Abstract
Background:
Surgical deactivation of extracranial nerve trigger sites is now well established as an effective treatment for migraine headache. Parallels have been drawn to median nerve decompression for carpal tunnel syndrome (CTS), and 2 previous studies have demonstrated an association between migraine and CTS. The authors sought to (1) substantiate these findings in a considerably larger UK cohort, and (2) investigate potential genetic associations between the 2 disorders.
Methods:
Nested case-control studies were conducted in the UK Biobank cohort of 401,656 individuals. Odds ratios were calculated for the association between migraine and CTS in the overall cohort and sex-stratified subsets. Genetic correlation between migraine and CTS was interrogated by linkage disequilibrium score regression, leveraging data from published genomewide association studies. Regions of genetic overlap were identified by multitrait analysis of genomewide association studies and cross-phenotype association.
Results:
Migraine and CTS show a significant epidemiologic association within UK Biobank (OR, 1.14, 95% CI, 1.04 to 1.25; P = 0.0058), which is specific to women (OR, 1.15; 95% CI, 1.04 to 1.28; P = 0.0057) and not men (OR, 1.07; 95% CI, 0.82 to 1.40; P = 0.61). Genetic analysis demonstrated a significant positive genetic correlation between the 2 disorders (rg = 0.13; P = 0.0039), and implicated the TRIM32 locus on chromosome 9 as a region of genetic overlap.
Conclusions:
This study replicates past reports of an epidemiologic association between CTS and migraine, albeit in women only. This association is underpinned by a genetic correlation, with shared genetic susceptibility at the TRIM32 locus. The authors’ data add credibility to the notion that an element of entrapment neuropathy underlies migraine pathophysiology.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Risk, III.
Migraine headache is a disabling chronic neurologic disorder that affects 14.6% of the U.S. population,1 and is the second largest contributor to neurologic disability-adjusted life-years after stroke.2 It has an annual health care cost per patient of $11,000.3 Pharmacotherapy for acute episodes and prophylaxis form the mainstays of migraine treatment; however, in up to 50% of cases, medication fails to relieve symptoms or produces significant side effects.4,5
Surgical nerve release for migraine has emerged over the past 20 years, based on the theory that entrapment of head and neck sensory nerves trigger migraine headaches.6 Anatomical studies have identified 4 major trigger sites (ie, frontal, temporal, occipital, and nasal), with trigger-site deactivation involving one or a combination of musculofascial decompression, removal of impinging vessels, and resection of nerve segments.6 Histologic studies support a natural history of chronic entrapment or irritation, with extracranial nerves removed from migraineurs exhibiting axonal abnormalities and dysregulated myelination.7 Notably, trigeminal sensory and pain fibers pass through cranial sutures between the calvarial bones.8,9 This provides an anatomical substrate for the intracranial transmission of peripheral triggers to meningeal nociceptors, with potential for the subsequent activation of the trigeminovascular system.
The efficacy of trigger-site deactivation is appreciable: 68.3% to 100% of patients show at least a 50% reduction in migraine episodes, and 8.3% to 86.5% of patients experience complete elimination of symptoms, and symptomatic improvement persists for at least 5 years after intervention.10–14 Considering the anatomical, histologic, and clinical evidence, migraine may be thought of as having an element of entrapment neuropathy, akin to carpal tunnel syndrome (CTS), which is treated effectively by median nerve decompression. A recent intraoperative study in migraineurs undergoing greater occipital nerve trigger-site deactivation found the trapezius fascia encasing the nerve to be thickened and fibrotic in the majority of patients, similar to changes to the subsynovial connective tissues surrounding the median nerve seen in CTS.15
Noting their pathophysiologic similarity, Law et al. reported an epidemiologic association between CTS and migraine in the U.S. population: CTS prevalence in patients with migraine was 8% compared with 3% in those without migraine headache (adjusted OR, 2.67; 95% CI, 2.22 to 3.22).16 Corroborating this, Gfrerer et al. found CTS prevalence to be 1.8- to 3.8-fold more common in a cohort of 137 patients who underwent surgical trigger-site deactivation for migraine than in the general population.17 However, the case ascertainment question of Law et al. was not specific to migraine (individuals experiencing “severe headaches or migraine”) and the study by Gfrerer et al. is limited by its modest cohort size and selection of surgical candidates that likely represent those migraineurs most susceptible to peripheral nerve irritability.
The present study sought to validate these findings by leveraging the power of a large UK cohort of over 400,000 individuals, and by using a phenotypic definition specific for migraine. We then interrogate the genetic underpinnings of this association by using summary data from genomewide association studies (GWAS) in migraine and CTS, and reveal a significant genetic correlation between the 2 disorders.
PATIENTS AND METHODS
Ethical Approval
UK Biobank has approval from the North West Multi-Centre Research Ethics Committee (11/NW/0382). All 23andMe, Inc. research participants included in this study provided informed consent for their genotype data to be used for research purposes under a protocol approved by the external Association for the Accreditation of Human Research Protection Programs–accredited institutional review board, ethical, and independent review services.
Data Set
The UK Biobank prospective cohort comprises approximately 500,000 individuals aged 40 to 69 years who have undergone whole-genome genotyping and linkage of these data to their medical records.18 We have previously conducted quality control of the raw Biobank data set for a related study of the genetic origins of CTS, and have described this in detail elsewhere.19 The final cohort consisted of 401,656 individuals (184,499 men and 217,157 women) of White British ancestry. Participant age was calculated on December 31, 2019, based on year of birth, and participant sex was determined by genotypic rather than self-reported sex.
Phenotyping
CTS and migraine cases were identified using diagnostic and operation codes from the UK Biobank showcase. CTS cases (n = 12,312) were those with one or more of the following codes:
International Classification of Diseases, 10th Revision, code for CTS (G56.0).
OPCS code for carpal tunnel release (A65.1) or revision of carpal tunnel release (A65.2).
Self-reported operation code for carpal tunnel surgery (1501).
Self-reported noncancer illness code for CTS (1541).
Migraine (n=14,409) cases were those with one or more of the following codes:
International Classification of Diseases, 10th Revision, code for migraine (G43.0).
Self-reported noncancer illness code for migraine (1265).
Epidemiologic Analysis
A nested case-control study was conducted to assess epidemiologic associations between CTS and migraine in UK Biobank. In this study design, cases and controls are drawn from the population of a fully enumerated cohort, which confers several advantages to a case-control study, including the ability to select cases and controls from the same underlying population, and to minimize the effect of potential confounding variables through matching.
Matching of cases to controls was performed at a 1:5 ratio using the nearest neighbor matching method in R package MatchIt,20 with matching variables “sex” (exact match) and “year of birth” (nearest match). In a separate set of matching, body mass index (BMI) (nearest match) was also included given that BMI is a risk factor for CTS and may act as a potentially significant confounder. The cobalt R package was used to assess covariate balance after matching. For the 2 case-control data sets (eg, with and without BMI matching), 3 analyses were performed: (1) for the whole cohort; and subgroup analyses for (2) men only, and (3) women only. ORs were calculated using 2 × 2 contingency tables with migraine as the exposure and CTS status as the outcome. Fisher exact tests were performed in R, and 95% CIs were calculated.
Genetic Analysis
Summary statistics for migraine were obtained from a meta-analysis of 22 individual GWAS of approximately 375 000 individuals (59,674 cases and 316,078 controls)21 provided by the International Headache Genetics Consortium and 23andMe, with the latter contributing approximately half of the cases and controls to the meta-analysis (30,465 cases and 134,147 controls). Summary statistics for CTS were obtained from our previous GWAS of carpal tunnel syndrome.19 The genomewide summary statistics for the 2 phenotypes were used to perform 3 independent analyses:
Linkage disequilibrium score correlation (LDSC) analysis: a genetic correlation (rg) value for CTS and migraine was calculated using LDSC version 1.0.022 using the full summary association statistics. Summary statistics from CTS and migraine were standardized using the “munge_sumstats.py” script in the LDSC package for python, with an INFO score threshold of 0.8 and minor allele frequency threshold of 0.01. Genetic correlation was performed using the “ldsc.py” script in the LDSC package for python, according to the authors’ tutorial23 using a UK Biobank–specific linkage disequilibrium score regression from the Pan-UK Biobank project.24 A value of P < 0.05 indicated a significant association.
Multitrait analysis of GWAS (MTAG)25 enables joint analysis of GWAS summary statistics by combining several genetically correlated traits to augment statistical power and identify genomic regions of overlap. We used this analysis with default parameters to identify genetic variants (ie, single nucleotide polymorphisms [SNPs]) that are strongly associated with both CTS and migraine. Summary statistics from CTS and migraine were standardized using the “MungeSumstats” package for R. MTAG was run according to the authors’ tutorial with default parameters.26
Cross-phenotype association27 is a complementary analytic approach to MTAG, which also uses summary-level data from GWAS to detect variants associated with at least one trait; its statistical power is improved by analyzing multiple phenotypes.28 Individual SNP overlaps between migraine and CTS were assessed using the beta-coefficients and standard error values for the SNPs in the two GWAS summary statistics, using the SHet function in cross-phenotype association (CPASSOC). We used this analysis to identify genetic variants that were suggestive of association but not genomewide significant in the individual CTS/migraine GWAS, but which reached statistical significance only on CPASSOC meta-analysis. We therefore conservatively prioritized SNPs with association P values between 1 × 10−5 and 5 × 10−8 in both the migraine and CTS GWAS to avoid discovering genomewide significant variants for the joint migraine-CTS phenotype that are driven solely by one of the phenotypes. A value of P < 5 × 10−8 on CPASSOC meta-analysis indicated a significant association.
RESULTS
Epidemiologic Analysis
Case ascertainment within the post–quality control cohort of 401,656 individuals identified 12,312 CTS cases and 14,409 migraine cases. The matching algorithm produced 2 well-matched case-control data sets (Table 1).
Table 1.
Characteristics of the Case-Control Cohortsa
| No. | Mean BMI ± SD, kg/m2 | Mean Age ± SD (yr) | Men (%) | Women (%) | CTS (%) | |
|---|---|---|---|---|---|---|
| Migraine cases | 14,409 | 27.1 ± 5.0 | 65.6 ± 7.8 | 3368 (33.4) | 11,041 (76.6) | 551 (3.82) |
| Controls (prematching) | 387,247 | 27.3 ± 5.0 | 66.9 ± 8.0 | 181,142 (46.8) | 206,105 (53.2) | 11,761 (3.04) |
| Controls (postmatching) | 72,045 | 27.2 ± 5.0 | 65.6 ± 7.8 | 16,840 (33.4) | 55,205 (76.6) | 2424 (3.36) |
Table 1 shows the total number of individuals, mean BMI, mean age, sex distribution, and numbers of individuals with CTS for (1) the migraine cases, (2) the nonmigraine controls (prematching), and (3) the nonmigraine controls (postmatching). Matching of migraine cases to controls was performed at a 1:5 ratio, using the R package MatchIt.
Table 2 reports the odds ratios for the association of migraine with CTS for the whole cohort (both sexes) with and without BMI matching, and for sex-stratified subgroups. Within UK Biobank, the overall odds ratio for association with CTS in migraine patients was 1.14 (95% CI, 1.04 to 1.25; P = 0.0058). Sex-stratified analysis revealed a significant association in women (OR, 1.15; 95% CI, 1.04 to 1.28; P = 0.0057) but not in men (OR, 1.07; 95% CI, 0.82 to 1.40; P = 0.61). These data suggest that there is a significant epidemiologic association between migraine and CTS in women only. Matching on BMI in addition to age and sex had the effect of marginally increasing the odds ratio in the female-specific (OR, 1.17; 95% CI, 1.06 to 1.29; P = 0.0023) and overall cohort (OR, 1.15; 95% CI, 1.04 to 1.26; P = 0.0044). Thus, despite reports in the literature of migraine and CTS both being associated with increased BMI, this was not the mediating factor.
Table 2.
ORs for Having a CTS Diagnosis in the 2 Nested Case-Control Cohorts
| Disease | OR of CTS (95% CI) | Z-Score | P |
|---|---|---|---|
| Migraine | |||
| Whole cohort | 1.14 (1.04–1.25) | 2.76 | 0.0058 |
| Men | 1.07 (0.82–1.40) | 0.51 | 0.61 |
| Women | 1.15 (1.04–1.28) | 2.77 | 0.0057 |
| Migraine BMIa | |||
| Whole cohort | 1.15 (1.04–1.26) | 2.85 | 0.0044 |
| Men | 1.01 (0.77–1.31) | 0.045 | 0.96 |
| Women | 1.17 (1.06–1.29) | 3.05 | 0.0023 |
The “BMI” suffix refers to matching on body mass index as an additional matching variable in addition to year of birth and sex.
Genetic Analysis
LDSC estimates the genetic correlation between 2 traits, with a correlation coefficient ranging from –1 to +1. The LDSC analysis between the CTS and migraine GWAS summary statistics yielded a statistically significant positive genetic correlation coefficient of 0.13 (Z-score = 2.89; P = 0.0039), providing strong evidence for a shared genetic architecture between CTS and migraine underlying the epidemiologic association.
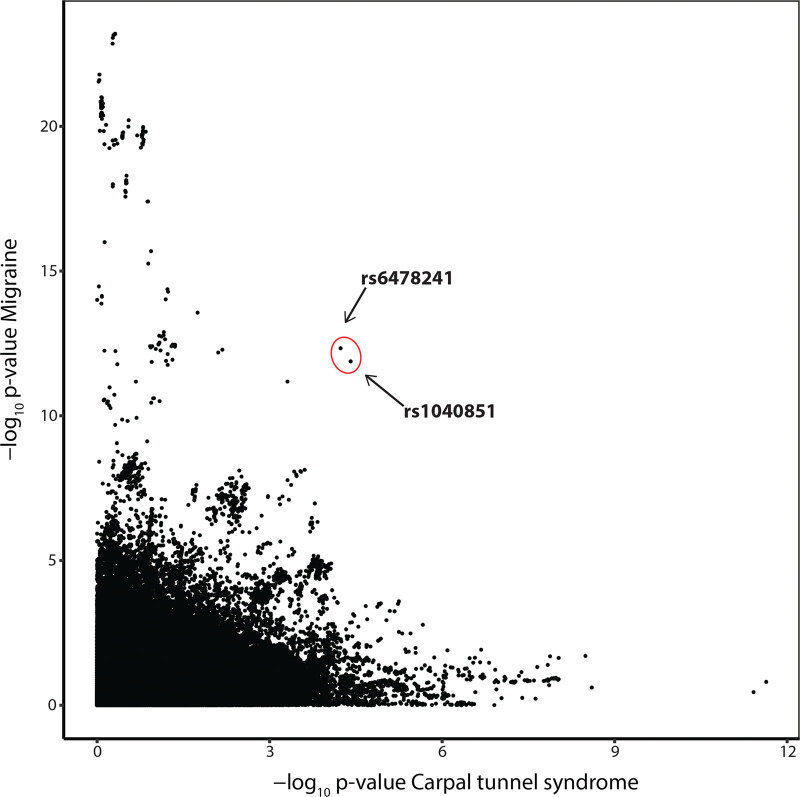
MTAG analysis, which jointly analyzes genetically correlated traits, demonstrated 2 SNPs on chromosome 9—rs1040851 and rs6487241—that were clear visual outliers when their strength of association with both migraine and CTS were plotted following meta-analysis (Fig. 1). For both SNPs, the strength of the statistical association with both phenotypes increased on MTAG meta-analysis (Table 3). The SNP rs1040851 is strongly correlated with rs6478241 (r2 = 0.87 in British ancestry populations; P < 0.0001), and both lie approximately 200 kb downstream of, and are expression quantitative trait loci of, the TRIM32 gene,29 meaning that these variants alter the expression levels of this gene.
Fig. 1.
MTAG analysis. Output of MTAG meta-analysis, demonstrating 2 SNPs, rs1040851 and rs6487241 on chromosome 9, that are clear visual outliers when the strength of association with migraine (y axis) is plotted against CTS (x axis).
Table 3.
Association P Values for the Single Nucleotide Variants rs1040851 and rs6478241 with Migraine and CTS, before and after Meta-Analysis Using MTAG
| P | rs1040851 | rs6478241 | ||
|---|---|---|---|---|
| Pre-MTAG | Post-MTAG | Pre-MTAG | Post-MTAG | |
| CTS | 1.40 × 10−3 | 3.87 × 10−5 | 0.0063 | 5.80 × 10−5 |
| Migraine | 3.86 × 10−12 | 1.32 × 10−12 | 1.22 × 10−12 | 4.67 × 10−13 |
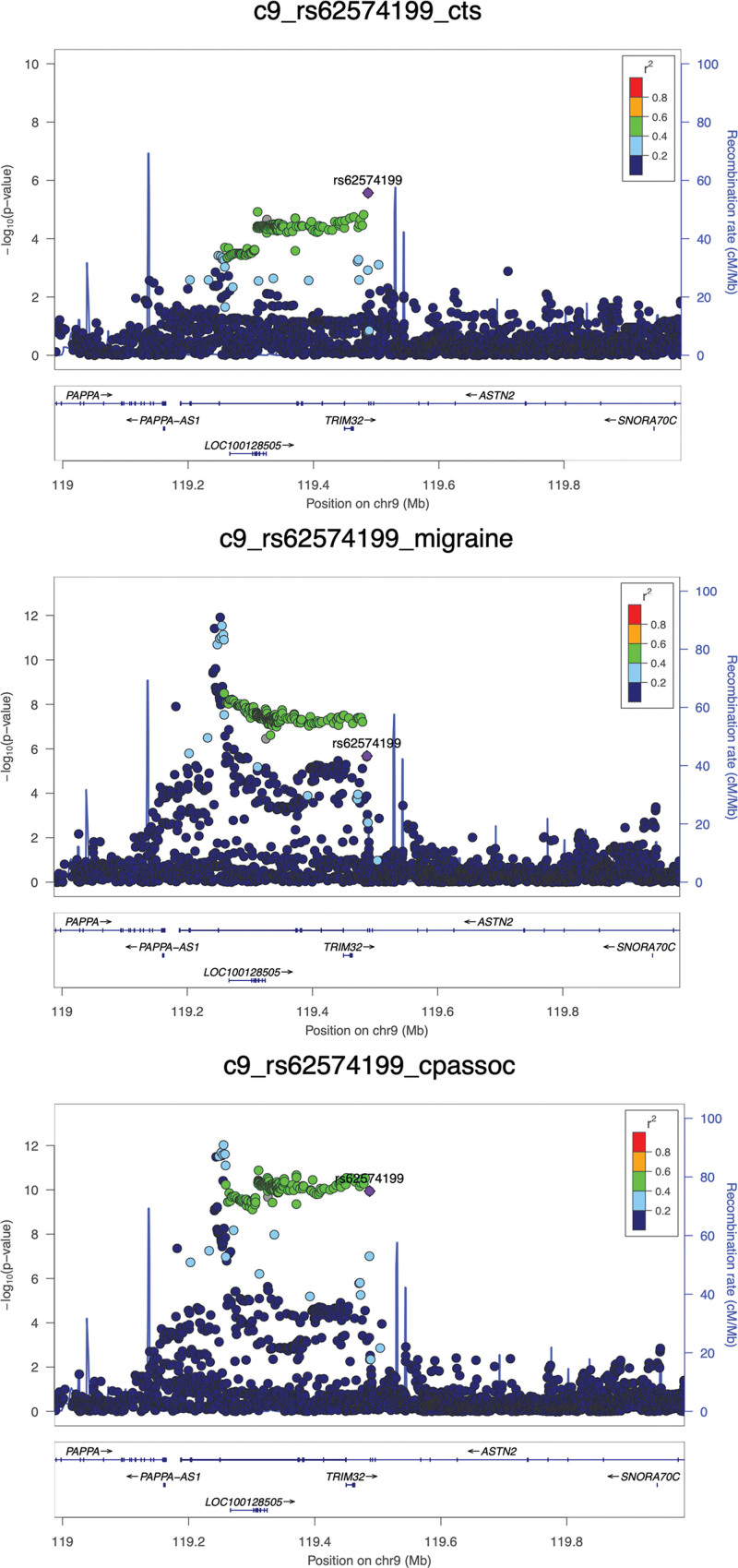
Our complementary analytic approach, CPASSOC, corroborated the importance of this genomic region in terms of CTS-migraine overlap; there was just a single SNP that satisfied the predetermined P value criteria of having a suggestive association in the individual GWAS of CTS and migraine, and reaching genomewide significance only on meta-analysis: rs62574199. This SNP is an intronic (ie, noncoding) variant in the gene ASTN2 (Fig. 2), and although it is not strongly correlated with rs1040851 (r2 = 0.09; P < 0.0001), it is also an expression quantitative trait locus of TRIM32, and therefore also affects the expression of this gene. The 2 results, considered together, suggest the importance of TRIM32 in mediating the shared genetics between CTS and migraine.
Fig. 2.
CPASSOC analysis. LocusZoom plots of the variant rs62574199, showing its association with carpal tunnel syndrome (above, left) and migraine (above, right) in their respective GWAS, and with the joint CTS-migraine phenotype on CPASSOC meta-analysis (below). The variant rs62574199 is shown in purple, and each of the circles represents an SNP. The degree of correlation (linkage disequilibrium) between the SNPs and rs62574199 is demonstrated by color-coding. The x axis indicates the genomic position on chromosome 9, with genes in the region shown, including TRIM32.
DISCUSSION
Anatomical, histologic, and clinical evidence accumulated over the past 2 decades strongly suggests that migraine exhibits features of a nerve entrapment disorder that may be successfully treated by surgical trigger-site deactivation. Two previous studies have reported an epidemiologic association between migraine and CTS, the quintessential entrapment neuropathy. The present study sought to test the veracity of this association in a substantially larger cohort of 401,656 individuals, and to leverage GWAS data to investigate the genetic foundations of this association.
A clear epidemiologic association between migraine and CTS was found in the UK Biobank cohort in this study (OR, 1.14; 95% CI, 1.04 to 1.25; P = 0.0058). Sex stratification revealed that this association was specific to women (OR, 1.15, 95% CI, 1.04 to 1.28; P = 0.0057), being not significant in men (OR, 1.07; 95% CI, 0.82 to 1.40; P = 0.61). Although both migraine and CTS are associated with raised BMI,30,31 this was not a mediating factor, as matching cases and controls on BMI had little material effect on the strength of association in both the overall and female-specific cohorts.
Comparing the present findings with previous studies, both Law et al. and Gfrerer et al. report a notably stronger association between migraine and CTS, between 1.8- and 3.8-fold. Differences in inclusion criteria likely explain this discrepancy. The use by Law et al. of a broad case definition of migraine (“migraine or severe headache”) led to a self-reported diagnosis of migraine in 16.3% of respondents, whereas only 3.6% (n = 14,409) of individuals had a migraine diagnosis in our UK Biobank cohort. The proportion of CTS cases was similar between the studies (3.7% and 3.1%, respectively), and thus the large difference in odds ratios between the studies is likely attributable to differences in the ascertainment of migraine cases. In contrast to the broad inclusion criteria of Law et al., Gfrerer et al. only included patients who had undergone trigger-site deactivation surgery for migraine, which may have enriched for migraineurs who are susceptible to entrapment neuropathy.
The case ascertainment for migraine and CTS in our study also had its limitations. First, our case/control classification was based on hospital diagnostic codes, self-report, or both. Individuals who we designated as cases based on self-report alone are more likely to have been misclassified than individuals with a hospital diagnostic code. Even the latter group may have been misclassified—for instance, not all individuals with a CTS hospital diagnostic code will have had confirmatory electrodiagnostic studies. Second, given that our data set did not include diagnostic codes from primary care (family physicians)—where the majority of health care delivery in the UK occurs32—a proportion of true cases will have been misclassified as controls, thereby underestimating the true population prevalence of both diseases. Third, our study was limited to individuals of White British ancestry to avoid confounding through the variable prevalence of migraine/CTS in different ancestry groups; transancestral analysis of the migraine-CTS association will be a focus of future studies. Despite the discrepancies in the reported odds ratios of migraine-CTS co-occurrence across the 3 studies, what is consistent is the existence of a statistically significant epidemiologic association between migraine and CTS.
Analysis of the genetic overlap between migraine and CTS by linkage disequilibrium score regression indicated a strong genetic correlation, with a positive genetic correlation coefficient (rg) of 0.13. By way of comparison, a recently published study substantiating a genetic correlation between migraine and blood pressure (for which an epidemiologic relationship has been reported33) found lower rg values than that reported here.34 To our knowledge, we report the first demonstration of a genetic association between migraine and CTS, and this bolsters the epidemiologic association reported in our study and others.
Scrutinizing the loci of genetic overlap from our 2 complementary genetic analyses, a region on chromosome 9 was found, implicating the gene TRIM32. We identified at least 3 SNPs in the vicinity of this gene that show the strongest association with both migraine and CTS, all of which alter the expression levels of TRIM32. TRIM32 encodes human tripartite motif family of proteins 32 (TRIM32), a ubiquitous multifunctional protein that has roles in muscle homeostasis, glucose metabolism, and both tumor suppression and tumourigenesis.35 Neurons from the brains of TRIM32 knockout mice have reduced neurofilament protein expression, suggesting a role for TRIM32 in neuronal maintenance.36 As extracranial nerves isolated from migraineurs exhibit neurofilament disruption and dysregulated myelination,7 TRIM32 may be tentatively implicated in entrapment neuropathies by means of a role in neuronal maintenance. Of note, a recent study reporting a highly significant genetic correlation between endometriosis and migraine (rg = 0.38; P = 2.30 × 10−25) also implicated TRIM32 as an overlapping gene between the 2 disorders.37 The role of TRIM32 as a potential risk variant in migraine, CTS, and endometriosis is yet unclear and requires further study, although it is intriguing to note its association with 3 disorders that predominantly affect women.38–40
The notion of migraine as a peripheral nerve disorder remains debatable, as it conflicts with longstanding theories of central generation of migraine. However, and consistent with a polygenic model of migraine susceptibility,21 it may be that only a subset of migraineurs have peripheral cause, and this may be the subgroup who benefit from trigger-site deactivation surgery.41 Accepting this does not devalue the contribution of central mechanisms (trigeminovascular system activation and sensitization, cortical spreading depression, parasympathetic input) to migraine generation, and medications targeting these events will continue to be critical to treatment, alongside surgical trigger-site deactivation in selected patients. Ideally, our study would have been able to stratify patients with peripherally generated migraines to examine the strength of association of CTS with this subset of migraine alone; however, surgery for migraine is not yet routinely undertaken in the UK, so we were unable to make the distinction between surgical and nonsurgical migraine patients based on the data set available to us.
SUMMARY
In this article, we provide the first-ever demonstration of a significant genetic association between migraine and CTS, suggesting shared susceptibility or pathophysiology. We also validate the previously related epidemiologic association between the 2 disorders in a substantially larger cohort. CTS is the archetypal entrapment neuropathy that is successfully treated by surgical decompression. By demonstrating the genetic underpinnings to the epidemiologic association between migraine and CTS, our findings add further credibility to the idea that migraine pathophysiology may be in part mediated by peripheral nerve entrapment or a triggering mechanism, thus providing a new lens for considering the value of migraine surgery.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
DISCLAIMER
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. This represents independent research funded by the NIHR. The views expressed in this article are the authors’ own and are not necessarily those of the National Institute for Health and Care Research, National Health Service, or Department of Health and Social Care.
ACKNOWLEDGMENTS
Dr. Wiberg was supported by a clinical research training fellowship from the Medical Research Council (MR/N001524/1) when this work was undertaken, and is currently supported by a clinical lectureship from the National Institute for Health and Care Research (NIHR). Dr. Furniss is supported by the NIHR Biomedical Research Centre, Oxford. The authors would like to thank the research participants and employees of 23andMe for making this work possible.
Footnotes
The first two authors contributed equally.
Presented at Plastic Surgery The Meeting 2022, the 91st Annual Meeting of the American Society of Plastic Surgeons, in Boston, Massachusetts, October 27 through 30, 2022.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonafede M, Sapra S, Shah N, Tepper S, Cappell K, Desai P. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache 2018;58:700–714. [DOI] [PubMed] [Google Scholar]
- 4.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the Second International Burden of Migraine Study (IBMS-II). Headache 2013;53:644–655. [DOI] [PubMed] [Google Scholar]
- 5.Lipton RB, Munjal S, Buse DC, Fanning KM, Bennett A, Reed ML. Predicting inadequate response to acute migraine medication: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache 2016;56:1635–1648. [DOI] [PubMed] [Google Scholar]
- 6.Hatef DA, Gutowski KA, Culbertson GR, Zielinski M, Manahan MA. A comprehensive review of surgical treatment of migraine surgery safety and efficacy. Plast Reconstr Surg. 2020;146:187e–195e. [DOI] [PubMed] [Google Scholar]
- 7.Guyuron B, Yohannes E, Miller R, Chim H, Reed D, Chance MR. Electron microscopic and proteomic comparison of terminal branches of the trigeminal nerve in patients with and without migraine headaches. Plast Reconstr Surg. 2014;134:796e–805e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schueler M, Neuhuber WL, De Col R, Messlinger K. Innervation of rat and human dura mater and pericranial tissues in the parieto-temporal region by meningeal afferents. Headache 2014;54:996–1009. [DOI] [PubMed] [Google Scholar]
- 9.Schueler M, Messlinger K, Dux M, Neuhuber WL, De R. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain 2013;154:1622–1631. [DOI] [PubMed] [Google Scholar]
- 10.Guyuron B, Kriegler JS, Davis J, Amini SB. Comprehensive surgical treatment of migraine headaches. Plast Reconstr Surg. 2005;115:1–9. [PubMed] [Google Scholar]
- 11.Guyuron B, Reed D, Kriegler JS, Davis J, Pashmini N, Amini S. A placebo-controlled surgical trial of the treatment of migraine headaches. Plast Reconstr Surg. 2009;124:461–468. [DOI] [PubMed] [Google Scholar]
- 12.Kurlander DE, Ascha M, Sattar A, Guyuron B. In-depth review of symptoms, triggers, and surgical deactivation of frontal migraine headaches (site I). Plast Reconstr Surg. 2016;138:681–688. [DOI] [PubMed] [Google Scholar]
- 13.Guyuron B, Kriegler JS, Davis J, Amini SB. Five-year outcome of surgical treatment of migraine headaches. Plast Reconstr Surg. 2011;127:603–608. [DOI] [PubMed] [Google Scholar]
- 14.ElHawary H, Barone N, Baradaran A, Janis JE. Efficacy and safety of migraine surgery. Ann Surg. 2022;275:e315–e323. [DOI] [PubMed] [Google Scholar]
- 15.Gfrerer L, Hansdorfer MA, Ortiz R, Chartier C, Nealon KP, Austen WG. Muscle fascia changes in patients with occipital neuralgia, headache, or migraine. Plast Reconstr Surg. 2021;147:176–180. [DOI] [PubMed] [Google Scholar]
- 16.Law H-Z, Amirlak B, Cheng J, Sammer DM. An association between carpal tunnel syndrome and migraine headaches—National Health Interview Survey, 2010. Plast Reconstr Surg Glob Open 2015;3:e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gfrerer L, Chartier C, Lans J, Eberlin KR, Austen WG. A correlation between upper extremity compressive neuropathy and nerve compression headache. Plast Reconstr Surg. 2021;148:1308–1315. [DOI] [PubMed] [Google Scholar]
- 18.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018;562:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiberg A, Ng M, Schmid AB, et al. A genome-wide association analysis identifies 16 novel susceptibility loci for carpal tunnel syndrome. Nat Commun. 2019;10:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28. [Google Scholar]
- 21.Gormley P, Anttila V, Winsvold BS, et al.; International Headache Genetics Consortium. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet. 2016;48:856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulik-Sullivan B, Finucane HK, Anttila V, et al.; ReproGen Consortium. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Github. Heritability and genetic correlation. Available at: https://github.com/bulik/ldsc/wiki/Heritability-and-Genetic-Correlation. Accessed May 8, 2024. [Google Scholar]
- 24.Pan-UK Biobank. Pan-UK Biobank. Available at: https://pan.ukbb.broadinstitute.org/. Accessed May 8, 2024. [Google Scholar]
- 25.Turley P, Walters RK, Maghzian O, et al.; 23andMe Research Team. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat Genet. 2018;50:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Github. Tutorial 1: The Basics. Available at: https://github.com/JonJala/mtag?tab=readme-ov-file#readme. Accessed May 8, 2024. [Google Scholar]
- 27.Zhu X, Feng T, Tayo BO, et al.; COGENT BP Consortium. Meta-analysis of correlated traits via summary statistics from GWASs with an application in hypertension. Am J Hum Genet. 2015;96:21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Zhu X. Cross-phenotype association analysis using summary statistics from GWAS. Methods Mol Biol. 2017;1666:455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghoussaini M, Mountjoy E, Carmona M, et al. Open targets genetics: systematic identification of trait-associated genes using large-scale genetics and functional genomics10.1093/nar/gkaa840. Nucleic Acids Res. 2021;49:D1311–D1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiri R, Pourmemari MH, Falah-Hassani K, Viikari-Juntura E. The effect of excess body mass on the risk of carpal tunnel syndrome: a meta-analysis of 58 studies. Obes Rev. 2015;16:1094–1104. [DOI] [PubMed] [Google Scholar]
- 31.Gelaye B, Sacco S, Brown WJ, Nitchie HL, Ornello R, Peterlin BL. Body composition status and the risk of migraine. Neurology 2017;88:1795–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The King’s Fund. Understanding pressures in general practice. Available at: https://assets.kingsfund.org.uk/f/256914/x/62ae34157d/understanding_pressures_general_practice_2016.pdf. Accessed May 8, 2024. [Google Scholar]
- 33.Scher AI, Terwindt GM, Picavet HSJ, Verschuren WMM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology 2005;64:614–620. [DOI] [PubMed] [Google Scholar]
- 34.Guo Y, Rist PM, Daghlas I, Giulianini F, Kurth T, Chasman DI; International Headache Genetics Consortium. A genome-wide cross-phenotype meta-analysis of the association of blood pressure with migraine. Nat Commun. 2020;11:3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bawa S, Piccirillo R, Geisbrecht ER. TRIM32: a multifunctional protein involved in muscle homeostasis, glucose metabolism, and tumorigenesis. Biomolecules 2021;11:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudryashova E, Wu J, Havton LA, Spencer MJ. Deficiency of the E3 ubiquitin ligase TRIM32 in mice leads to a myopathy with a neurogenic component. Hum Mol Genet. 2009;18:1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adewuyi EO, Sapkota Y, Auta A, et al. Shared molecular genetic mechanisms underlie endometriosis and migraine comorbidity. Genes (Basel) 2020;11:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. 2017;16:76–87. [DOI] [PubMed] [Google Scholar]
- 39.Wiberg A, Smillie RW, Dupré S, Schmid AB, Bennett DL, Furniss D. Replication of epidemiological associations of carpal tunnel syndrome in a UK population-based cohort of over 400,000 people. J Plast Reconstr Aesthetic Surg. 2021;75:1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rei C, Williams T, Feloney M. Endometriosis in a man as a rare source of abdominal pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2018;2018:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gfrerer L, Hulsen JH, McLeod MD, Wright EJ, Austen WG. Migraine surgery: an all or nothing phenomenon? Prospective evaluation of surgical outcomes. Ann Surg. 2019;269:994–999. [DOI] [PubMed] [Google Scholar]