Abstract
Background
Obesity and metabolic syndrome are global health concerns associated with development of different types of diseases and serious health threats in the long term. Their metabolic imbalance can be attributable to inherited and environmental factors. As a considerable environmental agent, heavy metals exposure can predispose individuals to diseases like obesity. This systematic review and meta-analysis aimed to evaluate the association between heavy metals exposure and the risk of obesity.
Methods
PubMed/MEDLINE, EMBASE and Web of Science were systematically searched until December 17, 2022. Only observational studies that evaluated heavy metals exposure and obesity were included. Studies were excluded if they assessed maternal or prenatal exposure, the mixture of heavy metals and other chemicals, reported the association with overweight or other diseases, and undesirable study designs. The Joanna Briggs Institute checklist was used for quality assessment. The pooled adjusted odds ratio (aOR) and the pooled standardized mean difference (SMD) with their 95% confidence intervals (CIs) were calculated, respectively. The publication bias was evaluated using Egger's and Begg's tests.
Results
Twenty studies (n = 127755), four case–control and sixteen analytical cross-sectional studies, were included. Lead exposure was significantly associated with a lower risk of obesity (aOR: 0.705, 95% CI: 0.498–0.997), while mercury (aOR: 1.458, 95% CI: 1.048–2.031) and barium (aOR: 1.439, 95% CI: 1.142–1.813) exposure increased the risk of obesity. No significant publication bias was found and the studies had a low risk of bias.
Conclusion
Overall, lead exposure reduced obesity risk, while mercury and barium exposure raised it. Further large-scale observational studies are recommended to determine the roles of heavy metals in obesity.
Study registration ID: CRD42023394865.
Supplementary information
The online version contains supplementary material available at 10.1007/s40200-023-01307-0.
Keywords: Heavy Metal; Obesity; Excess Body Weight; Mercury; Lead; Cadmium; Manganese; Barium; Chromium; Zinc; Systematic Review, Meta-analysis
Introduction
According to the World Health Organization (WHO), "Overweight and obesity are defined as abnormal or excessive fat accumulation that presents a health risk". In this regard, body mass index (BMI) over 25 is considered overweight, and more than 30 is sub grouped in the obese category [1].
The global prevalence of overweight and obesity has been twofold since 1980 [2]. On March 4, 2022, WHO declared that around the world, 650 million adults, 340 million teenagers, and 39 million children are obese, reaching 1 billion obese people worldwide [3]. Over the past 50 years, obesity has developed into a global pandemic due to its prevalence. Obesity is a substantial public health concern because it significantly increases the risk of diseases such as hypertension, myocardial infarction [4], type 2 diabetes, fatty liver disease, stroke, dementia, osteoarthritis, obstructive sleep apnea [5, 6], and various cancers [7] all of which lead to a decline in both life expectancy and quality of life. Beyond that, obesity is companied by low social competence, low self-esteem, social isolation, unemployment and low socioeconomic status, which aggravate the quality of life in the patients moreover [8].
The pathogenesis of obesity entails regulating calorie use, appetite, and physical activity. Still, it has complicated interplay with the accessibility of healthcare systems, the role of socioeconomic conditions, and underlying hereditary and environmental factors [9].
Environmental endocrine-disrupting chemicals are "exogenous factors that intervene with synthesis, secretion, transport, metabolism, binding action or removal of natural blood-borne hormones present in the body and are responsible for homeostasis, reproduction and developmental processes" [10]. Several chemical compounds have been identified as environmental risk factors for obesity, including phthalates [11], bisphenols, parabens, and persistent organic pollutants [12]. Heavy metals are naturally present in the environment and can cause systemic diseases like chronic kidney disease and have a tremendous attributable burden [13, 14]. As such, metabolic syndrome has been shown to be linked with exposure to mercury [15], lead [16], and arsenic [17] exposure. On the other hand, the results of a study showed that serum mercury levels have a negative relationship with body mass index (BMI) in adults, while no correlation was observed between serum mercury levels and BMI in children [18]. A negative relationship between the urinary levels of lead and cadmium with BMI and waist circumference (WC) was also found in another study [19].
Previous studies have evaluated heavy metals exposure and the development of osteopenia/osteoporosis [20] and chronic kidney disease [13]. Also, considering that several studies have investigated the relationship between heavy metals and metabolic disorders, including obesity, and have reached controversial findings regarding the effect of exposure to heavy metals and obesity, a systematic review and meta-analysis study is necessary to find an accurate statistical relationship. Herein, this systematic review and meta-analysis study was conducted to shed light on the association between heavy metals exposure and the risk of obesity.
Methods
This study was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [21]. The study was registered in the Systematic Review Registration: PROSPERO (registration ID: CRD42023394865).
Search strategy
We searched PubMed/Medline, EMBASE and Web of Science for studies reporting the association between heavy metals exposure and risk of obesity published up to December 17, 2022. Observational studies written in English were selected. We used the following Medical Subject Heading (MeSH) terms: ("Environmental Exposure" OR "Inhalation Exposure" OR "Occupational Exposure" OR "Dietary Exposure") AND ("Obesity" OR "Pediatric Obesity" OR "Obesity, Abdominal" OR "Obesity, Morbid" OR "Overweight") AND ("Metals, Heavy" OR "Cadmium" OR "Arsenic" OR "Mercury" OR "Lead") (Table S1-S3). Backwards and forwards citation searching was performed.
Study selection
The records found through database searching were merged, and the duplicates were removed using EndNote X8 (Thomson Reuters, Toronto, ON, Canada). Two reviewers (MZ and AGJ) independently screened the records by title/abstract and full texts according to the inclusion/exclusion criteria. Any disagreements were resolved by the lead investigator (MS). Included studies met the following criteria: (i) participants with exposure to heavy metals that were divided into high and low exposure groups according to serum and/or urine heavy metals levels, (ii) identification of patients with general or abdominal obesity and lean individuals, and (iii) comparison of the number of obese participants or levels of heavy metals in each group.
According to the World Health Organization (WHO) definitions [22], general obesity was defined as BMI ≥ 30 kg/m2 in adults and ≥ 95th percentile for children and adolescents. On the other hand, the definition of abdominal obesity was WC greater than 102 cm for men and greater than 88 cm for women according to the WHO definition [22], WC ≥ 90 cm in men and WC ≥ 80 cm in women according to the criteria of the International Diabetes Federation [23] or waist-to-height ratio ≥ 0.5. Heavy metal was defined as an element with an atomic number greater than 20 and an atomic density of more than 5 g per cubic meter, with metal-like properties [24].
The exclusion criteria were as follows: (i) assessing the maternal or prenatal (early life) exposure or pregnant women with obesity, (ii) assessing the exposure through heavy metals levels in soil, water, or participants' nail or hair, (iii) mixture of heavy metals with other chemicals, (iv) reporting the association between heavy metals and overweight or combination of overweight and obesity, (v) combined effect of heavy metals and obesity on other diseases, (vi) association between heavy metals and BMI, not obesity, and (vii) studies with common datasets and participants. Also, editorials, reviews, study protocols, meeting or conference abstracts and animal and molecular articles, were excluded.
Data extraction
Two reviewers (MZ and AGJ) designed a data extraction form. These reviewers collected data from all relevant studies, and consensus settled disagreements. The following data were extracted: first author name; year of publication; study design; duration and data source; countries where the research was conducted; demographics (i.e., age and sex); obesity type; source of sampling; heavy metals detection limit; the lowest and highest levels of heavy metals or the mean level in samples; the total number of participants, and the number of participants with obesity.
Quality assessment
Two reviewers (MZ and AGJ) assessed the quality of the studies using the Joanna Briggs Institute (JBI) critical appraisal tools for case controls and analytical cross-sectional studies [25]. A third reviewer (MS) was consulted if there were any discrepancies. Items such as study population, the measure of exposures, confounding factors, the extent of outcomes, follow-up data, and statistical analysis were evaluated.
Statistical analysis
The pooled adjusted odds ratio (aOR) with 95% confidence intervals (CIs) for dichotomous data and the pooled standardized mean difference (SMD) with 95% CIs for continuous data were assessed using random or fixed-effects models. Multiple effect sizes in a study with different groups were pooled with a fixed-effects model. The random-effects model was used because of the estimated methodological heterogeneity of the true effect sizes. The between-study heterogeneity was assessed by Cochran's Q and the I2 statistic. I2 values of more than 50% were considered high heterogeneity [26]. Subgroup and subset analyses were performed to compare the role of age, sampling source and type of obesity in statistical heterogeneity and final effect size. The median and inter-quartile range (IQR) were converted to mean and standard deviation (SD) for SMD calculation [27]. In order to avoid data overlap, different heavy metals from studies with common data sets were chosen. Meta-analysis was done for heavy metals with at least three studies. Effect sizes with the most adjustments were pooled to modify potential confounding factors. Publication bias was evaluated statistically by using Egger's and Begg's tests (p < 0.05 was considered indicative of statistically significant publication bias) [28]. The funnel plot was not used for publication bias assessment because fewer than ten studies were in each analysis [29]. All analyses were performed using "Comprehensive Meta-Analysis" software, Version 3.7 (Biostat, Englewood, NJ).
Results
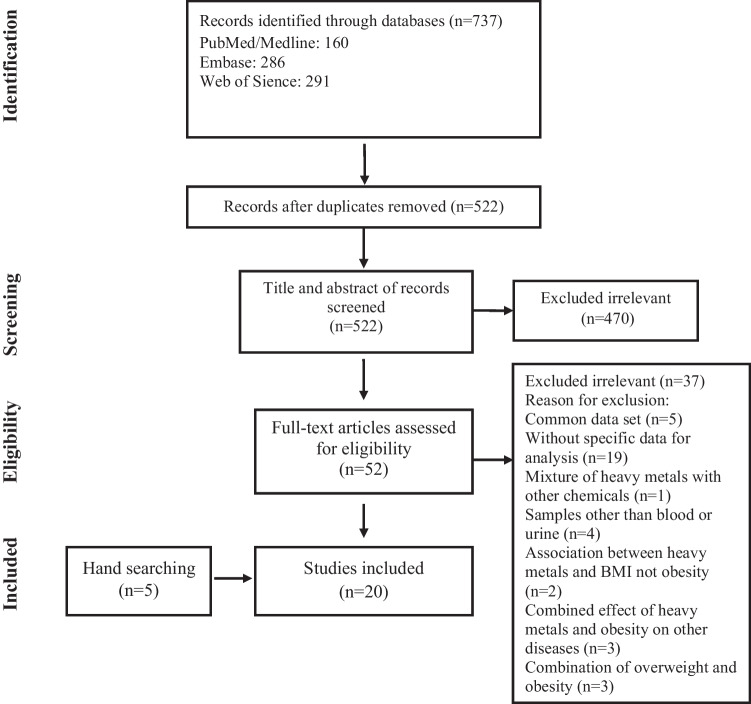
We identified 737 papers through online database searching and screened 522 papers after removing duplicates. First, we excluded 470 papers by title and abstract screening. Then, we assessed 52 studies by full-text review. Fifteen articles were selected, and five studies were found by hand searching and backward/forward citation searching. Finally, four case–control [30–33] and sixteen analytical cross-sectional studies [34–49] were included (Fig. 1).
Fig. 1.
Flow chart of study selection for inclusion in the systematic review and meta-analysis
Study characteristics
Seven studies were conducted in the United States of America (USA) [37, 39, 43–46, 48], five in South Korea [34–36, 38, 40], four in China [33, 42, 47, 49] and others in Taiwan [41], Turkey [32], Egypt [31], and Russia [30]. All the USA studies [37, 39, 43–46, 48] were from US National Health and Nutrition Examination Survey data sources in the period from 1999 to 2016. The data source of the four Korean studies [34, 35, 38, 40] and one Egyptian article [31] was the Korean National Health and Nutrition Examination Survey from 2005 to 2017. Two Chinese studies [42, 47] were from the Survey on Prevalence in East China for Metabolic Diseases and Risk Factors 2014. Ten studies [30–32, 37, 42, 44–48] had assessed general obesity, and ten articles [33–36, 38–41, 43, 49] had reported abdominal obesity (Table 1).
Table 1.
Study characteristics
| First author | Publication year | country | Study design | Study duration | Data source | Type of obesity |
|---|---|---|---|---|---|---|
| Tascilar et al. [32] | 2011 | Turkey | Case control | NA | Healthy children | General |
| Scinicariello et al. [44] | 2013 | USA | Analytical cross-sectional | 1999–2006 | NHANES | General |
| Lee and Kim. [38] | 2013 | South Korea | Analytical cross-sectional | 2005–2010 | KNHANES | Abdominal |
| moon et al. [40] | 2014 | South Korea | Analytical cross-sectional | 2009–2010 | KNHANES | Abdominal |
| Eom et al. [36] | 2014 | South Korea | Analytical cross-sectional | 2010–2011 | Healthy adult | Abdominal |
| Azab et al. [31] | 2014 | Egypt | Case control | 2011–2013 | KNHANES | General |
| Wang et al. [47] | 2015 | China | Analytical cross-sectional | 2014 | SPECT-China | General |
| Nie et al. [42] | 2016 | China | Analytical cross-sectional | 2014 | SPECT-China | General |
| Shao et al. [45] | 2017 | USA | Analytical cross-sectional | 1999–2012 | NHANES | General |
| Fan et al. [37] | 2017 | USA | Analytical cross-sectional | 2011–2014 | NHANES | General |
| Noor et al. [43] | 2018 | USA | Analytical cross-sectional | 2001–2014 | NHANES | Abdominal |
| Wang et al. [48] | 2018 | USA | Analytical cross-sectional | 2003–2014 | NHANES | General |
| Zhang et al. [33] | 2020 | China | Case control | 2017 | Beijing Population Health Cohort | Abdominal |
| Lo et al. [39] | 2021 | USA | Analytical cross-sectional | 2001–2016 | NHANES | Abdominal |
| Duc et al. [35] | 2021 | South Korea | Analytical cross-sectional |
2009–2013 2016–2017 |
KNHANES | Abdominal |
| Swayze et al. [46] | 2021 | USA | Analytical cross-sectional | 1999–2016 | NHANES | General |
| Cho et al. [34] | 2021 | South Korea | Analytical cross-sectional | 2010–2013 | KNHANES | Abdominal |
| Tinkov et al. [30] | 2021 | Russia | Case control | NA | Healthy adult | General |
| Zhao et al. [49] | 2022 | China | Analytical cross-sectional | 2017–2019 | Healthy adult | Abdominal |
| Ngu et al. [41] | 2022 | Taiwan | Analytical cross-sectional | NA | Healthy adult | Abdominal |
NA: not available/ USA: United States of America/ NHANES: National Health and Nutrition Examination Survey/ KNHANES: Korean National Health and Nutrition Examination Survey/ SPECT-China: Survey on Prevalence in East China for Metabolic Diseases and Risk Factors
Participant characteristics
There were 127,755 participants in the included studies that 81% were adults. According to nineteen studies [30–45, 47–49], 48.4% (46,421/95743) were males and 23.8% (12,273/51502) of participants were obese according to nine studies [30–32, 35, 41, 42, 44, 45, 47] (Table 2).
Table 2.
Participant characteristics
| First author | Total participants | Age (year) |
Obese participants (%) |
||
|---|---|---|---|---|---|
| Male (%) | Female (%) | ||||
| Tascilar et al. [32] | 67 | 10.6 | 34 (50.7) | ||
| 36 (53.7) | 31 (46.3) | ||||
| Scinicariello et al. [44] | 26,592 | 30.7 | 7299 (27.4) | ||
| 13,291 (49.9) | 13,301 (50.1) | Children: 11 | Adult: 44 | ||
| Lee and Kim. [38] | 7559 | ≥ 20 | - | ||
| 3783 (50.0) | 3776 (50.0) | ||||
| moon et al. [40] | 3950 | ≥ 20 | - | ||
| 1961 (49.6) | 1989 (50.4) | ||||
| Eom et al. [36] | 2114 | 45.5 | - | ||
| 920 (43.5) | 1194 (56.5) | ||||
| Azab et al. [31] | 160 | 7.9 | 80 (50.0) | ||
| 84 (52.5) | 76 (47.5) | ||||
| Wang et al. [47] | 5558 | 53.3 | 300 (5.3) | ||
| 2235 (40.2) | 3323 (59.8) | ||||
| Nie et al. [42] | 5544 | ≥ 18 | 299 (5.3) | ||
| 2229 (40.2) | 3315 (59.8) | ||||
| Shao et al. [45] | 6602 | 12.8 | 1127 (17.0) | ||
| 3443 (52.1) | 3159 (47.9) | ||||
| Fan et al. [37] | 5404 | 11.9 | - | ||
| 2745 (50.7) | 2659 (49.3) | ||||
| Noor et al. [43] | 3981 | 44.7 | - | ||
| 1996 (50.1) | 1985 (49.9) | ||||
| Wang et al. [48] | 9537 | 49.2 | - | ||
| 4841 (50.7) | 4696 (49.3) | ||||
| Zhang et al. [33] | 4134 | 60.0 | - | ||
| 2043 (49.4) | 2091 (50.6) | ||||
| Lo et al. [39] | 5048 | > 18 | - | ||
| 2452 (48.5) | 2596 (51.5) | ||||
| Duc et al. [35] | 6434 | 61.6 | 2870 (44.6) | ||
| 3063 (47.6) | 3371 (52.4) | ||||
| Swayze et al. [46] | 32,012 | ≥ 20 | - | ||
| - | - | ||||
| Cho et al. [34] | 1327 | 14.3 | - | ||
| 672 (50.6) | 655 (49.4) | ||||
| Tinkov et al. [30] | 395 | 45.2 | 196 (49.6) | ||
| 103 (26.0) | 292 (74) | ||||
| Zhao et al. [49] | 1187 | 62.4 | - | ||
| 418 (28.3) | 769 (71.7) | ||||
| Ngu et al. [41] | 150 | 42.6 | 68 (45.3) | ||
| 106 (70.7) | 44 (29.3) | ||||
Exposure characteristics
Eight studies [37, 38, 40, 44–47, 49] reported the effect of lead exposure on obesity. This number was eight for cadmium exposure [38, 40–43, 45, 46, 49], six for mercury exposure [33, 34, 36, 37, 40, 41] and zinc exposure [30–33, 37, 41], five for manganese exposure [33, 37, 39, 41, 49], four for exposure to the mixture of heavy metals, three for barium exposure [33, 45, 46], and three for the exposure to chromium [30–32]. Thirteen studies assessed the exposure through blood samples [31–38, 40–42, 44, 47], urine samples were evaluated in three articles [43, 45, 49], and four studies assessed both samples [30, 39, 46, 48] (Table 3).
Table 3.
Exposure characteristics
| First author | Heavy metal (unit) | Detection limit (unit) | sample | |
|---|---|---|---|---|
| Lower or case level | Higher or control level | |||
| Tascilar et al. [32] |
Zn (μg/dl) Cr (μg/L) |
– | Blood | |
|
Case: 67.45 ± 18.42 Case: 13.59 ± 2.47 |
Control: 74.45 ± 18.42 Control: 13.84 ± 1.61 |
|||
| Scinicariello et al. [44] | Pb (μg/dL) | 0.25–0.30 (μg/dl) | Blood | |
|
Children (q1): ≤ 0.7 Adult (q1): ≤ 1 |
Children(q4): ≥ 1.61 Adult(q4): ≥ 2.4 |
|||
| Lee and Kim. [38] |
Pb (μg/L) Cd (μg/L) |
Pb: 0.12 (mg/dl) Cd: 0.056 (mg/L) |
Blood | |
|
Q1: ≤ 2.362 Q1: ≤ 0.819 |
Q3: ≥ 3.282 Q3: ≥ 1.359 |
|||
| moon et al. [40] |
Pb (μg/dl) Cd (μg/L) Hg (μg/L) |
≥ 20 | Blood | |
|
Q1: 1.23 ± 1.01 Q1: 0.37 ± 1.01 Q1: 1.88 ± 1.01 |
Q4: 3.79 ± 1.01 Q4: 1.94 ± 1.01 Q4: 8.73 ± 1.01 |
|||
| Eom et al. [36] | Hg (μg/L) | 0.2 (μg/L) | Blood | |
| Q1: < 2.99 | Q3: ≥ 4.88 | |||
| Azab et al. [31] |
Zn (μg/dl) Cr (ng/ml) |
– | Blood | |
|
Case: 57 ± 14 Case: 0.2 ± 0.08 |
Control: 75 ± 17 Control: 0.18 ± 0.05 |
|||
| Wang et al. [47] | Pb (μg/L) | – | Blood | |
|
Men (q1): ≤ 29 Women(q1): ≤ 25.13 |
Men (q4): ≥ 62.17 Women(q4): ≥ 54.36 |
|||
| Nie et al. [42] | Cd (μg/L) | 0.01 (μg/L) | Blood | |
| Q1: ≤ 0.8 | Q3: ≥ 2.95 | |||
| Shao et al. [45] |
Cd (ng/ml) Pb (ng/ml) Ba (ng/ml) |
– | Urine | |
|
Q1: ≤ 0.049 Q1: ≤ 0.35 Q1: ≤ 0.9 |
Q4: > 0.173 Q4: > 1.04 Q4: > 3.25 |
|||
| Fan et al. [37] |
Pb Zn Mn Hg |
– | Blood | |
| Q1 | Q4 | |||
| Noor et al. [43] | Cd (μg/L) | 0.056 (μg/L) | Urine | |
| Q1: < 0.13 | Q4: > 0.60 | |||
| Wang et al. [48] | 18 heavy metals | – |
Blood (3 metals) Urine (15 metals) |
|
| 10th % | 90th % | |||
| Zhang et al. [33] |
Zn (μg/L) Hg (μg/L) Mn (μg/L) Ba (μg/L) |
Zn: 11.75 (μg/L) Hg: 0.0112 (μg/L) Mn: 0.0347 (μg/L) Ba: 0.1087 (μg/L) |
Blood | |
|
Q1: < 763.87 Q1: < 0.32 Q1: < 0.95 Q1: < 2.17 |
Q4: > 973.89 Q4: > 0.90 Q4: > 1.69 Q4: > 4.89 |
|||
| Lo et al. [39] |
Blood Mn (μg/L) Urine Mn (10–2 μg/g creatinine) |
– | Blood & Urine | |
|
Q1: < 7.63 Q1: < 6.97 |
Q4: > 11.91 Q4: > 18.60 |
|||
| Duc et al. [35] |
Cd (μg/L) Pb (μg/dl) Hg (μg/L) |
Cd: 0.087 μg/L Pb: 0.223 μg/dL Hg: 0.05 μg/L |
Blood | |
|
5th %: 0.55 5th %: 1.14 5th %: 1.31 |
95th %: 2.55 95th %: 4.41 95th %: 11.36 |
|||
| Swayze et al. [46] |
Blood Cd (ng/ml) Urine Cd (ng/ml) Urine Pb (ng/ml) Urine Ba (ng/ml) |
– | Blood & Urine | |
|
Lower level: 2.4 Lower level: 0.16 Lower level: 0.4 Lower level: 0.88 |
Higher level: 11.2 Higher level: 1.01 Higher level: 1.8 Higher level: 4.76 |
|||
| Cho et al. [34] | Hg (μg/L) | 0.158 (µg/L) | Blood | |
| Q1 | Q4 | |||
| Tinkov et al. [30] |
Blood Zn (μg/dl) Blood Cr (ng/ml) |
– | Blood & Urine | |
|
Case: 91 ± 10 Case: 0.89 ± 0.56 |
Control: 92 ± 10 Control: 1.64 ± 0.82 |
|||
| Zhao et al. [49] |
Cd (μg/L) Pb (μg/L) Mn (μg/L) |
1 (μg/L) | Urine | |
|
Q1: 1.30 Q1: 1.20 Q1: 2.84 |
Q4: 3.65 Q4: 2.90 Q4: 7.13 |
|||
| Ngu et al. [41] |
Zn (μg/L) Cd (μg/L) Hg (μg/L) Mn (μg/L) |
– | Blood | |
|
Q1: < 764.5 NA Q1: < 0.9 Q1: < 1.4 |
Q3: > 951.9 NA Q3: > 1.4 Q3: > 1.7 |
|||
Zn: zinc/ Cr: chromium/ Pb: lead/ Cd: cadmium/ Hg: mercury/ Ba: barium/ Mn: manganese/ NA: not available
Meta-analysis results
Lead exposure
Obesity was significantly lower in participants with high exposure to lead (aOR: 0.705, 95% CI: 0.498–0.997, Begg's test: 0.265, Egger's test: 0.398) (Fig. 2). This association remained significant in children and general obesity. Subset analysis showed that children had the most role in statistical heterogeneity in lead exposure (Table S4).
Fig. 2.
Forest plot of the adjusted odds ratio for the association between lead exposure and obesity
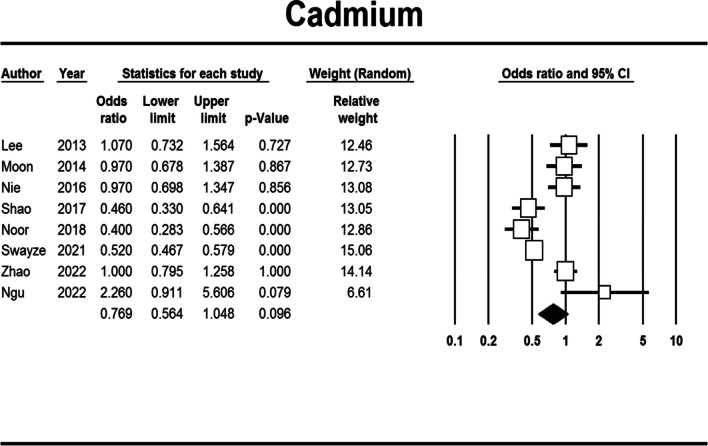
Cadmium exposure
Those with high exposure to cadmium had non-significantly lower odds of obesity development compared to those with low exposure (aOR: 0.769, 95% CI: 0.564–1.048, Begg's test: 0.536, Egger's test: 0.123) (Fig. 3). But after subgroup and subset analysis, a statistically significant association between general obesity, urine sampling and cadmium exposure was found. Also, the difference between adults and children was significant (Table S5).
Fig. 3.
Forest plot of the adjusted odds ratio for the association between cadmium exposure and obesity
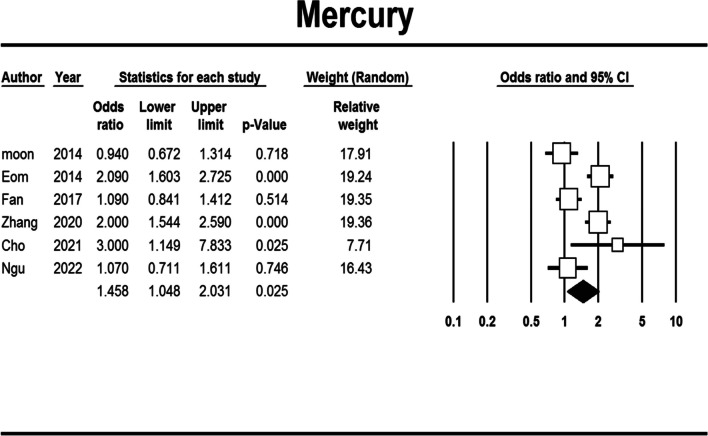
Mercury exposure
Mercury exposure was significantly related to an increased risk of obesity (aOR: 1.458, 95% CI: 1.048–2.031, Begg's test: 1.000, Egger's test: 0.987) (Fig. 4). This association remained significant only for abdominal obesity (Table S6).
Fig. 4.
Forest plot of the adjusted odds ratio for the association between mercury exposure and obesity
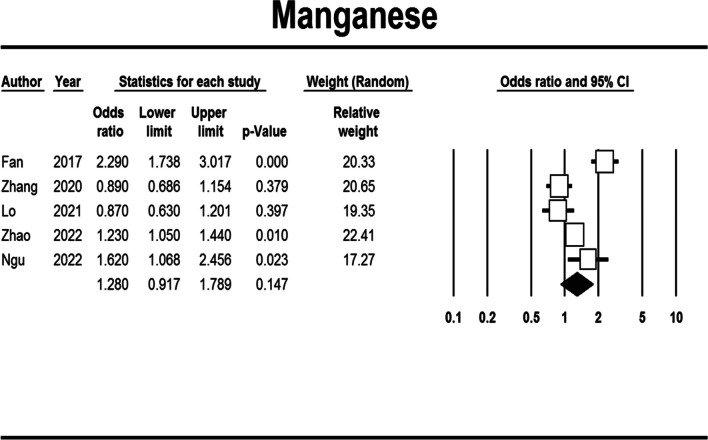
Manganese exposure
Manganese exposure could insignificantly increase the risk of obesity (aOR: 1.280, 95% CI: 0.917–1.789, Begg's test: 0.806, Egger's test: 0.867) (Fig. 5). This relation was inverted for a urine sample, and there were significant differences between adult/children age groups and general/abdominal obesity (Table S7).
Fig. 5.
Forest plot of the adjusted odds ratio for the association between manganese exposure and obesity
Other heavy metals
Obesity was about 10% more in participants with high zinc exposure, but there was no statistical significance (Figure S1 and Table S8). On the other hand, barium exposure could significantly increase the risk of obesity by up to 44% (Figure S2 and Table S9). The mean of serum zinc (SMD: -0.542, 95% CI: -1.245/0.161, Begg's test: 1.000, Egger's test: 0.597) and chromium (SMD: -0.303, 95% CI: -1.258/0.651, Begg's test: 1.000, Egger's test: 0.493) levels were insignificantly lower in obese participants than lean people (Figures S3-S4 and Tables S10-S11).
The mixture of heavy metals
The mixture of different heavy metals could insignificantly increase the risk of obesity (aOR: 1.508, 95% CI: 0.748–3.041, Begg's test: 0.574, Egger's test: 0.734) (Fig. 6).
Fig. 6.
Forest plot of the adjusted odds ratio for the association between exposure to heavy metals mixture and obesity
Quality assessment
Overall, the included analytical cross-sectional and case–control studies had a low risk of bias (Table 4 and Table 5) except Wang et al. [47] study in the cases of sampling inclusion criteria, the definition of the study setting and exposure measurement along with the Azab et al. [31], and Tascilar et al. [32] studies for dealing with confounding factors.
Table 4.
Quality assessment of analytical cross-sectional studies
| Author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Moon et al. [40] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Lee and Kim. [38] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Scinicariello et al. [44] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Shao et al. [45] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Wang et al. [47] | No | No | No | Yes | Yes | Yes | Yes | Yes |
| Ngu et al. [41] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Eom et al. [36] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Nie et al. [42] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Fan et al. [37] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Noor et al. [43] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Wang et al. [48] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Cho et al. [34] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Swayze et al. [46] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Duc et al. [35] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Zhao et al. [49] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Lo et al. [39] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
1. Were the criteria for inclusion in the sample clearly defined?
2. Were the study subjects and the setting described in detail?
3. Was the exposure measured in a valid and reliable way?
4. Were objective, standard criteria used for measurement of the condition?
5. Were confounding factors identified?
6. Were strategies to deal with confounding factors stated?
7. Were the outcomes measured in a valid and reliable way?
8. Was appropriate statistical analysis used?
Table 5.
Quality assessment of case control studies
| Author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Tinkov et al. [30] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes |
| Azab et al. [31] | Yes | Yes | Yes | Yes | Yes | No | Unclear | Yes | Yes | Yes |
| Tascilar et al. [32] | Yes | Yes | Yes | Yes | Yes | No | Unclear | Yes | Unclear | Yes |
| Zhang et al. [33] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
1. Were the groups comparable other than the presence of disease in cases or the absence of disease in controls?
2. Were cases and controls matched appropriately?
3. Were the same criteria used for identification of cases and controls?
4. Was exposure measured in a standard, valid and reliable way?
5. Was exposure measured in the same way for cases and controls?
6. Were confounding factors identified?
7. Were strategies to deal with confounding factors stated?
8. Were outcomes assessed in a standard, valid and reliable way for cases and controls?
9. Was the exposure period of interest long enough to be meaningful?
10. Was appropriate statistical analysis used?
Discussion
The present study's findings showed that mercury and barium exposure significantly increased the odds of obesity, while lead exposure significantly decreased the risk. The subgroup and subset analysis results showed that lead exposure in children and participants with general obesity, in addition to cadmium exposure in urine samples and participants with general obesity, was significantly associated with decreased obesity development. However, exposure to mercury in participants with abdominal obesity and exposure to barium in those with general obesity, urine samples, and adults significantly increased the risk of obesity. Also, the overall quality of the included studies was high.
Heavy metal poisoning often arises as a result of exposure in the workplace, like in mining and metallurgical activities, or due to interaction with industrial waste. There are also specific sources of heavy metal exposure in the general population [50]. For example, inorganic arsenic is taken into through pesticides, the smelting of copper and lead, wood preservation methods, and volcanic emissions [50]. Also, significant origins of lead exposure include lead-based paint, materials in the production of batteries, and the use of certain cosmetics [51]. Cadmium exposure sources are smoking, vegetables, seeds, shellfish, the manufacturing of plastics, as well as nickel–cadmium battery production [52]. Fish contaminated due to pollution is the primary origin of mercury, mainly in the form of methylmercury. Moreover, in countries that still permit the production of amalgam used for dental fillings, mercury poses a professional risk for dentists [50, 53].
Results of a survey of 884 women showed a significant association between blood lead levels and body mass index (B = 0.66; 95% CI = 0.09–1.22, p = 0.02) [54]. A prospective birth cohort study on 1442 mother–child pairs showed that increased red blood cell lead levels were associated with 1.65 times increased odds of overweight or obesity in children (95% CI: 1.18–2.32) [55]. On the other hand, several studies pointed out that lead exposure during the prenatal period has been associated with lower body weight in human infants [56, 57]. Complex interactions between neurodevelopmental effects of lead exposure on appetite and energy utilization, gut microbiome dysbiosis, and epigenetic changes have been suggested for the development of obesity [58]. A study by Pilsner et al. demonstrated that the methylation of genomic DNA in cord blood is inversely correlated with prenatal lead exposure. These findings indicate that maternal cumulative lead burden can impact the developing fetus' epigenome [59]. The animal study revealed that lead exposure causes gene expression alterations of methyltransferases and methyl cytosine-binding proteins, both of which regulate the methylation of DNA [60] and alter insulin and leptin gene expression associated with food intake and body glucose [61].
Our results showed that lead exposure decreased the risk of obesity (OR = 0.71; 95% CI: 0.50–1.00), and the results were also significant for children and those with general obesity subgroup analysis. It should be noted that there was a remarkable heterogeneity between the studies (I-square = 93.24%), and the effect size was so close to the significant cut-off (p = 0.048). Furthermore, the inverse association between lead exposure and obesity might be due to the not inclusion of cohort studies in the meta-analysis. As a result, further large-scale prospective cohort studies and in-vitro and in-vivo studies are recommended to determine whether there is an association between lead exposure and obesity.
We found that mercury exposure significantly increased the odds of obesity development by 1.5 times. In this regard, a systematic review and meta-analysis showed that participants with metabolic syndrome had higher mercury exposure (pooled effect size: 1.26; 95% CI: 1.06–1.48) [62]. Furthermore, the findings of a meta-analysis of 14 studies with 34,000 participants revealed a non-significant higher risk of nonfatal ischemic heart disease (risk ratio: 1.21; 95% CI: 0.98–1.50) [63]. Moreover, no significant association was found between mercury exposure and type 2 diabetes mellitus (OR: 1.00; 95 CI: 0.85–1.17) [64]. Overall, there is controversy about the association between mercury and the development of the metabolic syndrome and other metabolic diseases. Another study also emphasized the epidemiological studies' lack of consistency about the association between mercury exposure and diabetes mellitus and metabolic syndrome [65]. Mercury may play a role in affecting the adipogenesis, specifically, when 3T3-L1 preadipocytes were exposed to MeHg, the lipid content increased, while the expression of certain markers related to adipogenesis showed varying patterns. Additionally, mercury can influence the activity of genes associated with adipogenesis when combined with TCDD in BEAS-2B cells. The impact of mercury on adipogenesis was also observed in terms of adipokine secretion. Moreover, mature adipocytes exposed to MeHg showed increased production of vascular endothelial growth factor which play roles in metabolic syndrome [66]. Additionally, mercury can induce oxidative stress by activating free radical oxidation processes and suppressing the antioxidant system in biological systems regarding its physic-chemical properties [67]. Mercury can cause a significant decrease in Glutathione production and activity and decreased catalase and Thioredoxin system activity [68]. All of these transformations can aggravate oxidative stress. Mercury is also supposed to induce an inflammatory response by increasing the pro-inflammatory cytokines. Some cytokines which play a crucial role in inflammation, like (IL)-1b and tumor necrosis factor a (TNFa), are upregulated in the elevated levels of mercury [69]. Also, it was demonstrated in the animal studies that peroral administration of mercuric chloride resulted in a dose-dependent manner can induce TNFa, IL-2 and IFN concentration in liver [70]. Several studies have been demonstrated a direct correlation between mercury levels and serum total cholesterol, and trig and LDL concentration [71]. Also in a study by Melt a negative correlation between mercury and HDL has been revealed [72]. Previously it was established that mercury exposure increases the risk of cardiovascular disease [73]. So, mercury’s adverse effects by inducing oxidative stress, inflammation development and metabolic syndrome and another related condition, can be supposed to relate with obesity but more explicit investigations are needed to conduct.
The article by Xu and colleagues showed an increased non-significant association between cadmium exposure and metabolic syndrome (pooled effect size = 1.10; 95% CI: 0.95–1.27) [62]. In addition, a cohort study on mothers in their first trimester revealed a 25-time increased risk of obesity development at age five of their children for every one ng/g elevation in the blood weight of cadmium [74]. We found a protective non-significant role for cadmium in obesity (aOR = 0.77; 95% CI: 0.56–1.05). The findings implicate the limited number of primary studies that evaluated the effects of cadmium on metabolic disorders and obesity. Also, data from human studies on the interplay between Cd exposure and obesity are somewhat contradictory, ranging from negative to positive. However, in regression models incorporating different heavy metals, cadmium content was inversely associated with anthropometric indices of obesity [19]. A recent study found that cadmium significantly reduced the size of adipocytes in metallothionein-null mice [75]. Also, the inhibition of adipogenesis at a concentration of 3 μM of Cd, which resulted in a gradual reduction in the protein expression of C/EBPα and PPARγ in 3T3-L1 preadipocytes can explain the protective roles of Cd against obesity [76]. Furthermore, the inconsistency in the results can be due to the evaluation of different diseases and variations in inclusion criteria and sample populations.
Regarding manganese exposure and metabolic syndrome, a systematic review and meta-analysis of cross-sectional and case–control studies showed a non-significant association between metabolic syndrome and manganese level from the diet (OR = 0.83; 95% CI: 0.57–1.21), serum (OR = 0.87; 95% CI: 0.66–1.14), urine (OR = 0.84; 95% CI: 0.59–1.19), and whole blood (OR = 0.92; 95% CI: 0.53–1.60) [77]. A longitudinal prospective cohort study of 1478 participants showed a non-significant association between manganese level quartiles and the body mass index (p = 0.61) [78]. Following the findings of the previously mentioned studies, we found a non-significant association between manganese exposure and obesity (aOR = 1.28; 95% CI: 0.92–1.79). On the other hand, Ngu et al. found that manganese levels can predict abdominal obesity (OR = 1.62; 95% CI: 1.07–2.46) [79]. One of the reasons for the differences could be the low sample size in the original study, which is 150, while our meta-analysis has a much greater number of participants. Additionally, we included both urine and blood samples, children and adults, while the abovementioned study merely included blood samples of middle-aged adults [79].
Manganese is essential for synthesizing and activating several enzymes and regulating human glucose and lipid metabolism. Mn is also needed for Mn superoxide dismutase (MnSOD), which scavenges ROS in mitochondrial oxidative stress. Mn insufficiently and intoxication both affect metabolism and neuropsychiatry. Metabolic diseases, including type 2 diabetes mellitus, insulin resistance, hyperlipidemia, atherosclerosis, and obesity, correlate considerably with oxidative stress and inflammation. So more investigation of the probable effect of Manganese on metabolic syndrome and obesity is inevitable [80].
Previous studies found a positive association between arsenic, cadmium, iron, lead, mercury, and fatty liver disease [81]. Moreover, a significantly increased risk of proteinuria was found for exposures to lead (p = 0.02) and cadmium (p < 0.001) [13]. Jalili et al. also revealed that lead (p = 0.05) and cadmium (p ≤ 0.001) exposure, unlike mercury, were associated with an elevated risk of osteopenia or osteoporosis [20]. Also, those patients with breast cancer had higher levels of copper, cadmium, and lead levels than healthy individuals, based on findings of a meta-analysis [82]. Overall, we also found a non-significant increased risk of obesity in those exposed to a combination of different heavy metals (aOR = 1.51; 95% CI: 0.75–3.04). From the molecular point of view, there is an upregulated of adipogenic factors (i.e., CCAAT/enhancer-binding protein and peroxisome proliferator-activated receptor gamma), which lead to adipose tissue hypertrophy and expansion and finally obesity [66]. On the other hand, exposure to a high dose of heavy metals can inhibit adipogenesis because of the toxic effects of heavy metals [66].
Our study also evaluated the effects of other heavy metals like zinc, barium and chromium on the occurrence of obesity, while none of them showed a significant result, except for barium (aOR = 1.44; 95% CI: 1.14–1.81). However, primary studies are very limited on them, and to our knowledge, no previous meta-analysis has evaluated the association between exposure to them and the development of obesity.
Our systematic review and meta-analysis study is one of the most recent and up-to-date secondary studies that comprehensively evaluated the effects of different types of heavy metals separately and in combination on obesity development by age groups and type of obesity. Moreover, the serum and/or urine levels of heavy metals were compared among those with high or low exposure to them. Although it might not be helpful for clinical practice, it provides some valuable insights for health policymakers, environmental epidemiologists, and researchers. Nevertheless, the study has different limitations that should be considered in interpreting the results. First, the included studies in the meta-analysis were case–control and cross-sectional. So, it might lead to recall and selection bias in the findings and results should be interpreted with caution. Although we performed backwards and forward citation searching and hand searching for grey literature, searching Google Scholar and some other online databases like Scopus was not done. Therefore, we cannot rule out the possibility of not finding some relevant articles. Second, the study only focused on obesity, while other types of excess body weight, like overweight, were not evaluated in the present study. Third, different definitions for obesity and exposure cut-offs were used in the included studies, and it can lead to the inclusion of a heterogeneous population in the analysis. Fourth, due to the limited number of included studies for each heavy metal, the subgroup analysis only included one study for some. We tried to search and include all heavy metals, while some heavy metals like arsenic were not evaluated in at least three studies.
Conclusion
Although barium and mercury exposure were associated with increased odds of obesity occurrence, lead exposure decreased the odds of obesity development. Lead and cadmium exposure in children and individuals with general obesity were substantially associated with lower obesity development. On the other hand, mercury and barium exposure in participants with abdominal obesity and general obesity, respectively, significantly elevated the risk of obesity. However, no significant association was found for other heavy metals. The controversy between the findings could be due to the low number of included studies that evaluated each type of heavy metal and the high heterogeneity between the study findings. Further large-scale observational studies and meta-analysis are recommended.
Key points
Obesity is significantly lower in participants with high exposure to lead.
Those with high exposure to cadmium have non-significantly lower odds of obesity development.
Mercury and barium exposure are significantly related to an increased risk of obesity.
Manganese and zinc exposure could insignificantly increase the risk of obesity.
The mean serum zinc and chromium levels are insignificantly lower in obese population.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
The present study was supported by the Kerman University of Medical Sciences, Kerman, Iran.
Author contribution
MZ and SAN conceptualized the topic; MZ searched the databases; MZ and AGJ performed screening and full-text review; MZ and AGJ performed data extraction; MZ and AGJ performed quality assessment; MZ, SAN and LA prepared the first draft of the manuscript; MS critically revised and edited the manuscript; MS supervised this project. All authors reviewed and approved the final version of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethical approval
The present study was approved by the ethics committee of Kerman University of Medical Sciences, Kerman, Iran (Ethics code: IR.KMU.REC.1401.396).
Conflict of interests
No conflict of interest declared.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ORGANIZATION WH. Obesity 2017 [Available from: https://www.who.int/health-topics/obesity#tab=tab_1. Accessed 02/05/2023.
- 2.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ORGANIZATION WH. World Obesity Day 2022 – Accelerating action to stop obesity 2022 [Available from: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity. Accessed 02/05/2023.
- 4.Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev. 2011;12(9):680–687. doi: 10.1111/j.1467-789X.2011.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS ONE. 2013;8(7):e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 7.Safiri S, Karamzad N, Kaufman JS, Nejadghaderi SA, Bragazzi NL, Sullman MJM, et al. Global, regional, and national burden of cancers attributable to excess body weight in 204 countries and territories, 1990 to 2019. Obesity. 2022;30(2):535–545. doi: 10.1002/oby.23355. [DOI] [PubMed] [Google Scholar]
- 8.Kazim AH, Al-Ruwaybiah AM, Al-Naami MY, Aldohayan A, Binjaloud AA, Alarfaj MA. The Social Problems of Morbidly Obese Patients on a Community Level: A Cross-Sectional Study in Saudi Arabia. Diabetes Metab Syndr Obes. 2022;15:2061–2075. doi: 10.2147/DMSO.S366358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin X, Li H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front Endocrinol (Lausanne) 2021;12:706978. doi: 10.3389/fendo.2021.706978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115(6):876–82. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29(7):1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- 13.Jalili C, Kazemi M, Cheng H, Mohammadi H, Babaei A, Taheri E, et al. Associations between exposure to heavy metals and the risk of chronic kidney disease: a systematic review and meta-analysis. Crit Rev Toxicol. 2021;51(2):165–182. doi: 10.1080/10408444.2021.1891196. [DOI] [PubMed] [Google Scholar]
- 14.Rezaee M, Esfahani Z, Nejadghaderi SA, Abbasi-Kangevari M, SaeediMoghaddam S, Ghanbari A, et al. Estimating the burden of diseases attributable to lead exposure in the North Africa and Middle East region, 1990–2019: a systematic analysis for the Global Burden of Disease study 2019. Environ Health. 2022;21(1):105. doi: 10.1186/s12940-022-00914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tinkov AA, Ajsuvakova OP, Skalnaya MG, Popova EV, Sinitskii AI, Nemereshina ON, et al. Mercury and metabolic syndrome: a review of experimental and clinical observations. Biometals. 2015;28(2):231–254. doi: 10.1007/s10534-015-9823-2. [DOI] [PubMed] [Google Scholar]
- 16.Rhee SY, Hwang YC, Woo JT, Sinn DH, Chin SO, Chon S, et al. Blood lead is significantly associated with metabolic syndrome in Korean adults: an analysis based on the Korea National Health and Nutrition Examination Survey (KNHANES), 2008. Cardiovasc Diabetol. 2013;12:9. doi: 10.1186/1475-2840-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JW, Wang SL, Wang YH, Sun CW, Huang YL, Chen CJ, et al. Arsenic methylation, GSTO1 polymorphisms, and metabolic syndrome in an arseniasis endemic area of southwestern Taiwan. Chemosphere. 2012;88(4):432–438. doi: 10.1016/j.chemosphere.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 18.Rothenberg SE, Korrick SA, Fayad R. The influence of obesity on blood mercury levels for U.S. non-pregnant adults and children: NHANES 2007–2010. Environ Res. 2015;138:173–80. doi: 10.1016/j.envres.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padilla MA, Elobeid M, Ruden DM, Allison DB. An Examination of the Association of Selected Toxic Metals with Total and Central Obesity Indices: NHANES 99–02. Int J Environ Res Public Health. 2010;7(9):3332–47. doi: 10.3390/ijerph7093332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalili C, Kazemi M, Taheri E, Mohammadi H, Boozari B, Hadi A, et al. Exposure to heavy metals and the risk of osteopenia or osteoporosis: a systematic review and meta-analysis. Osteoporos Int. 2020;31(9):1671–1682. doi: 10.1007/s00198-020-05429-6. [DOI] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. WHO IRIS: Obes.: Prev. Manag. Glob. epidemic: Rep. a WHO Consult. 2000, pp.18–30
- 23.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet (London, England) 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 24.Raychaudhuri SS, Pramanick P, Talukder P, Basak A. Polyamines, metallothioneins, and phytochelatins—Natural defense of plants to mitigate heavy metals. Stud Nat Prod Chem. 2021;69:227–261. [Google Scholar]
- 25.Institute JB. Critical Appraisal Tools JBIUoA, South Australia; 2021 [Available from: https://jbi.global/critical-appraisal-tools. Accessed 02/05/2023.
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;1088–101. [PubMed]
- 29.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 30.Tinkov AA, Skalnaya MG, Ajsuvakova OP, Serebryansky EP, Chao JC, Aschner M, et al. Selenium, zinc, chromium, and vanadium levels in serum, hair, and urine samples of obese adults assessed by inductively coupled plasma mass spectrometry. Biol Trace Elem Res. 2021;199(2):490–499. doi: 10.1007/s12011-020-02177-w. [DOI] [PubMed] [Google Scholar]
- 31.Azab SF, Saleh SH, Elsaeed WF, Elshafie MA, Sherief LM, Esh AM. Serum trace elements in obese Egyptian children: a case–control study. Ital J Pediatr. 2014;40(1):1–7. doi: 10.1186/1824-7288-40-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tascilar ME, Ozgen IT, Abaci A, Serdar M, Aykut O. Trace elements in obese Turkish children. Biol Trace Elem Res. 2011;143(1):188–195. doi: 10.1007/s12011-010-8878-8. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Du J, Li H, Yang Y, Cai C, Gao Q, et al. Multiple-element exposure and metabolic syndrome in Chinese adults: A case-control study based on the Beijing population health cohort. Environ Int. 2020;143:105959. doi: 10.1016/j.envint.2020.105959. [DOI] [PubMed] [Google Scholar]
- 34.Cho KY. Association of Blood Mercury Levels with the Risks of Overweight and High Waist-to-Height Ratio in Children and Adolescents: Data from the Korean National Health and Nutrition Examination Survey. Children-Basel. 2021;8(12):17. doi: 10.3390/children8121087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duc HN, Oh H, Kim MS. The Effect of Mixture of Heavy Metals on Obesity in Individuals >= 50 Years of Age. Biol Trace Elem Res. 2022;200(8):3554–3571. doi: 10.1007/s12011-021-02972-z. [DOI] [PubMed] [Google Scholar]
- 36.Eom SY, Choi SH, Ahn SJ, Kim DK, Kim DW, Lim JA, et al. Reference levels of blood mercury and association with metabolic syndrome in Korean adults. Int Arch Occup Environ Health. 2014;87(5):501–513. doi: 10.1007/s00420-013-0891-8. [DOI] [PubMed] [Google Scholar]
- 37.Fan Y, Zhang C, Bu J. Relationship between Selected Serum Metallic Elements and Obesity in Children and Adolescent in the U.S. Nutrients. 2017;9(2):104. doi: 10.3390/nu9020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee BK, Kim Y. Blood cadmium, mercury, and lead and metabolic syndrome in South Korea: 2005–2010 Korean National Health and Nutrition Examination Survey. Am J Ind Med. 2013;56(6):682–692. doi: 10.1002/ajim.22107. [DOI] [PubMed] [Google Scholar]
- 39.Lo K, Yang JL, Chen CL, Liu L, Huang YQ, Feng YQ, et al. Associations between blood and urinary manganese with metabolic syndrome and its components: Cross-sectional analysis of National Health and Nutrition Examination Survey 2011–2016. Sci Total Environ. 2021;780:9. doi: 10.1016/j.scitotenv.2021.146527. [DOI] [PubMed] [Google Scholar]
- 40.Moon SS. Additive effect of heavy metals on metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocrine. 2014;46(2):263–271. doi: 10.1007/s12020-013-0061-5. [DOI] [PubMed] [Google Scholar]
- 41.Ngu YJ, Skalny AV, Tinkov AA, Tsai CS, Chang CC, Chuang YK, Nikolenko VN, Zotkin DA, Chiu CF, Chang JS. Association between essential and non-essential metals, body composition, and metabolic syndrome in adults. Biol Trace Elem Res. 2022;1–3. [DOI] [PubMed]
- 42.Nie X, Wang N, Chen Y, Chen C, Han B, Zhu C, et al. Blood cadmium in Chinese adults and its relationships with diabetes and obesity. Environ Sci Pollut Res Int. 2016;23(18):18714–18723. doi: 10.1007/s11356-016-7078-2. [DOI] [PubMed] [Google Scholar]
- 43.Noor N, Zong G, Seely EW, Weisskopf M, James-Todd T. Urinary cadmium concentrations and metabolic syndrome in U.S. adults: The National Health and Nutrition Examination Survey 2001–2014. Environ Int. 2018;121(Pt 1):349–56. doi: 10.1016/j.envint.2018.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scinicariello F, Buser MC, Mevissen M, Portier CJ. Blood lead level association with lower body weight in NHANES 1999–2006. Toxicol Appl Pharmacol. 2013;273(3):516–523. doi: 10.1016/j.taap.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao W, Liu Q, He X, Liu H, Gu A, Jiang Z. Association between level of urinary trace heavy metals and obesity among children aged 6–19 years: NHANES 1999–2011. Environ Sci Pollut Res Int. 2017;24(12):11573–11581. doi: 10.1007/s11356-017-8803-1. [DOI] [PubMed] [Google Scholar]
- 46.Swayze S, Rotondi M, Kuk JL. The associations between blood and urinary concentrations of metal metabolites, obesity, hypertension, type 2 diabetes, and dyslipidemia among US adults: NHANES 1999–2016. J Environ Public Health. 2021;25(2021):1–3. doi: 10.1155/2021/2358060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang N, Chen C, Nie X, Han B, Li Q, Chen Y, et al. Blood lead level and its association with body mass index and obesity in China - Results from SPECT-China study. Sci Rep. 2015;5:18299. doi: 10.1038/srep18299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Mukherjee B, Park SK. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ Int. 2018;121:683–94. doi: 10.1016/j.envint.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao M, Ge X, Xu J, Li A, Mei Y, Zhao J, et al. Negatively interactive effect of chromium and cadmium on obesity: Evidence from adults living near ferrochromium factory. Ecotoxicol Environ Saf. 2022;231:113196. doi: 10.1016/j.ecoenv.2022.113196. [DOI] [PubMed] [Google Scholar]
- 50.Fisher RM, Gupta V. Heavy Metals. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023 Available from: https://www.ncbi.nlm.nih.gov/books/NBK557806/. Accessed 02/05/2023.
- 51.Hanna-Attisha M, Lanphear B, Landrigan P. Lead Poisoning in the 21st Century: The Silent Epidemic Continues. Am J Public Health. 2018;108(11):1430. doi: 10.2105/AJPH.2018.304725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim H, Lee HJ, Hwang JY, Ha EH, Park H, Ha M, et al. Blood cadmium concentrations of male cigarette smokers are inversely associated with fruit consumption. J Nutr. 2010;140(6):1133–1138. doi: 10.3945/jn.109.120659. [DOI] [PubMed] [Google Scholar]
- 53.Bjørklund G, Hilt B, Dadar M, Lindh U, Aaseth J. Neurotoxic effects of mercury exposure in dental personnel. Basic Clin Pharmacol Toxicol. 2019;124(5):568–574. doi: 10.1111/bcpt.13199. [DOI] [PubMed] [Google Scholar]
- 54.Park S, Choi N-K. The relationships of blood lead level, body mass index, and osteoarthritis in postmenopausal women. Maturitas. 2019;125:85–90. doi: 10.1016/j.maturitas.2019.04.215. [DOI] [PubMed] [Google Scholar]
- 55.Wang G, DiBari J, Bind E, Steffens AM, Mukherjee J, Azuine RE, et al. Association Between Maternal Exposure to Lead, Maternal Folate Status, and Intergenerational Risk of Childhood Overweight and Obesity. JAMA Netw Open. 2019;2(10):e1912343–e1912343. doi: 10.1001/jamanetworkopen.2019.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berkowitz Z, Price-Green P, Bove FJ, Kaye WE. Lead exposure and birth outcomes in five communities in Shoshone County. Idaho Int J Hyg Environ Health. 2006;209(2):123–132. doi: 10.1016/j.ijheh.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Sanín LH, González-Cossío T, Romieu I, Peterson KE, Ruíz S, Palazuelos E, et al. Effect of maternal lead burden on infant weight and weight gain at one month of age among breastfed infants. Pediatrics. 2001;107(5):1016–1023. doi: 10.1542/peds.107.5.1016. [DOI] [PubMed] [Google Scholar]
- 58.Park SS, Skaar DA, Jirtle RL, Hoyo C. Epigenetics, obesity and early-life cadmium or lead exposure. Epigenomics. 2016;9(1):57–75. doi: 10.2217/epi-2016-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pilsner JR, Hu H, Ettinger A, Sánchez BN, Wright RO, Cantonwine D, et al. Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Environ Health Perspect. 2009;117(9):1466–1471. doi: 10.1289/ehp.0800497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider JS, Kidd SK, Anderson DW. Influence of developmental lead exposure on expression of DNA methyltransferases and methyl cytosine-binding proteins in hippocampus. Toxicol Lett. 2013;217(1):75–81. doi: 10.1016/j.toxlet.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun H, Wang N, Nie X, Zhao L, Li Q, Cang Z, et al. Lead Exposure Induces Weight Gain in Adult Rats, Accompanied by DNA Hypermethylation. PLoS ONE. 2017;12(1):e0169958. doi: 10.1371/journal.pone.0169958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu P, Liu A, Li F, Tinkov AA, Liu L, Zhou J-C. Associations between metabolic syndrome and four heavy metals: A systematic review and meta-analysis. Environ Pollut. 2021;273:116480. doi: 10.1016/j.envpol.2021.116480. [DOI] [PubMed] [Google Scholar]
- 63.Hu XF, Lowe M, Chan HM. Mercury exposure, cardiovascular disease, and mortality: A systematic review and dose-response meta-analysis. Environ Res. 2021;193:110538. doi: 10.1016/j.envres.2020.110538. [DOI] [PubMed] [Google Scholar]
- 64.GhorbaniNejad B, Raeisi T, Janmohammadi P, Mehravar F, Zarei M, Dehghani A, et al. Mercury Exposure and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int J Clin Pract. 2022;2022:7640227. doi: 10.1155/2022/7640227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roy C, Tremblay P-Y, Ayotte P. Is mercury exposure causing diabetes, metabolic syndrome and insulin resistance? A systematic review of the literature. Environ Res. 2017;156:747–760. doi: 10.1016/j.envres.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 66.Tinkov AA, Aschner M, Ke T, Ferrer B, Zhou JC, Chang JS, et al. Adipotropic effects of heavy metals and their potential role in obesity. Fac Rev. 2021;10:32. doi: 10.12703/r/10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82(2):291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 68.Tinkov AA, Ajsuvakova OP, Skalnaya MG, Popova EV, Sinitskii AI, Nemereshina ON, et al. Mercury and metabolic syndrome: a review of experimental and clinical observations. Biometals. 2015;28(2):231–254. doi: 10.1007/s10534-015-9823-2. [DOI] [PubMed] [Google Scholar]
- 69.Gardner RM, Nyland JF, Silbergeld EK. Differential immunotoxic effects of inorganic and organic mercury species in vitro. Toxicol Lett. 2010;198(2):182–190. doi: 10.1016/j.toxlet.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim SH, Johnson VJ, Sharma RP. Oral exposure to inorganic mercury alters T lymphocyte phenotypes and cytokine expression in BALB/c mice. Arch Toxicol. 2003;77(11):613–620. doi: 10.1007/s00204-003-0497-0. [DOI] [PubMed] [Google Scholar]
- 71.Lim S, Choi MC, Joh KO, Paek D. The Health Effects of Mercury on the Cardiac Autonomic Activity According to the Heart Rate Variability. kjoem. 2019;20(4):302–13. [Google Scholar]
- 72.Meltzer HM, Mundal HH, Alexander J, Bibow K, Ydersbond TA. Does dietary arsenic and mercury affect cutaneous bleeding time and blood lipids in humans? Biol Trace Elem Res. 1994;46(1–2):135–153. doi: 10.1007/BF02790074. [DOI] [PubMed] [Google Scholar]
- 73.Downer MK, Martínez-González MA, Gea A, Stampfer M, Warnberg J, Ruiz-Canela M, et al. Mercury exposure and risk of cardiovascular disease: a nested case-control study in the PREDIMED (PREvention with MEDiterranean Diet) study. BMC Cardiovasc Disord. 2017;17(1):9. doi: 10.1186/s12872-016-0435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green AJ, Hoyo C, Mattingly CJ, Luo Y, Tzeng J-Y, Murphy SK, et al. Cadmium exposure increases the risk of juvenile obesity: a human and zebrafish comparative study. Int J Obes. 2018;42(7):1285–1295. doi: 10.1038/s41366-018-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawakami T, Nishiyama K, Kadota Y, Sato M, Inoue M, Suzuki S. Cadmium modulates adipocyte functions in metallothionein-null mice. Toxicol Appl Pharmacol. 2013;272(3):625–636. doi: 10.1016/j.taap.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 76.Lee EJ, Moon JY, Yoo BS. Cadmium inhibits the differentiation of 3T3-L1 preadipocyte through the C/EBPα and PPARγ pathways. Drug Chem Toxicol. 2012;35(2):225–231. doi: 10.3109/01480545.2011.591401. [DOI] [PubMed] [Google Scholar]
- 77.Wong MM, Chan KY, Lo K. Manganese Exposure and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutrients. 2022;14(4):825. doi: 10.3390/nu14040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riseberg E, Chui K, James KA, Melamed R, Alderete TL, Corlin L. A Longitudinal Study of Exposure to Manganese and Incidence of Metabolic Syndrome. Nutrients. 2022;14(20):4271. doi: 10.3390/nu14204271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ngu YJ, Skalny AV, Tinkov AA, Tsai C-S, Chang C-C, Chuang Y-K, et al. Association Between Essential and Non-essential Metals, Body Composition, and Metabolic Syndrome in Adults. Biol Trace Elem Res. 2022;200(12):4903–4915. doi: 10.1007/s12011-021-03077-3. [DOI] [PubMed] [Google Scholar]
- 80.Li L, Yang X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid Med Cell Longev. 2018;2018:7580707. doi: 10.1155/2018/7580707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sadighara P, Abedini AH, Irshad N, Ghazi-Khansari M, Esrafili A, Yousefi M. Association between non-alcoholic fatty liver disease and heavy metal exposure: a systematic review. Biol Trace Elem Res. 2023;1–9. [DOI] [PubMed]
- 82.Liu L, Chen J, Liu C, Luo Y, Chen J, Fu Y, et al. Relationships Between Biological Heavy Metals and Breast Cancer: A Systematic Review and Meta-Analysis. Front Nutr. 2022;9:838762. doi: 10.3389/fnut.2022.838762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.