Abstract
Objectives
The aims of this study were to evaluate the response, outcome and prognostic factors in cats with clinically presumed relapsed low-grade alimentary lymphoma (LGAL) receiving cyclophosphamide as a first-line rescue therapy after failing chlorambucil treatment.
Methods
The medical records of 20 cats (from three institutions, between 2002 and 2017) treated with cyclophosphamide for relapsed LGAL after initial treatment with chlorambucil were retrospectively reviewed. Progression-free survival (PFS), overall survival time (OST) and the association of select variables with measures of outcome were assessed. Adverse events (AEs) were also described.
Results
Eighteen cats (90%) achieved a complete clinical response (CR) for a median duration of 239 days. The median PFS was 215 days. The median OST was 1065 days. The only clinical factor associated with a longer PFS was achievement of a CR with cyclophosphamide treatment. Cyclophosphamide was associated with few and reversible constitutional, gastrointestinal and hematologic AEs.
Conclusions and relevance
Cyclophosphamide appears to be a safe and effective first-rescue therapeutic option for cats with relapsed LGAL.
Keywords: Cyclophosphamide, lymphoma, alimentary, gastrointestinal, GI, relapsed, recurrent, rescue
Introduction
In cats, the alimentary tract is the most common anatomical location of lymphoma.1–5 Alimentary lymphoma is classified according to lesion distribution, histologic characteristics, immunophenotypic markers and biologic activity.4–18 Low-grade alimentary lymphoma (LGAL) is histologically described as an infiltrate of small T-cell lymphocytes, primarily affecting the epitheliotropic layer of the small intestinal mucosa.5,11,13,14,17,19 LGAL typically follows an indolent clinical course, with cats displaying non-specific clinical signs, including anorexia, weight loss, vomiting and/or diarrhea.8,11–13,20–22 LGAL often responds to less intensive chemotherapy protocols and is associated with a favorable outcome.11,13,19,20,22–24 Combination chlorambucil and prednisolone is recommended as a first-line therapy, with reported response rates between 69% and 96%, and median durations of response between 567 and 1078 days.11,20,22–24 However, cats can relapse several times during the course of disease, and limited information exists to guide therapeutic recommendations at the time of relapse.20,22–24
Rescue therapy for LGAL is inconsistently and sparsely described in the extant literature.20,23,24 When describing treatments, response rates and survival durations in cats with relapsed lymphoma, investigators have not uniformly differentiated between the anatomic, histologic and/or immunophenotypic subtypes.25–27 Studies that have focused on LGAL emphasize initial therapy, only summarily recounting any rescue therapies employed and associated measures of outcome.11,20,23,24 However, the utilization of cyclophosphamide in the rescue setting seems common.20,23,24 In one study, 12 cats with relapsed LGAL treated with cyclophosphamide after failing chlorambucil had a longer disease-free interval and survival time vs eight cats that did not receive cyclophosphamide. 20 This positive outcome is supported by another study in which 7/7 (100%) cats with similarly managed relapsed LGAL responded for a median clinical remission duration of 241 days. 24 Despite their small numbers, these studies are promising and have likely contributed to the utilization of cyclophosphamide in the rescue setting.
Cyclophosphamide is an alkylating agent commonly employed in the management of various types of lymphoma in cats,28–37 as well as other feline malignancies.38–41 Until recently,42,43 the maximum tolerated dose of cyclophosphamide in cats was not well established, and the spectrum of published dosage regimens was diverse.28–41 Even so, cyclophosphamide appeared to be well-tolerated, with adverse events (AEs) uncommonly reported.28–42 The principal dose-limiting toxicity in cats is myelosuppression, primarily affecting neutrophils.42–44 Additional clinically significant toxicities, including gastrointestinal and neurologic toxicities, are rarely reported, even with very high doses.42,45,46 In conjunction with this negligible AE profile, and similar to chlorambucil, cyclophosphamide may be preferred over alternative rescue agents because it is conveniently available as an oral formulation that can be administered by the client at home, sparing the cats and clients from hospital-associated stress and costs, respectively.
Its tolerability and convenience have positioned cyclophosphamide to be a relevant therapeutic option for cats with relapsed LGAL, although data regarding its efficacy and toxicity in this setting remain lacking. The objective of this study was to describe the utilization of cyclophosphamide as first-line rescue therapy in cats with clinically presumed relapsed LGAL, specifically documenting response and measures of outcome, any identifiable AEs and prognostic factors potentially associated with response and outcome.
Materials and methods
Cats with a definitive ante-mortem diagnosis of LGAL were identified from a medical record search of three institutions (University of Wisconsin–Madison, Hospital Aúna Especialidades Veterinarias and Kansas State University). Inclusion criteria were: (1) a histologic diagnosis of small-cell, lymphocytic or low-grade alimentary lymphoma (LGAL), as originally reported by a board-certified pathologist; or (2) a presumptive cytologic diagnosis of LGAL, accompanied by a clonal PCR for antigen receptor rearrangement result and consistent ultrasonographic findings; (3) initial antineoplastic therapy with chlorambucil ± a glucocorticoid; (4) clinical confirmation of relapse as reasonably determined by the overseeing clinician based on progression of clinical signs and/or ultrasonographic findings; (5) subsequent rescue therapy with cyclophosphamide ± a glucocorticoid; and (6) follow-up evaluations for a minimum of 30 days. Cases were excluded because of: (1) the absence of a histologic or molecularly confirmed diagnosis of LGAL; (2) prior chemotherapy other than chlorambucil ± a glucocorticoid; (3) concurrent disease(s) confounding interpretation of relapse, response, outcome and AE data; and/or (4) inadequate follow-up duration and/or information. No exclusions were made for any supportive therapies.
Information collated from the medical records included: demographic data; clinical signs and physical examination findings at the time of initial diagnosis, presumed first clinical relapse and during cyclophosphamide therapy; laboratory test results and imaging findings throughout therapy at various time point(s) as determined by the overseeing clinician; histologic, cytologic and/or immunologic details; dose, response and response duration with cyclophosphamide therapy; details of any comorbidities and concomitant medications; and details of clinical progression and/or death. The referring veterinarian(s) and/or owner(s) were contacted for follow-up, where additional details were required. All relevant material from the medical records was evaluated at all available time points, although physical examination findings (including weight), and laboratory and imaging results were a particular focus at the times of diagnosis, first relapse (ie, start of cyclophosphamide therapy), any available time points during the course of cyclophosphamide therapy and cyclophosphamide therapy failure. Body condition scores were not reliably available for evaluation; therefore, weights were instead assessed.
Response to cyclophosphamide therapy was determined based predominantly on the resolution, reduction, stabilization or worsening of the cats’ presenting clinical signs. As there is no standardized method of reporting or grading untreated disease, and to facilitate objective comparisons over time, the Veterinary Cooperative Oncology Group – common terminology criteria for adverse events (VCOG–CTCAE v1.1) following chemotherapy or biological antineoplastic therapy in dogs and cats (summarized in Table 1) 47 was retrospectively utilized. This approach provided a qualitative framework for grading clinical signs and laboratory abnormalities. Similar to other studies documenting response to therapy for feline LGAL,20,22–24 a complete clinical response (CR) was defined as complete resolution of clinical signs (grade 0) for at least 30 days, a partial clinical response (PR) as improvement in clinical signs (decrease in grade) for at least 30 days, progressive disease (PD) as worsening of clinical signs (increase in grade) and stable disease (SD) as no change in clinical signs. Imaging findings and laboratory results were taken into account where available. AEs were graded by the overseeing clinician at the time or retrospectively by the investigators according to the same VCOG-CTCAE v1.1 criteria. 47
Table 1.
VCOG-CTCAE v1.1 grading criteria applied to both pretreatment and toxicity-related clinical and laboratory abnormalities in cats with relapsed low-grade alimentary lymphoma receiving rescue cyclophosphamide after failing chlorambucil treatment
| Parameter | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Weight loss | <10% from baseline; intervention not indicated | 10–15% from baseline; nutritional dietary modification indicated | >15% of baseline | – |
| Anorexia | Coaxing or dietary change required to maintain appetite | Oral intake altered (⩽3 days) without significant weight loss; oral nutritional supplements/appetite stimulants may be indicated | Of >3 days’ duration; associated with significant weight loss (⩾10%) or malnutrition; IV fluids, tube feeding or force feeding indicated | Life-threatening consequences; TPN indicated; >5 days’ duration |
| Vomiting | <3 episodes in 24 h, medical intervention not indicated | 3–10 episodes in 24 h; <5 episodes/day for ⩽48 h; parenteral fluids (IV or SC) indicated ⩽48 h; medications indicated | Multiple episodes >48 h and IV fluids or PPN/TPN indicated >48 h | Life-threatening (eg, hemodynamic collapse) |
| Diarrhea | Increase of up to two stools per day over baseline; no increase in frequency; however, consistency decreased over baseline | Increase of 3–6 stools per day over baseline; medications indicated; parenteral (IV or SC) fluids indicated ⩽48 h; not interfering with ADL | Increase of >6 stools per day over baseline; incontinence >48 h; IV fluids >48 h; hospitalization; interfering with ADL | Life-threatening (eg, hemodynamic collapse) |
| Lethargy | Mild lethargy over baseline; diminished activity from pre-disease level, but able to function as an acceptable pet | Moderate lethargy causing some difficulty with performing ADL; ambulatory only to the point of eating, sleeping and consistently defecating and urinating in acceptable areas | Compromised, severely restricted in ADL; unable to confine urinations and defecation to acceptable areas; will consume food if offered in place | Disabled, must be force fed and helped to perform ADL |
| Dehydration | Increased oral fluids indicated; dry mucous membranes; <skin turgor | Parenteral (IV or SC) fluids indicated <48 h | IV fluids indicated >48 h | Life-threatening (eg, hemodynamic collapse) |
| Fever | 103.5–104°F (39.7–40°C) | >104–105.8°F (40–41°C), transient (<6 h), not requiring hospitalization | >105.8–107.6°F (41–42°C), prolonged (>6 h); requiring hospitalization | >107.6ºF (42ºC) |
| Ascites | Clinical or diagnostic observations only; intervention not indicated | Symptomatic; medical intervention indicated | Severe clinical signs; invasive intervention indicated | Life-threatening consequences; urgent operative intervention indicated |
| PCV | 25% to <LLN | 20 to <25% | 15 to <20% | <15% |
| Neutropenia | 1500 μl–¹ to <LLN | 1000−1499 μl–¹ | 500−999 μl–¹ | <500 μl–¹ |
| Thrombocytopenia | 100,000 μl–¹ to <LLN | 50,000−99,000 μl–¹ | 25,000−49,000 μl–¹ | <25,000 μl–¹ |
| BUN | >1–1.5 × baseline; >ULN to 1.5 × ULN | >1.5–3 × baseline; >1.5–2.0 × ULN | >3 × baseline; >2.0–3 × ULN | >3 × ULN |
| Creatinine | >1–1.5 × baseline; >ULN to 1.5 × ULN | >1.5–3 × baseline; >1.5–2.0 × ULN | >3 × baseline; >2.0–3 × ULN | >3 × ULN |
| ALT | >ULN to 1.25 × ULN | >1.25–1.5 × ULN, transient (<2 weeks) | >1.5–2.0 × ULN | >2 × ULN |
| ALP | >ULN to 1.25 × ULN | >1.25–1.5 × ULN, transient (<2 weeks) | >1.5–2.0 × ULN | >2 × ULN |
| Potassium, high | >ULN–5.5 mmol l–¹ | >5.5–6.0 mmol l–¹ | >6.0–7.0 mmol l–¹ | >7.0 mmol l–¹ |
| Bilirubin | >ULN to 1.5 × ULN | >1.5–3.0 × ULN | >3.0–10 × ULN | >10 × ULN |
| Glucose, high | >ULN–200 mg dl–¹ | >200–250 mg dl–¹ | >250–500 mg dl–¹ | >500 mg dl–¹ |
| Thyroid function, hyperthyroidism | Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Symptomatic; thyroid suppression therapy indicated; limiting ADL | Severe clinical signs; limiting ADL, hospitalization indicated | Life-threatening consequences; urgent intervention indicated |
| Potassium, low | <LLN–3.0 mmol l–¹ | – | <3.0–2.5 mmol l–¹ | <2.5 mmol l–¹ |
| Calcium, low | <LLN–8.0 mg dl–¹; ionized: <LLN–0.9 nmol l–¹ | <8.0–7.0 mg dl–¹; ionized: <0.9–0.8 nmol l–¹ | <7.0–6.0 mg dl–¹; ionized: <0.8–0.7 nmol l–¹ | <6.0 mg dl–¹; ionized: <0.7 nmol l–¹ |
| Albumin, low | <LLN–2.0 g dl–¹ | <2.0–1.5 g dl–¹ | <1.5 g dl–¹ | – |
VCOG–CTCAE = Veterinary Cooperative Oncology Group – common terminology criteria for adverse events; IV = intravenous; TPN = total parenteral nutrition; SC = subcutaneous; PPN = partial parenteral nutrition; ADL = activities of daily living (eating, sleeping, defecating and urinating); PCV = packed cell volume; LLN = lower limit of normal; BUN = blood urea nitrogen; ULN = upper limit of normal; ALT = alanine aminotransferase; ALP = alkaline phosphatase
Statistical methods
Descriptive statistics were undertaken for the overall study population. Two measures of outcome were assessed. Progression-free survival (PFS) was calculated as the days elapsing between the first dose of cyclophosphamide and disease progression or death. Cats were censored from PFS if progression or death failed to be reported during cyclophosphamide treatment. Overall survival time (OST) was calculated as the days elapsing between the initial diagnosis and death or loss to follow-up. Cats were censored from OST if they remained alive at the conclusion of the study period or were lost to follow-up. Kaplan–Meier survival analyses estimated both measures of outcome, with median survival times and confidence intervals (CIs). The log-rank test was utilized for any association of PFS and OST with categorical variables of interest (sex, presence and number of clinical signs, tumor location, presence of epitheliotropism, previous steroid treatment, presence of concurrent disease and magnitude of response to cyclophosphamide [CR vs others]). Univariate Cox proportional hazard modeling was utilized for any association of PFS and OST with continuous variables (weight, age, dose of cyclophosphamide and duration of response to cyclophosphamide). For all analyses, P ⩽0.05 was considered significant. Statistical analyses were performed utilizing commercially available software (SPSS version 25).
Results
Study population
Twenty cats fulfilled the aforementioned inclusion and exclusion criteria. Cats were treated at the University of Wisconsin–Madison (n = 15), Hospital Aúna Especialidades Veterinarias (n = 4) and Kansas State University (n = 1), between May 2002 and February 2017. Patient demographic data is reported in Table 2. LGAL features are reported in Table 3. Clinical signs and laboratory test results at diagnosis are reported in Tables 4 and 5, respectively. Although not a requirement for study inclusion, all 20 (100%) cats had previously achieved a CR with prior chlorambucil and glucocorticoid therapy for a median duration of response of 295 days (range 42–1107 days; 95% CI 252–602).
Table 2.
Demographic data of cats with relapsed low-grade alimentary lymphoma receiving rescue cyclophosphamide after failing chlorambucil treatment
| Breed (n) | |
| Domestic shorthair | 15 |
| Domestic longhair | 3 |
| Domestic mediumhair | 1 |
| Siamese | 1 |
| Median (range) age (years) | |
| At initial diagnosis | 12.9 (6.1–18.6) |
| At cyclophosphamide initiation | 14.8 (6.2–19.8) |
| Median (range) weight (kg) | |
| At initial diagnosis | 4.04 (2.8–5.1) |
| At cyclophosphamide initiation | 3.64 (2.6–4.4) |
| Sex (n) | |
| Castrated male | 14 |
| Spayed female | 6 |
| FeLV/FIV status (n) | |
| Negative/negative | 3 |
| Not reported | 17 |
FeLV = feline leukemia virus; FIV = feline immunodeficiency virus
Table 3.
Anatomic, histologic and immunologic descriptions of initial low-grade alimentary lymphoma (LGAL) diagnosis in cats with relapsed LGAL receiving rescue cyclophosphamide after failing chlorambucil treatment
| Mode of diagnosis (n) | |
| Histology (surgical biopsy) | 15 |
| Histology (endoscopic biopsy) | 4 |
| PARR and reactive cytology of mesenteric LN with ultrasonographically thickened SI | 1 |
| Anatomic location (n) | |
| SI only | 13 |
| Stomach only | 1 |
| SI and mesenteric LN | 4 |
| SI, stomach and mesenteric LN | 2 |
| Epitheliotropism (n) | |
| Present | 13 |
| Absent | 3 |
| Not reported | 4 |
| Immunophenotype (via IHC in n = 5 or PARR in n = 1) (n) | |
| T cell | 5 |
| B cell | 1 |
| Non-B/non-T cell | 1 |
| Not reported | 13 |
PARR = PCR for antigen receptor rearrangements; LN = lymph node; SI = small intestine; IHC = immunohistochemistry
Table 4.
Clinical signs at initial diagnosis, presumed first relapse and during cyclophosphamide therapy in cats with relapsed low-grade alimentary lymphoma (LGAL) receiving rescue cyclophosphamide after failing chlorambucil treatment
| Clinical sign | At initial diagnosis, n (n = 20 total*) | At first presumed relapse, n (n = 16 total † ) | During cyclophosphamide therapy, n (n = 20 total ‡ ) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 or 2 | Grade 3 or 4 | Grade unknown | Total | Grade 1 or 2 | Grade 3 or 4 | Grade unknown | Total | Grade 1 or 2 | Grade 3 or 4 | Grade unknown | Total | |
| Weight loss § | 0 | 0 | 15 | 15 | 9 | 1 | 0 | 10 | 5 | 4 | 0 | 9 ¶ |
| Anorexia | 1 | 0 | 6 | 7 | 3 | 1 | 0 | 4 | 13 | 1 | 2 | 16 |
| Vomiting | 5 | 2 | 3 | 10 | 6 | 1 | 0 | 7 | 9 | 0 | 0 | 9 |
| Diarrhea | 5 | 1 | 2 | 8 | 6 | 0 | 0 | 6 | 7 | 0 | 0 | 7 |
| Lethargy | 2 | 1 | 2 | 5 | 2 | 1 | 0 | 3 | 4 | 1 | 2 | 7 |
| Dehydration | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 2 |
| Fever | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ascites | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Number of clinical signs | n | n | n | |||||||||
| 0 | 0 | 1 | 0 | |||||||||
| 1 | 8 | 5 | 5 | |||||||||
| 2 | 2 | 5 | 5 | |||||||||
| 3 | 7 | 4 | 6 | |||||||||
| >3 | 3 | 1 | 4 | |||||||||
Clinical signs were recorded from a physical examination performed at the time of initial diagnosis
Clinical signs were recorded from a physical examination performed at the time of first presumed relapse (ie, start of cyclophosphamide treatment)
Clinical signs were recorded at various time points throughout cyclophosphamide therapy at the discretion of the specific overseeing clinician
The comparator for weight loss was the weight noted at the previous time point. For the initial diagnosis, the comparator was from a previous weight that was not necessarily dated. At first presumed relapse (the start of cyclophosphamide therapy), the comparator weight was from initial diagnosis. During cyclophosphamide therapy, the baseline weight was from the start of cyclophosphamide therapy and was compared with the lowest documented weight during cyclophosphamide therapy
In three of these cats, weight gain was also noted during cyclophosphamide therapy
Table 5.
Laboratory results* at initial diagnosis, first presumed relapse and during cyclophosphamide therapy in cats with relapsed low-grade alimentary lymphoma receiving rescue cyclophosphamide after failing chlorambucil treatment
| Hematology | At initial diagnosis, n (n = 16 total † ) | At first presumed relapse, n (n = 16 total ‡ ) | During cyclophosphamide therapy, n (n = 15 total § ) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1 or 2 | Grade 3 or 4 | Total | Grade 1 or 2 | Grade 3 or 4 | Total | Grade 1 or 2 | Grade 3 or 4 | Total | |
| Anemia | 3 | 0 | 3 | 5 | 0 | 5 | 8 | 0 | 8 |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 4 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biochemistry ¶ | At initial diagnosis, n (n = 11 total † ) | At first presumed relapse, n (n = 10 total ‡ ) | During cyclophosphamide therapy, n (n = 12 total § ) | ||||||
| Grade 1 or 2 | Grade 3 or 4 | Total | Grade 1 or 2 | Grade 3 or 4 | Total | Grade 1 or 2 | Grade 3 or 4 | Total | |
| Increased BUN | 3 | 0 | 3 | 2 | 0 | 2 | 4 | 0 | 4 |
| Increased creatinine | 3 | 0 | 3 | 1 | 0 | 1 | 1 | 0 | 1 |
| Increased ALT | 2 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 |
| Increased ALP | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 0 |
| Increased potassium | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Increased bilirubin | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 0 |
| Increased glucose | 1 | 0 | 1 | 2 | 0 | 2 | 1 | 0 | 1 |
| Increased T4 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Decreased potassium | 0 | 0 | 0 | 2 | 0 | 2 | 1 | 0 | 1 |
| Decreased calcium | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 2 |
| Decreased albumin | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
Seven cats had a urinalysis performed only at initial diagnosis; aside from trace to 1 + proteinuria in four cats, all results were unremarkable
Laboratory results were collected at the time of initial diagnosis
Laboratory results were collected at the time of first presumed relapse (ie, start of cyclophosphamide treatment)
Laboratory results were collected at various time points throughout cyclophosphamide therapy at the discretion of the specific overseeing clinician
Four cats were reported to have any combination of increased serum folate, trypsin-like immunoreactivity, pancreatic lipase immunoreactivity and/or decreased serum cobalamin that were subjectively mild-to-moderate in severity (no grading system exists for these parameters) and were only evaluated at initial diagnosis. The cats with decreased serum cobalamin received cobalamin supplementation
BUN = blood urea nitrogen; ALT = alanine transaminase; ALP = alkaline phosphatase; T4 = thyroxine
Clinical signs and diagnostic tests at first presumed relapse
The clinical signs and laboratory test results at first presumed relapse (ie, the start of cyclophosphamide therapy) are also described in Tables 4 and 5, respectively. Either progression of clinical signs alone (n = 12), progression of abdominal ultrasound findings alone (n = 2), or progression of both clinical signs and ultrasound findings (n = 6) were reported reasons for discontinuation of chlorambucil and initiation of cyclophosphamide. Eleven cats (55%) that had abdominal ultrasound re-evaluation at the time of first presumed relapse had ultrasonographic findings suggestive of lymphoma (Table 6). Eight cats were considered to have progressive changes, while the three cats with stable ultrasound findings had progressive clinical signs. One cat also had thoracic radiographs that were unremarkable.
Table 6.
Abdominal ultrasound findings at first presumed relapse (after receiving chlorambucil) and second presumed relapse (after receiving cyclophosphamide) of cats with relapsed low-grade alimentary lymphoma receiving rescue cyclophosphamide after failing chlorambucil treatment
| At first presumed relapse, n (n = 11 total) | At second presumed relapse, n (n = 6 total) | |
|---|---|---|
| SI wall thickening | 11 | 5 |
| Mesenteric LN enlargement | 5 | 3 |
| Peritoneal effusion | 2 | 2 |
| No lymphoma-related changes | 0 | 1 * |
This cat was diagnosed with oral squamous cell carcinoma
SI = small intestine; LN = lymph node
Cyclophosphamide treatment
The median time between chlorambucil discontinuation and cyclophosphamide initiation was 14 days (range 3–32 days) in the 15 cats for which this information was recorded. The median dose of cyclophosphamide was 206.9 mg/m2 (range 161.3–281.8 mg/m2), administered orally, every 2 weeks. The median number of cyclophosphamide treatments was 13 (range 3–54). Nineteen cats received ongoing concurrent prednisolone (n = 16) or prednisone (n = 3) orally, either continuously (n = 16) at doses between 1–2 mg/kg q24–48h, or tapered (n = 3) over 7–10 days from 1–2 mg/kg q24h to a maintenance dosage of 1 mg/kg q48h. Comorbidities and concomitant medications are outlined in Table 7.
Table 7.
Comorbidities and concomitant medications of cats with relapsed low-grade alimentary lymphoma receiving rescue cyclophosphamide after failing chlorambucil treatment
| n | |
|---|---|
| Comorbidity* † | |
| Hyperthyroidism | 4 |
| Hypocobalaminemia | 4 |
| Heart murmur ‡ | 4 |
| Renal pseudocyst | 1 |
| Chronic kidney disease | 1 |
| Restrictive cardiomyopathy | 1 |
| Pyoderma/skin allergy | 1 |
| Concomitant medications § ¶ | |
| Cobalamin | 4 |
| Mirtazapine | 4 |
| Subcutaneous fluids | 3 |
| Cyproheptadine | 2 |
| Famotidine | 2 |
| Maropitant | 2 |
| Methimazole | 2 |
| Metronidazole | 2 |
| Potassium gluconate | 2 |
| Amlodipine | 1 |
| Amoxicillin/clavulanic acid | 1 |
| Ciprofloxacin ophthalmic drops | 1 |
| Dexamethasone otic drops | 1 |
| Dolasetron | 1 |
| Enalapril | 1 |
| L-lysine | 1 |
| Metoclopramide | 1 |
| Mupirocin ointment | 1 |
| Omega-3 fatty acids | 1 |
| Ondansetron | 1 |
| Probiotics | 1 |
| S-adenosylmethionine | 1 |
| Sucralfate | 1 |
Eight cats were reported to have one or more of the listed comorbidities
With the exception of the renal pseudocyst and pyoderma/skin allergy, all other comorbidities were existing during prior chlorambucil treatment
Heart murmurs were not otherwise described or graded
Fourteen cats received concomitant medications, six of which received only one and eight of which received 2–6 concomitant medications. Six cats did not receive any concomitant medications
With the exception of mupirocin ointment, ciprofloxacin ophthalmic drops, L-lysine, sucralfate and cyproheptadine, all other concomitant medications were used during prior chlorambucil treatment
Outcomes
Eighteen cats (90%) achieved a CR for a median duration of 239 days. One cat had SD for 91 days. The other cat experienced PD. The median PFS was 215 days (range 0–823 days; 95% CI 102–328 [Figure 1]); one cat was censored from PFS analysis. The median OST was 1065 days (95% CI 974–1156; Figure 2); 10 cats were censored from OST analysis. The factor associated with longer PFS was achievement of a CR with cyclophosphamide therapy (hazard ratio [HR] 0.14, 95% CI 0.025–0.768; P = 0.02). No other factors were found to be associated with PFS or OST. Net weight gain (n = 6 cats; 3.3–13% weight gain) or a combination of weight gain and loss (n = 3 cats) was noted during cyclophosphamide therapy.
Figure 1.
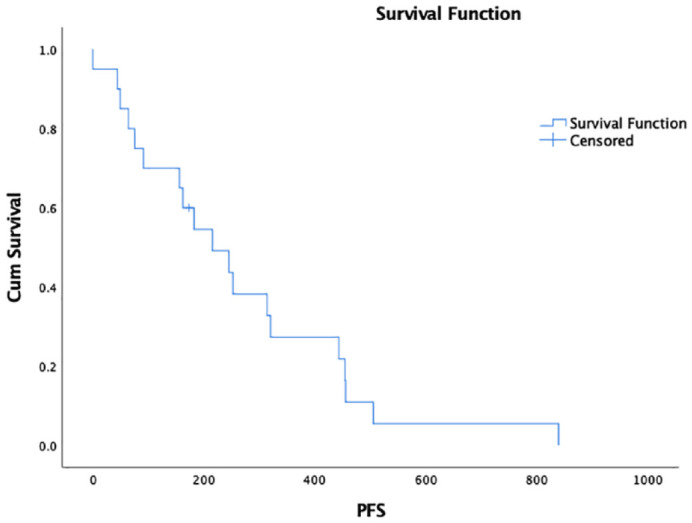
Kaplan–Meier survival curve depicting progression-free survival (PFS) in cats with relapsed low-grade alimentary lymphoma receiving rescue cyclophosphamide. Median PFS was 215 days (95% CI 102–328)
Figure 2.
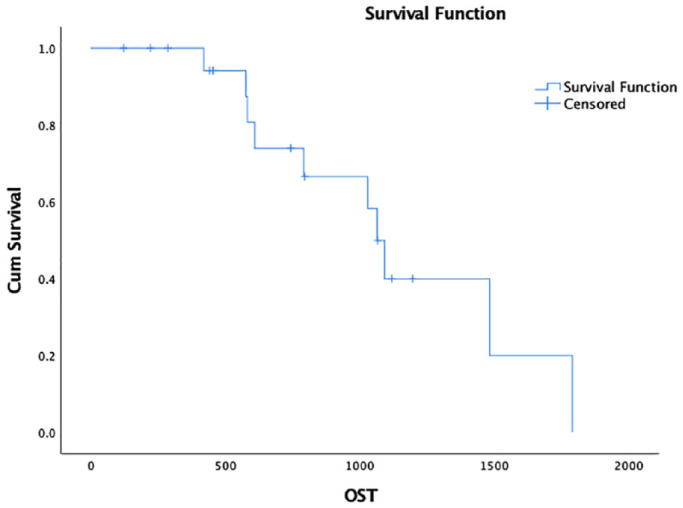
Kaplan–Meier survival curve depicting overall survival time (OST) in cats with relapsed low-grade alimentary lymphoma receiving rescue cyclophosphamide. Median OST was 1065 days (95% CI 974–1156)
Cyclophosphamide was ultimately discontinued owing to progression of clinical signs alone (n = 12), progression of abdominal ultrasound findings alone (n = 1), progression of both clinical signs and ultrasound findings (n = 4), or other findings (n = 3) presumably unrelated to lymphoma (oral squamous cell carcinoma, n = 1; acute conscious proprioceptive deficits and inability to stand, n = 1; acute death, n = 1). Abdominal ultrasound was undertaken in six cats at presumed second relapse (Table 6). One cat also had thoracic radiographs that revealed a mild bronchial pattern.
Eleven (55%) cats went on to receive further rescue therapy after cyclophosphamide at the discretion of the overseeing clinician(s). Eight cats received one further rescue protocol, and three cats received two further rescue protocols. Three cats achieved a CR with either CCNU (lomustine; n = 2) or vinblastine (n = 1). Three cats had a PR with CCNU. Two cats maintained SD with CCNU/L-asparaginase or CCNU/vinblastine. One cat had PD despite L-asparaginase. Two cats were lost to follow-up after receiving CCNU.
Ten cats (50%) had died or were euthanized by the conclusion of the study period. In nine of these cats, death was reasonably presumed to be tumor-related. The other cat discontinued cyclophosphamide with unspecified neurologic deficits and was euthanized as a result. Nine cats (45%) were lost to follow-up. The median time from cyclophosphamide initiation to censoring of these nine cats was 279 days (range 44–633 days). One cat (5%) remained alive.
Three cats (15%) underwent necropsy. One cat had diffuse intra-abdominal lymphoma, involving the omentum, mesentery, small intestines, pancreas, ileocolic junction and diaphragm. Another cat had lymphoma identified only in the small intestine. The third cat, which had displayed neurologic deficits, did not have evidence of lymphoma, and the neurologic deficits remained unexplained.
AEs
Clinical signs and laboratory test results during cyclophosphamide therapy are described in Tables 4 and 5, respectively. As recheck examinations during cyclophosphamide therapy were at the discretion of the overseeing clinician, the recheck schedule could not be generalized for the entire study population. Two cats had cyclophosphamide dose reductions. One of these cats had a dose reduction from 250 mg/m2 to 200 mg/m2 and a 7-day dose delay due to grade 3 neutropenia, while the other cat had a dose reduction from 250 mg/m2 to 220 mg/m2 due to grade 3 anorexia and grade 2 vomiting, lethargy, dehydration and weight loss. Both cats recovered from their AEs. One cat had a 7-day dose delay without a dose reduction at the discretion of the referring clinician, with no reason reported.
Discussion
To our knowledge, this study is the first to specifically describe measures of outcome and potential prognostic factors in a population of cats with LGAL receiving cyclophosphamide as a first-line rescue agent, having failed initial therapy with chlorambucil and a glucocorticoid.
The complete CR rate to cyclophosphamide rescue therapy reported in this study was high (90%), with clinical benefit for a median of 215 days, despite prior alkylating therapy. This response rate may have been aided by confounding factors. One was the study’s unintentional selection bias for patients that are likely to be favorable responders; although not an inclusion criterion, all cats in this study had all previously achieved a CR with initial chlorambucil and glucocorticoid therapy. Another was the use of concomitant medications that may have improved clinical signs, masked lymphoma progressive disease and/or unintentionally interfered with cyclophosphamide bioavailability. The authors believe, however, that these concomitant medications are unlikely to have significantly affected the interpretation of response, given that most medications were used during the prior chlorambucil therapy, presumed relapse and then also during cyclophosphamide therapy.
The comparison of response rates and measures of outcome across non-contemporaneous studies is not advisable, given differences in patient selection, diagnostic efforts, staging procedures, treatment regimens, monitoring schedules and owner populations. However, with these potential confounders in mind, the results of this study are consistent and compare favorably with previous studies that have secondarily evaluated analogous rescue therapies in cats with LGAL.20,23,24 Fondacaro et al reported that 12 cats receiving cyclophosphamide rescue therapy had a significantly longer OST compared with eight cats that did not receive rescue therapy (29 months vs 18.8 months). 20 Stein et al reported an overall response rate of 100% in seven cats with LGAL treated with cyclophosphamide ± prednisolone as rescue therapy. 24 Somewhat divergent, a study by Pope et al documented only a 59% response rate for first attempt rescue chemotherapy, albeit the median rescue specific survival for all cats in this study was 861 days. 23 However, that study was structured differently, consisting of a finite 6-month chlorambucil and prednisolone protocol as first-line therapy, the rescue regimens included a spectrum of various chemotherapeutic protocols, including the re-institution of chlorambucil and prednisolone, and the response rates and comparisons were not specifically reported for the cyclophosphamide-based protocols. 23
In the present study, achieving a complete response to cyclophosphamide was associated with a longer PFS. This positive association between treatment response and improved measures of outcome has been reported in previous feline lymphoma studies.4,11,22,26–28,36,37 The lack of a corresponding association between cyclophosphamide treatment response and OST in this study is likely a consequence of the small sample size and lack of statistical power, a confounding effect of the inclusion of the chlorambucil and subsequent treatment periods in calculating the OST, and the owners’ individual perspectives. Additionally, the CR that all cats had achieved with prior chlorambucil treatment could have falsely increased the OST given the expected increased survival with cats achieving CR over PR, SD or PD. These confounding effects impacting OST are why it is considered a less robust measurement of outcome.
Cyclophosphamide was associated with few constitutional, hematologic and gastrointestinal AEs in this study. While the monitoring schedule varied during the rescue therapy, physical examinations were performed throughout rescue therapy in all cats, and blood tests and imaging were undertaken intermittently in most cats. However, since re-evaluations during cyclophosphamide therapy were dependent on the overseeing clinician, AEs may have been missed and subsequently underestimated in our study. The attribution of AEs to an effect of therapy vs disease was obviously complicated. However, such ascription was based upon the reasonable evaluation of the overseeing clinician, as well as the duration of the AE and response to supportive therapy vs unremitting signs. Overall, the AE profile was negligible in this study.
Given the response rate and AE profile, cyclophosphamide seems a reasonable therapeutic option in cats with recurrent LGAL, especially considering the additional logistical advantage of oral administration. However, it must also be acknowledged that alternative oral agents are available, also with suggested efficacy in the rescue setting.23,25 Lomustine was found to markedly improve the median progression-free interval for cats with small-to-intermediate cell lymphoma over cats with large cell lymphoma (169 days vs 39 days). 25 However, the frequency of higher-grade AEs need to be considered.48,49 Moreover, the findings reported by Pope et al might imply lomustine is less efficacious in the rescue setting, although this supposition would require further investigation. 23 With the potential malabsorptive nature of LGAL, another therapeutic consideration is parenteral cyclophosphamide administration. Since cyclophosphamide has been shown to have similar bioavailability between intravenous (IV), intraperitoneal (IP) and oral routes of administration,50–52 it is possible that IV or IP administration of cyclophosphamide may be of benefit, at least initially at relapse when gastrointestinal absorption of cyclophosphamide may be most compromised.
A substantial challenge of this retrospective study was indisputably denoting response to therapy and relapse, and this remains the most consequential limitation of the study. Accurate evaluation of response to treatment and relapse is always troublesome in cats with LGAL because affected cats have internal disease, rendering the objective determination of relative change in tumor burden difficult without repeating invasive and expensive diagnostic tests. The majority of cats diagnosed with LGAL have clinical signs referable to the alimentary tract; therefore, it seems reasonable to incorporate improvement or recurrence or progression of these clinical signs as a component of response assessment in these cats as was done in this study. Moreover, the clinical criteria relied upon in this study are those commonly applied by clinicians in practice when evaluating response and relapse in cats with LGAL, rendering the data presented here clinically relevant despite its limitations. Finally, these criteria have been solely utilized in previously published studies to decide response and relapse in feline LGAL.5,10–12 Arguably, however, the clinical signs, largely relied upon to decide response and relapse in this study, could be an AE of chemotherapy, the onset of a novel disease or lymphoma, and misattribution of one or more clinical signs is possible. For example, the chronic administration of oral cyclophosphamide could have caused anorexia and weight loss, and both could have been misconstrued as disease progression, not cyclophosphamide toxicity. Additionally, transient anorexia secondary to cyclophosphamide bolus could also have been misattributed to disease progression. The likelihood that the constitutional and gastrointestinal AEs were an effect of therapy is low, however, given the low frequency of such AEs in cats receiving even over double the median dose of cyclophosphamide utilized in this study. 42 However, the challenges associated with clinically deciding response and relapse means that it is possible that the PFS was under- or overestimated if AEs were erroneously attributed to PD or AEs, respectively.
There are additional limitations to the present study. Limitations inherent to any multi-institutional and/or retrospective study include the absence of a standardized approach to case management, incomplete diagnostic and staging tests, variable treatment regimens, inconsistent monitoring and follow-up, as well as the lack of appropriate control groups. Furthermore, in an attempt to address these issues and standardize the study population, cases were excluded, resulting in a smaller study population, which precluded the statistically robust evaluation of prognostic variables. Finally, cats were excluded if they were lost to follow-up within 30 days of commencing cyclophosphamide, and since cats were not included on an intent-to-treat basis, this may have caused a bias that falsely increased PFS or OST by removing cats with rapid disease progression. Consequently, the results of this study should be interpreted cautiously and utilized to inform future prospective randomized and ideally blinded studies, with expanded study populations and appropriate control groups.
Conclusions
Within the limited parameters of this study, the findings support the widespread clinical practice of recommending cyclophosphamide as rescue therapy for cats with relapsed LGAL, demonstrating a good response rate, duration of response and minimal AEs. Future prospective randomized and ideally blinded studies, comprising larger populations and appropriate control groups, would be required to better delineate the measurable value of cyclophosphamide in the rescue setting.
Acknowledgments
The authors wish to thank the University of Wisconsin–Madison Department of Statistics for their assistance in the analysis and interpretation of the statistical methods utilized herein.
Footnotes
Accepted: 19 January 2021
Author note: This paper was presented, in part, at the University of Wisconsin–Madison School of Veterinary Medicine Phi Zeta Research Day in Madison, Wisconsin in April 2018, and the European Society of Veterinary Oncology Congress in Frankfurt, Germany in May 2019.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not specifically required for publication in JFMS.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Esther Chon  https://orcid.org/0000-0002-0717-8157
https://orcid.org/0000-0002-0717-8157
References
- 1. Gabor LJ, Malik R, Canfield PJ. Clinical and anatomical features of lymphosarcoma in 118 cats. Aust Vet J 1998; 76: 725–732. [DOI] [PubMed] [Google Scholar]
- 2. Kristal O, Lana SE, Ogilvie GK, et al. Single agent chemotherapy with doxorubicin for feline lymphoma: a retrospective study of 19 cases (1994–1997). J Vet Intern Med 2001; 15: 125–130. [DOI] [PubMed] [Google Scholar]
- 3. Louwerens M, London CA, Pedersen NC, et al. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med 2005; 19: 329–335. [DOI] [PubMed] [Google Scholar]
- 4. Vail DM, Moore AS, Ogilvie GK, et al. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med 1998; 12: 349–354. [DOI] [PubMed] [Google Scholar]
- 5. Vezzali E, Parodi AL, Marcato PS, et al. Histopathologic classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO. Vet Comp Oncol 2010; 8: 38–49. [DOI] [PubMed] [Google Scholar]
- 6. Evans SE, Bonczynski JJ, Broussard JD, et al. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc 2006; 229: 1447–1450. [DOI] [PubMed] [Google Scholar]
- 7. Ezura K, Ezura K, Nomura I, et al. Natural killer-like T cell lymphoma in a cat. Vet Rec 2004; 154: 268–270. [DOI] [PubMed] [Google Scholar]
- 8. Kiupel M, Smedley RC, Pfent C, et al. Diagnostic algorithm to differentiate lymphoma from inflammation in feline small intestinal biopsy samples. Vet Pathol 2011; 48: 212–222. [DOI] [PubMed] [Google Scholar]
- 9. Kleinschmidt S, Harder J, Nolte I, et al. Chronic inflammatory and non-inflammatory diseases of the gastrointestinal tract in cats: diagnostic advantages of full-thickness intestinal and extraintestinal biopsies. J Feline Med Surg 2010; 12: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krick EL, Little L, Patel R, et al. Description of clinical and pathological findings, treatment and outcome of feline large granular lymphocyte lymphoma (1996–2004). Vet Comp Oncol 2008; 6: 102–110. [DOI] [PubMed] [Google Scholar]
- 11. Lingard AE, Briscoe K, Beatty JA, et al. Low-grade alimentary lymphoma: clinicopathological findings and response to treatment in 17 cases. J Feline Med Surg 2009; 11: 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahony OM, Moore AS, Cotter SM, et al. Alimentary lymphoma in cats: 28 cases (1988–1993). J Am Vet Med Assoc 1995; 207: 1593–1598. [PubMed] [Google Scholar]
- 13. Moore PF, Rodriguez-Bertos A, Kass PH. Feline gastrointestinal lymphoma: mucosal architecture, immunophenotype, and molecular clonality. Vet Pathol 2012; 49: 658–668. [DOI] [PubMed] [Google Scholar]
- 14. Pohlman LM, Higginbotham ML, Welles EG, et al. Immunophenotypic and histologic classification of 50 cases of feline gastrointestinal lymphoma. Vet Pathol 2009; 46: 259–268. [DOI] [PubMed] [Google Scholar]
- 15. Roccabianca P, Vernau W, Caniatti M, et al. Feline large granular lymphocyte (LGL) lymphoma with secondary leukemia: primary intestinal origin with predominance of a CD3/CD8(alpha)(alpha) phenotype. Vet Pathol 2006; 43: 15–28. [DOI] [PubMed] [Google Scholar]
- 16. Zwingenberger AL, Marks SL, Baker TW, et al. Ultrasonographic evaluation of the muscularis propria in cats with diffuse small intestinal lymphoma or inflammatory bowel disease. J Vet Intern Med 2010; 24: 289–292. [DOI] [PubMed] [Google Scholar]
- 17. Briscoe KA, Krockenberger M, Beatty JA, et al. Histopathological and immunohistochemical evaluation of 53 cases of feline lymphoplasmacytic enteritis and low-grade alimentary lymphoma. J Comp Pathol 2011; 145: 187–198. [DOI] [PubMed] [Google Scholar]
- 18. Valli VE, Jacobs RM, Norris A, et al. The histologic classification of 602 cases of feline lymphoproliferative disease using the National Cancer Institute working formulation. J Vet Diagn Invest 2000; 12: 295–306. [DOI] [PubMed] [Google Scholar]
- 19. Carreras JK, Goldschmidt M, Lamb M, et al. Feline epitheliotropic intestinal malignant lymphoma: 10 cases (1997–2000). J Vet Intern Med 2003; 17: 326–331. [DOI] [PubMed] [Google Scholar]
- 20. Fondacaro JV, Richter KP, Carpenter JL, et al. Feline gastrointestinal lymphoma: 67 cases (1988–1996). Eur J Comp Gastroenterol 1999; 4: 69–74. [Google Scholar]
- 21. Gieger T. Alimentary lymphoma in cats and dogs. Vet Clin North Am Small Anim Pract 2011; 41: 419–432. [DOI] [PubMed] [Google Scholar]
- 22. Kiselow MA, Rassnick KM, McDonough SP, et al. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995–2005). J Am Vet Med Assoc 2008; 232: 405–410. [DOI] [PubMed] [Google Scholar]
- 23. Pope KV, Tun AE, McNeill CJ, et al. Outcome and toxicity assessment of feline small cell lymphoma: 56 cases (2000–2010). Vet Med Sci 2015; 1: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stein TJ, Pellin M, Steinberg H, et al. Treatment of feline gastrointestinal small-cell lymphoma with chlorambucil and glucocorticoids. J Am Anim Hosp Assoc 2010; 46: 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dutelle AL, Bulman-Fleming JC, Lewis CA, et al. Evaluation of lomustine as a rescue agent for cats with resistant lymphoma. J Feline Med Surg 2012; 14: 694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin OA, Price J. Mechlorethamine, vincristine, melphalan and prednisolone rescue chemotherapy protocol for resistant feline lymphoma. J Feline Med Surg 2018; 20: 934–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oberthaler KT, Mauldin E, McManus PM, et al. Rescue therapy with doxorubicin-based chemotherapy for relapsing or refractory feline lymphoma: a retrospective study of 23 cases. J Feline Med Surg 2009; 11: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collette SA, Allstadt SD, Chon EM, et al. Treatment of feline intermediate- to high-grade lymphoma with a modified university of Wisconsin-Madison protocol: 119 cases (2004–2012). Vet Comp Oncol 2016; 14 Suppl 1: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fabrizio F, Calam AE, Dobson JM, et al. Feline mediastinal lymphoma: a retrospective study of signalment, retroviral status, response to chemotherapy and prognostic indicators. J Feline Med Surg 2014; 16: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gouldin ED, Mullin C, Morges M, et al. Feline discrete high-grade gastrointestinal lymphoma treated with surgical resection and adjuvant CHOP-based chemotherapy: retrospective study of 20 cases. Vet Comp Oncol 2017; 15: 328–335. [DOI] [PubMed] [Google Scholar]
- 31. Hadden AG, Cotter SM, Rand W, et al. Efficacy and toxicosis of VELCAP-C treatment of lymphoma in cats. J Vet Intern Med 2008; 22: 153–157. [DOI] [PubMed] [Google Scholar]
- 32. Jeglum KA, Whereat A, Young K. Chemotherapy of lymphoma in 75 cats. J Am Vet Med Assoc 1987; 190: 174–178. [PubMed] [Google Scholar]
- 33. Limmer S, Eberle N, Nerschbach V, et al. Treatment of feline lymphoma using a 12-week, maintenance-free combination chemotherapy protocol in 26 cats. Vet Comp Oncol 2016; 14 Suppl 1: 21–31. [DOI] [PubMed] [Google Scholar]
- 34. Simon D, Eberle N, Laacke-Singer L, et al. Combination chemotherapy in feline lymphoma: treatment outcome, tolerability, and duration in 23 cats. J Vet Intern Med 2008; 22: 394–400. [DOI] [PubMed] [Google Scholar]
- 35. Teske E, van Straten G, van Noort R, et al. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. J Vet Intern Med 2002; 16: 179–186. [DOI] [PubMed] [Google Scholar]
- 36. Waite AH, Jackson K, Gregor TP, et al. Lymphoma in cats treated with a weekly cyclophosphamide-, vincristine-, and prednisone-based protocol: 114 cases (1998–2008). J Am Vet Med Assoc 2013; 242: 1104–1109. [DOI] [PubMed] [Google Scholar]
- 37. Zwahlen CH, Lucroy MD, Kraegel SA, et al. Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993–1997). J Am Vet Med Assoc 1998; 213: 1144–1149. [PubMed] [Google Scholar]
- 38. Barber LG, Sorenmo KU, Cronin KL, et al. Combined doxorubicin and cyclophosphamide chemotherapy for nonresectable feline fibrosarcoma. J Am Anim Hosp Assoc 2000; 36: 416–421. [DOI] [PubMed] [Google Scholar]
- 39. Cannon CM, Knudson C, Borgatti A. Clinical signs, treatment, and outcome in cats with myeloma-related disorder receiving systemic therapy. J Am Anim Hosp Assoc 2015; 51: 239–248. [DOI] [PubMed] [Google Scholar]
- 40. Leo C, Stell A, Borrego J, et al. Evaluation of low-dose metronomic (LDM) cyclophosphamide toxicity in cats with malignant neoplasia. J Feline Med Surg 2014; 16: 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mauldin GN, Matus RE, Patnaik AK, et al. Efficacy and toxicity of doxorubicin and cyclophosphamide used in the treatment of selected malignant tumors in 23 cats. J Vet Intern Med 1988; 2: 60–65. [DOI] [PubMed] [Google Scholar]
- 42. Chan CM, Rassnick KM, Frimberger AE, et al. Phase I dose escalating study of oral cyclophosphamide in tumour-bearing cats. Vet J 2020; 258: 105450. [DOI] [PubMed] [Google Scholar]
- 43. Moore AS, Frimberger AE, Chan CM. Dosage escalation of intravenous cyclophosphamide in cats with cancer. Vet J 2018; 242: 39–43. [DOI] [PubMed] [Google Scholar]
- 44. Okamura T, Kurashige A, Hanahachi A, et al. Thiazole orange-positive platelets in cats with thrombocytopenia induced by cyclophosphamide. Vet Rec 2003; 152: 506–507. [DOI] [PubMed] [Google Scholar]
- 45. Bosnjak S, Beleslin DB. Emesis: antiemetic effect of cyclophosphamide at central receptors of multitransmitter system in the cat. Pharmacol Res 2002; 46: 425–434. [DOI] [PubMed] [Google Scholar]
- 46. Fetting JH, McCarthy LE, Borison HL, et al. Vomiting induced by cyclophosphamide and phosphoramide mustard in cats. Cancer Treat Rep 1982; 66: 1625–1629. [PubMed] [Google Scholar]
- 47. Veterinary Cooperative Oncology Group – common terminology criteria for adverse events (VCOG–CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol 2016; 14: 417–446. [DOI] [PubMed] [Google Scholar]
- 48. Rassnick KM, Gieger TL, Williams LE, et al. Phase I evaluation of CCNU (lomustine) in tumor-bearing cats. J Vet Intern Med 2001; 15: 196–199. [DOI] [PubMed] [Google Scholar]
- 49. Rassnick KM, Williams LE, Kristal O, et al. Lomustine for treatment of mast cell tumors in cats: 38 cases (1999–2005). J Am Vet Med Assoc 2008; 232: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 50. Stroda KA, Murphy JD, Hansen RJ, et al. Pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide in cats after oral, intravenous, and intraperitoneal administration of cyclophosphamide. Am J Vet Res 2017; 78: 862–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Teske E, van Lankveld AJ, Rutteman GR. Intraperitoneal antineoplastic drug delivery: experience with a cyclophosphamide, vincristine and prednisolone protocol in cats with malignant lymphoma. Vet Comp Oncol 2014; 12: 37–46. [DOI] [PubMed] [Google Scholar]
- 52. Voorhorst MJ, van Maarseveen EM, van Lankveld AJ, et al. Bioavailability of cyclophosphamide and vincristine after intraperitoneal administration in cats. Anticancer Drugs 2014; 25: 1211–1214. [DOI] [PubMed] [Google Scholar]


