Abstract
The in vivo persistence of gene-modified cells may be limited by the development of a host immune response to vector-encoded proteins. Herpesviruses evade cytotoxic T-lymphocyte (CTL) recognition by expressing genes which interfere selectively with presentation of viral antigens by class I major histocompatibility complex (MHC) molecules. Here, we studied the use of retroviral vectors encoding herpes simplex virus ICP47, human cytomegalovirus (HCMV) US3, or HCMV US11 to decrease presentation of viral proteins and transgene products to CD8+ CTL. Human fibroblasts and T cells transduced to express the ICP47, US3, or US11 genes alone exhibited a decrease in cell surface class I MHC expression. The combination of ICP47 and US11 rendered fibroblasts negative for surface class I MHC and allowed a class I MHC-low population of T cells to be sorted by flow cytometry. Fibroblasts and T cells expressing both ICP47 and US11 were protected from CTL-mediated lysis and failed to stimulate specific memory T-cell responses to transgene products in vitro. Our findings suggest that expression of immunoregulatory viral gene products could be a potential strategy to prolong transgene expression in vivo.
The development of methods to introduce genes into somatic cells has led to clinical applications of this technology including the treatment of genetic or acquired diseases, marking transferred cells to evaluate their in vivo persistence and migration, and the introduction of a suicide gene to address safety concerns in cell therapy (5, 20). However, a major obstacle to the in vivo persistence of cells modified by retroviral or adenoviral vectors is the development of a host immune response to transgene or vector-encoded proteins (47, 62).
Early reports demonstrated long-term in vivo persistence of gene-modified cells transduced with retroviral vectors encoding a therapeutic gene and/or an antibiotic selection marker. However, in these studies the gene-modified cells were administered to immunocompromised hosts such as patients with primary immunodeficiency or those undergoing bone marrow transplantation and cancer chemotherapy (7, 11, 12, 25, 34, 49, 50). More recent studies in which gene-modified cells have been inoculated into immunocompetent animals and humans have shown that potent host immune responses to transgene-encoded proteins such as neomycin phosphotransferase, hygromycin phosphotransferase (Hy), herpes simplex virus (HSV) thymidine kinase (TK), and therapeutic genes may limit the in vivo persistence of transferred cells (9, 13, 23, 37, 47, 57). The immune mechanisms responsible for eliminating genetically altered cells included antibody responses to transgene products that were secreted or expressed at the cell surface and CD8+ cytotoxic T-cell responses to peptide fragments derived from intracellular proteins. These findings suggest that immunomodulatory strategies to render gene-modified cells less susceptible to host immune surveillance will be required for successful gene therapy in immunocompetent hosts.
Long-term persistence of gene-modified cells could potentially be achieved by administering immunosuppressive regimens commonly used in the prevention of solid organ graft rejection, graft-versus-host disease, and autoimmune disorders. The continuous administration of both cyclosporin and cyclophosphamide, but not cyclosporin alone, prolonged in vivo gene expression following adenovirus-mediated gene transfer, although the ability of this regimen to facilitate secondary gene delivery was not tested (17, 18). However, this strategy is limited by incomplete efficacy, the risk of infectious complications, and other regimen-related toxicities. Transient and less toxic immunomodulatory approaches such as the inhibition of costimulatory interactions between T cells and antigen-presenting cells by blockade of CD28- and CD40 signaling with CTLA4Ig and monoclonal antibody (MAb) against CD40 ligand, respectively, have been evaluated in animal studies of adenovirus-mediated gene transfer to the liver and airways. This transient immunomodulation resulted in a significant prolongation of transgene expression, a decrease in the magnitude of neutralizing antibody titers to the vector, and successful secondary gene transfer in some animals (33, 61). However, these studies indicated that cellular and humoral immune responses were markedly reduced but not completely abrogated.
An alternative approach to decrease the immunogenicity of gene-modified cells which do not secrete their transgene products is to inhibit the pathway by which antigens are presented to CD8+ cytotoxic T lymphocytes (CTL). This antigen presentation pathway requires intracellular degradation of the antigenic protein to peptide fragments, transport of these peptides into the endoplasmatic reticulum (ER) by the heterodimeric transporter of antigen presentation (TAP), assembly of class I major histocompatibility complex (MHC) heavy chain, β2-microglobulin, and peptide complexes within the ER lumen, and transport of this trimeric complex to the plasma membrane, where it is displayed for recognition by CTL (22). Several human herpesviruses evade recognition by CD8+ CTL by selectively interfering at discrete sites in the class I MHC antigen processing pathway (29, 44). HSV produces a cytosolic protein, ICP47, which prevents transport of peptides into the ER by the TAP complex (19, 26, 63). Human cytomegalovirus (HCMV) interferes with antigen presentation at several sites (2, 30). The HCMV US2 and US11 gene products cause reverse translocation of class I heavy chains from the ER to the cytosol, leading to their rapid degradation (58, 59). HCMV US3 binds to and retains class I MHC molecules in the ER (31) and the US6 protein blocks the TAP complex from the ER-lumenal side (3, 24).
In this study, we developed retroviral vectors encoding HSV ICP47, HCMV US3, or HCMV US11 and evaluated their ability to inhibit class I MHC presentation of antigenic epitopes derived from viral proteins and transgene products expressed in gene-modified cells. Our results demonstrate that expression of ICP47 and US11 in human fibroblasts and primary T cells dramatically decreases class I MHC expression, protects from CTL-mediated lysis, and renders these cells ineffective for stimulating memory CTL responses in vitro. Thus, constitutive expression of viral inhibitory gene products could be a potential strategy to prolong transgene expression in vivo.
MATERIALS AND METHODS
Cell lines and viruses.
The retroviral packaging cell lines PE501, PA317, and PG13 were grown in Dulbecco's modified Eagle medium with 10% heat-inactivated fetal bovine serum (FBS; HyClone Laboratories, Logan, Utah) (41, 42). Epstein-Barr virus-transformed lymphoblastoid cell lines were generated from peripheral blood mononuclear cells (PBMC) obtained from normal volunteer donors and cultured in RPMI 1640 with 10% FBS. The class I MHC-negative human B-cell line 721.221 (54) was kindly provided by Dan Geraghty (Fred Hutchinson Cancer Research Center, Seattle, Wash.). A human HCMV pp65-specific and HLA-A24-restricted CD8+ CTL clone was isolated and grown as described previously (48). CD8+ human immunodeficiency virus (HIV) Gag-specific T lymphocyte clones were isolated from HIV-seropositive patients as described elsewhere (47). CD8+ cytotoxic T-cell clones specific for Hy were derived from these same HIV-infected patients who received treatment on an adoptive immunotherapy protocol with Hy- and TK-marked (HyTK-marked) CD8+ HIV Gag-specific CTL (47). These T-cell clones were cultured in medium consisting of RPMI 1640 supplemented with 25 mM HEPES, 11% human AB serum, 25 μM 2-mercaptoethanol (Sigma Chemical Co., St. Louis, Mo.), and 4 mM l-glutamine (GIBCO-BRL, Gaithersburg, Md.). Dermal fibroblast cell lines were established from these patients and normal HCMV-seropositive volunteer donors and were grown in Waymouth's medium supplemented with 15% FBS. AD169 strain HCMV was obtained from the American Type Culture Collection (ATCC, Manassas, Va.), propagated in human foreskin fibroblasts, and used as supernatant virus to infect dermal fibroblast lines. AdICP47-1, a replication-deficient recombinant adenovirus encoding for HSV ICP47, was described previously (63).
Retroviral vectors.
Recombinant DNA manipulations were performed according to standard protocols (51). Plasmids pLXSN and pLXSH were obtained from A. D. Miller (Fred Hutchinson Cancer Research Center, Seattle, Wash.) and have been previously described (42). Plasmids containing cDNA encoding HSV ICP47 (p47BE-P), HCMV US3 (pT7proUS3), and HCMV US11 (pCA13US11) were provided by D. C. Johnson (Oregon Health Science University). pLICP47SN was constructed by excising an XbaI-EcoRI fragment from plasmid p47BE-P containing the ICP47 cDNA (333 bp), blunting the ends, and ligating this into the XhoI-digested and blunt-ended plasmid pLXSN. For pLUS3SN, a BamHI-PvuII fragment containing the US3 cDNA (570 bp) was blunt ended and ligated into HpaI-digested pLXSN. pLUS11SH was constructed by digesting pCA13US11 with EcoRI to remove the US11 gene (708 bp), blunting the ends, and ligating into HpaI-digested pLXSH. Correct orientation was ascertained by restriction enzyme digest.
Retroviruses encoding ICP47, US3, or US11 were generated using standard methods (42). Briefly, PE501 packaging cells were transfected over 5 h with 2 μg of plasmid DNA from pLICP47SN, pLUS3SN, or pLUS11SH, using 24 μg of Lipofectamine (GIBCO-BRL) in serum-free conditions. Virus-containing supernatants of these cells were used to infect PA317 or PG13 cells in the presence of Polybrene (5 μg/ml; Sigma). From each packaging cell line, 6 to 12 individual producer clones resistant to Geneticin (G418 sulfate; GIBCO-BRL) or hygromycin B (Hm) (Boehringer Mannheim, Indianapolis, Ind.) were isolated and expanded. The Hy retroviral vector was produced in PA317 packaging cells as described. Vector titers were determined using NIH 3T3 cells for virus packaged in PA317 cells or canine D17 cells for virus packaged in PG13 cells according to established procedures and ranged between 3 × 105 to 8 × 105 CFU/ml (42).
Stable retroviral transductions of human fibroblasts and primary T cells.
Individual fibroblast cultures in log phase of growth were transduced by culturing for 12 h with retroviral supernatant from LXSH, LICP47SN, LUS3SN, or LUS11SH packaging cells in the presence of Polybrene (5 μg/ml). This procedure was repeated after 12 h. One day later, transduced cells were selected with G418 (0.75 mg/ml, 100% active) or Hm (0.1 mg/ml) for 14 days. Cell lines expressing both ICP47 and US11 were obtained by first culturing fibroblasts with LICP47SN supernatant and selecting in G418 and then culturing with LUS11SH supernatant and selecting with Hm.
Human CD8+ HIV Gag-specific T-cell clones were transduced as previously described (47). Briefly, T cells were stimulated with anti-CD3 MAb (30 ng/ml; Orthoclone OKT3; Ortho Biotech Inc., Raritan, N.J.) in the presence of allogeneic γ-irradiated PBMC and lymphoblastoid cell lines. Recombinant interleukin-2 (50 U/ml; Chiron Corporation, Emeryville, Calif.) was added on day +1. Three and five days after restimulation, aliquots of the T cells were exposed for 24 h to retroviral supernatant from LXSH, LICP47SN, or LUS11SH, respectively, in a 1:1 (volume-to-volume) ratio of CTL medium with Polybrene (5 μg/ml). One day later, T cells were pelleted and resuspended in medium containing interleukin-2 and G418 (1.0 mg/ml, 100% active) or Hm (0.25 mg/ml). T cells expressing both ICP47 and US11 were obtained by exposing LICP47SN-transduced T lymphocytes to retroviral supernatant from LUS11SH packaging cells and selecting with Hm. Analysis of transgene expression and functional assays were performed after T cells had been selected with drug for >21 days.
Flow cytometry and cell sorting.
Class I MHC surface expression was determined by indirect immunofluorescence using as the primary antibody the murine immunoglobulin G2a MAb W6/32, which recognizes the complex of class I MHC heavy chain and β2-microglobulin, followed by a fluorescein isothiocyanate-conjugated goat-anti-mouse immunoglobulin G2a secondary antibody (Tago Immunologicals, Camarillo, Calif.) (43). In some experiments, we used a fluorescein isothiocyanate-conjugated MAb which recognizes HLA-A, -B, and -C (Pharmingen, San Diego, Calif.). CD8 expression of T cells was assessed by staining with phycoerythrin (PE)-conjugated CD8 MAb (Becton Dickinson, Mountain View, Calif.). Isotype-matched MAbs (Tago Immunologicals) were used as controls. All fluorescence analyses were performed on a FACScan flow cytometer (Becton Dickinson), and data were analyzed using CellQuest software.
To sort-purify a class I MHC-low T-cell population, viable CD8+ T lymphocytes transduced with both LICP47SN and LUS11SH were stained on day 14 poststimulation with MAb W6/32 as described above. Cells expressing the lowest levels of class I MHC were then sorted on a Vantage Becton Dickinson instrument by gating on a region where the fluorescence intensity of transduced cells stained for class I MHC overlapped with T cells stained with isotype-matched control antibody.
Generation of cytotoxic T cells and cytotoxicity assays.
The ability of cells expressing ICP47, US3, and/or US11 to present viral and transgene target antigens to CD8+ CTL was assayed using in vitro stimulation and cytotoxicity assays. Human fibroblasts, either parental or transduced with LICP47SN, LUS3SN, or LUS11SH alone or both LICP47SN and LUS11SH, were infected with HCMV for 12 h at a multiplicity of infection of 5:1 or mock infected and assessed as targets for a CD8+ CTL clone which is specific for the structural HCMV matrix protein pp65 and restricted by HLA-A24. The ability of HCMV-infected fibroblasts expressing ICP47 and US11 to stimulate HCMV-specific memory T-cell responses was assayed as described previously (48). Briefly, aliquots of responder PBMC derived from an HCMV-seropositive donor were cocultured with autologous HCMV-infected parental fibroblasts or HCMV-infected fibroblasts transduced to express the ICP47 and US11 genes. On day +7, cells from these cultures were assayed in a chromium release assay at various effector-to-target (E/T) ratios against HCMV-infected or mock-infected fibroblasts as described previously (48).
Recognition of fibroblasts and CD8+ T lymphocytes expressing the Hy transgene alone, Hy with US11, or Hy with both ICP47 and US11 was assayed by chromium release assay at various E/T ratios using an autologous CD8+ CTL clone specific for epitopes derived from Hy. In these experiments, T cells expressing ICP47 and US11 were used as target cells prior to cell sorting and after sorting into a class I MHC-low fraction.
Autologous T cell clones expressing Hy alone or Hy with ICP47 and US11 were also evaluated as stimulators to elicit a CD8+ Hy-specific cytotoxic T-cell response from PBMC derived from an HIV-infected patient who received immunotherapy with Hy-marked HIV-specific CD8+ T lymphocyte clones (47). Briefly, T cells transduced either with both LICP47SN and LUS11SH and sorted for low class I MHC expression or with LXSH were γ-irradiated (3,300 rad) and cocultured with PBMC at a responder-to-stimulator ratio of 2:1. After 7 days, the cultures were assayed at various E/T ratios in a chromium release assay for recognition of autologous parental T cells and T cells expressing Hy.
Recognition of parental and gene-modified CD8+ T cells by natural killer (NK) cells was assessed by using autologous NK cells obtained from one of the HIV-infected patients who received adoptive immunotherapy with HyTK-marked HIV Gag-specific CD8+ CTL. CD3− CD16+ CD56+ NK cells were enriched from PBMC preparations as described previously (39). Briefly, aliquots of PBMC were cocultured with γ-irradiated (3,000 rad) 721.221 cells at a ratio of 3:1. After 5 to 6 days, pure preparations of NK cells were obtained by depletion of contaminating T cells by using magnetic immunobeads precoated with specific MAb to CD3 and CD4 (Dynabeads; Dynal Inc., Lake Success, N.Y.) according to the manufacturer's directions. In some experiments, NK cells were directly isolated from PBMC by negative selection using Dynabeads precoated with specific MAb to CD3, CD14, or CD19. The purity of the NK cell population was confirmed by flow cytometry. NK cells were then assayed in a chromium release assay at various E/T ratios against T cells expressing ICP47 or US11 alone, both ICP47 and US11 either unsorted or sort-purified into a class I MHC-low population, T cells transduced to express Hy alone, parental T cells, and K562 cells.
Immunoprecipitations.
Equal numbers of cells either untransduced, transduced with LICP47SN, or infected with AdICP47-1 at 100 PFU/cell were preincubated for 2 h in methionine-free Dulbecco's modified Eagle medium (Sigma) followed by addition of [35S]methionine (150 μCi/ml) for 4 h. Cells were then lysed for 10 min in Nonidet P-40 lysis buffer (1% Nonidet P-40, 0.5% deoxycholic acid, 5 mg of bovine serum albumin per ml, 1 mM phenylmethylsulfonyl fluoride 1 mM EDTA, 1 mM EGTA, 0.1 trypsin inhibitor units of aprotinin per ml) on ice. Lysates were centrifugated at 1,200 rpm for 8 min and preabsorbed with normal rabbit serum (ImmunoPure; Pierce, Rockford, Ill.) and 25 μl of protein A-agarose beads (Santa Cruz Biotechnology, Santa Cruz, Calif.) for 1 h at 4°C. For immunoprecipitations, rabbit antisera specific for ICP47 and 20 μl of protein A agarose beads were added for at least 1 h at 4°C. Samples were washed four times with wash buffer and loaded for sodium dodecyl sulfate-polyacrylamide acrylamide gel electrophoresis (SDS-PAGE). Gels were analyzed by Coomassie blue staining and autoradiography using a Kodak X-Omat AR film.
RESULTS
Expression of ICP47, US11, or US3 in human fibroblasts decreases class I MHC expression.
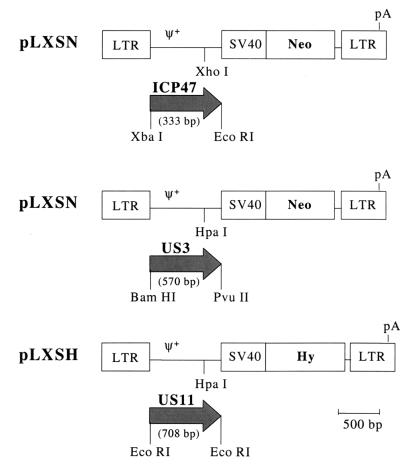
Three retroviral vectors in which the complete coding sequence of ICP47, US3, or US11 was inserted under the transcriptional control of the Moloney murine leukemia virus (Mo-MuLV) long terminal repeat (LTR) were constructed and packaged in PA317 or PG13 cells (Fig. 1).
FIG. 1.
Schematic representation of the Mo-MuLV-based retroviral vector constructs encoding viral immune evasion genes. Abbreviations: LTR, retroviral long terminal repeat; ψ+, extended packaging signal; SV40, SV40 early promoter and enhancers; Neo, bacterial neomycin phosphotransferase cDNA; Hy, hygromycin phosphotransferase cDNA; pA, polyadenylation site; ICP47, HSV ICP47 cDNA; US3, HCMV US3 cDNA; US11, HCMV US11 cDNA.
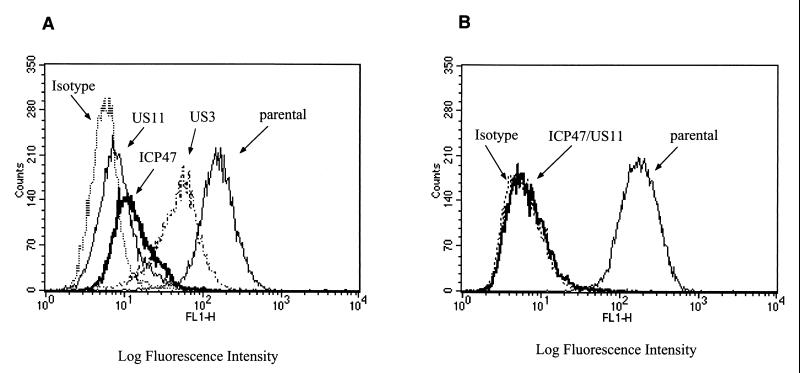
We assessed the expression and function of herpesvirus genes encoded by these vectors by transducing human fibroblasts and, after at least 14 days of drug selection, analyzing class I MHC surface expression, cell growth, and capacity to present antigen via the class I MHC pathway. Fibroblasts expressing ICP47, US11, or US3 alone exhibited a decrease of class I MHC expression, which was most marked for cells expressing US11 or ICP47 (Fig. 2A). When both ICP47 and US11 were expressed in fibroblasts, complete class I MHC downregulation was achieved, and this phenotype was stable on repeated evaluation over several weeks of culture (Fig. 2B). No decrease in class I MHC surface expression was observed in fibroblasts transduced with LXSH alone, and expression of herpesvirus genes was not detrimental to cell growth since gene-modified fibroblasts had in vitro proliferation comparable to that of parental fibroblasts (data not shown).
FIG. 2.
Efficient downregulation of class I MHC expression in human fibroblasts expressing ICP47, US3, and/or US11. (A) Aliquots of fibroblasts either untransduced or transduced with LICP47SN, LUS3SN, or LUS11SH alone were stained with the HLA class I-specific MAb W6/32 or isotype-matched control MAb and analyzed by flow cytometry. (B) Fibroblasts were either mock transduced or transduced with both LICP47SN and LUS11SH and stained as described above.
Human fibroblasts expressing ICP47 and US11 fail to present antigen to CD8+ HCMV-specific T cells.
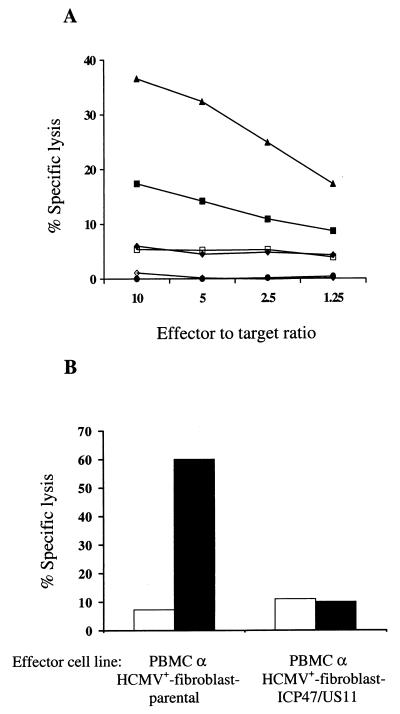
Viruses circumvent specific CTL responses by interfering with class I MHC presentation of viral antigens (29, 44). However, HCMV-seropositive individuals maintain strong HCMV-specific CTL responses which are predominantly specific for structural virion proteins such as pp65 that are processed and presented immediately after viral entry and prior to expression of US2, US3, US6, and US11 (40, 48, 60). To determine if the expression of either ICP47, US3, or US11 alone or both ICP47 and US11 prior to infection with HCMV could interfere with presentation of the structural HCMV matrix protein pp65 to CD8+ T cells, fibroblasts transduced with LICP47SN, LUS3SN, or LUS11SH alone or with both LICP47SN and LUS11SH, along with parental autologous and HLA-mismatched fibroblasts, were infected for 12 h with HCMV or left uninfected and assessed as targets for a CD8+ pp65-specific CTL clone in a chromium release assay. Twelve hours of infection was selected for these experiments because parental cells infected with HCMV for only 12 h exhibit no decrease of surface class I MHC expression (data not shown) and are effective targets for pp65-specific CD8+ CTL. Thus, the effect of the introduced ICP47 or US11 transgenes on T-cell recognition could be evaluated. Expression of ICP47, US3, or US11 inhibited lysis of HCMV-infected fibroblasts comparly to parental cells; however, the blockade in antigen presentation with any single gene was not complete. HCMV-infected fibroblasts which were rendered class I MHC negative by expression of both ICP47 and US11 were not recognized at all by the CD8+ CTL clone (Fig. 3A). Lysis of HCMV-infected HLA-mismatched fibroblasts did not differ from that of uninfected autologous cells (data not shown).
FIG. 3.
(A) HCMV-infected fibroblasts expressing both ICP47 and US11 are not recognized by HCMV-specific CD8+ T cells. HCMV-infected fibroblasts, either parental (▴) or transduced to express the ICP47 (⧫), US3 (■), or US11 (□) genes alone or both ICP47 and US11 (●) and mock-infected fibroblasts (◊), were assessed as targets for a pp65-specific CD8+ CTL clone in a chromium release assay. (B) HCMV-infected fibroblasts expressing ICP47 and US11 fail to stimulate HCMV-specific T-cell responses. Aliquots of PBMC derived from an HCMV-seropositive donor were cocultivated with autologous HCMV-infected fibroblasts, either parental or transduced to express the ICP47 and US11 genes. On day +7, the cultures were assayed for HCMV-specific CTL activity against autologous HCMV-infected (■) and mock-infected (□) parental fibroblasts in a chromium release assay. Data are shown for an E/T ratio of 10:1.
To determine if the blockade in antigen presentation induced by expression of both ICP47 and US11 was sufficient to prevent T-cell activation even after prolonged exposure between virus-infected cells and T cells, HCMV-infected fibroblasts expressing ICP47 and US11 were cocultured with autologous PBMC derived from an HCMV-seropositive donor (10, 48). After 7 days, strong HCMV-specific CTL responses were elicited in control cocultures of parental HCMV-infected fibroblasts with PBMC, whereas the HCMV-infected fibroblasts expressing ICP47 and US11 failed to elicit detectable CTL activity (Fig. 3B). These results indicated that fibroblasts transduced to express the ICP47 and US11 genes fail to present antigens to effector CD8+ CTL in short-term cytotoxic assays and fail to activate memory CTL responses in long-term cocultures in vitro.
Fibroblasts expressing Hy and both ICP47 and US11 fail to present the Hy transgene product to CD8+ CTL.
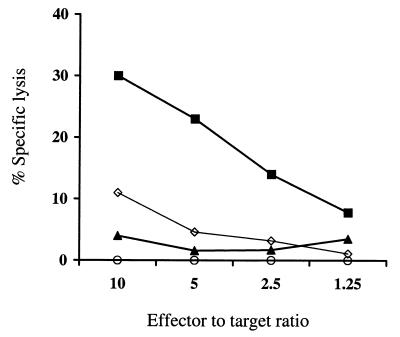
The expression of ICP47 and US11 effectively prevented the presentation of introduced viral proteins to CD8+ CTL, but it was anticipated that inhibition of CTL activation may be more difficult if a transgene was constitutively expressed. Thus, autologous fibroblasts expressing Hy alone, Hy and US11, or Hy and both US11 and ICP47 were evaluated as target cells for a CD8+ Hy-specific CTL clone derived from one of the patients treated with HyTK-marked HIV-specific CD8+ cytotoxic T cells (47). Control fibroblasts expressing Hy alone were lysed efficiently by the Hy-specific CTL clone, but fibroblasts expressing US11 and Hy were recognized poorly and only at high E/T ratios. In contrast, fibroblasts expressing both ICP47 and US11 with Hy were not lysed significantly at any E/T ratio, demonstrating that gene-modified cells could be protected from CTL attack even when the target antigen was constitutively expressed (Fig. 4).
FIG. 4.
Hy-expressing fibroblasts transduced with ICP47 and US11 are protected from CTL-mediated lysis. Fibroblasts expressing Hy alone (■), Hy and US11 (◊), or Hy and both US11 and ICP47 (▴) as well as parental fibroblasts (○) were evaluated as target cells for a CD8+ Hy-specific CTL clone derived from a patient sensitized to Hy after immunotherapy with HyTK-marked CD8+ HIV-specific CTL.
Expression and function of herpesvirus genes in primary human T cells.
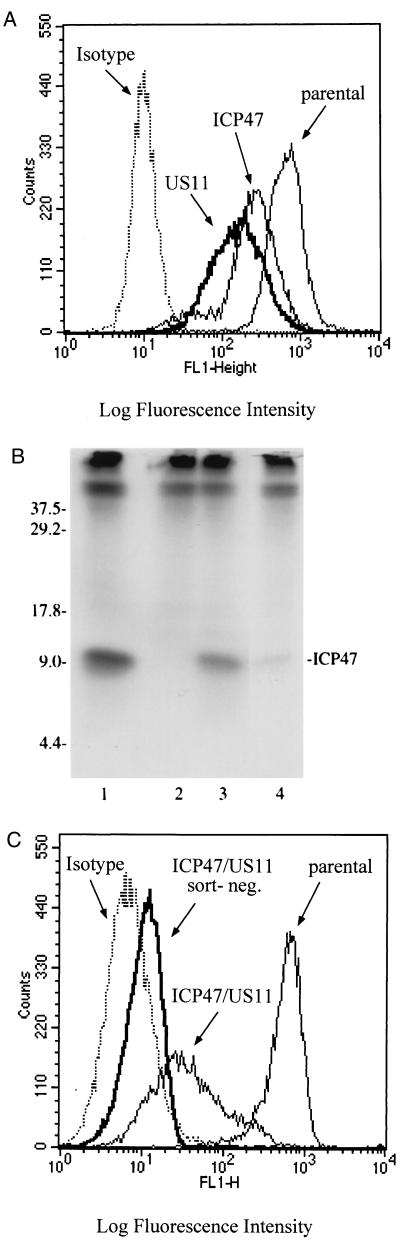
The successful use of herpesvirus genes to interfere with class I MHC presentation of viral and transgene proteins in fibroblasts suggested that this approach might also be applied to human T cells. To evaluate expression and function of herpesvirus genes in primary T lymphocytes, HIV Gag-specific CD8+ T-cell clones were transduced with LXSH, LICP47SN, and/or LUS11SH. The phenotype of transduced cells was assessed by monitoring both class I MHC and CD8 surface expression. T cells expressing either ICP47 or US11 alone exhibited a decrease of class I MHC expression, although the reduction of class I MHC was less than observed in fibroblasts (Fig. 5A). No changes in class I MHC expression was seen in T cells transduced with LXSH alone compared with parental T cells (data not shown). Transduced T cells grew efficiently in vitro, maintained a stable phenotype with regard to class I MHC and CD8 expression, respectively, and exhibited comparable levels of HIV-specific cytolytic function following multiple restimulations (data not shown).
FIG. 5.
(A) Downregulation of class I MHC expression in human T cells expressing ICP47 and/or US11. Aliquots of a CD8+ T-lymphocyte clone either untransduced or transduced with LICP47SN or LUS11SH were stained with the HLA class I-specific MAb W6/32 and isotype-matched control MAb on day 14 after stimulation. (B) Immunoprecipitation of ICP47 protein in T cells and fibroblasts. [35S]methionine-labeled lysates were immunoprecipitated with anti-ICP47 antisera and analyzed by SDS-PAGE. Transgene expression in LICP47SN-transduced T cells (lane 4) is compared to that for fibroblasts transduced with adenoviral vector AdICP47-1 (lane 1) or retroviral vector LICP47SN (lane 3) and untransduced control fibroblasts (lane 2). Sizes are indicated in kilodaltons. (C) Efficient downregulation of class I MHC in T cells expressing both ICP47 and US11. CD8+ T lymphocytes expressing ICP47 and US11 were stained on day 14 after stimulation with MAb W6/32 or isotype-matched control MAb, sort-purified for lowest levels of class I MHC expression, and analyzed by flow cytometry. Unsorted CD8+ T cells expressing ICP47 and US11 and parental CD8+ T cells were stained as described above and analyzed for surface class I MHC expression.
One potential mechanism for the inferior downregulation of class I MHC in T cells compared to fibroblasts was less efficient expression of the transgene by the retroviral LTR in T cells (1). The expression levels of ICP47 protein in fibroblasts and T cells were compared by radioimmunoprecipitation. ICP47 was detected in cell lysates of fibroblasts transduced with LICP47SN or infected with AdICP47-1, and in LICP47SN-transduced T cells, but transduced T cells exhibited a lower level of transgene expression (Fig. 5B).
To further decrease the level of cell surface class I MHC in transduced T cells, we examined the effect of expressing both ICP47 and US11. T cells expressing both ICP47 and US11 exhibited a much greater decrease in class I MHC surface expression, which allowed the easy purification of a T-cell population that was almost completely class I negative by flow cytometry (Fig. 5C).
T cells expressing both ICP47 and US11 are protected from CTL-mediated lysis.
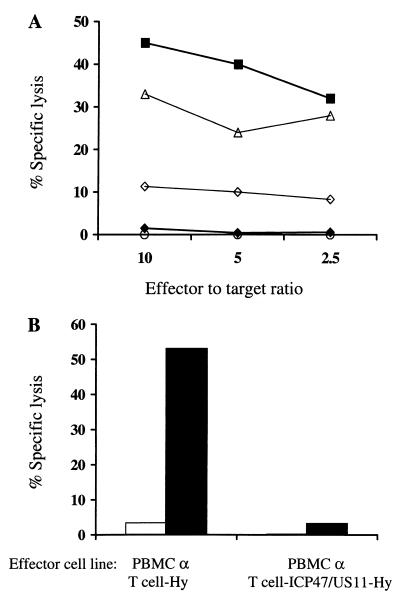
To determine whether T cells expressing ICP47 and US11 were protected from CTL-mediated lysis, CD8+ T lymphocytes expressing Hy alone, Hy and US11, or Hy and both ICP47 and US11, and untransduced controls were assayed for recognition by an autologous Hy-specific CD8+ CTL clone. Expression of US11 alone provided a slight protection from CTL lysis, but significantly better protection was provided by both ICP47 and US11 (Fig. 6A). Moreover, the subpopulation of T cells transduced with LICP47SN and LUS11SH and sorted for low levels of class I MHC expression were not lysed at all by the Hy-specific CTL clone.
FIG. 6.
(A) Class I MHC-low T cells expressing ICP47 and US11 are protected from CTL-mediated lysis. T cells derived from a patient sensitized to Hy after receiving adoptive immunotherapy with HyTK-marked CD8+ HIV-specific CTL were gene modified to express Hy alone (■), Hy and US11 (▵), or Hy and both ICP47 and US11 either unsorted (◊) or sort-purified class I MHC-low (⧫). These cells and parental T cells (○) were evaluated in a chromium release assay as target cells for a CD8+ Hy-specific CTL clone. (B) Class I MHC-low Hy-marked T cells expressing both ICP47 and US11 fail to stimulate Hy-specific memory T-cell responses. Responder PBMC derived from one of the patients immunized against Hy were cocultured for 7 days with irradiated autologous T cells expressing Hy alone or Hy and both ICP47 and US11 and then assayed at various E/T ratios in a chromium release assay for recognition of Hy-expressing T cells (■) and parental T cells (□). T cells expressing ICP47 and US11 fail to stimulate a CTL response to Hy after 7 days of in vitro culture. In contrast, a strong Hy-specific CTL response was elicited when responder PBMC were stimulated with Hy-transduced T cells. Data are shown for an E/T ratio of 5:1.
Hy-marked T cells coexpressing both ICP47 and US11 failed to stimulate Hy-specific T-cell responses in vitro.
The cytotoxicity assays involve a relatively short duration of exposure between antigen-presenting cell and the cytotoxic T cell and could underestimate the ability of gene-modified T cells to activate Hy-specific CD8+ CTL. Therefore, aliquots of responder PBMC derived from one of the patients who developed CD8+ CTL responses against Hy after receiving adoptive immunotherapy with HyTK-marked CD8+ HIV-specific T lymphocyte clones were cocultured for 7 days with γ-irradiated autologous T cells transduced with LXSH or transduced with LICP47SN and LUS11SH (47). The T cells expressing ICP47 and US11 were as resistant to Hm as those transduced with LXSH and were sorted by flow cytometry to obtain a population with low expression of class I MHC prior to use as stimulators. After 7 days, the cultures were assayed for recognition of autologous LXSH-transduced or parental T cells. Gene-modified T cells expressing Hy and both ICP47 and US11 failed to stimulate a CTL response to Hy. In contrast, a strong Hy-specific CTL response was elicited from the same responder PBMC stimulated with T cells transduced with LXSH only (Fig. 6B). Thus, expression of herpesvirus genes can inhibit antigen presentation of constitutively expressed transgene products in gene-modified T cells, suggesting that this might be a potential strategy to prolong the persistence of gene-modified cells in vivo.
CD8+ T lymphocytes expressing ICP47 and US11 exhibit increased susceptibility to NK cell lysis.
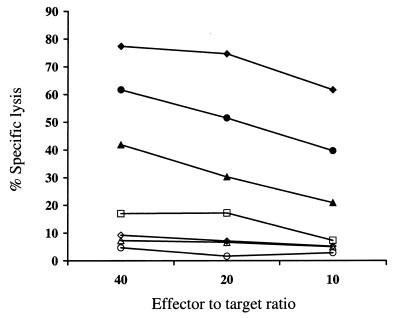
Cells deficient in class I MHC molecules would be less able to provide inhibitory signals to NK cells via the NK cell surface receptors that bind to class I MHC. Thus, attempts to downregulate class I MHC expression as a strategy to circumvent T-cell recognition of gene-modified cells might render the cells more susceptible to recognition by NK cells (32, 35). To evaluate whether T cells expressing ICP47 and/or US11 alone exhibited an increased susceptibility to NK cell-mediated lysis, CD8+ T lymphocytes transduced to express the ICP47 or US11 genes alone or both ICP47 and US11, either unsorted or sorted for low class I MHC expression, parental CD8+ T lymphocytes, T cells expressing Hy alone, and K562 cells were assayed as target cells for NK cells. T cells expressing both ICP47 and US11 exhibited an increased susceptibility to NK cell-mediated lysis compared to parental or Hy-transduced T lymphocytes, and this was most marked in T cells sorted for the lowest class I MHC expression (Fig. 7). T cells expressing either ICP47 or US11 alone and exhibiting a much less profound decrease in class I MHC expression were lysed less efficiently. These data show a correlation between the degree of class I MHC reduction and NK-mediated lysis and suggest that NK cell recognition may be a potential limitation of the expression of viral genes which downregulate class I MHC.
FIG. 7.
T cells expressing ICP47 and US11 exhibit an increased susceptibility to NK cell-mediated lysis. Autologous NK cells were assayed in a chromium release assay at various E/T ratios against T cells expressing ICP47 (◊) or US11 (□) alone or both ICP47 and US11, either unsorted (▴) or sort-purified into a class I MHC-low population (●), T cells expressing Hy alone (○), parental T cells (▵), and K562 cells (⧫).
DISCUSSION
A major obstacle to the in vivo persistence of gene-modified cells is the development of a host immune response to transgene or vector-encoded proteins (47, 62). Only limited success in facilitating long-term persistence of gene-modified cells in immunocompetent hosts has been achieved using immunosuppressive or immunomodulatory regimens (17, 18, 33, 61). Herpesviruses are remarkably successful in establishing persistent infections in immunocompetent hosts. A hallmark of most of the herpesviruses is the presence of genes which encode proteins that interfere with class I MHC antigen presentation, presumably to evade recognition by CD8+ CTL. In this study, we developed and evaluated retroviral vectors which encode herpesvirus genes that inhibit the class I MHC antigen processing pathway as a potential approach for reducing the immunogenicity of gene-modified cells.
Our studies using human fibroblasts and T cells demonstrate that expression of US11 or ICP47 and to a lesser extent US3 results in a decrease of class I MHC expression. The expression of a combination of ICP47 and US11 resulted in undetectable levels of class I MHC on fibroblasts and a large fraction of T cells. Our results indicate that the less efficient decrease in class I MHC expression in primary T cells compared with fibroblasts might be due to lower levels of transgene expression in T cells. This may in part reflect the lesser efficacy of the Mo-MuLV LTR promoter in T cells or, as indicated by recent studies, the activation dependence of the LTR in T cells (1, 45, 46). However, this could potentially be overcome by using alternative constitutively active promoters such as the T-cell-specific CD2 promoter or by the incorporation of the human beta interferon scaffold attachment region elements into the retroviral vector which have been proposed to facilitate the generation of open chromatin and allow access of transcription factors to neighboring enhancer-promoter elements and thus confer activation-independent transgene expression in primary T cells (1, 6, 8, 27, 28, 64). Newer-generation vectors encoding ICP47 or US11 are now being constructed to determine if a better reduction of class I MHC can be achieved in T cells.
Our experiments demonstrate that cells expressing both ICP47 and US11 have a profound defect in the ability to present antigens to primed CD8+ T cells. Importantly, we demonstrate that the expression of viral inhibitory genes can protect fibroblasts and T cells from CTL attack even if the antigen is a constitutively expressed transgene-encoded protein. However, complete downregulation of class I MHC expression was required for optimal protection from CTL recognition. Thus, fibroblasts and T cells expressing only a single immune evasion gene and with incomplete downmodulation of class I MHC expression were still targets for CTL. These findings are in line with previous reports demonstrating that as few as 1 to 100 MHC-peptide complexes can be sufficient to trigger T-cell activation (14, 21, 55). Thus, although expression of the ICP47 or US11 genes individually markedly decreased class I MHC expression, the critical threshold to protect from CTL-mediated lysis may have not been reached in all cells. There are potential disadvantages to express two immune evasion genes, including increased immunogenecity. Thus, it is possible that a single viral gene would be superior if improved transgene expression could be achieved.
The results of our in vitro experiments demonstrate that herpesvirus genes can protect gene-modified cells from recognition by CTL, but there are several factors that may limit the efficacy of this approach in vivo. First, there is evidence that the initial priming of CD8+ CTL in vivo involves presentation of antigens by dendritic cells which may take up apoptotic cells and process the intracellular protein for presentation to CD8+ CTL (4). This cross-priming might limit the capacity of viral inhibitory genes to prevent in vivo priming of CTL against gene-modified cells. However, our data suggest that gene-modified cells expressing ICP47 and US11 would still be poor targets for primed CTL and may persist despite the presence of CTL directed at the transgene product.
A second potential obstacle for this strategy in vivo might be recognition by NK cells which are normally inhibited by the expression of class I MHC (32, 35). Indeed, we observed an increased susceptibility of cells expressing both ICP47 and US11 to NK cell lysis in vitro which was most marked in cells expressing the lowest levels of class I MHC. These findings are in line with previous reports indicating that there is a correlation between the level of class I MHC expression and the susceptibility to NK cell attack (53). The development of strategies to prevent both NK cell- and CTL-mediated recognition might be difficult, but possible approaches are suggested by recent studies of the immunobiology of HIV and HCMV, respectively. The HIV Nef protein decreases expression of HLA-A and -B and interferes with CTL recognition, but it does not significantly affect HLA-C or -E (15, 16). NK cells express inhibitory receptors that are specific for HLA-C and HLA-E, and cells expressing these MHC molecules are protected from NK attack (35, 36). Thus, expressing HIV Nef may prevent CTL-mediated lysis without increasing the susceptibility to NK cell recognition. Allelic preferences have also been described for HCMV US2 and US11. Schust et al. demonstrated that in human trophoblast cells, HLA-C and -G were resistant to the effects of HCMV US11 and US2, suggesting that preserved expression of these molecules on HCMV-infected cells could block NK recognition (52). Our data for primary T cells demonstrated that these cells exhibited an increased susceptibility to NK cell lysis. However, we did not examine the expression of HCMV US2, and it is possible that expression of this gene would maintain resistance to NK cell recognition.
In conclusion, we have shown that the constitutive expression of the herpesvirus genes ICP47 and US11 inhibits the class I MHC presentation of antigenic epitopes derived from viral proteins and transgene products expressed in gene-modified cells and that these cells are protected from CTL-mediated lysis and fail to stimulate CTL in vitro. Unfortunately, murine models cannot be used to evaluate the efficacy of this approach for improving persistence of gene-modified cells in vivo since ICP47 does not interfere with peptide transport in rodent cells and US11 induces degradation of only a subset of murine class I MHC molecules (38, 56, 63). However, both ICP47 and US11 decrease class I MHC expression in nonhuman primates, and evaluation of this approach in vivo in nonhuman primates may provide insights into the efficacy and potential limitations of this strategy for prolonging transgene expression (44; C. Berger, unpublished data).
ACKNOWLEDGMENTS
C.B. is supported by the Deutsche Krebshilfe, Dr. Mildred-Scheel-Stiftung für Krebsforschung. This work was supported by National Institutes of Health grants AI43650 (S.R.R.), AI41754 (S.R.R.), CA18029 (S.R.R.), and EY11245 (D.C.J.).
REFERENCES
- 1.Agarwal M, Austin T W, Morel F, Chen J, Böhnlein E, Plavec I. Scaffold attachment region-mediated enhancement of retroviral vector expression in primary T cells. J Virol. 1998;72:3720–3728. doi: 10.1128/jvi.72.5.3720-3728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn K, Angulo A, Ghazal P, Peterson P A, Yang Y, Früh K. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc Natl Acad Sci USA. 1996;93:10990–10995. doi: 10.1073/pnas.93.20.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn K, Gruhler A, Galocha B, Jones T R, Wiertz E J H J, Ploegh H L, Peterson P A, Yang Y, Früh K. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 4.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 5.Anderson W F. Human gene therapy. Science. 1992;256:808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- 6.Auten J, Agarwal M, Chen J, Sutton R, Plavec I. Effect of scaffold attachment region on transgene expression in retrovirus vector-transduced primary T cells and macrophages. Hum Gene Ther. 1999;10:1389–1399. doi: 10.1089/10430349950018058. [DOI] [PubMed] [Google Scholar]
- 7.Blaese R M, Culver K W, Miller D A, Carter C S, Fleisher T, Clerici M, Shearer J, Chang L, Chiang Y, Tolstoshev P, Greenblatt J J, Rosenberg S A, Klein H, Berger M, Mullen C A, Ramsey W J, Muul L, Morgan R A, Anderson W F. T lymphocyte-directed gene therapy for ADA− SCID: inital trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 8.Bode J, Schlake T, Ríos-Ramírez M, Mielke C, Stengert M, Kay V, Klehr-Wirth D. Scaffold/matrix-attached regions: structural properties creating transcriptionally active loci. In: Berezney R, Jeon K W, editors. Structural and functional organization of the nuclear matrix. San Diego, Calif: Academic Press; 1995. pp. 389–454. [Google Scholar]
- 9.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, Bordignon C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 10.Borysiewicz L K, Morris S, Page J D, Sissons J G P. Human cytomegalovirus-specific cytotoxic T lymphocytes: requirements for in vitro generation and specificity. Eur J Immunol. 1983;13:804–809. doi: 10.1002/eji.1830131005. [DOI] [PubMed] [Google Scholar]
- 11.Brenner M K, Rill D R, Holladay M S, Heslop H E, Moen R C, Buschle M, Krance R A, Santana V M, Anderson W F, Ihle J N. Gene marking to determine whether autologous marrow infusion restores long-term haemopoiesis in cancer patients. Lancet. 1993;342:1134–1137. doi: 10.1016/0140-6736(93)92122-a. [DOI] [PubMed] [Google Scholar]
- 12.Brenner M K, Rill D R, Moen R C, Krance R A, Mirro J, Jr, Anderson W F, Ihle J N. Gene-marking to trace origin of relapse after autologous bone-marrow transplantation. Lancet. 1993;341:85–86. doi: 10.1016/0140-6736(93)92560-g. [DOI] [PubMed] [Google Scholar]
- 13.Brodie S J, Lewinsohn D A, Patterson B K, Jiyamapa D, Krieger J, Corey L, Greenberg P D, Riddell S R. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med. 1999;5:34–41. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- 14.Christinck E R, Luscher M A, Barber B H, Williams D B. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature. 1991;352:67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- 15.Cohen G B, Gandhi R T, Davis D M, Mandelboim O, Chen B K, Strominger J L, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 16.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:401–404. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 17.Dai Y, Schwarz E M, Gu D, Zhang W-W, Sarvetnick N, Verma I M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelhardt J F, Ye X, Doranz B, Wilson J M. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frueh K, Ahn K, Djaballah H, Sempé P, van Endert P M, Tampé R, Peterson P A, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 20.Hanania E G, Kavanagh J, Hortobagyi G, Giles R E, Champlin R, Deisseroth A B. Recent advances in the application of gene therapy to human disease. Am J Med. 1995;99:537–552. doi: 10.1016/s0002-9343(99)80232-0. [DOI] [PubMed] [Google Scholar]
- 21.Harding C V, Unanue E R. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990;346:574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- 22.Heemels M-T, Ploegh H. Generation, translocation, and presentation of MHC class I-restricted peptides. Annu Rev Biochem. 1995;64:463–491. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 23.Heim D E, Hanazono Y, Childs R, Metzger M, Donahue R E, Dunbar C E. In vivo persistence of rhesus monkey lymphocytes transduced with a non-expressing retroviral vector compared to rapid clearance of lymphocytes transduced with a Neo-expressing vector. Blood. 1998;92:688a. [Google Scholar]
- 24.Hengel H, Koopmann J-O, Flohr T, Muranyi W, Goulmy E, Hämmerling G J, Koszinowski U H, Momburg F. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity. 1997;6:623–632. doi: 10.1016/s1074-7613(00)80350-7. [DOI] [PubMed] [Google Scholar]
- 25.Heslop H E, Ng C Y C, Li C, Smith C A, Loftin S K, Krance R A, Brenner M K, Rooney C M. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 26.Hill A B, Jugovic P, York I A, Russ G, Bennink J, Yewdell J, Ploegh H L, Johnson D C. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 27.Jacob J, Baltimore D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature. 1999;399:593–597. doi: 10.1038/21208. [DOI] [PubMed] [Google Scholar]
- 28.Jenuwein T, Forrester W G, Fernández-Herrero L A, Laible G, Dull M, Grosschedl R. Extension of chromatin accessibility by nuclear matrix attachment regions. Nature. 1997;385:269–272. doi: 10.1038/385269a0. [DOI] [PubMed] [Google Scholar]
- 29.Johnson D C, Hill A B. Herpesvirus evasion of the immune system. Curr Top Microbiol Immunol. 1998;232:149–177. doi: 10.1007/978-3-642-72045-1_8. [DOI] [PubMed] [Google Scholar]
- 30.Jones T R, Hanson L K, Sun L, Slater J S, Stenberg R M, Campbell A E. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J Virol. 1995;69:4830–4841. doi: 10.1128/jvi.69.8.4830-4841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones T R, Wiertz E J H J, Sun L, Fish K N, Nelson J A, Ploegh H L. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc Natl Acad Sci USA. 1996;93:11327–11333. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaerre K. How to recognize a foreign submarine. Immunol Rev. 1997;155:5–9. doi: 10.1111/j.1600-065x.1997.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 33.Kay M A, Meuse L, Gown A M, Linsley P, Hollenbaugh D, Aruffo A, Ochs H D, Wilson C B. Transient immunomodulation with anti-CD40 ligand antibody and CTLA41g enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohn D B, Weinberg K I, Nolta J A, Heiss L N, Lenarsky C, Crooks G M, Hanley M E, Annett G, Brooks J S, El-Khoueiry A, Lawrence K, Wells S, Moen R C, Bastian J, Williams-Herman D E, Elder M, Wara D, Bowen T, Hershfield M S, Mullen C A, Blaese R M, Parkman R. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat Med. 1995;1:1017–1023. doi: 10.1038/nm1095-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanier L L, Corliss B, Phillips J H. Arousal and inhibition of human NK cells. Immunol Rev. 1997;155:145–154. doi: 10.1111/j.1600-065x.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee N, Llano M, Carretero M, Ishitani A, Navarro F, López-Botet M, Geraghty D E. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci USA. 1998;95:5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lutzko C, Kruth S, Abrams-Ogg A C G, Lau K, Li L, Clark B R, Ruedy C, Nanji S, Foster R, Kohn D, Shull R, Dubé I D. Genetically corrected autologous stem cells engraft, but host immune responses limit their utility in canine α-l-iduronidase deficiency. Blood. 1999;93:1895–1905. [PubMed] [Google Scholar]
- 38.Machold R P, Wiertz E J H J, Jones T R, Ploegh H L. The HCMV gene products US11 and US2 differ in their ability to attack allelic forms of murine major histocompatibility complex (MHC) class I heavy chains. J Exp Med. 1997;185:363–366. doi: 10.1084/jem.185.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandelboim O, Reyburn H T, Valés-Goméz M, Pazmany L, Colonna M, Borsellino G, Strominger J L. Protection from lysis by natural killer cells of group 1 and 2 specificity is mediated by residue 80 in human histocompatibility leukocyte antigen C alleles and also occurs with empty major histocompatibility complex molecules. J Exp Med. 1996;184:913–922. doi: 10.1084/jem.184.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLaughlin-Taylor E, Pande H, Forman S J, Tanamachi B, Li C-R, Zaia J A, Greenberg P D, Riddell S R. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J Med Virol. 1994;43:103–110. doi: 10.1002/jmv.1890430119. [DOI] [PubMed] [Google Scholar]
- 41.Miller A D, Garcia J V, von Suhr N, Lynch C N, Wilson C, von Eiden M. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–988. [PMC free article] [PubMed] [Google Scholar]
- 43.Parham P, Barnstable C J, Bodmer W F. Use of monoclonal antibody (W6/32) in structural studies of HLA-A,B,C antigens. J Immunol. 1979;123:342–349. [PubMed] [Google Scholar]
- 44.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 45.Pollok K E, Hanenberg H, Noblitt T W, Schroeder W, Kato I, Emanuel D, Williams D A. High-efficiency gene transfer into normal and adenosine deaminase-deficient T lymphocytes is mediated by transduction on recombinant fibronectin fragments. J Virol. 1998;72:4882–4892. doi: 10.1128/jvi.72.6.4882-4892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quinn E R, Lum L G, Trevor K T. T cell activation modulates retrovirus-mediated gene expression. Hum Gene Ther. 1998;9:1457–1467. doi: 10.1089/hum.1998.9.10-1457. [DOI] [PubMed] [Google Scholar]
- 47.Riddell S R, Elliott M, Lewinsohn D A, Gilbert M J, Wilson L, Manley S A, Lupton S D, Overell R W, Reynolds T C, Corey L, Greenberg P D. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–223. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 48.Riddell S R, Rabin M, Geballe A P, Britt W J, Greenberg P D. Class I MHC-restricted cytotoxic T lymphocyte recognition of cells infected with human cytomegalovirus does not require endogenous viral gene expression. J Immunol. 1991;146:2795–2804. [PubMed] [Google Scholar]
- 49.Rooney C M, Smith C A, Ng C Y C, Loftin S K, Li C, Krance R A, Brenner M K, Heslop H E. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg S A. The immunotherapy and gene therapy of cancer. J Clin Oncol. 1992;10:180–199. doi: 10.1200/JCO.1992.10.2.180. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 52.Schust D J, Tortorella D, Seebach J, Phan C, Ploegh H L. Trophoblast class I major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11. J Exp Med. 1998;188:497–503. doi: 10.1084/jem.188.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimizu Y, DeMars R. Demonstration by class I gene transfer that reduced susceptibility of human cells to natural killer cell-mediated lysis is inversely correlated with HLA class I antigen expression. Eur J Immunol. 1989;19:447–451. doi: 10.1002/eji.1830190306. [DOI] [PubMed] [Google Scholar]
- 54.Shimizu Y, DeMars R. Production of human cells expressing individual transferred HLA-A, -B, -C genes using an HLA-A, -B, -C null human cell line. J Immunol. 1989;142:3320–3328. [PubMed] [Google Scholar]
- 55.Sykulev Y, Joo M, Vturina I, Tsomides T J, Eisen H N. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 56.Tomazin R, Hill A B, Jugovic P, York I, van Endert P, Ploegh H L, Andrews D W, Johnson D C. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–3266. [PMC free article] [PubMed] [Google Scholar]
- 57.Walker R E, Carter C S, Muul L, Natarajan V, Herpin B R, Leitman S F, Klein H G, Mullen C A, Metcalf J A, Baseler M, Falloon J, Davey Jr R T, Kovacs J A, Polis M A, Masur H, Blaese R M, Lane H C. Peripheral expansion of pre-existing mature T cells is an important means of CD4+ T-cell regeneration HIV-infected adults. Nat Med. 1998;4:852–856. doi: 10.1038/nm0798-852. [DOI] [PubMed] [Google Scholar]
- 58.Wiertz E J H J, Jones T R, Lei S, Bogyo M, Geuze H J, Ploegh H L. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmatic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 59.Wiertz E J H J, Tortorella D, Bogyo M, Yu J, Mothes W, Jones T R, Rapoport T A, Ploegh H L. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 60.Wills M R, Carmichael A J, Mynard K, Jin X, Weekes M P, Plachter B, Sissons J G P. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson C B, Embree L J, Schowalter D, Albert R, Aruffo A, Hollenbaugh D, Linsley P, Kay M A. Transient inhibition of CD28 and CD40 ligand interactions prolongs adenovirus-mediated transgene expression in the lung and facilitates expression after secondary vector administration. J Virol. 1998;72:7542–7550. doi: 10.1128/jvi.72.9.7542-7550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y, Li Q, Ertl H C J, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T-lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 64.Zhumabekov T, Corbella P, Tolaini M, Kioussis D. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J Immunol Methods. 1995;185:133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]