Abstract
Introduction
Injectable extended-release formulations of luteinizing hormone-releasing hormone agonists (LHRHa) have simplified the treatment of prostate cancer with a satisfactory level of androgen castration. This study aims to determine the percentage of patients whose initial LHRHa prescription was renewed during follow-up, how many changed formulation and how their quality of life evolved.
Methods
This is an observational, prospective, multicentre study of men with prostate cancer who were to receive treatment with LHRHa (triptorelin every 3 or 6 months, leuprorelin every 3 or 6 months, or goserelin every 3 months) for 24 months. The treatment used was recorded and quality of life was assessed (QLQ-PR25 questionnaire) at four follow-up visits.
Results
A total of 497 men (median age 75 years) were evaluated. The median exposure to LHRHa was 24 months. The initial prescription was renewed in 95.7% at follow-up 1 and 75% at follow-up 4. The main reason for changing from a 6-month to a 3-month formulation was a preference for sequential treatment (according to the investigator) and to see the physician more frequently (according to the patient). The main reason for switching from the 3-month to 6-month formulation was simplification of treatment (according to the investigator) and for convenience (according to the patient). Findings in the QLQ-PR25 questionnaire revealed no changes in urinary or bowel symptoms, though an improvement in sexual activity was reported. Practically all investigators and patients were satisfied/very satisfied with the treatment.
Conclusion
Changes in formulation were scarce and generally justified by convenience factors or personal preferences. Patients maintained a good health status, with a high rate of retention of LHRHa treatment.
Clinical Trial Registration
Study number: A-ES-52014-224.
A plain language summary is provided as supplementary material (available at: https://www.drugsincontext.com/wp-content/uploads/2024/05/dic.2024-2-2-Suppl.pdf).
Keywords: androgen deprivation, concomitant, hormone therapy, quality of life, radiotherapy
Introduction
Prostate cancer is considered one of the most prevalent male malignancies.1 Despite advances in treatment and early detection through improved screening,2,3 a subset of men diagnosed with prostate cancer progress to an advanced or metastatic stage requiring systemic therapy.4 Androgen deprivation therapy (ADT) has been the only therapeutic strategy for men with metastatic disease. However, a multitude of treatments can now be combined with ADT to provide an overall survival benefit in both newly diagnosed metastatic and castration- resistant disease. Nevertheless, ADT continues to be the backbone therapy for prostate cancer.4–11 The efficacy of ADT on prostate volume, progression of disease and survival outcomes has been well established.12–14 When undergoing radiotherapy, clinical experience has shown that neoadjuvant ADT increases disease-specific and overall survival in men with localized or advanced disease who undergo radiotherapy.15,16
First-line therapies to reduce testosterone levels include bilateral orchiectomy, oestrogens, luteinizing hormone-releasing hormone (LHRH) analogues (agonists and antagonists), antiandrogens, and long-acting LHRH agonists (LHRHa). LHRHa are the most widely used ADT for advanced prostate cancer.8,17 With the development of injectable depot formulations, chemical castration has progressively supplanted surgical castration for effective reduction of circulating testosterone.18 LHRHa have been shown to improve survival and progression-related outcomes in a similar way to bilateral orchiectomy, a procedure that many men find psychologically difficult to accept.19,20 LHRHa are available in different formulations, thus enabling them to be administered every 1, 2, 3, 6 or 12 months.21
Both the European Association of Urology and the American Urological Association guidelines recommend follow-up visits every 3–6 months. Furthermore, prostate-specific antigen (PSA) levels should be checked every 6 months in most patients with stable disease receiving long-term ADT.8–10 Because adherence to ADT, according to current guidelines, is not optimal and must be balanced against possible adverse effects,22 matching the administration of hormone therapy with PSA monitoring is reasonable, even if it is not necessary.
The main objective of this study was to determine the percentage of men for whom the initial LHRHa prescription had been renewed at their follow-up visits as well as the percentage of patients who switched between the 3-month and 6-month formulations and the reasons for this decision, along with any changes in quality of life.
Methods
Study design
This was a prospective, non-interventional study, conducted at 28 centres in Spain between July 2017 and June 2021. Its primary objective was to determine the percentage of men with prostate cancer for whom the initial LHRHa prescription had been renewed.
The decision to prescribe an LHRHa as a 3-month or 6-month formulation was part of routine clinical practice and was made prior to and independently of the decision to enrol the patient. The treatment used was recorded, and quality of life was assessed using the QLQ-PR25 questionnaire at four follow-up visits after initiation of treatment [V1: at 3 or 6 months (according to the initial formulation); V2: 12 months; V3: 18 months; and V4: 24 months]. No additional assessments or tests were required.
Patients were treated with one of the following LHRHa regimens: triptorelin every 3 or 6 months (3M or 6M), leuprorelin 3M or 6M, or goserelin 3M. As this was a non-interventional study, investigators were free to choose the product and routes of administration in accordance with the local summary of product characteristics.
Ethics of approval statement
This study was approved by the Clinical Research Ethics Committee of the participating centres and was conducted in compliance with the recommendations of the Declaration of Helsinki (2013) and the International Ethical Guidelines for Epidemiological Studies of the Council for International Organizations of Medical Sciences (2009).23 The Ethics Committee of reference was that of Hospital del Mar (Barcelona, Spain). Informed consent was obtained before enrolment and prior to data collection.
Patient consent statement
The patients provided their written informed consent.
Participants
Patients were eligible for participation in the study if they were adults who had been diagnosed with prostate cancer (local and/or advanced disease) and were scheduled to receive a 3-month or 6-month LHRHa formulation, including those requiring neoadjuvant or adjuvant ADT in association with radiotherapy. In addition, patients had to be sufficiently mentally fit to complete a self-administered questionnaire and provide their written informed consent.
Patients were excluded if they had a life expectancy of <12 months, had already been treated with an LHRHa within the previous year from inclusion, were participating in another clinical trial at the time of inclusion or had another severe malignant disease. As this was a non-interventional study, no specific withdrawal criteria were specified.
Endpoints
The primary effectiveness endpoint was the percentage of patients for whom the initial LHRHa prescription (the prescription at baseline) had been renewed at the first follow-up visit (same product and same formulation amongst all patients enrolled with a treatment recorded at baseline).
Secondary endpoints included the percentage of patients for whom the initial LHRHa prescription had been renewed at each visit, the percentage of patients who changed the formulation at each visit and the reasons leading to the switch, and the change in the QLQ-PR25 score compared with baseline and at each visit.
Demographics and baseline characteristics were recorded as follows: age, time since diagnosis of prostate cancer, comorbidities, Gleason score, PSA levels, local or metastatic disease, relapses, concomitant treatments and duration of exposure to LHRHa.
The QLQ-PR25 consists of 30 items and utilizes a 1–4 Likert-type scale (1 = ‘not at all’ to 4 = ‘very much’) to answer items within a question format. These scores are linearly converted and summed into a scaled score ranging from 0 to 100. The items are categorized into 6 scales: 2 functional scales (sexual activity and sexual functioning) and 4 symptom scales (urinary symptoms, use of incontinence aids, bowel symptoms and hormone treatment-related symptoms). A higher score on the functional scales indicates a higher level of functioning, though on the symptom scales, a higher score indicates greater severity of symptoms. A difference of ≥10 on the 0- to 100-point scale is considered a clinically significant difference, and a difference of more than 20 points is considered particularly significant. A difference of 5 points should be considered only as a possible direction of change, that is, improvement or deterioration.24
Statistical analysis
Continuous variables were presented as the number of available observations (n), mean and standard deviation (SD), median and range (minimum, maximum), and 95% confidence intervals (CIs). Categorical (discrete) variables were presented as absolute values and relative values (percentage) and 95% CIs.
It was expected that approximately 75% of patients with prostate cancer would renew treatment with LHRHa at the first follow-up visit. Thus, a sample size of 510 patients would enable 3.96% precision in estimating the proportion for whom the initial LHRHa prescription was renewed with a 95% CI and considering that approximately 10% would not be evaluable owing to premature discontinuation or missing data.
No statistical testing was performed. The analyses were descriptive only. The two-sided 95% CIs of the proportions were calculated (the approximate binomial CI was estimated using the Agresti–Coull method). Statistical analyses were performed using Statistical Analysis System (SAS)® (version 9.4).
Results
Baseline characteristics
A total of 510 patients were screened in the study. The signed informed consent was missing for 13 patients, who were therefore not enrolled. Accordingly, a total of 497 patients were enrolled across 28 sites and included in data collection. Table 1 summarizes the baseline characteristics of these patients according to the LHRHa received, namely triptorelin 3M (n=45), triptorelin 6M (n=430), leuprorelin 3M (n=8), leuprorelin 6M (n=12) and goserelin (n=2). Of note, each investigator used 3M or 6M LHRHa formulation depending on their experience with the different drugs (not driven by hospital formulary) and as per clinical practice.
Table 1.
Baseline characteristics of the study population (n=497).
| Variable, median (IQR) or n (%) | Triptorelin 3M (n=45) | Triptorelin 6M (n=430) | Leuprorelin 3M (n=8) | Leuprorelin 6M (n=12) | Goserelin 3M (n=2) | Total (n=497) |
|---|---|---|---|---|---|---|
| Age, years | 75 (68–81) | 75 (70–80) | 81 (72.5–85.5) | 76.5 (72–83) | 83 (82–84) | 75 (70–80) |
| Time since diagnosis of prostate cancer | 1.93 (0.12–4.55) | 0.18 (0.08–0.53) | 6.52 (1.15–10.90) | 0.11 (0.04–0.42) | 5.40 (0.02–10.77) | 0.19 (0.08–0.79) |
| Without comorbidities | 10 (22.2%) | 139 (32.3%) | 2 (25.0%) | 2 (16.7%) | 0 | 153 (30.8%) |
| Life expectancy >10 yearsa | 10 (22.2%) | 144 (33.5%) | 4 (50.0%) | 1 (8.3%) | 0 | 159 (32.0%) |
| Gleason score at diagnosis | 7 (7–8) | 7 (7–8) | 7 (6–7) | 8 (7–9) | 8 (7–9) | 7 (7–8) |
| PSA at diagnosis, ng/mL | 14.80 (7.00–24.55) | 12.60 (6.90–26.20) | 8.60 (6.50–16.90) | 18.10 (10.50–47.00) | 141.13 (0.05–282.20) | 12.75 (6.90–27.80) |
| Local disease (adjuvant treatment) | 8 (17.8%) | 87 (20.2%) | 1 (12.5%) | 1 (8.3%) | 0 | 97 (19.5%) |
| Local and advanced disease (local treatment) | 11 (24.4%) | 176 (40.9%) | 2 (25.0%) | 3 (25.0%) | 0 | 192 (38.6%) |
| With relapse after local treatment | 12 (26.7%) | 46 (10.7%) | 4 (50.0%) | 1 (8.3%) | 1 (50.0%) | 64 (12.9%) |
| With metastases | 11 (24.4%) | 58 (13.5%) | 1 (12.5%) | 3 (25.0%) | 1 (50.0%) | 74 (14.9%) |
| Immediately metastaticb | 2 (4.4%) | 53 (12.3%) | 0 | 3 (25.0%) | 0 | 58 (11.7%) |
| Concomitant treatments used: | ||||||
| Antiandrogens | 23 (63.9%) | 349 (93.1%) | 4 (50.0%) | 9 (81.8%) | 1 (100%) | 386 (89.6%) |
| Treatment to protect bone density | 8 (22.2%) | 98 (26.1%) | 3 (37.5%) | 4 (36.4%) | 1 (100%) | 114 (26.5%) |
| Radiotherapy | 11 (30.6%) | 142 (37.9%) | 1 (12.5%) | 3 (27.3%) | 1 (100%) | 158 (36.7%) |
| Chemotherapy | 0 | 23 (6.1%) | 0 | 0 | 0 | 23 (5.3%) |
| LHRHa exposure, months (95% CI) | 24.05 (21.00–24.10) | 24.00 (24.00–24.10) | 23.75 (18.10–28.10) | 23.90 (6.00–30.30) | 7.05 (3.00–11.10) | 24.00 (24.00–24.10) |
At the physician’s discretion.
Metastases within 6 months of diagnosis of the primary tumour.
3M, every 3 months; 6M, every 6 months; LHRHa, luteinizing hormone-releasing hormone agonist; PSA, prostate-specific antigen.
Renewal of initial LHRHa prescription at V1 (3 or 6 months)
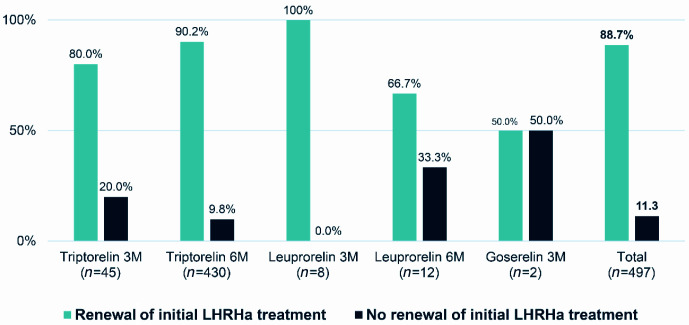
The percentage of patients with a renewed prescription at V1 was 88.7% (441/497). The initial treatment was not renewed at V1 in 11.3% (56/497): 20 patients whose initial treatment was not renewed (7 patients who changed LHRHa product and/or formulation and 13 who stopped their treatment) and 36 for whom no information was available and whose initial treatment was not considered for renewal. Figure 1 details the percentage of patients whose prescription for each LHRHa administered was renewed.
Figure 1.
Renewal of initial LHRHa treatment at the first follow-up visit (3 or 6 months).
3M, every 3 months; 6M, every 6 months; LHRHa, luteinizing hormone-releasing hormone agonist.
A supportive analysis was performed for patients who maintained LHRHa treatment at V1 (n=448) (i.e. 49 patients were excluded: 36 who did not attend V1 and 13 who stopped their treatment). This supportive analysis revealed that the prescription was maintained at V1 in 98.4% (95% CI 96.7–99.3%) of patients.
Renewal of initial LHRHa treatment at each follow-up visit
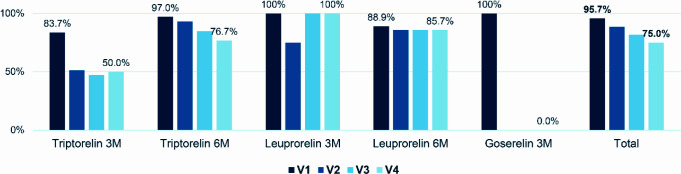
This analysis was performed only on the patients who attended each visit. At V1, 95.7% (441/461) of patients had their initial LHRHa prescription renewed. This percentage decreased to 88.5% (376/425) at V2, 81.6% (315/386) at V3 and 75.0% (270/360) at V4. Figure 2 details the percentage of patients whose LHRHa prescription was renewed at each follow-up visit.
Figure 2.
Renewal of initial LHRHa treatment at each follow-up visit.
The percentages were calculated based on the patients who attended each visit.
3M, every 3 months; 6M, every 6 months; LHRHa, luteinizing hormone-releasing hormone agonist; V1, at 3 or 6 months from the start of treatment; V2, at 12 months from the start of treatment; V3, at 18 months from the start of treatment; V4, at 24 months from the start of treatment.
Switching of treatment and formulation at each follow-up visit and reasons for switching
Very few patients switched treatment and formulation with respect to the preceding visit. Treatment was switched from a 3M to a 6M formulation in 1.1% of patients at V1 (5/448), 1.3% (5/385) at V2 and 0.3% (1/317) at V3; there was no switch at V4. Treatment was switched from a 6M to a 3M formulation in 0.4% (2/448) of patients at V1 and 0.6% (2/317) at V3; there was no switch at V2 or V4. The highest proportion of switching was in the triptorelin 3M group at V1 (versus baseline), where 5 patients (1.1% of the total population) switched from triptorelin 3M to triptorelin 6M. For the other visits and groups, the proportion of patients who switched was even lower.
According to the investigators, the main reason for switching from a 3M formulation to a 6M formulation was ‘simplification of the treatment regimen’ (56.3% (9/16) of patients switching from a 3M to a 6M formulation). According to the patients, the main reason for this switch was that they found it more practical and would receive fewer injections (62.5% (5/8) and 50.0% (4/8) of patients switching from a 3M to a 6M formulation, respectively; Table 2).
Table 2.
Reasons for switching from one formulation to another.
| Reasons leading to switch | n (%) |
|---|---|
| From a 3M to a 6M formulation | |
| Reasons for 6M formulation based on the investigator’s opiniona (n=16) | |
| To avoid unnecessary visits | 5 (31.3%) |
| Simplification of the treatment regimen | 9 (56.3%) |
| More efficacious than the 3M form | 1 (6.3%) |
| Corresponds to the patient’s selection | 6 (37.5%) |
| Criteria for 6M formulation based on the patient’s opiniona (n=8) | |
| The patient finds it more practical | 5 (62.5%) |
| There are fewer injections | 4 (50%) |
| The patient finds it less restrictive | 1 (12.5%) |
| From a 6M to a 3M formulation | |
| Reasons for 3M formulation based on the investigator’s opiniona (n=4) | |
| Better adherence (less likely to forget to take medication) | 1 (25%) |
| More closely supervised patient management | 1 (25%) |
| Preference for sequential treatment | 2 (50%) |
| Reassuring effect for the patient | 2 (25%) |
| Enables the patient to be seen more frequently | 1 (25%) |
| Corresponds to the patient’s selection | 1 (25%) |
| Reasons for 3M formulation based on the patient’s opiniona (n=2) | |
| The patient prefers to see the doctor every 3 months | 2 (100%) |
| The patient prefers to see the nurse every 3 months | 1 (50%) |
| The patient has the impression that the treatment is more efficacious | 1 (50%) |
More than one reason could be chosen.
3M, every 3 months; 6M, every 6 months.
According to the investigators, the main reason for switching from a 6M to a 3M formulation was ‘preference for sequential treatment’ (2 patients switching from a 6M to a 3M formulation). According to these two patients, the main reason for this switch was that they preferred to meet the investigator every 3 months (Table 2).
Criteria for choice of formulation at initiation of hormone treatment, taking into consideration the patients’ characteristics and disease status
The main criteria for choosing a 3M formulation were ‘more closely supervised patient management’, reported in 45.5% of the patients treated with the 3M formulation (n=55), and ‘preference for sequential treatment’, reported in 34.5% (Table 3).
Table 3.
Criteria for choice of formulation at initiation of hormone treatment taking into consideration patient characteristics.
| Criteria for choice of 3M formulation, n (%)a | Triptorelin 3M (n=45) | Leuprorelin 3M (n=8) | Goserelin 3M (n=2) | Total with 3M formulation (n=55) |
|---|---|---|---|---|
| Habit | 5 (11.1%) | 1 (12.5%) | 2 (100%) | 8 (14.5%) |
| Better adherence (less likely to forget to take medication) | 4 (8.9%) | 2 (25.0%) | 0 | 6 (10.9%) |
| Manageability | 8 (17.8%) | 0 | 0 | 8 (14.5%) |
| More efficacious than the 6M form | 0 | 0 | 0 | 0 |
| More closely supervised patient management | 25 (55.6%) | 0 | 0 | 25 (45.5%) |
| Preference for sequential treatment | 13 (28.9%) | 6 (75.0%) | 0 | 19 (34.5%) |
| Reassuring effect for the patient | 1 (2.2%) | 0 | 0 | 1 (1.8%) |
| Enables the patient to be seen more frequently | 2 (4.4%) | 0 | 0 | 2 (3.6%) |
| Corresponds to the patient’s selection | 3 (6.7%) | 0 | 0 | 3 (5.5%) |
| Criteria for choice of 6M formulation, n (%) a | Triptorelin 6M ( n =430) | Leuprorelin 6M ( n =12) | Total with 6M formulation ( n =442) | |
| More efficacious than the 3M form | 3 (0.7%) | 0 | 3 (0.7%) | |
| To avoid unnecessary visits | 165 (38.4%) | 3 (25.0%) | 168 (38.0%) | |
| Innovation: try something new | 1 (0.2%) | 0 | 1 (0.2%) | |
| Simplification of the treatment regimen | 369 (85.5%) | 11 (91.7%) | 380 (86.0%) | |
| Less nursing work | 50 (11.6%) | 0 | 50 (11.3%) | |
| Corresponds to the patient’s selection | 57 (13.3%) | 1 (8.3%) | 58 (13.1%) |
More than 1 reason could be chosen.
3M, every 3 months; 6M, every 6 months.
The main criteria for choosing a 6M formulation were ‘simplification of the treatment regimen’, reported in 86.0% of the patients treated with the 6M formulation (n=442), and ‘to avoid unnecessary visits’, reported in 38.0% (Table 3).
Change in QLQ-PR25 score between baseline and each follow-up visit
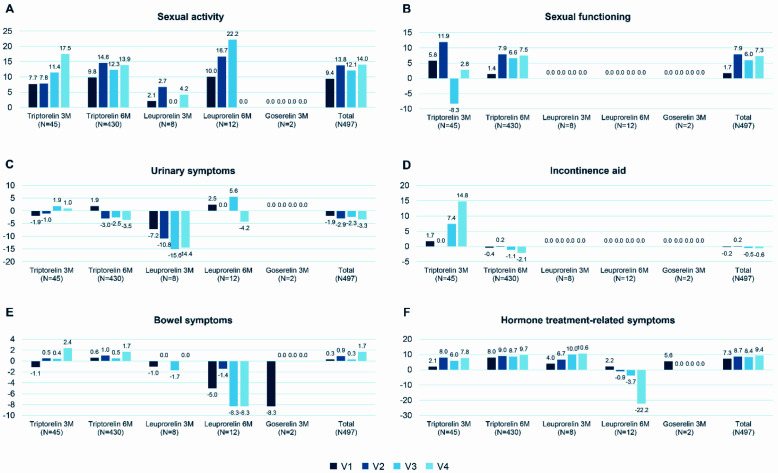
Figure 3 shows the change in the QLQ-PR25 score from baseline to each visit in the six domains assessed: sexual activity, sexual functioning, urinary symptoms, use of incontinence aids, bowel symptoms and hormone treatment-related symptoms.
Figure 3.
Change in QLQ-PR25 score from baseline to each follow-up visit (mean): (A) sexual activity; (B) sexual functioning; (C) urinary symptoms; (D) incontinence aid; (E) bowel symptoms; and (F) hormone treatment-related symptoms.
3M, every 3 months; 6M, every 6 months; LHRHa, luteinizing hormone-releasing hormone agonist; V1, at 3 or 6 months from the start of treatment; V2, at 12 months from the start of treatment; V3, at 18 months from the start of treatment; V4, at 24 months from the start of treatment.
The mean total score for sexual activity at baseline was 77.3 (SD 26.4), which increased at the follow-up visits. The mean total score increased by 9.4 (SD 21.0) at V1, 13.8 (SD 23.4) at V2, 12.1 (SD 25.8) at V3 and 14.0 (SD 26.0) at V4. The extent of the change from V2 is considered clinically relevant, that is, sexual activity improved.
The mean total score for sexual functioning at baseline was 39.0 (SD 26.0), which increased at the follow-up visits. The mean total score increased by 1.7 (SD 23.8) at V1, 7.9 (SD 26.3) at V2, 6.0 (SD 28.5) at V3 and 7.3 (SD 27.6) at V4. The extent of the changes is considered limited, that is, not clinically relevant, though tending towards improvement.
The mean total score for urinary symptoms at baseline was 22.4 (SD 16.6), which decreased at the follow-up visits. The mean change from baseline in total score was −1.9 (SD 14.7) at V1, −2.9 (SD 17.1) at V2, −2.3 (SD 17.8) at V3 and −3.3 (SD 18.3) at V4. The extent of the changes is considered limited, that is, not clinically relevant.
The mean total score for use of incontinence aids at baseline was 10.3 (SD 23.3), which decreased at V1, V3 and V4 (−0.2 (SD 24.9), −0.5 (SD 25.9) and −0.6 (SD 29.7), respectively) and increased by 0.2 (SD: 26.2) at V2. There were no measurements in the leuprorelin 3M/6M and goserelin 3M groups. The extent of the changes is considered limited, that is, not clinically relevant.
The mean total score for bowel symptoms at baseline was 3.5 (SD 7.6), which increased at the follow-up visits. The total score increased by 0.3 (SD 7.8) at V1, 0.9 (SD 10.6) at V2, 0.3 (SD 9.7) at V3 and 1.7 (SD 10.0) at V4. The extent of the changes is considered limited, that is, not clinically relevant.
The mean total score for hormone treatment-related symptoms at baseline was 12.4 (SD 10.1), which increased during the follow-up visits. The mean total score increased by 7.2 (SD 12.7) at V1, 8.7 (SD 14.2) at V2, 8.4 (SD 14.5) at V3 and 9.4 (SD: 14.1) at V4. The extent of the changes is considered clinically relevant, that is, the patients’ experienced increased severity of symptoms related to LHRHa treatment.
Association between investigator and patient satisfaction and progress of PSA
The proportion of investigators satisfied with the treatment at the follow-up visits was 98.3–99.8%. In the same way, the proportion of patients ‘satisfied’ or ‘very satisfied’ with the treatment at the different follow-up visits was 86.2–91.2%. Most patients (at least 94.6% at each follow-up visit) were satisfied with the injection rate. In most cases, the injection was not painful (at least 72.0% at each follow-up visit).
The median PSA at baseline was 10.60 ng/mL (95% CI 9.30–12.90), which decreased at the follow-up visits. The median PSA was 0.20 ng/mL (0.20–0.30) at V1, 0.10 (0.03–0.10) at V2, 0.01 ng/mL (0.00–0.10) at V3 and 0.00 ng/mL (0.00–0.04) at V4. Median PSA decreased in all treatment groups at each follow-up visit compared with baseline.
Considering the association between investigators’ overall satisfaction with the treatment (categories: ‘Yes’ and ‘No’) and the changes in PSA levels, the results revealed a decrease in PSA levels at each follow-up visit (−84.1 ng/mL at V1, −74.1 ng/mL at V2, −93.6 ng/mL at V3 and −55.0 ng/mL at V4) in cases where the investigators were satisfied with the treatment. Considering the association between the patients’ overall satisfaction with treatment (categories: ‘very unsatisfied’, ‘unsatisfied’, ‘no opinion’, ‘satisfied’, ‘very satisfied’) and changes in PSA levels, the results revealed a decrease in PSA levels at each follow-up visit (−116.1 to −78.1 ng/mL at V1, −137.2 to −41.4 ng/mL at V2, −154.6 to 68.5 ng/mL at V3 and −77.3 to −33.0 ng/mL at V4) in patients who were satisfied with the treatment (i.e. answered either ‘satisfied’ or ‘very satisfied’, as their overall satisfaction with treatment).
Intermittent treatment
In total, between 2 and 4 patients stopped the initial LHRHa treatment and later renewed it (intermittent treatment) at each follow-up visit (2 patients in the triptorelin 6M group at V2; 1 in each of the triptorelin 3M and the triptorelin 6M groups at V3; and 2 in each of the triptorelin 3M and the triptorelin 6M groups at V4).
Safety evaluation
A total of 80 adverse events (AEs) were reported by 43 patients during the study: 3 AEs were considered as non-serious and 77 as serious adverse events (SAEs). Amongst the SAEs reported, 4 were assessed as related and 47 led to death, these last concerning 33 patients treated with triptorelin. However, none of the fatal AEs were considered related to treatment. The majority of SAEs by preferred term (i.e. events reported at least two times) were as follows: respiratory failure (8 events); respiratory tract infection and death (4 events each); neoplasm progression (3 events); and respiratory arrest, dizziness, cardiac arrest, cardio-respiratory arrest, and febrile neutropenia (2 events each). Of note, only 30.8% of patients had no comorbidities.
Discussion
This observational, prospective and multicentre study was designed to determine the percentage of patients with prostate cancer whose initial LHRHa prescription was renewed during follow up, how many changed treatment formulation and how their health-related quality of life progressed. The results showed that the initial prescription was renewed in most of the patients evaluated. In addition, changes in formulation were scarce and generally justified or motivated by convenience factors or personal preferences. The retention rate for LHRHa was high, probably because the patients’ good health status remained unchanged throughout the study, leading to a high degree of satisfaction amongst both patients and investigators.
According to Chung et al., sustained release of LHRHa has eased complex treatment regimens by providing patients with more flexibility and convenience, whilst at the same time maintaining adherence and satisfactory ADT.18 The results of their study showed that patients preferred synchronous PSA monitoring with the depot injections and that longer intervals between the depot administrations are preferable owing to perceived needle pain. More than 70% of the patients surveyed would like to receive their depot injections whilst their PSA is being assessed. The authors found that patients who prefer a 6-monthly injection schedule are 3 times more likely to have their PSA checked every 6 months and are 9.8 times more likely to have been diagnosed with prostate cancer more than 5 years previously than those who receive the 3-month injection regimen. The preference for a 6-month interval over a 3-month interval was primarily due to fear of frequent painful injections.18
In the present study, most of the patients continued to receive the initial formulations prescribed. Only 1.1% switched from a 3M to a 6M formulation at V1, and 0.4% switched from a 6M to a 3M formulation at V1. Consistent with previous studies, the main reasons why patients preferred a 6M formulation were that it was more convenient and required fewer injections.18,25 Six-month dosing is usually preferred in patients who are treated with LHRHa for at least 3 years when compared with those who have been treated for less than 12 months.26 On the other hand, patients who chose the 3M formulation argued that they preferred to see their physician or nurse every 3 months. This is consistent with other studies, which reported that a higher level of contact with healthcare providers helps patients to cope with disease and therapy, providing an opportunity to reassure them about safety and efficacy, and has beneficial effects on quality of life.25,26 Although less frequent LHRHa therapy may help to lower the pressure on healthcare systems and may benefit some patients, this ADT cannot be prescribed blindly without a potential effect on patient satisfaction.27 Therefore, the choice of treatment intervals should be decided by both the physician and the patient.
It is important to highlight the baseline characteristics of the study population. Almost 15% had metastases, more than 38% had locally advanced disease and relapse had occurred after local treatment in almost 13%. Nevertheless, the patients adhered to the initially prescribed treatment. In this regard, the study was initiated in 2017, when combined therapy in metastatic hormone-sensitive prostate cancer had not yet been approved. This may have affected the results for the study patients, whose survival is low despite hormone treatment.28 Once the study was completed at 24 months, the patients were no longer followed up; therefore, the survival of those with metastases is unknown.
Although this study only considered differences in dosing regimens, different LHRHa formulations could affect the results obtained and impact clinical practice, for example, ready-to-use implants and microspheres or powder for reconstitution.21 Healthcare providers must be aware of these formulations to ensure that LHRHa therapy is tailored to the patient, taking into consideration their preferences, disease stage and treatment duration.
According to the QLQ-PR25 questionnaire, participants had few symptoms at the start of treatment, a situation that remained stable throughout follow up, with no changes in urinary or bowel symptoms. However, the results revealed a clinically relevant improvement in sexual activity, albeit with a limited change suggesting a possible improvement in sexual functioning. The results recorded for sexual functioning, especially between V1 and V2, might be explained by the lack of data because not all patients responded to this question. Sexual functioning may have worsened between the baseline visit and the following visit and then improved. In addition, the data may only apply to a minority of sexually active patients. Additionally, it is possible that some young patients may have received treatments for erectile dysfunction.
The main limitation of this study lies in its non-interventional observational design. Therefore, no conclusions can be drawn based on the specific LHRHa chosen. Moreover, despite the considerable sample size, it is not possible to draw appropriate conclusions according to the sub-groups because the number of patients in some of them is very small. In addition, the same patient could have received more than one concomitant treatment. Finally, radiotherapy and other concomitant treatments may have affected the results of the QLQ-PR25, specifically with respect to urinary and bowel symptoms.
Conclusion
The initial LHRHa prescription was renewed for most patients, whose health status remained unchanged throughout the 24 months of the study. Likewise, very few patients switched treatment and formulation compared with the initial prescription. This high retention rate for all the treatments coincided with a high degree of satisfaction, and very few changes in formulation were justified or motivated by convenience factors or personal preferences. The safety data captured within this study are as expected in this population with prostate cancer and with a study duration of 4 years. No new and unexpected safety issues arose from this study, which requires further investigation.
Supplementary Information
Acknowledgements
The authors wish to thank Content Ed Net (Madrid) and Fernando Sánchez Barbero PhD for their support in the preparation of this manuscript, and Pippa McIlwaine, PhD, of Oxford PharmaGenesis, Oxford, UK, for providing medical writing support for the plain language summary, which was sponsored by Ipsen in accordance with Good Publication Practice guidelines. In addition, authors would like to thank the following researchers and participating sites in Spain: Miguel Ángel Alonso (Hospital Universitario de León, León); Manuel Carballo (Hospital Álvaro Cunqueiro, Vigo); José Manuel De la Morena (Hospital Universitario Infanta Sofía, San Sebastián de los Reyes); Julio Fernández (Hospital Universitario de Torrejón, Torrejón de Ardoz); Ivone Freitas (Hospital Universitario Doctor Negrín, Las Palmas de Gran Canaria); Guillermo Gómez (Hospital Universitario Virgen de la Arrixaca, El Palmar); Francisco Lozano (Hospital Virgen del Camino, Pamplona); Francisco Javier Madrid (Hospital del Sureste, Arganda del Rey); Luis Martínez (Hospital Universitario La Paz, Madrid); Concepción Masip (Hospital Universitario de Canarias, San Cristóbal de La Laguna); Serenella Monagas (Hospital Universitario San Agustín, Avilés); Juan Moreno (Hospital General Universitario Santa Lucía, Cartagena); Carlos Olivier (Hospital de la Princesa, Madrid); Paula Portela (Hospital Ntra. Señora del Cristal, Ourense); Luis Rodríguez (Hospital Universitario de Cabueñes, Gijón); Ignacio Romero (Hospital Universitario de Getafe, Getafe); José Rosa (Hospital Comarcal Santiago Apóstol, Miranda de Ebro); Manuel Ruibal (Hospital Montecelo, Pontevedra); Miguel Sánchez (Hospital Universitario Rey Juan Carlos, Móstoles).
Footnotes
Contributions: FG-V, VC-A, RPdC, MP-S and A-SG: conception and design of the study, data collection, data analysis and interpretation, critical revision of the article, and final approval of the version to be published. JC-E, VB, AR-A, JB and AG-C: Data collection, critical revision of the article and final approval of the version to be published. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Correct attribution: Copyright © 2024 Calleja-Escudero J, Barrondo V, Rodriguez-Alonso A, Gómez-Veiga F, Bestard J, Gómez-Caamaño A, Grandoulier AS, Pérez-Sampietro M, Chantada-Abal V, Poza de Celis R, on behalf of ANAREN Study Group. https://doi.org/10.7573/dic.2024-2-2. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights, and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Disclosure and potential conflicts of interest: MP-S and A-SG are employees of Ipsen. JB has received honoraries for patient recruitment, follow-up and data collection from Ipsen; honoraries for lectures and presentations from Astellas; economic support for attending congresses and events from Ipsen, Astellas and Recordati. The other authors have no relevant financial or non-financial interests to disclose. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2024/06/dic.2024-2-2-COI.pdf
Funding declaration: The study was funded by Ipsen Pharma S.A.U.
Data availability statement
Where patient data can be anonymized, Ipsen will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to DataSharing@Ipsen.com and will be assessed by a scientific review board. Data are available beginning 6 months and ending 5 years after publication; after this time, only raw data may be available.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med. 2017;167(7):449–455. doi: 10.7326/m16-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19(2):175–181. doi: 10.1007/s10552-007-9083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479–499. doi: 10.1146/annurev-med-051517-011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huggins C. Effect of orchiectomy and irradiation on cancer of the prostate. Ann Surg. 1942;115(6):1192–1200. doi: 10.1097/00000658-194206000-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huggins C. The treatment of cancer of the prostate: the 1943 address in surgery before the Royal College of Physicians and Surgeons of Canada. Can Med Assoc J. 1944;50(4):301–307. [PMC free article] [PubMed] [Google Scholar]
- 7.Perlmutter MA, Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev Urol. 2007;9(Suppl 1):S3–S8. [PMC free article] [PubMed] [Google Scholar]
- 8.Mottet N, van den Bergh RCN, Briers E, et al. [Accessed February 1, 2024];EANM – ESTRO – ESUR – ISUP – SIOG guidelines on prostate cancer. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP_SIOG-Guidelines-on-Prostate-Cancer-2022_2022-04-25-063938_yfos.pdf . [Google Scholar]
- 9.Lowrance WT, Breau RH, Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline part I. J Urol. 2021;205(1):14–21. doi: 10.1097/ju.0000000000001375. [DOI] [PubMed] [Google Scholar]
- 10.Lowrance WT, Breau RH, Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline part II. J Urol. 2021;205(1):22–29. doi: 10.1097/ju.0000000000001376. [DOI] [PubMed] [Google Scholar]
- 11.Parker C, Gillessen S, Heidenreich A, Horwich A ESMO Guidelines Committee. Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v69–v77. doi: 10.1093/annonc/mdv222. [DOI] [PubMed] [Google Scholar]
- 12.Ross RW, Xie W, Regan MM, et al. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate cancer: association between Gleason score, prostate-specific antigen level, and prior ADT exposure with duration of ADT effect. Cancer. 2008;112(6):1247–1253. doi: 10.1002/cncr.23304. [DOI] [PubMed] [Google Scholar]
- 13.Tsai HT, Penson DF, Makambi KH, et al. Efficacy of intermittent androgen deprivation therapy vs conventional continuous androgen deprivation therapy for advanced prostate cancer: a meta-analysis. Urology. 2013;82(2):327–333. doi: 10.1016/j.urology.2013.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadono Y, Yaegashi H, Izumi K, et al. Efficacy of androgen deprivation therapy for localized prostate cancer: analysis of pT0 evaluated by radical prostatectomy specimen. Anticancer Res. 2013;33(3):1147–1151. [PubMed] [Google Scholar]
- 15.Roach M, 3rd, DeSilvio M, Lawton C, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21(10):1904–1911. doi: 10.1200/jco.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Ciezki JP, Klein EA, Angermeier K, et al. A retrospective comparison of androgen deprivation (AD) vs. no AD among low-risk and intermediate-risk prostate cancer patients treated with brachytherapy, external beam radiotherapy, or radical prostatectomy. Int J Radiat Oncol Biol Phys. 2004;60(5):1347–1350. doi: 10.1016/j.ijrobp.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 17.Saad F, Fizazi K. Androgen deprivation therapy and secondary hormone therapy in the management of hormone-sensitive and castration-resistant prostate cancer. Urology. 2015;86(5):852–861. doi: 10.1016/j.urology.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Chung E, Watt H, Glasgow A, et al. Patient rationale in selecting androgen deprivation (PRISAD): do we give patients what they want? Med Oncol. 2009;26(4):420–423. doi: 10.1007/s12032-008-9139-y. [DOI] [PubMed] [Google Scholar]
- 19.Seidenfeld J, Samson DJ, Hasselblad V, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med. 2000;132(7):566–577. doi: 10.7326/0003-4819-132-7-200004040-00009. [DOI] [PubMed] [Google Scholar]
- 20.Labrie F. Medical castration with LHRH agonists: 25 years later with major benefits achieved on survival in prostate cancer. J Androl. 2004;25(3):305–313. doi: 10.1002/j.1939-4640.2004.tb02791.x. [DOI] [PubMed] [Google Scholar]
- 21.Meani D, Solaric M, Visapaa H, et al. Practical differences between luteinizing hormone-releasing hormone agonists in prostate cancer: perspectives across the spectrum of care. Ther Adv Urol. 2018;10(2):51–63. doi: 10.1177/1756287217738985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oderda M, Bertetto O, Barbera G, et al. Appropriateness and complications of androgen deprivation therapy for prostate cancer: can we do better? A retrospective observational analysis from a referral center. Urologia. 2023;90(1):100–108. doi: 10.1177/03915603221149502. [DOI] [PubMed] [Google Scholar]
- 23.Council for International Organizations of Medical Sciences (CIOMS) [Accessed February 1, 2024];International ethical guidelines for epidemiological studies. https://cioms.ch/wp-content/uploads/2017/01/International_Ethical_Guidelines_LR.pdf . [Google Scholar]
- 24.Chu D, Popovic M, Chow E, et al. Development, characteristics and validity of the EORTC QLQ-PR25 and the FACT-P for assessment of quality of life in prostate cancer patients. J Comp Eff Res. 2014;3(5):523–531. doi: 10.2217/cer.14.41. [DOI] [PubMed] [Google Scholar]
- 25.Montorsi F, Tomlinson P. Which luteinising hormone-releasing hormone agonist injection schedule do men with prostate cancer prefer? Results of a European patient survey. Eur Urol. 2015;67(1):177–179. doi: 10.1016/j.eururo.2014.08.055. [DOI] [PubMed] [Google Scholar]
- 26.Lebret T, Bouregba A. Roles of the urologist and nurse from the perspective of patients with prostate cancer receiving luteinizing hormone-releasing hormone analogue therapy. BJU Int. 2008;102(10):1419–1424. doi: 10.1111/j.1464-410x.2008.07785.x. [DOI] [PubMed] [Google Scholar]
- 27.Fode M, Nielsen TK, Al-Hamadani M, et al. Preferred treatment frequency in patients receiving androgen deprivation therapy for advanced prostate cancer. Scand J Urol. 2014;48(2):183–188. doi: 10.3109/21681805.2013.820789. [DOI] [PubMed] [Google Scholar]
- 28.Davis ID. Combination therapy in metastatic hormone-sensitive prostate cancer: is three a crowd? Ther Adv Med Oncol. 2022;14:17588359221086827. doi: 10.1177/17588359221086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Where patient data can be anonymized, Ipsen will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to DataSharing@Ipsen.com and will be assessed by a scientific review board. Data are available beginning 6 months and ending 5 years after publication; after this time, only raw data may be available.