Abstract
Background
Dietary choices can affect human health through alterations in gut microbial metabolism, and gut microbial metabolites could serve as biomarkers for disease risk conferred by dietary intake. However, self-reported dietary intake may not reflect true intake.
Objectives
We identified circulating metabolites, including gut microbiome-related metabolites, associated with adherence to a healthy diet in the ChooseWell 365 study. In this randomized clinical trial, the dietary choices of hospital employees were assessed over 24 mo using not only 24-h dietary recalls but also electronic records of hospital cafeteria purchases.
Methods
Plasma metabolites were profiled from 470 participants. Two targeted metabolomics methods were developed and implemented to expand detection coverage for metabolites related to gut microbial activity. Linear regression models were used to associate metabolites with Healthy Purchasing Scores (HPSs) derived from cafeteria purchases and Healthy Eating Index-2015 (HEI-15) scores derived from dietary recalls.
Results
Fourteen metabolites were concordantly associated with the HPS and HEI-15 scores in multivariable models adjusted for age, gender, and race, including the gut microbiome-related metabolites indole-3-propionic acid (HPS, β: 0.16, 95% CI: 0.07, 0.26, P = 7.32 × 10−4; HEI-15, β: 0.16, 95% CI: 0.07, 0.25, P = 6.79 × 10−4), hippuric acid (HPS, β: 0.11, 95% CI: 0.02, 0.21, P = 1.97 × 10−2; HEI-15, β: 0.10, 95% CI: 0.01, 0.19, P = 3.14 × 10−2), and indoxyl sulfate (HPS, β = −0.13, 95% CI: −0.23, −0.03, P = 8.21 × 10−3; HEI-15, β: −0.12, 95% CI: −0.22, −0.03, P = 8.50 × 10−3). These gut microbial metabolites were associated with the intake of specific food groups, such as whole fruits. These metabolites were also associated with clinical variables, including blood pressure, diabetes or prediabetes, and body mass index.
Conclusions
In a secondary analysis of the ChooseWell 365 study, associations between circulating gut microbiome–related metabolites and a healthy diet were confirmed using both objective and subjective measures of consumption. Accurate identification of diet-associated metabolites may help guide dietary or microbiome-based interventions aimed at disease prevention.
Keywords: ChooseWell 365, dietary recall, gut microbiome, Healthy Eating Index, metabolomics, purchasing data
Introduction
Diet is a major modifiable risk factor in the development of common chronic diseases [[1], [2], [3]]. Indices that consolidate intake of individual items into dietary patterns, such as the Healthy Eating Index-2015 (HEI-15) [4], have been associated with altered risk of cardiovascular disease and other illnesses [[5], [6], [7]]. Diet can affect host disease states through the direct absorption of nutrients and non-nutrients that impart biological activity and indirectly through alterations in the gut microbiome, which is increasingly understood to play an important role in human health [[8], [9.], [10]]. Diet plays a critical role in the function of the gut microbiome, and alterations in diet can induce shifts in the composition of gut microbiota [[11], [12], [13], [14]]. Metabolism of diet-derived compounds by intestinal microorganisms generates metabolites that are released locally and into circulation, which have been linked to host disease states such as immune system homeostasis [8], cardiovascular disease [9], and neurodegenerative disorders [10].
As dietary patterns are associated with alterations in the circulating metabolome, previous studies have investigated whether metabolomic signatures can serve as reliable readouts of dietary intake [15]. The discovery of surrogate markers that could guide dietary interventions would facilitate the development of personalized targets for disease prevention. Furthermore, because metabolites can integrate activity in biological pathways modulated by dietary intake [16], these markers can shed light on underlying mechanisms by which diet patterns affect disease risk.
In most observational studies, dietary patterns are derived using predetermined scoring systems or data-driven methods based on self-reported data from questionnaires [17]. However, self-reported dietary intake is subject to limitations, including selective energy underreporting [18]. Given these limitations, metabolomic markers of dietary intake have primarily been identified and validated in small interventional studies, with limited replication in larger observational cohorts [15,19,20]. Nonetheless, markers from feeding studies may not be reliably associated with usual food intake in community-dwelling populations. This can occur with metabolites that have shorter half-lives or that are associated with less-frequently consumed foods [21]. Discordance between metabolites associated with dietary intake in feeding studies and community-dwelling populations has been reported previously [22].
In this study, we sought to link plasma metabolites to dietary intake measured using objective food purchasing data as well as dietary recall instruments. We studied the ChooseWell 365 randomized clinical trial, in which the dietary choices of hospital employees were tracked with both 24-h dietary recalls and electronic records of hospital cafeteria purchases over 24 mo [23]. The ChooseWell trial thus represents a unique source of matched data on subjective and objective measures of dietary habits. We implemented a metabolomics method [24] to target metabolites related to gut microbiome activity (e.g., short-chain fatty acids, odd-chain fatty acids, indoles, and other aromatic amino acid derivatives). To our knowledge, this is the first study to apply a targeted gut microbiome metabolomics technique to identify metabolites associated with dietary intake. Our findings highlight associations between gut microbiome-related metabolites and dietary patterns that were validated using both subjective dietary recalls and objective food purchasing data.
Methods
Study population and design
The methods and study design of the ChooseWell 365 trial have been described in detail elsewhere [23]. The ChooseWell study was an individual-level randomized clinical trial to test an automated behavioral intervention among employees of Massachusetts General Hospital (Boston, Massachusetts) who purchased food at on-site cafeterias that displayed traffic light labels for menu items (i.e., green labels indicating healthy purchases; yellow, less healthy; red, unhealthy). A total of 602 employees were enrolled from September 2016 to February 2018. Employees were eligible for the study if they were between 20 and 75 y of age and used their hospital badge for cafeteria purchases on the campus ≥4 times per week for ≥6 wk for 12 wk before enrollment. Exclusion criteria included a plan to leave employment in the next year; current pregnancy; the desire to gain weight; history of an eating disorder; weight loss surgery in the prior 6 mo or planned in the upcoming year; current enrollment in a weight loss program; and working in the cafeteria or clinical trial facility where study visits took place [23]. The ChooseWell study protocol was approved by the institutional review board at Mass General Brigham, and all participants provided written informed consent.
The randomized trial included a 12-mo intervention period with 12 mo of additional follow-up. During the intervention, participants in the treatment group received 2 automatically generated emails per week with feedback on previous cafeteria purchases and personalized health and lifestyle tips, along with 1 letter per month with peer comparisons and modest financial incentives for healthier purchases. Control group participants received 1 letter per month with general healthy lifestyle information. Outcomes assessed in the trial included change in weight from baseline, along with shifts over time in the healthfulness of cafeteria purchases and HEI-15 scores obtained from 24-h dietary recalls [23].
Dietary and clinical variables
At baseline, participants completed a survey in which they provided information about their demographic characteristics (e.g., age, race, and gender) and past medical history. For self-reported race, participants could choose from the following categories: “White,” “Asian,” “Black,” “Native Hawaiian/Pacific Islander,” “More than one race,” or “Prefer not to answer.” A baseline physical examination included the measurement of each participant’s weight, height, and blood pressure [23]. The mean arterial pressure was estimated from systolic blood pressure (SBP) and diastolic blood pressure (DBP) using a standard formula: DBP + 1/3(SBP – DBP). Participants were also categorized as having hypertension, hyperlipidemia, and diabetes, or prediabetes. Hypertension was defined as 1) a self-reported hypertension/high blood pressure diagnosis, 2) a self-reported prescription for antihypertensive medications, or 3) SBP ≥ 150 mmHg or DBP ≥ 90 mmHg. Diabetes/prediabetes was defined as 1) a self-reported diabetes or prediabetes diagnosis, 2) a self-reported prescription medication for diabetes, or 3) HbA1c ≥ 5.7%. Hyperlipidemia was defined as 1) a self-reported high cholesterol/hyperlipidemia diagnosis, 2) a self-reported prescription medication for high cholesterol, or 3) a fasting total cholesterol ≥ 220 mg/dL, LDL ≥ 160 mg/dL, or triglycerides ≥ 180 mg/dL [25].
The traffic light labeling system was developed by hospital dietitians based on the United States Department of Agriculture guidelines and has been described in detail elsewhere [[26], [27], [28]]. Red, yellow, and green labels were assigned to food and beverage items based on an algorithm that factored in calories, saturated fat, and nutrient density. A green rating indicates the highest level of healthfulness and a red rating is the lowest. Traffic light labels were readily visible on menu boards, shelf labels, and directly on packages for prepared foods. To summarize workplace food purchases into a single metric, a Healthy Purchasing Score (HPS) [25] was derived for each participant over the 24-mo duration of the study by weighting purchases of red items to be 0, purchases of yellow items to be 0.5, and purchases of green items to be 1. This weighted average was converted to a percentage by multiplying by 100 (range, 0%–100% healthy). A higher HPS has previously been shown to negatively correlate with the prevalence of multiple cardiometabolic risk factors [25].
The HEI-15 measures overall dietary quality, consistent with the United States Department of Agriculture guidelines, and possible scores similarly range from 0 (least healthy) to 100 (healthiest) [4]. For most participants, HEI-15 scores were calculated based on 2 automated self-administered 24-h (ASA24) dietary recalls completed at baseline and each follow-up time point (6, 12, and 24 mo) [23]. ASA24 is a free Web-based tool for dietary intake assessment developed by the National Cancer Institute [29]. If a participant did not complete a second ASA24, the HEI-15 score was calculated based on one ASA24 assessment. Because plasma samples were collected at the 24-mo follow-up time point, HEI-15 scores were derived from ASA24 dietary recalls completed at 24 mo.
Targeted metabolomics analyses
Targeted metabolomics analyses were performed on plasma samples collected from ChooseWell participants at the 24-mo follow-up time point [23]. Plasma was obtained from fasting morning blood samples. Targeted metabolomics analyses were performed using liquid chromatography triple quadrupole mass spectrometry (LC-QQQ). Derivatization-free and derivatization-based metabolomics methods were implemented to expand the detection coverage. Standards were purchased from several sources, including Sigma–Aldrich, Cayman Chemical, Thermo-Fisher Scientific and VWR. Detection parameters and a complete list of reference standards and sources for each metabolite are shown in Supplemental Table 1. All plasma samples were stored at −80°C until analysis and sample preparation procedures were carried out over ice. Samples went through 2 freeze–thaw cycles; subaliquoting for both metabolomics methods was conducted at the time of the second thaw.
Derivatization-free approach
Protein precipitation was carried out to extract polar metabolites as previously described [30,31]. Briefly, 30 μL of K2EDTA plasma was mixed with 30 μL of internal solution mixture containing proline (13C5, 15N), glutamine (13C5, 15N2), deuterated leucine-d10, and phenylalanine-d8 [15 μM each in methanol:water (50:50)]. Proteins were precipitated with 110 μL of ice-cold acetonitrile:methanol (75:25), and samples were vortexed for 5 min at 1400 × g. Samples were centrifuged at 4000 × g for 10 min at 4°C, and 100 μL of the clean supernatant was transferred to clean 96-well plates for injection. Human pooled plasma (HPP) samples purchased from BioIVT were also extracted and injected after every 10 study samples (for normalization) and after every 20 study samples (for quality control assessments) using previously described approaches [[30], [31], [32]]. Briefly, the nearest pooled plasma normalization approach involves the normalization of metabolite signals based on the average of the 2 closest normalization HPP samples; values for each metabolite therefore represent area–ratio values rather than concentrations. To assess precision, the coefficient of variation (%CV) for each metabolite was calculated using the area–ratio values of quality control HPP samples. As multiple individual HPP aliquots were prepared, %CV values captured the variability of the extraction protocols and instrumentation.
The extracted metabolites were separated on Xbridge Amide columns (2.1 × 100 mm; 3.5 μm; Waters) using dual Infinity II 1290 HLPC pumps and a 6495 QQQ tandem mass spectrometer (Agilent). The QQQ was optimized for the detection of 162 compounds using scheduled multiple reaction monitoring (MRM) events. Metabolites in well-known and biologically important pathways were targeted. Mobile phase A was 20 mM ammonium acetate + 20 mM ammonium hydroxide (aq) in acetonitrile (95:5); mobile phase B was acetonitrile. The gradient on the analytical pump was 90% B to 35% over 6 min and 2% B over 0.5 min, held for 1 min. The column was maintained at 30°C. The flow rate was 0.25 mL/min. The autosampler was kept at 5°C, and samples were analyzed on the same day as extraction. Each sample was injected twice to obtain data in positive and negative electrospray ionization (ESI) mode using dynamic MRM events. Metabolite peaks were integrated and reviewed using Mass Hunter QQQ Quantitative Analysis software (Agilent). Across all metabolites profiled using a derivatization-free approach, the %CV for interassay variability had a median (IQR) of 8.1% (5.1) (Supplemental Table 1).
Derivatization-based approach
A derivatization-based method was developed to capture additional gut microbiome–associated metabolites. Samples were derivatized with 3-nitrophenylhydrazine (3-NPH) following previously published methods with modifications [24,33]. Derivatization approaches using 3-NPH have been demonstrated to be effective in improving sensitivity and coverage for numerous compounds, including gut microbiota–derived metabolites [[34], [35], [36]]. The derivatization reagents, 3-NPH, coupling reagent N-(3-(dimethylamino)propyl)-N′-ethyl carbodiimide, and pyridine were purchased from Sigma–Aldrich.
Briefly, 15 μL aliquots of plasma were used. Metabolite quenching was carried out with the addition of 45 μL propionic acid-d6 (100 μM in acetonitrile), and samples were vortex mixed for 5 min at 1400 × g. Each sample first received 10 μL of 3-NPH HCl [200 mM in acetonitrile:water (50:50)] and was vortex mixed for 1 min at 1400 × g. Subsequently, 10 μL of 3-NPH, coupling reagent N-(3-(dimethylamino)propyl)-N′-ethyl carbodiimide HCl [120 mM in 6% pyridine acetonitrile:water (50:50)] was added, and each sample was vortex mixed for a further 1 min at 1400 × g. Derivatization was carried out for 30 min at 40°C. Samples were centrifuged at ∼4000 × g for 5 min at 4°C to remove reagent condensation from sealing mats. After centrifugation, 195 μL of ice-cold water was added, and samples were vortex mixed for 5 min at 1400 × g. Plates were centrifuged at ∼4000 × g for an additional 10 min at 4°C, and 120 μL of the supernatant was transferred to a clean 96-well plate for LC-QQQ analysis.
The LC-QQQ setup consisted of a 1290 UHPLC system coupled with a 6490 triple quadrupole mass spectrometer (Agilent). Scheduled MRM events were optimized for 77 metabolites using negative ESI mode. Separation was achieved on ACE Excel C18-AR column (150 mm × 2.1 mm ID; 2 μm; MAC-MOD Analytical). Mobile phase A was water, and mobile phase B was acetonitrile. The column was maintained at 45°C. The flow rate was 0.35 mL/min, and the sample injection volume was 10 μL. The initial gradient conditions were 20% B held over 3 min; B was increased to 99% over 6 min and held for 4 min. Finally, the mobile phase composition was returned to the initial conditions, and the column was re-equilibrated over 2 min. The total run time was 15 min, and the overall cycle time was 17 min. Across all metabolites profiled using a derivatization-based approach, the %CV for interassay variability had a median (IQR) of 7.7% (5.2) (Supplemental Table 1).
Statistical methods
Statistical analyses and the generation of figure panels were performed in R (R Project for Statistical Computing, version 4.2). In all statistical analyses, the Benjamini–Hochberg procedure was used to control the false discovery rate (FDR).
Consistent with previous studies, the data for each metabolite underwent rank-based inverse normal transformation before analyses due to the skewed distribution of metabolite levels [37,38]. For inverse normal transformations, sample measurements were mapped to the probability scale by replacing the observed values with fractional ranks and then transformed into z-scores. Values for the HPS and HEI-15 scores were also z-scored before regression analyses to enable the calculation of standardized (β) coefficients.
Multivariable linear regression models were used to identify associations between normalized values for metabolites and the HPS or HEI-15 scores after adjustment for demographic characteristics. These demographic characteristics were age in years, gender (either male, female, or not reported), and race (see categories listed above). Metabolites with statistically significant (FDR < 0.10) associations with the HPS and that were nominally associated (unadjusted P < 0.05) with HEI-15 scores in linear regressions (diet-associated metabolites) were carried forward in subsequent analyses. These analyses included the identification of associations between diet-associated metabolites and both individual HEI-15 categories and clinical variables using similar multivariable linear regressions. In all regression analyses, metabolite levels were treated as dependent variables, and dietary scores or clinical variables were treated as independent variables. The threshold for statistical significance was set as FDR< 0.10 for identifying diet-associated metabolites and for all subsequent analyses. When applicable, listwise deletion was used to account for missing data in multivariable models.
Results
Study cohort
Plasma samples were obtained from hospital employees enrolled in the control and intervention groups of the ChooseWell study (n = 470) who provided plasma samples at the 24-mo follow-up time point [23] (Supplemental Figure 1). Characteristics of participants with samples available for metabolomics profiling are shown in Table 1. Notably, the majority of participants in the cohort self-identified as White (81.7%) and female (79.6%). The mean age of subjects was 43.9 ± 12.0 y. A proportion of participants were categorized as having hypertension (16.6%), hyperlipidemia (20.2%), and prediabetes or diabetes (8.1%). The mean baseline BMI was 28.0 ± 6.3 kg/m2. The mean HEI-15 score at the 24-mo time point was 60.2 ± 12.0 out of 100.
TABLE 1.
Characteristics of the metabolomics cohort (n = 470 participants).
| Demographic or clinical variable1 | |
|---|---|
| Group assignment | |
| Control | 48.9% (n = 230) |
| Intervention | 50.0% (n = 235) |
| Not assigned | 1.1% (n = 5) |
| Age, y (mean ± SD) | 43.9 ± 12.0 (n = 470) |
| Gender | |
| Male | 19.4% (n = 91) |
| Female | 79.6% (n = 374) |
| Not reported | 1.1% (n = 5) |
| Race | |
| White | 81.7% (n = 384) |
| Black | 8.9% (n = 42) |
| Asian | 4.7% (n = 22) |
| Other or not reported | 4.7% (n = 22) |
| Current smoker | 3.4% (n = 16) |
| Ever smoker | 22.6% (n = 106) |
| Prediabetes or diabetes | 8.1% (n = 38) |
| Hypertension | 16.6% (n = 78) |
| Systolic blood pressure, mmHg (mean ± SD) | 120.3 ± 12.8 (n = 470) |
| Diastolic blood pressure, mmHg (mean ± SD) | 69.7 ± 8.7 (n =470) |
| Hyperlipidemia | 20.2% (n = 95) |
| Body mass index (mean ± SD) | 28.0 ± 6.3 (n = 470) |
| Normal weight (<25 kg/m2) | 38.3% (n = 180) |
| Overweight (25–29.9 kg/m2) | 32.1% (n = 151) |
| Obese (≥30 kg/m2) | 29.4% (n = 138) |
| Cardiovascular disease | 2.3% (n = 11) |
| Previous stroke | 0.9% (n = 4) |
| Healthy Eating Index-2015 (HEI-15) at 24 mo (mean ± SD) | 60.2 ± 12.0 (n = 465) |
| Healthy Purchasing Score (HPS) (mean ± SD) | 67.9 ± 13.0 (n = 470) |
Abbreviations: kg/m2, kilograms per meter squared; mmHg, millimeters of mercury; SD, standard deviation.
Demographic and clinical features of participants from the ChooseWell trial with metabolomics data at the 24-mo follow-up time point are shown.
Metabolites associated with healthy dietary intake
We applied 2 distinct targeted metabolomics approaches to profile 264 metabolites from plasma samples (Supplemental Table 1). We first used linear regression models to identify metabolites associated with the HPS throughout the study (Figure 1). In multivariable models adjusted for age, gender, and race, 52 metabolites were associated with the HPS after correction for multiple comparisons (Supplemental Table 2). Fourteen metabolites associated with the HPS were concordantly associated with HEI-15 scores derived from 24-h dietary recalls obtained from the same participants at the 24-mo follow-up time point (Table 2, Supplemental Table 3). Metabolites associated with both dietary indices in multivariable regressions were carried forward in subsequent analyses. For select metabolites that were measured via both derivatization-free and derivatization-based targeted metabolomics methods, associations with the HPS and HEI-15 scores using each method were consistent (Supplemental Tables 2 and 3).
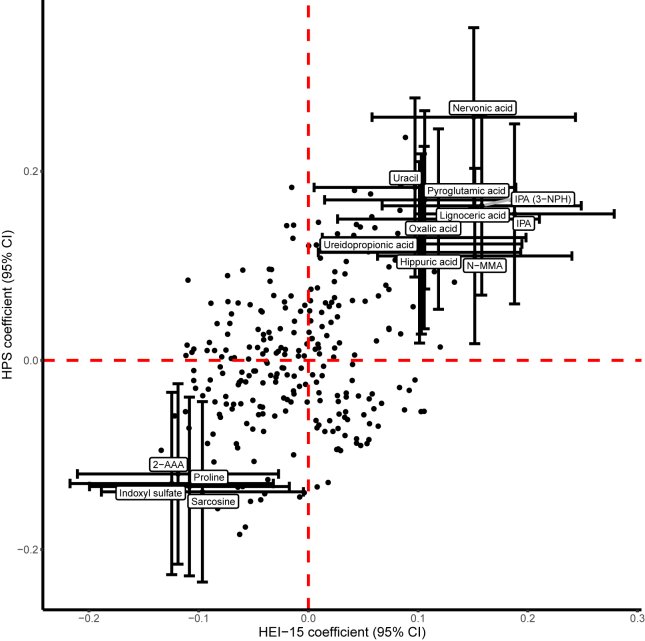
FIGURE 1.
Metabolites concordantly associated with the Healthy Purchasing Score (HPS) and Healthy Eating Index-2015 (HEI-15) in the ChooseWell 365 study (n = 470 participants). Linear regression coefficients and 95% confidence intervals (CIs) are shown for all metabolites associated with the HPS at a false discovery rate < 0.10 and concordantly associated with HEI-15 scores. Multivariable models were adjusted for age, gender, and race. β-Coefficients represent the change in standard deviations of the HPS (y-axis) or HEI-15 scores (x-axis) per standard deviation increase in the normalized level of each metabolite. Metabolites profiled using a 3-nitrophenylhydrazine (3-NPH) derivatization-based method rather than an amide-based (derivatization-free) method are indicated in parentheses. Abbreviations: IPA, indole-3-propionic acid; N-MMA, N-monomethylarginine; 2-AAA, 2-aminoadipic acid.
TABLE 2.
Metabolites concordantly associated with the Healthy Purchasing Score (HPS) and Healthy Eating Index-2015 (HEI-15) scores in multivariable analyses (n = 470 participants).
| HPS |
HEI-15 |
|||
|---|---|---|---|---|
| Metabolite1 | Coefficient (95% CI) | P value | Coefficient (95% CI) | P value |
| Nervonic acid [3-NPH] | 0.26 (0.16, 0.35) | 1.48 × 10−7 | 0.15 (0.06, 0.24) | 1.49 ×10−3 |
| Uracil | 0.18 (0.09, 0.28) | 1.68 ×10−4 | 0.10 (0.01, 0.19) | 3.84 ×10−2 |
| Pyroglutamic acid | 0.17 (0.08, 0.26) | 4.44 ×10−4 | 0.11 (0.01, 0.20) | 2.29 ×10−2 |
| Indole-3-propionic acid [3-NPH] | 0.16 (0.07, 0.26) | 7.32 ×10−4 | 0.16 (0.07, 0.25) | 6.79 ×10−4 |
| Indole-3-propionic acid | 0.15 (0.06, 0.25) | 1.49 ×10−3 | 0.19 (0.10, 0.28) | 5.68 ×10−5 |
| Lignoceric acid [3-NPH] | 0.15 (0.05, 0.24) | 2.21 ×10−3 | 0.12 (0.03, 0.21) | 1.16 ×10−2 |
| Sarcosine | −0.14 (−0.23, −0.04) | 4.36 × 10−3 | −0.10 (−0.19, 0.00) | 3.98 × 10−2 |
| Proline | −0.13 (−0.23, −0.04) | 5.79 × 10−3 | −0.11 (−0.20, −0.02) | 1.97 × 10−2 |
| Indoxyl sulfate | −0.13 (−0.23, −0.03) | 8.21 × 10−3 | −0.12 (−0.22, −0.03) | 8.50 × 10−3 |
| Oxalic acid | 0.13 (0.03, 0.23) | 8.42 × 10−3 | 0.11 (0.01, 0.20) | 2.60 × 10−2 |
| Ureidopropionic acid [3-NPH] | 0.12 (0.03, 0.22) | 1.15 × 10−2 | 0.10 (0.01, 0.19) | 2.78 × 10−2 |
| 2-Aminoadipic acid | −0.12 (−0.22, −0.02) | 1.37 × 10−2 | −0.12 (−0.21, −0.03) | 1.12 × 10−2 |
| Hippuric acid | 0.11 (0.02, 0.21) | 1.97 × 10−2 | 0.10 (0.01, 0.19) | 3.14 × 10−2 |
| N-monomethylarginine | 0.11 (0.02, 0.20) | 1.98 × 10−2 | 0.15 (0.06, 0.24) | 8.31 × 10−4 |
Abbreviation: CI, confidence interval.
β-Coefficients represent the change in SDs of the normalized level of each metabolite per standard deviation increase in the weighted proportion of healthy purchases or HEI-15 scores. Linear regression models were adjusted for age, gender, and race. Metabolites shown were associated with the HPS at a false discovery rate < 0.10 and concordantly associated with HEI-15 scores. Metabolites profiled using a 3-nitrophenylhydrazine (3-NPH) derivatization-based method rather than an amide-based (derivatization-free) method are indicated in brackets.
Several metabolites previously shown to be derived from gut microbial metabolism [[39], [40], [41]] were concordantly associated with the HPS and HEI-15 scores in multivariable analyses. These gut microbial metabolites included indole-3-propionic acid (IPA) (HPS, β: 0.16, 95% CI: 0.07, 0.26, P = 7.32 × 10−4; HEI-15, β: 0.16, 95% CI: 0.07, 0.25, P = 6.79 × 10−4), hippuric acid (HPS, β: 0.11, 95% CI: 0.02, 0.21, P = 1.97 × 10−2; HEI-15, β: 0.10, 95% CI: 0.01, 0.19, P = 3.14 10−2), and indoxyl sulfate (HPS, β: −0.13, 95% CI: −0.23, −0.03, P = 8.21 × 10−3; HEI-15, β: −0.12, 95% CI: −0.22, −0.03], P = 8.50 × 10−3). Additional metabolites that were positively associated with the HPS and HEI-15 scores included nervonic acid, lignoceric acid, N-monomethylarginine (N-MMA), pyroglutamic acid, oxalic acid, uracil, and ureidopropionic acid. Other metabolites that were negatively associated with the HPS and HEI-15 scores included 2-aminoadipic acid, proline, and sarcosine (Table 2). Although numerous metabolites associated with the HPS were not similarly associated with HEI-15 scores derived from dietary recalls, no metabolites were significantly associated with the HPS and HEI-15 scores in opposing directions (Supplemental Tables 2 and 3).
Although the HPS has previously been demonstrated to correlate with lower rates of obesity, hypertension, and prediabetes or diabetes—and allows summarizing the healthfulness of purchases into a single measure—the score is based on the proportion of purchased items with different traffic light labels rather than the energy obtained from different types of purchases [23,25]. We confirmed that metabolites associated with the HPS were also associated with the proportion of calories from cafeteria items with different traffic light labels (Supplemental Tables 4 and 5). To formalize this analysis, we compared associations between metabolites and the HPS to associations between metabolites and caloric intake from cafeteria purchases. In multivariable models adjusted for age, gender, and race, metabolites associated with a higher relative intake of calories from green-labeled (healthy) items were also strongly associated with higher HPS scores (Pearson’s r = 0.95, 95% CI: 0.93, 0.96) (Supplemental Figure 2A). Metabolites associated with a higher relative intake of calories from red-labeled (unhealthy) items were likewise strongly associated with lower HPS scores (Pearson’s r = −0.92, 95% CI: −0.94, −0.90) (Supplemental Figure 2B).
Metabolites associated with specific food groups
We next explored whether metabolites associated with the objective and subjective metrics of dietary intake were associated with specific food groups (Supplemental Table 6). Food groups incorporated into HEI-15 scores include vegetables, greens and beans, total fruits, whole fruits, whole grains, refined grains, dairy, total protein, seafood and plant protein, fatty acids, sodium, saturated fats, and added sugars [4]. After adjustment for multiple comparisons, several significant associations were observed between metabolites and specific components of HEI-15 scores in multivariable models adjusted for age, gender, and race (Figure 2, Table 3).
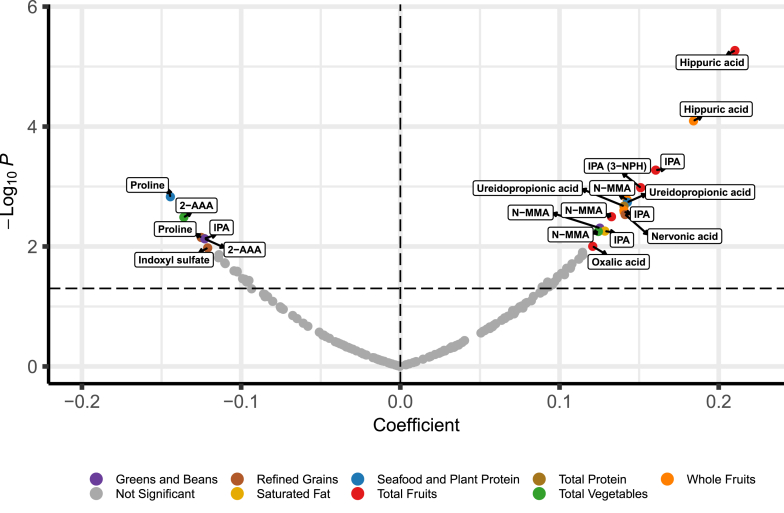
FIGURE 2.
Metabolites associated with Healthy Eating Index-2015 (HEI-15) components (n = 465 participants). Metabolites concordantly associated with the Healthy Purchasing Score (HPS) and HEI-15 were associated with individual HEI-15 categories in multivariable models adjusted for age, gender, and race. Labeled metabolites were significant at a false discovery rate < 0.10. Abbreviations: IPA, indole-3-propionic acid; N-MMA, N-monomethylarginine; 2-AAA, 2-aminoadipic acid; 3-NPH, 3-nitrophenylhydrazine.
TABLE 3.
Associations between diet-associated metabolites and Healthy Eating Index-2015 (HEI-15) categories (n = 465 participants).
| HEI-15 category1 | Metabolite | Coefficient (95% CI) | P value |
|---|---|---|---|
| Total fruit | Hippuric acid | 0.21 (0.12, 0.30) | 5.40 × 10−6 |
| Indole-3-propionic acid | 0.16 (0.07, 0.25) | 5.33 × 10−4 | |
| Indole-3-propionic acid [3-NPH] | 0.15 (0.06, 0.24) | 1.04 × 10−3 | |
| N-monomethylarginine | 0.13 (0.04, 0.22) | 3.19 × 10−3 | |
| Oxalic acid | 0.12 (0.03, 0.21) | 9.94 × 10−3 | |
| Whole fruit | Hippuric acid | 0.18 (0.09, 0.28) | 8.04 × 10−5 |
| N-monomethylarginine | 0.14 (0.05, 0.23) | 1.66 × 10−3 | |
| Indole-3-propionic acid [3-NPH] | 0.14 (0.05, 0.23) | 2.50 × 10−3 | |
| Indole-3-propionic acid | 0.14 (0.05, 0.23) | 2.64 × 10−3 | |
| Refined grains | Nervonic acid [3-NPH] | 0.14 (0.05, 0.23) | 2.94 × 10−3 |
| Proline | −0.13 (−0.22, −0.03) | 7.08 × 10−3 | |
| Indoxyl sulfate | −0.12 (−0.21, −0.03) | 1.06 × 10−2 | |
| Saturated fats | Indole-3-propionic acid | 0.13 (0.04, 0.22) | 5.54 × 10−3 |
| Seafood and plant protein | Proline | −0.14 (−0.23, −0.06) | 1.48 × 10−3 |
| Ureidopropionic acid [3-NPH] | 0.14 (0.05, 0.23) | 1.83 × 10−3 | |
| Total protein | Ureidopropionic acid [3-NPH] | 0.14 (0.05, 0.23) | 2.13 × 10−3 |
| Indole-3-propionic acid | −0.12 (−0.21, −0.03) | 7.38 × 10−3 | |
| Total vegetables | 2-Aminoadipic acid | −0.14 (−0.23, −0.05) | 3.25 × 10−3 |
| N-monomethylarginine | 0.12 (0.04, 0.21) | 5.62 × 10−3 | |
| Greens and beans | N-Monomethylarginine | 0.13 (0.04, 0.21) | 4.94 × 10−3 |
| 2-Aminoadipic acid | −0.12 (−0.21, −0.03) | 7.45 × 10−3 |
Abbreviations: CI, confidence interval; 3-NPH, 3-nitrophenylhydrazine.
β-Coefficients represent the change in SDs of the normalized level of each metabolite per standard deviation increase in HEI-15 scores. Linear regression models were adjusted for age, gender, and race. Results are shown for diet-associated metabolites that were associated with the Healthy Purchasing Score (HPS) and concordantly associated with HEI-15 scores. All P values shown are significant at a false discovery rate < 0.10.
Among gut microbial metabolites, hippuric acid (β: 0.21, 95% CI: 0.12, 0.30, P = 5.40 × 10−6) and IPA (β: 0.16, 95% CI: 0.07, 0.25, P = 5.33 × 10−4) were associated with higher total fruit intake in multivariable models. IPA was also positively associated with intake of saturated fats (β: 0.13, 95% CI: 0.04, 0.22, P = 5.54 × 10−3) and negatively associated with total protein intake (β: −0.12, 95% CI: −0.21, −0.03, P = 7.38 × 10−3). Indoxyl sulfate was negatively associated with intake of refined grains (β: −0.12, 95% CI: −0.21, −0.03, P = 1.06 × 10−2).
Other diet-associated metabolites associated with higher total fruit intake included N-MMA (β: 0.13, 95% CI: 0.04, 0.22, P = 3.19 × 10−3) and oxalic acid (β: 0.12, 95% CI: 0.03, 0.21, P = 9.94 × 10−3). Total intake of vegetables was positively associated with N-MMA (β: 0.12, 95% CI: 0.04, 0.21, P = 5.62 × 10−3) and negatively associated with 2-aminoadipic acid (β: −0.14, 95% CI: −0.23, −0.05, P = 3.25 × 10−3). Protein intake from seafood and plant-based sources was positively associated with ureidopropionic acid (β: 0.14, 95% CI: 0.05, 0.23, P = 1.83 × 10−3) and negatively with proline β: −0.14, 95% CI: −0.23, −0.06, P = 1.48 × 10−3). Nervonic acid (β: 0.14, 95% CI: 0.05, 0.23, P = 2.94 × 10−3) was positively associated with intake of refined grains, whereas proline (β: −0.13, 95% CI: −0.22, −0.03, P = 7.08 × 10−3) was negatively associated with refined grains (Figure 2, Table 3).
The data collected in this study did not allow for the assessment of relative intake for most HEI-15 categories to be derived from information on cafeteria purchases. However, the proportion of purchased calories from whole fruits was readily identifiable from electronic purchasing records. We found that hippuric acid (β: 0.12, 95% CI: 0.03, 0.22, P = 8.44 × 10−3) and IPA (β: 0.10, 95% CI: 0.00, 0.19, P = 4.14 × 10−2) were each associated with the proportion of calories from cafeteria purchases of whole fruit (Supplemental Table 7). These results were consistent with the association between hippuric acid (β: 0.18, 95% CI: 0.09, 0.28, P = 8.04 × 10−5) and IPA (β: 0.14, 95% CI: 0.05, 0.23, P = 2.64 × 10−3) and the HEI-15 category of whole fruits (Figure 2, Table 3).
Clinical variables associated with diet-related metabolites
We investigated whether diet-associated metabolites were associated with clinical variables, including BMI, blood pressure (estimated mean arterial pressure), hyperlipidemia, hypertension, diabetes or prediabetes, and cardiovascular disease. Several significant associations were observed in multivariable models adjusted for age, gender, and race after adjustment for multiple comparisons (Table 4).
TABLE 4.
Associations between diet-associated metabolites and clinical comorbidities (n = 470 participants).
| Variable1 | Metabolite | Coefficient (95% CI) | P value |
|---|---|---|---|
| Hypertension | Indole-3-propionic acid [3-NPH] | −0.29 (−0.54, −0.04) | (x) 2.13 × 10−2 |
| Indoxyl sulfate | 0.24 (−0.01, 0.49) | (x) 5.92 × 10−2 | |
| Mean arterial pressure (per mmHg) | Pyroglutamic acid | −0.02 (−0.03, −0.01) | 3.13 × 10−4 |
| Proline | 0.02 (0.01, 0.03) | 3.60 × 10−4 | |
| Oxalic acid | −0.02 (−0.03, −0.01) | 1.34 × 10−3 | |
| Sarcosine | 0.01 (0.01, 0.02) | 2.73 × 10−3 | |
| Indole-3-propionic acid | −0.01 (−0.02, 0.00) | 1.24 × 10−2 | |
| Indole-3-propionic acid [3-NPH] | −0.01 (−0.02, 0.00) | 1.52 × 10−2 | |
| Hyperlipidemia | Pyroglutamic acid | −0.27 (−0.51, −0.04) | (x) 2.22 × 10−2 |
| Diabetes/prediabetes | Sarcosine | 0.64 (0.31, 0.98) | 1.61 × 10−4 |
| Proline | 0.47 (0.14, 0.80) | 5.58 × 10−3 | |
| Lignoceric acid [3-NPH] | −0.47 (−0.81, −0.14) | 5.96 × 10−3 | |
| Indole-3-propionic acid [3-NPH] | −0.44 (−0.77, −0.11) | 1.01 × 10−2 | |
| Hippuric acid | −0.36 (−0.70, −0.02) | 3.72 × 10−2 | |
| Uracil | −0.34 (−0.67, 0.00) | (x) 4.96 × 10−2 | |
| Indole-3-propionic acid | −0.32 (−0.66, 0.02) | (x) 6.15 × 10−2 | |
| Current smoking | Indole-3-propionic acid | −0.75 (−0.24, −0.25) | 3.10 × 10−3 |
| Indole-3-propionic acid [3-NPH] | −0.73 (−0.22, −0.24) | 3.41 × 10−3 | |
| Hippuric acid | −0.68 (−0.18, −0.18) | 7.92 × 10−3 | |
| Ureidopropionic acid [3-NPH] | −0.52 (−0.01, −0.02) | (x) 4.16 × 10−2 | |
| Cardiovascular disease | Uracil | −0.63 (−0.23, −0.04) | (x) 3.79 × 10−2 |
| Body mass index (per point) | Indole-3-propionic acid [3-NPH] | −0.04 (−0.05, −0.02) | 3.32 × 10−7 |
| Indole-3-propionic acid | −0.04 (−0.05, −0.02) | 5.84 × 10−7 | |
| Pyroglutamic acid | −0.03 (−0.05, −0.02) | 1.89 × 10−5 | |
| 2-aminoadipic acid | 0.03 (0.02, 0.04) | 8.38 × 10−5 | |
| Lignoceric acid [3-NPH] | −0.03 (−0.04, −0.01) | 1.45 × 10−4 | |
| Sarcosine | 0.03 (0.01, 0.04) | 2.15 × 10−4 | |
| Proline | 0.03 (0.01, 0.04) | 2.69 × 10−4 | |
| Indoxyl sulfate | 0.02 (0.01, 0.04) | 6.46 × 10−3 | |
| Oxalic acid | −0.02 (−0.04, −0.01) | 7.92 × 10−3 | |
| Hippuric acid | −0.02 (−0.03, 0.00) | 2.74 × 10−2 | |
| Nervonic acid | −0.02 (−0.03, 0.00) | 3.12 × 10−2 |
Abbreviations: CI, confidence interval; mmHg, millimeters of mercury; 3-NPH, 3-nitrophenylhydrazine.
β-Coefficients represent the change in standard deviations of the normalized level of each metabolite per unit increase in the variables listed. Linear regression models were adjusted for age, gender, and race. Results are shown for diet-associated metabolites that were associated with the HPS and concordantly associated with HEI-15 scores. All P values except those marked by an (x) are significant at a false discovery rate < 0.10.
Among gut microbial metabolites, IPA was associated with lower blood pressure (β: −0.01, 95% CI: −0.02, 0.00], P = 1.24 × 10−2), risk of diabetes or prediabetes (β: −0.44, 95% CI: −0.77, −0.11], P =1.01 × 10−2), and BMI (β: −0.04, 95% CI: −0.05,−0.02, P = 3.32 × 10−7). Hippuric acid was also associated with lower BMI (β: −0.02, 95% CI: −0.03, 0.00, P = 2.74 × 10−2) and risk of diabetes or prediabetes (β: −0.36, 95% CI: −0.70, −0.02], P = 3.72 × 10−2). In contrast, indoxyl sulfate was associated with greater BMI (β: 0.02, 95% CI: 0.01, 0.04, P = 6.46 × 10−3).
Several additional diet-associated metabolites were associated with multiple clinical variables. Sarcosine and proline were each associated with higher risk of diabetes or prediabetes (sarcosine, β: 0.64, 95% CI: 0.31, 0.98, P = 1.61 × 10−4; proline, β: 0.47, 95% CI: 0.14, 0.80, P = 5.58 × 10−3), blood pressure (sarcosine, β: 0.01, 95% CI: 0.01, 0.02, P = 2.73 × 10−3; proline, β: 0.02, 95% CI: 0.01, 0.03, P = 3.60 × 10−4), and BMI (sarcosine, β: 0.03, 95% CI: 0.01, 0.04, P = 2.15 × 10−4; proline, β: 0.03, 95% CI: 0.01, 0.04, P = 2.69 × 10−4).
In contrast, pyroglutamic acid was associated with lower blood pressure (β: −0.02, 95% CI: −0.03, −0.01, P = 3.13 × 10−4) and BMI (β: −0.03, 95% CI: −0.05, −0.02, P = 1.89 × 10−5). Oxalic acid was similarly associated with lower blood pressure (β: −0.02, 95% CI: −0.03, −0.01, P = 1.34 × 10−3) and BMI (β: −0.02, 95% CI: −0.04, −0.01, P = 7.92 × 10−3). Lignoceric acid was associated with lower risk of diabetes or prediabetes (β: −0.47, 95% CI: −0.81, −0.14, P = 5.96 × 10−3) and BMI (β: −0.03, 95% CI: −0.04, −0.01, P = 1.45 × 10−4) (Table 4).
Discussion
Although dietary intake can affect the composition of the gut microbiome and circulating metabolome [[42], [43], [44], [45]], self-reported dietary intake may not accurately reflect true dietary intake. We used targeted metabolomics to profile an expanded panel of plasma metabolites in the ChooseWell 365 trial. We identified 14 metabolites concordantly associated with subjective and objective measures of adherence to a healthy diet, including gut microbial metabolites (IPA, hippuric acid, and indoxyl sulfate). These gut microbial metabolites were associated with both intake of specific food groups (e.g., whole fruits) and clinical variables (e.g., BMI, diabetes, blood pressure). Our findings highlight associations between gut microbiome-related metabolites and a healthy diet while establishing purchasing data as an effective tool for identifying dietary biomarkers in community-dwelling individuals.
Several diet-associated metabolites are relevant in predicting disease development and may capture disease risk conferred by upstream dietary exposures. For instance, plasma concentrations of 2-aminoadipic acid—which was negatively associated with a healthy diet in this study—have been found to predict the risk of diabetes among normoglycemic individuals [46]. Although 2-aminoadipic acid was not associated with prevalent diabetes in this study (Table 4), it was associated with higher BMI (β: 0.03, 95% CI: 0.02, 0.04, P = 8.38 × 10−5). Concentrations of dimethylguanidino valeric acid—which was also negatively associated with a healthy diet (Supplemental Tables 2 and 3)—have been associated with intake of sugar-sweetened beverages and predict the risk of incident cardiovascular disease, diabetes, and stroke [30,47,48]. Because large-scale cohort studies aimed at assessing disease risk will continue to rely on self-reported dietary data in practice, our results may be useful in identifying metabolites whose associations with dietary exposures are invariant to the measurement approach. These metabolites could serve as biomarkers of dietary intake that are sufficiently robust to overcome the limitations of self-reported data and even purchasing scores (e.g., limited assessment of intake outside of the workplace).
Identification of metabolites associated with a healthy diet may also provide insight into how diet influences disease risk specifically through effects on the gut microbiome. For example, IPA is produced exclusively from tryptophan metabolism by human gut microorganisms [40,49]. Consistent with our findings, previous work has demonstrated that plasma IPA concentrations are positively associated with adherence to healthy, plant-based dietary patterns [50,51] and negatively associated with the conditions that comprise metabolic syndrome [52]. IPA has also been associated with a reduced risk of incident stroke [50]. Indoxyl sulfate is a metabolite derived from the host modification of indole, which is also exclusively produced from the gut microbial metabolism of tryptophan [40,53]. Indoxyl sulfate is known to accumulate in plasma as kidney function declines and contributes to the progression of chronic kidney disease, including the risk of vascular complications [[54], [55], [56]]. Circulating levels of metabolites involved in tryptophan metabolism, including IPA and indoxyl sulfate, can be altered by probiotic supplementation [57,58]. Importantly, indoxyl sulfate has not been associated with adherence to healthy dietary patterns in previous studies.
Although circulating hippuric acid has not been associated with health outcomes in humans, it has been associated with greater adherence to recommended healthy dietary patterns in multiple cohorts [59,60]. Hippuric acid can be derived from host modification of phenylpropionic acid, which can be derived from the reduction of phenylalanine by gut bacteria [39,40]. Hippuric acid can also originate from the gut microbial metabolism of polyphenols, which are abundant in fruits, and associations between hippuric acid and fruit intake have been reported [[61], [62], [63]].
Our study design and methods have several strengths. Although not all cafeteria purchases are necessarily consumed, electronic tracking of purchases can enable relatively accurate monitoring of dietary habits in a free-living cohort. Notably, metabolites in this study were not always concordantly associated with subjective and objective measures of a healthy diet (Supplemental Tables 2 and 3). This finding underscores the extent to which measurement tools influence the identification of biomarkers that are associated with dietary intake. Evidence suggests that metabolite associations with food groups may be greater in magnitude when measuring intake objectively, such as in a controlled feeding study [64]. Similar to previous studies, we leveraged a tandem quadrupole design that enabled the quantification of numerous biologically relevant metabolites with high specificity and sensitivity [30,31,37,38,50]. In this work, the implementation of a derivatization-based method enabled sensitive quantification of numerous additional gut microbiome–associated metabolites [[34], [35], [36]].
Nonetheless, our results are subject to limitations. The generalizability of observed diet–metabolite associations may be limited by the study cohort, which only included participants employed in a hospital setting and displayed limited diversity in terms of race and gender. The available data also restricted our ability to identify the proportion of purchased calories or grams that were obtained from specific nutrients and most HEI-15 food groups. In addition, associations between clinical variables and metabolites may have been affected by numerous factors—such as lifestyle variables—unrelated to dietary intake. For example, smoking status was associated with both IPA and hippuric acid (Table 4). Finally, our inability to monitor intake outside of the cafeteria limited our capacity to objectively monitor overall diet quality. However, HPS and HEI-15 scores from dietary recalls are correlated in the ChooseWell 365 cohort [25], and metabolites associated with the HPS tended to be similarly associated with HEI-15 scores (Figure 1). Both purchasing data and self-reported dietary intake—which in large part reflects intake outside of the cafeteria—are therefore likely to track underlying dietary habits.
Our findings suggest several avenues for future studies. In both observational and interventional studies, evaluating changes in the metabolome with changes in diet will help establish the utility of circulating metabolites as reliable surrogates for dietary intake. Longitudinal cohort studies will also be critical in establishing which plasma metabolites can be understood as mediators that connect dietary exposures to disease risk [50]. Ultimately, the identification of diet-associated metabolites that are reliably associated with disease development may lead to the establishment of personalized biomarker targets for disease prevention. These targets could guide the implementation of dietary interventions and direct manipulations of the gut microbiome.
Author contributions
The authors’ responsibilities were as follows—VMB, ZA, WTK: designed research, VMB, ZA: conducted research; DEL,ANT provided essential databases; VMB, ZA: analyzed data; VMB, WTK: wrote the paper; WTK: primary responsibility for final content; and all authors: read and approved the final manuscript.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding
This work was supported by the National Institutes of Health (NIH) R01 NS099209 (WTK). The ChooseWell 365 study was funded by the NIH R01 grants HL125486 and DK114735; the project was also supported by NIH grant 1UL1TR001102. ANT is supported by NIH grant K24 HL163073.
Data availability
Data described in the manuscript will not be made available because these data contain potentially identifying participant information. The study population is a relatively small cohort of employees at Massachusetts General Hospital (which is named in the manuscript) who were enrolled in the ChooseWell 365 trial during a recent time. Sharing information on individuals' age, gender, timing of cafeteria purchases, and BMI could compromise participant privacy. All analytic code will be made available upon reasonable request to the corresponding author.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2024.04.022.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.GBD 2017 Diet Collaborators Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulze M.B., Martínez-González M.A., Fung T.T., Lichtenstein A.H., Forouhi N.G. Food-based dietary patterns and chronic disease prevention. BMJ. 2018;361:k2396. doi: 10.1136/bmj.k2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micha R., Peñalvo J.L., Cudhea F., Imamura F., Rehm C.D., Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317:912. doi: 10.1001/jama.2017.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krebs-Smith S.M., Pannucci T.E., Subar A.F., Kirkpatrick S.I., Lerman J.L., Tooze J.A., et al. Update of the healthy eating index: HEI-2015. J. Acad. Nutr. Diet. 2018;118:1591–1602. doi: 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morze J., Danielewicz A., Hoffmann G., Schwingshackl L. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: a second update of a systematic review and meta-analysis of cohort studies. J. Acad. Nutr. Diet. 2020;120:1998–2031. doi: 10.1016/j.jand.2020.08.076. [DOI] [PubMed] [Google Scholar]
- 6.Shan Z., Li Y., Baden M.Y., Bhupathiraju S.N., Wang D.D., Sun Q., et al. Association between healthy eating patterns and risk of cardiovascular disease. JAMA Intern. Med. 2020;180:1090–1100. doi: 10.1001/jamainternmed.2020.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu E.A., Steffen L.M., Coresh J., Appel L.J., Rebholz C.M. Adherence to the Healthy Eating Index-2015 and other dietary patterns may reduce risk of cardiovascular disease, cardiovascular mortality, and all-cause mortality. J. Nutr. 2020;150:312–321. doi: 10.1093/jn/nxz218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16(6):341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkowski M., Weeks T.L., Hazen S.L. Gut microbiota and cardiovascular disease. Circ. Res. 2020;127:553–570. doi: 10.1161/CIRCRESAHA.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogt N.M., Kerby R.L., Dill-McFarland K.A., Harding S.J., Merluzzi A.P., Johnson S.C., et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh R.K., Chang H.W., Yan D., Lee K.M., Ucmak D., Wong K., et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothschild D., Weissbrod O., Barkan E., Kurilshikov A., Korem T., Zeevi D., et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 13.Zmora N., Suez J., Elinav E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 14.Valdes A.M., Walter J., Segal E., Spector T.D. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:36–44. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rafiq T., Azab S.M., Teo K.K., Thabane L., Anand S.S., Morrison K.M., et al. Nutritional metabolomics and the classification of dietary biomarker candidates: a critical review. Adv. Nutr. 2021;12(6):2333–2357. doi: 10.1093/advances/nmab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guasch-Ferré M., Bhupathiraju S.N., Hu F.B. Use of metabolomics in improving assessment of dietary intake. Clin. Chem. 2018;64:82–98. doi: 10.1373/clinchem.2017.272344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuhouser M.L., Prentice R.L., Tinker L.F., Lampe J.W. Enhancing capacity for food and nutrient intake assessment in population sciences research. Annu. Rev. Public Health. 2023;44:37–54. doi: 10.1146/annurev-publhealth-071521-121621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subar A.F., Freedman L.S., Tooze J.A., Kirkpatrick S.I., Boushey C., Neuhouser M.L., et al. Addressing current criticism regarding the value of self-report dietary data. J. Nutr. 2015;145:2639–2645. doi: 10.3945/jn.115.219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barton S., Navarro S.L., Buas M.F., Schwarz Y., Gu H., Djukovic D., et al. Targeted plasma metabolome response to variations in dietary glycemic load in a randomized, controlled, crossover feeding trial in healthy adults. Food Funct. 2015;6:2949–2956. doi: 10.1039/c5fo00287g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galié S., García-Gavilán J., Papandreou C., Camacho-Barcía L., Arcelin P., Palau-Galindo A., et al. Effects of Mediterranean diet on plasma metabolites and their relationship with insulin resistance and gut microbiota composition in a crossover randomized clinical trial. Clin. Nutr. 2021;40:3798–3806. doi: 10.1016/j.clnu.2021.04.028. [DOI] [PubMed] [Google Scholar]
- 21.Guertin K.A., Moore S.C., Sampson J.N., Huang W.Y., Xiao Q., Stolzenberg-Solomon R.Z., et al. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am. J. Clin. Nutr. 2014;100(1):208–217. doi: 10.3945/ajcn.113.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pujos-Guillot E., Hubert J., Martin J.F., Lyan B., Quintana M., Claude S., et al. Mass spectrometry-based metabolomics for the discovery of biomarkers of fruit and vegetable intake: citrus fruit as a case study. J. Proteome. Res. 2013;12(4):1645–1659. doi: 10.1021/pr300997c. [DOI] [PubMed] [Google Scholar]
- 23.Thorndike A.N., McCurley J.L., Gelsomin E.D., Anderson E., Chang Y., Porneala B., et al. Automated behavioral workplace intervention to prevent weight gain and improve diet: the ChooseWell 365 randomized clinical trial. JAMA Netw. Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han J., Lin K., Sequeira C., Borchers C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta. 2015;854:86–94. doi: 10.1016/j.aca.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 25.McCurley J.L., Levy D.E., Rimm E.B., Gelsomin E.D., Anderson E.M., Sanford J.M., et al. Association of worksite food purchases and employees’ overall dietary quality and health. Am. J. Prev. Med. 2019;57:87–94. doi: 10.1053/j.jrn.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy D.E., Gelsomin E.D., Rimm E.B., Pachucki M., Sanford J., Anderson E., et al. Design of ChooseWell 365: randomized controlled trial of an automated, personalized worksite intervention to promote healthy food choices and prevent weight gain, Contemp. Clin. Trials. 2018;75:78–86. doi: 10.1016/j.cct.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorndike A.N., Riis J., Sonnenberg L.M., Levy D.E. Traffic-light labels and choice architecture. Am. J. Prev. Med. 2014;46:143–149. doi: 10.1016/j.amepre.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorndike A.N., Sonnenberg L., Riis J., Barraclough S., Levy D.E. A 2-phase labeling and choice architecture intervention to improve healthy food and beverage choices. Am. J. Public Health. 2012;102:527–533. doi: 10.2105/AJPH.2011.300391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subar A.F., Kirkpatrick S.I., Mittl B., Zimmerman T.P., Thompson F.E., Bingley C., et al. The automated self-administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J. Acad. Nutr. Diet. 2012;112:1134–1137. doi: 10.1016/j.jand.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ament Z., Patki A., Chaudhary N., Bhave V.M., Garcia Guarniz A.L., Gao Y., et al. Nucleosides associated with incident ischemic stroke in the REGARDS and JHS cohorts. Neurology. 2022;98:e2097–e2107. doi: 10.1212/WNL.0000000000200262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ament Z., Patki A., Bhave V.M., Chaudhary N.S., Garcia Guarniz A.L., Kijpaisalratana N., et al. Gut microbiota-associated metabolites and risk of ischemic stroke in REGARDS. J. Cereb. Blood Flow Metab. 2023;43:1089–1098. doi: 10.3390/ijms23031222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimberly W.T., O’Sullivan J.F., Nath A.K., Keyes M., Shi X., Larson M.G., et al. Metabolite profiling identifies anandamide as a biomarker of nonalcoholic steatohepatitis. JCI Insight. 2017;2(9) doi: 10.1172/jci.insight.92989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.García-Rivera M.A., Fernández-Ochoa Á., Brüning U., Fritsche-Guenther R., Kirwan J.A. Identification and validation of small molecule analytes in mouse plasma by liquid chromatography–tandem mass spectrometry: a case study of misidentification of a short-chain fatty acid with a ketone body. Talanta. 2022;242 doi: 10.1016/j.talanta.2022.123298. [DOI] [PubMed] [Google Scholar]
- 34.Shafaei A., Vamathevan V., Pandohee J., Lawler N.G., Broadhurst D., Boyce M.C. Sensitive and quantitative determination of short-chain fatty acids in human serum using liquid chromatography mass spectrometry. Anal. Bioanal. Chem. 2021;413:6333–6342. doi: 10.1007/s00216-021-03589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao H.Y., Wang C.Y., Lee C.H., Kao H.L., Wu W.K., Kuo C.H. Development of an efficient and sensitive chemical derivatization-based LC-MS/MS method for quantifying gut microbiota-derived metabolites in human plasma and its application in studying cardiovascular disease. J. Proteome Res. 2021;20:3508–3518. doi: 10.1021/acs.jproteome.1c00147. [DOI] [PubMed] [Google Scholar]
- 36.Meng X., Pang H., Sun F., Jin X., Wang B., Yao K., et al. Simultaneous 3-nitrophenylhydrazine derivatization strategy of carbonyl, carboxyl and phosphoryl submetabolome for LC-MS/MS-based targeted metabolomics with improved sensitivity and coverage. Anal. Chem. 2021;93:10075–10083. doi: 10.1021/acs.analchem.1c00767. [DOI] [PubMed] [Google Scholar]
- 37.Kijpaisalratana N., Ament Z., Patki A., Bhave V.M., Garcia-Guarniz A.L., Judd S.E., et al. Association of circulating metabolites with racial disparities in hypertension and stroke in the REGARDS study. Neurology. 2023;100:e2312–e2320. doi: 10.1212/WNL.0000000000207264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kijpaisalratana N., Ament Z., Patki A., Bhave V.M., Jones A.C., Garcia Guarniz A.L., et al. Acetylglutamine differentially associated with first-time versus recurrent stroke, Transl. Stroke. Res. 2023 doi: 10.1007/s12975-023-01181-1. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pruss K.M., Chen H., Liu Y., Van Treuren W., Higginbottom S.K., Jarman J.B., et al. Host–microbe co-metabolism via MCAD generates circulating metabolites including hippuric acid. Nat. Commun. 2023;14(1):512. doi: 10.1038/s41467-023-36138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dodd D., Spitzer M.H., Van Treuren W., Merrill B.D., Hryckowian A.J., Higginbottom S.K., et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen L., Zhernakova D.V., Kurilshikov A., Andreu-Sánchez S., Wang D., Augustijn H.E., et al. Influence of the microbiome, diet and genetics on inter-individual variation in the human plasma metabolome. Nat Med. 2022;28:2333–2343. doi: 10.1038/s41591-022-02014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Visconti A., Le Roy C.I., Rosa F., Rossi N., Martin T.C., Mohney R.P., et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019;10:4505. doi: 10.1038/s41467-019-12476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asnicar F., Berry S.E., Valdes A.M., Nguyen L.H., Piccinno G., Drew D.A., et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 2021;27:321–332. doi: 10.1038/s41591-020-01183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bar N., Korem T., Weissbrod O., Zeevi D., Rothschild D., Leviatan S., et al. A reference map of potential determinants for the human serum metabolome. Nature. 2020;588:135–140. doi: 10.1038/s41586-020-2896-2. [DOI] [PubMed] [Google Scholar]
- 46.Wang T.J., Ngo D., Psychogios N., Dejam A., Larson M.G., Vasan R.S., et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J. Clin. Invest. 2013;123(10):4309–4317. doi: 10.1172/JCI64801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ottosson F., Ericson U., Almgren P., Smith E., Brunkwall L., Hellstrand S., et al. Dimethylguanidino valerate: a lifestyle-related metabolite associated with future coronary artery disease and cardiovascular mortality. J. Am. Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Sullivan J.F., Morningstar J.E., Yang Q., Zheng B., Gao Y., Jeanfavre S., et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J. Clin. Invest. 2017;127:4394–4402. doi: 10.1172/JCI95995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agus A., Planchais J., Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1002/ana.26552. [DOI] [PubMed] [Google Scholar]
- 50.Bhave V.M., Ament Z., Patki A., Gao Y., Kijpaisalratana N., Guo B., et al. Plasma metabolites link dietary patterns to stroke risk. Ann. Neurol. 2023;93:500–510. doi: 10.1002/ana.26552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu C., Sawrey-Kubicek L., Beals E., Rhodes C.H., Houts H.E., Sacchi R., et al. Human gut microbiome composition and tryptophan metabolites were changed differently by fast food and Mediterranean diet in 4 days: a pilot study. Nutr. Res. 2020;77:62–72. doi: 10.1016/j.nutres.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Konopelski P., Mogilnicka I. Biological effects of indole-3-propionic acid, a gut microbiota-derived metabolite, and its precursor tryptophan in mammals’ health and disease. Int. J. Mol. Sci. 2022;23:1222. doi: 10.3390/ijms23031222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devlin A.S., Marcobal A., Dodd D., Nayfach S., Plummer N., Meyer T., et al. Modulation of a circulating uremic solute via rational genetic manipulation of the gut microbiota. Cell Host Microbe. 2016;20:709–715. doi: 10.1016/j.chom.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu I.W., Hsu K.H., Lee C.C., Sun C.Y., Hsu H.J., Tsai C.J., et al. Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant. 2011;26:938–947. doi: 10.1093/ndt/gfq580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hung S., Kuo K., Wu C., Tarng D. Indoxyl sulfate: a novel cardiovascular risk factor in chronic kidney disease. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barreto F.C., Barreto D.V., Liabeuf S., Meert N., Glorieux G., Temmar M., et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim P.S., Wang H.F., Lee M.C., Chiu L.S., Wu M.Y., Chang W.C., et al. The efficacy of lactobacillus-containing probiotic supplementation in hemodialysis patients: a randomized, double-blind, placebo-controlled trial. J. Ren. Nutr. 2021;31:189–198. doi: 10.1053/j.jrn.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Kim C.S., Jung S., Hwang G.S., Shin D.M. Gut microbiota indole-3-propionic acid mediates neuroprotective effect of probiotic consumption in healthy elderly: a randomized, double-blind, placebo-controlled, multicenter trial and in vitro study. Clin. Nutr. 2023;42:1025–1033. doi: 10.1016/j.clnu.2023.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Kim H., Anderson C.A., Hu E.A., Zheng Z., Appel L.J., He J., et al. Plasma metabolomic signatures of healthy dietary patterns in the Chronic Renal Insufficiency Cohort (CRIC) study. J. Nutr. 2021;151(10):2894–2907. doi: 10.1093/jn/nxab203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walker M.E., Song R.J., Xu X., Gerszten R.E., Ngo D., Clish C.B., et al. Proteomic and metabolomic correlates of healthy dietary patterns: the Framingham Heart Study. Nutrients. 2020;12(5):1476. doi: 10.3390/nu12051476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pallister T., Jackson M.A., Martin T.C., Zierer J., Jennings A., Mohney R.P., et al. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walsh M.C., Brennan L., Pujos-Guillot E., Sébédio J.L., Scalbert A., Fagan A., et al. Influence of acute phytochemical intake on human urinary metabolomic profiles. Am. J. Clin. Nutr. 2007;86(6):1687–1693. doi: 10.1093/ajcn/86.5.1687. [DOI] [PubMed] [Google Scholar]
- 63.Gonthier M.P., Verny M.A., Besson C., Rémésy C., Scalbert A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003;133(6):1853–1859. doi: 10.1093/jn/133.6.1853. [DOI] [PubMed] [Google Scholar]
- 64.Playdon M.C., Tinker L.F., Prentice R.L., Loftfield E., Hayden K.M., Van Horn L., et al. Measuring diet by metabolomics: a 14-d controlled feeding study of weighed food intake. Am. J. Clin. Nutr. 2024;119(2):511–526. doi: 10.1016/j.ajcnut.2023.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will not be made available because these data contain potentially identifying participant information. The study population is a relatively small cohort of employees at Massachusetts General Hospital (which is named in the manuscript) who were enrolled in the ChooseWell 365 trial during a recent time. Sharing information on individuals' age, gender, timing of cafeteria purchases, and BMI could compromise participant privacy. All analytic code will be made available upon reasonable request to the corresponding author.