Abstract
Objectives
Isopropyl alcohol 70% as a rinse agent for chlorhexidine scrub has been shown to decrease body temperature more quickly than chlorhexidine solution in mice prepared aseptically prior to surgery. For this reason, some high-quality, high-volume (HQHV) surgical sterilization clinics use chlorhexidine solution rather than alcohol. We sought to determine if temperature upon entry to recovery, heat loss per kg and rate of temperature decline during surgery were different between cats rinsed with chlorhexidine solution vs 70% isopropyl alcohol following surgical scrub, and if there were significant predictors of recovery temperature.
Methods
Female cats admitted for surgery to trap–neuter–return (TNR) clinics at a veterinary college were assigned chlorhexidine solution or alcohol rinse agents via block randomization. Veterinary students and veterinarians performed spay surgeries using HQHV techniques. In recovery, heat support and reversal agents were available for cats with a low body temperature or that were slow to recover. Baseline values, outcome variables and duration of each stage (preparation, surgery, recovery) were assessed using Wilcoxon rank-sum and t-tests. Recovery temperature was evaluated using random effects multiple linear regression.
Results
The recovery temperature, heat loss per kg, heat loss per min, need for reversal and need for heat support in recovery were not significantly different between rinse groups. Weight <2.3 kg, body condition score <4, duration of surgery and postinduction temperature were predictors of recovery temperature. The rate of heat loss in the first 30 mins of surgery was slightly lower for cats in the alcohol rinse group and the recovery duration was shorter for cats weighing less <2.3 kg in the alcohol rinse group.
Conclusions and relevance
There were no clinically meaningful differences in body temperature between chlorhexidine and alcohol rinses. Both chlorhexidine solution and isopropyl alcohol 70% are appropriate rinse agents for aseptic preparation of feline spay surgeries.
Keywords: Chlorhexidine solution, isopropyl alcohol, surgical scrub, aseptic preparation, temperature
Introduction
Preoperative surgical site asepsis serves as a routine method for prevention of surgical site infection (SSI). 1 Veterinarians prepare surgical sites by alternating a scrub with a rinse with the goal of chemically killing and mechanically removing microorganisms and other contaminants. 2 Surgical scrubs are antiseptic detergents, such as chlorhexidine or povidone iodine, which are typically applied to the skin of the surgical site using a gauze pad unidirectionally in a concentric pattern to mechanically remove organic matter. The rinse is then applied in the same manner to remove the suds and any matter trapped within. This pattern is typically repeated in triplicate. 3
Temperature decreases during three phases in anesthesia. In the first hour the main driver is peripheral vasodilation that results in redistribution of heat from the core. For the following 2 h there is a less dramatic decline from decrease in metabolism because of the anesthetic drugs. After this the temperature is relatively stable.4,5 Anesthesia causes hypothermia by directly inducing vasodilation and by decreasing the threshold temperature for reflex vasoconstriction by the hypothalamus. 5 Radiation, evaporation, convection and conduction are sources of heat loss. 4 Hypothermia in cats has been defined as having a temperature <99°F and is classified as mild at 90–99°F, moderate at 82–90°F and severe at <82°F with normothermia ranging from 100.0°F to 102.5°F.6,7 Even mild hypothermia has been shown to cause adverse effects such as poor cutaneous perfusion owing to vasoconstriction, which can increase the risk of SSI.5,8 Severe hypothermia can cause more critical reactions such as bradycardia, hypotension, decreased cardiac output, arrhythmias, reduced respiratory rate and tidal volume, possibly leading to hypoxia.5,7 Other contributing factors include patient size and age, location of the surgical site, duration of anesthesia, American Association of Anesthesiologists’ status, basal body temperature and the surgical procedure that is being performed.7,8
The use of 70% isopropyl alcohol and 0.5% chlorhexidine aqueous solutions are widely accepted as surgical rinses with no significant difference in rate of SSI. 9 However, textbooks and laboratory manuals have advised against the use of alcohol in surgical preparations of rodents such as mice owing to concern for hypothermia following rapid evaporation.10–13 In the clinical experience of one author (RK) some high-quality, high-volume spay–neuter (HQHVSN) clinicians use a chlorhexidine rinse instead of alcohol in an effort to reduce the risk of perioperative hypothermia, particularly in their pediatric patients. However, the effect of alcohol rinses on mice is nuanced, with a rapid decrease in temperature followed by a rebound effect,14,15 and it is unclear whether the use of an alcohol rinse would affect cats similarly to mice.
The goal of this study was to determine whether the choice of surgical rinse affects the core body temperature of cats as measured upon entry to recovery in a clinically meaningful manner (a difference of at least 1°F). Exploratory outcomes included rate of heat loss, overall heat loss per minute, heat loss per kg, need for reversal in recovery and need for rescue heat support in recovery.
Materials and methods
Female cats presented for sterilization surgery at trap–neuter–return clinics at a veterinary college between 9 November 2019 and 9 March 2020 were included in this study. To induce anesthesia, cats were given an intramuscular (IM) injection of tiletamine/zolazepam 3 mg/kg, dexmedetomidine 7.5 µg/kg and butorphanol at 0.15 mg/kg (based on visually estimated weight) through their trap by a single technician and monitored by veterinary students. Once recumbent and unresponsive to stimuli, the cats were placed on a stainless steel table covered by a standard terry cloth towel and rectal temperature (postinduction temperature) was taken immediately with a digital thermometer (Vet One; MWI Veterinary Supply), which was left in place for subsequent monitoring. Temperature was recorded every 5 mins and no specific instruction in the use of rectal thermometers was provided. Cats were assigned to rinse treatment A (chlorhexidine solution) or B (isopropyl alcohol 70%) arms via block randomization (http://www.randomization.com/). A physical examination was performed by veterinary students assisted by attending faculty; this included estimation of age based on dentition, body condition score (BCS) and actual weight. Students then shaved the cats from xiphoid to pubis, applied eye lubrication, emptied the bladder and inserted a supraglottic airway control device (V-gel; Docsinnovent).
Following gross preparation, cats were moved to a surgical table with active heat support provided by an electrically resistant conductive fabric blanket (HotDog; Augustine Surgical) set at 109.4°F and connected to a non-rebreathing anesthetic circuit (T-Piece or Bain), capnograph and pulse oximeter by a dedicated student anesthetist. Anesthesia was maintained with isoflurane set at 1.5% unless there was a clinical indication for modification and oxygen at 2 l/min. The anesthetist took rectal temperatures every 5 mins, as well as a temperature recording at the start of surgery and end of surgery. As a final step cats were aseptically prepared using alternating chlorhexidine scrub and assigned rinse in triplicate by a research team member using the standard technique of circular motion starting from the anticipated surgical site and rotating outward. Owing to the different appearance of the rinse agents, as well as distinct smell of the alcohol, the research team members could not be blinded to the treatment. All scrub and rinses were at room temperature, which was targeted to 70°F, though not confirmed by environmental monitoring. Chlorhexidine scrub was kept in a standard stainless steel bin and prepared from a stock 2% chlorhexidine scrub diluted with water to approximately 1.5% with gauze sponges placed inside. The rinses were provided in either pre-made baggies labeled A and B for large events of >100 cats or in similar stainless steel bins labeled A and B. All baggies or bins were prepared on the morning of surgery during surgical set up giving them time to equilibrate to room temperature, although the temperature of the scrub and solution was not monitored. The gauze applied to the animals was saturated with scrub or rinse but in a quantity that did not result in pooling on the animal.
Surgeries were performed mainly by veterinary students using the HQHVSN technique. Specifically, 1 cm incisions were located on the ventral midline midway between the umbilicus and pubis, the uterus exteriorized with a spay hook, pedicle ties (auto-ligation) 16 of both ovarian pedicles, a single miller’s knot on the uterine body, the body wall closed with a cruciate, and the skin closed with a purse string or similar closure. For pregnant cats the procedure was modified so that the incision was extended as necessary, stick ties 17 applied to the uterine arteries distal to the miller’s knot, the body wall closed in a simple continuous pattern, and the subcutaneous tissue and skin closed with a modified Colorado pattern. On one of the surgical days a flank approach was used as part of a flank spay laboratory. For the flank spay, a similar technique was followed, except that the left side of the abdomen between the last rib and the iliac crest was shaved and scrubbed, and the incision located caudal to the midpoint between the last rib and iliac crest. The surgical stage was considered to span the time between the initial incision through the final closure.
After surgery, cats were moved to an ear-tipping station and then to recovery. Temperature was taken immediately in recovery and the temperature monitored every 5 mins. Cats were returned to their trap when they began to lift their heads. Rescue heat support was available in recovery, as well as reversal of dexmedetomidine with atipamezole for cats (0.04 mg/kg IM) with a low body temperature or that were slow to recover. Rescue heat support was deployed when the temperature at entry to recovery was <97°F or temperature declined over three measurements. Atipamezole was administered if they did not regain a palpebral reflex within 10 mins, did not begin to lift their head within 30 mins or had a declining temperature despite rescue heat support. Atipamezole was also administered for clinical indications unrelated to recovery or body temperature for indications such as mild respiratory disease where respiratory depression due to butorphanol or tiletamine/zolazepam may be potentiated by dexmedetomidine. All decisions were made by the attending veterinarian blind to treatment group.
Statistical methods
Baseline values (age, weight, BCS, incision size, surgical duration and postinduction temperature), the primary outcome measure (recovery temperature) and exploratory outcome measures (heat loss per kg, need for rescue heat support and need for reversal) were evaluated using Wilcoxon rank-sum tests for non-normal and t-tests for normal data. Normality was assessed via tests of skewness and kurtosis. The proportion of cats in each group that were hypothermic at recovery (<99°F), required heat support or required reversal was compared using two-sided tests of proportion. Predictors of recovery temperature were evaluated using multiple linear regression with robust standard errors. BCS was coded as <4 (unthrifty BCS) or ⩾4 on a 1–9 scale and weight was coded as less than (small) or greater or equal (large) to 2.3 kg based on determination of an inflection point around this weight. Temperature over time for each stage was analyzed longitudinally using mixed-effects linear regression models clustered on patient. Models were created using backwards stepwise regression and competing models evaluated using Akaike information criterion (AIC) and Bayesian information criterion (BIC). Potential clusters (patient and date) were evaluated with a likelihood ratio test. Model residuals were evaluated via scatterplots. All statistical analysis was performed using standard statistical software (STATA version 16) and significance was set at P <0.05.
Results
Over six surgical days there were 158 cats enrolled, 79 in the chlorhexidine arm and 79 in the alcohol arm. One cat in the alcohol arm was noted to be a neutered male after enrollment but before surgery and was excluded. Median weight was 2.7 kg (interquartile range [IQR] 2.2–3.1 kg), median estimated age was 12 months (IQR 8–24 months) and mean ± SD BCS 5 ± 1. All baselines were not different (Table 1). The mean postinduction temperature was 101.7 ± 1.2°F and the median time between induction and the first temperature reading (postinduction temperature) was 8 mins (IQR 5–13). Median duration of surgery was 30 mins (IQR 18–43 mins) and the median incision length was 1 cm (IQR 1–1.5 cm). At recovery, median temperature was 99.0°F (IQR 98.1–100.2°F), with 48% of cats hypothermic (<99°F), atipamezole administered in 22% of cats and 12% requiring rescue heat support. Median heat loss per kg was 1.09 (IQR 1.5–0.7), while mean heat loss per min was 0.05 ± 0.03.
Table 1.
Variables for the chlorhexidine and alcohol treatment groups with P values
| Chlorhexidine solution (n = 79) | Isopropyl alcohol 70% (n = 78) | P value | |
|---|---|---|---|
| Age (months) | 12 (7–24) | 12 (8–24) | 0.3977 |
| Weight (kg) | 2.6 (2.2–2.9) | 2.7 (2.1–3.2) | 0.0921 |
| Weight <2.3 kg | 20 (25) | 21 (27) | 0.8188 |
| BCS | 4.4 ± 0.7 | 4.5 ± 0.7 | 0.4102 |
| BCS <4 | 4 (5) | 4 (5) | 0.9852 |
| Postinduction temperature (°F) | 101.5 (100.7–102.3) | 101.9 (101.0–102.7) | 0.1872 |
| Incision size (cm) | 1 (1–1.5) | 1 (1–1.5) | 0.8068 |
| Flank incision | 11 (14) | 9 (12) | 0.6540 |
Median (interquartile range) for non-normally distributed data; mean ± SD for normally distributed data; or n (% of total). All variables were not different between groups
BCS = body condition score
A Wilcoxon signed-rank test indicated that the primary outcome – temperature at recovery (Figure 1) – was not significantly different between chlorhexidine solution (median 98.9°F, IQR 97.7–99.9°F) and alcohol (median 99.3°F, IQR 98.2–100.4°F) rinses (P = 0.2686). Exploratory outcomes, including heat loss per kg (P = 0.9316), overall heat loss per min (t(154) = 1.3; P = 0.1926), need for rescue heat support (P = 0.7840) and need for reversal (P = 0.2640) were also not significantly different between arms. A two-sided test of proportions showed that the proportion of cats in the chlorhexidine arm that were administered atipamezole (25%) was not different (P = 0.2625) than the proportion of cats in the alcohol arm (18%), nor was the proportion of cats requiring rescue heat support in the chlorhexidine arm (11%) different (P = 0.7608) than the proportion in the alcohol arm (13%). The proportion of cats hypothermic at recovery was not different between the chlorhexidine (53%) and alcohol (42%) arms (P = 0.1733). The proportion of small cats that were administered atipamezole (27%) was not different (P = 0.7608) than the proportion of large cats (20%); however, the proportion of small cats requiring rescue heat support (32%) was greater (P <0.00001) than large cats (5%), as was the proportion (P = 0.0006) of small cats (71%) that were hypothermic at recovery vs large cats (40%).
Figure 1.
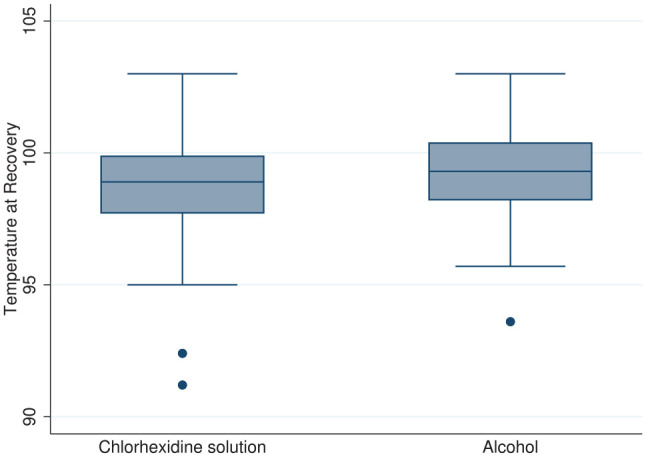
Temperature at recovery for the chlorhexidine (n = 79) and alcohol (n = 78) treatment groups. Line at median, box between 25th and 75th percentile, whiskers to lower and upper adjacent values, dot at outlier values
Linear regression to determine predictors of recovery temperature showed that total duration of time since induction (beta [β] = −0.2, P <0.0001), temperature after induction (β = 0.26, P = 0.009), temperature at start of surgery (β = 0.65, P <0.0001), unthrifty BCS (β = −1.44, P <0.0001) and small size (β = −0.57, P = 0.003) were significant. Rinse group and incision size were not significant, and models using total anesthetic time had lower AIC and BIC scores than those incorporating preparation and surgical duration as separate variables.
The duration, and start and end temperatures between arms for each stage were not different for any stage (preparation, surgery or recovery), both for small and large cats, with the exception of recovery duration (Table 2). The recovery duration was significantly shorter (P = 0.0453) for alcohol rinse cats (median 12 mins) compared with chlorhexidine rinse cats (median 16 mins), which was driven by the shorter (P = 0.0015) recovery for small cats, which in the alcohol group had a median recovery duration of 10 mins and the chlorhexidine group, 25 mins.
Table 2.
Duration, start temperature and end temperature for the stages of preparation, surgery and recovery, as well as the P values for their comparison
| All | Preparation | Surgery | Recovery | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Duration | Start temperature | End temperature | Duration | Start temperature | End temperature | Duration | Start temperature | End temperature | |
| Chlorhexidine solution | 79 | 11 (6–15) | 101.5 (100.7–102.3) | 101.0 (100.4–102.1) | 30 (18–40) | 100.7 (100.0–101.9) | 99.2 (98.3–100.3) | 16 (10–24) | 98.9 (97.7–99.9) | 98.8 (98.1–99.5) |
| Isopropyl alcohol 70% | 78 | 12 (7–20) | 101.9 (101.0–102.7) | 101.4 (100.5–102.1) | 30 (19–44) | 101.0 (100.3–101.7) | 99.6 (98.7–100.4) | 12 (6–19) | 99.3 (98.2–100.4) | 98.9 (98.1–99.5) |
| P value | 0.3193 | 0.2306 | 0.4247 | 0.7269 | 0.9152 | 0.2199 | 0.0451 | 0.2574 | 0.8114 | |
| Weight <2.3 kg | Preparation | Surgery | Recovery | |||||||
| Duration | Start temperature | End temperature | Duration | Start temperature | End temperature | Duration | Start temperature | End temperature | ||
| Chlorhexidine solution | 20 | 14 (8–20) | 101.2 (99.9–101.7) | 100.4 (99.8–101.1) | 31 (25–40) | 99.9 (99.2–101.8) | 98.4 (96.8–99.0) | 25 (16–40) | 97.5 (96.0–98.6) | 98.2 (97.4–98.4) |
| Isopropyl alcohol 70% | 21 | 10 (7–19) | 101.6 (100.2–103.0) | 101.3 (100.2–102.3) | 37 (21–44) | 100.6 (100.0–101.6) | 99.1 (97.2–100.4) | 10 (4–16) | 98.8 (97.3–99.5) | 98.9 (97.8–99.5) |
| P value | 0.4457 | 0.2101 | 0.1076 | 0.6731 | 0.2321 | 0.0544 | 0.0015 | 0.0678 | 0.1039 | |
| Weight ⩾2.3 kg | Preparation | Surgery | Recovery | |||||||
| Duration | Start temperature | End temperature | Duration | Start temperature | End temperature | Duration | Start temperature | End temperature | ||
| Chlorhexidine solution | 59 | 10 (6–15) | 101.8 (100.9–102.5) | 101.4 (100.6–102.3) | 30 (16–40) | 101.0 (100.3–102.1) | 99.5 (98.8–100.5) | 14 (7–20) | 99.2 (98.7–100.4) | 99.0 (98.0–100.0) |
| Isopropyl alcohol 70% | 57 | 13 (7–20) | 101.9 (101.2–102.6) | 101.5 (100.6–102.0) | 28 (15–44) | 101.0 (100.3–101.7) | 99.9 (98.9–100.4) | 14 (9–19) | 99.3 (98.5–100.5) | 98.9 (98.2–99.5) |
| P value | 0.1416 | 0.5269 | 0.9187 | 0.8682 | 0.6042 | 0.6836 | 0.9119 | 0.8294 | 0.3678 | |
Median (interquartile range) for non-normally distributed data. Values for all cats, as well as cats broken down by weight <2.3 kg and ⩾2.3 kg. Significant P values shown in bold. Durations are given in mins, and temperatures are in °F
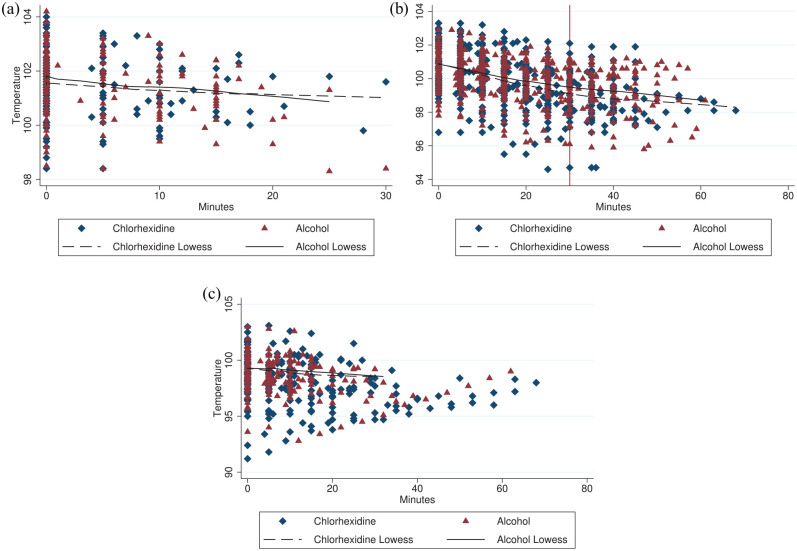
Visual analysis of each stage using a scatterplot overlaid by LOWESS curve showed that the temperature decreased linearly for the preparation stage, while there was an inflection point in the decrease for the surgical stage with a change in slope around 30 mins (Figure 2). For the preparation stage a mixed-effects model clustered by cat showed that postinduction temperature (β = 0.91, P <0.0001), small size (β = −0.12, P = 0.031) and minutes of preparation (β = −0.06 per min, P <0.0001) predicted the recovery temperature, while unthrifty BCS was not significant. For surgical time at less than 30 mins a mixed-effects model clustered by cat showed that temperature at start of surgery (β = 0.80, P <0.0001), post-induction temperature (β = 0.10, P = 0.023), unthrifty BCS (β = −0.45, P <0.0001), small size (β = −0.35, P <0.0001), minutes of surgical time for the chlorhexidine group (β = −0.07, P <0.0001) and minutes of surgical time for the alcohol group (β = −0.06, P <0.0001) were significant predictors of recovery temperature. Duration of preparation, flank approach and incision size were not significant predictors of recovery temperature. For surgical times at ⩾30 mins, temperature at start of surgery (β = 0.54, P <0.0001), postinduction temperature (β = 0.35, P <0.0001), unthrifty BCS (β = −1.24, P <0.0001) and minutes of surgical time (β = −0.02, P <0.0001) were significant, while treatment group and small size were no longer significant. For recovery of cats that were not administered atipamezole, the temperature at the start of recovery (β = 0.86, P <0.0001) and minutes in recovery (β = −0.02, P = 0.006) were significant, while the treatment group, small size, postinduction temperature, unthrifty BCS, flank approach and incision size were not significant predictors of recovery temperature. No model was improved by clustering by date.
Figure 2.
Scatterplot of temperature (ºF) by minute for chlorhexidine (n = 79) and alcohol (n = 78) rinses by the phases (a) preparation, (b) surgery and (c) recovery overlaid with their respective locally weighted scatterplot smoothing (LOWESS) lines. Time 0 is the start of the respective phase. Vertical line at 30 mins in the (b) surgical phase demonstrates inflection point
Discussion
The impact of surgical rinse on mice has been inconclusive. In one study comparing various aqueous and alcohol-based agents for aseptic preparation of mice prior to a 15 min surgery, it was found that use of a 70% isopropyl alcohol rinse resulted in a more rapid decrease in body temperature than other rinses, including chlorhexidine solution. 14 However, all mice ended up at a similar temperature by the conclusion of the surgery. Unlike this study, no surgical drapes were used. In comparison, our study also found that the end temperature of cats was the same at the conclusion of surgery, although for small cats there was a difference of 0.7°F that just missed statistical significance (P = 0.0544). However, the chlorhexidine rinse group rather than the alcohol group was noted to decrease faster.
In another study, the surface and core body temperature of mice was examined as they underwent aseptic preparation and 38 mins of anesthesia. The use of 70% isopropyl alcohol resulted in rapid decrease in both core and body surface area readings. However, these mice experienced a rebound effect within minutes, which resulted in a more rapid recovery to baseline temperature than unscrubbed animals. 15 No surgery or draping was performed. They also found that heavier mice in the alcohol group had a shorter duration of recovery to baseline temperature than lighter mice. In our study the recovery duration was shorter for small alcohol rinse cats. These cats recovered 15 mins faster than chlorhexidine rinse cats (more than twice as fast). This may echo the rebound effect observed with the mice, 15 though here the effect was observed in smaller, not larger, cats.
Duration of anesthesia, starting temperature, low weight and low BCS were predictors of recovery temperature, which is consistent with previous studies.7,8 The impact of the initial postinduction temperature highlights the importance of ensuring that patients are kept warm prior to induction and minimizing time under anesthesia, particularly for smaller or unthrifty cats. In this study, the temperature of approximately three-quarters of the cats was likely impacted primarily by heat redistribution as time under anesthesia was <1 h. 4 This may have been affected by the use of dexmedetomidine which, per the package insert, causes a peripheral vasoconstriction with peak sedative effects at 30 mins after a single-agent IM injection and waning of sedative effects after 3 h, 18 although a study found peak effects following IM injection to be sooner. 19 As the induction cocktail was dosed on body weight and dexmedetomidine is dosed in species having a wide range of body weight based on body surface area, it is possible that small cats received proportionately less dexmedetomidine than larger cats given their larger surface area-to-weight ratio. The potential for this effect may be minimal given the small range of weights in this study. Isoflurane also causes vasodilation that may have counteracted some of these effects. 20
Data were also analyzed as preparation, surgical time <30 mins, surgical time ⩾30 mins and recovery phases in order to better examine the effect of the rinse. In the preparation phase, postinduction temperature, small size and minutes of preparation were significant, while unthrifty BCS was not. This is consistent with the main source of initial heat loss coming from the redistribution of heat from the core to the periphery. 4 Small cats may have experienced greater losses from radiation and convection owing to their larger exposed surface area-to-weight ratio after being shaved or from the difference in energy required to evaporate the water-based rinse vs the alcohol rinse.
For the initial 30 mins of the surgical phase, which occurred immediately after cats were scrubbed, the impact of small size increased and unthrifty BCS became significant, accounting for nearly half of a degree. Temperature at the start of surgery was significant as was postinduction temperature, although to a lesser degree than in the preparation stage. Cats in the alcohol group decreased at the same rate observed during preparation (0.06°F/min), while cats in the chlorhexidine group decreased at a slightly greater rate (0.07°F/min). The difference in the rate of heat loss may have been due to the chlorhexidine rinse providing evaporative cooling for a longer duration of time, or vasoconstriction of the abdominal skin in the alcohol group due to rapid evaporation that decreased cutaneous heat loss. 15 A flank approach vs ventral midline was not significant, nor was incision size, which may be due to incisions being of minimal length. After 30 mins of surgical time, the impact of time decreased to 0.02°F/min for both groups and the impact of unthrifty BCS increased to 1.3°F of additional loss. This may have been due to a switch to metabolic sources of heat loss and highlights the importance of minimizing surgical times for animals with low BCS.
In HQHVSN, cat spay surgeries performed by veterinarians are typically 5–10 mins in length. 21 In this study, surgeries were performed by student veterinarians with a median time of 30 mins, though the median incision length of 1 cm was similar to HQHVSN surgeons. The expected heat loss for an experienced HQHVSN surgeon can be estimated from this study’s linear regression with cats expected to lose 0.3–0.7°F in the surgical phase.
Limitations
The scrub and rinse were applied pragmatically with minimal attempts to standardize the process beyond a common triplicate scrub protocol. There was also no standardization of when the scrub was applied in relation to temperature taking or from application of scrub to start of surgery. However, there should have been no systematic bias between rinse groups, and there were no differences noted between surgical dates. The digital thermometers were not calibrated before use. The study was only performed in female cats owing to the assumption that the brief duration of surgery for male cats and relatively small area of rinse application would minimize any differences in body temperature. The research team members who scrubbed the cats could not be blinded to treatment group owing to the distinct appearance and smell of the alcohol rinse. However, the team members were not responsible for collecting the temperature data during surgery nor for any medical decisions in recovery. Finally, there may be limited generalizability beyond this operating environment as ambient temperature, preanesthetic body temperature, active and passive warming techniques, and differing drug protocols might all impact the patient’s body temperature and rate of heat loss.
Conclusions
There was no clinically meaningful difference in any of the outcome variables between chlorhexidine and alcohol rinses. The temperature at the start of recovery was not different between groups. None of the exploratory endpoints, including heat loss per kg, overall heat loss per min, proportion of hypothermic cats in recovery, need for reversal in recovery or need for rescue heat support in recovery were different. The rate of heat loss in the first 30 mins of surgery was slightly lower for cats in the alcohol rinse group and the recovery duration was shorter for cats weighing <2.3 kg in the alcohol rinse group. Both chlorhexidine solution and isopropyl alcohol 70% are appropriate rinse agents for aseptic preparation of feline spay surgeries.
Acknowledgments
We would like to thank Lexi Hechtman for her assistance with data collection and entry.
Footnotes
Accepted: 16 November 2020
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Rachael E Kreisler  https://orcid.org/0000-0002-5562-5521
https://orcid.org/0000-0002-5562-5521
References
- 1. Anggrahita T, Wardhana A, Sudjatmiko G. Chlorhexidine-alcohol versus povidone-iodine as preoperative skin preparation to prevent surgical site infection: a meta-analysis. Med J Indones 2017; 26: 54–61. [Google Scholar]
- 2. Zubrod CJ, Farnsworth KD, Oaks JL. Evaluation of arthrocentesis site bacterial flora before and after 4 methods of preparation in horses with and without evidence of skin contamination. Vet Surg 2004; 33: 525–530. [DOI] [PubMed] [Google Scholar]
- 3. Fossum TW. Small animal surgery textbook. 3rd ed. St Louis, MO: Elsevier Health Sciences, 2007. [Google Scholar]
- 4. Sessler DI. Perioperative thermoregulation and heat balance. Lancet 2016; 387: 2655–2664. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong SR, Roberts BK, Aronsohn M. Perioperative hypothermia. J Vet Emerg Crit Care 2005; 15: 32–37. [Google Scholar]
- 6. Kavanagh T, Buggy DJ. Can anaesthetic technique effect postoperative outcome? Curr Opin Anaesthesiol 2012; 25: 185–198. [DOI] [PubMed] [Google Scholar]
- 7. Redondo JI, Suesta P, Serra I, et al. Retrospective study of the prevalence of postanaesthetic hypothermia in dogs. Vet Rec 2012; 171: 374. [DOI] [PubMed] [Google Scholar]
- 8. Grimm KA. Perioperative thermoregulation and heat balance. In: Grimm KA, Lamont LA, Tranquilli WJ, et al. (eds). Veterinary anesthesia and analgesia: the fifth edition of Lumb and Jones. Chichester: John Wiley & Sons, 2017, pp 327–379. [Google Scholar]
- 9. Charles D, Heal CF, Delpachitra M, et al. Alcoholic versus aqueous chlorhexidine for skin antisepsis: the AVALANCHE trial. CMAJ 2017; 189: E1008–E1016. DOI: 10.1503/cmaj.161460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Capello V. Common surgical procedures in pet rodents. J Exot Pet Med 2011; 20: 204–307. [Google Scholar]
- 11. Bennett R. Soft tissue surgery. In: Quesenberry K, Carpenter J. (eds). Ferrets, rabbits, and rodents: clinical medicine and surgery. 3rd ed. St Louis, MO: Elsevier, 2012, pp 373–391. [Google Scholar]
- 12. Muir WW, de Morais H. Acid–base physiology. In: Tranquilli WJ, Thurmon JC, Grimm KA. (eds). Lumb and Jones’ veterinary anesthesia and analgesia. Ames, IA: Wiley-Blackwell, 2007, p 173. [Google Scholar]
- 13. Sessler DI, Sessler AM, Hudson S, et al. Heat loss during surgical skin preparation. Anesthesiology 1993; 78: 1055–1064. [DOI] [PubMed] [Google Scholar]
- 14. Del Valle JM, Fisk EA, Noland EL, et al. Comparison of aqueous and alcohol-based agents for presurgical skin preparation methods in mice. J Am Assoc Lab Anim Sci 2018; 57: 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skorupski AM, Zhang J, Ferguson D, et al. Quantification of induced hypothermia from aseptic scrub applications during rodent surgery preparation. J Am Assoc Lab Anim Sci 2017; 56: 562–569. [PMC free article] [PubMed] [Google Scholar]
- 16. Miller KP, Rekers W, Ellis K, et al. Pedicle ties provide a rapid and safe method for feline ovariohysterectomy. J Feline Med Surg 2016; 18: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herold LV, Devey JJ, Kirby R, et al. Clinical evaluation and management of hemoperitoneum in dogs. J Vet Emerg Crit Care 2008; 18: 40–53. [Google Scholar]
- 18. Dechra Veterinary Products. Dexmedesed. https://www.dechra-us.com/Files/Files/SupportMaterialDownloads/us/07PG-DEX50062-0117.pdf (2001, accessed November 24, 2020).
- 19. Siao KT, Pypendop BH, Honkavaara J, et al. Hemodynamic effects of dexmedetomidine, with and without MK-467, following intramuscular administration in cats anesthetized with isoflurane. Vet Anaesth Analg 2017; 44: 1101–1015. [DOI] [PubMed] [Google Scholar]
- 20. Sessler DI, McGuire J, Moayeri A, et al. Isoflurane-induced vasodilation minimally increases cutaneous heat loss. Anesthesiology 1991; 74: 226–232. [DOI] [PubMed] [Google Scholar]
- 21. Semick DN, Shaver SL, Cornell HN, et al. Perioperative blood glucose concentrations in kittens following overnight fasting and gonadectomy. J Feline Med Surg 2018; 20: 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]