Abstract
OBJECTIVES:
To describe the utilization of early ketamine use among patients mechanically ventilated for COVID-19, and examine associations with in-hospital mortality and other clinical outcomes.
DESIGN:
Retrospective cohort study.
SETTING:
Six hundred ten hospitals contributing data to the Premier Healthcare Database between April 2020 and June 2021.
PATIENTS:
Adults with COVID-19 and greater than or equal to 2 consecutive days of mechanical ventilation within 5 days of hospitalization.
INTERVENTION:
The exposures were early ketamine use initiated within 2 days of intubation and continued for greater than 1 day.
MEASUREMENTS:
Primary was hospital mortality. Secondary outcomes included length of stay (LOS) in the hospital and ICUs, ventilator days, vasopressor days, renal replacement therapy (RRT), and total hospital cost. The propensity score matching analysis was used to adjust for confounders.
MAIN RESULTS:
Among 42,954 patients, 1,423 (3.3%) were exposed to early ketamine use. After propensity score matching including 1,390 patients in each group, recipients of ketamine infusions were associated with higher hospital mortality (52.5% vs. 45.9%, risk ratio: 1.14, [1.06–1.23]), longer median ICU stay (13 vs. 12 d, mean ratio [MR]: 1.15 [1.08–1.23]), and longer ventilator days (12 vs. 11 d, MR: 1.19 [1.12–1.27]). There were no associations for hospital LOS (17 [10–27] vs. 17 [9–28], MR: 1.05 [0.99–1.12]), vasopressor days (4 vs. 4, MR: 1.04 [0.95–1.14]), and RRT (22.9% vs. 21.7%, RR: 1.05 [0.92–1.21]). Total hospital cost was higher (median $72,481 vs. $65,584, MR: 1.11 [1.05–1.19]).
CONCLUSIONS:
In a diverse sample of U.S. hospitals, about one in 30 patients mechanically ventilated with COVID-19 received ketamine infusions. Early ketamine may have an association with higher hospital mortality, increased total cost, ICU stay, and ventilator days, but no associations for hospital LOS, vasopressor days, and RRT. However, confounding by the severity of illness might occur due to higher extracorporeal membrane oxygenation and RRT use in the ketamine group. Further randomized trials are needed to better understand the role of ketamine infusions in the management of critically ill patients.
Keywords: COVID-19, critical care, critical care outcomes, ketamine, mechanical, ventilators
KEY POINTS.
Question: What was the utilization pattern of early ketamine for sedation among mechanically ventilated patients with COVID-19, and what was the association of early ketamine exposure with in-hospital mortality?
Findings: In this retrospective cohort study of 42,954 patients, 1,423 (3.3%) received early ketamine. After propensity score matching, early ketamine use was associated with higher in-hospital mortality, compared to those who did not receive early ketamine.
Meaning: Early ketamine use among mechanically ventilated patients with COVID-19 was used in a small proportion of patients. Early ketamine exposure was associated with higher in-hospital mortality, but due to the concern for residual confounding, future randomized controlled trials are likely needed.
Despite the rapidly evolving evidence base for the treatment of mechanically ventilated patients with COVID-19, the in-hospital mortality associated with this high-acuity disease remains at approximately 40% (1). As our understanding of COVID-19 acute respiratory distress syndrome improved, several commonly used critical care therapies have been shown to improve survival, including dexamethasone (2), prone positioning (3), and low tidal volume ventilation (4). Other therapies that have been shown to impact symptoms in the early stages of COVID-19 (such as antiviral therapy) have shown conflicting or limited evidence for efficacy (5). As the pandemic continues to significantly impact healthcare, it is important to review therapies and assess possible impact on outcomes.
Ketamine is an N -methyl- D -aspartate receptor antagonist and is commonly used as an adjunct for pain management or anesthetic agents. It is less frequently used as an adjunct for sedation in critically ill patients requiring mechanical ventilation, and therefore its impact on outcomes in critically ill patients remains unknown. Despite a lack of data from prospective, randomized controlled trials of ketamine sedation in this population, the use of ketamine for analgosedation during mechanical ventilation continues to increase (6). The trend may be a reflection of recent expert opinion and guidelines broadly supporting the use of ketamine as one of the nonopioid analgesics in the ICU setting (7, 8).
There are several plausible mechanisms by which ketamine may influence outcomes during mechanical ventilation. First, it is a known bronchodilator through presumed anticholinergic-mediated relaxation of bronchial smooth muscles. Ketamine sedation has been shown to improve dynamic compliance and Pao2/Fio2 ratio while reducing peak inspiratory pressures during mechanical ventilation (9). In vitro, ketamine has been found to have immunoinhibitory effects and reduced proinflammatory production (10), a sigma-1 receptor agonist that could plausibly reduce COVID-19 viral replication (11), and potent anti-inflammatory effects. In vivo, ketamine has been shown to produce a dose-dependent survival benefit in animal models of sepsis, with corresponding reductions in tumor necrosis factor-alpha and interleukin-6 levels (12, 13). Similar reductions in inflammatory mediators following ketamine administration have been shown in select surgical populations (14–16), but data on such anti-inflammatory effects of ketamine in critically ill patients remain lacking. The use of ketamine infusions during mechanical ventilation has been associated with reduced opioid exposure (17), which may have meaningful implications for patient recovery following critical illness with COVID-19.
To better define the impact of ketamine administered during mechanical ventilation for COVID-19, our objectives were to: 1) describe ketamine infusion utilization patterns and 2) explore the association of early ketamine infusion exposure with hospital mortality among mechanically ventilated patients with COVID-19.
MATERIALS AND METHODS
Study Design and Data Source
We conducted a retrospective cohort study utilizing data from 610 hospitals included in the Premier Healthcare Database for patients admitted between April 2020 and June 2021. This inpatient database contains patient demographics, admission type (elective, emergent, or unknown), hospital characteristics, patient-specific billing information, as well as diagnosis and procedure codes (including mechanical ventilation), and date-stamped inpatient drug administration. This study was approved by the Duke University Healthcare System Institutional Review Board (no. Pro00107287, approval date: June 23, 2021, study title: Outcomes of critically ill COVID-19 patients) and was determined to be exempt from informed patient consent in accordance with the Health Insurance Portability and Accountability Act, as patient data were fully de-identified before analysis.
Study Population
All adult (> 18 yr) patients who were admitted between April 2020 and June 2021 with either a primary, secondary, or contributing diagnosis of COVID-19 (identified using International Classification of Diseases 10th Edition [ICD-10] code U07.1) were evaluated, who also received early mechanical ventilation (within 5 d of admission). The need for early mechanical ventilation was used as inclusion criteria to restrict the cohort to patients with high disease severity at hospital presentation. Exclusion criteria included primary admissions for pregnancy.
Exposure, Outcomes, and Covariates
The exposure of interest was the receipt of ketamine for early sedation following mechanical ventilation. This was defined as the presence of a hospital charge code for greater than 2 consecutive days and within 2 days of intubation. This definition was selected to distinguish those who received ketamine as a single dose in the setting of intubation (presence of a charge code on a single day) from those who received ketamine for sedation purposes during mechanical ventilation (presence of a charge code on multiple consecutive days). Additionally, early ketamine sedation (within 2 d of intubation) was selected to distinguish patients receiving ketamine as part of the primary sedation regimen, as opposed to patients receiving ketamine as a “rescue” agent (i.e., severe pain/agitation despite the primary sedation regimen). The primary outcome was hospital mortality (defined as death during hospitalization or discharge to hospice care). Secondary outcomes included: hospital length of stay (LOS) based on calendar day, ICU LOS, ventilator days, vasopressors days, dialysis utilization, extracorporeal membrane oxygenation (ECMO) utilization, and total cost. The total cost included both fixed and variable costs. Fixed costs, such as depreciation, management, and maintenance, are expenses that do not vary with departmental activity. Conversely, variable costs, including supplies and direct patient care, fluctuate with the activity of the department. For our comparative effectiveness aim, only hospitals that used ketamine were included in the study population. Covariates included: patient demographic and clinical characteristics, hospital characteristics, comorbidities, and co-treatments as described in Supplemental Table 1 (http://links.lww.com/CCX/B359).
Statistical Analysis
A power calculation based on Fisher exact test with a baseline mortality rate of 50% finds that a sample size of 1390 per group (2780 total) provides at least 80% power to detect an absolute difference in mortality of 5.4%. With the assumed baseline mortality rate, this corresponds to a minimum detectable risk ratio (RR) of 1.11.
Demographic and clinical characteristics of the cohort are reported, stratified by early ketamine exposure. Descriptive statistics were used to examine the study population with categorical variables reported as counts and frequencies, and continuous data reported as median with interquartile range (IQR). Because of the skewness of hospital costs, we reported a median with an IQR, and compared between groups using a Wilcoxon rank-sum test. Propensity score matching (using a logistic regression model to define propensity scores for early ketamine exposure and 1:1 matching) was used to compare outcomes among patients exposed to early ketamine vs. unexposed patients. Covariates for the logistic regression models were selected a priori based on known maternal morbidity risk factors, prior literature, and subject-matter expertise of the authors and included in Supplemental Table 1 (http://links.lww.com/CCX/B359). Treatments that occurred during the hospitalization (after the exposure period) were reported descriptively but were not included as covariates in the statistical models, as these variables were considered mediators (occurring on the causal pathway from exposure to outcome) rather than confounders. To examine the robustness of our results, we conducted a sensitivity analysis among patients receiving early neuromuscular blockade (within 2 d of starting invasive mechanical ventilation), to further restrict the cohort to patients with severe respiratory failure. Statistical analyses were performed in the SAS System, Version 9.4 (SAS Institute, Cary, NC). A two-sided alpha level of 0.05 was prespecified as statistically significant.
RESULTS
Demographic and Clinical Characteristics
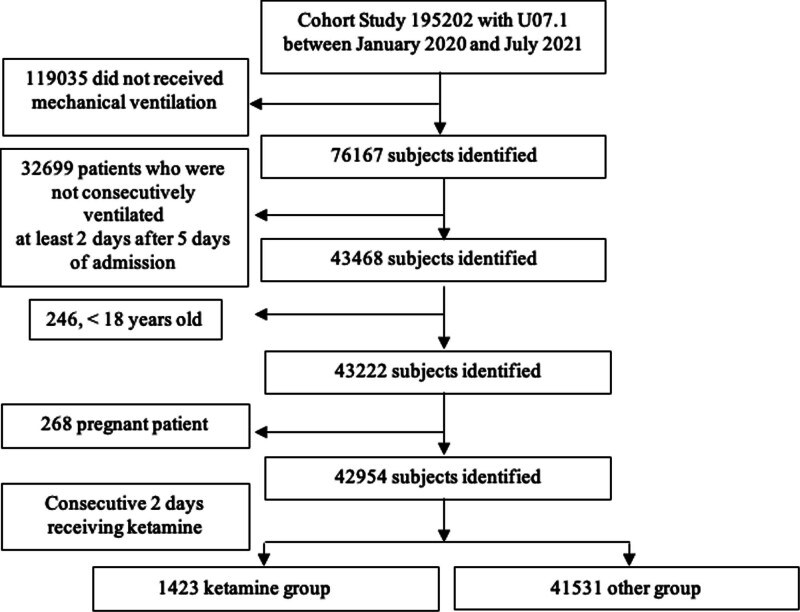
Within the Premier Healthcare Database, 42,954 patients were identified as meeting general study criteria as shown in Figure 1. Of these patients, 1,423 (3.3%) were exposed to early ketamine infusion as a part of the primary sedation regimen. Demographic and clinical characteristics of the study population are displayed in Supplemental Table 1 (http://links.lww.com/CCX/B359). Before propensity score matching, the ketamine-exposed patients were slightly younger than unexposed patients (59 yr vs. 65 yr, respectively), and more likely to be male (64.5% vs. 60%, respectively). Patients receiving ketamine were less likely to be Black and White race categories, but more likely to be “other” and “unknown” race categories, compared with those who did not receive ketamine. Patient pre-admission comorbidities were largely balanced between groups. The group receiving ketamine contained more patients identified as obese and slightly lower van Walraven Score. Patients unexposed to ketamine had a slightly greater proportion of congestive heart failure (24.0% vs. 16.8%), other neurologic disorders (12.8% vs. 8.2%), renal failure (19.8% vs. 13.0%) and hypertension (73.5% vs. 66.5%). There was similar usage of dexamethasone, monoclonal antibodies, and concomitant vasopressors between the two groups.
Figure 1.
Flow diagram of the study.
Ketamine Utilization
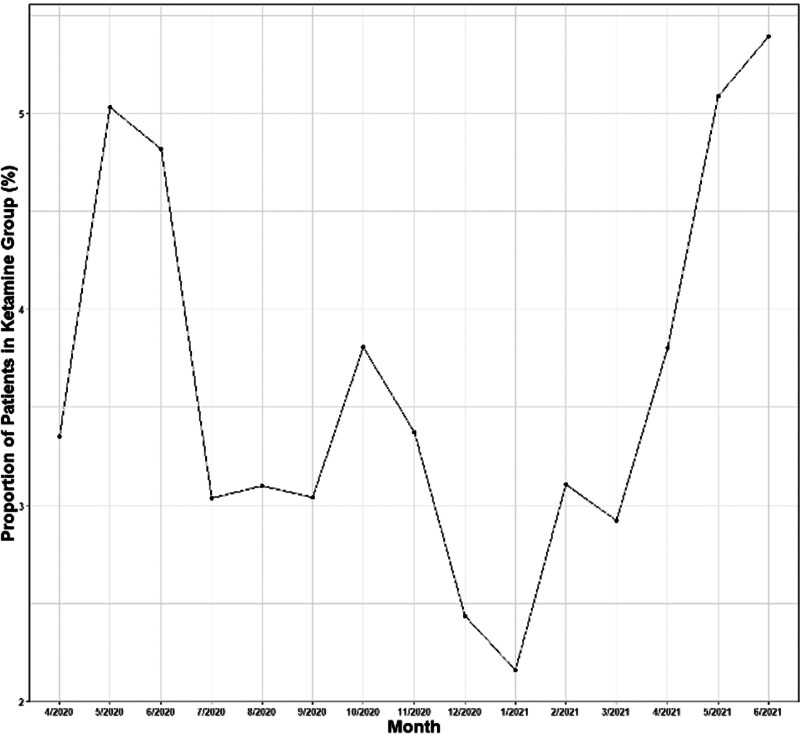
Higher utilization of ketamine was noted in large urban (93.4%) and academic medical centers (65.6%). Ketamine use was also more predominant in the southern region (55.4%). The proportion of patients by month across all hospitals receiving ketamine is shown in Figure 2. There was an early peak of ketamine usage in May 2020 and subsequent peaks of ketamine utilization were observed in October 2020 and June 2021.
Figure 2.
Proportion of patients by month across all hospitals receiving ketamine.
Association of Early Ketamine Exposure with Clinical Outcomes
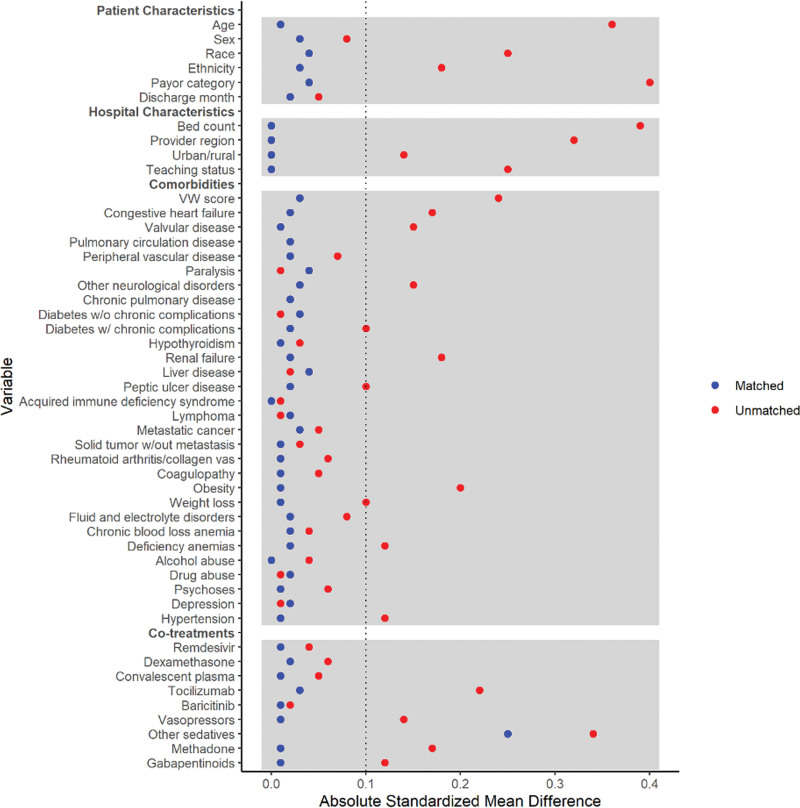
Covariate balance before and after propensity score matching is presented in Table 1, and a covariate plot is displayed in Figure 3. Following propensity score matching, in-hospital mortality was significantly higher in patients who received ketamine than those who did not receive ketamine (50.4% vs. 43.9%, RR 1.15 [1.06–1.24], p = 0.001), and it was also higher for total mortality (death during hospitalization or discharge to hospice care) (52.5% vs. 45.9%, RR 1.14 [1.06–1.23], p = 0.001). The survival curves of the two groups are shown in Supplemental Figure 1 (http://links.lww.com/CCX/B359). Notably, despite similar use of dexamethasone, monoclonal antibodies, and concomitant vasopressors between the two groups, a mortality difference persisted. Total costs incurred by the group receiving ketamine were significantly higher than those who did not receive ketamine ($72,481 vs. $65,584, mean ratio [MR] 1.11 [1.05–1.19], p = 0.001). ICU LOS was longer for patients receiving ketamine than those who did not receive ketamine (13 vs. 12 d, MR 1.15 [1.08–1.23], p < 0.001). Patients receiving ketamine also had more ventilator days (12 vs. 11 d, MR 1.19 [1.12–1.27], p < 0.001) and were more likely to receive ECMO (6.5% vs. 4.1%, RR 1.6 [1.16–2.20], p = 0.004). However, total LOS was the same in both groups (17 d, MR 1.05 [0.99–1.12], p = 0.123). The distribution of LOS among the survivors and nonsurvivors is shown in Supplemental Figure 2 (http://links.lww.com/CCX/B359).
TABLE 1.
Clinical Outcome Before and After Propensity Score Matched Analysis
| Outcomes | Patients, N% | Risk Ratio/Mean Ratio | 95% CI |
p |
|||
|---|---|---|---|---|---|---|---|
| Before Matching | After Matching | ||||||
| Ketamine Group (n = 1,423) | Other Group (n = 41,531) | Ketamine Group (n = 1,390) | Matched Group (n = 1,390) | ||||
| In-hospital mortality | 714 (50.2%) | 21,005 (50.6%) | 700 (50.4%) | 610 (43.9%) | 1.15 | 1.06–1.24 | 0.001 |
| Discharge to hospice | 29 (2.0%) | 1,202 (2.9%) | 29 (2.1%) | 28 (2.0%) | 1.04 | 0.62–1.73 | 0.894 |
| Hospital mortality | 743 (52.2%) | 22,207 (53.5%) | 729 (52.5%) | 638 (45.9%) | 1.14 | 1.06–1.23 | 0.001 |
| Hospital length of stay (median, IQR) | 17 (10, 27) | 14 (8, 24) | 17 (10, 27) | 17 (9, 28) | 1.05 | 0.99–1.12 | 0.123 |
| Total cost (median, IQR) ($) | 72,494 (44,481, 121,432) | 52,317 (29,828, 90,754) | 72,481 (44,253, 121,411) | 65,584 (37,687, 117,901) | 1.11 | 1.05–1.19 | 0.001 |
| ICU length of stay (median, IQR) | 13 (8, 21) | 10 (5, 17) | 13 (8, 21) | 12 (6, 21) | 1.15 | 1.08–1.23 | < 0.001 |
| Ventilator days (mean, IQR) | 12 (7, 20) | 9 (5, 16) | 12 (7, 20) | 11 (5, 19) | 1.19 | 1.12–1.27 | < 0.001 |
| Vasopressor days (among those with vasopressors) (median, IQR) | 4 (2, 9) | 4 (2, 7) | 4 (2, 9) | 4 (2, 9) | 1.04 | 0.95–1.14 | 0.43 |
| Renal replacement therapy | 323 (22.7%) | 9,331 (22.5%) | 318 (22.9%) | 302 (21.7%) | 1.05 | 0.92–1.21 | 0.466 |
| Extracorporeal membrane oxygenation | 94 (6.6%) | 477 (1.1%) | 91 (6.5%) | 57 (4.1%) | 1.6 | 1.16–2.20 | 0.004 |
IQR = interquartile range.
Figure 3.
Standardized mean differences for covariates plot. vW score = van Walraven score.
Sensitivity Analysis
A subsequent sensitivity analysis of patients who received paralytics revealed a higher in-hospital mortality among those who received ketamine compared with those who did not receive ketamine during mechanical ventilation (49.5% vs. 45.1%, RR 1.10 [1.00–1.20], p = 0.042). Additionally, the ketamine-exposed group showed significantly longer ICU LOS (14 vs. 12 d, MR 1.09 [1.01–1.17], p = 0.029) and more ventilator days (13 vs. 11 d, MR 1.13 [1.06–1.21], p = 0.001). Despite propensity score matching, more patients who received ketamine and concomitant paralysis also received ECMO than in the group who received paralysis but no ketamine (7.8% vs. 4.7%, RR 1.66 [1.18–2.33], p = 0.003) subgroup analysis is shown in Table 2.
TABLE 2.
The Outcomes of the Group Who Had Paralytics Within 2 Days of Starting Mechanical Ventilation After Propensity Score Matched Analysis
| Outcomes | Ketamine Group (n = 1,067) | Matched Group (n = 1,067) | Risk Ratio/Mean Ratio | 95% CI | p |
|---|---|---|---|---|---|
| Patients, N% | |||||
| In-hospital mortality | 528 (49.5%) | 481 (45.1%) | 1.1 | 1.00–1.20 | 0.042 |
| Discharge to hospice | 19 (1.8%) | 23 (2.2%) | 0.83 | 0.45–1.51 | 0.533 |
| Mortality or discharge to hospice | 547 (52.1%) | 504 (48.0%) | 1.09 | 1.00–1.18 | 0.063 |
| Hospital length of stay (median, IQR) | 17 (10, 27) | 18 (11, 28) | 1 | 0.94–1.07 | 0.961 |
| ICU length of stay (median, IQR) | 14 (8, 22) | 12 (7, 21) | 1.09 | 1.01–1.17 | 0.029 |
| Ventilator days (median, IQR) | 13 (7, 21) | 11 (6, 20) | 1.13 | 1.06–1.21 | 0.001 |
| Vasopressor days (among those with vasopressors) (median, IQR) | 4 (2, 9) | 5 (2, 9) | 1.01 | 0.91–1.12 | 0.816 |
| Renal replacement therapy | 252 (23.6%) | 253 (23.7%) | 1 | 0.86–1.16 | 0.959 |
| Extracorporeal membrane oxygenation | 83 (7.8%) | 50 (4.7%) | 1.66 | 1.18–2.33 | 0.003 |
| Patient cost (median, IQR) ($) | 77,712 (47,753, 127,343) | 71,618 (46,069, 125,495) | 1.05 | 0.98–1.12 | 0.178 |
IQR = interquartile range.
DISCUSSION
This study aimed to study ketamine utilization and the impact of ketamine exposure on mortality among patients with COVID-19 who received mechanical ventilation. We showed that ketamine utilization varied nationally. The pattern of ketamine utilization also changed over time due to the increased demand for sedative drugs during the pandemic (18, 19). Despite similar baseline demographics and comorbidities, patients who were exposed to ketamine had a higher hospital mortality than those who were unexposed to ketamine during mechanical ventilation. Our findings are novel, as this is the first large cohort study of outcomes associated with ketamine use during the COVID-19 pandemic.
The biological effects of ketamine make it a potentially useful adjunct for the sedation of critically ill patients. Ketamine is known for providing excellent analgesia without respiratory depression, which may be of significant benefit to patients receiving mechanical ventilation during critical illness. Ketamine also has a more favorable hemodynamic profile when compared with other more commonly used sedative medications (notably propofol and dexmedetomidine), which may be beneficial in shock states. It has been shown to reduce opioid consumption in mechanically ventilated patients (17), which may help in reducing iatrogenic opioid dependence. Similarly, it is a known, powerful antidepressant, which may have a role to play in mitigating the negative mental health effects of postintensive care syndrome.
Despite these potential benefits, relatively few ICU patients are given ketamine infusions during mechanical ventilation. The potential detrimental effects of ketamine warrant discussion. Ketamine has a sympathomimetic effect, which may cause a significant increase in cardiac output (20, 21), in patients with adequate catecholamine reserves. However, when tested in critically ill patients, ketamine elicited a myocardial depressant effect in some patients, perhaps in those whose catecholamine reserves had already been depleted (22) Also, the increased sympathetic tone from ketamine may precipitate or worsen tachyarrhythmias during critical illness (23, 24). Ketamine is also associated with hallucinations, which may potentiate ICU delirium. Additionally, prolonged high-dose ketamine use (1.4 mg/kg/hr for 9 d) in critically ill patients with COVID-19 showed an association with cholestatic liver injury (25). Also, one of the frequently observed side effects of ketamine is the heightened production of bronchial and oral secretions (26, 27). For COVID-19 patients, this occurrence may contribute to an elevated demand for recurrent oral and bronchial suctioning (28). Additionally, the accumulation of secretions in the airway could initiate a cycle leading to ventilation-perfusion mismatch and impaired gas exchange (29). Furthermore, retained secretions may serve as a medium for bacterial growth, thereby amplifying the risk of developing pneumonia and perpetuating this detrimental cycle (30, 31). However, due to the limited evidence, drawing a definitive conclusion may be challenging.
Our study has several limitations. First, the Premier database is structured for administrative and billing purposes, it lacks additional clinical information. For example, the Premier database limits the details of the indications for ketamine, the dosage, and the duration of ketamine use, socioeconomic status, and the causes of mortality. It is also important to note that many factors associated with ICU care are unable to be captured, including patient vital signs, laboratory data, adherence to recommended ventilation strategies, location of ICU care (i.e., in a temporary ICU location), doses of medications, ventilator-free days, and the severity scores such as Simplified Acute Physiology Score or Acute Physiology and Chronic Health Evaluation scores. Coding errors in the ICD-10 codes used may have resulted in misrepresentations of preexisting comorbidities. Another major limitation is the potential underreporting of COVID-19 cases inherent to ICD-10 coding, such as missed diagnoses or coding errors. Unfortunately, the Premier data we have access to lacks laboratory or culture data. The lack of laboratory confirmation hinders our ability to further evaluate the results and identify potential missed COVID-19 cases. Second, despite the inclusion of many confounders in our regression models, we were not able to control for unknown confounders. Despite the similar hospital LOS, the significantly higher total costs incurred by the group receiving ketamine may indicate a higher acuity of care and more complex illness than can be captured by the other measured outcomes, such as increased resource utilization like ECMO and renal replacement therapy, in the ketamine group. It is important to note that the hospital costs were reported in this database, which is skewed, hence our choice to report a median with an IQR. Furthermore, the geographic variation in ketamine use, with a higher rate observed in urban and southern regions, introduces a potential bias. Therefore, a cautious interpretation of these findings is necessary. The nature of this study design, as a retrospective analysis, makes causation challenging to confirm. Although we attempted to control for incidental COVID-19 infection by requiring mechanical ventilation within 48 hours of COVID-19 diagnosis, we may not have eliminated all the patients in that category. Despite these limitations, our methodology comprised restriction, as well as propensity matching on demographics, comorbidities, severity of illness, and month of admission (to help reduce confounding associated with surge status), to mitigate the influences of unmeasured confounding.
CONCLUSIONS
This observational study found variation in the utilization of ketamine due to demographic, clinical, facility, and time characteristics. Early ketamine sedation during mechanical ventilation for COVID-19 may be associated with higher hospital mortality, total costs, ICU LOS, ventilator days, and ECMO utilization, although the acuity difference between the groups could not fully be accounted for in the analysis. Further randomized trials are needed to better understand the role of ketamine sedation in the management of critically ill patients.
Supplementary Material
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Contributor Information
Galen Royce-Nagel, Email: galen.roycenagel@duke.edu.
Mary Jarzebowski, Email: mjarzebo@med.umich.edu.
Pattrapun Wongsripuemtet, Email: pattrapun.wongsripuemtet@duke.edu.
Vijay Krishnamoorthy, Email: vijay.krishnamoorthy@duke.edu.
Matthew Fuller, Email: matthew.fuller@duke.edu.
Miriam Treggiari, Email: miriam.treggiari@duke.edu.
Miguel Yaport, Email: miguel.yaport@duke.edu.
Julien Cobert, Email: julien.cobert@ucsf.edu.
Ethan Garrigan, Email: ethan.garrigan@duke.edu.
Raquel Bartz, Email: rbartz@bwh.harvard.edu.
REFERENCES
- 1.Nolley EP, Sahetya SK, Hochberg CH, et al. : Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023; 6:e2250401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021; 384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shelhamer MC, Wesson PD, Solari IL, et al. : Prone positioning in moderate to severe acute respiratory distress syndrome due to COVID-19: A cohort study and analysis of physiology. J Intensive Care Med 2021; 36:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nijbroek SGLH, Hol L, Ivanov D, et al. ; PRoVENT-COVID Collaborative Group: Low tidal volume ventilation is associated with mortality in COVID-19 patients—insights from the PRoVENT-COVID study. J Crit Care 2022; 70:154047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amstutz A, Speich B, Mentré F, et al. : Effects of remdesivir in patients hospitalised with COVID-19: A systematic review and individual patient data meta-analysis of randomised controlled trials. Lancet Respir Med 2023; 11:453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gershengorn HB, Wunsch H: Temporal trends and variability in ketamine use for mechanically ventilated adults in the United States. Ann Am Thorac Soc 2022; 19:1534–1542 [DOI] [PubMed] [Google Scholar]
- 7.Hurth KP, Jaworski A, Thomas KB, et al. : The reemergence of ketamine for treatment in critically ill adults. Crit Care Med 2020; 48:899–911 [DOI] [PubMed] [Google Scholar]
- 8.Devlin JW, Skrobik Y, Gelinas C, et al. : Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018; 46:e825–e873 [DOI] [PubMed] [Google Scholar]
- 9.Miller AC, Jamin CT, Elamin EM: Continuous intravenous infusion of ketamine for maintenance sedation. Minerva Anestesiol 2011; 77:812–820 [PubMed] [Google Scholar]
- 10.Welters ID, Hafer G, Menzebach A, et al. : Ketamine inhibits transcription factors activator protein 1 and nuclear factor-kappaB, interleukin-8 production, as well as CD11b and CD16 expression: studies in human leukocytes and leukocytic cell lines. Anesth Analg 2010; 110:934–941 [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto K: Repurposing of CNS drugs to treat COVID-19 infection: Targeting the sigma-1 receptor. Eur Arch Psychiatry Clin Neurosci 2021; 271:249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loix S, De Kock M, Henin P: The anti-inflammatory effects of ketamine: State of the art. Acta Anaesthesiol Belg 2011; 62:47–58 [PubMed] [Google Scholar]
- 13.Hirota K, Lambert DG: Ketamine: new uses for an old drug? Br J Anaesth 2011; 107:123–126 [DOI] [PubMed] [Google Scholar]
- 14.Roytblat L, Talmor D, Rachinsky M, et al. : Ketamine attenuates the interleukin-6 response after cardiopulmonary bypass. Anesth Analg 1998; 87:266–271 [DOI] [PubMed] [Google Scholar]
- 15.Beilin B, Rusabrov Y, Shapira Y, et al. : Low-dose ketamine affects immune responses in humans during the early postoperative period. Br J Anaesth 2007; 99:522–527 [DOI] [PubMed] [Google Scholar]
- 16.Dale O, Somogyi AA, Li Y, et al. : Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth Analg 2012; 115:934–943 [DOI] [PubMed] [Google Scholar]
- 17.Chan K, Burry LD, Tse C, et al. : Impact of ketamine on analgosedative consumption in critically ill patients: A systematic review and meta-analysis. Ann Pharmacother 2022; 56:1139–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orser BA, Wang DS, Lu WY: Sedating ventilated COVID-19 patients with inhalational anesthetic drugs. EBioMedicine 2020; 55:102770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dabestani A, DeAngelo D, Chhay SR, et al. : Medication utilization in patients in New York hospitals during the COVID-19 pandemic. Am J Health Syst Pharm 2020; 77:1885–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigtermans M, Dahan A, Mooren R, et al. : S(+)-ketamine effect on experimental pain and cardiac output: A population pharmacokinetic-pharmacodynamic modeling study in healthy volunteers. Anesthesiology 2009; 111:892–903 [DOI] [PubMed] [Google Scholar]
- 21.Timm C, Linstedt U, Weiss T, et al. : Sympathomimetic effects of low-dose S(+)-ketamine. Effect of propofol dosage. Anaesthesist 2008; 57:338–346 [DOI] [PubMed] [Google Scholar]
- 22.Lippmann M, Appel PL, Mok MS, et al. : Sequential cardiorespiratory patterns of anesthetic induction with ketamine in critically ill patients. Crit Care Med 1983; 11:730–734 [DOI] [PubMed] [Google Scholar]
- 23.Traber DL, Wilson RD: Involvement of the sympathetic nervous system in the pressor response to ketamine. Anesth Analg 1969; 48:248–252 [PubMed] [Google Scholar]
- 24.Traber DL, Wilson RD, Priano LL: The effect of bea-adrenergic blockade on the cardiopulmonary response to ketamine. Anesth Analg 1970; 49:604–613 [PubMed] [Google Scholar]
- 25.Wendel-Garcia PD, Erlebach R, Hofmaenner DA, et al. : Long-term ketamine infusion-induced cholestatic liver injury in COVID-19-associated acute respiratory distress syndrome. Crit Care 2022; 26:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinz P, Geelhoed GC, Wee C, et al. : Is atropine needed with ketamine sedation? A prospective, randomised, double blind study. Emerg Med J 2006; 23:206–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ammar MA, Sacha GL, Welch SC, et al. : Sedation, analgesia, and paralysis in COVID-19 patients in the setting of drug shortages. J Intensive Care Med 2021; 36:157–174 [DOI] [PubMed] [Google Scholar]
- 28.Suri A, Sindwani G: Ketamine use in the COVID-19 era: Be cautious! Korean J Anesthesiol 2020; 73:568–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volpe MS, Guimaraes FS, Morais CC: Airway clearance techniques for mechanically ventilated patients: Insights for optimization. Respir Care 2020; 65:1174–1188 [DOI] [PubMed] [Google Scholar]
- 30.Hess DR: Airway clearance: Physiology, pharmacology, techniques, and practice. Respir Care 2007; 52:1392–1396 [PubMed] [Google Scholar]
- 31.Karamchandani K, Dalal R, Patel J, et al. : Challenges in sedation management in critically ill patients with COVID-19: A brief review. Curr Anesthesiol Rep. 2021; 11:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]