Abstract
Woodchuck hepatitis virus (WHV), similar to human hepatitis B virus, causes acute liver inflammation that can progress to chronic hepatitis and hepatocellular carcinoma. WHV also invades cells of the host lymphatic system, where it persists for life. We report here that acute and chronic hepadnavirus hepatitis is characterized by a profound difference in the expression of class I major histocompatibility complex (MHC) molecules on the surface of infected hepatocytes and, notably, lymphoid cells. While acute WHV infection is accompanied by the enhanced hepatocyte surface presentation of class I MHC antigen and upregulated transcription of the relevant hepatic genes, inhibition of class I antigen display on liver cells is a uniform hallmark of chronic WHV infection. This inhibition in chronic hepatitis occurs despite augmented (as in acute infection) expression of hepatic genes for class I MHC heavy chain, β2-microglobulin, and transporters associated with antigen processing (TAP1 and TAP2). Further, the class I antigen inhibition is not related to the histological severity of hepatocellular injury, the extent of lymphocytic infiltrations, the level of intrahepatic gamma interferon induction, or the hepatic WHV load. Importantly, the antigen expression is also inhibited on organ lymphoid cells of chronically infected hosts. The results obtained in this study demonstrate that the defective presentation of class I MHC molecules on cells supporting persistent WHV replication is due to viral posttranscriptional interference. This event may diminish the susceptibility of infected hepatocytes to virus-specific T-cell-mediated elimination, hinder virus clearance, and deregulate the class I MHC-dependent functions of the host immune system. This multifarious effect could be critical for perpetuation of liver damage and evasion of the antiviral immunological surveillance in chronic infection and therefore could be supportive of hepadnavirus persistence.
The hepatitis B virus (HBV) is a small, noncytopathic DNA virus, the prototype of the hepadnavirus family. This virus causes acute and chronic liver inflammation and hepatocellular carcinoma in humans. The host cellular immune responses directed against HBV peptides displayed on infected hepatocytes, particularly those mediated by cytotoxic T lymphocytes (CTL), are considered to be crucial in the induction of hepatic damage and probably contribute to both cytopathic and noncytopathic elimination of the virus from infected livers (5, 18). A strong CTL response specific to multiple HBV epitopes has been identified during and years after recovery from acute hepatitis (AH) (39, 42, 44). Conversely, CTL specific for HBV have been found infrequently in peripheral blood and at low precursor levels in livers in patients with chronic hepatitis (CH) (2, 12), even though sustained liver cell damage coexists with intrahepatic lymphomononuclear infiltrations and high virus loads in many chronically infected patients. Although a diminished antiviral CTL response is thought to be the main contributor to the pathogenesis of CH type B, the basis of this hindrance remains uncertain. Since triggering and the strength of antiviral CTL responsiveness depend to a significant degree on the efficient cell surface presentation of viral peptides by class I major histocompatibility complexes (MHC), delineation of the changes and the mechanism(s) modulating their expression in the course of viral hepatitis might be decisive for understanding the pathogenesis of protracted liver disease and virus persistence in hepadnavirus infection. The above reasoning has proven to be correct for other viruses. These investigations have shown that virus-induced alterations in the class I MHC surface display play an important role in viral pathogenesis and persistence (13, 37, 40, 45).
Lymphotropism is a common feature of many viruses capable of induction of long-term infection in the host. It is now evident that although the liver is the main site of HBV replication and hepatic tissue injury is a leading source of clinical manifestations, the virus also infects the host lymphatic system. Different forms of HBV DNA, including the covalently closed circular DNA and replicative intermediates, as well as viral transcripts, have been identified in lymphoid cells (reviewed in references 29 and 30). The direct link between HBV lymphotropism and chronic infection is not yet established. However, it is conceivable that, as in other viral infections, invasion of the lymphatic system may have a detrimental effect on a variety of the host immune responses. Among others, the virus may alter the display of class I MHC molecules on lymphoid cells and, in consequence, impair a variety of cell immune functions.
The woodchuck (Marmota monax) is a natural model of hepadnavirus hepatitis that reflects, with a high degree of accuracy, virological and pathobiological events occurring in HBV-infected patients (29, 50). Similar to HBV, woodchuck hepatitis virus (WHV) induces acute infection that can progress to CH and hepatocellular carcinoma. In this study, we investigated the WHV-woodchuck model to elucidate the relationship between the acute and chronic phases of hepadnavirus infection and the class I MHC presentation on cells naturally supporting WHV replication. We have also searched for a molecular basis of the discovered disparities in class I MHC expression. In contrast to past evaluations, which reached conflicting conclusions based on immunohistochemical stainings of liver tissue from HBV-infected patients (6, 26, 43), purified cell plasma membranes, quantitative and highly sensitive immunoblotting techniques, and molecular methods measuring class I MHC-affiliated gene activity were used in this study. We report that acute and chronic WHV infections are characterized by a profound difference in presentation of class I antigen on hepatocytes and organ lymphoid cells. Hence, while AH is accompanied by an augmented expression of class I molecules on liver cells but an unaltered display on lymphoid cells, inhibition of the antigen presentation on both cell types is a uniform characteristic of chronic WHV infection. Interestingly, this inhibition in CH occurs despite upregulated transcription of the hepatic genes encoding class I MHC heavy (α) and light (β2-microglobulin) chains and transporters associated with antigen presentation (TAP1 and TAP2), implying that the defect in class I molecule presentation occurs posttranscriptionally.
MATERIALS AND METHODS
Animals and categories of WHV infection.
Fourteen woodchucks constituted the main study group (study group 1). In this group, eight animals (WM 2070, WF 2112, WF 2114, WF 2131, WM 2121, WF 2160, WM 2167, and WM 2171) were infected intravenously with WHV (33, 35). Four others (WF 2020, WF 2030, WM 2040, and WM 2150) had naturally acquired, WHV surface antigen (WHsAg)-positive CH, which was monitored for up to 21 months prior to the start of the experiment. Two healthy animals (WM 2075 and WF 2078) were examined as controls (Table 1). All the woodchucks except WF 2112 and WM 2121 were a part of our previous study aimed at identification of molecular species of WHV structural proteins and the nature of their interactions with hepatocyte plasma membranes (HPM) in AH and CH and in recovery (33).
TABLE 1.
Immunovirological and histological characteristics of WHV infection in animals studied at the time of analysis of class I MHC expression
| Category of disease and animala | Duration of WHs antigenemia (wk) | WHV serology and DNA
|
Histological degree of hepatitisc | |||
|---|---|---|---|---|---|---|
| WHsAg | Anti-WHs | Anti-WHc | WHV DNAb | |||
| Group 1 | ||||||
| Healthy | ||||||
| WM 2075 | 0 | − | − | − | − | 0 |
| WF 2078 | 0 | − | − | − | − | 0 |
| Resolution of AH | ||||||
| WF 2131 | 3 | − | − | + | (+)d | 0/I |
| WF 2160 | 2 | − | + | + | (+)d | 0/I |
| AH | ||||||
| WM 2070 | 8 | + | − | + | + | I |
| WM 2167 | 8 | + | − | + | + | II |
| WM 2121 | 6 | + | − | + | + | III |
| WM 2171 | 10 | + | − | + | + | III |
| CH | ||||||
| WF 2114 | 46 | + | − | + | + | I |
| WF 2030 | >60 | + | − | + | + | II |
| WM 2040 | >26 | + | − | + | + | II |
| WF 2020 | >84 | + | − | + | + | III |
| WF 2112 | 26 | + | − | + | + | III |
| WM 2150 | >49 | + | − | + | + | III |
| Group 2 | ||||||
| Healthy | ||||||
| WM 3069 | 0 | − | − | − | − | 0 |
| WF 3299 | 0 | − | − | − | − | 0 |
| AH | ||||||
| WF 3392 | 1 | + | − | + | + | I |
| WF 3838 | 3 | + | − | + | + | II |
| WM 3158 | 6 | + | − | + | + | III |
| CH | ||||||
| WF 4832 | >72 | + | − | + | + | I |
| WF 3349 | >83 | + | − | + | + | II |
| WF 4980 | >65 | + | − | + | + | II |
| WF 4751 | >55 | + | − | + | + | III |
WM, woodchuck male; WF, woodchuck female.
Evaluated by slot blot hybridization with full-length, cloned WHV DNA as a probe (sensitivity, 106 to 107 vge/ml).
Severity of hepatitis from 0 to III reflects the degree of liver injury determined on the basis of grades separately assigned for hepatocellular, extracellular intralobular, and portal alterations and taking into consideration a global impression of the pathological picture of liver injury as a whole (34).
Detected by nested PCR with WHV core gene-specific primers and Southern blot hybridization of the amplified virus sequences (sensitivity, 10 to 102 vge/ml).
The serological status of WHV infection was determined by testing sequentially collected sera for WHsAg, antibodies to WHsAg (anti-WHs), and antibodies to WHV core antigen (anti-WHc) by immunoassays as described previously (9, 33, 35). The serum WHV DNA level was determined by slot blot hybridization (35) and, when negative, by PCR using WHV core gene specific primers, as described previously (7, 35).
Histological examination of liver samples, obtained by laparotomy 3 to 4 weeks prior to the experiment or at the time of liver perfusion, was done after conventional processing to paraffin. Paraffin sections (4 μm) were stained with hematoxylin and eosin, Masson-trichrome, or periodic acid-Schiff or impregnated with silver (33). The morphologic assessment of liver damage, referred to as the histological degree of hepatitis, was based on criteria described in our previous works (33, 34).
Based on serological and histological assessments, woodchucks in study group 1 were classified into three categories: (i) healthy or recovered from AH (n = 4), (ii) with AH (n = 4), and (iii) persistently infected, with serologically and histologically evident CH (n = 6) (Table 1). Histological examination showed a highly variable degree of inflammatory liver injury in the animals examined (Table 1). The changes ranged from minor lesions (grade I; WM 2070 and WF 2114) through mild AH or CH (grade II; WF 2030, WM 2040, and WM 2167) to severe liver injury with heavy lymphocytic infiltrations and prominent necrosis of parenchyma (grade III; WF 2020, WF 2112, WM 2121, WM 2150, and WM 2171). Liver biopsy specimens from healthy animals (WM 2075 and WF 2078) did not show morphological alterations, whereas hepatic changes in woodchucks which had recovered from AH (WF 2131 and WF 2160) were minimal and consisted mainly of minor lymphomononuclear infiltrations in some portal areas and scanty intralobular infiltrations surrounding singular degenerating hepatocytes, as reported previously (35).
Liver and spleen specimens from seven other woodchucks with detailed characterization of serological and histological profiles of AH (n = 3) or CH (n = 4) and from two healthy animals (study group 2) were also investigated (Table 1). Hepatic histological lesions in this group varied from none in healthy animals (WM 3069 and WF 3299) through minor (grade I; WF 3392 and WF 4832) and moderate (grade II; WF 3349, WF 3838, and WF 4980) to severe (grade III; WM 3158 and WF 4751) in animals with either AH or CH. In addition, several other woodchucks with a well-defined status of WHV infection were used as a source of peripheral blood mononuclear cells (PBMC) to test the class I MHC display on the surface of circulating lymphoid cells. For this purpose, freshly isolated PBMC from two animals with AH, two animals convalescent from AH, four animals with CH, and four healthy animals were examined by flow cytometry (see below). In a parallel experiment, PBMC collected from two woodchucks prior to WHV infection and then during AH and from two other animals before WHV administration, during AH, and then during advanced CH were used for isolation of PBMC plasma membranes and evaluation of class I MHC heavy-chain presentation by immunoblotting.
Cells and plasma membranes.
Hepatocytes were isolated by two-step collagenase perfusion of livers from animals in study group 1 using methods reported previously (31, 33, 36). HPM were purified from the isolated hepatocytes by differential fractionation in sucrose gradients (31). The purity of HPM was determined by measuring the activities of marker enzymes for plasma membranes (5′-nucleotidase), microsomes (glucose-6-phosphatase), and mitochondria (cytochrome c oxidase) (31, 33). These evaluations showed that the HPM were essentially free from subcellular contamination and of comparable purity. Kidney plasma membranes (KPM) were isolated from the woodchuck kidney homogenates by using the HPM isolation procedure.
Splenic lymphomononuclear cells (splenocytes), containing mainly lymphocytes, were prepared by two sequential density gradient centrifugations in Histopaque 1119 (Sigma Chemical Co., St. Louis, Mo.) as described previously (32). After depletion of residual erythrocytes, spleen plasma membranes (SPM) were purified by hypotonic treatment and sucrose gradient centrifugation (32).
PBMC were isolated from freshly drawn blood treated with EDTA by a gradient centrifugation in Histopaque (22, 32). Plasma membranes were prepared by hypotonic shock, brief sonication, and subsequent removal of nuclei and cellular debris by centrifugation, as described previously (23). The protein content was determined by a bicinchoninic acid protein assay (Sigma).
MAb to woodchuck class I MHC heavy chain.
Mouse monoclonal antibody (B1b.B9 MAb) against a nonpolymorphic epitope of the woodchuck class I MHC heavy chain was generated and characterized in our previous study (32). This antibody recognizes two polypeptide species of woodchuck class I heavy chains with molecular masses of 43 and 39 kDa.
Dot and Western immunoblotting.
Plasma membranes, subcellular fractions, or tissue homogenates were immobilized at the desired protein concentration onto nitrocellulose (pore size, 0.45 μm; Bio-Rad Laboratories, Richmond, Calif.); exposed to a blocking solution containing 3% bovine serum albumin, 1% normal goat serum, 0.05% Tween 20, and 0.001% sodium azide in phosphate-buffered saline (pH 7.4) (PBS); and incubated with B1b.B9 MAb under conditions described previously (32). After incubation and washing, the blots were exposed to goat anti-mouse antibody conjugated with alkaline phosphatase (Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.) and washed, and the reactions were developed (9).
For Western immunoblotting, purified membrane preparations were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 20 μg of protein/lane and separated proteins were electrotransferred onto nitrocellulose and immunoblotted as described previously (9, 33). The efficiency of protein transfer and the molecular masses of the detected polypeptide species were determined using prestained molecular weight markers (Bio-Rad Laboratories). The relative expression of class I MHC heavy and light chains was determined by densitometry using a computerized Chemi-Imager 4000 System (Canberra-Packard Canada Ltd., Mississauga, Ontario, Canada).
Immunohistochemical staining.
Cryostat sections 4 μm thick were cut from frozen liver and spleen tissue blocks, air dried, and fixed in cold acetone-chloroform (1:1) mixture for 5 min at ambient temperature (32). The sections were hydrated in PBS, incubated for 45 min with B1b.B9 MAb or PBS (control), and washed for 30 min in three changes of PBS. Subsequently, sections were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (IgG) (H+L) antibody (Jackson ImmunoResearch Laboratories Inc.) for 30 min, washed three times for 10 min each in PBS, mounted in 20% glycerol buffered in PBS, and examined using a Leitz-Diaplan epifluorescence microscope.
Flow cytometry.
Freshly isolated PBMC, approximately 5 × 105 cells/sample with viability greater than 95% by trypan blue exclusion, were incubated with B1b.B9 MAb or PBS (control) and then with anti-mouse antibody labeled with FITC (Jackson ImmunoResearch Laboratories Inc.) by a procedure described previously (32). Cell analysis was done using a FACS Star-Plus flow cytometer (Becton-Dickinson, Mississauga, Ontario, Canada).
DNA and RNA extractions.
DNA was isolated by proteinase K digestion, phenol-chloroform extraction, and precipitation with ethanol by standard methods (49). Total RNA was extracted from mechanically pulverized frozen tissue using TRIzol reagent (Gibco BRL, Grand Island, N.Y.). Nucleic acids were quantitated by standard spectroscopic analysis and stored in small aliquots at −80°C prior to use.
Dot blot detection of tissue WHV DNA.
For WHV DNA hybridization, 5 μg of liver or 10 μg of spleen DNA was denatured by boiling for 10 min in 200 μl of 6× SSC (pH 7.0) (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), chilled on ice, and immobilized on a nylon membrane (Hybond-N; Amersham Life Sciences, Arlington Heights, Ill.) using a BioDot SF apparatus (Bio-Rad Laboratories). The membrane was hybridized for 16 h at 65°C to a full-length, linearized, cloned WHV DNA (41) labeled with [32P]dCTP by a random primer method (Rediprime; Amersham Life Sciences). The blot was washed to final stringency of 0.2× SSC–0.1% SDS for 30 min at 65°C and exposed to X-ray film (XRP-1 or XAR-5; Eastman Kodak Co., Rochester, N.Y.) with an intensifying screen or to a phosphor screen (Canberra-Packard Canada Ltd.). For estimation of the levels of WHV DNA expression, autoradiographic or phosphor images of hybridization signals were quantitated for equivalence with 10-fold serial dilutions of recombinant, complete WHV DNA using a chemi-image analyzer or a Cyclone phosphorimaging system (Canberra-Packard Canada Ltd.), respectively.
Cloning of woodchuck class I MHC heavy chain, β2-microglobulin, TAP1, TAP2, CD3 and IFN-γ.
Total RNA isolated from the spleen of a healthy woodchuck was reverse transcribed to cDNA as described previously (35). The resulting cDNA was amplified by PCR with oligonucleotide primers which were deduced through interspecies comparison of human, mouse, rat, and rabbit (when available) mRNA sequences using PC Gene software (IntelliGenetics Inc., Mountain View, Calif.). For amplification of the woodchuck class I MHC heavy-chain sequence, sense primer MHC-W (5′-AGTCTTTCCGAGTGAACCTGCGGAC) and antisense primer W-CHM (5′-TCCTTTCCCATCTGAGCTGTGCTTC) were used. The woodchuck β2-microglobulin was amplified with the sense and antisense degenerative primers β2M-plus (5′-ATGKCTCGCTCSGTGRCC) and β2M-minus (5′-TTACATGTCTCGRTCCCAS), respectively. The woodchuck TAP1 sequence was amplified with sense primer APT-1 (5′-TTCTTYACRGGCCGCMTCACTGAC) and antisense primer 1-PAT (5′-AGGGCACTGGTGGCATCRTC), whereas the TAP2 sequence was amplified with sense primer APT-2 (5′-TTCGGGTCGTGTRATTGACATCC) and antisense primer 2-PAT (5′-CTTSACAGAACCSGAGAACAGCAC). For identification of the woodchuck CD3 gene, whose transcripts are specific for T lymphocytes, primers CD3P (5′-CTGGGACTCTGCCTCTTATC) and CD3M (5′-GCTGGCCTTTCCGGATGGGCTC), with sequences essentially identical to those reported by others (38), were used. Woodchuck gamma interferon (IFN-γ) was amplified with primers designed in this laboratory, W-IFNG (5′-GGCCTAACTCTCTCTGAAACG) and W-GNFI (5′-GAGGACTGTTATTTGGATGC). In addition, an approximately 315-bp fragment of woodchuck β-actin and a 570-bp fragment of glyceraldehyde-3-phosphate dehydrogenase (GADPH) were generated by PCR using woodchuck liver cDNA and oligonucleotide primers published previously for human β-actin (16) and mouse GADPH (24). For PCR amplification of cDNA to be cloned, samples were denatured at 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, 52°C for 2 min, and 72°C for 3 min. The last cycle was followed by an elongation step at 72°C for 10 min. PCR amplifications were carried out in a TwinBlock thermal cycler (Ericomp Inc., San Diego, Calif.) using 5 μl of the reverse transcription reaction product and a standard reagent mixture described previously (35). The specificity of the amplified woodchuck DNA fragments was verified by Southern blot analysis using internal oligonucleotide probes, except for the class I MHC heavy chain, which was probed with a 32P-labeled fragment of rabbit class I MHC (exon 4) excised from plasmid pUC12-RLA-A (ATCC 77230) (28). After confirmation of specificity, the DNA amplicons were purified from low-melting-point agarose using the Wizard PCR Preps DNA purification system (Promega Corp.) and cloned into vector pCRII using a TA cloning kit (Invitrogen, Carlsbad, Calif.). After plasmid amplification, the specificity and orientation of the fragments cloned were validated by sequencing by using either the fmol DNA sequencing system (Promega Corp.) or a fluorescence-based automated sequence analyzer (LI-COR; LiCor Inc., Lincoln, Neb.).
Analysis for class I MHC-affiliated RNAs.
Liver RNA was analyzed for class I MHC heavy chain, β2-microglobulin, TAP1, and TAP2, as well as for CD3, WHV, β-actin, and GADPH expression, by Northern blot hybridization. Transcription of the above genes was also assessed in the spleens of the animals examined. Briefly, 10 or 20 μg of total liver or spleen RNA was separated electrophoretically using a standard formaldehyde–denaturing-agarose method (3), blotted onto a nylon membrane (Hybond-XL; Amersham Life Sciences) by download capillary transfer, and baked for 2 h at 80°C. The blots were hybridized for 18 h at 42°C to probes excised from relevant clones by EcoRI digestion and labeled with 32P using the Strip-EZ DNA kit (Ambion Inc., Austin, Tex.). After hybridization, the membranes were washed to a final stringency of 0.2× SSC–0.1% SDS at 42°C and exposed for autoradiography or phosphorimager analysis. Prior to rehybridization, probes were stripped from the membranes as specified by the manufacturer of the Strip-EZ DNA kit. The signal intensity was quantified and equalized to β-actin expression by densitometry.
Intrahepatic IFN-γ RNA expression was estimated by relative PCR using woodchuck liver cDNAs and oligonucleotide primers listed above. PCR was performed in the linear amplification range under the following conditions: 94°C for 5 min, 51°C for 2 min, and 72°C for 1 min in the first cycle; then 94°C for 1 min, 52°C for 1.5 min, and 72°C for 1.5 min for 34 cycles; and a final extension at 72°C for 10 min. As loading controls, the same cDNA samples were amplified with β-actin primers. The resulting PCR products were analyzed by Southern blot hybridization with appropriate cloned probes and compared to β-actin expression with a phosphorimager analyzer.
Nucleotide sequences accession numbers.
The accession numbers for the woodchuck nucleotide sequences derived in this study submitted to GenBank were as follows: class I MHC heavy chain, AF232723; TAP1, AF232724; TAP2, AF232725; β2-microglobulin, AF232726; CD3, AF232727; IFN-γ, AF232728; GADPH, AF232729; and β-actin, AF232730.
RESULTS
Upregulated expression of class I MHC on the hepatocyte surface is a hallmark of acute but not chronic WHV infection.
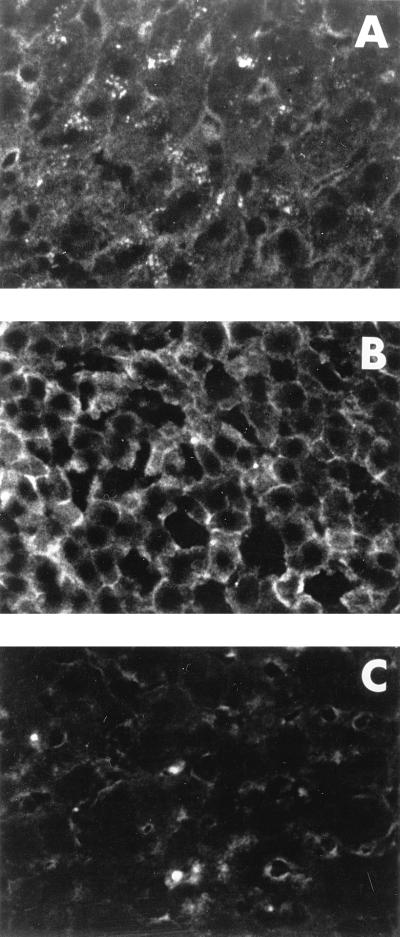
Liver sections from healthy and convalescent animals showed immunofluorescent staining of sinusoidal lining cells and the bile duct epithelia but little or no expression of class I MHC heavy chain at hepatocyte outer membranes (Fig. 1A). In the livers of animals with AH, a strong staining of periportal and intralobular inflammatory infiltrates and membranes of the hepatocytes adjacent to these infiltrates were seen. The lobular hepatocytes, not associated with inflammatory cells, showed a membranous staining of part or the entire surface, while their cytoplasm essentially remained negative (Fig. 1B). Woodchucks chronically infected with WHV had an enhanced display of the class I antigen on hepatocytes only in the areas of inflammatory infiltrations. Hepatocytes distant from the infiltrates were nonreactive (Fig. 1C); however, some cells, usually occurring in clusters, had weak staining at their outer membranes. Overall, the class I MHC pattern was noticeably different in AH and CH, but there was no relation between this display and the overall histological severity of liver injury. There also was no differences in the MHC antigen staining on sinusoidal lining endothelium or on the bile duct epithelium in livers from infected, healthy, or recovered animals. Sections incubated with the second layer antibody alone showed the same minimal background staining in all livers examined.
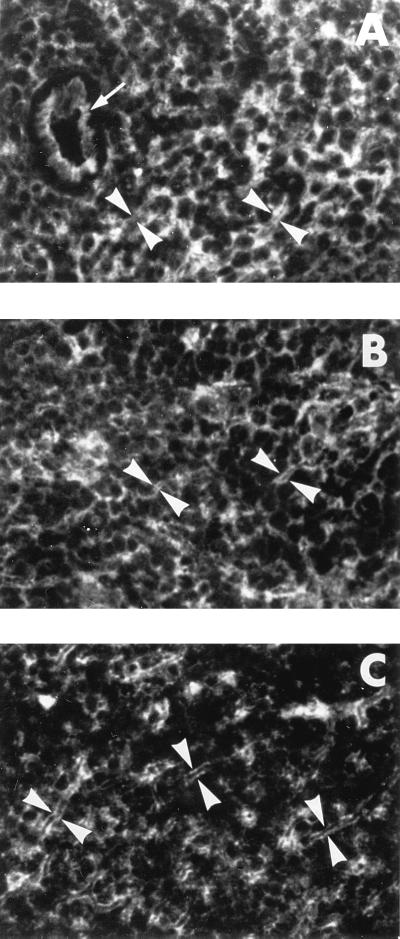
FIG. 1.
Immunofluorescent identification of the class I MHC expression in livers of healthy and WHV-infected woodchucks. Cryostat sections from hepatic tissue of a normal woodchuck (WM 2075) (A) and livers of animals with AH (WM 2121) (B) and CH (WF 2114) (C) were incubated with B1b.B9 MAb directed against woodchuck class I MHC heavy chain followed by FITC-labeled anti-mouse IgG. A plasma membrane-associated pattern of the class I MHC staining of intralobular hepatocytes is evident in the liver of the animal with AH but not in the livers of the healthy woodchuck and that with CH. Magnification, ×400.
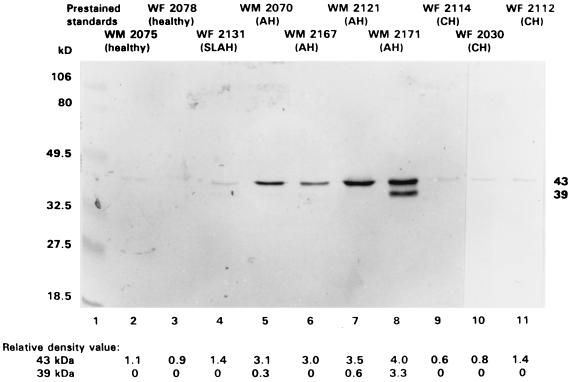
All HPM preparations isolated from study group 1 and probed with B1b.B9 MAb by Western blotting demonstrated the class I heavy-chain 43-kDa polypeptide, although the protein was displayed at markedly higher density in HPM from animals with AH (Table 2 and Fig. 2). The HPM from healthy and convalescent woodchucks, as well as those from animals with CH, showed noticeably lower expression of the polypeptide. In addition to the 43-kDa species, the 39-kDa polypeptide was detected on hepatocyte outer membranes from animals with AH (Fig. 2 and Table 2). This band was not identifiable on blots of the membranes from healthy, recovered, or chronically infected woodchucks. The detection of the 39-kDa polypeptide was consistent with our previous finding that only woodchuck cells displaying surface class I MHC molecules at the highest densities (e.g., normal splenic lymphoid cells) show both 43- and 39-kDa heavy-chain species (32).
TABLE 2.
Hepatocyte surface membrane expression of class I MHC heavy chain in woodchucks with WHV hepatitis and in control animals
| Category of disease and animalb | Histologic degree of hepatitis | HPM class I MHC heavy-chain expressiona
|
|
|---|---|---|---|
| 43-kDa protein | 39-kDa protein | ||
| Healthy | |||
| WM 2075 | 0 | 1.1 | 0 |
| WF 2078 | 0 | 0.9 | 0 |
| Resolution of AH | |||
| WF 2131 | 0/I | 1.4 | 0 |
| WF 2160 | 0/I | 1.1 | 0 |
| AH | |||
| WM 2070 | I | 3.1 | 0.3 |
| WM 2167 | II | 3.0 | 0 |
| WM 2121 | III | 3.5 | 0.6 |
| WM 2171 | III | 4.0 | 3.3 |
| CH | |||
| WF 2114 | I | 0.6 | 0 |
| WF 2030 | II | 0.8 | 0 |
| WM 2040 | II | 1.0 | 0 |
| WF 2020 | III | 0.7 | 0 |
| WF 2112 | III | 1.4 | 0 |
| WM 2150 | III | 0.9 | 0 |
Assessed by Western blotting with B1b.B9 MAb against woodchuck class I MHC heavy chain and expressed using a scale from 0 to 4, comparatively presenting the densities of the 39- and 42-kDa polypeptide bands based on integrated chemi-image density values.
WM, woodchuck male; WF, woodchuck female.
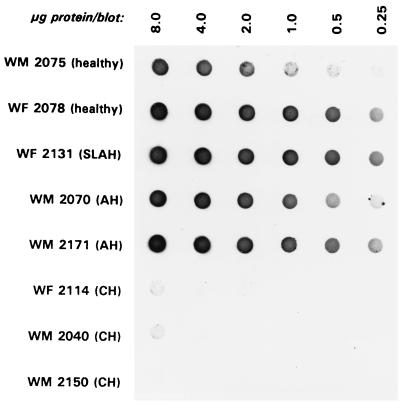
FIG. 2.
Expression of the class I MHC heavy chain on HPM in animals with AH and CH, healthy woodchucks, and an animal convalescent from self-limiting AH (SLAH). Purified hepatocyte outer membranes were separated at 20 μg of protein/lane on SDS-PAGE (12% polyacrylamide gel), electrotransferred onto nitrocellulose, and probed by Western blotting with B1b.B9 MAb against woodchuck class I MHC heavy chain. The positions of the class I 43- and 39-kDa heavy-chain polypeptides and the prestained protein standards (lane 1) are indicated on the right and left side, respectively. The relative density values of the identified heavy-chain protein bands (scale from 0 to 4) were assigned based on integrated chemi-image scanning values. The heavy-chain display is augmented in HPM from animals with AH, whereas HPM from woodchucks with CH, as well as those from healthy or recovered animals, have comparably very low contents.
Densitometric quantitation of the class I heavy-chain signals detected by Western (Fig. 2 and Table 2) or immunodot (Table 3) blotting confirmed a significant difference in the hepatocyte surface presentation of class I MHC between acutely infected and healthy, recovered, or chronically infected animals. Thus, HPM from either healthy or convalescent woodchucks, which had normal (WM 2075 and WF 2078) or nearly normal (WF 2131 and WF 2160) liver histology, displayed approximately the same amounts of class I heavy chain. In contrast, the quantity of MHC on HPM from AH was on average 3.5-fold greater than that on the membranes from healthy and convalescent woodchucks (Table 3). When HPM from animals with AH and CH were compared, a 3.2-fold-lower content of the heavy chain was found on the membranes derived from chronically infected animals (Table 3). Taken together, the hepatocyte surface expression of class I MHC was evidently elevated in woodchucks with AH but was essentially the same in woodchucks with CH and in the healthy or convalescent woodchucks.
TABLE 3.
Relative expression of cell surface class I MHC heavy chain and its RNA in woodchucks with AH and CH
| Category | MHC heavy-chain expressiona in:
|
MHC heavy-chain mRNA levelb in:
|
|||
|---|---|---|---|---|---|
| HPM | SPM | KPM | Liver | Spleen | |
| Healthy and convalescent animals (n = 4) | 100 | 100 | 100 | 100 | 100 |
| Animals with AH (n = 4) | 350 | 100 | 95 | 330 | 120 |
| Animals with CH (n = 6) | 110 | <5 | 100 | 350 | 95 |
The dot blots of the indicated plasma membrane preparations were probed with B1b.B9 MAb for class I heavy-chain expression, and the resulting signals were quantitated by chemi-image densitometry. The average integrated density values were calculated for each animal group and membrane type and are presented as percentages of the average amounts detected in HPM, SPM, or KPM derived from healthy and convalescent woodchucks, which were taken as 100%.
The class I MHC heavy-chain mRNA levels were normalized to the β-actin RNA signal in each tissue tested and are presented as percentages of the average amounts detected in livers or spleens of healthy and convalescent animals, which were taken as 100%.
It is of note that probing of the whole-liver homogenates with B1b.B9 MAb by immunodot and Western blotting did not show detectable variation in the hepatic class I MHC content between infected and healthy animals. This observation supported the conclusion that the identified difference was restricted predominantly to the hepatocyte surface. There was no variation in the class I heavy-chain display on KPM purified from the animals examined (Table 3).
Inhibition of hepatocyte surface class I MHC expression is associated with chronic WHV infection but not with hepatic virus load, severity of hepatitis, or intrahepatic level of IFN-γ induction.
The level of the class I heavy-chain expression on HPM was not related to the amount of WHV present in the liver. The average hepatic content of WHV DNA was 6.1 × 107 ± 1.2 × 107 (standard error of the mean) virus genome equivalents (vge)/μg of liver DNA in acutely infected animals and 8.2 × 107 ± 4.5 × 107 vge/μg in animals with CH. Also, the hepatic expression of WHV-specific mRNA, determined by Northern hybridization and densitometric analysis, was not meaningfully different between woodchucks with AH and CH (see Fig. 6). In animals which had recovered from AH (WF 2131 and WF 2160), traces of WHV genome were identifiable in the livers by nested PCR followed by Southern hybridization of the amplified products (sensitivity, <102 WHV vge/ml). This result corroborates our previous findings which demonstrated that traces of replicating WHV persist in the liver for life after resolution of AH (35). In these serologically silently infected animals, hepatocyte membrane expression of class I antigen was not appreciably different from that in healthy woodchucks. Collectively, these data showed that comparable hepatic loads of WHV were accompanied by strikingly distinct hepatocyte surface display of class I MHC molecules that depended on whether HPM originated from acutely or chronically infected animals.
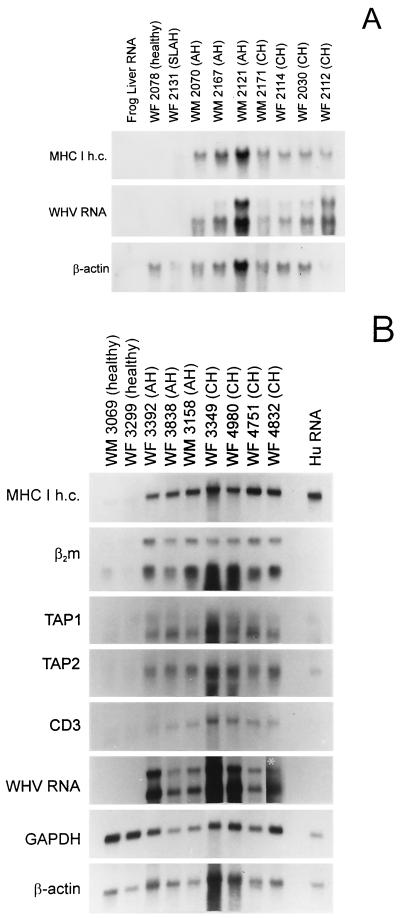
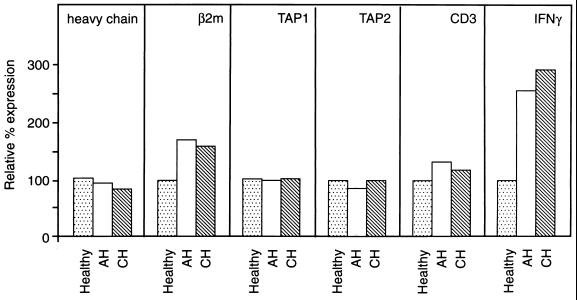
FIG. 6.
Effect of acute and chronic WHV infection on hepatic expression of class I MHC-affiliated genes. Total hepatic RNA was isolated from woodchucks with different histological severity of AH or CH, an animal convalescent from AH (SLAH), and healthy woodchucks in study group 1 (A) or 2 (B) (for details, see Table 1). The RNA was probed by Northern blotting with 32P-labeled woodchuck class I MHC heavy chain (MHC I h.c.), β2-microglobulin (β2m), TAP1, TAP2, CD3 cDNA, complete recombinant WHV DNA, or cloned woodchuck GADPH cDNA and/or human β-actin cDNA as housekeeping genes. Frog liver RNA (A) and human RNA (Hu RNA) (B) were used as species specificity references. The white asterisk in panel B depicts a signal that required increased exposure. The expression of class I MHC heavy and light chains, TAP1, TAP2, and CD3 RNAs is augmented to the same extent in the livers of both acutely and chronically infected animals.
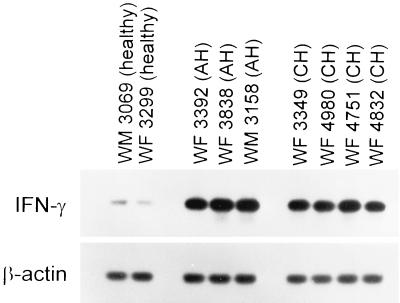
As illustrated in Fig. 2 and Table 2, there was also no correlation between the hepatocyte surface presentation of class I molecules and the histological severity of liver disease. Albeit, HPM from animals with the most severe AH (WM 2121 and WM 2171) tended to display larger amounts of class I heavy chain than did HPM from woodchucks with mild or moderate AH (WM 2070 and WM 2167). HPM from animals with chronic infection, which had histologically very mild (WF 2114), moderate (WF 2030 and WM 2040), or severe (WF 2020, WF 2112, and WM 2150) hepatitis, showed comparably low levels of surface class I heavy chain. The intrahepatic CD3 RNA, an indicator of T-lymphocyte infiltrations, was barely detectable in healthy animals but was elevated to the same level in woodchucks with AH and CH (see Fig. 6). Similarly, the levels of IFN-γ RNA in the liver were comparable in acutely and chronically infected woodchucks but were on average approximately 4.5-fold greater than those in healthy animals (Fig. 3). Overall, these data pointed out that the status of the class I MHC expression on hepatocyte surface in actively progressing hepatitis was not related to the histological severity of hepatocellular injury, the degree of lymphocytic infiltrations, or the intrahepatic IFN-γ activity but was clearly connected with chronicity of WHV infection.
FIG. 3.
Expression of IFN-γ mRNA in livers of woodchucks with AH or CH. Total liver RNA was reverse transcribed to cDNA and amplified with woodchuck IFN-γ- and β-actin-specific primers, as described in Materials and Methods. The amplified PCR products were detected by Southern blot hybridization. The signals showed that the hepatic levels of IFN-γ induction are comparable in animals with AH and CH.
Chronic but not acute WHV infection is associated with downregulation of class I MHC antigen expression in lymphoid cells.
Because WHV replicates in both hepatocytes and lymphoid cells, it was of interest to establish whether the class I antigen display differs in the lymphatic tissue in AH and CH. Immunohistochemical staining of spleen sections from healthy and WHV-infected animals showed the same intensity of class I MHC expression on the cells lining splenic sinuses and blood vessels. The staining of the periarteriolar lymphoid sheaths, which are enriched in lymphoid cells, also was similar in healthy (Fig. 4A), recovered (data not shown), and acutely infected (Fig. 4B) woodchucks. However, lymphoid cells in the same periarteriolar regions in animals with CH showed recognizably less intense and sometimes almost absent staining for the class I heavy chain, even though the endothelial cells remained positive (Fig. 4C).
FIG. 4.
Splenic distribution of class I MHC in healthy and WHV-infected woodchucks. (A and B) Sections from spleens of a healthy animal (WM 2075) (A) and from a woodchuck with AH (WM 2167) (B) incubated with B1b.B9 MAb and FITC-labeled anti-mouse IgG show immunofluorescent staining of lymphocytes as well as endothelium lining intrafollicular capillaries (arrowheads) and the central arteriole (arrow). (C) The same staining of a spleen section from a woodchuck with CH (WM 2150) demonstrates class I MHC expression on endothelium of blood capillaries (arrowheads) but not on lymphoid cells. Magnification, ×400.
Western blotting of SPM isolated from WHV-infected, recovered, or healthy woodchucks showed both 43- and 39-kDa heavy-chain polypeptide bands. The same protein bands, or in some cases only the 43-kDa species, were exhibited at lower densities in SPM from animals with CH (data not shown). Determination of the class I heavy-chain display by immunodot blotting and subsequent densitometric analysis revealed closely comparable levels of class I heavy chain in SPM from healthy, convalescent, and acutely infected animals, similar to the results of Western blot analyses (Table 3). In contrast, the class I heavy-chain content was evidently reduced in SPM from woodchucks with CH. Overall, SPM from chronically infected animals displayed more than a 20-fold-lower level of class I MHC than did SPM from woodchucks with AH or healthy controls (Table 3). Interestingly, identical results were obtained when the whole-spleen homogenates, instead of SPM, were probed with B1b.B9 MAb, as illustrated in Fig. 5. This finding suggested that class I MHC expression in chronic infection is not confined to the lymphoid cell surface, as seems to be the case in WHV-infected hepatocytes, but, rather, has a pancellular character. In contrast to splenic tissue, immunodot blots of KPM preparations (Table 3), as well as whole-kidney homogenates from the same animals (data not shown), did not show any variation in the class I MHC heavy-chain content.
FIG. 5.
Expression of class I MHC heavy chain in spleens of woodchucks with AH or CH and from control animals. Serial twofold dilutions of whole-spleen homogenates prepared from woodchucks with AH or CH, healthy animals, and a woodchuck convalescent from self-limited AH (SLAH) were immobilized onto nitrocellulose at the indicated protein concentrations and probed with woodchuck class I heavy-chain-specific B1b.B9 MAb. The heavy-chain expression is faint in splenic tissue from animals with CH but is intense and not altered in animals with AH and in convalescent or healthy animals.
The WHV genome levels in the spleens of animals with AH and CH were comparable, with an average viral DNA content of 8.4 × 105 ± 3.4 × 105 vge/μg of splenic DNA for animals with AH and 1.9 × 106 ± 1.1 × 106 vge/μg for woodchucks with CH, while WHV mRNA was not detectable by Northern blotting. Renal tissue was WHV DNA and mRNA nonreactive by the same assays in the animals examined.
In separate experiments, the density of the class I MHC heavy-chain expression on intact, freshly isolated PBMC and on purified PBMC surface membranes was determined. Analysis of comparable numbers of PBMC by flow cytometry and the same amounts of PBMC membrane proteins by immunodot blotting with B1b.B9 MAb failed to demonstrate any consistent difference between healthy animals and those with AH or CH (data not shown). PBMC membranes probed for class I heavy chain by Western blotting displayed the 43-kDa protein only (data not shown).
Both acute and chronic WHV infection upregulates class I MHC-linked gene transcription in hepatic but not in splenic tissue.
To determine whether the detected variation in the cell surface presentation of class I MHC molecules between AH and CH reflects a difference in the transcriptional activity of the relevant gene loci, RNA from the livers and spleens of the animals investigated was probed for class I MHC heavy and light chains and TAP1 and TAP2 transcripts. In addition, to learn about possible differences in the T-lymphocyte contents, RNA preparations from livers and spleens of animals belonging to study group 2 were analyzed for expression of CD3 gene transcripts. Northern blot hybridization of hepatic RNA showed markedly elevated levels of heavy-chain mRNA in both acutely and chronically infected woodchucks compared to those in healthy or convalescent animals (Fig. 6), as well as increased expression of β2-microglobulin, TAP1, and TAP2 mRNA (Fig. 6B). Quantitation of the hybridization signals, corrected to β-actin gene expression, revealed a greater than threefold-enhanced intrahepatic transcription of the class I heavy-chain gene in woodchucks with AH and a similar increase in animals with CH compared to normal or recovered woodchucks (Table 3). In general, the hepatic class I MHC-affiliated genes were upregulated to the same extent in AH and CH, indicating that this augmentation was not related to the duration of WHV infection. Also, the intrahepatic levels of the CD3 RNA were the same in animals with acute and chronic liver disease, revealing that the magnitude of lymphocytic infiltrations was comparable in these two phases of WHV-induced necroinflammation in the animals studied (Fig. 6B). Similarly, as already presented (Fig. 3), the extent of induction of IFN-γ was the same in the livers from acutely and chronically infected woodchucks. Taken together, these results imply that while overexpression of class I molecules on hepatocytes in animals with AH could be explained as a direct consequence of activation of class I MHC genes, the defect in presentation of these molecules in CH was certainly related to posttranscriptional suppression.
Splenic tissue showed comparable levels of the class I heavy chain, TAP1, and TAP2 RNAs, as well as CD3 RNA, both in acutely or chronically infected woodchucks and in the healthy or recovered animals (Fig. 7). The exception was β2-microglobulin RNA, which was moderately elevated in WHV-infected in comparison to healthy animals. In addition, the splenic level of IFN-γ RNA was identical in acute and chronic infection but at least twice that detected in healthy controls (Fig. 7). Overall, the findings showed that in contrast to the diseased livers, neither acute nor chronic WHV infection meaningfully upregulates transcription of the class I MHC-linked genes in splenic tissue. These data imply that the defective expression of class I MHC molecules in splenic lymphocytes in chronic WHV infection does not result from inhibited transcription of the relevant cellular genes and therefore has to occur at the posttranscriptional level.
FIG. 7.
Relative expression of class I MHC heavy-chain and related gene mRNA in splenic tissue from woodchucks with acute or chronic WHV hepatitis. Total RNA isolated from spleens of animals with AH (n = 3) and CH (n = 4) and from healthy animals (n = 2) (study group 2) were probed with 32P-labeled woodchuck class I heavy chain, β2-microglobulin (β2m), TAP1, TAP2, CD3, or β-actin by Northern blotting, whereas IFN-γ was assessed by PCR and Southern blotting as described in Materials and Methods. Hybridization signals were quantitated by phosphoimager densitometry, normalized to the β-actin, and presented as a percentage of the average amount detected in the spleens of healthy woodchucks, which were taken as 100%.
DISCUSSION
Analysis of hepatocyte surface membranes in this study revealed a significant difference in hepatocyte presentation of class I MHC molecules in acute and chronic phases of hepadnavirus hepatitis. While acute WHV infection is accompanied by increased hepatocyte surface display of class I MHC, chronic disease, independent of the severity of liver injury and the duration of chronicity, is uniformly associated with a decrease in the expression of these molecules similar to that seen in healthy animals and those convalescent from AH. This defective class I antigen display in CH occurs in spite of the enhanced transcription of relevant class I MHC-affiliated hepatic genes, equal to that observed in the livers of acutely infected hosts. Considering the above findings, our study demonstrates that hepatocyte presentation of the class I MHC molecules is evidently enhanced in acute disease but dramatically suppressed at the posttranscriptional level in chronic stage of WHV hepatitis. Importantly, this impairment in chronic infection also affects lymphoid cells, another site of virus propagation. However, in contrast to the diseased livers, transcription of the class I MHC-linked genes is not altered in the infected splenic tissue compared to healthy animals. Also, the splenic display of the class I MHC molecules is essentially identical in animals with AH and in the healthy or convalescent woodchucks. In addition, neither acute nor chronic WHV infection affects the class I MHC antigen expression in kidneys, which were found to be WHV nonreactive. Collectively, the present findings show that (i) the liver-restricted, augmented transcription of the class I MHC-affiliated genes is an invariable characteristic of any active liver inflammation induced by WHV; (ii) the severely impaired presentation of class I MHC molecules is a unique feature of chronic WHV infection; (iii) the decreased cellular presentation of class I MHC in chronic disease appears to be restricted to tissues in which WHV actually replicates; and (iv) the deficient hepatocellular and lymphoid cell display of class I MHC in CH is a consequence of a virus-dependent posttranscriptional inhibition. Overall, the data from our study reveal a transparent although multifarious interplay among phases of hepadnavirus hepatitis, the status of class I MHC-affiliated gene transcription in virus infected organs, and the expression of the class I MHC molecules on cells of these organs.
The class I MHC antigen processing and presentation pathway involves formation of the trimeric complexes constituted by class I heavy chain, β2-microglobulin, and a proteolytically generated peptide (19). The assembly of the stable class I MHC molecules requires the translocation of cytosolically produced peptides into the lumen of the endoplasmic reticulum. This process is facilitated by transmembrane transporter proteins, referred to as TAP1 and TAP2, which deliver peptides into the endoplasmic reticulum to bind to empty class I heavy-chain–light-chain heterodimers (48). The created trimeric complexes are transported to the cell surface for interaction with specific CTL. The current model also includes auxiliary molecules, such as proteasomes, in the generation of peptides for loading onto class I molecules and emphasizes a role for IFN-γ in the modulation of peptide processing and in upregulation of genes involved in the antigen class I MHC presentation pathway (reviewed in references 14 and 51).
As uncovered in this study, progressive acute and chronic WHV hepatitis is accompanied by augmentation of hepatic class I heavy-chain, light-chain, and TAP genes, as well as by the increased levels of hepatic T-cell and IFN-γ RNAs. Since lymphomononuclear cell infiltrations are an invariable feature of active liver inflammation in hepadnavirus infection and since activated T lymphocytes and NK cells, secreting inflammatory cytokines including IFN-γ (17, 47), are constituents of these infiltrates, induction of intrahepatic IFN-γ could be mainly responsible for activation of class I MHC-affiliated genes in WHV hepatitis. This is supported by the fact that regulation of class I MHC genes by IFN-γ occurs primarily at the level of gene transcription (52). In addition to the indirect augmentation through IFN-γ, direct upregulation of the class I MHC genes by viral proteins might be possible. It has been shown in vitro that the HBV X protein can transactivate the class I MHC heavy-chain promoter in the virus-transfected HepG2 and related liver cell lines, leading to a three- to fourfold increase in the heavy-chain RNA and protein levels (53). Since this event has been observed in the absence of T cells and independently of IFN-γ, this may suggest that the HBV X protein can directly modulate class I MHC gene activity in cultured liver cells. It has also been postulated that HBV X, by interfering with proteasome functions, may prevent viral peptide interaction and presentation by class I molecules (21). Determining whether these hypothetical mechanisms operate in in vivo-infected hepatocytes requires further studies.
In contrast to the livers, the activity of class I MHC genes was not altered in the spleens of WHV-infected animals, except for that of β2-microglobulin RNA. The divergent effect of WHV infection on the class I MHC gene transcription in the liver and the spleen could be due to an innate difference in the levels of the gene expression between these two organs. Quantitative analysis performed in this study showed that class I heavy-chain mRNA is present at three- to fourfold-greater levels in the splenic than in the hepatic tissue of healthy woodchucks. It is possible that while viral infection is capable of upregulating class I genes in hepatocytes, where their transcriptional activities are naturally low, it is not able to exert this additive effect in lymphatic organs like the spleen, where the gene expression is inherently high. Therefore, WHV infection may act as a conditional transcriptional inducer whose indirect or direct modulatory effect depends on the microenvironment regulating the local activity of class I MHC genes. On the other hand, although perhaps less likely, the lack of class I gene upregulation in the infected spleens might be a consequence of a 10- to 100-fold-lower WHV DNA and RNA content than that observed in the livers of these animals. Conceivably, virus replicating less efficiently or occurring at low levels in invaded cells might be unable to induce an identifiable increase in transcription of the class I MHC-linked genes. This situation seems to be true for livers of woodchucks convalescent from AH, which support persistent WHV replication at low levels (35) but which do not show any noticeable change in class I heavy-chain RNA or protein expression. In addition, the splenic T-cell and IFN-γ RNA levels were identical in acutely and chronically infected animals. Therefore, a diminished T-cell number or a decrease in the local activity of IFN-γ cannot account for the observed class I antigen inhibition in splenocytes in chronic WHV infection.
The equally augmented expression of hepatic class I MHC-affiliated genes in AH and CH should imply enhanced synthesis of class I heterodimers and the availability of processed viral peptides for their loading. Consequently, this should lead to the increased presentation of class I complexes on liver cells irrespective of the phase of hepatitis. However, this situation exists only in acute infection, where the HPM class I heavy-chain expression was evidently higher than that detected on HPM from healthy or convalescent animals and appeared to be proportional to the elevated transcription from the respective cellular genes. In contrast, the reduced hepatocyte display of class I heavy chain in chronic infection was accompanied by an increased level of RNA like that in AH (Table 3). In spleens of chronically infected animals, unaltered expression of the class I MHC-affiliated genes was associated with a dramatic (more than 20-fold) reduction in class I heavy-chain presentation when assessed by immunoblotting. In general, the results of these quantitative analyses of class I display in isolated hepatocyte and splenocyte outer membranes agreed with the results of immunofluorescent staining of tissue sections, but, as anticipated, they were substantially more sensitive and objective. In summary, the obtained data clearly document that the disparity between class I-affiliated gene activity and class I antigen display in chronic infection is not related to downregulation of gene transcription; therefore, it must be due to a virus-dependent posttranscriptional interference that is unique for cells persistently supporting WHV replication. However, they do not exclude the possibility that the inhibited presentation of class I molecules can be a consequence of their impaired trafficking to the cell surface or enhanced recycling. The observed lack of heavy-chain accumulation in the cytoplasm of the chronically infected hepatocytes by immunohistochemical staining must be interpreted with caution, considering the relatively low sensitivity of this method.
Many viruses inhibit the class I surface molecules on invaded cells to avoid cytopathic or noncytopathic elimination initiated by specific CTLs (reviewed in references 13, 37, 40, and 45). It is known that viral proteins may induce posttranscriptional inhibition of class I antigen by interfering with the generation or transport of peptides predestined for interaction with class I heterodimers in the endoplasmic reticulum. They may also disrupt the class I complex assembly, trafficking, and cell surface presentation or increase their degradation (reviewed in reference 37). Frequently, the same virus uses a variety of strategies mediated by more than one viral factor. In the context of the ability of WHV to suppress class I MHC antigen display in different cell types, it is conceivable that the virus may also utilize multifactorial mechanisms acting at different posttranscriptional levels of the class I MHC presentation pathway.
The data collected so far reveal that the deficient expression of class I molecules on hepatocytes coincides with another unique feature of hepatocyte surface that is characteristic of chronic WHV hepatitis (31, 33, 36). We have previously shown that HPM from chronically infected woodchucks, independent of the histological severity of hepatitis and the duration of chronicity, contain large quantities of WHV envelope proteins irreversibly incorporated into the membrane lipid bilayer (31, 36). Assessments of the WHs antigenic content and the binding of exogenous WHsAg to HPM from different forms of WHV-induced liver pathology revealed that HPM from animals with CH have the largest amounts of the integrated antigen and are characterized by an inability to bind exogenous WHsAg (36), indicating that a massive (saturable) quantity of the virus envelope material has been inserted. We have hypothesized that this explicit feature of hepatocyte surface membrane in chronic disease, which naturally coexists with the abundant amounts of the same viral proteins in the circulation, may constitute an important element of protection of infected hepatocytes against immunoelimination and therefore contributes to prolonged liver disease and virus persistence (31, 33; reviewed in reference 29). Since the same HPM preparations were analyzed to determine the expression of class I MHC in this study (study group 1) and WHV envelope polypeptides in our previous work (33), we can conclusively state that suppression of class I molecules on hepatocytes occurs only in the context of a massive incorporation of WHV envelope proteins into HPM. This association, coexisting with heavy deposits of viral envelope material in the endoplasmic reticulum, which are typical for hepatocytes in chronically infected livers (33, 36), may exert a severe constraint on intracellular assembly and transport, as well as on presentation of class I molecules at the hepatocyte surface. This mechanism provides a reasonable explanation for the deficiency in class I antigen display on chronically infected hepatocytes; however, it is rather unlikely that it also operates in lymphoid cells, which, at best, express WHsAg in minute quantities. Therefore, it is possible that class I expression in the lymphatic system is downregulated by WHV through an alternative pathway.
Independent of the mechanism involved, defective expression of class I MHC complexes on hepatocytes in chronically infected hosts must have significant immunopathogenic consequences. Among them, as in other viral infections, the foremost could be evasion of immune surveillance by virus-specific CTL, leading to suppression of cytolytic and noncytolytic elimination of virus governed by these cells. This alone can contribute to the perpetuation of liver disease and facilitates virus persistence. However, there could also be other implications that are potentially important for WHV pathogenesis during chronic infection. Since virally infected cells with reduced class I antigen expression are considered to be inherently susceptible to attack from NK cells (4, 27), this may imply that non-class I MHC-dependent, cell-mediated cytotoxicity might play a role in controlling virus spread and induction of hepatocellular injury in chronic hepadnavirus infection. We have recently tested this intriguing possibility by evaluating perforin and Fas ligand-based cytotoxicity of circulating lymphoid cells from woodchucks with AH and CH hepatitis (20). The data from this study showed that the levels of perforin-dependent killing, which is the principal mechanism of cell elimination by NK cells (25, 46), are significantly enhanced in animals with AH but essentially the same in animals with CH and healthy animals. Although intrahepatic NK cells were not examined in this study, the above findings suggest that NK cell activity could be inhibited during the chronic phase of WHV hepatitis, despite suppressed hepatocyte class I MHC surface expression. In this context, of note are the past observations postulating that HBV surface antigen (HBsAg) depresses NK cell cytotoxicity, presumably by interfering with their binding to target cells (1, 8). If this is the case, it is conceivable that massive quantities of hepadnavirus envelope proteins inserted into hepatocyte outer membrane, coexisting with the large amounts of the same antigenic material in serum, may act as a negative modulator on intrahepatic NK cells during chronic infection. This mechanism, together with inhibition of hepatocyte surface class I MHC presentation, might constitute a strategy that the virus employs to escape elimination by both CTL and NK cells.
At least one more issue requires comment with regard to the findings of the present study. Identification of severely reduced expression of class I molecules in splenic lymphoid cells probably reflects a situation existing in other lymphatic organs in chronic WHV infection, although we did not see its evidence in circulating lymphoid cells. It is known that class I molecules are involved in the elaborate network of interactions between cells of the immune system and that they play key roles in regulating immune cell functions. Among these, it has been shown that interruption of the class I MHC presentation on lymphoid cells is sufficient to induce autoimmune reactions (15), which are a very common consequence of WHV infection (9–11). Therefore, downregulation of class I MHC presentation on lymphoid cells by hepadnavirus infection could deregulate a variety of host immune reactions whose effects, although not directly apparent, may profoundly diminish overall effectiveness of antiviral immune responses. This important issue awaits future studies. The identified defect in class I MHC presentation on both hepatocyte and lymphoid cells in chronic infection in this study once again exemplifies the complexity of the strategies utilized by hepadnavirus to survive within the host. Unraveling the molecular essence of this defect and its functional consequences will be necessary to fully understand the mechanisms underlying the perpetuation of liver disease and virus persistence in hepadnavirus infections.
ACKNOWLEDGMENTS
We thank Colleen L. Trelegan for expert assistance and Judy Foote and Edward Evelly for processing of tissues and histological stainings.
This work was supported by operating grants MT-14818 and RO-15174 to T.I.M. from the Medical Research Council of Canada. P.D.H. is a recipient of a Memorial University Fellowship.
REFERENCES
- 1.Azzari C, Rossi M E, Resti M, Caldini A L, Carbonella R, Ciappi S, Vierucci A. VIP restores natural killer cell activity depressed by hepatitis B surface antigen. Viral Immunol. 1992;5:195–200. doi: 10.1089/vim.1992.5.195. [DOI] [PubMed] [Google Scholar]
- 2.Barnaba V, Franco A, Balsano A, Alberti C, Benvenuto R, Balsano F. Recognition of hepatitis B envelope proteins by liver-infiltrating T lymphocytes in chronic HBV infection. J Immunol. 1989;143:2650–2655. [PubMed] [Google Scholar]
- 3.Brown T, Mackey K. Northern hybridization of RNA fractionated by agarose-formaldehyde gel electrophoresis. In: Ausebel F A, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1997. pp. 4.9.2–4.9.8. [Google Scholar]
- 4.Brutkiewicz R R, Welsh R M. Major histocompatibility complex class I antigens and the control of viral infections by natural killer cells. J Virol. 1995;69:3967–3971. doi: 10.1128/jvi.69.7.3967-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chisari F V, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 6.Chu C-M, Shyu W-C, Kuo R-W, Liaw Y-F. HLA class I antigen display on hepatocyte membrane in chronic hepatitis B virus infection: its role in the pathogenesis of chronic type B hepatitis. Hepatology. 1988;8:712–717. doi: 10.1002/hep.1840080358. [DOI] [PubMed] [Google Scholar]
- 7.Coffin C S, Michalak T I. Persistence of infectious virus in the offspring of woodchuck mothers recovered from viral hepatitis. J Clin Investig. 1999;104:203–212. doi: 10.1172/JCI5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Martino M, Rossi M E, Muccioli A T, Resti M, Vierucci A. Interference of hepatitis B surface antigen with natural killer cell function. Clin Exp Immunol. 1985;61:90–95. [PMC free article] [PubMed] [Google Scholar]
- 9.Diao J, Michalak T I. Virus-induced anti-asialoglycoprotein receptor autoimmunity in experimental hepadnaviral hepatitis. Hepatology. 1997;25:689–696. doi: 10.1002/hep.510250333. [DOI] [PubMed] [Google Scholar]
- 10.Diao J, Churchill N D, Michalak T I. Complement-mediated cytotoxicity and inhibition of ligand binding to hepatocytes by woodchuck hepatitis virus-induced autoantibodies to asialoglycoprotein receptor. Hepatology. 1998;27:1623–1631. doi: 10.1002/hep.510270623. [DOI] [PubMed] [Google Scholar]
- 11.Dzwonkowski P, Michalak T I. Autoantibody pattern in a woodchuck model of hepatitis B. Clin Investig Med. 1990;13:322–328. [PubMed] [Google Scholar]
- 12.Ferrari C, Penna A, Bertoletti A, Valli A, Antoni A D, Giuberti T, Cavali A, Petit M-A, Fiaccadori F. Cellular and immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442–3449. [PubMed] [Google Scholar]
- 13.Früh K, Gruhler A, Murli Krishna R, Schoenhals G J. A comparison of viral immune escape strategies targeting the MHC class I assembly pathway. Immunol Rev. 1999;168:157–166. doi: 10.1111/j.1600-065x.1999.tb01290.x. [DOI] [PubMed] [Google Scholar]
- 14.Früh K, Yang Y. Antigen presentation by MHC class I and its regulation by interferon γ. Curr Opin Immunol. 1999;11:76–81. doi: 10.1016/s0952-7915(99)80014-4. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y, Nathan D M, Li F, Li X, Faustman D L. Defective major histocompatibility complex class I expression on lymphoid cells in autoimmunity. J Clin Investig. 1993;91:2301–2307. doi: 10.1172/JCI116459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuqua S A, Fitzgerald S D, McGuire W L. A simple polymerase chain reaction method for detection and cloning of low-abundance transcripts. BioTechniques. 1990;9:206–211. [PubMed] [Google Scholar]
- 17.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 18.Guidotti L G, Rochford R, Chung J, Shapiro M, Purcell R, Chisari F V. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 19.Hill A, Ploegh H. Getting the inside out: the transporter associated with antigen processing (TAP) and the presentation of viral antigen. Proc Natl Acad Sci USA. 1995;92:341–343. doi: 10.1073/pnas.92.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgson P D, Grant M D, Michalak T I. Perforin and Fas/Fas ligand-mediated cytotoxicity in acute and chronic woodchuck viral hepatitis. Clin Exp Immunol. 1999;117:63–70. doi: 10.1046/j.1365-2249.1999.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Kwong J, Sun E C-Y, Liang T J. Proteasome complex as a potential cellular target of hepatitis B virus X protein. J Virol. 1996;70:5582–5591. doi: 10.1128/jvi.70.8.5582-5591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Y-M, Churchill N D, Michalak T I. Protease-activated lymphoid cell and hepatocyte recognition site in the preS1 domain of the large woodchuck hepatitis virus envelope protein. J Gen Virol. 1996;77:1837–1846. doi: 10.1099/0022-1317-77-8-1837. [DOI] [PubMed] [Google Scholar]
- 23.Jin Y-M, Pardoe I U, Burness A T H, Michalak T I. Identification and characterization of the cell surface 70-kilodalton sialoglycoprotein(s) as a candidate receptor for encephalomyocarditis virus on human nucleated cells. J Virol. 1994;68:7308–7319. doi: 10.1128/jvi.68.11.7308-7319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ju S-T, Panka D J, Cui H, Ettinger R, El-Khatib M, Sherr D H, Stanger B Z, Marshak-Rothstein A. Fas (CF95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 25.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 26.Lau J Y N, Bird G L, Naoumov N V, Williams R. Hepatic HLA antigen display in chronic hepatitis B virus infection: relation to hepatic expression of HBV genome/gene products and liver histology. Dig Dis Sci. 1993;38:888–895. doi: 10.1007/BF01295916. [DOI] [PubMed] [Google Scholar]
- 27.Ljunggren H-G, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 28.Marche P N, Tykocinski M L, Max E E, Kindt T J. Structure of a functional rabbit class I MHC gene: similarity to human class I genes. Immunogenetics. 1985;21:71–82. doi: 10.1007/BF00372243. [DOI] [PubMed] [Google Scholar]
- 29.Michalak T I. The woodchuck animal model of hepatitis B. Viral Hepatitis Rev. 1998;4:139–165. [Google Scholar]
- 30.Michalak, T. I. Occult persistence and lymphotropism of hepadnaviral infection: insights from the woodchuck viral hepatitis model. Immunol. Rev., in press. [DOI] [PubMed]
- 31.Michalak T I, Churchill N D. Interaction of woodchuck hepatitis virus surface antigen with hepatocyte plasma membrane in woodchuck chronic hepatitis. Hepatology. 1988;8:499–506. doi: 10.1002/hep.1840080312. [DOI] [PubMed] [Google Scholar]
- 32.Michalak T I, Churchill N D, Codner D, Drover S, Marshall W H. Identification of woodchuck class I MHC antigens using monoclonal antibodies. Tissue Antigens. 1995;45:333–342. doi: 10.1111/j.1399-0039.1995.tb02463.x. [DOI] [PubMed] [Google Scholar]
- 33.Michalak T I, Lin B. Molecular species of hepadnavirus core and envelope polypeptides in hepatocyte plasma membrane of woodchucks with acute and chronic viral hepatitis. Hepatology. 1994;20:275–286. [PubMed] [Google Scholar]
- 34.Michalak T I, Lin B, Churchill N D, Dzwonkowski P, Desousa J R B. Hepadna virus nucleocapsid and surface antigens and the antigen-specific antibodies associated with hepatocyte plasma membranes in experimental woodchuck acute hepatitis. Lab Investig. 1990;62:680–689. [PubMed] [Google Scholar]
- 35.Michalak T I, Pardoe I U, Coffin C S, Churchill N D, Freake D S, Smith P, Trelegan C L. Occult life-long persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology. 1999;29:928–938. doi: 10.1002/hep.510290329. [DOI] [PubMed] [Google Scholar]
- 36.Michalak T I, Snyder R L, Churchill N D. Characterization of the incorporation of woodchuck hepatitis virus surface antigen into hepatocyte plasma membrane in woodchuck hepatitis and in the virus-induced hepatocellular carcinoma. Hepatology. 1989;10:44–55. doi: 10.1002/hep.1840100111. [DOI] [PubMed] [Google Scholar]
- 37.Miller D M, Sedmak D D. Viral effects on antigen processing. Curr Opin Immunol. 1999;11:94–99. doi: 10.1016/s0952-7915(99)80017-x. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura I, Nupp J T, Rao B S, Buckler-White A, Engle R E, Casey J L, Gerin J L, Cote P J. Cloning and characterization of partial cDNAs for woodchuck cytokines and CD3 epsilon with applications for the detection of RNA expression in tissues by RT-PCR assay. J Med Virol. 1997;53:85–95. doi: 10.1002/(sici)1096-9071(199709)53:1<85::aid-jmv15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 39.Nayersina R, Fowler P, Guilhot S, Missale G, Cerny A, Schlicht H-J, Vitiello A, Chesnut R, Person J L, Redeker A G, Chisari F V. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol. 1993;150:4659–4671. [PubMed] [Google Scholar]
- 40.Oldstone M B. How viruses escape from cytotoxic T lymphocytes: molecular parameters and players. Virology. 1997;234:179–185. doi: 10.1006/viro.1997.8674. [DOI] [PubMed] [Google Scholar]
- 41.Pardoe I U, Michalak T I. Detection of hepatitis B and woodchuck hepatitis viral DNA in plasma and mononuclear cells from heparinized blood by the polymerase chain reaction. J Virol Methods. 1995;51:277–288. doi: 10.1016/0166-0934(94)00116-x. [DOI] [PubMed] [Google Scholar]
- 42.Penna A, Artini M, Cavalli A, Levero M, Bertoletti A, Pilli M, Chisari F V, Rehermann B, Del Prete G, Fiaccadori F, Ferrari C. Long-lasting memory T cell responses following self-limited acute hepatitis B. J Clin Investig. 1996;98:1185–1194. doi: 10.1172/JCI118902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pignatelli M, Waters J, Brown D, Lever A, Iwarson S, Schaff Z, Gerety R, Thomas H C. HLA class I antigens on the hepatocyte membrane during recovery from acute hepatitis B virus infection and during interferon therapy in chronic hepatitis B virus infection. Hepatology. 1986;6:349–353. doi: 10.1002/hep.1840060303. [DOI] [PubMed] [Google Scholar]
- 44.Rehermann B, Fowler P, Sidney J, Person J, Redeker A, Brown M, Moss B, Sette A, Chisari F V. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995;181:1047–1058. doi: 10.1084/jem.181.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rinaldo C R., Jr Modulation of major histocompatibility complex antigen expression by viral infection. Am J Pathol. 1994;144:637–650. [PMC free article] [PubMed] [Google Scholar]
- 46.Sayers T J, Brooks A D, Lee J K, Fenton R G, Komschlies K L, Wigginton J M, Winkler-Pickett R, Wiltrout R H. Molecular mechanisms of immune-mediated lysis of murine renal cancer: differential contributions of perforin-dependent versus Fas-mediated pathways in lysis by NK and T cells. J Immunol. 1998;161:3957–3965. [PubMed] [Google Scholar]
- 47.Scharton T M, Scott P. Natural killer cells are a source of interferon-γ that drives differentiation of CD4+ cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spies T, Bresnahan M, Bahram S, Arnold D, Blanck G, Mellins E, Pious D, DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990;348:744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- 49.Strauss W. Preparation of genomic DNA from mammalian tissue. In: Ausebel F A, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1997. pp. 2.2.1–2.2.3. [Google Scholar]
- 50.Tennant B C, Gerin J L. The woodchuck model of hepatitis B virus infection. In: Arias I M, Boyer J L, Fausto N, Jakoby W B, Schachter D A, Shafritz D A, editors. The liver: biology and pathobiology. New York, N.Y: Raven Press; 1994. pp. 1455–1466. [Google Scholar]
- 51.Van Endert P M. Genes regulating MHC class I processing of antigen. Curr Opin Immunol. 1999;11:82–88. doi: 10.1016/s0952-7915(99)80015-6. [DOI] [PubMed] [Google Scholar]
- 52.Wallach D, Fellous M, Revel M. Preferential effect of gamma interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature. 1982;299:833–836. doi: 10.1038/299833a0. [DOI] [PubMed] [Google Scholar]
- 53.Zhou D-X, Taraboulos A, Ou J-H, Yen T S B. Activation of class I major histocompatibility complex gene expression by hepatitis B virus. J Virol. 1990;64:4025–4028. doi: 10.1128/jvi.64.8.4025-4028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]