Abstract
Aims/Introduction
Defective insulin signaling in the brain may disrupt hippocampal neuroplasticity resulting in learning and memory impairments. Thus, this study investigated the effect of aerobic exercise training on cognitive function and synaptic protein markers in diabetic rats.
Materials and methods
Twenty male Wistar rats (200–250 g), were fed on high-fat diet and received a low dose of streptozotocin (35 mg/kg, i.p) to induce type 2 diabetes. Then diabetic animals were randomly divided into sedentary and training groups. The exercise training program was treadmill running at 27 m/min for 60 min/day for 8 weeks. One day after the last training session, Morris Water Maze (MWM) task was performed to evaluate spatial learning and memory. Then, the hippocamp and prefrontal cortex tissues were instantly dissected for immunoblotting assay of BDNF, GSK-3β, p-GSK-3β, P38, p-P38, ERK1/2, p-ERK1/2, heat shock protein-27 (HSP27), SNAP-25, synaptophysin, and PSD-95. Independent t-test analysis and two-way ANOVA was used to determine the differences under significance level of 0.05 using the 26th version of IBM SPSS statistical software.
Results
The results showed that aerobic exercise improved memory as assessed in the MWM task. Moreover, aerobic exercise up-regulated HSP27 and BDNF protein levels in the prefrontal cortex, and hippocampus coincided with robust elevations in SNAP25 and PSD-95 levels. Moreover, exercise reduced phosphorylated P38, while increased p-ERK1/2 and p-GSK-3β (p).
Conclusion
Our findings suggest that aerobic exercise may debilitate the harmful effects of diabetes on the cognitive function possibly through enhancing synaptic protein markers.
Keywords: Exercise, Memory, Synaptophysin, BDNF, Hippocampus
Introduction
Diabetes is a global health problem with widespread deleterious effects and chronic complications. The current prevalence of type 2 diabetes mellitus (T2DM) is more than 463 million people and without sufficient action predicted to reach 578 million by 2030 and 629 million by 2045 [1]. This metabolic disorder is characterized by sustained hyperglycemia resulting from inappropriate insulin secretion and, or signaling [2, 3].
In the central nervous system (CNS), insulin receptors are widely expressed in the cognitive-related areas like the cortex and hippocampus. Studies show that insulin signaling facilitates cognitive abilities by promoting synaptic maturation and plasticity, synaptogenesis, neuronal maturation, and neurogenesis [4]. Animal models of T2DM have demonstrated that defective insulin signaling in the brain may disrupt hippocampal neuroplasticity resulting in learning and memory impairments [5] .
Synapses are the most dynamic structures in the neuronal networks that permit neurons to communicates with a target cell [6]. In the synaptic junctions, electrical signals are converted into the release of signaling molecules called neurotransmitters. These transmitters bind to specific receptors that transform the message forward to electrical signals in the postsynaptic cell [7]. The loss of synapses and synaptic proteins deficits are strongly correlated with psychological impairment [8]. Several studies have also established that diabetes damages synaptic structure and function, and changes neurotransmitter release in the different brain regions. Previous studies have shown a significant decrease in presynaptic proteins such as synaptosome-associated protein-25 (SNAP-25) and synaptophysin, and the postsynaptic protein such as postsynaptic density-95 (PSD-95) levels in the cerebral cortex and hippocampus of diabetic mice [2].
Nevertheless, clinical and experimental researches have demonstrated that physical activity enhances brain health and cognitive function by releasing neurotrophic factors [9] and increasing neurogenesis and synaptic efficacy [10]. Furthermore, recent investigations recommend that aerobic exercise has beneficial effects on diabetes-induced cognitive impairments [10, 11]. However, it is still poorly understood how exercise may affect synaptic proteins involved in cognitive performances in diabetes.
The present study aimed to elucidate the effect of aerobic treadmill exercise training (ATET) on cognitive function and synaptic protein markers in the HIP and PFC in a rat model of type 2 diabetes.
Materials and methods
Animals and study design
Twenty male Wistar rats, approximately 3-month-old, weighing 200–250 g, were obtained from the Pasture Institute (Tehran, Iran). Animals were housed in pairs under temperature 22 ± 2◦C and humidity 60%, with a 12-h light/dark cycle (7:00 a.m. − 7:00 p.m.) with free access to food and tap water in the animal facility of Neurosciences Research Center (NSRC), Tabriz University of Medical Sciences. Before experiments, animals were handled daily to reduce stress and adapt to the animal facilities for one week [12]. All procedures were performed in accordance with the research ethics committee (REC) of Tabriz University of Medical Sciences (TUOMS) (IR.TBZMED.REC.1395.1225) and carried out under veterinary supervision.
Diabetes induction
For induction of T2DM, animals were fed on high fat diet (HFD) (5.7 kcal/g; 57% lipids, 23% protein, 20% carbohydrate as a percentage of total kcal) [13] for 4 weeks. HFD was prepared by adding 25 gr of tallow and 25 gr of clarified butter to 100 gr of powdered standard pellets and re-pelleting it. Preferably, HFD was prepared once a week to prevent food from spoiling. In the next step, the animals were received a low dose of STZ injection (35 mg/kg, intraperitoneally, i.p) freshly dissolved in 10 mM sodium citrate buffer (Sigma, St. Louis, MO, USA). Hyperglycemic status (blood glucose levels exceeding 250 mg/dl) was confirmed 3 days after injection with a glucometer (Roche, Germany). Following confirmation of diabetes, animals were randomly assigned to the sedentary group and training group and were fed by standard chow pellets until the last day of intervention.
Exercise training protocol
All exercise sessions were supervised by a professional exercise physiologist. Following 2 days of treadmill familiarization (40 min once a day), animals in the training group ran on a flattened motorized treadmill at 0˚ slope (Model T510E, Diagnostic and Research Instruments Co., Taoyuan, Taiwan). Electric shock was applied to motivate the animals to run (intensity = 0.25 mA). The speed and duration of training were progressively enhanced until each rat was running continuously for 60 min/day at 27 m/min for 8 weeks [14]. Animals in the sedentary group were placed on the treadmill for 10 min each day without any exercise training.
Morris water maze (MWM)
One day after the last training session, Morris Water Maze (MWM) task was performed to evaluate spatial learning and memory. The water maze was consisted of a black circular pool, 136 cm in diameter and 80 cm in-depth, with a small black escape platform (10 cm×10 cm) placed in the center of one quadrant of the tank submerged 1.5 cm under the water surface. The testing room contained extra-maze visual cues on the wall, and their locations were not changed during the testing period. The pool was divided into four equal quadrants with four starting points (N, S, W, and E), in which the order was changed every day. The behaviors of the animals were recorded by a fixed digital video camera recorder and then interpreted using Noldus tracking software (Ethovision XT, Noldus Information Technology, Wageningen, Netherlands).
Animals were trained on the hidden platform task, 1.5 cm below the water surface, to assess spatial acquisition. The rats were subjected to four effort a day for three consecutive days. In each attempt, rats were placed in the water in one of the four starting locations (N, S, W, and E) and given a maximum of 60 s to find the platform. At the end of 60 s, if they couldn’t find the platform within 60 s, the rat was calmly hand-guided to the platform and allowed to wait there for 15 s. A day after the last acquisition trial, a probe test was carried out to assess spatial memory retention. In this test, the platform was removed from the pool, and rats were placed in the water and allowed to search for it for 60 s. The time spent in the target quadrant in which the platform had been placed during acquisition trails was recorded [15].
Sampling
At the end of the experiment, rats were anesthetized with ketamine (75 mg/kg) and xylazine (2.5 mg/kg) and sacrificed by decapitation. Then, the hippocampus (HIP) and prefrontal cortex (PFC) tissues were instantly dissected on dry ice and stored at − 70 °C for further analysis.
Immunoblotting assay
Frozen samples were homogenized with a lysis buffer cocktail (200 µl) containing protease and phosphatase inhibitors using a Polytron homogenizer PRO250 (PRO Scientific, Oxford, CT), and centrifuged at 12,000 rpm for 15 min at 4 °C. Bradford method (Bio-Rad, Hercules, CA, USA) was used to estimate the amount of protein in each sample. Equal amounts of total protein were loaded onto 10% SDS-polyacrylamide gel for electrophoresis. Subsequently, proteins were transferred onto the polyvinylidene fluoride (PVDF) membrane (GE Healthcare Bioscience, Arlington Heights, IL, USA). They were then incubated with blocking solution in phosphate-buffered saline containing 0.1% Tween 20 to block nonspecific bindings for 2 h at room temperature. Next, blots were exposed to different rabbit polyclonal primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) of BDNF (sc-546), GSK-3β (sc-7291), p-GSK-3β (sc-373,800), P38 (sc-535), p-P38 (sc-17,852), ERK1/2 (sc-292,838), p-ERK1/2 (sc-16,981), HSP27 (sc-1048), SNAP-25 (SP12): sc-20,038, synaptophysin (D-4): sc-17,750, PSD-95 (7E3): sc-32,290, and β-Actin (sc-130,657) overnight at 4 °C. After three times washing the membrane with PBS, blots were incubated with horseradish peroxidase (HRP) conjugated secondary anti-rabbit antibody for 2 h at room temperature. The enhanced chemiluminescence (ECL) kit (Bio-Rad) was used to detect the target protein bands development and the density of bands was quantified using Image J software. β-Actin was used as an internal loading control [16].
Statistical analysis
Data were analyzed using SPSS (version 26.0) software. In all cases, statistical differences were considered significant at p < 0.05. After confirmation of the normality of variables by the Shapiro-Wilk test, independent t-test analysis was applied to compare the differences between the two groups. The result of escape latency time was analyzed using two-way ANOVA. Data were expressed as mean ± S.E.M.
Results
Effect of ATET on memory in MWM
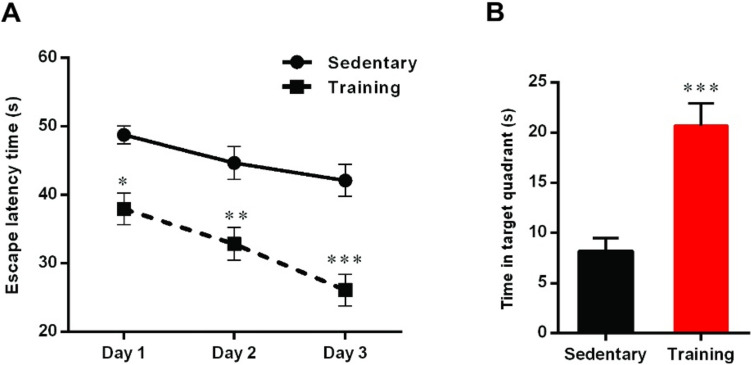
A two-way ANOVA of escape latency time in acquisition trails using group and day as factors showed a main effect of group (F (1, 42) = 51.04, p < 0.001), day (F (2, 42) = 8.74, p < 0.001), and group × day interaction (F (2, 42) = 0.77, p > 0.05). Intergroup analysis indicated that aerobic training in the diabetic rats significantly decreased escape latency time compared to the sedentary group (day 1: p < 0.05, day 2: p < 0.01, and day 3: p < 0.001) (Fig. 1A).
Fig. 1.
The effect of aerobic training on (A) escape latency time on the hidden platform of MWM task in different groups. Data are shown as mean ± SEM (n = 8): Two-way ANOVA *p < 0.05, **p < 0.01, ***p < 0.001. B Time spent in the target quadrant in probe test. Data are shown as mean ± SEM (n = 8): Un-paired t-test, ***p < 0.001
The result of the probe test also showed a significant difference in the time spent in the target quadrant between groups (t = 4.86, df = 12, p < 0.001, Fig. 1B) and time spent in the target quadrant in the sedentary group was lower than the training group.
Effects of ATET on small heat shock protein (HSP27) and BDNF
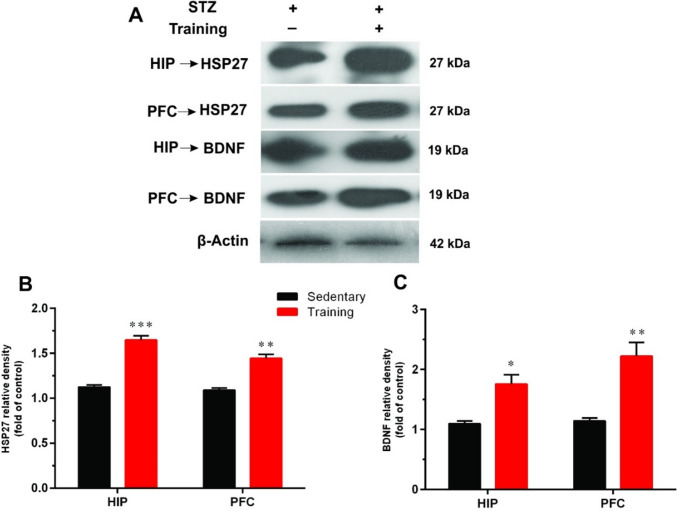
As shown in Fig. 2, the expression of HSP27 was significantly lower in the sedentary group versus trained rats in the HIP (t = 9.45, df = 4, p < 0.001) and PFC (t = 6.75, df = 4, p < 0.01).
Fig. 2.
The effects of aerobic training on heat shock protein-27 (HSP27) and brain-derived neurotrophic factor (BDNF) in the hippocampus (HIP) and prefrontal cortex (PFC) of diabetic rats. A A representative immunoblotting image of HSP27, BDNF, and β-Actin in different groups. Graphs show the protein expression of HSP27 (B) and BDNF (C) in the HIP and PFC of sedentary and training groups (n = 3). Data shown represent mean ± SEM: Un-paired t-test, *p < 0.05, **p < 0.01, ***p < 0.001
Moreover, aerobic exercise training significantly up-regulated BDNF protein expression levels in both HIP (t = 4.24, df = 4, p < 0.05) and PFC (t = 4.63, df = 4, p < 0.01) in diabetic animals (Fig. 2B).
Effects of ATET on protein kinases
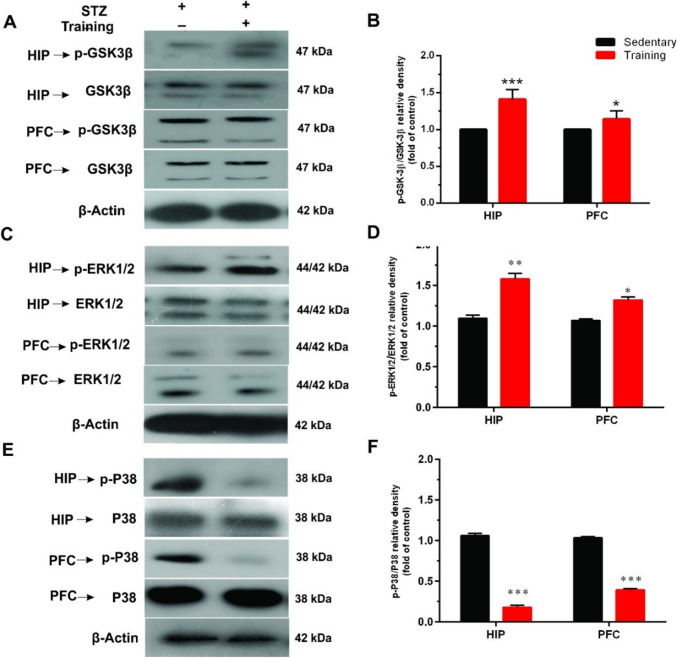
We also measured phosphorylation of ERK1/2 and P38 (MAPKs) as well as GSK-3β to see which intracellular signals was affected after exercise in diabetic animals (Fig. 3). Results showed that exercise significantly increased p-GSK-3β (t = 5.25, df = 4, p < 0.01 for HIP; t = 4.23, df = 4, p < 0.05 for PFC) and p-ERK1/2 (t = 6.37, df = 4, p < 0.01 for HIP; t = 6.22, df = 4, p < 0.05 for PFC) while decreased p-P38 (t = 22.97, df = 4, p < 0.001 for HIP; t = 29.34, df = 4, p < 0.001 for PFC) in the HIP and PFC of diabetic rats.
Fig. 3.
The effects of aerobic training on mitogen-activated protein (MAP) kinases in the hippocampus (HIP) and prefrontal cortex (PFC) of diabetic rats. A A representative immunoblotting image of phosphorylated GSK-3β (p-GSK-3β), total GSK-3β, C phosphorylated extracellular signal-regulated kinases 1 and 2 (p-ERK1/2), total ERK1/2, E phosphorylated P38 (p-P38), total P38, and β-Actin of different groups. Graphs show the ratio of p-GSK-3β/ GSK-3β (B), p-ERK1/2/ERK1/2 (D), and p-P38/P38 (F) in the HIP and PFC of sedentary and training groups (n = 3). Data shown represent mean ± SEM: Un-paired t-test, *p < 0.05, **p < 0.01, ***p < 0.001
Effects of ATET on synaptic proteins
SNAP-25
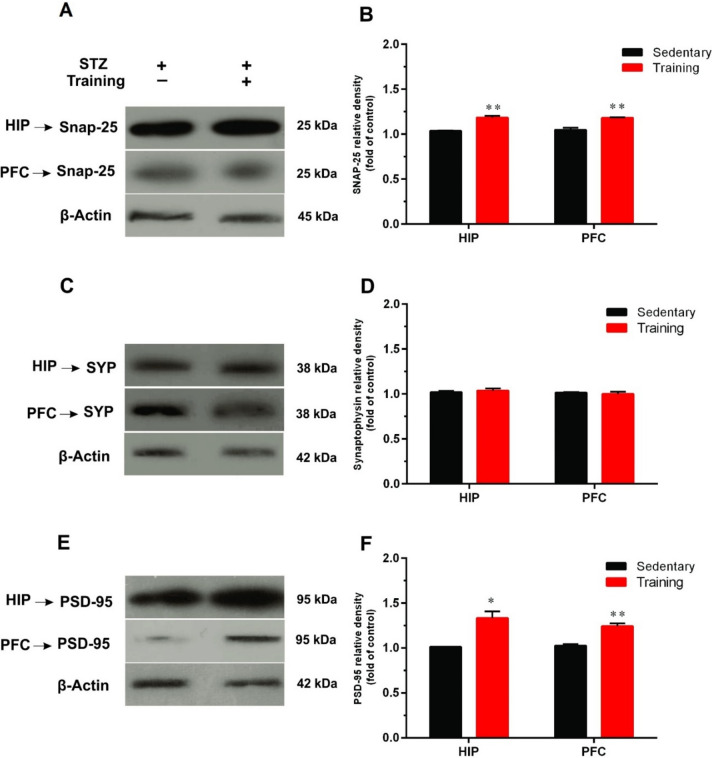
The results of Western blotting also revealed that 8 weeks of ATET significantly increased protein expression of SNAP-25 in the HIP (t = 8.23, df = 4, p < 0.01) and PFC (t = 5.80, df = 4, p < 0.01) as compared to the sedentary group (Fig. 4A and B).
Fig. 4.
The effects of aerobic training on synaptic protein markers including (B) SNAP-25, D synaptophysin (SYP), and (F) postsynaptic protein-95 (PSD-95) in the hippocampus (HIP) and prefrontal cortex (PFC) of diabetic rats. A representative immunoblotting image of SNAP-25 (A), SYP (C), PSD-95 (E), and β-Actin in the HIP and PFC of sedentary and training groups (n = 3). Data are shown as mean ± SEM: Un-paired t-test, *p < 0.05, **p < 0.01
Synaptophysin (SYP)
We also evaluated the effects of ATET on protein expression levels of SYP in the HIP and PFC. No significant differences were observed between the SYP levels of the sedentary group and training group (Fig. 4C and D) in both HIP (t = 1.73, df = 4, p > 0.05) and PFC (t = 0.020, df = 4, p > 0.05).
PSD-95
Our results indicated that 8 weeks of ATET significantly increased the postsynaptic protein level of PSD-95 in the HIP (t = 4.35, df = 4, p < 0.05) and PFC (t = 6.24, df = 4, p < 0.01) as compared to the sedentary group (Fig. 4E and F).
Discussion
The main findings of the present study showed that ATET enhanced the cognitive function of the diabetic rats as assessed in the MWM task. Moreover, this result was accompanied by increased synaptic proteins in the HIP and PFC. Nowadays, the potential benefits of exercise on overall brain health and functions are pretty well-known. The proposed mechanisms by which exercise enhances cognitive functions are through the modulation of synaptic structure, neuronal plasticity, and synaptic proteins involved in regulating brain function [10]. Moreover, studies showed that cognitive function and synaptic plasticity are impaired by diabetes [4].
HSP27 has been found to be localized to synaptic sites as well as to peri-synaptic glial processes in rat cerebellum following hyperthermia [17]. Previous studies have reported reduced tissue levels of HSP in type 1 and type 2 diabetes, which results in secondary complications and delayed wound healing [18]. Evidence also shows that HSP27 is involved in preserving nerve function and low expression of HSP27 in diabetes promotes neuropathy [19]. Therefore, therapies aimed at enhancing HSP27 expression may have beneficial effects against secondary complications of diabetes. Tóth et al. have also reported that overexpression of HSP27 protein alleviates learning and memory loss in Alzheimer’s disease (AD) in mice models [20]. In the present study, exercise apparently up-regulated HSP27 expression in the HIP and PFC of trained diabetic rats compared with the sedentary group. Studies have also shown that exercise training increases HSP72 expression in the heart and brain in type 1 diabetic rats [21]. It is likely that up-regulation of heat shock proteins during stressful conditions such as exercise is likely a compensatory mechanism to mitigate the disturbance of synaptic function.
Several studies have reported low levels of BDNF in diabetic patients associated with cognitive deficits [22]. In addition, experimental and clinical studies showed that inhibition or down-regulation of BDNF is associated with learning and cognitive impairments [23]. Several studies have also proven the significant aerobic exercise benefits on cognition in rodents and humans [24]. Indeed, exercise promotes neuronal function through the release of growth and neurotrophic factors such as BDNF. Previous studies have demonstrated that exercise up-regulates BDNF expression in the motor cortex and hippocampus accompanied by enhanced cognitive performance supposedly through improving hippocampal synaptic plasticity [25]. In the present study, diabetes induced low expression of BDNF which was enhanced by 8 weeks of aerobic exercise in the training group. In line with our results, previous studies have also demonstrated that exercise increases BDNF levels in type 2 diabetes [26]. Therefore, it seems that aerobic exercise facilitates cognitive performance in diabetic patients through up-regulation of BDNF in brain regions involved in learning and memory.
The ERK pathway belongs to the mitogen-activated protein kinase (MAPK) superfamily is implicated in the regulation of synapse formation and plasticity, as well as learning and memory [27]. Indeed, ERK1/2 signaling mediates the effects of BDNF on synaptic plasticity and memory formation [27]. Moreover, activation of ERK1/2 has been shown to regulate the formation of new dendritic spines [28]. Several studies showed that phosphorylated ERK translocates into the nucleus and regulates phosphorylation of target proteins in both dendritic and axonal compartments [27, 28]. In our study, exercise also increased p-ERK1/2 in both HIP and PFC of diabetic rats.
The p38 MAPKs, stress-activated protein kinases, are activated by extracellular stresses and cytokines and are involved in synaptic plasticity and neurodegenerative disease [29]. Phosphorylated (activated) P38 is known to reduce synaptic density, and its inhibition can attenuate synaptic dysfunction and behavioral deficits [30]. Munoz et al. have shown that administration of P38 inhibitor, 069 A, attenuates the loss of synaptophysin induced by amyloid beta (Aβ) exposure and improved spatial memory deficit in the AD mouse model [31]. Dai et al. have also demonstrated that knockdown of p38 MAPK in the hippocampus improves memory and synaptic plasticity in angiotensin II-dependent hypertensive mice [30]. In this study, induction of diabetes increased p-P38 in the HIP and PFC of the sedentary group, which was reduced by aerobic exercise. Since oxidative stress is an activator of P38 [32] and diabetes is associated with oxidative damage, we suggest this mechanism may be involved in increased p-P38 in non-trained diabetic rats.
GSK-3𝛽 contributes to cellular metabolism and synaptic plasticity and is a target of BDNF [33]. Unlike other kinases, GSK3 is active at baseline and inhibited upon phosphorylation by Akt. Ochs et al. have demonstrated that knockout of GSK3β decreases dendritic spine stability and attenuates excitatory synaptic transmission [34]. In this study, exercise also increased p-GSK-3β in both HIP and PFC of trained rats.
To investigate whether the BDNF and kinases changes were related to the hippocampal and PFC synaptic changes, we also measured pre- and postsynaptic protein including SNAP-25, synaptophysin, and PSD-95, respectively. Although many aspects of mechanisms underlying exercise-induced cognition improvement are understood, little is known about the effects of aerobic exercise on synaptic proteins in the HIP and PFC following diabetes.
The synaptic connections are crucial to the effective synchronization of all brain functions, such as cognition. Several complex molecular interactions regulate synapses formation and plasticity by controlling the assembly and functions of pre- and postsynaptic proteins. Alteration of the fundamental synaptic proteins directly affects synaptic transmission [35].
SNAP-25 is a component of the trans-SNARE complex, which negatively modulates neuronal voltage-gated calcium channels. Besides, SNAP-25 has an essential role in regulating exo-endocytic processes at the presynaptic terminal and also regulates postsynaptic receptor trafficking. Reports have also shown that reduced SNAP-25 expression may impair synaptic plasticity and result in psychiatric and neurodegeneration diseases [35]. In this study, induction of diabetes resulted in decreased expression of SNAP-25 in both HIP and PFC. Several studies have consistently reported increased protein or mRNA levels of SNAP-25 in the hippocampus following routine exercise upon activation of BDNF/TrkB signaling [36]. Previous studies have shown that SNAP-25 and PSD-95 are involved in exercise-induced cognition improvement [36]. However, ATET enhanced the expression of both pre- and postsynaptic proteins, SNAP-25 and the PSD 95, in the PFC and HIP of diabetic animals. In agreement with our results, Hu et al. demonstrated that 7 days of voluntary exercise increases NR2b, PSD95, synaptophysin, and SNAP-25 expressions in the hippocampus [37]. In contrast to our results, Liu et al. have reported that treadmill exercise training did not increase the hippocampal protein expression of SNAP-25 [38]. This inconsistency may stem from differences in study design (diabetic and non-diabetic animals), exercise protocols, and animal species (mice or rat).
Synaptophysin is a marker of synaptic density, which plays crucial role in the biogenesis of synaptic vesicles, neurotransmitters release and endocytosis, and recycling synaptic vesicles [39]. Moreover, previous in vivo and in vitro studies have shown that inhibition of neuronal p38 MAPK inhibited decreases in synaptophysin levels [40]. Our results demonstrated that treadmill exercise training did not significantly change the expression of this protein in the PFC and HIP of diabetic animals. According to the previous report, down-regulation of synaptophysin expression decrease neurotransmitters release contributing to diabetes-induced cognitive dysfunction and dementia [41]. Our finding is consistent with the previous study that demonstrated one-month of aerobic exercise did not significantly change synaptophysin expression [42]. Moreover, it has been reported that forced and voluntary exercise did not alter hippocampal levels of this protein [43]. On the contrary, some studies have reported the increased hippocampal expression of synaptophysin after different exercise regimens [44]. These inconsistencies are possibly due to differences in the exercise protocol in intensity, and duration of exercise training. Although both aerobic and resistance trainings can improve learning, spatial memory and plasticity in a similar manner, these effects are done through different molecular mechanisms [44]. On the other hand, even aerobic training in different periods of time can lead to different responses; that is, in the face of increased protein synthesis after short periods of training, the system may downregulate mRNA expression after longer training periods to balance the increased protein levels and return protein production to basal levels [45]. In addition, the techniques employed in different studies may don’t have the same sensitivity to detect SYP changes in various areas. All in all, it seems that an aerobic exercise program can reduce the detrimental effects of diabetes on memory function, but this effect is not directly related to the changes in SYP protein expression analyzed in the present study.
The PSD-95, a most abundant postsynaptic scaffolding protein, is localized at excitatory synapses and plays a crucial role in synapse development and function [46]. PSD-95 is also a potent regulator of ion-channel function, and synaptic activity. Moreover, the amount of PSD-95 controls the balance between the number of inhibitory and excitatory synapses [46]. Overexpression of PSD-95 is associated with increased spine numbers, synaptic efficacy, and hippocampal synaptic plasticity [47]. In this study, treadmill exercise significantly up-regulated PSD-95 expression in the HIP and PFC as compared to diabetic sedentary animals. Previous studies have also shown that diabetes decreases hippocampal PSD-95 and synaptophysin levels [48]. Arnold et al. have also reported that HFD decreased PSD-95 expression and impaired spatial working memory [49]. However, Grillo et al. have demonstrated that induction of diabetes by STZ injection up-regulated hippocampal synaptophysin and PSD-95 expressions [50]. Moreover, BDNF has been shown to regulate the levels of PSD-95, and its blockade impairs the hippocampal PSD-95 expression and decreases the dendritic growth [51]. Furthermore, GSK-3 β is required for phosphorylation of PSD-95, which is essential for AMPA receptor trafficking in dendritic spines and synaptic function [52]. Therefore, it is likely that exercise training increased PSD-95 levels in the HIP and PFC through the up-regulation of BDNF and GSK-3β.
Conclusion
Our results demonstrated that ATET improved the cognitive performance of diabetic rats in the MWM task. Moreover, exercise training up-regulated BDNF and its downstream kinases in both HIP and PFC coincided with considerable elevations in pre-synaptic protein, SNAP-25, and postsynaptic protein, PSD-95, but not synaptophysin. These findings may indicate the molecular mechanisms by which treadmill running improved the deleterious effect of diabetes on cognitive function.
Acknowledgements
We are grateful to the Neurosciences Research Center (NSRC), Tabriz University of Medical Sciences, especially Dr. Farhoudi for their laboratory equipment, exercise facilities, and technical support.
Authors’ contributions
I.S. designed and performed research, analyzed data, and wrote the manuscript. S.D.N. contributed new reagents, and analytical tools and analyzed data and reviewed and edited the manuscript. P.K. analyzed data, and contributed to the discussion and reviewed and edited the manuscript. M.K. designed research, analyzed data, contributed to the discussion, and reviewed and edited the manuscript. S.N. Cooperated in running exercise protocol and data extraction, analyzed data, and wrote the manuscript I.S. is the sponsor of this work and has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
The fund for this study was provided by Dr. Iraj Sadri. This fund was used to purchase the necessary supplies. Authors also declare that no funds, grants, or other support were received during the preparation of this manuscript from other organizations or institutes.
Data availability
Data are all contained within the article.
Declarations
Ethics approval
This study was approved by the research ethics committee (REC) of Tabriz University of Medical Sciences (TUOMS) (IR.TBZMED.REC.1395.1225).
Consent to participate
Not applicable.
Consent for publication
All the participants gave their consent for the publication of identifiable details, which can include photograph(s) and/or videos and/or case history and/or details within the text (“Material”) to be published in the Journal of diabetes and metabolic disorders and the present article.
Conflicts of interest/Competing interests
There are no competing conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Iraj Sadri and Mostafa Khani contributed equally to this work.
Contributor Information
Iraj Sadri, Email: iraj.sadri@gmail.com.
Mostafa Khani, Email: khani_ms@tabrizu.ac.ir.
References
- 1.International Diabetes Federation . IDF Diabetes Atlas 9th edn. Belgium: Brussels; 2019. [Google Scholar]
- 2.Carvalho C, et al. Alzheimer’s disease and type 2 diabetes-related alterations in brain mitochondria, autophagy and synaptic markers. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2015;1852(8):1665–1675. doi: 10.1016/j.bbadis.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Piralaiy E, et al. Cardiac autonomic modulation in response to three types of Exercise in patients with type 2 Diabetic Neuropathy. J Diabetes Metab Diosord. 2021;20(2):1469–1478. doi: 10.1007/s40200-021-00889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrario CR, Reagan LP. Insulin-mediated synaptic plasticity in the CNS: anatomical, functional and temporal contexts. Neuropharmacology. 2018;136:182–191. doi: 10.1016/j.neuropharm.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fadel JR, Reagan LP. Stop signs in hippocampal insulin signaling: the role of insulin resistance in structural, functional and behavioral deficits. Curr Opin Behav Sci. 2016;9:47–54. doi: 10.1016/j.cobeha.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Zee EA. Synapses, spines and kinases in mammalian learning and memory, and the impact of aging. Neurosci Biobehav Rev. 2015;50:77–85. doi: 10.1016/j.neubiorev.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Hooper PL, et al. The central role of heat shock factor 1 in synaptic fidelity and memory consolidation. Cell Stress Chaperones. 2016;21(5):745–753. doi: 10.1007/s12192-016-0709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson JL, et al. Perforant path synaptic loss correlates with cognitive impairment and Alzheimer’s Disease in the oldest-old. Brain. 2014;137(9):2578–2587. doi: 10.1093/brain/awu190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kujach S, et al. Acute sprint interval exercise increases both cognitive functions and peripheral neurotrophic factors in humans: the possible involvement of lactate. Front Neurosci. 2020;13:1455. doi: 10.3389/fnins.2019.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahalakshmi B, et al. Possible neuroprotective mechanisms of physical exercise in neurodegeneration. Int J Mol Sci. 2020;21(16):5895. doi: 10.3390/ijms21165895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karimi SA, et al. Effects of regular Exercise on Diabetes-Induced memory deficits and biochemical parameters in male rats. J Mol Neurosci. 2021;71(5):1023–1030. doi: 10.1007/s12031-020-01724-3. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Miguel A, et al. Exercise prevents downregulation of hippocampal presynaptic proteins following olanzapine-elicited metabolic dysregulation in rats: distinct roles of inhibitory and excitatory terminals. Neurosci. 2015;301:298–311. doi: 10.1016/j.neuroscience.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Diba R, et al. Protective effects of troxerutin on maternal high-fat diet-induced impairments of spatial memory and apelin in the male offspring. Iran J Basic Med Sci. 2018;21(7):682. doi: 10.22038/IJBMS.2018.28170.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khani M, et al. Effect of thyme extract supplementation on lipid peroxidation, antioxidant capacity, PGC-1α content and endurance exercise performance in rats. J Int Soc Sports Nutr. 2017;14(1):1–8. doi: 10.1186/s12970-017-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shima T, et al. Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 Diabetes. Diabetologia. 2017;60(3):597–606. doi: 10.1007/s00125-016-4164-4. [DOI] [PubMed] [Google Scholar]
- 16.Farajdokht F, et al. Inhibition of PTEN protects PC12 cells against oxygen-glucose deprivation induced cell death through mitoprotection. Brain Res. 2018;1692:100–109. doi: 10.1016/j.brainres.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 17.de Reyes L, Casas-Tintó S. Neural functions of small heat shock proteins. Neural Regen Res. 2022;17(3):512. doi: 10.4103/1673-5374.320975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakhotia S, et al. Circulating levels of Hsp27 in microvascular Complications of Diabetes: prospects as a biomarker of diabetic Nephropathy. J Diabetes Compl. 2018;32(2):221–225. doi: 10.1016/j.jdiacomp.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Pourhamidi K, et al. Heat shock protein 27 is associated with better nerve function and fewer signs of neuropathy. Diabetologia. 2011;54(12):3143–3149. doi: 10.1007/s00125-011-2303-5. [DOI] [PubMed] [Google Scholar]
- 20.Tóth ME, et al. Overexpression of Hsp27 ameliorates symptoms of Alzheimer’s Disease in APP/PS1 mice. Cell Stress Chaperones. 2013;18(6):759–771. doi: 10.1007/s12192-013-0428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henstridge DC, Febbraio MA, Hargreaves M. Heat shock proteins and exercise adaptations. Our knowledge thus far and the road still ahead. J Appl Physiol. 2016;120(6):683–691. doi: 10.1152/japplphysiol.00811.2015. [DOI] [PubMed] [Google Scholar]
- 22.Kim OY, Song J. The importance of BDNF and RAGE in diabetes-induced Dementia. Pharmacol Res. 2020;160:105083. doi: 10.1016/j.phrs.2020.105083. [DOI] [PubMed] [Google Scholar]
- 23.He S, et al. Burnout and cognitive impairment: associated with serum BDNF in a Chinese Han population. Psychoneuroendocrinology. 2017;77:236–243. doi: 10.1016/j.psyneuen.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Kim T-W, et al. High-intensity exercise improves cognitive function and hippocampal brain-derived neurotrophic factor expression in obese mice maintained on high-fat diet. J Exerc Rehabilitation. 2020;16(2):124. doi: 10.12965/jer.2040050.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews SC, et al. Intensity matters: high-intensity interval exercise enhances motor cortex plasticity more than moderate exercise. Cereb Cortex. 2020;30(1):101–112. doi: 10.1093/cercor/bhz075. [DOI] [PubMed] [Google Scholar]
- 26.Jamali A, Shahrbanian S, Tayebi SM. The effects of exercise training on the brain-derived neurotrophic factor (BDNF) in the patients with type 2 Diabetes: a systematic review of the Randomized controlled trials. J Diabetes Metabolic Disorders. 2020;19(1):633–643. doi: 10.1007/s40200-020-00529-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert-Gascó H, et al. MAP/ERK signaling in developing cognitive and emotional function and its effect on pathological and neurodegenerative processes. Int J Mol Sci. 2020;21(12):4471. doi: 10.3390/ijms21124471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medina JH, Viola H. ERK1/2: a key cellular component for the formation, retrieval, reconsolidation and persistence of memory. Front Mol Neurosci. 2018;11:361. doi: 10.3389/fnmol.2018.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutigliano G, et al. An isoform-selective p38αMAPK inhibitor rescues early entorhinal cortex dysfunctions in a mouse model of Alzheimer’s Disease. Neurobiol Aging. 2018;70:86–91. doi: 10.1016/j.neurobiolaging.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai H-l, et al. 38 MAPK inhibition improves synaptic plasticity and memory in angiotensin II-dependent hypertensive mice. Sci Rep. 2016;6:27600. doi: 10.1038/srep27600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munoz L, et al. A novel p38α MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer’s Disease mouse model. J Neuroinflammation. 2007;4(1):21. doi: 10.1186/1742-2094-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song C, et al. AMPK/p38/Nrf2 activation as a protective feedback to restrain oxidative stress and inflammation in microglia stimulated with sodium fluoride. Chemosphere. 2020;244:125495. doi: 10.1016/j.chemosphere.2019.125495. [DOI] [PubMed] [Google Scholar]
- 33.Gupta V, et al. Brain derived neurotrophic factor is involved in the regulation of glycogen synthase kinase 3β (GSK3β) signalling. Biochem Biophys Res Commun. 2014;454(3):381–386. doi: 10.1016/j.bbrc.2014.10.087. [DOI] [PubMed] [Google Scholar]
- 34.Ochs S, et al. Loss of neuronal GSK3β reduces dendritic spine stability and attenuates excitatory synaptic transmission via β-catenin. Mol Psychiatry. 2015;20(4):482. doi: 10.1038/mp.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irfan M, et al. SNAP-25 isoforms differentially regulate synaptic transmission and long-term synaptic plasticity at central synapses. Sci Rep. 2019;9(1):1–14. doi: 10.1038/s41598-019-42833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikukheslat SD, Karimi P, Sadri I. Effect of aerobic training on Synaptic Integrity Proteins in Hippocampus and Prefrontal Cortex of type II Diabetic rats. Med J Tabriz Univ Med Sci. 2020;42(2):160–167. [Google Scholar]
- 37.Hu S, et al. Exercise can increase small heat shock proteins (sHSP) and pre- and post-synaptic proteins in the hippocampus. Brain Res. 2009;1249:191–201. doi: 10.1016/j.brainres.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y-F, et al. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol Learn Mem. 2008;90(1):81–89. doi: 10.1016/j.nlm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Perdigão C, et al. Intracellular trafficking mechanisms of synaptic dysfunction in Alzheimer’s Disease. Front Cell Neurosci. 2020;14:72. doi: 10.3389/fncel.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye Q, et al. Ferrostatin-1 mitigates cognitive impairment of epileptic rats by inhibiting P38 MAPK activation. Epilepsy Behav. 2020;103:106670. doi: 10.1016/j.yebeh.2019.106670. [DOI] [PubMed] [Google Scholar]
- 41.Yaribeygi H, et al. Neuromodulatory effects of anti-diabetes medications: a mechanistic review. Pharmacol Res. 2020;152:104611. doi: 10.1016/j.phrs.2019.104611. [DOI] [PubMed] [Google Scholar]
- 42.Fernandes J, et al. Aerobic exercise attenuates inhibitory avoidance memory deficit induced by paradoxical sleep deprivation in rats. Brain Res. 2013;1529:66–73. doi: 10.1016/j.brainres.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Ferreira AF, et al. Short-term, moderate exercise is capable of inducing structural, BDNF-independent hippocampal plasticity. Brain Res. 2011;1425:111–122. doi: 10.1016/j.brainres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Cassilhas R, et al. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neurosci. 2012;202:309–317. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira AF, et al. Moderate exercise changes synaptic and cytoskeletal proteins in motor regions of the rat brain. Brain Res. 2010;1361:31–42. doi: 10.1016/j.brainres.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 46.Nie J, Yang X. Modulation of synaptic plasticity by exercise training as a basis for ischemic Stroke rehabilitation. Cell Mol Neurobiol. 2017;37(1):5–16. doi: 10.1007/s10571-016-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dore K, et al. PSD-95 protects synapses from β-amyloid. Cell Rep. 2021;35(9):109194. doi: 10.1016/j.celrep.2021.109194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang R, et al. Quercetin attenuates diabetic neuropathic pain by inhibiting mTOR/p70S6K pathway-mediated changes of synaptic morphology and synaptic protein levels in spinal dorsal horn of db/db mice. Eur J Pharmacol. 2020;882:173266. doi: 10.1016/j.ejphar.2020.173266. [DOI] [PubMed] [Google Scholar]
- 49.Arnold SE, et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014;67:79–87. doi: 10.1016/j.nbd.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grillo C, et al. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005;136(2):477–486. doi: 10.1016/j.neuroscience.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 51.Kratschke MM. Centre for clinical Brain sciences. Edinburgh: University of Edinburgh; 2018. Investigating PSD-95 turnover at the synapse using the HaloTag technology; p. 322. [Google Scholar]
- 52.Jaworski T, Banach-Kasper E, Gralec K. GSK-3β at the intersection of neuronal plasticity and neurodegeneration. Neural Plast 2019; 2019:14. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are all contained within the article.