Abstract
Background and Aims:
The role of preoperative pharmacological prophylaxis in preventing aspiration pneumonitis under general anesthesia (GA) in patients at low risk of aspiration pneumonitis is still under debate. We addressed the need for routine pharmacological aspiration prophylaxis in at-risk population by assessing the change in gastric volume using ultrasound with and without pharmacological acid aspiration prophylaxis.
Material and Methods:
A single-center, randomized double-blinded trial, with 200 adult patients scheduled for elective surgical procedures under GA, were randomized into a prophylaxis group, in which the patients received oral famotidine and metoclopramide, and a no prophylaxis group, in which the patients did not receive any prophylaxis. Gastric volume derived from preinduction measurement of gastric antral volume by ultrasound, postinduction gastric pH, and incidences of aspiration pneumonitis were compared. Bland–Altman plot was used to determine the level of agreement between measured gastric volume and ultrasonography based on calculated gastric volume.
Results:
The gastric antral cross-sectional area (CSA) and volume in the no prophylaxis group (3.12 cm2 and 20.11 ml, respectively) were comparable to the prophylaxis group (2.56 cm2 and 19.67 ml, respectively) (P-values 0.97 and 0.63, respectively). Although there was a statistically significant decrease in gastric pH in the no prophylaxis group (P-value 0.01), it was not clinically significant to increase the risk of aspiration pneumonitis based on Roberts and Shirley criteria (P-value 0.39).
Conclusion:
In an adequately fasted low-risk population, the amount of residual gastric volume was similar and below the aspiration threshold, regardless of the aspiration prophylaxis status.
Keywords: Aspiration pneumonia, aspiration prophylaxis, gastric volume, general anesthesia, H2 receptor antagonists, prokinetics, ultrasonography
Introduction
Aspiration of gastric contents is considered one of the most fearsome complications under anesthesia in patients undergoing various surgical procedures. After Mendelson’s description of aspiration pneumonitis under anesthesia, various recommendations have been proposed to minimize the risk and incidence. One among them is to administer preoperative pharmacological acid aspiration prophylaxis. Although the American Society of Anesthesiologists (ASA) and the European Society of Anesthesiologists (ESA) guidelines recommend avoiding the routine use of prophylactic pharmacological prophylaxis in patients with low risk of aspiration pneumonitis, their recommendations are based on consensus opinion rather than high-quality evidence.[1,2] So, we decided to conduct a clinical trial to assess the role of acid aspiration prophylaxis in the population at risk using preinduction gastric ultrasonography (USG). Among the methods available, USG has been proved to be an effective noninvasive tool for quantitative and qualitative gastric volume assessment.[3,4,5] The aim of this study was to show that optimal preoperative fasting alone would be sufficient to prevent aspiration in the low-risk surgical population. The primary aim was to compare the difference in residual gastric volume calculated based on gastric antral cross-sectional area (CSA) with and without acid aspiration prophylaxis. The secondary outcomes were to compare the gastric pH and incidence of aspiration pneumonitis between both the groups.
Material and Methods
This study was a single-center, double-blinded, randomized controlled trial conducted in a tertiary care hospital and had two arms. The scientific and ethical approval for the conduct of the study was obtained from the Institute Ethics Committee (human studies JIP/IEC/2016/27/920, 25/05/2016.). The trial was registered prospectively in the clinical trials registry of India (CTRI/2017/08/009290). Patients above the age of 18 years who were scheduled for elective surgical procedures under general anesthesia (GA) and belonging to the ASA physical status classification I and II were deemed eligible to participate in this trial. Patients with a history of diabetic autonomic neuropathy, previous gastrointestinal surgery, gastroesophageal reflux disease, preoperative continuous nasogastric drainage, obesity (body mass index >30 kg/m2), and pregnant patients were excluded from this study. Patients with a preoperative intake of proton pump inhibitors, H2 receptor antagonists, and prokinetics were also excluded. Informed written consent was obtained from each study participant by the anesthesiologist during the preoperative evaluation on the day before surgery. Then the study participants were randomized into either prophylaxis (group P) or no-prophylaxis (group NP) group using computer-generated block randomization table using variable block sizes in the ratio of 1:1. Randomization was performed by a research coordinator using random allocation software 2.0. Allocation concealment was done using the sequentially numbered opaque sealed envelope (SNOSE) technique. The anesthesiologist performing ultrasound and the data collector were blinded to the group allocation by restraining their access to the patient medical records and also restraining them from obtaining history regarding premedication intake. Study participants were grouped according to their allocation into P and NP groups. They were randomly allocated to one of the groups on the night before surgery by an anesthesiologist not involved in this study. All patients in both the study groups were kept fasting for 8 h with no intake of solid food and allowed intake of 200 ml of clear fluid until 2 h before the scheduled surgery.[2,6]
The patients in the P group received oral famotidine 20 mg on the night before surgery and oral famotidine 20 mg and metoclopramide 10 mg, 2 h before the surgery. The NP group did not receive any pharmacological prophylaxis. Both the groups received oral diazepam (0.1 mg/kg) on the night before surgery and 2 h before on the day of surgery. On the day of surgery, after verifying the fasting status, patients were shifted inside the operation theater and standard monitors like pulse oximetry, electrocardiogram, and noninvasive blood pressure were attached and monitored. The anesthesiologist who performed gastric USG had been trained by the consultant anesthesiologists experienced in gastric USG before starting the study. Patients in both the groups underwent ultrasound scanning of the gastric antrum as follows. While patients were lying in the right lateral decubitus position, the gastric antrum was identified by placing a 2–5 MHz curvilinear Sonosite S-ICU™ (FUJIFILM, Sonosite, Inc.) ultrasound probe in a parasagittal plane over the left upper abdominal quadrant by an anesthesiologist experienced in point-of-care ultrasound. The ultrasound probe was placed in the midline of the upper abdomen in the sagittal plane and moved toward the left to identify the left lobe of the liver. After visualizing the left lobe of the liver, the probe was changed to oblique view to visualize either inferior vena cava or superior mesenteric artery. Then, the gastric antrum was visualized either as a bull eye pattern (closed antrum) with no residual gastric contents or as a circular pattern (open antrum) if the gastric contents were present. The free tracing method was used to measure the gastric antral CSA. The images were recorded and verified by another anesthesiologist. The following formula was used subsequently to calculate the predicted gastric volume: gastric volume (ml) = 27.0 + 14.6 (right lateral gastric antral CSA) −1.28 (age), as proposed by Bolondi and subsequently by Perlas et al.[7,8,9,10,11,12] Then, we converted the calculated gastric volume to the patient body weight-based gastric volume to assess the risk of aspiration. If a patient in any of the two groups was found to have a predicted gastric volume of more than or equal to 1.5 ml/kg, the patients would receive rescue premedication with intravenous metoclopramide 10 mg before the induction of GA and followed by rapid sequence intubation. The induction and airway management of choice was as per the decision of the primary anesthesiologist. In both groups of patients, after the induction of GA, an appropriate-sized orogastric tube (OGT) was inserted. The OGT position was confirmed by auscultating the epigastric region with a stethoscope while injecting 10 ml of air through its proximal end. Aspiration of the gastric volume was attempted through OGT with a 50-ml syringe in supine, Trendelenburg, and bilateral tilt positions. The volume of the aspirate was noted. From the aspirated sample, gastric pH was measured with the help of a pH meter (VantaKool auto-calibrated pH meter with 0.01 accuracy, Vanta Kool, Inc.). Postoperatively, all study participants were followed up for 48 h for the evidence of aspiration pneumonitis. The presence of fever, cough, breathlessness, pleuritic chest pain, and infiltrates in chest X-ray defined aspiration pneumonitis. The incidence of aspiration pneumonitis was noted in patients with acute inflammatory signs such as fever, tachycardia, tachypnea, leukocytosis, along with the respiratory signs and symptoms such as cough, sputum production, hypoxia combined with new or worsened infiltrates that can be identified on the chest radiograph.[13]
The sample size was calculated using n-master software version 2.0 and based on a study by Vila et al.[14] to detect a mean difference in the gastric volume of 9 ml with a standard deviation of 13 ml between the two groups. For a 95% confidence interval, a power of 80%, and an attrition rate of 5%, the sample size required was 100 in each group. The Statistical Package for the Social Sciences (SPSS) for Windows statistical package (version 19, IBM PASW statistics) was used for statistical analysis. The data’s normality was assessed using the one-sample Kolmogorov–Smirnov test, and the remaining non-normally distributed descriptive data were expressed as median with interquartile range (IQR). The study participants’ age, weight, height, gastric antral CSA, and gastric pH were expressed as mean with standard deviation and analyzed between the two groups using the independent t-test. Categorical data like gender were expressed as number and percentage, whereas comparison between the two groups was carried out using the Chi-square test. The total gastric volume calculated from gastric antral CSA, measured gastric volume by OGT aspiration, and weight-based calculated gastric volume were expressed as median with IQR, and they were compared between the two groups using the Mann–Whitney test. A Bland–Altman plot was used to determine the level of agreement between the measured gastric volume and USG based on calculated gastric volume. To reject the null hypothesis, a P-value ≤0.05 was considered significant.
Results
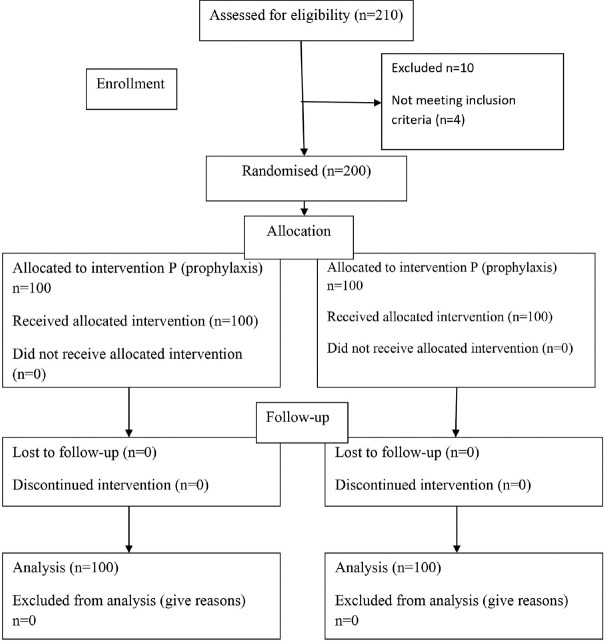
A total of 200 patients were studied. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram detailing their allocation and analysis is shown in Figure 1. The demographic characteristics were comparable between both the groups [Table 1]. The calculated gastric volume did not show statistically significant difference between both the groups (P = 0.634). The measured gastric pH was statistically more acidic in the NP group. Recent evidence suggests that the minimal gastric volume required to produce aspiration-induced pulmonary changes is 1.5 ml/kg.[5,15] In our study, none of the patients in either group had a gastric volume of more than 1.5 ml/kg [Table 2]. Since all patients were found to be at no risk of aspiration pneumonia based on the criteria mentioned above, we compared the risk of aspiration based on Roberts and Shirley criteria [Table 3]. Animal experiments proposed that the minimal gastric volume required to produce pulmonary changes was 0.4 ml/kg.[16] Based on their criteria, the patients at risk of aspiration between the two groups were comparable and had no statistically significant difference, with a Chi-square value of 0.744 (P = 0.39). The gastric antral status between the study groups is depicted in Figure 2. USG-guided predicted gastric volume and the gastric volume measured through OGT were analyzed by the Bland–Altman plot to assess the level of agreement between the two measurements. The USG and OGT aspiration yielded similar volume assessments within a difference of 25 ml in 92% of cases and within 40 ml in 96% of cases. It showed a significant level of agreement between the two techniques with a mean difference of 8.3 ml [Figure 3].
Figure 1.
CONSORT flow diagram showing patient progress through the study phases. CONSORT = Consolidated Standards of Reporting Trials, NP = no prophylaxis group, P = prophylaxis group
Table 1.
Demographic characteristics of patients
| Variables | Group P (n=100) | Group NP (n=100) | P |
|---|---|---|---|
| Age (years) | 41.54±14.9 | 40.35±12.49 | 0.54 |
| Height (cm) | 156.6±7.82 | 156.9±7.9 | 0.80 |
| Weight (kg) | 55.12±10.6 | 55.01±10.74 | 0.94 |
| BMI (kg/m2) | 22.3±3.3 | 22.2±3.4 | 0.77 |
| Gender (M/F) | 46/54 | 50/50 | 0.572 |
| ASA-PS (I/II) | 47/53 | 44/56 | 0.20 |
ASA-PS=American Society of Anesthesiologists physical status class, BMI=Body mass index, NP=No prophylaxis group, P=Prophylaxis group, SD=Standard deviation. Age, height, weight, and BMI are expressed as mean±SD; gender and ASA-PS class are expressed as a percentage
Table 2.
Comparison of calculated gastric volume and measured gastric volume
| Variables (unit) | Group P (n*=100) | Group NP (n=100) | P |
|---|---|---|---|
| Calculated gastric volume (USG†) (ml) | 19.67 (6.01, 33.86) | 20.11 (8.79, 33.74) | 0.634 |
| Gastric volume (ml/kg) | 0.37 (0.11, 0.65) | 0.34 (0.16, 0.64) | 0.786 |
| Measured gastric volume (OGT‡) (ml) | 10 (10, 15) | 14.5 (9, 20) | 0.056 |
| Gastric pH | 4.35±1.12 | 3.88±0.85 | 0.01* |
| Gastric antral CSA§ | 3.13±0.96 | 3.12±0.99 | 0.97 |
*p-value < 0.05 considered statistically significant. Calculated gastric volume, measured gastric volumes, and weight-based gastric volume (ml/kg) were expressed in median with interquartile range. Gastric antral CSA and gastric pH were expressed as mean±SD. *n=number of participants in the group, †USG-ultrasonography, ‡OGT-orogastric tube, §CSA-cross sectional
Table 3.
Comparison of risk of aspiration based on Roberts and Shirley criteria
| Risk possibility (based on gastric volume) | Group P (n=100) | Group NP (n=100) | Total | P |
|---|---|---|---|---|
| No (<0.4 ml/kg) | 62 | 56 | 118 (59%) | 0.39 |
| Yes (≥0.4 ml/kg) | 38 | 44 | 82 (41%) |
NP=No prophylaxis group, P=Prophylaxis group
Figure 2.
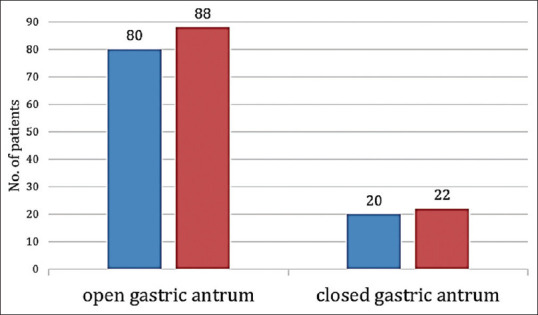
Comparison of gastric antral status between the study groups: group P,
group P,  group NP. NP = no prophylaxis, P = prophylaxis
group NP. NP = no prophylaxis, P = prophylaxis
Figure 3.
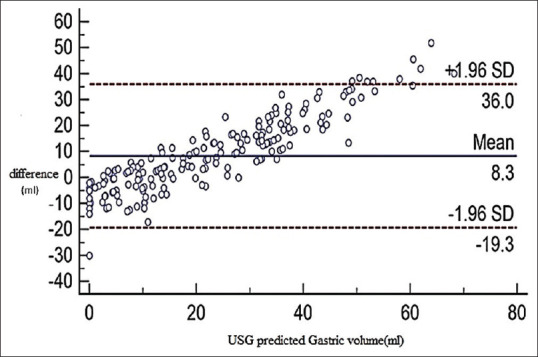
Bland–Altman analysis. USG guided predicted gastric volume (ml) with the difference between the two volumes (i.e., calculated from gastric ultrasonogram minus measured by orogastric aspiration) USG minus the volume measured by orogastric aspiration) (Solid line: observed mean agreement; dashed lines: represent the 95% limits of agreement) USG: ultrasonography
Discussion
In our study, there was no difference in gastric volume between the study groups, that is, 20.11 ml (8.79, 33.74) in the NP group compared to 19.67 ml (6.01, 33.86) in the P group. But it showed a statistically significant difference in gastric pH in the P group (4.35 ± 1.12) compared to the NP group (3.85 ± 0.85). Although the study showed significant decrease in gastric pH without famotidine, it was not found to be clinically significant. The mean gastric pH was 3.8 in the NP group, which was sufficiently high enough to overcome the gastric content induced pneumonitis as per the traditional criteria which still hold pH less than 2.5 as a risk factor.
Routine preoperative administration of antacids, H2 receptor blockers, proton pump inhibitors, and prokinetics to surgical patients with a low risk of aspiration pneumonitis is a questionable practice. Although no direct evidence is available to recommend against their routine use, various guidelines recommend not using them in non-obstetric low-risk patients, based on consensus and expert opinion.[1,2] We planned a study to add an evidence basis to their recommendation. We found that gastric volume measured using ultrasound or aspirated through OGT was not elevated but comparable between patients, irrespective of their preoperative H2 blocker and prokinetics medication intake. In healthy surgical individuals, preoperative liberal fluid intake and acid aspiration prophylaxis influence the residual gastric volume and pH.[17,18,19,20] But the results of the previous studies are limited by the methods to measure the residual gastric volume. We attempted to directly measure the gastric volume through an OGT to prove this point. Despite the measured gastric volume being comparable between the study groups and showing an excellent correlation between the calculated gastric volumes, it underestimates the risk of aspiration. Many studies have shown that gastric contents aspirated through nasal or OGTs underestimate the gastric volume and can be assessed accurately with the help of USG before the induction of anesthesia.[8,10,20,21,22] Also, it was found that there was no difference in the fluid intake 2 h before surgery and it did not affect the gastric volume measured by ultrasound.
A study by Sharma et al.[23] showed that there were cases reported with gastric volume >1.5 ml/kg even after they fasted adequately for hours before surgery. This was even seen in patients with no comorbidities like diabetes or chronic kidney disease. But the intake of aspiration prophylaxis was not mentioned in the study. In our study, 100% of participants with optimal fasted state showed gastric volume less than 1.5 ml/kg. So, we analyzed the minimal gastric volume required to produce pulmonary changes as 0.4 ml/kg based on the Roberts and Shirley criteria.[16] Though 38% of patients in group P and 44% of patients in group NP had gastric volume ≥0.4 ml/kg, there was no clinical evidence of aspiration pneumonitis on postoperative follow-up and the difference in the risk of aspiration was not found be statistically significant (P = 0.390). It is interesting to note that despite the dual pharmacological intervention, group P was still at risk of aspiration as per the Roberts and Shirley criteria.
The pulmonary changes secondary to avoid aspiration depend on both residual gastric volume and gastric pH (pH <2.5).[5,16] In our study, the mean difference in the gastric pH was found to be significant between the groups (3.88 ± 0.85 vs. 4.35 ± 1.12), yet it remained high enough to avoid aspiration-induced pneumonitis. Prolonged fasting results in endogenous gastric hydrochloric acid secretion, which is the principal determinant for gastric volume and pH. However, the gastric volume is contributed by salivary secretion (1–2 ml/kg/h) and other gastric secretions (0.4–0.8 ml/kg/h).[16,17] It may be the other reason for the significant change observed in the pH of gastric content in a patient on overnight fasting, even if the patient had not been treated with H2 blockers. Though metoclopramide is a dopamine antagonist which carries extrapyramidal side effects,[24] omitting it in a healthy surgical population will also avoid this risk.
There were no cases reported with the incidence of aspiration pneumonitis in our study. Patients presenting with any signs and symptoms of aspiration pneumonitis are at risk of aspiration-induced lung injury. Gastric ultrasound may be useful in many clinical situations such as lack of patient adherence to fasting instructions (e.g., due to emergency/urgent procedure or miscommunication), unreliable fasting history (e.g., altered sensorium, language barrier, or cognitive dysfunction), and potential delay in gastric emptying (e.g., pregnancy, diabetes mellitus, severe liver or kidney dysfunction, or neuromuscular disorders) in which the aspiration risk is unclear or undetermined.[25] But the role of gastric ultrasound-based detection of gastric volume following aspiration prophylaxis has not been studied till now, which makes our study unique.
There are a few limitations to our study. Firstly, the formula to calculate the residual gastric volume proposed by Bolondi and Perlas et al. validated up to 500 ml of gastric volume in a non–high-risk population with a body mass index of <40 kg/m2.[3,8,26,27] Hence, the same formula was not validated and cannot be used to assess gastric volume in the pregnant and pediatric population. Further studies will be required to validate the same formula in special populations. Second limitation of the study is that the position of the OGT may affect gastric pH. The pH was measured from the aspirated sample, assuming that the OGT position would be in the stomach. If the tube crosses the pyloric antrum, an improperly positioned OGT may lead to false-high gastric pH. Previous studies have shown that blind nasogastric aspiration overestimates the gastric pH.[28] Third, in our study, we did not find any cases with aspiration pneumonitis, as we studied the usual surgical population with adequate preoperative fasting status, who were at less risk of aspiration. Hence, studies with a larger sample size may be required in future to evaluate the aspiration risk in these individuals.
Conclusion
We conclude that in a usual surgical population with adequate fasting as per the ASA fasting guidelines, the amount of gastric volume and the pH were similar and below the threshold to cause aspiration pneumonitis, irrespective of their acid aspiration prophylaxis status.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Smith I, Kranke P, Murat I, Smith A, O’Sullivan G, Søreide E, et al. Perioperative fasting in adults and children: Guidelines from the European Society of anaesthesiology. Eur J Anaesthesiol. 2011;28:556–69. doi: 10.1097/EJA.0b013e3283495ba1. [DOI] [PubMed] [Google Scholar]
- 2.Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An Updated Report by the American Society of Anesthesiologists Task Force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126:376–93. doi: 10.1097/ALN.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 3.Bouvet L, Mazoit XJ, Chassard D, Allaouchiche B, Boselli E, Benhamou D. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;114:1086–92. doi: 10.1097/ALN.0b013e31820dee48. [DOI] [PubMed] [Google Scholar]
- 4.Perlas A, Davis L, Khan M, Mitsakakis N, Chan VWS. Gastric sonography in the fasted surgical patient: A prospective descriptive study. Anesth Analg. 2011;113:93–7. doi: 10.1213/ANE.0b013e31821b98c0. [DOI] [PubMed] [Google Scholar]
- 5.Van de Putte P, Perlas A. Ultrasound assessment of gastric content and volume. Br J Anaesth. 2014;113:12–22. doi: 10.1093/bja/aeu151. [DOI] [PubMed] [Google Scholar]
- 6.Maltby JR, Pytka S, Watson NC, Cowan RA, Fick GH. Drinking 300 mL of clear fluid two hours before surgery has no effect on gastric fluid volume and pH in fasting and non-fasting obese patients. Can J Anaesth. 2004;51:111–5. doi: 10.1007/BF03018767. [DOI] [PubMed] [Google Scholar]
- 7.Bolondi L, Bortolotti M, Santi V, Calletti T, Gaiani S, Labo G. Measurement of gastric emptying time by real-time ultrasonography. Gastroenterology. 1985;89(4):752–9. doi: 10.1016/0016-5085(85)90569-4. [DOI] [PubMed] [Google Scholar]
- 8.van de Putte P, van Hoonacker J, Perlas A. Gastric ultrasound to guide anesthetic management in elective surgical patients non-compliant with fasting instructions: A retrospective cohort study. Minerva Anestesiol. 2018;84:787–95. doi: 10.23736/S0375-9393.17.12305-9. [DOI] [PubMed] [Google Scholar]
- 9.Kruisselbrink R, Arzola C, Jackson T, Okrainec A, Chan V, Perlas A. Ultrasound assessment of gastric volume in severely obese individuals: A validation study. Br J Anaesth. 2017;118:77–82. doi: 10.1093/bja/aew400. [DOI] [PubMed] [Google Scholar]
- 10.Perlas A, Mitsakakis N, Liu L, Cino M, Haldipur N, Davis L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357–63. doi: 10.1213/ANE.0b013e318274fc19. [DOI] [PubMed] [Google Scholar]
- 11.Arzola C, Perlas A, Siddiqui NT, Carvalho JCA. Bedside gastric ultrasonography in term pregnant women before elective cesarean delivery: A prospective cohort study. Anesth Analg. 2015;121:752–8. doi: 10.1213/ANE.0000000000000818. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz A, Schmidt AR, Buehler PK, Schraner T, Frühauf M, Weiss M, et al. Gastric ultrasound as a preoperative bedside test for residual gastric contents volume in children. Pediatr Anesth. 2016;26:1157–64. doi: 10.1111/pan.12993. [DOI] [PubMed] [Google Scholar]
- 13.Son YG, Shin J, Ryu HG. Pneumonitis and pneumonia after aspiration. J Dent Anesth Pain Med. 2017;17:1–12. doi: 10.17245/jdapm.2017.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vila P, Espachs P, Echevarria V, Garcia M, Rincón R, Vidal F. Acid aspiration prophylaxis in elective biliary surgery. A comparison of omeprazole and famotidine using manually aided gastric aspiration. Anaesthesia. 1994;49:909–11. doi: 10.1111/j.1365-2044.1994.tb04274.x. [DOI] [PubMed] [Google Scholar]
- 15.Perlas A, Chan VWS, Lupu CM, Mitsakakis N, Hanbidge A. Ultrasound assessment of gastric content and volume: Anesthesiology. 2009;111:82–9. doi: 10.1097/ALN.0b013e3181a97250. [DOI] [PubMed] [Google Scholar]
- 16.Roberts RB, Shirley MA. Reducing the risk of acid aspiration during cesarean section. Anesth Analg. 1974;53:859–68. doi: 10.1213/00000539-197453060-00010. [DOI] [PubMed] [Google Scholar]
- 17.Phillips S, Hutchinson S, Davidson T. Preoperative drinking does not affect gastric contents. Br J Anaesth. 1993;70:6–9. doi: 10.1093/bja/70.1.6. [DOI] [PubMed] [Google Scholar]
- 18.Siu L, Yee KH, Agarwal S, Monagle J, Canty DJ. The Impact of preoperative oral ingestion of water on intraoperative gastric fluid volume for elective gastroscopy: A randomized controlled trial. J Anesth Clin Res. 2014;05:423–5. [Google Scholar]
- 19.Van de Putte P, Perlas A. The link between gastric volume and aspiration risk. In search of the holy grail? Anaesthesia. 2018;73:274–9. doi: 10.1111/anae.14164. [DOI] [PubMed] [Google Scholar]
- 20.Van de Putte P. Bedside gastric ultrasonography to guide anesthetic management in a nonfasted emergency patient. J Clin Anesth. 2013;25:165–6. doi: 10.1016/j.jclinane.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Kruisselbrink R, Gharapetian A, Chaparro LE, Ami N, Richler D, Chan VWS, et al. Diagnostic accuracy of point-of-care gastric ultrasound. Anesth Analg. 2019;128:89–95. doi: 10.1213/ANE.0000000000003372. [DOI] [PubMed] [Google Scholar]
- 22.Koenig SJ, Lakticova V, Mayo PH. Utility of ultrasonography for detection of gastric fluid during urgent endotracheal intubation. Intensive Care Med. 2011;37:627–31. doi: 10.1007/s00134-010-2125-9. [DOI] [PubMed] [Google Scholar]
- 23.Sharma G, Jacob R, Mahankali S, Ravindra MN. Preoperative assessment of gastric contents and volume using bedside ultrasound in adult patients: A prospective, observational, correlation study. Indian J Anaesth. 2018;62:753–8. doi: 10.4103/ija.IJA_147_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandit SK, Kothary SP, Pandit UA, Mirakhur RK. Premedication with cimetidine and metoclopramide. Effect on the risk factors of acid aspiration. Anaesthesia. 1986;41:486–92. doi: 10.1111/j.1365-2044.1986.tb13272.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaydu A, Gokcek E. Preoperative assessment of ultrasonographic measurement of antral area for gastric content. Med Sci Monit. 2018;24:5542–8. doi: 10.12659/MSM.908520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouvet L, Chassard D. Contribution of ultrasonography for the preoperative assessment of gastric contents. Ann Fr Anesth Reanim. 2014;33:240–7. doi: 10.1016/j.annfar.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Song I-K, Kim H-J, Lee J-H, Kim E-H, Kim J-T, Kim H-S. Ultrasound assessment of gastric volume in children after drinking carbohydrate-containing fluids. Br J Anaesth. 2016;116:513–7. doi: 10.1093/bja/aew031. [DOI] [PubMed] [Google Scholar]
- 28.Gouda BB, Lydon AM, Badhe A, Shorten GD. A comparison of the effects of ranitidine and omeprazole on volume and pH of gastric contents in elective surgical patients. Eur J Anaesthesiol. 2004;21:260–4. doi: 10.1017/s0265021504004028. [DOI] [PubMed] [Google Scholar]