Abstract
Analysis of C57BL/6 mice acutely infected with lymphocytic choriomeningitis virus (LCMV) by using intracellular cytokine staining revealed a high frequency (2 to 10%) of CD4+ T cells secreting the Th1-associated cytokines interleukin-2 (IL-2), gamma interferon (IFN-γ), and tumor necrosis factor alpha, with no concomitant increase in the frequency of CD4+ T cells secreting the Th2-associated cytokines IL-4, IL-5, and IL-10 following stimulation with viral peptides. In LCMV-infected C57BL/6 CD8−/− mice, more than 20% of the CD4+ T cells secreted IFN-γ after viral peptide stimulation, whereas less than 1% of the CD4+ T cells secreted IL-4 under these same conditions. Mice persistently infected with a high dose of LCMV clone 13 also generated a virtually exclusive Th1 response. Thus, LCMV induces a much more profound virus-specific CD4+ T-cell response than previously recognized, and it is dramatically skewed to a Th1 phenotype.
Many viruses are potent inducers of T-cell responses (1, 3, 22), and the infection of mice with lymphocytic choriomeningitis virus (LCMV) is one of the most well-characterized model systems for studying T-cell responses to viruses. Most work in the LCMV system has focused on CD8+ T cells, but by day 7 of an acute LCMV infection, approximately 25% of the CD4+ T cells are blast sized and 20 to 30% express activation markers and adhesion molecules (16, 18). Interleukin-2 (IL-2)-based LDA of LCMV-specific CD4+ T-cell frequencies account for only a small percentage (<1%) of the activated CD4+ T cells (17, 18), but intracellular gamma interferon (IFN-γ) staining following stimulation with either of only two LCMV-encoded major histocompatibility complex (MHC) class II-restricted peptides (10) has revealed that as many as 10% of the CD4+ T cells can be defined as LCMV specific (17). CD4+ T cells may be separated into distinct subsets based on the cytokines that they secrete, with Th1 responses characterized by the production of IL-2, IFN-γ, and tumor necrosis factor β (TNF-β), and Th2 responses characterized by the production of IL-4, IL-5, IL-6, IL-10, and IL-13 (7–9). In this study we sought to examine the frequencies and phenotypes of acute and memory LCMV-specific CD4+ T cells capable of making Th1 cytokines versus those of cells capable of making Th2 cytokines. We show here that LCMV induces exclusively a Th1 cytokine response.
Intracellular cytokine analysis during acute LCMV infection into memory.
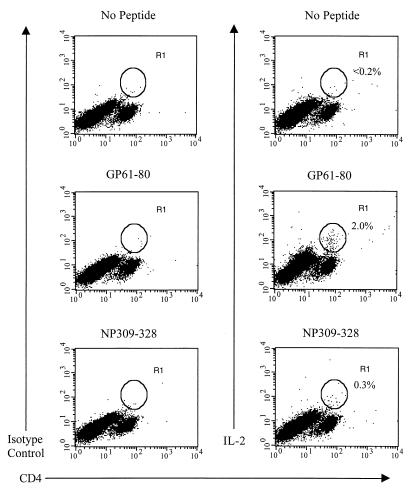
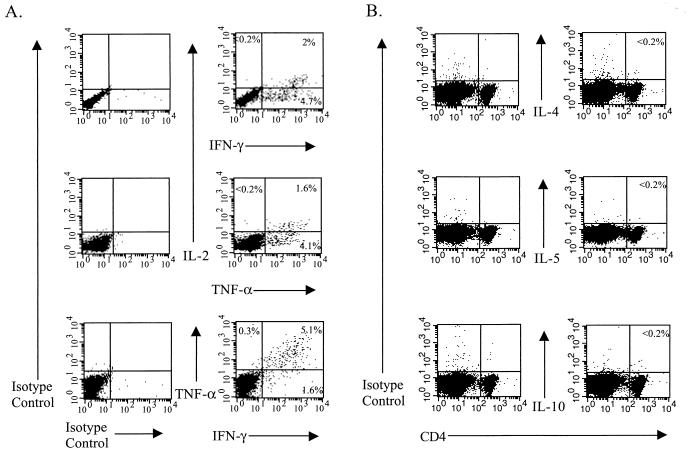
Intracellular cytokine staining was performed as described previously (17), except that monensin instead of brefeldin A was used in some experiments to allow cytokines to accumulate intracellularly. Table 1 shows the frequencies of CD4+ T cells that produce the Th1-associated cytokines IL-2, IFN-γ, or TNF-α when stimulated with the LCMV GP61-80 MHC class II-restricted peptide at various times during the acute LCMV infection and into the memory state, and Fig. 1 shows that approximately 2% of the CD4+ T cells from C57BL/6 mice at day 9 postinfection (p.i.) with LCMV produced IL-2 following stimulation with this peptide. Others have failed to show IL-2 production in these assays (14, 19), but we were able to do so by substituting monensin for brefeldin A. Multicolor flow cytometric analysis revealed that those CD4+ T cells that expressed IL-2 also produced either IFN-γ or TNF-α, and most of the cells that expressed IFN-γ also produced TNF-α (Fig. 2A). However, we failed to detect any increase, compared to the levels obtained with the appropriate isotype-matched control monoclonal antibody (MAb), in the intracellular expression of the Th2 cytokines IL-4, IL-5, and IL-10 in CD4+ T cells during an acute LCMV infection following viral peptide stimulation in the presence of monensin (Fig. 2B) or following stimulation with phorbol myristate acetate and ionomycin (reference 17 and data not shown).
TABLE 1.
Frequency of cytokine-secreting CD4+ T cells during the acute LCMV infection following GP61-80 peptide stimulationa
| Days p.i. | Frequency of CD4+ cells secreting:
|
||
|---|---|---|---|
| IL-2 | IFN-γ | TNF-α | |
| 0 | <0.2 | <0.2 | 0.3 ± 0.2 |
| 7 | 0.6 ± 0.4 | 2.2 ± 1.4 | 1.4 ± 0.9 |
| 9 | 1.7 ± 0.7 | 4.2 ± 2.2 | 4.1 ± 0.7 |
| 11 | 1.3 ± 1.0 | 3.1 ± 1.7 | 2.9 ± 1.1 |
| 15 | 0.6 ± 0.5 | 1.1 ± 0.8 | 1.1 ± 0.5 |
| 180 | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.5 ± 0.3 |
Intracellular cytokine assays were performed on splenocytes from the indicated days p.i. with LCMV that were stimulated with the LCMV MHC class II-restricted peptide GP61-80 in the presence of IL-2 and monensin for 5 h. Background staining with the appropriate isotype-matched control MAb was not included in the results. Data are the mean percentages of CD4+ T cells that stained positive for the indicated cytokine ± standard deviations from three separate experiments with two mice per experiment (n = 6 per group).
FIG. 1.
Intracellular IL-2 expression of LCMV peptide-specific CD4+ T cells. Splenocytes from LCMV-infected (day 9) C57BL/6 mice were stimulated in the presence of IL-2 (Pharmingen, San Diego, Calif.) and monensin (Sigma, St. Louis, Mo.) with or without one of the two LCMV MHC class II-restricted peptides, GP61-80 and NP309-328. The cells were subsequently harvested, blocked with purified anti-FcγRII/III MAb (clone 2.4G2; Pharmingen), and stained with fluorescein isothiocyanate- or PerCP-conjugated anti-CD4 (clone RM4-5; Pharmingen). After fixation the cells were stained for intracellular cytokines using directly conjugated anti-cytokine MAbs or the appropriate isotype-matched control MAb (all from Pharmingen) in buffer containing 0.5% saponin (Sigma). Control cells known to express IL-2, IL-4, IL-10, or IFN-γ (MiCK-1 and MiCK-2; Pharmingen) were used in all experiments as positive controls. The percentages shown each indicate the number of CD4+ T cells that stained positive for intracellular IL-2 as represented by the R1 gate. Data shown are representative of five experiments, with two mice per experiment.
FIG. 2.
Th1 (A) and Th2 (B) cytokine expression of LCMV peptide-specific CD4+ T cells. Splenocytes from LCMV-infected (day 9) C57BL/6 mice were prepared and analyzed by flow cytometry after stimulation with the GP61-80 peptide as described in the legend for Fig. 1. For panel A, the cells were gated on CD4+ cells, and the numbers shown represent the percentage of CD4+ cells that fall into each quadrant. Data shown are representative of five experiments, with two mice per experiment. For panel B, the percentages shown each indicate the number of CD4+ T cells that stained positive for the indicated cytokine. Data shown for IL-4 are representative of two experiments, with two individual mice per experiment, and the data shown for IL-5 and IL-10 are from one experiment with two mice.
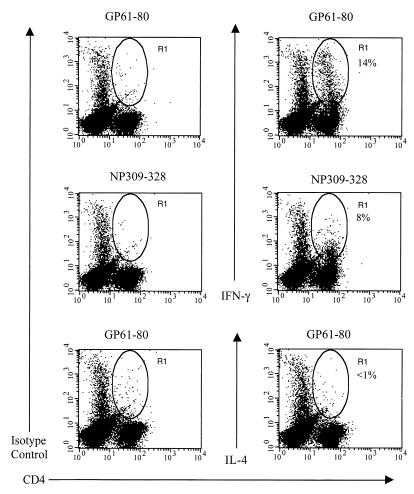
The expression of intracellular IFN-γ and IL-4 was also examined following LCMV infection of C57BL/6 mice genetically deficient in CD8+ T cells; these mice mount a vigorous LCMV-specific CD4+ T-cell response. Figure 3 shows a high frequency of IFN-γ-producing CD4+ T cells at day 9 p.i., with no increase in the frequency of IL-4-producing CD4+ T cells. Thus, there is no significant increase in the frequency of IL-4-secreting CD4+ T cells even in mice that develop a very strong LCMV-specific CD4+ T-cell response.
FIG. 3.
Intracellular IFN-γ and IL-4 expression of LCMV peptide-specific CD4+ T cells from CD8 knockout mice. Splenocytes from LCMV-infected (day 9) C57BL/6 CD8 knockout mice were stimulated with one of the two LCMV MHC class II-restricted peptides in the presence of IL-2 and brefeldin A for 5 h and subsequently stained as described in the legend to Fig. 1. The percentages shown each indicate the number of CD4+ T cells that stained positive for intracellular IFN-γ or IL-4 as represented by the R1 gate. Data are representative of two experiments, with two mice per experiment.
Cell surface phenotype of LCMV-specific memory CD4+ T cells.
In order to analyze the LCMV-specific memory CD4+ Thp cell phenotype, CD4+ T cells obtained from LCMV-immune mice were sorted for CD4+ T cells expressing one of a panel of activation and memory markers (Table 2). We utilized the IL-2-based LDA (18) to examine the memory CD4+ T-cell phenotype, as the LDA is able to detect lower frequencies of virus-specific CD4+ T cells more reliably than the intracellular cytokine assay presented above is (17). The virus-specific memory CD4+ Thp cells expressed predominantly a CD44hi CD45RBlo CD49dhi CD62Llo CZ-1hi phenotype. A fairly high frequency of LCMV-specific memory CD4+ Thp cells were found in both the CD62Llo and CD62Lhi subsets. CD49d and CZ-1 were two of the best markers for identifying LCMV-specific CD4+ Thp cells. We have previously shown CZ-1 to be a CD4+ T-cell activation and memory marker (16), and expression of CD49d on a high frequency of Sendai virus-specific memory CD4+ Thp cells has been reported previously (4).
TABLE 2.
Phenotype of LCMV-immune IL-2-producing CD4+ T-cell precursorsa
| Group | Reciprocal of Thp cell frequencyb
|
|
|---|---|---|
| Uninfected APC | LCMV-infected APC | |
| CD4+ | 7,905 (5,699–12,897) | 1,650 (1,345–2,135) |
| CD4+ CD44hi | 9,709 (6,329–20,831) | 633 (541–764) |
| CD4+ CD44lo | 61,610 (40,713–126,586) | 21,526 (17,196–28,770) |
| CD4+ CD62Lhi | 11,189 (8,734–15,563) | 2,242 (1,893–2,749) |
| CD4+ CD62Llo | 10,620 (6,915–22,879) | 651 (546–807) |
| CD4+ | 34,437 (18,340–281,475) | 3,390 (2,727–4,476) |
| CD4+ CD44hi | 14,696 (6,764–85,070) | 1,978 (1,510–2,862) |
| CD4+ CD44lo | 13,504 (10,914–17,704) | 11,782 (9,747–14,894)c |
| CD4+ CD62Lhi | 14,338 (11,761–18,362) | 5,911 (4,829–7,617) |
| CD4+ CD62Llo | 5,315 (2,860–37,616) | 1,408 (1,162–1,788) |
| CD4+ CD45RBhi | 36,042 (23,139–81,475) | 3,267 (2,450–4,903) |
| CD4+ CD45RBlo | NRd | 1,542 (1,222–2,089) |
| CD4+ CD45RBhi | 63,620 (46,075–102,744) | 2,982 (2,303–4,227) |
| CD4+ CD45RBlo | 5,341 (4,123–7,579) | 7,094 (5,339–10,568)c |
| CD4+ CZ-1hi | 30,085 (20,012–60,573) | 467 (392–577) |
| CD4+ CZ-1lo | 11,668 (9,812–14,389) | 2,350 (1,805–3,369) |
| CD4+ CD45RBhi | 45,168 (32,309–75,034) | 8,621 (6,514–12,739) |
| CD4+ CD45RBlo | NR | 38,020 (21,980–140,663) |
| CD4+ CZ-1hi | NR | 4,690 (3,766–6,216) |
| CD4+ CZ-1lo | NR | 55,873 (35,602–129,746) |
| CD4+ CD49dhi | 7,414 (5,803–10,268) | 349 (277–473) |
| CD4+ CD49dlo | 22,306 (18,843–27,331) | 2,442 (1,964–3,228) |
Splenocytes from a pool of four LCMV-immune mice (6 to 8 months) were stained and sorted into two populations based on CD4 and either CD44, CD62L, CD45RB, CD49d, or CZ-1 expression. CD4 LDA were then performed with these two FACS-purified populations.
Values in parentheses are 95% confidence limits.
Not significantly different (within the 95% confidence limits) from uninfected APC controls.
NR, no response.
Intracellular cytokine analysis during persistent LCMV clone 13 infection.
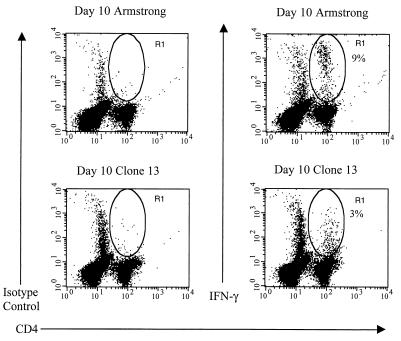
Infection of mice with a high dose of LCMV clone 13 has been shown to selectively delete the virus-specific CD8+ T cells by day 7 or 8 p.i. due to the overwhelming virus infection (6). The fate of the virus-specific CD4+ T cells under these conditions has been less studied. A recent report has suggested that Th1 cells are more sensitive to activation-induced cell death than Th2 cells are (21). Therefore, we postulated that during a high-dose LCMV clone 13 infection there may be a selective loss of virus-specific CD4+ T cells secreting Th1-associated cytokines and, if LCMV-specific Th2-like CD4+ T cells were present, they might be less susceptible to deletion in an environment with a high viral antigen concentration and therefore represent a higher proportion of the remaining virus-specific CD4+ T-cell population. We again utilized the intracellular cytokine assay to follow the fate of the IL-4-secreting CD4+ T cells but detected no increase (data not shown). Figure 4 shows the percentage of viral peptide-specific IFN-γ+ CD4+ T cells present following a high-dose LCMV clone 13 infection. The percentage of IFN-γ+ CD4+ T cells never reached the percentage observed during LCMV Armstrong infection, and the CD4+ T cells that stained brightest for IFN-γ in the LCMV Armstrong-infected mice were absent in the LCMV clone 13-infected animals (Fig. 4). This may suggest that high-affinity LCMV-specific CD4+ T cells are rapidly lost following a high-dose LCMV clone 13 infection. The remaining low-affinity LCMV-specific CD4+ T cells may then slowly become anergic or unresponsive due to chronic stimulation by virus-infected antigen-presenting cells (APC), as suggested recently by others (12).
FIG. 4.
Intracellular IFN-γ expression of LCMV peptide-specific CD4+ T cells from LCMV clone 13-infected C57BL/6 mice. Splenocytes from LCMV Armstrong- or clone 13-infected mice (both day 10) were stimulated with the LCMV MHC class II-restricted peptide GP61-80 in the presence of IL-2 and brefeldin A for 5 h and subsequently stained as described in the legend to Fig. 1. The percentages shown each indicate the number of CD4+ T cells that stained positive for intracellular IFN-γ as represented by the R1 gate. Data are representative of three separate experiments, with three mice per experiment.
In this report we demonstrate that the LCMV-induced CD4+ T-cell response is virtually exclusively a Th1 response associated with the production of IL-2, IFN-γ, and TNF-α but not IL-4, IL-5, or IL-10 (Fig. 1 to 3 and Table 1). During the peak of the acute LCMV response, approximately 2% of the CD4+ T cells make IL-2 following stimulation with either of the two known LCMV-encoded MHC class II-restricted peptides (Fig. 1 and Table 1). Two recent reports of studies done with the LCMV system showed that fluorescence-activated cell sorter (FACS) analysis following either peptide stimulation (19) or anti-CD3 stimulation (14) of splenocytes in the presence of brefeldin A, an inhibitor of protein transport between the endoplasmic reticulum and the Golgi body, failed to detect IL-2-secreting CD4+ T cells. Here, we show that using monensin, an inhibitor of trans-Golgi function, allows for the detection of a high frequency of IL-2-producing CD4+ T cells following viral peptide stimulation. This frequency is significantly greater than previous estimates by us using LDA (18) and others using enzyme-linked immunospot (ELISPOT) assays (19). Multicolor FACS analysis revealed that the majority of the CD4+ T cells that expressed intracellular IL-2 also synthesized IFN-γ (and/or TNF-α) following viral peptide stimulation (Fig. 2A). This suggests that the IL-2- and IFN-γ-secreting CD4+ T cells are not separate populations of virus-specific CD4+ T cells.
Previous studies examining mice infected intracranially with LCMV revealed the expression of easily detectable IFN-γ mRNA but not IL-4 mRNA in the brain and spleen (2). More recent studies from our laboratory (17, 18) and others (5, 11) have shown that LCMV-specific CD4+ T cells make IFN-γ but not IL-4 following stimulation with virus-infected APC or viral peptides, using several different techniques, including LDA, enzyme-linked immunosorbent assay (ELISA), and ELISPOT assay. Moreover, analysis of antibody isotypes produced following acute LCMV infection has demonstrated a significant skewing of antibodies toward the immunoglobulin G2a isotype (13, 20), which is promoted by IFN-γ (15). All of these results indicate that the LCMV-specific CD4+ T-cell response is predominantly of a Th1 phenotype. However, two recent studies have reported elevated frequencies of CD4+ T cells secreting IL-4 following LCMV infection using ELISAs and ELISPOT assays (14, 19). We cannot completely exclude the possibility that there is a very low frequency of LCMV-specific CD4+ T cells of a Th2 phenotype, but we could not detect any in this study. It should be noted that we have found that the inclusion of 10% fetal calf serum in the viral inoculum drives a strong IL-4 response that is absent if the viral inoculum is purified apart from serum, and serum was present in the inocula in the two studies showing Th2 responses (14, 19). The data presented here and in another recent report (5) clearly establish that the LCMV-specific CD4+ T-cell response is dominated by cells that secrete Th1-associated cytokines.
Acknowledgments
We thank Keith Daniels for his technical assistance and Tammy Krumpoch and Barbara Fournier for help with the FACS analysis.
This study was supported by Public Health Service Training Grant AI07439 to Steven M. Varga and Research Grants AI17672, AR35506, and CA34461 to Raymond M. Welsh.
REFERENCES
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–59. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Campbell I L, Hobbs M V, Kemper P, Oldstone M B A. Cerebral expression of multiple cytokine genes in mice with lymphocytic choriomeningitis. J Immunol. 1994;152:716–723. [PubMed] [Google Scholar]
- 3.Doherty P C, Topham D J, Tripp R A. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 4.Ewing C, Topham D J, Doherty P C. Prevalence and activation phenotype of Sendai virus-specific CD4+ T cells. Virology. 1995;210:179–185. doi: 10.1006/viro.1995.1329. [DOI] [PubMed] [Google Scholar]
- 5.Kamperschroer C, Quinn D G. Quantification of epitope-specific MHC class-II-restricted T cells following lymphocytic choriomeningitis virus infection. Cell Immunol. 1999;193:134–146. doi: 10.1006/cimm.1999.1458. [DOI] [PubMed] [Google Scholar]
- 6.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 7.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 8.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann T R, Schumacher J H, Street N F, Budd R, O'Garra A, Fong T A T, Bond M W, Moore K W M, Sher A, Fiorentino D F. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev. 1991;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 10.Oxenius A, Bachmann M F, Ashton-Rickardt P G, Tonegawa S, Zinkernagel R M, Hengartner H. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur J Immunol. 1995;25:3402–3411. doi: 10.1002/eji.1830251230. [DOI] [PubMed] [Google Scholar]
- 11.Oxenius A, Campbell K A, Maliszewski C R, Kishimoto T, Kikutani H, Hengartner H, Zinkernagel R M, Bachmann M F. CD40-CD40 ligand interactions are critical in T-B cooperation but not for other anti-viral CD4+ T cell functions. J Exp Med. 1996;183:2209–2218. doi: 10.1084/jem.183.5.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oxenius A, Zinkernagel R M, Hengartner H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 1998;9:449–457. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- 13.Slifka M K, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995;69:1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su H C, Cousens L P, Fast L D, Slifka M K, Bungiro R D, Ahmed R, Biron C A. CD4+ and CD8+ T cell interactions in IFN-γ and IL-4 responses to viral infections: requirements for IL-2. J Immunol. 1998;160:5007–5017. [PubMed] [Google Scholar]
- 15.Utermohlen O, Dangel A, Tarnok A, Lehmann-Grube F. Modulation by gamma interferon of antiviral cell-mediated immune responses in vivo. J Virol. 1996;70:1521–1526. doi: 10.1128/jvi.70.3.1521-1526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varga S M, Welsh R M. The CD45RB-associated epitope defined by monoclonal antibody CZ-1 is an activation and memory marker for mouse CD4 T cells. Cell Immunol. 1996;167:56–62. doi: 10.1006/cimm.1996.0007. [DOI] [PubMed] [Google Scholar]
- 17.Varga S M, Welsh R M. Cutting edge: detection of a high frequency of virus-specific CD4+ T cells during acute infection with lymphocytic choriomeningitis virus. J Immunol. 1998;161:3215–3218. [PubMed] [Google Scholar]
- 18.Varga S M, Welsh R M. Stability of virus-specific CD4+ T cell frequencies from acute infection into long term memory. J Immunol. 1998;161:367–374. [PubMed] [Google Scholar]
- 19.Whitmire J K, Asano M S, Murali-Krishna K, Suresh M, Ahmed R. Long-term CD4 Th1 and Th2 memory following acute lymphocytic choriomeningitis virus infection. J Virol. 1998;72:8281–8288. doi: 10.1128/jvi.72.10.8281-8288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitmire J K, Slifka M A, Grewal I S, Flavell R A, Ahmed R. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J Virol. 1996;70:8375–8381. doi: 10.1128/jvi.70.12.8375-8381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Brunner T, Carter L, Dutton R W, Rogers P, Bradley L, Sato T, Reed J C, Green D, Swain S L. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinkernagel R M. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]