further confirmed that MV replication up-regulated Fas expression on DCs.
FIG. 1.
Fas-dependent apoptosis of MV-infected DCs in MV DC-T cell cocultures. (A) At day 5, apoptosis was analyzed by FACS in cocultures of MV-infected DCs and syngeneic activated T cells. MHC-II-FITC–CD3-PE doubling staining confirmed the FSC/SSC gates for DCs and T cells. These gates were used to analyze the DiOC6-PI double staining in the presence of ZB4 blocking anti-Fas antibody or related isotype control. Apoptotic dead cells have decreased mitochondrial transmembrane potential and permeabilized membranes that render them DiOC6 negative and PI positive, respectively. In contrast, viable cells are DiOC6 positive and PI negative. Results are representative of three experiments; standard deviations were below 10%. (B) Immature DCs were not infected or MV infected and then cultured for 24 h. RNAs were extracted and used for RNase protection using the hAPO-3 probe kit and developed by a PhosphorImager system for 6 h. Local background has been subtracted from each signal. The highest value (1,000) was attributed to the highest signal; then the levels of mRNAs were quantified by densitometry and scanning comparison with control probes (GAPDH and L32). Data shown are representative of three experiments. (C) DCs were not infected (thick line) or MV infected (gray histogram), cultured for 3 days, stained with antibodies against Fas (UB2-FITC) or a control antibody, and analyzed by FACS. The expression of Fas protein on gated viable DCs is shown. Data shown are representative of three experiments; standard deviations were below 10%.
Thus, DC apoptosis was mainly Fas dependent, whereas T-cell apoptosis was Fas independent. In conclusion, MV-infected DCs develop increased sensitivity to FasL and undergo Fas-mediated apoptosis when cocultured with activated T cells.
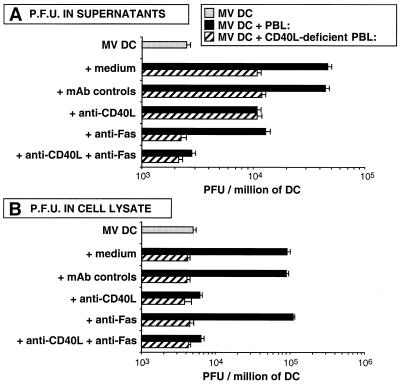
CD40 activation enhances MV replication in DCs, while Fas-mediated apoptosis facilitates MV release.
At day 5, PFU were measured in supernatants of culture and in cell lysate (Fig. 2). In MV DC-PBL cocultures, anti-CD46 antibodies abrogated the weak (10%) T-cell infection during the culture, whereas total PFU were only 2% decreased; thus, infectious virus were mainly produced by DCs in MV DC-PBL cocultures (data not shown). We have previously shown that CD40L+ T cells enhance viral production by DCs (18). In MV DC–CD40L-deficient PBL cocultures, the absence of CD40L decreased PFU measured in supernatant. Anti-CD40L antibodies but also blocking anti-Fas antibodies decreased PFU measured in supernatant. The combination of anti-CD40L and anti-Fas antibodies completely abrogated the enhancement of viral production induced by activated T cells (Fig. 2A). Thus, viral production measured in supernatant both results from CD40 and Fas ligation on DCs. By contrast, measurement of PFU in cell lysates showed that CD40L-deficient PBL or anti-CD40L abrogated the enhancement of viral production, whereas blocking anti-Fas antibodies had no effect (Fig. 2B). Thus, viral replication in infected DCs results from CD40 activation but not from Fas ligation which participates in MV release in the supernatant.
FIG. 2.
Comparison of PFU measured in culture supernatants with PFU measured after cell lysis. DCs were MV infected and cultured for 5 days alone, with activated allogeneic PBL, or with activated allogeneic CD40L-deficient PBL in the presence of blocking anti-CD40L, blocking anti-Fas (ZB4), or MAb controls. Then either supernatants (A) or cells (B) were frozen and PFU were measured. Results are means of three experiments.
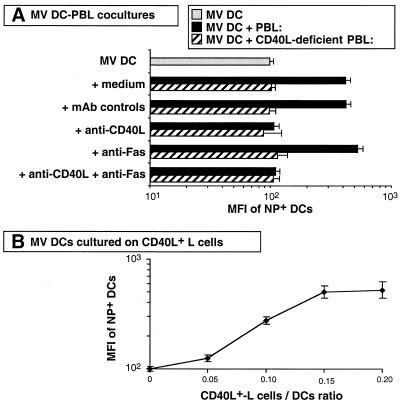
This conclusion was confirmed by measuring MV NP in infected DCs. NP is the earliest MV protein transcribed during viral cell cycle, and its amount, measured by mean fluorescence intensity (MFI), reflects the intensity of viral replication in infected cells (Fig. 3A). At day 3 of culture, 50% of DCs were NP+ with or without activated T cells (data not shown), but activated T cells enhanced MFI of NP+ DCs. This enhancement was abrogated with CD40L-deficient PBL or with anti-CD40L antibodies, whereas incubation with blocking anti-Fas antibodies did not increase MFI of NP+ DCs. When both anti-CD40L and anti-Fas were added to MV DC-T cell coculture, MFI of NP+ DCs remained low. Furthermore, like CD40L+ T cells, CD40L+ L cells enhanced MFI of NP+ DCs, which increased proportionally to the amount of CD40L signal delivered to the DCs (Fig. 3B).
FIG. 3.
MFI of NP depends on CD40 activation in MV-infected DCs. (A) DCs were MV infected and cultured for 3 days alone, with activated allogeneic PBL, or with activated allogeneic CD40L-deficient PBL in the presence of blocking anti-CD40L, blocking anti-Fas (ZB4), or MAb controls. (B) DCs were MV infected and cultured for 3 days with the indicated CD40L+ L cell/DC ratio. The number of transfected L cells was constant, as CD40L+ cells were replaced by CD32-transfected L-cell controls. Cells were then stained with antibodies against NP plus PE-conjugated anti-mouse Ig and analyzed by FACS. The MFI of NP+ DCs was plotted. Results are means of three experiments.
Thus, after CD40-dependent enhancement of MV replication in DCs, Fas-dependent DC apoptosis participates in MV release.
MV-infected DCs induce bystander maturation of uninfected DCs after contact and/or engulfment of apoptotic DCs.
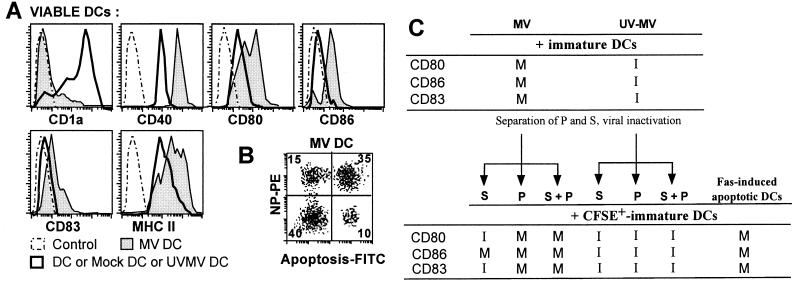
As previously described (34), immature DCs isolated from peripheral blood and then infected with MV up-regulated MHC-II, CD83, and CD86, thus demonstrating that MV induces DC maturation. DC maturation was also obtained with monocyte-derived DCs: MV infection down-regulated CD1a expression up-regulated CD40, CD80, and MHC-II expression, and induced CD86 and CD83 expression in all viable DCs (Fig. 4A). However, double NP-apoptosis staining showed that only a minority (27%) of these viable DCs were positive for NP staining (Fig. 4B). Besides, replication was required for DC maturation since mock supernatant or UVMV had no effect. Therefore, MV replication in DCs (NP+ DCs) induced bystander maturation of uninfected viable DCs (NP− DCs). As MV replication and not UVMV (18) is responsible for DC apoptosis, we have hypothesized that apoptotic cells produced in MV-infected DC cultures could induce DC maturation. Supernatants and pellets of 3-day primary cultures of MV-infected or UVMV-pulsed DCs were harvested. Secondary cultures were performed by culturing immature DCs either with supernatant, with pellet, or with a reconstituted mix after neutralization of infectious viral particles by UV and paraformaldehyde fixation of the pellet. Freshly added immature DCs were monitored by their green fluorescence after CFSE staining. After 3 days of culture, more than 90% of CFSE+ DCs were viable (data not shown). Supernatant of MV-infected DCs induced only CD86 expression in CFSE+ DCs, while the pellet induced up-regulation of CD80 together with CD86 and CD83 as MV infection or reconstituted mix did (Fig. 4C). None of the control conditions with UVMV primary culture induced DC maturation. In addition, Fas-induced apoptotic DCs triggered maturation of freshly cocultured CFSE+ DCs. CD1a expression was also down-regulated, while MHC-II expression was up-regulated (data not shown). Moreover, CFSE labeling showed that CFSE+ DCs had engulfed CFSE− apoptotic DCs (data not shown). Thus, bystander maturation of uninfected DCs mainly results from engulfment or cellular contact with apoptotic DCs induced by MV replication.
FIG. 4.
Percentages of NP+ apoptotic cells and phenotype of MV-infected immature DCs. (A) Immature DCs were not infected (thick line), infected with MV (gray histogram), or incubated with UVMV or with mock supernatant for 3 h, then cultured for 3 days, stained with antibodies against CD1a, CD40, CD80, CD86, CD83, or MHC-II HLA-DR or control antibodies, and analyzed by FACS. The expression of these proteins on gated viable DCs is shown. Data shown are representative of eight experiments; standard deviations were below 15%. (B) FACS apoptosis and NP+ cell analysis of MV-infected DCs at day 3. Cells were stained with anti-NP followed by PE-anti-mouse and FITC-antidigoxigenin. The number in each quadrant represent the percentages of gated DCs. Quadrant limits were positioned on the negative control (not shown). Results are representative of five experiments; standard deviations were below 10%. (C) A two-step culture was performed. First, DCs were MV infected or UVMV pulsed; then the supernatants (S) and pellets (P) of these primary cultures were separately harvested at day 3. P were fixed for 6 h with 1% para-formaldehyde and washed. Medium was added to P to reconstitute the 1-ml original volume. S were frozen, UV inactivated, and passed through a 0.2-μm-pore-size filter. In addition, apoptotic DCs were generated by a 3-day culture of immature DCs in the presence of Fas inducer antibodies (CH11). In a second culture, S, P, and Fas-apoptotic DCs were placed on immature syngeneic DCs. The immature DCs used in this second culture were previously stained with CFSE (green fluorescence), then either not infected, MV infected, UVMV pulsed, cultured with 10% (vol/vol) S, 10% (vol/vol) P, or 10% P–10% S (columns 3 to 5 and 7 to 9), or cultured with 10% (vol/vol) Fas-apoptotic DCs. After 3 days, CFSE-positive DCs were analyzed by cytometry after CD80, CD86, and CD83 stainings. I or M denotes immature (CD80low CD86− CD83−) or mature (CD80high CD86+ CD83+) state of cells for each marker. Data shown are representative of three experiments; standard deviations on cytometry analysis were below 15%.
DISCUSSION
We document here that MV-infected DCs develop increased sensitivity to FasL and undergo Fas-mediated apoptosis when they are cocultured with activated T cells. The role of CD40L, expressed by activated T cells, which enhances MV replication in DCs was confirmed, while two novel consequences of DC apoptosis induced during MV infection were identified. First, Fas-mediated apoptosis of DCs facilitates MV release in MV-infected DC-T cell cocultures; second, apoptotic DCs induce bystander maturation of uninfected DCs.
MV-induced apoptosis has been observed in various cell types: (i) in Vero fibroblasts and monocytic cell lines (16); (ii) in human thymocytes (2); (iii) in PMA-plus-ionomycin-activated T cells, but not in phytohemagglutinin-activated T cells, thus demonstrating that apoptosis of T cells depends on their activation status (23); (iv) in DCs (18); and (v) in thymic epithelial cells after their terminal differentiation (38). MV-induced apoptosis in these various experimental models originates from unknown mechanisms, but apoptosis of cells from the immune system has been proposed as one mechanism of immunosuppression. We observed a massive apoptosis of both DCs and T cells in MV-infected DC-activated T-cell cocultures. We showed here that MV-induced DC apoptosis was Fas mediated. As FasL mRNA was not detected in MV-infected DCs, we propose that MV infection renders DCs susceptible to FasL expressed by activated T cells. In contrast, T-cell apoptosis induced by MV-infected DCs was shown to be Fas independent. As cytotoxic activity of MV-infected DC was demonstrated to be TRAIL mediated (39), this death ligand may be responsible for T-cell apoptosis in the cocultures. In vivo, the terminal fate of DCs remains uncertain and probably depends both on maturation signal received and on signal provided by the microenvironment. In vitro, LPS activation induces DC maturation and stimulates DC survival. Maturation and survival are regulated through two different signaling pathways (31). In vivo, after LPS activation, mature DCs would be programmed to die unless they receive a survival signal from T cells (12). This survival signal may be CD40L expressed by activated T cells, which protects DCs from Fas apoptosis (25, 26). Splenic murine DCs or murine DC line XS52 undergo apoptosis after ex vivo antigen-specific interaction with T cells. The authors propose that T-cell-induced DC apoptosis serves as a down-regulatory mechanism that prevents the continued activation of T cells by antigen-bearing DCs (28). Thus, following a danger signal received in the periphery, DCs undergo maturation and migrate to secondary lymphoid organs, where they could receive CD40L survival signal from T cells, then initiate T-cell responses, and finally die by apoptosis. According to this model, early apoptosis of MV-infected DCs would prevent efficient T-cell activation and could account for in vivo suppression of cell-mediated immunity. Following lymphocytic choriomeningitis virus infection in mice, immune system-mediated destruction of DCs results in generalized immune suppression (6). Unlike uninfected DCs, MV-infected DCs cannot be rescued from Fas-mediated apoptosis by CD40L+ T cells. We have recently demonstrated that MV replication modifies CD40 signaling in DCs, leading to impaired maturation (36). Modification of CD40 signaling by MV infection in DCs could be also responsible for the inability of CD40L to induce survival in MV-infected DCs.
We next studied the consequences of MV-induced DC apoptosis. Detailed studies of NP+ cells and viral production permit us to propose that FasL expressed by activated T cells facilitates the release of MV in supernatant of DC-T cell cocultures by inducing DC apoptosis. Such a relationship between viral production and viral budding has been previously documented with two viruses: expression of bcl-2, which blocks influenza virus-induced apoptosis, also reduces the spread of virus (29); and in bovine herpesvirus 1 infection, inhibition of caspase activity delays cytotoxic activity and virus release but increases the overall virus yield in bovine kidney cells (13). Thus, MV-induced DC apoptosis contributes to the release of MV infectious particles in vitro. Nevertheless, as efficient engulfment of apoptotic cells occurs in vivo, Fas-mediated DCs apoptosis may not lead to the release of infectious MV particles but rather contribute to contaminating resident phagocytes. It would be interesting to study whether apoptotic bodies from MV-infected cells can contaminate phagocytes in vitro. In vitro infection studies indicate that CD46 is a major host cell factor involved in the MV-induced fusion process and MV entry, but the high efficiency of the replicative cycles and virus propagation requires additional factors (20). Budding itself involves vectorial growth of actin filaments, since alteration of actin microfilament structure with cytochalasin inhibits MV release (5, 37). In certain tissues such as the brain, propagation of MV infectivity in human infections may occur principally by cell contacts (7). Moreover, using persistently infected U937 monocytes and HeLa cells, uptake of viral material was recently demonstrated after microfusion events at the cell contacts and without syncytium formation (17). In the case of MV-infected DCs, carriage of MV by DCs which undergo maturation may facilitate virus spreading to secondary lymphoid organs, where they encounter CD40L+ T cells. Indeed, we confirm that CD40L signal induces a burst of MV production by infected DCs (18).
In vitro, MV replication induced the apoptosis of 45% of the DCs. One intriguing feature was that phenotypic maturation involved the whole DC population whereas only 27% of viable DCs had replicated MV as assessed by NP staining. Complete phenotypic maturation of DCs was achieved by culturing immature DCs with MV-induced apoptotic DCs or Fas-induced apoptotic DCs. Thus, bystander uninfected DCs undergo maturation as a result of engulfment and/or cellular contact with MV-induced apoptotic DCs. We also observed that supernatant of MV-infected DCs partially induced DC maturation. As MV infection induces IFN-α/β (14) and IFN-α/β enhances terminal differentiation of human DCs (9, 27), we propose that IFN-α/β secretion by MV-infected DCs enhances bystander DC maturation.
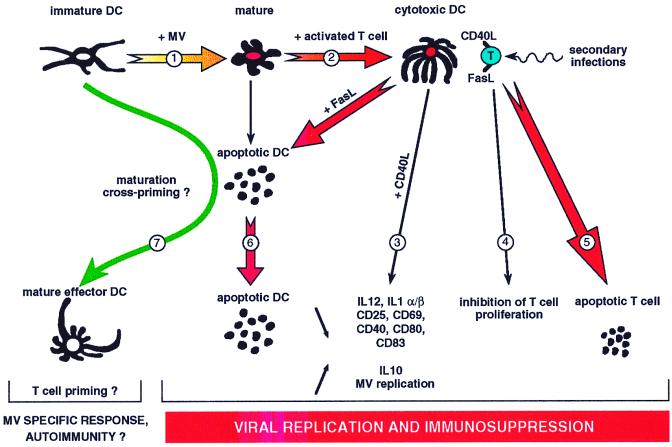
We propose a model that highlights the respective role of Fas-mediated apoptosis and CD40L activation of DCs both in specific immune response and in immunosuppression observed after MV infection (Fig. 5). MV infection of peripheral immature DCs may induce their maturation and migration (1) to the secondary lymphoid organs, where they receive the CD40L signal expressed by activated T cells. Instead of culminating in DC differentiation into mature effector DCs, CD40L activation of MV-infected DCs generates cytotoxic DCs (2) unable to prime naive T cells because of defective IL-12 production (18), defective IL-1α/β mRNA synthesis, and no or low expression of cosignal membrane molecules (3, 36) but able to synthesize IL-10 mRNA (3), to inhibit activated T-cell proliferation (4, 18), and to delete activated T cells (5). At the same time, DCs may highly replicate MV (3) and then release infectious virus when they die by a Fas-dependent mechanism (6). A putative pathway to organize MV-specific immune response would be the cross-presentation (1, 21) of MV protein antigens after engulfment of MV-infected apoptotic cells by living DCs (7). Finally, the persistence of immune suppression after the clearance of MV may be related to the time necessary to recolonize peripheral tissues with an efficient number of DCs. Such a model would require that the majority of DCs, at least those which are localized in the secondary lymphoid organs, be infected.
FIG. 5.
Model of immunosuppression induced by MV-infected DCs. 1, peripheral immature DCs mature and migrate when they are MV infected; 2, MV-infected CD40-activated DCs become cytotoxic DCs; 3, cytotoxic DCs show low IL-12 secretion and defective cosignal membrane molecules that render them unable to prime naive T cells, but they highly replicate MV; 4, cytotoxic DCs inhibit T-cell proliferation; 5, cytotoxic DCs induce the death of activated T cells; 6, cytotoxic DCs undergo Fas-dependent apoptosis; 7, uninfected DCs engulf apoptotic DCs coming from MV-infected surrounding cells and initiate an MV-specific immune response.
ACKNOWLEDGMENTS
We thank B. Horvat and H. Valentin for critical reading of the manuscript, M. Perret for technical assistance, and A. Thomas and S. Mouradian for FACS settings.
This work was supported by institutional grants from the INSERM and from MENESR and by additional support from ARC (CRC 6108), Ligue Nationale Contre le Cancer, Programme PRFMMIP, and Region Rhone-Alpes.
REFERENCES
- 1.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 2.Auwaerter P G, Kaneshima H, McCune J M, Wiegand G, Griffin D E. Measles virus infection of thymic epithelium in the SCID-hu mouse leads to thymocyte apoptosis. J Virol. 1996;70:3734–3740. doi: 10.1128/jvi.70.6.3734-3740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Bennett S R, Carbone F R, Karamalis F, Flavell R A, Miller J F, Heath W R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 5.Bohn W, Rutter G, Hohenberg H, Mannweiler K, Nobis P. Involvement of actin filaments in budding of measles virus: studies on cytoskeletons of infected cells. Virology. 1986;149:91–106. doi: 10.1016/0042-6822(86)90090-5. [DOI] [PubMed] [Google Scholar]
- 6.Borrow P, Evans C F, Oldstone M B. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cathomen T, Mrkic B, Spehner D, Drillien R, Naef R, Pavlovic J, Aguzzi A, Billeter M A, Cattaneo R. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J. 1998;17:3899–3908. doi: 10.1093/emboj/17.14.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, de Saint-Vis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 11.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Smedt T, Pajak B, Klaus G G, Noelle R J, Urbain J, Leo O, Moser M. Antigen-specific T lymphocytes regulate lipopolysaccharide-induced apoptosis of dendritic cells in vivo. J Immunol. 1998;161:4476–4479. [PubMed] [Google Scholar]
- 13.Devireddy L R, Jones C J. Activation of caspases and p53 by bovine herpesvirus 1 infection results in programmed cell death and efficient virus release. J Virol. 1999;73:3778–3788. doi: 10.1128/jvi.73.5.3778-3788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhib-Jalbut S S, Cowan E P. Direct evidence that interferon-beta mediates enhanced HLA-class I expression in measles virus-infected cells. J Immunol. 1993;151:6248–6258. [PubMed] [Google Scholar]
- 15.DiSanto J P, Bonnefoy J Y, Gauchat J F, Fischer A, de Saint Basile G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993;361:541–543. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 16.Esolen L M, Park S W, Hardwick J M, Griffin D E. Apoptosis as a cause of death in measles virus-infected cells. J Virol. 1995;69:3955–3958. doi: 10.1128/jvi.69.6.3955-3958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Firsching R, Buchholz C J, Schneider U, Cattaneo R, ter Meulen V, Schneider-Schaulies J. Measles virus spread by cell-cell contacts: uncoupling of contact-mediated receptor (CD46) downregulation from virus uptake. J Virol. 1999;73:5265–5273. doi: 10.1128/jvi.73.7.5265-5273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan M C, Liu Y J, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horvat B, Rivailler P, Varior-Krishnan G, Cardoso A, Gerlier D, Rabourdin-Combe C. Transgenic mice expressing human measles virus (MV) receptor CD46 provide cells exhibiting different permissivities to MV infections. J Virol. 1996;70:6673–6681. doi: 10.1128/jvi.70.10.6673-6681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, Albert M, Bhardwaj N, Mellman I, Steinman R M. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inaba K, Witmer-Pack M, Inaba M, Hathcock K S, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley P S, Ikehara S, et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito M, Watanabe M, Ihara T, Kamiya H, Sakurai M. Measles virus induces apoptotic cell death in lymphocytes activated with phorbol 12-myristate 13-acetate (PMA) plus calcium ionophore. Clin Exp Immunol. 1997;108:266–271. doi: 10.1046/j.1365-2249.1997.d01-995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koppi T A, Tough-Bement T, Lewinsohn D M, Lynch D H, Alderson M R. CD40 ligand inhibits Fas/CD95-mediated apoptosis of human blood-derived dendritic cells. Eur J Immunol. 1997;27:3161–3165. doi: 10.1002/eji.1830271212. [DOI] [PubMed] [Google Scholar]
- 26.Ludewig B, Graf D, Gelderblom H R, Becker Y, Kroczek R A, Pauli G. Spontaneous apoptosis of dendritic cells is efficiently inhibited by TRAP (CD40-ligand) and TNF-alpha, but strongly enhanced by interleukin-10. Eur J Immunol. 1995;25:1943–1950. doi: 10.1002/eji.1830250722. [DOI] [PubMed] [Google Scholar]
- 27.Luft T, Pang K C, Thomas E, Hertzog P, Hart D N, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- 28.Matsue H, Edelbaum D, Hartmann A C, Morita A, Bergstresser P R, Yagita H, Okumura K, Takashima A. Dendritic cells undergo rapid apoptosis In vitro during antigen-specific interaction with CD4+ T cells. J Immunol. 1999;162:5287–5298. [PubMed] [Google Scholar]
- 29.Olsen C W, Kehren J C, Dybdahl-Sissoko N R, Hinshaw V S. bcl-2 alters influenza virus yield, spread, and hemagglutinin glycosylation. J Virol. 1996;70:663–666. doi: 10.1128/jvi.70.1.663-666.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson S, English N R, Longhurst H, Balfe P, Helbert M, Pinching A J, Knight S C. Analysis of human immunodeficiency virus type 1 (HIV-1) variants and levels of infection in dendritic and T cells from symptomatic HIV-1-infected patients. J Gen Virol. 1998;79:247–257. doi: 10.1099/0022-1317-79-2-247. [DOI] [PubMed] [Google Scholar]
- 31.Rescigno M, Martino M, Sutherland C L, Gold M R, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridge J P, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 33.Rima B K, Earle J, Yeo R P, Herlihy L, Baczko K, Ter Meulen V, Carabaña J, Caballero M, Celma M L, Fernandez-Muñoz R. Temporal and geographical distribution of measles virus genotypes. J Gen Virol. 1995;76:1173–1180. doi: 10.1099/0022-1317-76-5-1173. [DOI] [PubMed] [Google Scholar]
- 34.Schnorr J J, Xanthakos S, Keikavoussi P, Kampgen E, ter Meulen V, Schneider-Schaulies S. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc Natl Acad Sci USA. 1997;94:5326–5331. doi: 10.1073/pnas.94.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoenberger S P, Toes R E, van der Voort E I, Offringa R, Melief C J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 36.Servet-Delprat C, Vidalain P O, Bausinger H, Manie S, Le Deist F, Azocar O, Hanau D, Fischer A, Rabourdin-Combe C. Measles virus induces abnormal differentiation of CD40L-activated human dendritic cells. J Immunol. 2000;164:1753–1760. doi: 10.4049/jimmunol.164.4.1753. [DOI] [PubMed] [Google Scholar]
- 37.Stallcup K C, Raine C S, Fields B N. Cytochalasin B inhibits the maturation of measles virus. Virology. 1983;124:59–74. doi: 10.1016/0042-6822(83)90290-8. [DOI] [PubMed] [Google Scholar]
- 38.Valentin H, Azocar O, Horvat B, Williems R, Garrone R, Evlashev A, Toribio M L, Rabourdin-Combe C. Measles virus infection induces terminal differentiation of human thymic epithelial cells. J Virol. 1999;73:2212–2221. doi: 10.1128/jvi.73.3.2212-2221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vidalain P O, Azocar O, Lamouille B, Astier A, Rabourdin-Combe C, Servet-Delprat C. Measles virus induces functional TRAIL production by human dendritic cells. J Virol. 2000;74:556–559. doi: 10.1128/jvi.74.1.556-559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weston S A, Parish C R. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J Immunol Methods. 1990;133:87–97. doi: 10.1016/0022-1759(90)90322-m. [DOI] [PubMed] [Google Scholar]
- 41.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere J L, Petit P X, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]