Abstract
We have identified the etiological agent of hemorrhagic nephritis enteritis of geese (HNEG), a fatal disease of European geese. HNEG has been recognized in almost all goose breeding areas, with an epizootic pattern, and up to now, the infectious agent has remained unknown. In order to identify the causative agent, infected tissues from HNEG-affected geese were inoculated to 1-day-old goslings, which then developed clinical signs typical of HNEG. Tissue homogenates from these birds were subjected to Freon extraction followed by sucrose density gradient ultracentrifugation. The resulting main band was examined by electron microscopy and consisted of spherical, naked, papovavirus-like particles approximately 45 nm in diameter. The virus was isolated and propagated in goose kidney cell primary culture. Tissue- or culture-purified virus allowed the experimental reproduction of the disease in goslings. Random PCR amplification of viral nucleic acid produced a 1,175-bp fragment which was shown to be associated with field samples collected from geese affected by HNEG on commercial farms in France. Sequence analysis of the PCR product revealed a unique open reading frame, showing 63 to 72% amino acid similarity with the major capsid protein (VP1) of several polyomaviruses. Finally, based on phylogenetic analysis, we conclude that the causative agent of HNEG is closely related to but clearly distinct from other polyomaviruses; we thus have named this newly identified virus Goose hemorrhagic polyomavirus.
The Papovaviridae family consists of two genera, Papillomavirus and Polyomavirus. Polyomaviruses appear as nonenveloped 40- to 50-nm icosahedral virions with a capsid composed of 72 pentameric capsomers and contain a 4.8- to 5.5-kbp circular double-stranded DNA. The viral genome is functionally divided into two regions on opposite DNA strands, encoding five main proteins: the early region, which codes for large and small T antigens (multifunctional regulatory proteins), and the late region, which encodes three structural proteins, VP1, VP2, and VP3. These viruses are widely distributed among mammals and birds and are generally species specific (20). Avian polyomaviruses APVs were first identified in various psittacine species. The prototype avian polyomavirus is the budgerigar fledgling disease virus (BFDV) and is responsible for a fulminating disease in neonate budgerigars (2, 11, 16). Similar viruses have been isolated from different psittacine species and from other wild birds, such as finches and falconiformes (6, 8, 14).
Since 1969, several outbreaks of a fulminating disease have been reported in goose flocks in Hungary (1), Germany (18), and southern France (17, 18, 25). This disease, called hemorrhagic nephritis enteritis of geese (HNEG), has spread through almost all goose breeding areas with an epizootic pattern.
HNEG is characterized by high morbidity and mortality rates in geese 4 to 10 weeks old. Under field conditions, death is the most common outcome, generally preceded by coma. Experimental inoculation of 1-day-old goslings results in 100% mortality, and a few hours before coma and death, nervous signs are frequently observed. The necropsic findings are edema of subcutaneous tissues, gelatinous ascites, inflammation of the kidneys, and often hemorrhagic enteritis (19). Geese which recover from HNEG are supposed to be persistently infected (19). Although HNEG has been quite well characterized at a clinical level, its agent has remained unknown to date, even if it is generally admitted to be a virus (17, 18).
In this paper, we present the purification, isolation, and identification of the HNEG agent. Homogenates of HNEG-infected tissues purified by sucrose gradient ultracentrifugation produced a dense visible band. Electron microscopy examination of this material showed spherical, naked, papovavirus-like particles approximately 45 nm in diameter. The virus was adapted to goose kidney primary cells. Concentrated virus fractions purified either from infected tissue or from culture allowed us to reproduce the disease in 1-day-old goslings. Using a random PCR approach, we identified a 1,175-bp fragment in our preparations as well as in several field samples collected during two distinct HNEG epizootics. After sequencing of the fragment, a comparison of the deduced amino acid sequence indicated that this newly identified virus was a novel polyomavirus. We thus propose that the agent of HNEG should be named Goose hemorrhagic polyomavirus (GHPV).
MATERIALS AND METHODS
Birds.
One-day-old goslings were obtained from a local hatchery. Birds were housed in biocontainement facilities according to the guidelines of the European Community Council on Animal Care, in wire-floored cages with infrared lamps for heating, and were provided with feed and water ad libitum. Inoculations were performed subcutaneously. Birds were monitored daily for clinical signs of HNEG.
Collection of serum samples and field virus isolates.
Serum samples were obtained from 1-day-old goslings hatched from a commercial breeding stock formerly affected by HNEG. These goslings were proved HNEG resistant in an attempt to reproduce the disease, as described below (data not shown). Field isolates of HNEG virus were derived from clinically affected geese on commercial farms in France. Negative controls consisted of samples collected from unaffected geese on farms where the disease had never been identified.
Experimental reproduction of HNEG.
The liver and spleen of five goslings naturally dead from HNEG were homogenized at a 1:5 dilution (wt/vol) in buffer A (10 mM Tris-HCl, 100 mM NaCl, 1 mM EDTA [pH 7.2]), pooled, and clarified by centrifugation at 10,000 × g for 30 min. Penicillin (10,000 IU/ml) and streptomycin (1 mg/ml) were added to the inoculum.
Aliquots (400 μl) of the clarified solution were inoculated subcutaneously to 20 goslings. Ten goslings were mock infected. Goslings of either group were inspected daily for signs of disease, and birds that either had died or were sacrificed when moribund were necropsied. Gross lesions were recorded, and samples of liver, spleen, and kidney were collected and frozen at −80°C. Samples of different tissues (liver, kidney, spleen, brain, gut, and bursa of Fabricius) were fixed in 10% neutral buffered formalin and processed for routine histological examination.
Cells and medium.
Goose embryo fibroblast (GEF) and goose kidney cell (GKC) primary cultures were prepared from 20-day-old embryos and 1-day-old goslings, respectively. A standard protocol of trypsinization (3 g of Trypsine [Difco] per liter) was used for cell disaggregation. Cells were grown in Eagle's minimal essential medium (Gibco BRL) with 10% fetal calf serum and antibiotics (penicillin [100 IU/ml] and streptomycin [100 μg/ml]). Cells were trypsinized and split every 5 days at a ratio of 1:2.
Virus culture.
Early passage GEF and GKC (passage 1 to 5) cultures were seeded in 25-cm2 flasks and inoculated the following day when the cell monolayers were subconfluent. Ten microliters of tissue-derived purified virus (see below) diluted in 1 ml of culture medium was used as the inoculum. The inoculum was allowed to adsorb for 2 h, after which it was removed and replaced by 10 ml of culture medium containing 5% fetal calf serum. Cell cultures were observed daily for cytopathic effect (CPE). Flasks were frozen 7 to 10 days postinoculation (dpi), and the viral inoculum for the next passage was a crude lysate prepared by three consecutive freeze-thaw cycles and vigorous shaking.
Virus concentration and purification.
Spleen, liver, and kidney specimens collected during experimental reproduction of the disease were pooled, homogenized in buffer A, and clarified as described above. The method used for virus sucrose gradient purification was derived from that described for the purification of rubella virus (24). Briefly, the suspension was centrifuged at 10,000 × g for 30 min. The supernatant was collected and purified twice by homogenization with trichlorotrifluoroethane (Freon) in order to eliminate lipids. Sucrose solutions used in the next steps of purification were prepared in buffer A. Supernatant was layered onto a 30% (wt/wt) sucrose cushion and ultracentrifuged. The pellet was resuspended in buffer A, layered onto a 25-to-60% discontinuous sucrose gradient, and then subjected to isopycnic ultracentrifugation at 120,000 × g for 16 h. After centrifugation, a dense visible band was collected, and an aliquot was kept for viral buoyant density determination by refractometry. Finally, the band was diluted 1:3 in buffer A and centrifuged at 120,000 × g for 2 h, and the pellet was resuspended in 200 μl of water.
After viral production in GKCs, GHPV purification was carried out with a modified protocol. Three 225-cm2 flasks of 1-day-old GKCs were inoculated with a lysate of the fourth passage of the virus on cell culture. At 8 dpi, the medium was collected and cell monolayers were disrupted by three freeze-thaw cycles in 20 ml of buffer B (10 mM Tris-HCl, 50 mM NaCl, 0.01 mM CaCl2, 0.5% desoxycholate, 1% Triton X-100 [pH 7.2]) and vigorous shaking. The medium and cell lysate were pooled and centrifuged at 10,000 × g for 30 min. The supernatant was collected, and pellets were reextracted two more times in 10 ml of buffer B. Extraction supernatants were pooled and ultracentrifuged at 100,000 × g for 2 h. The resulting pellets were resuspended in 30 ml of buffer C (10 mM Tris-HCl, 50 mM NaCl, 0.01 mM CaCl2, 0.01% Triton X-100 [pH 7.2]) and subjected to a sucrose cushion followed by a sucrose gradient as described above, with buffer C replacing A.
Indirect immunofluorescence assays.
Cells were grown on tissue culture-treated glass slides (Falcon), fixed, and permeabilized in acetone-ethanol (1:1) at −20°C for at least 2 h. GHPV antigens were detected with the serum of 1-day-old goslings hatched from HNEG-infected breeders. Fluorescein isothiocyanate-conjugated rabbit anti-duck immunoglobulins (Nordic Immunological Laboratories) were used as secondary antibodies.
Electron microscopy.
In order to observe viral particles in situ, infected cells were trypsinized, fixed with 2% glutaraldehyde in 0.2 M Sorensen phosphate buffer (pH 7.4) on ice for 1 h, and then postfixed with 1% osmium tetroxide in 333 mM saccharose and 66 mM Sorensen buffer (pH 7.4). After dehydration, cells were embedded in Embed 812 (Electron Microscopy Sciences) according to current commercial protocols. Finally, cells were sliced into 70-nm-thick sections prior to staining with 5% uranyl acetate (20 min) and 0.3% lead citrate (10 min).
Purified viral particles were negatively stained as follows: 10 μl of purified fractions was allowed to adsorb on Formvar-coated copper grids stabilized with evaporated carbon (300 mesh; Electron Microscopy Sciences) for 1 min; excess liquid was removed, and the grids were negatively stained with 2% phosphotungstic acid for 30 s to 1 min and finally dried on a filter paper. Examinations were carried out on a transmission electronic Hitachi HU-12A microscope at an accelerating voltage of 75 kV.
Nucleic acid extraction and DNA amplification.
Viral DNA was extracted from tissue or purified viral particles with a High Pure PCR template preparation kit (Roche Diagnostic). DNA amplifications were carried out in a GeneAmp PCR System 2400 thermal cycler (Perkin-Elmer) in a volume of 20 μl with 1 U of recombinant Taq DNA polymerase (Gibco BRL). A first random PCR was performed using 10-mer random primers (Pharmacia) at a low stringency (2.5 mM MgCl2) for 5 min at 94°C; 40 cycles of 30 s at 94°C, 30 s at 45°C, and 30 s at 72°C; and a final elongation step of 5 min at 72°C. PCR products were cloned in a pGEMT phagemid vector (Promega Corp.) and sequenced (Genome Express, Grenoble, France). A first sequence analysis led us to choose a 244-bp fragment sharing homology with the polyomavirus VP1 sequence, in which we have designed a set of primers (VP1F, 5′-GAGGTTGTTGGAGTGACCACAATG-3′; VP1R, 5′-ACAACCCTGCAATTCCAAGGGTTC-3′) to specifically amplify a 144-bp fragment in the open reading frame believed to code for the GHPV VP1 protein. The specific amplification was carried out at a high stringency (1 mM MgCl2) for 5 min at 94°C; 35 cycles of 30 s at 94°C, 30 s at 60°C, and 30 s at 72°C; and a final elongation step of 5 min at 72°C. A second round of amplification at a low stringency was carried out using 10-mers as random primers mixed either with VP1F or VP1R in order to walk on the GHPV genome. Finally a 1,175-bp fragment encoding the complete GHPV VP1 was cloned and sequenced.
Phylogenetic analysis.
The GHPV VP1 coding sequence (1,062 bp) was compared to those from other polyomaviruses using the multiple sequence alignment software CLUSTAL W, version 1.7 (23). The subsequent phylogenetic analysis of the VP1 sequences was performed using the PHYLIP programs NEIGHBOR, DNApars, and DNAml from the PHYLIP package, version 3.752c (4). Neighbor-joining, parsimony, and maximum-likelihood methods gave similar results. In order to ascertain the robustness of the constructed trees, bootstrapping was done to generate 1,000 resamplings of the original data.
Nucleotide sequence accession number.
The 1,175-bp GHPV nucleotide sequence and the deduced complete VP1 amino acid sequence have been deposited in GenBank under accession no. AF226991.
RESULTS
Experimental reproduction of HNEG.
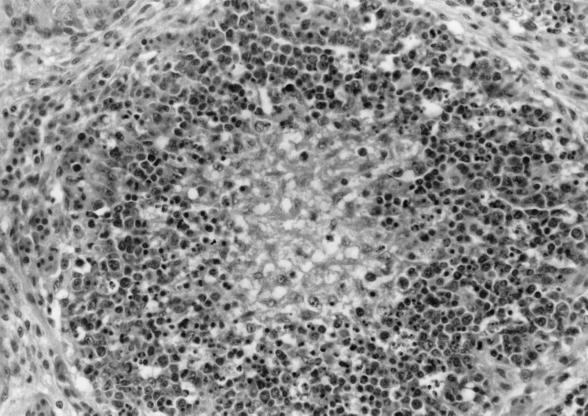
In order to reproduce the disease and obtain enough working stock with which to establish purification, virus was propagated in goslings. Crude liver and spleen extracts from five goslings naturally dead from HNEG were used as inoculum. The 20 HNEG-inoculated 1-day-old goslings died within 8 dpi (Table 1). Clinical and postmortem signs matched those described in field cases (10): gelatinous ascites, edema, and swollen kidneys were observed in all birds, and hemorrhagic enteritis was recorded in 16 goslings. Histologic analysis showed classical lesions associated with HNEG (1, 19). Typical lesions seen in all birds were found in the bursa of Fabricius: moderate to severe lympholysis was seen in cortical and medular regions of bursal follicles (Fig. 1). The spleens, livers, and kidneys of dead birds were collected for virus purification.
TABLE 1.
Experimental reproduction of HNEG
| Inoculum (400 μl) | Total no. of goslings | No. of surviving goslings at:
|
|
|---|---|---|---|
| 8 dpi | 21 dpi | ||
| Control crude extracta | 10 | 10 | 10 |
| GKC lysateb | 10 | 10 | 10 |
| HNEG crude extractc | 20 | 0 | 0 |
| Tissue-purified virusd | 20 | 0 | 0 |
| Culture-purified virusd | 20 | 0 | 0 |
| Heat-treated viruse | 9 | 0 | 0 |
Organs from control geese homogeneized in buffer A, and clarified by centrifugation at 10,000 × g for 30 min.
Lysate of 6-day-old GKCs prepared by three consecutive freeze-thaw cycles and vigorous shaking.
Homogenized HNEG-infected organs in buffer A, clarified by centrifugation at 10,000 × g for 30 min.
Purified virus (10 μl) diluted in 400 μl of culture medium.
Purified virus (10 μl) diluted in 400 μl of culture medium and heat treated at 55°C for 1 h.
FIG. 1.
Photomicrograph of bursa of Fabricius with hematoxylin-eosin staining. The follicle with depleted center and severe cortical lympholysis associated with a light histiocytic infiltration can be seen. Magnification, ×170.
Purification of HNEG virus.
The purification process was derived from those described elsewhere (24). At the equilibrium, the isopycnic gradient yielded a main visible band in sucrose with a buoyant density of 1.20 g/cm3 as determined by refractometry. Concentrated virus fractions purified either from infected tissue or from cell cultures were tested for infectivity. Twenty goslings were inoculated with each fraction, and 9 goslings were inoculated with a heat-treated (55°C for 1 h) culture-purified viral fraction in order to test the thermal resistance of the virus. The infectivity was confirmed; actually, the 69 inoculated goslings died in the week following inoculation and presented the typical signs of HNEG infection, whereas no clinical signs or pathological changes were seen in control birds during the 3-week period of observation (Table 1). Furthermore, the virions seem to be heat resistant, since goslings inoculated with the heat-treated fraction also died in the week postinoculation.
Cell culture and virus isolation.
The first attempts to adapt the HNEG virus to a cell culture system such as duck fibroblasts or duck embryos were unsuccessful (A. Vuillaume, unpublished data). Therefore, we decided to develop culture systems derived from two goose cell types, GEF and GKC. Early passages of GEFs and GKCs (passage 1 to 5) were used in order to isolate the virus. We attempted to adapt the virus to GEFs, but even after five passages we could not detect any CPE, and lysates from the last passage were uninfectious in goslings (data not shown). During the first passage on GKCs, a very mild effect was observed at 7 dpi, and the CPE became more distinct in the following days, with a maximum around 10 to 12 dpi. The cytopathic alterations consisted of granulations and vesicles in the cytoplasm, budding of the cell, and finally cell detachment from the monolayer. One of the more typical features was an aggregation of detached cells, vesicles, and other fragments, in a formation like a bunch of grapes, weakly attached to the monolayer. Through the next passages the CPE became more distinct and at the fifth passage, the first sign of viral replication could be detected 3 dpi; a maximal CPE was detected 7 to 10 dpi. GKCs were still susceptible at passage 30.
Indirect immunofluorescence analysis.
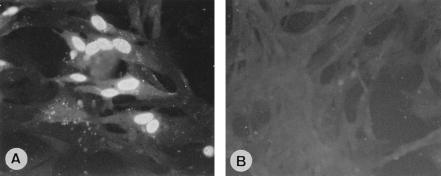
In order to investigate the subcellular localization of viral antigens, 5-dpi GHPV-infected GKCs were fixed and stained by immunofluorescence with serum from 1-day-old HNEG-resistant goslings. Microscopic examination showed the presence of viral antigens almost exclusively in the nucleus (Fig. 2A). In contrast, mock-infected cells showed no significant staining (Fig. 2B).
FIG. 2.
Immunofluorescence assays. (A) Dense fluorescent bodies in the nucleus of GHPV-infected GKCs revealing the presence of viral antigens. (B) Mock-infected cells. Magnification, ×400.
Observation of virus particles by electron microscopy.
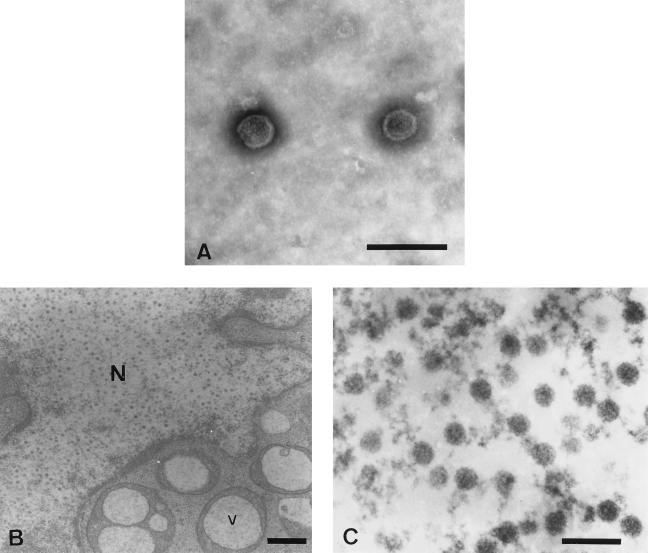
To study the morphology of the isolated virions, purified virus preparations were analyzed by electron microscopy after negative staining. Examination of tissue- or culture-purified virus preparations revealed spherical, naked particles approximately 45 nm in diameter that resembled the Papovaviridae in structure and size (5, 7, 12, 20, 21). A typical picture of virions purified from infected tissues is presented in Fig. 3A.
FIG. 3.
Electron microscopic examination of GHPV. (A) Negatively stained micrograph of purified particles from isopycnic gradient visible band. Shown are naked virion particles 45 nm in diameter (bar = 100 nm). (B) Electron micrograph of GHPV-infected GKCs containing virus particles in the nucleus (N) and large vesicles (V) in the cytoplasm (bar = 400 nm). (C) Polyomavirus-like particles in the nucleus (bar = 100 nm).
Subcellular localization of the virus particles was determined by in situ examination of GHPV-infected GKCs at 5 dpi that were fixed, embedded in resin, sliced into thin sections, and finally positively stained with uranyl acetate and lead citrate. Preparation analysis revealed the presence of large vesicles of dense material, including an optically clear center in the cytoplasm of infected cells (Fig. 3B). This feature, observed in about 20% of the cells, was always associated with papovavirus-like particles in the nucleus (Fig. 3C). Such vacuoles or particles were not observed in mock-infected cells.
Amplification of viral nucleic acid.
Nucleic acids extracted from purified particles were amplified by PCR to identify the virus at a molecular level. Initial results, especially electron microscopy, suggested that the HNEG agent could be papovavirus-like. In a first experiment, we failed to amplify the viral nucleic acid with a set of primers developed for the detection of avian polyomavirus (13). Thus, we used a random amplification approach, which led us to produce a 1,175-bp fragment. This PCR product was cloned, and sequence analysis showed a unique open reading frame.
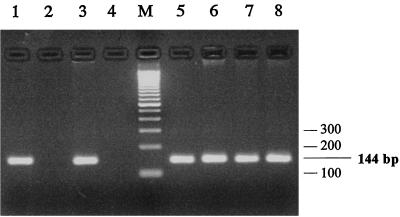
A set of internal specific primers (VP1F and VP1R) was designed. PCR performed with these primers on nucleic acids extracted from purified virus showed that this sequence was strictly associated with tissue- or culture-purified virus (Fig. 4, lanes 1 to 4). Furthermore, PCR performed on various field isolates collected during different epizootics showed that all geese that died of HNEG were positive for this sequence (Fig. 4, lanes 5 to 8), and no amplification was obtained with control, uninfected geese from farms where the disease had never been described (Fig. 4, lane 2).
FIG. 4.
PCR detection of viral DNA. The set of primers VP1F-VP1R produced a 144-bp fragment. Nucleic acids were extracted from tissue-purified virus (lane 1), uninfected liver (lane 2), culture-purified virus (lane 3), uninfected GKCs (lane 4), and infected tissue (liver and spleen) from field samples collected during epizootics in February 1991, January 1998, March 1998, and March 1999 (lanes 5 to 8, respectively). Lane M, molecular size marker.
Sequence analysis.
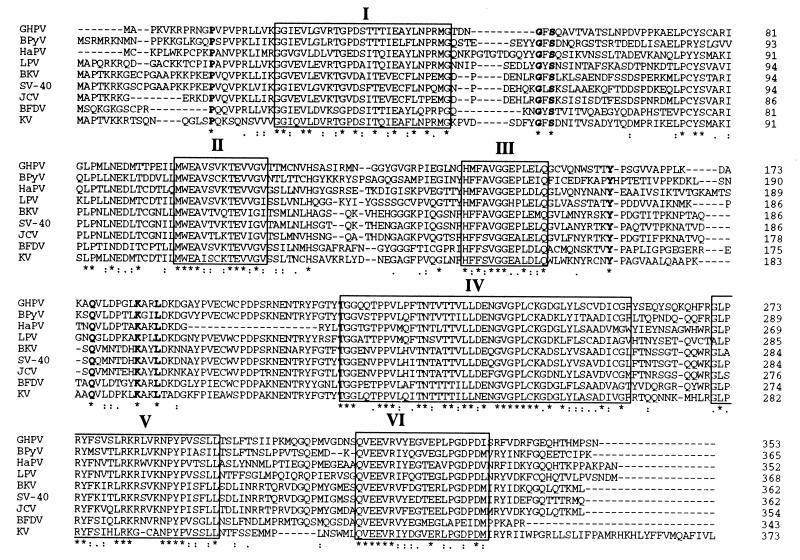
The 353-amino-acid sequence deduced from the large open reading frame of the 1,175-bp GHPV genome fragment was first analyzed by consulting the GenBank database and showed between 50 and 59% identity and 63 to 72% similarity with the VP1 sequences of eight different polyomaviruses (data not shown). In order to highlight the common features between GHPV and polyomaviruses, VP1 sequences were aligned (Fig. 5). The GHPV VP1 presents typical amino acid blocks (Fig. 5, blocks I to VI) conserved in all members of the genus. Furthermore, isolated amino acids conserved among different polyomaviruses, such as P12, G51, S53, Y161, Q186, K193, and L196 (Fig. 5, characters in boldface type) were also present in the GHPV.
FIG. 5.
Alignment of VP1 sequence of GHPV and eight polyomaviruses. The blocks labeled with roman numerals highlight conserved sequence between the different VP1 sequences. Characters in boldface type are isolated conserved amino acids. Below the sequence alignment are shown conserved positions (defined in CLUSTAL W) represented by the following symbols: ∗, single, fully conserved residue; :, one of the strong groups fully conserved (STA, NEQK, NHQK, NDEQ, QHRK, MILV, MILF, HY, or FYW); ., one of the weaker groups fully conserved (CSA, ATV, SAG, STNK, STPA, SGND, SNDEQK, NDEQHK, NEQHRK, FVLIM, or HFY). Polyomavirus VP1 sequences used for comparison and their GenBank accession numbers were as follows: simian virus 40 (SV-40), AF038616; BK virus (BKV), Z19536; JC virus (JCV), AF015537, murine polyomavirus Kilham strain (KV), M55904; bovine polyomavirus (BPyV), M74843; BFDV, M20775; LPV, M14494; hamster polyomavirus (HaPV), P03092; GHPV, AF226991.
Phylogenetic analysis.
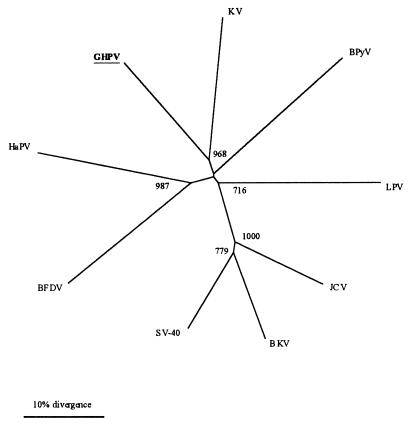
The sequence analysis strongly suggests that the GHPV is a novel member of the Polyomavirus genus. In order to examine the relationships between GHPV and polyomaviruses, we carried out a phylogenetic analysis on the nucleic acid sequence of the GHPV VP1 and those of eight polyomaviruses. Dendrograms were constructed using neighbor-joining, parsimony, or maximum-likelihood methods, all of which gave similar results. The unrooted dendrogram presented in Fig. 6 shows that GHPV is not a variant of the APV BFDV and indicates that the newly identified virus is a novel member of the Polyomavirus genus.
FIG. 6.
An unrooted dendrogram based on VP1 nucleic acid sequence. The tree was constructed by using the neighbor-joining method. The numbers at the nodes are bootstrap values; 1,000 resamplings of the original sequence alignment were generated. Abbreviations of the polyomavirus VP1 sequences used for comparison and their GenBank accession numbers are given in the legend to Fig. 5.
DISCUSSION
Our attempts to isolate the agent of HNEG led to the discovery, using electron microscopy and sequence analysis, of a novel virus belonging to the genus Polyomavirus, which we hence named GHPV.
The successful reproduction of HNEG with purified particles isolated from HNEG-infected goslings suggests that these virions have a causal relationship to this disease. The infectivity of the heat-treated fraction indicates a thermal resistance of the agent, a characteristic of the Papovaviridae (12). The purified virions were shown to be 45 nm in diameter, naked, with a buoyant density in sucrose of 1.20 g · cm−3, which matched with physical properties of the Papovaviridae (12), and thus were identified as papovavirus-like. HNEG virus was efficiently propagated on epithelial GKCs: the first cytopathic changes occurred 3 to 7 dpi with a pattern characterized by vesicles in the cytoplasm and budding from the cell membrane. Surprisingly, the virus showed no capacity to grow on GEFs, although this cell type is elective for replication of APVs (2). Subcellular localization of the viral antigens was investigated on GHPV-infected GKCs by immunofluorescence with the serum of goslings hatched from HNEG-infected breeders. The nuclear staining observed suggests that the virus replicates in the nuclei, a common feature of all polyomaviruses (20). In addition, the detection of viral antigens was efficient with field-collected serum, which confirms the association of this virus with the occurrence of HNEG in the field. Furthermore, electron microscopy performed on infected cells showed slightly enlarged nuclei with marginated heterochromatin, containing a high number of 45-nm-diameter papovavirus-like virions, consistent with those described in BFDV-infected cells (3). The exact nature of the large vesicles observed in infected cells remains unknown, and further examination may discriminate between a necrosis or apoptosis origin of these inclusions.
These results suggesting that the HNEG agent is related to papovaviruses were confirmed by molecular data, which led us to conclude that the HNEG agent is a novel polyomavirus. A 1,175-bp product of the random PCR performed on purified particles was specifically detected in samples collected during two distinct epizootics from geese that died of HNEG but not those from uninfected geese. This PCR fragment showed about 70% amino acid similarity with the major capsid protein (VP1) of several polyomaviruses. Interestingly, GHPV VP1 shared only 59% amino acid identity with BFDV VP1, which suggests that HNEG virus is strongly divergent from BFDV. Retrospectively, this poor sequence conservation between GHPV and BFDV is consistent with the inefficient amplification of viral DNA obtained with the set of primers designed in conserved regions of BFDV VP1 and specifically developed for the detection of such an avian polyomavirus APV (13).
The relationships of GHPV across the Polyomavirus genus are reflected by phylogenetic analysis, confirming that this virus is neither located within any of the clusters identified so far nor strongly related to another polyomavirus. Furthermore, no particular relationship with APVs could be evidenced. Together, these results strongly suggest that the HNEG agent, namely, GHPV, is a novel species within the Polyomavirus genus.
Polyomaviruses have been isolated from many mammals, in which they are associated with persistent nonclinical infections leading to disease in immunocompromised hosts. An acute nature is therefore highly unusual in the Polyomavirus genus (5). Only BFDVs were shown to be associated with fulminant diseases in psittacines, finches, and falconides, with a clinical evolution, lesions, and epidemiologic features that are dramatically different among susceptible species (6, 9, 15). Clinical signs and gross lesions associated with these infections show an amazing likeness with descriptions of HNEG: the most reliable features are an acute death with poor or no premonitory signs and lesions, including ascites, subcutaneous edema, nephritis, and intestinal hemorrhages (1, 15, 19).
A key feature of APV infections is the occurrence, in most infected cells, of intranuclear, large basophilic inclusions, mainly with karyomegaly. There is a great diversity in cell types concerned by these inclusions, but they are never totally absent in avian hosts (15). Various examinations performed previously (1, 18) and during this work failed to identify such inclusions in birds diagnosed with HNEG. This absence of inclusions is, in our knowledge, only shared with lymphotropic papovavirus (LPV) among polyomaviruses. Nevertheless, it is important that unlike GHPV, LPV, which affects monkeys and probably humans, is only associated with nonclinical carriage (20, 26).
All the APVs already described are characterized by their wide host range, which contrasts with the highly specific host range of mammalian polyomaviruses (20). Investigations relative to infections by GHPV in other host species are just beginning, but field experience suggests a very narrow host range, even among waterfowl species (J.-L. Guerin and J.-L. Pingret, unpublished data). At the cellular level, GHPV probably actively replicates in B cells, as suggested by the medullar lympholysis observed in the bursa of Fabricius. This elective replication in B cells is described in other polyomavirus infections such as BK virus, JC virus, and BFDV infections (6, 15, 20). In addition, histopathology suggests that GHPV shows a wide tropism for various cell types, like all APVs (15). In contrast, most mammalian polyomaviruses display a restricted tropism for a few cell types (12).
To date, all polyomaviruses isolated from birds are designated so-called APVs. It has been proposed that these viruses be considered a distinct subgenus within the Polyomavirus genus (22), on the basis of the structure of their genome, host range, and innate acute pathogenicity. Actually, all APVs isolated so far were closely related to the BFDV originally described (2, 16) and shared more than 99% nucleotide identity (14). This suggests that they might even be variants of one single species. Our molecular data reveal that BFDV and GHPV VP1 share only 59% amino acid identity, which indicates that GHPV and BFDV are indeed distinct viruses, even though they share a similar acute pathogenicity, unusual among members of the Polyomavirus genus. Thus, since GHPV is an APV which does not match all the criteria to be classified as a true APV, we suggest that the significance of the term APV be reconsidered.
The complete GHPV genome sequence and its molecular characterization are in progress. The preliminary results confirm both its relation to the Polyomavirus genus and its originality. All the polyomaviruses infecting birds share an acute pathogenicity for their respective hosts, with mostly similar clinical patterns, which might reflect common features at the molecular level. Among APV variants, point mutations observed in the continuous open reading frame of two minor capsid proteins (VP2 and VP3) have been cited as the reason for differences in histopathological appearance or, more putatively, restricted host range (14). The molecular characterization of the GHPV genome will focus on structural features of early and late genes and especially on molecular relationships between GHPV and APV. Such studies should highlight molecular features shared by both GHPV and APV but not by other polyomaviruses and thus might correlate molecular data to pathobiological properties.
ACKNOWLEDGMENTS
This work was supported by the Régions Aquitaine and Midi-Pyrénées, the Comité Interprofessionnel des Palmipèdes à Foie Gras, and the Office Interprofessionnel des Viandes, de l'Elevage et de l'Aviculture.
We are grateful to Pierre Sans for his scientific contribution to the HNEG study (17) and for his continuous involvement in field investigations. We gratefully thank Isabelle Raymond, Jérôme Braun, and Olivier Andreoletti (Ecole Nationale Vétérinaire de Toulouse) for histologic examinations and Jean-Luc Duteyra and Bruno Payré (Centre de microscopie électronique appliquée à la biologie, Faculté de médecine Toulouse III) for excellent technical assistance with electron microscopy. We are also particularly grateful to Christophe Pasquier (Laboratoire de Virologie, Hôpital Purpan Toulouse) for his guidance in phylogenetic analysis. Finally, thanks to many colleagues who contributed constructive criticisms during the preparation of the manuscript, and in particular, Stéphane Bertagnoli, Christelle Camus, and Frédérique Messud-Petit.
REFERENCES
- 1.Bernath S, Szalai F. Investigations for clearing the etiology of the disease appeared among goslings in 1969. Magyar Alla Lap. 1970;25:531–536. [Google Scholar]
- 2.Bozeman L H, Davis R B, Gaudry D, Lukert P D, Fletcher O J, Dykstra M J. Characterization of a papovavirus isolated from fledgling budgerigars. Avian Dis. 1981;25:972–980. [PubMed] [Google Scholar]
- 3.Dykstra M J, Bozeman L H. A light and electron microscopic examination of budgerigar fledgling disease virus in tissue and in cell culture. Avian Pathol. 1982;11:11–28. doi: 10.1080/03079458208436078. [DOI] [PubMed] [Google Scholar]
- 4.Felsenstein J. PHYLIP (phylogeny inference package) version 3.572c. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 5.Fenner F J, Frederick D O, Gibbs E P, Murphy F A, Rott R, Studdert M J, White D O, editors. Veterinary virology. 2nd ed. San Diego, Calif: Academic Press Incorporated; 1993. [Google Scholar]
- 6.Graham D L, Calnek B W. Papovavirus infection in hand-fed parrots: virus isolation and pathology. Avian Dis. 1987;31:398–410. [PubMed] [Google Scholar]
- 7.Haun G, Keppler O T, Bock C T, Herrmann M, Zentgraf H, Pawlita M. The cell surface receptor is a major determinant restricting the host range of the B-lymphotropic papovavirus. J Virol. 1993;67:7482–7492. doi: 10.1128/jvi.67.12.7482-7492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johne R, Müller H. Avian polyomavirus in wild birds: genome analysis of isolates from Falconiformes and Psittaciformes. Arch Virol. 1998;143:1501–1512. doi: 10.1007/s007050050393. [DOI] [PubMed] [Google Scholar]
- 9.Kingston R S. Budgerigar fledgling disease (papovavirus) in pet birds. J Vet Diagn Investig. 1992;4:455–458. doi: 10.1177/104063879200400417. [DOI] [PubMed] [Google Scholar]
- 10.Kisary J. Haemorragic nephritis and enteritis of geese. In: McFerran J-B, McNulty M S, editors. Virus infections of birds. London, United Kingdom: Elsevier Editions; 1993. pp. 513–514. [Google Scholar]
- 11.Müller H, Nitschke R. A polyoma-like virus associated with an acute disease of fledgling budgerigars. Med Microbiol Immunol. 1986;175:1–13. doi: 10.1007/BF02123124. [DOI] [PubMed] [Google Scholar]
- 12.Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. Virus taxonomy. Classification and nomenclature of viruses. Sixth report of the international committee on taxonomy of viruses. Vienna, Austria: Springer-Verlag; 1995. [Google Scholar]
- 13.Phalen D N, Wilson V G, Graham D L. Polymerase chain reaction assay for avian polyomavirus. J Clin Microbiol. 1991;29:1030–1037. doi: 10.1128/jcm.29.5.1030-1037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phalen D N, Wilson V G, Gaskin J M, Derr J N, Graham D L. Genetic diversity in twenty variants of the avian polyomavirus. Avian Dis. 1999;43:207–218. [PubMed] [Google Scholar]
- 15.Ritchie B W. Avian polyomavirus: an overview. J Am Avian Vet. 1991;3:147–153. [Google Scholar]
- 16.Rott O, Kroger M, Muller H, Hobom G. The genome of budgerigar fledgling disease virus, an avian polyomavirus. Virology. 1988;165:74–86. doi: 10.1016/0042-6822(88)90660-5. [DOI] [PubMed] [Google Scholar]
- 17.Sans P. Contribution à l'étude de la maladie des jeunes oies. DVM thesis. Toulouse, France: Ecole Nationale Vétérinaire de Toulouse; 1992. [Google Scholar]
- 18.Schettler C H. Détection en France de la néphrite hémorragique et entérite de l'oie (NHEO) Rec Med Vet. 1977;153:353–355. [Google Scholar]
- 19.Schettler C H. Clinical picture and pathology of haemorrhagic and enteritis in geese. Tierartzl Prax. 1980;8:313–320. [PubMed] [Google Scholar]
- 20.Shah K V. Polyomaviruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2027–2043. [Google Scholar]
- 21.Siray H, Özel M, Jandrig B, Voronkova T, Jia W, Zocher R, Arnold W, Scherneck S, Krüger D H, Ulrich R. Capsid protein-encoding genes of hamster polyomavirus and properties of the viral capsid. Virus Genes. 1998;18:39–47. doi: 10.1023/a:1008017201999. [DOI] [PubMed] [Google Scholar]
- 22.Stoll R, Luo D, Kouwenhoven B, Hobom G, Müller H. Molecular and biological characteristics of avian polyomaviruses: isolates from different species of birds indicate that avian polyomaviruses form a distinct subgenus within the polyomavirus genus. J Gen Virol. 1993;74:229–237. doi: 10.1099/0022-1317-74-2-229. [DOI] [PubMed] [Google Scholar]
- 23.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trudel M, Payment P. Concentration and purification of rubella virus hemagglutinin by hollow fiber ultrafiltration and sucrose density gradient. Can J Microbiol. 1980;26:1334–1339. doi: 10.1139/m80-221. [DOI] [PubMed] [Google Scholar]
- 25.Vuillaume A, Tournut J, Banon H. A propos de la maladie des oisons d'apparition tardive ou néphrite hémorragique-entérite de l'oie (N.H.E.O.) Rev Med Vet. 1982;133:341–346. [Google Scholar]
- 26.Zur Hausen H, Gissmann L. Lymphotropic papovaviruses isolated from African green monkey and human cells. Med Microbiol Immunol. 1979;167:137–153. doi: 10.1007/BF02121180. [DOI] [PubMed] [Google Scholar]