Abstract
High-intensity interval training (HIIE) may present unique challenges to the cerebrovascular system in individuals post-stroke. We hypothesized lower middle cerebral artery blood velocity (MCAv) in individuals post-stroke: 1) during 10 minutes of HIIE, 2) immediately following HIIE, and 3) 30 minutes after HIIE, compared to age- and sex-matched controls (CON). We used a recumbent stepper submaximal exercise test to determine workloads for high-intensity and active recovery. Our low volume HIIE protocol consisted of 1-minute intervals for 10 minutes. During HIIE, we measured MCAv, mean arterial pressure (MAP), heart rate (HR), and end tidal carbon dioxide (PETCO2). We assessed carotid-femoral pulse wave velocity as a measure of arterial stiffness. Fifty participants completed the study (25 post-stroke, 76% ischemic, 32% moderate disability). Individuals post-stroke had lower MCAv during HIIE compared to CON (p = 0.03), which remained 30 minutes after HIIE. Individuals post-stroke had greater arterial stiffness (p = 0.01) which was moderately associated with a smaller MCAv responsiveness during HIIE (r = −0.44). No differences were found for MAP, HR, and PETCO2. This study suggests individuals post-stroke had a lower MCAv during HIIE compared to their peers, which remained during recovery up to 30 minutes. Arterial stiffness may contribute to the lower cerebrovascular responsiveness post-stroke.
Keywords: Cerebral autoregulation, cerebral blood flow, cerebral hemodynamics, rehabilitation, physiotherapy
Introduction
Individuals post-stroke demonstrate an impaired cerebrovascular response during moderate-intensity continuous exercise,1,2 potentially due to the presence of vascular disease, poor cerebrovascular reactivity to end tidal carbon dioxide (PETCO2), poor endothelial function, presence of stroke, or a combination of these factors.1,3 –6 While moderate-intensity continuous exercise is the current aerobic exercise recommendation for individuals post-stroke, 7 high-intensity interval exercise (HIIE) has recently gained interest as a promising therapeutic intervention.8 –10 HIIE includes repetitive bouts of high-intensity exercise followed by active or passive recovery.8,11,12 HIIE causes rapid physiological changes in heart rate (HR), mean arterial pressure (MAP), and PETCO2, which may challenge the cerebrovascular system in individuals post-stroke who have an underlying cerebrovascular injury and poor vascular health.13 –15
Previous reviews have postulated that individuals post-stroke may not have a “typical” cerebrovascular response,13,14 including the potential for heightened mean arterial pressure and arterial stiffness, which could in turn lead to an inability to vasoconstrict or vasodilate downstream arterioles.5,14,16 Previous studies in acute (<7 days) 17 and subacute (7 days – 6 months) 17 stroke revealed impaired cerebral autoregulation at rest18,19 as well as during passive limb movement compared to healthy controls.20,21 Individuals at 3-months post-stroke have also shown a reduced cerebrovascular response to moderate-intensity continuous exercise compared to their age- and sex-matched controls. 1 While few studies have examined the cerebrovascular system in individuals with chronic stroke (>6 months),17,22,23 one study reported lower cerebral blood velocity at rest and during visual stimuli in individuals 2 months to 7 years post-stroke. 24 Therefore, the cerebrovascular response to an aerobic exercise stimulus, such as HIIE, may also be lower in individuals with chronic stroke.
To our knowledge, no studies have examined the MCAv during an acute bout of HIIE in individuals post-stroke.1,5,25 Two studies have examined MCAv recovery following 1-minute interval HIIE and reported decreased MCAv immediately following HIIE that returned back to resting values at 30-minutes after HIIE in young adults as well as prepubertal children.15,26 However, due to prolonged blood pressure recovery following aerobic exercise in individuals post-stroke, 27 the time to recovery of the cerebrovascular system may be longer than previously found in young adults and prepubertal children. Therefore, the recovery of the MCAv following HIIE may also provide valuable information in individuals post-stroke.
To address the gap in knowledge, the aim of this study was to characterize the mean middle cerebral artery blood velocity (MCAv), a surrogate measure of cerebral blood flow,1,3,5,25,28 –30 to a single bout of low-volume HIIE in individuals with chronic stroke compared to age- and sex-matched controls (CON). We hypothesized that individuals post-stroke would have lower MCAv: 1) during an acute 10-minute bout of low-volume HIIE, 2) immediately following HIIE, and 3) 30 minutes after HIIE, compared to CON.
Due to young healthy adults showing large increases and decreases in MCAv during HIIE, 15 we wanted to determine whether this “responsiveness”, or MCAv coefficient of variation (CoV), was lower in individuals post-stroke compared to CON. As an exploratory analysis, we also wanted to determine potential participant and stroke characteristics that may be related to greater changes in MCAv or MCAv “responsiveness” during HIIE.
Methods
Study procedures were approved by the University of Kansas Medical Center Human Subjects Committee. Research was conducted in accordance with the Declaration of Helsinki and local statutory requirements. This study was registered on clinicaltrials.gov (NCT04673994). STROBE guidelines were followed. Data is available upon request to corresponding author.
We recruited individuals with chronic stroke between 6-months to 5 years and adults without stroke who were matched to sex and age ±5 years. Inclusion criteria: 1) 40–85 years old, 2) performed <150 minutes of moderate-intensity exercise per week within the past month, and 3) answered consenting questions and followed 2-step command. Exclusion criteria: 1) COVID-19 stroke etiology, 2) symptoms of COVID-19 and/or currently positive, 3) could not stand from a seated position without physical assistance from another person, 4) unable to perform exercise on a recumbent stepper, 5) currently insulin-dependent, 6) currently using supplementary oxygen, and 7) history of another neurologic disorder.
Power analysis
Due to our study being the first to examine MCAv during HIIE in individuals post-stroke, our sample size was calculated based on our prior work reporting the MCAv response to moderate-intensity continuous exercise in individuals post-stroke compared to CON, with an effect size of d = 1.36 1 between groups with no hypothesized group-by-time interaction. We simulated data for 50 participants (1:1 allocation) for every time point with an assumed effect size of 1.36. Data sets were simulated 1,000 times and a linear mixed model was fit for every simulated data set. With n = 50 and if our assumptions were correct, we would have 98% power. Therefore, if our effect size is slightly lower than previously seen during moderate-intensity, we would still be sufficiently powered.
Visit 1
Participants were informed of study procedures, benefits, and risks, and provided voluntary written informed consent. Next, we collected demographics, current medications, modified Rankin scale (mRS), 31 and the Fugl Meyer Lower Extremity Subscale. 32 Participants were screened for MCAv signal by an assessor blinded to group.
Submaximal exercise test
The total body recumbent stepper (TBRS, T5XR NuStep, Inc. Ann Arbor, MI) submaximal exercise test is a valid and reliable measure of fitness.33 –36 We have previously published on the use of the TBRS submaximal exercise test in stroke34,37 and to determine the workload for HIIE. 15 Following the published protocol, participants began exercising at 30 watts and stepped at a constant pace between 95–100 steps per minute (spm). We followed the TBRS submaximal exercise test’s protocol for workload progression.33 –35 Heart rate (HR, Polar electro Inc, New York) and rating of perceived exertion (RPE) were collected every minute. To maintain similar conditions between the submaximal exercise test and our recordings, participants were asked to only exercise with their legs. As performed in our prior work,1,38 the participants’ arms rested on adjustable height tables to ensure the arms were at heart-level for blood pressure readings. Previous test termination criteria were followed.15,33,37 After the exercise test, participants performed a 2-minute cool-down and rested for ∼15 minutes to allow HR and MAP to return to resting values.
The TBRS submaximal exercise test linear regression equation was used to calculate the estimated maximal oxygen consumption (estimatedV̇O2max). 33 The linear relationship between workload and HR (i.e. slope) was plotted to determine the estimated maximal watts (estimatedWattmax) to determine the appropriate workload for the high-intensity and active recovery bouts. 36 For individuals on beta-blockers, predicted maximal HR was calculated from the beta-blocker equation (164 – (0.7 × age)). 39 Similar to previous HIIE protocols in older adults and clinical populations, high-intensity was 70% estimatedWattmax and active recovery was 10% estimatedWattmax.8,40 –45
Exercise familiarization
HIIE familiarization included practicing switching every minute between high-intensity (70% estimatedWattmax) and active recovery (10% estimatedWattmax), while maintaining a consistent step rate between 95–100 spm, for no more than 10 minutes. 15 Based on previously published HIIE protocols in individuals with chronic stroke, a HR limit of 85% of age-predicted maximum HR was used. 46
Visit 2
All participants were asked to refrain from caffeine for 8 hours, 47 food for 2 hours, 48 and vigorous exercise 49 and alcohol 50 for 24 hours. The room was dimly lit with a constant temperature (21–23 °C).15,38
Carotid ultrasound
Participants laid supine for 20 minutes while we conducted bilateral Doppler ultrasound scans (GE Healthcare LOGIQ Ultrasound, Chicago, IL) of the common and internal carotid artery. A blinded physician (SE) reviewed images offline and determined the degree of carotid stenosis based on classifications from the Society of Radiologists in Ultrasound. 51
Next, we collected carotid-femoral pulse wave velocity (PWV, SphygmoCor, Itasca, IL), a measure of arterial stiffness. 52 A thigh cuff was placed on the upper right leg while a sensor was placed on the right carotid pulse. The aortic distance was estimated by taking the difference between the distance from the carotid artery pulse to the sternal notch and the distance from the sternal notch to the femoral artery site. 53 Approximately ten cardiac cycles were collected via the piezoelectric sensors. The aortic distance was then divided by the average time difference from the carotid and femoral diastolic troughs, or wavefoot. 54 PWV was performed for 2 separate trials and averaged. A higher average PWV indicated greater arterial stiffness. 52
Experimental procedure
Participants sat quietly for 20 minutes while instrumented with the following equipment: 1) bilateral TCD probes (2-MHz, Multigon Industries Inc, Yonkers, New York) to measure mean MCAv, 2) a 5-lead electrocardiogram (ECG; Cardiocard, Nasiff Associates, Central Square, New York) to measure HR, 3) a nasal cannula attached to a capnograph (BCI Capnocheck Sleep 9004 Smiths Medical, Dublin, Ohio) to measure PETCO2 and respiratory rate (RR), 4) a left middle finger Finometer (Finapres Medical Systems, Amsterdam, the Netherlands) to measure beat-to-beat MAP, and 5) a gold-standard microphoned, brachial automated sphygmomanometer (Tango M2; Suntech, Morrisville, NC) on the right arm to calibrate the Finometer. For participants with left upper extremity spasticity, the Finometer was placed on the right middle finger.
Recordings started with 5 minutes of baseline (BL) seated rest. Participants performed 10 minutes of low-volume HIIE with 1-minute high-intensity (70% estimatedWattmax) separated by 1-minute active recovery (10% estimatedWattmax). As performed previously, we started with 10% estimatedWattmax to avoid a Valsalva maneuver. 15 RPE was collected immediately after HIIE. Following HIIE, participants performed a 2-minute active cool-down with decreased resistance and step rate. After the cool-down, we continued our recording for another 5 minutes of seated rest to measure recovery immediately following HIIE and then 30 minutes after HIIE.
Data acquisition
As previously published, raw data was sampled at 500 Hz and collected via an analog-to-digital unit (NI-USB-6212, National Instruments) and custom-written software within MATLAB (v2014a, TheMathworks Inc, Natick, Massachusetts).15,38 Our prior work showed no significant difference between right and left MCAv in healthy adults. 38 Therefore, the left MCAv signal was used for CON. If the left MCA signal was not obtainable, then right MCAv was used. 38 In individuals post-stroke, we acquired both left and right MCAv. The ipsilesional hemisphere’s MCAv in individuals post-stroke was used to compare to CON. For individuals post-stroke with clinical imaging of bilateral strokes (n = 3), the MCAv side used for analysis compared to CON was determined by the hemisphere with the larger lesion.
We calculated 5-minute averages for the variables of interest at BL, immediately following HIIE, and 30 minutes after HIIE. During HIIE, 1-minute averages were calculated for a total of 10 separate time points.15,26,55
Data analysis
Normality was determined using a Shapiro-Wilk test. Participant characteristics were compared using t-tests or Mann-Whitney U tests for non-normally distributed continuous variables, and Fisher’s exact test or Chi-Square test for categorical variables. BL values were compared between groups using independent t-tests or Mann-Whitney U tests.
To analyze the primary aim, we fit a linear mixed model with MCAv during HIIE (10 time points) as the response, fixed effects for time and quadratic time (minutes) and group (stroke/CON), and a random intercept for each participant. Residual analyses showed lack of model fit with only linear time effect and the residual plots indicated a quadratic relationship. Thus, we included a quadratic effect for time which resulted in a better model fit. We used an autoregressive-1, AR(1), correlation structure to model the dependencies across time. The mixed model has significant advantages over more traditional techniques for analyzing longitudinal data, including better modeling dependencies across time and allowing for more flexible time effects. The primary between group comparison was tested as a one-sided test with α = 0.05. 56 After our primary analysis, a sensitivity analysis which included BL MCAv was performed. To determine whether MCAv differed between groups during recovery immediately following HIIE and at 30 minutes after HIIE, we used ANCOVA models adjusted for BL. These models were chosen instead of mixed models due to the hypothesis being specifically on the differences between groups immediately after HIIE and 30 minutes after HIIE.
For secondary outcomes (MAP, HR, PETCO2, and RR) we performed the same statistics as the primary aim. We fit a linear mixed model with fixed effects for time and group (stroke/CON), and a random intercept. Residual analysis for these secondary aims did not suggest a quadratic time effect was necessary for model fit, so only a linear time effect was included. During recovery, we examined differences between groups in secondary outcomes using separate ANCOVAs immediately following HIIE and 30-minutes after HIIE, adjusted for BL. Assumptions of all the models were assessed using residual analyses, e.g., QQ-plots, predicted vs. fitted.
As an exploratory aim, we calculated each individual’s coefficient of variation of the 10 time points during HIIE (CoV = (MCAv SD/MCAv average)*100) to determine each participant’s MCAv “responsiveness” to HIIE. We used bivariate Pearson Correlations to determine the association of MCAv CoV with age, PWV, estimatedV̇O2max, absolute high-intensity watts, and with stroke specific variables such as months since stroke, and Fugl Meyer lower extremity score. To estimate the association between MCAv CoV and dichotomous variables of sex, history of stroke, carotid stenosis, beta-blocker use, statin use and stroke specific variables of ischemic stroke and MCA stroke we used Point-Biserial Correlations. For mRS score, which is an ordinal measure, we used Spearman’s rank correlation due to concerns over the approximate normality assumption.
Results
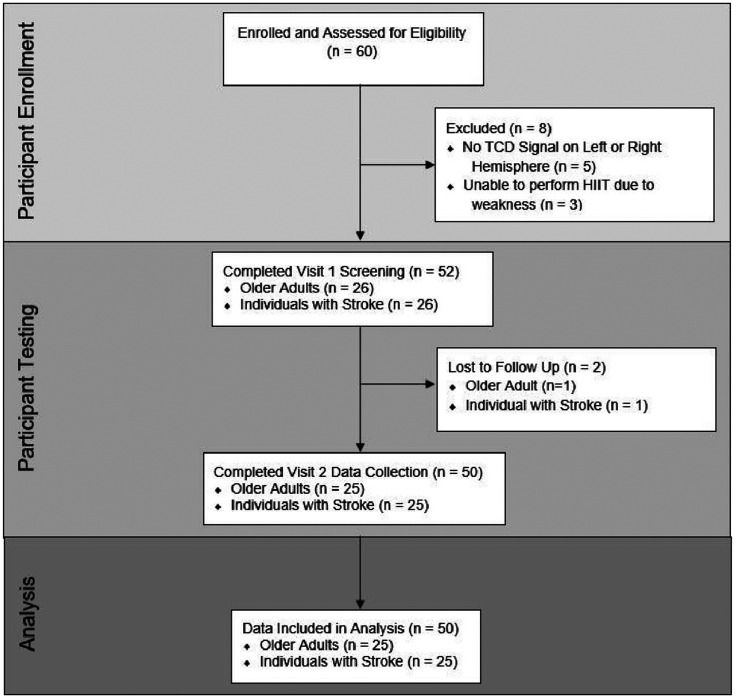
Sixty individuals were enrolled within the study. Fifty participants completed this study and were included in the primary analysis, shown in Figure 1. Comparisons of participant demographics between individuals post-stroke (n = 25) and CON (n = 25) are shown in Table 1. No adverse events occurred during the study. Model assumptions were adequately met using residual analyses.
Figure 1.
Flow diagram.
Table 1.
Participant characteristics.
| Individuals post-stroke(n = 25) | CON(n = 25) | p-value | |
|---|---|---|---|
| Age (years) | 60 ± 12 | 60 ± 13 | 0.92 |
| Female n (%) | 9 (36%) | 9 (36%) | 1.00 |
| BMI (kg/m2) | 30.6 ± 5.8 | 28.6 ± 6.6 | 0.27 |
| Race n (%) | 0.54 | ||
| Asian | 0 | 1 | |
| Black/African American | 4 | 2 | |
| White/Caucasian | 20 | 22 | |
| White & Native American | 1 | 0 | |
| Beta-blocker medication n (%) | 10 (40%) | 1 (4%) | 0.01 a |
| Statin medication n (%) | 22 (88%) | 11 (44%) | 0.002 a |
| Carotid stenosis n (%) | 0.67 | ||
| Normal | 23 (92%) | 21 (84%) | |
| <50% | 2 (8%) | 4 (16%) | |
| Carotid-femoral pulse wave velocity (m/s) b | 9.18 ± 1.86 | 8.00 ± 1.89 | 0.01 a |
| TBRS estimatedV̇O2max (ml·kg−1·min−1) | 29.5 ± 9.1 | 32.3 ± 10.0 | 0.32 |
| Absolute high-intensity (Watts) b | 80 ± 27 | 116 ± 35 | 0.001 a |
| Absolute active recovery (Watts) b | 16 ± 2 | 18 ± 4 | 0.04 a |
| Rating of perceived exertion (6–20) b | 14 ± 2 | 14 ± 2 | 0.73 |
| Stroke characteristics | |||
| Months post-stroke | 31 ± 16 | ||
| Type of Stroken, % (ischemic, hemorrhagic) | 19 (76%), 6 (24%) | ||
| Stroke Location n (%) | |||
| MCA & Territories | 10 (40%) | ||
| ACA & Territories | 3 (12%) | ||
| PCA & Territories | 6 (24%) | ||
| Vertebrobasilar | 2 (8%) | ||
| Cerebellar | 1 (4%) | ||
| Combined MCA & ACA | 3 (12%) | ||
Means ± SD. BMI: body mass index; TBRS: total body recumbent stepper; V̇O2max: maximal oxygen consumption.
Significant between groups.
Not normally distributed, used Mann-Whitney U test.
Visit 1
For individuals with stroke, 40% reported no significant disability on the mRS, while 28% reported slight disability, and 32% reported moderate disability. The lower extremity Fugl-Meyer Score was 23.2 ± 5.3, indicating our participants with stroke had mild to moderate lower extremity impairment. 57 Although participants with stroke reported higher use of beta blocker and statin medications (Table 1), pulse wave velocity, a measure of arterial stiffness, was higher in those with stroke indicating greater arterial stiffness. No group differences were observed for estimated V̇O2 max from the submaximal exercise test. However, the workload in watts prescribed for the HIIE exercise bout was significantly greater in the CON group compared to the individuals post-stroke for both the high-intensity and active recovery bouts.
Visit 2
Participants returned to the laboratory 8 ± 6 days after their first study visit. At rest, BL MCAv was significantly lower in individuals post-stroke compared to CON (Cohen’s d = 0.61), shown in Table 2. No group differences were observed for BL MAP, HR, PETCO2, and RR.
Table 2.
Baseline hemodynamics.
| Individuals post-stroke(n = 25) | CON(n = 25) | p-value | |
|---|---|---|---|
| Mean MCAv (cm/s) a | 41 ± 11 | 49 ± 13 | 0.04 b |
| MAP (mmHg) | 82 ± 12 | 80 ± 11 | 0.45 |
| HR (bpm) c | 69 ± 12 | 65 ± 8 | 0.32 |
| PETCO2 (mmHg) | 36 ± 4 | 36 ± 4 | 0.95 |
| RR (breaths/min) c | 14 ± 4 | 15 ± 3 | 0.27 |
Means ± SD. MCAv: middle cerebral artery blood velocity; MAP: mean arterial pressure; HR: heart rate; PETCO2: expired end tidal carbon dioxide; RR: respiratory rate.
(n = 24) MCAv signal on the ipsilesional hemisphere could not be obtained in one individual post-stroke.
Significant between groups.
Not normally distributed, used Mann-Whitney U test.
MCAv during HIIE
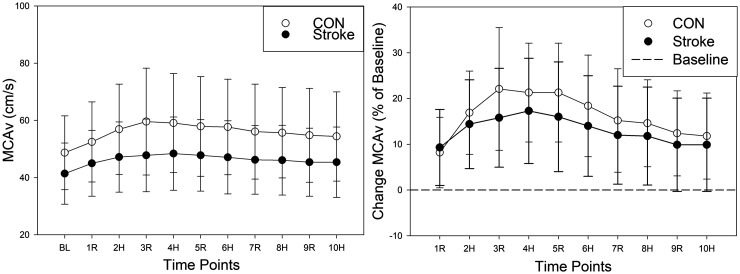
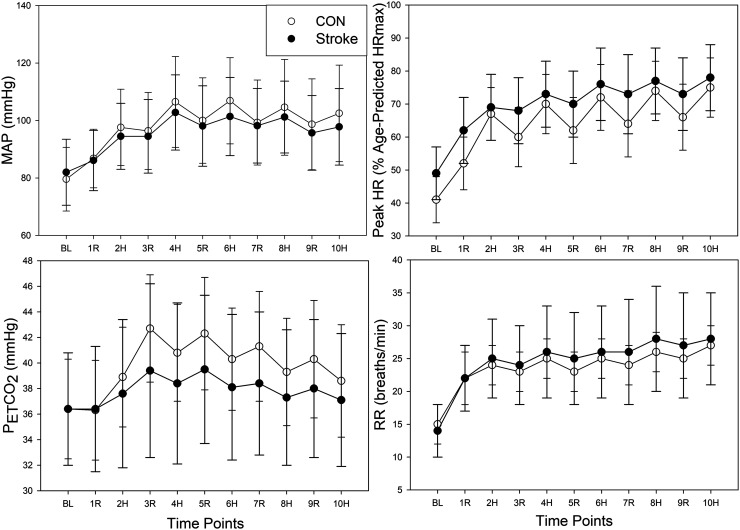
During HIIE there was evidence for a strong time (p < 0.001) and quadratic time (p < 0.001) effect and a difference between groups (p = 0.03) with individuals post-stroke having MCAv values 9.33 cm/s lower on average, shown in Figure 2. As a sensitivity analysis, we included BL MCAv as a covariate in the mixed model. Time and quadratic time were both still associated with MCAv (p < 0.001). However, the group effect was attenuated ( = −1.01, p = 0.41). No group differences were observed during HIIE for MAP, HR, PETCO2, and RR, shown in Figure 3. See Supplementary Table 1 for the mean ± SD of MCAv and secondary outcomes (MAP, HR, PETCO2, and RR) during HIIE and recovery. The ipsilesional and contralesional MCAv to HIIE in individuals post-stroke is also shown in Supplementary Figure 1.
Figure 2.
Cerebrovascular Response to HIIE in Individuals Post-Stroke and CON. Controls (CON) = open circle. Stroke Ipsilesional hemisphere = closed circle. BL: baseline; R: active recovery; H: high-intensity; MCAv: middle cerebral artery blood velocity.
Figure 3.
Peripheral Response to HIIE in Individuals Post-Stroke and CON. Controls (CON) = open circles; Individuals post-stroke = closed circles. BL: baseline; R: active recovery; H: high-intensity; MAP: mean arterial pressure; HR: heart rate; PETCO2: expired end tidal carbon dioxide; RR: respiratory rate.
MCAv during recovery
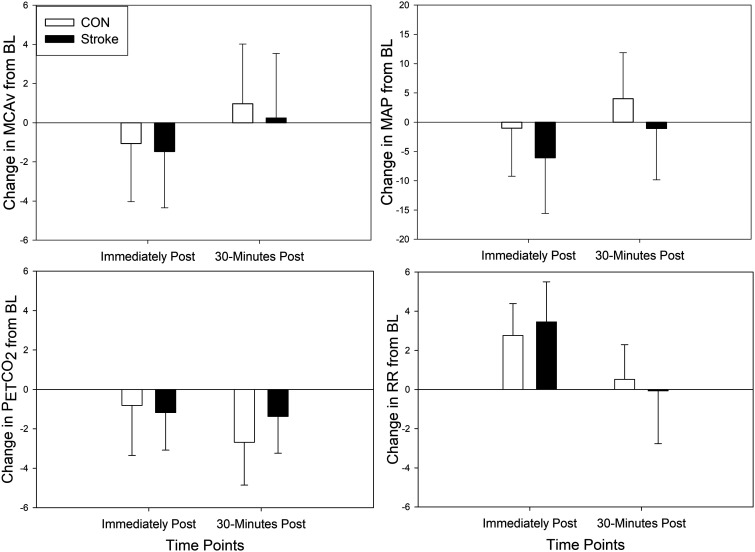
No group differences existed immediately following HIIE (p = 0.42) or 30 minutes after HIIE (p = 0.46), when controlling for BL MCAv, shown in Figure 4. We also found no significant difference between groups in MAP, HR, and RR immediately following HIIE or 30 minutes after HIIE when controlling for BL. When examining PETCO2 between groups, it was not different immediately following HIIE (p = 0.22) but was significantly different at 30 minutes after HIIE (p = 0.02).
Figure 4.
Absolute Change in Cerebrovascular and Peripheral Response during Recovery Immediately Following HIIE and 30 minutes after HIIE. Controls (CON) = open bars. Individuals post-stroke = closed bars. Solid horizontal line = baseline. R: active recovery; H: high-intensity; MAP: mean arterial pressure; HR: heart rate; PETCO2: expired end tidal carbon dioxide; RR: respiratory rate.
MCAv responsiveness
During HIIE, MCAv CoV was greater in CON ( = 5.16% ± 1.80%, p = 0.01) compared to individuals post-stroke ( = 3.84% ± 1.39%). Correlation coefficients between MCAv responsiveness with participant characteristics and stroke-specific characteristics are shown in Table 3. A lower MCAv responsiveness was moderately correlated to males (r = −0.31), history of stroke (r = −0.39), and greater arterial stiffness (r = −0.44). When examining only individuals post-stroke, MCAv responsiveness was only moderately correlated with having an ischemic stroke (r = −0.34).
Table 3.
Associations between MCAv responsiveness and participant characteristics.
| Participant characteristics (n = 50) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1MCAv responsiveness | 1.00 | |||||||||
| 2Age | −0.04 | 1.00 | ||||||||
| 3Sex (male) | −0.31 | 0.28 | 1.00 | |||||||
| 4Stroke history | −0.39 | 0.02 | 0.00 | 1.00 | ||||||
| 5Beta-blocker | −0.22 | 0.19 | 0.23 | 0.47 | 1.00 | |||||
| 6Statin use | −0.27 | 0.41 | 0.34 | 0.46 | 0.30 | 1.00 | ||||
| 7Arterial stiffness | −0.44 | 0.27 | 0.24 | 0.31 | 0.32 | 0.44 | 1.00 | |||
| 8Carotid stenosis | −0.12 | 0.28 | 0.02 | −0.12 | 0.08 | 0.01 | 0.21 | 1.00 | ||
| 9EstimatedV̇O2max | 0.18 | −0.15 | 0.16 | −0.15 | −0.12 | −0.21 | −0.42 | −0.12 | 1.00 | |
|
10High-intensity Watts |
0.13 |
−0.18 |
0.23 |
−0.51 |
−0.34 |
−0.12 |
−0.20 |
−0.04 |
0.34 |
1.00 |
|
Stroke characteristics (n = 25) |
1 |
2 |
3 |
4 |
5 |
6 |
|
|
|
|
| 1MCAv responsiveness | 1.00 | |||||||||
| 2Months since stroke | 0.19 | 1.00 | ||||||||
| 3Ischemic stroke | −0.34 | −0.18 | 1.00 | |||||||
| 4MCA stroke | 0.19 | 0.34 | −0.21 | 1.00 | ||||||
| 5mRS score | 0.17 | 0.06 | 0.16 | 0.20 | 1.00 | |||||
| 6Fugl Meyer lower extremity score | 0.07 | −0.02 | 0.21 | −0.43 | −0.30 | 1.00 |
Correlation coefficients (r). Low correlation = 0–0.3, Moderate correlation = 0.3–0.5. High correlation = 0.5–1.0.
Discussion
This study was the first to characterize MCAv during an acute bout of HIIE in individuals post-stroke and addresses a critical gap in knowledge.13,14,25,27 Our main findings are: 1) MCAv was uniformly lower throughout HIIE and recovery in individuals post-stroke compared to age- and sex-matched adults. However, when accounting for BL MCAv, differences between groups no longer remained, 2) the hemodynamic response was not different between groups (i.e. MAP, HR, PETCO2, and RR) during HIIE or recovery, despite CON working at a higher absolute workload, and 3) greater MCAv responsiveness during HIIE was moderately correlated to being female, no history of stroke, and having lower arterial stiffness. 58
We report a lower MCAv at rest in individuals with chronic stroke (>6 months) compared to CON which is supported by a previous study in individuals within the subacute (7 days to 6 months) and chronic stage post-stroke reporting lower MCAv at rest compared to age-matched adults. 59 Another study using magnetic resonance imaging (MRI) also reported lower resting regional blood flow in individuals with chronic stroke compared to younger and older healthy adults. 22 Our results also showed lower MCAv during HIIE in individuals post-stroke compared to CON, which was no longer significant when accounting for BL. Furthermore, individuals post-stroke had lower MCAv responsiveness to HIIE, which was moderately correlated with arterial stiffness. Arterial stiffening creates an inability of downstream arterioles to vasodilate or vasoconstrict, 60 which is essential to increasing and decreasing MCAv during exercise. 61 Our results support this hypothesis by showing an association between MCAv responsiveness during HIIE and arterial stiffness. Individuals post-stroke, who had greater arterial stiffness compared to CON, also had a smaller MCAv responsiveness during HIIE. One way to potentially reduce arterial stiffness is through statin medication, 62 which has been shown to improve cerebral vessel health4,63 and restore the vasomotor abilities of the downstream vessels. 3 While our exploratory analysis reported low correlations between statin use and beta-blocker use with a greater MCAv responsiveness during HIIE, we believe that future analyses is warranted.3,63 Exercise is also a non-pharmacological intervention known to reduce blood pressure and improve peripheral vascular health after stroke. 64 Future work should explore whether exercise such as HIIE can improve cerebrovascular health in people post stroke.
The peripheral hemodynamic response (i.e. MAP, HR) during HIIE was not significantly different between individuals post-stroke and CON. The CON group worked at a higher absolute workload during HIIE which should have increased MAP and HR in response to greater skeletal muscle use. 65 However, by performing the TBRS submaximal exercise test we were able to account for lower muscle strength and activation post-stroke by prescribing the exercise bout as a percentage of estimated workload.65 –67 During recovery immediately following HIIE, MAP was below BL. The decrease in MAP immediately following HIIE did not meet the criteria of post-exercise hypotension in our participants. 68 However, this finding is important since previous work suggests the brain is most sensitive to decreases in MAP,27,69 –71 and may challenge cerebrovascular homeostasis during blood pressure variations, also known as cerebral autoregulation. 49 Future work exploring the cerebral autoregulation response to HIIE in individuals post-stroke is needed.25,49
Cerebrovascular reactivity to carbon dioxide has a critical role in the MCAv during HIIE in healthy young adults.3,15,61,63,72,73 Based on our prior work performing low-volume HIIE in young healthy adults, MCAv decreased during high-intensity as a potential result of arterial acidemia leading to hyperventilation, decreased PETCO2, and downstream arteriole vasoconstriction.15,38 During active recovery, healthy young adults were able to recover their breathing which increased PETCO2, causing downstream arteriole vasodilation which “rebounded” or increased MCAv. 15 While the work presented here shows that both individuals post-stroke and CON hyperventilated during high-intensity and recovered their breathing during the active recovery bouts of HIIE, MCAv did not follow the pattern of PETCO2. Previous studies have shown reduced cerebrovascular reactivity to PETCO2 in individuals post-stroke as well as healthy aging adults.3,72,73 Though our study provides foundational information, further studies should examine resting cerebrovascular reactivity to carbon dioxide and pulmonary function in individuals post-stroke to better understand its contribution to the MCAv response to HIIE.
Our exploratory analysis revealed potential sex-differences in the MCAv responsiveness to HIIE.4,74,75 Our results confirm the need to sex-match individuals between groups, due to women having higher MCAv responsiveness compared to men. 15 Our findings are supported by previous work showing young women have a greater MCAv to HIIE compared to young men. 15 While not measured within the study, estrogen and its protective role in vascular health could have contributed to greater MCAv responsiveness.76 –78 We did control for pre-menopausal women to be tested during days 1–7 of the follicular cycle. 76 We included both pre-menopausal (44%) and post-menopausal women (hormone replacement n = 2) within our study, as the effects of hormones on MCAv was not the intended objective but warrants further study.
Although our exploratory analysis reported low correlations between MCAv responsiveness during HIIE with age or estimatedV̇O2max, we controlled for the potential influence of these confounding variables within the study design.74,75,77 –79 This study included age-matched (±5 years) adults who reported being physically inactive (<150 minutes of moderate-intensity exercise per week). Physical inactivity is associated with increased vascular risk in healthy adults6,80 and could have caused a floor effect within the CON group. Therefore, including only physically inactive CON individuals may explain our findings of no differences between groups in the MCAv response to HIIE when accounting for BL MCAv. A larger sample of healthy adults across a larger spectrum of age and physical activity levels may be needed to show an association between MCAv responsiveness during HIIE with age and physical fitness.77,78
Limitations
TCD is currently the best method to examine the cerebrovascular response to exercise with high temporal resolution. 81 While there is controversy as to how much MCA diameter changes with a specific exercise stimulus,82,83 a previous study in older adults using 4 D Flow Magnetic Resonance Imaging (MRI) showed no significant change in MCA diameter during an 8 mmHg change in PETCO2. 73 While our study reported an average change in PETCO2 < 8 mmHg, we could not directly measure the MCA diameter during exercise and therefore blood velocity cannot be assumed to be an exact measure of cerebral blood flow.
While the TBRS submaximal exercise test allows individuals to perform our HIIE protocol without the need for a maximal exercise test, the prescription of HIIE was based on an estimation rather than directly measuring maximal heart rate and power output. 11 While HIIE performed in young healthy adults may reach higher HRs, 84 we followed previous recommendations of a heart limit of 85% age-predicted maximal HR.33,46 However, it is worth noting that in individuals post-stroke “prescription based on [age-predicted] max HR may overestimate HR max, resulting in a higher intensity than expected”.64,85,86 While submaximal exercise testing overcomes barriers to aerobic exercise prescription in individuals post-stroke,34,87 performing maximal graded exercise tests and HIIE above 85% HR max may elicit differing cerebrovascular and hemodynamic responses. The TBRS submaximal exercise test was developed to be a clinically feasible way to estimate fitness, therefore, oxygen uptake was not collected. However, the estimatedV̇O2max calculated from the regression equation may have overestimated physical fitness for the individuals post-stroke due to the linear regression equation not accounting for beta-blocker use.
While our study was powered for the primary analysis, all other analyses were unadjusted for multiplicity and should be interpreted with caution. Due to a small sample size (n = 22) we did not statistically compare individuals post-stroke ipsilesional and contralesional hemispheres, instead we presented this data visually in Supplementary Figure 1.
Clinical relevance
HIIE is an exercise used worldwide and is an upcoming mode of exercise for stroke rehabilitation.8,10,14,88 Our study addressed the critical need that is currently unknown regarding the cerebrovascular and hemodynamic response of HIIE and could be used to guide clinical implementation of HIIE in stroke rehabilitation.13,14,25,27 While we report no difference in MCAv to HIIE between individuals post-stroke and CON after accounting for BL, our data show a decreased MCAv responsiveness, or variation in MCAv, when switching between high-intensity and active recovery in individuals post-stroke. As observed in Figure 4, MAP and PETCO2 demonstrated a responsiveness to the high-intensity and active recovery. In our previous work, we highlighted these physiologic variables are known to impact MCAv during exercise through either pressure driven influence or downstream arteriolar resistance. 38 Our work and others reported a lower MCAv response during moderate-intensity continuous exercise. 25 The results presented here combined with prior work regarding the lack of MCAv response during moderate-intensity continuous exercise1,5,38 suggest an impaired cerebrovascular response after stroke and may impact overall brain health. The MCAv response to exercise in healthy individuals is strongly correlated to cerebrovascular regulation and reactivity 61 and while not directly measured within this study may provide mechanistic insight to why the MCAv response to exercise is reduced post-stroke. 18 Given the strong emphasis on healthy brain aging,89,90 the present investigation provided valuable information regarding cerebrovascular response after stroke. Although the cerebrovascular system was less responsive to the alternating high and low-intensity bouts, we report no sudden drops or surges in MCAv with fluctuations in blood pressure during HIIE whereas one report previously hypothesized this could be of concern in individuals post-stroke. 14 Our findings provide initial support that large fluctuations in MCAv weren’t observed with HIIE suggesting intact regulation in chronic stroke. These findings are encouraging despite data in acute stroke suggesting impaired cerebral autoregulation. 18 Exercise, especially HIIE, challenges the system to maintain optimal cerebral blood flow and ensure adequate nutrient delivery and removal of cellular waste. The management of cerebral blood flow during physiological stressors such as HIIE requires a range of interconnected and redundant regulatory mechanisms in the management of cerebral blood flow. 91 Future research is needed to determine whether HIIE or high-intensity locomotor training can improve MCAv following an intervention in people living with chronic stroke as well as earlier stages of stroke recovery when cerebral autoregulation may be impaired.18,19,92 Further, we need to understand whether moderate intensity continuous exercise or high intensity interval exercise is optimal for improving MCAv and other measures of cerebrovascular health in people after stroke.
Our protocol describes a feasible method to prescribe and perform HIIE on a recumbent stepper in individuals with chronic stroke. Our protocol was successfully implemented in 25 individuals with minor to moderate stroke severity as well as 25 inactive middle-age and older adults with no adverse events. While treadmill HIIE protocols must take into account walking capacity and neuromuscular fatigue to reach high-intensity HR zones,8,93 we have shown the ability to reach ∼75% age-predicted HRmax on a recumbent stepper when performing 1-minute bouts of high-intensity at 70% power output. Individuals post-stroke were also able to perform HIIE and maintain blood pressures values that follow the American College of Sports Medicine’s exercise guidelines.58,94 Therefore, we provide the foundational knowledge on the physiological response during HIIE in individuals with chronic stroke and inactive adults.
Conclusion
Our data showed lower MCAv during HIIE in individuals post-stroke when compared to their age- and sex-matched peers. However, when controlling for BL MCAv in individuals post-stroke, differences between groups during HIIE and recovery no longer remained. Lower MCAv responsiveness, or the variation in MCAv, to HIIE may be associated with stroke pathology, poor vascular health, or sedentary behavior. 4 While we provide the foundational knowledge on the cerebrovascular response in individuals post-stroke, further characterization of other metrics such as cerebrovascular reactivity, cerebral autoregulation, 19 endothelial function, and blood-brain barrier permeability following an acute bout of HIIE are warranted.18,91 Future studies should also examine whether the greater vascular challenge during HIIE 95 could improve cerebrovascular health in individuals post-stroke and inactive adults after a chronic exercise intervention. 95,96
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231201472 for Lower middle cerebral artery blood velocity during low-volume high-intensity interval exercise in chronic stroke by Alicen A Whitaker, Saniya Waghmare, Robert N Montgomery, Stacey E Aaron, Sarah M Eickmeyer, Eric D Vidoni and Sandra A Billinger in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
Thank you, Andrew Geise, Katherine Nguyen, Jake Buchanan, Kailee Carter, Katelyn Struckle, and Emily Hazen for data collection.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AW was supported by the National Heart, Lung and Blood Institute [T32HL134643], Cardiovascular Center’s A.O. Smith Fellowship Scholars Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development [T32HD057850], American Heart Association [898190], and Kansas Partners in Progress Inc. SEA was supported by National Center for Advancing Translational Sciences [TL1TR002368]. SB and EV were supported in part by the National Institute on Aging [P30 AG072973]. REDCap at University of Kansas Medical Center was supported by National Center for Research Resources [ULTR000001]. The REACH laboratory was supported by Georgia Holland Endowment. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: AW, RM, SE, EV, SB conceived and designed research; AW, SW, SA performed experiments; AW, SW, RM, SA, SE, EV, SB analyzed data; AW, SW, RM, SA, SAB interpreted results of experiments; AW, RM, SB drafted manuscript; AW, SW, RM, SA, SE, EV, SB edited and revised manuscript; AW, SW, RM, SA, SE, EV, SB approved final version of manuscript.
ORCID iDs: Stacey E Aaron https://orcid.org/0000-0002-2532-8278
Sandra A Billinger https://orcid.org/0000-0002-1618-7207
Supplementary material
Supplemental material for this article is available online.
References
- 1.Kempf KS, Whitaker AA, Lui Yet al. The effect of stroke on middle cerebral artery blood flow velocity dynamics during exercise. J Neurol Phys Ther 2019; 43: 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufman CS, Bai SX, Ward JLet al. Middle cerebral artery velocity dynamic response profile during exercise is attenuated following multiple ischemic strokes: a case report. Physiol Rep 2019; 7: e14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivey FM, Ryan AS, Hafer-Macko CEet al. Improved cerebral vasomotor reactivity after exercise training in hemiparetic stroke survivors. Stroke 2011; 42: 1994–2000. [DOI] [PubMed] [Google Scholar]
- 4.Billinger SA, Whitaker AA, Morton Aet al. Pilot study to characterize middle cerebral artery dynamic response to an acute bout of moderate intensity exercise at 3- and 6-months poststroke . J Am Heart Assoc 2021; 10: e017821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson AD, Atwi S, Kostoglou Ket al. Cerebrovascular pulsatility during rest and exercise reflects hemodynamic impairment in stroke and cerebral small vessel disease. Ultrasound Med Biol 2019; 45: 3116–3127. [DOI] [PubMed] [Google Scholar]
- 6.Perdomo SJ, Ward J, Liu Yet al. Cardiovascular disease risk is associated with middle cerebral artery blood flow velocity in older adults. Cardiopulm Phys Ther J 2020; 31: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billinger SA, Arena R, Bernhardt Jet al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American heart association/American stroke association. Stroke 2014; 45: 2532–2553. [DOI] [PubMed] [Google Scholar]
- 8.Crozier J, Roig M, Eng JJet al. High-intensity interval training after stroke: an opportunity to promote functional recovery, cardiovascular health, and neuroplasticity. Neurorehabil Neural Repair 2018; 32: 543–556. [DOI] [PubMed] [Google Scholar]
- 9.Hornby TG, Reisman DS, Ward IGet al. Clinical practice guideline to improve locomotor function following chronic stroke, incomplete spinal cord injury, and brain injury. J Neurol Phys Ther 2020; 44: 49–100. [DOI] [PubMed] [Google Scholar]
- 10.Boyne P, Billinger SA, Reisman DSet al. Optimal Intensity and Duration of Walking Rehabilitation in Patients With Chronic Stroke A Randomized Clinical Trial. JAMA Neurology 2023; 80: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weston KS, Wisloff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 2014; 48: 1227–1234. [DOI] [PubMed] [Google Scholar]
- 12.Taylor JL, Holland DJ, Spathis JGet al. Guidelines for the delivery and monitoring of high intensity interval training in clinical populations. Prog Cardiovasc Dis 2019; 62: 140–146. [DOI] [PubMed] [Google Scholar]
- 13.Calverley TA, Ogoh S, Marley CJet al. HIITing the brain with exercise: mechanisms, consequences and practical recommendations. J Physiol 2020; 598: 2513–2530. [DOI] [PubMed] [Google Scholar]
- 14.Lucas SJ, Cotter JD, Brassard Pet al. High-intensity interval exercise and cerebrovascular health: curiosity, cause, and consequence. J Cereb Blood Flow Metab 2015; 35: 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitaker AA, Aaron SE, Kaufman CSet al. Cerebrovascular response to an acute bout of low-volume high-intensity interval exercise and recovery in young healthy adults. J Appl Physiol (1985) 2022; 132: 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefferts WK, Smith KJ. Let's talk about sex, let's talk about pulsatility, let's talk about all the good things and the bad things of MCAv. J Appl Physiol (1985) 2021; 130: 1672–1674. [DOI] [PubMed] [Google Scholar]
- 17.Bernhardt J, Hayward KS, Kwakkel Get al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke 2017; 12: 444–450. [DOI] [PubMed] [Google Scholar]
- 18.Fan JL, Brassard P, Rickards CAet al. Integrative cerebral blood flow regulation in ischemic stroke. J Cereb Blood Flow Metab 2022; 42: 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueira RC, Aries M, Minhas JSet al. Review of studies on dynamic cerebral autoregulation in the acute phase of stroke and the relationship with clinical outcome. J Cereb Blood Flow Metab 2022; 42: 430–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salinet AS, Panerai RB, Robinson TG. The longitudinal evolution of cerebral blood flow regulation after acute ischaemic stroke. Cerebrovasc Dis Extra 2014; 4: 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoi MC, Hu K, Lo MTet al. Impaired cerebral autoregulation is associated with brain atrophy and worse functional status in chronic ischemic stroke. PLoS One 2012; 7: e46794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brumm KP, Perthen JE, Liu TTet al. An arterial spin labeling investigation of cerebral blood flow deficits in chronic stroke survivors. Neuroimage 2010; 51: 995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez G, Nobili F, Carli FDet al. Regional cerebral blood flow in chronic stroke patients. Stroke 1993; 24: 94–99. [DOI] [PubMed] [Google Scholar]
- 24.Lin WH, Hao Q, Rosengarten Bet al. Impaired neurovascular coupling in ischaemic stroke patients with large or small vessel disease. Eur J Neurol 2011; 18: 731–736. [DOI] [PubMed] [Google Scholar]
- 25.Moncion K, Allison EY, Al-Khazraji BKet al. What are the effects of acute exercise and exercise training on cerebrovascular hemodynamics following stroke? A systematic review and meta-analysis. J Appl Physiol (1985) 2022; 132: 1379–1393. [DOI] [PubMed] [Google Scholar]
- 26.Tallon CM, Simair RG, Koziol AVet al. Intracranial vascular responses to high-intensity interval exercise and moderate-intensity steady-state exercise in children. Pediatr Exerc Sci 2019; 31: 290–295. [DOI] [PubMed] [Google Scholar]
- 27.Marzolini S, Robertson AD, Oh Pet al. Aerobic training and mobilization early post-stroke: cautions and considerations. Front Neurol 2019; 10: 1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jørgensen LG. Transcranial doppler ultrasound for cerebral perfusion. Acta Physiol Scand Suppl 1995; 625: 1–44. [PubMed] [Google Scholar]
- 29.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 1982; 57: 769–774. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki M, Kato K. Measurement of cerebral blood flow by ultrasonic doppler technique; hemodynamic comparison of right and left carotid artery in patients with hemiplegia. Jpn Circ J 1965; 29: 383–386. [DOI] [PubMed] [Google Scholar]
- 31.Jurca R, Jackson AS, LaMonte MJet al. Assessing cardiorespiratory fitness without performing exercise testing. Am J Prev Med 2005; 29: 185–193. [DOI] [PubMed] [Google Scholar]
- 32.Park EY, Choi YI. Psychometric properties of the lower extremity subscale of the Fugl-Myer assessment for community-dwelling hemiplegic stroke patients. J Phys Ther Sci 2014; 26: 1775–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Billinger SA, VAN Swearingen E, McClain Met al. Recumbent stepper submaximal exercise test to predict peak oxygen uptake. Med Sci Sports Exerc 2012; 44: 1539–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson DR, Mattlage AE, Seier NMet al. Recumbent stepper submaximal test response is reliable in adults with and without stroke. PLoS One 2017; 12: e0172294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herda AA, Lentz AA, Mattlage AEet al. Cross-validation of the recumbent stepper submaximal exercise test to predict peak oxygen uptake in older adults. Phys Ther 2014; 94: 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.YMCA. Y's way to physical fitness. 3rd ed. Geneva, Switzerland: Author, 1989. [Google Scholar]
- 37.Mattlage AE, Ashenden AL, Lentz AAet al. Submaximal and peak cardiorespiratory response after moderate-high intensity exercise training in subacute stroke. Cardiopulmonary Physical Therapy Journal 2013; 24: 14–20. [PMC free article] [PubMed] [Google Scholar]
- 38.Billinger SA, Craig JC, Kwapiszeski SJet al. Dynamics of middle cerebral artery blood flow velocity during moderate-intensity exercise. J Appl Physiol (1985) 2017; 122: 1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brawner CA, Ehrman JK, Schairer JRet al. Predicting maximum heart rate among patients with coronary heart disease receiving beta-adrenergic blockade therapy. Am Heart J 2004; 148: 910–914. [DOI] [PubMed] [Google Scholar]
- 40.Boyne P, Dunning K, Carl Det al. High-intensity interval training and moderate-intensity continuous training in ambulatory chronic stroke: feasibility study. Phys Ther 2016; 96: 1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Currie KD, Dubberley JB, McKelvie RSet al. Low-volume, high-intensity interval training in patients with CAD. Med Sci Sports Exerc 2013; 45: 1436–1442. [DOI] [PubMed] [Google Scholar]
- 42.Nepveu JF, Thiel A, Tang Aet al. A single bout of high-intensity interval training improves motor skill retention in individuals with stroke. Neurorehabil Neural Repair 2017; 31: 726–735. [DOI] [PubMed] [Google Scholar]
- 43.Bailey TG, Perissiou M, Windsor Met al. Cardiorespiratory fitness modulates the acute flow-mediated dilation response following high-intensity but not moderate-intensity exercise in elderly men. J Appl Physiol (1985) 2017; 122: 1238–1248. [DOI] [PubMed] [Google Scholar]
- 44.Bailey TG, Perissiou M, Windsor MTet al. Effects of acute exercise on endothelial function in patients with abdominal aortic aneurysm. Am J Physiol Heart Circ Physiol 2018; 314: H19–H30. [DOI] [PubMed] [Google Scholar]
- 45.Windsor MT, Bailey TG, Perissiou Met al. Cytokine responses to acute exercise in healthy older adults: the effect of cardiorespiratory fitness. Front Physiol 2018; 9: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carl DL, Boyne P, Rockwell Bet al. Preliminary safety analysis of high-intensity interval training (HIIT) in persons with chronic stroke. Appl Physiol Nutr Metab 2017; 42: 311–318. [DOI] [PubMed] [Google Scholar]
- 47.Addicott MA, Yang LL, Peiffer AMet al. The effect of daily caffeine use on cerebral blood flow: how much caffeine can we tolerate? Hum Brain Mapp 2009; 30: 3102–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marley CJ, Hodson D, Brugniaux JVet al. Post-prandial hyperlipidaemia results in systemic nitrosative stress and impaired cerebrovascular function in the aged. Clin Sci (Lond) 2017; 131: 2807–2812. [DOI] [PubMed] [Google Scholar]
- 49.Burma JS, Copeland P, Macaulay Aet al. Dynamic cerebral autoregulation across the cardiac cycle during 8 hr of recovery from acute exercise. Physiol Rep 2020; 8: e14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathew RJ, Wilson WH. Regional cerebral blood flow changes associated with ethanol intoxication. Stroke 1986; 17: 1156–1159. [DOI] [PubMed] [Google Scholar]
- 51.Grant EG, Benson CB, Moneta GLet al. Carotid artery stenosis: gray-scale and doppler US diagnosis – society of radiologists in ultrasound consensus conference. Radiology 2003; 229: 340–346. [DOI] [PubMed] [Google Scholar]
- 52.Gurovich AN, Braith RW. Pulse wave analysis and pulse wave velocity techniques: are they ready for the clinic? Hypertens Res 2011; 34: 166–169. [DOI] [PubMed] [Google Scholar]
- 53.Townsend RR, Wilkinson IB, Schiffrin ELet al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 2015; 66: 698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weber T, Ammer M, Rammer Met al. Noninvasive determination of carotid-femoral pulse wave velocity depends critically on assessment of travel distance: a comparison with invasive measurement. J Hypertens 2009; 27: 1624–1630. [DOI] [PubMed] [Google Scholar]
- 55.Whitaker AA, Alwatban M, Freemyer Aet al. Effects of high intensity interval exercise on cerebrovascular function: a systematic review. PLoS One 2020; 15: e0241248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Labrecque L, Drapeau A, Rahimaly Ket al. Comparable blood velocity changes in middle and posterior cerebral arteries during and following acute high-intensity exercise in young fit women. Physiol Rep 2020; 8: e14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwong PWH, Ng SSM. Cutoff score of the lower-extremity motor subscale of Fugl-Meyer assessment in chronic stroke survivors: a cross-sectional study. Arch Phys Med Rehabil 2019; 100: 1782–1787. [DOI] [PubMed] [Google Scholar]
- 58.Thompson PD, Arena R, Riebe Det al. ACSM's new preparticipation health screening recommendations from ACSM's guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep 2013; 12: 215–217. [DOI] [PubMed] [Google Scholar]
- 59.Hu K, Lo M-T, Peng C-Ket al. A nonlinear dynamic approach reveals a long-term stroke effect on cerebral blood flow regulation at multiple time scales. PLOS Comput Biol 2012; 8: e1002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes JN, Pearson AG, Corkery ATet al. Exercise, arterial stiffness, and cerebral vascular function: potential impact on brain health. J Int Neuropsychol Soc 2021; 27: 761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith KJ, Ainslie PN. Regulation of cerebral blood flow and metabolism during exercise. Exp Physiol 2017; 102: 1356–1371. [DOI] [PubMed] [Google Scholar]
- 62.Upala S, Wirunsawanya K, Jaruvongvanich Vet al. Effects of statin therapy on arterial stiffness: a systematic review and meta-analysis of randomized controlled trial. Int J Cardiol 2017; 227: 338–341. [DOI] [PubMed] [Google Scholar]
- 63.Aaron SE, Tomoto T, Zhang Ret al. Statin contribution to middle cerebral artery blood flow velocity in older adults at risk for dementia. Eur J Appl Physiol 2022; 122: 2417–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Billinger SA, Mattlage AE, Ashenden ALet al. Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. J Neurol Phys Ther 2012; 36: 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grotle AK, Macefield VG, Farquhar WBet al. Recent advances in exercise pressor reflex function in health and disease. Auton Neurosci 2020; 228: 102698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunnicutt JL, Gregory CM. Skeletal muscle changes following stroke: a systematic review and comparison to healthy individuals. Top Stroke Rehabil 2017; 24: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Estrada JA, Ducrocq GP, Kaufman MP. The magnitude of the exercise pressor reflex is influenced by the active skeletal muscle mass in the decerebrate rat. Am J Physiol Regul Integr Comp Physiol 2020; 318: R30–r37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Juraschek SP, Daya N, Rawlings AMet al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in Middle-aged adults. JAMA Intern Med 2017; 177: 1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brassard P, Ferland-Dutil H, Smirl JDet al. Evidence for hysteresis in the cerebral pressure-flow relationship in healthy men. Am J Physiol Heart Circ Physiol 2017; 312: H701–h704. [DOI] [PubMed] [Google Scholar]
- 70.Roy M-A, Labrecque L, Perry BGet al. Directional sensitivity of the cerebral pressure–flow relationship in young healthy individuals trained in endurance and resistance exercise. Exp Physiol 2022; 107: 299–311. [DOI] [PubMed] [Google Scholar]
- 71.Labrecque L, Smirl JD, Brassard P. Utilization of the repeated squat-stand model for studying the directional sensitivity of the cerebral pressure-flow relationship. J Appl Physiol (1985) 2021; 131: 927–936. [DOI] [PubMed] [Google Scholar]
- 72.Maeda H, Matsumoto M, Handa Net al. Reactivity of cerebral blood flow to carbon dioxide in various types of ischemic cerebrovascular disease: evaluation by the transcranial doppler method. Stroke 1993; 24: 670–675. [DOI] [PubMed] [Google Scholar]
- 73.Miller KB, Howery AJ, Rivera-Rivera LAet al. Age-related reductions in cerebrovascular reactivity using 4D flow MRI. Front Aging Neurosci 2019; 11: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Witte E, Liu Y, Ward JLet al. Exercise intensity and middle cerebral artery dynamics in humans. Respir Physiol Neurobiol 2019; 262: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ward JL, Craig JC, Liu Yet al. Effect of healthy aging and sex on middle cerebral artery blood velocity dynamics during moderate-intensity exercise. Am J Physiol Heart Circ Physiol 2018; 315: H492–H501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krejza J, Mariak Z, Huba Met al. Effect of endogenous estrogen on blood flow through carotid arteries. Stroke 2001; 32: 30–36. [DOI] [PubMed] [Google Scholar]
- 77.Alwatban MR, Aaron SE, Kaufman CSet al. Effects of age and sex on middle cerebral artery blood velocity and flow pulsatility index across the adult lifespan. J Appl Physiol (1985) 2021; 130: 1675–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeller NP, Miller KB, Zea RDet al. Sex-specific effects of cardiorespiratory fitness on age-related differences in cerebral hemodynamics. J Appl Physiol (1985) 2022; 132: 1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ainslie PN, Cotter JD, George KPet al. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 2008; 586: 4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lavie CJ, Ozemek C, Carbone Set al. Sedentary behavior, exercise, and cardiovascular health. Circ Res 2019; 124: 799–815. [DOI] [PubMed] [Google Scholar]
- 81.Ainslie PN, Hoiland RL. Transcranial doppler ultrasound: valid, invalid, or both? J Appl Physiol (1985) 2014; 117: 1081–1083. [DOI] [PubMed] [Google Scholar]
- 82.Valdueza JM, Balzer JO, Villringer Aet al. Changes in blood flow velocity and diameter of the middle cerebral artery during hyperventilation: assessment with MR and transcranial doppler sonography. AJNR Am J Neuroradiol 1997; 18: 1929–1934. [PMC free article] [PubMed] [Google Scholar]
- 83.Hoiland RL, Ainslie PN. CrossTalk proposal: the middle cerebral artery diameter does change during alterations in arterial blood gases and blood pressure. J Physiol 2016; 594: 4073–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kravitz L. High-Intensity Interval Exercise, www.acsm.org/docs/default-source/files-for-resource-library/high-intensity-interval-training.pdf. 2014. (accessed January 2023).
- 85.Billinger SA, Boyne P, Coughenour Eet al. Does aerobic exercise and the FITT principle fit into stroke recovery? Curr Neurol Neurosci Rep 2015; 15: 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacKay-Lyons MJ, Howlett J. Exercise capacity and cardiovascular adaptations to aerobic training early after stroke. Top Stroke Rehabil 2005; 12: 31–44. [DOI] [PubMed] [Google Scholar]
- 87.Inness EL, Aqui A, Foster Eet al. Determining safe participation in aerobic exercise early after stroke through a graded submaximal exercise test. Phys Ther 2020; 100: 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gjellesvik TI, Becker F, Tjonna AEet al. Effects of high-intensity interval training after stroke (the HIIT-Stroke study) – a multicenter randomized controlled trial. Arch Phys Med Rehabil 2020; 101: 939–947. [DOI] [PubMed] [Google Scholar]
- 89.Gorelick PB, Furie KL, Iadecola Cet al. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke 2017; 48: e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lazar RM, Howard VJ, Kernan WNet al. A primary care agenda for brain health: a scientific statement from the American Heart Association. Stroke 2021; 52: e295–e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Claassen JAHR, Thijssen DHJ, Panerai RBet al. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev 2021; 101: 1487–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fan J-L, Nogueira RC, Brassard Pet al. Integrative physiological assessment of cerebral hemodynamics and metabolism in acute ischemic stroke. J Cereb Blood Flow Metab 2022; 42: 454–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller A, Reisman DS, Billinger SAet al. Moderate-intensity exercise versus high-intensity interval training to recover walking post-stroke: protocol for a randomized controlled trial. Trials 2021; 22: 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Medicine ACoS. ACSM's guidelines for exercise testing and prescription. Philadelphia: Lippincott Williams & Wilkins, 2021. [Google Scholar]
- 95.Ramos JS, Dalleck LC, Tjonna AEet al. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med 2015; 45: 679–692. [DOI] [PubMed] [Google Scholar]
- 96.Hsu CC, Fu TC, Huang SCet al. Increased serum brain-derived neurotrophic factor with high-intensity interval training in stroke patients: a randomized controlled trial. Ann Phys Rehabil Med 2021; 64: 101385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231201472 for Lower middle cerebral artery blood velocity during low-volume high-intensity interval exercise in chronic stroke by Alicen A Whitaker, Saniya Waghmare, Robert N Montgomery, Stacey E Aaron, Sarah M Eickmeyer, Eric D Vidoni and Sandra A Billinger in Journal of Cerebral Blood Flow & Metabolism