Abstract
Changes in the envelope glycoprotein ectodomains of a nonpathogenic simian-human immunodeficiency virus (SHIV-89.6) that was serially passaged in vivo have been shown to be responsible for the increased pathogenicity of the resulting virus, SHIV-KB9 (G. B. Karlsson, et al., J. Exp. Med. 188:1159–1171, 1998). The 12 amino acid changes in the envelope glycoprotein ectodomains resulted in increased chemokine receptor-binding and syncytium-forming abilities. Here we identify the envelope glycoprotein determinants of these properties. A single amino acid change in the gp120 third variable (V3) loop was both necessary and sufficient for the observed increase in the binding of the SHIV-KB9 gp120 glycoprotein to the CCR5 chemokine receptor. The increased syncytium-forming ability of SHIV-KB9 involved, in addition to the V3 loop change, changes in the second conserved (C2) region of gp120 (residue 225) and in the gp41 ectodomain (residues 564 and 567). The C2 and gp41 ectodomain changes influenced syncytium formation in a cooperative manner. Changes in the V1/V2 gp120 variable loops exerted a negative effect on syncytium formation and chemokine receptor binding, supporting a previously described role of these changes in immune evasion. The definition of the passage-associated changes that determine the efficiency of chemokine receptor binding and membrane fusogenicity will allow evaluation of the contribution of these properties to in vivo CD4-positive lymphocyte depletion.
Human immunodeficiency viruses types 1 and 2 (HIV-1 and HIV-2, respectively) and the related simian immunodeficiency viruses cause AIDS in humans and monkeys, respectively (2, 9, 12, 23, 38). HIV-1 infection is accompanied by the progressive loss of CD4+ lymphocytes, leading to the development of AIDS (18, 19). The mechanisms underlying the destruction of CD4+ lymphocytes in HIV-1-infected humans still remain to be elucidated. Studies in infected humans indicate that the infected, virus-producing cell is prone to rapid destruction; in contrast, most latently infected cells exhibit normal half-lives (26, 55). This result suggests that mechanisms such as viral cytopathic effects and immunologic clearance may make important contributions to CD4+ lymphocyte decline (26, 55). HIV-1-induced cytopathic effects, which include syncytium formation and single-cell lysis (4, 6, 39, 42, 50), primarily result from membrane fusion mediated by the viral envelope glycoproteins (4, 31, 42, 50).
The HIV-1 envelope glycoproteins, gp120 and gp41, play an essential role in virus attachment and entry (60). The exterior envelope glycoprotein, gp120, contains five variable (V1 to V5) and five conserved (C1 to C5) regions (37, 45). The gp120 envelope glycoprotein binds to the CD4 receptor on the cell surface (10), and the gp120-CD4 complex subsequently binds to one member of the family of chemokine receptors (1, 8, 11, 13, 14, 20, 58). Chemokine receptor binding is believed to trigger additional conformational changes in the viral envelope glycoproteins that ultimately lead to the fusion of the viral and target cell membranes. HIV-1 envelope glycoproteins expressed on the surface of infected cells also initiate receptor binding and membrane fusion events involving adjacent CD4+ cells, resulting in the formation of multinucleated syncytia (42, 50). Previous studies have shown that disease progression in HIV-1-infected individuals correlates with the emergence of HIV-1 variants that are more cytopathic in a variety of human cell lines (6, 32, 54). Furthermore, differences in in vitro cytopathic properties of HIV-1 isolates have been found to correlate with severe immunodeficiency in vivo (21, 53); primary isolates with high syncytium-inducing capacity were found more frequently in individuals that had progressed to AIDS. The ability of primary HIV-1 isolates to induce syncytia efficiently in a wide range of CD4-positive cells is related to the use of the ubiquitously expressed CXCR4 chemokine receptor.
The observations made in HIV-1-infected individuals have advanced our understanding of HIV-1 pathogenesis, but because of the obvious limitations of human studies, the importance of HIV-1 cytopathicity in vitro to CD4+ lymphocyte depletion in vivo remains uncertain. Animal models allow the control of variables related to the virus inoculum, and several models in which HIV-like viruses induce AIDS-like illness in primates have been established (12, 28, 38, 47, 49). Chimeric simian-human immunodeficiency viruses (SHIVs) that bear several HIV-1 genes, including that encoding the envelope glycoproteins, provide a useful tool for studies of viral pathogenesis in Old World monkeys (27, 40, 41, 43). One molecularly cloned SHIV that replicates to high levels in vivo and induces acute CD4+ lymphocyte depletion was generated after serial animal passage of a nonpathogenic SHIV clone (SHIV-89.6) (47, 48). The resulting virus, SHIV-89.6P, caused rapid and severe depletion of CD4+ lymphocytes in rhesus macaques within 2 weeks of inoculation (47). A molecular clone of SHIV-89.6P was used to generate an infectious virus, designated SHIV-KB9 (29). SHIV-KB9 also induced rapid depletion of CD4+ lymphocytes in rhesus monkeys (29, 30). Interestingly, the in vivo viremia in SHIV-89.6P- and SHIV-KB9-infected animals during the first weeks of infection was not dramatically increased compared to that seen in SHIV-89.6-infected animals (47). This observation suggested the possibility that animal passage had resulted in an increase in the ability of the virus to deplete CD4+ lymphocytes in vivo, independently of viral replication and turnover.
Sequence comparison of SHIV-KB9 and the original SHIV-89.6 shows that most of the passage-associated changes occurred in the envelope gene (29). Thirteen single amino acid substitutions arose in the envelope glycoproteins. In addition, a 140-base-pair deletion in the 3′ end of the SHIV-KB9 env gene created an in-frame junction between the HIV-1 and the SIVmac239 env sequences, resulting in a new, longer gp41 protein containing the SIVmac239 carboxy-terminal cytoplasmic tail (29). Two amino acid substitutions in Tat, and two nucleotide alterations in the long terminal repeats, also resulted from animal passage of SHIV-89.6.
Chimeric viruses containing sequences from SHIV-89.6 and SHIV-KB9 were constructed to evaluate the effect of the passage-associated changes on replication and in vivo CD4+ lymphocyte-depleting ability during the acute infection (30). The study identified the HIV-1 envelope glycoprotein ectodomain changes as determinants of CD4+ T-cell loss in vivo. The envelope glycoproteins of recombinant SHIVs that efficiently caused CD4+ T-cell depletion in vivo exhibited increased chemokine receptor-binding affinity and membrane-fusing capacity, compared to those of less pathogenic viruses. Both of these properties were shown to be specified solely by the envelope glycoprotein ectodomain changes (30).
In this study, we further dissect the genetic determinants of the increased membrane fusogenicity of the SHIV-KB9 envelope glycoproteins. We identify single amino acid changes that occurred during animal passage that play a crucial role in syncytium induction and chemokine receptor binding.
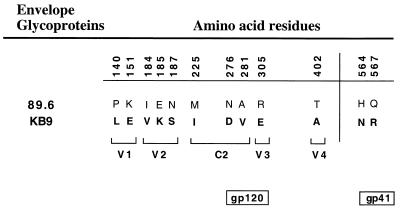
Previous analyses showed that the ectodomain changes that occurred in the passaged KB9 envelope glycoproteins are responsible for a striking increase in the number and size of syncytia in cultured T-cell lines infected with SHIV-KB9, when compared to SHIV-89.6 (30). The increased fusogenicity of the KB9 envelope glycoproteins, relative to that of the SHIV-89.6 envelope glycoproteins, was confirmed by using a more controlled syncytium formation assay where envelope-glycoprotein-expressing cells were cocultivated with target CEMx174 lymphocytes (30). To assess which of the amino acid changes in the KB9 envelope glycoproteins are necessary for the increased fusogenic activity, we constructed a collection of recombinant KB9 envelope glycoproteins in which individual amino acids that were changed during animal passage were reverted, individually or in combination, to the amino acid originally found in the parental 89.6 envelope glycoproteins (16) (Fig. 1). All 10 amino acid changes in the gp120 envelope glycoprotein were reverted individually, and each new envelope glycoprotein was designated KB9(−amino acid number). Note that the residue numbers correspond to those of the prototypic HXBc2 envelope glycoproteins, according to current convention (33), and are different from those previously published (16). We also created a few selected envelope glycoproteins in which the amino acid changes found in the KB9 envelope glycoproteins were introduced into the 89.6 envelope glycoproteins. These recombinant envelope glycoproteins were designated 89.6(+amino acid number). Additional recombinant envelope glycoproteins were also created by reverting or introducing more than one change; for example, in KB9(−V1/V2), all the passage-associated changes in the V1 and V2 variable loops of SHIV-KB9 were reverted to the amino acids originally found in SHIV-89.6. Mutations were introduced into the HIV-1(89.6) or HIV-1(KB9) env sequences by using the QuickChange site-directed mutagenesis kit (Stratagene) and were confirmed by DNA sequencing. To assess expression of the various envelope glycoproteins, 293T cells were cotransfected with the designated pSVIIIenv plasmid and with a plasmid expressing the HIV-1 Tat protein. Thirty-six hours after transfection, a portion of the cells was used to measure envelope glycoprotein expression by immunoprecipitation with the serum from an HIV-1-infected individual. All of the envelope glycoproteins exhibited comparable processing of the gp160 envelope glycoprotein precursor, gp120-gp41 association, and cell-surface expression of the envelope glycoproteins (29; data not shown). The ΔKS clone, which contains an env gene with a large deletion, was used as a negative control. For the syncytium formation assay, the envelope-glycoprotein-expressing 293T cells were detached 36 h after transfection by washing once with 10 mM EDTA in phosphate-buffered saline and were resuspended into 10 ml of Dulbecco minimal essential medium plus fetal calf serum. The envelope-glycoprotein-expressing 293T cells were subsequently mixed in a 1:10 ratio with 106 target cells, in a final volume of 1 ml, in 48-well plates. Wells were scored for syncytia after 6 h of cocultivation at 37°C. At this time point, the size and number of syncytia were adequate for counting, yet extensive cell death did not occur.
FIG. 1.
Amino acid sequences of the 89.6 and KB9 envelope glycoproteins. The identity and position of the amino acid residues that were altered during animal passage of SHIV-89.6 are indicated. The locations of the amino acid residues within one of the variable (V) or conserved (C) regions of the gp120 envelope glycoprotein are also indicated. Numbering of envelope glycoprotein residues is according to current convention (33).
The target cells in the syncytium formation assay were either CEMx174, SupT1, or human peripheral blood CD4+ lymphocytes. CD4+ lymphocytes were selected from Ficoll-enriched human peripheral blood mononuclear cells (PBMC) by using a magnetic cell-sorting column (Miltenyi Biotec). The CD4+ lymphocytes were stimulated with 1 μg of phytohemagglutinin (Murex Diagnostics Inc., Dartfield, United Kingdom) per ml for 48 h and were subsequently incubated with human interleukin-2 (final concentration of 10%) (Hemagen Diagnostics Inc., Columbia, Md.) for 24 to 48 h before cocultivation with envelope-glycoprotein-expressing 293T cells.
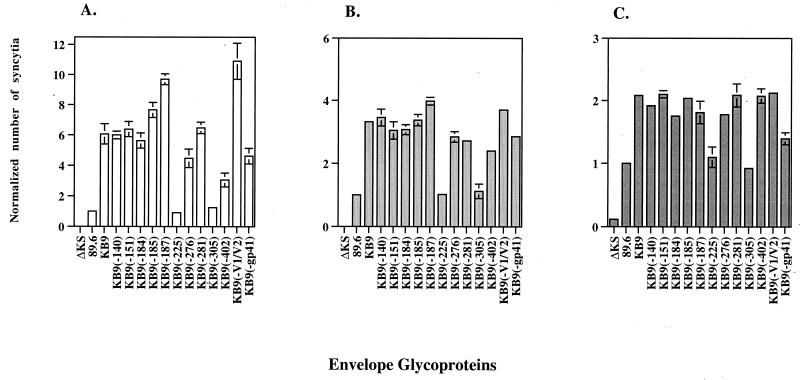
Figure 2A shows the results of the syncytium formation assay using CEMx174 target cells. Most of the single-amino-acid reversions in the KB9 envelope glycoproteins did not exert an effect on syncytium formation. However, single amino acid reversions in the 225 and 305 residues attenuated the fusogenic activity of the envelope glycoproteins to levels comparable to that of the 89.6 envelope glycoproteins. This result suggests that these two single-amino-acid changes may play an important role in the increased fusogenicity associated with the KB9 envelope glycoproteins. Other single-amino-acid changes exhibited a more subtle effect on syncytium formation: a single-amino-acid change in the V4 loop [KB9(−402)] and the two gp41 ectodomain changes [KB9(−gp41)] also decreased the fusogenic activity of KB9. On the other hand, the KB9(−185), KB9(−187), and KB9(−V1/V2) envelope glycoproteins formed more syncytia than the KB9 envelope glycoproteins, suggesting that these V2 loop changes may exert a negative effect on fusogenicity.
FIG. 2.
Syncytium-forming ability of envelope glycoprotein recombinants. 293T cells, transiently expressing the 89.6, KB9, or recombinant envelope glycoproteins, were cocultivated with either CEMx174 (A), SupT1 (B), or CD4+-enriched human PBMC (C) for 6 h at 37°C. The number of syncytia was scored and normalized to that observed for the wild-type 89.6 envelope glycoproteins (assigned a value of 1). The latter value corresponds to an average of 150, 200, and 112 syncytia/ml in assays performed with CEMx174, SupT1, and CD4+-enriched human PBMC, respectively. The number of syncytia observed with the negative control (ΔKS) was close to zero when either CEMx174 or SupT1 cell lines were used as targets and was 10-fold lower than that associated with the 89.6 envelope glycoproteins when CD4+-enriched human PBMC were used as target cells. The mean values and standard deviations for at least two independent experiments are shown.
To determine the generality of the results from our syncytium formation analysis, we repeated the assay with a different T-cell line, SupT1, as well as with primary CD4+ lymphocytes as targets. The differences between the syncytium-forming ability of the 89.6 and KB9 envelope glycoproteins were more subtle when SupT1 or CD4+-selected PBMC were used as target cells in the assay (Fig. 2B and C). Nevertheless, the pattern of syncytium induction by the various recombinant envelope glycoproteins was similar when either SupT1 or CEMx174 lymphocytes were used. In the assay using the primary human CD4+ lymphocytes, the effects of the single amino acid reversions in the KB9(−225), KB9(−305), and KB9(−gp41) envelope glycoproteins on syncytium formation were consistent with the data obtained by using the two T-cell lines. However, we detected no significant difference in fusogenic activity among the KB9(−185), KB9(−187), KB9(−V1/V2), and KB9 envelope glycoproteins with the CD4+-selected PBMC as target cells.
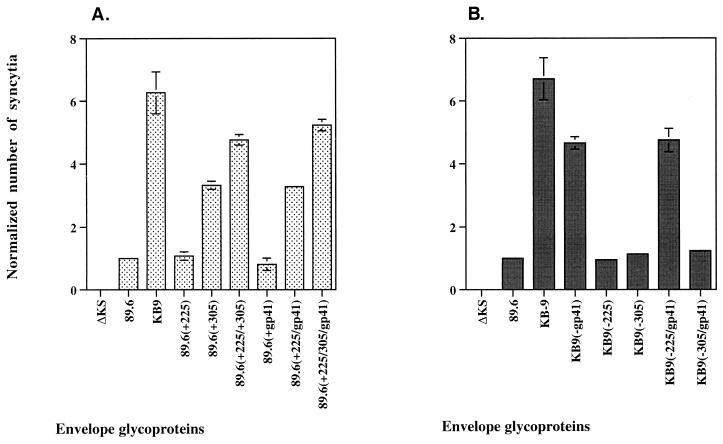
To examine further the role of the passage-associated changes in residues 225 and 305 and in the gp41 ectodomain, we introduced the changes singly, or in combination, in the background of the 89.6 envelope glycoproteins (Fig. 3A). The 89.6(+225) and 89.6(+gp41) envelope glycoproteins exhibited phenotypes indistinguishable from that of the 89.6 envelope glycoproteins. The combination of both changes in the 89.6(+225/gp41) glycoproteins resulted in an increased syncytium-forming ability, relative to that of the 89.6 glycoproteins. The 89.6(+305) envelope glycoproteins also formed more syncytia than the 89.6 envelope glycoproteins. The 89.6(+225/305) envelope glycoproteins formed even more syncytia, although not as many as the KB9 envelope glycoproteins. The 89.6(+225/305/gp41) envelope glycoproteins formed almost as many syncytia as the KB9 envelope glycoproteins. Thus, these four amino acid changes that occurred during animal passage are sufficient to account for most of the increased fusogenic activity observed in vitro.
FIG. 3.
Ability of selected amino acid changes to enhance syncytium formation. (A) Selected passage-associated changes were introduced singly, or in combination, into the 89.6 envelope glycoproteins. The syncytium-forming ability of these envelope glycoprotein variants was investigated. (B) The syncytium-forming ability of KB9 recombinant envelope glycoproteins with selected reversions, alone or in combination, was tested. Syncytium formation analysis was performed with CEMx174 cells as targets, and the number of syncytia was normalized to that observed for the 89.6 envelope glycoproteins (assigned a value of 1).
The results shown in Fig. 3A indicate that the phenotype of the residue 225 change and the gp41 ectodomain changes is more pronounced when both sets of changes are present, suggesting functional cooperativity between these regions. To evaluate this possibility further, we created KB9 envelope glycoproteins that lacked various combinations of the residue 225 and gp41 ectodomain changes. We also included various combinations involving the change at residue 305, to assess the specificity of any observed effects. Consistent with previous results (Fig. 2), when residue 225 or the gp41 ectodomain sequences were individually reverted to those found in the 89.6 envelope glycoproteins, the syncytium-forming ability of the KB9 envelope glycoproteins was decreased (Fig. 3B). However, in the presence of the gp41 ectodomain changes, the changes in residue 225 exhibited no negative phenotype with respect to syncytium formation [compare KB9(−gp41) and KB9(−225/gp41) in Fig. 3B]. Conversely, in the presence of the change in residue 225, the gp41 ectodomain changes exhibited a beneficial effect on syncytium-forming ability [compare KB9(−225) and KB9(−225/gp41) in Fig. 3B]. These results indicate that the presence of both 89.6 or both KB9 sequences at residue 225 and the gp41 ectodomain represent optimal configurations for fusogenicity.
By contrast, the reversion at position 305 to the 89.6 sequence exerted a negative effect on the ability of the KB9 envelope glycoproteins to induce the formation of syncytia, regardless of the presence of the gp41 ectodomain changes. This is consistent with the results shown in Fig. 3A, in which positive effects on fusogenicity were observed for substitution of the KB9-associated changes at residue 305 irrespective of the sequence changes at residue 225 or the gp41 ectodomain. Thus, the passage-associated changes at residue 305 appear to influence fusogenicity in a manner independent of that mediated by the residue 225 and gp41 ectodomain changes.
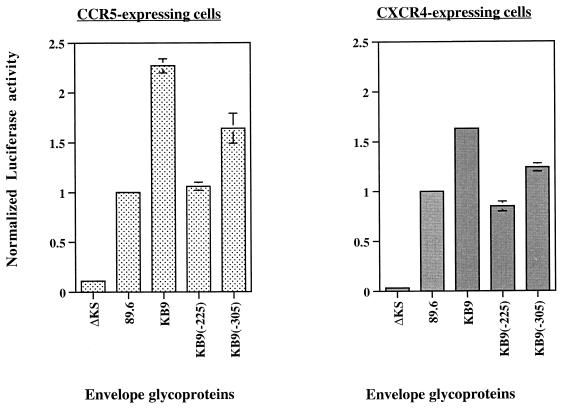
The 89.6 and KB9 envelope glycoproteins both utilize CXCR4 and CCR5 chemokine receptors for entry into CD4+ lymphocytes (30). Because the T-cell lines we used in the syncytium formation assay described above express the CXCR4 chemokine receptor but not CCR5, we tested the importance of the identity of the chemokine receptor in our syncytium formation assay by using target cells that express either chemokine receptor. Figure 4 shows that the pattern of syncytium-forming abilities associated with the various recombinant envelope glycoproteins was independent of the chemokine receptor expressed on the target cells. In these assays, KB9(−305) displayed an intermediate fusogenic phenotype, in contrast to the phenotype seen in the assays described above. This difference may be due to the use of a different cell line as a target and suggests once more that the 225 and 305 residue changes affect different aspects of envelope-glycoprotein-mediated cell-cell fusion.
FIG. 4.
Effect of chemokine receptor usage on syncytium formation. The stable Cf2Th-luciferase cell line used in the assay was made by cotransfection of Cf2Th canine thymocytes with the pLTR-Luc plasmid, which expresses luciferase under the control of the HIV-1 long terminal repeat (61) and a plasmid conferring resistance to Neomycin. Stable transfectants were selected with 0.8 mg of Neomycin per ml. The Cf2Th-luciferase cell line was then transiently cotransfected with a pCEP4 plasmid encoding CD4 and a pcDNA3 plasmid encoding either CCR5 or CXCR4 (8). Approximately 36 h after transfection, the cells were used as targets in the syncytium formation assays by cocultivating them with 293T cells expressing the envelope glycoproteins and Tat. Luciferase activity was quantified by using the Luciferase Assay System (Promega). A defective envelope glycoprotein (ΔKS) was used as a negative control to account for any baseline luciferase activity in the Cf2Th-luciferase cells and for potential effects of Tat secreted from the transfected 293T cells. The background luciferase activity observed with ΔKS was 10- and 50-fold lower with the CCR5 and CXCR4-expressing cells, respectively. Luciferase activity in the lysate was calculated and normalized to that observed for the 89.6 envelope glycoproteins.
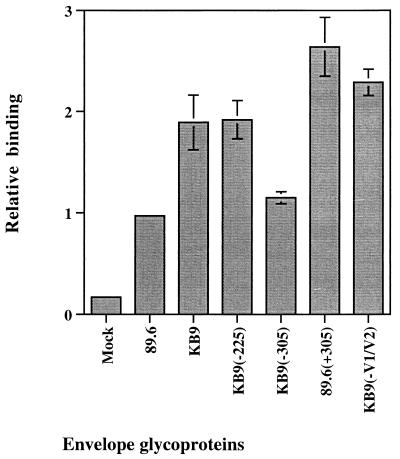
The KB9 gp120 glycoprotein exhibits an increase in affinity for the CCR5 chemokine receptor compared to the 89.6 glycoprotein (30). We examined the potential role of the two single-amino-acid changes in residues 225 and 305 in the enhanced binding of KB9 gp120 to CCR5 expressed at the cell surface. The binding of the KB9(−225) gp120 glycoprotein was indistinguishable from that of KB9 gp120 in our assay, whereas reversion of the 305 residue created a gp120 glycoprotein that displayed an affinity for CCR5 comparable to that of 89.6 gp120 (Fig. 5). Furthermore, the passage-associated substitution of an arginine for a glutamate residue at position 305 in 89.6 gp120 created an envelope glycoprotein that bound CCR5 even more efficiently than the KB9 envelope glycoprotein. These results indicate that the change in residue 305 is sufficient to confer increased affinity for CCR5. Our assay also suggests that the passage-associated changes in the V1/V2 loops exert a mild negative effect on CCR5 binding. The increased CCR5-binding affinity of the KB9(−V1/V2) gp120 glycoprotein, compared with that of the KB9 gp120 glycoprotein, was confirmed by using a range of gp120 concentrations in the binding assay (G. Karlsson and J. Sodroski, unpublished observations). Binding experiments were also attempted by using the CXCR4 coreceptor, but the binding affinities of the 89.6 and KB9 gp120 glycoproteins for this chemokine receptor were too low to detect in our assay (data not shown).
FIG. 5.
CCR5-binding ability of soluble recombinant envelope glycoproteins. Equivalent amounts of recombinant soluble gp120 envelope glycoproteins labelled with [35S]cysteine and [35S]methionine were preincubated with 0.5 μg of sCD4 per ml for 1 h at room temperature. Cf2Th-CCR5 cells (106 cells/well in six-well plates), which stably express human CCR5 (17), were overlayed with the gp120-sCD4 mixture and were incubated at 37°C for 2 h. The cells were subsequently washed with phosphate-buffered saline plus 2% fetal calf serum and were lysed with NP-40 buffer. The cell lysates were then immunoprecipitated with serum from an HIV-1-infected individual, and the amount of precipitated gp120 was determined by densitometry of sodium dodecyl sulfate-polyacrylamide gels. As a control, the binding assay was also performed with the 89.6 gp120 glycoprotein in the absence of sCD4 (Mock). Two independent clones of each recombinant envelope glycoprotein were used in the analysis, and the experiments were carried out at least twice.
SHIV-KB9, which causes rapid CD4+ lymphocyte depletion and AIDS in rhesus macaques, was derived by in vivo passage of the nonpathogenic SHIV-89.6 (29, 47). Subsequent studies identified the passage-associated changes in the HIV-1 envelope glycoprotein ectodomains as determinants of CD4+ T-cell loss in vivo (30). The envelope ectodomain structure of SHIV-KB9 was shown to specify an increase in syncytium-forming ability, chemokine receptor binding, and resistance to neutralizing antibodies. In tissue culture systems, viral cytopathic effects, including syncytium formation, are dependent on the efficiency with which the HIV-1 envelope glycoproteins fuse membranes (4, 34). An understanding of the genetic determinants of the passage-associated changes in SHIV-KB9 envelope glycoprotein function represents a first step in clarifying the relationship between in vivo CD4+ T-cell loss and in vitro properties. Therefore, in this study we dissected the KB9 envelope glycoprotein determinants of increased fusogenicity and chemokine receptor binding.
Syncytium formation requires proteolytic processing of the gp160 envelope precursor, cell-surface expression, stable association of gp120 and gp41 subunits within a trimeric structure, CD4 and chemokine receptor binding, and postbinding membrane fusion events (35). Earlier studies indicated that there were no differences in processing, cell-surface expression, or gp120-gp41 association between the 89.6 and KB9 envelope glycoproteins (30). Furthermore, compared with the SHIV-89.6 envelope glycoproteins, the KB9 envelope glycoproteins exhibited no increase in affinity for soluble CD4 glycoproteins. Thus, the differences in the efficiency with which the 89.6 and KB9 envelope glycoproteins negotiate post-CD4 binding events apparently account for the observed differences in syncytium-forming ability. The location of the changes identified in this study as important for these functional differences is consistent with this assertion. Two sets of envelope glycoprotein changes, which appear to modulate syncytium formation in a mutually independent fashion, were identified. One change involves V3 residue 305, which is altered from a basic residue, arginine, in SHIV-89.6 to an acidic residue, glutamic acid, in SHIV-KB9. This change accounts for the increased CCR5 binding of the KB9 gp120 glycoprotein, which presumably represents the mechanism by which fusogenicity is enhanced. The second set of changes involve residue 225 in the second conserved (C2) region of gp120 and residues 546 and 567 in the gp41 ectodomain. The phenotypic effects of changes in residue 225 were dependent upon the particular sequences found at positions 546 and 567 in the gp41 ectodomain, suggesting functional cooperativity between these regions. The crystal structure of an HIV-1 gp120 core (36) suggests that amino acid residues 225 and 305 lie on opposite surfaces of the glycoprotein. Residue 305 is located in the V3 loop, which projects towards the target cell, whereas residue 225 is buried in the inner gp120 domain, which is proposed to interact with the gp41 ectodomain to maintain the integrity of the assembled trimer (36, 59). The distant location and the distinct environments of residues 225 and 305 are consistent with the different proposed mechanisms by which changes in these amino acids modulate the process of syncytium formation.
The third variable region of the HIV-1 gp120 envelope glycoprotein has been shown to be important for virus entry and syncytium formation. Alterations in the V3 loop can decrease the efficiency of these processes without affecting gp120-CD4 interaction (22, 35). The gp120 V3 loop is an important determinant of chemokine receptor choice; a laboratory-adapted virus that uses CXCR4 as a receptor was converted to an efficient CCR5-using virus simply by substituting the V3 loop of an R5 virus (8). Furthermore, deletions or amino acid substitutions affecting the V3 loop can dramatically alter chemokine receptor binding (7, 51). The basis for the ability of a single residue substitution at position 305, accompanied by a charge reversal, to increase chemokine receptor binding is still uncertain. Three contiguous arginines are located in this region of the 89.6 V3 loop, and the middle arginine is altered in the KB9 envelope glycoproteins. Apparently, the ability of the KB9 envelope glycoproteins to utilize at least the CCR5 and CXCR4 chemokine receptors is enhanced by this alteration. It is noteworthy that the effects of reversion of residue 305 to the 89.6 sequence were less pronounced when the formation of syncytia involved cells expressing high levels of the CXCR4 and CCR5 receptors, compared with the effects observed in cells expressing more typical levels of chemokine receptors. A lowered affinity of the KB9(−305) glycoprotein for chemokine receptors may be partly compensated by high levels of receptor expression, a result consistent with previous data (3, 46).
The gp120 and gp41 envelope glycoproteins are maintained in an assembled trimer by noncovalent interactions between the gp41 ectodomain and discontinuous structures in the gp120 sequence (25). The X-ray crystal structure of the HIV-1 gp120 core (36, 59) revealed that amino acid residue 225 is proximal to the gp120 interface that is thought to contact the gp41 ectodomain in the trimeric structure. Although the fractional solvent accessibility of isoleucine 225 in the gp120 core structure was low, it is possible that, in the assembled trimer, this residue modulates the interaction of gp120 with gp41. In the prefusogenic state of the HIV-1 envelope glycoproteins, interactions among the N-terminal halves of the gp41 ectodomains, which can form trimeric coiled coils (5, 52, 56, 57), are believed to stabilize the assembled oligomer. Residues 564 and 567 are located at positions f and b, respectively, of the heptad repeat and are thus on the exposed outer surface of the coiled coil. This would render these residues potentially available for interactions with gp120. Cooperative interactions between gp120 and gp41 involving sequences at or near positions 225, 564, and 567 may help promote conformational changes following receptor binding that ultimately allow insertion of the gp41 fusion peptide into the membrane of the target cell (5, 57).
Passage-associated changes in the V2 loop of the KB9 gp120 glycoprotein actually exerted a negative effect on syncytium formation in some target cells. Previous studies demonstrated the contribution of these V2 loop changes to the relative resistance of SHIV-KB9 to neutralization by antibodies (15, 16). Thus, two independent selective forces may have influenced the in vivo evolution of the viral envelope glycoproteins: the requirement to evade the neutralizing antibody response and a preference for increased fusogenic activity. The modest loss in fusogenicity associated with the V2 loop changes may be offset by the decreased sensitivity to neutralizing antibodies. The passage-associated changes in the gp120 V1/V2 region were also shown to exert a negative effect on CCR5 binding, suggesting a mechanistic basis for the down-regulation of syncytium-forming ability. Several previous studies have suggested that the V2 loop can mask the chemokine receptor binding site on the gp120 glycoprotein (44, 59, 60), a model supported by X-ray crystallographic analysis of the HIV-1 gp120 core (36, 59). Thus, a modulation of the chemokine receptor binding affinity and envelope glycoprotein fusogenicity by V2 loop alterations has ample precedent.
The identification of the specific determinants of the increased fusogenicity and chemokine receptor binding of the SHIV-KB9 envelope glycoproteins should allow an examination of the contribution of these properties to the in vivo pathogenicity of virulent SHIV.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 3.Brand D, Srinivasan K, Sodroski J. Determinants of human immunodeficiency virus type 1 entry in the CDR2 loop of the CD4 glycoprotein. J Virol. 1995;69:166–171. doi: 10.1128/jvi.69.1.166-171.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao J, Park I W, Cooper A, Sodroski J. Molecular determinants of acute single-cell lysis by human immunodeficiency virus type 1. J Virol. 1996;70:1340–1354. doi: 10.1128/jvi.70.3.1340-1354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 6.Cheng-Mayer C, Seto D, Tateno M, Levy J A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988;240:80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 7.Chesebro B, Wehrly K, Nishio J, Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992;66:6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 9.Clavel F. HIV-2, the West African AIDS virus. AIDS. 1987;1:135–140. [PubMed] [Google Scholar]
- 10.Dalgleish A G, Beverley P C, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 11.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 12.Desrosiers R C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 13.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 14.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 15.Etemad-Moghadam B, Sun Y, Nicholson E K, Karlsson G B, Schenten D, Sodroski J. Determinants of neutralization resistance in the envelope glycoproteins of a simian-human immunodeficiency virus (SHIV) passaged in vivo. J Virol. 1999;73:8873–8879. doi: 10.1128/jvi.73.10.8873-8879.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etemad-Moghadam B, Karlsson G B, Halloran M, Sun Y, Schenten D, Fernandes M, Letvin N L, Sodroski J. Characterization of simian-human immunodeficiency virus envelope glycoprotein epitopes recognized by neutralizing antibodies from infected monkeys. J Virol. 1998;72:8437–8445. doi: 10.1128/jvi.72.10.8437-8445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard N P, Gerard C, Sodroski J, Choe H. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–676. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 18.Fauci A S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- 19.Fauci A S, Macher A M, Longo D L, Lane H C, Rook A H, Masur H, Gelmann E P. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 21.Fenyo E M, Morfeldt-Manson L, Chiodi F, Lind B, von Gegerfelt A, Albert J, Olausson E, Asjo B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988;62:4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freed E O, Myers D J, Risser R. Identification of the principal neutralizing determinant of human immunodeficiency virus type 1 as a fusion domain. J Virol. 1991;65:190–194. doi: 10.1128/jvi.65.1.190-194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 24.Helseth E, Kowalski M, Gabuzda D, Olshevsky U, Haseltine W, Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990;64:2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helseth E, Olshevsky U, Furman C, Sodroski J. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J Virol. 1991;65:2119–2123. doi: 10.1128/jvi.65.4.2119-2123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi T, Shibata R, Hasebe F, Ami Y, Shinohara K, Komatsu T, Stahl-Hennig C, Petry H, Hunsmann G, Kuwata T, et al. Persistent infection with SIVmac chimeric virus having tat, rev, vpu, env and nef of HIV type 1 in macaque monkeys. AIDS Res Hum Retrovir. 1994;10:1021–1029. doi: 10.1089/aid.1994.10.1021. [DOI] [PubMed] [Google Scholar]
- 28.Joag S V, Li Z, Foresman L, Pinson D M, Raghavan R, Zhuge W, Adany I, Wang C, Jia F, Sheffer D, Ranchalis J, Watson A, Narayan O. Characterization of the pathogenic KU-SHIV model of acquired immunodeficiency syndrome in macaques. AIDS Res Hum Retrovir. 1997;13:635–645. doi: 10.1089/aid.1997.13.635. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson G B, Halloran M, Li J, Park I W, Gomila R, Reimann K A, Axthelm M K, Iliff S A, Letvin N L, Sodroski J. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J Virol. 1997;71:4218–4225. doi: 10.1128/jvi.71.6.4218-4225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsson G B, Halloran M, Schenten D, Lee J, Racz P, Tenner-Racz K, Manola J, Gelman R, Etemad-Moghadam B, Desjardins E, Wyatt R, Gerard N P, Marcon L, Margolin D, Fanton J, Axthelm M K, Letvin N L, Sodroski J. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J Exp Med. 1998;188:1159–1171. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koga Y, Sasaki M, Nakamura K, Kimura G, Nomoto K. Intracellular distribution of the envelope glycoprotein of human immunodeficiency virus and its role in the production of cytopathic effect in CD4+ and CD4− human cell lines. J Virol. 1990;64:4661–4671. doi: 10.1128/jvi.64.10.4661-4671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koot M, Vos A H, Keet R P, de Goede R E, Dercksen M W, Terpstra F G, Coutinho R A, Miedema F, Tersmette M. HIV-1 biological phenotype in long-term infected individuals evaluated with an MT-2 cocultivation assay. AIDS. 1992;6:49–54. doi: 10.1097/00002030-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Korber B T, Foley B, Kuiken C, Pillai S, Sodroski J G. Numbering position in HIV relative to HXB2. Human retroviruses and AIDS 1999. A compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.Mex: Los Alamos National Laboratories; 1998. [Google Scholar]
- 34.Kowalski M, Bergeron L, Dorfman T, Haseltine W, Sodroski J. Attenuation of human immunodeficiency virus type 1 cytopathic effect by a mutation affecting the transmembrane envelope glycoprotein. J Virol. 1991;65:281–291. doi: 10.1128/jvi.65.1.281-291.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowalski M, Potz J, Basiripour L, Dorfman T, Goh W C, Terwilliger E, Dayton A, Rosen C, Haseltine W, Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987;237:1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- 36.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard C K, Spellman M W, Riddle L, Harris R J, Thomas J N, Gregory T J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- 38.Letvin N L, Daniel M D, Sehgal P K, Desrosiers R C, Hunt R D, Waldron L M, MacKey J J, Schmidt D K, Chalifoux L V, King N W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 39.Levy J A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Lord C I, Haseltine W, Letvin N L, Sodroski J. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J Acquir Immune Defic Syndr. 1992;5:639–646. [PubMed] [Google Scholar]
- 41.Li J T, Halloran M, Lord C I, Watson A, Ranchalis J, Fung M, Letvin N L, Sodroski J G. Persistent infection of macaques with simian-human immunodeficiency viruses. J Virol. 1995;69:7061–7067. doi: 10.1128/jvi.69.11.7061-7067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lifson J D, Feinberg M B, Reyes G R, Rabin L, Banapour B, Chakrabarti S, Moss B, Wong-Staal F, Steimer K S, Engleman E G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986;323:725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- 43.Luciw P A, Pratt-Lowe E, Shaw K E, Levy J A, Cheng-Mayer C. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV) Proc Natl Acad Sci USA. 1995;92:7490–7494. doi: 10.1073/pnas.92.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore J P, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers G, Berzofsky J, Korber B, Smith R, Pavia G. Human Retroviruses and AIDS. A compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.Mex: Los Alamos National Laboratories; 1992. [Google Scholar]
- 46.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reimann K A, Li J T, Veazey R, Halloran M, Park I-W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D C, Lee-Parritz D E, Lu Y, Collman R G, Sodroski J, Letvin N L. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shibata R, Maldarelli F, Siemon C, Matano T, Parta M, Miller G, Fredrickson T, Martin M A. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J Infect Dis. 1997;176:362–373. doi: 10.1086/514053. [DOI] [PubMed] [Google Scholar]
- 50.Sodroski J, Goh W C, Rosen C, Campbell K, Haseltine W A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature. 1986;322:470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- 51.Speck R F, Wehrly K, Platt E J, Atchison R E, Charo I F, Kabat D, Chesebro B, Goldsmith M A. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J Virol. 1997;71:7136–7139. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan K, Liu J, Wang J, Shen S, Lu M. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad Sci USA. 1997;94:12303–12308. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tersmette M, Gruters R A, de Wolf F, de Goede R E, Lange J M, Schellekens P T, Goudsmit J, Huisman H G, Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989;63:2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tersmette M, Lange J M, de Goede R E, de Wolf F, Eeftink-Schattenkerk J K, Schellekens P T, Coutinho R A, Huisman J G, Goudsmit J, Miedema F. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989;i:983–985. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- 55.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 56.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 57.Weissenhorn W, Wharton S A, Calder L J, Earl P L, Moss B, Aliprandis E, Skehel J J, Wiley D C. The ectodomain of HIV-1 env subunit gp41 forms a soluble, alpha- helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J. 1996;15:1507–1514. [PMC free article] [PubMed] [Google Scholar]
- 58.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardosa A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interactions of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;184:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 59.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 60.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 61.Yang X, Chen Y, Gabuzda D. ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-kappaB. J Biol Chem. 1999;274:27981–27988. doi: 10.1074/jbc.274.39.27981. [DOI] [PubMed] [Google Scholar]