Abstract
Background
Acute exacerbations of chronic obstructive pulmonary disease (COPD) are a major cause of hospital admission and mortality. They contribute to long‐term decline in lung function, physical capacity and quality of life. The most common causes are infective, and treatment includes antibiotics, bronchodilators and systemic corticosteroids as anti‐inflammatory agents.
Objectives
To assess the effects of corticosteroids administered orally or parenterally for treatment of acute exacerbations of COPD, and to compare the efficacy of parenteral versus oral administration.
Search methods
We carried out searches using the Cochrane Airways Group Specialised Register of Trials, MEDLINE and CENTRAL (Cochrane Central Register of Controlled Trials), and checked references of included studies and trials registries. We conducted the last search in May 2014.
Selection criteria
Randomised controlled trials comparing corticosteroids administered orally or parenterally with an appropriate placebo, or comparing oral corticosteroids with parenteral corticosteroids in the treatment of people with acute exacerbations of COPD. Other interventions (e.g. bronchodilators and antibiotics) were standardised for both groups. We excluded clinical studies of acute asthma.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration.
Main results
Sixteen studies (n = 1787) met inclusion criteria for the comparison systemic corticosteroid versus placebo and 13 studies contributed data (n = 1620). Four studies (n = 298) met inclusion criteria for the comparison oral corticosteroid versus parenteral corticosteroid and three studies contributed data (n = 239). The mean age of participants with COPD was 68 years, median proportion of males 82% and mean forced expiratory volume in one second (FEV1) per cent predicted at study admission was 40% (6 studies; n = 633). We judged risk of selection, detection, attrition and reporting bias as low or unclear in all studies. We judged risk of performance bias high in one study comparing systemic corticosteroid with control and in two studies comparing intravenous corticosteroid versus oral corticosteroid.
Systemic corticosteroids reduced the risk of treatment failure by over half compared with placebo in nine studies (n = 917) with median treatment duration 14 days, odds ratio (OR) 0.48 (95% confidence interval (CI) 0.35 to 0.67). The evidence was graded as high quality and it would have been necessary to treat nine people (95% CI 7 to 14) with systemic corticosteroids to avoid one treatment failure. There was moderate‐quality evidence for a lower rate of relapse by one month for treatment with systemic corticosteroid in two studies (n = 415) (hazard ratio (HR) 0.78; 95% CI 0.63 to 0.97). Mortality up to 30 days was not reduced by treatment with systemic corticosteroid compared with control in 12 studies (n = 1319; OR 1.00; 95% CI 0.60 to 1.66).
FEV1, measured up to 72 hours, showed significant treatment benefits (7 studies; n = 649; mean difference (MD) 140 mL; 95% CI 90 to 200); however, this benefit was not observed at later time points. The likelihood of adverse events increased with corticosteroid treatment (OR 2.33; 95% CI 1.59 to 3.43). Overall, one extra adverse effect occurred for every six people treated (95% CI 4 to 10). The risk of hyperglycaemia was significantly increased (OR 2.79; 95% CI 1.86 to 4.19). For general inpatient treatment, duration of hospitalisation was significantly shorter with corticosteroid treatment (MD ‐1.22 days; 95% CI ‐2.26 to ‐0.18), with no difference in length of stay the intensive care unit (ICU) setting.
Comparison of parenteral versus oral treatment showed no significant difference in the primary outcomes of treatment failure, relapse or mortality or for any secondary outcomes. There was a significantly increased rate of hyperglycaemia in one study (OR 4.89; 95% CI 1.20 to 19.94).
Authors' conclusions
There is high‐quality evidence to support treatment of exacerbations of COPD with systemic corticosteroid by the oral or parenteral route in reducing the likelihood of treatment failure and relapse by one month, shortening length of stay in hospital inpatients not requiring assisted ventilation in ICU and giving earlier improvement in lung function and symptoms. There is no evidence of benefit for parenteral treatment compared with oral treatment with corticosteroid on treatment failure, relapse or mortality. There is an increase in adverse drug effects with corticosteroid treatment, which is greater with parenteral administration compared with oral treatment.
Keywords: Female; Humans; Male; Acute Disease; Administration, Oral; Disease Progression; Glucocorticoids; Glucocorticoids/administration & dosage; Glucocorticoids/adverse effects; Infusions, Intravenous; Pulmonary Disease, Chronic Obstructive; Pulmonary Disease, Chronic Obstructive/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Do systemic corticosteroids improve treatment outcomes in flare‐ups of chronic obstructive pulmonary disease?
Why is this question important?
Chronic obstructive pulmonary disease (COPD), also referred to as emphysema or chronic bronchitis, is a long‐term lung condition commonly associated with smoking. People with COPD usually have persistent symptoms of breathlessness and may experience flare‐ups (exacerbations) on occasion, often precipitated by infection, in which symptoms become markedly worse and further medical intervention is required beyond regular treatment by inhalers.
Systemic (i.e. not inhaled corticosteroids) such as prednisolone, prednisone and cortisone, are anti‐inflammatory drugs commonly used in the treatment of exacerbations. We wanted to assess the effectiveness of systemic corticosteroids and whether different routes of administration have impacts on response to treatment of COPD exacerbations.
How did we answer the question?
We looked for all studies that compared corticosteroid, given either by injections (parenterally) or tablets (orally), with matching dummy injections or tablets and all studies that compared corticosteroid given by injections with corticosteroid given by tablets.
What did we find?
We found 16 studies including over 1700 people with COPD who experienced a flare‐up that required additional medical treatment that compared corticosteroid given by injections or tablets with dummy treatment. Four studies with nearly 300 people compared corticosteroid injections with corticosteroid tablets. More men than women took part in the studies and they were usually in their late 60s, with moderately severe symptoms of COPD. Most studies took place in hospitals, two in intensive care units with people who needed breathing support, and three studies involved people who were treated at home. The last search for studies to include in the review was done in May 2014.
There were three studies where people knew which treatment they were getting, but otherwise studies were generally well designed.
People treated with either corticosteroid injections or tablets compared with dummy treatment were less likely to experience treatment failure, 122 fewer people per 1000 treated, with a lower rate of relapse by one month. They had shorter stays in hospital if they did not require assisted ventilation in an intensive care unit, and their lung function and breathlessness improved more quickly during treatment. However, they had more adverse events while taking treatment, especially a temporary increase in glucose levels in blood. Corticosteroid treatment did not reduce the number of people who died within one month of their flare‐up.
In studies comparing two ways of giving corticosteroid, either by injections or tablets, there were no differences in treatment failure, the time in hospital or number of deaths after discharge; however, a temporary increase in glucose levels in blood was more likely with injections than tablets.
Conclusion
There is high‐quality evidence that is unlikely to be changed by future research that people who experience flare‐ups of COPD benefit from treatment with corticosteroid given by injections or tablets with the increased risk of some temporary side effects.
Summary of findings
Summary of findings for the main comparison. Systemic corticosteroid compared with placebo for acute exacerbations of COPD.
| Systemic corticosteroid compared with placebo for acute exacerbations of COPD | ||||||
|
Patient or population: acute exacerbations COPD
Settings: outpatient, inpatient and people in ICU Intervention: systemic corticosteroid Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic corticosteroid | ||||||
| Treatment failure Need to intensify therapy/ED or hospital admission Follow‐up: 3‐30 days | 276 per 1000 | 154 per 1000 (118 to 203) | OR 0.48 (0.35 to 0.67) | 917 (9 studies) | ⊕⊕⊕⊕ high | ‐ |
| Relapse Treatment for AE of COPD or hospital re‐admission Follow‐up: 1‐4 months | 215 per 1000 | 174 per 1000 (122 to 242) | OR 0.77 (0.51 to 1.17) | 596 (5 studies) | ⊕⊕⊕⊝ moderate1 | In 2 studies (n = 415) relapse to 1 month was lower with systemic corticosteroid compared with placebo (HR 0.78; 95% CI 0.63 to 0.97 |
| Improvement in lung function ‐ early effect FEV1 (L) as absolute or change Follow‐up: 3 days | The mean FEV1 in control groups ranged from 0.77 to 0.91 L | The mean early improvement in lung function in the intervention group was 0.14 L higher (0.09 to 0.20 higher) | ‐ | 649 (7 studies) | ⊕⊕⊕⊕ high | ‐ |
| Decreased breathlessness ‐ early effect Borg scale or VAS Follow‐up: 3 days | The mean change in breathlessness in control group was 1.8 units using the Borg scale and 1.5 units on the VAS scale | The mean early decrease in breathlessness in the intervention group was 0.35 standard deviations higher (0.05 to 0.64 higher) | ‐ | 178 (3 studies) | ⊕⊕⊕⊝ moderate2 | Effect size on Borg scale 0.93 units; 95% CI 0.18 to 1.7 (MCID = 2); effect size on VAS scale 5.24; 95% CI 0.75 to 9.59 (MCID = 10). |
| Adverse drug effect Follow‐up: 2‐26 weeks | 285 per 1000 | 481 per 1000 (388 to 577) | OR 2.33 (1.59 to 3.43) | 736 (8 studies) | ⊕⊕⊕⊕ high | ‐ |
| Hyperglycaemia | 124 per 1000 | 282 per 1000 (208 to 371) | OR 2.79 (1.86 to 4.19) | 804 (6 studies) | ⊕⊕⊕⊕ high | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: acute exacerbation; CI: confidence interval; COPD: chronic obstructive pulmonary disease; ED: emergency department; FEV1: forced expiratory volume in 1 second; HR: hazard ratio; MCID: minimum clinically important difference; OR: odds ratio; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Wide CIs include significant benefit and harm (‐1 for imprecision). 2 Upper or lower CI of effect size crosses 0.5 (‐1 for imprecision).
Summary of findings 2. Treatment route: intravenous corticosteroid compared with oral corticosteroid for acute exacerbations of COPD.
| Intravenous corticosteroid compared with oral corticosteroid for acute exacerbations of COPD | ||||||
| Patient or population: acute exacerbations of COPD Settings: inpatient Intervention: intravenous corticosteroid Comparison: oral corticosteroid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral corticosteroid | Intravenous corticosteroid | |||||
| Treatment failure Need to intensify therapy Follow‐up: 7‐14 days | 162 per 1000 | 115 per 1000 (62 to 201) | OR 0.67 (0.34 to 1.3) | 298 (3 studies) | ⊕⊕⊕⊝ moderate1 | ‐ |
| Relapse Hospital readmission for COPD Follow‐up: 4‐12 weeks | 155 per 1000 | 149 per 1000 (84 to 249) | OR 0.95 (0.5 to 1.8) | 298 (3 studies) | ⊕⊕⊕⊝ moderate1 | ‐ |
| Breathlessness ‐ early effect VAS 0‐10. Scale from: 0 to 10. Follow‐up: 3 days | The early breathlessness VAS in control groups ranged from mean 4.4 to 7.5 units | The early mean breathlessness in the intravenous corticosteroid group was 0.62 higher (0.55 lower to 1.78 higher) | ‐ | 75 (2 studies) | ⊕⊕⊝⊝ low1,2 | ‐ |
| Mortality after discharge (1‐3 months) Follow‐up: 1‐3 months | 27 per 1000 | 37 per 1000 (12 to 111) | OR 1.4 (0.44 to 4.51) | 298 (3 studies) | ⊕⊕⊕⊝ moderate1,3 | ‐ |
| Duration of hospitalisation Days Follow‐up: mean 14 days | Mean duration in the control group ranged from 10.6 to 11.2 days | The mean duration of hospitalisation in the intravenous corticosteroid group was 1.54 longer (0.09 lower to 3.17 higher) | ‐ | 298 (3 studies) | ⊕⊕⊝⊝ low1,2 | ‐ |
| Adverse drug effect ‐ hyperglycaemia Follow‐up: mean 12 days | 200 per 1000 | 550 per 1000 (231 to 833) | OR 4.89 (1.2 to 19.94) | 40 (1 study) | ⊕⊕⊕⊝ moderate4 | ‐ |
| PaO2 mmHg Follow‐up: 7 days | The mean PaO2 in the control group was 66.5 mmHg | The mean PaO2 in the intravenous corticosteroid group was 1.2 mmHg lower (8.61 lower to 6.21 higher) | ‐ | 38 (1 study) | ⊕⊕⊝⊝ low1,4,5 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease; OR: odds ratio; PaO2: partial pressure of oxygen dissolved in arterial blood; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Wide CIs include significant benefit and harm (‐1 for imprecision). 2 Participants and physicians not blinded to treatment in 2 studies (‐1 for risk of bias). 3 Participants and physicians not blinded to treatment in 2 studies; however, the risk of bias for the event mortality was considered to be low. 4 Single inpatient study (‐1 indirectness). 5 Participants and physicians not blinded to treatment; however, the risk of bias for the outcome measurement was considered to be low.
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is characterised by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to noxious particles or gases (GOLD 2013). A diagnosis of COPD is considered on a clinical basis in the presence of symptoms such as dyspnoea, chronic cough or sputum production and exposure to known risk factors. Confirmation of COPD diagnosis is based on demonstration of persistent airflow limitation with spirometry, according to the criterion post‐bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio less than 0.7, as specified in guidelines including GOLD: Global Strategy for Diagnosis, Management, and Prevention of COPD (GOLD 2013).
COPD is an important and increasing cause of mortality, estimated to be the fifth leading cause of death in 2000, responsible for 2.75 million deaths (Lopez 2006). Worldwide mortality due to COPD is projected to rise to 4.5 million deaths in 2020, and become the third leading cause (Murray 1997). Morbidity is high and worldwide in 2000 COPD resulted in 16.5 million years of life lost, almost 10 million years lived with disability and 26.5 million disability‐adjusted life years (Lopez 2006).
COPD prevalence measured in a worldwide study to estimate the Burden of Lung Disease (BOLD) showed the prevalence of stage II or higher severity (FEV1 less than 80% predicted) is 10.1% (standard error (SE) 4.8%) overall, 11.8% (SE 7.9%) for men and 8.5% (SE 5.8%) for women (Buist 2007). International variation in prevalence and severity stage of COPD is partially explained by variation in smoking prevalence and other risk factors (Buist 2007).
Exacerbations and co‐morbidities contribute to the varying natural history of COPD in individual people (GOLD 2013). Exacerbations contribute to long‐term decline in lung function (Donaldson 2002), and reduced physical activity (Donaldson 2005), and are associated with increased risk of death (Soler‐Cataluna 2005). They also have a profound and long‐lasting effect on quality of life (QoL) (Seemungal 1998; Groenewegen 2001; Wilkinson 2004); in 10% of exacerbations, pre‐exacerbation QoL was not recovered after three months (Seemungal 2000).
COPD exacerbations may require hospitalisation, although exacerbations with less severe symptoms and signs are often managed as an outpatient (NICE 2010). Hospital‐at‐home, or early discharge services if available, may be used as an alternative way of caring for people with exacerbations of COPD who would otherwise need to be admitted or stay in hospital (Jeppesen 2012; NICE 2010). The treatment of exacerbations is a large contributor to the economic burden of COPD (Sullivan 2000; Schermer 2002), with a high proportion of costs being due to hospitalisations (Crockett 2001; Oostenbrink 2004).
Studies on the frequency of exacerbations usually use an 'event‐based' definition based on healthcare utilisation (Effing 2009), and different events may be a proxy for severity, with unscheduled clinic or emergency department visits rated 'moderate' and those requiring hospitalisation labelled 'severe' (Rodriguez‐Roisin 2000). However, the clinical onset of an acute exacerbation is defined according to symptoms, although there is no universally agreed definition (Rodriguez‐Roisin 2000). Type 1 exacerbations were defined by Anthonisen on the basis of three major symptoms; increased dyspnoea, sputum volume and sputum purulence; Type 2 exacerbations had only two of the major symptoms and Type 3 exacerbations had one major symptom plus cough, wheeze or symptoms of an upper respiratory tract infection (Anthonisen 1987). A later definition required an increase in two of the 'major symptoms' of dyspnoea, sputum volume or sputum purulence, or one major symptom with an increase in one 'minor symptom' for two days (wheeze, sore throat, cough or common cold symptoms) (Seemungal 2000). More recently, a standardised measure for assessing the frequency, severity and duration of exacerbations of COPD using participant‐reported outcomes has been developed for use in clinical studies (Leidy 2010).
COPD exacerbations can be precipitated by several factors, the most common causes being infective, with bacterial pathogens identified in just over 50% and viral causes in around 25% of people (Sherk 2000). Non‐infective causes such as air pollution and other environmental conditions that increase airway inflammation may account for 15% to 20% of exacerbations (Sethi 2008). Exacerbations become more frequent and more severe as the severity of COPD increases (Suissa 2012), although the rate at which they occur may reflect an independent susceptibility phenotype, the 'frequent exacerbator' (Hurst 2010).
Description of the intervention
The acute inflammatory response to airway infection is influenced by both pathogenic and host factors, resulting in increased airway and systemic inflammation (Sethi 2008). Airway inflammation is significantly increased during exacerbations of COPD, with evidence of increased neutrophils, lymphocytes and eosinophils seen in airways and in sputum (Papi 2006; Bathoorn 2008; Falk 2008). Systemic inflammation is also present in COPD; many circulating inflammatory mediators are elevated both in stable COPD and during exacerbations. C‐reactive protein is a known marker of systemic inflammation whose levels are elevated during exacerbations and it is a likely participant in the inflammatory cascade (Falk 2008).
How the intervention might work
Theoretical mechanisms for clinical improvement in lung function in people treated with corticosteroids during exacerbations may include reduction in airway inflammation or a decrease in airway oedema (Wedzicha 2000).
Corticosteroid use may be associated with a number of adverse effects, including fluid retention, hypertension, diabetes mellitus, adrenal suppression, osteoporosis and increased fracture risk (Vestergaard 2007). Although the risks are greater with longer‐term use than short‐term use (Henzen 2000), their benefits in the management of acute COPD exacerbations must be balanced against adverse effects (McEvoy 1997).
Why it is important to do this review
Previous versions of this systematic review showed beneficial effects of treatment with systemic corticosteroids in acute exacerbations of COPD on treatment failure (Wood‐Baker 2001; Walters 2009), and reduced length of hospital stay, reduced dyspnoea, improved oxygen saturation and improved lung function measures (Walters 2009). The findings of these systematic reviews (based on searches of the literature to 2008) are reflected in current guidelines for the treatment of acute exacerbations of COPD that recommend the use of systemic corticosteroids (GOLD 2013), both within and outside hospital (NICE 2010).
Long‐term follow‐up of a population‐based inception cohort of people with COPD suggested the need to investigate the effects of systemic corticosteroids in severe exacerbations requiring non‐invasive ventilation (Suissa 2012). However, in previous versions of this systematic review, studies in which participants received assisted ventilation for a severe exacerbation were excluded and we wish to update the evidence and extend the review to include the setting of assisted ventilation. With the finding of increased risk of subsequent exacerbations following first hospitalisation (Suissa 2012), and observation of higher individual risk of exacerbations in frequent exacerbators (Hurst 2010), we wished to include subgroup analysis by frequency and severity of exacerbations in this version of the review.
Objectives
To assess the effects of corticosteroids administered orally or parenterally for treatment of acute exacerbations of COPD, and to compare the efficacy of parenteral versus oral administration.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in which treatment with oral or parenteral corticosteroids was compared with appropriate placebo, or in which treatment with oral corticosteroids was compared with parenteral corticosteroid for acute exacerbations of COPD.
Types of participants
We included studies that recruited people with a guideline‐typical clinical diagnosis of COPD of any severity (GOLD 2013), based on persistent (post‐bronchodilator) airflow limitation, confirmed for example by FEV1/FVC ratio less than 0.7 and history of exposure to risk factors (tobacco smoke, smoke from home cooking and heating fuels, occupational dusts and chemicals).
Participants must have experienced an acute functional deterioration, thus allowing a wide definition of an acute exacerbation, which could include any combination of increased breathlessness or sputum volume, sputum purulence, cough or wheeze and symptoms or overt respiratory tract infection. Participants could be treated in primary care, or hospital secondary care, including when requiring assisted ventilation. We excluded trials of people with acute asthma.
Types of interventions
We included studies comparing:
corticosteroid, administered either parenterally or orally with placebo‐control injections or tablets as appropriate;
oral corticosteroid with parenteral corticosteroid.
We permitted other non‐corticosteroid co‐interventions (e.g. bronchodilators and antibiotics) as long as they were not part of the randomised treatments.
Types of outcome measures
We divided outcome data into early (defined as up to and including 72 hours from study entry) and late (occurring after 72 hours and up to the last available measurement during study treatment) time points.
Primary outcomes
Treatment failure: defined as necessity to intensify pharmacological treatment, hospital admission during outpatient treatment or return to emergency department during outpatient treatment.
Relapse: defined as treatment or hospital admission for a COPD exacerbation after completion of study treatment.
Mortality.
Secondary outcomes
Adverse drug effects.
Arterial blood gas (ABG) measurements (partial pressure of oxygen dissolved in arterial blood (PaO2) and partial pressure of carbon dioxide dissolved in arterial blood (PaCO2)).
Symptom scores: measuring breathlessness, symptoms of cough, wheeze, sputum production ; preferably using validated scales.
Lung function, pre‐ and post‐bronchodilator including FEV1, FVC), peak expiratory flow (PEF).
Health status: QoL assessments using validated scales.
Physical capacity: timed walking tests, endurance tests,
Duration of hospitalisation.
Duration of assisted ventilation.
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (see the Airways Group Module for further details). We searched all records in the Specialised Register coded as 'COPD' (Airways Trials Search Co‐ordinator) most recently in June 2013 using the following terms:
"adrenal cortex hormone*" or steroid* or glucocorticoid* or corticoid* or corticosteroid* or beclomethasone or betamethasone or fluticasone or cortisone or dexamethasone or hydrocortisone or prednisolone or prednisone or methylprednisolone or methylprednisone or triamcinolone.
We performed the most recent search (DT/CW) of MEDLINE for the period 12 months from June 2012 (Appendix 1) and of EMBASE for the period 2010 to June 2013 (Appendix 2).
We ran previous searches of these databases for the review published in 1999, and updated versions in 2004 and in 2007.
Searching other resources
In addition, we searched the bibliographies of each RCT and any review articles identified for additional papers. We searched the registers of ongoing clinical trials ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/).
Data collection and analysis
Selection of studies
At least two review authors (DT, CW) assessed all potentially relevant trials for relevance. We screened the full text to independently select trials for inclusion and identify and record reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third person (JW). We identified and excluded duplicates and collated multiple reports of the same study so that each study (rather than each report) was the unit of interest in the review. We recorded the selection process as a PRISMA flow diagram (Figure 1).
1.
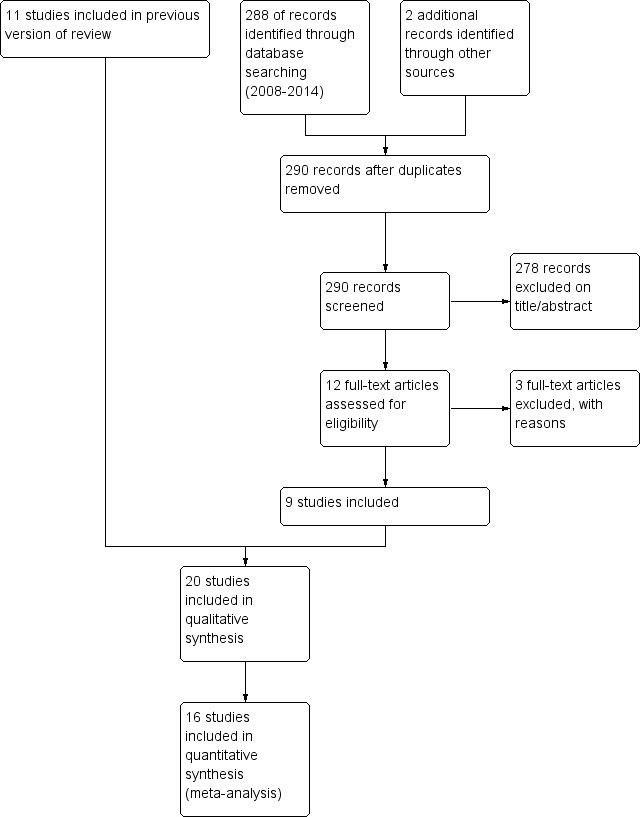
Study flow diagram for 2008‐2014 literature searches.
Data extraction and management
We used a data collection form for study characteristics and outcome data. Two review authors (DT, CW) independently extracted the following study characteristics from included studies.
Methods: study design, total duration of study, number of study centres and location, study setting, withdrawals and date of study.
Participants: n, mean age, age range, gender, diagnostic criteria for exacerbation, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: study treatment, comparison, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (two of JW, DT, CW) independently extracted outcome data from included studies. We entered data into the Review Manager 5 (JW, DT, CW) and a second review author double‐checked entries. We checked that data were entered correctly by comparing the data presented in the systematic review with the study reports.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study (two of JW, CW, DT) using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias(es).
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
In addition, we assessed each study for the basis of the diagnosis of COPD using the following criteria.
Was the age of participants over 45 years?
Was the smoking history greater than 10 pack‐years?
Were participants with a previous physician diagnosis of asthma excluded?
Was there evidence of fixed airflow obstruction?
Measures of treatment effect
For continuous variables, we analysed data as mean difference (MD), with 95% confidence interval (CI). We used standardised mean difference (SMD) with 95% CI if different scales of measurement had been used for an outcome. The SMD is a statistic that expresses the difference in means between treatment groups in units of the pooled standard deviation (SD). We analysed dichotomous outcomes using Mantel‐Haenszel odds ratio (OR) with a 95% CI. Where events were rare, we employed the Peto OR. We entered scale data with a consistent direction of effect.
We undertook meta‐analyses only where it was meaningful; when treatments, participants and the underlying clinical question were similar.
When skewed data were available (reported as medians and interquartile ranges), we described them narratively.
For 'time‐to‐event' outcomes such as log hazard ratios (HR), we used the fixed‐effect generic inverse variance outcome to combine results. This method gives a weighted mean of the effect estimates of separate studies (Higgins 2011). We calculated number needed to treat for an additional beneficial outcome (NNTB) from the pooled OR and its CI using the baseline risk in the control group.
Unit of analysis issues
We analysed dichotomous data using participants as the unit of analysis. For continuous data, the MD based on change from baseline was preferred over MD based on absolute when both were available.
Dealing with missing data
We contacted investigators to obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results using a sensitivity analysis.
Assessment of heterogeneity
We carried out an assessment of possible heterogeneity, where the null hypothesis is that all studies are evaluating the same effect, for pooled effects using a Breslow‐Day test of heterogeneity; a P value > 0.05 was considered to indicate a significant difference between studies. In addition, we used the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance (Higgins 2003). Interpretation of statistical heterogeneity was as follows: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity (Higgins 2011).
We assessed clinical and methodological heterogeneity by recording differences in study design and participant characteristics between individual studies. When we found substantial heterogeneity, we reported it and explored possible causes by pre‐specified subgroup analysis.
Assessment of reporting biases
We tried to minimise reporting bias from non‐publication of studies or selective outcome reporting by using a broad search strategy, checking references of included studies and relevant systematic reviews, and contacting authors for additional outcome data. We visually inspected funnel plots when 10 or more studies contributed to analysis for an outcome.
Data synthesis
We used a fixed‐effect model, and, in addition, we performed a sensitivity analysis with a random‐effects model if there was unexplained heterogeneity. We presented the findings of our primary outcomes in a 'Summary of findings' table according to recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (generated with the use of GRADEPRO software).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were performed by:
duration of corticosteroid treatment (number of days' treatment is reported in the user‐defined order in analysis tables);
setting (i.e. primary care, hospital secondary care or requiring assisted ventilation).
Analysis by frequency and severity of exacerbations was not possible owing to lack of data in studies.
Sensitivity analysis
In the assessment of heterogeneity, we considered possible causes arising from details of study design. We performed sensitivity analyses using random‐effects models versus fixed‐effect models, by risk of bias and by other potential confounders.
Results
Description of studies
Results of the search
We carried out an initial search in 1996, using the Cochrane Airways Group COPD RCT register and MEDLINE; the search retrieved 108 references and we subsequently identified 85 papers as suitable for consideration in the review, with eight RCTs included in the original review. We located two additional studies in searches up to August 2004 and included them in the 2005 update of the review (Maltais 2002; Aaron 2003). In the 2009 review update, from searches to August 2008, we identified 10 studies for potential inclusion of which Chen 2005 was included.
For this review update in 2014, we undertook searches on 28 June 2013 and 23 May 2014 (see Figure 1). The 2003 search yielded 140 and the 2014 search yielded 148 references after duplicates were removed and we identified two studies for the new comparison of route of treatment from those previously excluded. We excluded 278 references on title or abstract. We excluded three references as they did not randomise participants to corticosteroid treatment or did not use placebo control (Roede 2008; Wang 2011; Bafadhel 2012). We included five additional studies comparing systemic corticosteroids with placebo (Cordero 1996; Gunen 2007; Alia 2011: Zheng 2011; Abroug 2014). We included two studies comparing oral and parenteral corticosteroids (Ridha 2006; Ceviker 2014), with two studies for this treatment comparison that had previously been excluded now included (Willaert 2002; de Jong 2007).
Included studies
See Characteristics of included studies table for details.
For the comparison systemic corticosteroid versus placebo, we included 16 studies in the review. Four were published in abstract form only (Rostom 1994; Cordero 1996; Wood‐Baker 1998; Zheng 2011). Three studies contained no data that could be included and no responses to requests for data have been received (Rostom 1994; Cordero 1996; Zheng 2011). Unpublished data were available for Wood‐Baker 1998 and data were sought and supplied by the authors for Chen 2005. Authors of eight published studies supplied additional data (Emerman 1989; Bullard 1996; Thompson 1996; Davies 1999; Niewoehner 1999; Maltais 2002; Aaron 2003; Alia 2011). Thus, 13 studies contribute some outcome data for this comparison.
For the comparison parenteral corticosteroid versus oral corticosteroid, we included three published studies in the review that contributed outcome data (de Jong 2007; Willaert 2002; Ceviker 2014), while one study was only published as an abstract and no response has been received to a request for data for inclusion in the review (Ridha 2006). We requested additional data for Ceviker 2014, but we received no response.
Table 3 summarises across the 20 studies: setting; exclusion of people with asthma, prior inhaled corticosteroid use, exacerbation definition, duration of study treatment, participants per cent male, mean age, FEV1, pack‐years' smoking history, number of withdrawals.
1. Summary of study characteristics.
| Study ID | Setting/exacerbation definition | n (% male) | Systemic corticosteroid (treatment duration) | Age mean (SD) | FEV1 mean (SD) | PYH smoking mean (SD) | Withdrawals (n/n) | History asthma excluded | Previous ICS use |
| Systemic corticosteroid vs. placebo | |||||||||
| Aaron 2003 | Outpatient/AO criteria used | 147 (57%) | Oral prednisone 40 mg (10 days) | 69.4 (10.5) | 1.0 (0.5) | 50 (38) | 3/147 | Yes | 52% |
| Abroug 2014 | ICU/AO criteria used | 217 (88%) | Prednisone 1 mg/kg daily until discharge or maximum 10 days | 69 (9) | 0.8 (0.4) | Not known | 0/217 | Not known | Not known |
| Albert 1980 | Inpatient/AO criteria not used | 45 (100%) | IV methylprednisolone 0.5 mg/kg 4 hourly x 72 hours (3 days) | 61.5 (9) | 0.8 (0.3) | 80 (30) | 3/45 | Yes | Not known |
| Alia 2011 | ICU/AO criteria not used | 83 (80%) | IV methylprednisolone 0.5 mg/kg every 6 hours for first 72 hours, 0.5 mg/kg 12 hourly days 4‐6, and 0.5 mg/kg days 7‐10 | 68.4 (10.2) | Not known | Not known | 0/83 | Yes | Not known |
| Bullard 1996 | ED‐inpatient 76%/AO criteria used | 138 (86%) | IV hydrocortisone x 96 hours + 4 days oral prednisone 40 mg (5‐8 days) | 66 (10.8) | 0.53 (0.53) | Not known | 27/138 | Not known | Not known |
| Chen 2005 | Inpatient/AO criteria not used | 130 (75%) | 1. Prednisolone 30 mg/day 7 days + placebo 7 days (7 days) vs. 2. prednisolone 30 mg/day 10 days + 15 mg/day 5 days vs. 3. placebo 14 days (14 days) (data from group 2 used) | 72 (6.7) | 0.73 (0.25) | Not known | 9/130 | Not known | Not known |
| Cordero 1996 [abstract only] | Outpatient/AO criteria used | 30 (100%) | Oral prednisolone 40 mg/day for 10 days | Not known | Not known | Not known | Not known | Yes | Not known |
| Davies 1999 | Inpatient/AO criteria used | 60 (68%) | Oral prednisone 30 mg/day (14 days) | 67 (8.5) | 1.7 | 55 (35) | 10/60 | Yes | 80% |
| Emerman 1989 | ED‐inpatient 63%/AO criteria used | 100 (52%) | IV methylprednisolone 100 mg single dose (1 day) | 64 (7.8) | 64% (35) | 59 (50) | 4/100 | Yes | Not known |
| Gunen 2007 | Inpatient/AO criteria not used | 159 (85%) | IV prednisolone 40 mg/day for days 1‐15 if not discharged, oral methylprednisolone 32 mg/day for days 11‐15 if discharged | 64.1 (9) | 37.2% (12.2) | 45 (20.8) | 38/159 | Not known | Not known |
| Maltais 2002 | Inpatient/AO criteria used | 199 (82%) | Oral prednisone 40 mg x 3 days then 30 mg/day x 7 days (10 days) | 70 (8) | 0.91 (0.4) | 56 (27) | 28/199 | Yes | 59% |
| Niewoehner 1999 | Inpatient/AO criteria not used | 271 (99%) | IV methylprednisolone 72 hours + oral prednisolone 60 mg/day tapering over 57 days (group 1) or 12 days (group 2) or IV placebo + oral placebo 57 days (group 3) (15 or 60 days) | 67.4 (10) | 0.76 (0.27) | 70 (33) | 20/191 | Yes | 45% |
| Rostom 1994 [abstract only] | Inpatient/AO criteria not used | 30 (not known) | IV methyl prednisolone 72 hours + oral prednisolone 15 days (19 days) | Not known | Not known | Not known | Not known | Not known | Not known |
| Thompson 1996 | Outpatient/AO criteria specified | 27 (96%) | Oral prednisone 60 mg tapering 9 days (9 days) | 67.5 (8) | 1.35 (0.5) | 65 (30) | 0 | Yes | 30% |
| Wood‐Baker 1998 [abstract and data supplied] | Inpatient/AO criteria not used | 47 (64%) | Oral prednisone high dose 2.5 mg/kg/day x 3 days OR medium dose 0.6‐0.3 mg/kg/day x 14 days (14 days or 3 day high dose) | 72 (6.3) | 0.6 | > 10 | 3/38 | Yes | Not known |
| Zheng 2011 [abstract only] | Inpatient/not known | 107 (not known) | Group 2 methylprednisolone 40 mg, IV and nebulised normal saline 4 mL 6 hourly x 7 days Group 3 (placebo): nebulised normal saline 4 mL and normal saline 10 mL IV 6 hourly x 7 days |
Not known | Not known | Not known | Not known/107 | Not known | Not known |
| IV corticosteroids vs. oral corticosteroids | |||||||||
| Ceviker 2014 | Inpatient/AO criteria not used | 40 (not known) | 1. Oral methylprednisolone 32 mg/day 2. IV methylprednisolone 1 mg/kg/day for 4 days, then 0.5 mg/kg/day for 3 days |
68 (9.4) | 1.03 (0.37) | 63 (38.7) | 2/40 | Not known | Not known |
| de Jong 2007 | Inpatient/AO criteria used | 157 (75%) | 1. IV prednisolone 60 mg + placebo 5 days then tapering oral prednisolone 30 mg up to day 12 vs. 2. oral prednisolone 60 mg + placebo 5 days then tapering oral prednisolone 30 mg up to day 12 |
71 (8.4) | 1.0 (0.4) | 38 (21) | 17/210 | Yes | 85% |
| Ridha 2006 [abstract only] | Not known/not known | 52 (not known) | 1. Oral prednisone 40 mg/day for 10 days vs. 2. IV hydrocortisone 400 mg/day for 10 days |
Not known | Not known | Not known | Not known | Not known | Not known |
| Willaert 2002 | Inpatient/AO criteria not used | 42 (87.5%) | 1. IV methylprednisolone 40 mg/day days 1‐10, decreased to 20 mg/day then to oral treatment 4 mg for 4 days vs. 2. oral methylprednisolone 32 mg/day days 1‐7, decreased to 24 mg/day for days 8‐11, then decrease in dosage by 4 mg/week, 14 days total |
71.5 (7) | 1.12 (0.47) | 31.7 (17.5) | 11/48 | Yes | Not known |
AO: airflow obstruction; ED: emergency department; FEV1: forced expiratory volume in 1 second; ICU: intensive care unit; IV: intravenous; PYH: pack‐years' history; SD: standard deviation.
The setting for participant recruitment and intervention delivery was hospital inpatients in 12 studies (Albert 1980; Rostom 1994; Wood‐Baker 1998; Davies 1999; Niewoehner 1999; Maltais 2002; Willaert 2002Chen 2005; de Jong 2007; Gunen 2007; Zheng 2011; Ceviker 2014), hospital intensive care units (ICU) in two studies (Alia 2011; Abroug 2014), and, in two studies, the intervention was initiated in the emergency department with subsequent admission only if required clinically (Emerman 1989; Bullard 1996). In Bullard 1996, participants were kept in the emergency department for six hours, and 26 of 113 participants were discharged within 24 hours. In Emerman 1989, a single intravenous infusion of methylprednisolone was given in the emergency department and participants were observed over a minimum of four hours. Subsequently 30 of 96 participants required admission and for some participants allocation to treatment group was not maintained after re‐admission. Three studies recruited outpatients (Cordero 1996; Thompson 1996; Aaron 2003), and the setting was not specified for Ridha 2006.
We attempted to verify the diagnosis of COPD using the criteria age of people over 40 years, smoking history greater than 10 pack‐years, exclusion of people with a previous physician diagnosis of asthma and evidence of fixed airflow obstruction for all studies (see notes in Characteristics of included studies). The important criterion of irreversible airflow obstruction determined by spirometry was specified in 12 studies (Bullard 1996; Cordero 1996; Thompson 1996; Wood‐Baker 1998; Davies 1999; Niewoehner 1999; Maltais 2002; Aaron 2003; de Jong 2007; Gunen 2007; Alia 2011; Abroug 2014). Where specified, the mean length of smoking history ranged from 32 to 80 pack‐years. The demographics of participants in these studies, particularly age, smoking history and severity of airflow obstruction suggests low likelihood of contamination with people with asthma, although this cannot be verified in Rostom 1994; Bullard 1996; Chen 2005; Ridha 2006; Gunen 2007; Zheng 2011; and Ceviker 2014, as they made no reference to the exclusion of people with asthma or the smoking history of participants. Of 2078 participants randomised to treatment in the 20 included studies, the mean age was 68 years and median proportion of males included was 82% (range 52% to 100%). For six studies reporting lung function, the mean FEV1 per cent predicted at study admission was 40% (range 27% to 64%).
Definition of an exacerbation of chronic obstructive pulmonary disease
In 11 studies, some combination of worsening symptoms was specified, including dyspnoea or cough, or increase in sputum volume or purulence. Niewoehner 1999 and Aaron 2003 used a clinical diagnosis of an exacerbation of COPD without specifying the criteria. Albert 1980; Emerman 1989; Bullard 1996; and Ceviker 2014 specified acute respiratory insufficiency as an inclusion criterion.
Interventions
For studies included in the comparison systemic corticosteroid versus placebo, systemic corticosteroid treatment varied. Two studies used only short intravenous courses (less than four days' length) (Albert 1980; Emerman 1989), four studies used intravenous administration followed by oral treatment (Rostom 1994; Bullard 1996; Niewoehner 1999; Gunen 2007), and two studies used intravenous administration (Alia 2011; Zheng 2011). Among the seven studies using oral corticosteroids throughout treatment, the initial dose of prednisolone used varied from 30 mg to 60 mg fixed dose daily or 1 mg/kg in Abroug 2014, with higher initial doses being tapered in Niewoehner 1999 and Thompson 1996. The length of treatment with oral corticosteroids varied from five to 15 days although Niewoehner 1999 included an arm in which treatment was continued for eight weeks.
Primary outcomes
The primary outcome, treatment failure occurring during the treatment period, met the review definition in a variety of ways during periods varying in length from two to 30 days (Table 4). Five studies used attendance or return to the emergency department or doctor's clinic (Emerman 1989; Bullard 1996; Thompson 1996; Niewoehner 1999; Aaron 2003). Eight studies used deterioration leading to intensification of pharmacological treatment (Thompson 1996; Wood‐Baker 1998; Davies 1999; Niewoehner 1999; Maltais 2002; Willaert 2002; de Jong 2007; and Ceviker 2014). Six studies used the requirement for assisted ventilation or ICU admission (Niewoehner 1999; Maltais 2002; Willaert 2002; de Jong 2007; Gunen 2007; and Ceviker 2014).
2. Definition of treatment failure by study.
| Study ID | Treatment failure definition used in study | Time period | Data |
| Inpatient (treatment > 3 days) | |||
| Bullard 1996 | Returned to ED | < 14 days | 5/60, 14/53 |
| Chen 2005 | Not known | Not known | 5/43, 6/43 |
| Davies 1999 | Withdrawal due to unsatisfactory clinical improvement (specialist), or participant not satisfied with progress, or pH < 7.26 | < 14 days | 1/29, 5/27 |
| Maltais 2002 | Deterioration of COPD while participant hospitalised defined as need for treatment intensification according to the treating physician, the development of confusion, lethargy, acute respiratory acidosis (PaCO2 > 70 mmHg with a pH < 7.30 or an increase in PaCO2 > 10 mmHg) or need for ventilatory assistance | < 11 days | 3/62, 8/66 |
| Niewoehner 1999 | Intubation and mechanical ventilation, re‐admission because of COPD or intensification of pharmacological therapy | < 30 days | 35/160, 37/111 |
| Wood‐Baker 1998 | Lack of progress according to attending physician during treatment | < 7 days | 1/13, 4/13 |
| Inpatient (treatment 1 day) | |||
| Emerman 1989 | Required unscheduled visit to ED | < 2 days | 8/38, 4/32 |
| Outpatient | |||
| Aaron 2003 | Unscheduled visit to doctor's surgery or return to ED because of worsening dyspnoea | < 30 days | 19/70, 30/70 |
| Thompson 1996 | Failure of outpatient therapy defined as hospitalisation for deteriorating respiratory status or lack of improvement of subjective dyspnoea requiring treatment with open‐label prednisone | < 14 days | 0/13, 8/14 |
COPD: chronic obstructive pulmonary disease; ED: emergency department; PaCO2: partial pressure of carbon dioxide dissolved in arterial blood.
Relapse occurred at a later time point to treatment failure, after completion of treatment during varied periods of follow‐up from one to four months, and was based on treatment for an acute exacerbation in two studies (Davies 1999; Aaron 2003), or hospital re‐admission for COPD in four studies (Davies 1999; Niewoehner 1999; de Jong 2007; Ceviker 2014), and hospital admission for which the cause was not specified in three studies (Willaert 2002; Chen 2005; Gunen 2007).
Excluded studies
We excluded 18 studies from this review; reasons for exclusion are shown in the Characteristics of excluded studies table.
Risk of bias in included studies
Full details of our judgements for the included studies, with supporting information for each judgement, can be found in Characteristics of included studies table. A summary of risk of bias across all studies is shown in Figure 2. We rated all studies either low or unclear risk of selection bias. We rated risk of performance bias as either low or unclear in all studies comparing systemic corticosteroid versus placebo, with the exception of Abroug 2014, which was an open‐label study without placebo control that we judged at high risk. We judged two of three studies comparing intravenous corticosteroid versus oral corticosteroid at high risk of performance bias. Detection bias was either low or unclear in 19 studies. Attrition bias risk was low in 60% of studies and high in 15% of studies. We rated all studies as either low or unclear risk of reporting bias.
2.
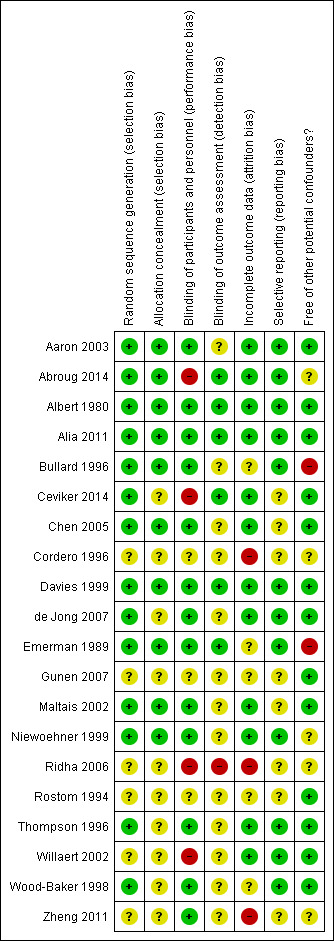
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
All 20 studies were described as randomised; in 14 studies assessment of random sequence generation indicated low risk of bias and in the remaining six studies information was lacking and risk of bias was unclear. In 10 studies, concealment of allocation was assessed at low risk of bias, with lack of information in 10 studies considered at unclear risk.
Blinding
Blinding of participants and treating personnel was adequate with low risk of bias in 13 of 19 studies; in three studies, risk of bias was unclear and we judged four studies at high risk of bias due to lack of blinding (Willaert 2002; Ridha 2006; Ceviker 2014; Abroug 2014). Blinding for outcome assessment indicated low risk of bias in six studies, unclear risk in 13 studies and high risk of bias one study (Ridha 2006).
Incomplete outcome data
We rated 12 studies as low risk of bias due to incomplete outcome data because the number of drop‐outs per group was low and even. In five studies, bias risk was unclear and three studies only published as abstracts were rated at high risk (Cordero 1996; Ridha 2006; Zheng 2011).
Selective reporting
We rated 12 studies as low risk of bias due to selective reporting as all likely outcomes were reported. The risk of bias was unclear in eight studies when there was insufficient information.
Effects of interventions
Comparison 1: systemic corticosteroid versus placebo
Twelve studies with 1620 participants contribute outcome data for systemic corticosteroids versus placebo (Table 1).
Primary outcomes
Treatment failure (Analysis 1.1)
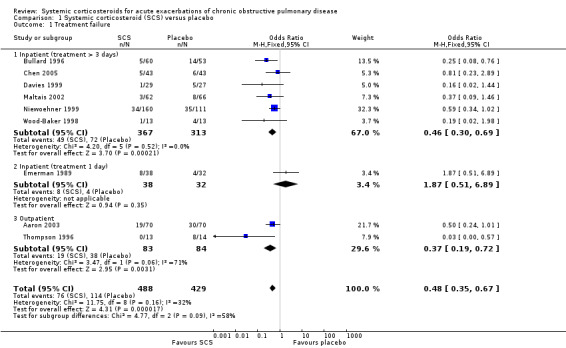
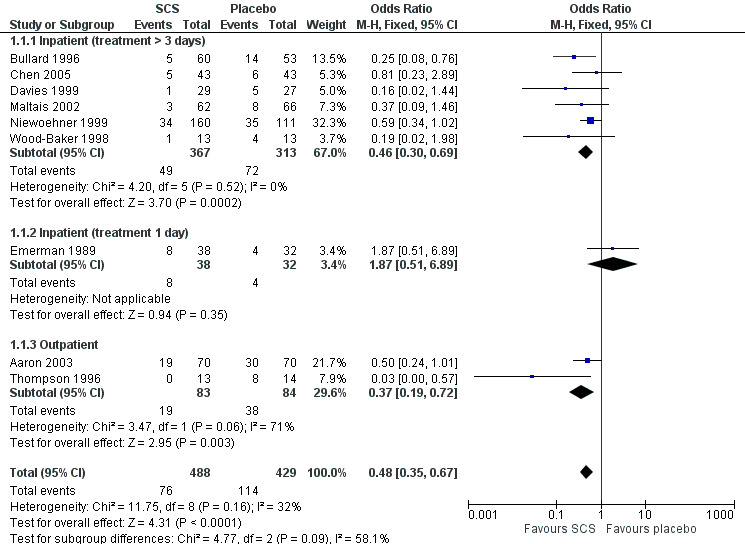
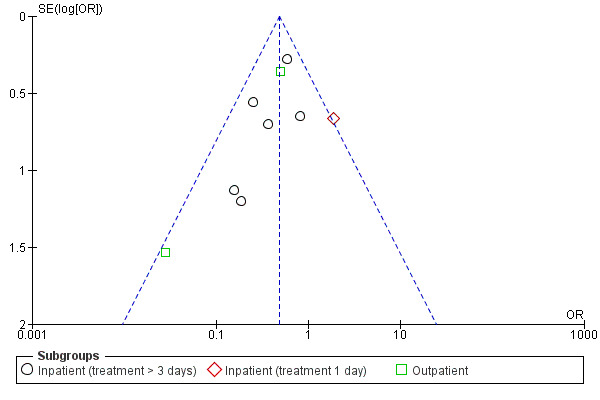
Definition of treatment failure and the time period during which an event occurred varied across studies (Table 4). Systemic corticosteroids reduced the risk of treatment failure by over half when compared with placebo in nine studies (n = 917), of which seven studies contributed data, with median treatment duration of 14 days (OR 0.48; 95% CI 0.35 to 0.67; Analysis 1.1; Figure 3). There was only minor heterogeneity between studies (Chi2 = 11.75; degrees of freedom (df) = 8; P value = 0.16); I2 = 32%). It would have been necessary to treat nine people (95% CI 7 to 14) with systemic corticosteroids to avoid one treatment failure during the treatment period. The funnel plot did not indicate a strong likelihood of publication bias (Figure 4). We rated the quality of evidence for this outcome as high.
1.1. Analysis.
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 1 Treatment failure.
3.
4.
In subgroup analyses, the reduction in likelihood of treatment failure between seven and 30 days for six inpatient studies (n = 680) with treatment duration greater than three days, was similar to the pooled overall result (OR 0.46; 95% CI 0.30 to 0.69), with no heterogeneity. For two outpatient studies (n = 167), the likelihood of treatment failure between 14 and 30 days with systemic corticosteroid compared with placebo was also lower (OR 0.37; 95% CI 0.19 to 0.72) (Aaron 2003; Thompson 1996. There was substantial heterogeneity in the fixed‐effect analysis (Chi2 = 3.47; df = 1; P value = 0.06; I2 = 71%). Oral corticosteroid treatment differed in these studies, with the dose used being higher in Thompson 1996.
In the Emerman 1989 study (n = 70), which used a single intravenous dose of systemic corticosteroid and assessed treatment failure over two days, the likelihood of treatment failure was not lower with systemic corticosteroid treatment (OR 1.87; 95% CI 0.51 to 6.89).
When Emerman 1989 was excluded from the meta‐analysis, the test for subgroup differences showed no difference between inpatient and outpatient studies (Chi2 = 0.28, df = 1; P value = 0.59; I2 = 0%). However, the result with Emerman 1989 included indicated some difference although it was not statistically significant (Chi2 = 4.77, df = 2; P value = 0.09); I2 = 58.1%).
Relapse (Analyses 1.2 and 1.3)
The HR for relapse up to 30 days in two large studies (n = 415) showed a significant reduction for treatment with systemic corticosteroid with no heterogeneity (HR 0.78; 95% CI 0.63 to 0.97; Analysis 1.2) (Aaron 2003; Niewoehner 1999). However, the reduced likelihood of relapse on treatment with systemic corticosteroid in five studies (n = 596) over periods of one to four months was not statistically significant with no heterogeneity between studies (OR 0.67; 95% CI 0.42 to 1.07; Analysis 1.3). We rated the quality of evidence for this outcome as moderate, which was downgraded once as wide CI values include significant benefit and harm.
1.2. Analysis.
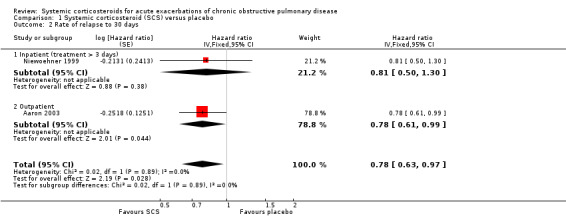
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 2 Rate of relapse to 30 days.
1.3. Analysis.
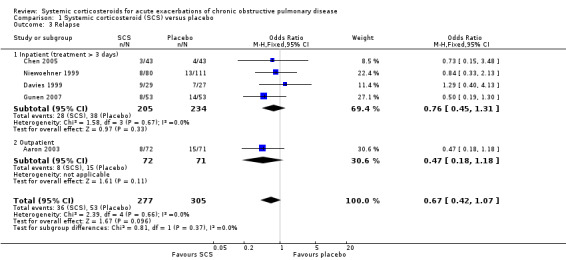
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 3 Relapse.
Mortality (Analysis 1.4)
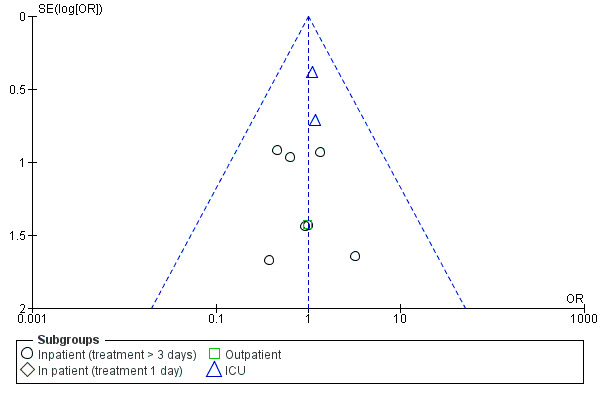
Mortality up to 30 days was not reduced by treatment with systemic corticosteroid compared with placebo in 11 studies (n = 1319; OR 1.00; 95% CI 0.60 to 1.66; Analysis 1.4; Figure 5). There were four subgroups, inpatient studies greater or less than three days' treatment, ICU treatment and outpatient treatment. No heterogeneity was found overall or in any subgroup analysed. The funnel plot did not indicate a strong likelihood of publication bias (Figure 6). We rated the quality of evidence for this outcome as moderate, which was downgraded once as wide CI values include significant benefit and harm.
1.4. Analysis.
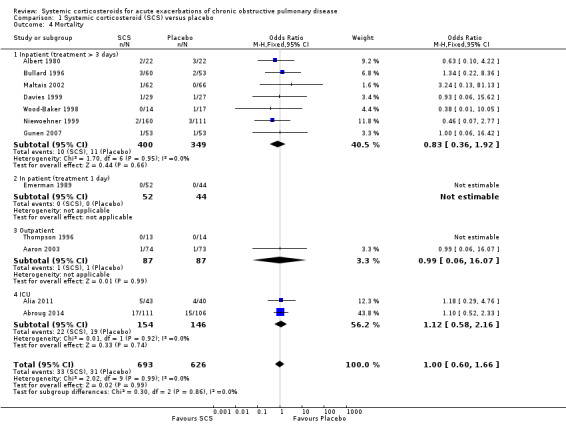
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 4 Mortality.
5.
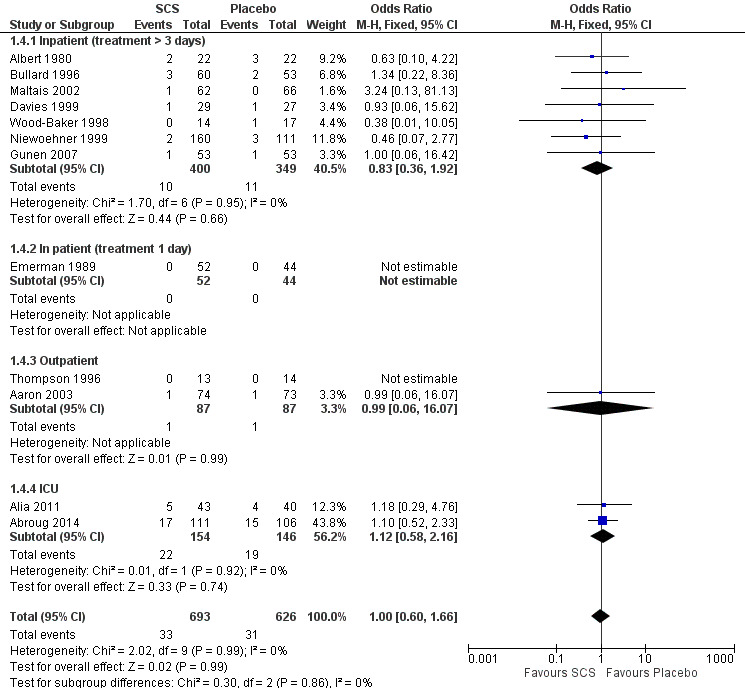
6.
Early outcomes: 72 hours or less
Lung function (Analyses 1.5 to 1.10)
FEV1 was significantly increased with corticosteroid treatment in seven studies (n = 649), with no heterogeneity between studies (MD 140 mL; 95% CI 90 to 200; Analysis 1.5). This effect size is thought to be clinically meaningful (Donohue 2005). The FEV1 per cent predicted was also greater at this time point in three studies, with no significant heterogeneity (n = 231; MD 3.85%; 95% CI 0.18 to 7.52; Chi2 = 2.62; df = 2; P value = 0.27; I2 = 24%; Analysis 1.6). There was no increase in FVC with corticosteroid treatment in three studies (n = 123; MD 200 mL; 95% CI ‐50 to 450). PEF was significantly increased with corticosteroid treatment in two studies with significant heterogeneity (n = 137; MD 22.52 L/minute; 95% CI 5.02 to 40.03; Chi2 = 4.48, df = 1; P value = 0.03; I2 = 78%; Analysis 1.10).
1.5. Analysis.
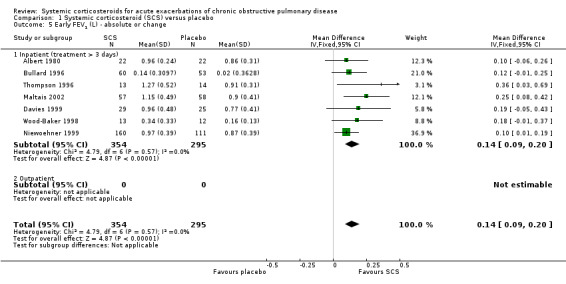
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 5 Early FEV1 (L) ‐ absolute or change.
1.6. Analysis.
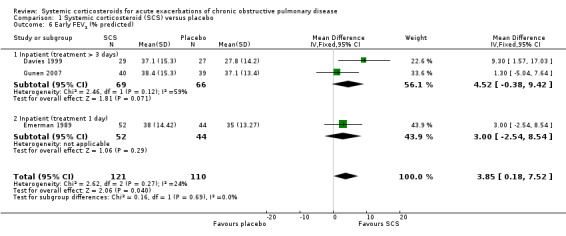
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 6 Early FEV1 (% predicted).
1.10. Analysis.
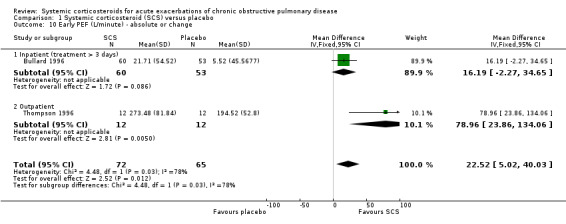
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 10 Early PEF (L/minute) ‐ absolute or change.
Symptom scores (Analysis 1.11)
Breathlessness was assessed using the Borg dyspnoea scale (Maltais 2002), and visual analogue scales (VAS) for overall dyspnoea (Thompson 1996; Wood‐Baker 1998), and dyspnoea related to specific activities (talking, dressing, washing, walking) (Wood‐Baker 1998). Corticosteroid treatment significantly decreased dyspnoea when results were pooled using the SMD in three studies, with some heterogeneity that might not be important (n = 178; SMD 0.35; 95% CI 0.05 to 0.64; Chi2 = 2.89; df = 2; P value = 0.24; I2 = 31%; Analysis 1.11). This equates to an effect size on the Borg scale of 0.93 units (95% CI 0.18 to 1.7) or on the VAS scale of 5.24 (95% CI 0.75 to 9.59). The effect sizes are less than the suggested minimal clinically important differences of 2 units for the Borg scale and 10 units for the VAS (Ries 2005). We rated the quality of evidence for this outcome as moderate, being downgraded once as the upper CI crossed an effect size of 0.5.
1.11. Analysis.
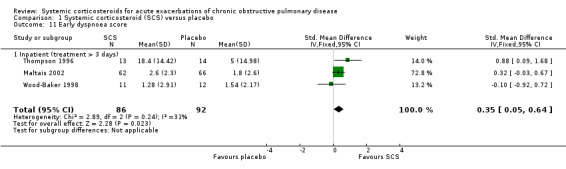
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 11 Early dyspnoea score.
Arterial blood gas measurements (Analyses 1.12 and 1.13)
Conditions for measurement of ABGs varied across studies. In Thompson 1996 and Gunen 2007, ABGs were measured while breathing room air at rest and, in Maltais 2002, conditions were variable, with some participants using supplementary oxygen and some breathing room air. Corticosteroid treatment compared with placebo significantly increased arterial oxygenation in three studies, with no heterogeneity (n = 233; PaO2 MD 3.71 mmHg; 95% CI 0.55 to 6.88; Analysis 1.12). In these three studies of inpatients and one study in ICU (Alia 2011), PaCO2 was decreased with corticosteroid treatment compared with placebo, with moderate heterogeneity between studies in different settings (n = 316; MD ‐2.21 mmHg; 95% CI ‐3.84 to ‐0.58; Chi2 = 7.23, df = 3; P value = 0.06; I2 = 58%; Analysis 1.13).
1.12. Analysis.
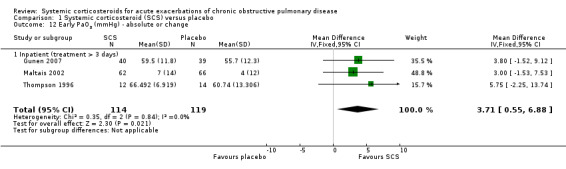
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 12 Early PaO2 (mmHg) ‐ absolute or change.
1.13. Analysis.
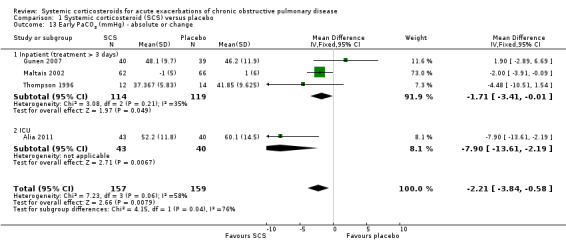
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 13 Early PaCO2 (mmHg) ‐ absolute or change.
Late outcomes: end of treatment
Lung function (Analyses 1.14 to 1.18)
FEV1 was not significantly increased with corticosteroid treatment compared with placebo in seven studies (n = 669; MD 90 mL; 95% CI ‐10 to 190; Analysis 1.14). There was little heterogeneity between inpatient studies (Chi2 = 6.21, df = 4; P value = 0.18; I2 = 36%) and moderate heterogeneity in pooled inpatient and outpatient studies (Chi2 = 11.28; df = 6; P value = 0.08; I2 = 47%). There was an increase in FEV1 per cent predicted in two studies with corticosteroid treatment, with no significant heterogeneity (n = 129; MD 6.14; 95% CI 1.32 to 10.96; Analysis 1.15). PEF improved with corticosteroid treatment in two studies, with moderate heterogeneity (n = 112; MD 119.06 L/minute; 95% CI 64.39 to 173.73; Chi2 = 2.27; df = 1; P value = 0.13; I2 = 56%; Analysis 1.19). There was no increase in FVC, FVC per cent predicted or the ratio of FEV1/FVC with corticosteroid treatment compared with placebo at the end of treatment.
1.14. Analysis.
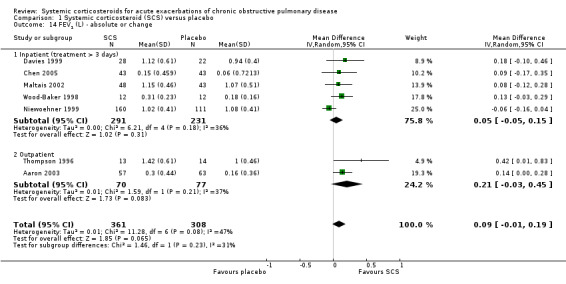
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 14 FEV1 (L) ‐ absolute or change.
1.15. Analysis.
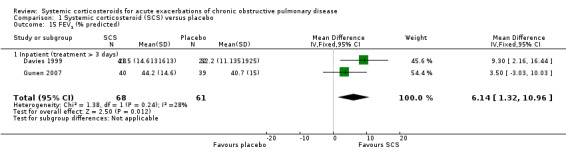
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 15 FEV1 (% predicted).
1.19. Analysis.
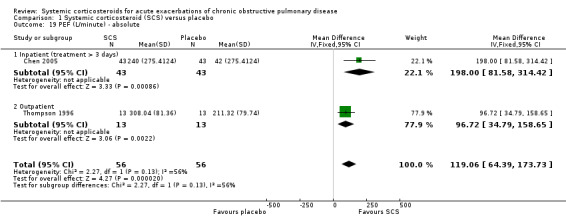
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 19 PEF (L/minute) ‐ absolute.
Symptom scores (Analyses 1.20 to 1.22)
Dyspnoea was measured using the transitional dyspnoea index (Aaron 2003), with a positive score indicating improvement and a change of one unit considered clinically significant (Mahler 1984). There was a significant benefit with corticosteroid treatment in Aaron 2003 (effect size MD 1.88 units; 95% CI 0.23 to 3.53; Analysis 1.20). VAS symptom scores were reported by Wood‐Baker 1998; Davies 1999; and Chen 2005. The improvement in dyspnoea with corticosteroid treatment in four studies was not significant, with no heterogeneity (n = 301; SMD 0.18; 95% CI ‐0.05 to 0.41; Analysis 1.22).
1.20. Analysis.
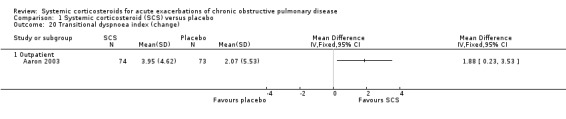
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 20 Transitional dyspnoea index (change).
1.22. Analysis.
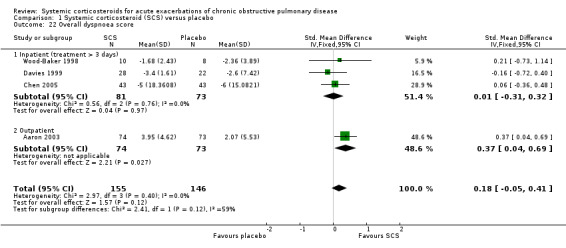
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 22 Overall dyspnoea score.
Arterial blood gas measurements (Analyses 1.23 and 1.24)
Corticosteroid treatment significantly improved PaO2 compared with placebo in four inpatient studies, with no heterogeneity between studies (n = 200; PaO2 MD 6.86 mmHg; 95% CI 2.75 to 10.96; Analysis 1.23). There was no significant decrease in PaCO2 with corticosteroid treatment in three studies, with moderate heterogeneity: (n = 188; MD ‐1.81 mmHg; 95% CI ‐5.06 to 1.44; Chi2 = 4.89; df = 2; P value = 0.09; I2 = 59%; Analysis 1.24).
1.23. Analysis.
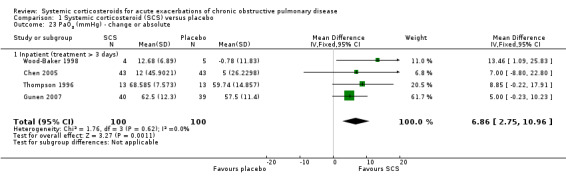
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 23 PaO2 (mmHg) ‐ change or absolute.
1.24. Analysis.
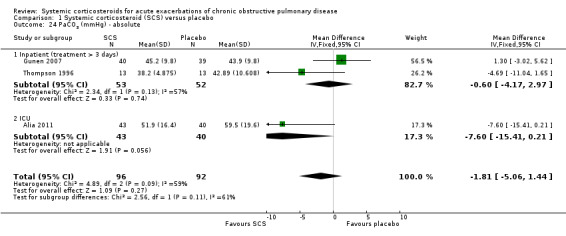
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 24 PaCO2 (mmHg) ‐ absolute.
Health‐related quality of life (Analysis 1.25)
Only Aaron 2003 (n = 147) reported data on quality of life with the total Chronic Respiratory Disease index and did not find a significant improvement with corticosteroid treatment after 10 days.
Functional capacity (Analysis 1.26)
Wood‐Baker 1998 reported data on six‐minute walk tests in 18 participants at 14 days and found no improvement with corticosteroid treatment (Wise 2005).
Length of stay and duration of ventilation (Analyses 1.27 and 1.28)
There was substantial heterogeneity in the outcome 'length of stay' between studies in a general inpatient setting versus an ICU setting for people who required assisted ventilation. The test for subgroup differences was significant (P value = 0.04; I2 = 75.4%) and we decided not to pool the subgroups. In the general inpatient setting, there was a shorter duration of hospital stay for corticosteroid treatment compared with placebo in two studies, with no heterogeneity (n = 296; MD ‐1.22 days; 95% CI ‐2.26 to ‐0.18). Davies 1999 also reported a lower median stay of seven days for corticosteroid treatment and nine days for placebo, while in Maltais 2002, the corticosteroid treatment group median length of stay was six days and eight days for placebo. We rated the quality of evidence for this outcome as high. However, in the ICU setting for people requiring assisted ventilation, there was no difference in length of ICU stay for corticosteroid treatment compared with control, with moderate heterogeneity (n=300; MD 0.65 days; 95% CI ‐0.84 to 2.15; P value = 0.17; I2 = 46%; Analysis 1.27).
1.27. Analysis.
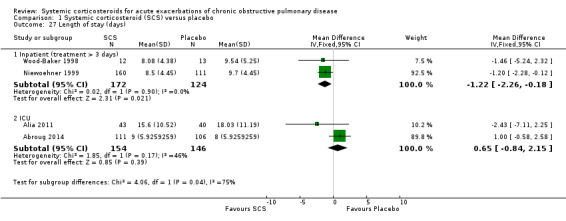
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 27 Length of stay (days).
The duration of assisted ventilation in two studies in an ICU setting was not significantly reduced with corticosteroid treatment compared with control (n = 300; MD ‐1.03 days; 95% CI ‐3.44 to 1.38).
Adverse effects (Analyses 1.29 to 1.41)
An adverse drug reaction was more than twice as likely with corticosteroid treatment compared with placebo in eight studies (n = 736; OR 2.33; 95% CI 1.59 to 3.43; Analysis 1.29). Overall, one extra adverse effect occurred for every six people treated (95% CI 4 to 10). Six studies reported data specifically on hyperglycaemia (Davies 1999; Niewoehner 1999; Maltais 2002; Aaron 2003; Alia 2011; Abroug 2014). There was an increased likelihood of hyperglycaemia with corticosteroid compared with placebo treatment, with no significant heterogeneity (n = 804; OR 2.79; 95% CI 1.86 to 4.19; P value = 0.25; I2 = 24%; Analysis 1.30). Overall, one extra participant developed hyperglycaemia for every seven treated with systemic corticosteroids (95% CI 5 to 12). We rated the quality of evidence for this outcome as high.
1.29. Analysis.
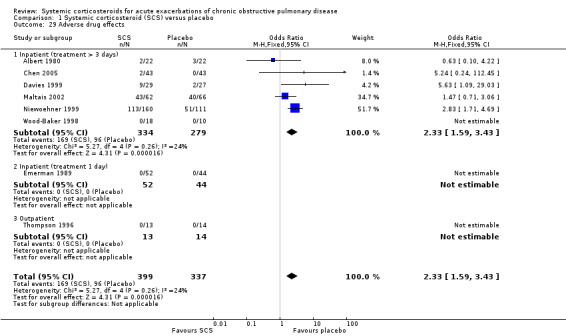
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 29 Adverse drug effects.
1.30. Analysis.
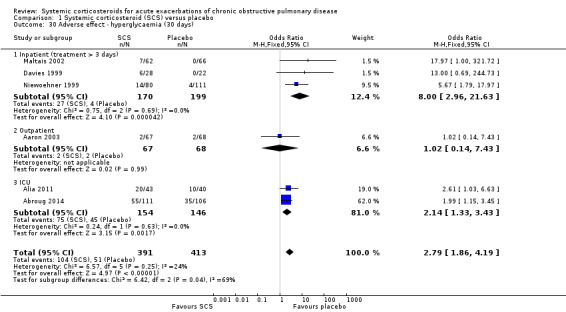
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 30 Adverse effect ‐ hyperglycaemia (30 days).
The risk of hypertension did not differ with corticosteroid treatment compared with placebo in two studies in inpatients and ICU, although there was moderate heterogeneity (n = 274; OR 1.20; 95% CI 0.44 to 3.25; Chi2 = 2.06; df = 1; P value = 0.15; I2 = 51%; Analysis 1.31). The risk of gastrointestinal bleeding did not differ with corticosteroid treatment compared with placebo in two studies in ICU (n = 300; OR 0.93; 95% CI 0.12 to 6.91; Analysis 1.32).
1.31. Analysis.
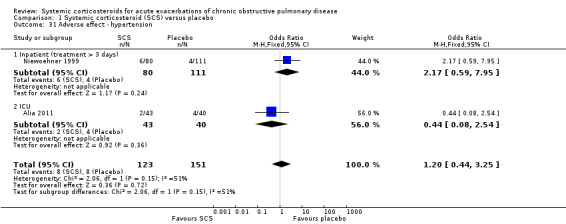
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 31 Adverse effect ‐ hypertension.
1.32. Analysis.
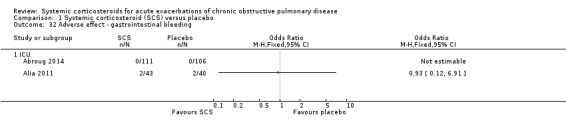
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 32 Adverse effect ‐ gastrointestinal bleeding.
In the ICU‐based studies of Alia 2011 and Abroug 2014, there was no increased likelihood of ventilator‐associated pneumonia with corticosteroid treatment (n = 300; OR 1.23; 95% CI 0.44 to 3.40).
There was a non‐significant two‐fold increase in likelihood of an adverse psychiatric event with corticosteroid treatment compared with placebo in two studies (n = 331; OR 2.15; 95% CI 0.95 to 4.88; Analysis 1.37).
1.37. Analysis.
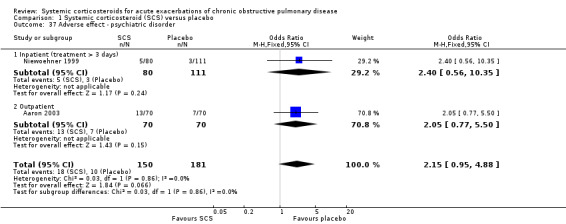
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 37 Adverse effect ‐ psychiatric disorder.
The outpatient study of Aaron 2003 and the inpatient study of Niewoehner 1999 reported incidences of other specific adverse effects. In single studies, there were significantly increased risks of weight gain and insomnia with corticosteroid treatment compared with placebo. In these studies, there were no significantly increased risks with corticosteroid treatment compare with placebo for anxiety, depression, dyspepsia, delirium or secondary infection.
Comparison 2: intravenous corticosteroid versus oral corticosteroid
Three studies with 298 participants contributed outcome data for intravenous corticosteroids versus oral corticosteroids (see Table 2).
Primary outcomes
Treatment failure (Analysis 2.1)
There was no significant reduction in risk of treatment failure with intravenous corticosteroid treatment and oral corticosteroid treatment in three studies in inpatients, with no heterogeneity (n = 298; OR 0.67; 95% CI 0.34 to 1.30; Analysis 2.1). We rated the quality of evidence for this outcome as moderate, being downgraded once as wide CI values included significant benefit and harm.
2.1. Analysis.
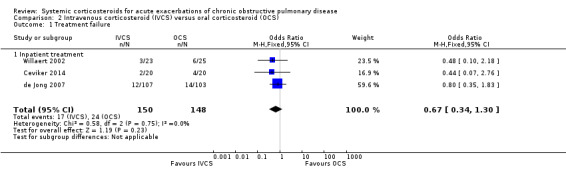
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 1 Treatment failure.
Relapse (Analysis 2.2)
The odds of relapse after completion of treatment was not significantly reduced by treatment with intravenous corticosteroid compared with oral corticosteroid in three studies, with no heterogeneity between studies (n = 298; OR 0.95; 95% CI 0.50 to 1.80; Analysis 2.2). We rated the quality of evidence for this outcome as moderate, being downgraded once as wide CI values included significant benefit and harm.
2.2. Analysis.
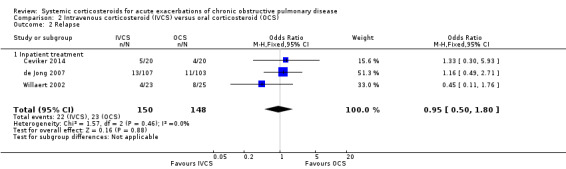
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 2 Relapse.
Mortality (Analysis 2.3)
Mortality after discharge between one and three months was not reduced by treatment with intravenous corticosteroid compared with oral corticosteroid in three studies, with no significant heterogeneity (n = 298; OR 1.40; 95% CI 0.44 to 4.51; Chi2 = 2.41; df = 2; P value = 0.30; I2 = 17%; Analysis 2.3). We rated the quality of evidence for this outcome as moderate, being downgraded once as wide CI values included significant benefit and harm.
2.3. Analysis.
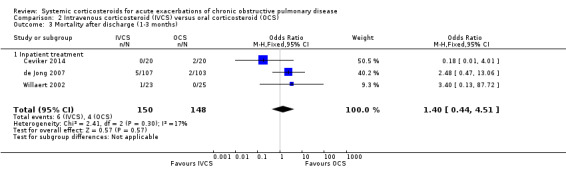
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 3 Mortality after discharge (1‐3 months).
Early outcomes: 72 hours or less
Lung function (Analyses 2.4 and 2.5)
Only one study reported lung function measures at the early time point, and neither FEV1 nor FVC was significantly increased with intravenous corticosteroid treatment compared with oral corticosteroid.
Symptom scores (Analyses 2.5 to 2.8)
Dyspnoea was not significantly decreased with intravenous corticosteroid treatment compared with oral corticosteroid in two inpatient studies using a VAS measure, with no heterogeneity (n = 75; MD 0.62; 95% CI ‐0.55 to 1.78; Analysis 2.6). We rated the quality of evidence for this outcome as low, being downgraded twice as wide CI values included significant benefit and harm and participants and physicians were not blinded to treatment in the two studies. In one single study (n = 38), there were no differences in VAS scores for cough or sputum volume.
2.6. Analysis.
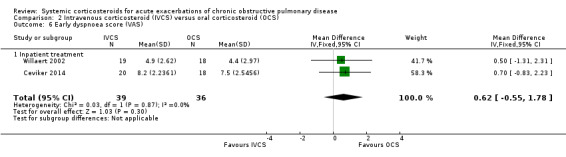
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 6 Early dyspnoea score (VAS).
Late outcomes: end of treatment
Lung function (Analyses 2.9 to 2.11)
FEV1 did not differ significantly at the end of treatment with intravenous corticosteroid compared with oral corticosteroid treatment in three studies, with no heterogeneity present (n = 285; MD ‐20 mL; 95% CI ‐80 to 30; Analysis 2.9). There was no difference in FVC in two studies with intravenous corticosteroid compared with oral corticosteroid treatment, with no heterogeneity present (n = 75; MD ‐50 mL; 95% CI ‐33 to 22; Analysis 2.10).
2.9. Analysis.
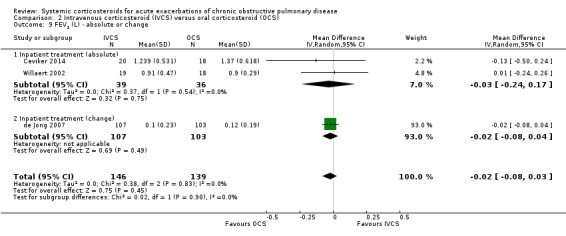
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 9 FEV1 (L) ‐ absolute or change.
2.10. Analysis.
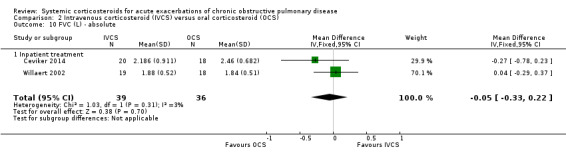
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 10 FVC (L) ‐ absolute.
Symptom scores (Analyses 2.12 to 2.14)
Dyspnoea was not significantly improved with intravenous corticosteroid treatment compared with oral corticosteroid in two inpatient studies using a VAS measure, with no heterogeneity (n = 75; MD 1.28; 95% CI ‐0.24 to 2.80; Analysis 2.12), and in one single study (n = 38) there were no differences in VAS scores for cough or sputum volume.
2.12. Analysis.
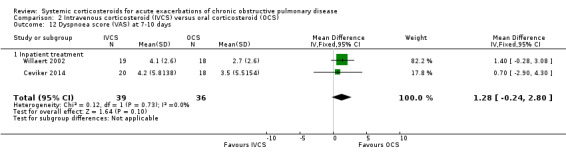
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 12 Dyspnoea score (VAS) at 7‐10 days.
Arterial blood gas measurements (Analyses 2.15 and 2.16)
There were no significant differences in between intravenous corticosteroid compared with oral corticosteroid treatment in a single study in PaO2 (n = 38; MD ‐1.20 mmHg; 95% CI ‐8.61 to 6.21; Analysis 2.15) or PaCO2 (MD 5.50 mmHg; 95% CI ‐0.79 to 11.79; Analysis 2.16).
2.15. Analysis.
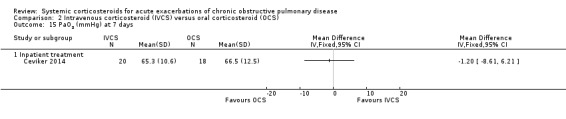
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 15 PaO2 (mmHg) at 7 days.
2.16. Analysis.
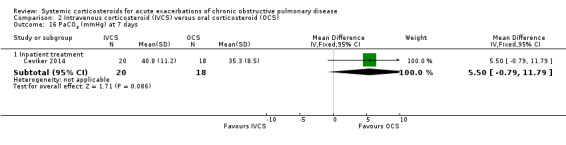
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 16 PaCO2 (mmHg) at 7 days.
Health status and quality of life (Analyses 2.17 to 2.22)
The Clinical COPD Questionnaire (CCQ) measuring health status (symptoms, functional state and mental state) and the St George's Hospital Respiratory Questionnaire (SGRQ) measuring respiratory‐related QoL were reported in one study (n = 210), with no differences between intravenous corticosteroid and oral corticosteroid treatment found for either measure after one week (n = 210; CCQ: MD 0.10; 95% CI ‐0.17 to 0.37; Analysis 2.17; SGRQ: MD ‐0.70; 95% CI ‐4.33 to 2.93; Analysis 2.18). There were no differences in the Dyspnoea, Fatigue, Mastery or Emotion domains of the Chronic Respiratory Questionnaire (CRQ) between intravenous corticosteroid and oral corticosteroid treatment in one study (n = 21) after four weeks.
2.17. Analysis.
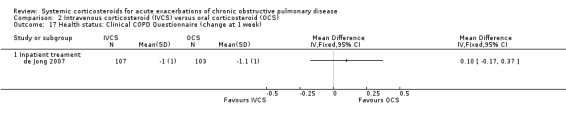
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 17 Health status: Clinical COPD Questionnaire (change at 1 week).
2.18. Analysis.
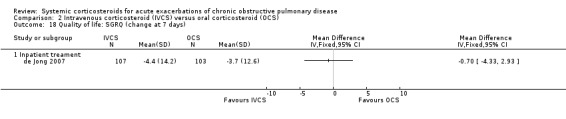
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 18 Quality of life: SGRQ (change at 7 days).
Length of stay (Analysis 2.23)
There was a non‐significant increase in duration of hospital stay for intravenous corticosteroid compared with oral corticosteroid treatment in three studies, with no significant heterogeneity (n = 298; MD 1.54 days; 95% CI ‐0.09 to 3.17; Chi2 = 2.93; df = 2; P value = 0.23; I2 = 32%; Analysis 2.23). We rated the quality of evidence for this outcome as low, being downgraded twice as wide CI values included significant benefit and harm and participants and physicians were not blinded to treatment in the two studies.
2.23. Analysis.
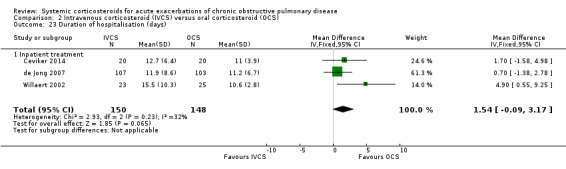
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 23 Duration of hospitalisation (days).
Adverse events (Analyses 2.24 and 2.25)
In one single inpatient study, there was an increased likelihood of hyperglycaemia with intravenous corticosteroid compared with oral corticosteroid treatment (n = 40; OR 4.89; 95% CI 1.20 to 19.94; Analysis 2.24) (Ceviker 2014). We rated the quality of evidence for this outcome as moderate, being downgraded once for indirectness based on a single study. The risk of hypertension was not significantly increased in this study (OR 8.20; 95% CI 0.40 to 169.90; Analysis 2.25).
2.24. Analysis.
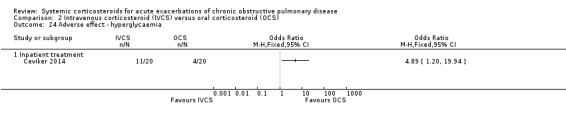
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 24 Adverse effect ‐ hyperglycaemia.
2.25. Analysis.
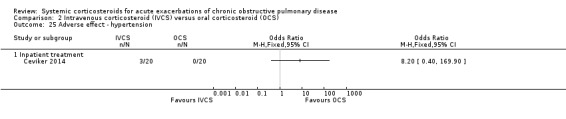
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 25 Adverse effect ‐ hypertension.
Discussion
Summary of main results
This updated review addressed the use of systemic corticosteroid (oral or parenteral) in the treatment of acute exacerbations of COPD. The review included 16 studies with 1787 participants, mostly conducted with inpatients, that compared systemic corticosteroid with placebo and four studies with 298 inpatients comparing parenteral corticosteroid versus oral corticosteroid.
Systemic corticosteroid treatment compared with placebo significantly decreased treatment failure up to one month and relapse after treatment for inpatients and outpatients, and improved lung function (FEV1, per cent predicted FEV1, PEF), symptoms of breathlessness and blood gases (PaO2, PaCO2) within three days. At the end of treatment, systemic corticosteroid treatment compared with placebo significantly improved some lung function measures (per cent predicted FEV1, PEF) and blood gases (PaO2) and reduced the length of hospital stay by around one to two days in people who did not require treatment with assisted ventilation in an ICU. In terms of adverse events, no significant difference was observed in rates of mortality up to one month but there was at least a two‐fold increase in adverse drug effects, especially for hyperglycaemia, which showed a four‐fold increase.
Parenteral corticosteroid treatment compared with oral corticosteroid treatment did not significantly decrease treatment failure, or improve lung function, QoL, respiratory symptoms or blood gases. There was no significant difference in the rate of mortality between one and three months of follow‐up but parenteral corticosteroid treatment was associated with a significantly increased rate of the adverse drug effect hyperglycaemia compared with oral treatment.
Overall completeness and applicability of evidence
The criteria for an acute exacerbation were explicit in most studies, either an increase in respiratory symptoms or respiratory insufficiency. Most studies used accepted criteria for COPD diagnosis. There was imbalance in the gender ratio of participants in the included studies, ranging from 52% to 100% males. This reflects the historically higher incidence of COPD in men; however, the increasing smoking rates among women means that the incidence of COPD is increasing in women (Chapman 2001). There seems to be no good reason to expect a different response to treatment in women based on studies in asthma and corticosteroid use, so the findings are applicable to all people with COPD.
Most studies explicitly excluded participants with asthma, as is generally the case in efficacy studies in COPD. However, excluding participants with a history of asthma excludes a population with increased risk of developing COPD. There is an association between fixed airflow obstruction in adults aged 40 to 44 years and early‐onset current clinical asthma, equivalent to a 33 pack‐year history of smoking (OR 3.7; 95% CI 1.5 to 9.3) (Perret 2013). This may limit the generalisability of the findings.
The current review expanded and updated a previous version of the review that excluded people requiring assisted ventilation treated in ICU and additionally compared the route of delivery of systemic corticosteroid, parenteral or oral. The decision to treat an acute exacerbation of COPD at home or admit for inpatient treatment is related to clinical assessment of severity of the person's condition. The findings of this review may be generalisable to both outpatient and inpatient settings and thus to exacerbations of a range of severity. Two studies of inpatients requiring assisted ventilation in ICU contributed data. We did not pool data on length of stay in participants who required assisted ventilation in ICU with data from studies in the general hospital setting.
Randomised trials in this review do not provide data on the use of repeated treatment with systemic corticosteroid in people with COPD and we were unable to perform subgroup analysis by frequency and severity of exacerbations.
The sensitivity analysis removing studies in which a significant proportion of participants were crossed over to active treatment (i.e. Emerman 1989; Bullard 1996), gave results that were very similar to those of the original pooled analysis for treatment failure, mortality and lung function.
Quality of the evidence
The methodological quality of the included trials that contributed data was good, and the three conference abstracts of unknown quality did not contribute any data to the analysis. In the comparison of systemic corticosteroid and placebo treatment, only one study was not double blind. This study contributed to analysis of objectively measured outcomes and results were unlikely to be compromised by detection or performance biases. Two studies comparing routes of administration of systemic corticosteroid were not double blind, and results for objective outcomes such as mortality were unlikely to be compromised by detection or performance biases. The evidence for symptoms and duration of hospitalisation was downgraded for possible detection or performance bias. The studies judged at high risk of attrition bias did not contribute data to any analyses.
Potential biases in the review process
Review authors made every effort to identify all relevant published and unpublished studies by using additional methods to identify studies that might not have been found in the main electronic search (e.g. searching drug company databases and clinical trial registration sites, checking reference lists). We did not routinely contact individual trial authors for additional data unless outcomes were clearly selectively reported. All authors adhered to the most recent best practice guidelines in terms of study selection, resolution of disagreements, data extraction and analysis to reduce bias and errors.
Agreements and disagreements with other studies or reviews
The results of one cohort study, conducted at 414 US hospitals in 2006 to 2007 in a non‐intensive care setting, showed oral corticosteroid treatment for acute exacerbations of COPD was not associated with worse outcomes than when high‐dose intravenous corticosteroid treatment was given during the first two hospital days (Lindenauer 2010). Another Cochrane review comparing systemic corticosteroid treatment for seven days or less with longer treatment in acute exacerbations of COPD concluded there were insufficient data to base firm conclusions on the optimal duration of corticosteroid therapy (Walters 2011).
Authors' conclusions
Implications for practice.
This updated review and meta‐analysis provide high‐quality evidence to support the use of systemic corticosteroid by the oral or parenteral route for exacerbations of chronic obstructive pulmonary disease (COPD). Treatment reduces the likelihood of treatment failure, shortens hospital stay when assisted ventilation is not required, and improves lung function and symptoms. There is no evidence that corticosteroid administration by a parenteral route is more effective than oral treatment. There is an increase in adverse drug effects with corticosteroid treatment, which is greater with parenteral administration compared with oral treatment. The specific adverse drug effects are known pharmacological effects of corticosteroids and are unlikely to persist after treatment ceases.
Implications for research.
Future studies of systemic corticosteroid use for exacerbation of COPD should report results for participants by previous exacerbation frequency to enable assessment of repeated systemic corticosteroid treatment. There is a need for more studies in severe exacerbations of COPD in people who require assisted ventilation. Data for cost‐effectiveness comparisons should be collected.
What's new
| Date | Event | Description |
|---|---|---|
| 23 May 2014 | New search has been performed | Literature search run. |
| 23 May 2014 | New citation required and conclusions have changed | Objectives of review amended. Participants: to include studies in which participants received assisted ventilation. Interventions: Comparison 1‐ corticosteroids, parenteral or oral administration versus placebo control injections or tablets as appropriate; Comparison 2‐ corticosteroids, parenteral versus oral administration. |
History
Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 22 August 2012 | New search has been performed | Review updated. Changes to criteria for study inclusion; studies with participants requiring assisted ventilation included |
| 1 August 2008 | New search has been performed | Literature searches re‐run |
| 11 July 2008 | Amended | Converted to new review format. |
| 2 January 2008 | New citation required and conclusions have changed | This review, originally published in 1999 was updated in 2004 and for this version in 2007. One additional study (Chen 2005) has been added and additional data have been received from two authors (Aaron 2003 and Wood‐Baker 1998). The conclusions of the previous version on the beneficial effect of treatment with systemic corticosteroids in acute exacerbations of COPD on the primary outcome of treatment failure have been strengthened. This update of the review found a significant reduction in length of hospital stay for treatment with systemic corticosteroids. The beneficial effects of treatment with systemic corticosteroids on the secondary outcomes of dyspnoea, blood gases and lung function, previously only significant at an early time point, have also been confirmed at the end of treatment. The increased risk of adverse effects associated with treatment, particularly the risk of hyperglycaemia, with systemic corticosteroids was confirmed. |
Acknowledgements
We are grateful to authors of studies, Bullard 1996; Wood‐Baker 1998; Davies 1999; Niewoehner 1999; Maltais 2002; Aaron 2003; Chen 2005; Alia 2011, who responded to our requests for data and information. We acknowledge the contribution of M Hannay to previous updates of the review in 2004 and 2008. We are also indebted to the editorial team and Cochrane Airways Group staff for performing searches, and their help and support.
Chris Cates was the Editor for this review and commented critically on the review.
Appendices
Appendix 1. MEDLINE search strategy
#1 COPD[MeSH Terms] #2 "adrenal cortex hormone*" #3 steroid #4 steroids #5 glucocorticoid* #6 corticoid* #7 corticosteroid* #8 beclomethasone #9 betamethasone #10 fluticasone #11 cortisone #12 dexamethasone #13 hydrocortisone #14 prednisolone #15 prednisone #16 methylprednisolone #17 methylprednisone #18 triamcinolone #19 (#2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) #20 randomised controlled trial [pt] #21 controlled clinical trial [pt] #22 randomised [tiab] #23 placebo [tiab] #24 clinical trials as topic [mesh: noexp] #25 randomly [tiab] #26 trial [ti] #27 (#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26) #28 (animals [mh] NOT humans [mh]) #29 (#27 NOT #28) #30 ("2012/01/01"[Date ‐ Publication] : "3000"[Date ‐ Publication]) #31 (#1 AND #19 AND #29 AND #30)
Appendix 2. EMBASE search strategy
#1 chronic AND obstructive AND pulmonary AND 'disease'/exp AND [embase]/lim AND [2010‐2013]/py
#2 'triamcinolone'/exp OR 'methylprednisone'/exp OR 'methylprednisolone'/exp OR 'prednisone'/exp OR 'prednisolone'/exp OR 'hydrocortisone'/exp OR 'dexamethasone'/exp OR 'cortisone'/exp OR 'fluticasone'/exp OR 'betamethasone'/exp OR 'beclomethasone'/exp OR 'corticosteroid'/exp OR 'corticoid'/exp OR 'glucocorticoid'/exp OR 'steroids'/exp OR 'steroid'/exp OR 'adrenal'/exp AND cortex AND 'hormone'/exp AND [embase]/lim AND [2010‐2013]/py
#1 AND #2 AND ([controlled clinical trial]/lim OR [randomised controlled trial]/lim) AND [humans]/lim AND [embase]/lim AND [2010‐2013]/py
Data and analyses
Comparison 1. Systemic corticosteroid (SCS) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 9 | 917 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.35, 0.67] |
| 1.1 Inpatient (treatment > 3 days) | 6 | 680 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.30, 0.69] |
| 1.2 Inpatient (treatment 1 day) | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.51, 6.89] |
| 1.3 Outpatient | 2 | 167 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.19, 0.72] |
| 2 Rate of relapse to 30 days | 2 | Hazard ratio (Fixed, 95% CI) | 0.78 [0.63, 0.97] | |
| 2.1 Inpatient (treatment > 3 days) | 1 | Hazard ratio (Fixed, 95% CI) | 0.81 [0.50, 1.30] | |
| 2.2 Outpatient | 1 | Hazard ratio (Fixed, 95% CI) | 0.78 [0.61, 0.99] | |
| 3 Relapse | 5 | 582 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.42, 1.07] |
| 3.1 Inpatient (treatment > 3 days) | 4 | 439 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.45, 1.31] |
| 3.2 Outpatient | 1 | 143 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.18, 1.18] |
| 4 Mortality | 12 | 1319 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.60, 1.66] |
| 4.1 Inpatient (treatment > 3 days) | 7 | 749 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.36, 1.92] |
| 4.2 In patient (treatment 1 day) | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Outpatient | 2 | 174 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 16.07] |
| 4.4 ICU | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.58, 2.16] |
| 5 Early FEV1 (L) ‐ absolute or change | 7 | 649 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [0.09, 0.20] |
| 5.1 Inpatient (treatment > 3 days) | 7 | 649 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [0.09, 0.20] |
| 5.2 Outpatient | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Early FEV1 (% predicted) | 3 | 231 | Mean Difference (IV, Fixed, 95% CI) | 3.85 [0.18, 7.52] |
| 6.1 Inpatient (treatment > 3 days) | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | 4.52 [‐0.38, 9.42] |
| 6.2 Inpatient (treatment 1 day) | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [‐2.54, 8.54] |
| 7 Early FVC (L) ‐ absolute or change | 3 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.45] |
| 7.1 Inpatient (treatment > 3 days) | 3 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.45] |
| 8 Early FVC (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Inpatient (treatment > 3 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Early FEV1/FVC (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Inpatient (treatment > 3 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Early PEF (L/minute) ‐ absolute or change | 2 | 137 | Mean Difference (IV, Fixed, 95% CI) | 22.52 [5.02, 40.03] |
| 10.1 Inpatient (treatment > 3 days) | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | 16.19 [‐2.27, 34.65] |
| 10.2 Outpatient | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 78.96 [23.86, 134.06] |
| 11 Early dyspnoea score | 3 | 178 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.05, 0.64] |
| 11.1 Inpatient (treatment > 3 days) | 3 | 178 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.05, 0.64] |
| 12 Early PaO2 (mmHg) ‐ absolute or change | 3 | 233 | Mean Difference (IV, Fixed, 95% CI) | 3.71 [0.55, 6.88] |
| 12.1 Inpatient (treatment > 3 days) | 3 | 233 | Mean Difference (IV, Fixed, 95% CI) | 3.71 [0.55, 6.88] |
| 13 Early PaCO2 (mmHg) ‐ absolute or change | 4 | 316 | Mean Difference (IV, Fixed, 95% CI) | ‐2.21 [‐3.84, ‐0.58] |
| 13.1 Inpatient (treatment > 3 days) | 3 | 233 | Mean Difference (IV, Fixed, 95% CI) | ‐1.71 [‐3.41, ‐0.01] |
| 13.2 ICU | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐7.90 [‐13.61, ‐2.19] |
| 14 FEV1 (L) ‐ absolute or change | 7 | 669 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.01, 0.19] |
| 14.1 Inpatient (treatment > 3 days) | 5 | 522 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.05, 0.15] |
| 14.2 Outpatient | 2 | 147 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.03, 0.45] |
| 15 FEV1 (% predicted) | 2 | 129 | Mean Difference (IV, Fixed, 95% CI) | 6.14 [1.32, 10.96] |
| 15.1 Inpatient (treatment > 3 days) | 2 | 129 | Mean Difference (IV, Fixed, 95% CI) | 6.14 [1.32, 10.96] |
| 16 FVC (L) ‐ absolute or change | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.28, 0.32] |
| 16.1 Inpatient (treatment > 3 days) | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.28, 0.32] |
| 17 FVC (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 Inpatient (treatment > 3 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 FEV1/FVC (%) | 3 | 189 | Mean Difference (IV, Fixed, 95% CI) | 1.07 [‐2.11, 4.26] |
| 18.1 Inpatient (treatment > 3 days) | 3 | 189 | Mean Difference (IV, Fixed, 95% CI) | 1.07 [‐2.11, 4.26] |
| 19 PEF (L/minute) ‐ absolute | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 119.06 [64.39, 173.73] |
| 19.1 Inpatient (treatment > 3 days) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 198.0 [81.58, 314.42] |
| 19.2 Outpatient | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 96.72 [34.79, 158.65] |
| 20 Transitional dyspnoea index (change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.1 Outpatient | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 Dyspnoea score walking (change, VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 21.1 Inpatient (treatment > 3 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 Overall dyspnoea score | 4 | 301 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.05, 0.41] |
| 22.1 Inpatient (treatment > 3 days) | 3 | 154 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.31, 0.32] |
| 22.2 Outpatient | 1 | 147 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.37 [0.04, 0.69] |
| 23 PaO2 (mmHg) ‐ change or absolute | 4 | 200 | Mean Difference (IV, Fixed, 95% CI) | 6.86 [2.75, 10.96] |
| 23.1 Inpatient (treatment > 3 days) | 4 | 200 | Mean Difference (IV, Fixed, 95% CI) | 6.86 [2.75, 10.96] |
| 24 PaCO2 (mmHg) ‐ absolute | 3 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐1.81 [‐5.06, 1.44] |
| 24.1 Inpatient (treatment > 3 days) | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐4.17, 2.97] |
| 24.2 ICU | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐7.60 [‐15.41, 0.21] |
| 25 Overall quality of life score (CRQ) (change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 25.1 Outpatient | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26 Physical capacity (m) (6‐minute walk distance) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 26.1 Inpatient (treatment > 3 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 27 Length of stay (days) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 27.1 Inpatient (treatment > 3 days) | 2 | 296 | Mean Difference (IV, Fixed, 95% CI) | ‐1.22 [‐2.26, ‐0.18] |
| 27.2 ICU | 2 | 300 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [‐0.84, 2.15] |
| 28 Duration of mechanical ventilation (days) | 2 | 300 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐3.44, 1.38] |
| 28.1 ICU | 2 | 300 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐3.44, 1.38] |
| 29 Adverse drug effects | 8 | 736 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.59, 3.43] |
| 29.1 Inpatient (treatment > 3 days) | 6 | 613 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.59, 3.43] |
| 29.2 Inpatient (treatment 1 day) | 1 | 96 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 Outpatient | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Adverse effect ‐ hyperglycaemia (30 days) | 6 | 804 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.79 [1.86, 4.19] |
| 30.1 Inpatient (treatment > 3 days) | 3 | 369 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.00 [2.96, 21.63] |
| 30.2 Outpatient | 1 | 135 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.14, 7.43] |
| 30.3 ICU | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.33, 3.43] |
| 31 Adverse effect ‐ hypertension | 2 | 274 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.44, 3.25] |
| 31.1 Inpatient (treatment > 3 days) | 1 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.59, 7.95] |
| 31.2 ICU | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.08, 2.54] |
| 32 Adverse effect ‐ gastrointestinal bleeding | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 32.1 ICU | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 33 Adverse effect ‐ dyspepsia | 2 | 185 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.45, 3.21] |
| 33.1 Inpatient (treatment > 3 days) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.18, 7.89] |
| 33.2 Outpatient | 1 | 135 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.38, 3.79] |
| 34 Adverse effect ‐ weight gain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 34.1 Outpatient | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 35 Adverse effect ‐ depression | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 35.1 Outpatient | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 36 Adverse effect ‐ anxiety | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 36.1 Outpatient | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 37 Adverse effect ‐ psychiatric disorder | 2 | 331 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.95, 4.88] |
| 37.1 Inpatient (treatment > 3 days) | 1 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.4 [0.56, 10.35] |
| 37.2 Outpatient | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.77, 5.50] |
| 38 Adverse effect ‐ insomnia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 38.1 Outpatient | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 39 Adverse effect ‐ delirium | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 39.1 ICU | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 40 Adverse effect ‐ secondary infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 40.1 ICU | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 41 Adverse effect ‐ ventilator‐associated pneumonia | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.44, 3.40] |
| 41.1 ICU | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.44, 3.40] |
1.7. Analysis.
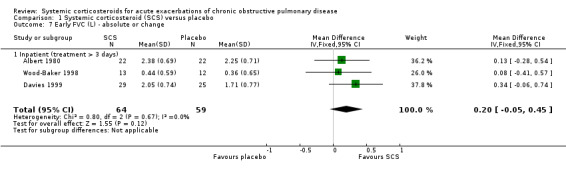
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 7 Early FVC (L) ‐ absolute or change.
1.8. Analysis.
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 8 Early FVC (% predicted).
1.9. Analysis.
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 9 Early FEV1/FVC (%).
1.16. Analysis.
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 16 FVC (L) ‐ absolute or change.
1.17. Analysis.
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 17 FVC (% predicted).
1.18. Analysis.
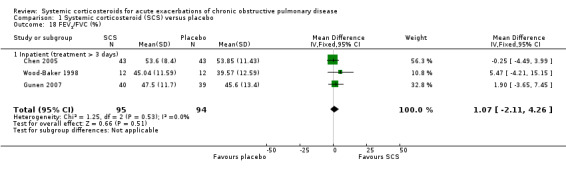
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 18 FEV1/FVC (%).
1.21. Analysis.
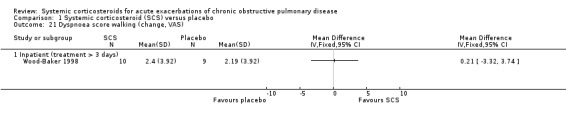
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 21 Dyspnoea score walking (change, VAS).
1.25. Analysis.
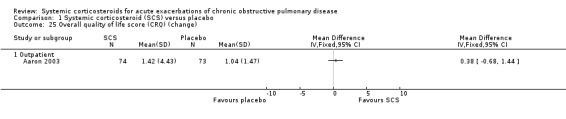
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 25 Overall quality of life score (CRQ) (change).
1.26. Analysis.
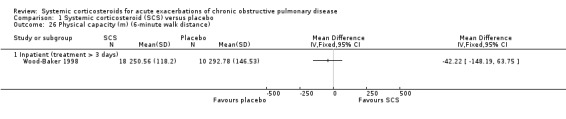
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 26 Physical capacity (m) (6‐minute walk distance).
1.28. Analysis.
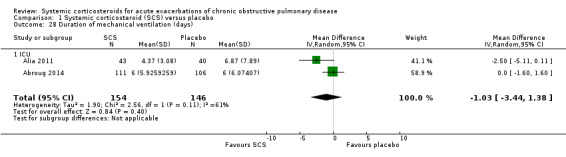
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 28 Duration of mechanical ventilation (days).
1.33. Analysis.
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 33 Adverse effect ‐ dyspepsia.
1.34. Analysis.
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 34 Adverse effect ‐ weight gain.
1.35. Analysis.
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 35 Adverse effect ‐ depression.
1.36. Analysis.
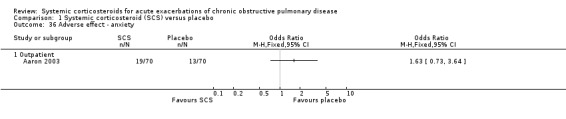
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 36 Adverse effect ‐ anxiety.
1.38. Analysis.
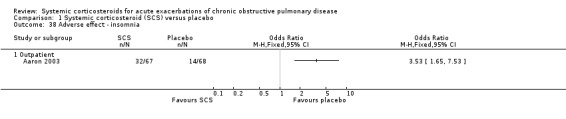
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 38 Adverse effect ‐ insomnia.
1.39. Analysis.
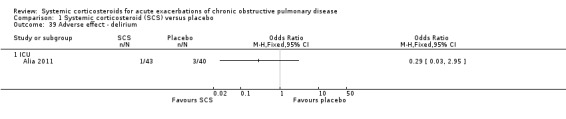
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 39 Adverse effect ‐ delirium.
1.40. Analysis.
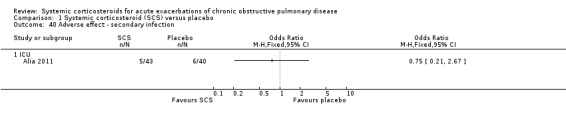
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 40 Adverse effect ‐ secondary infection.
1.41. Analysis.
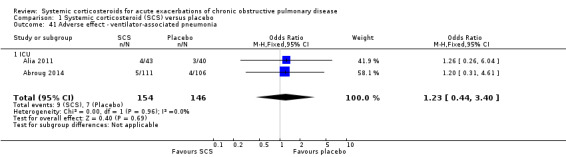
Comparison 1 Systemic corticosteroid (SCS) versus placebo, Outcome 41 Adverse effect ‐ ventilator‐associated pneumonia.
Comparison 2. Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 3 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.30] |
| 1.1 Inpatient treatment | 3 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.30] |
| 2 Relapse | 3 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.50, 1.80] |
| 2.1 Inpatient treatment | 3 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.50, 1.80] |
| 3 Mortality after discharge (1‐3 months) | 3 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.44, 4.51] |
| 3.1 Inpatient treatment | 3 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.44, 4.51] |
| 4 Early FEV1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Early FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Early dyspnoea score (VAS) | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [‐0.55, 1.78] |
| 6.1 Inpatient treatment | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [‐0.55, 1.78] |
| 7 Early cough score (VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Early sputum volume score (VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 FEV1 (L) ‐ absolute or change | 3 | 285 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.08, 0.03] |
| 9.1 Inpatient treatment (absolute) | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.24, 0.17] |
| 9.2 Inpatient treatment (change) | 1 | 210 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.08, 0.04] |
| 10 FVC (L) ‐ absolute | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.33, 0.22] |
| 10.1 Inpatient treatment | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.33, 0.22] |
| 11 FEV1/FVC ratio | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Dyspnoea score (VAS) at 7‐10 days | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [‐0.24, 2.80] |
| 12.1 Inpatient treatment | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [‐0.24, 2.80] |
| 13 Cough score (VAS) at 7 days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Sputum volume score (VAS) at 7 days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 PaO2 (mmHg) at 7 days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 PaCO2 (mmHg) at 7 days | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 Inpatient treatment | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 5.5 [‐0.79, 11.79] |
| 17 Health status: Clinical COPD Questionnaire (change at 1 week) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 Inpatient treament | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Quality of life: SGRQ (change at 7 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 Inpatient treament | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Quality of life: CRQ Dyspnoea (change at 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1 Inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Quality of life: CRQ Fatigue (change at 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.1 Inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 Quality of life: CRQ Mastery (change at 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 21.1 Inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 Quality of life: CRQ Emotion (change at 4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 22.1 Inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 Duration of hospitalisation (days) | 3 | 298 | Mean Difference (IV, Fixed, 95% CI) | 1.54 [‐0.09, 3.17] |
| 23.1 Inpatient treatment | 3 | 298 | Mean Difference (IV, Fixed, 95% CI) | 1.54 [‐0.09, 3.17] |
| 24 Adverse effect ‐ hyperglycaemia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24.1 Inpatient treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 Adverse effect ‐ hypertension | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 25.1 Inpatient treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.4. Analysis.
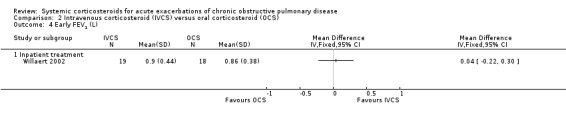
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 4 Early FEV1 (L).
2.5. Analysis.
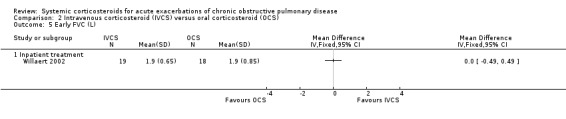
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 5 Early FVC (L).
2.7. Analysis.
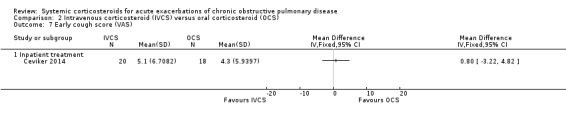
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 7 Early cough score (VAS).
2.8. Analysis.
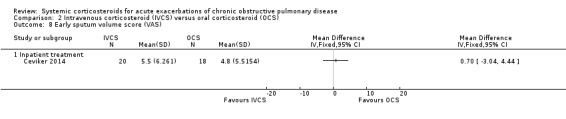
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 8 Early sputum volume score (VAS).
2.11. Analysis.
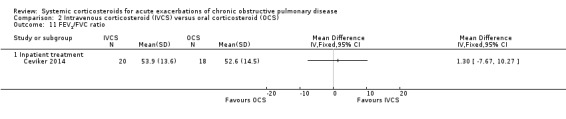
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 11 FEV1/FVC ratio.
2.13. Analysis.
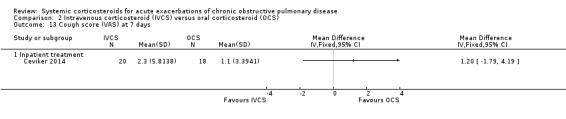
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 13 Cough score (VAS) at 7 days.
2.14. Analysis.
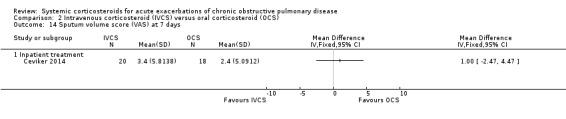
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 14 Sputum volume score (VAS) at 7 days.
2.19. Analysis.
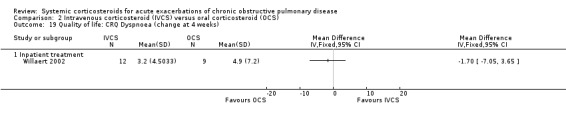
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 19 Quality of life: CRQ Dyspnoea (change at 4 weeks).
2.20. Analysis.
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 20 Quality of life: CRQ Fatigue (change at 4 weeks).
2.21. Analysis.
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 21 Quality of life: CRQ Mastery (change at 4 weeks).
2.22. Analysis.
Comparison 2 Intravenous corticosteroid (IVCS) versus oral corticosteroid (OCS), Outcome 22 Quality of life: CRQ Emotion (change at 4 weeks).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aaron 2003.
| Methods | Design: parallel group
Duration: treatment for 10 days Setting: ED of 10 participating hospitals in Canada |
|
| Participants | Number screened: 1087 of which 202 eligible Number randomised: 147 (74 prednisone/2 withdrew, 2 hospitalised; 73 placebo/1 withdrew, 1 hospitalised, 1 lost to follow‐up) Number completed: 140 evaluated at 30 days Baseline details: Mean age (years): prednisone group: 68.9 ± 11.2; placebo group: 69.9 ± 10.4 Gender: M = 84, F = 63 Diagnosis COPD: evidence of irreversible airflow obstruction in the ED, with FEV1/FVC < 0.70, an FEV1 < 70% predicted value, post‐bronchodilator improvement in FEV1 of < 20% AE criteria: clinical diagnosis of recent exacerbation of COPD Inclusion criteria: age > 35 years; 15 pack‐years' smoking history; evidence of irreversible airflow obstruction in the ED, with FEV1/FVC < 0.70, an FEV1 < 70% predicted value, post‐bronchodilator improvement in FEV1 of < 20% Exclusion criteria: subsequent admission to hospital from ED; diagnosis of asthma or atopy; use of oral or IV corticosteroids within the preceding 30 days; received oral or IV corticosteroids in the ED; findings on chest radiography consistent with the presence of pneumonia or congestive heart failure; adverse reactions to oral corticosteroids; severe uncontrolled diabetes mellitus or renal, hepatic or cardiac failure |
|
| Interventions | Experimental: prednisone 40 mg capsule (oral admin), once daily for 10 days (10D SS course)
Control: matched placebo, identical in taste and appearance once daily for 10 days
Co‐interventions: trimethoprim 160 mg with sulphamethoxazole 800 mg twice daily or if allergic doxycycline 100 mg twice daily. Inhaled albuterol and inhaled ipratropium bromide Confounders: inhaled corticosteroids and all other medications used by the participants at the time of enrolment were continued throughout the study in both groups |
|
| Outcomes | Primary outcome: treatment failure defined as an unscheduled visit to a doctor's clinic or a return to the ED because of worsening dyspnoea within 30 days after randomisation Secondary outcomes: change from day 1 to day 10 in FEV1, severity of dyspnoea and disease‐specific QoL Measurement: treatment failure participants assessed 3, 10 and 30 days after randomisation. Secondary outcomes assessed on days 1 and 10. Analysed: relapse rates, FEV1 (post‐bronchodilator measurements), severity of dyspnoea (transitional dyspnoea index), disease‐specific QoL (Chronic Respiratory Disease Index Questionnaire); adverse effects of prednisone Reported: hyperglycaemia as an adverse effect Mortality: 2 in prednisone group, 1 in placebo group Adverse effects: increased appetite, weight gain, insomnia, hyperglycaemia (subjectively reported but not objectively measured as not part of protocol) |
|
| Notes | Likelihood of COPD: age > 35 years, > 15 pack‐years' smoking history, diagnosis of asthma exclusion, evidence of fixed airflow obstruction at presentation Funding: grants from the Canadian Institutes of Health Research (MCT‐41545), the Ontario Ministry of Health Emergency Health Services Research Advisory Committee (13098), the Ontario Thoracic Society and the Canadian Institute of Health Research 21st Century Chairs Program (to Dr. Rowe). Authors declared previous support from pharmaceutical companies; no notable conflict |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random listing of the 2 treatment assignments blocked in groups of 4 and stratified according to the ED |
| Allocation concealment (selection bias) | Low risk | Central allocation of a randomisation schedule prepared through a computer‐generated random listing of the 2 treatment assignments blocked in groups of 4 and stratified according to the ED. Randomisation occurred at the time of discharge from the ED. Neither research staff nor participants were aware of the treatment assignment before randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither research staff nor participants were aware of the treatment assignment before or after randomisation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals accounted for with reasons, 4 in each group |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods all reported |
| Free of other potential confounders? | Low risk | Inhaled corticosteroids and all other medications used by the participants at the time of enrolment were continued throughout the study in both groups. Characteristics of both groups were similar at baseline |
Abroug 2014.
| Methods | Design: parallel group open‐label randomised study
Duration: conducted 2008‐2011 Setting: ICU in 2 tertiary teaching hospitals: CHU Fattouma Bourguiba, Monastir and CHU Tahar Sfar, Mahdia, affiliated with the University of Monastir, Tunisia |
|
| Participants | Number screened: 518 Number randomised: 217, 111 prednisone, 106 control Number completed: 217 Baseline details: Age median (IQR) (years): prednisone 70 (63‐75), control 68 (63‐75) Gender: prednisone M = 99, F = 12; control M = 92, F = 14 Baseline FEV1 median (IQR) (L): prednisone 0.82 (0.59‐1.12), control 0.75 (0.57‐0.95) GOLD stage COPD: prednisone stage III = 30% stage IV = 70%; control stage III = 27%, stage IV = 73% LTOT: prednisone 72%, control 67% Diagnosis COPD: airflow obstruction post‐bronchodilator ratio FEV1/FVC < 0.7, previously documented or present on discharge from ICU AE criteria: change in participant's baseline dyspnoea, cough or sputum (or both) requiring a change in regular medication with acute respiratory failure defined by severe hypoxaemia PaO2 < 60 mmHg or arterial oxygen saturation < 90% on room air associated with hypercapnia (or both), PaCO2 ≥ 45 mmHg associated with pH ≤ 7.35 and clinical signs of excessive respiratory muscle activity (contraction of accessory respiratory muscles and respiration rate ≥ 25 breaths/minute) Inclusion criteria: all participants aged > 40 years, ≥ 10 pack‐years' smoking history, with known or strongly suspected COPD admitted to participating ICU for AE COPD with hypercapnic acute respiratory failure requiring ventilatory support Exclusion criteria: evidence of pneumonia, treated for COPD, people with asthma defined by a reversible obstructive disease following nebulised bronchodilators, uncontrolled left heart failure, systemic corticosteroids for exacerbation within 30 days prior to screening, absolute contraindication to corticosteroids (active gastroduodenal ulcer, severe uncontrolled sepsis, hepatitis or other active viral disease or neuromuscular disease (or both)) |
|
| Interventions | Experimental: oral prednisone 1 mg/kg daily either until discharge or for a maximum of 10 days, median duration 8 days (IQR 5‐10) Control: usual care (undefined) Co‐interventions: all included participants received ventilatory support (NIV or conventional) prednisone NIV 76%, conventional 24%: control NIV 76%, conventional 24% Nebulised beta2‐agonists (terbutaline, 5 mg every 6 hours) and ipratropium bromide (0.5 mg every 8 hours) Antibiotics prescribed at discretion of physician in charge: prednisone 82%, control 79%. Treatment period: up to 10 days Follow‐up period: maximum 30 days |
|
| Outcomes | Primary outcome: ICU mortality Secondary outcomes: duration of ventilator support (sum of conventional and NIV if ventilated with both); length of ICU stay; rate of NIV failure (intubation rate in participants managed initially with NIV); corticosteroidal complications: occurrence of secondary infections, hyperglycaemic episodes necessitating initiation of insulin therapy (corresponding to a blood glucose level ≥180 mg/dL in participants without pre‐existing diabetes) or increase in initial insulin therapy, ICU‐acquired muscular weakness, or significant gastrointestinal bleeding inducing a fall in the haemoglobin level ≥ 2 g/dL | |
| Notes | Trial registration: Clinicaltrials.gov/NCT01353235. Date of registration 17 May 2011 Protocol: not available. Trial approved by the ethics committee of both centres. Sample size target 300, but study was ended before completion of the planned sample size because of the slow inclusion rate Length of stay and duration of mechanical ventilation published as median and IQR. Mean and SD assumptions based on Cochrane Handbook for Systematic Reviews of Interventions Section 7.7.3.5. The width of the IQR is approximately 1.35 SDs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed at each centre by a random number table, and was stratified according to the type of mechanical ventilation (either conventional or NIV) |
| Allocation concealment (selection bias) | Low risk | Participants were randomised (by means of sealed envelopes that were opened sequentially) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study, control group received usual care, no placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open‐label study, control group received usual care. Outcomes of mortality and duration of ICU stay were objective endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were included in outcome measures |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in methods and trial registration were reported |
| Free of other potential confounders? | Unclear risk | Study halted early because of the slow inclusion rate |
Albert 1980.
| Methods | Design: parallel group
Setting: people with chronic airflow limitation hospitalised due to acute respiratory insufficiency from acute and chronic bronchitis, Veterans Hospital, Washington, USA Duration: treatment for 72 hours |
|
| Participants | Number screened: 45 Number randomised: 44 Number completed: 40 Baseline details: Mean age (years): 61.5 Gender: M = 44, F = 0 Diagnosis COPD: chronic bronchitis defined as cough and sputum production most days for at least 3 months of the 2 previous years, chronic airflow obstruction ‐FEV1 < 60% predicted or FEV1/FVC < 60% AE criteria: hospitalised with acute respiratory insufficiency, acute bronchitis defined as an increase in cough and sputum production within 5 days Inclusion criteria: acute respiratory insufficiency, PaO2 < 65 mmHg on room air (after correcting for hyperventilation) or PaCO2 > 50 mmHg with pH < 7.35 or alveolar‐arterial oxygen difference > 10 mmHg above that within the previous 2 years; chronic bronchitis defined as cough and sputum production most days for at least 3 months of the 2 previous years; FEV1 < 60% predicted or FEV1/FVC < 60% Exclusion criteria: personal or family history of asthma, increase in FEV1 of > 30% after inhaled bronchodilator when clinically stable; history of eczema or allergic rhinitis, consolidation on admission chest X‐ray, corticosteroid therapy within the last 30 days |
|
| Interventions | Experimental: methylprednisolone (Upjohn) 0.5 mg/kg, IV, 6 hourly, for 72 hours (3D Systemic Steroid course)
Control: matched placebo, IV, 6 hourly, for 72 hours Co‐interventions: IV aminophylline, inhaled isoproterenol, IV ampicillin or oral tetracycline, supplemental oxygen to maintain SaO2 above 85% |
|
| Outcomes | Analysed: pre‐ and post‐bronchodilator bedside spirometry. Reported: ABG, serum theophylline concentration, blood glucose concentration Mortality: 2 participants in the corticosteroid group (at 30 hours and 9 days) Morbidity: experimental group; psychosis (n = 1), gastrointestinal bleed (n = 1). Control group; dermatitis (n = 1), pneumonia (n = 1), gastrointestinal bleed (n = 1) In comparison of treatments, the post‐bronchodilator spirometric measurements at 72 hours were used for analysis. The spirometric measurements were estimated from the baseline values and graphical changes at 72 hours. The baseline SD was used for all time points |
|
| Notes | Likelihood of COPD: no age limitations, no smoking history required, history of asthma exclusion, airflow obstruction previously or 1 month after acute presentation Funding: no external sources declared. In‐house study assistance from the Veterans Administration Hospital, Washington |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by table of random numbers using 20 subject blocks. Participants allocated to consecutive numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation by table of random numbers using 20 subject blocks |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants, investigators, respiratory therapists and other hospital personnel were unaware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, respiratory therapists and other hospital personnel were unaware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was not randomised. Data from 2 participants in the placebo group were not analysed |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in methods are reported |
| Free of other potential confounders? | Low risk | None identified |
Alia 2011.
| Methods | Design: parallel group Duration: recruitment began in June 2005 and concluded in July 2009. Participants were enrolled during a mean time of 19.6 months (range 5‐49 months). Setting: 8 hospitals in 4 countries (Hospital Universitario de Getafe, Hospital Fundación Alcorcón, Hospital Clinic de Barcelona, Consorci Hospitalari Parc Taulí, and Hospital Principe de Asturias in Spain; Hospital ABC in Mexico; Clinica Universitaria Bolivariana in Colombia and University of Texas Health Science Center in the USA (University Hospital and Audie L. Murphy Veterans Affairs Hospital)) |
|
| Participants | Number screened: 354 Number randomised: 83 Number completed: 83 Baseline details: Age (years): methylprednisolone 69.1 (SD 9.7), control 67.6 (SD 10.7) Gender: M = methylprednisolone 32 (74%), control 34 (85%) Diagnosis COPD: known COPD and hospitalised with exacerbation that required ventilator support either conventional with intubation or NIV AE criteria: defined as the presence of ≥ 2 of: worsening dyspnoea, increase in sputum purulence or increase in sputum volume Inclusion criteria: acute hypercapnic respiratory failure (pH < 7.35, with a PaCO2 > 45 mmHg) requiring invasive or non‐invasive mechanical ventilation Exclusion criteria: asthma or atopy; use of systemic corticosteroids within the preceding month; use of systemic corticosteroids for the treatment of COPD exacerbation for > 24 hours at the time of randomisation; clinical or radiological evidence of pneumonia; uncontrolled LVF requiring the use of inotropes or vasoactive drugs; uncontrolled arterial hypertension; uncontrolled diabetes mellitus; neuromuscular disease; allergy or adverse reaction (or both) to corticosteroid therapy |
|
| Interventions | Experimental: methylprednisolone 0.5 mg/kg every 6 hours for 72 hours, 0.5 mg/kg every 12 hours on days 4‐6, and 0.5 mg/kg/day on days 7‐10 Control: placebo normal saline 50 mL IV Co‐interventions: inhaled beta2‐ agonist (salbutamol 2.5 mg every 6 hours or 2 puffs from an MDI at least 4 times daily) and inhaled ipratropium bromide (0.5 mg every 6 hours or 2 puffs from an MDI at least 4 times daily). Any participant who was receiving inhaled corticosteroid therapy before randomisation was continued on this therapy. Systemic antibiotics were used at the judgement of the treating physicians Treatment period: 10 days Follow‐up period: not reported |
|
| Outcomes | Primary outcomes: duration of mechanical ventilation, length of ICU stay, need for intubation in people treated with NIV Secondary outcomes: length of hospital stay, ICU mortality Adverse events: secondary infection, gastrointestinal bleeding, arterial hypertension, hyperglycaemia, hospital acquired pneumonia |
|
| Notes | Likelihood of COPD: no age restrictions, no criterion for required pack history, diagnosis of asthma exclusion, required ventilator support although no specified threshold for fixed airflow obstruction at presentation Trial registration: ClinicalTrials.gov/NCT01281748 Financial disclosure: none reported. Funding/Support: study funded in part by grant PI041233 from Fondo de Investigación Sanitaria Study lasted 5 years because of a lower enrolment rate, mainly due to a reduction in ICU admissions of people with COPD exacerbations and a high rate of exclusion Protocol: days 1‐5: ABG analysis, plasma C‐reactive protein level, white blood cell count, maximal blood glucose level, daily dose insulin, intrinsic positive end expiratory pressure (intubated participants) Emailed data request to authors ‐ 7 September 2013 (Esteban). Data provided for outcomes ‐ length of hospital stay, ICU stay and mechanical ventilation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed by hospital pharmacy at each centre by a random number table with permuted blocks of 4, with stratification according to the type of mechanical ventilation (conventional or NIV) |
| Allocation concealment (selection bias) | Low risk | Allocation schedule was concealed with sealed envelopes that were opened sequentially |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Pharmacists dispensed the IV medications in a blinded manner. Nurses who were administering the medications, the physicians who were caring for the participants, were unaware of the treatment assignments |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The local investigators and research personnel who collected the data were unaware of the treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants' data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. ClinicalTrials.gov/NCT01281748 |
| Free of other potential confounders? | Low risk | Study lasted 5 years because of a lower enrolment rate, mainly due to a reduction in ICU admissions of people with COPD exacerbations and a high rate of exclusion. Authors do not believe that this limitation affected the study findings, main change in practice over time was higher use of NIV |
Bullard 1996.
| Methods | Design: parallel group Duration: recruited March‐August 1993, and November 1993‐February 1994. Treatment for up to 8 days then follow‐up for 2 weeks Setting: single‐centre tertiary hospital, Chang Gung Memorial Hospital, Linkou, Taiwan, ED with subsequent admission if required |
|
| Participants | Number screened: not reported Number randomised: 138 (18 excluded on chest X‐ray ‐ 6 cardiac asthma, 2 pneumothoraces, 5 pneumonia, 3 severe bronchiectasis, 2 bronchogenic cancer. 1 participant in placebo group died, 2 incorrect diagnosis, 2 withdrew consent). Number completed: 113 26 participants were discharged from the ED within 24 hours (16 in the corticosteroid group and 10 in the placebo group) Baseline details: Mean age (years): 66 Gender: M = 97, F = 16 Diagnosis COPD: no details of previous clinical COPD diagnosis AE criteria: moderate‐to‐severe dyspnoea presenting to ED, FEV1 < 60% predicted or FEV1/FVC < 60% Inclusion criteria: > 40 years of age; suspected chronic airflow limitation, presenting with dyspnoea and a FEV1 < 60% predicted or FEV1/FVC < 60% Exclusion criteria: pneumothorax, pneumonia, intubation within 2 hours, hospitalisation for a co‐existent disease, known corticosteroid use |
|
| Interventions | Experimental: hydrocortisone 100 mg, IV, within 15 minutes of arrival. Hydrocortisone 100 mg IV 4 hourly for 4 days for those participants hospitalised. Participant received prednisolone 40 mg, orally, daily for 4 days either on discharge from the ED or if admitted having completed the IV therapy (5D‐8D systemic steroid treatment)
Control: IV placebo administered within 15 minutes of arrival. Subsequent treatment not clearly reported Co‐interventions: inhaled fenoterol, inhaled ipratropium bromide, IV aminophylline Confounders: IV hydrocortisone could be administered at the discretion of chest medicine physicians. 12 participants received cross‐over treatment or had their treatment stopped after 24 hours |
|
| Outcomes | Analysed: FEV1 (at 6 hours) published results for lower limit of 95% CI control group missing a minus sign as 95% CI in the paper does not include the point estimate as reported; PEF (at 6 hours) published results for lower limit of 95% CI control group missing a minus sign as 95% CI in the paper does not include the point estimate as reported Reported: admissions, discharges from the ED within 24 hours, serum aminophylline concentration, re‐attendance to the ED, subjective assessment of dyspnoea. Others: none reported Mortality: 3 deaths in corticosteroid group, 2 in the placebo. Morbidity: not reported |
|
| Notes | Likelihood of COPD: people > 40 years of age, no requirement for past smoking, asthma not an exclusion, chronic airflow limitation suspected Funding: no sources of funding declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was used to generate randomisation codes |
| Allocation concealment (selection bias) | Low risk | Allocation was performed using consecutive allocation of participants to pre‐packaged treatments based on random code |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants were blinded to treatment allocation. Investigators did not break the code during the course of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16/60 corticosteroid group and 10/53 of placebo group were discharged from ED within 24 hours. 12 participants had a physician‐directed change to their study intervention following admission to hospital (either given corticosteroids or corticosteroids stopped). Treatment failure defined as a return to the ED within 14 days. The author of this study noted that results of treatment administered in the ED and measured in the first 24 hours were reliable, later measurement of outcomes was affected by breaks in protocol after admission |
| Selective reporting (reporting bias) | Low risk | All outcomes supplied by author |
| Free of other potential confounders? | High risk | IV hydrocortisone could be administered at the discretion of chest medicine physicians. 12 participants received cross‐over treatment or had their treatment stopped after 24 hours |
Ceviker 2014.
| Methods | Design: parallel group Duration: 3 months (following discharge) Setting: State Hospital, Gumushane, Turkey | |
| Participants | Number screened: not available Number randomised: 40 Number completed: 38 Number withdrawals: 2 (developed severe respiratory failure) Baseline details: Mean age (years): oral group: 69.0 (± 10.5), IV group: 67.1 (±8.4) Smoking history: oral group: 56.3 (±38.8), IV group: 69.0 (±38.5) Diagnosis COPD: previous documented diagnosis of COPD AE criteria: presence of an exacerbation, which, in the opinion of the attending physician, necessitated admission to hospital Inclusion criteria: aged > 40 years, previous documented diagnosis of COPD, ≥ 10 pack‐years' smoking history, exacerbation requiring hospitalisation based on opinion of attending doctor Exclusion criteria: the presence of pneumonia, uncontrolled hypertension or diabetes mellitus, previously diagnosed bronchiectasis, need for mechanical ventilation, use of systemic corticosteroids during the preceding month and a history of gastrointestinal bleeding during the preceding 3 months |
|
| Interventions | Group 1: oral methylprednisolone 32 mg/day Group 2: IV methylprednisolone 1 mg/kg/day for 4 days, then 0.5 mg/kg/day for 3 days Co‐interventions: salbutamol 2.5 mg and Ipratropium 0.5 mg ‐ every 6 hours, antibiotics (if meet Anthonisen criteria), nasal oxygen (according to attending physician's decision) Treatment period: 7 days Follow‐up period: 3 months |
|
| Outcomes | Reported:
During treatment period:
Pre‐ and post‐bronchodilator spirometry: days 7, 90
ABGs: days 2, 4, 7, 90
Symptom scores: days 2, 4, 7.
Adverse events: daily questioning of the participants and daily measurements of blood pressure and blood glucose levels during the treatment period During 3 months' follow‐up: visits to the ED or unplanned visits to family physicians and admission to hospital Treatment failure defined as severe respiratory failure requiring mechanical ventilation or need for systemic corticosteroids beyond 7 days intervention period Relapse defined as re‐admission for COPD during 12‐week follow‐up No legend was provided for certain outcomes (ABG, symptom scores, lung function tests, length of hospital stay). Data for symptom score were obtained from a graph ‐ confirmation is required |
|
| Notes | Likelihood of COPD: aged > 40 years for inclusion, > 10 pack‐years' smoking history, asthma not an exclusion, previously documented COPD diagnosis, no criteria specified for airflow obstruction Funding: no sources of funding declared Trial registration: not located, pre‐study protocol not available Emailed data request to authors ‐ 7 September 2013, 30 September 2013 (Ceviker). No response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomised to 1 of 2 treatment regimens according to a previously prepared randomisation list |
| Allocation concealment (selection bias) | Unclear risk | This list was kept by 1 of the investigators (AS), who was also in contact with the nurses responsible for the treatment of the participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No use of placebo, participants and hospital staff not blind to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participant interviews and measurements were performed by another investigator (YC), who remained blinded to the treatment regimens |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals (2 participants) accounted for (reasons supplied), otherwise, all data reported |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in methods are reported but protocol not available |
| Free of other potential confounders? | Low risk | Characteristics of both groups were similar at baseline |
Chen 2005.
| Methods | Design: parallel group Duration: 14 days Setting: inpatients, unknown number of centres, China | |
| Participants | Number screened: not reported Number randomised: 130 Number completed: 121 Baseline details: Mean age (years): 7‐day corticosteroids 70.3, 14‐day corticosteroids 71.7, placebo 73.0 (not significantly different) Age range: not reported Gender: M = 98, F = 32 Diagnosis COPD: criteria for COPD not reported AE criteria: not reported Diagnosis and severity: FEV1 (L) baseline 7‐day corticosteroids 0.79 (SD 0.25), 14‐day corticosteroids 0.74 (SD 0.21), placebo 0.74 (SD 0.32), baseline PaO2 (mmHg) 7‐day corticosteroids 69.06 (SD 11.54), 14‐day corticosteroids 68.07 (SD 13.53), placebo 67.48 (SD 11.61) Current smokers: 7‐day corticosteroids 18/44 (41%), 14‐day corticosteroids 20/43 (47%), placebo 18/43 (42%) (not significantly different) Inclusion criteria: not reported Exclusion criteria: not reported, people with asthma excluded: not reported, bronchodilator reversibility tested: not reported |
|
| Interventions | Group 1 prednisolone 30 mg/day 7 days + placebo 7 days Group 2 prednisolone 30 mg/day 10 days + 15 mg/day 5 days Group 3 placebo 14 days Delivery: oral Co‐interventions permitted/dose: not reported Co‐interventions not permitted: not reported | |
| Outcomes | Outcomes measured: lung function, ABG measurement, days hospitalisation, symptoms scores, rate of treatment failure, side effects corticosteroids, rate of relapse Outcomes reported: FEV1, FEV1/FVC ratio, PEF, PaO2, number relapsed, number failed treatment, number side effect Follow‐up assessment points: not reported Prednisolone 14‐day treatment group data used in this review |
|
| Notes | Full paper published in Chinese 2008. Abstract publication 2005. Study and outcome data also provided by authors
Likelihood of COPD: unknown if age threshold, unknown pack‐years' smoking threshold, asthma an exclusion, fixed airflow obstruction, FEV1/FVC < 70%, FEV1 < 80% predicted Funding: no sources disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stochastic function used to create 150 numbers 0‐1. Randomisation code prepared by an assistant not involved in other parts of study. Sealed envelopes prepared before study initiation by assistant |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used and allocated by third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of identical boxes and medications. Allocated by third party. Researchers blinded to medications |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Description of withdrawals/drop‐outs given. Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Not fully published, no protocol |
| Free of other potential confounders? | Low risk | Information not reported |
Cordero 1996.
| Methods | Design: parallel group randomised controlled study Duration: conducted April‐September 1995 Setting: outpatients, University Hospital La Fe, Valencia, Spain | |
| Participants | Number screened: not reported Number randomised: 30 Number completed: Baseline details: M = 30, F = 0 Median age (range) (years): not reported Diagnosis COPD: airflow obstruction FEV1 > 70% predicted or FEV1/FVC < 60% predicted AE criteria: not described Inclusion criteria: male, > 50 years, airflow obstruction Exclusion criteria: asthma or atopy |
|
| Interventions | Experimental: oral prednisone 40 mg daily 10 days Control: placebo 10 days Co‐interventions: standardised treatment, inhaled beta‐agonists, anticholinergics, inhaled corticosteroids, antibiotics, physiotherapy Treatment period: 10 days Follow‐up period: 14 days |
|
| Outcomes | Reported: spirometry, PEF, ABG, PaO2, Borg dyspnoea scale Measurements: days 1, 7, 14 | |
| Notes | Likelihood of COPD: > 50 years for inclusion, unknown pack‐years' smoking history, unknown if asthma an exclusion, fixed airflow obstruction, FEV1/FVC < 60%, FEV1 < 70% predicted Funding: no sources disclosed Protocol: not available, no registration located Published as conference abstract only. Emailed data request to authors 20 September 2012, 9 August 2013 (Cordero), 16 August 2013 (Sole). No response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised controlled study, method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Results data incomplete in abstract |
| Selective reporting (reporting bias) | Unclear risk | No details, protocol not available |
| Free of other potential confounders? | Unclear risk | No information on group characteristics at baseline |
Davies 1999.
| Methods | Design: parallel group Duration: follow‐up for 6 weeks Setting: single centre, people presenting to an ED of a university hospital, Liverpool UK | |
| Participants | Number screened: 246 Number randomised: 60; prednisolone 29, placebo 29 Number completed: 27; prednisolone (1 withdrew, 1 died), 22 placebo (5 withdrew, 1 died, 1 lost to follow‐up) Baseline details: Mean age (years): 67 Gender: M = 34, F = 16 Diagnosis COPD: not specified AE criteria: increased breathlessness and 2 of following criteria: increased sputum ‐ volume or purulence, cough frequency or severity, increased wheeze Inclusion criteria: diagnosis of COPD, aged 40‐80 years. FEV1 < 70% and FEV1/FVC < 75% Exclusion criteria: personal/family history of asthma, uncontrolled LVF, pneumonia, oral corticosteroids within last month, pH < 7.26 |
|
| Interventions | Experimental: prednisolone 30 mg orally for 14 days
Control: matched placebo Treatment for 14 days Co‐interventions: nebulised salbutamol and ipratropium bromide, oxygen and antibiotics at physician's judgement |
|
| Outcomes | FEV1; FVC; visual analogue scale of symptoms; length of hospitalisation data removed due to skewed data, was previously mentioned in text, but not data analysis; ABG, sputum culture Treatment failure: defined arterial PH falling < 7.26, physician or participant decision lack of progress requiring further treatment, QoL (SGRQ) Measurements: daily symptoms, lung function 5 days, 6 weeks SGRQ, AE Mortality: 2 deaths, 1 in each treatment group Adverse drug effects: heartburn and glycosuria |
|
| Notes | Likelihood of COPD: aged > 40 years, > 20 pack‐years' smoking history, history of asthma exclusion, evidence of fixed airflow obstruction Funding: Glaxo Wellcome supplied oral corticosteroids and placebo. Fazakerley Foundation for funding research of L. Davies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by hospital pharmacy according to random number table |
| Allocation concealment (selection bias) | Low risk | Randomisation by random number table by hospital pharmacy and allocation to consecutive participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants, investigators, respiratory physicians, technicians and other hospital staff were masked to treatment status until the end of the study. Use of identical placebo tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants, investigators, respiratory physicians, technicians and other hospital staff were masked to treatment status until the end of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals accounted for, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Free of other potential confounders? | Low risk | Characteristics of both groups were similar at baseline |
de Jong 2007.
| Methods | Design: parallel group prospective, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group clinical study with treatment failure as the primary outcome Duration: June 2001 to June 2003 Setting: inpatients, single centre, Isala Klinieken, Zwolle, the Netherlands | |
| Participants | Number screened: 435 Number randomised: 210; 107 IV group, 103 oral group Number completed: 193 Number withdrawals: 17 (6 withdrew consent before day 4 of the study, 2 in IV group and 4 in oral group 11 excluded from the per‐protocol analysis because they did not fulfil inclusion or exclusion criteria, 6 IV group, 5 oral group) Baseline details: Smoking history (mean) (pack‐years): IV group: 37.2 (SD 20.2) oral group: 40.5 (SD 23.1) Baseline lung function (mean) (L): IV group: 1.0 (SD 0.43), 36% predicted (SD 14%); oral group: 1.0 (SD 0.40), 39% predicted (SD 17%) Mean age (years): IV group: 69.8 (SD 8.6), oral group 71.6 (SD 8.1) Gender: IV group: M = 76.6%, oral group: M = 72.8% Diagnosis COPD: airflow limitation was defined as an FEV1/FVC ratio of < 70% and FEV1 of < 80% predicted (at least GOLD severity stage II) AE criteria: defined as a history of increased breathlessness and the presence of at least 2 of the following symptoms for at least 24 6 hours: increased cough frequency or severity, increased sputum volume or purulence and increased wheeze Inclusion criteria: aged > 40 years, > 10 pack‐years' smoking history, evidence of airflow limitation Exclusion criteria: people who had signs of a very severe exacerbation on hospital admission (arterial pH < 7.26 or PaCO2 > 9.3 kPa), significant or unstable co‐morbidity, history of asthma, participated in another study within the 4 weeks before hospital admission, previously randomised into this study, had clinically significant findings on chest radiography other than COPD, known hypersensitivity to prednisolone, known to be totally non‐compliant |
|
| Interventions | IV group: IV prednisolone 60 mg + placebo medication 5‐day course, up to 7 days tapering course oral prednisolone 30 mg Oral group: oral prednisolone 60 mg + placebo medication 5‐day course, up to 7 days tapering course oral prednisolone 30 mg Co‐interventions: nebulised ipratropium bromide and albuterol 4 times daily together with oral amoxicillin/clavulanate. In case of allergy to this regimen, doxycycline was prescribed Treatment period: 12 days Follow‐up period: 90 days |
|
| Outcomes | Reported: by early < 2 weeks, late 2‐12 weeks; death from any cause, admission to the ICU, re‐admission to the hospital because of COPD, necessity to intensify pharmacological treatment Spirometry days 1 and 7 Health status SGRQ days 1 and 7. Minimal clinically important difference ⩾0.4, range 0‐100 Health‐related QoL 24‐hour version CCQ daily on days 1‐7. Minimal clinically important difference ⩾0.4, range 0‐6 Treatment failure defined as intensification of pharmacological treatment with open‐label corticosteroids Relapse defined as re‐admission to the hospital for COPD between 2 and 12 weeks Analysis: per‐protocol IV group 99 (of 107 participants), oral group 94 (of 103 participants) |
|
| Notes | Likelihood of COPD: aged > 40 years, > 10 year‐packs' smoking history, history of asthma exclusion, evidence of fixed airflow obstruction Funding: sources not disclosed; authors reported on 23 January 2007 to the ACCP that no significant conflicts of interest existed with any companies/organisations whose products or services may be discussed in the article Trial registration: ClinicalTrials.gov/NCT00311961 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a computer minimisation programme for the following 10 parameters: use of oral prednisolone, use of inhaled corticosteroids, theophylline use 30 days before hospital admission, admission to the hospital because of an exacerbation of COPD in the last year, age, gender, smoking history, use of supplemental oxygen at home, pCO2 and time since the diagnosis of COPD (i.e. < 5, 5‐10, 10‐15 or > 15 years, or unknown) |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding: double blind. Active and placebo medication had a similar appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals accounted for, per‐protocol analysis |
| Selective reporting (reporting bias) | Low risk | Registration protocol available, all outcomes reported |
| Free of other potential confounders? | Low risk | Characteristics of both groups were similar at baseline |
Emerman 1989.
| Methods | Design: parallel group Duration: Setting: single centre, ED of a urban county general hospital, Cleveland USA | |
| Participants | Number screened: not reported Number randomised: 100 Number completed: 96 (4 lost to follow‐up) Baseline details: Mean age (years): 64 (SD6.7) Gender: M = 50, F = 46 Diagnosis COPD: clinical history of emphysema or chronic bronchitis AE criteria: presenting to ED with respiratory distress, initial spirometry FEV1 < 70% predicted or FEV1/FVC < 60% Inclusion criteria: aged > 50 years Exclusion criteria: history of asthma, episodes of respiratory distress before 35 years of age, oral or IV corticosteroids within 1 month, pneumonia, acute congestive heart failure, other conditions requiring hospitalisation |
|
| Interventions | Experimental: methylprednisolone 100 mg IV (1D systemic corticosteroid treatment)
Control: matched placebo solution IV
Co‐interventions: inhaled isoetharine, nasal oxygen and IV aminophylline Exclusions: no antibiotics given |
|
| Outcomes | Analysed: spirometry, outcomes reported as percentage of predicted lung function measurements, hospitalisation rate Reported: return to the ED. Active intervention was a single IV infusion of methylprednisolone given in the ED with participants observed over a minimum of 4 hours. Subsequently 30 of 96 participants required admission and for some participants allocation to treatment group was not maintained after re‐admission Follow‐up measurements: 48 hours Others: none reported Mortality: none reported Adverse drug effects: none reported |
|
| Notes | Likelihood of COPD: aged > 50 years; past smoking not required, but mean smoking history > 50 pack‐years so most participants will have a significant smoking history; asthma excluded; chronic airflow obstruction not known Funding: no sources disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to treatment arms, randomisation was by allocation of participants to receive corticosteroid or placebo that were prepared by the hospital pharmacy in a pre‐determined random order, identical in appearance and labelling |
| Allocation concealment (selection bias) | Low risk | Order of treatment prepared by the hospital pharmacy in a pre‐determined random order |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical in appearance and labelling. Participants, treating physicians and investigators blinded to allocation until termination of study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators blinded to allocation until termination of study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 30 of 96 participants required admission and for some participants allocation to treatment group was not maintained after re‐admission |
| Selective reporting (reporting bias) | Low risk | Results reported for outcomes in methods |
| Free of other potential confounders? | High risk | Allocation to treatment group was not maintained for an unknown number of participants after re‐admission |
Gunen 2007.
| Methods | Design: parallel group Duration: treatment 15 days, follow‐up 1 month Setting: inpatients, single centre, Turgut Ozal Research Centre of Inonu University, Malatya, Turkey | |
| Participants | Number screened: not reported Number randomised: 159 (group 1 = 53, group 2 = 53, group 3 = 53) Number completed: 121 (group 1 = 39, group 2 = 40, group 3 = 42) Number withdrawals: 11 participants lost to follow‐up post discharge group 1 = 4, group 2 = 3, group 3 = 4. Deaths group 1 = 1, group 2 = 1 Baseline details: Age (years): 64.1 (SD 8.9) Gender: group 1: M = 35, F = 4; group 2: M = 33, F = 7; group 3: M = 35, F = 7 FEV1 at admission: 37.2% predicted (SD 12.2) Diagnosis COPD: people with COPD hospitalised for an exacerbation prospectively enrolled, COPD according to the criteria set by the ATS AE criteria: presence of worsening in at least 2 of the following symptoms: cough, purulent sputum and dyspnoea. Inclusion criteria: level II exacerbation Exclusion criteria: hospitalised for pneumonia, pulmonary emboli, congestive heart failure and pneumothorax. Risk of imminent respiratory failure requiring mechanical ventilation or direct admission to the ICU (level III exacerbation). Utilised systemic corticosteroids or had an exacerbation in the preceding month |
|
| Interventions | Group 1: salbutamol nebulised 2.5 mg 4 times daily and ipratropium bromide nebulised 0.5 mg 4 times daily Group 2 prednisolone IV 40 mg plus nebulised salbutamol 2.5 mg 4 times daily and nebulised ipratropium bromide 0.5 mg 4 times daily Group 3 budesonide nebulised 1500 mg 4 times daily; combined nebulised solution of salbutamol and ipratropium bromide 4 times daily ipratropium bromide 0.5 mg and salbutamol 2.5 mg/2.5 mL Co‐interventions: nebulised salbutamol as rescue medication. All participants were given supplemental oxygen and systemic methylxanthines, antibiotics were used where signs of bacterial infection existed: group 1 = 59, group 2 = 63, group 3 = 57% Treatment period: 15 days; at least 10 days in hospital remainder continued after discharge: group 2 systemic corticosteroids methylprednisolone 32 mg tablets in the morning or group 3 or nebulised inhaled budesonide 1500 mg 4 times daily Follow‐up period: 1‐month. Combivent aerosol, ipratropium bromide 20 µg plus salbutamol 100 µg x 2 puffs 4 times daily; and methylxanthines |
|
| Outcomes | Reported: complete blood counts, detailed biochemical analysis, spirometric measurements and ABG analysis at admission, 24 hours, 72 hours, 7 days and 10 days Status after discharge was assessed by telephone calls and home visits every week during a 1‐month period after discharge Adverse effects: COPD deterioration, admission to the ICU, respiratory failure, participant withdrawal for any reason, delayed discharge (beyond 15 days), inhospital deaths, deaths after discharge within 1 month, exacerbation, rehospitalisation rates within 1 month after discharge. Paper reports total number of exacerbations in 30 days and no response received to request for author clarification on whether data refer to number of participants or number of events. Data not used |
|
| Notes | Likelihood of COPD: age inclusion not known; smoking history threshold not known, but mean smoking history > 40 pack‐years so most participants will have a significant smoking history; asthma not an exclusion; required COPD diagnosis according to 1087 ATS standards Funding: no sources of funding disclosed Trial registration: ClinicalTrials.gov/NCT00274222 Protocol: not available Data on treatment failure and adverse effects were sought from author. Emailed data request to authors 30 September 2013 (Gunen). No response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly sorted into 1 of 3 groups ‐ method not specified |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly sorted into 1 of 3 groups. No details on allocation method |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants received apparently identical treatment, given same number of scheduled nebulised solutions every day (8 times a day) and were infused with a single physiological saline solution (50 mL) IV in the morning |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details on investigator or assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data from 38 enrolled participants were not evaluated due to exclusion during hospitalisation period of the study; group 1 = 39, group 2 = 40, group 3 = 42. The main reasons for exclusion were: the inability to perform 2 consecutive spirometric examinations (n = 10), non‐compliance with the treatment (n = 8), adverse effects (n = 6), treatment failure or admission to the ICU (n = 5), withdrawal of consent (n = 5) and request for early discharge (n = 4). Not clear which group these drop‐outs occurred in. Rates of drop‐outs similar: group 1 = 26%, group 2 = 24%, group 3 = 21% |
| Selective reporting (reporting bias) | Unclear risk | Protocol: not available |
| Free of other potential confounders? | Low risk | Characteristics of both groups were similar at baseline |
Maltais 2002.
| Methods | Design: parallel group Duration: recruited over 20 months, treatment for 10 days Setting: 34 centres (Belgium n = 3, Canada n = 11; France n = 20) participated; people in clinic or ED with deterioration in their respiratory status subsequently admitted to hospital |
|
| Participants | Number screened: 687 in 11 months, 75 enrolled Number randomised: 199 (prednisone n = 62, placebo n = 66) Number completed: 147 assessed at day 10 (prednisone n = 55, placebo n=55) Baseline details: Mean age (years): 70 Gender: M= 162; F = 37 Diagnosis COPD: based on clinical evaluation compatible with chronic bronchitis or emphysema as defined by the ATS and when available, baseline spirometry had to confirm the presence of irreversible airflow obstruction (post‐bronchodilator FEV1 70% normal predicted value and FEV1/FVC < 70% AE criteria: recent (i.e. within 14 days) history of acute COPD exacerbation defined as increased breathlessness Inclusion criteria: requiring admission to hospital; recent acute COPD exacerbation (within 14 days), defined as increased breathlessness; aged > 50 years; > 20 pack‐years' smoking history Exclusion criteria: history of asthma, allergic rhinitis or atopy; exposure to systemic corticosteroids within 1 month; if used, inhaled beclomethasone equivalent > 1500 g/day; at risk of imminent acute respiratory failure requiring mechanical ventilation or admission to ICU; diagnosis of a specific underlying cause for the exacerbation (e.g. pneumonia, pneumothorax, heart failure) |
|
| Interventions | Experimental: prednisone 30 mg orally every 12 hours and placebo Pulmicort Respules/Nebuamp for 72 hours followed by prednisone 40 mg/day orally and placebo turbuhaler for 7 days (10‐day systemic corticosteroid course)
Control: placebo nebulisation and tablets for 72 hours and placebo turbuhaler for 7 days Co‐interventions: nebulised ‐ agonists, ipratropium bromide, oral antibiotics (used in 83% budesonide, 84% prednisone, 80% placebo), and supplemental oxygen. Methylxanthines allowed, if prescribed before the study as a regular medication |
|
| Outcomes | Analysed: post‐bronchodilator FEV1; pre‐bronchodilator FEV1; ABGs (0‐72 hours); dyspnoea score; duration of hospitalisation; length of hospitalisation data removed due to skewed data, was previously given in text, but not in data analysis; occurrence of adverse events Reported: primary variable to assess treatment efficacy was the change in post‐bronchodilator FEV1 from 0 to 72 hours Secondary endpoints included: changes in pre‐bronchodilator FEV1; dyspnoea score; ABGs from 0 to 72 hours; duration of hospitalisation; occurrence of adverse events, defined as any medical event reported by the participants from study entry to day 10 Measurements: participants were assessed every 12 hours during the acute phase (from 0 to 72 hours), at hospital discharge, and at day 10 Mortality: 1 death in prednisone group Adverse drug effects: hyperglycaemia |
|
| Notes | Likelihood of COPD: aged > 50 years, > 20 pack‐years' smoking history, diagnosis of asthma exclusion, evidence of fixed airflow obstruction at presentation if spirometry available Funding: financial support from AstraZeneca Canada Dr. Robert Jenkins and Ms. Joanna Lee, AstraZeneca Canada assisted in reviewing and preparing the manuscript, and AstraZeneca R&D Lund, Kurt Nikander advised |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised; computerised single list using blocks of consecutive participant number, proportion 1 : 1 : 1 |
| Allocation concealment (selection bias) | Low risk | Allocated on site next available sequential participant number |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study described as placebo‐controlled, double‐blind, double dummy; identical nebuliser ampoules and placebo tablets used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details on assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 28 participants were withdrawn from the study during the acute phase for the following reasons: adverse events (n = 12), treatment failure according to the evaluation of the attending physician (n = 9), inclusion or exclusion criteria not fulfilled (n = 4), need for a prohibited medication (n = 2) and withdrawal of participant's consent (n = 1). The proportion of drop‐outs during the acute phase was similar in the 3 groups: 10 (14%) participants in the budesonide group, 7 (11%) in the prednisone group, and 11 (17%) in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported |
| Free of other potential confounders? | Low risk | Characteristics of both groups were similar at baseline |
Niewoehner 1999.
| Methods | Design: parallel group Duration: November 1994 to October 1996. Treatment for 57 days, follow‐up for 6 months Setting: hospitalised people with exacerbations of COPD, 20 centres, Veterans Affairs USA |
|
| Participants | Number screened: 1840 Number recruited: 271 Number withdrawals: oral prednisolone 2 weeks, n = 10, oral prednisolone 8 weeks n = 8, placebo n = 10. Follow‐up data complete for 19 withdrawals Mean age (years): oral prednisolone 2 weeks: 67.1 (SD10.6); oral prednisolone 8 weeks: 68.1 (SD 6.8); placebo group: 67.8 (SD 10.0) Gender: M = 268, F = 3 Diagnosis COPD: clinical diagnosis. Likelihood of COPD: aged > 50 years, > 30 pack‐years' smoking history, diagnosis of asthma exclusion, evidence of airflow obstruction AE criteria: clinical diagnosis of exacerbations of COPD Inclusion criteria: aged > 50 years, > 30 pack‐years' smoking history, FEV1 < 1.5 L or inability to perform spirometry due to dyspnoea Exclusion criteria: diagnosis of asthma, use of systemic corticosteroids within the preceding 30 days, co‐morbidities making survival for 1 year unlikely, inability to give informed consent Smoked in past 3 months: oral prednisolone 2 weeks 52%, oral prednisolone 8 weeks 50%, placebo 50% FEV1 (L): oral prednisolone 2 weeks 0.77 (SD 0.29); oral prednisolone 8 weeks 0.79 (SD 0.29); placebo 0.75 (SD 0.27) |
|
| Interventions | Group 1: IV methyl prednisolone 125 mg 6 hourly for 72 hours followed by oral prednisolone 60 mg day 4‐ day 7, 40 mg day 8 to day 11, 20 mg day 12 to day 43, 10 mg day 44 to day50, 5 mg day 51 to day 56 (60‐day SS course). Treatment 8 weeks
Group 2: IV methyl prednisolone 125 mg 6 hourly for 72 hours followed by oral prednisolone 60 mg day 4 to day 7, 40 mg day 8 to day 11, 20 mg day 12 to day 15 followed by oral placebo day 16 to day 57 (15‐day systemic corticosteroid course). Placebo for weeks 3‐8
Group 3: IV placebo (5% dextrose) 6 hourly for 72 hours followed by oral placebo day 3 to day 57. Treatment 8 weeks Co‐interventions: antibiotics for 7 days. Beta‐agonists, ipratropium bromide and triamcinolone administered to all during the 6 months of the study Exclusions: theophyllines, high dose inhaled corticosteroid > 1600 μg/day equivalent beclomethasone |
|
| Outcomes | Analysed: time to treatment failure (death, need for intubation and mechanical ventilation, re‐admission for COPD, intensification of pharmacological therapy), length of hospital stay, FEV1, death, complications
Reported: theophylline use, prior hospitalisation
Outcomes measured: 2, 8, 26 weeks
Mortality: 13 in experimental arm, 11 in placebo arm
Adverse events: 51 in placebo arm and 113 in experimental arm Data from intervention groups 1 and 2 combined in analyses |
|
| Notes | Likelihood of COPD: aged > 50 years, > 30 pack‐years' smoking history, diagnosis of asthma exclusion, FEV1 < 1.50 L or an inability to undergo spirometry because of dyspnoea Funding: conducted under the aegis of the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development and supported by a grant from Boehringer Ingelheim Protocol: published (Erbland 1998) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation, stratified randomisation with a permuted‐block scheme, stratified according to hospital |
| Allocation concealment (selection bias) | Low risk | Blinded pharmacists responsible for drug administration |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medication dispensed in blinded fashion, identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: 10 oral prednisolone 2 week, 5 oral prednisolone 8 week, 10 placebo. Follow‐up data complete for 19 withdrawals |
| Selective reporting (reporting bias) | Low risk | Protocol published |
| Free of other potential confounders? | Unclear risk | There were differences between groups at baseline in pack‐years' smoking, previous use of systemic corticosteroids and proportion with diabetes |
Ridha 2006.
| Methods | Design: parallel group, 3 groups Duration: 10 days' treatment, 90 days' follow‐up Setting: inpatients, Mami Hospital, Ariana‐Tunisia | |
| Participants | Number screened: not reported Number randomised: 52; group 1 n = 18, group 2 n = 17, group 3 n = 17 Number completed: not reported Baseline details: not reported Age: not reported Gender: not reported Diagnosis COPD: not reported AE criteria: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Group 1 (n = 18): hydrocortisone 400 mg daily IV for 10 days Group 2 (n = 17): prednisone 40 mg once daily for 10 days Group 3 (n = 17): no corticosteroids (control group) no placebo use reported Co‐interventions: not known Treatment period: 10 days Follow‐up period: 90 days |
|
| Outcomes | Primary outcomes: PEF, spirometric measures (FEV1 level) Secondary outcomes: symptom scores (dyspnoea, cough, sputum), treatment failure Adverse events: hyperglycaemia, epigastric pain Assessment: days 3, 10, 30 and 90 Reported: no results in abstract |
|
| Notes | Likelihood of COPD: age limit for inclusion unknown, unknown pack‐years' smoking, unknown if asthma an exclusion, fixed airflow obstruction criteria unknown Funding: no sources disclosed Protocol: not available. Abstract only Emailed data request to authors ‐ 4 September 2012, 9 August 2013, 30 September 2013 (Ridha). No response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospective controlled study randomised in 3 groups. Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control group "no corticosteroids" and placebo use not specified in abstract |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No details on assessor blinding and as placebo use was not specified in abstract assessors were unlikely to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No results or details in abstract |
| Selective reporting (reporting bias) | Unclear risk | No results or details in abstract. No protocol or registration found |
| Free of other potential confounders? | Unclear risk | No results or details in abstract |
Rostom 1994.
| Methods | Design: parallel group Duration: treatment for 19 days, follow‐up over 1 month Setting: hospitalised people, Canada Publication: abstract only |
|
| Participants | Number recruited: 30 (6 withdrawn, data incomplete in a further 5) Mean age: not reported Gender: not reported Diagnosis COPD: not reported AE criteria: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Experimental: methylprednisolone 40 mg IV, 6 hourly for 3 days followed by methylprednisolone 32 mg orally daily reducing by 4 mg every second day
Control: saline IV followed by oral placebo Duration treatment: 19 days Co‐interventions: oxygen, inhaled (nebulised) beta‐agonists, theophylline (route not detailed) and oral antibiotics (amoxicillin, trimethoprim/sulphamethoxazole or doxycycline) |
|
| Outcomes | Analysed: spirometry Reported: spirometry Others: none reported Mortality: not reported Adverse events: hypertension (n = 1), hyperglycaemia (n = 1) and congestive heart failure (n = 1), but allocation not detailed |
|
| Notes | Likelihood of COPD: age limit for inclusion unknown, unknown pack‐years' smoking, unknown if asthma an exclusion, fixed airflow obstruction criteria for COPD unknown Funding: no sources disclosed Published as abstract. No objective measurements included in the report. No response to letter of request |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no other details of randomisation procedure available |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind; no other details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported on assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 withdrawals, no details given |
| Selective reporting (reporting bias) | Unclear risk | Not full publication |
| Free of other potential confounders? | Low risk | Characteristics of both groups were similar at baseline |
Thompson 1996.
| Methods | Design: parallel group Duration: May 1991 to April 1993 Setting: single centre, outpatients clinic and ED at a Veterans Affairs Medical Center, USA |
|
| Participants | Number screened: 140 clinic, 199 ED Number randomised 11 clinic, 16 ED Baseline details: Mean age (years): prednisolone 65 (SD 9), control 70 (SD 7) Gender: M = 26, F = 1 Diagnosis COPD: clinical diagnosis of chronic bronchitis or emphysema according to ATS criteria. Airflow obstruction, FEV1 < 60% predicted or FEV1/FVC ratio < 65% after bronchodilator AE criteria: subjective worsening of dyspnoea or cough for > 24 hours requiring a hospital visit, > 25% increase in inhaled short‐acting beta2 agonist use over 24 hours, or an increase in sputum production or purulence (or both). Inclusion criteria: > 20 pack‐years' smoking history. Exclusion criteria: family history of asthma; history of asthma, atopy, allergic rhinitis or nasal polyps; history of lung disease other than COPD; use of systemic corticosteroids within 1 month; uncompensated congestive heart failure; pneumonia; fever > 38.5 ºC; arterial pH < 7.35; hospitalisation for other reasons. |
|
| Interventions | Experimental: prednisolone 60 mg orally daily for 3 days, then 40 mg for 3 days, then 20 mg for 3 days. (9‐day systemic corticosteroid treatment) Control: vitamin B6 orally daily Treatment period: 9 days Co‐interventions: inhaled beta‐agonist (MDI or nebulised 4 hourly) in all. Ipratropium bromide, inhaled corticosteroids and theophylline continued unchanged. Oral antibiotics if evidence of infection on gram stain of sputum. | |
| Outcomes | Analysed: spirometry FEV1, dyspnoea using visual analogue scale (200 mm scale with improvement in dyspnoea represented by a higher score), failure of study treatment (hospitalisation or requirement for oral prednisolone), ABG. Reported: complete blood count Others: none reported Mortality: none reported Adverse events: none reported Author supplied lung function, ABGs and mortality and adverse events data Results: used result supplied by the trialist for day 3 and 10 PaCO2 and PaO2 |
|
| Notes | Likelihood of COPD: age limits not known, > 20 pack‐years' smoking pack history, diagnosis of asthma exclusion, FEV1 post‐ bronchodilator < 60% predicted or ratio FEV1/ FVC < 65% Funding: supported by the US Department of Veterans Affairs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment assigned by computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | information not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, use of similar appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details on assessor/investigator blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant withdrew from follow‐up |
| Selective reporting (reporting bias) | Low risk | Protocol not available. All outcomes in methods reported |
| Free of other potential confounders? | Low risk | Characteristics of both groups were similar at baseline |
Willaert 2002.
| Methods | Design: parallel group
Duration: July 1999 to March 2000. Decision to discharge from hospital based on physician judgement (after a minimum of a 10‐day inpatient therapeutic regimen) Follow‐ up period: 4 weeks Setting: single centre, casualty department of the University Hospital Gasthuisberg, Leuven, Belgium |
|
| Participants | Number screened: not reported Number randomised: 48 Number completed: 37 Baseline details: Age (years) IV group = 72 (SD 6), oral group 71 (SD 8) Gender: IV group M = 91.3%, oral group 84.0% Smoking history (pack‐years): IV group: mean 27 (SD 16); oral group: mean 36 (SD 18) Baseline lung function (mean): FEV1: IV group 1.14 L (SD 0.43), oral group 1.10 (SD 0.51) FVC: IV group 2.48 L (SD 0.64), oral group 2.55 L (SD 0.63) FEV1/FVC: IV group 47% (SD 16%), oral group 42% (SD 12%) Diagnosis COPD: clinical history of COPD AE criteria: increased dyspnoea, increased cough frequency or severity, increased production or purulence of sputum, increased wheeze, lasting for at least 3 days Inclusion criteria: ATS indications for hospitalisation of people with COPD exacerbations. People who had used inhaled or systemic corticosteroids prior to admission to casualty were not excluded. The cumulative dose over a period of 2 weeks prior to admission was recorded and used as a variable in the evaluation of the participants' characteristics Exclusion criteria: personal or family history of asthma (defined as episodic wheezing or dyspnoea that rapidly improved with treatment) or atopy, invasive or non‐invasive assisted ventilation, unable to use a MDI successfully as a device for administering bronchodilators |
|
| Interventions | IV group: methylprednisolone 40 mg/day IV with a decrease to 20 mg after 10 days and subsequent oral treatment with a further decrease of 4 mg. 4 days. Co‐intervention: aerosol therapy salbutamol 10 mg/day and ipratropium bromide 1 mg/day, aerosols Oral group: oral methylprednisolone 32 mg for 1 week followed by 24 mg/day for a period of 4 days and a subsequent decrease of 4 mg/week. Co‐intervention: Duovent1 4 x 4 puffs/day (cumulative dose of fenoterol 1.6 mg/day and ipratropium bromide 640 mg/day), MDI with spacer Treatment period: IV group: 14 days, oral group: 14 days plus tapering | |
| Outcomes | Treatment failure: required invasive or non‐invasive assisted ventilation, clinical improvement was not satisfactory and a change of treatment was deemed necessary by the treating physician, participant was not satisfied with his or her own clinical progress and demanded another treatment regimen (non‐compliance) Time point: while in hospital, minimum 10 days Relapse defined as re‐admission to hospital within 4 weeks Analysed: late re‐admission to hospital, followed up over 20 weeks; spirometry, FEV1, FVC daily for 10 days; Dyspnoea recorded on 10‐cm visual analogue scale daily for 10 days; daily use and additional need of bronchodilators; cumulative corticosteroid dose; length of stay; QoL (Chronic Respiratory Disease Index Questionnaire daily for 10 days) Follow‐up after discharge: 28 days' follow‐up, re‐admissions followed up over 20 weeks |
|
| Notes | Likelihood of COPD: age limits not known, mean 72 years; threshold pack‐ years' smoking history not known, mean 30 pack‐years' smoking history; diagnosis of asthma exclusion; airflow obstruction criteria not known Funding: the study was supported by the Research Foundation Katholieke Universiteit Leuven grant #OT98/27 and the "Fonds voor Wetenschappelijk Onderzoek Vlaanderen" grants #G.0/ 75.99 and #G.0237.01 Protocol: not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to 1 of the 2 therapy groups, method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation method not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded, no placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Physicians in charge received no information on the objectives or specific target variables of the trial, thereby minimising bias, although they could not be completely blinded. No information on outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. IV group: 4 withdrew; 3 ICU, 1 femur fracture. In oral group:,7 withdrew, ICU, 2 progress unsatisfactory, 3 non‐compliance 1 lung tumour |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods fully reported, protocol not available. Inclusion criteria for the study were established before the trial and strictly applied |
| Free of other potential confounders? | Low risk | Bronchodilators administered via different devices in IV group (aerosol) and oral group (MDI + spacer). Total dose recorded, data available, doses equivalent. Extra bronchodilator did not differ between groups. |
Wood‐Baker 1998.
| Methods | Design: parallel 3‐group design Duration: 2 weeks Setting: hospitalised people, 2 centres, Australia/New Zealand |
|
| Participants | Number recruited: 47 Number commenced treatment: 38 Number analysed: 35 Number completed: study 28. 3 withdrawals, 5 treatment failure, 2 adverse events, 10 protocol violation, 1 death Mean age (years): high‐dose arm: 69.3 (SD 5.5), moderate‐dose arm: 71.1 (SD 9.7), placebo 71.3 (SD 7.8). Range 61‐86 years. Gender: M = 24, F = 14 Diagnosis COPD: clinical diagnosis smoking‐related COPD AE criteria: increased breathlessness necessitating hospital admission Inclusion criteria: aged > 40 years, > 10 pack‐ years' smoking history, FEV1 FEV1 < 50% predicted. Exclusion criteria: long‐term corticosteroid therapy equivalent to > 5 mg prednisolone 5 mg/day or taking oral prednisolone for the current exacerbation; other co‐existent lung disease, pneumonia; inability to co‐operate with investigations; previous adverse drug reaction to corticosteroids, endoscopically or radiographically confirmed peptic ulcer disease within the past 2 years; history of cardiac failure, current hepatic or renal failure; inadequately treated hypertension |
|
| Interventions | High‐dose arm: prednisolone 2.5 mg/kg orally daily for 3 days followed by 11 days placebo (3D systemic steroid treatment) Moderate‐ dose arm: prednisolone 0.6 mg/kg orally daily for 7 days followed by prednisolone 0.3 mg/kg orally daily for 7 days (14D systemic steroid treatment) Control: matched placebo for 14 days Co‐interventions: inhaled bronchodilators, oral antibiotics, oral/IV xanthines and oxygen | |
| Outcomes | Measured: spirometry, 6‐minute walking distance, duration of hospitalisation, PaO2, PaCO2, visual analogue scale of breathlessness (100‐mm scale, more breathless represented by a higher number), treatment failure, mortality, adverse events
Reported or data available: spirometry, 6‐minute walking distance, duration of hospitalisation, visual analogue scale of breathlessness Mortality: 1 death in the placebo arm. Morbidity: no adverse biochemical effects |
|
| Notes | Likelihood of COPD: aged > 40 years, > 10 pack‐years' smoking history, diagnosis of asthma not exclusion, FEV1 < 50% predicted Funding: no funding source disclosed Data analysed by review authors in 3 groups. Data from 14 day treatment group used in review Study published as abstract. Protocol and study data available to review authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded to allocation until completion. Use of identical placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 participants withdrew from follow‐up (6%), no details on group allocation |
| Selective reporting (reporting bias) | Low risk | Study outcome data supplied to review authors |
| Free of other potential confounders? | Low risk | Characteristics of both groups were similar at baseline |
Zheng 2011.
| Methods | Design: parallel RCT Duration: not reported Setting: not reported |
|
| Participants | Number screened: not reported Number randomised: 153 Number completed: not reported Number withdrawals: not reported Baseline details: not reported Gender: not reported Age: not reported Diagnosis COPD: not reported AE criteria: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Group 1 (n = 53): 2 mg/4 mL nebulised budesonide, every 6 hours and normal saline 10 mL IV 4 times daily Group 2 (n = 54): methylprednisolone 40 mg, IV and nebulised normal saline 4 mL every 6 hours Group 3 (n = 46): nebulised normal saline 4 mL every 6 hours and normal saline 10 mL IV 4 times daily Treatment period: 7 days Follow‐up period: not reported |
|
| Outcomes | Treatment failure, FEV1, dyspnoea score, use of rescue medication and systemic glucocorticoids, length of hospital stay, adverse events |
|
| Notes | Likelihood of COPD: age limit for inclusion unknown, unknown pack‐ years' smoking, unknown if asthma an exclusion, fixed airflow obstruction criteria for COPD unknown. Funding: no sources disclosed Protocol: not available (abstract only). No trial registration located Emailed data request to authors ‐ 9 August 2013, 30 September 2013 (Zheng). No response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method for randomisation not known |
| Allocation concealment (selection bias) | Unclear risk | Allocation method not known |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind, double‐dummy and parallel controlled trial. Each group used nebulised medication (active or placebo) and had IV medication (active or saline) over same period and frequency |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Results data incomplete in abstract |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Free of other potential confounders? | Unclear risk | No data on baseline characteristics |
ABG: arterial blood gas; ACCP: American College of Chest Physicians; AE: acute exacerbation; ATS: American Thoracic Society; CCQ: Clinical COPD Questionnaire; CI: confidence interval; COPD: chronic obstructive pulmonary disease; ED: emergency department; F: female; FEV1 : forced expiratory volume in 1 second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; ICU: intensive care unit; IQR: interquartile range; IV: intravenous; LTOT: long‐term oxygen therapy; LVF: left ventricular failure; M: male; MDI: metered‐dose inhaler; NIV: non‐invasive ventilation; PaCO2: partial pressure of carbon dioxide dissolved in arterial blood; PaO2 : partial pressure of oxygen dissolved in arterial blood; PEF: peak expiratory flow; QoL: quality of life; SD: standard deviation; SGRQ: St. George Respiratory Questionnaire.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bafadhel 2012 | Parallel group, double‐blind trial, with randomisation to a biomarker‐directed arm (peripheral blood eosinophil count at exacerbation was used to guide corticosteroid treatment) or standard treatment control group receiving prednisolone 30 mg capsule once daily (irrespective of the blood eosinophil biomarker results) |
| Bingol 2005 | Participants did not have an acute exacerbation of COPD |
| Dahlen 2001 | Purpose of study was to analyse the changes seen in inflammatory markers after treatment with 1 of 2 corticosteroids, 1 given orally, the other inhaled. The study was not placebo‐controlled. Comparison between the effects of oral and inhaled corticosteroids |
| Gerogianni 2002 | Not reported to be randomised. Study published as abstract only. Comparison of people with COPD divided into 2 groups. 1 group received prednisolone 12 mg daily plus SABA and the other SABA only during exacerbations and for 12 weeks after. Not clear how participants allocated to groups. Sent letter requesting details ‐ no response within 4 months |
| Ghanei 2005 | Participants with exposure to mustard gas inducing "chronic bronchitis", all non‐smokers. Lung function indicates restriction present low FVC. Comparison of IV methylprednisolone and oral prednisolone in "acute exacerbation" of symptoms. Excluded as COPD not confirmed in participants |
| GSK FLIT98 | Mild or moderate stable COPD. group comparison. Group 1: placebo 14 days + inhaled fluticasone 500 mcg 12/52; group 2:prednisolone 20‐40 mg/day 14 days + placebo diskus 14 days and fluticasone 500 mcg 10/52; group 3: placebo 14 days + placebo diskus 12/52 . |
| Kunter 2006 | Consecutive participants with exacerbations enrolled without randomisation to treatment. No placebo used in control group |
| Li 2003 | RCT comparison of 2 corticosteroids, methylprednisolone and dexamethasone in hospital for 7‐14 days. Dose regimen was 3 steps. IV/oral/inhaled methylprednisolone and dexamethasone and matched co‐interventions. No placebo control group |
| Mirici 2002 | Systemic (IV) corticosteroids versus nebulised budesonide in acute exacerbations of COPD, no placebo group |
| Murata 1990 | Not an RCT. Retrospective study of 45 visits in which IV and oral corticosteroids were given (T visits) were compared with an equal number of matched visits in which they were withheld (N visits) |
| Plant 1999 | Abstract for conference paper not obtainable |
| Rahman 2004 | Oral corticosteroids 7 days versus 14 days in acute exacerbations of COPD, no placebo group |
| Rizzato 1998 | The aim of study was to evaluate and compare clinical tolerability and relative potency of a new corticosteroid drug, deflazacort against that of methylprednisolone. No placebo control |
| Roede 2008 | Cluster RCT of general practices, comparing high‐dose oral corticosteroid course + antibiotics in accordance with the national guideline versus treatment 'as usual' (general practitioner could use oral corticosteroid and 75% of participants received oral corticosteroid) |
| Sayiner 2001 | This study compared the likelihood of a link between duration of treatment with corticosteroids in COPD exacerbations and efficacy of treatment. Not a placebo‐controlled trial |
| Shortall 2002 | Participants screened with acute exacerbation COPD (n = 117), 50 recruited but 1 participant was admitted and included twice and 1 patent was admitted and included 3 times. Data not reported for participants on 1 occasion only. Comparison of oral corticosteroid methylprednisolone 40 mg + metered‐dose inhaler bronchodilators with IV methylprednisolone 40 mg until wheeze‐free then oral corticosteroid)/nebulised bronchodilators |
| Sin 2004 | Comparison of inhaled corticosteroids, oral corticosteroids and placebo in stable COPD |
| Wang 2011 | Observational study of high‐dose (60 mg) and, lower‐ dose (< 60 mg) corticosteroid treatment in exacerbations (protocol clinicaltrials.gov/show/NCT01215825) |
COPD: chronic obstructive pulmonary disease; FVC: forced vital capacity; IV: intravenous; RCT: randomised controlled trial; SABA: short‐acting beta agonist.
Differences between protocol and review
We amended the objectives of the review in 2014 to include studies in which participants received assisted ventilation, and interventions to include: comparison 1: corticosteroid, parenteral or oral administration versus placebo control injections or tablets as appropriate; comparison 2: corticosteroid, parenteral versus oral administration. We changed the order of the secondary outcomes. We updated the methods, including using the latest version of the Cochrane 'Risk of bias' tool and added a 'Summary of findings' table. We added a subgroup analysis on setting of the exacerbations.
Two new review authors joined the team, DJT and CJW, and one review author stepped down, M Hannay.
Contributions of authors
JAEW: updates 2004, 2008, 2014: study selection, grading and data management, data entry, author correspondence, analysis, 'Summary of findings' table. Primary author first draft of results, discussion and conclusions.
DT: 2014 update: study selection, grading and data management, data entry, author correspondence, reviewing results and discussion, drafting abstract and plain language summary.
CW: 2014 update: study selection, grading and data management, data entry, author correspondence, reviewing results and discussion.
RWB: protocol development; data extraction and entry; data verification; writing first version of review; editing updates in 2004, 2008 and 2014.
EHW: protocol development; data extraction and entry; writing first version of review; editing updates 2004, 2008 and 2014.
PG: editing review drafts 2004, 2008 and 2014.
Sources of support
Internal sources
University of Tasmania, Australia.
External sources
-
Commonwealth Department of Health and Aged Care, Australia.
Through the National Health and Medical Research Council Cochrane funding (2013‐2016)
Declarations of interest
R Wood‐Baker has undertaken research sponsored by a number of pharmaceutical companies including Marion Merrell Dow, ICI Australia, Pharmacia Upjohn, GlaxoSmithKline, Sanofi‐Synthélabo and Novartis.
E H Walters has undertaken research sponsored by a number of pharmaceutical companies including Glaxo Wellcome, Novartis, Astra, Boehringer, Schering‐Plough and Amgen. He has held consultancies for AstraZeneca, ICI Australia, Pfizer and GlaxoSmithKline.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aaron 2003 {published and unpublished data}
- Aaron SD, Vandemheen KL, Hebert P, Dales R, Stiell IG, Ahuja J, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. New England Journal of Medicine 2003;348(26):2618‐25. [DOI] [PubMed] [Google Scholar]
- Aaron SD, Vandemheen KL, Wells G, Dales R, Ahuja J, Dickinson G, et al. Oral prednisone for the prevention of relapse in outpatients with acute exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 2003;167(7):A947. [Google Scholar]
- Courtney AU. Oral prednisone prevents relapse in COPD exacerbations. Journal of Family Practice 2003;52(10):762‐4. [PubMed] [Google Scholar]
Abroug 2014 {published data only}
- Abroug F, Ouanes‐Besbes L, Fkih‐Hassen M, Ouanes I, Ayed S, Dachraoui F, et al. Prednisone in COPD exacerbation requiring ventilatory support: an open‐label randomised evaluation. European Respiratory Journal 2014;43(3):717‐24. [DOI] [PubMed] [Google Scholar]
Albert 1980 {published data only}
- Albert R, Martin T, Lewis S. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Annals of Internal Medicine 1980;92:753‐8. [DOI] [PubMed] [Google Scholar]
Alia 2011 {published and unpublished data}
- Alia I, Cal MA, Esteban A, Abella A, Ferrer R, Molina FJ, et al. Efficacy of corticosteroid therapy in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. Archives of Internal Medicine 2011;171(21):1939‐46. [DOI] [PubMed] [Google Scholar]
- Alia I, Cal MA, Esteban A, Ferrer R, Molina F, Pier R, et al. Evaluation of the efficacy of corticosteroids in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support: a prospective, international, randomized double blind clinical trial. Intensive Care Medicine 2010;36(Suppl 2):S205. [Google Scholar]
Bullard 1996 {published and unpublished data}
- Bullard M, Liaw S‐J, Tsai Y‐H, Min H. Early corticosteroid use in acute exacerbations of chronic airflow obstruction. American Journal of Emergency Medicine 1996;14:139‐43. [DOI] [PubMed] [Google Scholar]
Ceviker 2014 {published data only (unpublished sought but not used)}
- Ceviker Y, Sayiner A. Comparison of two systemic steroid regimens for the treatment of COPD exacerbations. Pulmonary Pharmacology and Therapeutics 2014;27(2):179‐83. [DOI] [PubMed] [Google Scholar]
Chen 2005 {published and unpublished data}
- Chen G, Xie C. The efficacy and treatment length of oral corticosteroids in patients with acute exacerbations of chronic obstructive pulmonary disease. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1966. [Google Scholar]
- Chen G, Xie CM, Luo YF. The effects and therapeutic duration of oral corticosteroids in patients with acute exacerbation of chronic obstructive pulmonary diseases [In Chinese]. Chinese Journal of Tuberculosis and Respiratory Diseases 2008;31(8):577‐80. [PubMed] [Google Scholar]
Cordero 1996 {published data only (unpublished sought but not used)}
- Cordero PJ, Benlloch E, SolÈ A, Morales P, MenÈndez R, Cebrian J. Corticosteroid therapy for COPD exacerbations at inpatient settings: a controlled‐randomized study. Archivos de Bronconeumologia 1996;32(Suppl 2):17. [Google Scholar]
- Cordero PJ, Sole A, Benlloch E, Morales P, Vallterra J, Menendez R, et al. A randomized controlled study of prednisone in outpatients with acute exacerbation of COPD. European Respiratory Journal 1996;9 Suppl 23:110s‐1s. [Google Scholar]
Davies 1999 {published and unpublished data}
- Davies L, Angus RM, Calverley PMA. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456‐60. [DOI] [PubMed] [Google Scholar]
de Jong 2007 {published data only}
- Kocks JW, Tuinenga MG, Uil SM, Berg JW, Stahl E, Molen T. Health status measurement in COPD: the minimal clinically important difference of the Clinical COPD Questionnaire. Respiratory Research 2006;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong Y, Uil M, Grotjohan P, Postma S, Kerstjens M, Berg J. A comparison of intravenous versus oral administration of corticosteroids in the treatment of patients admitted to the hospital with an exacerbation of COPD. European Respiratory Journal 2004;24(Suppl 48):64s. [Google Scholar]
- Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study. Chest 2007;132(6):1741‐7. [DOI] [PubMed] [Google Scholar]
Emerman 1989 {published and unpublished data}
- Emerman C, Connors A, Lukens T, May M, Effron D. A randomized controlled trial of methylprednisolone in the emergency treatment of acute exacerbations of COPD. Chest 1989;95:563‐7. [DOI] [PubMed] [Google Scholar]
Gunen 2007 {published data only (unpublished sought but not used)}
- Gunen H, Hacievliyagil SS, Yetkin O, Gulbas G. The role of nebulised budesonide in the treatment of acute exacerbations of COPD. European Respiratory Journal 2007;30(2):399‐400. [DOI] [PubMed] [Google Scholar]
- Gunen H, Hacievliyagil SS, Yetkin O, Gulbas G, Mutlu LC, In E. The role of nebulised budesonide in the treatment of exacerbations of COPD. European Respiratory Journal 2007;29(4):660‐7. [DOI] [PubMed] [Google Scholar]
Maltais 2002 {published and unpublished data}
- Maltais F, Ostinelli J, Bourbeau J, Tonnel AB, Jacquemet N, Haddon J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2002;165(5):698‐703. [DOI] [PubMed] [Google Scholar]
Niewoehner 1999 {published and unpublished data}
- Erbland ML, Deupree RH, Niewoehner DE. Systemic corticosteroids in chronic obstructive pulmonary disease exacerbations (SCCOPE): rationale and design of an equivalence trial. Veterans Administration Cooperative Trials SCCOPE Study Group. Controlled Clinical Trials 1998;19(4):404‐17. [DOI] [PubMed] [Google Scholar]
- Gross N. Is there a subgroup of steroid responders in acute exacerbations of COPD (AECOPD)? Further evaluation of SCCOPE trial data. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A271. [Google Scholar]
- Niewoehner DE, Collins D, Erbland ML. Relation of FEV(1) to clinical outcomes during exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(4 pt1):1201‐5. [DOI] [PubMed] [Google Scholar]
- Niewoehner DE, Erbland ML, Duepree RH, Collins D, Gross NJ, Light RW, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. New England Journal of Medicine 1999;340:1941‐7. [DOI] [PubMed] [Google Scholar]
Ridha 2006 {published data only (unpublished sought but not used)}
- Ridha M, Ferdaous Y, Sofia T, Amel C, Ali BK. Effect of systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. European Respiratory Journal 2006;28(Suppl 50):624s. [Google Scholar]
Rostom 1994 {published data only}
- Rostom R, Mink S, Hebert P, Cardinal P. The long term efficacy of methylprednisolone in the treatment of acute exacerbation of COPD. Chest 1994;106(2):161S. [Google Scholar]
Thompson 1996 {published and unpublished data}
- Thompson W, Nielson C, Carvalho P, Charan N, Crowley J. Controlled trial of oral prednisolone in outpatients with acute COPD exacerbation. American Journal of Respiratory and Critical Care Medicine 1996;154:407‐12. [DOI] [PubMed] [Google Scholar]
Willaert 2002 {published data only}
- Willaert W, Daenen M, Bomans P, Verleden G, Decramer M. Intravenous steroids and aerosol bronchodilators versus oral steroids and MDI bronchodilators in COPD exacerbations. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A578. [Google Scholar]
- Willaert W, Daenen M, Bomans P, Verleden G, Decramer M. What is the optimal treatment strategy for chronic obstructive pulmonary disease exacerbations?. European Respiratory Journal 2002;19:928‐35. [DOI] [PubMed] [Google Scholar]
Wood‐Baker 1998 {published and unpublished data}
- Wood‐Baker R, Wilkinson J, Pearce M, Ryan G. A double‐blind, randomised, placebo‐controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease [Abstract]. Australian and New Zealand Journal of Medicine 1998;28:262. [Google Scholar]
Zheng 2011 {published data only (unpublished sought but not used)}
- Zheng J, Lin J, Zhou X, Huang S, Xie C, Liang Z, et al. Nebulized budesonide in the treatment of acute exacerbations of chronic obstructive pulmonary disease (AECOPD): a randomized, double blind, double dummy, parallel controlled, multicenter trial. Chest 2011;140(4):526A. [Google Scholar]
References to studies excluded from this review
Bafadhel 2012 {published data only}
- Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Lomas D, et al. A double‐blind randomised control trial of peripheral blood eosinophils to direct prednisolone use in COPD exacerbations. European Respiratory Journal 2011;38(55):38s [357]. [Google Scholar]
- Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2012;186(1):48‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bingol 2005 {published data only}
- Bingol H, Cingoz F, Balkan A, Kilic S, Bolcal C, Demirkilic U. The effect of oral prednisolone with chronic obstructive pulmonary disease undergoing coronary artery bypass surgery. Journal of Cardiac Surgery 2005;20:252‐6. [DOI] [PubMed] [Google Scholar]
Dahlen 2001 {published data only}
- Dahlen I, Janson C, Björsson E, Stålenheim G, Peterson CG, Venge P. Changes in inflammatory markers following treatment of acute exacerbations of obstructive pulmonary disease. Respiratory Medicine 2001;95:891‐7. [DOI] [PubMed] [Google Scholar]
Gerogianni 2002 {published data only}
- Gerogianni A, Mathioudakis G, Koulouris N, Orphanidou D, Malamos N, Markozannes E, et al. The effect of oral steroid treatment in patients with chronic bronchitis. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A594. [Google Scholar]
Ghanei 2005 {published data only}
- Ghanei M, Khalili A, Arab M, Mojtahedzadeh M, Aslani J, Lessan‐Pezeshki M, et al. Diagnostic and therapeutic value of short‐term corticosteroid therapy in exacerbation of mustard gas‐induced chronic bronchitis. Basic & Clinical Pharmacology and Toxicology 2005;97(5):302‐5. [DOI] [PubMed] [Google Scholar]
GSK FLIT98 {unpublished data only}
- GSK FLIT98. Inhaled fluticasone propionate in an acute exacerbation of chronic obstructive pulmonary disease: a multi‐centre, randomised, double‐blind, parallel group study of the efficacy and safety of inhaled fluticasone propionate 2000 µg daily compared with placebo in chronic obstructive pulmonary disease (chronic bronchitis). GlaxoSmithKline Clinical Trial Register 2005.
Kunter 2006 {published data only}
- Ilvan A, Kunter E, Demirer E, Avsar K, Kartaloglu Z, Oztosun M, et al. Effect of systemic steroid treatment on serum leptin levels in patients with COPD exacerbation. European Respiratory Journal 2005;26(Suppl 49):284s. [Google Scholar]
- Kunter E, Ilvan A, Ozmen N, Demirer E, Ozturk A, Avsar K, et al. Effect of corticosteroids on hemostasis and pulmonary arterial pressure during chronic obstructive pulmonary disease exacerbation. Respiration 2006;75(2):145‐54. [DOI] [PubMed] [Google Scholar]
- Kunter E, Ilvan A, Ozturk A, Demirer E, Ozmen N, Avsar K, et al. Effects of steroid treatment on hemodynamic and haemostatic parameters in COPD exacerbation. European Respiratory Journal 2005;26(Suppl 49):298s. [Google Scholar]
Li 2003 {published data only}
- Li H, He G, Chu H, Zhao L, Yu H. A step‐wise application of methylprednisolone versus dexamethasone in the treatment of acute exacerbations of COPD. Respirology 2003;8(2):199‐204. [DOI] [PubMed] [Google Scholar]
Mirici 2002 {published data only}
- Mirici A, Meral M, Akgun M, Tutar U, Kiris S. Comparison of the effectiveness of parenteral and nebuliser steroid in COPD exacerbations. European Respiratory Journal 2002;20(Suppl 38):243s. [Google Scholar]
Murata 1990 {published data only}
- Murata GH, Gorby MS, Chick TW, Halperin AK. Intravenous and oral corticosteroids for the prevention of relapse after treatment of decompensated COPD. Effect on patients with a history of multiple relapses. Chest 1990;98:845‐94. [DOI] [PubMed] [Google Scholar]
Plant 1999 {unpublished data only}
- Plant PK, Watson JP, Muers MF, Pearson SB. Randomised controlled trial of oral corticosteroids in acute exacerbations of COPD. Proceedings of the European Respiratory Society Annual Congress; 1999 Oct 9‐13; Madrid.
Rahman 2004 {published data only}
- Rahman M, Rahman M, Mamun S, Abdullah A, Haque A. Role of 7‐day and 14‐day courses of oral prednisolone treatment in acute exacerbation of COPD. Chest 2004;126(Suppl 4):839s. [Google Scholar]
Rizzato 1998 {published data only}
- Rizzato G. Effects of deflazacort hemisuccinate vs methylprednisolone succinate in COPD exacerbations. L'internista 1998;6:35‐40. [Google Scholar]
Roede 2008 {published data only}
- Roede Berendina Maria. Treatment of COPD Exacerbations in Primary and Secondary Care. [PhD thesis]. Amsterdam: University of Amsterdam, 2008:Chapter 3, 31‐47. [Google Scholar]
Sayiner 2001 {published data only}
- Sayiner A, Aytemur ZA, Cirit M, Unsal I. Systemic glucocorticoids in severe exacerbations of COPD. Chest 2001;119:726‐30. [DOI] [PubMed] [Google Scholar]
Shortall 2002 {published data only}
- Shortall SP, Blum J, Oldenburg FA, Rodgerson L, Branscombe JM, Harrow EM. Treatment of patients hospitalised for exacerbations of chronic obstructive pulmonary disease: comparison of an oral/metered‐dose inhaler regime and an intravenous/nebulizer regimen. Respiratory Care 2002;47(2):154‐8. [PubMed] [Google Scholar]
Sin 2004 {published data only}
- Sin DD, Lacy P, Man SF. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2004;170(7):760‐5. [DOI] [PubMed] [Google Scholar]
Wang 2011 {published data only}
- Wang PH, Cheng SL, Wang HC, Chang HT, Hsu YL, Chen YS, et al. Systemic steroids in acute exacerbation of COPD ‐ from guidelines to bedside. International Journal of Clinical Pharmacology and Therapeutics 2011;49(11):705‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Anthonisen 1987
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Annals of Internal Medicine 1987;106:196‐204. [DOI] [PubMed] [Google Scholar]
Bathoorn 2008
- Bathoorn E, Kerstjens H, Postma D, Timens W, MacNee W. Airways inflammation and treatment during acute exacerbations of COPD. International Journal of Chronic Obstructive Pulmonary Disease 2008;3(2):217‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buist 2007
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (The BOLD Study): a population‐based prevalence study. Lancet 2007;370(9589):741‐50. [DOI] [PubMed] [Google Scholar]
Chapman 2001
- Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD. Chest 2001;119(6):1691‐5. [DOI] [PubMed] [Google Scholar]
Crockett 2001
- Crockett AJ, Cranston JM, Moss JR. Chronic Obstructive Pulmonary Disease (COPD) ‐ Economic Case Statement. Milton QLD: Lung Foundation Australia, 2001. [Google Scholar]
Donaldson 2002
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57(10):847‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donaldson 2005
- Donaldson GC, Wilkinson TMA, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2005;171:446‐52. [DOI] [PubMed] [Google Scholar]
Donohue 2005
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005;2(1):111‐24. [DOI] [PubMed] [Google Scholar]
Effing 2009
- Effing TW, Kerstjens HAM, Monninkhof EM, Valk PDLPM, Wouters EFM, Postma DS, et al. Definitions of exacerbations: does it really matter in clinical trials on COPD?. Chest 2009;136(3):918‐23. [DOI] [PubMed] [Google Scholar]
Falk 2008
- Falk JA, Minai OA, Mosenifar Z. Inhaled and systemic corticosteroids in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society; 2008 May 16‐20; Toronto. 2008. [DOI] [PMC free article] [PubMed]
GOLD 2013
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/guidelines‐global‐strategy‐for‐diagnosis‐management.html (accessed 10 August 2014).
Groenewegen 2001
- Groenewegen K, Schols A, Wouters EFM. Prognosis after hospitalization for acute exacerbations of COPD. European Respiratory Journal 2001;8(Suppl 33):209s. [Google Scholar]
Henzen 2000
- Henzen C, Suter A, Lerch E, Urbinelli R, Schorno XH, Briner VA. Suppression and recovery of adrenal response after short‐term, high‐dose glucocorticoid treatment. Lancet 2000;355:542‐45. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JD, Altman G. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hurst 2010
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal‐Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. New England Journal of Medicine 2010;363(12):1128‐38. [DOI] [PubMed] [Google Scholar]
Jeppesen 2012
- Jeppesen E, Brurberg KG, Vist GE, Wedzicha JA, Wright JJ, Greenstone M, et al. Hospital at home for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD003573.pub2] [DOI] [PubMed] [Google Scholar]
Leidy 2010
- Leidy NK, Wilcox TK, Jones PW, Murray L, Winnette R, Howard K, et al. Development of the EXAcerbations of Chronic Obstructive Pulmonary Disease Tool (EXACT): a patient‐reported outcome (PRO) measure. Value in Health 2010;13(8):965‐75. [DOI] [PubMed] [Google Scholar]
Lindenauer 2010
- Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbations of chronic obstructive pulmonary disease. JAMA 2010;303:2359‐67. [DOI] [PubMed] [Google Scholar]
Lopez 2006
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. European Respiratory Journal 2006;27(2):397‐412. [DOI] [PubMed] [Google Scholar]
Mahler 1984
- Mahler D, Weinberg D, Wells C, Feinstein A. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984;85:751‐8. [DOI] [PubMed] [Google Scholar]
McEvoy 1997
- McEvoy CE, Niewoehner DE. Adverse effects of corticosteroid therapy for COPD. Chest 1997;111:732‐43. [DOI] [PubMed] [Google Scholar]
Murray 1997
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990‐2020: Global Burden of Disease Study. Lancet 1997;349(9064):1498‐504. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update), 2010. www.guidance.nice.org.uk/cg101 (accessed 10 August 2014).
Oostenbrink 2004
- Oostenbrink JB, Rutten‐van Molken MP. Resource use and risk factors in high‐cost exacerbations of COPD. Respiratory Medicine 2004;98:883‐91. [DOI] [PubMed] [Google Scholar]
Papi 2006
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. American Journal of Respiratory and Critical Care Medicine 2006;173(10):1114‐21. [DOI] [PubMed] [Google Scholar]
Perret 2013
- Perret JL, Dharmage SC, Matheson MC, Johns DP, Gurrin LC, Burgess JA, et al. The interplay between the effects of lifetime asthma, smoking, and atopy on fixed airflow obstruction in middle age. American Journal of Respiratory and Critical Care Medicine 2013;187(1):42‐8. [DOI] [PubMed] [Google Scholar]
Ries 2005
- Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and visual analog scale. COPD 2005;2(1):105‐10. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Roisin 2000
- Rodriguez‐Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000;117:398s‐401. [DOI] [PubMed] [Google Scholar]
Schermer 2002
- Schermer T, Chavannes N, Saris C, Akkermans R, Schayck C, Weel C. Exacerbations and associated health care cost in patients with chronic obstructive pulmonary disease in general practice. Results from the COOPT trial. European Respiratory Journal 2002;20(Suppl 38):398s. [Google Scholar]
Seemungal 1998
- Seemungal TAR, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157:1418‐22. [DOI] [PubMed] [Google Scholar]
Seemungal 2000
- Seemungal T, Donaldson G, Bhowmik A, Jefries D, Wedizicha J. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(5):1608‐13. [DOI] [PubMed] [Google Scholar]
Sethi 2008
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. New England Journal of Medicine 2008;359(22):2355‐65. [DOI] [PubMed] [Google Scholar]
Sherk 2000
- Sherk PA, Grossman RF. The chronic obstructive pulmonary disease exacerbation. Clinics in Chest Medicine 2000;21(4):705‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soler‐Cataluna 2005
- Soler‐Cataluna JJ, Martinnez‐Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60(11):925‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suissa 2012
- Suissa S, Dell'Aniello S, Ernst P. Long‐term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012;67(11):957‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sullivan 2000
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000;117:5s‐9s. [DOI] [PubMed] [Google Scholar]
Vestergaard 2007
- Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in patients with chronic lung diseases treated with bronchodilator drugs and inhaled and oral corticosteroids. Chest 2007;132(5):1599‐607. [PUBMED: 17890464 ] [DOI] [PubMed] [Google Scholar]
Walters 2011
- Walters JA, Wang W, Morley C, Soltani A, Wood‐Baker R. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD006897.pub2] [DOI] [PubMed] [Google Scholar]
Wedzicha 2000
- Wedzicha JA. Oral corticosteroids for exacerbations of chronic obstructive pulmonary disease. Thorax 2000;55(Suppl 1):S23‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkinson 2004
- Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2004;169(12):1298‐303. [DOI] [PubMed] [Google Scholar]
Wise 2005
- Wise RA, Brown CD. Minimal clinically important differences in the six‐minute walk test and the incremental shuttle walking test. COPD 2005;2(1):125‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Walters 2009
- Walters JAE, Gibson PG, Wood‐Baker R, Hannay M, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001288.pub3] [DOI] [PubMed] [Google Scholar]
Wood‐Baker 2001
- Wood‐Baker R, Walters E, Gibson P. Oral corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001288] [DOI] [PubMed] [Google Scholar]


