Abstract
Objectives
The aim of the study was to document whether a proportion of non-diabetic cats with left ventricular hypertrophy (LVH) previously diagnosed with hypertrophic cardiomyopathy (HCM) have elevated circulating insulin-like growth factor 1 (IGF-1) concentrations.
Methods
A retrospective analysis of residual blood samples obtained at the time of echocardiographic diagnosis of HCM from a population of 60 non-diabetic cats were analysed for circulating IGF-1 concentrations using a validated radioimmunoassay and compared with a control group of 16 apparently healthy cats without LVH. Clinical and echocardiographic data for cats with an IGF-1 level >1000 ng/ml were compared with those with an IGF-1 level <800 ng/ml.
Results
In total, 6.7% (95% confidence interval 1.8–16.2%) of cats with HCM had an IGF-1 level >1000 ng/ml. The prevalence of an IGF-1 level >1000 ng/ml in the control group was zero.
Conclusions and relevance
A small proportion of non-diabetic cats previously diagnosed with HCM had an IGF-1 concentration at a level that has been associated with feline hypersomatotropism (fHS) in the diabetic cat population. Further prospective research is required to confirm or refute the presence of fHS in non-diabetic cats with LVH and increased IGF-1.
Keywords: Endocrinopathy, cardiac, hypersomatotropism, acromegaly, phenocopy, left ventricular hypertrophy
Introduction
Diagnosis of hypertrophic cardiomyopathy (HCM), as a primary condition, relies on accurately screening for and ruling out conditions that may cause an HCM phenotype. 1 An HCM phenotype may be caused by a small number of known disorders in cats; examples include fixed aortic stenosis, infiltrative myocardial disease, hyperthyroidism, systemic hypertension, transient myocardial thickening, myocarditis and hypersomatotropism.1–11 HCM, as a primary condition, has an average prevalence of 15%, with increasing prevalence occurring with age.
Feline hypersomatotropism (fHS), which causes the clinical syndrome of feline acromegaly, is increasingly recognised as an important endocrinopathy in cats and it may be present in approximately 17–25% of diabetic cats.12,13 fHS is typically associated with the concurrent presence of diabetes mellitus (DM),12,14,15 whereas fHS in non-diabetic cats has only been reported in a small number of cases.16,17 Conversely, in people, hypersomatotropism in the absence of DM is common.18,19 Furthermore, the presence of growth hormone-induced left ventricular hypertrophy (LVH) is also recognised in patients without concurrent DM.20–22
LVH is commonly seen in diabetic cats with fHS;23,24 importantly, this seems to be a reversible cause of cardiac remodelling in most cases following hypophysectomy. 10 This association of LVH to fHS has only been documented in cats with the typical phenotype of fHS, that is, fHS with concurrent DM. The prevalence of fHS in cats without DM has not been assessed and no current studies have explored the possibility of fHS in non-diabetic cats with LVH.
The aim of this study was to measure insulin-like growth factor 1 (IGF-1) in stored serum or plasma samples of non-diabetic cats previously diagnosed with HCM using a validated radioimmunoassay (RIA), 12 and to compare the prevalence of IGF-1 concentrations >1000 ng/ml, a cut-off associated with a high probability of fHS in diabetic cats, 12 with a control population of apparently healthy cats without LVH. We hypothesised that we would detect a number of non-diabetic cats previously diagnosed with HCM with IGF-1 concentrations above a threshold that could raise a tentative suspicion for fHS and provide grounds for further study. In addition, we hypothesised that clinically healthy cats without LVH would not have IGF-1 concentrations >1000 ng/ml.
Materials and methods
Study population
A population of non-diabetic cats previously diagnosed with HCM and with a banked residual blood sample, obtained at the time of echocardiographic diagnosis, were selected via review of computerised patient records from client-owned cats. This population had been referred to either of two veterinary teaching hospitals – the Queen Mother Hospital for Animals, Royal Veterinary College and Langford Vets, University of Bristol – over a period spanning 5 years (2012–2017). A group of apparently healthy control cats, without LVH on echocardiography, were recruited from a geriatric cat health clinic, performed at two first-opinion practices (a geriatric cat research outreach clinic run weekly at PDSA Bow and Blue Cross Victoria, London) over an 8-week period in 2013–2014 where blood samples were collected at the same time that echocardiography was performed. Analysis of IGF-1 concentrations from the stored serum/plasma samples from both groups was performed in 2017. Ethical approval by both institutions was granted (URN 2017 1734-2 and VIN/17/041 numbers, respectively) and, at both institutions, owner permission for the use of residual blood samples was obtained at the time the initial blood sample was taken.
Inclusion criteria
To be included, a residual blood sample (either serum or plasma) stored at −80°C for no longer than the 5-year period stated above had to be available. All cats must have had a documented history, physical examination, echocardiographic examination performed by a cardiology diplomate or resident under direct supervision using a standard protocol, 25 non-invasive blood pressure measurement (NIBP) and, if over the age of 7 years, a total thyroxine (TT4) result available for review. Echocardiographic diagnosis of control and HCM cats was confirmed by a cardiology diplomate (KB, JRP, XNC) according to current consensus guidelines. 1
Exclusion criteria
Cats were excluded if there was documented evidence of disorders likely to result in secondary LVH, specifically: hyperthyroidism, systemic hypertension or congenital heart disease such as aortic stenosis. Hyperthyroidism was excluded based on absence of compatible historical or physical examination findings in all cats (unexplained weight loss, increased appetite, hyperactivity, behavioural changes or palpable goitre) with the additional requirement of TT4 <40 nmol/l (Immulite 1000; Siemens Healthineers [laboratory reference interval 19–65 nmol/l]) in all cats older than 7 years of age. 26 Systemic hypertension was defined as at least three consecutive measurements of systolic NIBP exceeding 160 mmHg, as per current guidelines. 27 In cases with equivocal results (140–159 mmHg) the presence of retinal changes compatible with hypertensive retinopathy were used as a further exclusion criterion. DM was excluded based on review of clinical records for clinical signs and clinicopathological data consistent with DM. Therefore, patients with evidence of unexplained polyuria, polydipsia or polyphagia were excluded, as were patients with unexplained weight loss, despite normal or increased appetite. Additionally, cats with repeatable hyperglycaemia (>6.3 mmol/l) or glycosuria and/or serum fructosamine >400 µmol/l were assumed to be diabetic and were also excluded. 28
IGF-1 measurement
Total IGF-1 concentrations were measured using a previously validated, commercially available RIA (Nationwide Specialist Laboratories).12,24 A cut-off of >1000 ng/ml has previously been shown to result in a 95% positive predictive value for fHS using this particular assay when used in diabetic cats. 12 Although the cats in this present study were non-diabetic, values above this cut-off were considered to be elevated. Cats with results that may be considered equivocal (800–1000 ng/ml) were not included in statistical comparisons. 12
Patient data
Retrospective medical data were entered into an electronic database for analysis (Microsoft Excel for Mac, Version 16.5). Patient details (age, weight, breed and sex), physical examination findings (heart rate, resting respiratory rate, body weight, grade of heart murmur, and presence or absence of arrhythmia and gallop sound) were recorded. Patients were classified as being in congestive heart failure (CHF) based on a combination of compatible historical, physical examination or ancillary test findings that would suggest pulmonary oedema/pleural effusion with concurrent cardiac changes making CHF the most likely diagnosis. For example, dyspnoea in the presence of an enlarged left atrium (defined as a left atrial to aortic ratio, measured on a short-axis view [LA:Ao] >1.5 and/or left atrial diameter [LAD], measured from a right parasternal long-axis view in the last frame before mitral valve opening LAD >16.0 mm), point-of-care ultrasound for evidence of B-lines and/or pleural fluid with left atrial enlargement, or thoracic radiography demonstrating an alveolar pattern and or pleural effusion with cardiomegaly and/or pulmonary vessel congestion.29–31 A diagnosis of feline arterial thromboembolism (ATE) was recorded based on evidence of acute paresis or paralysis in one or more limbs, accompanied by limb pain, pulselessness or pallor in an affected limb or limbs, in combination with a compatible clinical history. 32 Standard echocardiographic measurements were included in the database for analysis.
Outcome data
For the cats with an IGF-1 >1000 ng/ml the referring practices were contacted to determine if there was any evidence of DM or fHS, defined as compatible clinical, morphological or clinicopathological signs, developed after the diagnosis of HCM. 12 The current status of the patient as alive/deceased/unknown was determined, along with the cause of death, if applicable. In addition, the time since diagnosis of LVH and blood sampling was recorded based on the date of the last contact in the clinical records.
Statistical analysis
Analysis was performed using commercially available software (IBM SPSS Statistics, Version 26). Data were assessed graphically and by Shapiro–Wilk tests for normality. Data are represented as median (range) due to the majority of data having skewed distribution. The proportion of cats with an IGF-1 >1000 ng/ml was calculated in both HCM and control groups, and confidence intervals (CIs) calculated (http://www.sample-size.net/confidence-interval-proportion/). HCM cats with an IGF-1 >1000 ng/ml were compared with those with an IGF-1 <800 ng/ml, to see if any associations could be made with an elevated IGF-1. Categorical variables were compared using χ2 or Fisher’s exact tests. Continuous variables were compared using Mann–Whitney U-test or an independent samples t-test. The significance level was set at 5% (P <0.05).
Results
Population characteristics
Records and blood samples of 60 cats with HCM meeting the inclusion criteria were available. Median age was 8 years (range 1–19 years). Pedigree breeds were represented in 20% (n = 12) of cats: three British Shorthair (5%), three Persian (5%), two British Blue (3%), and one each of Devon Rex, Sphynx, Maine Coon and Russian Blue. The remaining 80% (n = 48) of cats were non-pedigree. Sixty-three per cent (n = 38) of cats were male and 36% (n = 22) were female. All but one cat (female) was recorded as neutered. The median body weight for all cats in the study was 4.47 kg (range 3.10–8.80 kg). Clinical and echocardiographic data for cats with HCM and the healthy controls are summarised in Table 1. Table 2 details the medications being received by the 60 cats diagnosed with HCM in this study.
Table 1.
Population characteristics, clinical presentation data and echocardiographic data for healthy control cats with no evidence of left ventricular hypertrophy
| Cats diagnosed with HCM (n = 60) | Healthy control cats (n = 16) | |
|---|---|---|
| Age (years) | 8.0 (1.0–19.0) | 13.2 (10.0–20.0) |
| Weight (kg) | 4.55 (3.13–8.83) | 4.13 (3.05–6.46) |
| IGF-1 (ng/ml) | 501 (25–1179) | 439 (207–681) |
| Pedigree | 10 (16.7) | 0 |
| Male | 38 (63.3) | 8 (50) |
| Heart murmur grade III or louder | 30 (50) | 1 (6) |
| Arrhythmia | 16 (26.7) | 0 |
| LV maximum thickness (mm) | 7.0 (6.0–12.6) | 5.0 (4.1–5.8) |
| LVIDd (mm) | 14.0 (8.3–28.0) | 14.4 (11.3–16.9) |
| LA:Ao | 1.54 (1.02–3.61) | 1.22 (1.01–1.39) |
| LA diameter (mm) | 17.00 (2.00–30.00) | 13.94 (11.20–17.44) |
Data are given as median (range). Categorical variables are displayed as n (%) of cats with the clinical sign or echocardiographic criteria
HCM = hypertrophic cardiomyopathy; IGF-1 = insulin-like growth factor-1; LV = left ventricle; LVIDd = left ventricular internal diameter in diastole; LA:Ao = left atrium to aortic ratio in a short-axis view; LA = left atrium
Table 2.
Cardiac medications prescribed to cats diagnosed with hypertrophic cardiomyopathy (HCM)
| Medications | Number of HCM cats receiving medication |
|---|---|
| Atenolol | 5 (8) |
| Aspirin | 6 (10) |
| Benazepril | 5 (8) |
| Benazepril + spironolactone combination | 2 (3) |
| Clopidogrel | 10 (17) |
| Diltiazem | 1 (2) |
| Enalapril | 1 (2) |
| Frusemide | 12 (20) |
| Hydrochlorothiazide | 2 (3) |
| Pimobendan | 5 (8) |
| None | 37 (62) |
Data are displayed as n (%)
Distribution of IGF-1 results
Of the cats diagnosed with HCM, IGF-1 >1000 ng/ml was found in 6.7% (n = 4; 95% CI 1.8–16.2%) of cases. Three cats had an IGF-1 of 800–1000 ng/ml and were excluded from further analysis. The remainder (88%, n = 53) had an IGF-1 <800 ng/ml. The distribution of IGF-1 results across this population, as well as the healthy control cats, is shown in Figure 1. Of the healthy control cats, all samples measured <800 ng/ml.
Figure 1.
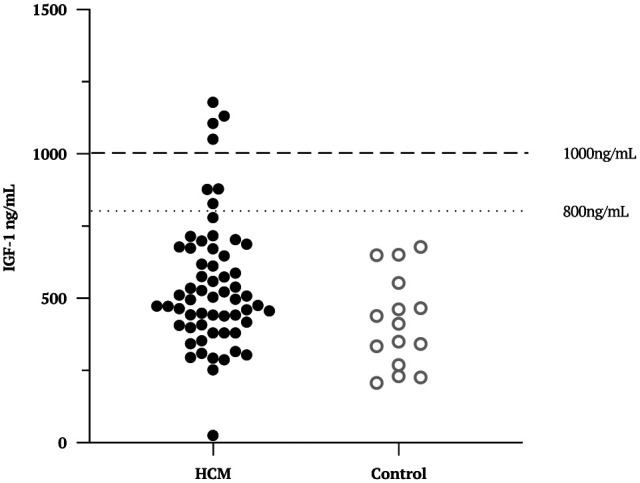
Scatter plot to show the distribution of insulin-like growth factor 1 (IGF-1) results in a population of cats previously diagnosed with hypertrophic cardiomyopathy (HCM; solid circles) vs healthy control cats (open circles)
Associations between clinical variables and IGF-1 results in cats diagnosed with HCM
Population data for HCM cats with an IGF-1 >1000 ng/ml, an IGF-1 <800 ng/ml and an IGF-1 of 800–1000 ng/ml are presented in Table 3. Comparisons were performed between cats with an IGF-1 >1000 ng/ml and cats with an IGF-1 <800 ng/ml only. HCM cats with an IGF-1 >1000 ng/ml were significantly heavier, had a larger LA:Ao and poorer LA function than those with an IGF-1 <800 ng/ml (Table 3). A greater proportion of cats with an IGF-1 >1000 ng/ml also presented with CHF vs those with an IGF-1 <800 ng/ml (Table 3). No other significant differences in population, clinical and echocardiographic data were found.
Table 3.
Population characteristics, clinical presentation data and echocardiographic data for cats according to circulating insulin-like growth factor 1 (IGF-1) concentration
| Circulating IGF-1 concentration | P value for comparisons of cats with IGF-1 <800 ng/ml vs IGF-1 >1000 ng/ml | |||
|---|---|---|---|---|
| IGF-1 <800 ng/ml | IGF-1 >1000 ng/ml | IGF-1 800–1000 ng/ml | ||
| Number | 53 | 4 | 3 | NA |
| Age (years) | 8.0 (1.0–19.0) | 8.5 (8.0–9.0) | 7.0 (2.0–11.0) | 0.730 |
| Weight (kg) | 4.63 (3.13–6.67) | 5.50 (5.40–5.50) | 4.70 (4.45–6.00) | 0.019* |
| IGF-1 (ng/ml) | 462 (25–779) | 1142 (1106–1179) | 877 (828–879) | NA |
| Pedigree | 11 (20) | 1 (25) | 0 | 0.669 |
| Male | 32 (60) | 4 (100) | 3 (100) | 0.332 |
| HR (beats/min) | 180 (100–260) | 170 (160–220) | 180 (160–180) | 0.736 |
| RR (breaths/min) | 34 (20–66) | 33 (22–44) | 48 (28–56) | 0.693 |
| Heart murmur grade III or louder | 9 (17) | 0 | 2 (67) | 0.102 |
| CHF | 14 (26) | 4 (100) | 2 (67) | 0.006* |
| ATE | 12 (23) | 3 (75) | 1 (34) | 0.135 |
| Arrhythmia | 12 (23) | 3 (75) | 1 (34) | 0.135 |
| DLVOTO | 23 (43) | 0 | 3 (100) | 0.140 |
| DRVOTO | 9 (17) | 0 | 0 | 0.497 |
| LV maximum thickness (mm) | 7.4 (6.0–12.6) | 6.9 (6.0–7.8) | 7.1 (6.0–8.8) | 0.467 |
| LVIDd (mm) | 13.4 (8.3–19.2) | 14.5 (12.0–17.0) | 14.1 (11.8–14.2) | 0.140 |
| LVFS% | 50.5 (22.5–78.6) | 38.8 (23.0–78.0) | 59.50 (49.97–62.00) | 0.795 |
| LA:Ao | 1.48 (1.02–2.89) | 3.13 (2.64–3.61) | 2.10 (1.22–3.06) | 0.0077* |
| LAFS% | 25 (4–47) | 10 (3.0–17.0) | 22 (11–33) | 0.0093* |
Data are given as median (range). Categoric variables are displayed as n (%) cats with the clinical sign or echocardiographic criteria compared with their respective group; that is, not as a percentage of the total population. Cats with an IGF-1 >1000 ng/ml and <800 ng/ml were compared statistically
Statistically significant differences (P <0.05) between these groups. Cats with an IGF-1 of 800–1000 ng/ml were excluded from statistical analysis
HR = heart rate; RR = respiratory rate; CHF = congestive heart failure; ATE = arterial thromboembolism; DLVOTO = dynamic left ventricular outflow tract obstruction; DRVOTO = dynamic right ventricular outflow tract obstruction; LV = left ventricle; LVIDd = left ventricular internal diameter in diastole; LVFS% = left ventricular fractional shortening; LA:Ao = left atrium to aortic ratio in a short-axis view; LAFS% = left atrial fractional shortening; NA = not applicable
Outcomes of cats with an IGF-1 >1000 ng/ml
One cat was euthanased because of suspected recurrent ATE 9 days after a diagnosis of HCM. Another cat died 3 months post-diagnosis, with no further information available related to the exact cause of death. The other two cats had an unknown status, with the last known contact following diagnosis being 1 month for one cat and 2 years for the other cat. None of these cats displayed any sign of DM or fHS in their clinical records up to the date of their last contact.
Discussion
In our population of cats diagnosed with HCM, 6.7% of cases had a circulating IGF-1 of >1000 ng/ml (95% CI 1.8–16.2%), none of the cats in the apparently healthy control group had an IGF-1 of >1000 ng/ml. This highlights that a small percentage of non-diabetic cats diagnosed with HCM had an IGF-1 level in a range that is consistent with fHS in a diabetic cat. The presence of fHS in these cats is unknown and warrants further investigation, but – if present – would imply that some cats with LVH could be misclassified as having primary HCM.
A measurement of IGF-1 >1000 ng/ml, using RIA, is reported to have good sensitivity for fHS. 33 The IGF-1 assay used in this study has previously been validated and shown to have a positive predictive value (PPV) of 95% for fHS in the diabetic cat population using this cutoff. 12 Given that there is some evidence in the veterinary literature of non-diabetic cats with fHS,16,17 it is possible that in cats with an IGF-1 >1000 ng/ml, fHS may, at least in part, have had an impact on their wall thickness, rather than HCM being solely responsible for the LVH. However, confirmation of the diagnosis of fHS in the cats with an IGF-1 >1000 ng/ml would be needed to provide definitive support for this hypothesis. This would require advanced intracranial imaging, such as CT or MRI, or a post-mortem examination to document pituitary acidophil hyperplasia.12,24,34 This was not possible due to the retrospective nature of this study. Such studies would be warranted given that if there is a population of non-diabetic cats with fHS-induced cardiomyopathy this may be a partially or fully reversible cause of LVH. 14
Caution should be applied in extrapolating the PPV of our assay from diabetic cats to the non-diabetic cats in our study, given that the PPV is affected by disease prevalence. Although there are case reports of non-diabetic cats with fHS and not all diabetic fHS cats exhibit the typical phenotype of poor glycaemic control, the prevalence of fHS in non-diabetic cats remains unknown and therefore the correct IGF-1 cutoff for a presumptive diagnosis of fHS in non-diabetic cats has not been determined. The cats in this study with an IGF-1 >1000 ng/ml cannot, without further diagnostic intervention, be assumed to have fHS.16,17,24 Furthermore, if these cases did have fHS we cannot definitively conclude that it was responsible for their LVH. The cats with an IGF-1 >1000 ng/ml in our study were 8.0–9.0 years old, an age range that has a relatively high prevalence of HCM; HCM and fHS could be present as concurrent but independent diseases in cats of this age range, both of which could have an impact on ventricular wall thickness. 25
Cats with an IGF-1 >1000 ng/ml were found to be in CHF and most (n = 3/4) had concurrent ATE. The presence of larger LA:Ao and reduced left atrial fractional shortening may also reflect a more advanced state of cardiac disease. 29 However, the reader is cautioned against drawing conclusions on any causal relationship between changes in IGF-1 and the progression of cardiac disease owing to the small number of cases. This could be explored in future studies.
The limitations of this study are inherent to the design of the included studies. The HCM population consisted of cats from two referral centres in the UK and therefore may not reflect the wider cat population. Furthermore, these cats represent a selected and small proportion of cats with HCM entering the hospitals and may not reflect these centre’s HCM cohorts as a whole. The control group was relatively small and, unfortunately, was not age matched. It was also from a general practice population; this is in contrast to the HCM cats, which were from a referral population. These limitations reflect difficulties in recruiting healthy control populations, and were unavoidable. Ageing is associated with reduced IGF-1 in cats, but this is a limited effect and would be unlikely to reduce an IGF-1 result to a degree that would be clinically relevant in ruling in or ruling out fHS. 35 Therefore, it is unlikely that lack of age matching in this study would change the conclusions we have drawn from our data. As this was a retrospective study, we were reliant on clinical records and diagnostic testing ordered by clinicians at the time, which may have been incomplete, inaccurate or subject to unknown confounding bias. Additionally, the diagnostic approach was not standardised, with the exception of the inclusion criteria of echocardiography performed without sedation, standardisation of echocardiography technique and a blood sample collected at the time of echocardiography. Sample handling at the time of collection or later storage may have affected measurable IGF-1 concentration. Finally, the effect of long-term storage on feline blood samples for IGF-1 measurement is currently unknown and could lead to falsely decreased or increased IGF-1 results.
Conclusions
In a cohort of cats previously diagnosed with HCM, 6.7% (95% CI 1.8–16.2%) were found to have an IGF-1 >1000 ng/ml; conversely, no cats in a healthy control population without LVH had an IGF-1 concentration >800 ng/ml. Future prospective studies are warranted to determine whether or not these cats with IGF-1 >1000 ng/ml have fHS and, if they do, whether their LVH is a direct result of fHS or represents concurrent HCM. Alternatively, these IGF-1 elevations may be a result of other factors that are not yet completely understood.
Footnotes
Accepted: 18 December 2020
Author note: An abstract of the current study with the title ‘Prevalence of hypersomatotropism in non-diabetic cats with left ventricular hypertrophy – a silent and curable phenocopy for hypertrophic cardiomyopathy’ was presented at the 2018 ECVIM-CA Congress.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animals (owned or unowned) and procedures that differed from established internationally recognised high standards (‘best practice’) of veterinary clinical care for the individual patient. The study therefore had ethical approval from an established committee as stated in the manuscript.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Matthew ME Steele  https://orcid.org/0000-0003-1395-6506
https://orcid.org/0000-0003-1395-6506
Kieran Borgeat  https://orcid.org/0000-0001-9199-4941
https://orcid.org/0000-0001-9199-4941
References
- 1. Luis Fuentes V, Abbott J, Chetboul V, et al. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J Vet Intern Med 2020; 34: 1062–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weichselbaum RC, Feeney DA, Jessen CR. Relationship between selected echocardiographic variables before and after radioiodine treatment in 91 hyperthyroid cats. Vet Radiol Ultrasound 2005; 46: 506–513. [DOI] [PubMed] [Google Scholar]
- 3. Watson N, Murray JK, Fonfara S, et al. Clinicopathological features and comorbidities of cats with mild, moderate or severe hyperthyroidism: a radioiodine referral population. J Feline Med Surg 2018; 20: 1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chetboul V, Lefebvre HP, Pinhas C, et al. Spontaneous feline hypertension: clinical and echocardiographic abnormalities, and survival rate. J Vet Intern Med 2003; 17: 89–95. [DOI] [PubMed] [Google Scholar]
- 5. Lesser M, Fox PR, Bond BR. Assessment of hypertension in 40 cats with left ventricular hypertrophy by Doppler-shift sphygmomanometry. J Small Anim Pract 1992; 33: 55–58. [Google Scholar]
- 6. Snyder PS, Sadek D, Jones GL. Effect of amlodipine on echocardiographic variables in cats with systemic hypertension. J Vet Intern Med 2001; 15: 52–56. [DOI] [PubMed] [Google Scholar]
- 7. Watson CE, Payne JR, Borgeat K. Valvular aortic stenosis in three cats. J Vet Cardiol 2019; 25: 1–6. [DOI] [PubMed] [Google Scholar]
- 8. Stepien RL, Bonagura JD. Aortic stenosis: clinical findings in six cats. J Small Anim Pract 1991; 32: 341–350. [Google Scholar]
- 9. Rolim VM, Casagrande RA, Wouters ATB, et al. Myocarditis caused by feline immunodeficiency virus in five cats with hypertrophic cardiomyopathy. J Comp Pathol 2016; 154: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borgeat K, Niessen SJM, Wilkie L, et al. Time spent with cats is never wasted: lessons learned from feline acromegalic cardiomyopathy, a naturally occurring animal model of the human disease. PLoS One 2018; 13: e0194342. DOI: 10.1371/journal.pone.0194342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Novo Matos J, Pereira N, Glaus T, et al. Transient myocardial thickening in cats associated with heart failure. J Vet Intern Med 2018; 32: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niessen SJM, Forcada Y, Mantis P, et al. Studying cat (Felis catus) diabetes: beware of the acromegalic imposter. PLoS One 2015; 10: e0127794. DOI: 10.1371/journal.pone.0127794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schaefer S, Kooistra HS, Riond B, et al. Evaluation of insulin-like growth factor-1, total thyroxine, feline pancreas-specific lipase and urinary corticoid-to-creatinine ratio in cats with diabetes mellitus in Switzerland and the Netherlands. J Feline Med Surg 2017; 19: 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peterson ME, Taylor RS, Greco DS, et al. Acromegaly in 14 cats. J Vet Intern Med 1990; 4: 192–201. [DOI] [PubMed] [Google Scholar]
- 15. Niessen SJM. Feline acromegaly. An essential differential diagnosis for the difficult diabetic. J Feline Med Surg 2010; 12: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fletcher JM, Scudder CJ, Kiupel M, et al. Hypersomatotropism in 3 cats without concurrent diabetes mellitus. J Vet Intern Med 2016; 30: 1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fracassi F, Salsi M, Sammartano F, et al. Acromegaly in a non-diabetic cat. JFMS Open Reports 2016; 2. DOI: 10.1177/2055116916646585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mestrón A, Webb SM, Astorga R, et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Español de Acromegalia, REA). Eur J Endocrinol 2004; 151: 439–446. [DOI] [PubMed] [Google Scholar]
- 19. Fieffe S, Morange I, Petrossians P, et al. Diabetes in acromegaly, prevalence, risk factors, and evolution: data from the French Acromegaly Registry. Eur J Endocrinol 2011; 164: 877–884. [DOI] [PubMed] [Google Scholar]
- 20. Hradec J, Marek J, Kral J, et al. Long-term echocardiographic follow-up of acromegalic heart disease. Am J Cardiol 1993; 72: 205–210. [DOI] [PubMed] [Google Scholar]
- 21. Sharma AN, Tan M, Amsterdam EA, et al. Acromegalic cardiomyopathy: Epidemiology, diagnosis, and management. Clin Cardiol 2018; 41: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nascimento GC, De Oliveira MT, Carvalho VC, et al. Acromegalic cardiomyopathy in an extensively admixed population: is there a role for GH/IGF-I axis? Clin Endocrinol 2013; 78: 94–101. [DOI] [PubMed] [Google Scholar]
- 23. Myers JA, Lunn KF, Bright JM. Echocardiographic findings in 11 cats with acromegaly. J Vet Intern Med 2014; 28: 1235–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niessen SJM, Petrie G, Gaudiano F, et al. Feline acromegaly: an underdiagnosed endocrinopathy? J Vet Intern Med 2007; 21: 899–905. [DOI] [PubMed] [Google Scholar]
- 25. Payne JR, Brodbelt DC, Fuentes VL. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J Vet Cardiol 2015; 17: S244–S257. [DOI] [PubMed] [Google Scholar]
- 26. Peterson ME, Melián C, Nichols R. Measurement of serum concentrations of free T4, total T4, and total T3 in cats with hyperthyroidism and cats with nonthyroidal disease. J Am Vet Med Assoc 2001; 218: 529–536. [DOI] [PubMed] [Google Scholar]
- 27. Acierno MJ, Brown S, Coleman AE, et al. ACVIM consensus statement: guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2018; 32: 1803–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crenshaw KL, Peterson ME, Heeb LA, et al. Serum fructosamine concentration as an index of glycemia in cats with diabetes mellitus and stress hyperglycemia. J Vet Intern Med 1996; 10: 360–364. [DOI] [PubMed] [Google Scholar]
- 29. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2013; 27: 1427–1436. [DOI] [PubMed] [Google Scholar]
- 30. Schober KE, Chetboul V. Echocardiographic evaluation of left ventricular diastolic function in cats: hemodynamic determinants and pattern recognition. J Vet Cardiol 2015; 17: S102–S133. [DOI] [PubMed] [Google Scholar]
- 31. Schober KE, Maerz I, Ludewig E, et al. Diagnostic accuracy of electrocardiography and thoracic radiography in the assessment of left atrial size in cats: comparison with transthoracic 2-dimensional echocardiography. J Vet Intern Med 2007; 21: 709–718. [DOI] [PubMed] [Google Scholar]
- 32. Fuentes VL. Arterial thromboembolism: risks, realities and a rational first-line approach. J Feline Med Surg 2012; 17: 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berg RIM, Nelson RW, Feldman EC, et al. Serum insulin-like growth factor-I concentration in cats with diabetes mellitus and acromegaly. J Vet Intern Med 2007; 21: 892–898. [DOI] [PubMed] [Google Scholar]
- 34. Niessen SJM, Church DB, Forcada Y. Hypersomatotropism, acromegaly, and hyperadrenocorticism and feline diabetes mellitus. Vet Clin North Am Small Anim Pract 2013; 43: 319–350. [DOI] [PubMed] [Google Scholar]
- 35. Campbell DJ, Rawlings JM, Heaton PR, et al. Insulin-like growth factor-I (IGF-I) and its association with lymphocyte homeostasis in the ageing cat. Mech Ageing Dev 2004; 125: 497–505. [DOI] [PubMed] [Google Scholar]


