Abstract
CD8+ lymphocytes from human immunodeficiency virus (HIV)-infected patients can suppress in vitro HIV replication in CD4+ T cells by a noncytolytic mechanism involving secreted CD8+-cell antiviral factor(s) (CAF). Using an HIV Nef-specific cytotoxic-T-lymphocyte (CTL) line and autologous CD4+ T cells infected with a nef-deleted HIV-1 virus, we demonstrated that, after a priming antigenic stimulation, this suppression does not require the presence of the specific antigen during the effector phase. Furthermore, using an Epstein-Barr virus (EBV)-specific CTL line from an HIV-seronegative donor, we demonstrated that the ability to inhibit HIV replication in a noncytolytic manner is not restricted to HIV-specific effector cells; indeed, EBV-specific CTL were as efficient as HIV-specific effectors in suppressing R5 or X4 HIV-1 strain replication in vitro. This HIV-suppressive activity mediated by a soluble factor(s) present in the culture supernatant was detectable for up to 14 days following stimulation of EBV-specific CD8+ cells with the cognate epitope peptide. Following acute infection of CEM cells with an X4 strain of HIV-1, EBV-specific CTL line supernatant containing HIV-suppressive activity did not block virus entry but was shown to interfere with virus replication after the first template switching of reverse transcription. Our results suggest that the noncytolytic control of HIV replication by EBV-specific CD8+ T lymphocytes corresponded to a CAF-like activity and thus demonstrate that CAF production may not be restricted to CTL induced during HIV disease. Moreover, CAF acts after reverse transcription at least for X4 isolate replication inhibition.
Infection with human immunodeficiency virus (HIV) is characterized by an initial acute phase with a high level of plasma viremia. Studies of the immune responses induced in HIV-infected patients suggest that cytotoxic T lymphocytes (CTL) play an important role in controlling the virus, since the emergence of antiviral CTL activity coincides with the clearance of viremia in primary infection (7, 28). Furthermore, a decline in this CTL response coincides with disease progression in infected individuals (12, 27). Two types of CD8+-cell-mediated antiviral functions have been described in HIV infection: one involves the classical antigen-specific HLA-restricted cytolysis of infected cells (54), and the other inhibits HIV replication in the absence of cell killing (33, 55, 59).
This noncytolytic antiviral response was first identified in vitro with lymphocytes from HIV-seropositive patients (57). It is detected by a reduction in HIV p24 production or a decrease in reverse transcriptase (RT) activity in supernatants from cocultures of HIV-infected CD4+ cells and activated CD8+ T lymphocytes. The effector cells carry the phenotype of activated CD8+ CTL (3, 36, 53), but the antiviral activity does not require HLA compatibility at the effector phase. This inhibition of HIV replication is mediated by soluble factor(s) referred to as CD8+-cell antiviral factor(s) (CAF) (8, 56); indeed, noncytotoxic HIV-suppressive activity can be mediated by using cell-free supernatants from cultures of activated CD8+ T cells (56). However, it has been reported that cell-to-cell contact (53, 56) and the use of autologous cell cultures (8) can enhance the effect. Furthermore, one CD8+ T cell could mediate both CAF secretion and HLA-restricted cytotoxicity, since we have previously demonstrated that a CD8+ CTL clone, specific for one HIV-Gag epitope, could also inhibit in vitro HIV replication by soluble-factor secretion (10). HIV-1 is not the only virus to be affected by this response since CD8+ T cells have also been shown to suppress HIV-2 or simian immunodeficiency virus replication; this noncytolytic antiviral activity has also been observed in nonhuman primate species (5, 13, 35, 58).
Recombinant cytokines have been tested independently for their ability to mediate CAF activity, and cytokine-specific monoclonal antibodies have been tested for their ability to block this effect (36, 39) but, to date, the identity of the CAF remains largely unknown. Recent studies have shown that HIV-suppressive factors produced by CD8+ T cells include the β-chemokines macrophage-inflammatory protein-1α (MIP-1α), MIP-1β, and RANTES (14), which block the entry of R5 HIV strains into CD4+ cells by competing for binding to the chemokine receptors CCR5 and CCR3 (1, 19, 20). In the same manner, the α-chemokine stromal-derivated factor 1 (SDF-1), for which CXCR4 is the receptor, inhibits the entry of T-tropic HIV isolates (44), whereas it has never been demonstrated to be produced by CD8+ lymphocytes (29). Furthermore, the β-chemokine MDC (45) and interleukin-16 (IL-16) (2) could be produced by CD8+ T cells and suppress HIV replication in vitro. Elsewhere, other studies indicate that CAF activity may block viral transcription. Using Jurkat cells transfected with an HIV-1 long-terminal-repeat (LTR)–luciferase construct (38) or an HIV-1 LTR-chloramphenicol acetyltransferase (CAT) construct (17), CD8+ T lymphocytes or their supernatants were shown to mediate specific inhibition of the LTR-driven activity, whereas the β-chemokines could not suppress HIV RNA transcription (22). Furthermore, β-chemokines appeared to be distinct from CAF (37).
The CD8+ antiviral noncytolytic activity has been evidenced with effector cells from HIV-seropositive patients and seems to correlate with the clinical status of infected individuals (4, 6, 24, 40). However, the ability of CD8+ cells from HIV-seronegative donors to inhibit HIV replication has been described but is controversial (26, 32, 49). Elsewhere, viral suppression by CD8+ lymphocytes from HIV-infected patients appears to be more efficient than the inhibition observed by effector cells from healthy donors (33). Thus, it is still unclear whether HIV infection of the host specifically induces in vivo the immune competence to elicit CAF activity or if infection with other viruses could have the same result as a consequence of activating CD8+ T cells.
In the present study, we have further analyzed this noncytolytic control of HIV replication using both HIV-specific and Epstein-Barr virus (EBV)-specific CD8+ T-cell lines. Our results indicate that antigen-specific T-cell receptor (TCR) stimulation is not required at the effector phase of the control and that noncytolytic inhibition of HIV replication is not restricted to HIV-specific CD8+ T cells. Finally, an EBV-specific CD8+ T-cell line could control replication of HIV-1 X4 strain by secretion of soluble factor(s) which interacts with the viral cycle, at least after the first template switching of reverse transcription.
MATERIALS AND METHODS
Cell lines.
B-lymphoblastoid cell lines (B-LCL) were established by in vitro transformation of peripheral blood B cells with the standard EBV isolate B95.8 (42). CEM cells (clone 13) were kindly provided by L. Montagnier (Institut Pasteur, Paris, France) (31). All of these cell lines were grown in RPMI 1640 (Whittaker, Gagny, France) supplemented with 2 mM l-glutamine (Gibco BRL, Cergy-Pontoise, France) and 10% heat-inactivated fetal calf serum (FCS; Dutscher, Strasbourg, France) (RPMI–10% FCS). All incubations and culture procedures were performed at 37°C in a water-saturated 5% CO2 atmosphere.
Isolation of CD8+ T-cell lines.
The P1-HIV CTL line was generated as described previously (21) from an HIV-1-seropositive adult who maintained a CTL response to the HIV-1 p27Nef protein during a 6-year study period (48). The HLA typing of this patient is A2.01, A11, B27, B51, and C1402. The line was stimulated every 2 to 3 weeks with irradiated (100 Gy) autologous B-LCL and the cognate epitope peptide HIV-1 Nef19 (representing amino acids 73 to 82 from p27Nef, QVPLRPMTYK, provided by Neosystem, Strasbourg, France, and obtained through the Agence Nationale de Recherche sur le Sida [ANRS]), which has been confirmed as the optimal specific epitope; fresh allogeneic irradiated peripheral blood mononuclear cells (PBMC) were also added as feeders. The P1-HIV line was maintained in RPMI 1640 (Whittaker) supplemented with 2 mM l-glutamine, 1 mM nonessential amino acids, 1 mM sodium pyruvate (Gibco BRL), 5% human AB serum (INTS, Bobigny, France), and 50 IU of recombinant human IL-2 (rIL2) (RU49637, a generous gift from D. Lando, Roussel-Uclaf, Romainville, France) per ml (cloning medium).
The 1.6-EBV CTL line was generated from an HIV-seronegative, EBV-seropositive individual as described previously (25). The HLA haplotype of this person is A2, A29, B8, B40, and Cw3. This cell line was restimulated every 2 to 3 weeks in the presence of fresh allogeneic irradiated PBMC as feeder cells and 0.5 μg of phytohemagglutinin (PHA-p; Difco, Detroit, Mich.) per ml. In addition, irradiated autologous B-LCL cells preincubated with 1 μg of a peptide representing amino acids 339 to 347 (FLRGRAYGL; Neosystem) per ml from the EBV nuclear antigen-3 (EBNA-3) protein of the BL74 EBV strain (9) were added at a stimulator/responder ratio of 1/10. This peptide represents the minimal target epitope recognized by the 1.6-EBV line. The cells were cultured in RPMI 1640 supplemented with 3% human AB serum (INTS), 10% FCS (Dutscher), 2 mM l-glutamine, 1 mM nonessential amino-acids, 1 mM sodium pyruvate (Gibco BRL), 20 IU of rIL2 (RU 49637) per ml, and 15% T-cell growth factor (Lymphocult T-LF; Biotest, Buc, France) as a source of cytokines (cloning mix).
Both cell lines were confirmed to be at least 99% CD3+ CD8+ by cytofluorometric analysis. The T-cell line Vβ repertoire was analyzed as described previously (46).
Isolation of CD4+ T-cell lines.
CD4+ T-cell lines were derived from the HIV-infected patient P1, from the EBV-seropositive individual 1.6, and from one additional HIV-seropositive donor (HLA typing: A2, A30, B44, C2, and C5). PBMC from these donors were depleted of CD8+ T cells by using magnetic beads coated with anti-CD8 monoclonal antibodies (Immunotech, Marseille, France) according to the manufacturer's instructions and then seeded at 10 or 25 cells/well in a 96-well plate (Costar) with fresh allogeneic irradiated PBMC and 2 μg of PHA (Difco) per ml. Cells were cultured in cloning medium and restimulated with allogeneic irradiated PBMC and PHA every 3 weeks. The lines were confirmed by cytofluorometric analysis to be at least 99% CD3+ CD4+ CD8−. Assays for RT activity (50) in the supernatant of uninfected activated CD4+ T-cell lines derived from HIV-infected patients were consistently negative.
Virus stocks.
The HIV-1 LAI strain was used as a lymphotropic (X4) virus; the monotropic (R5) molecular clone HIV-1 YU2b (34) and the nef frameshift mutant YU2bΔnef (43) were kindly provided by O. Schwartz (Institut Pasteur). The frameshift mutation suppresses completely the expression of the Nef protein by YU2bΔnef, as evidenced by immunoblotting (43). For production of virus stocks, PBMC from healthy donors were prepared by Ficoll-Paque (Pharmacia, Les Ulis, France) density gradient centrifugation, suspended in cloning medium, and stimulated for 3 days with 2 μg of PHA (Difco) per ml. PBMC cultures were then infected with the various isolates, and virus production was monitored by measuring RT activity in the culture supernatant as described previously (50). The coreceptor usage of these virus stocks was checked on HeLa cells bearing human CD4 alone, or human CD4 and human CCR5, as described previously (41).
The dual-tropic (X4R5) 89.6 strain was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH); HIV 89.6 was from Ronald Collman (16) and was propagated in CEMx174 cells.
The CEM-adapted HIV-1 LAI strain (31) was amplified on CEM clone 13 cells.
Recombinant vaccinia viruses (rVV) expressing individual HIV antigens were as described previously (48).
All virus stocks were stored at −80°C.
Reverse transcription assays.
RT activity was measured as described previously (50). Briefly, using a 96 well-plate, we incubated 50 μl of cell-free supernatant at 37°C for 1 h with 10 μl of a mix containing 0.5% of Triton X-100 (Sigma), 50 mM of dithiothreitol (Sigma), and 0.5 M of KCl (Sigma) and 40 μl of a mix containing 1.25 mM EGTA (Sigma) in 0.5 M Tris-HCl solution (pH 7.8), 12.5 mM MgCl2 (Sigma), 0.125 mg of Poly-rA-Oligo d/T (Pharmacia-LKB, Saint-Quentin-en-Yvelines, France) per ml, and 3 μCi of [3H]dTTP (ICN, Orsay, France). The reaction was stopped at 4°C with 20 μl of a mix containing 120 mM of Na4P2O7 (Merck, Darmstadt, Germany) in 60% trichloroacetic acid (Prolabo, Paris, France) solution. The supernatants were harvested on a DEAE-treated filter (EGG, Evry, France) and counted on a beta counter. RT activity was expressed as the counts per minute (cpm) per 50 μl of supernatant.
Cytotoxicity assays.
CTL activity was measured using a conventional 4-h 51Cr release assay as described previously (48). Briefly, autologous B-LCL were infected with rVV 16 h before the assay, labeled for 1 h with 3.7 MBq of Na251CrO4 (ICN) per 106 cells, washed three times, and then used as target cells. For peptide assays, B-LCL were labeled for 1 h with Na251CrO4 and washed, and then synthetic peptides were added at 1 μg/ml. These targets were then added to the effector cells at various effector-to-target ratios. After incubation, supernatants were removed and counted on a beta counter. The percentage of specific release was calculated as follows: % release = (experimental release − spontaneous release)/(total release − spontaneous release) × 100. Total release was measured by resuspending target cells in lysis buffer (5% Triton X-100–1% sodium dodecyl sulfate). Spontaneous release was obtained from targets incubated with medium alone and was usually less than 15% of the total release.
Direct coculture of CD8+ effectors with HIV-infected CD4+ T cells.
CD4+ T-cell lines stimulated for 5 days with PHA were incubated at 2 × 106 cells/ml with 5 × 104 cpm/106 cells of the HIV viral stock and 5 μg of polybrene per ml for 2 h at 37°C. An equal volume of fresh medium was then added, and cells were incubated at 37°C overnight. Infected cells were washed, seeded at 105 cells per well in a 48-well plate (Costar), and mixed with various concentrations of CD8+ T effector cells at CD8+/CD4+ ratios ranging from 0/1 to 4/1, in a final volume of 1 ml. Uninfected CD4+ and CD8+ T lymphocytes alone were used as negative controls, and HIV-infected CD4+ T cells alone were used as a positive control. Half of the culture supernatant was collected from each well two or three times a week and stored at −80°C, and cultures were refed with fresh medium. At the end of the coculture, the RT activity in the supernatant was measured as described previously (50) and was expressed as cpm per 50 μl of supernatant. The percent inhibition of viral replication was calculated as follows: [1 − (experimental RT/infected CD4+ cells alone RT)] × 100.
Inhibition of HIV replication by CTL supernatants. (i) Generation of inhibitory supernatants from CTL.
Supernatants from cultures of the 1.6-EBV line were tested for their inhibitory activity on HIV replication. After TCR stimulation with the cognate peptide epitope, cells were maintained at 106 cells/ml in a cloning mix. Supernatants were collected 9 (d9), 12 (d12), 14 (d14), or 15 (d15) days after stimulation (i.e., at least 2 days after the cultures were last fed with fresh medium). Supernatants from autologous PHA-stimulated uninfected CD4+ T-cell cultures (maintained at 106 cells/ml) were collected 9 days (d9) after the addition of PHA (i.e., 3 days after the cultures were last fed). Cloning mix or cloning medium alone was used as a negative control. The supernatants were stored at −20°C. For these experiments, cell lines were stimulated with the same batch of irradiated allogeneic PBMC feeder cells.
(ii) HIV infection of CEM cells.
Pelleted CEM clone 13 cells were incubated at 37°C for 1 h with the CEM-adapted HIV-1 LAI stock (11) at 150 ng of Gag p24 per 106 cells (corresponding to 12 × 104 cpm of RT activity). The cells were infected in 1 ml of culture medium and then washed. Infected cells were then cultured at 105 cells per well in a 48-well plate for 7 days in the presence of CD8+ T-cell supernatant. CEM cells preincubated with HIV but cultured without CD8+ supernatant were used as a positive control for HIV infection; uninfected CEM cells cultured alone acted as a negative control. Half of each culture supernatant was collected daily and stored at −80°C, and the cultures were refed. At the end of the assay, the RT activity in the supernatants was measured as described previously (50).
(iii) Assays for inhibition of HIV replication.
Target cells (PHA-activated CD4+ T lymphocytes or CEM cells) were acutely infected with HIV-1 as described above, in the presence or absence of CTL supernatants (see Results), and were plated at 105 cells per well (in 1 ml) in a 48-well plate. CTL supernatants were tested at a dilution of 1/2 and maintained at this concentration throughout the course of the assay. Supernatant fluid (0.5 ml) was removed for RT activity testing (50) every day for CEM cells and twice a week for CD4+ lymphocytes and then replaced with fresh medium containing 50% CTL supernatant fluid. Infected T cells alone were used as a positive control for HIV infection. PHA-stimulated CD4+ T-cell supernatants or cloning medium alone acted as negative controls for CAF activity. The percent inhibition of viral replication was calculated as follows: [1 − (experimental RT/infected CD4+ cells alone RT)] × 100.
PCR analysis.
DNA was extracted from CEM cells 24 h after HIV-1 LAI infection using the QIAamp blood kit (Qiagen, Hilden, Germany) as described by the manufacturer and then tested for proviral DNA using a PCR-based assay. PCR was performed with 770 ng of DNA matrix. Amplification (25 cycles) of viral DNA was carried out using primers corresponding to U3+ (5′-CACACAAGGCTACTTCCCTGA-3′) and U5− (5′-GATCTCTAGTTACCAGAGTCAC-3′) domains in the HIV-1 LTR to generate a 540-bp fragment as described previously (15). The plasmid pBru2 (47) containing the complete genome of HIV-1 was used to estimate the linearity of the HIV DNA amplification. As a control, part of the β-globin gene was amplified independently (30 cycles) using the primer β-globin+ from the region from 1614 to 1633 (exon) (5′-CCTTTGTTCCCTAAGTCCAA-3′), and the primer β-globin− from the region from 1851 to 1832 (intron) (5′-CCTCACCTTCTTTCATGGAG-3′) to generate a 238-bp fragment as described previously (15). PCR products were resolved in 2% agarose gels and quantified using NIH 1.61 software (Wayne Rasband, NIH, Bethesda, Md.).
RESULTS
Characterization of the CD8+ CTL lines.
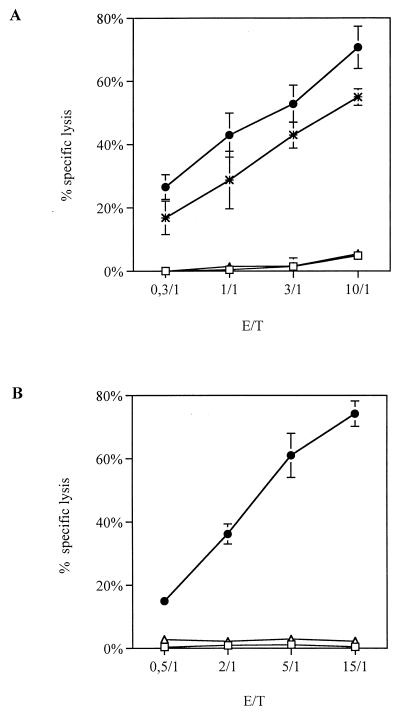
As tools to study CD8+-cell-mediated HIV-suppressive activity, we characterized two CD8+ CTL lines: P1-HIV obtained from an HIV-1-infected patient and 1.6-EBV isolated from an EBV-seropositive, HIV-1-seronegative individual. P1-HIV is an HIV-1 Nef-specific line, which lysed autologous B-LCL infected with rVV encoding the entire Nef protein, and B-LCL coated with peptide Nef19 (amino acids [aa] 73 to 82) from p27Nef (Fig. 1A). The irrelevant peptide Nef2 (aa 86 to 100 from p27Nef) did not elicit any cytotoxic response. The Nef-specific CTL response was restricted by HLA-A11 (data not shown). Flow cytometric analysis demonstrated that the P1-HIV line was at least 99% CD3+ CD4− CD8+ TCRαβ+γδ−. This line, as demonstrated by using antibodies specific for TCR Vβ chains, was composed of 80% Vβ8+ cells, while the remaining 20% of cells were not recognized by any of the available antibodies specific for 14 different TCR Vβ chains (data not shown). The Vβ8+ cell population was clonal, as confirmed in a runoff method (46) (data not shown), and mediated the Nef-specific cytotoxic activity, as demonstrated by depletion experiments (data not shown).
FIG. 1.
Cytotoxic activity of CD8+ T-cell lines. CTL assays were conventional 4-h 51Cr release assays. (A) P1-HIV line was tested against autologous B-LCL alone as a negative control (□), B-LCL previously infected with a rVV expressing the entire nef gene (✻), B-LCL preincubated with the specific peptide Nef19 (aa 73 to 82 of p27Nef; ●), or B-LCL preincubated with an irrelevant peptide (Nef2, aa 86 to 100 of p27Nef, ▵). (B) 1.6-EBV line was assayed against autologous B-LCL alone as a negative control (□), B-LCL previously incubated with the specific peptide (aa 339 to 347 of EBNA-3 from BL74 virus; ●), or B-LCL previously incubated with an irrelevant peptide (HIV 25-5; aa 168 to 184 of HIV p25Gag; ▵). Results represent the mean of triplicate cultures and are expressed as the percent specific lysis plus the standard deviation.
The 1.6-EBV line recognized the minimal epitope peptide FLRGRAYGL (aa 339 to 347) from the EBNA-3 protein of the EBV strain BL74 (Fig. 1B). The variant epitope sequence from the EBV strain B95.8 (FLRGRAYGI) was not recognized (9). Thus, the autologous B-LCL was not lysed in this assay, nor was it lysed if it was precoated with an irrelevant peptide 25-5 from HIV-1 p25Gag (aa 168 to 184) (Fig. 1B). This CTL response was restricted by HLA-B8 (51). By flow cytometric analysis the 1.6-EBV line was found to be at least 98% CD3+ CD8+ CD16− CD38+ HLA-DR+. Using a runoff method (46), we observed that the CTL line was composed of two clones, one expressing Vβ6B and one expressing Vβ3 (data not shown).
The P1-HIV line is a potent inhibitor of HIV-1 replication even in the absence of the cognate epitope.
We investigated the HIV-suppressive capacity of the P1-HIV line on autologous acutely infected CD4+ T cells. The CD4+ lymphocytes were infected with the R5 HIV-1 YU2b isolate and cocultured with the P1-HIV line at multiple CD8+/CD4+ ratios. Even at a ratio of 1/1, the P1-HIV line could completely suppress viral replication in target cells (Fig. 2A). In this experiment it is likely that the Nef-specific P1-HIV line was at least partly inhibiting virus replication through the classical HLA-restricted antigen-specific cytolytic mechanism. In order to assess HIV inhibition capacity of the line in the absence of this cytotoxicity, the P1-HIV line was cocultured with the same autologous CD4+ cell line infected with a mutant YU2b virus deleted for the nef gene (YU2bΔnef). These targets could not express the cognate epitope Nef aa 73 to 82 on their surface since Nef expression is completely suppressed in the molecular clone YU2bΔnef (43). In this coculture experiment, replication of YU2bΔnef was inhibited by 80% at a CD8+/CD4+ ratio of 1/1. Since the only HIV-specific cytotoxic activity mediated by P1-HIV was directed against Nef, this suggests that this HIV suppression was induced by a noncytotoxic activity. The viral replication inhibition was not due to the elimination of infected cells because CD4+ cells were present in the coculture with the CD8+ T cells at the peak of HIV replication, as confirmed by flow cytometry analysis (data not shown). In the absence of cytotoxic activity, this suppressive effect was less efficient than with the wild-type virus but acted in a dose-dependent manner (Fig. 2B). This anti-HIV response seemed to be inherent to the cell line since it was efficient even without TCR recognition of the specific HLA-peptide complex during the effector phase. Thus, P1-HIV line was able to control HIV replication in autologous CD4+ lymphocytes by a mechanism distinct to cytotoxicity. This antiviral activity dependent on the initial CD8+/CD4+ ratio could correspond to CAF activity as described initially (33). We next investigated the possibility that a non-HIV-specific CTL line could express the same anti-HIV activity.
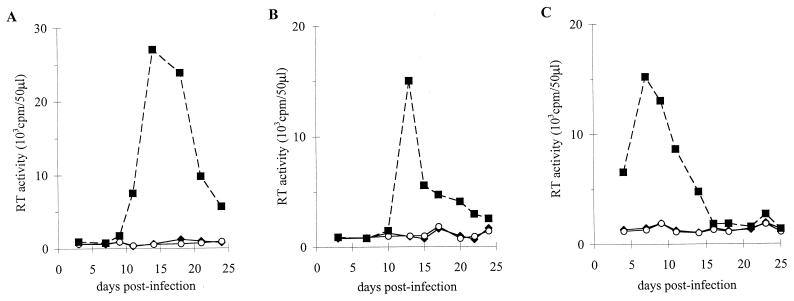
FIG. 2.
P1-HIV line suppression of viral replication in autologous CD4+ T cells. (A) CD4+ T lymphocytes were acutely infected with the HIV-1 R5 strain YU2b. (B) CD4+ T cells were infected with the nef frameshift mutant YU2bΔnef. Infected cells were either cultured alone (■) or cocultured with antigen-stimulated P1-HIV line at CD8+/CD4+ ratios of 4/1 (⧫) or 1/1 (○). Results are expressed as the RT activity (cpm)/50 μl of culture supernatant.
The 1.6-EBV line inhibited HIV replication as efficiently as the P1-HIV line.
We cocultivated the CD8+ 1.6-EBV line with autologous CD4+ T lymphocytes acutely infected with HIV-1 YU2b at various CD8+/CD4+ ratios. At the peak of viral replication, the 1.6-EBV line could inhibit at least 98% of HIV replication even at the lowest effector cell concentration (Fig. 3A). In YU2b-infected allogeneic CD4+ lymphocytes, the 1.6-EBV line suppressed viral replication by at least 93% (Fig. 3B). Thus, although the 1.6-EBV line is not capable of mediating HIV-specific cytotoxicity, it controlled HIV replication in vitro as efficiently as the P1-HIV line by a noncytotoxic mechanism. The ability of the 1.6-EBV line to suppress HIV replication in allogeneic CD4+ cells, as well as in autologous target cells, suggested that this effect was neither HLA class I nor class II restricted. In both cases, the presence of CD4+ T cells at the end of cocultures with CD8+ T lymphocytes was confirmed by flow cytometry analysis (data not shown). When cocultivated with allogeneic CD4+ lymphocytes infected with the X4 strain HIV-1 LAI, the 1.6-EBV line suppressed virus replication by 85% (Fig. 3C). Although this level of suppression was slightly lower than that seen with the R5 HIV-1 YU2b virus isolate, this difference was not considered significant. Therefore, the EBV-specific CTL line could suppress in a non-HLA-restricted manner replication of R5 and X4 HIV strains in acutely infected CD4+ lymphocytes. The ability to control HIV replication in vitro did not depend on recognition of HIV specificity.
FIG. 3.
1.6-EBV line control of HIV-1 replication in autologous or heterologous CD4+ lymphocytes. Autologous CD4+ T cells were infected with the molecular clone YU2b (A); heterologous CD4+ T lymphocytes were infected with YU2b (B) or LAI virus (C). Infected cells were cultured alone (■) or with antigen-stimulated 1.6-EBV line at CD8+/CD4+ ratios of 0.5/1 (○) or 1.5/1 (⧫) (A and B) or 0.5/1 (○) and 1/1 (⧫) (C). Results are expressed as the RT activity (cpm)/50 μl of culture supernatant. The results shown are from a representative experiment.
Inhibition of HIV replication by the 1.6-EBV line is mediated by a soluble factor(s).
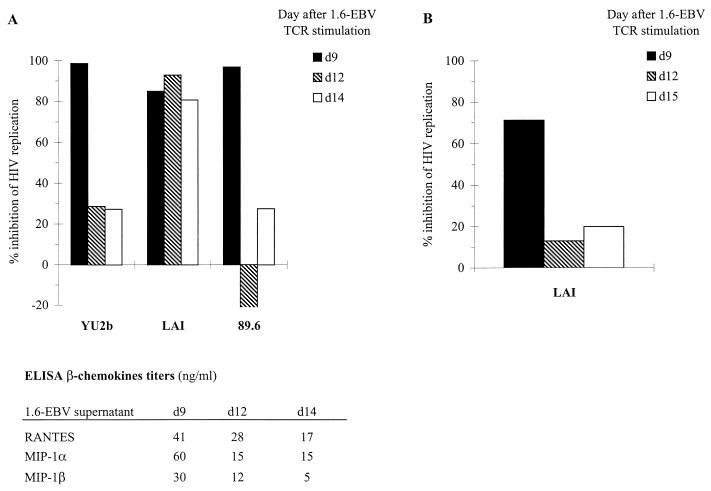
We next investigated the nature of the noncytotoxic anti-HIV suppressive activity of the anti-EBV CTL line. The 1.6-EBV line was stimulated with the cognate epitope peptide, and culture supernatants were collected at various time points. Autologous CD4+ lymphocytes were infected with three different HIV-1 strains overnight, washed, and then cultured in medium containing 50% cell-free supernatant from the 1.6-EBV line. The percent inhibition of RT activity was calculated at the peak of viral replication in each culture. Figure 4A shows that a supernatant taken 9 days after specific stimulation of the 1.6-EBV line completely suppressed replication of the R5 strain HIV-1 YU2b (98% inhibition of RT activity) and the X4R5 virus 89.6 (97% inhibition) and almost completely controlled replication of the X4 HIV-1 LAI isolate (85% inhibition) in autologous CD4+ T cells. Supernatants taken 12 and 14 days after specific stimulation had little effect on HIV-1 YU2b and 89.6 (<30% inhibition) but were still capable of inhibiting HIV-1 LAI replication (93 and 82% inhibition, respectively) (Fig. 4A). These results indicate that the efficiency of this HIV-suppressive activity depended upon the virus isolate and may be related to viral tropism, but these findings need to be confirmed with primary isolates. Chemokines produced by CD8+ T cells have been demonstrated to inhibit HIV replication. By enzyme-linked immunosorbent assay (ELISA) a significant level of β-chemokines in HIV-suppressive supernatants was detected (Fig. 4A). The lower β-chemokine titers in d12 and d14 supernatants could explain their reduced efficiency to control YU2b and 89.6 replication. But these factors could account in part only for the antiviral effect on R5 (YU2b) and X4R5 (89.6) isolates (22, 37). No SDF-1 mRNA was detected by RT-PCR in the activated 1.6-EBV line (data not shown). Ruling out a possible role of this α-chemokine in the inhibition of X4 strain replication, we conclude that 1.6-EBV line could control HIV replication in vitro by secretion of soluble mediators. β-chemokines could participate in this activity for R5 or X4R5 virus, but other unidentified factors need to be implicated in the X4 strain suppression. The noncytolytic control of HIV-1 LAI replication by 1.6-EBV cells or supernatants appeared to be functionally similar to CAF activity.
FIG. 4.
Effect of 1.6-EBV line supernatants on HIV-1 replication in CD4+ T cells. (A) Autologous CD4+ T lymphocytes were infected with HIV-1 strains YU2b, LAI, or 89.6 and were cultured with or without antigen-stimulated 1.6-EBV line supernatant collected at days 9 (d9, ■), 12 (d12,  ), and 14 (d14, □) after specific activation. ELISA titers of β-chemokines in 1.6-EBV line supernatants were evaluated according to the manufacturer's instructions (R&D). (B) The CEM cell line was infected with HIV-1 LAI CEM-adapted strain, cultivated with or without antigen-stimulated 1.6-EBV line supernatants collected at days 9 (d9, ■), 12 (d12,
), and 14 (d14, □) after specific activation. ELISA titers of β-chemokines in 1.6-EBV line supernatants were evaluated according to the manufacturer's instructions (R&D). (B) The CEM cell line was infected with HIV-1 LAI CEM-adapted strain, cultivated with or without antigen-stimulated 1.6-EBV line supernatants collected at days 9 (d9, ■), 12 (d12,  ), and 15 (d15, □) after specific activation. The final concentration of supernatants was 1/2 in both experiments. The peaks of RT activity in cultures of PHA-stimulated CD4+ lymphocytes alone (A) were 26,100 cpm/50 μl for YU2b-infected targets, 33,000 cpm/50 μl for LAI-infected lymphocytes, and 76,200 cpm/50 μl for 89.6-infected CD4+ T cells; in infected CEM cells alone (B) the peak RT activity was 17,100 cpm/50 μl. The amount of antiviral activity exhibited (percent inhibition of HIV replication) was calculated by comparing the RT activity levels with those in infected CD4+ cultures alone.
), and 15 (d15, □) after specific activation. The final concentration of supernatants was 1/2 in both experiments. The peaks of RT activity in cultures of PHA-stimulated CD4+ lymphocytes alone (A) were 26,100 cpm/50 μl for YU2b-infected targets, 33,000 cpm/50 μl for LAI-infected lymphocytes, and 76,200 cpm/50 μl for 89.6-infected CD4+ T cells; in infected CEM cells alone (B) the peak RT activity was 17,100 cpm/50 μl. The amount of antiviral activity exhibited (percent inhibition of HIV replication) was calculated by comparing the RT activity levels with those in infected CD4+ cultures alone.
Inhibition of HIV replication by the 1.6-EBV line occurs after viral reverse transcription.
To further characterize the mechanism of HIV-suppressive activity mediated by the 1.6-EBV line, CEM cells were infected with an HIV-1 LAI CEM-adapted strain as a model system. This experimental model allows the generation of a synchronous infection in CEM cells, i.e., all cells were infected by the input virus, and at 2 to 3 days postinfection more than 90% of the cells produced HIV proteins (31). In addition, the inhibition of T-tropic virus infection does not depend upon antiviral effects of β-chemokines. The infected CEM cells were grown for 1 week in medium containing 50% supernatant from the antigen-stimulated 1.6-EBV line and were assessed for viral replication by measuring the RT activity in the culture medium (Fig. 4B). Supernatant collected from 1.6-EBV line 9 days after antigenic stimulation (d9) suppressed HIV-1 LAI replication by 71%, but supernatants harvested at later time points (d12 and d15) were only very weakly effective (Fig. 4B). Since these supernatants were derived from the same culture of 1.6-EBV line as those used in the previous experiment (Fig. 4A), these results indicated that 1.6-EBV line inhibition of HIV-1 LAI replication was less efficient in CEM cells than in PHA-stimulated CD4+ lymphocytes (Fig. 4A). It could be due to the faster viral replication in infected CEM cells or to the nature of the target cells. Since CEM cells divide and grow more quickly than PHA-stimulated lymphocytes, the suppressive activity could interfere differently with HIV replication in these two cell types.
To determine which stage in the HIV life cycle is sensitive to anti-HIV activity, supernatant from antigen-stimulated 1.6-EBV line was added at different times during the culture of acutely infected CEM cells. Following HIV infection and washing of CEM cells, addition of T-cell supernatants resulted in 67% inhibition of virus replication (Table 1) compared to virus-infected CEM cells cultured without supernatant. This level of suppression compared favorably with that observed in the previous experiment (Fig. 4B). However, if supernatant was added only during the virus-to-cell contact period and was then completely removed, there was no inhibition of virus replication, suggesting that anti-HIV factors in the supernatant had little or no effect on virus entry. Furthermore, if the T-cell supernatant was not subsequently removed, HIV replication was inhibited to the same extent regardless of whether T-cell supernatant was added before or after virus infection of CEM cells (Table 1). In this model of synchronous infection of CEM cells zidovudine (AZT) had, on the one hand, no effect on HIV replication when added more than 24 h after infection. AZT inhibits specifically viral reverse transcription (31): 24 h after synchronous infection all of the viral genomes had been reverse transcribed. On the other hand, supernatant from the antigen-stimulated 1.6-EBV line was still able to suppress viral replication in CEM cells even when added 2 days after infection (data not shown). These observations suggested that the inhibition of viral replication observed with soluble factors produced by the 1.6-EBV CTL line was not due to a decrease in viral entry but rather acted after HIV reverse transcription.
TABLE 1.
Inhibition of viral replication of HIV-1 LAI strain in CEM cells by a TCR-stimulated 1.6-EBV line supernatant added during or after cell-to-virus contacta
| Presence of 1.6-EBV supernatant during:
|
RT activity
|
||
|---|---|---|---|
| Infection | Culture | cpm | % HIV infection |
| − | − | 17,772 | 100 |
| − | + | 5,915 | 33 |
| + | − | 20,519 | 115 |
| + | + | 7,100 | 35 |
A pool of the supernatant of 1.6-EBV line from 5 and 12 days post-TCR stimulation was added (+) or not added (−) with virus on CEM cells. Culture of infected cells was maintained in the presence (+) or absence (−) of supernatant for 7 days. The results are expressed as the RT activity (cpm)/50 μl of culture supernatant at the peak of viral replication (day 4), and the percentage of HIV infection reflects the extent of RT activity in the cell culture fluid compared to the activity in the infected CEM cells cultured alone (i.e., “infection −, culture −”) at this time point. The RT activity in supernatants of uninfected CEM cells during all of the culture and in all of the supernatants at day 1 did not exceed 600 cpm/50 μl.
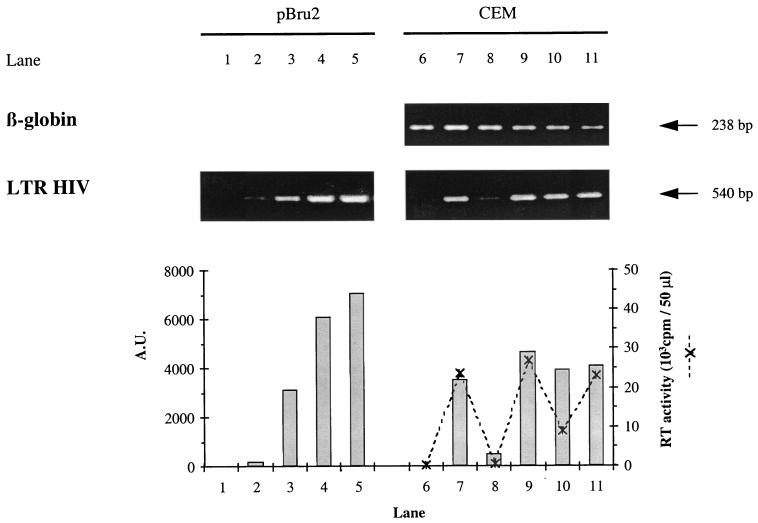
To confirm these conclusions, CEM cells were infected with HIV-1 LAI and grown in the presence of culture supernatant taken 9 days after antigenic stimulation of the 1.6-EBV line. After 24 h DNA was extracted from the infected cells and full-length HIV-1 LTR was detected by PCR. Since the PCR was performed using primers located in the U3 and the U5 regions of the HIV LTR, it only allowed detection of newly reverse transcribed viral DNA obtained after the first template switching (52). As shown in Fig. 5, the 1.6-EBV line supernatant had no effect in this system (lane 10) compared with HIV-1 LAI-infected CEM cells cultured in medium alone (lane 7) or in medium supplemented with supernatant from a PHA-activated CD4+ T-lymphocyte culture (lane 9). In contrast, AZT treatment of CEM cells before and during virus infection greatly reduced the amount of newly reverse-transcribed viral DNA that could be detected (lane 8). In this experiment, the infected CEM cells were also monitored 4 days after infection (which corresponded to the peak of HIV replication) for RT activity. As shown in Fig. 5, although supernatant from the 1.6-EBV line did not reduce the level of newly reverse-transcribed viral DNA detected by PCR, it did inhibit RT activity by 60% (lane 10) when compared to CEM cells infected with virus in the presence of control culture medium (lane 7) or in the presence of a nonsuppressive PHA-activated CD4+ T-lymphocyte culture supernatant (lane 9). AZT treatment of CEM cells during virus infection almost completely blocked RT activity (lane 8). Altogether, these data suggest that noncytolytic suppression of HIV-1 LAI replication in CEM cells by the 1.6-EBV line did not act at the level of virus entry, but rather occurred following the first template switching of viral reverse transcription.
FIG. 5.
Estimation of HIV proviral DNA in CEM cells infected with the HIV-1 LAI CEM-adapted virus. At 24 h after infection, DNA was extracted from uninfected CEM (lane 6), infected CEM alone (lane 7), infected CEM cultivated with 5 μM AZT (lane 8), with supernatant from a 9-day-old culture of PHA-activated CD4+ cells from the EBV-positive donor (lane 9), with supernatant from a 9-day-old culture of antigen-stimulated 1.6-EBV line (lane 10), and with 1.6-EBV line cloning mix (lane 11). The quality of DNA extracts was monitored with a β-globin-specific PCR. A fourfold range of dilutions of the pBru2 plasmid, encoding the full-length HIV-1 Bru genome, from 1 × 105 to 6.4 × 106 copies per sample (lanes 2 to 5) was used as a calibration curve. Lane 1 corresponds to the negative control containing no DNA. The products obtained after the first template switching of the reverse transcription were amplified by a PCR-specific for the complete HIV-1 LTR. PCR products were quantified by using NIH 1.61 software, and their intensities were expressed as arbitrary units (A.U.) on a histogram. For CEM cells (lanes 6 to 11), the RT activity in supernatant fluids was expressed as the cpm/50 μl at the peak of viral replication (day 4, ---✻---).
DISCUSSION
In the present study, we have analyzed the potent antiviral effects of two virus-specific CTL lines on HIV replication. We have shown that both a CD8+ cytotoxic cell line specific for HIV-1 Nef and an EBV-specific CTL line could suppress in vitro HIV-1 replication by a noncytolytic mechanism in acutely infected CD4+ cells. We focused the study on the anti-HIV noncytotoxic activity of the EBV-specific CTL line and demonstrated that this activity was mediated by soluble factor(s). Using synchronous infection of CEM cells we demonstrated that this inhibition acted after viral reverse transcription regarding X4 strain replication. All of the characteristics of this HIV replication control by the 1.6-EBV line are in agreement with the functional definition of CAF activity described previously (33). Therefore, we conclude that CAF-like activity is not restricted to HIV-specific CD8+ T cells.
The HIV-1 Nef-specific CTL line, P1-HIV, exerted in vitro a potent antiviral effect on HIV replication in autologous CD4+ T cells infected with the R5 strain YU2b (Fig. 2A), in part due to major histocompatibility complex (MHC) class I-restricted cytolysis of infected cells. However, P1-HIV could mediate also a noncytolytic HIV-1 suppression, as demonstrated by using the same autologous CD4+ cell line infected with the R5 strain YU2bΔnef (Fig. 2B) devoid of the nef gene (43). Compared to wild-type virus inhibition, the control of deleted virus replication was less effective in the absence of conventional cytotoxicity. This system allows us to dissociate HIV-specific cytotoxicity from noncytolytic HIV suppression. Yang et al. (60) attempted to estimate the relative importance of CAF activity in the global HIV suppression by using HLA-mismatched target cells. CAF activity was shown to be less efficient in an HLA-mismatched setting (8), and therefore this approach may underestimate the importance of CAF-mediated control. In our study, we have been able to assess the relative contribution of both anti-HIV cytotoxic and noncytolytic mechanisms within an HLA-matched system using the same target CD4+ population.
Surprisingly, we observed that the 1.6-EBV CTL line, obtained from an HIV-seronegative individual and specific for EBV, could also suppress in vitro replication of both R5 and X4 HIV-1 isolates in acutely infected CD4+ T cells. This CD8+ T-cell line controlled R5 virus replication as efficiently as the HIV-1-specific CTL line P1-HIV (Fig. 2 and 3). This anti-HIV function is distinct from cytolysis of infected cells given that the conventional cytotoxic activity expressed by the 1.6-EBV line is specific for a defined epitope of EBV and that this HIV inhibition appeared to be not restricted by MHC class I (Fig. 3). Since this HIV-suppressive effect could be mimicked using cell-free supernatants, it could be considered as a CAF-like activity at a functional level. Altogether, we conclude that CAF production is not restricted to HIV-specific CD8+ effector cells. Further studies of additional CTL lines with other recognition specificities, however, are required to extend this observation.
Our data provide an example of HIV suppression by CD8+ lymphocytes from an uninfected individual, devoid of HIV specificity. Previous attempts to identify CAF activity in HIV-seronegative donors have yielded conflicting results (26, 32, 49). According to these observations the proportion of HIV-suppressive CD8+ clones in an HIV-seronegative donor may be relatively low. In our experimental model, 5 of 6 CD8+ PHA-stimulated lines from two HIV-seronegative individuals were not able to inhibit HIV (YU2b and LAI) replication in vitro, whereas 5 of 13 CD8+ PHA-stimulated lines from one HIV-1 infected patient could control HIV (YU2b and LAI) replication (data not shown). Furthermore, the HIV-suppressive activity of CD8+ T cells needed to be primed by cellular activation, although the presence of the cognate epitope was not required during the effector phase of the inhibition. Indeed, in HIV-infected patients the anti-HIV activity was associated with an increase in activated CD8+ T cells (23, 30). Furthermore the efficiency of CAF activity increased following strong stimulation of the effector CD8+ cells (with CD28) (3), and cells mediating this activity presented an activated cell phenotype (36). Since an EBV-specific CTL line could control HIV replication, the immune ability to produce CAF activity is not exclusively induced by HIV infection. We propose that CAF secretion is a consequence of an activation state of the immune system and of the CD8+ effector cells in particular that is induced by the HIV infection but which could eventually occur during other types of activation of the immune system.
The β-chemokines RANTES, MIP-1α, and MIP-1β have been shown to inhibit R5 isolate infection by blocking virus entry (1, 19). The two factors MDC (45) and IL-16 (2) have also been demonstrated to suppress HIV replication. By ELISA we detected significant amounts of β-chemokines in HIV-suppressive supernatants which could account in part for the control of R5 and X4R5 virus replication by the 1.6-EBV line (Fig. 4A) (37). However, to characterize the mechanism of CAF-like activity mediated by this cell line, we studied the synchronous infection of CEM cells with the X4 strain HIV-1 LAI. In this experimental model, no known chemokines produced by CD8+ T cells could be implicated in potent control of viral replication. Supernatants from the 1.6-EBV line were then demonstrated not to affect viral entry on CEM cells (Table 1). Furthermore, we observed that the number of LTR copies detected in infected CEM cells was not affected by the presence of fluids containing CAF-like activity (Fig. 5). Thus, this CAF-like activity may not interfere with HIV replication until the first template switching of reverse transcription. These results are in agreement with previous studies demonstrating that CAF mediates specific inhibition of LTR-driven activity (17, 38). Copeland et al. also showed that this inhibition may be mediated via the NF-κB or the NFAT-1 element (18). Therefore, our data combined with these previous observations indicate that CAF is active at a post-reverse transcriptional level, and especially at the transcription level.
In summary, we have demonstrated that an EBV-specific CTL clone could express a CAF-like activity. In the case of X4 strains, this HIV suppression is distinct from a blocking on viral entry and acts after the first template switching of the reverse transcription. Thus, CAF-mediated inhibition of HIV replication is not restricted to HIV-specific CD8+ cells. In HIV disease, production of antiviral soluble factors by CD8+ T cells could be of importance in the in vivo control of viral latency. Our data imply that CD8+ T lymphocytes, regardless of their recognition specificity, may contribute to this effect by an in vivo bystander CAF secretion after TCR stimulation.
ACKNOWLEDGMENTS
We thank Mandaleshwar K. Singh for critical review of the manuscript and Geneviève Janvier for excellent technical assistance. We also thank Alan B. Rickinson for his interest in this work.
This work was supported by grants from the Agence Nationale de Recherche sur le SIDA, the Pediatric AIDS Foundation, and the Institut Pasteur. Sylvie Le Borgne is a fellow of the “Fondation Roux.” Yves Rivière is an Elisabeth Glaser scientist.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1 alpha, MIP-1 beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Baier M, Werner A, Bannert N, Metzner K, Kurth R. HIV suppression by interleukin-16. Nature. 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 3.Barker E, Bossart K N, Fujimura S H, Levy J A. CD28 costimulation increases CD8+ cell suppression of HIV replication. J Immunol. 1997;159:5123–5131. [PubMed] [Google Scholar]
- 4.Barker E, Bossart K N, Locher C P, Patterson B K, Levy J A. CD8+ cells from asymptomatic human immunodeficiency virus-infected individuals suppress superinfection of their peripheral blood mononuclear cells. J Gen Virol. 1996;77:2953–2962. doi: 10.1099/0022-1317-77-12-2953. [DOI] [PubMed] [Google Scholar]
- 5.Blackbourn D J, Locher C P, Ramachandran B, Barnett S W, Murthy K K, Carey K D, Brasky K M, Levy J A. CD8+ cells from HIV-2-infected baboons control HIV replication. AIDS. 1997;11:737–746. doi: 10.1097/00002030-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Blackbourn D J, Mackewicz C E, Barker E, Hunt T K, Herndier B, Haase A T, Levy J A. Suppression of HIV replication by lymphoid tissue CD8+ cells correlates with the clinical state of HIV-infected individuals. Proc Natl Acad Sci USA. 1996;93:13125–13130. doi: 10.1073/pnas.93.23.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinchmann J E, Gaudernack G, Vartdal F. CD8+ T cells inhibit HIV replication in naturally infected CD4+ T cells. Evidence for a soluble inhibitor. J Immunol. 1990;144:2961–2966. [PubMed] [Google Scholar]
- 9.Burrows S R, Rodda S J, Suhrbier A, Geysen H M, Moss D J. The specificity of recognition of a cytotoxic T lymphocyte epitope. Eur J Immunol. 1992;22:191–195. doi: 10.1002/eji.1830220128. [DOI] [PubMed] [Google Scholar]
- 10.Buseyne F, Février M, Garcia S, Gougeon M-L, Rivière Y. Dual function of HIV specific CTL clone: inhibition of HIV replication by non-cytolytic mechanisms and lysis of HIV-infected CD4+ cells. Virology. 1996;225:248–253. doi: 10.1006/viro.1996.0597. [DOI] [PubMed] [Google Scholar]
- 11.Callebaut C, Jacotot E, Guichard G, Krust B, Rey-Cuille M, Cointe D, Benkirane N, Blanco J, Muller S, Briand J, Hovanessian A G. Inhibition of HIV infection by pseudopeptides blocking viral envelope glycoprotein-mediated membrane fusion and cell death. Virology. 1996;218:181–192. doi: 10.1006/viro.1996.0178. [DOI] [PubMed] [Google Scholar]
- 12.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro B A, Walker C M, Eichberg J W, Levy J A. Suppression of human immunodeficiency virus replication by CD8+ cells from infected and uninfected chimpanzees. Cell Immunol. 1991;132:246–255. doi: 10.1016/0008-8749(91)90023-5. [DOI] [PubMed] [Google Scholar]
- 14.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 15.Collin M, James W, Gordon S. Development of techniques to analyse the formation of HIV provirus in primary human macrophages. Res Virol. 1991;142:105–112. doi: 10.1016/0923-2516(91)90045-5. [DOI] [PubMed] [Google Scholar]
- 16.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Copeland K F, McKay P J, Rosenthal K L. Suppression of activation of the human immunodeficiency virus long terminal repeat by CD8+ T cells is not lentivirus specific. AIDS Res Hum Retrovir. 1995;11:1321–1326. doi: 10.1089/aid.1995.11.1321. [DOI] [PubMed] [Google Scholar]
- 18.Copeland K F T, McKay P J, Rosenthal K L. Suppression of the human immunodeficiency virus long terminal repeat by CD8+ T cells is dependent on the NFAT-1 element. AIDS Res Hum Retrovir. 1996;12:143–148. doi: 10.1089/aid.1996.12.143. [DOI] [PubMed] [Google Scholar]
- 19.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane domain, G-protein coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 21.Garcia S, Fevrier M, Dadaglio G, Lecoeur H, Riviere Y, Gougeon M L. Potential deleterious effect of anti-viral cytotoxic lymphocyte through the CD95 (FAS/APO-1)-mediated pathway during chronic HIV infection. Immunol Lett. 1997;57:53–58. doi: 10.1016/s0165-2478(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 22.Garzino-Demo A, Arya S K, Devico A L, Cocchi F, Lusso P, Gallo R C. C-C chemokine RANTES and HIV long terminal repeat-driven gene expression. AIDS Res Hum Retrovir. 1997;13:1367–1371. doi: 10.1089/aid.1997.13.1367. [DOI] [PubMed] [Google Scholar]
- 23.Giorgi J V, Ho H N, Hirji K, Chou C C, Hultin L E, O'Rourke S, Park L, Margolick J B, Ferbas J, Phair J P. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: development of HLA-DR+ CD38− CD8+ cells is associated with subsequent stable CD4+ cell levels. The Multicenter AIDS Cohort Study Group. J Infect Dis. 1994;170:775–781. doi: 10.1093/infdis/170.4.775. [DOI] [PubMed] [Google Scholar]
- 24.Gomez A M, Smaill F M, Rosenthal K L. Inhibition of HIV replication by CD8+ T cells correlates with CD4 counts and clinical stage of disease. Clin Exp Immunol. 1994;97:68–75. doi: 10.1111/j.1365-2249.1994.tb06581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill A B, Lee S P, Haurum J S, Murray N, Yao Q Y, Rowe M, Signoret N, Rickinson A B, McMichael A J. Class I major histocompatibility complex-restricted cytotoxic T lymphocytes specific for Epstein-Barr virus (EBV) nuclear antigens fail to lyse the EBV-transformed B lymphoblastoid cell lines against which they were raised. J Exp Med. 1995;181:2221–2228. doi: 10.1084/jem.181.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsueh F W, Walker C M, Blackbourn D J, Levy J A. Suppression of HIV replication by CD8+ cell clones derived from HIV-infected and uninfected individuals. Cell Immunol. 1994;159:271–279. doi: 10.1006/cimm.1994.1313. [DOI] [PubMed] [Google Scholar]
- 27.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacey S F, McDanal C B, Horuk R, Greenberg M L. The CXC chemokine stromal cell-derived factor 1 is not responsible for CD8+ T cell suppression of syncytia-inducing strains of HIV-1. Proc Natl Acad Sci USA. 1997;94:9842–9847. doi: 10.1073/pnas.94.18.9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landay A L, Mackewicz C E, Levy J A. An activated CD8+ T cell phenotype correlates with anti-HIV activity and asymptomatic clinical status. Clin Immunol Immunopathol. 1993;69:106–116. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 31.Laurent-Crawford A G, Hovanessian A G. The cytopathic effect of human immunodeficiency virus is independent of high levels of unintegrated viral DNA accumulated in response to superinfection of cells. J Gen Virol. 1993;74:2619–2628. doi: 10.1099/0022-1317-74-12-2619. [DOI] [PubMed] [Google Scholar]
- 32.Levy J A, Hsueh F, Blackbourn D J, Wara D, Weintrub P S. CD8 cell noncytotoxic antiviral activity in human immunodeficiency virus-infected and -uninfected children. J Infect Dis. 1998;177:470–472. doi: 10.1086/517378. [DOI] [PubMed] [Google Scholar]
- 33.Levy J A, Mackewicz C E, Barker E. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Hui H, Burgess C J, Price R W, Sharp P M, Hahn B H, Shaw G M. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation. J Virol. 1992;66:6587–6600. doi: 10.1128/jvi.66.11.6587-6600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locher C P, Blackbourn D J, Barnett S W, Murthy K K, Cobb E K, Rouse S, Greco G, Reyes-Teran G, Brasky K M, Carey K D, Levy J A. Superinfection with human immunodeficiency virus type 2 can reactivate virus production in baboons but is contained by a CD8 T cell antiviral response. J Infect Dis. 1997;176:948–959. doi: 10.1086/516544. [DOI] [PubMed] [Google Scholar]
- 36.Mackewicz C, Levy J A. CD8+ cell anti-HIV activity: nonlytic suppression of virus replication. AIDS Res Hum Retrovir. 1992;8:1039–1050. doi: 10.1089/aid.1992.8.1039. [DOI] [PubMed] [Google Scholar]
- 37.Mackewicz C E, Barker E, Levy J A. Role of beta-chemokines in suppressing HIV replication. Science. 1996;274:1393–1395. doi: 10.1126/science.274.5291.1393. [DOI] [PubMed] [Google Scholar]
- 38.Mackewicz C E, Blackbourn D J, Levy J A. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc Natl Acad Sci USA. 1995;92:2308–2312. doi: 10.1073/pnas.92.6.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackewicz C E, Ortega H, Levy J A. Effect of cytokines on HIV replication in CD4+ lymphocytes: lack of identity with the CD8+ cell antiviral factor. Cell Immunol. 1994;153:329–343. doi: 10.1006/cimm.1994.1032. [DOI] [PubMed] [Google Scholar]
- 40.Mackewicz C E, Ortega H W, Levy J A. CD8+ cell anti-HIV activity correlates with the clinical state of the infected individual. J Clin Investig. 1991;87:1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marechal V, Arenzana-Seisdedos F, Heard J-M, Schwartz O. Opposite effects of SDF-1 on human immunodeficiency virus type 1 replication. J Virol. 1999;73:3608–3615. doi: 10.1128/jvi.73.5.3608-3615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller G, Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci USA. 1973;70:190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 45.Pal R, Garzino-Demo A, Markham P D, Burns J, Brown M, Gallo R C, DeVico A L. Inhibition of HIV-1 infection by the beta-chemokine MDC. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 46.Pannetier C, Even J, Kourilsky P. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today. 1995;16:176–181. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 47.Peden K, Emerman M, Montagnier L. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1LAI, HIV-1MAL, and HIV-1ELI. Virology. 1991;185:661–672. doi: 10.1016/0042-6822(91)90537-l. [DOI] [PubMed] [Google Scholar]
- 48.Robertson M N, Buseyne F, Schwartz O, Riviere Y. Efficient antigen presentation to cytotoxic T lymphocytes by cells transduced with a retroviral vector expressing the HIV-1 Nef protein. AIDS Res Hum Retrovir. 1993;9:1217–1223. doi: 10.1089/aid.1993.9.1217. [DOI] [PubMed] [Google Scholar]
- 49.Rosok B, Voltersvik P, Larsson B-M, Albert J, Brinchmann J, Asjo B. CD8+ T cells from HIV type 1-seronegative individuals suppress virus replication in acutely infected cells. AIDS Res Hum Retrovir. 1997;13:79–85. doi: 10.1089/aid.1997.13.79. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz O, Henin Y, Marechal V, Montagnier L. A rapid and simple colorimetric test for the study of anti-HIV agents. AIDS Res Hum Retrovir. 1988;4:441–448. doi: 10.1089/aid.1988.4.441. [DOI] [PubMed] [Google Scholar]
- 51.Sutton J, Rowland-Jones S, Rosenberg W, Nixon D, Gotch F, Gao X M, Murray N, Spoonas A, Driscoll P, Smith M, et al. A sequence pattern for peptides presented to cytotoxic T lymphocytes by HLA B8 revealed by analysis of epitopes and eluted peptides. Eur J Immunol. 1993;23:447–453. doi: 10.1002/eji.1830230222. [DOI] [PubMed] [Google Scholar]
- 52.Trono D. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J Virol. 1992;66:4893–4900. doi: 10.1128/jvi.66.8.4893-4900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsubota H, Lord C I, Watkins D I, Morimoto C, Letvin N L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989;169:1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker B D, Plata F. Cytotoxic T lymphocytes against HIV. AIDS. 1990;4:177–184. doi: 10.1097/00002030-199003000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Walker C M, Erickson A L, Hsueh F C, Levy J A. Inhibition of human immunodeficiency virus replication in acutely infected CD4+ cells by CD8+ cells involves a noncytotoxic mechanism. J Virol. 1991;65:5921–5927. doi: 10.1128/jvi.65.11.5921-5927.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker C M, Levy J A. A diffusible lymphokine produced by CD8+ T lymphocytes suppresses HIV replication. Immunology. 1989;66:628–630. [PMC free article] [PubMed] [Google Scholar]
- 57.Walker C M, Moody D J, Stites D P, Levy J A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 58.Walker C M, Thomson-Honnebier G A, Hsueh F C, Erickson A L, Pan L Z, Levy J A. CD8+ T cells from HIV-1-infected individuals inhibit acute infection by human and primate immunodeficiency viruses. Cell Immunol. 1991;137:420–428. doi: 10.1016/0008-8749(91)90090-x. [DOI] [PubMed] [Google Scholar]
- 59.Wiviott L D, Walker C M, Levy J A. CD8+ lymphocytes suppress HIV production by autologous CD4+ cells without eliminating the infected cells from culture. Cell Immunol. 1990;128:628–634. doi: 10.1016/0008-8749(90)90054-u. [DOI] [PubMed] [Google Scholar]
- 60.Yang O O, Kalams S A, Rosenzweig M, Trocha A, Jones N, Koziel M, Walker B D, Johnson R P. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J Virol. 1996;70:5799–5806. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]