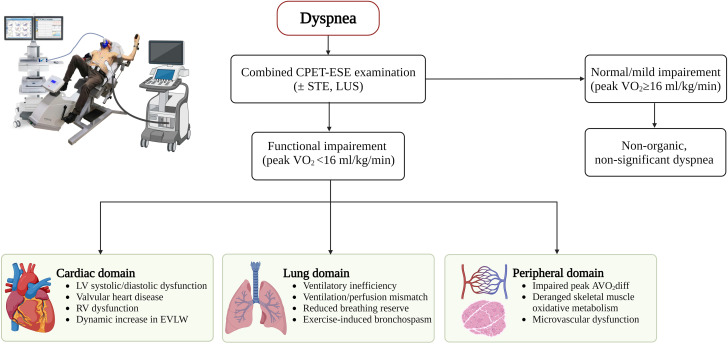
Abstract
Exercise intolerance is a prominent feature of several cardiovascular conditions. However, the physical effort requires the intertwined adaptation of several factors, namely the cardiovascular system, the lungs, and peripheral muscles. Several abnormalities in each domain may be present in a given patient. Cardiopulmonary exercise testing (CPET) has been used to investigate metabolic and ventilatory alterations responsible for exercise intolerance but does not allow for direct evaluation of cardiovascular function. However, this can readily be obtained by concomitant exercise-stress echocardiography (ESE). The combined CPET-ESE approach allows for precise and thorough phenotyping of the pathophysiologic mechanisms underpinning exercise intolerance. Thus, it can be used to refine the diagnostic workup of patients with dyspnoea of unknown origin, as well as improve risk stratification and potentially guide the therapeutic approach in specific conditions, including left and right heart failure or valvular heart disease. However, given its hitherto sporadic use, both the conceptual and technical aspects of CPET-ESE are often poorly known by the clinician. Improving knowledge in this field could significantly aid in anticipating individual disease trajectories and tailoring treatment strategies accordingly. Therefore, we designed this review to revise the pathophysiologic correlates of exercise intolerance, the practical principles of the combined CPET-ESE examination, and its main applications according to current literature.
Keywords: ecocardiography, stress echocardiography, cardiopulmonary function, exercise, heart failure, heart valve disease
Graphical Abstract
Graphical abstract.
The combined CPET-ESE approach to a patient with dyspnoea. AVO2diff, peripheral oxygen extraction; CPET, cardiopulmonary exercise testing; EVLW, extravascular lung water; ESE, exercise-stress echocardiography; LV, left ventricle; RV, right ventricle; VO2, oxygen consumption.
Introduction
Exercise intolerance, defined as the failure to perform physical activities without experiencing dyspnoea and/or fatigue, is a prominent clinical feature of cardiovascular abnormalities.1 In healthy individuals, functional capacity results from the consonant interplay between cardiac and extracardiac responses to stress, including vascular and pulmonary reserve, adequate plasma haemoglobin concentration, and skeletal muscle structure and function.1,2 Cardiopulmonary exercise testing (CPET) can investigate all these contributors simultaneously, allowing for an initial differentiation of cardiac and pulmonary alterations underlying exercise intolerance. However, CPET alone does not allow for a direct evaluation of cardiac determinants of impaired functional capacity [e.g. left or right ventricular (LV and RV, respectively) dysfunction, LV- or RV-arterial uncoupling, worsening valvular heart disease], which can limit the identification of the leading cause of effort intolerance, i.e. cardiac disease, lung disease, physical deconditioning, or any combination of these factors. Combining exercise-stress echocardiography (ESE) with CPET yields further data to investigate the pathophysiology behind effort intolerance.3,4 The multiparametric nature of CPET-ESE provides a deep pathophysiologic characterization of the patient with dyspnoea and can refine its diagnosis and prognosis.3–5 Thus, a more precise understanding of the combined CPET-ESE approach could aid the clinician in anticipating individual disease trajectories and tailoring treatment strategies accordingly. This review aims to revise the theoretical and technical aspects of CPET-ESE and dissect the main parameters analysed during a CPET-ESE evaluation.
Pathophysiology of effort intolerance: a brief overview
The cardiovascular contributors to exercise capacity are summarized by Fick’s principle, stating that oxygen consumption (VO2) is equal to the product of cardiac output (CO) times the arteriovenous oxygen difference (AVO2diff); in turn, CO is the product of heart rate (HR) and stroke volume (SV). Thus, at any given time, the use of oxygen as an energy substrate depends not only on oxygen exchange across the alveolar-capillary membrane in the lungs and on cardiac pump function (i.e. CO) but also on the capacity of peripheral muscles to extract oxygen from the blood and metabolize it.2 This complex system requires a coordinated interplay of numerous factors in resting conditions and is under further stress when physical effort is undertaken.2,3 CPET-ESE provides essential information to understand the causes of exercise intolerance in any subject by simultaneously analysing cardiovascular, metabolic, and respiratory responses to exercise. Indeed, the patient could suffer from a dominant limitation in cardiac function, lung impairment, or inadequate peripheral oxygen extraction (Graphical abstract). However, the pathophysiology of effort intolerance is often multifactorial, and several abnormalities in more than one system may be present in the same patient. For example, while a marked impairment in cardiac reserve (i.e. CO increase during exercise) is a typical feature of conditions such as coronary artery disease, heart failure (HF) with reduced LV ejection fraction (HFrEF), and severe aortic stenosis (AS), subjects with HF and preserved LV ejection fraction (HFpEF) have also been found to display impaired CO increase during exercise compared with healthy subjects.6 Similarly, poor oxygen extraction (i.e. reduced peak AVO2diff) is typical of HFpEF, primarily due to the high prevalence of comorbidities (e.g. arterial hypertension and diabetes mellitus) driving deranged skeletal muscle oxidative metabolism and peripheral microvascular dysfunction, but it can also be observed in advanced HFrEF when skeletal muscle wasting develops due to cardiac cachexia.3,7
The combined CPET-ESE approach
At present, there is no standardized method to perform combined CPET-ESE. However, general indications should be followed to increase the reliability and reproducibility of the findings. First, anaemia should be excluded as a potential cofactor of impaired oxygen delivery and AVO2diff.2 It is reasonable to target 8–12 min as the optimal exercise duration for the patient to achieve peak VO2, as reported in current guidelines regarding CPET.8 Similarly, a functional pulmonary evaluation with spirometry should precede CPET-ESE, in order to identify lung abnormalities associated with exercise intolerance, specifically more than moderate airflow obstruction [forced expiratory volume in the first second (FEV1) to forced vital capacity (FVC) ratio <0.70 and FEV1 < 50% of predicted] and/or restrictive pattern (FVC <80% of predicted) or exercise-induced bronchospasm.9
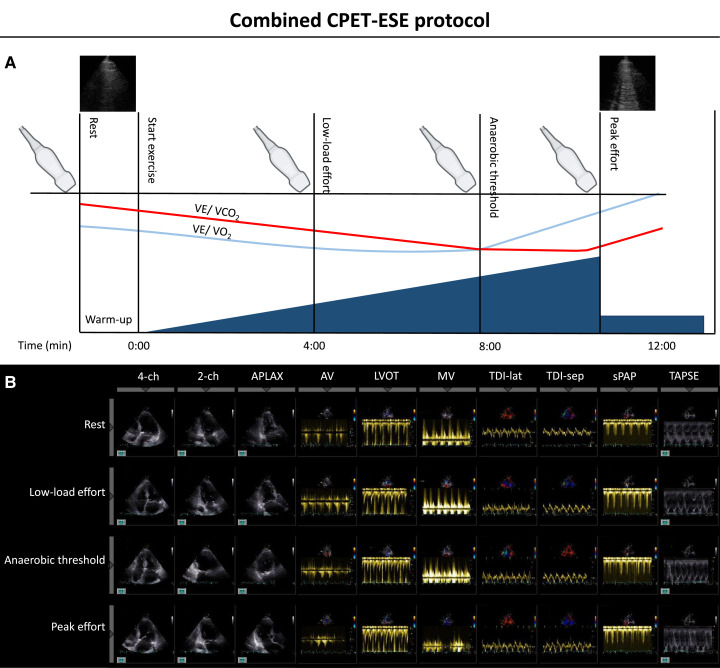
Incremental ramps allow for a steady, low-grade workload increase (8–15 W/min) instead of the brisk workload increments observed with the Bruce protocol stress testing. It should be remembered that the CPET-ESE examination aims to dissect the pathophysiologic mechanisms of exercise intolerance rather than investigate the presence of coronary artery disease (which is, instead, the purpose of the Bruce protocol). This method has been extensively applied to CPET-ESE in different settings.6,10–17 In addition to ensuring that every patient performs an adequately challenging exercise at a constant pedalling rate, the clinician must focus on obtaining adequate echocardiographic images, taking into account each patient’s acoustic window and potential modifying factors (e.g. heart movements, hyperventilation, and body motion). For this reason, the patient is usually asked to exercise in a semirecumbent position, which slightly contrasts with standard CPET protocols employing an upright cycle ergometer or treadmill. However, there seems to be little or no variation between these approaches regarding peak VO2 achieved during exercise.2 The incremental ramp allows the patient to adapt to the exercise gradually and gives the clinician time to store all required echocardiographic images. Albeit the echocardiographic evaluation at rest and peak effort is mandatory, we suggest acquiring images multiple times during exercise. We usually employ a four-stage protocol3: (i) rest; (ii) low-load effort (i.e. within 4 min from the start when HR is usually <100 bpm); (iii) anaerobic threshold, i.e. when the respiratory exchange ratio [expressed by the carbon dioxide production (VCO2)/VO2 ratio] is steadily ≥1.00; and (4) peak effort (i.e. when the patient reports effort-limiting symptoms; Figure 1).
Figure 1.
Combined CPET-ESE protocol outline. (A) Before the beginning of the effort, an echocardiographic evaluation at rest is performed, including baseline lung ultrasound. Then, after a brief warm-up, the symptom-limited graded ramp test starts with continuous breath-by-breath gas exchange measurements. ESE images are acquired at three different stages during exercise: low load (HR <100 bpm), after reaching the anaerobic threshold, and at peak effort (when lung ultrasound is also performed). (B) ESE-derived image acquisition during each stage. Adapted from Pugliese NR, de Biase N, Balletti A, Filidei F, Pieroni A, D’Angelo G et al. Characterisation of hemodynamic and metabolic abnormalities in the heart failure spectrum: the role of combined cardiopulmonary and exercise echocardiography stress test. Minerva Cardiol Angiol 2022;370–84.
During the whole exercise, blood pressure and HR are continuously monitored. Inadequate (both hypotensive18 and hypertensive11) blood pressure response to effort and chronotropic incompetence (defined as the failure to achieve ≥80% of the difference between age-predicted maximal HR and resting HR—i.e. HR reserve—or >62% in patients taking beta-blockers),19 contribute to impaired functional capacity and carry poor prognostic significance in patients with and without cardiovascular disease.
The CPET module allows breath-by-breath gas exchange measurements to analyse the respiratory determinants of effort intolerance (Table 1). It investigates ventilatory (in)efficiency by measuring exercise-induced changes in the dead space volume to tidal volume (VD/VT) ratio and breathing reserve, i.e. the difference between maximum voluntary ventilation (MVV) and minute ventilation (VE). MVV is the total volume of air exhaled during 12 s of rapid, deep breathing and can be either directly measured or estimated from FEV1 352; MVV can be impaired in primary lung disease, especially obstructive lung disease. CPET also gives indirect evidence of ventilation/perfusion mismatch via the slope of VE to carbon dioxide production (VE/VCO2 slope). It also highlights the presence of exercise oscillatory ventilation (EOV), namely a cyclic fluctuation of ventilation and respiratory exchanges (VO2 and VCO2) during the exercise. The EOV sign is not a normal ventilatory response under any circumstances, and it is observed in patients with advanced HFrEF. Despite a controversial underlying mechanism, EOV is invariably associated with poor prognosis.2 Other noteworthy CPET-derived parameters are the VO2/work ratio and oxygen pulse (i.e. the VO2/HR ratio). The VO2/work trajectory plots the relationship between VO2 and workload; in physiological conditions, the VO2/work presents a continual linear rise. In normal subjects, the average slope is 10 mL/min/W, independently of the load imposed and slightly changing according to exercise duration. An abnormally flat VO2/work ratio is often observed in physical deconditioning and/or LV dysfunction.2 On the other hand, the oxygen pulse represents a proxy for SV, assuming a constant AVO2diff. It also has a linear rise throughout the exercise, with a possible plateau approaching maximal exertion. Normal peak values for the O2 pulse at peak exercise are >11 mLO2/min, while they are reduced in patients with exercise-induced left ventricular dysfunction due to myocardial ischaemia.2
Table 1.
Significant CPET parameters
| Parameter | Significance | Normal value |
|---|---|---|
| Assessment of exercise intolerance | ||
| Peak VO2 | The highest VO2 observed during exercise, usually calculated as a 30 s average.10 Index of global performance by central (i.e. cardiopulmonary) and peripheral (i.e. skeletal muscle) components,12 resulting in a robust prognostic marker in HF.20,21 | Values >20 mL/kg/min indicate good functional capacity and prognosis.21 To account for age, sex, weight, and height-dependent differences, peak VO2 can also be expressed as a percentage of the predicted value derived from Wassermann–Hansen equations.22 Normal values are >80% of predicted peak VO2 (the equations might underperform in HFpEF23). |
| RER | The ratio between VCO2 and VO2 increases during exercise. | Reliable index of exercise effort. Psychogenic hyperventilation can cause false increases, especially in the first minutes of exercise. Values ≥1.1 indicate maximal effort. |
| VO2/work | Correlates with exercise limitation due to physical deconditioning or left ventricular dysfunction (inducible myocardial ischaemia and HF). | In physiological conditions, the relationship is characterized by a 10 mL/min/W slope independently of workload, with minor changes according to exercise duration. In advanced HF, the slope is reduced. VO2/work flattening and downsloping indicate a significant reduction in CO (e.g. myocardial ischaemia and severe aortic stenosis). |
| Oxygen pulse | The ratio between VO2 and HR. According to Fick’s principle, the O2 pulse is an indirect index of stroke volume and cardiac performance during exercise, presuming a constant AVO2diff. | Steady rise during the exercise, possibly reaching a plateau in the final moments. Observed values are deemed normal if ≥80% of predicted. |
| Assessment of ventilatory efficiency | ||
| VE/VCO2 slope | Expression of ventilation/perfusion matching in the lung,24 depending on CO2 production (VCO2), physiological dead space/tidal volume ratio (VD/VT), and arterial CO2 partial pressure (PaCO2). VE/VCO2 slope portends prognostic value in HF,25 also at submaximal levels of effort.2 | Optimal when <30. Values >36 indicate significant ventilation/perfusion mismatch.25 |
| PETCO2 | Indicator of ventilation/perfusion matching in the lung and a reliable non-invasive estimate of PaCO2. | Normal values are 36–42 mmHg at rest, with a 3–8 mmHg increase before AT. Following AT, PETCO2 decreases due to hyperventilation. |
| BR | Difference between maximal voluntary ventilation (MVV, either directly measured or calculated as 35 · FEV1 or 40 · FEV1) and VE. Index of the ventilatory response to exercise. | Normal if BR >11 L/min Low BR (i.e. VE almost equal to MVV) is typical of patients with primary lung disease (especially obstructive lung disease). |
| VD/VT | The ratio between physiological dead space (VD) and tidal volume (VT) is another indicator of ventilation/perfusion matching in the lung; it decreases during exercise due to alveolar recruitment and vasodilation in the pulmonary circulation. Its regulation is impaired in numerous respiratory disorders and in left-sided pulmonary hypertension. | Normal values at rest are 33–34%. During exercise, the decrease is appropriate if peak VD/VT ≤25%. |
| EOV | An oscillatory pattern of VE and expired gas kinetics is typically observed in patients with advanced HF and represents a poor prognostic indicator.2 | No agreed-upon definition is available at present. It is usually defined as an oscillatory pattern at rest persisting for ≥60% of the exercise, at an amplitude ≥15% of the average resting value.26 |
| Haemodynamic and peripheral response to exercise | ||
| Heart rate | HR modifications during exercise are a good index of cardiac performance. HR reserve is the HR difference from rest to peak exercise, divided by the difference between age-predicted maximal HR and resting HR.19,27 Chronotropic incompetence is defined as the inability to reach >80% of HR reserve (or >62% in patients taking beta-blockers). | HR continuously increases during exercise in healthy subjects; values >85% of age-predicted maximal HR indicate maximal effort. |
| Blood pressure | Blood pressure (BP) variations reflect the cardiovascular response to exercise. | Systolic BP increases to 200 mmHg in healthy subjects (slightly lower in women than men). Diastolic BP may stay the same or even decrease due to vasodilation in metabolically active muscles. |
| AVO2diff | It describes the difference between arterial and venous oxygen content. AVO2diff can be indirectly estimated from Fick’s principle as VO2/CO, using CO measurement by invasive catheterization or ESE. | Normal resting values are in the range of 5 mL/100 mL. At peak exercise, healthy subjects can increase AVO2diff to 16 mL/100 mL by working muscles.2 |
AVO2diff, arteriovenous oxygen difference; BP, blood pressure; BR, breathing reserve; CO, cardiac output; CPET, cardiopulmonary exercise testing; EOV, exercise oscillatory ventilation; FEV1, forced expiratory volume in the first second; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; MVV, maximal voluntary ventilation; PaCO2, arterial partial pressure of carbon dioxide; PETCO2, end-tidal partial pressure of carbon dioxide; RER, respiratory exchange ratio; SV, stroke volume; VCO2, carbon dioxide production; VD, physiological dead space volume; VE, minute ventilation; VO2, oxygen consumption; VT, tidal volume.
Key echocardiographic parameters that should be acquired during each stage include (Figure 1 and Table 2):
Table 2.
Significant ESE parameters
| Parameter | Significance | Normal value |
|---|---|---|
| LVEF | Primary parameter to classify patients with HF and robust prognostic indicator when <50%.28 However, it only highlights net differences in end-diastolic and end-systolic volumes, being highly load and geometry dependent.29 It does not evaluate true myocardial contractility. | Normal range (mean ± standard deviation) for resting LVEF is 62 ± 5%, slightly higher in women than in men.29 An exercise-induced increase ≥7.5% in patients is defined as contractile reserve. The absence of contractile reserve indicates limited coronary flow reserve and myocardial damage in HF, even in patients with normal resting LVEF.30 |
| LV SV | Blood volume ejected from the LV during a single systolic phase. It can be directly calculated by multiplying the LV outflow tract area by the LV outflow tract velocity-time integral measured by pulsed-wave Doppler. It should be indexed to body surface area. | An exercise-induced increase ≥20% is defined as flow reserve30 and subtends an efficient boost in myocardial contractility via the Frank–Starling mechanism. |
| TDI-S′ | Measured by pulsed wave-TDI, it can be expressed as a single value for each LV wall or averaged. As an index of early systolic velocity (i.e. related to the pre-ejection phase), it may be a more reliable marker of LV contractility than end-systolic indices such as LVEF. It is related to peak VO2.12 | No established cut point, especially for values at peak effort. |
| Diastolic function | For a thorough evaluation of diastole, pulsed-wave Doppler of mitral inflow (E, A, E/A ratio), TDI-derived septal and lateral mitral annular velocities (e′, E/e′ ratio), and TRV must be integrated. In particular, E/e′ ratio is usually considered a reliable non-invasive estimation of LV filling pressures.31 However, evidence for this assumption has been questioned, especially during exercise.32 | Abnormal diastolic response to exercise includes an E/e′ ratio >14, septal e′ velocity <7 cm/s, TRV >3.1 m/s and sPAP >50 mmHg.30 |
| TAPSE | Valuable measure of RV longitudinal systolic function, significantly correlated with RV global function. | Values <17 mm indicate RV systolic dysfunction.29 |
| sPAP | Calculated by estimated right atrial pressure (usually estimated by the inferior vena cava diameter and inspiratory collapse) to continuous-wave Doppler-derived TRV. The TAPSE/sPAP ratio is a valuable index of RV-PA coupling and is related to both peak VO2 and VE/VCO2 slope at peak exercise.33 | Values >50–60 mmHg during exercise are associated with exercise-induced PH30; however, this is best evaluated by analysing the slope of the multipoint mPAP/CO relationship (with values ≥3 mmHg/L/min virtually diagnostic for exercise-induced PH34,35). |
| LV GLS | LV myocardium length change between end-diastole and end-systole, normalized for end-diastolic length (thus conventionally a negative number). GLS is best evaluated by STE and averaged from the three apical views.36 It is an index of LV global longitudinal function and resembles true myocardial contractility more closely than LVEF.29 | GLS ≤−20% is usually deemed normal in healthy subjects. An increase of ≥2% in the absolute value of GLS during exertion indicates good LV functional reserve.30 |
| LARS | Evaluated by STE and averaged from six segments in the apical four- and two-chamber views. It is a reliable index of LA performance and is related to RV-PA coupling,37 impaired ventilatory efficiency during exercise38; finally, it has substantial prognostic significance in both HFrEF and HFpEF.39,40 The LARS/E/e′ ratio has been proposed as a novel measure of LA compliance, as it seems to correlate well with LV filling pressures measured by cardiac catheterization.12 | At present, there is no established cut-point for abnormal LARS at rest and during exercise. |
| LUS B-lines | B-lines are signs of EVLW.41 A dynamic increase in B-lines during exercise highly suggests cardiogenic pulmonary congestion.42,43 | No defined cut-point has been established for peak B-lines or rest-peak DB-lines to diagnose increased EVLW accurately. Nevertheless, their increase demonstrated an adverse prognostic value in HFrEF44 and HFpEF.13,45 |
CO, cardiac output; EF, ejection fraction; ESE, exercise-stress echocardiography; EVLW, extravascular lung water; GLS, global longitudinal strain; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; LA, left atrium; LUS, lung ultrasound; LV, left ventricle; mPAP, mean pulmonary artery pressure; PA, pulmonary artery; PH, pulmonary hypertension; RV, right ventricle; sPAP, systolic pulmonary artery pressure; STE, speckle-tracking echocardiography; SV, stroke volume; TAPSE, tricuspid annular plane systolic excursion; TDI, tissue Doppler imaging; TRV, tricuspid regurgitation velocity; VCO2, carbon dioxide production; VE, minute ventilation; VO2, oxygen consumption.
Two-dimensional (2D) images. The 2D echocardiographic examination at rest should be carried out according to current guidelines.29 Throughout the exercise, apical four-, two-, and three-chamber views should be used to evaluate LV global and regional contractile function.
Doppler evaluation. Measurements should include transvalvular aortic velocity, left ventricular outflow tract velocity-time integral to calculate SV, mitral inflow and tissue Doppler-derived mitral e′ to evaluate the diastolic function, and tricuspid regurgitation (TR) velocity (TRV), to estimate systolic pulmonary arterial pressure (sPAP). Any valvular defect deemed significant at colour Doppler imaging should be evaluated by semiquantitative or quantitative criteria according to current guidelines,46 at least at rest and peak exercise.
M-mode evaluation. Tricuspid annular plane systolic excursion (TAPSE) should be measured as an index of right ventricular systolic function,29 and the TAPSE/sPAP ratio should be calculated to evaluate right ventricular-pulmonary arterial (RV-PA) coupling.33
The following ultrasound techniques can add further significant information:
Speckle tracking echocardiography (STE). STE evaluates LV systolic and left atrial (LA) function [e.g. global longitudinal strain (GLS) and LA reservoir, respectively]. This technique finds its greater application at rest and low-load effort when HR is <100–120 bpm. At higher HRs, STE is prone to algorithm undersampling, and hyperventilation-related through-plane motion artefacts are usually associated with insufficient image quality for STE analysis.37,47
Lung ultrasound (LUS). LUS allows for evaluating and quantifying extravascular lung water (EVLW) during exercise, counting B-lines at rest and peak effort. A dynamic increase in B-lines is virtually always diagnostic of a cardiogenic increase in EVLW.41 While the complete LUS protocol at rest requires careful examination of both the anterior and posterior thorax,41 simplified protocols of four- or eight-site scan acquisitions only on the anterior thorax41 have been proposed during exertion and successfully integrated into the CPET examination.13,48,49
Noteworthy, three- to five-beat loops should be recorded to average parameters over different cardiac cycles, especially in patients with irregular HR, such as those with atrial fibrillation or a significant extrasystolic burden.
CPET-ESE in specific conditions
HFrEF and HFpEF
One of the main fields of application of CPET-ESE is HF (Figure 2).3,4,7,10–15,17,23,35,50–53 CPET-ESE can aid the clinician in investigating the relative pathophysiologic and prognostic weight of the diverse cardiovascular and ventilatory alterations, resulting in exercise intolerance in a given patient with HF, a difficult task to perform relying only on clinical data, as these patients are often ailed by multiple comorbidities.54 CPET is paramount in evaluating peak VO2 in patients with advanced HFrEF for transplant eligibility purposes8; at the same time, ESE can be used to highlight exercise-induced LV wall motion abnormalities and/or CO reduction. However, the combined approach has several other applications, particularly in refining the diagnosis and prognosis of patients with (or with a suspect of) HFpEF. Indeed, CPET-ESE has been used to investigate the relationship between exercise-induced pulmonary hypertension, EVLW accumulation, ventilation/perfusion mismatch, abnormal peripheral oxygen extraction, and patient prognosis across the HF spectrum.13,49,55–57 It has also been used to evaluate the role of LA function and RV-PA uncoupling on effort intolerance and prognosis.12,38 More recently, the impact of impaired LV-arterial coupling14 and hypertensive response to exercise11 on functional capacity has been addressed by the combined approach.
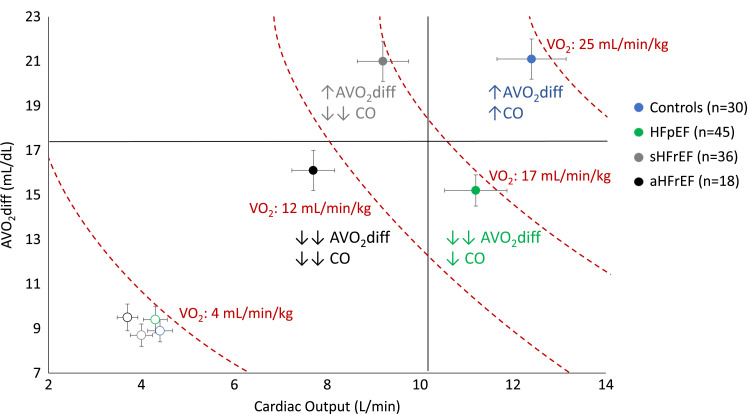
Figure 2.
Patient distribution from rest (empty dots) to peak effort (full dots) plotting AVO2diff vs. CO; error bars indicate standard error of the mean. Dashed lines join points of equal values of VO2. Compared with controls, HFrEF is characterized by reduced peak CO, with preserved peak AVO2diff in stable HFrEF (sHFrEF: peak VO2 > 14 mL/min/kg) and impaired peak AVO2diff in advanced HFrEF (aHFrEF: peak VO2 ≤ 14 mL/min/kg). Patients with HFpEF show preserved peak CO and reduced peak AVO2diff. Adapted from Pugliese NR, Fabiani I, Santini C, Rovai I, Pedrinelli R, Natali A et al. Value of combined cardiopulmonary and echocardiography stress test to characterise the haemodynamic and metabolic responses of patients with heart failure and mid-range ejection fraction. Eur Heart J Cardiovasc Imaging 2019;20:828–36.
Finally, attention has been drawn to the potential relationship between HF severity and acetone, an end product of beta-oxidation, in exhaled breath. This pathway is impaired in patients with acute HF due to mitochondrial energy metabolism alterations.58 Therefore, implementing breath analysis during exercise with a dedicated gas chromatography coupled with mass spectrometry or a selected ion flow tube mass spectrometer could provide additional data to CPET-ESE, unravelling early and/or worsening disease, potentially underestimated at rest.59
Aortic stenosis
The presence of severe symptomatic AS represents per se an indication for aortic valve replacement (Figure 3). However, most patients with degenerative AS in western countries are elderly, asymptomatic (usually due to self-limitation at home) or with non-specific dyspnoea due to multiple extravalvular and extracardiac comorbidities.46 CPET-ESE can be particularly useful in this scenario, thanks to the unique advantage of quantifying objectively effort intolerance measuring peak VO2, which in turn can be related to the other CPET-ESE-derived parameters to better identify the cause of the dyspnoea. Indeed, the detection of effort-induced AS worsening, impaired functional capacity and/or onset of typical symptoms can change the therapeutic course for a given patient.46,60 Thus, the ESE examination should focus on the evaluation of the haemodynamic severity of AS (peak transvalvular velocity, mean transvalvular pressure gradient, and estimated aortic valve area), including the presence or absence of LV functional flow and/or contractile reserve (i.e. adequate SV or LV ejection fraction increase during effort30). ESE can also highlight signs of dynamic increases in sPAP and pulmonary congestion (LUS B-lines) during the whole exercise, which carry an adverse prognostic significance in AS.61 At the same time, CPET can indirectly assess SV response (VO2/work slope) and correlate echographic evidence of pulmonary congestion with data regarding ventilation/perfusion mismatch (VE/VCO2 slope).
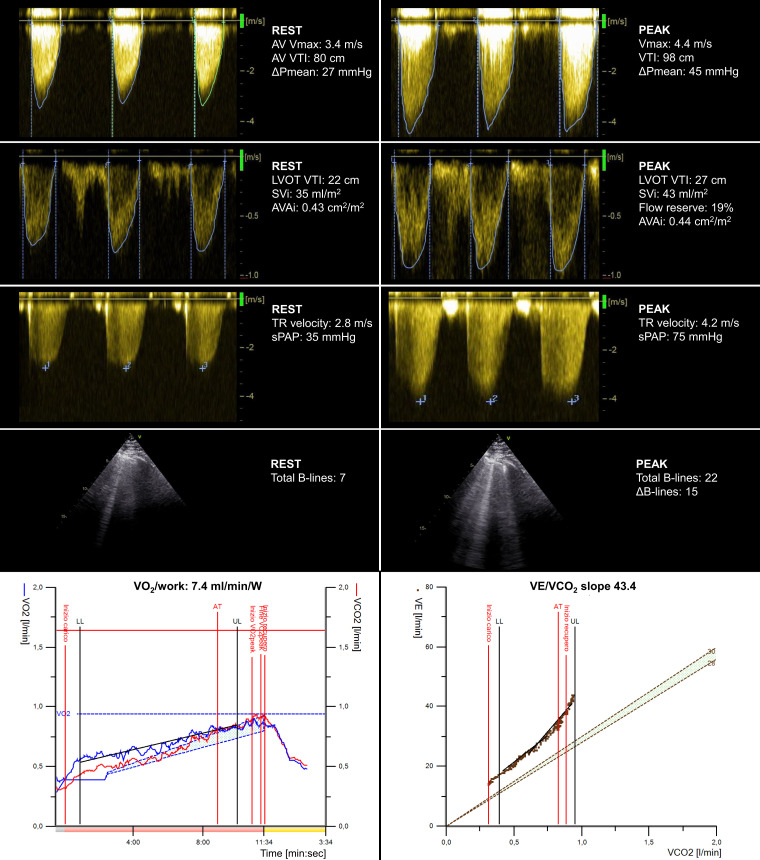
Figure 3.
Applying the combined CPET-ESE protocol to a male patient (75 years old) with moderate AV stenosis at rest and preserved left ventricle ejection fraction. ESE unravelled severe AV stenosis, significant TR worsening, and increased extravascular lung water. CPET showed a moderate reduction in peak oxygen consumption (VO2 = 13.2 mL/kg/min), a reduced VO2/work trajectory (7.4 mL/min/W), and a steep ventilation (VE)/carbon dioxide production slope (43.4). AVAi, indexed aortic valve area; DPmean, mean aortic transvalvular gradient; LVOT, left ventricular outflow tract; sPAP, systolic pulmonary arterial pressure; SVi, indexed stroke volume; Vmax, peak aortic transvalvular velocity; VTI, velocity-time integral.
Mitral regurgitation
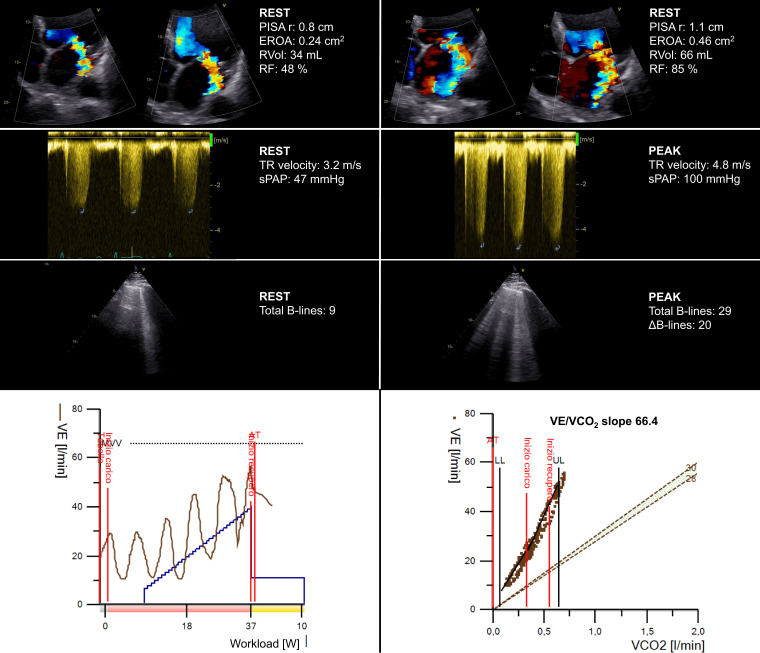
In patients with asymptomatic or paucisymptomatic mitral regurgitation (MR), CPET-ESE can measure an impaired functional capacity and elucidate the mechanisms behind it (Figure 4). Indeed, MR severity can be evaluated with semiquantitative (i.e. vena contracta) or quantitative methods (i.e. effective regurgitant orifice area, regurgitant volume, and fraction) at rest and peak exercise to highlight the worsening of the valvular defect. Also, MR worsening is often associated with multiple supportive indirect signs, as the rise in LV filling pressure is reflected upstream in the pulmonary circulation,30 leading to increased sPAP, EVLW accumulation (measured by LUS), and ventilation/perfusion mismatch (expressed by steeper VE/VCO2 slope). In most severe cases, EOV can be observed, especially in patients with secondary MR (e.g. tethering of the mitral valve leaflets in a severely dilated and dysfunctional LV).
Figure 4.
Applying the combined CPET-ESE protocol to a female patient (72 years old) with dilated cardiomyopathy (left ventricular ejection fraction 45%, diffuse hypokinesia) and moderate MR due to the tethering of the mitral valve posterior leaflet at rest. At peak exercise, ESE showed significant MR and TR worsening and increased extravascular lung water. CPET showed a moderate-to-severe reduction in peak oxygen consumption (VO2 = 10.3 mL/kg/min), an oscillatory ventilation pattern throughout the exercise and a very steep ventilation (VE)/carbon dioxide production slope (66.4). EROA, effective regurgitant orifice area; PISA r, proximal isovelocity surface area radius; RF, regurgitant fraction; RVol, regurgitant volume; sPAP, systolic pulmonary artery pressure.
Right ventricle dysfunction
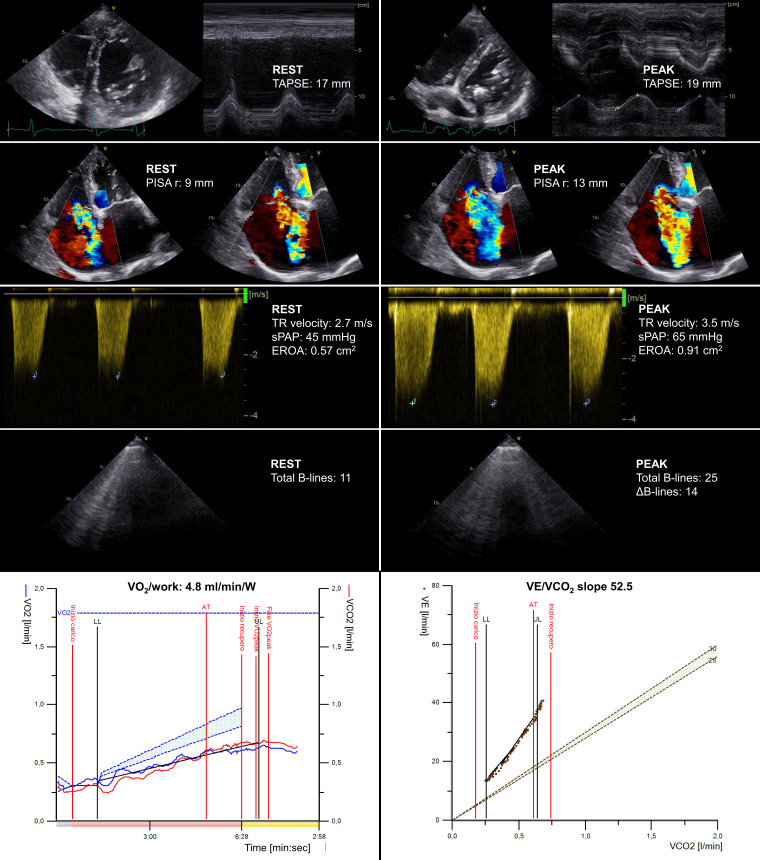
CPET-ESE can be helpful for both the evaluation of functional capacity and the risk stratification of patients with RV dysfunction (Figure 5).62 During the examination, several red flags can be observed, such as the flattening of the interventricular septum (leading to D-shaped LV), suggesting increased RV pressure and/or volume overload, impaired RV-PA coupling (TAPSE/sPAP ratio), TR worsening by semiquantitative or quantitative methods, and increased LUS-derived B-lines denoting increased LV filling pressures associated with enhanced ventricular interdependence. Invasive haemodynamic exercise testing has shown impaired functional capacity in severe TR is associated with elevated systemic and pulmonary venous pressures. Also, increased ventricular interdependence leads to low LV preload and impaired CO increase.63 The CPET module can non-invasively provide multiple findings supporting a significant RV dysfunction, including VO2/work slope flattening and a steeper VE/VCO2 slope.
Figure 5.
Applying the combined CPET-ESE protocol to a male patient (77 years old) with right ventricle dilation (mildly D-shaped left ventricle during diastole) and systolic dysfunction, with severe TR at rest. At peak exercise, ESE showed a more marked D-shaped left ventricle, a significant TR worsening (which became torrential), and increased extravascular lung water. CPET showed a severe reduction in peak oxygen consumption (VO2 = 9.7 mL/kg/min), a reduced VO2/work trajectory (4.8 mL/min/W) and a very steep ventilation (VE)/carbon dioxide production slope (52.5). EROA, effective regurgitant orifice area; PISA r, proximal isovelocity surface area radius; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion.
Other applications and future perspectives
CPET, with or without echocardiography, has been shown to refine the characterization and clinical management of other complex conditions. Indeed, the combined CPET-ESE approach has been used to investigate the pathophysiology and prognostic significance of exercise-induced pulmonary hypertension in hypertrophic cardiomyopathy,64 to refine risk assessment in PA hypertension,65 and to identify patients with poor functional capacity after pulmonary embolism, potentially deserving closer follow-up and/or advanced therapeutic interventions.66
Conclusion
Combined CPET-ESE melds multiple data regarding a patient’s haemodynamic response to exercise with specific insights into its cardiovascular and respiratory determinants. This allows for refined pathophysiologic characterization and prognostic stratification in several cardiovascular conditions. While it is not readily available in all clinical settings due to some intrinsic limitations (e.g. time-consuming, expensive, need for specialized equipment and expertise), the thoroughness of the CPET-ESE examination and its non-invasive nature may suggest a wider acknowledgement and application of the technique (e.g. cardiomyopathies, pulmonary hypertension).
Contributor Information
Lavinia Del Punta, Department of Clinical and Experimental Medicine, University of Pisa, Via Roma, 67, 56126 Pisa, Italy.
Nicolò De Biase, Department of Clinical and Experimental Medicine, University of Pisa, Via Roma, 67, 56126 Pisa, Italy.
Silvia Armenia, Department of Clinical and Experimental Medicine, University of Pisa, Via Roma, 67, 56126 Pisa, Italy.
Valerio Di Fiore, Department of Clinical and Experimental Medicine, University of Pisa, Via Roma, 67, 56126 Pisa, Italy.
Davide Maremmani, Department of Clinical and Experimental Medicine, University of Pisa, Via Roma, 67, 56126 Pisa, Italy.
Luna Gargani, Department of Surgical, Medical and Molecular Pathology and Critical Care Medicine, University of Pisa, Pisa, Italy.
Matteo Mazzola, Department of Surgical, Medical and Molecular Pathology and Critical Care Medicine, University of Pisa, Pisa, Italy.
Marco De Carlo, Cardiac, Thoracic and Vascular Department, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy.
Alessandro Mengozzi, Department of Clinical and Experimental Medicine, University of Pisa, Via Roma, 67, 56126 Pisa, Italy; Department of Cardiology, University Heart Center, Zurich, Switzerland.
Tommaso Lomonaco, Department of Chemistry and Industrial Chemistry, University of Pisa, Pisa, Italy.
Gian Giacomo Galeotti, Department of Surgical, Medical and Molecular Pathology and Critical Care Medicine, University of Pisa, Pisa, Italy.
Frank L Dini, Istituto Auxologico IRCCS, Centro Medico Sant’Agostino, Milan, Italy.
Stefano Masi, Department of Clinical and Experimental Medicine, University of Pisa, Via Roma, 67, 56126 Pisa, Italy.
Nicola Riccardo Pugliese, Department of Clinical and Experimental Medicine, University of Pisa, Via Roma, 67, 56126 Pisa, Italy.
Lead author biography
 I obtained my Master’s degree in ‘Medical Sciences’ from the Sant'Anna School of Advanced Studies, and afterwards, I became a cardiology specialist at Pisa University Hospital. I undertook specific training in cardiovascular imaging, achieving multiple international certifications. I completed my PhD in ‘Clinical and Translational Science’ at Pisa University, where I applied several imaging techniques in the field of heart failure. My main interest and contribution to the literature lie with cardiovascular physiology and pathophysiology, combining research with intensive clinical activity. In particular, I am focusing on novel measures of cardiovascular damage to refine the stratification of patients who present different cardiovascular risk factors.
I obtained my Master’s degree in ‘Medical Sciences’ from the Sant'Anna School of Advanced Studies, and afterwards, I became a cardiology specialist at Pisa University Hospital. I undertook specific training in cardiovascular imaging, achieving multiple international certifications. I completed my PhD in ‘Clinical and Translational Science’ at Pisa University, where I applied several imaging techniques in the field of heart failure. My main interest and contribution to the literature lie with cardiovascular physiology and pathophysiology, combining research with intensive clinical activity. In particular, I am focusing on novel measures of cardiovascular damage to refine the stratification of patients who present different cardiovascular risk factors.
References
- 1.Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DLet al. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2209–25. [DOI] [PubMed] [Google Scholar]
- 2.Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary exercise testing: what is its value? J Am Coll Cardiol 2017;70:1618–36. [DOI] [PubMed] [Google Scholar]
- 3.Pugliese NR, De Biase N, Balletti A, Filidei F, Pieroni A, D’Angelo Get al. Characterisation of haemodynamic and metabolic abnormalities in the heart failure spectrum: the role of combined cardiopulmonary and exercise echocardiography stress test. Minerva Cardiol Angiol 2022;70:370–84. [DOI] [PubMed] [Google Scholar]
- 4.Verwerft J, Soens L, Wynants J, Meysman M, Jogani S, Plein Det al. Heart failure with preserved ejection fraction: relevance of a dedicated dyspnoea clinic. Eur Heart J 2023;44:1544–56. [DOI] [PubMed] [Google Scholar]
- 5.Verwerft J, Bertrand PB, Claessen G, Herbots L, Verbrugge FH. Cardiopulmonary exercise testing with simultaneous echocardiography: blueprints of a dyspnea clinic for suspected HFpEF. JACC Heart Fail 2023;11:243–9. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BDet al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 2010;56:845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pugliese NR, Fabiani I, Santini C, Rovai I, Pedrinelli R, Natali Aet al. Value of combined cardiopulmonary and echocardiography stress test to characterize the haemodynamic and metabolic responses of patients with heart failure and mid-range ejection fraction. Eur Heart J Cardiovasc Imaging 2019;20:828–36. [DOI] [PubMed] [Google Scholar]
- 8.Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 Focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2016;133:e694–711. [DOI] [PubMed] [Google Scholar]
- 9.Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic Iet al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2022;60:2101499. [DOI] [PubMed] [Google Scholar]
- 10.Pugliese NR, Mazzola M, Fabiani I, Gargani L, De Biase N, Pedrinelli Ret al. Haemodynamic and metabolic phenotyping of hypertensive patients with and without heart failure by combining cardiopulmonary and echocardiographic stress test. Eur J Heart Fail 2020;22:458–68. [DOI] [PubMed] [Google Scholar]
- 11.Pugliese NR, De Biase N, Del Punta L, Balletti A, Armenia S, Buralli Set al. Deep phenotype characterization of hypertensive response to exercise: implications on functional capacity and prognosis across the heart failure spectrum. Eur J Heart Fail 2023;25:497–509. [DOI] [PubMed] [Google Scholar]
- 12.Pugliese NR, De Biase N, Conte L, Gargani L, Mazzola M, Fabiani Iet al. Cardiac reserve and exercise capacity: insights from combined cardiopulmonary and exercise echocardiography stress testing. J Am Soc Echocardiogr 2021;34:38–50. [DOI] [PubMed] [Google Scholar]
- 13.Pugliese NR, De Biase N, Gargani L, Mazzola M, Conte L, Fabiani Iet al. Predicting the transition to and progression of heart failure with preserved ejection fraction: a weighted risk score using bio-humoural, cardiopulmonary, and echocardiographic stress testing. Eur J Prev Cardiol 2021;8:1650–61. [DOI] [PubMed] [Google Scholar]
- 14.Pugliese NR, Balletti A, Armenia S, De Biase N, Faita F, Mengozzi Aet al. Ventricular-arterial coupling derived from proximal aortic stiffness and aerobic capacity across the heart failure spectrum. JACC Cardiovasc Imaging 2022;15:1545–59. [DOI] [PubMed] [Google Scholar]
- 15.Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani Let al. Impact of epicardial adipose tissue on cardiovascular hemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail 2021;23:1858–71. [DOI] [PubMed] [Google Scholar]
- 16.Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J 2018;39:2810–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 2018;39:2825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Neal WT, Qureshi WT, Blaha MJ, Keteyian SJ, Brawner CA, Al-Mallah MH. Systolic blood pressure response during exercise stress testing: the Henry Ford ExercIse Testing (FIT) project. J Am Heart Assoc 2015;4:e002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation 2011;123:1010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991;83:778–86. [DOI] [PubMed] [Google Scholar]
- 21.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilisation and ventilation during exercise in patients with chronic cardiac failure. Circulation 1982;65:1213–23. [DOI] [PubMed] [Google Scholar]
- 22.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis 1984;129:S49–55. [DOI] [PubMed] [Google Scholar]
- 23.Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail 2018;6:665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumminello G, Guazzi M, Lancellotti P, Piérard LA. Exercise ventilation inefficiency in heart failure: pathophysiological and clinical significance. Eur Heart J 2007;28:673–8. [DOI] [PubMed] [Google Scholar]
- 25.Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase Pet al. Development of a ventilatory classification system in patients with heart failure. Circulation 2007;115:2410–7. [DOI] [PubMed] [Google Scholar]
- 26.Guazzi M, Boracchi P, Labate V, Arena R, Reina G. Exercise oscillatory breathing and NT-proBNP levels in stable heart failure provide the strongest prediction of cardiac outcome when combining biomarkers with cardiopulmonary exercise testing. J Card Fail 2012;18:313–20. [DOI] [PubMed] [Google Scholar]
- 27.Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol). Am J Cardiol 2005;96:1328–33. [DOI] [PubMed] [Google Scholar]
- 28.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJSet al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 29.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande Let al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–71. [DOI] [PubMed] [Google Scholar]
- 30.Lancellotti P, Pellikka PA, Budts W, Chaudhry FA, Donal E, Dulgheru Ret al. The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2016;17:1191–229. [DOI] [PubMed] [Google Scholar]
- 31.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen Tet al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- 32.Sharifov OF, Gupta H. What is the evidence that the tissue Doppler index E/e’ reflects left ventricular filling pressure changes after exercise or pharmacological intervention for evaluating diastolic function? A systematic review. J Am Heart Assoc 2017;6:e004766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio Set al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 2013;305:H1373–81. [DOI] [PubMed] [Google Scholar]
- 34.Bossone E, Naeije R. Exercise-induced pulmonary hypertension. Heart Fail Clin 2012;8:485–95. [DOI] [PubMed] [Google Scholar]
- 35.Pugliese NR, Mazzola M, Madonna R, Gargani L, De Biase N, Dini FLet al. Exercise-induced pulmonary hypertension in HFpEF and HFrEF: different pathophysiologic mechanism behind similar functional impairment. Vascul Pharmacol 2022;144:106978. [DOI] [PubMed] [Google Scholar]
- 36.Lang RM, Badano LP, Mor-avi V, Afilalo J, Armstrong A, Ernande Let al. Recommendations for cardiac chamber quantification by echocardiography in adults : an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 37.Reddy YNV, Obokata M, Egbe A, Yang JH, Pislaru S, Lin Get al. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail 2019;21:891–900. [DOI] [PubMed] [Google Scholar]
- 38.Sugimoto T, Bandera F, Generati G, Alfonzetti E, Bussadori C, Guazzi M. Left atrial function dynamics during exercise in heart failure: pathophysiological implications on the right heart and exercise ventilation inefficiency. JACC Cardiovasc Imaging 2017;10:1253–64. [DOI] [PubMed] [Google Scholar]
- 39.Carluccio E, Biagioli P, Mengoni A, Francesca Cerasa M, Lauciello R, Zuchi Cet al. Left atrial reservoir function and outcome in heart failure with reduced ejection fraction. Circ Cardiovasc Imaging 2018;11:e007696. [DOI] [PubMed] [Google Scholar]
- 40.Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol 2020;76:1051–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gargani L. Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound 2011;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gargani L. Ultrasound of the lungs: more than a room with a view. Heart Fail Clin 2019;15:297–303. [DOI] [PubMed] [Google Scholar]
- 43.Miglioranza MH, Gargani L, Sant’Anna RT, Rover MM, Martins VM, Mantovani Aet al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging 2013;6:1141–51. [DOI] [PubMed] [Google Scholar]
- 44.Scali MC, Cortigiani L, Simionuc A, Gregori D, Marzilli M, Picano E. Exercise-induced B-lines identify worse functional and prognostic stage in heart failure patients with depressed left ventricular ejection fraction. Eur J Heart Fail 2017;19:1468–78. [DOI] [PubMed] [Google Scholar]
- 45.Coiro S, Simonovic D, Deljanin-Ilic M, Duarte K, Carluccio E, Cattadori Get al. Prognostic value of dynamic changes in pulmonary congestion during exercise stress echocardiography in heart failure with preserved ejection fraction. Circ Heart Fail 2020;13:e006769. [DOI] [PubMed] [Google Scholar]
- 46.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs Jet al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561–632. [DOI] [PubMed] [Google Scholar]
- 47.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann Ret al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015;16:1–11. [DOI] [PubMed] [Google Scholar]
- 48.Pugliese NR, Pellicori P, Filidei F, Del Punta L, De Biase N, Balletti Aet al. The incremental value of multi-organ assessment of congestion using ultrasound in outpatients with heart failure. Eur Heart J Cardiovasc Imaging 2023;24:961–71. [DOI] [PubMed] [Google Scholar]
- 49.Reddy YNV, Obokata M, Wiley B, Koepp KE, Jorgenson CC, Egbe Aet al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 2019;40:3721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guazzi M, Wilhelm M, Halle M, Van Craenenbroeck E, Kemps H, de Boer RAet al. Exercise testing in heart failure with preserved ejection fraction: an appraisal through diagnosis, pathophysiology and therapy—a clinical consensus statement of the Heart Failure Association and European Association of Preventive Cardiology of the European Society of Cardiology. Eur J Heart Fail 2022;24:1327–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nesti L, Pugliese NR, Sciuto P, De Biase N, Mazzola M, Fabiani Iet al. Mechanisms of reduced peak oxygen consumption in subjects with uncomplicated type 2 diabetes. Cardiovasc Diabetol 2021;20:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using non-invasive methods. JACC Cardiovasc Imaging 2020;13:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 2016;37:3294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich Tet al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation 2016;134:e535–78. [DOI] [PubMed] [Google Scholar]
- 55.Lewis GD, Murphy RM, Shah R V, Pappagianopoulos PP, Malhotra R, Bloch KDet al. Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ Heart Fail 2011;4:276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis GD, Bossone E, Naeije R, Grünig E, Saggar R, Lancellotti Pet al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013;128:1470–9. [DOI] [PubMed] [Google Scholar]
- 57.Lewis GD, Gona P, Larson MG, Plehn JF, Benjamin EJ, O’Donnell CJet al. Exercise blood pressure and the risk of incident cardiovascular disease (from the Framingham Heart Study). Am J Cardiol 2008;101:1614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biagini D, Lomonaco T, Ghimenti S, Bellagambi FG, Onor M, Scali MCet al. Determination of volatile organic compounds in exhaled breath of heart failure patients by needle trap micro-extraction coupled with gas chromatography-tandem mass spectrometry. J Breath Res 2017;11:047110. [DOI] [PubMed] [Google Scholar]
- 59.Biagini D, Pugliese NR, Vivaldi FM, Ghimenti S, Lenzi A, De Angelis Fet al. Breath analysis combined with cardiopulmonary exercise testing and echocardiography for monitoring heart failure patients: the AEOLUS protocol. J Breath Res 2023;17:acec08. [DOI] [PubMed] [Google Scholar]
- 60.Magne J, Lancellotti P, Piérard LA. Exercise testing in asymptomatic severe aortic stenosis. JACC Cardiovasc Imaging 2014;7:188–99. [DOI] [PubMed] [Google Scholar]
- 61.Szabó IA, Gargani L, Morvai-Illés B, Polestyuk-Németh N, Frigy A, Varga Aet al. Prognostic value of lung ultrasound in aortic stenosis. Front Physiol 2022;13:838479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ricci F, Bufano G, Galusko V, Sekar B, Benedetto U, Awad WIet al. Tricuspid regurgitation management: a systematic review of clinical practice guidelines and recommendations. Eur Heart J Qual Care Clin Outcomes 2022;8:238–48. [DOI] [PubMed] [Google Scholar]
- 63.Andersen MJ, Nishimura RA, Borlaug BA. The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ Heart Fail 2014;7:911–7. [DOI] [PubMed] [Google Scholar]
- 64.Re F, Halasz G, Moroni F, Beltrami M, Baratta P, Avella Aet al. Exercise-induced pulmonary hypertension in hypertrophic cardiomyopathy: a combined cardiopulmonary exercise test-echocardiographic study. Int J Cardiovasc Imaging 2022;38:2345–52. [DOI] [PubMed] [Google Scholar]
- 65.Badagliacca R, Rischard F, Giudice F L, Howard L, Papa S, Valli Get al. Incremental value of cardiopulmonary exercise testing in intermediate-risk pulmonary arterial hypertension. J Heart Lung Transplant 2022;41:780–90. [DOI] [PubMed] [Google Scholar]
- 66.Farmakis IT, Valerio L, Barco S, Alsheimer E, Ewert R, Giannakoulas Get al. Cardiopulmonary exercise testing during follow-up after acute pulmonary embolism. Eur Respir J 2023;61:2300059. [DOI] [PMC free article] [PubMed] [Google Scholar]