Abstract
Purpose
Thyroid cancer is recognized as the predominant form of endocrine cancer. The likelihood of cancer recurrence and the development of distant metastases varies depending on the cancer’s pathology and stage. Iran currently lacks country-specific data on thyroid cancer, which can potentially result in clinicians deviating from the optimal treatment. The primary objectives of establishing such a registry are to determine the incidence, identify risk factors, and evaluate treatment outcomes for thyroid cancer within the Iranian population. Ultimately, the overarching goal of this protocol study is to reduce mortality and morbidity rates among thyroid cancer patients by implementing appropriate interventions based on the findings derived from this registration system.
Methods
The study will enroll all individuals aged 18 years and older who have received a diagnosis of primary thyroid carcinoma based on pathology criteria. Data will be collected from various thyroid clinic centers. The participating centers include the Endocrinology Clinic at Shariati Hospital, the Thyroid Clinic in the Nuclear Medicine Center at Shariati Hospital, as well as pathology and nuclear medicine centers in Kerman and Bushehr. Patient records comprise information on outpatient visits to the clinic.
Conclusion
The registry aims to enhance treatment approaches and follow-up protocols while serving as a foundation for conducting clinical, epidemiological, and basic science studies based on robust evidence-based data.
Keywords: Registry, Thyroid cancer, Iran, Study protocol
Introduction
According to multiple studies, thyroid cancer is recognized as the predominant form of endocrine cancer [1, 2]. In Iran, it ranks as the 12th most prevalent cancer among men and the 9th most prevalent cancer among women [3]. Furthermore, there has been a global upsurge in the incidence of thyroid cancer in recent years [4, 5]. This increase in thyroid carcinoma can be attributed to improved detection of small and early-stage tumors resulting from advancements in diagnostic technologies, as well as changes in environmental risk factors [6].
Thyroid cancer encompasses various subtypes, namely papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), medullary thyroid cancer, and anaplastic cancer, comprising approximately 75–85%, 10–20%, 5%, and less than 5% of cases, respectively [7]. Among all non-cutaneous malignancies, thyroid cancer exhibits the highest 5-year survival rate, reaching 98% [8].
Treatment for thyroid cancer typically involves surgical intervention, such as a total thyroidectomy or hemithyroidectomy. In cases where patients face an elevated risk, radioactive iodine (RAI) may be administered to eliminate any remaining thyroid tissue following a total thyroidectomy. Additionally, central neck dissection may be necessary in certain instances. The combination of total thyroidectomy and RAI ablation necessitates lifelong levothyroxine treatment for the patient [9].
While thyroidectomy has demonstrated favorable long-term outcomes, it is important to acknowledge the potential for both physical and psychological complications [10]. These complications encompass temporary alterations in voice quality and the occurrence of permanent vocal cord palsy [11]. Another possible complication is postoperative hypocalcemia, which might necessitate extended hospitalization and lifelong treatment with calcitriol and calcium supplementation. Furthermore, the use of RAI treatment can lead to xerostomia. It is also crucial to recognize the potential risks of hemorrhage and wound infections [12]. The likelihood of cancer recurrence and the development of distant metastases varies depending on the cancer’s pathology and stage [13].
Previous studies conducted in Iran include the following:
In 1973, a cancer registry focusing on esophageal cancer was carried out in the Caspian Littoral region of Iran. This research collaboration involved the International Agency for Research on Cancer (IARC), the Institute of Public Health Research of Tehran University, and other institutes [14].
The Tehran Cancer Institute Data System Registry (TCIDSR) was utilized to identify individuals in Iran who had various histological types of thyroid cancer. A total of 438 cases of thyroid cancer identified by the TCIDSR during the period of 1998-99 were analyzed [15].
Between 1996 and 2001, a registry recorded 429 cases of primary thyroid cancer in residential areas of Tehran [16].
In 2015, a study focusing on endocrine cancers was conducted in four provinces of Iran. Among the 319 cases of primary endocrine cancer, there were 313 cases of thyroid cancer and 6 cases of adrenal cancer [17].
Rationale
Iran currently lacks country-specific data on thyroid cancer, which can potentially result in clinicians deviating from established international treatment guidelines [18]. Given the rarity of the disease, treatment recommendations primarily rely on small-scale retrospective observational studies that are subject to limitations and potential biases. Furthermore, individual studies often lack the necessary clinical data to draw conclusive findings. In contrast, a comprehensive registry can provide a large dataset that enables meaningful research and offers definitive answers regarding thyroid cancer treatment.
The primary objectives of establishing such a registry are to determine the incidence, identify risk factors, and evaluate treatment outcomes for thyroid cancer within the Iranian population. Additionally, the registry aims to enhance treatment approaches and follow-up protocols while serving as a foundation for conducting clinical, epidemiological, and basic science studies based on robust evidence-based data. Ultimately, the overarching goal of this protocol study is to reduce mortality and morbidity rates among thyroid cancer patients by implementing appropriate interventions based on the findings derived from this registration system.
Methods
Objectives of the registry
Main Objectives.
To monitor the incidence of thyroid cancers.
To identify risk factors associated with thyroid cancer.
To expedite the treatment process and enhance follow-up care for thyroid cancer patients.
To evaluate the quality and effectiveness of medical services provided for thyroid cancer.
Research-related Objectives.
To establish a comprehensive database for conducting clinical, epidemiological, and basic science studies, including cellular, molecular, and genetic investigations related to thyroid cancer.
To establish a framework for research aimed at identifying novel and suitable treatments for thyroid cancer.
To analyze patient survival rates based on specific cancer types and the treatments administered.
To evaluate the short- and long-term effects of exposure to RAI, including the occurrence of secondary cancers.
To investigate hypotheses pertaining to the etiology of thyroid cancer.
Inclusion criteria
The study will enroll all individuals aged 18 years and older who have received a diagnosis of primary thyroid carcinoma based on pathology criteria. Both males and females will be included in the study.
Sources of data collection
Data pertaining to patients meeting the aforementioned inclusion criteria will be collected from various thyroid clinic centers. Information regarding patient records, particularly the administration of RAI, is documented in the hospital information system (HIS). The participating centers include the Endocrinology Clinic at Shariati Hospital, the Thyroid Clinic in the Nuclear Medicine Center at Shariati Hospital, as well as pathology and nuclear medicine centers in Kerman and Bushehr. Patient records comprise information on outpatient visits to the clinic. The specific data elements collected by the registry are outlined in Table 1.
Table 1.
Data items collected by the registry
| Demographics | Disease history | Paraclinical results | Surgery | Follow-up visit | Medical management | risk and stage calculation | Complications |
|---|---|---|---|---|---|---|---|
| Identification | History related to thyroid cancer | Sonography of thyroid | Surgical pathology characteristics (tumor type, histological grade, lymph node involvement, and invasion) | Levothyroxine therapy dose | Resurgery | TNM stage | surgical |
| Residence | past medical/ surgical history including genetic disorders related to thyroid cancer, hospital admission history, and radiation therapy | Thyroid scan with technetium | Laboratory test results | Imaging (whole body scan, CT scan, bone scan, PET scan, MRI, and BMD) | Dynamic risk stratification | TKI complications | |
| Nationality | Drug history | FNA result | Medical therapy (radioiodine dose, TKI, EBR, RFA, and alcohol injection) | Radioactive iodine | |||
| Education, job, and insurance | Family history (thyroid cancer, neck or thyroid surgery, radioiodine ablation, head and neck radiotherapy, and others) | Molecular study | Levothyroxine | ||||
| Family style | Physical examination (general characteristics, thyroid exam, and others) | Gene translocations | External beam radiation | ||||
| Others like physical activity and nutrition | Other complications |
FNA, fine needle aspiration; PET, positron emission tomography; MRI, magnetic resonance imaging; BMD, bone mineral density; TKI, tyrosine kinase inhibitor; EBR, external beam radiation; RFA, radiofrequency ablation; TNM, tumor, node, metastasis
Collection tools
The collection tools employed in this registry encompass a questionnaire for patient interviews, archived medical documents, and an electronic form utilized by medical staff. The data collection process takes place when the patient arrives at the clinic, involving the administration of the questionnaire through an interview and the completion of the form based on the accompanying medical documents. Subsequently, the information gathered from the questionnaire is entered into a dedicated software program designed specifically for this purpose. All patients are monitored and recorded on a monthly basis. The registry recording is planned to span a period of five years. In the initial stages of this endeavor, information from patients since 2021 will be utilized, and as the project progresses, data from new patients will be added. The platform employed for this registry is called Bioarch.
Site investigators
Site investigators responsible for recording information actively include nurses or general practitioners based in medical, university, and nuclear medicine centers.
Follow-up
Follow-up procedures are conducted through telephone calls, patient referrals to treatment and registration centers, or by utilizing databases.
Data collection, storage, analysis, and quality assessment
The methodology adheres to the cancer registration system protocol, ensuring the elimination of duplicate entries. Data analysis is performed by a team of experts using specialized cancer registration software. Drawing upon experience and scientific principles, the thyroid cancer registration team at the Endocrine Science Research Institute has developed software that aligns with the registry’s objectives, with the initial stages of software development already underway. Consequently, the information collected will be registered in the database. The expert team comprises individuals responsible for executing their assigned duties, monitoring the registry, and conducting quality control. This methodology encompasses the formation of an executive committee, the establishment of a software infrastructure, the evaluation of the initial phase at the Department of Endocrinology and Nuclear Medicine of Shariati Hospital, Tehran University of Medical Sciences, and the subsequent completion and nationwide expansion of the registration system.
Management Committee
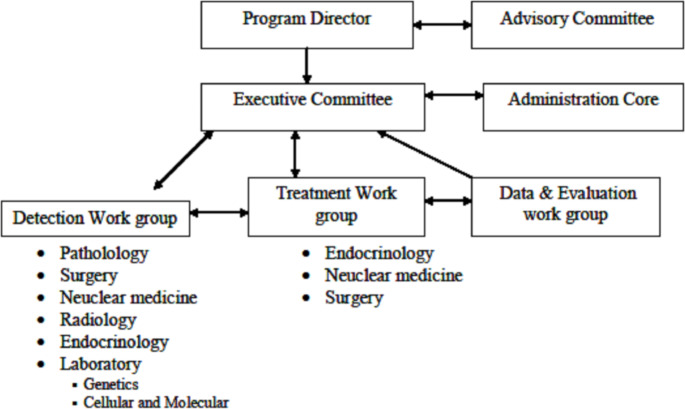
The management structure of the registry is illustrated in Fig. 1. The program director assumes overall supervision of all committees, including the executive and advisory committees. The management team is responsible for conducting regular meetings and overseeing the functioning of the registry system. The executive committee comprises the administrative core, treatment work group, and specialists involved in the cancer registry, such as the data and evaluation work group and the detection work group. The administrative core is entrusted with managing the registry project under the program director’s guidance.
Fig. 1.
Flowchart of registry management
Ethical considerations, principles of confidentiality, ownership, and data release
The confidentiality of data encompasses the protection of patient identities and other identifiable information collected directly or indirectly within the registry system. Adequate electronic security measures will be implemented to safeguard confidential data. All staff members will receive appropriate training based on the developed guidelines. Written informed consent from patients is a mandatory requirement. This study has obtained approval from the ethics committee of the Endocrine and Metabolism Research Institute, with the ethics code of IR.TUMS.EMRI.REC.1402.026, and adhered to the ethical guidelines of the hospital’s ethics committee. Large Language Models for summarizing several contents were used. No personally identifiable information was included in this article.
Determining data ownership will adhere to established global standards and will be determined in consultation with the executive registration committee. Consequently, the data will be published accordingly. A protocol has been devised and implemented for data release.
Access to patient identity information will only be granted in necessary circumstances. Data will be made available to researchers in accordance with specified instructions. The guidelines for authorship of published reports and articles derived from the registry will align with the ethics guidelines outlined by the Ministry of Health. Otherwise, individuals will be acknowledged solely for their cooperation.
Discussion
A meta-analysis conducted by Salari et al. in 2021, analyzing 28 articles, revealed a prevalence of thyroid cancer in Iran of 3.5% among a sample size of 100,869 individuals. These findings highlight the high prevalence of thyroid cancer in Iran. To address this issue, it is recommended to implement appropriate strategies such as providing feedback to hospitals, monitoring the situation, and troubleshooting at all levels [19, 20]. While several countries have established systems to register thyroid tumors, some of these systems primarily focus on specific subgroups based on age, primary or secondary cancer status, and benign or malignant classification.
We launched this comprehensive registry of thyroid cancers among adults to address the limitations of small-scale retrospective observational studies in guiding treatment recommendations for this rare disease. The registry aims to provide a large dataset that can facilitate meaningful research and offer definitive answers regarding thyroid cancer treatment. Its establishment will help overcome existing knowledge gaps and optimize treatment strategies for affected individuals.
The ped-DTC registry was launched in 2018 with the primary objective of collecting clinical information for individuals aged 18 and below who have been diagnosed, evaluated, or treated for differentiated thyroid carcinoma (DTC) at participating medical facilities. This registry is part of the broader European Registries for Rare Endocrine Conditions project [21]. The Australian and New Zealand Thyroid Cancer Registry (ANZTCR), established in 2017, aims to develop a joint clinical quality registry covering both Australia and New Zealand to monitor and improve the standard of care for individuals diagnosed with thyroid cancer [22]. In Iran, the national cancer registry system, initiated in 2014, focuses on gathering data related to childhood cancer [23].
Sustained funding is necessary for long-term sustainability, national progress, extended clinical follow-up, and assessment of patient-reported outcomes. Obtaining approval for patient recruitment poses a challenge in the development of the patient registry. It is crucial to ensure continuous commitment from investigators in the field. Physicians are encouraged to enter follow-up data for each patient on scheduled dates. The registration system faces challenges related to the scope and level of cooperation among data collection centers and potential overlap. These challenges are mitigated through the training of staff and regular information control. Future goals include obtaining longer-term follow-up data, involving multidisciplinary clinicians, establishing data linkage, and focusing on cancer survival issues and the treatment of cancers with poorer prognosis and recurrences.
Participating medical centers will have the opportunity to compare patient outcomes with those of other centers and may benefit from participating in multicenter clinical trials to improve patient care. Implementation of this registry system will significantly reduce the burden of patient follow-up and lead to improved treatment outcomes.
Conclusion
The registry aims to enhance treatment approaches and follow-up protocols while serving as a foundation for conducting clinical, epidemiological, and basic science studies based on robust evidence-based data. Ultimately, it will help reduce mortality and morbidity rates among individuals diagnosed with thyroid cancer.
Funding
No funding was received to assist with the preparation of this manuscript.
Declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This research was in compliance with ethical standards. The Medical Research Ethics Committee of the Shariati Hospital granted ethical approval for this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schweppe RE. Thyroid cancer cell lines: critical models to study thyroid cancer biology and new therapeutic targets. Front Endocrinol. 2012;3:81. doi: 10.3389/fendo.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LiVolsi VA. Papillary thyroid carcinoma: an update. Mod Pathol. 2011;24:1–S9. doi: 10.1038/modpathol.2010.129. [DOI] [PubMed] [Google Scholar]
- 3.Alireza S, Mehdi N, Ali M, Alireza M, Reza M, Parkin D. Cancer occurrence in Iran in 2002, an international perspective. Asian Pac J Cancer Prev. 2005;6(3):359. [PubMed] [Google Scholar]
- 4.Enyioha C, Roman SA, Sosa JA. Central lymph node dissection in patients with papillary thyroid cancer: a population level analysis of 14,257 cases. Am J Surg. 2013;205(6):655–61. doi: 10.1016/j.amjsurg.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23(7):885–91. doi: 10.1089/thy.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Reviews Endocrinol. 2016;12(11):646–53. doi: 10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Milas M, Nasr C, Brainard JA, Khan MJ, Burkey BB, et al. Anaplastic thyroid cancer in young patients: a contemporary review. Am J Otolaryngol. 2013;34(6):636–40. doi: 10.1016/j.amjoto.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson AB et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. JNCI: Journal of the National Cancer Institute. 2017;109(9):djx030. [DOI] [PMC free article] [PubMed]
- 9.Husson O, Haak HR, Mols F, Nieuwenhuijzen GA, Nieuwlaat W-A, Reemst PH, et al. Development of a disease-specific health-related quality of life questionnaire (THYCA-QoL) for thyroid cancer survivors. Acta Oncol. 2013;52(2):447–54. doi: 10.3109/0284186X.2012.718445. [DOI] [PubMed] [Google Scholar]
- 10.Mendoza A, Shaffer B, Karakla D, Mason ME, Elkins D, Goffman TE. Quality of life with well-differentiated thyroid cancer: treatment toxicities and their reduction. Thyroid. 2004;14(2):133–40. doi: 10.1089/105072504322880373. [DOI] [PubMed] [Google Scholar]
- 11.Serpell JW, Lee JC, Yeung MJ, Grodski S, Johnson W, Bailey M. Differential recurrent laryngeal nerve palsy rates after thyroidectomy. Surgery. 2014;156(5):1157–66. doi: 10.1016/j.surg.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Lee JC, Chang P, Grodski S, Yeung M, Johnson W, Serpell J. Temporal analysis of thyroid cancer management in a Melbourne tertiary centre. ANZ J Surg. 2019;89(1–2):38–42. doi: 10.1111/ans.13792. [DOI] [PubMed] [Google Scholar]
- 13.Nixon IJ, Whitcher MM, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid. 2012;22(9):884–9. doi: 10.1089/thy.2011.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahboubi E, Kmet J, Cook PJ, Day NE, Ghadirian P, Salmasizadeh S. Oesophageal cancer studies in the Caspian Littoral of Iran: the Caspian cancer registry. Br J Cancer. 1973;28(3):197–214. doi: 10.1038/bjc.1973.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larijani B, Shirzad M, Mohagheghi MA, Haghpanah V, Mosavi-Jarrahi AR, Tavangar SM, et al. Epidemiologic analysis of the Tehran Cancer Institute Data System Registry (TCIDSR) Asian Pac J Cancer Prev. 2004;5(1):36–9. [PubMed] [Google Scholar]
- 16.Larijani B, Mohagheghi MA, Bastanhagh MH, Mosavi-Jarrahi AR, Haghpanah V, Tavangar SM, et al. Primary thyroid malignancies in Tehran. Iran Med Principles Pract. 2005;14(6):396–400. doi: 10.1159/000088112. [DOI] [PubMed] [Google Scholar]
- 17.Larijani MA, Malekzadeh R, Haghpanah V, Soleymanpour B, Heshmat R, Tavangar S, CANCER, RECENTLY KNOWN AS THE THIRD MORTALITY FACTOR, IS ONE OF THE HEALTH PROBLEMS IN IRAN J Sabzevar Univ Med Sci. 2007;13(4):190. [Google Scholar]
- 18.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 american thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salari N, Kazeminia M, Mohammadi M. The prevalence of thyroid Cancer in Iran: a systematic review and Meta-analysis. Indian J Surg Oncol. 2022;13(1):225–34. doi: 10.1007/s13193-021-01465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohouli MH, Almuqayyid F, Alfardous Alazm A, Ziamanesh F, Izze da Silva Magalhães E, Bagheri SE et al. A comprehensive review and meta-regression analysis of randomized controlled trials examining the impact of vitamin B12 supplementation on homocysteine levels. Nutr Rev. 2023. [DOI] [PubMed]
- 21.Clement SC, Visser WE, Lebbink CA, Albano D, Claahsen-van der Grinten HL, Czarniecka A et al. Development of a pediatric differentiated thyroid carcinoma registry within the EuRRECa project: rationale and protocol. Endocr Connections. 2023;12(3). [DOI] [PMC free article] [PubMed]
- 22.Ioannou LJ, Serpell J, Dean J, Bendinelli C, Gough J, Lisewski D, et al. Development of a binational thyroid cancer clinical quality registry: a protocol paper. BMJ open. 2019;9(1):e023723. doi: 10.1136/bmjopen-2018-023723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modirian M, Rahimzadeh S, Cheraghi Z, Khosravi A, Salimzadeh H, Kompani F et al. Quality evaluation of national cancer registry system in Iran: study protocol. 2014. [PubMed]