Abstract
Studies consistently showed that sodium-glucose cotransporter inhibitors (SGLTi) have cardiovascular and renal benefits, independent of their glucose lowering effects. Recent studies showed that SGLTi might influence gut microbiota. We performed a narrative review of publications focusing on use of SGLTi and changes in gut microbiota. Most studies showed that use of SGLTi re-shapes gut microbiota. These studies are heterogeneous regarding in study designs, doses and types of drugs used (SGLT1i vs. SGLT2i, or SGLT1/2i in combination) and the methods used to determine gut microbiota. However, existing data showed that SGLTi might alter food fermentation and gut permeability, which might translate into clinical outcomes. Thus the objective of this review is to summarize and discuss the updated data regarding SGLTi and changes in gut microbiota for the first time and suggest further study points that needs to be discovered.
Graphical Abstract
Keywords: Canagliflozin, Dapagliflozin, Empogliflozin, Gut permeability, Microbiota, Sodium glucose cotransporter
Introduction
Sodium-glucose cotransporter inhibitors (SGLTi) are a new class of drugs to treat diabetes mellitus. Especially SGLT2i, but also the combination of SGLT1/2i, have cardiovascular and renal benefits, independent of their glucose lowering effects [1]. SGLT2i have favorable effects on inflammation, hypoxia, oxidative stress, metabolic functions and renin-angiotensin system (RAS), which altogether reduce cardiovascular complications [2]. Although the above mentioned mechanisms have been elucidated, other beneficial mechanisms of SGLTi are emerging such as interactions with gut microbiota [3]. In physiologic conditions, humans live in harmony with gut microbiota, which have many beneficial actions for homeostasis, including food fermentation, vitamin production, balancing immune response, and protection against pathogens [4, 5]. Indeed, the pathologic alterations in the gut microbiota are linked to the development of many pathologic entities such as cardiovascular disease, cancer, diabetes, obesity, inflammatory bowel disease and kidney dysfunction [6]. There is reciprocal interaction between microbiota and host and gut microbiota is mutually shaped by humans via lifestyle factors (stress, smoking etc.) and food intake beginning from early stage of life. Additionally, studies have already shown that prebiotics and probiotics are beneficial in various disease conditions [7]. Apart from foods and various prebiotic and probiotic drugs, medications including proton pump inhibitors, metformin, selective serotonin reuptake inhibitors and laxatives, can change and re-shape the gut microbiota [8]. Thus, microbiota may be changed and modulated by diet, health status, age, ethnicity, genetics and medications [9]. Recently, some studies have shown that SGLTi might influence gut microbiota. This finding seems logical since SGLT1 is located at luminal site on the small intestinal brush border and inhibits transport of glucose into enterocytes. On the other hand, major effect of SGLT2i is to inhibit glucose absorption in the kidney tubule. The important question is whether SGLT2i have also actions in the small intestine. This question is important because SGLT2i, but not SGLT1i, are mostly used in clinical practice. The answer to this question is ‘’yes’’ since SGLT2i have varying SGLT1i activity and SGLT2i can reduce intestinal glucose uptake [10]. For example, in humans, transient inhibition of intestinal SGLT1 occurs with 300 mg canagliflozin [11]. Other studies have shown that SGLT2i re-shapes composition of gut microbiota (Table 1). Thus, SGLT2i also have gastrointestinal actions though smaller extent compared to SGLT1i [12, 13]. By blocking upper small intestinal glucose absorption, SGLTi facilitates the delivery of glucose into the ileum and colon, and increases glucose fermentation in the colon. The augmentation of glucose fermentation in distal intestine and colon in tur alters gut microbiota composition [14]. Given the fact that SGLTi have beneficial health effects and these drugs effect gut microbiota, it is important to know whether the change in gut microbiota by these drugs is responsible for these effects. In the current manuscript, we summarized the studies investigating the impact of SGLTi on gut microbiota composition and subsequent outcomes in healthy and disease states.
Table 1.
Studies summarizing the impacts of SGLT inhibition on gut microbiota and related outcomes
| Ref | Methods | Effects on microbiota | Additional findings |
|---|---|---|---|
| [3] |
-Diabetic mice randomized to groups: (I): Control (II): Control + Dapa (60 mg /kg diet; 0.006%) (III): DM (IV): DM + Dapa for 8 weeks -Microbiota analysis was by 16S rRNA gene sequencing |
-Group IV showed reduced richness and diversity vs. Group I and Group II -Firmicutes Bacteroidetes ratio (F/B) and Oscillospira were lower in Group IV vs. other groups -Akkermansia muciniphila decreased in group III vs. Group I, and had an increased trend in Group IV -Enterococcus was higher Group IIII vs. all groups -Lactobacillus were higher in Group I vs. other groups |
-No relationship between microbiota changes and vascular improvements in diabetic mice, although Akkermansia muciniphila improved endothelium-dependent dilation and aortic pulse wave velocity |
| [12] |
-Wild type, C57Bl/6 mice heterozygous null (lack one allele) for SGLT1 (slc5a1) and homozygous null (lack both alleles) for SGLT1 mice were fed with: (I): Chow + vehicle (II): Chow + 1 mg/kg of cmpd 8 (dual SGLT1/2 inhibitor) for 5 days (III): high sucrose (35%) diet + 1 mg/kg of cmpd 8 for 5 days -16S rRNA used for cecal analysis |
-Cecal bacterial diversity was not influenced by cmpd 8 |
Cmpd reduced intestinal glucose absorption -No difference in villus-crypt ratio or villus length with cmpd |
| [19] |
-C57BL/6 mice randomized into groups: (I): Control (II): Adenine induced RF mice (adenine 0.2% for 5 weeks then vehicle) (III): Adenine induced RF mice (adenine 0.2% for 5 weeks) then Cana (10 mg/kg by daily gavage for 2 wk.) -Fecal microbial contents assessed by 16S rRNA |
- (B/F decreased in Groups II and III vs. Group I -Cana did not change B/F -Actinobacteria and TM7 phyla increased in the group II, but were recovered by Cana -Bifidobacterium and unclassified genus in the F16 family increased in Group II but were restored by Cana -Cana decreased the abundance of Oscillospira |
-Cana increased colonic SCFA -Cana did not influence the impaired renal function although it reduced p-cresyl sulfate indoxyl sulfate asymmetric dimethylarginine, symmetric dimethylarginine, and guanidinosuccinic acid |
| [18] |
- db/db mice were assigned to four treatment groups for 6 weeks: (I): Vehicle with normal chow diet (NCD) (II): Dapa with NCD (III): Vehicle with 5% sodium butyrate-supplemented NCD (NaB) (IV): Dapa with 5% NaB -Fecal microbiota was analyzed with rRNA |
-In Group IV (F/B), Adlercreutzia and Alistipes decreased and Streptococcus increased - F/B increased in Group II vs. group I but decreased Groups III and IV vs. Group I and II -Anaerotruncus, Mucispirillum, Alistipes and Oscillospira were decreased in Group IV vs. Group I and II |
- Alistipes and Adlercreutzia showed a positive correlation with total fat gain, whereas Streptococcus showed a negative correlation –In Groups I, III, and IV increase of body weight was attenuated The Dapa + NaB group gained the least total and abdominal fat from baseline -The main contributor for microbiota change was butyrate, but not Dapa |
| [27] |
-A human study of 4-week run-in phase following 12-week intervention period -All patients were already on metformin -44 patients were randomized to either Dapa (N:24) or gliclazide (N:20) -At baseline and after 12 weeks, fecal samples and 24-h urine were collected -Microbiome analyses was by 16S rRNA |
-Neither Dapa nor gliclazide affected gut microbiome diversity and composition | -Dapa reduced fasting insulin, body mass index, fat mass and waist circumference, gliclazide increased these parameters |
| [20] |
-Sprague Dawley rats were fed a high-fat diet for 8 weeks and then were given a single dose of streptozotocin injection (30 mg/kg, i.p) to induce diabetes -Diabetic rats were then randomized into 3 groups receiving (I): Normal saline (2 ml, 0.9%) (II): Metformin (215.15 mg/kg/day) (III): Dapa (1 mg/kg/day) for 4 weeks -Fecal microbiome content was assessed by 16S rRNA |
-Beneficial Lactobacillaceae and Bifidobacteriaceae increased with metformin, but not with Dapa -Proteobacteria (especially Desulfovibrionaceae), Oscillibacter Lachnoapiraceae, Ruminiclostridium were enriched in Group III -Shannon index was higher in Group III vs. Group II, but indices indicating richness, including Sobs, Ace, and Chao1 were similar -Firmicutes and Bacteroidetes did not change in either group -Actinobacteria and Spriochaetes were lower in Group III vs. Group II |
-Blood glucose decreased similarly between Dapa vs. metformin groups -Dapa decreased the levels of HOMA-IR when compared with metformin and the control |
| [14] |
Mice divided into 3 groups: (I): Control (II): RF mice (induced by 0.2% adenine (0.2% adenine diet for at least 6 weeks) (III): Adenine induced RF mice + low-absorbable SGLT1 inhibitor, SGL5213 (100 mg/kg) -Group II and Group III were administered a 10 mg/kg glucose in a 100 μl volume after 20–22 h of fasting -Fecal microbial contents were assessed by 16S rRNA |
-Chao 1 and Shannon indices showed lower richness and diversity in the RF group, but were restored by SGL5213 -Firmicutes and F/B increased and Bacteroidetes decreased in Groups II and III vs. Group I -SGL5213 rebalanced the dysbiotic gut and reduced F/B ratio (increased Bacteroidetes and decreased Firmicutes) -Actinobacteria increased in Groups II and II, but this increase was attenuated by SGL5213 in Group III -At the genus level within Firmicutes, Allobaculum was increased in Group II group, but was ameliorated by SGL5213 in Group III -Turicibacter increased in Group II and more in Group III -Within Bacteroidetes, Bacteroides and S24-7; and Rikenellaceae decreased in Group II, but reduction was canceled by SGL5213 in Group III -Bifidobacterium increased in Group II which was canceled by SGL5213 -Corynebacterium increased only in Group III |
-Plasma levels of indoxyl sulfate, phenyl sulfate and trimethylamine N-oxide increased in Group II, but decreased significantly in Group III except forindoxyl sulfate -SGL5213 ameliorated renal damage and reduced fibrosis and macrophage infiltration in an adenine induced RF model -Effect of SGTl5213 on phenyl sulfate reduction may depend mainly on the changes in the gut microbiome |
| [21] |
-C57BL/6 mice received a high-fat-high-fructose (HFHC) diet with a surplus of cholesterol for 16 weeks to induce Nonalcoholic steatohepatitis Mice then were treated with either dulaglutide, Empa or their combination -16S rRNA gene was used for stool analysis |
-Empa and dulaglutide and their combination increased Bacteriodetes with a concomitant reduction of Firmicutis | -Dulaglutide alone and in combination with Empa led to significant weight loss, improved glucose homeostasis and diminished anti-inflammatory and anti-fibrotic pathways |
| [44] |
-C57BL/6 J mice were randomized into 4 groups: (I): Control: Received normal-chow diet (NCD) (II): High fed diet (HFD) Group (III): HFD + metformin (200 mg/kg) (IV): HFD + Cana (50 mg/kg) for 6 weeks -Total bacterial DNA was isolated from colonic contents by the QIAamp DNA Stool Mini Kit and the colonic bacterial 16S rDNA gene was amplified by PCR, then the PCR products were purified by the QIAquick Gel Extraction Kit |
-At the phylum level, Cana group had a higher abundance of Bacteroidetes with a lower abundance of Firmicutes, and an increased ratio of F/B compared to HFD group -Proteobacteria increased with HFD, decreased with Cana, but was not changed with metformin -Cana enriched the abundance of Actinobacteria and decreased the abundance of Deferribacteres which occurred with HFD -At genus level, Cana increased relative abundance of Olsenella, Alistipes, and Alloprevotella suggesting a healthier microbiome |
-In cardiac tissue, Cana decreased lipid accumulation, inflammation and oxidative stress induced by HFD -Alteration in gut microbiota with Cana was associated with a decrease in systemic inflammation, oxidative stress and lipid accumulation |
| [29] |
-Randomized, open-label, included 76 treatment-naïve patients with T2DM divided into 2 groups: -(I): Empa (10 mg/d) for 3 months (n:40) -(II): Metformin (1700 mg/d) for 3 months (n:36) -Microbiota was analyzed by 16S rRNA and plasma metabolites were analyzed by liquid chromatography-tandem mass spectrometry |
-Empa, but not metformin, increased richness and diversity (Shannon index) of gut microbiota after 1 month of treatment, which was maintained until the end of the trial -Empa, but not metformin, increased SCFA-producing bacteria such as Eubacterium, Roseburia and Faecalibacterium, and reduced harmful bacteria including Escherichia, Shigella, Bilophila, and Hungatella |
-HbA1c, glucose, body weight, waist circumference, and waist-hip circumference ratio were decreased both in Empa and metformin groups -Interleukin-6 was decreased more in Empa vs. metformin group -Blood pressure and uric acid were decreased, hematocrit and adipokine were increased only with Empa -Sphingomyelin and capric acid were increased, glycochenodeoxycholate, cis-aconitate and erythritol were decreased only with Empa -Empa modified plasma metabolites and gut bacteria related to clinical parameters, including blood glucose levels and inflammatory factors |
| [35] |
- C57BL/6 J mice were randomized into: Group I: Normal fat diet Group II: Diabetic group (induced by high-fat diet + streptozocin injection) -Group III: Diabetic group + Empa (10 mg/kg/day) for 13 weeks -Microbiome was analyzed by 16S rRNA -Fecal SCFAs and fecal and serum LPS were determined |
-Alpha diversity was reduced in Group II vs. Group I, but was restored by Empa in Group III -LPS-producing bacteria, Oscillibacter was increased in Group II but was decreased in Group III - SCFA-producing bacteria, Bacteroid and Odoribacter were decreased in Group II and were increased in Group III |
–Empa reduced glucose and urinary albumin creatinine ratio in diabetic mice -Empa inhibited thickening of the colonic crypt, restored goblet cells and the expressions of zonulin 1 and occludin - The decrease in fecal SCFA and increase in LPS in group II were alleviated by Empa in group III -Especially acetic acid and butyric acid were increased by Empa -LPS levels were positively correlated with LPS-producing bacteria, Oscillibacter and Bilophila -SCFA levels were positively correlated with SCFA-producing bacteria, Bacteroides and Odoribacter -Therapeutic effect of Empa was related to the metabolic products such as SCFA and LPS of gut microbiota |
| [36] |
-db/db mice fed either with standard diet (4 kcal/g, carbohydrate kcal 64%, fat kcal 16%) or low-carbohydrate diet (4 kcal/g, carbohydrate kcal 51%, fat kcal 21%) for 8 weeks were divided into 3 -(I): Normal diet (6.0 g/day) -(II): Low-carbohydrate diet -(III): Normal diet + 0.01% luseogliflozin -Metagenomic deep shut gun analysis was performed to stool samples -LEfSe analysis was performed to compare the gut microbiota among groups |
-Enterobacterales, Escherichia, Gammaproteobacteria and Bacteroides increased in Group II vs. Groups I and III -Syntrophothermus lipocalidus, Parabacteroidesdistasonis distasonis Syntrophomonadaceae family and the genus Anaerotignum increased in Group III |
-Skeletal muscle mass was decreased in Group II -Amino acid content in skeletal muscle and liver were decreased in the Group II, but was increased in Group III -Fecal SCFAs (propanoic acid, acetic acid, and butanoic acid) were higher in Group III vs. Groups I and II -Saturated fatty acids (palmitic, lauric, stearic and myristic acids) in the skeletal muscle were lower, whereas unsaturated FA (oleic acid) was higher in Group III vs. Groups I and II -Increased SCFA in Group III was associated with an increase in the family of Syntrophomonadaceae, Syntrophothermus lipocalidus and genus Anaerotignum |
| [45] |
-Sprague–Dawley rats were induced diabetic by i.p streptozotocin injection -Mice (8 mice in each group) were divided according to combination Drugs: -Crocin, Dapa solely and with or without Lactobacillus for 6 weeks -Change in microbiota abundance was investigated using real-time PCR |
-In diabetic rats, Lactobacillus, Bifidobacteria, E. Faecium were decreased, and E. coli, Bacteroides, Clostridium, Providencia, Fusobacterium, and P. Aeruginosa were increased compared to controls -Dapa, crocin and Lactobacillus treatment increased P. aeruginosa, Fusobacterium, Bacteroides E. coli Providencia and Clostridium species |
-Addition of Lactobacillus or crocin to dapa decreased blood glucose compared to Dapa alone -Combination of Lactobacillus, crocin and Dapagliflozin showed a significant amelioration of adiponectin when compared to crocin-treated group -Crocin, dapa, lactobacillus all decreased tumor necrosis factor-α and interleukin-1β and increased interleukin 10 -Diabetic rats exhibited cardiac fibrous, which was most efficiently healed by a combination of Dapa + crocin + lactobacillus -Dapa as a monotherapy failed to restore the normal histological characterization of the cardiac tissue |
| [46] |
- C57BL/6 J mice divided into (I): Control: fed with a chow diet (320 kcal/100 g) for 20 weeks, (II): High Fed Diet Group: Fed with an HFD (54.05% fat, 529.8 kcal/100 g) for 12 weeks and with saline for another 8 weeks (III) EMPA group: Fed with HFD (54.05% fat, 529.8 kcal/100 g) for 12 weeks and then administered EMPA (10 mg/kg/d) for another 8 weeks - RNA-seq and data analysis, Metagenomic sequencing and analysis performed |
- Compared with the CT group, the HFD group had a higher Simpson index (0.14 ± 0.04 vs. 0.40 ± 0.01, p < 0.05); however, no significant differences in alpha diversity (Shannon and Simpson - In the HFD and EMPA groups, Faecalibaculum rodentium was elevated at the species level, and there was a higher proportion of Faecalibaculum at the genus level compared with the CT group - Bacteroidota was the dominant phylum in the CT group, accounting for over half of all microbes, and Firmicutes dominated in the HFD and EMPA groups - Compared with obese mice, the empagliflozin-treated mice had a higher species diversity of gut microbiota, characterized by a reduction in the Firmicutes/Bacteroides ratio |
- HFD induced metabolic disturbance but ameliorated by Empa - RNA sequencing results showed that immunoglobulin A and peroxisome proliferator-activated receptor signaling pathways in the intestinal immune network were activated after empagliflozin treatment. This integrative analysis highlighted that empagliflozin maintains intestinal homeostasis by modulating gut microbiota diversity and tryptophan metabolism |
| [23] |
- C57BL/6 mice were randomized into 3 groups: -(I): Sham -(II): MI (induced by left anterior descending ligation), 0.9% saline (15 mL/kg/day) for 4 weeks -(III): Dapa (1.5 mg/kg/day) for 4 weeks -Echocardiography was obtained on day 28 post MI -Masson's trichrome staining was used to determine the degree of fibrosis -Microbiota was analyzed by 16S rRNA gene sequencing |
-Dapa increased beneficial bacteria like Lactobacillaceae, while decreased other beneficial bacteria like Bifidobacteriaceae -Proteobacteria (especially Desulfovibrionaceae) increased in Group III -Ace index and chao1 index were both lower in the Group III vs. Group II -No differences observed in the indexes indicating diversity, such as Shannon and Simpson indices - F/B were similar among groups -Dapa increased Rumonicoccaceae, Desulfovibrionaceae, Eggerthellaceae, Lachnospiraceae, but decreased Bifidobacteriaceae and Erysipelotrichaceae -Compared with the MI group, dapa enriched Lactobacillaceae and Akkermansiaceae, while reduced Bifidobacteriaceae |
-Group II exhibited decreased ejection fraction, increased intestinal villi atrophy and reduced goblet cells, which were alleviated in Group III with dapa -Occludin and claudin decreased post MI, and dapa partially restored occludin and zonulin levels -Positive correlation was found between Lactobacillaceae and left ventricular systolic function parameters |
| [47] |
-21 treatment naïve patients with T2DM received 30 days of Cana (100 mg/d), 10 healthy volunteers served as controls -Stool, oral, ocular and blood samples were collected after 8 h of overnight fasting at pre- and post-Cana treatment -Stool and oral surface samples were processed for DNA extraction and PCR amplification -16S rRNA sequencing was applied to gut and oral bacteria |
-Cana resulted in an increase of SCFA-producing bacteria including Lachnospiraceae UCG 004, Bacteroides and Lachnospiraceae NK4A136 -In oral mucosa, Cana treatment increased in Prevotella and Veillonella, which are known to increase production after Cana tx |
-Gut microbiota alterations with Cana resulted in a significant decrease in glycated serum protein -FPG negatively correlated with microbiota like Phocea and Lachnospiraceae NK4A136 which increased after Cana treatment, |
| [48] |
- Male 8-week-old C57BL/6N mice randomly assigned to three groups: (I): Control: thoracotomy without ligation of left anterior descending coronary artery (II): Heart Failure (induced via thoracotomy with permanent ligation of left anterior descending coronary artery (III): HF + Dapa (1 mg/kg/day) - At the end of the 8-week period, mice were euthanized - Fecal collection and 16S rDNA sequencing and microbiome bioinformatics was performed |
-Dapa decreased the HF-induced increase in the Firmicutes/Bacteroidetes ratio - At the genus level, Desulfovibrio, AF12, and Paraprevotella were enriched in the HF + dapagliflozin group |
-Dapa decreased Monocyte chemoattractant Protein-1 IL-1β, IL-6, and IL-17 which are elevated in HF group - Dapa increased endothelial-dependent dilation - Dapa ameliorated cardiac hypertrophy and cardiac fibrosis induced by HF |
| [49] |
- ApoE−/− mice fed a HFD received either Empa (10 mg/kg) or saline for 12 weeks -Feces collected from both groups for fecal microbiota transplantation (FMT) Another 12 six-week-old male ApoE− /− mice were fed HFD and received FMT with feces either from Empa (FMT-Empa group) or from Ctrl (FMT-saline) - intestinal flora we assessed in feces collected from Ctrl and SGLT2i groups by 16S ribosomal RNA (16S rRNA) gene sequencing |
-Firmicutes/Bacteroidetes (F/B) ratio in Empa group lower vs. saline -Muribaculaceae and Lachnospiraceae higher in SGLT2i group -Lactobacillus, Subdoligranulum, and Clostridium were enriched in feces of mice treated with Empa than in their counterparts. In contrast, pathogenic bacteria Dorea were more enriched in the saline group |
- Atherosclerosis was less severe in the Empa vs saline group -Systemic inflammatory parameters decreased with Empa |
| [50] |
- C57BL/6 mice and BKS.Cg-Dock7m + / + Leprdb/J (db/db) mice used Group (I): Control- C57BL/6 mice received saline Group II: db/db mice + saline Group II: db/db mice treated with DAPA [1.0 mg/kg/day) at 14 weeks,18 weeks and 22 weeks - 16S rRNA gene analysis was used for a-diversity and b-diversity of the gut microbiota |
- At 14 weeks at the phylum level, harmful proteobacteria, Firmicutes/Bacteroidota (F/B) ratio increased but beneficial Patescibacteria diminished in dm/dm mice -At genus level, pathogenic bacteria such as Escherichia, Shigella expanded; beneficial bacteria such as Roseburia, unclassified_Lachnospiraceae, Alistipes, Akkermansia, and Bifidobacterium diminished in dm/dm mice - After Dapa treatment for 16 weeks, Bacteroidetes increased, and Firmicutes decreased at 22 weeks - At both 18 and 22 weeks, Dapa consistently reversed the abundance changes of Lactobacillus and Muribaculaceae |
- Dapa delayed the progression of diabetic kidney disease |
| [51] |
- Spontaneously hypertensive rats assigned to (I): Sham (II): 5/6th nephrectomized (Nx) (III): Nx + Cana administered 0.024% in standard chow (IV): Nx + Tofogliflozin administered 0.015% tofogliflozin in standard chow - The abundance of specific bacterial groups in the rat fecal samples were measured by real-time quantitativePCR |
- Bacteroides species were more abundant in Nx rats and Cana had no effect on this difference - Lactobacillus species were less abundant in Nx rats, and this phenotype was reversed by Cana |
- Claudin-1 and ZO-1 were decreased in Nx rats, which were ameliorated by Cana - Uremic toxins of indoxyl sulfate, P-cresyl sulfate hippuric acid, and indole acetic acid were significantly increased by 5/6th nephrectomy -Cana decreased indoxyl sulfate, and hippuric acid, but had no effects on P-cresyl sulfate, and indole acetic acid - There was no significant difference in the abundance of Lactobacillus these bacteria between the Nx + T group and the Nx group |
| [52] |
- C57BL/6 mice received chow diet and HFD for 8 weeks and HFD mice intraperitoneally injected with streptozotocin 40 mg/kg/day for 5 days thereafter -Normal chow diet group, mice were intraperitoneally injected with the same amount of citrate buffer for 5 days -Mice then divided into 3 groups: (I): Normal chow group (II): HFD/ streptozotocin group (and an (III): HFD/ streptozotocin + Empa (10 mg/kg/days, dissolved in normal saline) by gavage daily for 6 weeks -For the control and HFD/STZ groups, mice were administered the same amount of normal saline by gavage daily for 6 weeks -Fecal sample collection and 16S rRNA amplicon sequencing for microbiome analysis |
- α diversity reduced in HFD/STZ remarkably reduced the abundance and diversity of the gut microbiota vs. controls and Empa administration reversed this effect -Lactobacillus increased but Ruminococcus and Adlercreutzia were reduced in the Empa group vs. HFD/ streptozotocin group |
Empa improved glucose metabolism and liver function with reduced liver fibrosis, |
Dapa Dapagliflozin; DM Diabetes Mellitus; SGLT Sodium-Glucose Cotransporter; Cana Canagliflozin; HOMA-IR Homeostatic Model Assessment for Insulin Resistance; Empa Empagliflozin; LPS Lipopolysaccharide; SCFA Short Chain Fatty Acids; Luseo, Luseogliflozin
Methods
We performed review of the literature in August 2023 using major online databases (PubMed, Web of Science, Scopus, Cochrane Library and Google Scholar) using the key words "sodium-glucose cotransporter inhibitors and gut microbiota", "sodium-glucose cotransporter inhibitors and gut dysbiosis", "sodium-glucose cotransporter inhibitors and intestinal metabolism", "Canagliflozin and microbiota", "dapagliflozin and microbiota", "empogliflozin and microbiota" and, "luseogliflozin and microbiota". We included full-text articles, observational and experimental studies which were only published in English language. Two independent researchers analyzed the titles and abstracts and selected relevant articles. The flow chart of the articles is shown in Fig. 1.
Fig. 1.
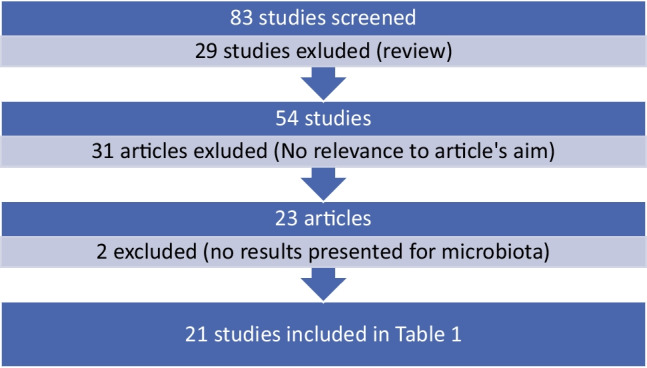
Flow chart of the study selection process
Results
Studies summarizing the impacts of SGLTi on gut microbiota
There is a consistent increase in the number of studies investigating the impact of SGLTi on gut microbiota, yet, the data is still scarce and conflicting (Table 1). While some studies showed that SGLTi increased beneficial bacteria, others showed no effect and a few studies showed detrimental effects on gut microbiota. On the phyla level, Firmicutes and Bacteroidetes take predominant occupation in gut microbiota, where they account for nearly 90% of the known phylogenetic categories [15]. Firmicutes to Bacteroidetes ratio (F/B) was a commonly used marker of gut dysbiosis and was associated with fecal short chain fatty acids (SCFAs) production [16]. Both an increased (as seen in obesity) and a decreased (as seen in inflammatory bowel disease) F/B have been associated with gut dysbiosis [17]. Some studies showed that F/B was increased by SGLTi [18], but others showed no effect of SGLTi on F/B [19, 20], or decreased F/B by SGLTi [14, 21]. Lactobacillaceae has been traditionally recognized as a potential health-promoting microbe species in the human gastrointestinal tract [22] and SGLTi increased lactobacillus in some [23], but not in other studies [20]. Even in the same study, it was demonstrated that SGLTi may increase one type of beneficial bacteria, while decreasing another beneficial bacterium. For example, dapagliflozin increased beneficial bacteria such as Lactobacillaceae, but decreased Bifidobacteriaceae which is other beneficial bacteria [23]. As shown in Tablel 1, various other changes in microbiota composition occurs which was associated with SGLT inhibition.
Most important question is why is there a difference between results of these studies? The exact answer is not known, but some factors may play a role for these inconsistent findings. First of all, the duration and the dosage of SGLT inhibitors are different in these studies (Table 1). These studies are mostly short term, thus the long term effects of the SGLT inhibition on gut microbiota are not known. Secondly, the SGLT1 inhibitor activity of SGLT2i differs among different SGLT2i [10]. For example, dapagliflozin is a much weaker SGLT1 inhibitor, as compared to canagliflozin [24], which suppresses SGLT1 by 40–60% [25]. Thirdly, the methods used for the determination of gut microbiota may also explain the different findings. For instance, while most of the studies used 16S rRNA gene sequencing, however, metagenomics sequencing may also be used to assess gut microbiota and these two different methods might have yielded different findings to some extend [23]. Fourthly, mice bred in the same facility vs. mice purchased from other sources might display differences in their baseline gut microbiota [26]. Lastly, and most importantly, food and concomitantly used medications, both of which are mutually related with gut microbiota, might have influenced the results. For example, in one study, dapagliflozin had no effects on microbial composition of gut in patients with type 2 diabetes. However, all of the enrolled patients were previously on metformin therapy and the metformin use might influence the results, as appropriately acknowledged by the authors [27]. Indeed, metformin could restore SGLT1 expression and glucose sensing to alter the upper small intestinal microbiota [28]. To further support this notion, in 76 treatment naïve type 2 diabetic patients, empagliflozin, but not metformin, increased SCFA producing bacteria and decreased harmful bacteria [29].
Potential benefits of blocking small intestinal glucose reabsorption by SGLT inhibitors
As suggested above and demonstrated in Table 1, SGLTi have potential to change gut microbiota. However, the clinical consequences of this change is not fully appreciated currently. One of the potential reasons for this data deficit is the fact that the SGLT inhibitors are relatively newer drugs tor treatment of diabetes and also the studies are only emerging about the impact of SGLTi on gut microbiota. Still, there are some quite data emerging which one of them impact on metabolism including carbohydrate and protein metabolism. Indeed, SGLT inhibition suppresses dietary carbohydrate absorption in the upper small intestine and increases delivery to the lower gastrointestinal tract. Consequently, the gut microbiota and microbiota-derived metabolites are altered with potential benefits including decreased gut permeability (Fig. 2). During fermentation, P-cresyl sulfate (PCS) and Indoxyl sulfate (IS) are derived from fermentation of dietary amino acids in the colon. By contrast, SCFAs (acetate, butyrate, and propionate) are generated from the fermentation of carbohydrates that are not digested in the small intestine [30]. An increase in carbohydrates in the large intestine could promote the production of SCFAs by carbohydrate fermentation, suppressing protein fermentation [31]. SCFAs participate in the maintenance of intestinal mucosal integrity, improve glucose and lipid metabolism, increase the secretions of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), and regulate the immune system. Besides, SCFAs, have anti-tumor activity, they decrease oxidative stress and inflammatory responses, as such, they confer protection against cardiovascular and kidney diseases [32, 33]. Furthermore, SCFAs in the intestine enhance the intestinal barrier function by increasing the mucin layer of the intestinal mucosa [34].
Fig. 2.
The potential benefits of sodium glucose transport inhibition in the Gut. Sodium Glucose co-transport Inhibitors mostly SGLT1i but to a lesser extend SGLT2i decrease glucose abortion in proximal small intestine which cause increased glucose surge and glucose fermentation in the distal small intestine and colon. The increased surge of glucose and colonic fermentation reduces gut dysbiosis and increase beneficial microorganisms. These microorganisms increase beneficial molecules such as short chain fatty acids (SCFA) and decrease harmful substances such as indoxyl sulfate (IS), phenyl sulfate (PS), P-cresyl sulfate (PCS) and trimethylamine N-oxide (TMAO). Furthermore, these changes are accompanied by restoration of gut permeability with reduced lipopolysaccharide translocation from gut lumen to systemic circulation. These changes all end-up with lower systemic inflammation with potential cardiovascular and kidney benefits
Some studies showed that SGLT inhibition increases SCFA production by gut microbiota [19, 29, 35, 36], which might explain their beneficial effects. It has been reported that gut dysbiosis and disruption of the tight junctions in the intestinal and colonic epithelium were observed in cardiovascular diseases and renal failure. This results in increased permeability of the intestinal mucosal barrier and translocation of gut bacteria-derived lipopolysaccharides into bloodstream to various organs throughout the body, causing systemic inflammation [35, 37, 38]. The proper function of intestinal tight junctions depends on tight junction proteins including zonulin, occludin and claudin, and gut microbiota has important functions that in turn regulate functions of tight junction [39]. In diabetic rats, expressions of zonulin-1 and occludin were decreased, which were recovered by empagliflozin [35]. Furthermore, dapagliflozin restored the decreased occludin and claudin levels [23].
In renal failure, intestinal fermentation of amino acids, by gut microbiota produce substances such as PCS and IS which play a role for progression of chronic kidney disease [40]. Importantly, canagliflozin treatment even for 2 weeks decreased PCS and IS and increased SCFAs, which were associated with beneficial gut bacterial change [19]. Treatment with SGLT1 inhibitor, SGL5213, for 6 weeks decreased the elevated plasma levels of trimethylamine N-oxide (TMAO) and phenyl sulfate in mice. Yet, the elevated level of IS did not change in adenine induced renal failure model. Nonetheless, these changes were associated with gut microbiome [14]. Empagliflozin, but not metformin, increased sphingomyelin but reduced glycochenodeoxycholate, cisaconitate and uric acid levels. Concurrently, empagliflozin elevated levels of SCFA-producing bacteria, such as species from Roseburia, Eubacterium, and Faecalibacterium, and reduced harmful bacteria including Escherichia, Shigella, Bilophila, and Hungatella [29]. These findings are important since sphingomyelin prevents translocation of gut bacteria derived lipopolysaccharides [41], and glycochenodeoxycholate acid is correlated with insulin resistance and hepatic steatosis [42, 43].
Perspectives and conclusions
SGLT inhibitors are relatively new anti-diabetic agents which have beneficial cardiovascular and renal effects independent of their blood glucose lowering effects. Recently, some, but not all studies, have shown that these drugs re-shape gut microbiota, and they alter food fermentation and intestinal permeability. The clinical reflections of these findings are not clear. The inconsistency of the findings of different studies might be explained by the variations in drug formulations and dosing, affinities for receptors, treatment duration, drug-drug interactions and the methods used to determine gut microbiota. As the literature is newly emerging, more studies are needed to highlight the impacts of SGLT inhibition on gut microbiota and associated clinical findings. It is especially important to explore whether the cardiovascular and renal benefits of SGLTi might partly be explained by changes in gut microbial diversity and their metabolism. Another important issue is the impact of gut microbiota on SGLTi absorption, metabolism or biotransformation as a bi-directional effect. Unfortunately, there are no specific studies in this area. Lastly, studies are needed to highlight the specific impact of SGLT1i, SGLT2i, and SGLT1/2i combination on gut microbiota.
In conclusion, preliminary fındings suggest that SGLTi change gut microbiome diversity and colonic fermentation. Whether these changes are related to hard outcomes are not currently known. Future studies should investigate whether SGLTi consistently decrease toxin production, repair intestinal permeability and favor metabolic health through gut microbiota.
Author contributions
Baris Afsar: Conceptualization, database search. tables and writing (original draft), Rengin Elsurer Afsar: Graphs and writing (manuscript revision). All authors approved the final version of the article. Krista L Lentine: Supervision, writing and reviewing manuscript.
Funding
None declared.
Declarations
Conflict of interest
None declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Salah HM, Al'Aref SJ, Khan MS, et al. Effects of sodium-glucose cotransporter 1 and 2 inhibitors on cardiovascular and kidney outcomes in type 2 diabetes: A meta-analysis update. Am Heart J. 2021;233:86–91. doi: 10.1016/j.ahj.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Afsar B, Afsar RE. Sodium-glucose cotransporter inhibitors and kidney fibrosis: review of the current evidence and related mechanisms. Pharmacol Rep. 2023;75(1):44–68. doi: 10.1007/s43440-022-00442-4. [DOI] [PubMed] [Google Scholar]
- 3.Lee DM, Battson ML, Jarrell DK, et al. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol. 2018;17(1):62. doi: 10.1186/s12933-018-0708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillman ET, Lu H, Yao T, Nakatsu CH. Microbial ecology along the gastrointestinal tract. Microbes Environ. 2017;32(4):300–313. doi: 10.1264/jsme2.ME17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 6.Hou K, Wu ZX, Chen XY, et al. Microbiota in health and diseases. Signal Transduct Target Ther. 2022;7(1):135. doi: 10.1038/s41392-022-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7(1):17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69(8):1510–1519. doi: 10.1136/gutjnl-2019-320204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montandon SA, Jornayvaz FR. Effects of antidiabetic drugs on gut microbiota composition. Genes (Basel). 2017;8(10):250. [DOI] [PMC free article] [PubMed]
- 10.Evenepoel P, Meijers B, Masereeuw R, Lowenstein J. Effects of an SGLT inhibitor on the production, toxicity, and elimination of gut-derived uremic toxins: a call for additional evidence. Toxins (Basel). 2022;14(3):210. [DOI] [PMC free article] [PubMed]
- 11.Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36(8):2154–2161. doi: 10.2337/dc12-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du F, Hinke SA, Cavanaugh C, et al. Potent sodium/glucose cotransporter SGLT1/2 Dual inhibition improves glycemic control without marked gastrointestinal adaptation or colonic microbiota changes in rodents. J Pharmacol Exp Ther. 2018;365(3):676–687. doi: 10.1124/jpet.118.248575. [DOI] [PubMed] [Google Scholar]
- 13.Sayour AA, Oláh A, Ruppert M, Barta BA, Merkely B, Radovits T. Effect of pharmacological selectivity of SGLT2 inhibitors on cardiovascular outcomes in patients with type 2 diabetes: a meta-analysis. Sci Rep. 2024;14(1):2188. doi: 10.1038/s41598-024-52331-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho HJ, Kikuchi K, Oikawa D, et al. SGLT-1-specific inhibition ameliorates renal failure and alters the gut microbial community in mice with adenine-induced renal failure. Physiol Rep. 2021;9(24):e15092. doi: 10.14814/phy2.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4(6):e121. doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8(11):1715. [DOI] [PMC free article] [PubMed]
- 18.Oh TJ, Sul WJ, Oh HN, et al. Butyrate attenuated fat gain through gut microbiota modulation in db/db mice following dapagliflozin treatment. Sci Rep. 2019;9(1):20300. doi: 10.1038/s41598-019-56684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishima E, Fukuda S, Kanemitsu Y, et al. Canagliflozin reduces plasma uremic toxins and alters the intestinal microbiota composition in a chronic kidney disease mouse model. Am J Physiol Renal Physiol. 2018;315(4):F824–f833. doi: 10.1152/ajprenal.00314.2017. [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Shi FH, Liu W, et al. Dapagliflozin modulates the fecal microbiota in a type 2 diabetic rat model. Front Endocrinol (Lausanne) 2020;11:635. doi: 10.3389/fendo.2020.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hupa-Breier KL, Dywicki J, Hartleben B, Wellhöner F, Heidrich B, Taubert R, Mederacke YE, Lieber M, Iordanidis K, Manns MP, Wedemeyer H, Hardtke-Wolenski M, Jaeckel E. Dulaglutide alone and in combination with empagliflozin attenuate inflammatory pathways and microbiome dysbiosis in a non-diabetic mouse model of NASH. Biomedicines. 2021;9(4):353. [DOI] [PMC free article] [PubMed]
- 22.Kleerebezem M, Vaughan EE. Probiotic and gut lactobacilli and bifidobacteria: molecular approaches to study diversity and activity. Annu Rev Microbiol. 2009;63:269–290. doi: 10.1146/annurev.micro.091208.073341. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Wang K, Ding Y, et al. Dapagliflozin modulates the faecal microbiota after myocardial infarction in non-diabetic mice. Clin Exp Pharmacol Physiol. 2023;50(1):68–81. doi: 10.1111/1440-1681.13727. [DOI] [PubMed] [Google Scholar]
- 24.Papakitsou I, Vougiouklakis G, Elisaf MS, Filippatos TD. Differential pharmacology and clinical utility of dapagliflozin in type 2 diabetes. Clin Pharmacol. 2019;11:133–143. doi: 10.2147/CPAA.S172353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori K, Saito R, Nakamaru Y, Shimizu M, Yamazaki H. Physiologically based pharmacokinetic-pharmacodynamic modeling to predict concentrations and actions of sodium-dependent glucose transporter 2 inhibitor canagliflozin in human intestines and renal tubules. Biopharm Drug Dispos. 2016;37(8):491–506. doi: 10.1002/bdd.2040. [DOI] [PubMed] [Google Scholar]
- 26.Franklin CL, Ericsson AC. Microbiota and reproducibility of rodent models. Lab Anim (NY) 2017;46(4):114–122. doi: 10.1038/laban.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Bommel EJM, Herrema H, Davids M, Kramer MHH, Nieuwdorp M, van Raalte DH. Effects of 12-week treatment with dapagliflozin and gliclazide on faecal microbiome: Results of a double-blind randomized trial in patients with type 2 diabetes. Diabetes Metab. 2020;46(2):164–168. doi: 10.1016/j.diabet.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Bauer PV, Duca FA, Waise TMZ, et al. Metformin alters upper small intestinal microbiota that impact a glucose-SGLT1-sensing glucoregulatory pathway. Cell Metab. 2018;27(1):101–117.e105. doi: 10.1016/j.cmet.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Deng X, Zhang C, Wang P, et al. Cardiovascular benefits of empagliflozin are associated with gut microbiota and plasma metabolites in type 2 diabetes. J Clin Endocrinol Metab. 2022;107(7):1888–1896. doi: 10.1210/clinem/dgac210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 31.Furuse SU, Ohse T, Jo-Watanabe A, Shigehisa A, Kawakami K, Matsuki T, Chonan O, Nangaku M. Galacto-oligosaccharides attenuate renal injury with microbiota modification. Physiol Rep. 2014;2(7):e12029. [DOI] [PMC free article] [PubMed]
- 32.Li YJ, Chen X, Kwan TK, et al. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid-mediated activation of g protein-coupled receptors GPR43 and GPR109A. J Am Soc Nephrol. 2020;31(6):1267–1281. doi: 10.1681/ASN.2019101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 34.Okamura T, Hamaguchi M, Mori J, Yamaguchi M, Mizushima K, Abe A, Ozeki M, Sasano R, Naito Y, Fukui M. Partially hydrolyzed guar gum suppresses the development of sarcopenic obesity. Nutrients. 2022;14(6):1157. [DOI] [PMC free article] [PubMed]
- 35.Deng L, Yang Y, Xu G. Empagliflozin ameliorates type 2 diabetes mellitus-related diabetic nephropathy via altering the gut microbiota. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867(12):159234. doi: 10.1016/j.bbalip.2022.159234. [DOI] [PubMed] [Google Scholar]
- 36.Hata S, Okamura T, Kobayashi A, Bamba R, Miyoshi T, Nakajima H, Kitagawa N, Hashimoto Y, Majima S, Senmaru T, Okada H, Ushigome E, Nakanishi N, Takakuwa H, Sasano R, Hamaguchi M, Fukui M. Gut microbiota changes by an SGLT2 inhibitor, luseogliflozin, alters metabolites compared with those in a low carbohydrate diet in db/db mice. Nutrients. 2022;14(17):3531. [DOI] [PMC free article] [PubMed]
- 37.Lewis CV, Taylor WR. Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease. Am J Physiol Heart Circ Physiol. 2020;319(6):H1227–h1233. doi: 10.1152/ajpheart.00612.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaziri ND, Yuan J, Nazertehrani S, Ni Z, Liu S. Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junction. Am J Nephrol. 2013;38(2):99–103. doi: 10.1159/000353764. [DOI] [PubMed] [Google Scholar]
- 39.Barbara G, Barbaro MR, Fuschi D, et al. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front Nutr. 2021;8:718356. doi: 10.3389/fnut.2021.718356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu IW, Hsu KH, Lee CC, et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26(3):938–947. doi: 10.1093/ndt/gfq580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norris GH, Blesso CN. Dietary sphingolipids: potential for management of dyslipidemia and nonalcoholic fatty liver disease. Nutr Rev. 2017;75(4):274–285. doi: 10.1093/nutrit/nux004. [DOI] [PubMed] [Google Scholar]
- 42.Sun L, Pang Y, Wang X, et al. Ablation of gut microbiota alleviates obesity-induced hepatic steatosis and glucose intolerance by modulating bile acid metabolism in hamsters. Acta Pharm Sin B. 2019;9(4):702–710. doi: 10.1016/j.apsb.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Wang Z, Liu D, et al. Canagliflozin prevents lipid accumulation, mitochondrial dysfunction, and gut microbiota dysbiosis in mice with diabetic cardiovascular disease. Front Pharmacol. 2022;13:839640. doi: 10.3389/fphar.2022.839640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalaf EM, Hassan HM, El-Baz AM, et al. A novel therapeutic combination of dapagliflozin, Lactobacillus and crocin attenuates diabetic cardiomyopathy in rats: Role of oxidative stress, gut microbiota, and PPARγ activation. Eur J Pharmacol. 2022;931:175172. doi: 10.1016/j.ejphar.2022.175172. [DOI] [PubMed] [Google Scholar]
- 46.Shi J, Qiu H, Xu Q, et al. Integrated multi-omics analyses reveal effects of empagliflozin on intestinal homeostasis in high-fat-diet mice. iScience. 2023;26(1):105816. doi: 10.1016/j.isci.2022.105816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Liang C, Song X, et al. Canagliflozin alters the gut, oral, and ocular surface microbiota of patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne) 2023;14:1256292. doi: 10.3389/fendo.2023.1256292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bao N, Liu X, Zhong X, et al. Dapagliflozin-affected endothelial dysfunction and altered gut microbiota in mice with heart failure. PeerJ. 2023;11:e15589. doi: 10.7717/peerj.15589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hao H, Li Z, Qiao SY, et al. Empagliflozin ameliorates atherosclerosis via regulating the intestinal flora. Atherosclerosis. 2023;371:32–40. doi: 10.1016/j.atherosclerosis.2023.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Wu J, Chen Y, Yang H, et al. Sodium glucose co-transporter 2 (SGLT2) inhibition via dapagliflozin improves diabetic kidney disease (DKD) over time associatied with increasing effect on the gut microbiota in db/db mice. Front Endocrinol (Lausanne) 2023;14:1026040. doi: 10.3389/fendo.2023.1026040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsui A, Yoshifuji A, Irie J, et al. Canagliflozin protects the cardiovascular system through effects on the gut environment in non-diabetic nephrectomized rats. Clin Exp Nephrol. 2023;27(4):295–308. doi: 10.1007/s10157-022-02312-y. [DOI] [PubMed] [Google Scholar]
- 52.Huang C, Qian J, Liu Y, Zhang L, Yang Y. Empagliflozin attenuates liver fibrosis in high-fat diet/streptozotocin-induced mice by modulating gut microbiota. Clin Exp Pharmacol Physiol. 2024;51(3):e13842. doi: 10.1111/1440-1681.13842. [DOI] [PubMed] [Google Scholar]




