Abstract
The envelope protein of human immunodeficiency virus type 1 HIV-1 undergoes proteolytic cleavage in the Golgi complex to produce subunits designated gp120 and gp41, which remain noncovalently associated. While gp41 has a well-characterized oligomeric structure, the maintenance of gp41-independent gp120 intersubunit contacts remains a contentious issue. Using recombinant vaccinia virus to achieve high-level expression of gp120 in mammalian cells combined with gel filtration analysis, we were able to isolate a discrete oligomeric form of gp120. Oligomerization of gp120 occurred intracellularly between 30 and 120 min after synthesis. Analysis by sedimentation equilibrium unequivocally identified the oligomeric species as a dimer. In order to identify the domains involved in the intersubunit contact, we expressed a series of gp120 proteins lacking various domains and assessed the effects of mutation on oligomeric structure. Deletion of the V1 or V3 loops had little effect on the relative amounts of monomer and dimer in comparison to wild-type gp120. In contrast, deletion of either all or part of the V2 loop drastically reduced dimer formation, indicating that this domain is required for intersubunit contact formation. Consistent with this, the V2 loop of the dimer was less accessible than that of the monomer to a specific monoclonal antibody. Previous studies have shown that while the V2 loop is not an absolute requirement for viral entry, the absence of this domain reduces viral resistance to neutralization by monoclonal antibodies or sera. We propose that the quaternary structure of gp120 may contribute to resistance to neutralization by limiting the exposure of conserved epitopes.
The envelope (env) proteins of human immunodeficiency virus type 1 (HIV-1) mediate viral entry and are the primary targets of neutralizing antibodies. Following synthesis and posttranslational modifications in the endoplasmic reticulum, including N-linked glycosylation, disulfide bond formation, and oligomerization, the env protein precursor gp160 passes through the Golgi complex where it is cleaved to form subunits designated gp120 and gp41 (11, 41). A noncovalently associated oligomeric gp120-gp41 complex is transported to the surface of infected cells, where incorporation into budding virions occurs. The binding of virion-associated or infected-cell-associated gp120 to the CD4 receptor induces conformational changes that promote subsequent interaction with one of a number of chemokine receptors (19, 46, 49). Movement of the variable (V) loop structure V1/V2 following CD4 binding has been shown to result in increased exposure of an antibody epitope which overlaps with the chemokine receptor binding site (44, 52). Receptor binding-induced env conformational changes are believed to culminate in the exposure of the gp41 fusion peptide and its repositioning toward the target cell prior to fusion of the membranes of the infected cell or virion and the target cell (5, 14, 26, 48).
Despite numerous investigations, the oligomeric structure of the native env protein is still poorly understood. It has been demonstrated that an amphipathic α-helix within the N-terminal portion of the gp41 segment mediates gp160 oligomerization (10, 30). Analysis by sucrose gradient sedimentation and/or chemical cross-linking suggests that mammalian-cell-expressed gp160 exists as a mixture of dimers and higher-order oligomers (6, 8, 35). Scanning transmission electron microscopy also revealed a dimeric gp160 molecule (45). Following cleavage in the Golgi complex, the gp41 subunit retains an oligomeric structure, with tetramer being the highest-order oligomer observed (6, 29, 35). Crystal-derived structures of bacterial-cell-expressed N- and C-terminal gp41 fragments revealed that the molecular basis of oligomerization was a coiled coil composed of the N-terminal α-helices (5, 48), although in this case trimer was formed. The C-terminal helices were packed in grooves on the outside of the coiled-coil core. This structural arrangement is thought to mimic that which occurs after receptor binding-induced activation and fusion with the target cell membrane.
While the role of the gp41 N-terminal α-helix in the oligomerization of gp160 and gp41 is well established, the question of whether the intersubunit contacts of gp120 are sufficient for it to adopt and maintain an oligomeric structure independently of gp41 remains unclear. Where gp120 was expressed in the absence of gp41, scanning transmission electron microscopy data led to the suggestion that gp120 was solely monomeric (45), and coimmunoprecipitation experiments demonstrated that gp120 could not form hetero-oligomers with gp160 (30). Furthermore, a gp120 core protein (with deletions at the C and N termini and of the V1, V2, and V3 domains) crystallized as a monomer (18). In contrast, gp120 within gp120-gp41 complexes expressed on the cell or virion surface revealed an oligomeric structure when analyzed by chemical cross-linking and/or sucrose gradient sedimentation (8, 47). Cell surface-expressed gp120 which had been shed into the medium has been shown to have an oligomeric structure under some experimental conditions (27) but not others (8). The observation that oligomeric structure could be disrupted during ultracentrifugation suggests that the interaction may be relatively labile (8, 47). Subunit interactions have been inferred from the differential reactivity of epitopes in cell surface-expressed env versus purified soluble gp120. Reduced exposure of epitopes within the V2, C1, C4, and C5 domains of cell surface-expressed gp120 has been reported (24, 34, 37, 39). While it is likely that reduced exposure of the C1 and C5 epitopes is due at least in part to the involvement of these domains in association between gp120 and gp41 (16, 50), the occlusion of epitopes in other domains is presumably caused by the close proximity of gp120 subunits within the oligomeric gp120-gp41 complex. The V3 domain appears to be well exposed in cell surface-expressed gp120 derived from T-cell-line-adapted strains but less well exposed in gp120 derived from a macrophage-tropic strain (24, 34, 39). Importantly, exposure of cell surface-expressed gp120 epitopes more accurately predicts the neutralizing activity of monoclonal antibodies (MAb) than the epitope exposure of purified soluble gp120 (12, 28, 33, 34, 43), although MAb binding may not always be sufficient for neutralization (13, 40).
Here, we report on the isolation of a soluble oligomeric form of gp120 expressed in the absence of gp41 in mammalian cells. Intersubunit association occurred intracellularly and was stable during repeated gel filtration. Sedimentation equilibrium analysis demonstrated that dimer was the predominant oligomeric species. Deletion mutants lacking all or part of the V2 loop were deficient in the production of gp120 dimers, suggesting a role for this domain in intersubunit contact formation. The relative inaccessibility of the V2 loop of the dimer to a specific MAb is consistent with this model.
MATERIALS AND METHODS
Recombinant vaccinia viruses.
The recombinant vaccinia virus vPE50 was made by excising a SmaI/EcoRI fragment containing the gp120 coding sequence (BH8 clone, IIIB/LAI strain) (31) followed by a downstream stop codon (9) and ligating it into the StuI/EcoRI sites of pSC59 (4). The resultant plasmid pPE50, which directs expression of the gp120 gene from the strong synthetic early/late promoter, was used to produce the recombinant vaccinia virus vPE50 by standard techniques (7). In order to produce the vRCΔV1 and vRCΔV3 or vRCwt viruses, the NdeI/AocI fragments were excised from mutated or wild-type pSVIIIenv plasmids (52) and used to replace the corresponding fragment in pPE50. The resultant plasmids were used to produce recombinant vaccinia viruses. The pSVIIIenv plasmid directs expression of the wild-type HXBc2 env clone (IIIB/LAI strain), and the mutant derivatives direct expression of HXBc2 env lacking amino acids Asn-136 to Lys-151 (ΔV1) or Thr-303 to Ile-323 (ΔV3). In order to express gp120 with V2 loop deletions, an overlap PCR strategy was employed. PCRs were performed using Pfu polymerase (Stratagene, La Jolla, Calif.), pPE50 as template, and the following oligonucleotide primer pairs: reaction 1, 5′-GCTAAAGCATATGATACAGAGG (5′ outer) and 5′-CAACTTGTAGAGCAGTTTTTTATCTC; reaction 2, 5′-CCATGTGTACATTGTACTGTGC (3′ outer) and 5′-AACTGCTCTACAAGTTGTAACACC; reaction 3, 5′ outer and 5′-CTATTGGTTCTTTCTGCACCTTACC, and reaction 4, 3′ outer and 5′-GCAGAAAGAACCAATAGATAATGATAC. The products of these reactions were purified from residual oligonucleotides by agarose gel electrophoresis and band excision. The products from reactions 1 and 2 and reactions 3 and 4 were then used as templates in secondary reactions with both 5′ outer and 3′ outer primers. The products of these reactions were digested with NdeI and StuI and ligated into the corresponding sites of pPE50 to produce pRCΔV2 and pRCΔV2T, respectively. The recombinant virus derived from these plasmids directs the expression of gp120 lacking amino acids Phe-159 to Leu-193 (ΔV2) or Tyr-173 to Ile-182 (ΔV2T). Mutations in all plasmids used to produce recombinant vaccinia viruses were confirmed by DNA sequencing.
Antibodies.
The MAbs T54 and D47 have been described previously (42). T54 binds to a conformation-sensitive V2-dependent epitope, while D47 recognizes a linear epitope within the V3 loop.
HIV-1 env expression, purification, and gel filtration.
BS-C-1 cells were infected with recombinant vaccinia virus at a multiplicity of infection (MOI) of 5 in 850-cm2 roller bottles (108 cells per bottle). Cells were overlaid with serum-free Opti-MEM (Gibco BRL, Grand Island, N.Y.) and incubated for 1.5 to 2 days at 37°C. After centrifugation to remove cellular debris, the supernatant was adjusted to 0.2% Triton X-100 in order to reduce nonspecific binding and then allowed to pass over lentil-lectin–Sepharose beads (Amersham Pharmacia Biotech AB, Uppsala, Sweden) by gravity flow. After sequential washing with phosphate-buffered saline (PBS) supplemented to 0.2% Triton X-100 and an additional 300 mM NaCl and with PBS alone, glycoproteins were eluted with PBS–0.5 M methyl-α-d-mannopyranoside and concentrated using a Centriprep 30 (Amicon, Beverly, Mass.). The sample (in a 1-ml volume) was subject to gel filtration chromatography using a Superdex 200 column (Amersham Pharmacia Biotech AB) with PBS as the buffer. A flow rate of 0.5 ml/min was used, and individual 1-ml fractions of between 40 and 80 ml (total volume) were collected.
Immunoblotting and chemical cross-linking.
Proteins in individual gel filtration fractions to be immunoblotted were subject to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% gels) under reducing conditions and transferred to nitrocellulose membranes. After blocking with 4% bovine serum albumin, membranes were sequentially probed with a polyclonal rabbit serum raised against gp140 (for 2 h at room temperature in PBS–0.2% Tween 20) and iodinated protein A (Amersham Pharmacia Biotech AB) (1 h at room temperature in PBS–0.2% Tween 20), with washing between incubations. Signal was revealed and quantitated by phosphor screen autoradiography using a scanner and Imagequant software (Molecular Dynamics, Sunnyvale, Calif.). Proteins to be cross-linked were incubated in the presence of ethylene glycol-bis(succinimidylsuccinate) (EGS; Pierce, Rockford, Ill.) at a final concentration of 5 mM for 30 min at room temperature and then quenched by adjusting the samples to 100 mM glycine and incubating them for a further 15 min prior to SDS-PAGE (6% gels) and immunoblotting.
Metabolic labeling, pulse-chase analysis, and sucrose gradient sedimentation.
Prior to metabolic labeling, three tissue culture wells with 106 BS-C-1 cells each were infected with the recombinant vaccinia virus vPE50 at an MOI of 10 for 2 h. After a further 3 h, the cells were incubated in methionine free Eagle minimal essential medium (EMEM) for 30 min, followed by incubation in methionine-free EMEM with the addition of 300 μCi of [35S]methionine (NEN, Boston, Mass.) for 15 min. After being washed with PBS, the cells in one well were immediately lysed in PBS–1% Triton X-100 (pulse). Cells in the other two wells were washed in PBS and overlaid with EMEM–10% fetal bovine serum. After either 30 min or 2 h, these cells were lysed as before. Cell lysates were loaded onto 5 to 20% sucrose gradients and centrifuged at 40,000 rpm for 20 h at 4°C. Gradients were fractionated, and each 0.5-ml fraction was precleared by the addition of nonimmune rabbit sera and protein A-Sepharose (Amersham Pharmacia Biotech AB) with overnight incubation at 4°C. Following the removal of the protein A-Sepharose, labeled gp120 was immunoprecipitated by the addition of polyclonal rabbit sera raised against gp140 and fresh protein A-Sepharose with overnight incubation at 4°C. After the protein A-Sepharose beads were washed with wash buffer (300 mM NaCl, 50 mM Tris [pH 7.4], 0.1% Triton X-100, 0.02% azide), bound proteins were subjected to SDS-PAGE (10%) as described above. Gels were dried, and the signal was revealed and quantified by using phosphor screen autoradiography.
Sedimentation equilibrium and velocity measurements.
Sedimentation equilibrium was used to determine the molecular mass of the monomeric and oligomeric forms of gp120. Sedimentation equilibrium analysis was performed in the XL-A analytical ultracentrifuge using a four-cell An-60 Ti rotor. In separate experiments, absorbance values versus radial position data were obtained at 11,000 and 15,000 rpm at 10°C for the gp120 monomer fraction and at 7,600, 9,600, and 11,600 rpm at 10°C for the gp120 oligomer fraction. Scans were obtained at 280 nm at radial increments of 0.001 cm, with each datum point representing the average of 10 repeats. The gp120 sedimentation equilibrium data were considered as a heterogeneous system, since the monomer and oligomer gel filtration fractions likely contained some oligomers and monomers, respectively. This system was modeled by global nonlinear regression fitting of the five data sets (for a review, see reference 20). This was accomplished using a two-species monomer and oligomer model. The variables determined by the computational fitting are the molecular weight of the monomer, which also determines the oligomer value, and the respective concentrations of the monomer and oligomer at a selected reference position. The total concentration of each component was calculated by integration of the monomer and oligomer exponential functions. Goodness-of-fit was determined from the residuals. The computational analysis was performed by using the commercial software package MLAB (Civilized Software, Bethesda, Md.).
Band sedimentation velocity was used to determine the sedimentation coefficients of the gp120 monomeric and oligomeric forms (21). Measurements were made using a Beckman Instruments XL-A analytical ultracentrifuge and a sample sector solution of 50% by volume D2O-H2O containing PBS. The band centerpiece was filled with 20 to 25 μl of gp120 monomer or oligomer solution in PBS (protein concentration, 0.10 to 0.20 mg/ml). Centrifugation was performed at 48,000 rpm with absorbance scanning at 230 nm. The Windows program Sedband (J. Lebowitz, P. Schuck, S. R. Kar, G. Howlett, and R. W. Ott, unpublished data) was used for the determination of band sedimentation coefficients. This program globally fits the absorbance band profiles using a Gaussian model. Experimental s values were corrected to s20,w values using the standard correction equation (20). The relative viscosity and density values for 50% D2O-PBS are 1.1417 and 1.0593 as determined by direct viscometric measurement and the use of a Paar density meter, respectively. The partial specific volume value of gp120 was estimated by using the analysis of glycoproteins developed by Lewis and Junghans (23). The molecular mass of the peak of the mass distribution as determined by mass spectral analysis of gp120 was 92 kDa (P. Earl and K. Parker, unpublished data). This value allows calculation of the weight fraction of protein (0.575) and carbohydrate (0.425), and the sum of each weight fraction times the respective vbar values for the protein and carbohydrate component (vbar,p and vbar,c) allows for the calculation of a range of vbar,gp values for gp120. A protein vbar,p of 0.730 was determined from amino acid composition. The Sednterp program 1.01 (http://www.bbri.harvard.edu/rasmb/rasmb.html) was used to perform hydrodynamic modeling of gp120.
RESULTS
gp120 intersubunit contacts are sufficient to form stable oligomers.
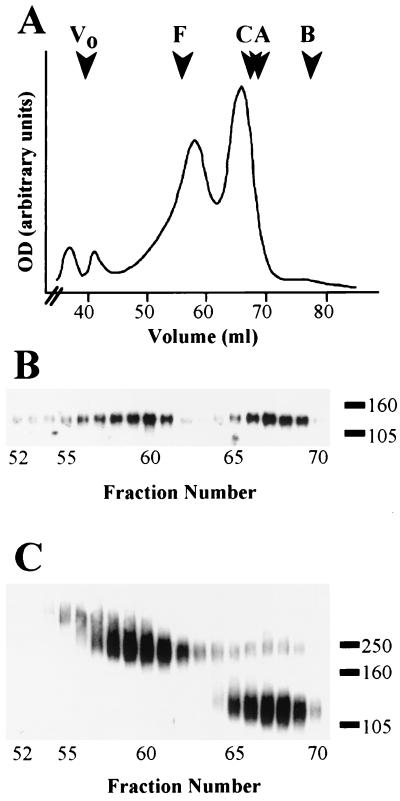
In order to assess the quaternary structure of gp120, a recombinant form of this protein lacking the gp41 domain was expressed in mammalian cells by using a vaccinia virus vector. Glycoproteins present in the serum-free medium were purified by lentil-lectin affinity chromatography at physiological pH and then analyzed by gel filtration. Two major protein peaks at 58.5 and 66 ml were resolved (Fig. 1A). Immunoblotting with an env-specific antiserum confirmed that peaks observed on the UV trace contained gp120 (Fig. 1B). Moreover, SDS-PAGE followed by Coomassie blue staining (data not shown) indicated the absence of significant levels of contaminating protein in the peak fractions. Treatment with the nonreducible cross-linker EGS was used to reveal the oligomeric state of gp120. gp120 from fractions corresponding to the more slowly eluting peak (65 to 69 ml) showed limited propensity to cross-link with EGS, mostly migrating during SDS-PAGE to a position consistent with that expected for monomer, between the 105- and 160-kDa standards (Fig. 1C). In contrast, gp120 from fractions corresponding to the more rapidly eluting peak (57 to 62 ml) migrated as a broad band overlapping the 250-kDa standard, with little or no monomer present. The gp120 in the 55- and 56-ml fractions appeared to migrate to a position above the 250-kDa standard after cross-linking, suggesting the possible presence of a higher-order species. These results demonstrated that gp120 expressed in the absence of gp41 attained quaternary structure.
FIG. 1.
Gel filtration analysis of secreted gp120. B-SC-1 cells were infected with a recombinant vaccinia virus encoding gp120 (vPE50). Secreted gp120 was purified by lentil-lectin affinity chromatography and analyzed by gel filtration over Superdex 200. (A) UV trace of gel filtration experiment. The molecular radius standards were given by: ferritin, F, 61.0 Å; catalase C, 52.2 Å; aldolase A, 48.1 Å; and bovine serum albumin, B, 35.5 Å. Void volume (Vo) was given by blue dextran 2000. (B) Aliquots of 1-ml gel filtration fractions were analyzed by SDS-PAGE (10% gel) and immunoblotting with env-specific antiserum. (C) Samples as in panel B but treated with 5 mM EGS prior to SDS-PAGE (6% gel). In panels B and C, the numbers on the right represent the positions and masses (in kilodaltons) of marker proteins.
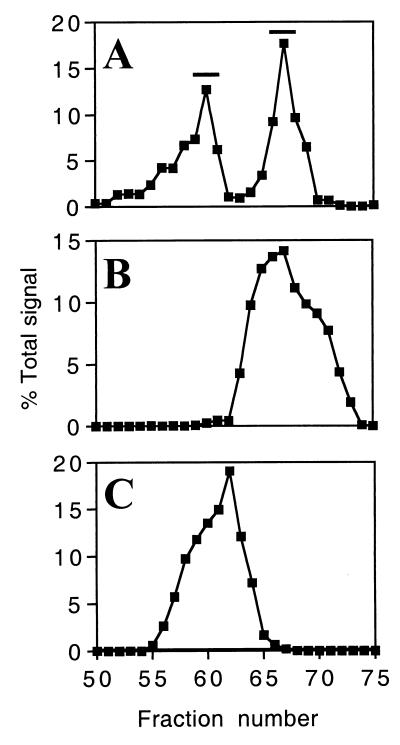
We sought to determine whether the gel filtration peaks represented distinct gp120 species or, alternatively, were products of an equilibrium reaction between associated and nonassociated states. In order to distinguish between these possibilities, individual fractions corresponding to the two gel filtration peaks were identified by quantitation of an immunoblot probed with an env-specific antiserum (Fig. 2A). Fractions 59 to 61 and 66 to 68 (indicated by the bars) were separately pooled, concentrated, and subjected to repeat gel filtration. Aliquots of fractions from these experiments were immunoblotted with a specific antiserum as before. Quantitation values of repeat gel filtration of the more slowly and more rapidly eluting peaks are shown in Fig. 2B and C, respectively. In both cases, a single peak was observed, with elution volumes corresponding to the original pooled fractions (Fig. 2A). This result demonstrated that gp120 intersubunit contacts were stably maintained but not formed during gel filtration, indicating that the two peaks observed represent distinct forms of the protein present in the sample prior to gel filtration.
FIG. 2.
Repeat gel filtration of secreted gp120. (A) Signal quantitation of immunoblotted gel filtration fractions. Bars indicate the fractions that were pooled and concentrated prior to repeat gel filtration. (B) Signal quantitation of fractions from repeat gel filtration of pooled monomer. (C) Signal quantitation of fractions from repeat gel filtration of pooled oligomer. Note that panel A is derived from the immunoblot displayed in Fig. 1B.
gp120 oligomers form intracellularly.
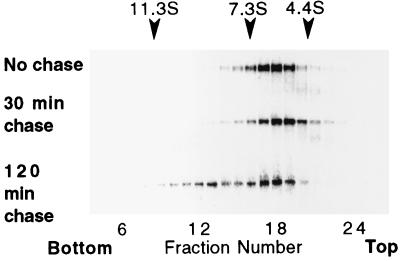
To determine if gp120 intersubunit contacts were made within the cell prior to secretion, recombinant virus-infected BS-C-1 cells were metabolically labeled for 15 min, and intersubunit contact formation within cells was assessed at subsequent time points by subjecting cell lysates to sucrose gradient sedimentation. The latter procedure was used rather than gel filtration because of the small sample sizes used for metabolic labeling. Preliminary work showed that gp120 which sedimented on gradients to a position between 7.3S and 11.3S could be chemically cross-linked, whereas gp120 which sedimented between 4.4S and 7.3S remained monomeric after the same treatment (data not shown). When cells were lysed immediately after the pulse or after a chase period of 30 min, most gp120 sedimented to fractions 17 to 19, which fell between the 4.4S and 7.3S sedimentation calibration standards (Fig. 3). Following a 120-min chase period, a bimodal distribution of gp120 molecules across the gradient was observed (Fig. 3). Along with the material sedimenting to fractions 17 to 19, a portion of the gp120 molecules sedimented to fractions 12 and 13 (between 7.3S and 11.3S). Therefore, the acquisition of intersubunit contacts that were stable during sedimentation occurred within the cells between 30 and 120 min after synthesis.
FIG. 3.
Intracellular gp120 oligomer formation. B-SC-1 cells were infected with a recombinant vaccinia virus encoding gp120 (vPE50), metabolically labeled for 15 min, and then lysed immediately (upper panel) or chased for 30 or 120 min prior to lysis (middle and lower panels, respectively). Lysates were sedimented through sucrose density gradients, and gp120 from gradient fractions was immunoprecipitated with an env-specific antiserum and subjected to SDS-PAGE (10% gels). The direction of sedimentation was right to left. Gradients were calibrated with catalase (11.3S), aldolase (7.3S), and bovine serum albumin (4.4S).
gp120 self-associates mainly as a dimer.
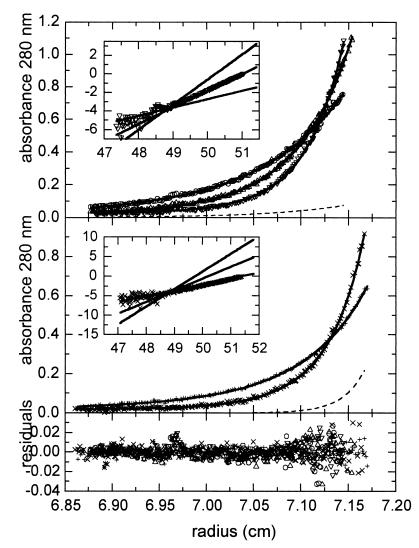
The name given to gp120 reflects its relative mobility during SDS-PAGE rather than its true mass. A mass of 89 kDa was determined by scanning transmission electron microscopy (45), and a mass of 92 kDa was determined by mass spectroscopy (P. Earl and K. Parker, unpublished data). Using the gel filtration standards noted above to calibrate the elution profile shown in Fig. 1A, we calculated the molecular radii of monomeric and oligomeric gp120 to be 49.9 and 62.3 Å, respectively. These values suggest nonglobular shapes for both the monomeric and oligomeric species, precluding the accurate determination of molecular masses by this method. We therefore chose to use sedimentation equilibrium analysis for oligomer mass determination. This technique provides a rigorous analytical methodology for determining molecular masses of proteins and protein complexes independent of shape. In order to minimize cross-contamination of the two gp120 species, protein from peak fractions of the repeat gel filtration experiments (Fig. 2B and C) were used for sedimentation equilibrium. Fractions 59 to 62 and 66 to 69 of the repeat gel filtration of oligomeric and monomeric gp120 respectively were separately pooled and concentrated to approximately 1 mg/ml. Sedimentation equilibrium data were obtained for both the gp120 monomer and oligomer. Initial single component modeling of the sedimentation equilibrium data using the separate monomer and oligomer data indicated that the gp120 oligomer was a dimer (results not shown). Modeling by two exponentials (monomer component and putative dimer component) using global nonlinear regression fitting of the five data sets is displayed in Fig. 4. To determine the molecular weight from the fitting analysis, evaluation of the partial specific volume of gp120 was required. Lewis and Junghans (23) determined that the vbar,c of the carbohydrate component of most substituted glycoproteins was in the range of 0.602 to 0.642 ml/g. Selecting the midpoint of this vbar,c range, we calculated a gp120 vbar,gp value of 0.684 ml/g, which when incorporated into the fitting model gave best-fit molecular weights of 92,490 ± 1,445 for the monomer and a corresponding doubling for the dimer. The residuals of the fit were small and randomly distributed, strongly supporting the heterogeneous monomer and dimer model. To further assess the validity of the model, we transformed the highest centrifugal speed exponential data to a linear plot in each sedimentation equilibrium data set. A linear plot of the exponential data readily allows comparison of the best fit using a monomer, dimer, or trimer model for the predominant gp120 form under analysis. For dimer (Fig. 4, upper panel inset), the expected dimer line closely fits to the experimental data, whereas neither the lower sloping line expected for monomer nor the higher sloping line expected for trimer fit the experimental results. In the case of monomer (Fig. 4, middle panel inset), the expected monomer line closely fits to the experimental line, whereas neither the dimer nor the trimer lines fit the experimental results. Overall, the sedimentation equilibrium results demonstrate that the higher-order oligomer of gp120 is predominantly dimeric.
FIG. 4.
Sedimentation equilibrium concentration profiles for the dimer fraction (top panel) and for the monomer fraction (middle panel). Rotor speeds were 7,600 (○), 9,600 (▵), and 11,600 (▿) rpm for the dimer fraction and 11,000 (+) and 15,00 (×) rpm for the monomer fraction, respectively. Solid lines show the best-fit distributions (root mean square error, 0.0078 optical density) after global modeling with the monomer molecular weight and with the relative amounts of monomer and dimer of each sample as unknowns. The best fit gave an average molecular weight of 92,490 ± 1,445 for the monomer with a corresponding doubling for the dimer. Residuals of the fitted lines to the experimental data are shown in the lower panel. The calculated absorbance contributions of the contaminating species are indicated by dashed lines for the experiments at 11,600 and 15,000 rpm. Their calculated relative total amounts were 5 ± 4% monomer contamination of the dimer fraction and 27 ± 4% dimer contamination of the monomer fraction. Incorporated in each sedimentation equilibrium data panel is an inset in which the highest-centrifugal-speed exponential data have been transformed into a linear plot in the upper panel (▿) and the middle panel (×). This transformation was achieved by subtracting the baseline offset first, converting the x axis to a radius2 axis, and then taking the derivative (dlnA/dr2) of the exponential data. Solid lines of increasing slope for either a monomer, dimer, or trimer model are given in order to compare the experimental data with the best model for the quaternary state of the predominant gp120 form under analysis.
Band sedimentation velocity analysis (analytical zone centrifugation) produced s20,w values of 5.79S (standard deviation [SD] = 0.0093) and 8.75S (SD = 0.013) for monomeric and dimeric gp120, respectively (data not shown). If the gp120 monomer and dimer were spherical in shape, the corresponding s values would be 8.77S and 13.99S, respectively, using the equation s = 0.012M2/3 (1 − vbar,gp)/(vbar,gp)1/3. The experimental-to-spherical frictional ratios (f/fo = ssphere/s20,w) were 1.52 and 1.60 for the monomer and dimer samples, respectively. From the frictional ratios the hydrodynamic shape asymmetry was evaluated using a prolate ellipsoid as a model for gp120. The major (a)-to-minor (b) axial ratio for the monomer is 9.59 (2a = 26 nm and 2b = 2.8 nm), and for the dimer the ratio was 11.1 (2a = 36.6 nm and 2b = 3.3 nm). These shape estimates did not include hydration and consequently are the maximum asymmetry for the prolate model. Since the monomer sample contains approximately 27% dimer, the measured s20,w value was higher than the true s20,w for a pure monomer, and consequently the actual frictional ratio should be greater than that cited above. However, for the dimer sample the fraction of monomer was approximately 5%, and consequently the estimate of the shape asymmetry was close to the true dimer value. This large asymmetry plus the contribution from carbohydrate chains accounted for the errors in molecular mass estimates based on size exclusion analysis from the gel filtration data. Of importance from a molecular perspective, the gp120 dimer appears to be arranged as a side-to-side dimer based on the frictional ratios.
The V2 loop is required for gp120 intersubunit association.
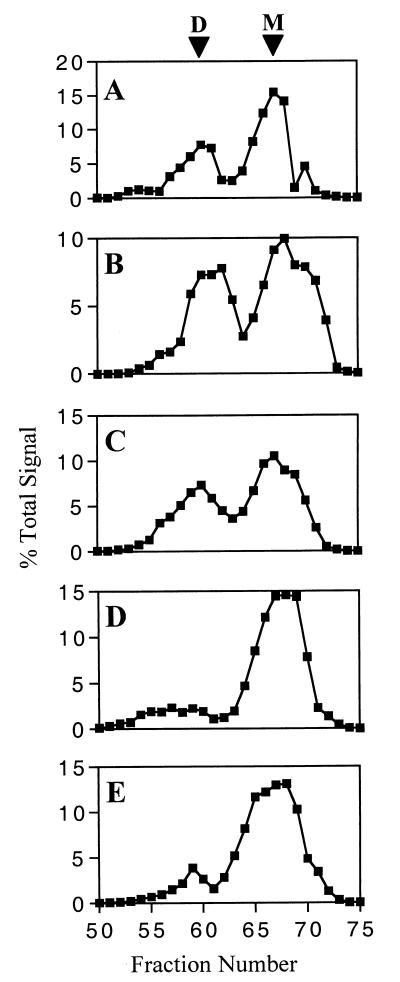
We reasoned that intersubunit contacts would likely involve regions exterior to the core of monomeric gp120. We focused on the variable loop domains based on their presumed exterior placement (24, 51) and relatively modular nature as defined by the disulfide-bonding pattern of gp120 (22). It has also been shown that deletion of V1/V2 or V3 from IIIB/LAI strain-derived env does not have a major impact on the global conformation of gp120, as gauged by the ability to bind CD4 and associate with gp41 (53). The conformational intactness of all the mutants used in this study was directly verified by their ability to interact with sCD4 and conformation-dependent MAb to a C1 epitope and a complex epitope not dependent on V1, V2, or V3 (data not shown). Accordingly, mutant gp120 lacking the V1, V2, or V3 domain was expressed, purified by lentil-lectin affinity chromatography, and subjected to gel filtration as before. Signal quantitation obtained from immunoblotted aliquots of gel filtration fractions are shown for wild-type gp120 and for gp120 lacking V1, V3, or V2 (Fig. 5A, B, C, and D, respectively). The two peaks corresponding to dimeric and monomeric species were present in comparable amounts in wild-type gp120 and in gp120 lacking V1 or V3. In contrast, the profile of gp120 lacking V2 showed a marked reduction in the amount of gp120 in the more rapidly eluting peak, indicating that this domain plays a major role in intersubunit association. In order to more precisely define the region involved, a gp120 deletion mutant lacking amino acids Tyr-173 to Ile-182 at the tip of the V2 loop was expressed as above. This region contains a number of relatively conserved residues (17) and is hydrophobic in nature, with Phe, Tyr, Leu, or Ile at 7 of 10 positions. When subjected to gel filtration, this mutant gp120 also showed a marked reduction in intersubunit association (Fig. 5E), although slightly more gp120 was seen in the more rapidly eluting peak than was the case when the entire V2 domain was deleted. Overall, analysis of the deletion mutants indicates that the V2 loop is required for gp120 intersubunit association.
FIG. 5.
Effect of variable domain deletion on gp120 oligomer formation. B-SC-1 cells were infected with recombinant vaccinia virus encoding wild-type or mutated gp120. Secreted gp120 was purified by lentil-lectin affinity chromatography and analyzed by gel filtration over Superdex 200. Aliquots of 1-ml fractions were immunoblotted with an env-specific antiserum. Signal quantitation is shown for vRCwt (A), vRCΔV1 (B), vRCΔV3 (C), vRCΔV2 (D), and vRCΔV2T (E). D and M indicate the dimer and monomer, respectively.
An epitope within the V2 loop is occluded in dimeric gp120.
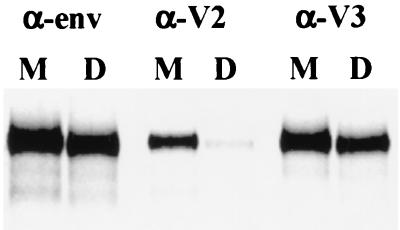
The data above are consistent with V2-V2 interactions or interactions between the V2 domain of one subunit and a different domain of the second subunit within the dimer. In order to evaluate these possibilities, the exposure of a V2-specific MAb epitope was assessed for both monomeric and dimeric gp120 by the immunoprecipitation of metabolically labeled proteins. A V2-V2 interaction would be expected to result in a more complete occlusion of this domain. The V3 loop has been shown to be well exposed in cell surface-expressed gp120 derived from T-cell-line-adapted strains (24, 34), and therefore exposure of a V3-specific MAb epitope was also examined. The V2-specific MAb epitope was markedly occluded within dimeric gp120 compared with monomer (Fig. 6). Signal quantitation corrected for total amounts of monomer and dimer present, as measured by immunoprecipitation with a polyclonal anti-env antiserum, revealed that exposure of this V2-specific epitope was approximately eightfold lower for dimeric gp120 compared with the monomeric form. In contrast, the V3-specific epitope was shown to have a similar level of exposure regardless of oligomeric status.
FIG. 6.
Comparison of epitope exposure of monomeric and dimeric gp120. B-SC-1 cells were infected with a recombinant vaccinia virus encoding gp120 (vPE50), metabolically labeled overnight, and lysed. Lysates were sedimented through sucrose density gradients, and fractions containing monomeric or dimeric gp120 were separately pooled. Monomeric and dimeric gp120 were immunoprecipitated with env-specific antiserum (α-env), the anti-V2 loop MAb T54 (α-V2), or the anti-V3 loop MAb D47 (α-V3).
DISCUSSION
We have shown that gp120 synthesized in the absence of gp41 can form and maintain intersubunit contacts. Analysis of cell lysates following pulse-labeling demonstrated that oligomers were formed intracellularly between 30 and 120 min after synthesis. The fact that secreted gp120 monomers did not form intersubunit contacts after concentration indicates that dimerization occurs as part of the protein folding process and that folding of the monomeric form is essentially irreversible once complete. The dimer was stable to repeated gel filtration as well as ultracentrifugation. Moreover, dissociation of the dimer by SDS in the absence of reducing agent was inefficient. However, treatment with 5.4 M guanidine hydrochloride prior to nonreducing SDS-PAGE resulted in dissociation (data not shown). Together, these data indicate that the intersubunit interactions are noncovalent but that either prior treatment with a strong denaturant or reduction of intramolecular disulfide bonds together with SDS treatment are required for efficient dissociation. Dimerization was not unique to one specific env protein, since gel filtration analysis of gp120 derived from the primary HIV-1 strain CM235 also suggested the existence of higher-order oligomers (P. Earl, unpublished data). A number of previous studies failed to detect appreciable amounts of oligomeric gp120 following the loss of association with gp41 (8, 47) or when synthesis occurred in the absence of the gp41 oligomerization domain (10). These studies used sucrose gradient sedimentation to gauge quaternary structure. We found in preliminary experiments that gp120 oligomers were less well maintained during this procedure than during gel filtration. Furthermore, differences in the conditions used in this and previous studies, including the detergent present, may have influenced the level of gp120 quaternary structure observed (27). In a study in which gp160 and gp120 were coexpressed, it was found that no hetero-oligomers were produced (30), indicating an absence of interaction between gp120 and the gp120 moiety of gp160. This may have been due to differences in the conformation of gp120 sequences within gp160 prior to cleavage or to lower intracellular concentrations of gp120 in the previous study compared with those obtained here. It is likely that high expression levels of gp120 may be required for efficient intersubunit contact formation given the fact that in the absence of the gp41 subunit the protein is not membrane bound.
Sedimentation equilibrium analysis indicated that the more rapidly eluting gel filtration peak was comprised mainly of gp120 dimers. A dimeric form of HIV-1 envelope is consistent with reports of gp160 dimers observed using sucrose density gradients and chemical cross-linking (6, 8) or scanning transmission electron microscopy (45). Furthermore, a soluble form of env containing the gp41 N-terminal oligomerization domain (gp140) was found using methods described here to be a mixture of dimers and tetramers (P. Earl and J. Lebowitz, unpublished data). Mixing N- and C-terminal transmembrane subunit fragments of HIV-1 (gp41) or HIV-2 which were derived by synthetic peptide synthesis or bacterial-cell expression led to trimer formation. Crystal-derived structures showed that the oligomeric interface consisted of a coiled-coil interaction between the N-terminal α-helical domains of each subunit (5, 48). It seems unlikely that gp120 and gp41 on the cell surface would self-associate with different numbers of subunits. To accommodate a trimeric structure for gp120 from our data it would be necessary to propose that the interaction between two of the subunits is stronger and therefore more stable during isolation and purification. This would imply that the contacts between the three subunits are asymmetrical. The NC1 domain of collagen type X presents an example of a highly stable dimeric component of a trimeric protein (2). Alternatively, it is possible that either the presence of gp120 modulates the oligomeric conformation adopted by the gp41 coiled coil or vice versa. The potential conformational flexibility of coiled-coil domains is demonstrated by the ability of a GCN4 leucine-zipper mutant to adopt both dimeric and trimeric forms (15). A further possibility is that the structural rearrangement that occurs in the transition from the native to the fusion-activated or postfusion conformation (mimicked by the mixed N- and C-terminal transmembrane subunit fragments) includes a change in oligomeric valency. The envelope protein E of the flavivirus tick-borne encephalitis virus provides an example of a dimer-to-trimer transition induced by lowered pH, which is believed to induce fusion of viral and endosomal membranes (1).
Analysis of deletion mutants indicated that the V2 domain was required for intersubunit contact formation. Deletion of the entire V1 or V3 domain had little impact on intersubunit association as assessed by gel filtration, whereas deletion of the entire V2 domain largely eliminated gp120 dimer formation and/or stability. Deletion of a conserved and hydrophobic segment at the tip of the V2 domain (amino acids Tyr-173 to Ile-182) also resulted in substantially less gp120 dimer, suggesting that residues within this segment form part of the intersubunit contact site. However, the possibility that the deletion of this segment affects the local V2 conformation and inhibits intersubunit association indirectly cannot be ruled out. We intend to perform further mutational analysis to more precisely define the amino acids directly involved in association. The fact that a V2-specific MAb epitope was eightfold less well exposed in dimeric gp120 compared with monomer suggests that the intersubunit contact involves the interaction of the V2 domains of both subunits within the dimer. It has been hypothesized that the ability of a peptide based on the sequence of a second conserved domain segment of gp120 to block the formation of gp120 oligomers (as assessed by gel filtration) demonstrated the involvement of this domain in gp120 oligomer formation (36). While this does not conflict with our findings, we point out that the latter study examined the self-association of purified gp120, which was dependent on high concentrations of calcium, and therefore may not reflect the intracellular process which produces functional cell or virion surface-expressed env. Furthermore, the involvement of the variable loops in gp120 intersubunit association is consistent with the crystallization of a gp120 molecule lacking these domains as a monomer (18).
An involvement of the V2 loop in the gp120 intersubunit contact site is also consistent with a number of reports suggesting that epitopes within this domain are less accessible to MAb binding to cell surface-expressed versus soluble purified gp120 (34, 37, 39). Moreover, it has also been shown that sera from infected individuals have few antibodies against V1/V2 epitopes (25). Within the V2 domain, it appears that epitopes located at the tip of the loop are relatively more occluded in cell surface-expressed gp120 than those in the N-terminal flank (37), supporting the role of the tip segment in association. The relative occlusion of a V2-specific epitope in dimeric versus monomeric gp120, together with the equal exposure of a V3-specific epitope irrespective of oligomeric status observed in this study, is in accord with the epitope exposure pattern reported for cell surface-expressed gp120 derived from T-cell-line-adapted strains (34, 37). This is consistent with a similar oligomeric organization for purified dimeric gp120 and gp120 within gp120-gp41 complexes. A more complete study of epitope exposure in dimeric and monomeric gp120 is being performed and will be reported elsewhere. It is well established that MAb binding to cell surface-expressed gp120 is more predictive of neutralization than is binding to purified soluble gp120 (12, 28, 33, 34, 43). It therefore seems likely that the quaternary structure of gp120 contributes to resistance to neutralization by limiting the exposure of potentially neutralizing epitopes. This is supported by the finding that removal of the V2 loop does not abrogate viral entry but can markedly increase viral sensitivity to neutralization by MAbs or patient sera (3, 38). Epitopes which are induced by CD4 binding and form part of the chemokine receptor binding site (18, 32) may be among those occluded at least in part by gp120 intersubunit association. Occlusion of CD4-induced epitopes of both purified soluble or cell surface-expressed gp120 prior to CD4 binding is substantially diminished upon deletion of the V1/V2 domain (44, 52).
In summary, we have shown that gp120 expressed in the absence of gp41 can form and maintain intersubunit contacts within cells, leading to the secretion of stable dimers. Furthermore, an important determinant of this association lies within the V2 domain. There is good evidence that the quarternary structure of gp120 on the cell surface modulates viral sensitivity to neutralization by antibody and probably influences processes such as cell surface-receptor binding and the induction of the conformational changes that culminate in fusion. Further elaboration of the quaternary structure of gp120 may allow a greater understanding of these processes than that gained by the study of monomeric forms of this protein.
ACKNOWLEDGMENTS
We thank J. Sodroski for providing the pSVIIIenv plasmid and mutant derivatives and N. Cooper for cells. Density and viscosity measurements for band sedimentation were performed by M. Vaske and M. Lewis, Molecular Interactions Resource, Office of Research Services, National Institutes of Health.
R.J.C. was supported by C. J. Martin Fellowship 987004 provided by the National Health & Medical Research Council of Australia.
REFERENCES
- 1.Allison S L, Schalich J, Stiasny K, Mandl C W, Kunz C, Heinz F X. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J Virol. 1995;69:695–700. doi: 10.1128/jvi.69.2.695-700.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber R E, Kwan A P L. Partial characterization of the C-terminal non-collagenous domain (NC1) of collagen type X. Biochem J. 1996;320:479–485. doi: 10.1042/bj3200479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao J, Sullivan N, Desjardin E, Parolin C, Robinson J, Wyatt R, Sodroski J. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J Virol. 1997;71:9808–9812. doi: 10.1128/jvi.71.12.9808-9812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarti S, Sisler J R, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 5.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 6.Doms R W, Earl P L, Moss B. The assembly of the HIV-1 env glycoprotein into dimers and tetramers. Adv Exp Med Biol. 1991;300:203–219. doi: 10.1007/978-1-4684-5976-0_13. [DOI] [PubMed] [Google Scholar]
- 7.Earl P L, Moss B, Wyatt L S, Carroll M W. Generation of recombinant vaccinia viruses. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1998. pp. 16.17.1–16.17.9. [Google Scholar]
- 8.Earl P L, Doms R W, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci USA. 1990;87:648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Earl P L, Koenig S, Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991;65:31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earl P L, Moss B. Mutational analysis of the assembly domain of the HIV-1 envelope glycoprotein. AIDS Res Hum Retrovir. 1993;9:589–594. doi: 10.1089/aid.1993.9.589. [DOI] [PubMed] [Google Scholar]
- 11.Earl P L, Moss B, Doms R W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991;65:2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouts T R, Binley J M, Trkola A, Robinson J E, Moore J P. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J Virol. 1997;71:2779–2785. doi: 10.1128/jvi.71.4.2779-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouts T R, Trkola A, Fung M S, Moore J P. Interactions of polyclonal and monoclonal anti-glycoprotein 120 antibodies with oligomeric glycoprotein 120-glycoprotein 41 complexes of a primary HIV type 1 isolate: relationship to neutralization. AIDS Res Hum Retrovir. 1998;14:591–597. doi: 10.1089/aid.1998.14.591. [DOI] [PubMed] [Google Scholar]
- 14.Furuta R A, Wild C T, Weng Y, Weiss C D. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol. 1998;5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez L, Jr, Brown R A, Richardson D, Alber T. Crystal structures of a single coiled-coil peptide in two oligomeric states reveal the basis for structural polymorphism. Nat Struct Biol. 1996;3:1002–1009. doi: 10.1038/nsb1296-1002. [DOI] [PubMed] [Google Scholar]
- 16.Helseth E, Olshevsky U, Furman C, Sodroski J. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J Virol. 1991;65:2119–2123. doi: 10.1128/jvi.65.4.2119-2123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korber B, Kuiken C, Foley B, Hahn B, McCutchan F, Mellors J W, Sodroski J. Human retroviruses and AIDS 1998. Los Alamos, N. Mex: Los Alamos National Laboratory; 1998. [Google Scholar]
- 18.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapham C K, Ouyang J, Chandrasekhar B, Nguyen N Y, Dimitrov D S, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 20.Laue T M, Stafford W F., III Modern applications of analytical ultracentrifugation. Annu Rev Biophys Biomol Struct. 1999;28:75–100. doi: 10.1146/annurev.biophys.28.1.75. [DOI] [PubMed] [Google Scholar]
- 21.Lebowitz J, Teale M, Schuck P W. Analytical band centrifugation of proteins and protein complexes. Biochem Soc Trans. 1998;26:745–749. doi: 10.1042/bst0260745. [DOI] [PubMed] [Google Scholar]
- 22.Leonard C K, Spellman M W, Riddle L, Harris R J, Thomas J N, Gregory T J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- 23.Lewis, M. S., and R. P. Junghans. A strategy for ultracentrifugal analysis of the molecular mass of glycoproteins of unknown or ill-defined carbohydrate composition. Methods Enzymol., in press. [DOI] [PubMed]
- 24.Moore J P, Sattentau Q J, Wyatt R, Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore J P, Sattentau Q J, Yoshiyama H, Thali M, Charles M, Sullivan N, Poon S-W, Fung M S, Traincard F, Pinkus M, Robey G, Robinson J E, Ho D D, Sodroski J. Probing the structure of the V2 domain of human immunodeficiency virus type 1 surface glycoprotein gp120 with a panel of eight monoclonal antibodies: human immune response to the V1 and V2 domains. J Virol. 1993;67:6136–6151. doi: 10.1128/jvi.67.10.6136-6151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz-Barroso I, Durell S, Sakaguchi K, Appella E, Blumenthal R. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J Cell Biol. 1998;140:315–323. doi: 10.1083/jcb.140.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owens R J, Compans R W. The human immunodeficiency virus type 1 envelope glycoprotein precursor acquires aberrant intermolecular disulfide bonds that may prevent normal proteolytic processing. Virology. 1990;179:827–833. doi: 10.1016/0042-6822(90)90151-g. [DOI] [PubMed] [Google Scholar]
- 28.Parren P W H I, Mondor I, Naniche D, Ditzel H J, Klasse P J, Burton D R, Sattentau Q J. Neutralization of human immunodeficiency virus type 1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J Virol. 1998;72:3512–3519. doi: 10.1128/jvi.72.5.3512-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinter A, Honnen W J, Tilley S A, Bona C, Zaghouani H, Gorny M K, Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989;63:2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poumbourios P, Wilson K A, Center R J, El Ahmar W, Kemp B E. Human immunodeficiency virus type 1 envelope glycoprotein oligomerization requires the gp41 amphipathic α-helical/leucine zipper-like sequence. J Virol. 1997;71:2041–2049. doi: 10.1128/jvi.71.3.2041-2049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratner L, Haseltine W, Patarca R, Livak K J, Starcich B, Josephs S F, Doran E R, Rafalski J A, Whitehorn E A, Baumeister K, Ivanoff L, Petteway J S R, Jr, Pearson M L, Lautenberger J A, Papas T S, Ghrayeb J, Chang N T, Gallo R C, Wong-Staal F. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985;313:277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- 32.Rizzuto C D, Wyatt R, Hernández-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 33.Roben P, Moore J P, Thali M, Sodroski J, Barbas III C F, Burton D R. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J Virol. 1994;68:4821–4828. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schawaller M, Smith G E, Skehel J J, Wiley D C. Studies with crosslinking reagents on the oligomeric structure of the env glycoprotein of HIV. Virology. 1989;172:367–369. doi: 10.1016/0042-6822(89)90142-6. [DOI] [PubMed] [Google Scholar]
- 36.Seddiki N, Bouhlal H, Rabehi L, Benjouad A, Devaux C, Gluckman J-C, Gattegno L. Involvement of the HIV-1 external envelope glycoprotein 120 (gp120) C2 region in gp120 oligomerization. Biochim Biophys Acta. 1997;1340:277–282. doi: 10.1016/s0167-4838(97)00052-6. [DOI] [PubMed] [Google Scholar]
- 37.Shotton C, Arnold C, Sattentau Q, Sodroski J, McKeating J A. Identification and characterization of monoclonal antibodies specific for polymorphic antigenic determinants within the V2 region of the human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1995;69:222–230. doi: 10.1128/jvi.69.1.222-230.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamatatos L, Cheng-Mayer C. An envelope modification that renders a primary, neutralization-resistant clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J Virol. 1998;72:7840–7845. doi: 10.1128/jvi.72.10.7840-7845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamatatos L, Cheng-Mayer C. Structural modulations of the envelope gp120 glycoprotein of human immunodeficiency virus type 1 upon oligomerization and differential V3 loop epitope exposure of isolates displaying distinct tropism upon virion-soluble receptor binding. J Virol. 1995;69:6191–6198. doi: 10.1128/jvi.69.10.6191-6198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamatatos L, Zolla-Pazner S, Gorny M K, Cheng-Mayer C. Binding of antibodies to virion-associated gp120 molecules of primary-like human immunodeficiency virus type 1 (HIV-1) isolates: effect on HIV-1 infection of macrophages and peripheral blood mononuclear cells. Virology. 1997;229:360–369. doi: 10.1006/viro.1997.8443. [DOI] [PubMed] [Google Scholar]
- 41.Stein B S, Engleman E G. Intracellular processing of the gp160 HIV-1 envelope precursor. J Biol Chem. 1990;265:2640–2649. [PubMed] [Google Scholar]
- 42.Sugiura W, Broder C C, Moss B, Earl P L. Characterization of conformation-dependent anti-gp120 murine monoclonal antibodies produced by immunization with monomeric and oligomeric human immunodeficiency virus type 1 envelope proteins. Virology. 1999;254:257–267. doi: 10.1006/viro.1998.9549. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan N, Sun Y, LI J, Hofmann W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan N, Sun Y, Sattentau Q, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. CD4-Induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J Virol. 1998;72:4694–4703. doi: 10.1128/jvi.72.6.4694-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas D J, Wall J S, Hainfeld J F, Kaczorek M, Booy F P, Trus B L, Eiserling F A, Steven A C. gp160, the envelope glycoprotein of human immunodeficiency virus type 1, is a dimer of 125-kilodalton subunits stabilized through interactions between their gp41 domains. J Virol. 1991;65:3797–3803. doi: 10.1128/jvi.65.7.3797-3803.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trkola A, Dragic T, Arthos J, Biney J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 47.Weiss C D, Levy J A, White J M. Oligomeric organization of gp120 on infectious human immunodeficiency virus type 1 particles. J Virol. 1990;64:5674–5677. doi: 10.1128/jvi.64.11.5674-5677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 49.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 50.Wyatt R, Desjardin E, Olshevsky U, Nixon C, Binley J, Olshevsky V, Sodroski J. Analysis of the interaction of the human immunodeficiency virus type 1 gp120 envelope glycoprotein with the gp41 transmembrane glycoprotein. J Virol. 1997;71:9722–9731. doi: 10.1128/jvi.71.12.9722-9731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 52.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyatt R, Sullivan N, Thali M, Repke H, Ho D, Robinson J, Posner M, Sodroski J. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J Virol. 1993;67:4557–4565. doi: 10.1128/jvi.67.8.4557-4565.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]