Abstract
Transition metal chalcogenides have been identified as low-cost and efficient electrocatalysts to promote the hydrogen evolution reaction in alkaline media. However, the identification of active sites and the underlying catalytic mechanism remain elusive. In this work, we employ operando X-ray absorption spectroscopy and near-ambient pressure X-ray photoelectron spectroscopy to elucidate that NiS undergoes an in-situ phase transition to an intimately mixed phase of Ni3S2 and NiO, generating highly active synergistic dual sites at the Ni3S2/NiO interface. The interfacial Ni is the active site for water dissociation and OH* adsorption while the interfacial S acts as the active site for H* adsorption and H2 evolution. Accordingly, the in-situ formation of Ni3S2/NiO interfaces enables NiS electrocatalysts to achieve an overpotential of only 95 ± 8 mV at a current density of 10 mA cm−2. Our work highlighted that the chemistry of transition metal chalcogenides is highly dynamic, and a careful control of the working conditions may lead to the in-situ formation of catalytic species that boost their catalytic performance.
Subject terms: Electrocatalysis, Electrocatalysis
Transition metal chalcogenides are effective and economical electrocatalysts for the hydrogen evolution reaction in alkaline media, yet active sites and catalytic mechanisms remain unclear. Here the authors use operando spectroscopy to study the in-situ conversion of NiS to highly active Ni3S2/NiO dual-site catalysts for the alkaline hydrogen evolution reaction.
Introduction
Electrolysis of water powered by renewable electricity to produce green hydrogen is widely considered as a promising pathway to a global clean and sustainable energy future1,2. The key to enable this technology is the development of low-cost and highly efficient electrocatalysts to accelerate the water splitting reactions, i.e., oxygen evolution reaction (OER) and hydrogen evolution reaction (HER)3. In particular, the design of high-performance electrocatalysts for HER in alkaline conditions has received intensive attention, because the alkaline water electrolysis is the most commonly used route in the industry, and the alkaline HER is also a key step in the chlor-alkali process4,5. However, the alkaline HER still suffers from sluggish reaction kinetics compared to that in acidic solution, due to the additional water dissociation step (Volmer step: H2O + M* + e− → M*H + OH−) in order to supply the absorbed H* intermediate for subsequent H2 generation6. The water dissociation has a higher activation barrier and is considered to be the rate determining step that limits the overall reaction6,7. Even the state-of-the-art Pt-based catalysts show about two orders of magnitude lower HER activity in alkaline than in acidic media3,8,9. Significant progress has been made to improve the HER activity of Pt and other noble metals in alkaline environments by constructing dual active site heterostructures, e.g., Pt-Ni(OH)2, in which the Ni(OH)2 functions as a promotor to accelerate the water dissociation10. Improving the activity of noble metal-based catalysts with a single active site has been challenging mainly due to the scaling relationships in the formation energy of intermediates, which affects not only the HER but also the OER and the oxygen reduction reaction (ORR)11,12. Dual active site catalysts provide alternative reaction mechanisms that may break such scaling relationships, offering more chance for achieving better catalytic performance, compared with single active site catalysts12,13.
Nevertheless, the scarcity and high cost of Pt group materials restrict their large-scale application14. In this regard, earth-abundant transition metal (TM)-based chalcogenides, phosphides, nitrides, and carbides have been recently emerging as cost-effective alkaline HER electrocatalysts15–21. Many TM-based catalysts exhibit comparable alkaline HER activity as Pt group materials22. In addition, doping, defect, and strain engineering have been further explored to modulate their electronic structure to enhance the activity and stability23–27. Nickel sulfides such as NiS, NiS2, and Ni3S2 have drawn significant attention because of their high catalytic activity, as well as easy and scalable methodologies for the preparation28–30. Despite this rapid development of alkaline HER catalysts, the underlying reaction mechanism and the active sites for the alkaline HER, which are different from the well-documented acidic HER, are still under considerable debate6,7,31. Furthermore, it has been demonstrated that TM-based compounds catalysts for the OER undergo structural or compositional reconstruction, or even transform into a new phase under OER working condition32–36. On the other hand, the structural and chemical conversion of catalysts under HER conditions has been less explored, and it has been even assumed HER catalysts are stable during the HER process37. However, recent works indicated that some transition metal chalcogenides may indeed undergo a phase transition under alkaline HER working condition38–40. Therefore, real-time monitoring of the catalytic processes and identifying the real active sites under HER operation conditions are of vital importance to uncover the catalytic mechanism.
In this study, we present an in-depth elucidation of the phase conversion process based on operando X-ray absorption spectroscopy (XAS) and operando Raman spectroscopy under HER working conditions. We found that NiS electrodes undergo a significant HER performance enhancement due to an in-situ transformation into a mixed phase of Ni3S2 and NiO, which is identified to be the actual active catalyst phase for alkaline HER. The ex-situ spectroscopy and theoretical investigations further reveal that the interfacial Ni (O-Ni-S) of Ni3S2/NiO provides the active sites to accelerate water dissociation while the interfacial S (O-Ni-S) facilitates the adsorption of hydrogen intermediates and its subsequent association to form molecular hydrogen. As a result, the in-situ formed Ni3S2/NiO exhibits a superior HER activity, requiring a low overpotential of 95 ± 8 mV to reach a current density of 10 mA cm−2 in our performance tests.
Results and discussion
Phase transition of NiS to Ni3S2/NiO induces enhanced HER activity
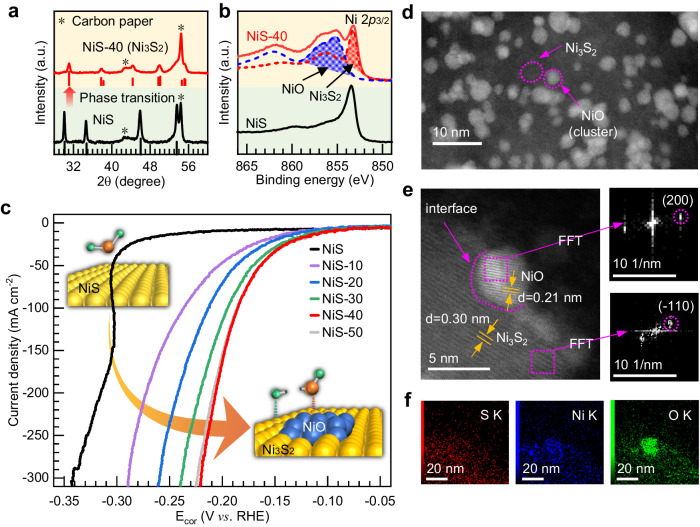
The NiS samples were synthesized on carbon paper using one-step hydrothermal method (Supplementary Fig. 1a). Details of the synthesis and characterization are provided in the Methods section. X-ray diffraction (XRD) pattern shown in Fig. 1a and X-ray photoelectron spectroscopy (XPS) of Ni 2p core level in Fig. 1b suggests that the as-synthesized samples are characteristic of the hexagonal α-phase of NiS28. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images in Supplementary Fig. 1 show that the samples consist of NiS particles with a size of ~200 nm uniformly coating on the carbon paper substrate.
Fig. 1. HER performance and structural characterizations of NiS catalyst before and after measurement.
a XRD patterns of the as-synthesized NiS and NiS after HER measurement (marked as NiS-40). b Ni 2p XPS spectra for NiS and NiS after HER measurement (marked as NiS-40), along with NiO and Ni3S2 as reference. c iR-corrected Linear sweep voltammetry (LSV) polarization curves of as-synthesized NiS, NiS-10, NiS-20, NiS-30, NiS-40, and NiS-50 samples measured in 1 M KOH solution. NiS-10, NiS-20, NiS-30, NiS-40, and NiS-50 are NiS samples after 10, 20, 30, 40, and 50 minute chronoamperometry measurements at −1.00 V vs. RHE, respectively. d The large-area TEM image, e high-resolution TEM image (the right panel shows the FFT images of both regions) and f the corresponding elemental mapping of the NiS-40 sample after conducting 25-h chronopotentiometric measurement at a current density of 200 mA cm−2.
The HER activity was evaluated in 1 M KOH using a three-electrode system (details in the Methods section). Cyclic voltammetry (CV) measurements were carried out in order to adjust the conditions for the evaluation of the HER catalytic activity (Supplementary Fig. 2a, b). In the first cycle of as-synthesized samples, there is a sharp onset of the current density for the HER at around −0.30 V vs. RHE, which continues to increase steadily as the sample is further polarized to negative potentials. After that sharp onset of the current density, the CV profile is reproducible in the second cycle, with an upper limit of the potential at 0.05 V vs. RHE. However, as the upper limit of the potential is extended to 0.40 V vs. RHE in the 3rd and 4th cycles, there is an oxidation wave with a peak at +0.27 V vs. RHE and a 15% increase of the current density when the sample is polarized back to negative potentials. Remarkably, the current density keeps on increasing as the sample is subject to chronoamperometry at −1.00 V vs. RHE (Supplementary Fig. 2c). Linear sweep voltammetry (LSV) of the sample after the chronoamperometry measurement shows that the current density increases 25% compared with the pristine sample, and there is also a clear shift of the onset to lower overpotential (Supplementary Fig. 2d). These electrochemical studies clearly demonstrate that in-situ modifications of NiS electrodes lead to a significantly enhanced HER catalytic activity.
On these bases, we carried out a more detailed study of the chronoamperometric conditions of NiS electrodes on their HER catalytic activity. Figure 1c shows the LSV curves of the as-synthesized NiS, as well as NiS samples after 10, 20, 30, 40, and 50 minute chronoamperometry measurements at −1.00 V vs. RHE, labeled as NiS-10, NiS-20, NiS-30, NiS-40, and NiS-50, respectively. In order to avoid deviations caused by ohmic drops, the potential was adjusted by an iR factor (Supplementary Fig. 3a, b). The as-synthesized NiS initially exhibits a low HER activity, but the HER activity gradually increases with the chronoamperometry measurement time until it is conducted for 40 minutes. A similar trend of HER activity is also observed when the current density is normalized by electrochemical active surface area (ECSA) (Supplementary Figs. 4 and 5). As shown in Fig. 1c, the NiS-40 sample exhibits a high HER catalytic activity, requiring an overpotential of 95 ± 8 mV to reach a current density of 10 mA cm−2 (acquisition of error bar can be seen in Supplementary Fig. 3c.). Further electrochemical characterizations showed that the Tafel slope and charge transfer resistance (Supplementary Fig. 6a, b) decrease in the order of NiS > NiS-10 > NiS-20 > NiS-30 > NiS-50 > NiS-40, indicating that the performance enhancement after the chronoamperometric treatments results from an intrinsic faster HER kinetics. Compared with previously reported HER catalysts, electrochemically treated NiS electrocatalysts exhibit competitive HER activity in terms of overpotentials at 10 mA cm−2 and Tafel slope (Supplementary Fig. 7 and Supplementary Table 1). Additionally, the catalytic activity of the NiS-40 sample is very stable, showing no overpotential increase over 25 h of continuous operation at a current density of 200 mA cm−2 in 1 M KOH (Supplementary Fig. 6c).
A series of ex-situ characterizations of the highly active samples after performance tests were conducted. The as-synthesized NiS is converted into a mixed phase of Ni3S2 and NiO after HER measurement. Figure 1a shows an XRD pattern of the NiS-40 sample, from which a pattern characteristic of Ni3S2 can be identified, suggesting a full-phase conversion of NiS to crystalline Ni3S241,42. The XPS in the Ni 2p3/2 region for the NiS-40 sample exhibits a pronounced spectral feature possibly arising from NiO (Fig. 1b). Additionally, the Ni K-edge XAS of sample NiS-40 (Supplementary Fig. 8) can be fitted by a linear combination of spectra of Ni3S2 and NiO, which further confirms the formation of Ni3S2 and NiO. We further characterized the NiS-40 sample after 25-h chronopotentiometric measurement at a current density of 200 mA cm-2, the XRD, XPS and XAS confirm the HER-post catalysts are present in the form of Ni3S2 and NiO (Supplementary Fig. 9). Spherical Aberration Corrected Transmission Electron Microscope (SAC-TEM) was used to further examine the microstructure of the HER-post catalysts. As shown in Fig. 1d, the large-area TEM image reveals numerous small NiO clusters anchored onto the large Ni3S2. The HR-TEM image exhibits a distinct interface that partitions the image into two regions (Fig. 1e). By analyzing the FFT images corresponding to these two regions, the cluster region has an interplanar spacing of 0.21 nm corresponding well to the (200) lattice plane of NiO, while the other region has an interplanar spacing of 0.30 nm which matches well with (–110) lattice plane of Ni3S2. More importantly, the elemental mapping shows the preferential enrichment of oxygen elements on the small cluster (Fig. 1f), which strongly reveals the small cluster is assigned to NiO. It should be noted that the formed NiO may exist in the form of a very small cluster, which is hard to determine by XRD patterns. To investigate the influence of the ratio of Ni3S2 to NiO on the catalytic activity, NiS samples received by 1, 5, 10, and 50 LSVs in the range from 0.00 V to –1.00 V vs. RHE were prepared. The Ni K-edge XAS of these samples, shown in Supplementary Figs. 10a–d, consists of a linear combination of spectra of Ni3S2 and NiO, which indicates that a full transformation of NiS occurs after a single LSV. However, the NiO to Ni3S2 ratio increases upon increasing the number of LSVs. We extracted the NiO to Ni3S2 ratio based on the XAS spectra (Supplementary Figs. 10a–d and Supplementary Table 2) and found that the HER activity increases with the amount of NiO (Supplementary Fig. 10e), which clearly indicates the beneficial effect of the Ni oxide species on the HER process.
Phase transition dynamics revealed by operando XAS and operando Raman spectroscopy
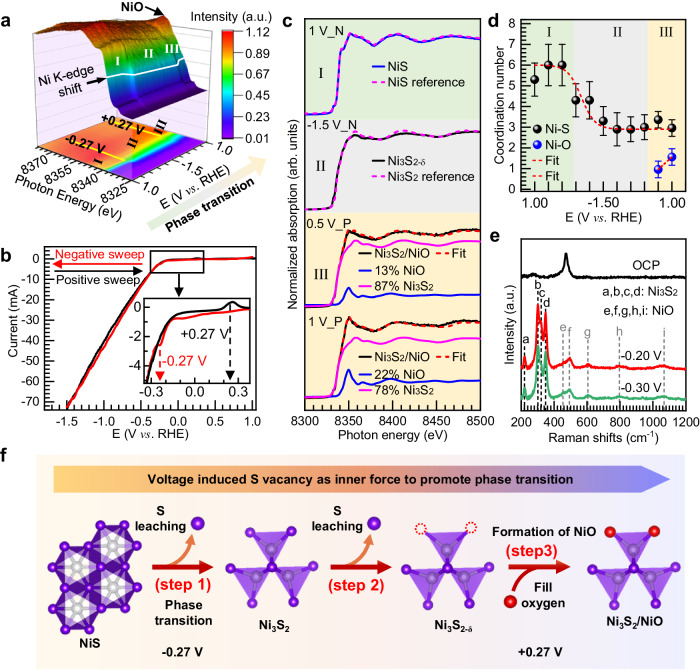
An operando XAS spectroscopy was employed to gain detailed insights into the NiS→Ni3S2/NiO transition process, and details of the experimental method are provided in the Methods section. Figure 2a shows a three-dimensional (3D) plot of the Ni K-edge XAS of the NiS electrode as a function of the applied potentials during cyclic CV measurement (Fig. 2b). Note that two important changes in the spectral profile occur at applied voltages of −0.27 V and +0.27 V vs. RHE, dividing the 3D plot into three regions as I (applied voltage from +1.00 V to −0.27 V), II (−0.27 V to −1.50 V and back to +0.27 V) and III (from +0.27 V to 1.00 V). Selected Ni K-edge XAS spectra at specific applied voltages within regions I, II and III are shown in Fig. 2c. In region I, with applied voltage from +1.00 V to −0.27 V, the spectrum of the sample remains as that of the NiS phase. The transition from region I to region II at −0.27 V can be identified as the phase transition NiS→Ni3S2, based on spectral fitting and comparisons with reference samples of NiS and Ni3S2 (region I and II in Fig. 2c). The associated reduction of the formal oxidation state of Ni2+ in NiS to Ni1.33+ in Ni3S2 can be observed as a cathodic wave peak in the CV profile just at the onset of the HER current shown in Fig. 2b (see the inset), clearly showing that the HER catalytic activity is associated with the formation of Ni3S2. After the phase transition, the Ni3S2 phase is stable under HER conditions. However, with a more negative voltage applied (region II in Fig. 2a), a continuous decrease of the spectral intensity of the white line of the Ni K-edge XAS spectra suggests a further reduction of the oxidation state of Ni. Consistently, the fitting of Fourier Transform moduli of spectra in region II revealed a decrease of the average coordination number of Ni coordinated by S (Fig. 2d, Supplementary Figs. 11 and 12, and Supplementary Tables 3 and 4, and the simulations procedure in Supplementary Note 1), suggesting the formation of S vacancies, i.e., Ni3S2−δ. As the sample is polarized from negative back to positive potentials, the transition from II to III at +0.27 V can be associated with an oxidation process observed in the CV profile shown in Fig. 2b, consistent with a constant increase of the white line of the spectrum as the sample is further polarized (region III in Fig. 2a). The spectrum can be fitted with a linear combination of Ni3S2 and an increasing contribution of NiO (region III in Fig. 2c), and details of the fitting are discussed in Supplementary Notes 2–5. Based on this analysis, we estimate that the NiO contribution at +0.50 V is about 13%, which further increases to 22% when the sample potential reaches +1.00 V (Fig. 2c and Supplementary Table 5). Thus, the coordination of Ni by O occurs gradually in region III (Fig. 2d), probably caused by the filling of the sulfur vacancies in Ni3S2−δ with water or OH− ions, leading to an intimately mixed phase of Ni3S2 and NiO, which is further verified by the Wavelet transform (WT) analysis of extended X-ray absorption fine structure (EXAFS) and Fourier-transformed k3-weighted EXAFS at applied voltage of +1.00 V in region III (Supplementary Fig. 13). The corresponding results of fitting at +1.00 V were shown in Supplementary Table 4, and the Ni-O show a bond length of ∼2.00 Å. Highly crystalline NiO has a characteristic Ni-O distances around 2.09 Å; however, the short-range order in NiO reveals that the Ni-O distances for amorphous and/or nanocrystalline NiO may decrease to 2.00–2.06 Å43,44. This result further confirms the formed NiO is in the form of a very small cluster.
Fig. 2. Operando characterizations and the mechanism of phase transition.
a Operando Ni K-edge XAS for NiS catalyst as a function of applied voltages. Top panel shows a 3-dimensional view of Ni K-edge XAS spectra; bottom shows a 2-dimensional projection from a 3-dimensional view. b The corresponding CV profile used for the operando XAS measurement. The inset shows the magnified region marked in the rectangular box. c The Ni K-edge XAS spectra at specific applied voltages of +1.00 V (1 V_N means +1.00 V in negative sweep of CV), −1.50 V (−1.50 V_N means −1.50 V in negative sweep of CV), and +0.50 V, +1.00 V (+0.50 V_P and +1.00 V_P mean +0.50 V and +1.00 V in positive sweep of CV) in region I, II, and III respectively, extracted from Fig. 2a, and their linear combination of Ni3S2 and NiO. d The coordination number of Ni as a function of applied voltages. e Operando Raman spectroscopy of NiS at different applied voltages. f Schematic illustration for phase transition of NiS catalyst during HER measurement.
The formation of mixed-phase of Ni3S2 and NiO was further verified by an operando Raman spectroscopy study (the details of the experiment in the Methods section). The top Raman spectrum in Fig. 2e can be assigned to pristine NiS, with characteristic bands appearing at Raman shifts 281 cm−1 and 473 cm−1. As the sample was polarized to negative potentials, the characteristic peaks assigned to NiS disappear, while the characteristic peaks assigned to Ni3S2 appear at 221, 303, 322, and 349 cm−1 (marked as a, b, c, and d, respectively), indicating the complete phase transition from NiS to Ni3S245. In addition, the newly emerged peaks e, f, g, h, and i located at 453, 493, 600, 800, and 1060 cm−1 can be assigned to Ni-O46–49, suggesting the formation of NiO under HER conditions. Raman spectra features associated with NiS, Ni3S2, and NiO are very well resolved so that even small trace concentrations of NiO species can be detected.
Based on the analysis of the operando XAS and operando Raman spectroscopy data, we propose a three-step mechanism for the phase transition of NiS→Ni3S2/NiO under alkaline HER conditions, as schematically shown in Fig. 2f 50. The electrochemical reduction of NiS to Ni3S2 at −0.27 V vs. RHE can be driven by the thermodynamic instability of NiS under HER conditions (step 1). As more negative potentials are set to reach higher HER current densities, S leaching leads to the formation of S vacancies in Ni3S2−δ (step 2). As the sample is polarized to more positive potentials, substantial oxidation occurs at +0.27 V vs. RHE, and S vacancies are occupied by the oxygen species from the media (water/OH− ions), leading to the formation of an intimately mixed phase of Ni3S2/NiO (step 3).
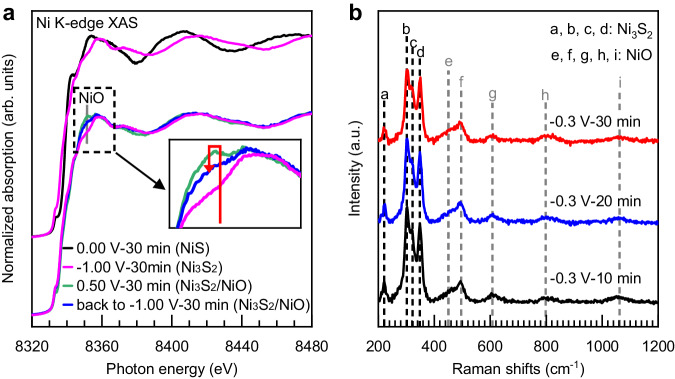
The stability of NiS, Ni3S2, and NiO under different conditions relevant to the HER catalytic performance was further investigated by operando XAS spectroscopy. Figure 3a shows operando XAS at Ni K-edge of the NiS electrode in chronoamperometry for 30 minutes at consecutively applied potentials: 0.00 V, −1.00 V, 0.50 V, and back to −1.00 V (all potentials are vs. RHE). As expected, the sample retains its pristine NiS structure at 0.00 V and converts to Ni3S2 when polarized to −1.00 V (Fig. 3a), due to the electrochemical reduction at −0.27 V as discussed above. Then, as the sample is polarized to +0.50 V, the white line of 8350 eV assigned to Ni-O increases noticeably (the inset in Fig. 3a) and the spectrum can be fitted with a linear combination of 90% of Ni3S2 features and 10% of NiO spectral features (Supplementary Fig. 14a and Supplementary Table 6), due to the bulk oxidation that occurs at +0.27 V as previously discussed. When the sample is brought back to HER conditions at −1.00 V, the content of NiO seems to decrease but still exist (the inset in Fig. 3a) as the spectrum can be fitted with a linear combination of 94% of Ni3S2 spectral features and 6% of NiO spectral features after 30 minutes under the reaction (Supplementary Fig. 14b and Supplementary Table 6). In addition, Fig. 3b shows operando Raman spectra of a NiS electrode in chronoamperometry at −0.30 V vs. RHE for 30 minutes, further confirming the existence of NiO under the HER process. In order to demonstrate the long-term stability of the Ni3S2/NiO mixed phase, we extended the operando Raman experiment at an applied potential of −0.30 V vs. RHE to 25 h. The results demonstrate Ni3S2 and NiO remain stable under HER conditions over an extended period of time (Supplementary Fig. 15).
Fig. 3. The long-time existence of reconstructed Ni3S2/NiO proven by operando characterizations.
a Operando XAS at Ni K-edge of a NiS electrode in chronoamperometry for 30 minutes at 4 consecutively applied potentials: 0.00 V, −1.00 V, 0.50 V, and back in −1.00 V. b Operando Raman spectra of NiS electrode in chronoamperometry for 30 minutes at a constant applied potential of −0.30 V. (All potentials vs. RHE).
Insight into the HER mechanism
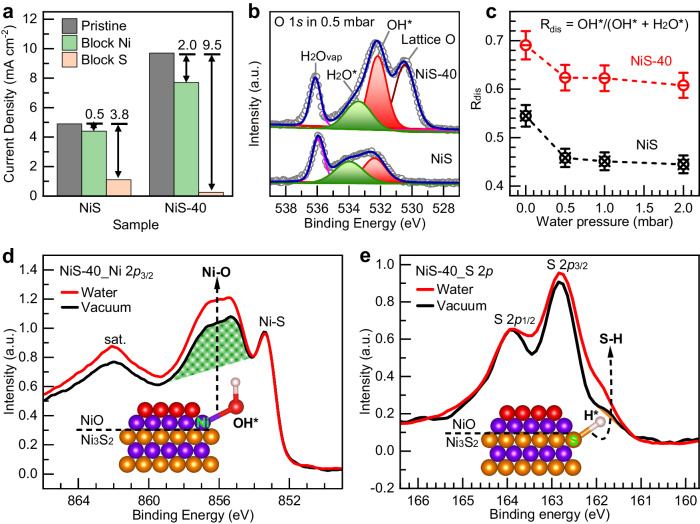
The HER in alkaline media mainly involves the step for water dissociation to form H* and OH*, i.e., Volmer step, followed by the coupling of H* to form H2 in the Heyrovsky and Tafel steps. The Volmer step has been shown to be the rate-determining step for alkaline HER6,7. Therefore, to identify the active sites formed after the electrochemical treatment of the samples, we carried out a study for the dissociative adsorption of water on NiS and NiS-40 (Ni3S2/NiO) catalysts by means of near-ambient-pressure (NAP)-XPS (experimental details in the Methods section). NAP-XPS is a commonly used spectroscopic technique to study the dissociative adsorption of water on catalysts in a humid environment51,52. Figure 4b shows the XPS spectra of NiS and NiS-40 (Ni3S2/NiO) in the O 1s region in the presence of 0.5 mbar water vapor. Oxygen related species including lattice oxygen in NiO (lattice O), adsorbed OH*, adsorbed H2O* and vapor H2O (H2Ovap) can be identified, and their relative amounts can be quantified by fitting the peak area (Supplementary Fig. 16 and Supplementary Table 7). In particular, the adsorbed OH* originates from the dissociative H2O adsorption, and therefore can be used as a measure of the degree of water dissociation on the catalyst surface, i.e., the higher the value, the better the ability to dissociate water. As shown in Fig. 4c, the ratio (Rdis) of OH* to (H2O* + OH*) in NiS-40 (Ni3S2/NiO) is much higher than that in NiS in the presence of different water vapor pressure, suggesting a stronger ability of dissociative adsorption of water on the NiS-40 (Ni3S2/NiO). In addition, the surface chemistry of the catalyst was further investigated by monitoring changes in the Ni 2p and S 2p core levels upon water exposure. For the NiS-40 (Ni3S2/NiO) sample, water exposure induces a pronounced change in the Ni 2p3/2 and S 2p. As shown in Fig. 4d, compared with the Ni 2p3/2 spectrum in vacuum, the spectral intensity associated with Ni-O increases significantly upon water exposure, which may result from the preferential adsorption of OH* at Ni sites. On the other hand, for S 2p shown in Fig. 4e, after water exposure, a shoulder feature appears at the lower binding energy side, which may result from the reduction of sulfur ions when H* adsorbs at S sites in Ni3S2. In contrast, for NiS (Supplementary Fig. 17), there is no obvious change in Ni 2p and S 2p after water exposure, which suggests that there is much less OH* and H* intermediates generated on NiS surfaces, in accordance with the weak water dissociation ability of NiS observed for this sample.
Fig. 4. Identification of the active sites by poisoning and NAP-XPS experiments.
a The current density of NiS and NiS-40 (NiO/Ni3S2) at a constant applied potential of −0.15 V vs. RHE before and after blocking the Ni sites and S sites. b O 1s XPS spectra and their peak fitting for NiS and NiS-40 at 0.5 mbar water pressure. c The ratio of OH* to H2O* + OH* (Rdis) as a function of different water pressure (0.0 mbar, 0.5 mbar, 1.0 mbar and 2.0 mbar). d The Ni 2p3/2 XPS spectra of NiS-40 in vacuum and 0.5 mbar water vapor. e The S 2p XPS spectra of NiS-40 in vacuum and 0.5 mbar water vapor.
Considering the involvement of Ni and S sites in the water dissociation process, the influence of each site on the alkaline HER was investigated by a systematic poison experiment (experimental details in the Methods section). Specifically, we used thiocyanate ions (SCN−) and dodecanethiol to selectively block the Ni and S sites, respectively53–55. Figure 4a shows the change in current density at −0.15 V vs. RHE before and after blocking Ni and S sites in as-synthesized NiS and NiS-40 (Ni3S2/NiO). The change in current density is a measure of the activity of a specific site; essentially, the greater the change after blocking a site, the higher the catalytic activity of the site in the non-poisoned state. For as-synthesized NiS, there is only a 0.5 mA cm–2 decrease in the HER current density after blocking Ni sites, while 3.8 mA cm–2 after blocking S sites; suggesting that S sites are the active sites. On the other hand, for the NiS-40 (Ni3S2/NiO) sample, it can be seen that the change is 2.0 mA cm–2 after blocking Ni sites, and 9.5 mA cm–2 after blocking S sites. This result indicates that both the Ni and S sites are the active sites in Ni3S2/NiO.
The above experiments indicate the synergistic effect of Ni3S2 and NiO in the transformed phase leading to the enhanced HER activity, in which the interfacial Ni sites play a crucial role in accelerating the rate determining step of water dissociation and producing H* intermediates while the interfacial S facilitates H* adsorption to generate H2.
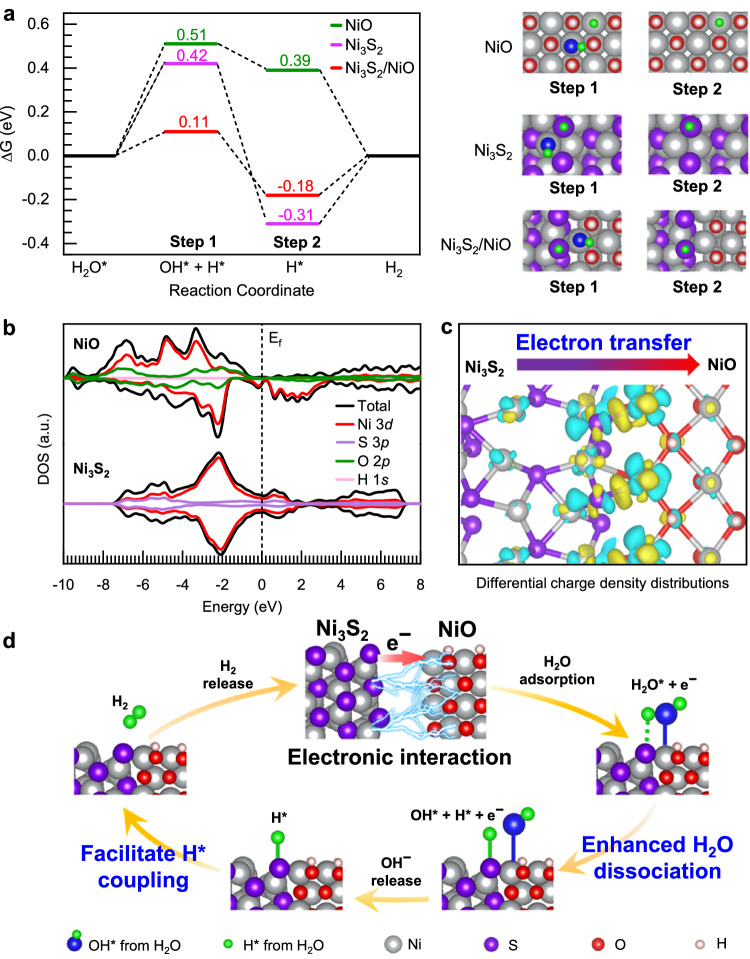
In order to support our experimental mechanistic studies for the enhanced HER activity after the formation of the mixed phase of Ni3S2/NiO, we performed theoretical calculations based on density functional theory (DFT) (computational method in the Methods section). In our model, we employ a heterostructure of Ni3S2 and NiO with O sites covered by H (marked as Ni3S2/NiO). We also construct models for individual Ni3S2 and NiO for comparison. Schematics and details of the models are shown in Supplementary Fig. 18, and the atomic coordinates of the optimized models are supplied in Supplementary Data 1. We used crystalline NiO with an FCC crystal structure in our model because our thermodynamic analyses suggest that the stable state of NiO is crystalline (see Supplementary Fig. 19, and corresponding atomic coordinates are supplied in Supplementary Data 1). The Ni3S2/NiO model with O sites covered by H can be rationalized because the adsorption of H at the unsaturated oxygen sites of NiO is spontaneous and very strong, as indicated by the large values of ΔGH* of up to –0.86 eV shown in Supplementary Fig. 20. We carried out our DFT mechanistic study based on comparisons of these three modes, drawing conclusion from the trends we found rather than from individual values.
Using these models, we calculated the Gibbs energy change toward HER for the Ni3S2/NiO heterostructure and the corresponding individual phases, and the results are graphically shown in Fig. 5a. For individual Ni3S2, the energy barrier for the water dissociation step is high (0.42 eV), which is mainly due to the weak adsorption of OH* on Ni sites (Supplementary Fig. 21). Similarly, the water dissociation energy for individual NiO catalyst is as high as 0.51 eV, due to the weak bonding strength between Ni and adsorption H*. On the other hand, for the Ni3S2/NiO heterostructure, by taking complementary advantages of stronger adsorption of OH* at Ni sites at the NiO side and stronger adsorption of H* at S sites at the Ni3S2 side, the energy barrier for water dissociation can be dramatically reduced to 0.11 eV. Furthermore, Ni3S2/NiO heterostructures also facilitate the coupling of H* to produce H2. The adsorption energy of H* on S sites at the Ni3S2/NiO heterostructure is −0.18 eV, which is closer to the optimal value (0 eV) for coupling of H* to form H2, compared with the corresponding value (−0.31 eV) for individual Ni3S2 (Fig. 5a). Interestingly, our DFT results reveals that a strong electronic interaction between Ni3S2 and NiO at the interface provide favorable energetics for coupling of H* to form H2. Figure 5b shows the density of states (DOS) of NiO and Ni3S2. NiO has low DOS at the Fermi level, and Ni3S2 has a metallic state, showing a high density of occupied electrons around the Fermi level. Therefore, electrons can easily transfer from Ni3S2 to the empty state of NiO. As shown in Fig. 5c, the charge differential analysis for the Ni3S2/NiO hetero-interface clearly reveals that electrons transfer from S sites of the Ni3S2 side to the NiO side. The depletion of electrons at S sites of the Ni3S2 side can tune the electronic states of S atoms further weaken the adsorption energy of H* from –0.31 eV to –0.18 eV, which provides favorable energetics for coupling of H* to form H2. Therefore, as shown in the schematic model in Fig. 5d, our experimental and theoretical mechanistic study results clearly reveal that the in-situ formed Ni3S2/NiO hetero-interface provides dual active sites to promote water dissociation, and the strong electronic interaction between Ni3S2 and NiO interface also modifies the electronic state of interfacial S sites and thus provide optimized energetics for coupling of H* to form H2.
Fig. 5. Theoretical investigations on the HER mechanism.
a Gibbs free energy diagrams of alkaline HER pathway at Ni-Ni sites of NiO, Ni-S sites of Ni3S2 and the interfacial Ni-S sites of Ni3S2/NiO. b The calculated total and partial density of states of NiO and Ni3S2. c Differential charge density distributions of Ni3S2/NiO. Yellow contours represent electron accumulation and cyan contours represent electron depletion. d The schematic diagram for HER mechanism of Ni3S2/NiO.
In summary, an operando XAS and operando Raman were employed to investigate the dynamic restructuring of NiS under alkaline HER conditions. It was found that NiS was electrochemically reduced to Ni3S2, which subsequently oxidized with water and OH– ions from the electrolyte, leading to the formation of a highly active Ni3S2/NiO hetero-interface. The combination of operando spectroscopy and theoretical investigations further unveiled that the Ni3S2/NiO hetero-interface would function as the actual active sites, i.e., the interfacial Ni sites provide the active sites to accelerate water dissociation while the interfacial S sites facilitate the adsorption of H* intermediates and its subsequent association to form H2. Our work shows that the chemistry of transition metal chalcogenides is highly dynamic, and careful control of the working conditions may lead to the in-situ formation of catalytic species that boot their catalytic performance.
Methods
Synthesis of NiS samples
Carbon paper was used as the substrate for NiS. Carbon paper was consecutively washed with HNO3 (1 M) and deionized water under sonication for 15 minutes in each solution to thoroughly remove impurities and improve hydrophilicity. 0.2448 g nickel acetate (99 wt%, Alfa Aesar) and 0.2300 g thioacetamide (99 wt%, Alfa Aesar) were dissolved in 35 mL deionized water. After stirring, the mixture was poured into a 50 ml autoclave with a piece of carbon paper (0.5 × 1.5 cm²). The growth was carried out at 120 °C in an electric oven for 12 h. After the autoclave cooled down naturally to room temperature, the samples were removed, washed with deionized water, and then dried in a vacuum oven at room temperature.
Preparation of electrolyte and electrode
1 M KOH can be obtained by dissolving 32.0058 g of KOH (85 wt%, Sinopharm Chemical Reagent) in 500 ml of deionized water. Store it in a plastic bottle. We usually prepare it freshly as needed. The pH value was 14.00 ± 0.03 measured by pH meter (details in the Supplementary Table 8 and Supplementary Fig. 22). As shown in Supplementary Fig. 1a, a portion of the carbon paper with 0.5 × 1.0 cm2 of geometric dimensions is covered by the NiS sample, while another portion with 0.5 × 0.5 cm2 of geometric dimensions is left bare for connection to the glassy carbon electrode. Clamping the exposed portion of the carbon paper with the glassy carbon electrode forms the working electrode.
Material characterizations
The crystal structure was analyzed using X-ray diffraction (XRD) employing Cu Kα radiation. Surface morphology was assessed using a ZEISS Sigma field emission scanning electron microscope (SEM). For analysis with transmission electron microscopy (TEM) and spherical aberration-corrected transmission electron microscopy (SAC-TEM), samples were suspended in absolute ethanol and subsequently deposited onto a Cu grid. High-resolution X-ray photoelectron spectroscopy (XPS) measurements were conducted using a monochromatic Al Kα1 X-ray source (hν = 1486.6 eV) equipped with a SPECS PHOIBOS 150 electron energy analyzer, achieving a total energy resolution of 0.50 eV. The binding energy was calibrated using the C 1s peak34.
Electrochemical measurements
All electrochemical measurements were carried out using a three-electrode configuration controlled by a CHI 750E electrochemical workstation, consisting of an as-prepared sample on carbon paper as working electrode, Hg/HgO (1 M KOH) as the reference electrode and graphite rode as the counter electrode (Supplementary Fig. 23). The reference electrode was calibrated by comparing it with the standard Hg/HgO (1 M KOH) purchased from Tianjin Aida Co., LTD. The potentials reported in this work were normalized versus the RHE using ERHE = EHg/HgO + 0.059 pH + 0.098 V. We conducted all HER measurements in 1 M KOH electrolyte. Prior to measurements, all fresh electrolytes were bubbled with pure nitrogen for 30 minutes. Linear sweep voltammetry (LSV) was employed to obtained polarization curves by sweeping the potential from 0.00 V to –1.00 V vs. RHE at a scan rate of 5 mV s–1. The electrochemical impedance spectroscopy (EIS) was performed in the same configuration at –0.20 V vs. RHE applied potential over frequency range from 100 kHz to 0.1 Hz at the amplitude of the sinusoidal voltage of 5 mV. To correct the ohmic drop, the measured potentials were calibrated using equation Ecor = E - iR, where Eor was corrected potentials, E was measured potential, i was current and R was the contact resistance determined either through iR measurement or by analyzing EIS curves. In this work, 85% iR was conducted. The polarization curves were replotted as overpotential (η) versus log current (log j) to get Tafel plots for assessing the HER kinetics of investigated catalysts. Capacitance measurements in the potential region of no faradaic process at different scan rates of 20, 40, 60, 80, 100, and 120 mV s–1 can be used to determine electrochemical active surface area (ECSA).
Operando electrochemical X-ray absorption experiment
Operando XAS measurements were carried out on NiS samples grown on carbon paper as the working electrode at the CLAESS beamline of the ALBA synchrotron in Spain, using a specially designed in-house electrochemical cell setup (see Supplementary Fig. 24). A platinum wire served as the counter electrode, and an Ag/AgCl (3 M KCl) electrode was used as the reference electrode in a 1 M KOH solution. These electrochemical experiments were done in a computer-controlled electrochemical workstation. Spectra at the Ni K-edge were continuously collected as the samples were subject to cyclic voltammetry (CV) from the open circuit potential to –1.50 V versus RHE and scan rate of 0.5 mV s–1. Spectra acquisition was performed using a Si (311) monochromator that offered an incident energy resolution of 0.3 eV. Appropriate angle and coating for the collimating and focusing mirrors were selected to ensure harmonic rejection. The measurements were done in fluorescence mode, and both NiO pellets and Ni foils were employed for energy calibration. XAS data processing was conducted using the ATHENA software package56.
Operando Raman experiment
Raman spectra were acquired using a Jobin-Yvon Horiba Xplora confocal Raman system. All experiments were done with an excitation wavelength of 638 nm and a ×50 microscope objective with a numerical aperture of 0.55. Laser power was maintained at ~1.5 mW. Operando experiments took place in a K006 Raman cell (see Supplementary Fig. 25), and Raman spectra were recorded at various applied potentials.
Near-ambient-pressure photoemission spectra experiment
Near-ambient-pressure photoemission spectra (NAP-XPS) were recorded on a lab-based spectrometer (SPECS GmbH, Berlin) using a monochromated Al Kα source (hν = 1486.6 eV) operating at 50 W. The analyzer is a SPECS PHOIBOS 150 NAP, a 180° hemispherical energy analyzer with a 150 mm mean radius. The entrance to the analyzer is a nozzle with a 300 μm diameter orifice. The analyzer is run in fixed analyzer transmission (FAT) mode. The pass energy was set to 40 eV for survey scans and 20 eV for high-resolution regions. Water vapor dosing was carried out via a leak valve, Ni 2p, S 2p, and O 1s NAP-XPS at all pressures were recorded after at least 15 minutes of pressure stabilization.
Poisoning experiment
In order to explore what is the active site during the HER process, the influence of thiocyanate ion (SCN–) and dodecanethiol on the HER activity of investigated catalysts was evaluated by adding 10 mM SCN– and 10 mM dodecanethiol in the electrolyte respectively, where SCN– and dodecanethiol are known to poison the Ni sites and S sites, respectively53–55.
Density functional theory calculations
For the density functional theory calculations (DFT), Perdew-Burke-Ernzenhof (PBE) method is used to obtain exchange correlation functional57, and projector-augmented wave (PAW) pseudopotentials are employed58,59. Hubbard-U and DFT-D3 (B-J) are also introduced in the calculations to correctthe electron correlation. The whole DFT calculations are implemented in the Vienna Ab initio Simulation Package (VASP) code60,61. The value of U-J term was set as 4.0 eV for Ni cations62,63. A Γ-centered Monkhorst-Pack grid with a 2 × 2 × 1 k-point mesh was used. The wave functions of valence electrons were expanded using a plane wave basis set with a cut-off energy of 500 eV. The convergence criterion was set as 10−6 eV between for electronic optimization. For the structural optimization loops, the maximum force on each atom was less than −0.01 eV/Å64. All calculations were spin-polarized.
Gibbs free energy calculations
The steps involved in HER in alkaline media are listed in Eqs. (1)–(3), as shown below:
| 1 |
| 2 |
| 3 |
Here, we applied a method developed previously for modeling the thermochemistry of electrochemical reactions based on density functional calculations64. Specifically, the Gibbs free energy of reactions, served as the criterion to determine the spontaneity of reactions, can be acquired based on DFT with corrections including entropic (TS) and zero-point energy (ZPE) contributions by the following equation:
| 4 |
Here, the EDFT represents the DFT energy computed for the respective systems, ZPE refers to the change in zero-point energy determined from the vibrational frequencies, and ΔS is the change in the entropy. For Gibbs free energy calculations, the free energy of a proton (GH*) is replaced by half of a hydrogen molecule in gaseous H2 (1/2GH2) for every step of electrochemical reactions3. The entropies of corresponding species were obtained from the vibrational frequencies. For gas molecules such as H2, the value of entropy can be described in the sum of the translational, vibrational, and rotational sections. Furthermore, the translational and rotational entropic values were not considered due to negligible contributions. The entropy is obtained by the following equation:
| 5 |
where R denotes the gas constant, kB is the Boltzmann constant, h represents Plank’s constant, NA corresponds to Avogadro’s number, vi signifies the frequency and N is the number of adsorbed atoms. Additionally, for frequency calculations, all substrate atoms were fixed and only adducts-related reaction intermediates are calculated, and the contributions from the catalyst phase to ZPE and ΔS are not involved in the Gibbs free energy of those reactions. The accuracy of calculations is provided in Supplementary Note 6.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
The authors acknowledge funding supports from National Natural Science Foundation of China under Grant Nos. 22075232, 22275154, and 52225104. K.H.L.Z., J.P.H., and X.D. also acknowledge the Mobility Program of the Sino-German Center for Research Promotion (Grant No. M-0377). R.W. is grateful for funding support by “Shuguang Program” supported by the Shanghai Education Development Foundation and Shanghai Municipal Education Commission (No. 20SG03), and the Science and Technology Commission of Shanghai Municipality (No. 22520710600). F.E.O. thanks to MINECO and European NextGenerationEU/PRTR Fund for the Ramón y Cajal contract (RyC2021-034254-I). V.A.P.O. is grateful for the funding supported by the EU (HYSOLCHEM project with grant agreement No. 101017928) and Spanish AEI (SOLFuture PLEC2021-007906). Giulio Gorni acknowledges Grant FJC2020-044866-I funded by MCIN/AEI/ 10.13039/501100011033, by Plan de recuperación, transformación y resiliencia, and by the “European Union NextGenerationEU/PRTR”. Mariam Barawi acknowledges NovaCO2 project (PID2020-118593RB-C22) and the RYC2022-038157-I Grant funded by MCIN/AEI/ 10.13039/501100011033. M.G.-T. acknowledges PEC2Change project (TED2021-129999A-C33) funded by MCIN/AEI/ 10.13039/501100011033 and the European Union Next Generation EU/PRTR. Additionally, the project that gave rise to these results received the support of a fellowship from “la Caixa” Foundation (ID 100010434). The fellowship code is LCF/BQ/PR23/11980046. Part of these experiments was performed at the CLAESS beamline of the ALBA synchrotron (proposal number AV-2021035069) with the help of the ALBA staff. The researchers would like to thank Iván García Dominguez and Francisco Martínez López, floor coordinators at ALBA Synchrotron, for their help with the design and 3D printing of the electrochemical cell used for in situ X-ray absorption measurements.
Author contributions
K.H.L.Z., R.W., and X.D. designed and supervised the research. X.D. and P.Z. prepared the samples. X.D. carried out electrochemical measurements and structure characterization, and analyzed the data. D.L. accomplished the DFT calculations. X.D., F.E.O., G.G., X.C., and V.A.P.O. contributed to all operando experiments and data analysis. D.L., H.W., M.B., M.G.-T., J.P.H., J.L., J.K., and S.C. participated in the discussion of the data. K.H.L.Z., R.W., and F.E.O. revised the manuscript. X.D. and D.L. wrote the manuscript. All authors contributed to the final version.
Peer review
Peer review information
Nature Communications thanks Yujin Chen, Alexei Kuzmin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Additional data supporting this study’s findings are available from the corresponding author upon request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xingyu Ding, Da Liu.
Contributor Information
Freddy E. Oropeza, Email: freddy.oropeza@imdea.org
Renbing Wu, Email: rbwu@fudan.edu.cn.
Kelvin H. L. Zhang, Email: kelvinzhang@xmu.edu.cn
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-49015-4.
References
- 1.Chu S, Cui Y, Liu N. The path towards sustainable energy. Nat. Mater. 2017;16:16–22. doi: 10.1038/nmat4834. [DOI] [PubMed] [Google Scholar]
- 2.Li X, et al. Latest approaches on green hydrogen as a potential source of renewable energy towards sustainable energy: Spotlighting of recent innovations, challenges, and future insights. Fuel. 2023;334:126684. doi: 10.1016/j.fuel.2022.126684. [DOI] [Google Scholar]
- 3.Seh Zhi W, et al. Combining theory and experiment in electrocatalysis: Insights into materials design. Science. 2017;355:eaad4998. doi: 10.1126/science.aad4998. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L-N, et al. Advanced hydrogen evolution electrocatalysts promising sustainable hydrogen and chlor-alkali co-production. Energy Environ. Sci. 2021;14:6191–6210. doi: 10.1039/D1EE02798K. [DOI] [Google Scholar]
- 5.Chatenet M, et al. Water electrolysis: from textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022;51:4583–4762. doi: 10.1039/D0CS01079K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu C, Zhang L, Gong J. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy Environ. Sci. 2019;12:2620–2645. doi: 10.1039/C9EE01202H. [DOI] [Google Scholar]
- 7.Mahmood N, et al. Electrocatalysts for hydrogen evolution in alkaline electrolytes: mechanisms, challenges, and prospective solutions. Adv. Sci. 2018;5:1700464. doi: 10.1002/advs.201700464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durst J, et al. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014;7:2255–2260. doi: 10.1039/C4EE00440J. [DOI] [Google Scholar]
- 9.Wei J, et al. Heterostructured electrocatalysts for hydrogen evolution reaction under alkaline conditions. Nanomicro Lett. 2018;10:75. doi: 10.1007/s40820-018-0229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subbaraman R, et al. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science. 2011;334:1256–1260. doi: 10.1126/science.1211934. [DOI] [PubMed] [Google Scholar]
- 11.You B, et al. Enhancing electrocatalytic water splitting by strain engineering. Adv. Mater. 2019;31:1807001. doi: 10.1002/adma.201807001. [DOI] [PubMed] [Google Scholar]
- 12.Shen X, et al. Dual-site cascade oxygen reduction mechanism on SnOx/Pt–Cu–Ni for promoting reaction kinetics. J. Am. Chem. Soc. 2019;141:9463–9467. doi: 10.1021/jacs.9b02286. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, et al. Emerging dual-atomic-site catalysts for efficient energy catalysis. Adv. Mater. 2021;33:2102576. doi: 10.1002/adma.202102576. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y-J, et al. Unlocking the door to highly active ORR catalysts for PEMFC applications: polyhedron-engineered Pt-based nanocrystals. Energy Environ. Sci. 2018;11:258–275. doi: 10.1039/C7EE02444D. [DOI] [Google Scholar]
- 15.Hu J, et al. Interface modulation of MoS2/metal oxide heterostructures for efficient hydrogen evolution electrocatalysis. Small. 2020;16:2002212. doi: 10.1002/smll.202002212. [DOI] [PubMed] [Google Scholar]
- 16.Zhong W, et al. Coupled vacancy pairs in Ni-doped CoSe for improved electrocatalytic hydrogen production through topochemical deintercalation. Angew. Chem. Int. Ed. 2020;59:22743–22748. doi: 10.1002/anie.202011378. [DOI] [PubMed] [Google Scholar]
- 17.Xu K, et al. Controllable surface reorganization engineering on cobalt phosphide nanowire arrays for efficient alkaline hydrogen evolution reaction. Adv. Mater. 2018;30:1703322. doi: 10.1002/adma.201703322. [DOI] [PubMed] [Google Scholar]
- 18.Duan J, Chen S, Ortíz-Ledón CA, Jaroniec M, Qiao S-Z. Phosphorus vacancies that boost electrocatalytic hydrogen evolution by two orders of magnitude. Angew. Chem. Int. Ed. 2020;59:8181–8186. doi: 10.1002/anie.201914967. [DOI] [PubMed] [Google Scholar]
- 19.Lin F, et al. Electrocatalytic hydrogen evolution of ultrathin Co-Mo5N6 heterojunction with interfacial electron redistribution. Adv. Energy Mater. 2020;10:2002176. doi: 10.1002/aenm.202002176. [DOI] [Google Scholar]
- 20.Sun H, et al. Boosting activity on Co4N porous nanosheet by coupling CeO2 for efficient electrochemical overall water splitting at high current densities. Adv. Funct. Mater. 2020;30:1910596. doi: 10.1002/adfm.201910596. [DOI] [Google Scholar]
- 21.Cui Y, et al. Tungsten oxide/carbide surface heterojunction catalyst with high hydrogen evolution activity. ACS Energy Lett. 2020;5:3560–3568. doi: 10.1021/acsenergylett.0c01858. [DOI] [Google Scholar]
- 22.He W, et al. Fluorine-anion-modulated electron structure of nickel sulfide nanosheet arrays for alkaline hydrogen evolution. ACS Energy Lett. 2019;4:2905–2912. doi: 10.1021/acsenergylett.9b02316. [DOI] [Google Scholar]
- 23.Kim B-J, et al. Functional role of Fe-doping in Co-based perovskite oxide catalysts for oxygen evolution reaction. J. Am. Chem. Soc. 2019;141:5231–5240. doi: 10.1021/jacs.8b12101. [DOI] [PubMed] [Google Scholar]
- 24.Zhou S, et al. Engineering electrocatalytic activity in nanosized perovskite cobaltite through surface spin-state transition. Nat. Commun. 2016;7:11510. doi: 10.1038/ncomms11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrie JR, Jeen H, Barron SC, Meyer TL, Lee HN. Enhancing perovskite electrocatalysis through strain tuning of the oxygen deficiency. J. Am. Chem. Soc. 2016;138:7252–7255. doi: 10.1021/jacs.6b03520. [DOI] [PubMed] [Google Scholar]
- 26.Du J, et al. Nonstoichiometric perovskite CaMnO3−δ for oxygen electrocatalysis with high activity. Inorg. Chem. 2014;53:9106–9114. doi: 10.1021/ic501631h. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, et al. Stabilizing the oxygen vacancies and promoting water-oxidation kinetics in cobalt oxides by lower valence-state doping. Nano Energy. 2018;53:144–151. doi: 10.1016/j.nanoen.2018.08.022. [DOI] [Google Scholar]
- 28.Jiang N, et al. Nickel sulfides for electrocatalytic hydrogen evolution under alkaline conditions: a case study of crystalline NiS, NiS2, and Ni3S2 nanoparticles. Catal. Sci. Technol. 2016;6:1077–1084. doi: 10.1039/C5CY01111F. [DOI] [Google Scholar]
- 29.Hung T-F, et al. Nickel sulfide nanostructures prepared by laser irradiation for efficient electrocatalytic hydrogen evolution reaction and supercapacitors. Chem. Eng. J. 2019;367:115–122. doi: 10.1016/j.cej.2019.02.136. [DOI] [Google Scholar]
- 30.da Silva MGS, Leite CM, Cordeiro MAL, Mastelaro VR, Leite ER. One-step synthesis of nickel sulfides and their electrocatalytic activities for hydrogen evolution reaction: a case study of crystalline h-NiS and o-Ni9S8 nanoparticles. ACS Appl. Energy Mater. 2020;3:9498–9503. doi: 10.1021/acsaem.0c01405. [DOI] [Google Scholar]
- 31.Zheng Y, Jiao Y, Jaroniec M, Qiao SZ. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem., Int. Ed. 2015;54:52–65. doi: 10.1002/anie.201407031. [DOI] [PubMed] [Google Scholar]
- 32.Jin S. Are metal chalcogenides, nitrides, and phosphides oxygen evolution catalysts or bifunctional catalysts? ACS Energy Lett. 2017;2:1937–1938. doi: 10.1021/acsenergylett.7b00679. [DOI] [Google Scholar]
- 33.Wygant BR, Kawashima K, Mullins CB. Catalyst or precatalyst? the effect of oxidation on transition metal carbide, pnictide, and chalcogenide oxygen evolution catalysts. ACS Energy Lett. 2018;3:2956–2966. doi: 10.1021/acsenergylett.8b01774. [DOI] [Google Scholar]
- 34.Ding X, et al. An Fe stabilized metallic phase of NiS2 for the highly efficient oxygen evolution reaction. Nanoscale. 2019;11:23217–23225. doi: 10.1039/C9NR07832K. [DOI] [PubMed] [Google Scholar]
- 35.Wan G, et al. Amorphization mechanism of SrIrO3 electrocatalyst: how oxygen redox initiates ionic diffusion and structural reorganization. Sci. Adv. 2021;7:eabc7323. doi: 10.1126/sciadv.abc7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fabbri E, et al. Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat. Mater. 2017;16:925–931. doi: 10.1038/nmat4938. [DOI] [PubMed] [Google Scholar]
- 37.Zhang G, et al. Enhanced catalysis of electrochemical overall water splitting in alkaline media by Fe doping in Ni3S2 nanosheet arrays. ACS Catal. 2018;8:5431–5441. doi: 10.1021/acscatal.8b00413. [DOI] [Google Scholar]
- 38.Ma Q, et al. Identifying the electrocatalytic sites of nickel disulfide in alkaline hydrogen evolution reaction. Nano Energy. 2017;41:148–153. doi: 10.1016/j.nanoen.2017.09.036. [DOI] [Google Scholar]
- 39.Zhu Y, et al. Operando unraveling of the structural and chemical stability of P-substituted CoSe2 electrocatalysts toward hydrogen and oxygen evolution reactions in alkaline electrolyte. ACS Energy Lett. 2019;4:987–994. doi: 10.1021/acsenergylett.9b00382. [DOI] [Google Scholar]
- 40.Zhai L, et al. In situ phase transformation on nickel-based selenides for enhanced hydrogen evolution reaction in alkaline medium. ACS Energy Lett. 2020;5:2483–2491. doi: 10.1021/acsenergylett.0c01385. [DOI] [Google Scholar]
- 41.Fleet ME. The crystal structure of heazlewoodite, and metallic bonds in sulfide minerals. Am. Mineral. 1977;62:341–345. [Google Scholar]
- 42.Vershinin AD, Selivanov EN, Gulyaeva RI, Sel’menskikh NI. Thermal expansion of Ni3S2 in Ni3S2-Ni alloys. Inorg. Mater. 2005;41:882–887. doi: 10.1007/s10789-005-0230-x. [DOI] [Google Scholar]
- 43.Li R, et al. Short-range order in amorphous nickel oxide nanosheets enables selective and efficient electrochemical hydrogen peroxide production. Cell Rep. Phys. Sci. 2022;3:100788. doi: 10.1016/j.xcrp.2022.100788. [DOI] [Google Scholar]
- 44.Denny YR, et al. Ni K-edge XAFS analysis of NiO thin film with multiple scattering theory. Surf. Interface Anal. 2014;46:997–999. doi: 10.1002/sia.5455. [DOI] [Google Scholar]
- 45.Cheng Z, Abernathy H, Liu M. Raman spectroscopy of nickel sulfide Ni3S2. J. Phys. Chem. C. 2007;111:17997–18000. doi: 10.1021/jp0770209. [DOI] [Google Scholar]
- 46.Fan L, et al. Molecular functionalization of NiO nanocatalyst for enhanced water oxidation by electronic structure engineering. ChemSusChem. 2020;13:5901–5909. doi: 10.1002/cssc.202001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bala N, Singh HK, Verma S, Rath S. Magnetic-order induced effects in nanocrystalline NiO probed by Raman spectroscopy. Phys. Rev. B. 2020;102:024423. doi: 10.1103/PhysRevB.102.024423. [DOI] [Google Scholar]
- 48.Sunny A, Balasubramanian K. Raman spectral probe on size-dependent surface optical phonon modes and magnon properties of NiO nanoparticles. J. Phys. Chem. C. 2020;124:12636–12644. doi: 10.1021/acs.jpcc.0c02638. [DOI] [PubMed] [Google Scholar]
- 49.Radinger H, et al. Importance of nickel oxide lattice defects for efficient oxygen evolution reaction. Chem. Mater. 2021;33:8259–8266. doi: 10.1021/acs.chemmater.1c02406. [DOI] [Google Scholar]
- 50.Momma K, Izumi F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011;44:1272–1276. doi: 10.1107/S0021889811038970. [DOI] [Google Scholar]
- 51.Stoerzinger KA, et al. Water reactivity on the LaCoO3 (001) surface: an ambient pressure X-ray photoelectron spectroscopy study. J. Phys. Chem. C. 2014;118:19733–19741. doi: 10.1021/jp502970r. [DOI] [Google Scholar]
- 52.Stoerzinger KA, Hong WT, Crumlin EJ, Bluhm H, Shao-Horn Y. Insights into electrochemical reactions from ambient pressure photoelectron spectroscopy. Acc. Chem. Res. 2015;48:2976–2983. doi: 10.1021/acs.accounts.5b00275. [DOI] [PubMed] [Google Scholar]
- 53.Yin J, et al. Ni–C–N nanosheets as catalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2016;138:14546–14549. doi: 10.1021/jacs.6b09351. [DOI] [PubMed] [Google Scholar]
- 54.Makarova M, Okawa Y, Aono M. Selective adsorption of thiol molecules at sulfur vacancies on MoS2 (0001), followed by vacancy repair via S-C dissociation. J. Phys. Chem. C. 2012;116:22411–22416. doi: 10.1021/jp307267h. [DOI] [Google Scholar]
- 55.Zhang J, et al. Copper dopants improved the hydrogen evolution activity of earth-abundant cobalt pyrite catalysts by activating the electrocatalytically inert sulfur sites. J. Mater. Chem. A. 2017;5:17601–17608. doi: 10.1039/C7TA05433E. [DOI] [Google Scholar]
- 56.Ravel B, Newville M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005;12:537–541. doi: 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
- 57.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 58.Blöchl PE. Projector augmented-wave method. Phys. Rev. B. 1994;50:17953–17979. doi: 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- 59.Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999;59:1758–1775. doi: 10.1103/PhysRevB.59.1758. [DOI] [Google Scholar]
- 60.Kresse G, Hafner J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B. 1993;47:558–561. doi: 10.1103/PhysRevB.47.558. [DOI] [PubMed] [Google Scholar]
- 61.Kresse G, Hafner J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B. 1994;49:14251–14269. doi: 10.1103/PhysRevB.49.14251. [DOI] [PubMed] [Google Scholar]
- 62.Peng L, et al. Atomic cation-vacancy engineering of NiFe-layered double hydroxides for improved activity and stability towards the oxygen evolution reaction. Angew. Chem. Int. Ed. 2021;60:24612–24619. doi: 10.1002/anie.202109938. [DOI] [PubMed] [Google Scholar]
- 63.Ma Y, et al. High-entropy energy materials: challenges and new opportunities. Energy Environ. Sci. 2021;14:2883–2905. doi: 10.1039/D1EE00505G. [DOI] [Google Scholar]
- 64.Yu, B., Jiang, H. & Zhang, Y. Superior specific capacity and energy density simultaneously achieved by Sr/In co-deposition behavior of Mg-Sr-In ternary alloys as anodes for Mg-Air cells. Preprint at 10.1016/j.jma.2024.02.005 (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Additional data supporting this study’s findings are available from the corresponding author upon request. Source data are provided with this paper.