In 1999 about 5.4 million people were newly infected with HIV.1 In some countries public health programmes have achieved modest gains in reducing HIV transmission through behavioural change, but the worldwide picture is one of increasing rates of infection. Although the use of condoms has slowly increased in countries most severely affected by the HIV epidemic, many vulnerable women are unable to ensure they are used. An effective and affordable vaginal microbicide, whose use could be controlled by women, would represent an important addition to the armamentarium against HIV infection. In this article we examine current progress in microbicide development and discuss their future role in HIV control.
Methods
We searched Medline using the key words and phrases “microbicides,” “virucides,” and “vaginal microbicides.” We also obtained the latest product information from the Alliance for Microbicide Development (P Harrison, personal communication),2 and we asked scientific colleagues involved in microbicide research for their comments on products in preclinical development.
Microbicides for controlling sexually transmitted infections
Microbicides act by disrupting or disabling organisms or block their entry into host cells by interfering with cell surface receptors. Chemical agents have a long history in the control of sexually transmitted infections and fertility. Penile antiseptics were widely promoted for controlling sexually transmitted disease in both world wars, although their efficacy and effectiveness remain uncertain.3 Intravaginal spermicides have been marketed for decades but have had limited popularity in the era of more reliable contraceptive methods.
The development of microbicides has drawn on existing contraceptive technology to develop safe, effective, acceptable, and accessible agents. As with HIV vaccines, progress with the development of effective microbicides has been slow, and the results of early trials of surfactants such as nonoxinol 9 were disappointing. The Microbicides 2000 conference in Washington, DC, provided new impetus to develop such compounds, and several promising products are now undergoing preclinical assessment.
Predicted developments
New microbicides will enter efficacy (phase III) trials in the next 5-10 years, and some may reach completion (such as carrageenan, Buffer Gel, dextrin sulphate, and PRO 2000)
One or more microbicides will have demonstrable efficacy against HIV infection sufficient to justify marketing the product
Research into low cost delivery systems will intensify to improve acceptability for female and male users
Careful consideration must be given to ethical issues and to ensuring appropriate procedures for informed consent
Microbicides will face the same challenges of distribution and implementation as condoms, which may ultimately remain more effective in preventing HIV transmission
Characteristics of an ideal microbicide
Laboratory evaluation of microbicidal activity must take account of the physiological changes that occur during sexual intercourse: compounds must retain activity in the presence of semen and over a broad pH range, ideally for several hours. Innovative methods to assess this include the use of “in vivo” cervical tissue from hysterectomy specimens.4 Activity against other sexually transmitted infections is an advantage, but products must not disrupt the normal vaginal flora, and non-spermicidal microbicides should not be teratogenic. Agents and vehicles for delivery must be latex compatible for use with condoms, and delivery systems must ensure adequate distribution and retention in the vagina. Methods to assess the latter are under development and include the use of fluorescent probes and magnetic resonance imaging. Topical microbicides should show negligible systemic absorption and be free from local toxic effects. The use of surfactants has been associated with disruption of genital epithelia, which may enhance HIV transmission.5,6
Acceptability of products is likely to be key to their widespread use. This will be enhanced by characteristics such as low cost, long shelf life, tolerance to high ambient temperature, and ease of use. Products must not be too messy, smelly, easily detectable, or strong tasting or interfere with sexual pleasure in other ways. Research indicates that women will want the choice of both contraceptive and non-contraceptive microbicides. Drawing on spermicide technologies, researchers are investigating a range of delivery systems including gels, films, sponges, and foaming tablets.
Status of microbicides tested in human trials
The first product to be tested in efficacy trials was nonoxinol 9. Although it was already widely used as a spermicide, safety studies were repeated in women in Europe, Thailand, and South Africa as the compound had a low selectivity index (activity against HIV compared with cytotoxicity against epithelial cells). The results of these studies justified proceeding to efficacy trials, and two such trials were reported by the end of 1999.7,8 Their results, and preliminary results from a study in commercial sex workers, suggest that nonoxinol 9 actually increases HIV transmission, probably because of local genital toxicity. The toxicity in these studies may have been associated with frequency of use, and analyses to address this are under way. However, these findings underline the importance of continuing to monitor safety in efficacy trials.
The table summarises the range of agents currently being assessed in human trials. Most are still at the early stages of safety studies. The next products likely to reach efficacy trials belong to the class that blocks HIV entry into host cells. These compounds have a much higher selectivity index than nonoxinol 9 in vitro, and toxicity experiments in animal models have been satisfactory. Preliminary safety studies have been completed in healthy female volunteers in the United Kingdom, Belgium, the United States, and South Africa for PRO 2000, a sulphonated polymer, and in the United Kingdom and Belgium for the sulphated polysaccharide dextrin sulphate. These two products will enter expanded safety studies in 2001 in Uganda (women attending a gynaecology clinic) and Côte d'Ivoire (commercial sex workers) as part of the microbicide programme funded by the European Commission. The Population Council (www.popcouncil.org) is similarly developing carrageenan and is sponsoring a large safety study in women attending a family planning clinic in South Africa. Family Health International plans to investigate Buffer Gel, a pH 4.5 vaginal buffering agent, and are setting up an efficacy trial in Malawi and Zimbabwe in collaboration with the National Institute of Health's HIV Prevention Network (www.fhi.org/en/aids/hptn/hptn.html).
Status of products in preclinical development
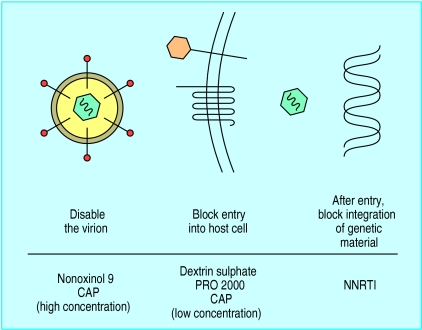
In vitro studies have identified many molecules with apparent potential as vaginal microbicides that have not yet been tested extensively in humans. Figure 1 details compounds that act specifically against HIV or block cell surface receptors. For compounds that act directly against the virus—for example, before integration of the virus into genetic material—it will be vital to check that drug resistance does not emerge, and for those that block cell surface receptors it will be necessary to ensure that they are not circumvented by the virus interacting with other receptors. Various microbicidal peptides with action against HIV have been described, both synthetic and those based on natural peptides such as defensins and magainins. Other compounds with encouraging antimicrobial profiles include cellulose acetate phthalate, a pharmaceutical additive already used in oral preparations, and CTC-96, an organocobalt compound.
Figure 1.
Modes of action of new vaginal microbicides. (CAP=cellulose acetate phthalate; NNRTI=non-nucleoside reverse transcriptase inhibitor)
Challenges for evaluation
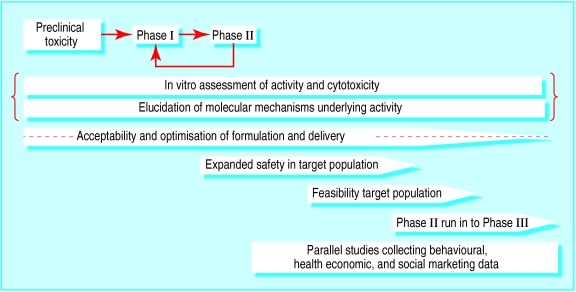
Like other new drugs, vaginal microbicides must be evaluated in preclinical studies, in small scale trials of safety and acceptability, and finally in large scale field trials of efficacy against target infections (fig 2 ). Careful consideration must be given to ethical issues and to ensuring appropriate procedures for informed consent. Since many of the trials are conducted in developing countries, ethical approval for microbicide studies is often sought from a developed country, where approval mechanisms are better established, as well as the host country.
Figure 2.
Stages of microbicide development
Testing safety and acceptability
Although initial trials of safety and acceptability focus on women, they must also include evaluation of male partners. Acceptable, practical, and standardised methods are needed for assessing toxicity to the vaginal mucosa by the naked eye and colposcopy, such as those proposed by the International Working Group on Microbicides.9 Risks to participants in trials must be minimised by protection from pregnancy and screening to exclude sexually transmitted infections and HIV. Microbicides are likely to be most effective at population level if they both protect uninfected women against HIV infection and reduce the infectiousness of women already infected. Trials therefore also need to check safety of microbicides in infected women; they could also study effects on levels of HIV RNA in vaginal swabs as a proxy for infectiousness. Because epidemiological and cultural conditions vary so greatly, studies of safety and acceptability are needed in all likely target populations. Other factors that need to be assessed before an efficacy trial are the distribution and retention of microbicide in the vagina.
Efficacy trials
Initial trials of efficacy against HIV acquisition involve women not infected with HIV. Effects on other sexually transmitted infections should also be measured, both as an important health issue in its own right and because such infections enhance HIV transmission10 and microbicides might affect HIV spread through this indirect mechanism.
A placebo control group is needed to reduce bias and to distinguish the product's effect from any changes in behaviour that might result from the provision of such a product. Investigators must therefore ensure that all participants understand they may receive an inactive product and should not assume they are protected. Condoms must be made available to participants if they choose to use them. Previous trials have shown increased condom use by participants, and trial size should allow for this. The ethical justification for a placebo group is that before the trial it is not known whether the product has any beneficial or adverse effect. Although lack of benefit may seem implausible on the basis of preclinical and early clinical studies, the experience with nonoxinol 9 shows that promising products may prove ineffective or even harmful in efficacy trials.7,8
In general, study populations with a high incidence of HIV infection are needed for efficacy trials in order to reduce the number of participants required. All trials will require baseline screening to identify women not infected with HIV. Potential study groups might include commercial sex workers,7 women attending clinics for sexually transmitted infections, women whose male partner is already infected, or women in the general population where incidence is sufficiently high (such as in South Africa11) who could be recruited postnatally or through maternal and child health clinics, family planning clinics, or voluntary HIV testing sites. All studies face the challenge of achieving adequate follow up rates.
Trials in women infected with HIV
Microbicides are initially being developed to protect uninfected women from HIV, but infected women would also use them if they reduced transmission of HIV and other sexually transmitted infections to male partners. The combined effect at population level of moderate protection against both acquisition and transmission of HIV could be substantial, as has been predicted for HIV vaccines.12 Effects on infectiousness could be assessed in an efficacy trial in couples where the woman is infected with HIV and the man is not. Innovative designs have been proposed for trials of HIV vaccines, and these might be adapted for microbicide trials.13
From efficacy to effectiveness
A distinction can be made between trials measuring the efficacy of an intervention when delivered optimally and those measuring its effectiveness when used in practical conditions. Even in a carefully conducted trial compliance with microbicide use may be suboptimal, so data interpretation must take account of trial records on product use and acceptability and concurrent condom use.
Use of a microbicide might also be associated with risk compensation.14 Women may perceive that they are protected by the product and consequently increase their exposure either through an increased number of sexual partners or decreased condom use. It would be important to seek evidence of any such effect in efficacy trials.
Challenges for implementation
To be widely used, microbicides must be both affordable and acceptable to women and men. Ease of delivery and lack of detectability will probably be crucial, and product delivery systems must be adapted to local cultural and sexual preferences, such as the use of dessicants for “dry sex.”15
Access to supplies is another issue. In some African countries condoms are provided freely or at very low cost, but many rural villages have no local outlet. Special programmes may be needed, such as through recruitment of villagers to become product marketers. Where there is a good network of primary health units, free supplies might be offered to women through such units. Supplies might be linked to established family planning programmes to offer a range of prevention options. However, it would be important not to have the unintended effect of decreasing condom use, as this may ultimately remain more effective in preventing HIV transmission.
Future directions
A combination of effective intervention strategies is needed to reduce transmission of HIV. This is important in all age groups, but particularly so in adolescents. Such strategies may include delay in sexual debut, reducing the number of casual sexual partners, use of condoms, and prompt and effective treatment of sexually transmitted infections, as well as use of microbicides.
Speeding up microbicide development and delivery to countries where HIV is the greatest threat will require a concerted multidisciplinary and international effort. Public and industrial investment in product development must be combined with an efficient and rapid system of evaluation through clinical studies involving basic scientists, epidemiologists, triallists, and behavioural scientists.
There is a good prospect that at least one safe and effective product will become available for widespread use within the next five to 10 years. This would be an important step forward in efforts to bring the HIV epidemic under control in Africa and other parts of the developing world.
Table.
Potential microbicides currently in human trials
| Activity
|
|||
|---|---|---|---|
| Product groups and active agents | Anti-STI | Spermicidal | Human trials |
| Broad spectrum activity | |||
| Non-ionic surfactants: | |||
| Nonoxinol 9* | ++ | + | Efficacy |
| Chlorhexidine* | ++ | + | Efficacy (vertical transmission) |
| Octoxinol 9* | ++ | + | Safety |
| Anionic surfactants: | |||
| Docusate sodium* | ++ | NA | Safety |
| Cationic surfactants: | |||
| Glyminox (C31G)* | ++ | + | Safety |
| Benzalkonium chloride* | ++ | NA | Safety |
| Geda plus* | ++ | + | Safety |
| Acid buffers: | |||
| Buffer gel | ++ | +/− | Safety |
| Plant extracts: | |||
| Praneem polyherbal | ++ | + | Safety |
| Gossypol | NA | + | Safety |
| Bacteria: | |||
| Lactobacilli | − | − | Safety |
| Peptides: | |||
| IB367 (protegrin) | + | NA | Safety |
| Inhibitors of viral entry | |||
| Sulphated polysaccharides: | |||
| Dextrin sulphate | + | − | Safety |
| Carrageenan | ++ | NA | Safety |
| Cellulose sulphate | ++ | − | Safety |
| Sulphonated polymers: | |||
| PRO 2000 | ++ | − | Safety |
| Inhibitors of viral replication | |||
| Reverse transcriptase inhibitors: | |||
| Tenofovir (PMPA) | − | − | Safety (oral administration) |
STI=sexually transmitted infection. NA=not assessed. *Known to be cytotoxic to HIV.
Acknowledgments
We thank Polly Harrison from the Alliance for Microbicide Development, Andrew Nunn from the MRC Clinical Trials Unit, and Robin Shattock from St George's Hospital Medical School for their invaluable comments. We acknowledge the contribution that the alliance has made to the promotion of microbicides for preventing spread of HIV and sexually transmitted infections. In addition, the International Working Group on Microbicides, currently chaired by Dr Alan Stone, has helped to set the standards for research in this area and to pool scientific expertise to facilitate the rapid development of candidate microbicides.
Footnotes
Competing interests: ML Laboratories and Interneuron, manufacturers of dextrin sulphate gel and PRO 2000 gel respectively, helped fund clinical safety studies of their products conducted at Imperial College School of Medicine (CL) and coordinated by the MRC Clinical Trials Unit (SMc).
References
- 1.United Nations Program for AIDS (UNAIDS). Report on the global HIV/AIDS epidemic—June 2000. www.unaids.org/epidemic_update/report/index.html (accessed Aug 2000).
- 2.Alliance for microbicide development. http://hometown.aol.com/ggkidder/alliance.html (accessed Jul 2000).
- 3.Allan M Brandt. No magic bullet. Oxford: Oxford University Press; 1987. [Google Scholar]
- 4.Greenhead P, Hayes P, Watts P, Laing K, Griffin G, Shattock R. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niruthisard S, Roddy RE, Chutivongse S. The effects of frequent N-9 use on the vaginal and cervical mucosa. Sex Transm Dis. 1991;18:176–179. doi: 10.1097/00007435-199107000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Stafford MK, Ward H, Flanagan A, Rosenstein IJ, Taylor-Robinson D, Smith RJ, et al. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–331. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339:504–510. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 8.Kreiss J, Ngugi E, Holmes K, Ndinya-Achola J, Waiyaki P, Roberts PL, et al. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268:477–482. [PubMed] [Google Scholar]
- 9.Contraceptive and Development Program (CONRAD), International Working Group on Microbicides (IWGM), United Nations Program for AIDS (UNAIDS). The role of colposcopy in assessing vaginal irritation in research. Proceedings. January 21-22, 1999. Washington, DC. www.conrad.org/workshops.html#colpo (dated 12 Oct 1999).
- 10.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willliams BG, Campbell CM. Understanding the epidemic of HIV in South Africa: analysis of the antenatal clinic survey data. S Afr Med J. 1998;88:247–251. [PubMed] [Google Scholar]
- 12.Anderson RM, Garnett GP. Low-efficacy HIV vaccines: potential for community-based intervention programmes. Lancet. 1996;348:1010–1013. doi: 10.1016/s0140-6736(96)07100-0. [DOI] [PubMed] [Google Scholar]
- 13.Datta S, Halloran ME, Longini IM. Augmented HIV vaccine trial design for estimating reduction in infectiousness and protective efficacy. Stat Med. 1998;17:185–200. doi: 10.1002/(sici)1097-0258(19980130)17:2<185::aid-sim732>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Richens J, Imrie J, Copas A. Condoms and seat belts: the parallels and the lessons. Lancet. 2000;355:400–403. doi: 10.1016/S0140-6736(99)09109-6. [DOI] [PubMed] [Google Scholar]
- 15.Pool R, Whitworth JA, Green G, Mbonye AK, Harrison S, Wilkinson J, et al. An acceptability study of female-controlled methods of protection against HIV and STDs in south-western Uganda. Int J STD AIDS. 2000;11:162–167. doi: 10.1258/0956462001915606. [DOI] [PubMed] [Google Scholar]