Abstract
Background
Urinary tract infection (UTI) is the most common hospital‐acquired infection. The major associated cause is indwelling urethral catheters. Several measures have been introduced to reduce catheter‐associated urinary tract infections (CAUTIs). One of these measures is the introduction of specialised urethral catheters that have been designed to reduce the risk of infection. These include antiseptic‐coated and antimicrobial‐impregnated catheters.
Objectives
The primary objective of this review was to compare the effectiveness of different types of indwelling urethral catheters in reducing the risk of UTI and to assess their impact on other outcomes in adults who require short‐term urethral catheterisation in hospitals.
Search methods
We searched the Cochrane Incontinence Group's Specialised Trials Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, ClinicalTrials.gov, WHO ICTRP and handsearching of journals and conference proceedings (searched 9 September 2014). We also examined the bibliographies of relevant articles and contacted catheter manufacturer representatives for trials.
Selection criteria
We included all randomised controlled trials (RCTs) and quasi‐RCTs comparing types of indwelling urethral catheters for short‐term catheterisation in hospitalised adults. 'Short‐term' is defined as a duration of catheterisation which is intended to be less than or equal to 14 days.
Data collection and analysis
At least two review authors independently screened abstracts, extracted data and assessed risk of bias of the included trials. Any disagreement was resolved by discussion or consultation with a third party. We processed data as described in the Cochrane Handbook for Systematic Reviews of Interventions. We assessed the quality of evidence using the GRADE approach.
Main results
Twenty‐six trials met the inclusion criteria involving 12,422 hospitalised adults in 25 parallel group trials, and 27,878 adults in one large cluster‐randomised cross‐over trial. No trials compared one antiseptic catheter versus another, nor an antimicrobial catheter versus another.
Antiseptic‐coated indwelling urethral catheters versus standard indwelling urethral catheters
The primary outcome, symptomatic CAUTI was reported in one large trial with a low risk of bias, comparing silver alloy hydrogel‐coated latex catheter (antiseptic‐coated) against a standard polytetrafluoroethylene (PTFE)‐coated latex catheter (control). The trial used a pragmatic, US Centers for Disease Control and Prevention (CDC)‐based definition for symptomatic CAUTI. For the comparison between silver alloy‐coated catheter versus standard catheter, there was no significant difference in symptomatic CAUTI incidence (RR 0.99, 95% CI 0.85 to 1.16).
For secondary outcomes, the included trials reported on two types of antiseptic catheters (coated with either silver oxide or silver alloy). For the outcome of bacteriuria, silver oxide catheters were not associated with any statistically significant reduction (RR 0.90, 95% CI 0.72 to 1.13). These catheters are no longer manufactured. Silver alloy catheters achieved a slight but statistically significant reduction in bacteriuria (RR 0.82, 95% CI 0.73 to 0.92). However, the one large trial with a low risk of bias did not support this finding (RR 0.99, 95% CI 0.85 to 1.16). The randomised cross‐over trial of silver alloy catheters versus standard catheters was excluded from the pooled results because data were not available prior to crossover. The results of this trial showed less bacteriuria in the silver alloy catheter group.
For the outcome of discomfort whilst the catheter was in‐situ, fewer patients with silver alloy catheters complained of discomfort compared with standard catheters (RR 0.84, 95% CI 0.74 to 0.96).
Antimicrobial‐impregnated indwelling urethral catheters versus standard indwelling urethral catheters
The primary outcome measure, symptomatic CAUTI was reported in one large trial with a low risk of bias, comparing nitrofurazone‐impregnated silicone catheter (antimicrobial‐impregnated) against a standard PTFE‐coated latex catheter (control). The nitrofurazone catheter achieved a reduction in symptomatic CAUTI incidence which was of borderline statistical significance (RR 0.84, 95% CI 0.71 to 0.99).
For secondary outcomes, the included trials reported on two types of antimicrobial catheters (impregnated with either nitrofurazone or minocycline/rifampicin). Antimicrobial‐impregnated catheters, compared with standard catheters, were found to lower the rate of bacteriuria in the antimicrobial group for both minocycline and rifampicin (RR 0.36, 95% CI 0.18 to 0.73), and nitrofurazone (RR 0.73, 95% CI 0.64 to 0.85). The minocycline and rifampicin catheter is no longer manufactured.
For the outcome of discomfort whilst the catheter was in‐situ, more patients with nitrofurazone catheters complained of pain whilst the catheter was in‐situ compared with standard catheters (RR 1.26, 95% CI 1.12 to 1.41). For the period after catheter removal, more patients with nitrofurazone catheters complained of pain than standard catheters (RR 1.43, 95% CI 1.30 to 1.57).
Antimicrobial‐impregnated indwelling urethral catheters versus antiseptic‐coated indwelling urethral catheters
One large trial compared antimicrobial‐impregnated (nitrofurazone) catheters versus silver alloy‐coated (antiseptic‐coated) catheters. The results showed people were less likely to have a symptomatic CAUTI with nitrofurazone‐impregnated catheters (228/2153, 10.6%) compared with silver alloy‐coated catheters (263/2097, 12.5%), but this was of borderline statistical significance (RR 0.84, 95% CI 0.71 to 1.00). They did, however, have significantly less bacteriuria (RR 0.78, 95% CI 0.67 to 0.91),
While the catheter was in‐situ (RR 1.50, 95% CI 1.32 to 1.70), and on removal (RR 1.32, 95% CI 1.20 to 1.45), nitrofurazone catheters were associated with more discomfort compared with silver‐coated catheters.
One type of standard indwelling urethral catheter versus another type of standard indwelling urethral catheter
None of the trials comparing standard catheters versus other types of standard catheters measured symptomatic CAUTI. In terms of reducing bacteriuria, individual trials were too small to show whether one type of standard catheter was superior to another type. For the outcome of urethral reactions, fully siliconised catheters appeared to be superior to latex‐based catheters. However, the trials involved small numbers of participants. There were no statistically significant differences between the different catheters for all other outcomes.
Authors' conclusions
Silver alloy‐coated catheters were not associated with a statistically significant reduction in symptomatic CAUTI, and are considerably more expensive. Nitrofurazone‐impregnated catheters reduced the risk of symptomatic CAUTI and bacteriuria, although the magnitude of reduction was low and hence may not be clinically important. However, they are more expensive than standard catheters. They are also more likely to cause discomfort than standard catheters.
Keywords: Adult; Humans; Alloys; Anti‐Infective Agents, Urinary; Anti‐Infective Agents, Urinary/administration & dosage; Catheter‐Related Infections; Catheter‐Related Infections/etiology; Catheter‐Related Infections/prevention & control; Catheters, Indwelling; Catheters, Indwelling/adverse effects; Minocycline; Minocycline/administration & dosage; Nitrofurazone; Nitrofurazone/administration & dosage; Randomized Controlled Trials as Topic; Rifampin; Rifampin/administration & dosage; Silver; Urinary Catheterization; Urinary Catheterization/adverse effects; Urinary Catheterization/instrumentation; Urinary Tract Infections; Urinary Tract Infections/etiology; Urinary Tract Infections/prevention & control; Urination Disorders; Urination Disorders/therapy
Plain language summary
Types of urethral catheters for management of short‐term voiding problems in hospitalised adults
Background on the condition
Urethral catheters are small tubes passed into the bladder via the urethra (outlet for urine). They are often used for a short time after major surgery. Urethral catheters are also used if a person is unable to empty the bladder when they need to (urinary retention). They are also used for monitoring urine output in hospitalised patients. About half of all hospitalised adults who have urethral catheters for longer than a week will get a urinary tract infection (UTI).
The main findings of the review
Twenty‐six trials were included in this systematic review involving 12,422 hospitalised adults in 25 parallel group trials, and 27,878 adults in one large cluster‐randomised cross‐over trial.The review of evidence from trials found that although antiseptic‐coated (silver alloy) catheters reduced the number of bacteria in the urine, they did not reduce the number of UTIs caused by the presence of the catheter. Catheters coated with antimicrobials (antibiotics, nitrofurazone) designed to kill or stop the growth of bacteria may reduce both the number of bacteria in the urine as well as number of people having UTI caused by the presence of the catheter. However, the evidence is relatively weak, and any benefit is likely to be small and hence unlikely to be meaningful to either patients or clinicians.
Adverse effects
These antibiotic catheters are also more likely to cause discomfort for patients compared with standard catheters, and they are more expensive.
Conclusions
The best approaches to reducing the risk of UTI include reducing the numbers of unnecessary catheterisations, or reducing the time period during which the catheter is used by removing it as early as possible.
Summary of findings
Summary of findings for the main comparison. Antispetic‐coated catheter versus standard catheter for management of short‐term voiding problems in hospitalised adults.
| Antiseptic‐coated catheter versus standard catheter for management of short‐term voiding problems in hospitalised adults | ||||||
| Patient or population: Patients with an indwelling urethral catheter of short‐term duration Settings: Hospital Intervention: antiseptic‐coated catheter versus standard catheter | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antiseptic‐coated catheter versus standard catheter | |||||
| Symptomatic CAUTI: without microbiological evidence ‐ silver alloy versus standard | See comment | See comment | Not estimable | 4241 (1 study) | ⊕⊕⊕⊕ high1,2 | |
| Symptomatic CAUTI: with microbiological evidence ‐ silver alloy versus standard | See comment | See comment | Not estimable | 4241 (1 study) | ⊕⊕⊕⊝ moderate1,2,3 | |
| Bacterial resistance towards antimicrobial agent ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Urinary sepsis ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Patient discomfort whilst catheter is in situ‐ silver oxide versus standard Number with pain with catheter in place | See comment | See comment | Not estimable | 34 (1 study) | ⊕⊝⊝⊝ very low1,2,4,5,6 | |
| Patient discomfort whilst catheter is in situ‐ silver alloy versus standard | See comment | See comment | Not estimable | 3718 (1 study) | ⊕⊕⊕⊕ high1,2,5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Only one trial. 2 Not applicable as there is only one trial. 3 95% confidence interval is wide (0.83 to 1.42) 4 Sequence generation and allocation concealment unclear. 5 GRADE‐specific outcome was patient reported discomfort whilst trial reported patient reported pain. 6 95% confidence interval is very wide (0.48 to 4.27)
Summary of findings 2. Antimicrobial‐impregnated catheter versus standard catheter for management of short‐term voiding problems in hospitalised adults.
| Antimicrobial‐impregnated catheter versus standard catheter for management of short‐term voiding problems in hospitalised adults | ||||||
| Patient or population: Patients with an indwelling urethral catheter of short‐term duration Settings: Hospital Intervention: antimicrobial‐impregnated catheter versus standard catheter | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antimicrobial‐impregnated catheter versus standard catheter | |||||
| Symptomatic CAUTI: without microbiological evidence ‐ nitrofurazone versus standard | See comment | See comment | Not estimable | 4297 (1 study) | ⊕⊕⊕⊕ high1 | |
| Symptomatic CAUTI: with microbiological evidence ‐ nitrofurazone versus standard | See comment | See comment | Not estimable | 4297 (1 study) | ⊕⊕⊕⊕ high1 | |
| Bacterial resistance towards the antimicrobial agent ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Urinary sepsis | Study population | Not estimable | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Patient discomfort whilst catheter is in situ ‐ nitrofurazone versus standard | See comment | See comment | Not estimable | 3768 (1 study) | ⊕⊕⊕⊕ high1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Not applicable because there was only one trial.
Summary of findings 3. Antimicrobial‐impregnated catheter versus antiseptic‐coated catheter for management of short‐term voiding problems in hospitalised adults.
| Antimicrobial‐impregnated catheter versus antiseptic‐coated catheter for management of short‐term voiding problems in hospitalised adults | ||||||
| Patient or population: Patients with an indwelling urethral catheter of short‐term duration Settings: Hospital Intervention: antimicrobial‐impregnated catheter versus antiseptic‐coated catheter | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antimicrobial‐impregnated catheter versus antiseptic‐coated catheter | |||||
| Symptomatic CAUTI: without microbiological evidence ‐ antibiotic versus silver alloy | Study population | RR 0.84 (0.71 to 1) | 4250 (1 study) | ⊕⊕⊕⊝ moderate1,2 | ||
| 125 per 1000 | 105 per 1000 (89 to 125) | |||||
| Bacterial resistance towards the antimicrobial agent ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Symptomatic CAUTI: with microbiological evidence ‐ antibiotic versus silver alloy | Study population | RR 0.64 (0.48 to 0.86) | 4250 (1 study) | ⊕⊕⊕⊕ high1 | ||
| 50 per 1000 | 32 per 1000 (24 to 43) | |||||
| Urinary sepsis | Study population | Not estimable | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Patient discomfort whilst catheter is in situ | Study population | RR 1.5 (1.32 to 1.7) | 3708 (1 study) | ⊕⊕⊕⊕ high1 | ||
| 176 per 1000 | 264 per 1000 (232 to 299) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Not applicable because there was only one trial. 2 95% Confidence interval is wide (0.71 to 1.00).
Summary of findings 4. One type of standard catheter versus another standard catheter for management of short‐term voiding problems in hospitalised adults.
| One type of standard catheter versus another standard catheter for management of short‐term voiding problems in hospitalised adults | ||||||
| Patient or population: Patients with an indwelling urethral catheter of short‐term duration Settings: Hospital Intervention: one type of standard catheter versus another standard catheter | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | One type of standard catheter versus another standard catheter | |||||
| Symptomatic CAUTI: with microbiological evidence ‐ nitrofurazone versus standard ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Bacterial resistance towards the antimicrobial agent ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Urinary sepsis | Study population | Not estimable | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Patient discomfort whilst catheter is in situ Number with burning sensation in urethra ‐ Silicone versus non‐silicone | See comment | See comment | Not estimable | 40 (1 study) | ⊕⊕⊝⊝ low1,2,3,4 | |
| Symptomatic CAUTI: without microbiological evidence ‐ nitrofurazone versus standard ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Did not specify the method of sequence generation and allocation concealment. 2 Only one trial. 3 Burning sensation in urethra is used as a proxy of patient discomfort. 4 Only one trial. Funnel plot not applicable.
Background
Description of the condition
Urinary tract infection (UTI) is the most common hospital‐acquired infection, accounting for between 20% and 40% of cases (Emmerson 1996; Haley 1981; Smyth 2008). Up to 80% of these UTIs occurring in hospitals can be attributed to the use of indwelling urethral catheters (Bryan 1984; Smyth 2008; Turck 1981). Catheter‐associated UTIs (CAUTIs) account for significant morbidity, with symptoms such as dysuria, urgency, frequency, haematuria, fever or bladder pain, or more serious complications such as bloodstream infection (Bryan 1984; Krieger 1983). In addition, healthcare costs increase by prolonging hospital stay, and this can adversely affect patients’ health‐related quality of life (Saint 2000; Tambyah 2002).
Urethral catheters are one of the most commonly applied medical devices. The indications for short‐term catheterisation include monitoring of urine output during the perioperative stage or in acutely unwell patients, as part of a urological procedure, or the treatment of patients with acute urinary retention. The prevalence of catheterisation is high, with up to a quarter of patients admitted to hospital requiring urethral catheterisation at some stage during their stay (Gould 2010; Haley 1981; Weinstein 1999). For patients with urethral catheters, there is a cumulative daily risk of 5% of developing bacteriuria (Haley 1981) (i.e. presence of bacteria in the urine), with the risk increasing to 35% and 70%, after 7 and 14 days of indwelling catheterisation, respectively. Whilst bacteriuria does not normally cause symptoms (i.e. asymptomatic bacteriuria), it does increase the risk of developing a symptomatic UTI; it has been estimated that symptomatic UTI occurs in 20% of patients with bacteriuria (Garibaldi 1982; Hartstein 1981). It is also associated with a small risk of bloodstream infection (< 1%) (Bryan 1984; Krieger 1983); whilst this risk is relatively low, bloodstream infection is associated with a high mortality rate of approximately 30%. The presence of bacteriuria in hospital patients with an indwelling catheter is also a potential source of cross‐infection, particularly in critical care units, with an estimated risk per episode of 15% (Johnson 2006). Other factors that increase the risk of infection include female gender, older age, impaired immunity, severity of illness (Stamm 1998), and care process factors, such as lack of antibiotic use, longer duration of catheterisation, catheter insertion or maintenance by poorly trained personnel, and deviation from catheter care protocols (CDC 2009).
In terms of the microbiology of UTIs, infections associated with short‐term catheterisation typically involve a single organism, in contrast with long‐term catheterisation, where polymicrobial infection is frequent (CDC 2009). Although a variety of micro‐organisms may be associated with CAUTI, the commonest pathogens are enteric Gram negative bacilli (Shuman 2010) Escherichia coli (E. Coli) is the most frequently isolated single species, but other species such as Klebsiella spp., Proteus spp. and Enterobacter spp. are also commonly identified. Enterococci, Pseudomonas aeruginosa (P. aeruginosa) and Candida spp. are also important causes of CAUTI, particularly in patients within critical care settings. Forty Staphylococci and other Gram‐negative bacilli are isolated less frequently (CDC 2009).
The criterion for diagnosis of a symptomatic CAUTI varies significantly in the literature. However, efforts have been made by healthcare organisations, such as the US Centers for Disease Control and Prevention (CDC) to standardise definitions, based on different scenarios (e.g. UTI in presence of a urethral catheter, symptomatic UTI, etc.) (CDC 2009). It is worthwhile noting that the CDC’s definitions for UTI have undergone several revisions in the past two decades. In line with CDC definitions, past and present, which reflect clinically relevant outcomes, for purposes of this review, a positive urine culture reported without any consideration for patient symptoms is defined as bacteriuria rather than a UTI.
Several strategies and policies aimed at reducing CAUTI have been introduced. These can be summarised as follows: (1) education of patients, their caregivers and healthcare personnel, in terms of hand hygiene and steps in preventing spread of infection; (2) reduction in the prevalence of catheterisation by assessing the need for catheterisation, and restricting the intervention to those who have no other alternative of achieving bladder drainage; (3) use of aseptic technique for catheter insertion; (4) use of antibiotic prophylaxis in selected high risk groups at insertion based on local antibiotic prescribing policies; (5) maintenance of a sterile, closed drainage system by obtaining urine specimens from the sampling port, positioning of drainage bag above floor level and below bladder level; (6) frequent emptying of drainage bag to maintain urine flow and prevent reflux, and daily washing of urethral meatus; (7) minimising the duration of catheterisation by regularly reviewing the need for catheterisation, and by aiming for early removal of catheter; and (8) coating or impregnation of catheter surface with antiseptic or antimicrobial substances (Brosnahan 2004; Parker 2009; Pratt 2007; Schumm 2008; Willson 2009).
Description of the intervention
Currently, there are many types of catheters available. Standard indwelling catheters are made from a variety of materials including: polyvinyl chlorine, plastic, plain latex, polytetrafluoroethylene (PTFE)‐coated latex, hydrogel‐coated latex, silicone elastomer, pure silicone hydrogel and polymer hydromer (Pomfret 2000; Robinson 2001). Specialised urethral catheters have been developed specifically to reduce the risk of infection. A common approach is to coat the catheter with antiseptic or antimicrobial agents either on the outer surface, the lumen, or both (Saint 1998), or impregnated into the catheter material.
How the intervention might work
Antiseptic agents are substances which kill bacteria and other micro‐organisms. The most common antiseptic agent used is silver. Silver has long been recognised as an antiseptic agent active against a variety of uropathogens through multiple mechanisms of action (Franken 2007). Silver exposure results in limited toxicity to human tissues (Gosheger 2004) and does not appear to induce microbial resistance (Percival 2005). Two types of silver‐based agents have been used to coat urethral catheters: silver alloy and silver oxide (Saint 1998).
Antibiotics are antimicrobial drugs which can either kill bacteria or inhibit their growth to stop or prevent infections. Antimicrobial‐impregnated catheters have also been developed using various types of antibiotics active against expected uropathogens. These antimicrobial agents are impregnated into the external and internal luminal catheter surfaces, and elute over time into the external surface‐urethral mucosa and internal lumen–urinary boundaries (Guay 2001).
Why it is important to do this review
Several systematic reviews have investigated the effectiveness of antiseptic and antimicrobial catheters in reducing CAUTIs (Drekonja 2008; Johnson 2006; Saint 1998; Schumm 2008), including the previous update of the present review (Shuman 2010). The results of these reviews suggest that silver alloy‐coated catheters and nitrofurazone‐impregnated catheters may reduce the incidence of bacteriuria in hospitalised patients catheterised for less than two weeks in comparison with standard catheters. The magnitude of relative risk reduction varied in each of the analyses due to different inclusion criteria, ranging from 16% to 48%. However, the far majority of included studies in those reviews defined CAUTI based solely on microbiological identification of bacteriuria without any patient‐driven or clinician‐defined contribution to the primary outcomes used. Since the last Cochrane review update, the results of a large RCT which compared silver alloy‐coated and nitrofurazone‐impregnated short‐term urethral catheters versus standard catheters and which assessed clinically relevant outcomes, have been published (Pickard 2012).
The aim of this review was to investigate the effects of these specialised catheters in comparison with standard ones in reducing the incidence of symptomatic CAUTI in hospitalised patients requiring short‐term catheterisation, but also taking into account other factors, such as ease of use, comfort and cost that may influence decision‐making regarding these catheters. For the purpose of this review, short‐term was defined as up to and including 14 days, or other temporary short‐term use as defined by the trialists.
The following are relevant Cochrane reviews that may be of interest to the reader.
Antibiotic policies for short‐term catheter bladder drainage in adults (Niël‐Weise 2005a).
Antibiotic prophylaxis for short‐term catheter bladder drainage in adults (Lusardi 2013).
Short‐term urethral catheter policies following urogenital surgery in adults (Phipps 2006).
Strategies for the removal of short‐term indwelling urethral catheters in adults (Griffiths 2007).
Urethral catheter policies for short‐term bladder drainage in adults (Niël‐Weise 2005b).
Objectives
The primary objective of this review was to compare the effectiveness of different types of indwelling urethral catheters in reducing the risk of UTI and to assess their impact on other outcomes in adults who require short‐term urethral catheterisation in hospitals.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs comparing types of indwelling urethral catheters for short‐term catheterisation in hospitalised adults. Short‐term is defined as duration of catheterisation which is intended to be less than or equal to 14 days.
Types of participants
Hospitalised adults (patients admitted to an adult hospital) with an indwelling urethral catheter of short‐term duration (less than or equal to 14 days duration, or other temporary short‐term use as defined by the trialists).
Exclusions
Children
Residential care facilities
Adult patients with chronic/long‐term catheterisation for more than 14 days
Patients anticipated as needing a catheter in the long‐term
Patients admitted with an indwelling catheter
Patients with suprapubic urethral catheters
Patients with pre‐existing UTIs
Types of interventions
Different types of indwelling urethral catheters:
antiseptic‐impregnated indwelling urethral catheters;
antimicrobial‐impregnated indwelling urethral catheters;
standard indwelling urethral catheters (defined as catheters that are not impregnated with antiseptics or antimicrobial)
We wished to make the following comparisons: 1. Antiseptic‐coated indwelling urethral catheters versus standard indwelling urethral catheters; 2. Antimicrobial‐impregnated indwelling urethral catheters versus standard indwelling urethral catheters; 3. Antimicrobial‐impregnated indwelling urethral catheters versus antiseptic‐coated indwelling urethral catheters; 4. One type of standard indwelling urethral catheter versus another type of standard indwelling urethral catheter; 5. One type of antiseptic‐coated indwelling urethral catheter versus another type of antiseptic‐coated indwelling urethral catheter; 6. One type of antimicrobial‐impregnated indwelling urethral catheter versus another type of antimicrobial‐impregnated indwelling urethral catheter
Types of outcome measures
Primary outcomes
The primary outcome of interest was the number of people with symptomatic CAUTI.
Secondary outcomes
Microbiological
Bacteriuria (defined by trialists)
Bacterial resistance
Patient‐reported
Patient discomfort
Patient satisfaction
Clinician‐reported
Length of time catheters used
Quality of life
Generic QoL measures (e.g. SF 36, Ware 1992)
Psychological outcome measures (e.g. HADS, Zigmond 1983)
Complications/adverse effects
Septicaemia
Death due to septicaemia
Allergic reactions to catheter materials
Other adverse effects of intervention (as described by trialists)
Co‐interventions
Use of prophylactic antibiotics
Use of rescue antibiotics
Economic outcomes
cost‐effectiveness
Other outcomes
Any other non‐pre‐specified outcomes judged to be important when performing the review.
We classified the primary and secondary outcomes above as critical, important or not important from patients' perspective for decision‐making. The GRADE working group strongly recommends including up to seven critical outcomes in a systematic review to be assessed via the GRADE approach (Guyatt 2011).
We contacted content experts to identify outcomes of importance to patients undergoing short‐term urethral catheterisation that could be included in a Cochrane systematic review. The content experts included clinicians, nurses, and a health economist. Subsequently, through the Urological Cancer Charity (UCAN), we identified five individuals who had undergone urethral catheterisation and invited them to take part in a group discussion to identify important outcomes from their perspective. The participants were not aware of the views of the content experts. On the whole, the participants were in agreement with the content experts regarding the key outcomes of importance. For example, they suggested that infections and discomfort were certainly important from their point of view. However, they also highlighted other outcomes as being meaningful and important such as length of hospital stay and the duration of catheterisation. Interestingly, participants also raised issues around being catheterised and the impact on self esteem and ability to wear clothes comfortably (Omar 2013). We selected the following critical outcomes to assess the quality of evidence in this systematic review, as suggested by patients and content experts.
Symptomatic CAUTI.
Patient discomfort whilst catheter is in‐situ.
Bacterial resistance towards the antimicrobial agent.
Septicaemia.
Search methods for identification of studies
We did not impose any language or other restrictions on any of the searches detailed below.
Electronic searches
This review has drawn on the search strategy developed for the Cochrane Incontinence Group. Relevant trials were identified from the Cochrane Incontinence Group Specialised Register of controlled trials. For more details of the search methods used to build the Specialised Register please see the Group's module in The Cochrane Library. The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and MEDLINE in process, ClinicalTrials.gov, WHO ICTRP and handsearching of journals and conference proceedings. Most of the trials in the Cochrane Incontinence Group Specialised Register are also contained in CENTRAL. The date of the last search was: 9 September 2014. (Please note: The first version of this review searched the Cochrane Renal Group Specialised Register (searched February 2003)).
The terms used to search the Cochrane Incontinence Group Specialised Register are given in Appendix 1.
Searching other resources
We searched the bibliographies of relevant articles. We also contacted catheter manufacturer representatives, however we did not identify any further trials for inclusion.
Data collection and analysis
Selection of studies
Three review authors (TL, MO or EF) independently assessed all titles and abstracts identified by the search. Where there was the possibility that the study might be included, the full paper was obtained. We resolved any disagreements through discussion. Another review author was available to resolve any disagreements related to study inclusion. We excluded studies that were not randomised or quasi‐randomised trials comparing types of indwelling urethral catheters in hospitalised adults.
Data extraction and management
One review author extracted trial data using a standardised form and this was independently verified by a second review author. Any disagreement which arose was resolved either by discussion or by arbitration with a third party. Where data in trials were not fully reported, clarification was sought directly from the trialists. We entered the extracted data into Review Manager software (RevMan 2012). We processed all data from included trials according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
At least two review authors (TL, MO and EF) investigated the included trials for risk of bias using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). We assessed a range of specific issues, including:
random sequence generation;
level of concealment of random allocation;
participant/therapist blinding;
outcome assessor blinding;
incomplete outcome data;
selective outcome reporting; and
any other potential sources of bias.
We resolved disagreements which arose either by discussion or by consultation with a third party.
Measures of treatment effect
We processed included trial data as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When appropriate, we undertook meta‐analysis. We categorised comparisons by type of intervention catheter. We performed sensitivity analyses that indicated that the results of silver oxide versus standard catheters and silver alloy versus standard catheters should not be combined. However, we combined data from different types of standard catheters for comparison with antimicrobial‐impregnated and antiseptic‐coated catheters. Where possible, we performed subgroup analyses, comparing outcomes by gender and according to whether the participants were receiving systemic antibiotics.
For categorical outcomes we related the numbers reporting an outcome to the numbers at risk in each group to derive a relative risk. For continuous variables we used means and standard deviations to derive a mean difference (MD). We used a fixed‐effect model to calculate the pooled relative risks, MD and their 95% confidence intervals (CIs). However, we also checked results using a random‐effects model. We compared trials to assess and investigate the likelihood of important clinical heterogeneity. Where we observed significant statistical heterogeneity, we offered an explanation in the text. We could not apply publication bias using a funnel plot as there were fewer than 10 trials in the meta‐analysis.
Unit of analysis issues
The primary analysis was per patient randomised.
Dealing with missing data
As far as possible, we analysed data on an intention‐to‐treat basis, meaning that analysis of patients was according to the groups to which they were originally randomised. However, if data were missing, we used the numbers as reported by the trialists. We contacted trialists for missing data or additional information.
If there had been evidence of differential dropout between the groups we would have considered imputing data for the missing results.
Assessment of heterogeneity
We assessed evidence of heterogeneity between trials by visual inspection of forest plots, the Chi2 test for heterogeneity and the I2 statistic (Higgins 2003; Higgins 2011). Thresholds for interpretation of the I2 statistic were defined according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We regarded statistical heterogeneity as substantial if either the I2 was greater than 50%, or there was a low P value (P < 0.10) in the Chi2 test for heterogeneity (Higgins 2011). In those outcomes, a random‐effects model would have been used.
Assessment of reporting biases
Owing to the difficulties involved in the detection and correction for publication bias, as well as other reporting biases, we aimed to minimise the potential impact of these biases by ensuring the implementation of a comprehensive search strategy and by being alert to data duplication.
Data synthesis
We only combined data from trials if the trials were clinically similar. We did this by meta‐analysis using a fixed‐effect approach.
Subgroup analysis and investigation of heterogeneity
We subgrouped data according to the following.
Type of catheter used (e.g. different types of antibiotics used to impregnate antimicrobial catheters).
Duration of catheter use (less than, compared with longer than one week).
Type of participant (diagnosis or condition).
Type of measurement unit for rate of bacteriuria (e.g. per 100 catheters used).
Results
Description of studies
Results of the search
We screened a total of 892 records produced by the literature search and retrieved the full‐text of 62 potentially eligible articles. After we assessed the articles, we considered 28 reports of 26 studies to be eligible for inclusion in the review; we excluded 31 reports of 28 studies. Additionally three ongoing studies were identified (NCT00482547 2007; NCT01681511 2012; NCT02198833 2014). The flow of literature through the assessment process is shown in the PRISMA diagram (Figure 1).
1.
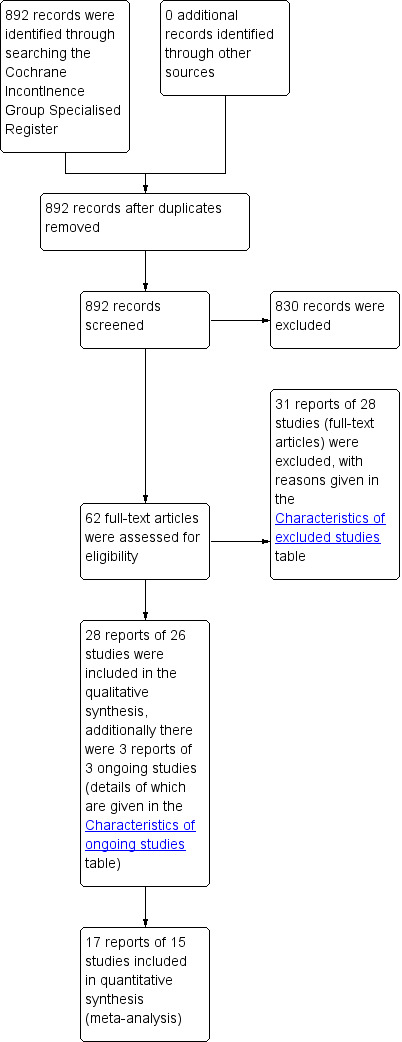
PRISMA study flow diagram.
Included studies
We included 26 trials involving 12,223 hospitalised adults in 25 parallel group trials (Al Habdan 2003; Chene 1990; Goodwin 1990; Darouiche 1999; Johnson 1990; Kalambheti 1965; Lee 2004; Liedberg 1990a; Liedberg 1990b; Liedberg 1993; Lundeberg 1986; Maki 1997; Maki 1998; Nacey 1984; Nickel 1989; Pickard 2012; Riley 1995; Stensballe 2007; Stenzelius 2011; Takeuchi 1993; Talja 1990; Thibon 2000; Tidd 1976; Verleyen 1999a; Verleyen 1999b) ) and 27,878 adults in one large cluster‐randomised cross‐over trial (Karchmer 2000) comparing two types of indwelling urethral catheters.
Of the 26 included trials, three were published in restricted format: one as a letter to the editor (Lundeberg 1986) and two as abstracts from scientific meetings (Maki 1998; Liedberg 1993). We were not able to contact any of these trialists, therefore additional information on the trials came from secondary sources (Niel‐Weise 2002; Saint 1998). Furthermore, one of the trials was an unpublished report (Maki 1997). All other trials were published as full‐text articles.
Participants
Conditions/populations
The trials involved heterogeneous population groups.
Fourteen trials included both women and men catheterised for haemodynamic monitoring or postoperative drainage for a variety of diagnoses (Al Habdan 2003; Chene 1990; Johnson 1990; Karchmer 2000; Liedberg 1990a; Liedberg 1990b; Maki 1998; Nickel 1989; Pickard 2012; Riley 1995; Takeuchi 1993; Thibon 2000; Verleyen 1999a; Verleyen 1999b).
Four trials included men with urological diagnoses (Darouiche 1999; Goodwin 1990; Kalambheti 1965; Tidd 1976).
Two other trials involved men only; one involved men postcardiac surgery (Nacey 1984) and the other included men with a variety of medical and surgical diagnoses (Talja 1990).
One trial included men and women undergoing elective orthopaedic surgery (Stenzelius 2011).
One trial included men and women from trauma and surgical wards, and patients with urinary incontinence (Maki 1997).
One trial included men and women from trauma centres only (Stensballe 2007).
One trial included men and women catheterised for more than 24 hours in five university hospitals (Lee 2004).
Two trials did not describe the characteristics of the population (Liedberg 1993; Lundeberg 1986).
Gender
The distribution of men and women was not even between groups in six trials (Lee 2004; Liedberg 1990a; Liedberg 1990b; Riley 1995; Stensballe 2007; Verleyen 1999b). In one trial, the intervention group (silver oxide catheter) had nearly twice the number of women than the comparison group (Riley 1995). The Lee trial included fewer women in the treatment group than the control group and more men in the treatment group than the control. One trial only included men after transurethral resection of the prostate or prostate cancer (Goodwin 1990).
Antiobiotic use
Six trials reported the number of participants on systemic antibiotics (Al Habdan 2003; Johnson 1990; Pickard 2012; Riley 1995; Stensballe 2007; Thibon 2000). The numbers were similar across the groups in the trials. Five trials reported on those taking systemic antibiotics prior to catheterisation (Al Habdan 2003; Pickard 2012; Riley 1995; Stensballe 2007; Thibon 2000) and one reported on the number given antibiotics for the final 48 hours of catheterisation (Johnson 1990). One trial (Pickard 2012) reported on the prescription of antibiotics immediately prior to, and during catheterisation, and within six weeks of catheter removal.
No trials included different types of populations, and so we were unable to subgroup the analysis by diagnosis.
Interventions and comparisons
The majority of trials randomised participants to an antiseptic‐coated catheter or a standard catheter. Four different types of antiseptic catheters were investigated:
silver oxide (Johnson 1990; Riley 1995; Takeuchi 1993);
silver alloy (Liedberg 1990a; Liedberg 1990b; Lundeberg 1986; Maki 1998; Thibon 2000; Verleyen 1999a);
silver alloy hydrogel (Liedberg 1993; Pickard 2012; Verleyen 1999b); and
noble metal alloy‐coated latex (Stenzelius 2011).
Two different types of antimicrobial‐impregnated catheters were studied:
rifampicin/minocycline combination (Darouiche 1999); and
nitrofurazone (Al Habdan 2003; Lee 2004; Maki 1997; Pickard 2012; Stensballe 2007).
The types of standard catheters used were heterogeneous:
hydrogel‐coated latex Foley catheter (Chene 1990; Karchmer 2000; Liedberg 1990a; Liedberg 1993; Talja 1990);
silicone‐coated latex Foley catheter (Al Habdan 2003; Chene 1990; Darouiche 1999; Maki 1997; Nacey 1984; Nickel 1989; Riley 1995; Stenzelius 2011; Talja 1990; Verleyen 1999a);
fully‐siliconised Foley catheter (Johnson 1990; Kalambheti 1965; Lee 2004; Stensballe 2007; Talja 1990; Thibon 2000);
hydrophilic polymer (Hydron)‐coated latex Foley catheter (Tidd 1976);
polyvinyl chloride (PVC) Foley catheter (Tidd 1976);
polyvinyl chloride (PVC) three‐way catheter (Goodwin 1990);
latex Foley catheter (Nacey 1984; Nickel 1989; Takeuchi 1993; Tidd 1976; Verleyen 1999b);
latex three‐way catheter (Goodwin 1990);
polytetrafluoroethylene (PTFE)‐coated latex Foley catheter (Pickard 2012; Liedberg 1990b); and
unspecified standard Foley catheter (Kalambheti 1965; Liedberg 1990a; Lundeberg 1986; Maki 1998).
Two trials randomised participants into three arms: Liedberg 1990a compared silver alloy, standard (hydrogel‐coated) and standard (non‐coated unspecified) catheters, whilst Pickard 2012 compared silver alloy hydrogel, nitrofurazone and standard PTFE‐coated latex catheters. Six trials randomised participants to two different types of standard catheters (Chene 1990; Kalambheti 1965; Nacey 1984; Nickel 1989; Talja 1990; Tidd 1976).
Types of catheters used are summarised in Table 5.
1. Types of catheters.
| Antiseptic | Antibiotic | Standard |
| Silver oxide | Silicone impregnated with minocycline and rifampin | Silicone |
| Silver alloy | Silicone impregnated with nitrofurazone | Latex |
| Noble metal alloy containing a mixture of gold, palladium and silver alloy | Hydrogel | |
| Siliconised latex | ||
| Teflonised latex | ||
| Hydrogel‐coated latex | ||
| Hydrophilic polymer‐coated latex | ||
| Polyvinyl chloride | ||
| Polytetrafluoroethylene |
Duration of catheterisation
Short‐term is defined as a duration of catheterisation which is intended to be less than 14 days. There was variation in the duration of catheterisation in the trials. The trials described the length of catheterisation in the following ways: total catheterisation time, total mean length of catheterisation for all participants, and mean or median length of catheterisation in the intervention and control groups.
In one trial the participants were catheterised for forty‐eight hours (Nacey 1984).
Another four trials had a total length of catheterisation of five to six days (Liedberg 1990a; Liedberg 1990b; Thibon 2000;Tidd 1976),
Two further trials had a total catheterisation time of fourteen days (Darouiche 1999; Verleyen 1999a).
Five trials recorded the length of catheterisation as total mean duration catheterised for all participants.The mean time catheterised varied from 44.9 hours in one trial (Nickel 1989) and 2.2 days (Maki 1997), three days (Goodwin 1990; Talja 1990) and five days (Verleyen 1999b).
Eight trials described the length of catheterisation as a separate mean for intervention and control groups. One trial reported a mean length of catheterisation of three days in the intervention group and two days in the control group (Stensballe 2007). Another trial reported a mean length of catheterisation of 3.5 days in the intervention group and 3.4 days in the control group (Kalambheti 1965). The mean length of catheterisation in both groups in four of the trials ranged from 3.4 to 4.6 days (Chene 1990; Johnson 1990; Lee 2004; Riley 1995), while one trial had a mean length of catheterisation in the intervention group of 7.7 days and 7.5 in the control group (Takeuchi 1993), and another of 7.9 in the intervention group and 7.2 in the control group (Al Habdan 2003).
Another trial (Pickard 2012) only recruited patients catheterised for up to 14 days, and reported the median duration of catheterisation for each of the three arms, which was balanced across all arms (two days); more than 96% of patients in each arm were catheterised for less than 14 days (balanced across all arms).
Stenzelius 2011 recruited patients who were catheterised during elective orthopaedic surgery, and reported the median duration of catheterisation for the two arms, which was balanced across both arms (two days).
Four trials did not clearly specify the length of catheterisation (Karchmer 2000; Liedberg 1993; Lundeberg 1986; Maki 1998).
Outcome measures
Primary outcome
The primary outcome measure, i.e. 'symptomatic CAUTI' was either not assessed or poorly defined in the great majority of studies. Very few studies defined symptomatic CAUTI based on standardised definitions. Pickard 2012 defined symptomatic CAUTI based on the development of UTI symptoms and signs, and prescription of antibiotics for a presumed UTI, at any time point during catheterisation, or up to six weeks postcatheter removal. This is a variation of a previous CDC symptomatic CAUTI definition, although the time point of six weeks was longer than any standard definitions. This primary outcome did not include any microbiological evidence of a UTI. However, the same study also included symptomatic CAUTI with microbiological evidence as a secondary outcome measure.
Another trial (Karchmer 2000) defined bacteriuria or symptomatic or non‐symptomatic UTI as ‘≥ 105 cfu/mL’, whilst Thibon 2000 defined UTI as ‘bacteriuria (> 105 cfu/mL) with > 10 leucocytes per mm3 of urine'. Darouiche and colleagues defined 'symptomatic bacteriuria' as being 'diagnosed by the healthcare provider' (Darouiche 1999).
The main outcome measure for most studies was bacteriuria without any consideration of patient symptoms. The exception to this was Stenzelius 2011, which assessed bacteriuria (> 105 cfu/mL) and urinary symptoms during and after the catheterisation period. There was significant heterogeneity in terms of the definition of bacteriuria across trials, ranging from 'greater than 102 colony forming units per mL' to 'greater than 106 colony forming units per mL'.
Secondary outcomes
Adverse effects
In terms of catheter‐related discomfort or symptoms, only five trials investigated this outcome (Nacey 1984; Pickard 2012; Stenzelius 2011; Takeuchi 1993; Talja 1990).
Pickard 2012 measured catheter‐related discomfort at four time points: catheter insertion, whilst the catheter remained in situ, during catheter removal, and within six weeks following catheter removal, using a self administered questionnaire completed by patients.
Nacey 1984 defined urethritis as ‘penile discharge and/or penile discomfort’.
Stenzelius 2011 measured various adverse effects such as pain, burning sensation and difficulty sleeping because of catheter.
Talja 1990 used measurement via scanning electron microscopic analysis to indicate urethral inflammatory reaction.
Takeuchi 1993 also measured catheter‐related pain, but did not provide a definition for this outcome.
Timing of outcome measurement
The timing of outcome measurement of the four trials that investigated outcomes related to comfort or urethritis, or both, differed considerably. The trial conducted by Nacey 1984 and colleagues investigated urethritis with assessment at eight weeks and six months postcatheterisation (length of catheterisation two days) by clinical examination and urethral swabs (Nacey 1984).
Definition of outcomes
The method for gathering the data on catheter‐related pain, urethral discharge and allergic reaction was not described in the Takeuchi 1993 report. The Talja 1990 trial investigated inflammatory reaction in the urethra assessed by cytological urethral swabs taken immediately after catheterisation, after removal of the catheter and on the second or third day after removal. Stenzelius 2011 measured urinary symptoms whilst the catheter remained in situ based on a questionnaire, and 7 to 10 days following catheter removal based on a telephone interview.
Other outcomes
Pickard 2012 also measured other outcomes, including the development of adverse events, serious events (including urinary sepsis and death), health‐related quality of life (based on the EQ‐5D questionnaire), and economic outcomes.
An outcome of interest that was included in the trials of standard catheters was catheter‐related infection. In addition, one small Japanese trial of antiseptic‐coated catheters reported data for: catheter‐associated pain, urethral discharge and catheter‐related hypersensitivity or allergy (Takeuchi 1993). The six trials that randomised participants to two different types of standard catheters (Chene 1990; Kalambheti 1965;Nacey 1984; Nickel 1989; Talja 1990; Tidd 1976) investigated outcomes of catheter‐related infection or catheter‐related urethritis and urethral inflammatory reaction. One trial only reported an adverse effect, meatal stricture (Goodwin 1990).
Apart from variation in the definition of infection, the timing of the outcome measurement and duration of follow‐up was also diverse. Only six trials (Darouiche 1999; Johnson 1990; Liedberg 1990b; Maki 1997; Nickel 1989; Stensballe 2007) monitored catheter care violations. The method of obtaining urine specimens was varied. The majority of trials acquired samples from the catheter sampling port (Darouiche 1999; Johnson 1990; Liedberg 1990b; Lundeberg 1986; Maki 1998; Stensballe 2007; Takeuchi 1993). However, two trials used suprapubic puncture to obtain urine samples from participants (Verleyen 1999a; Verleyen 1999b), and one trial took specimens from the catheter urine bag, which could have resulted in contamination (Liedberg 1990a).
Economic outcomes
An economic analysis was performed in two trials. Karchmer 2000 calculated the total catheter‐related costs by summing the cost of infections and the cost of catheters and their components, and cost savings were estimated by subtracting the total catheter‐related cost for silver‐coated catheters from the total cost for uncoated catheters. The analysis included an estimation of both a lower and higher approximation of costs. Pickard 2012 measured cost‐effectiveness using a decision‐analytical model, which compared three types of catheters in terms of both UK NHS costs, and quality‐adjusted life‐years (QALYs), based on responses to the EQ‐5D questionnaire.
Excluded studies
We excluded twenty‐eight reports (Andersson 1986; Bach 1990; Bologna 1999; Britt 1977; Cleland 1971; Day 2003; Domurath 2011; Erickson 2008; Ghoreishi 2003; Grocela 2010; Hakvoort 2011; Hart 1981; Lee 1996; Leone 2003; Leone 2007; Leriche 2006; Litherland 2007; Nakada 1996; Newton 2002; Pachler 1998; Ratahi 2005; Rigini 2006; Sallami 2011; Schaeffer 1988; Shafik 1993; Sun 2008; Teare 1992; Witjes 2008). The reasons for exclusion are listed in the table Characteristics of excluded studies.
Risk of bias in included studies
Details of the risk of bias assessment of the individual trials are mentioned in the Characteristics of included studies table. The results are graphically illustrated in Figure 2 and Figure 3. The results are summarised below.
2.
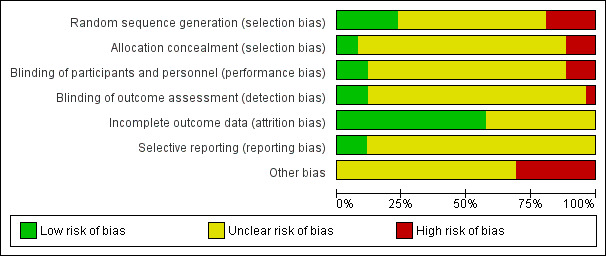
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
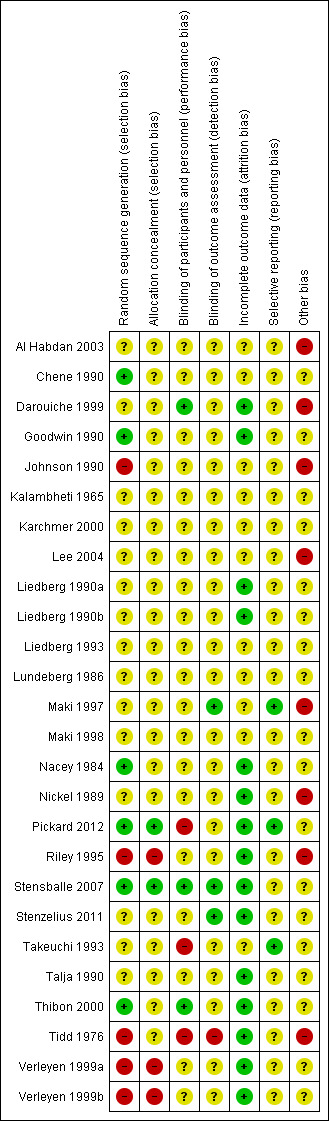
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We considered randomisation with allocation concealment to be adequate in six trials and judged it to be at low risk of bias (Chene 1990; Goodwin 1990; Nacey 1984; Pickard 2012; Stensballe 2007; Thibon 2000).
We judged the method of allocation concealment to be unclear in 15 trials (Al Habdan 2003; Darouiche 1999; Kalambheti 1965; Karchmer 2000; Lee 2004; Liedberg 1990a; Liedberg 1990b; Liedberg 1993; Lundeberg 1986; Maki 1998; Maki 1997; Nickel 1989; Stenzelius 2011; Takeuchi 1993; Talja 1990).
We found the method of allocation concealment to be inadequate in a further five trials and judged these at high risk of bias (Johnson 1990; Riley 1995; Tidd 1976; Verleyen 1999a; Verleyen 1999b).
Concealment of allocation
We considered allocation to be adequately concealed in two trials and judged these to be at low risk of bias (Pickard 2012; Stensballe 2007).
There was insufficient information to permit judgement in 21 trials (Al Habdan 2003; Chene 1990; Darouiche 1999; Goodwin 1990; Johnson 1990; Kalambheti 1965; Karchmer 2000; Lee 2004; Liedberg 1990a; Liedberg 1990b; Liedberg 1993; Lundeberg 1986; Maki 1997; Maki 1998; Nacey 1984; Nickel 1989; Stenzelius 2011; Takeuchi 1993; Talja 1990; Thibon 2000; Tidd 1976).
We judged three trials to be at high risk of bias (Riley 1995; Verleyen 1999a; Verleyen 1999b).
Blinding
Blinding of participants and personnel (performance bias)
We considered blinding to be adequate in three of the included trials (Darouiche 1999; Stensballe 2007; Thibon 2000); it was achieved in one trial by identical packaging (Thibon 2000).
We deemed blinding to be unclear in 20 of the included trials (Al Habdan 2003; Chene 1990; Goodwin 1990; Johnson 1990; Kalambheti 1965; Karchmer 2000; Lee 2004; Liedberg 1990a; Liedberg 1990b; Liedberg 1993; Lundeberg 1986; Maki 1997; Maki 1998; Nacey 1984; Nickel 1989; Riley 1995; Stenzelius 2011; Talja 1990; Verleyen 1999a; Verleyen 1999b) and inadequate in three of the included trials (Pickard 2012; Takeuchi 1993; Tidd 1976), with two cases being due to differences in catheter appearance (Pickard 2012; Tidd 1976).
Blinding of outcome assessment (detection bias)
We considered blinding of outcome assessment to be adequate in three of the included trials (Maki 1997; Stensballe 2007; Stenzelius 2011). We deemed blinding of outcome assessors to be unclear in 22 of the included trials (Al Habdan 2003; Chene 1990; Darouiche 1999; Goodwin 1990; Johnson 1990; Kalambheti 1965; Karchmer 2000; Lee 2004; Liedberg 1990a; Liedberg 1990b; Liedberg 1993; Lundeberg 1986; Maki 1998; Nacey 1984; Nickel 1989; Pickard 2012; Riley 1995; Takeuchi 1993; Talja 1990; Thibon 2000; Verleyen 1999a; Verleyen 1999b) and inadequate in one trial (Tidd 1976).
Incomplete outcome data
Data was either complete or accounted for, if missing, in 15 of the included trials and judged to be at low risk of bias (Darouiche 1999; Goodwin 1990; Liedberg 1990a; Liedberg 1990b; Nacey 1984; Nickel 1989; Pickard 2012; Riley 1995; Stenzelius 2011; Stensballe 2007; Talja 1990; Thibon 2000; Tidd 1976; Verleyen 1999a; Verleyen 1999b). It was judged to be at unclear risk of bias for the following 11 trials (Al Habdan 2003; Chene 1990; Johnson 1990; Kalambheti 1965; Karchmer 2000; Lee 2004; Liedberg 1993; Lundeberg 1986; Maki 1997; Maki 1998; Takeuchi 1993).
Selective reporting
We considered three of the included trials to be at low risk of selective reporting (Maki 1997; Pickard 2012; Takeuchi 1993) and the remaining 23 trials to be at unclear risk (Al Habdan 2003; Chene 1990; Darouiche 1999; Goodwin 1990; Johnson 1990; Kalambheti 1965; Karchmer 2000; Lee 2004; Liedberg 1990a; Liedberg 1990b; Liedberg 1993; Lundeberg 1986; Maki 1998; Nacey 1984; Nickel 1989; Riley 1995; Stensballe 2007; Stenzelius 2011; Talja 1990; Thibon 2000; Tidd 1976; Verleyen 1999a; Verleyen 1999b).
Other potential sources of bias
The risk of other bias having occurred was unclear in 18 of the included trials (Chene 1990; Goodwin 1990; Kalambheti 1965; Karchmer 2000; Liedberg 1990a; Liedberg 1990b; Liedberg 1993; Lundeberg 1986; Maki 1998; Nacey 1984; Pickard 2012; Stensballe 2007; Stenzelius 2011; Takeuchi 1993; Talja 1990; Thibon 2000; Verleyen 1999a; Verleyen 1999b) and we deemed eight of the included trials to be at high risk (Al Habdan 2003; Darouiche 1999; Johnson 1990; Lee 2004; Maki 1997; Nickel 1989; Riley 1995; Tidd 1976).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
1. Antiseptic‐coated indwelling urethral catheters versus standard indwelling urethral catheters
There were three types of antiseptic catheters compared with a standard catheter: silver oxide, silver alloy, and noble metal alloy. The trials for each were analysed in three subgroups depending on the type of intervention catheter.
Silver oxide versus standard catheter
Three trials compared silver oxide‐coated catheters with a standard catheter (Johnson 1990; Riley 1995; Takeuchi 1993) enrolling a total of 1828 patients. The trials used different standard catheters as the comparison catheter. Johnson 1990 used an all‐silicone catheter as the standard catheter, while Riley 1995 used silicone‐coated latex as the comparison catheter. Takeuchi 1993 did not define the standard catheter used in the trial. Eighty per cent of participants in one of the trials received systemic antibiotics (the reasons were not stated in the trial) (Riley 1995). All trials included men and women admitted to surgical or medical wards, or both, although Riley had more women in the treatment group (451/745) than in the control group (285/564). In the Takeuchi 1993 trial all the participants had bacteriuria when the trial ended at nine days catheterisation.
Symptomatic CAUTI
None of the studies assessed the primary outcome CAUTI as an outcome. Instead, all studies measured bacteriuria as the primary effectiveness outcome.
Bacteriuria and other secondary outcomes
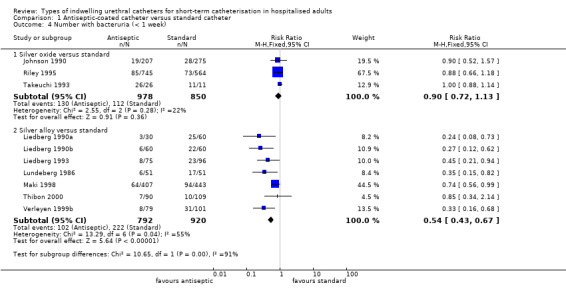
Pooling the results of all three trials using a fixed‐effect model did not provide enough evidence to show whether or not there was a reduction in risk of developing bacteriuria (RR 0.90, 95% CI 0.72 to 1.13, Analysis 1.3.1; all three trials measured this outcome at less than one week Analysis 1.4.1). There was no statistically significant difference in the number with bacteriuria between groups.
1.3. Analysis.
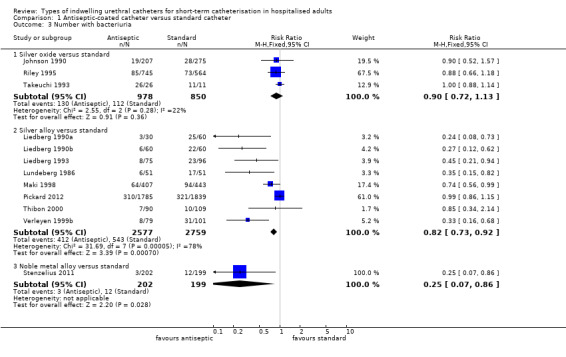
Comparison 1 Antiseptic‐coated catheter versus standard catheter, Outcome 3 Number with bacteriuria.
1.4. Analysis.
Comparison 1 Antiseptic‐coated catheter versus standard catheter, Outcome 4 Number with bacteruria (< 1 week).
Subgroup analysis
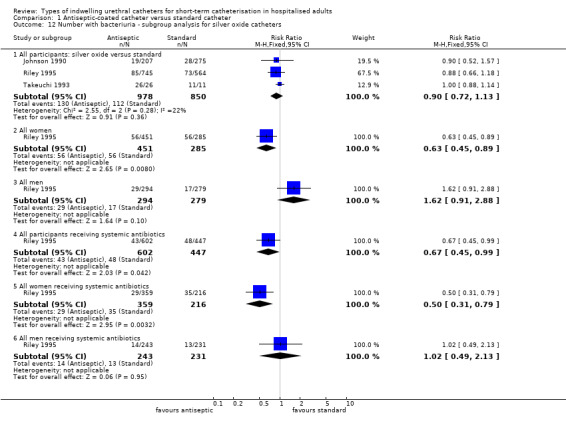
Subgroup analysis was possible in one trial (Riley 1995). For bacteriuria reported separately in women and men, there was a reduction of risk of almost one‐third with the silver oxide catheter for women (RR 0.63, 95% CI 0.45 to 0.89, Analysis 1.12.2), while for men there was not enough evidence to suggest whether or not there was a difference in risk with the standard catheter (RR 1.62, 95% CI 0.91 to 2.88, Analysis 1.12.3).
1.12. Analysis.
Comparison 1 Antiseptic‐coated catheter versus standard catheter, Outcome 12 Number with bacteriuria ‐ subgroup analysis for silver oxide catheters.
The trial reported separately on those participants commenced on antibiotics prior to catheterisation but did not state the reason for the antibiotics. Further subgroup analysis of all participants receiving systemic antibiotics indicated that combining antibiotics with silver oxide catheters may reduce the risk of bacteriuria (RR 0.67, 95% CI 0.45 to 0.99, Analysis 1.12.4). Further analysis of women and men separately who received systemic antibiotics suggested that women were protected from bacteriuria with silver oxide catheters (RR 0.50, 95% CI 0.31 to 0.79, Analysis 1.12.5), but there was not enough evidence either way for men (RR 1.02, 95% CI 0.49 to 2.13, Analysis 1.12.6).
Silver alloy versus standard catheter
Ten trials compared silver alloy catheter with a standard catheter (Karchmer 2000; Liedberg 1990a; Liedberg 1990b; Liedberg 1993; Lundeberg 1986; Maki 1998; Pickard 2012; Thibon 2000; Verleyen 1999a; Verleyen 1999b).
Most trials included both men and women except for one which included only men after radical prostatectomy (Verleyen 1999a), and two trials did not state information about the participants (Liedberg 1993; Lundeberg 1986).
Pickard 2012 was a three‐armed trial, comparing both silver alloy hydrogel‐coated latex catheter and nitrofurazone‐impregnated silicone catheter with a standard PTFE‐coated latex catheter (control), in hospitalised patients catheterised for 14 days or less. In the trial, 95% of patients were catheterised for perioperative monitoring purposes. The distribution of symptomatic CAUTI baseline risk factors was balanced across the study groups. 73% of patients received prophylactic antibiotics to cover the surgical procedure. The median duration of catheterisation was two days (interquartile range, one to three days).
Liedberg 1990a was a three‐armed trial comparing silver alloy catheters with two non‐antiseptic impregnated catheters; one defined in the trial only as a standard catheter and the other a hydrogel catheter (the results for both these standard catheter groups were combined in the meta‐analyses).
Maki 1998 also defined the usual care catheter as 'control' and did not provide any further details.
Liedberg 1990b compared the silver alloy catheter with a standard catheter defined as Teflonised latex Foley.
Verleyen 1999b used a silver alloy hydrogel catheter versus a latex catheter as the comparison standard catheter.
Two trials compared silver alloy versus a standard silicone catheter (Thibon 2000; Verleyen 1999a).
One trial compared the silver alloy hydrogel catheter versus a standard hydrogel‐coated catheter (Liedberg 1993).
Lundeberg did not define the standard catheter used as a comparison with the silver alloy catheters. Three trials monitored catheter care violations (Liedberg 1990b; Verleyen 1999a; Verleyen 1999b).
Karchmer 2000 compared silver alloy hydrogel‐coated latex catheter with a hydrogel‐coated latex standard catheter in a cluster randomised trial where hospital ward was the unit of randomisation. Data were not presented in a form suitable for meta‐analysis, therefore are reported in Other Data tables only Analysis 1.6.
1.6. Analysis.
Comparison 1 Antiseptic‐coated catheter versus standard catheter, Outcome 6 Cross‐over trial.
| Cross‐over trial | |||
|---|---|---|---|
| Study | Silver | Standard | Risk Ratio |
| Bacteriuria rate per 1000 patient days | |||
| Karchmer 2000 | 2.66 | 3.35 | 0.79, 95% CI 0.63 to 0.99 |
| Bacteriuria rate per 100 patients | |||
| Karchmer 2000 | 1.10 | 1.36 | 0.81, 95% CI 0.65 to 1.01 |
| Bacteriuria rate per 100 catheters | |||
| Karchmer 2000 | 2.13 | 3.12 | 0.68, 95% CI 0.54 to 0.86 |
Symptomatic CAUTI
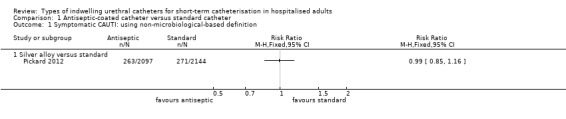
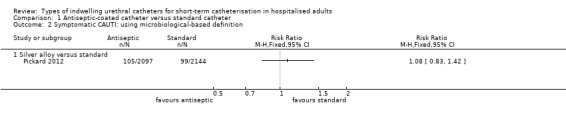
In terms of the primary review outcome, Pickard 2012 was the only trial which measured symptomatic CAUTI. The trial found no evidence that silver alloy‐coated catheters reduced symptomatic CAUTI risk, using either definitions (non‐microbiological‐based definition: 263/2097, 12.5% versus 271/2144, 12.6%; RR 0.99, 95% CI 0.85 to 1.16, Analysis 1.1.1; microbiological based definition: RR 1.08, 95% CI 0.83 to 1.42, Analysis 1.2.1).
1.1. Analysis.
Comparison 1 Antiseptic‐coated catheter versus standard catheter, Outcome 1 Symptomatic CAUTI: using non‐microbiological‐based definition.
1.2. Analysis.
Comparison 1 Antiseptic‐coated catheter versus standard catheter, Outcome 2 Symptomatic CAUTI: using microbiological‐based definition.
Bacteriuria and other secondary outcomes
For bacteriuria, nine trials measured the outcome (Liedberg 1990a; Liedberg 1990b; Liedberg 1993; Lundeberg 1986; Maki 1998; Pickard 2012; Thibon 2000; Verleyen 1999a; Verleyen 1999b). Eight trials defined ‘bacteriuria’ as ‘greater than 105 CFU/mL’, whilst Pickard 2012 defined it as ‘greater than 104 CFU/mL’. The timing of the outcome measurement varied considerably between trials. For the analysis, the outcome of bacteriuria was separated into two groups based on the time point of measurement: less than one week of catheterisation, and more than one week of catheterisation. Seven trials reported bacteriuria at less than one week (Liedberg 1990a; Liedberg 1990b; Liedberg 1993; Lundeberg 1986; Maki 1998; Thibon 2000; Verleyen 1999b). All the trials used a latex catheter as the control catheter except Thibon 2000 which used a silicone control catheter.
The results of the meta‐analysis on bacteriuria showed a slight reduction in bacteriuria achieved by silver alloy catheters (RR 0.82, 95% CI 0.73 to 0.92, Analysis 1.3.2).
For the seven trials which reported bacteriuria at less than one week, the slight reduction in bacteriuria achieved by silver alloy catheters was still significant (RR 0.54, 95% CI 0.43 to 0.67, Analysis 1.4.2).
For the outcome of bacteriuria after more than one week of catheterisation, four trials reported on this outcome (Liedberg 1993; Thibon 2000; Verleyen 1999a; Verleyen 1999b). Liedberg 1993 and Verleyen 1999b used a latex catheter as the control, whilst Verleyen 1999a and Thibon 2000 used a silicone control catheter. The pooled results showed a reduction in bacteriuria achieved by silver alloy catheters over standard catheters (RR 0.64, 95% CI 0.51 to 0.80, Analysis 1.5). Subgroup analysis showed that the reduction achieved by silver alloy was greater when latex was used as the control (RR 0.60, 95% CI 0.47 to 0.76, Analysis 1.5.1) compared with silicone as the control (RR 0.88, 95% CI 0.50 to 1.55, Analysis 1.5.2).
1.5. Analysis.
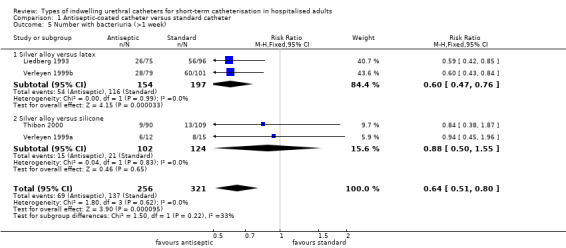
Comparison 1 Antiseptic‐coated catheter versus standard catheter, Outcome 5 Number with bacteriuria (>1 week).
The results of a cluster‐randomised cross‐over trial comparing silver alloy with standard (silicone) catheters (Karchmer 2000) were not included in the meta‐analyses because data were not available prior to crossover (Analysis 1.6), and also because of the heterogeneity of the outcome definition, which included patients with ‘bacteriuria or symptomatic or non‐symptomatic UTI’ (collectively defined as ‘≥ 105 cfu/m’). The results of the rate of bacteriuria per 1000 patient days were 2.66 versus 3.35 (RR 0.79, 95% CI 0.63 to 0.99), the rate of bacteriuria per 100 patients was 1.10 versus 1.36 (RR 0.81, 95% CI 0.65 to 1.01) and the rate of bacteriuria per 100 catheters was 2.13 versus 3.12 (RR 0.68, 95% CI 0.54 to 0.86) (Analysis 1.6).
The data from the cluster‐randomised cross‐over trial (Karchmer 2000) suggested that the sliver alloy catheter was better on two out of three outcome measures (Analysis 1.6).
Adverse effects
One trial (Pickard 2012) of silver alloy versus standard catheters reported on patient‐reported discomfort as a tertiary outcome. The results suggested that for the period whilst the catheter was in‐situ, silver alloy‐coated catheters were associated with less discomfort than standard catheters (RR 0.84, 95% CI 0.74 to 0.96) (Analysis 1.7.1). Although more people reported pain on removal of antiseptic catheters, this did not reach statistical significance (RR 1.08, 95% CI 0.97 to 1.20, Analysis 1.8).
1.7. Analysis.
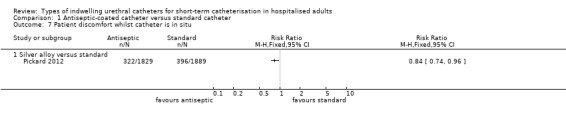
Comparison 1 Antiseptic‐coated catheter versus standard catheter, Outcome 7 Patient discomfort whilst catheter is in situ.
1.8. Analysis.
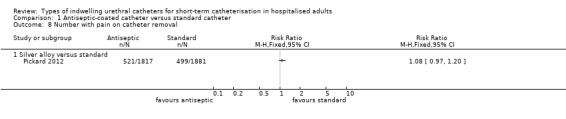
Comparison 1 Antiseptic‐coated catheter versus standard catheter, Outcome 8 Number with pain on catheter removal.
One trial of antiseptic catheters (silver oxide) versus standard catheters included secondary outcomes related to patient comfort and adverse effects of the catheters (Takeuchi 1993). They recorded outcome measurements for pain and urethral secretions. No statistically significant difference was found in either outcome but the CIs were wide:
reported urethral secretions (RR 0.72, 95% CI 0.25 to 2.03, Analysis 1.9.1);
patients reporting pain with catheters in place (RR 2.35, 95% CI 0.74 to 7.43, Analysis 1.10.1).
1.9. Analysis.
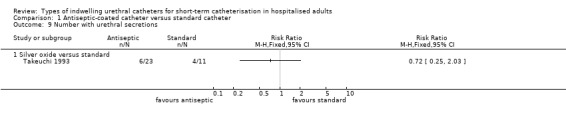
Comparison 1 Antiseptic‐coated catheter versus standard catheter, Outcome 9 Number with urethral secretions.
1.10. Analysis.
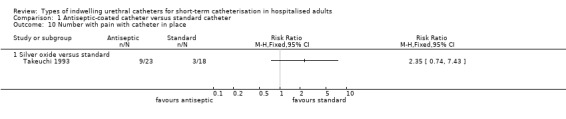
Comparison 1 Antiseptic‐coated catheter versus standard catheter, Outcome 10 Number with pain with catheter in place.
Economic outcomes
Two trials reported on economic outcomes (Karchmer 2000; Pickard 2012). Pickard 2012 undertook formal cost‐benefit analysis using a decision‐analytical model, comparing silver alloy‐coated catheters with standard PTFE‐coated catheters. The primary economic outcome was incremental cost per quality‐adjusted life‐year (QALY). Healthcare costs were estimated from UK National Health Service (NHS) sources with QALYs calculated from participant completion of the European Quality of Life‐5 Dimensions questionnaire (EQ‐5D). The analysis suggested that silver alloy catheters were unlikely to be cost‐effective for use within UK NHS hospitals at all incremental cost‐effectiveness ratio (ICER) threshold values of between GBP 0 to GBP 50,000. Karchmer 2000 reported that for the duration of the trial (one year) silver alloy hydrogel catheter usage resulted in a total estimated catheter‐related cost reduction of between 3.3% and 35.5%. This translated to savings of between USD 14,456 and USD 573,293.
Noble metal alloy versus standard catheter
One trial (Stenzelius 2011) compared a noble metal alloy‐coated (containing a mixture of gold, palladium and silver alloy) latex catheter versus standard silicone catheters in patients undergoing elective orthopaedic surgery (n = 439 patients). The outcomes measured were bacteriuria and urinary symptoms. The distribution of symptomatic CAUTI risk factors was balanced across both groups. Ninety‐three per cent of patients received prophylactic antibiotics to cover the surgical procedure. The median duration of catheterisation was two days (range 0 to 16 days).
The trialist did not report the primary outcome, symptomatic CAUTI. The trial found a significant reduction in bacteriuria achieved by the metal alloy‐coated latex catheter compared with control (RR 0.25, 95% CI 0.07 to 0.86, Analysis 1.3.3). There were no significant differences in urinary symptoms, either during the period while the catheter remained in situ or within 7 to 10 days following catheter removal between the two groups (RR 0.96, 95% CI 0.67 to 1.38, Analysis 1.11.1). None of the patients who developed bacteriuria complained of any adverse urinary symptoms.
1.11. Analysis.
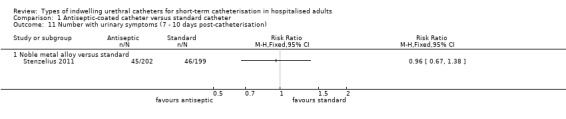
Comparison 1 Antiseptic‐coated catheter versus standard catheter, Outcome 11 Number with urinary symptoms (7 ‐ 10 days post‐catheterisation).
2. Antimicrobial‐impregnated indwelling urethral catheters versus standard indwelling urethral catheters
Two types of antimicrobial‐impregnated catheters were compared with a standard catheter: i) minocycline and rifampicin combined; or ii) nitrofurazone. The trials for each were analysed in two subgroups, depending on the type of intervention catheter, and grouped into separate outcomes, dependent upon duration of catheterisation, if this was reported.
Nitrofurazone versus standard
Five trials compared nitrofurazone‐impregnated catheters with standard catheters (Al Habdan 2003; Lee 2004; Maki 1997; Pickard 2012; Stensballe 2007). Three types of standard catheter were used.
Al Habdan 2003 used a latex catheter as the standard catheter whilst Lee 2004 and Stensballe 2007 both used silicone catheters as the comparison.
Pickard 2012 used a PTFE‐coated latex catheter as the control.
One study did not report which catheter they used as a comparator (Maki 1997).
All of the patients in one trial received prophylactic antibiotics pre‐ and postoperatively (Al Habdan 2003), three trials recorded antibiotic use (Maki 1997; Pickard 2012; Stensballe 2007), whilst two others did not record antibiotic use (Lee 2004; Liedberg 1993).
Only three of the trials adequately described the trial participants, and included both men and women (Lee 2004; Pickard 2012; Stensballe 2007). However, Lee 2004 included fewer women in the treatment group compared to the control (23 versus 40) and more men in the treatment group than the control (69 versus 45); the reasons for this were not stated in the trial. In Pickard 2012, the ratio of women to men (62%) was balanced across all arms.
Symptomatic CAUTI
In terms of the primary review outcome, Pickard 2012 was the only study which measured symptomatic CAUTI. The median duration of catheterisation was two days (interquartile range, one to three days). For the outcome of symptomatic CAUTI using a non‐microbiological‐based definition, people using nitrofurazone catheters had a slight but statistically significant lower chance of having a UTI compared with standard catheters (228/2153, 10.6% versus 271/2144, 12.6%; RR 0.84, 95% CI 0.71 to 0.99; Analysis 2.1.1). For the outcome of symptomatic CAUTI using a microbiological‐based definition, nitrofurazone catheters achieved a slightly higher reduction against standard catheters (RR 0.69, 95% CI 0.51 to 0.94; Analysis 2.2.1).
2.1. Analysis.
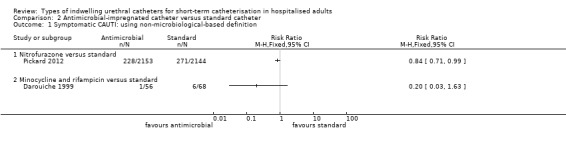
Comparison 2 Antimicrobial‐impregnated catheter versus standard catheter, Outcome 1 Symptomatic CAUTI: using non‐microbiological‐based definition.
2.2. Analysis.
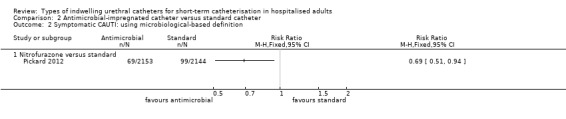
Comparison 2 Antimicrobial‐impregnated catheter versus standard catheter, Outcome 2 Symptomatic CAUTI: using microbiological‐based definition.
Bacteriuria and other outcomes
For the outcome of bacteriuria, all five trials reported it as an outcome. All but one trial (Pickard 2012) defined bacteriuria as ‘≥ 103 cfu/mL’; Pickard 2012 defined it as ‘≥ 104 cfu/mL’. Stensballe 2007 also included funguria as an outcome.
The trials differed in their timing of the outcome measurement. Three trials investigated the outcomes at less than one week (Lee 2004; Maki 1997; Stensballe 2007). Pickard 2012 did not report bacteriuria at different time periods, but since the majority of patients were catheterised for less than one week (median duration of catheterisation two days, interquartile range one to three days), the outcome for bacteriuria for the study was grouped under ‘less than one week’. Results were pooled using a fixed‐effect model and indicated that at less than one week of catheterisation, the risk of bacteriuria was statistically significantly reduced in the nitrofurazone impregnated catheter group (RR 0.73, 95% CI 0.64 to 0.85, Analysis 2.3.1).
2.3. Analysis.
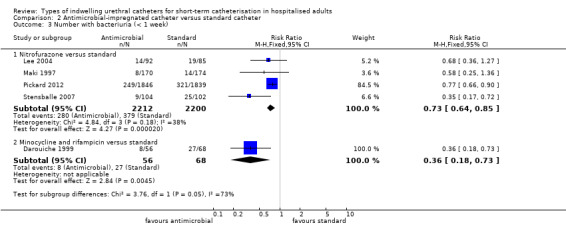
Comparison 2 Antimicrobial‐impregnated catheter versus standard catheter, Outcome 3 Number with bacteriuria (< 1 week).
For the outcome of bacteriuria at more than one week, only one study measured this outcome (Al Habdan 2003). The benefit from nitrofurazone impregnated catheters in preventing bacteriuria at more than one week was inconclusive, due to the small number of patients and relatively few events resulting in wide CIs (RR 0.08, 95% CI 0.00 to 1.33, Analysis 2.4.1)
2.4. Analysis.
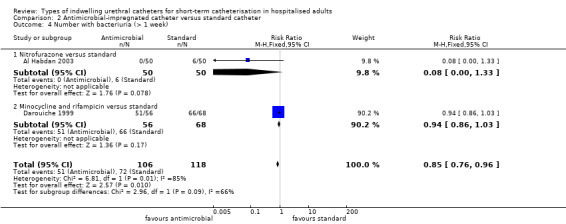
Comparison 2 Antimicrobial‐impregnated catheter versus standard catheter, Outcome 4 Number with bacteriuria (> 1 week).
Adverse effects
For adverse effects, one trial compared nitrofurazone catheters versus standard catheters in terms of patient‐reported discomfort as a tertiary outcome (Pickard 2012). The results suggested that for the period whilst the catheter was in place, and on removal, nitrofurazone catheters were associated with more discomfort, with more patients complaining of discomfort compared with standard catheters (RR 1.26, 95% CI 1.12 to 1.41 (Analysis 2.5.1); and RR 1.43, 95% CI 1.30 to 1.57 (Analysis 2.6.1) respectively).
2.5. Analysis.
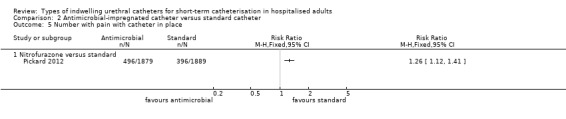
Comparison 2 Antimicrobial‐impregnated catheter versus standard catheter, Outcome 5 Number with pain with catheter in place.
2.6. Analysis.
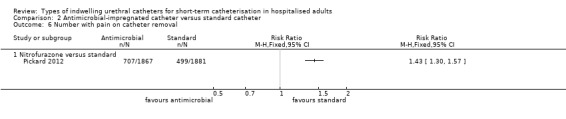
Comparison 2 Antimicrobial‐impregnated catheter versus standard catheter, Outcome 6 Number with pain on catheter removal.
Economic outcomes
One trial reported on economic outcomes comparing nitrofurazone catheters with standard PTFE‐coated catheters (Pickard 2012). A formal cost‐benefit analysis using a decision‐analytical model was undertaken. The primary economic outcome was incremental cost per quality‐adjusted life‐year (QALY). Healthcare costs were estimated from UK NHS sources with QALYs calculated from participant completion of the European Quality of Life‐5 Dimensions questionnaire (EQ‐5D). The analysis suggested that nitrofurazone catheters could potentially be cost‐saving, with an 84% probability that the incremental cost per QALY would be under GBP 30,000 (i.e. the willingness‐to‐pay threshold typically used in the UK).
Minocycline and rifampicin versus standard catheters
Only one small trial (n = 124) compared a minocycline and rifampicin‐impregnated catheter with a standard catheter (Darouiche 1999). This trial included men after radical prostatectomy for prostate cancer and compared a silicone catheter impregnated with minocycline and rifampicin with a standard (silicone) catheter (Darouiche 1999). Outcome measures included bacteriuria (greater than 104 colony forming units per mL) at days three, seven and 14, and symptomatic UTI (timing not stated) as defined by the healthcare provider (Darouiche 1999).
One of 56 men in the antimicrobial‐impregnated catheter group had symptomatic UTI compared with six of 68 men in the control group (RR 0.20, 95% CI 0.03 to 1.63, Analysis 2.1.2). For bacteriuria, at less than one week, the risk was about two‐thirds lower in the antimicrobial‐impregnated catheter group (RR 0.36, 95% CI 0.18 to 0.73, Analysis 2.3.2); however, at greater than one week the evidence was inconclusive (RR 0.94, 95% CI 0.86 to 1.03, Analysis 2.4.2).
3. Antimicrobial‐impregnated indwelling urethral catheters versus antiseptic‐coated indwelling urethral catheters
Only one trial addressed this comparison. Pickard 2012 was a three‐armed trial, comparing both silver alloy hydrogel‐coated latex catheter and nitrofurazone‐impregnated silicone catheter with a standard PTFE‐coated latex catheter (control), in hospitalised patients catheterised for 14 days or less. Although the study did not directly report on the comparison of antimicrobial (i.e. nitrofurazone) versus antiseptic (i.e. silver alloy) catheters, data from the trial were available to perform this direct comparison.
Symptomatic CAUTI
For the primary review outcome of symptomatic CAUTI, using a non‐microbiological‐based definition, people were less likely to have a UTI with nitrofurazone catheters (228/2153, 10.6%) compared with silver alloy (263/2097, 12.5%), but this was of borderline statistical significance (RR 0.84, 95% CI 0.71 to 1.00; Analysis 3.1.1). For the outcome of symptomatic CAUTI using a microbiological‐based definition, this difference was statistically significant in favour of nitrofurazone catheters versus silver alloy catheters (RR 0.64, 95% CI 0.48 to 0.86; Analysis 3.2.1).
3.1. Analysis.
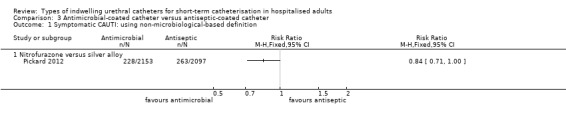
Comparison 3 Antimicrobial‐coated catheter versus antiseptic‐coated catheter, Outcome 1 Symptomatic CAUTI: using non‐microbiological‐based definition.
3.2. Analysis.
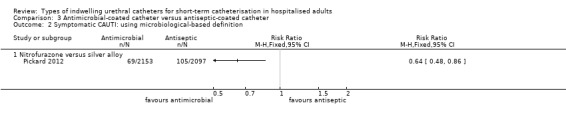
Comparison 3 Antimicrobial‐coated catheter versus antiseptic‐coated catheter, Outcome 2 Symptomatic CAUTI: using microbiological‐based definition.
Bacteriuria and other outcomes
For the outcome of bacteriuria, data from Pickard 2012 showed that people also had significantly less bacteriuria with nitrofurazone catheters compared with silver alloy catheters (RR 0.78, 95% CI 0.67 to 0.91, Analysis 3.3.1).
3.3. Analysis.
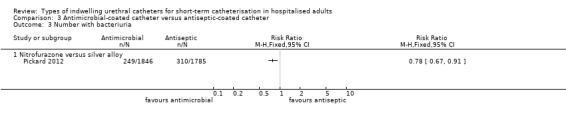
Comparison 3 Antimicrobial‐coated catheter versus antiseptic‐coated catheter, Outcome 3 Number with bacteriuria.
Adverse effects
For adverse effects, one trial (Pickard 2012) measured the incidence of any discomfort reported by patients, for patients catheterised with nitrofurazone catheters and silver alloy catheters.
For the period whilst the catheter was in‐situ, and on removal, nitrofurazone catheters were associated with more discomfort, with more patients complaining of discomfort while the catheter was in place (RR 1.50, 95% CI 1.32 to 1.70, Analysis 3.4.1); and on removal RR 1.32, 95% CI 1.20 to 1.45, Analysis 3.5.1) compared with silver alloy catheters.
3.4. Analysis.
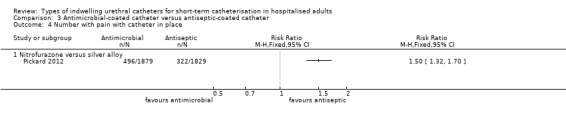
Comparison 3 Antimicrobial‐coated catheter versus antiseptic‐coated catheter, Outcome 4 Number with pain with catheter in place.
3.5. Analysis.
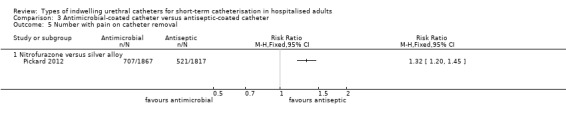
Comparison 3 Antimicrobial‐coated catheter versus antiseptic‐coated catheter, Outcome 5 Number with pain on catheter removal.
Economic outcomes
One trial reported on economic outcomes indirectly comparing nitrofurazone catheters with silver alloy catheters, with both types of catheters being compared directly with standard catheters (Pickard 2012). The methods and results have been described in earlier sections, under the main comparisons of antiseptic‐coated catheters versus standard catheters, and antimicrobial‐impregnated catheters versus standard catheters.
4. One type of standard indwelling urethral catheter versus another type of standard indwelling urethral catheter
Seven trials compared one type of standard catheter with another (Chene 1990; Goodwin 1990; Kalambheti 1965; Nacey 1984; Nickel 1989; Talja 1990; Tidd 1976).
Three trials using different outcome measurements compared two types of standard catheters to investigate infection (Chene 1990; Nickel 1989; Tidd 1976). The trials were not combined. All three trials compared different types of standard catheters.
The Nickel 1989 trial compared silicone with latex catheters, using the outcome asymptomatic bacteriuria (defined as greater than 106 colony forming units per mLL), with the final measurement recorded 96 hours postcatheterisation.
The Tidd 1976 trial compared three types of standard catheters: hydrophilic polymer‐coated latex, uncoated latex and PVC indwelling catheters. The outcome of interest was UTI defined as asymptomatic bacteriuria 103 per mL colony forming units. The final outcome measurement was recorded at day five to six postcatheterisation.
The Chene 1990 trial compared hydrogel with silicone catheters and the outcome measurement was asymptomatic bacteriuria.
In a further small trial, Goodwin 1990 compared a latex three‐way catheter (size 22G) with a PVC three‐way catheter (size 22G).
Three further trials compared different types of standard catheters to investigate urethral side effects in men (Kalambheti 1965; Nacey 1984; Talja 1990). The outcome measurements differed in all three trials.
The Kalambheti 1965 trial compared silicone with non‐silicone (not defined further) catheters with an outcome measurement of reported burning sensation in the urethra.
The Nacey 1984 trial compared silicone with latex catheters and the outcome was urethritis as measured by swabs of urethral discharge.
The Talja 1990 trial compared three types of standard catheters: hydrogel‐coated latex, siliconised latex and full silicone. The outcome of interest was urethral reaction measured from cytological urethral swab specimens using scanning electron microscopic analysis.
Symptomatic CAUTI
None of the trials reported the primary outcome.
Bacteriuria and other outcomes
One trial (Nickel 1989) found no evidence of difference in the risk of bacteriuria between two standard catheters, but they had wide CIs (RR 1.07, 95% CI 0.23 to 5.01, Analysis 4.1.1).
4.1. Analysis.
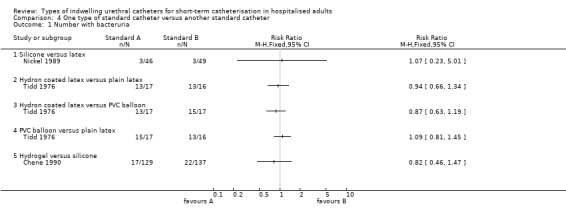
Comparison 4 One type of standard catheter versus another standard catheter, Outcome 1 Number with bacteruria.
The evidence from anther small trial (Tidd 1976) was insufficient to detect a difference in the risk of infection between any of the three standard catheters compared, with wide CIs: hydron‐coated latex versus plain latex (RR 0.94, 95% CI 0.66 to 1.34, Analysis 4.1.2); hydron‐coated latex versus PVC balloon (RR 0.87, 95% CI 0.63 to 1.19, Analysis 4.1.3); PVC balloon versus plain latex (RR 1.09, 95% CI 0.81 to 1.45, Analysis 4.1.4). This trial also had methodologically flawed randomisation in some cases.
The results in a further trial (Chene 1990) also had insufficient evidence to say whether or not there was a reduced risk of infection between the two standard catheters (RR 0.82, 95% CI 0.46 to 1.47, Analysis 4.1.5).
Adverse effects
In one trial, (Kalambheti 1965), results using a fixed‐effect model found that the risk of a burning sensation in the urethra was less in the silicone catheter group than the non‐silicone group (RR 0.28, 95% CI 0.13 to 0.60, Analysis 4.3.1).
4.3. Analysis.
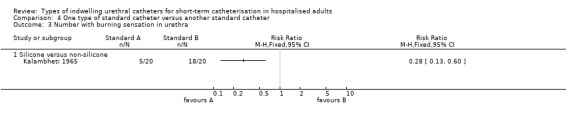
Comparison 4 One type of standard catheter versus another standard catheter, Outcome 3 Number with burning sensation in urethra.
In Nacey 1984, there were fewer cases of urethritis with a silicone catheter (1/50 versus 11/50; RR 0.09, 95% CI 0.01 to 0.68, Analysis 4.4.1).
4.4. Analysis.
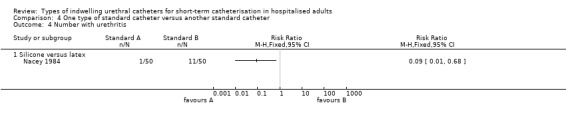
Comparison 4 One type of standard catheter versus another standard catheter, Outcome 4 Number with urethritis.
In Talja 1990, results using a fixed‐effect model in the comparison of hydrogel‐coated latex versus siliconised latex indicated no difference in urethral reaction (MD 0.00, 95% CI ‐3.51 to 3.51, Analysis 4.2.1). Results of the comparison of full silicone versus hydrogel‐coated latex and siliconised latex found that in both comparisons the risk of urethral reaction was less with a full silicone catheter: full silicone versus hydrogel‐coated latex (MD ‐16.00, 95% CI ‐18.84 to ‐13.16, Analysis 4.2.2); and full silicone versus siliconised latex (MD ‐16.00, 95% CI ‐18.96 to ‐13.04, Analysis 4.2.3).
4.2. Analysis.
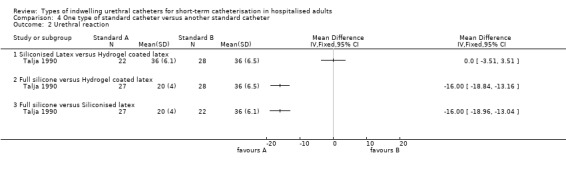
Comparison 4 One type of standard catheter versus another standard catheter, Outcome 2 Urethral reaction.
In a fourth small trial (Goodwin 1990), only one person in each group had meatal stricture (Analysis 4.5.1).
4.5. Analysis.
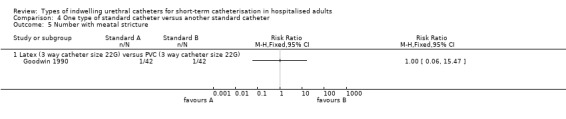
Comparison 4 One type of standard catheter versus another standard catheter, Outcome 5 Number with meatal stricture.
Economic outcomes
None of the trials reported economic outcomes.
5. One type of antiseptic‐coated indwelling urethral catheter versus another type of antiseptic‐coated indwelling urethral catheter
No trials were found that addressed this comparison.
6. One type of antimicrobial‐impregnated indwelling urethral catheter versus another type of antimicrobial‐impregnated indwelling urethral catheter
No trials were found that addressed this comparison.
Discussion
Summary of main results
This systematic review identified 26 eligible trials that addressed four of the six prestated hypotheses. In addition, three ongoing studies were identified (NCT00482547 2007; NCT01681511 2012; NCT02198833 2014). One trial compared an antiseptic catheter with an antibiotic catheter (Pickard 2012). No trials were identified that compared one type of antiseptic catheter with another type of antiseptic catheter, or one type of antibiotic catheter with another type of antibiotic catheter. Short‐term is defined as a duration of catheterisation which was intended to be less than 14 days.
Antiseptic‐coated indwelling urethral catheters versus standard indwelling urethral catheters.
This comparison included twelve trials that randomised a total of 8317 hospitalised adults (4133 catheterised with antiseptic catheters and 4184 with standard catheters) and one cross‐over trial that randomised hospital wards of 27,878 hospitalised adults. There were three types of antiseptic catheters: silver oxide, silver alloy and noble metal alloy (composed of a mixture of gold, palladium and silver alloy). Most of the trials included in the pooled analysis were small, with only three trials stating they used a power calculation (Pickard 2012; Stenzelius 2011; Thibon 2000).
For silver oxide (antiseptic‐coated) catheters, none of the included three trials measured the prespecified primary outcome i.e. symptomatic CAUTI. The catheters were not found to prevent bacteriuria in short‐term catheterised hospitalised adults in the three trials included in the analysis. Subgroup analysis by gender in one trial did suggest that women are less likely to develop bacteriuria if they use silver oxide catheters, whereas the evidence for men was inconclusive (Riley 1995). The same trial also suggested that systemic antibiotic use also decreased the rate of bacteriuria in the silver oxide group, particularly in women. These subgroup analyses should be interpreted cautiously, particularly when the overall result suggests no difference. However, silver oxide catheters are no longer manufactured and therefore these data are no longer clinically relevant.
For silver alloy (antiseptic‐coated) catheters, out of the 10 included studies, only one trial measured the primary outcome (Pickard 2012), which did not find any statistically significant reduction in symptomatic CAUTI. This finding was based on two different definitions of symptomatic CAUTI. In both instances, the experimental catheters did not demonstrate any benefit. The study was a well designed and robust RCT, specifically designed to determine the effectiveness of silver alloy catheters in comparison with standard catheters as used in UK National Health Service (NHS) hospitals. Although previous studies (including the present review) found a slight but statistically significant reduction in bacteriuria from the pooled analysis of nine studies (RR 0.83, 95% CI 0.75 to 0.92), the Pickard 2012 trial confirms that such a reduction is neither clinically significant nor meaningful because of the lack of impact on symptomatic UTI. However, the authors of this trial acknowledged that the trial reflected the way short‐term catheters were used in UK NHS hospitals, where the median duration of catheterisation for the entire cohort (n = 7102) was only two days.
To address the issue of whether duration of catheterisation had an effect on the outcome, the authors conducted a subgroup analysis, which showed that the risk of infection was not influenced by the duration of catheterisation. This demonstrates the importance of measuring outcomes which are clinically important and meaningful to patients in clinical trials, rather than surrogate outcomes such as bacteriuria.
Other limitations in the meta‐analysis of trials for the outcome of bacteriuria included clinical heterogeneity, such as diverse populations, use of antibiotics and differences in the standard catheter chosen as the comparison. Only one trial reported catheter care violations (Liedberg 1990b). The method of urine specimen collection also differed between the trials, from two trials using the gold standard method of suprapubic puncture (Verleyen 1999a; Verleyen 1999b), five using various methods to collect the specimen directly from the catheter and one trial collecting the specimen from the drainage bag, which increases the likelihood of bacterial contamination.
For noble metal alloy (antiseptic‐coated) catheters, one study (Stenzelius 2011) found a dramatic reduction in bacteriuria in comparison with standard silicone catheters (RR 0.27, 95% CI 0.08 to 0.95). Rather oddly, the primary outcome of the study was bacteriuria rather than symptomatic CAUTI, although there was an opportunity for the trialists to measure symptomatic UTI, considering they assessed for the presence of urinary symptoms. As such, the clinical significance of the findings remains uncertain.
Catheterised men and women generally develop bacteriuria in different ways due to their anatomical differences. Men are more likely to develop catheter‐related bacteriuria via the intraluminal route from a contaminated drainage bag, while in women contamination is more often transurethral when bacteria migrate from the periurethral region after faecal contamination. One major limitation of the silver alloy trials was that subgroup analysis was not possible by gender. Subgrouping of the results by duration of catheterisation was possible, however, and this indicated that the effect of silver alloy catheters on bacteriuria persisted beyond one week of catheterisation up to two weeks.
In terms of adverse effects or catheter‐related discomfort, only three trials assessed these outcomes on antiseptic catheters compared with standard catheters. Pickard 2012 found silver alloy catheters were no worse than standard catheters, whilst Stenzelius 2011 found no differences between noble metal alloy catheters and standard catheters. Although Takeuchi 1993 measured patient comfort and adverse effects related to silver oxide catheters, the trial was underpowered and poorly designed, and the methods for collecting the secondary outcome data were not described; this precluded any meaningful interpretation of the trial findings.
Economic evaluation
There have been no trials that investigated the issue of whether patients catheterised with antiseptic catheters may develop antimicrobial resistance. Two trials undertook economic evaluation. Karchmer 2000 was a large cluster‐randomised cross‐over trial which also included an economic analysis; the study found data in favour of silver alloy hydrogel catheters (Karchmer 2000). Silver alloy catheters were significantly more expensive than standard catheters. The cost estimates derived in the trial used both a low and high approximation of costs and calculated catheter‐related cost reduction of between 3.3% and 35.5%. The limitation of this trial was firstly, the randomisation of hospital wards rather than individual patients, and secondly, the risk of cross‐over of catheters leading to contamination between groups. (Pickard 2012 undertook formal economic analysis of silver alloy catheters versus standard catheters using an economic model. In the absence of any evidence of clinical effectiveness, the trial concluded that silver alloy catheters were unlikely to be cost‐effective for the UK NHS.
Elsewhere, outside this review, the issue of cost‐effectiveness of silver alloy catheters has also been covered by other studies, albeit in an indirect way. Plowman developed an illustrative model of the annual costs and benefits associated with the use of silver alloy catheters in hospitalised medical and surgical inpatients in NHS hospitals in England (Plowman 2001). The model suggested that a reduction in the incidence of UTI of 14.6% in catheterised medical patients and 11.4% in catheterised surgical patients would ensure that the cost of silver alloy catheters was the same as standard catheters. Any further reduction in incidence would then result in cost savings. In another study conducted in the USA, Saint 2000 developed a cost‐benefit decision model using a simulated cohort of 1000 hospitalised general medical, surgical, urologic and intensive care patients requiring short‐term catheterisation (two to ten days) comparing silver alloy with standard catheters (Saint 2000). The results were calculated using a relative risk reduction of bacteriuria with the use of silver alloy catheters of 25%. They calculated that the use of silver alloy catheters could lead to a 47% relative decrease in the incidence of symptomatic UTI (from 30 to 16 cases per 1000 patients), with a number needed to treat (NNT) of 74, as well as a relative decrease in resultant bacteraemia of 44% (from 4.5 to 2.5 cases), with a NNT of 500. Using a multivariate sensitivity analysis and Monte Carlo simulation, they indicated that silver alloy catheters could provide benefit in all cases and cost savings in 84% of cases. However, without any clinical benefit in terms of significant reductions in symptomatic CAUTI, it is hard to imagine how these potential economic benefits could have been achieved.
Antimicrobial‐impregnated indwelling urethral catheters versus standard indwelling urethral catheters
This comparison included the results of six trials that randomised a total of 5248 hospitalised adults (2625 catheterised with antimicrobial‐impregnated catheters and 2623 with standard catheters). There were two types of antimicrobial‐impregnated catheters: minocycline combined with rifampicin and nitrofurazone alone.
As with the antiseptic trials, the antimicrobial trials included in the pooled analysis were of small numbers of participants, with the exception of Pickard 2012. Only Pickard 2012 measured symptomatic CAUTI, whilst the rest measured bacteriuria as the main outcome measure.
Of the six studies, only one of these (Darouiche 1999) investigated minocycline‐ and rifampicin‐impregnated catheters compared with a standard catheter. This relatively small trial was not powered to detect differences in bacteriuria. It included a very limited population (men after radical prostatectomy) and therefore the benefit shown in reducing bacteriuria in those catheterised for less than a week may not be applicable to other groups of adult patients, particularly women. In fact, one of the inclusion criterion was sterile urine prior to catheterisation which would be unlikely to be found in more high risk groups. There was not enough evidence to show whether this difference persisted after the first week. Adverse effects, such as antimicrobial resistance to the catheters over time, were not investigated. Moreover, minocycline‐ and rifampicin‐impregnated catheters are no longer in clinical use and therefore these data are no longer clinically relevant.
There were five other antimicrobial trials that investigated nitrofurazone‐impregnated catheters against standard controls. Based on only one study (Pickard 2012), the review found that nitrofurazone catheters achieved a slight but statistically significant reduction in symptomatic CAUTI, depending on the definition of the outcome. For the outcome of bacteriuria, the pooled data from four studies also showed a significant reduction at one week. The data regarding duration of catheterisation beyond one week were inconclusive.
Of the four trials that investigated the effect of nitrofurazone‐impregnated catheters against standard catheters on bacteriuria at less than one week, three of these studies were well designed to minimise bias (Maki 1997; Pickard 2012; Stensballe 2007);Maki 1997 and Stensballe 2007 were relatively small trials. The other trial (Lee 2004) demonstrated bias in its selection criteria with an unequal distribution of men and women in the control and treatment groups.
In terms of catheter‐related discomfort, Pickard 2012 was the only study which assessed this outcome for nitrofurazone catheters in comparison with standard catheters. Up to 43% more patients who received the nitrofurazone catheter complained of catheter‐related discomfort for the period whilst the catheter was in situ and on catheter removal, compared with standard catheters. This finding is likely to be clinically relevant to patients, and is probably due to the different properties of the catheter material.
Taken together, these data indicate a small but statistically significant benefit regarding clinically relevant outcomes achieved by these catheters, whilst being associated with more discomfort.
Economic evaluation
Data regarding economic evaluation of nitrofurazone catheters were obtained from Pickard 2012, who undertook formal economic analysis of nitrofurazone catheters versus standard catheters using an economic model. Based on incremental cost per quality‐adjusted life‐year (QALY) calculated from the perspective of the UK NHS using self administered questionnaires, the study concluded that there was a high (84%) probability that the catheters could be cost‐effective within NHS hospitals, although this estimate was associated with some uncertainty. Part of the methodological problems related to the length of hospital stay, which appeared to be unbalanced within the study arms; in the majority of circumstances, duration of hospital stay was not related to the presence of the catheter or to CAUTI, but rather to underlying medical conditions or surgical procedures.
Antimicrobial‐impregnated indwelling urethral catheters versus antiseptic‐coated indwelling urethral catheters
Data for this comparison were provided by Pickard 2012, which compared nitrofurazone (antimicrobial‐impregnated) catheters and silver alloy (antiseptic‐coated) catheters against standard catheters in a three‐armed trial. The results showed that nitrofurazone catheters were superior to silver alloy catheters in reducing both symptomatic CAUTI and bacteriuria. However, up to 50% more patients who received nitrofurazone catheters complained of catheter‐related discomfort compared with those who received silver catheters. The potential clinical benefit of nitrofurazone catheters must be balanced against the likelihood that it will cause greater discomfort.
Economic evaluation
Pickard 2012 also performed formal economic evaluation of nitrofurazone catheters versus silver alloy catheters. Both catheters were compared with standard catheters, and the results suggested that silver alloy catheters were unlikely to be cost‐effective, whilst nitrofurazone catheters could potentially be cost‐effective for use in UK NHS hospitals.
One type of standard indwelling urethral catheter versus another type of standard indwelling urethral catheter
This comparison included six trials that randomised a total of 653 hospitalised adults to different types of standard catheters. Three small trials looked at the likelihood of infection between types of standard catheters. There were significant clinical differences between the trials, in terms of: comparison of different types of standard catheters, inclusion of different types of patients and variable outcome measurements. For these reasons, the data from these trials were not pooled. None of the trials, however, provided sufficient evidence to suggest whether any type of standard catheter was superior than another in terms of reducing the rate of bacteriuria.
Another three trials investigated adverse effects of standard catheters, in particular urethral side effects in men. As before, the trials were clinically heterogenous. All the trials included different outcome measures and the catheters compared were diverse. The results indicated that siliconised catheters were less likely to result in adverse urethral effects in men, but each outcome was addressed only in single small trials.
Overall completeness and applicability of evidence
The primary objective of this review was to determine the effectiveness of different types of indwelling urethral catheter in reducing the risk of UTI in adults who undergo short‐term urethral catheterisation in hospitals. Although 26 RCTs were included, only one trial ((Pickard 2012) measured symptomatic CAUTI using standardised definitions. The trial was designed to assess the catheters as they are used within UK NHS hospitals as short‐term catheters only. The majority of patients (95%) were patients undergoing elective surgery and were catheterised for perioperative monitoring purposes. As such, the study and review findings have to be interpreted accordingly.
It remains unknown if the effectiveness results regarding the experimental catheters would apply to longer‐term catheters, although the trial did not find any interaction between catheter duration and reduction of CAUTI risk. Equally, the impact of the catheters on different groups of patients, for instance those at high risk of developing CAUTIs (i.e. patients in Intensive Therapy Units, patients undergoing urological surgery, or patients with recurrent UTIs) remains uncertain.
In addition, none of the identified trials addressed the following potentially important question:
Is one type of antimicrobial‐impregnated indwelling urethral catheter better than another type of antimicrobial‐impregnated indwelling urethral catheter?
Consequently, further trials may be required to address the above uncertainties.
Quality of the evidence
We contacted content experts to identify critical outcomes relevant to patients undergoing short‐term urethral catheterisation that could be included in this systematic review. Subsequently, we identified five patients who had undergone urethral catheterisation and invited them to take part in a focus group to identify important outcomes from their perspective. The review authors believe that the current work demonstrates the importance of patient involvement when developing Cochrane reviews and for identifying critical outcomes in order to maximise their relevance (Omar 2013).
The assessment of the quality of the evidence was performed using GRADE, based on the four critical outcomes identified by this process: symptomatic CAUTI, patient discomfort whilst catheter is in‐situ, bacterial resistance towards the antimicrobial agent, and urinary sepsis (Omar 2013).
There was high quality evidence from one large trial (Pickard 2012) that silver alloy (antiseptic‐coated) catheters did not significantly reduce symptomatic CAUTI (RR 0.99, 95% CI 0.85 to 1.16). No trial reported CAUTI for silver oxide (antiseptic‐coated) catheters.
There was high quality evidence from the same trial (Pickard 2012) that nitrofurazone‐impregnated (antimicrobial‐impregnated) catheters achieved a slight but statistically significant reduction in symptomatic CAUTI compared with standard catheters (RR 0.84, 95% CI 0.71 to 0.99). No trial reported CAUTI for minocycline/rifampicin (antimicrobial‐impregnated) catheters.
The quality of evidence for the other outcomes is summarised in the following tables: Table 1; Table 2; Table 3; Summary of findings table 5; Summary of findings table 6; Table 4.
In general the risk of bias from the smaller trials was high due to lack of adequate reporting or use of standardised methods.
Potential biases in the review process
We searched all the important databases and imposed no language restriction in our search strategy. However, we were mindful that these databases might not have contained all the potentially eligible trials. We made every effort to ensure adherence to the methods as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Some of the authors of this review (TL and KG) were directly involved in the Catheter Trial (Pickard 2012) as co‐applicants. In order to minimise bias, the risk of bias assessment of Pickard 2012 and GRADE quality of evidence assessments were performed by other authors who were not involved with the trial (MO and EF).
Authors' conclusions
Implications for practice.
1. Are antiseptic‐coated indwelling urethral catheters better than standard indwelling urethral catheters?
The evidence suggests that silver alloy (antiseptic‐coated) catheters do not reduce symptomatic CAUTI, although they appear to reduce bacteruria, in the short‐term catheterised patient. With a few exceptions, the majority of trials dealing with this question were generally of poor quality.
2. Are antimicrobial‐impregnated indwelling urethral catheters better than standard indwelling urethral catheters?
The evidence suggests that antimicrobial‐impregnated catheters do reduce symptomatic CAUTI in hospitalised adults catheterised short‐term, although the margin of benefit appears to be small. They also reduce bacteriuria to a significant degree. However, they are associated with greater patient‐reported discomfort. Whilst these catheters may be cost‐effective, it remains unclear if the marginal benefits are clinically important. Some uncertainties also remain over how beneficial the catheters are beyond one week of catheterisation.
3. Are antimicrobial‐impregnated indwelling urethral catheters better than antiseptic‐coated indwelling urethral catheters?
The data from one well designed study indicated that antimicrobial catheters were more effective in reducing symptomatic CAUTIs than antiseptic‐coated catheters, and in reducing bacteriuria, in hospitalised adults catheterised short‐term. They were also more likely to be more cost‐effective than antiseptic‐coated catheters, although they were associated with more patient‐reported discomfort.
4. Is one type of standard indwelling urethral catheter better than another type of standard indwelling urethral catheter?
No standard catheter was found to be better than another in terms of reducing the risk of bacteriuria in hospitalised adults catheterised short‐term. Siliconised catheters may be less likely to cause urethral side effects in men, but this result should be interpreted with some caution as the trials were small and the outcome definitions and specific catheters compared differed.
5. Is one type of antiseptic‐coated indwelling urethral catheter better than another type of antiseptic‐coated indwelling urethral catheter?
None of the trials included in the review addressed this question.
6. Is one type of antimicrobial‐impregnated indwelling urethral catheter better than another type of antimicrobial‐impregnated indwelling urethral catheter?
None of the trials included in the review addressed this question.
Implications for research.
This review found no evidence which supports the use of antiseptic‐coated silver alloy‐coated catheters in reducing symptomatic CAUTI. There was some evidence which suggested that nitrofurazone (antimicrobial‐impregnated) catheters reduced symptomatic CAUTI, but the margin of benefit was small and such catheters were more uncomfortable for patients.
However, the following important questions and issues remain unresolved.
1. Need for standardised definitions for outcome measures in trials assessing CAUTIs
There is a distinct lack of consensus regarding the choice of outcomes which should be measured in trials, how they should be defined, how they should be measured in terms of the most appropriate measurement tools, time point of outcome measurement, and in their reporting. This leads to difficulty in systematically summarising or pooling the results of different trials, and renders the results of different trials incomparable. Another important knowledge gap is the lack of patient‐centred outcomes, such as satisfaction and discomfort/pain, measured in clinical trials. A core outcome set for trials of interventions for reducing symptomatic CAUTI, which encompasses the most important outcomes and which reflect the interests of patients and clinicians, should be developed (Gargon 2014).
2. Valuation of benefit
For the assessment of economic outcomes, there appear to be difficulties in capturing events which accurately reflect the true impact of interventions. For instance, assessment of health‐related QoL impact of experimental interventions, such as urethral catheterisation, which represents only a subsidiary part of overall care of patients undergoing more major intervention (e.g. major surgery), is fraught with difficulties, because any impact of the experimental intervention is likely to be masked by the greater impact of the major intervention. More precise and pragmatic methods of capturing any changes in well‐being specific to the subsidiary intervention, along with the resultant impact on costs, are needed.
3. Further exploration of antimicrobial devices
The exploration of different materials and antimicrobial agents constituting the catheter, or different ways or strategies of inhibiting the formation of catheter biofilms, remains appealing and deserves further research. New catheter materials are being introduced all the time, along with new ways of inhibiting bacterial biofilm formation within the catheter surface, such as the incorporation of drug‐eluting materials.
4. Alternative interventions to reduce CAUTI
Whilst much emphasis has been placed on innovative catheter designs (e.g. coating or impregnation with antiseptic or antimicrobial compounds), more pragmatic and intuitive strategies, based on minimising the incidence of catheterisations, shortening catheter duration, and using alternative techniques (e.g. intermittent self catheterisation catheters), warrant further research.
Feedback
Asymptomatic versus symptomatic UTI.
Summary
Basically it appears that there is an error in the data tables in that the same numbers appear in the symptomatic and asymptomatic groups. There is an assumption that a bacteria count of >105 CFU equates with symptomatic UTI rather than asymptomatic bacteriuria. The reference articles do not distinguish between asymptomatic bacteriuria and symptomatic bacteriuria but call both UTI. They do not look at indications for antibiotic use as these are all hospital patients who often receive antibiotics which could be prophylaxis or for other reasons.
The conclusions on silver catheters preventing symptomatic UTI have been arrived from analysing false data. There is insufficient evidence to claim that silver catheters reduce symptomatic UTI. Similarly it is odd that Maki's abstract is sufficient quality to be included for the UTI analysis but not the asymptomatic bacteriuria as none of these studies adequately distinguish between symptomatic and asymptomatic bacteriuria.
Reply
Addressed these issues within 2008 update (see published notes in Schumm 2008) .
Contributors
Katie Gillies and Thomas Lam.
What's new
| Date | Event | Description |
|---|---|---|
| 15 September 2014 | New citation required and conclusions have changed | Three new trials have been added (Goodwin 1990; Pickard 2012; Stenzelius 2011). Identified 3 ongoing trials (NCT00482547 2007; NCT01681511 2012; NCT02198833 2014). Risk of bias was re‐assessed on all the included trials in accordance with current methods. Conducted patient focus group for identifying the critical outcomes (Omar 2013) and applied GRADE for assessing the quality of evidence. |
| 15 September 2014 | New search has been performed | Three new trials have been added (Goodwin 1990; Pickard 2012; Stenzelius 2011). Identified 3 ongoing trials (NCT00482547 2007; NCT01681511 2012; NCT02198833 2014). Risk of bias was re‐assessed on all the included trials in accordance with current method. Conducted patient focus group for identifying the critical outcomes (Omar 2013) and applied GRADE for assessing the quality of evidence. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 26 July 2010 | New search has been performed | Updated review. No new studies added. Sub‐group analysis of silicone vs. silver alloy coated catheter conducted to address feedback. Other items from feedback incorporated elsewhere throughout review (Maki 1998; details of standard catheter changed to remove silicone from description). |
| 22 April 2008 | Amended | 1 study removed |
| 7 April 2008 | Amended | Converted to new review format. |
| 19 February 2008 | New citation required and conclusions have changed | Substantive amendment |
| 19 February 2008 | Amended | 5 new studies added |
| 19 February 2008 | Feedback has been incorporated | Comment on previous review regarding symptomatic UTI has been addressed and amended accordingly. |
Acknowledgements
This review is an updated version of the original review by Brosnahan 2004. We would like to acknowledge the substantial work of the original authors of this review
The review authors would like to acknowledge the participants who took part in a group discussion to identify critical outcomes from their perspective (Omar 2013).
Appendices
Appendix 1. Incontinence Group Specialised Register search terms
(({DESIGN.RCT} OR {DESIGN.CCT} ) AND ({INTVENT.MECH.CATHETER*} OR {INTVENT.SURG.POSTSURG*} OR {INTVENT.PREVENT.*} OR {INTVENT.SURG.INTRAOPERATIVE*})) Key: * = wildcard. (All searches were of the keywords field of Reference Manager 2012).
Data and analyses
Comparison 1. Antiseptic‐coated catheter versus standard catheter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic CAUTI: using non‐microbiological‐based definition | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Silver alloy versus standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Symptomatic CAUTI: using microbiological‐based definition | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Silver alloy versus standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with bacteriuria | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Silver oxide versus standard | 3 | 1828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 3.2 Silver alloy versus standard | 8 | 5336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.73, 0.92] |
| 3.3 Noble metal alloy versus standard | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.07, 0.86] |
| 4 Number with bacteruria (< 1 week) | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Silver oxide versus standard | 3 | 1828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 4.2 Silver alloy versus standard | 7 | 1712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.43, 0.67] |
| 5 Number with bacteriuria (>1 week) | 4 | 577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.51, 0.80] |
| 5.1 Silver alloy versus latex | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.47, 0.76] |
| 5.2 Silver alloy versus silicone | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.50, 1.55] |
| 6 Cross‐over trial | Other data | No numeric data | ||
| 6.1 Bacteriuria rate per 1000 patient days | Other data | No numeric data | ||
| 6.2 Bacteriuria rate per 100 patients | Other data | No numeric data | ||
| 6.3 Bacteriuria rate per 100 catheters | Other data | No numeric data | ||
| 7 Patient discomfort whilst catheter is in situ | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Silver alloy versus standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number with pain on catheter removal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Silver alloy versus standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Number with urethral secretions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Silver oxide versus standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number with pain with catheter in place | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Silver oxide versus standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Number with urinary symptoms (7 ‐ 10 days post‐catheterisation) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 Noble metal alloy versus standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Number with bacteriuria ‐ subgroup analysis for silver oxide catheters | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 All participants: silver oxide versus standard | 3 | 1828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 12.2 All women | 1 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.89] |
| 12.3 All men | 1 | 573 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.91, 2.88] |
| 12.4 All participants receiving systemic antibiotics | 1 | 1049 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.45, 0.99] |
| 12.5 All women receiving systemic antibiotics | 1 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.31, 0.79] |
| 12.6 All men receiving systemic antibiotics | 1 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.49, 2.13] |
Comparison 2. Antimicrobial‐impregnated catheter versus standard catheter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic CAUTI: using non‐microbiological‐based definition | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Nitrofurazone versus standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Minocycline and rifampicin versus standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Symptomatic CAUTI: using microbiological‐based definition | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Nitrofurazone versus standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with bacteriuria (< 1 week) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nitrofurazone versus standard | 4 | 4412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.64, 0.85] |
| 3.2 Minocycline and rifampicin versus standard | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.18, 0.73] |
| 4 Number with bacteriuria (> 1 week) | 2 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.76, 0.96] |
| 4.1 Nitrofurazone versus standard | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.33] |
| 4.2 Minocycline and rifampicin versus standard | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.86, 1.03] |
| 5 Number with pain with catheter in place | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Nitrofurazone versus standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number with pain on catheter removal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Nitrofurazone versus standard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Antimicrobial‐coated catheter versus antiseptic‐coated catheter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic CAUTI: using non‐microbiological‐based definition | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Nitrofurazone versus silver alloy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Symptomatic CAUTI: using microbiological‐based definition | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Nitrofurazone versus silver alloy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with bacteriuria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Nitrofurazone versus silver alloy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number with pain with catheter in place | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Nitrofurazone versus silver alloy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number with pain on catheter removal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Nitrofurazone versus silver alloy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. One type of standard catheter versus another standard catheter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with bacteruria | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Silicone versus latex | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Hydron coated latex versus plain latex | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Hydron coated latex versus PVC balloon | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 PVC balloon versus plain latex | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Hydrogel versus silicone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Urethral reaction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Siliconised Latex versus Hydrogel coated latex | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Full silicone versus Hydrogel coated latex | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Full silicone versus Siliconised latex | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with burning sensation in urethra | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Silicone versus non‐silicone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number with urethritis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Silicone versus latex | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number with meatal stricture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Latex (3 way catheter size 22G) versus PVC (3 way catheter size 22G) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al Habdan 2003.
| Methods | RCT | |
| Participants | n = 100 men and women Orthopaedic and trauma surgery ‐ postoperative catheterisation | |
| Interventions | I (50) Nitrofuroxone‐coated II (50) Silicone‐coated Foley catheter (control) | |
| Outcomes | Bacteriuria as defined as 100,000 cfu/mL I 0/50 II 6/50 | |
| Notes | Prophylactic antibiotics ‐ all patients given pre‐ and postop No inclusion or exclusion criteria Trial conducted in Saudi Arabia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Other bias | High risk | UTI was stated as outcome but bacteriuria was measured |
Chene 1990.
| Methods | RCT | |
| Participants | n= 266 men and women | |
| Interventions | I (129) Standard catheter (Hydrogel) II (137) Standard catheter (Silicone) | |
| Outcomes | UTI I 17/129 II 22/137 not statistically significant | |
| Notes | Analysis not ITT withdrawals = 24; 17 due to UTI at time of randomisation; 3 due to 'catheterised in inadequate manner'; 4 due to 'catheterisation was impossible with the catheter randomised to' Study conducted in neurological intensive care unit, France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | The report is in French with English abstract |
Darouiche 1999.
| Methods | RCT (double‐blind) Allocation by block randomisation | |
| Participants | n = 124 men Mean age 62 yrs Incl: Age > 35 yrs, prostate cancer requiring catheterisation during radical prostatectomy Excl: Allergy to tetracycline or rifampin, active UTI at time of surgery, dermatitis at site of catheter insertion, expected duration of catheterisation < 14 days, no informed consent | |
| Interventions | I (56) Silicone impregnated with minocycline and rifampin catheter II (68) Standard catheters (silicone). | |
| Outcomes | Bacteriuria as defined as 100,000 cfu/mL at >1 week : I 8/56, II 27/68; 2 weeks: I 51/56, 66/68 | |
| Notes | Not powered to investigate symptomatic UTI
Record application of local antimicrobials
Catheters held in place through a sutured safety button on the anterior abdominal wall
Catheter care violations monitored 48% in antimicrobial‐impregnated group and 51% in silicone group
Catheter specimens collected via needle aspiration Trial conducted in USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'Catheters assigned in a blind fashion' 'Catheter pouches removed from the box 1 at a time...' There is an assumption here however that both catheters look identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 121 excluded postrandomisation, therefore no cultures for these participants Reports pertaining to bacteriuria appear complete |
| Selective reporting (reporting bias) | Unclear risk | Primary/secondary outcomes not clearly specified |
| Other bias | High risk | Study was supported by Cook Urological |
Goodwin 1990.
| Methods | RCT | |
| Participants | Patients with benign prostatic hyperplasia (71) or prostatic carcinoma (13) | |
| Interventions | A (42): Latex three‐way catheter size 22G B (42): Polyvinyl chloride three‐way catheter size 22G Mean duration of catheterisation = 3 days |
|
| Outcomes | Meatal stricture at 24 weeks: A 1/42, B 1/42 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "......patients were randomised by drawing cards into two groups....." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not seen |
| Other bias | Unclear risk | Unclear risk |
Johnson 1990.
| Methods | RCT | |
| Participants | n = 482 women and men Mean age 49 Incl: Catheterised at least 24 hrs Excl: UTI at catheterisation, < 17 yrs, catheter removed < 24 hrs Specialties: ICU, neurology and surgery Length of catheterisation: mean of 3 days in silver and 4 in silicone, range 1 to 31 silver and 1 to 58 in silicone | |
| Interventions | I (207) Silver oxide‐coated catheters II (275) Standard catheters (fully‐siliconised) | |
| Outcomes | UTI as defined as 1000 cfu/mL: I 19/207, II 28/275; Women: I 0/93, II 5/74; Men: I 19/133, 23/182 | |
| Notes | Power calculation used to determine a sample size of 105 per group needed to detect 67% reduction in the incidence of UTI with silver oxide catheter at 5% significance and 80% power Specimen collection via catheter sampling port. Catheter care monitored, violations 56% silver group and 54% in silicone Trial conducted in USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by stocking supply carts on alternative weeks with intervention or control catheters |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although both catheters looked identical, it is unclear whether packages were identifiable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Other bias | High risk | Bacteriuria was defined as UTI; Study was supported by Baxter Pharmaseal Division. |
Kalambheti 1965.
| Methods | RCT | |
| Participants | n = 40 men post‐transurethral resection of the prostate Mean length of catheterisation silicone group 3.5 days and 3.4 days in the standard catheter group | |
| Interventions | I (20) Siliconised catheters II (20) Standard catheters (not defined) | |
| Outcomes | Adverse events: burning sensation in urethra: I 5/40, II 18/40; pus from urethra: I 7/40, II 18/40 | |
| Notes | No power calculation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
Karchmer 2000.
| Methods | Cross‐over trial with stratified randomisation Unit of randomisation: hospital ward or unit | |
| Participants | n = 27,878 men and women Incl: catheterised Excl: paediatrics, obstetrics, gynaecology and psychiatry | |
| Interventions | I (13945) Silver alloy hydrogel‐coated latex Foley catheters II (13933) Standard catheters (hydrogel‐coated latex Foley) | |
| Outcomes | Bacteriuria or symptomatic/nonsymptomatic UTI as defined as >/= 1,000,000 cfu/mL Infection rate per 1000 patient days: I 2.66, II 3.35 Infection rate per 100 patients: I 1.10, II 1.36 Infection rate per 100 catheters: I 2.13, II 3.12 | |
| Notes | Power calculation estimated a sample size of 29,184 hospital admissions necessary to detect 25 relative reduction in the rate of infection per 100 patients with silver catheters at 5% significance and 80% power Data from first arm of trial not available. Mean length of catheterisation 9 days prior to infection Catheter regimen or violations not reported Duration of follow‐up not reported Trial conducted in USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Exact method not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Outcome definition (CDC) appropriate (low risk of bias) Study and author were supported by C. R. Bard Inc (High risk of bias) |
Lee 2004.
| Methods | RCT | |
| Participants | n = 177 total (114 men and 63 women) Incl: > 18 years of age, catheterised for more than 24 hours Excl: Allergies, pregnancy, lactating, hospitalisation for more than 7 days, urinary diseases. Included but later excluded if positive urine culture before catheterisation or catheter removed | |
| Interventions | I (92) Nitrofurazone‐coated silicone II (85) Silicone | |
| Outcomes | Bacteriuria as defined as 10,000 cfu/mL I 14/92 II 19/85 | |
| Notes | Prophylactic antibiotic use Trial conducted in South Korea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Outcome defined as UTI but was actually bacteriuria. Study was supported by Pacific Pharmaceuticals |
Liedberg 1990a.
| Methods | RCT | |
| Participants | n = 90 men and women requiring catheterisation for haemodynamic monitoring or postoperative drainage Mean age 59 yrs Incl: > 18 yrs, catheterised > 5 days Excl: antibiotics, bacteruria at catheterisation, postinvasive urological procedure Length of catheterisation 6 days | |
| Interventions | I (30) Silver alloy catheters II (30) Standard catheters (hydrogel‐coated) III (30) Standard catheters (non‐coated) | |
| Outcomes | Bacteriuria as defined as 1,000,000 cfu/mL: I 3/30, II 10/30, III 15/30 | |
| Notes | No power calculation. Specimen collection via catheter drainage bag Catheter care regimen not monitored Trial conducted in Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified and reported as "The patients were randomised to receive either a silver alloy or hydrogel‐coated Foley catheter" |
| Allocation concealment (selection bias) | Unclear risk | Method of sequence generation not specified and reported as "The patients were randomised to receive either a silver alloy or hydrogel‐coated Foley catheter" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
Liedberg 1990b.
| Methods | RCT | |
| Participants | n = 120 women and men Mean age 50 Incl: > 18 yrs, Excl: Bacteriuria, antibiotics and invasive urological procedures prior to catheterisation, catheterised < 6 days Length of catheterisation 5 days | |
| Interventions | I (60) Silver alloy catheters II (60) Standard catheters (teflonised latex) | |
| Outcomes | Bacteriuria as defined as 1,000,000 cfu/mL: I 6/60, II 22/60 | |
| Notes | No power calculation Specimen collection via catheter sampling port Catheter violations monitored, 7 in silver group and 11 in latex Infecting organisms recorded Trial conducted in Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified and reported as "The patients were randomised to receive either a ..........." |
| Allocation concealment (selection bias) | Unclear risk | Method of sequence generation not specified and reported as "The patients were randomised to receive either a ..........." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
Liedberg 1993.
| Methods | RCT | |
| Participants | n = 171 patients men and women Incl: > 18 years of age, Abacteriuric, Not taking antibiotics, Not undergone invasive urinary tract procedures, catheterisation for at least 21 days | |
| Interventions | I (75) Silver‐coated Hydrogel II (96) Hydrogel‐coated | |
| Outcomes | Bacteriuria as defined as 1,000,000 cfu/mL: at 7 days I 8/75 II 23/96; at 14 days I 26/75 II 56/96 | |
| Notes | Published as abstract only Trial conducted in USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Other bias | Unclear risk | Outcome defined as bacteriuria (low risk), however other aspects of trial such as ethical approval and ITT analysis are not specified |
Lundeberg 1986.
| Methods | RCT (blinding unclear) | |
| Participants | n = 102 | |
| Interventions | I (51) silver‐coated catheter II (51) standard catheters | |
| Outcomes | Bacteriuria as defined as 100 cfu/mL: I 6/51, II 17/51 | |
| Notes | No power calculation noted Published as letter to the editor, unable to contact author for further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Other bias | Unclear risk | Bacteriuria measured and defined (low risk), however other aspects of trial such as ethical approval and ITT analysis are not specified |
Maki 1997.
| Methods | RCT | |
| Participants | n = 344 women and men Patients from Trauma, surgical procedures or urinary incontinence Incl: catheterised for at least 24 hours, >18 years of age, Catheter size: 16 Fr or 18 Fr Excl: Informed consent not obtained, pregnancy, allergies | |
| Interventions | I (170) Nitrofurazone‐coated silicone II (174) Standard silicone | |
| Outcomes | Bacteriuria as defined by greater than or equal to 10,000 cfu/mL: I 8/170, II 14/174 | |
| Notes | Unpublished report Trial conducted in USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding: Low risk Personnel blinding: High risk (person inserting catheter was aware of catheter type) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Low risk | Results reported consistent with study objectives |
| Other bias | High risk | Bacteriuria wrongly defined as UTI. This was a report prepared for the manufacturer |
Maki 1998.
| Methods | RCT | |
| Participants | n = 852 women and men | |
| Interventions | I (407) silver hydrogel‐coated catheters II (443 ) standard catheter | |
| Outcomes | UTI (definition not stated): I 64/407, II 94/443 | |
| Notes | Catheter specimens collected on insertion and then daily from the sampling port and collection bag Study published as abstract only Unable to contact trialists for further information on methods, participants and results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | 'Double‐blind' but no further information given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Other bias | Unclear risk | UTI definition not stated and other aspects of trial such as ethical approval and ITT analysis are not specified. Baseline comparability was favourable |
Nacey 1984.
| Methods | RCT | |
| Participants | n = 100 men postcardiac surgery Age range (20 to 73), Mean 54 Incl: > 18 yrs, male Excl: previous invasive urology procedure or surgery to lower urinary tract, Hx UTI, smaller calibre urethra Length of catheterisation 48 hours | |
| Interventions | I (50) Standard catheters (silicone) II (50) Standard catheters (latex) | |
| Outcomes | Urethritis as defined as penile discomfortor urethral discharge, or both: I 1/50, II 11/50 | |
| Notes | No power calculation Study conducted in New Zealand | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised using a "table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is reported that the catheters were identical, however, it is not specifically stated if the participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is reported that the catheters were identical, however, it is not specifically stated if the participants and personnel were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Conflict of interest of the authors not stated |
Nickel 1989.
| Methods | RCT | |
| Participants | n = 95 obstetric or urology men and women Median age 54 Excl: catheter removed within 24 hrs | |
| Interventions | I (46) Standard catheters (silicone) II (49) Standard catheters (latex) | |
| Outcomes | Bacteriuria as defined as 10,000,000 cfu/mL: I 3/46, II 3/49 | |
| Notes | No power calculation Specimen collection via catheter port and within 12 hours of catheter removal Catheter regimen monitored no violations noted Trial conducted in Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified and reported as "randomly selected obstetric and urologic patients......" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not specified and reported as "randomly selected obstetric and urologic patients......" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Rate of bacteriuria was not reported separately for each group and reported for all the participants (Table II) |
Pickard 2012.
| Methods | RCT | |
| Participants | Adults undergoing urethral catheterisation of </= 14 days duration in hospitals | |
| Interventions | A (2097) = Silver alloy hydrogel‐coated latex catheter; B (2153) = Nitrofurazone‐impregnated silicon catheter; C (2144) = Polytetrafluoroethylene‐ (PTFE) coated latex catheter |
|
| Outcomes | Symptomatic CAUTI; A 263/2097; B 228/2153; C 271/2144 Microbiologically confirmed symptomatic CAUTI: A 105/2097; B 69/2153; C 99/2144 Bacteruria (symptomatic and asymptomatic) at 3 days: A 310/1785; B 249/1846; C 321/1839 Asymptomatic bacteruria ? Urethral discomfort with catheter: A 322/1829; B 496/1879; C 395/1889 Urethral discomfort on catheter removal: A 521/1817; B 707/1867; C 499/1881 Economic outcomes |
|
| Notes | The Catheter Trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation using remote computer allocation" |
| Allocation concealment (selection bias) | Low risk | "Randomisation using remote computer allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Particpants, clinicians and the trial team were not blinded to the allocated intervention because of the distinctive appearances of each catheter..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Symptomatic UTI judged by symptoms and physician prescription; patient discomfort (participants were unaware about the type of catheter) Microbiological outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as specified in the trial protocol |
| Other bias | Unclear risk | Protocol was changed in 2008 as advised by the Data Monitoring Committee in light of higher than anticipated event rate of primary outcome (symptomatic CAUTI) |
Riley 1995.
| Methods | Randomised Clinical Trial | |
| Participants | n = 1309 men and women Median age 59 Excl: catheterised < 24 hrs, bacteriuric day 1, thoracic surgery Mean length of catheterisation 3.8 days Range 1 to 47 days | |
| Interventions | A (745) Silver oxide‐coated silicone catheters B (564) Standard catheters (silicone latex) | |
| Outcomes | Bacteriuria as defined as > 1000 cfu/mL: I 85/745, II 73/564 Antibiotic treatment: A 43/602, B 48/477; Women: A 56/451, B 56/285; Men: A 29/294, B 17/279 | |
| Notes | Power calculation used to determine a sample size of 686 per group needed to detect 33% reduction in the incidence of bacteriuria with silver oxide catheter at 5% significance and 80% power Specimen collection via catheter port via needle aspiration Significantly more women in the silver group. 166 catheter care violations recorded 96 in intervention group and 70 in control Trial conducted in the USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients were randomly assigned (by month of initial catheter insertion) to receive either a ........." |
| Allocation concealment (selection bias) | High risk | "Patients were randomly assigned (by month of initial catheter insertion) to receive either a ........." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | "... the sex distribution was uneven, with both a greater number and proportion of women in the silver oxide group" |
Stensballe 2007.
| Methods | RCT | |
| Participants | n = 212 men and women all trauma patients. Median age (I) 41 (II) 43 Duration of catheterisation < 7days (151), > 7days (41), >14days (15) Incl: > 18 years of age, admitted directly to trauma centre from accident Excl: HIV infection, preinjury corticosteroid treatment, pregnancy, primary burn injury, unattainable signed consent form | |
| Interventions | I (104) Nitrofurazone‐coated catheter II (102) Standard silicone catheter | |
| Outcomes | Catheter‐associated bacteriuria and funguria (CABF) as defined as >10000 cfu/mL: I (9/104), II (25/102). | |
| Notes | ITT Analysis Withdrawals: 5. 2 due to pregnancy, 2 < 18 years of age, 1 unable to attain informed consent Prophylactic antibiotics were given according to size and type of injury Catheter specimens were taken immediately after insertion and then daily until catheter removal Trial conducted in Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomisation list was computer‐generated, with a block size of 8 (Medstat, version 2.1; ASTRA Group A/S, Albertslund, Denmark), by a biostatistician who was independent of the investigators” |
| Allocation concealment (selection bias) | Low risk | "Two nurses from a team of nurses specially trained in the allocation and catheterization procedure performed the allocation by sequentially opening consecutively numbered, sealed randomisation envelopes that contained the name of the assigned catheter" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Catheterizing nurses were not formally blinded to catheter type, but both study catheters were new in the hospital. Thus, nurses caring for the patients did not know which of the new catheters was the nitrofurazone catheter. Patients were effectively blinded to their catheter assignment ......” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The main outcome was infection and the microbiologist was blinded "The microbiologist evaluating the urine cultures was blinded to the type of catheter used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data accounted for |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available |
| Other bias | Unclear risk | Unclear |
Stenzelius 2011.
| Methods | RCT | |
| Participants | n = 509, I = 254, II = 255. Men and women Mean age 67.2 (I: 67.6. II: 66.7) Incl: Adult patients undergoing elective orthopaedic surgery Excl: recent (within 3 weeks) Hx of catheterisation / UTI, previous radiation over lower pelvis, latex allergy, cognitive impairment, difficulties understanding Swedish language Median catheterisation (days): 2 (range 0 to 16) |
|
| Interventions | I: Noble metal alloy‐coated latex catheter. II: Standard silicone Foley catheter | |
| Outcomes | Incidence of bacteriuria (positive urinary culture => 100,000 cfu/mL). Indentification of patient characteristics that are risk factors for bacteriuria. To study urinary symptoms during and after catheterisation period | |
| Notes | 1st urine sample taken on day of operation. 2nd sample before catheter removal. Follow up interview regarding urinary symptoms at 7 to 10 days Size 12 Ch catheters used in both groups 'Oral cloxacillin given preoperatively to 93% of the patients....no differences between catheter groups (P = 0.96) 30% open drainage system, 70% closed drainage system Per‐protocol analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Closed envelope randomisation |
| Allocation concealment (selection bias) | Unclear risk | '...patients were randomly assigned...envelopes were then kept in the patient's journals until the time of catheterization' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patient blinding unclear risk: 'patients blinded'. Unclear of method Personnel blinding high risk: Differences in catheter appearance ‐ blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurses (who performed follow‐up telephone calls) and microbiologist blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All incomplete data accounted for |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
Takeuchi 1993.
| Methods | RCT | |
| Participants | n = 37 men and women Duration of follow up: 9 days | |
| Interventions | I (26) Silver protein‐ (oxide) coated catheters II (11) Standard latex Foley catheters | |
| Outcomes | Bacteriuria as defined as 1.000,000 cfu/mL, < 1 week: I 2/26, 2/11; > 1 week: 26/26, 11/11 Adverse effect: pain associated with catheterisation: I 9/23, II 3/11; urethral discharge: I 6/23, II 4/11; allergic reaction: I 0/26, II 0/11 | |
| Notes | No power calculation Methods for observing for adverse effects not described Trial conducted in Japan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not specified but unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The extent of missing data not described |
| Selective reporting (reporting bias) | Low risk | All reported outcomes are listed in methods section |
| Other bias | Unclear risk | Participant age was the only baseline data given |
Talja 1990.
| Methods | RCT | |
| Participants | n = 77 men Mean age 58.7 Incl: major surgery, ICU, acute myocardial infarction, vascular surgery to lower extremities Length of catheterisation mean 2.2 days, range 1 to 4 days | |
| Interventions | I (22) Standard catheter (Hydrogel‐coated latex) II (28) Standard catheter (Full silicone) III (27) Standard catheter (Siliconised latex) | |
| Outcomes | Urethral inflammatory reaction measured by scanning electron microscopic (SEM): Mean change I 36, II 36, III 20; standard deviation I 6.1, II 6.5, III 4.0 | |
| Notes | No power calculation Primary investigator contacted for clarification of methods Method of specimen collection penile swabs prior to catheterisation and directly postcatheterisation and 2 to 3 days after Trial conducted in Finland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as ".......catheterised randomly........" no further details provided |
| Allocation concealment (selection bias) | Unclear risk | Reported as ".......catheterised randomly........" no further details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Conflict of interest of the authors not stated |
Thibon 2000.
| Methods | RCT | |
| Participants | n = 199 men and women Mean age 60 Incl: requiring catheter for at least 3 days, hospitalised at least 10 days Excl: UTI or inflammation of the perineum/penis prior to catheterisation, allergy to silver/hydrogel, catheterised 48 hrs prior to inclusion, antibiotic therapy for UTI, urinary tract intervention (prostate or bladder) | |
| Interventions | I (90) Silver alloy hydrogel‐coated catheters II (109) Standard catheters (full silicone) | |
| Outcomes | UTI as defined as >1,000,000 cfu bacteria per mL % > 10 leucocytes per mm3: < 1 week: I 7/90, II 10/109; > 1 week : I 9/90, II 13/109 | |
| Notes | Power calculation used to determine a sample size of 90 per group needed to detect 50% reduction in the incidence of bacteriuria with silver alloy hydrogel catheter at 5% significance and 90% power Method of urine specimen dipsticks, urine collected from a dedicated opening in the drainage system that did not require disconnection Catheter care regimen or violations not described Antibiotic treatment at time of catheterisation recorded Trial conducted in France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomisation list was used to allocate the type of catheter to each patient in the study" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The trial was double‐blind: neither the patient not the medical staff knew what type of catheter was used; the packing of each type of catheter was the same.........." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | 75/274 participants were excluded. Reasons were provided for some but not all "Seventy‐five were excluded..............." |
Tidd 1976.
| Methods | RCT | |
| Participants | n = 54 men urology patients Age range 22 to 87, Mean 65 Incl: catheterised at least 72 hrs Excl: trauma during catheterisation, UTI on admission, antibiotics prior to admission | |
| Interventions | I (17) Standard catheters (Hydron‐coated latex catheters) II (17) Standard catheters (PVC balloon) III (16) Standard catheters (Plain latex) | |
| Outcomes | Bacteriuria as defined as 10,000 cfu per mL: I 13/17, II 15/17, III 13/16 | |
| Notes | Flawed randomisation in some cases No power calculation Between 79% and 81% of participants received antibiotics Catheter care regimen not described or monitored Trial conducted in the UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sealed envelope method. Randomisation not preserved however, as 'in the event of a subsequent need to withdraw a subject because of urinary infection on admission, the next suitable subject was allocated to the same catheter type to maintain the balance of the experiment' |
| Allocation concealment (selection bias) | Unclear risk | Opacity of envelopes not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 'Differences in catheter appearance made an open evaluation inevitable' |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 'Differences in catheter appearance made an open evaluation inevitable' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 50 of the 54 participants randomised were assessed. Reasons for non‐analysis were given |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Other bias | High risk | Bacteruria was defined as a UTI |
Verleyen 1999a.
| Methods | RCT | |
| Participants | n = 27 men postradical prostatectomy patients Excl: antibiotic therapy, violation of closed drainage system, early hospital discharge | |
| Interventions | I (12) Silver alloy hydrogel catheters II (15)Standard catheters (Silicone) | |
| Outcomes | Bacteriuria as defined as 1,000,000 cfu/mL at 2 weeks: I 6/12, II 8/15 | |
| Notes | No power calculation Specimen collection via suprapubic puncture Catheter care standardised and monitored (exclusion criteria) Trial conducted in Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization was done by changing the catheter type available in the operating theatre on a weekly basis" |
| Allocation concealment (selection bias) | High risk | "Randomization was done by changing the catheter type available in the operating theatre on a weekly basis" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
Verleyen 1999b.
| Methods | RCT | |
| Participants | n = 206 men and women Incl: urological surgery Excl: Bacteriuric at catheterisation, antibiotic therapy, gross haematuria | |
| Interventions | I (79) Silver alloy hydrogel catheters II (101)Standard catheters (Latex) | |
| Outcomes | Bacteriuria as defined as 1,000,000 cfu/mL < 1 week: I 8/79, II 31/101; 2 weeks: I 28/79, II 60/101 | |
| Notes | No power calculation Specimen collection via suprapubic puncture Catheter care standardised and monitored (exclusion criteria) Trail conducted in Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization was done by changing the catheter type available in the operating theatre on a weekly basis" |
| Allocation concealment (selection bias) | High risk | "Randomization was done by changing the catheter type available in the operating theatre on a weekly basis" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
CAUTI ‐ catheter‐associated urinary tract infection cfu ‐ colony forming units per mL Excl ‐ exclusion criteria Fr‐ French scale hrs ‐ hours ICU ‐ intensive care unit Incl ‐ inclusion criteria ITT ‐ intention to treat RCT ‐ randomised controlled trial UTI ‐ urinary tract infection yrs ‐ years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersson 1986 | Randomised controlled trial Intervention: Instillation of enzyme into the bladder (not a catheter) Long‐term catheterisation (not temporary) |
| Bach 1990 | Intervention not relevant (irrigation of catheters with different solution) |
| Bologna 1999 | Cross‐over study with no randomisation to first group (direct blind replacement) Length of catheterisation not defined |
| Britt 1977 | Type of intervention systemic antibiotics (not a catheter) |
| Cleland 1971 | Intervention catheter care (not type of catheter) |
| Day 2003 | Need for long‐term catheterisation using intermittent self catheterisation |
| Domurath 2011 | Intervention not relevant (All patients had intermittent self catheterisation) |
| Erickson 2008 | Duration of catheterisation was more than 14 days for all patients |
| Ghoreishi 2003 | Comparator arm not relevant (catheter versus no catheter) |
| Grocela 2010 | Outcomes not relevant (mucosal changes) |
| Hakvoort 2011 | Comparator not relevant (catheter versus intermittent self catheterisation) |
| Hart 1981 | Duration of catheterisation not reported Catheter size not standardised across trial arms Primary outcome measure is stricture rates |
| Lee 1996 | Audit (not randomised controlled trial) |
| Leone 2003 | Intervention not relevant (comparison of drainage system) |
| Leone 2007 | Majority of the patients had catheter beyond 14 days |
| Leriche 2006 | Intervention not relevant (all patients had intermittent self catheterisation) |
| Litherland 2007 | Intervention not relevant (all patients had intermittent self catheterisation) |
| Nakada 1996 | Length of catheterisation long‐term (not temporary) |
| Newton 2002 | Not randomised controlled trial Participants admitted catheterised |
| Pachler 1998 | Cross‐over study with no randomisation to first group (direct blind replacement) Intermittent catheterisation (not indwelling) |
| Ratahi 2005 | Comparator arm not relevant (catheter versus no catheter) |
| Rigini 2006 | Comparator arm not relevant (catheter versus intermittent self catheterisation) |
| Sallami 2011 | Intervention not relevant (all patients had intermittent self catheterisation) |
| Schaeffer 1988 | Length of catheterisation long‐term (not temporary). Co‐intervention instillation of antibiotics into the catheter bag |
| Shafik 1993 | Type of intervention electrified catheter (not impregnated) |
| Sun 2008 | Participants had neurological conditions and required long‐term catheterisation |
| Teare 1992 | Type of intervention silver cartridges or placebo cartridges inserted between the catheter and drainage bag at the time of catheterisation (not type of catheter) |
| Witjes 2008 | Intervention not relevant (all patients had intermittent self catheterisation) |
Characteristics of ongoing studies [ordered by study ID]
NCT00482547 2007.
| Trial name or title | Study of a Urethral Catheter Coated With Eluting Silver Salts (SUCCESS) |
| Methods | RCT |
| Participants | Age: 18 Years and older |
| Interventions | Experimental group:Silver‐coated catheter Comparator group: Silicone‐coated catheter |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | June 2007 |
| Contact information | Principal Investigator: Mark Rupp, MD Henry Ford Hospital, Detroit, Michigan, United States, 48202 |
| Notes |
NCT01681511 2012.
| Trial name or title | A Randomized Trial for the Safety and Effectiveness of a Novel Antimicrobial‐Coated Foley Catheter Attached to an Antimicrobial Anti‐Reflux Device for Reduction of Catheter‐Associated Urinary Tract Infection |
| Methods | RCT |
| Participants | Age: 18 Years and older Participants will be expected to be catheterized with 14 or 16 French Foley catheters for at least 72 hours. |
| Interventions | Experimental group: Silver‐based antimicrobial coated Foley catheter connected to an antimicrobial anti‐reflux accessory Comparator group: Foley Catheter |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | May 2012 |
| Contact information | Principal Investigator: Susan E Kline, MD University of Minnesota Fairview medical center Minneapolis,, Minnesota, United States, 55455 |
| Notes | Estimated Enrollment:100 |
NCT02198833 2014.
| Trial name or title | Efficacy of Micro‐Patterned Foley Catheter to Reduce Catheter‐Associated Urinary Tract Infection |
| Methods | RCT |
| Participants | Age: 21 Years and older Patients who require a Foley catheter for drainage of their urinary bladder |
| Interventions | Experimental group: Micro‐Patterned Foley Catheter Comparator group: Standard‐of‐Care Foley Catheter |
| Outcomes | Primary Outcome Measures:
Patients will be assessed daily for the occurrence of signs and symptoms of urinary tract infection. A single, independent evaluator (PI/co‐investigator) will determine whether the subject has a catheter associated urinary tract infection based on pre‐defined criteria that involve symptom reports and lab values without knowledge of or access to the catheter type randomly assigned to the patient. Secondary Outcome Measures:
Urine cultures will be obtained every third day to assess for the presence of microbial growth.
Patient will be assessed daily for signs and symptoms of infection. Catheter placement and patency will be confirmed. Insertion site will be evaluated for signs of inflammation and or trauma. |
| Starting date | September 2014 |
| Contact information | James A. Haley Veterans' Hospital Tampa, Florida, United States, 33612 Contact: Brittany Durant, RN 813‐972‐2000 ext 1014 Brittany.Durant@va.gov Contact: Theresa Schwartz, RN, MSN 8139722000 ext 7219 Theresa.schwartz1@va.gov Michael E. DeBakey Veterans Affairs Medical Center Houston, Texas, United States, 77030 Contact: Colleen A Cerra‐Stewart, RN, MN 713‐794‐7127 Colleen.Cerra‐Stewart@va.gov Contact: Debra Duncan, RN 7137911414 ext 26845 Debra.Duncan4@va.gov |
| Notes | Estimated Enrollment:300 |
Differences between protocol and review
An extra subgroup was added to the Methods section (Duration of catheter use ('less than' compared with 'longer than one week')), although the analysis had already been included in the previous version of the review.
Contributions of authors
For the 2014 update, Thomas BL Lam (TL), Muhammad Imran Omar (MO) and Euan Collin Fisher (EF) independently screened all the abstracts and full‐text reports.TL, MO and EF independently extracted data and performed risk of bias assessment. MO and EF assessed the quality of evidence. TL, MO and Sara Maclennan (SM) conducted the qualitative research for exploring the views of the participants (Omar 2013). TL, MO and CA reassessed the risk of bias of all the included trials in accordance with the current methods. All review authors contributed in the analysis of data and writing of the manuscript for this update.
For the 2008 update (Schumm 2008): KG and TL independently assessed all of the titles and abstracts included for the update and completed the data extraction and quality assessment of such trials. KG drafted the updated text and TL contributed to the text editing.
In the original review (Brosnahan 2004), all reviewers contributed to writing the protocol. JB and AJ independently assessed all titles and abstracts identified by the search. JB and CT completed the data extraction and quality assessment of all trials. JB drafted the text and all authors contributed to the editing of the text, and CT also provided a clinical perspective and interpretation.
Sources of support
Internal sources
Univeristy of Aberdeen, UK.
External sources
-
The National Institute for Health Research, UK.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Incontinence Group.
Declarations of interest
Thomas BL Lam was involved in the Catheter Trial, funded by the NIHR HTA Programme in the UK (Pickard 2012)
Muhammad Imran Omar: None known
Euan Fisher: None known
Katie Gillies was involved in the Catheter Trial, funded by the NIHR HTA Programme in the UK (Pickard 2012)
Sara MacLennan: None known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Al Habdan 2003 {published data only}
- Al Habdan I, Sadat‐Ali M, Corea JR, Al Othman A, Kamal BA, Shriyan DS. Assessment of nosocomial urinary tract infections in orthopaedic patients: a prospective and comparative study using two different catheters. International Surgery 2003;88(3):152‐4. [17332] [PubMed] [Google Scholar]
Chene 1990 {published data only}
- Chene G, Boulard G, Gachie JP. A controlled trial of a new material for coating urinary catheters [Essai controle d'un nouveau materiau pour enduire les sondes urnaires]. Agressologie 1990;31(8):499‐501. [PubMed] [Google Scholar]
Darouiche 1999 {published data only}
- Darouiche RO, Smith JA, Hanna H, Dhabuwala CB, Steiner MS, Babaian RJ, et al. Efficacy of antimicrobial‐impregnated bladder catheters in reducing catheter‐associated bacteriuria: A prospective, randomised, multicentre clinical trial. Urology 1999;54(6):976‐87. [DOI] [PubMed] [Google Scholar]
Goodwin 1990 {published data only}
- Goodwin MI, Chester JF. Meatal strictures after transurethral prostatectomy using latex or polyvinyl chloride three‐way catheters. Annals of the Royal College of Surgeons of England 1990;72(2):125‐7. [PMC free article] [PubMed] [Google Scholar]
Johnson 1990 {published data only}
- Johnson JR, Roberts PL, Olsen RJ, Moyer KA, Stamm WE. Prevention of catheter‐associated urinary tract infection with a silver oxide‐coated urinary catheter: clinical and microbiologic correlates. Journal of Infectious Diseases 1990;162:1145‐50. [DOI] [PubMed] [Google Scholar]
Kalambheti 1965 {published data only}
- Kalambheti K. Siliconized Foley catheters. American Journal of Surgery 1965;110(6):935‐6. [DOI] [PubMed] [Google Scholar]
Karchmer 2000 {published data only}
- Karchmer TB, Giannetta ET, Muto CA, Strain BA, Farr BM. A randomized crossover study of silver‐coated urinary catheters in hospitalized patients. Archives of Internal Medicine 2000;160(2):3294‐8. [DOI] [PubMed] [Google Scholar]
Lee 2004 {published data only}
- Lee SJ, Kim SW, Cho YH, Shin WS, Lee SE, Kim CS, et al. A comparative multicentre study on the incidence of catheter‐associated urinary tract infection between nitrofurazone‐coated and silicone catheters. International Journal of Antimicrobial Agents 2004;24(Suppl 1):S65‐9. [20993] [DOI] [PubMed] [Google Scholar]
Liedberg 1990a {published data only}
- Liedberg T, Lundeberg PE. Refinements in the coating of urethral catheters reduces the incidence of catheter‐associated bacteriuria. European Urology 1990;17(3):236‐40. [DOI] [PubMed] [Google Scholar]
Liedberg 1990b {published data only}
- Liedberg H, Lundeberg T. Silver alloy coated catheters reduce catheter‐associated bacteriuria. British Journal of Urology 1990;65(4):379‐81. [DOI] [PubMed] [Google Scholar]
Liedberg 1993 {published data only}
- Liedberg H, Lundeberg T. Prospective study of incidence of urinary tract infection in patients catheterized with Bard hydrogel and silver‐coated catheters or Bard hydrogel‐coated catheters (Abstract number 769). Journal of Urology 1993;149:405A. [21457] [Google Scholar]
Lundeberg 1986 {published data only}
- Lundeberg T. Prevention of catheter‐associated urinary tract infections by use of silver impregnated catheters. Lancet 1986;2(8514):1031. [DOI] [PubMed] [Google Scholar]
Maki 1997 {unpublished data only}
- Maki DG, Holcomb RG. A report on the randomized, controlled clinical trial of the nitrofurazone‐impregnated, antibacterial, indwelling urinary catheter [unpublished report]. Stewartville, Minnesota: Rochester Medical Corporation; 1997 Apr. Stewartville, Minnesota: Rochester Medical Corporation.
Maki 1998 {published data only}
- Maki DG, Knasinski V, Halvorson K, Tambyah PA. A novel silver‐hydrogel‐impregnated indwelling urinary catheter reduces CAUTIs: A prospective double‐blind trial. Infection Control and Hospital Epidemiology 1998;1992(19):682. [Google Scholar]
Nacey 1984 {published data only}
- Nacey JN, Tulloch AGS, Ferguson AS. Catheter‐induced urethritis: a comparison between latex and silicone catheters in a prospective clinical trial. British Journal of Urology 1984;57:325‐8. [DOI] [PubMed] [Google Scholar]
Nickel 1989 {published data only}
- Nickel JC, Feero P, Costerton W, Wilson E. Incidence and importance of bacteriuria in postoperative, short‐term urinary catheterization. Canadian Journal of Surgery 1989;32(2):131‐2. [PubMed] [Google Scholar]
Pickard 2012 {published data only}
- Pickard R, Lam T, MacLennan G, Starr K, Kilonzo M, McPherson G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short‐term catheterisation in hospital: a multicentre randomised controlled trial. Lancet 2012; Vol. 380, issue 1927:1935. [DOI: 10.1016/S0140-6736(12)61380-4] [DOI] [PubMed]
- Pickard R, Lam T, MacLennan G, Starr K, Kilonzo M, McPherson G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short‐term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial‐ and antiseptic‐impregnated urethral catheters (the CATHETER trial). Health Technology Assessment 2012;16(47):1‐197. [DOI] [PubMed] [Google Scholar]
- Schumm K, N'Dow J, Norrie J, CATHETER Study Group. Recruitment challenges in a large randomised controlled trial with complex processes (Abstract number P06). Proceedings of the 29th Society for Clinical Trials Annual Meeting; 2008 May 18‐21 May; St Louis. St Louis (MO), 2008.
Riley 1995 {published data only}
- Riley DK, Classen DC, Stevens LE, Burke JP. A large randomized clinical trial of a silver‐impregnated urinary catheter: lack of efficacy and staphylococcal superinfection. American Journal of Medicine 1995;98(4):349‐56. [DOI] [PubMed] [Google Scholar]
Stensballe 2007 {published data only}
- Stensballe J, Tvede M, Looms D, Lippert FK, Dahl B, Tonnesen E, et al. Infection risk with nitrofurazone‐impregnated urinary catheters in trauma patients: a randomized trial. Annals of Internal Medicine 2007;147(5):285‐93. [SR‐INCONT23739] [DOI] [PubMed] [Google Scholar]
Stenzelius 2011 {published data only}
- Stenzelius K, Persson S, Olsson UB, Stjarneblad M. Noble metal alloy‐coated latex versus silicone Foley catheter in short‐term catheterization: a randomized controlled study. Scandinavian Journal of Urology and Nephrology 2011;45(4):258‐64. [DOI] [PubMed] [Google Scholar]
Takeuchi 1993 {published data only}
- Takeuchi H, Hida S, Yoshida O, Ueda T. Clinical study on efficacy of a Foley catheter coated with silver‐protein in prevention of urinary tract infections. Hinyokika Kiyo 1993;39(3):293‐8. [PubMed] [Google Scholar]
Talja 1990 {published data only}
- Talja M, Korpela A, Jarvi K. Comparison of urethral reaction to full silicone hydrogel‐coated and siliconised latex catheters. British Journal of Urology 1990;66(6):652‐7. [DOI] [PubMed] [Google Scholar]
Thibon 2000 {published data only}
- Thibon P, Coutour X, Leroyer R, Fabry J. Randomized multi‐centre trial of the effects of a catheter coated with hydrogel and silver salts on the incidence of hospital‐acquired urinary tract infection. Journal of Hospital Infection 2000;45(2):117‐24. [DOI] [PubMed] [Google Scholar]
Tidd 1976 {published data only}
- Tidd MJ, Gow JG, Pennington JH, Shelton J, Scott MR. Comparison of hydrophilic polymer‐coated latex, uncoated latex and PVC indwelling balloon catheters in the prevention of urinary infection. British Journal of Urology 1976;48(4):285‐91. [DOI] [PubMed] [Google Scholar]
Verleyen 1999a {published data only}
- Verleyen P, Ridder D, Poppel H, Baert L. Clinical application of the Bardex IC Foley catheter. European Urology 1999;36(3):240‐6. [DOI] [PubMed] [Google Scholar]
Verleyen 1999b {published data only}
- Verleyen P, Ridder D, Poppel H, Baert L. Clinical application of the Bardex IC Foley catheter. European Urology 1999;36(3):240‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andersson 1986 {published data only}
- Andersson H. A double‐blind randomised comparison of the effect and tolerance of varidase versus saline when instilled in the urinary bladder in patients with catheter problems. Journal of International Medical Research 1986;14(2):91‐4. [DOI] [PubMed] [Google Scholar]
Bach 1990 {published data only}
- Bach D, Hesse A, Prange CH. Prevention of incrustations and urinary tract infections during transurethral continuous catheterization [German]. Therapiewoche Urologie Nephrologie 1990;2(1):25‐32. [Google Scholar]
Bologna 1999 {published data only}
- Bologna RA, Tu LM, Polansky M, Fraimow HD, Gordon DA, Whitmore KE. Hydrogel/silver ion‐coated urinary catheter reduces nosocomial urinary tract infection rates in intensive care unit patients: a multicenter study. Urology 1999;54(6):982‐7. [DOI] [PubMed] [Google Scholar]
Britt 1977 {published data only}
- Britt MR, Garibaldi RA, Miller WA, Herbertson RM, Burke JP. Antimicrobial prophylaxis for catheter‐associated bacteriuria. Antimicrobial Agents and Chemotherapy 1977;11(2):240‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cleland 1971 {published data only}
- Cleland V, Cox F, Berggren H, MacInnis MR. Prevention of bacteriuria in female patients with indwelling catheters. Nursing Research 1971;20(4):309‐18. [PubMed] [Google Scholar]
Day 2003 {published data only}
- Day RA, Moore KN, Albers MK. A pilot study comparing two methods of intermittent catheterization: limitations and challenges. Urologic Nursing 2003;23(2):143‐7, 158. [PubMed] [Google Scholar]
Domurath 2011 {published data only}
- Domurath B, Kutzenberger J, Kurze I, Knoth HS. Clinical evaluation of a newly developed catheter (SpeediCath Compact Male) in men with spinal cord injury: residual urine and user evaluation. Spinal Cord 2011;49(7):817‐21. [DOI] [PubMed] [Google Scholar]
Erickson 2008 {published data only}
- Erickson BA, Navai N, Patil M, Chang A, Gonzalez CM. A prospective, randomized trial evaluating the use of hydrogel coated latex versus all silicone urethral catheters after urethral reconstructive surgery. Journal of Urology 2008;179(1):203‐6. [DOI] [PubMed] [Google Scholar]
Ghoreishi 2003 {published data only}
- Ghoreishi J. Indwelling urinary catheters in cesarean delivery. International Journal of Gynecology and Obstetrics 2003;83(3):267‐70. [DOI] [PubMed] [Google Scholar]
Grocela 2010 {published data only}
- Grocela JA, Jura YH. Top‐vented urinary drainage catheters cause fewer epithelial and vascular changes in the bladder mucosa compared to conventional catheters and may reduce susceptibility to urinary tract infections. Current Urology 2010;4(3):136‐41. [Google Scholar]
Hakvoort 2011 {published data only}
- Hakvoort R, Roovers J. Comparing clean intermittent catheterisation and transurethral indwelling catheterisation for incomplete voiding after vaginal prolapse surgery: a multicentre randomised trial. BJOG: an International Journal of Obstetrics and Gynaecology 2012;119(1):113‐4. [DOI] [PubMed] [Google Scholar]
- Hakvoort R, Roovers J. Comparing clean intermittent catheterisation and transurethral indwelling catheterisation for incomplete voiding after vaginal prolapse surgery: a multicentre randomised trial. BJOG: an International Journal of Obstetrics and Gynaecology 2012;119(1):115‐6. [DOI] [PubMed] [Google Scholar]
- Hakvoort RA, Thijs SD, Bouwmeester FW, Broekman AM, Ruhe IM, Vernooij MM, et al. Comparing clean intermittent catheterisation and transurethral indwelling catheterisation for incomplete voiding after vaginal prolapse surgery: a multicentre randomised trial. BJOG: an International Journal of Obstetrics and Gynaecology 2011;118(9):1055‐60. [DOI] [PubMed] [Google Scholar]
Hart 1981 {published data only}
- Hart AJ, Fowler JW. Incidence of urethral stricture after transurethral resection of prostate. Effects of urinary infection, urethral flora, and catheter material and size. Urology 1981;18(6):588‐91. [SR‐INCONT26236] [DOI] [PubMed] [Google Scholar]
Lee 1996 {published data only}
- Lee J, Hernandez J. Silver/hydrogel‐coated catheters to reduce incidence of nosocomial urinary tract infection. American Journal of Infection Control 1996;224(2):117. [Google Scholar]
Leone 2003 {published data only}
- Leone M, Garnier F, Antonini F, Bimar MC, Albanese J, Martin C. Comparison of effectiveness of two urinary drainage systems in intensive care unit: a prospective, randomized clinical trial. Intensive Care Medicine 2003;29(3):410‐3. [DOI] [PubMed] [Google Scholar]
- Leone M, Garnier F, Antonini F, Bimar MC, Albanese J, Martin C. Comparison of effectiveness of two urinary drainage systems in intensive care unit: a prospective, randomized clinical trial. Intensive Care Medicine 2003;29(4):551‐4. [DOI] [PubMed] [Google Scholar]
Leone 2007 {published data only}
- Leone M, Perrin AS, Granier I, Visintini P, Blasco V, Antonini F, et al. A randomized trial of catheter change and short course of antibiotics for asymptomatic bacteriuria in catheterized ICU patients. Intensive Care Medicine 2007;33(4):726‐9. [DOI] [PubMed] [Google Scholar]
Leriche 2006 {published data only}
- Leriche A, Charvier K, Bonniaud V, Peyrat L, N'Guyen P, Soler JM, et al. Comparative study of the acceptability of the SpeediCath Set and Actreen set catheterization sets in patients performing self‐catheterization [French]. Progres en Urologie 2006;16(3):347‐51. [PubMed] [Google Scholar]
Litherland 2007 {published data only}
- Litherland AT, Schiotz HA. Patient‐perceived discomfort with two coated urinary catheters. British Journal of Nursing 2007;16(5):284‐7. [DOI] [PubMed] [Google Scholar]
Nakada 1996 {published data only}
- Nakada J, Kawahara M, Onodera S, Oishi Y. Clinical study of silver lubricath Foley catheter. Hinyokika Kiyo 1996;42:433‐8. [PubMed] [Google Scholar]
Newton 2002 {published data only}
- Newton T, Still JM, Law E. A comparison of the effect of early insertion of standard latex and silver‐impregnated latex Foley catheters on urinary tract infections in burn patients. Infection Control and Hospital Epidemiology 2002;23(4):217‐8. [DOI] [PubMed] [Google Scholar]
Pachler 1998 {published data only}
- Pachler J, Fromodt‐Moller C. Pre‐lubricated hydrophilic PVC urethral catheters compared to non‐hydrophilic PVC catheters. Neurology and Urodynamics 1998;14(4):325. [Google Scholar]
Ratahi 2005 {published data only}
- Ratahi E, Lynskey T. Preoperative urethral catheterisation in patients undergoing total hip and knee arthroplasty. A randomized controlled trial (Abstract). Journal of Bone and Joint Surgery ‐ British Volume 2005;87‐B(Suppl 1):21. [Google Scholar]
Rigini 2006 {published data only}
- Rigini N, Evron S, Sadan O, Szmuk P, Ezri T. Intermittent versus continuous bladder catheterization and labor epidural (Abstract). Anesthesiology 2006;105:A907. [Google Scholar]
Sallami 2011 {published data only}
- Sallami S, Mouine Y, Rhouma SB, Cherif K, Dahmani A, Horchani A. Clean intermittent catheterization following urethral stricture surgery using a low friction catheter versus conventional plastic catheter: a prospective, randomized trial. UroToday International Journal 2011;4:1. [Google Scholar]
Schaeffer 1988 {published data only}
- Schaeffer AJ, Story KO, Johnson SM. Effect of silver oxide/trichloroisocyanuric acid antimicrobial urinary drainage system on catheter‐associated bacteriuria. Journal of Urology 1988;139(1):69‐73. [DOI] [PubMed] [Google Scholar]
Shafik 1993 {published data only}
- Shafik A. The electrified catheter: role in sterilizing urine and decreasing bacteriuria. World Journal of Urology 1993;11(3):183‐5. [DOI] [PubMed] [Google Scholar]
Sun 2008 {published data only}
- Sun JL, Lu YP, Huang B, Tu HL, Zhou XY, Chen QM, et al. Effect of a novel analgesic disposable urinary catheter in prevention of restlessness caused by catheter‐related bladder discomfort in general anesthesia patients in recovery period [Chinese]. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2008;88(25):1750‐2. [PubMed] [Google Scholar]
Teare 1992 {published data only}
- Teare EL, Lewi H, Peacock A, Marshall S, Norton M, Robertson MB, et al. 'Silverline', a device for the prevention of nosocomial bacteriuria?. Journal of Hospital Infection 1992;21(2):154‐6. [DOI] [PubMed] [Google Scholar]
Witjes 2008 {published data only}
- Witjes A, Marberger M, Popolo G, Jonsson O, Kaps H, Chapple CR, et al. Is a PVC‐free catheter as well tolerated as a PVC catheter? A randomised multi‐centre double blind study in experienced users of clean intermittent catheterisation (Abstract number 100). Neurourology and Urodynamics 2008;27(7):688‐9. [Google Scholar]
References to ongoing studies
NCT00482547 2007 {published data only}
- NCT00482547, Rupp M, Dulin J. Study of a urethral catheter coated with eluting silver salts. http://clinicaltrials.gov/show/NCT00482547 2007.
NCT01681511 2012 {published data only}
- NCT01681511, Kline SE. A randomized trial for the safety and effectiveness of a novel antimicrobial‐coated Foley catheter attached to an antimicrobial anti‐reflux device for reduction of catheter‐associated urinary tract infection. http://clinicaltrials.gov/show/NCT01681511 2012.
NCT02198833 2014 {published data only}
- NCT02198833, Darouiche R, Fisher K. Clinical study to assess the efficacy of a novel micro‐patterned Foley catheter to reduce catheter‐associated urinary tract infection among spinal cord injury patients. http://clinicaltrials.gov/show/NCT02198833 2014.
Additional references
Bryan 1984
- Bryan CS, Reynolds KL. Hospital‐acquired bacteremic urinary tract infection: epidemiology and outcome. Journal of Urology 1984;132(3):494‐8. [DOI] [PubMed] [Google Scholar]
CDC 2009
- Centers for Disease Control and Prevention (CDC). Healthcare Infection Control Practices Advisory Committee. Guideline for Prevention of Catheter‐Associated Urinary Tract Infections. www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf (accessed July 2011).
Drekonja 2008
- Drekonja DM, Kuskowski MA, Wilt TJ, Johnson JR. Antimicrobial urinary catheters: a systematic review. Expert Review of Medical Devices 2008;5(4):495‐506. [DOI] [PubMed] [Google Scholar]
Emmerson 1996
- Emmerson AM, Enstone JE, Griffin M, Kelsey MC, Smyth ET. The Second National Prevalence Survey of infection in hospitals‐overview of the results. Journal of Hospital Infection 1996;32(3):175‐90. [DOI] [PubMed] [Google Scholar]
Franken 2007
- Franken A, Bosch EEM, Crespo‐Biel O, Loontjens JA, Dias AA. Anti‐microbial coatings for urological applications. European Cells and Materials 2007;14 Suppl 3:130. [Google Scholar]
Gargon 2014
- Gargon E, Williamson PR, Altman DG, Blazeby JM, Clarke M. The COMET Initiative database: progress and activities from 2011 to 2013. Trials 2014;10(15):279. [doi: 10.1186/1745‐6215‐15‐279] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garibaldi 1982
- Garibaldi RA, Mooney BR, Epstein BJ. An evaluation of daily bacteriologic monitoring to identify preventable episodes of catheter‐associated urinary tract infection. Infection Control 1982;3(6):466‐70. [DOI] [PubMed] [Google Scholar]
Gosheger 2004
- Gosheger G, Hardes J, Ahrens H, Streitburger A, Buerger H, Erren M, et al. Silver‐coated megaendoprostheses in a rabbit model‐an analysis of the infection rate and toxicological side effects. Biomaterials 2004;25(24):5547‐56. [DOI] [PubMed] [Google Scholar]
Gould 2010
- Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter‐associated urinary tract infections 2009. Infection Control and Hospital Epidemiology 2010;31(4):319‐26. [DOI] [PubMed] [Google Scholar]
Griffiths 2007
- Griffiths R, Fernandez R. Strategies for the removal of short‐term indwelling urethral catheters in adults. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004011.pub3; CD004011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guay 2001
- Guay DR. An update on the role of nitrofurans in the management of urinary tract infections. Drugs 2001;61(3):353‐64. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt 2011Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articlesin the Journal of Clinical Epidemiology. Journal of ClinicalEpidemiology 2011;64(4):380–2. [DOI] [PubMed] [Google Scholar]
Haley 1981
- Haley RW, Hooton TM, Culver DH, Stanley RC, Emori TG, Hardison CD, et al. Nosocomial infections in US hospitals, 1975–1976: estimated frequency by selected characteristics of patients. American Journal of Medicine 1981;70(4):947–59. [DOI] [PubMed] [Google Scholar]
Hartstein 1981
- Hartstein AI, Garber SB, Ward TT, Jones SR, Morthland VH. Nosocomial urinary tract infection: a prospective evaluation of 108 catheterized patients. Infection Control 1981;2(5):380‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnson 2006
- Johnson JR, Kuskowski MA, Wilt TJ. Systematic review: antimicrobial urinary catheters to prevent catheter‐associated urinary tract infection in hospitalized patients. Annals of Internal Medicine 2006;144(2):116‐26. [DOI] [PubMed] [Google Scholar]
Krieger 1983
- Krieger JN, Kaiser DL, Wenzel RP. Urinary tract etiology of bloodstream infections in hospitalized patients. Journal of Infectious Diseases 1983;148(1):57‐62. [DOI] [PubMed] [Google Scholar]
Lusardi 2013
- Lusardi G, Lipp A, Shaw C. Antibiotic prophylaxis for short‐term catheter bladder drainage in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD005428.pub2; CD005428] [DOI] [PMC free article] [PubMed] [Google Scholar]
Niel‐Weise 2002
- Niel‐Weise BS, Arend SM, Broek PJ. Is there evidence for recommending silver‐coated urinary catheters in guidelines?. Journal of Hospital Infection 2002;52(2):81‐7. [DOI] [PubMed] [Google Scholar]
Niël‐Weise 2005a
- Niël‐Weise BS, Broek PJ. Antibiotic policies for short‐term catheter bladder drainage in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005428; CD005428] [DOI] [PubMed] [Google Scholar]
Niël‐Weise 2005b
- Niël‐Weise BS, Broek PJ. Urinary catheter policies for short‐term bladder drainage in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004203.pub2; CD004203] [DOI] [PubMed] [Google Scholar]
Omar 2013
- Omar MI, Lam T, MacLennan. Critical outcomes in a Cochrane Systematic Review: patients’ perspective. Cochrane Database Syst Rev Suppl 1‐212. 2013:2. [DOI:10.1002/14651858.CD201300]
Parker 2009
- Parker D, Callan L, Harwood J, Thompson DL, Wilde M, Gray M. Nursing interventions to reduce the risk of catheter‐associated urinary tract infection. Part 1: Catheter selection. Journal of Wound, Ostomy and Continence Nursing 2009;36(1):23‐34. [DOI] [PubMed] [Google Scholar]
Percival 2005
- Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. Journal of Hospital Infection 2005;60(1):1‐7. [DOI] [PubMed] [Google Scholar]
Phipps 2006
- Phipps S, Lim YN, McClinton S, Barry C, Rane A, N'Dow JMO. Short term urinary catheter policies following urogenital surgery in adults. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004374.pub2; CD004374] [DOI] [PubMed] [Google Scholar]
Plowman 2001
- Plowman R, Graves N, Esquive J, Roberts JA. An economic model to assess the cost and benefits of the routine use of silver alloy coated urinary catheters to reduce the risk of urinary tract infections in catheterized patients. Journal of Hospital Infection 2001;48(1):38‐42. [DOI] [PubMed] [Google Scholar]
Pomfret 2000
- Pomfret I. Catheter care in the community. Nursing Standard 2000;14(27):46‐53. [DOI] [PubMed] [Google Scholar]
Pratt 2007
- Pratt RJ, Pellowe CM, Wilson JA, Loveday HP, Harper PJ, Jones SR, et al. epic2: National evidence‐based guidelines for preventing healthcare‐associated infections in NHS hospitals in England. Journal of Hospital Infection 2007;65 Suppl 1:S1‐S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reference Manager 2012 [Computer program]
- Thomson Reuters. Reference Manager Professional Edition. Version 12. New York: Thomson Reuters, 2012.
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Robinson 2001
- Robinson J. Urethral catheter selection. Nursing Standard 2001;15(25):39‐42. [DOI] [PubMed] [Google Scholar]
Saint 1998
- Saint S, Elmore JG, Sullivan SD, Emerson SS, Koepsell TD. The efficacy of silver alloy‐coated urinary catheters in preventing urinary tract infection: A meta‐analysis. American Journal of Medicine 1998;105:236‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saint 2000
- Saint S, Veenstra DL, Sullivan SD, Chenoweth C, Fendrick AM. The potential clinical and economic benefits of silver alloy urinary catheters in preventing urinary tract infection. Archives of Internal Medicine 2000;160(17):2670‐5. [DOI] [PubMed] [Google Scholar]
Shuman 2010
- Shuman EK, Chenoweth CE. Recognition and prevention of healthcare‐associated urinary tract infections in the intensive care unit. Critical Care Medicine 2010;38(8 Suppl):S373‐9. [DOI] [PubMed] [Google Scholar]
Smyth 2008
- Smyth ET, McIlvenny G, Enstone JE, Emmerson AM, Humphreys H, Fitzpatrick F, et al. Four country healthcare associated infection prevalence survey 2006: overview of the results. Journal of Hospital Infection 2008;69(3):230‐48. [DOI] [PubMed] [Google Scholar]
Stamm 1998
- Stamm WE. Urinary tract infection. In: Bennett JV, Brachmand PS editor(s). Hospital Infections. 4th Edition. Philadelphia: Lippincott‐Raven, 1998:477‐85. [Google Scholar]
Tambyah 2002
- Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter‐associated urinary tract infection in the era of managed care. Infection Control and Hospital Epidemiology 2002;23(1):27‐31. [DOI] [PubMed] [Google Scholar]
Turck 1981
- Turck M, Stamm W. Nosocomial infection of the urinary tract. American Journal of Medicine 1981;70(3):651‐4. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Snow KK, Kosinski M, Gandek B. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [MEDLINE: ] [PubMed] [Google Scholar]
Weinstein 1999
- Weinstein JW, Mazon D, Pantelick E, Reagan‐Cirincione P, Dembry LM, Hierholzer WJ Jr. A decade of prevalence surveys in a tertiary‐care center: trends in nosocomial infection rates, device utilization, and patient acuity. Infection Control and Hospital Epidemiology 1999;20(8):543‐8. [DOI] [PubMed] [Google Scholar]
Willson 2009
- Willson M, Wilde M, Webb ML, Thompson D, Parker D, Harwood J, et al. Nursing interventions to reduce the risk of catheter‐associated urinary tract infection: part 2: staff education, monitoring, and care techniques. Journal of Wound, Ostomy, and Continence Nursing 2009;36(2):137‐54. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Brosnahan 2004
- Brosnahan J, Jull A, Tracy C. Types of urethral catheters for management of short‐term voiding problems in hospitalised adults. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD004013.pub2] [DOI] [PubMed] [Google Scholar]
Schumm 2008
- Schumm K, Lam TBL. Types of urethral catheters for management of short‐term voiding problems in hospitalised adults. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD004013.pub3; CD004013] [DOI] [PubMed] [Google Scholar]


