Highlights
-
•
The genetic basis of B12 auxotrophy in dinoflagellates is examined systematically.
-
•
B12-dependent gene metH express responsively to B12 level in all dinoflagellates.
-
•
Most species have incomplete B12-independent metE lacking the N-terminal domain.
-
•
Other dinoflagellates (< 30%) are completely absent of metE.
-
•
The incompleteness/absence of metE explains B12 auxotrophy of all dinoflagellates.
Keywords: Harmful algal blooms (HABs), Dinoflagellates, Vitamin B12 (cobalamin), Auxotrophy, Methionine synthase genes, Phytoplankton ecology
Abstract
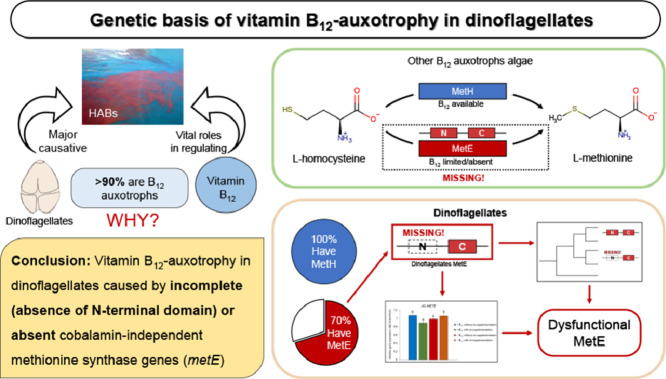
Dinoflagellates are responsible for most marine harmful algal blooms (HABs) and play vital roles in many ocean processes. More than 90% of dinoflagellates are vitamin B12 auxotrophs and that B12 availability can control dinoflagellate HABs, yet the genetic basis of B12 auxotrophy in dinoflagellates in the framework of the ecology of dinoflagellates and particularly HABs, which was the objective of this work. Here, we investigated the presence, phylogeny, and transcription of two methionine synthase genes (B12-dependent metH and B12-independent metE) via searching and assembling transcripts and genes from transcriptomic and genomic databases, cloning 38 cDNA isoforms of the two genes from 14 strains of dinoflagellates, measuring the expression at different scenarios of B12, and comprehensive phylogenetic analyses of more than 100 organisms. We found that 1) metH was present in all 58 dinoflagellates accessible and metE was present in 40 of 58 species, 2) all metE genes lacked N-terminal domains, 3) metE of dinoflagellates were phylogenetically distinct from other known metE genes, and 4) expression of metH in dinoflagellates was responsive to exogenous B12 levels while expression of metE was not responding as that of genuine metE genes. We conclude that most, hypothetically all, dinoflagellates have either non-functional metE genes lacking N-terminal domain for most species, or do not possess metE for other species, which provides the genetic basis for the widespread nature of B12 auxotrophy in dinoflagellates. The work elucidated a fundamental aspect of the nutritional ecology of dinoflagellates.
Graphical abstract
1. Introduction
Vitamins B12 (cobalamin) is a vital nutrient element [1], and the vitamin B12 auxotrophy of many microalgae is well known [2], [3], [4], [5], [6], [7]. Since the requirement of vitamins by microalgae is small (micronutrient) and since many bacteria are capable of synthesizing vitamin B12 [2,[8], [9], [10]], vitamins were once considered to be of little ecological significance [7], despite evidence that B12 had significant stimulating effects on the dinoflagellates (e.g., Karenia brevis) [11], [12], [13]. Recent field evidence has repeatedly demonstrated that B12 is of vital ecological importance as it may reach limiting levels in many ecosystems [14], [15], [16]. More strikingly, recent investigations have also found that most harmful algal bloom-forming species, dinoflagellates in particular, have absolute requirements for B-vitamins and these requirements may be high enough to deplete vitamin B12 stocks in coastal waters as rapid as from days to hours [3,17,18].
Dinoflagellates are a group of protists comprised of ∼2,400 modern species [19] that cause the majority of marine harmful algal blooms (HABs) [20], producing a variety of toxins that threaten the public and ecosystem health [21,22], and also support the growth of coral reefs due to endosymbiotic existence within corals [23]. Dinoflagellates are also biologically distinct regarding their genome sizes, histones, and condensed chromosomes during mitosis [24]. In addition, > 91% of dinoflagellates are vitamin B12 auxotrophs [3] and there is mounting evidence for that vitamin B12 can regulate the population dynamics of dinoflagellates and HABs [13,[15], [16], [17], [18],[25], [26], [27], [28]]. A recent field study of plankton dynamics across the Eastern North Atlantic found that Dinophyceae was the only phytoplankton group whose abundance positively correlated with vitamin B12 concentration [29]. Still, the biochemical and molecular basis for the widespread auxotrophy among dinoflagellates is presently unknown.
Vitamin B12 acts as a cofactor for methylmalonyl-CoA mutase (MCM) which is involved in odd-chain fatty acid metabolism, ribonucleotide reductase, and methionine synthase in algal species [2,30]. There are two enzymes responsible for methionine synthesis: cobalamin-independent (MetE, which can synthesize methionine without the involvement of B12) and cobalamin-dependent (MetH, which uses B12 as its cofactor). However, there has been no detailed investigation of the presence of these two enzymes in dinoflagellates, except for three dinoflagellates that were recently reported having metE homologs in Nef et al. [31]. Another recent study of 15 algal species, including one or more representatives from the Rhodophyta (red algae), Chlorophyta (green algae), diatoms, Haptophyta, and brown macroalgae, indicated that the B12 auxotrophy correlated with the absence of a functional metE [4]. Given that all algae have the B12-independent type I isoform of the ribonucleotide reductase [4] and that several cellular functions of MCM may be non-essential to B12-independent algal species (i.e., not a determining factor of B12 auxotrophy) [4], the genetic basis of B12 auxotrophy in algae is, therefore, largely or entirely controlled by the presence/absence and functioning of the isoform(s) of methionine synthase, MetH and MetE. Consequently, a parsimonious hypothesis for the widespread nature of B12 auxotrophy in dinoflagellates is that B12-auxotrophs in dinoflagellates have the B12-dependent metH gene but do not have the B12-independent metE gene.
The objective of this study was thus to test the hypothesis by determining the presence or absence of the two methionine synthase genes, metH and metE, in dinoflagellates as well as examining their sequence structures and how they transcriptionally respond to different vitamin B12 levels. Our results showed that while all 14 dinoflagellates tested required vitamin B12, 12 had homologs of both metH and metE. Moreover, phylogenetic and sequence analyses coupled with an examination of metH and metE transcription in dinoflagellates cultivated at different levels of vitamin B12 revealed that the absence of the functional N-terminal domain in metE (in 12 species) or an entire absence of metE (in 2 species) is the genetic basis for vitamin B12 auxotrophy in most, possibly all, dinoflagellates.
2. Materials and methods
2.1. Cultures
Fourteen dinoflagellates were investigated in the present study with the majority being HAB species in China [32] (Table 1). Species identifications were affirmed by PCR amplification of the partial large subunit ribosomal DNA, sequencing, and alignment with GenBank sequences (see Supplementary Material for more information). All cultures were grown in autoclaved and sterile filtered (0.22 μm) seawater with a salinity of ∼32 PSU enriched with f/2-Si medium [33] in incubators at 21 °C, ∼100 μmol quant•m−2•s−1 irradiance, and a 12:12 h light:dark photoperiod supplied by white fluorescent lights (Ningbo Jiangnan Instrument Factory, China). An antibiotic solution (a mixture of 10,000 I.U. penicillin and 10,000 μg mL−1 streptomycin, Solarbio, Beijing, China) was added to medium immediately before every time of inoculation (final concentration 2%) to prevent bacterial development. Samples of 50 mL for each culture in the logarithmic phase of growth were collected via centrifugation and then immediately frozen in liquid nitrogen and stored at −80°C until RNA extraction for full-length cloning of metE and metH.
Table 1.
Vitamin B12requirements for 14 dinoflagellates and the presence ofmetHandmetE.
| Species | Strain | Origin | Requires cobalamin?(pM) | metH (subclade) | metE (subclade) | metH expression (B12 limitation/re-supplementation) | metE expression (B12 limitation/re-supplementation) |
|---|---|---|---|---|---|---|---|
| Akashiwo sanguinea | CCMA256 | Xiamen, China | Y (> 1.46 × 10−4) | +(iii) | +(ii) | ↓/↑ | ↓/↑ |
| Alexandrium insuetum | AILYG-12 | Lianyungang, China | Y (> 2.71 × 10−6) | +(iii) | +(ii) | ↑/↓ | ↑/↓ |
| Alexandrium pacificum | APNJD-2 | Nanji Island, China | Y (> 0.34)* | +(iii) | +(ii) | - | - |
| Gymnodinium catenatum | TIO-527 | Xiamen, China | Y (> 1.52)* | +(iii) | +(ii/iii) | - | - |
| Heterocapsa rotundata | HRDH-13 | East China Sea, China | Y (> 1.58 × 10−2) | +(iii) | - | ↑/↓ | - |
| Karenia brevis | CCMA027 (CCMP2229) | Florida, USA | Y (> 1.05 × 10−2) | +(ii) | +(ii/iii) | ↑/↓ | ↑/↓ |
| Karenia mikimotoi | CCMA083 | Pingtan, China | Y (> 1.83 × 10−5) | +(ii) | +(ii/iii) | ↑/↓ | ↑/↓ |
| Karlodinium veneficum | KVND-1 | Ningde, China | Y (> 1.58 × 10−2) | +(i) | +(ii/iii) | ↑/↓ | ↑/↓ |
| Margalefidinium polykrikoides | CP1 | New York, USA | Y (> 0.001)* | +(iii) | +(ii) | - | - |
| Prorocentrum donghaiense | CCMA264 | East China Sea, China | Y (> 0.11)* | +(iii) | +(ii) | - | - |
| Prorocentrum minimum | CCMA262 | East China Sea, China | Y (> 2.44 × 10−5) | +(iii) | +(ii) | ↑/↓ | ↑/↑ |
| Scrippsiella acuminata (formerly Scrippsiella trochoidea) | STIOCAS | Unknown | Y (> 2.0 × 10−5)* | +(iii) | +(ii/iii) | - | - |
| Symbiodinium sp. | CCMA128 | Qingdao, China | Y (> 4.69 × 10−3) | +(iii) | - | ↑/↓ | - |
| Effrenium voratum | - | Unknown | N* | +(iii) | +(ii) | - | - |
The presence of metH and metE as derived from genomic or transcriptomic sequence data is denoted by (+). Subclades of metH and metE in phylogeny analysis are presented after (+). Twelve of the 14 dinoflagellate species have metE. Numbers in parentheses indicate the estimated vitamin B12 concentrations when the culture growth ceased or when the experiment was terminated. N, no (vitamin B12 auxotrophy was not observed); Y, yes (auxotrophy for vitamin B12). Upward or downward arrow (↑or↓) indicates the expression of metH and metE is up-regulated or down-regulated, respectively, under B12 limitation or B12 re-supplementation.
The B12 requirements for six species of the dinoflagellate were not established in the present study and the “No” result was taken from Tang et al, 2010 [3].
2.2. Sequence search and validation
TBLASTN [34] sequence similarity searches were performed to assess the presence of metE and metH in the transcriptomes and genomes of corresponding dinoflagellates, including Marine Microbial Eukaryote Transcriptome Sequencing Project (MMETSP, downloaded from iMicrobe database, http://data.imicrobe.us/project/view/104) [35], reefgenomics database (http://reefgenomics.org), and transcriptomes deposited in Sequence Read Archive (SRA). The organisms and sequence IDs of the proteins that were used to perform these searches are as follows: Chlamydomonas reinhardtii MetH (XP_001696420) and MetE (XP_001702934), Homo sapiens MetH (AAB58906), Escherichia coli MetH (AAA02995) and MetE (AAA23544), Arabidopsis thaliana MetE (OAO94672), Fragilariopsis cylindrus MetH (AIM62183) and MetE (AIL25366). Considering the insufficient annotations of sequence data, we increased the threshold of TBLASTN search to find as many metE sequences as possible. All sequences were identified through TBLASTN with an e-value threshold of < 10−5. To verify the identity of all putative orthologous proteins, conserved functional domains were identified by alignment of nucleic acid and amino acid sequences in NCBI (MetH:PF02965, PF02965, PF02607, PF00809, and PF02574; MetE: PF01717 and PF08267) via Pfam analysis [36] and the conserved domains analysis tool CD-search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Multiple sequences of metE and metH in one species were regarded as different isoforms or different genes depending on the identity (isoforms of metH share over 90% identity; isoforms of metE share over 50% identity) of multiple sequence alignment. A total of 24 species of dinoflagellates (see Supplementary Data 1) were searched for the purpose of full-length cDNA cloning of metE and metH. After validation and full-length cloning of metE and metH of 14 dinoflagellates, these sequences were used to perform a second search in other 34 species of dinoflagellates to examine the presence or absence of metE and metH in as many species of dinoflagellates as possible.
2.3. Phylogenetic analyses of methionine synthases (MetH and MetE) sequences
Methionine synthase sequences were also retrieved from both prokaryotes and eukaryotes of other phyla (non-dinoflagellate algae mostly) (see Supplementary Data 2) according to the phylogenetic analysis in Helliwell et al. [4], in combination with newly published transcriptomes, and transcriptomes from The Marine Microbial Eukaryote Transcriptome Sequencing Project (MMESTP) [35], and the short metE genes that have been recently reported to be presumably capable of synthesizing methionine from some prokaryotes [37]. TBLASTN and BLASTP sequence similarity searches were performed in the GenBank nonredundant database, SRA or MMETSP for appropriate sequences in prokaryotes and other eukaryotes. The methionine synthase genes identified in dinoflagellates as described above were used to perform these searches. An alignment of protein sequences of metE or metH was constructed using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) and manually corrected using BioEdit [38] where appropriate, to ensure only unambiguous residues were compared. Maximum likelihood (ML) phylogenetic analysis was performed using IQ-TREE [39] based on the LG+F+R6 model of evolution and with 1,000 ultrafast bootstrap.
2.4. Assessment of vitamin B12 requirements of dinoflagellates
The B12 requirements of dinoflagellates were assessed by culturing each alga in the appropriate f/2-Si medium with (“plus-B12”, as control) or without (“minus-B12”) addition of B12. All cultures were initially grown in f/2-Si medium made from natural seawater, then transferred into new f/2-Si medium with a salinity of ∼32 PSU that was prepared with Sea Salts (Sigma Chemicals, USA) based artificial sea water after filtering out the original medium using a suitable filter membrane (depending on cell size) to exclude a potential source of B12 from natural seawater. The initial concentrations of B12 in the medium after filtration were semi-quantitatively determined for each species according to Tang et al. [3]. Then cultures were transferred into two types of f/2-Si medium made from artificial seawater (ASW f/2-Si): the full-strength ASW f/2-Si medium containing 3.69 × 102 pM B12 (plus-B12), and the ASW f/2-Si medium without B12 addition (minus-B12), with addition of an antibiotics mixture (as above, final concentration 1–2%) to prevent bacterial growth in both plus-B12 and minus-B12 media, as bacteria may synthesize vitamins. All cultures were grown as above. Cell densities of cultures were monitored microscopically and cultures were transferred into fresh plus- or minus-B12 medium once stationary growth stage was reached, typically within two weeks. Cultures were continually transferred until a culture growth ceased in the minus-B12 medium treatments (final concentrations of B12 were calculated according to the initial concentration, dilution ratio, and the numbers of transfer, and listed in Table 1). Auxotrophy for B12 was declared when the following occurred: A culture ceased to grow in the minus-B12 medium whereas the growth in plus-B12 medium (control) was normal plus the growth of the culture in minus-B12 medium resumed upon the re-introduction of B12. A culture sample of 3 mL was taken and fixed with Lugol's solution (2% final concentration) for cell density enumeration with an inverted microscope (IX73, Olympus, Japan), using a 1.0-mL Sedgewick Rafter counting chamber.
2.5. Vitamin B12 re-supplementation experiments
When the culture growth ceased or slowed in minus-B12 medium, both the plus- and minus-B12 cultures were divided into two parallel samples and a B12 stock solution was added (3.69 × 102 pM) to one of the two sub-cultures, while an equal volume of minus-B12 medium was added to the other cultures as control. Samples (50 mL) of each culture were collected via centrifugation, immediately frozen in liquid nitrogen, and stored at −80 °C until RNA extraction for gene expression analysis when growth in the minus-B12 cultures resumed upon the addition of B12. These samples, therefore, included four categories: plus-B12 culture with B12 re-supplementation; plus-B12 culture without B12 re-supplementation; minus-B12 culture with B12 re-supplementation; and minus-B12 culture without B12 re-supplementation. Samples (3 mL) for cell quantification were obtained immediately before and after re-supplementation, respectively, as described above.
2.6. DNA and RNA extraction, full-length cDNA cloning qRT-PCR
Total DNA was extracted with a Plant Genomic DNA Kit following the manufacturer's protocol (Tiangen, China), while the total RNA was extracted with a RNeasy Plant Mini Kit (Qiagen, Germany). RNA concentration and quality were assessed using a NanoDrop Spectrophotometer (ND-2000, Thermo Fisher Scientific, USA) and analyzed on agarose gels. Two μg of each RNA sample with a DNase treatment done was reverse transcribed into cDNA using PrimeScript RT Kit (Takara, Japan) for full-length cDNA cloning and reverse transcription quantitative polymerase chain reaction (qRT-PCR; TB green, Takara, Japan). The full-length sequences of metH and metE for were obtained using rapid amplification of cDNA ends (RACE) using the 22-base dinoflagellate spliced leader sequence (DinoSL) [40] as the forward primer in 5’ end cloning of RACE to ensure that all transcriptomic sequences obtained were indeed exclusively from dinoflagellates [41,42] (see Supplementary Data 3 and 4 for details of primers).
2.7. Statistical analysis
The 2–△△CT method was used to analyze the expression level of metH and metE. To test for significant differences between gene expression among different samples, one-way ANOVA and Duncan's multiple range test were performed using the SPSS 20.0 at a significance level of P < 0.05. Refer to Supplementary material for more details of the molecular experiments.
3. Results
3.1. Presence of metH and metE in sequenced dinoflagellates genomes and transcriptomes
A survey of methionine synthase isoforms from 58 dinoflagellate species based on all publicly available databases of transcriptomes and genomes (see Supplementary Data 1) revealed that all 58 species contained unigenes annotated as metH with the same conserved functional domains. Unigenes annotated as metE were found in 40 of the 58 dinoflagellate species, while there was no evidence for the presence of metE in the other 18 species (see Supplementary Data 1). While all retrieved metE sequences contained the C-terminal domain (∼1,150 bases), they all lacked the N-terminal domain (∼1,150 bases) compared to known functional metE genes in model organisms, e.g., E. coli and A. thaliana [43,44]. Although most metE sequences were retrieved from transcriptomes, the metE sequences from complete genome assemblies of Fugacium kawagutii, Symbiodinium microadriaticum, and Polarella glacialis each also lacked the N-terminal domain in this region, suggesting that the absence of N-terminal in these species was not the result of missing or gap due to incomplete transcriptome or genome assembly. In addition, 10 of the 40 dinoflagellates had metE genes that lacked N-terminal domain but did contain other non-MetE conserved domains (e.g., Acyl transferase domain in polyketide synthase enzymes), as judged from the prediction using Pfam analysis and CD-search.
3.2. Full-length cloning of metH and metE
To verify the presence of metH and metE and the absence of N-terminal of metE in dinoflagellates as observed above, 14 species of dinoflagellates were selected for cloning the full-length cDNA sequences and function analyses of metH and metE. A total of 38 full-length cDNA sequences of metH and metE isoforms were obtained from these 14 species (Table 1; see Tables S1 and S2 for detailed information of sequences). Whereas the metE of Effrenium voratum had acyl transferase domain similar to that in polyketide synthase (PKS) enzymes and a conserved domain relevant to inorganic ion transport and metabolism in the upstream and downstream of the domain encoding metE sequence, only the C-terminal CDS of metE conserved domain was found. Using the CD-search tool, all metH genes of 14 species were confirmed to have the four known metH conserved functional domains found in other model organisms, e.g., E. coli and Homo sapiens [45,46] (see Fig. 1b). In contrast, 12, but not the other two, of the 14 species had metE (Table 1), with all of them having the C-terminal domain and lacking the N-terminal domain in the 5′ termini, indicating a loss of function as a methionine synthase gene (see below for more information).
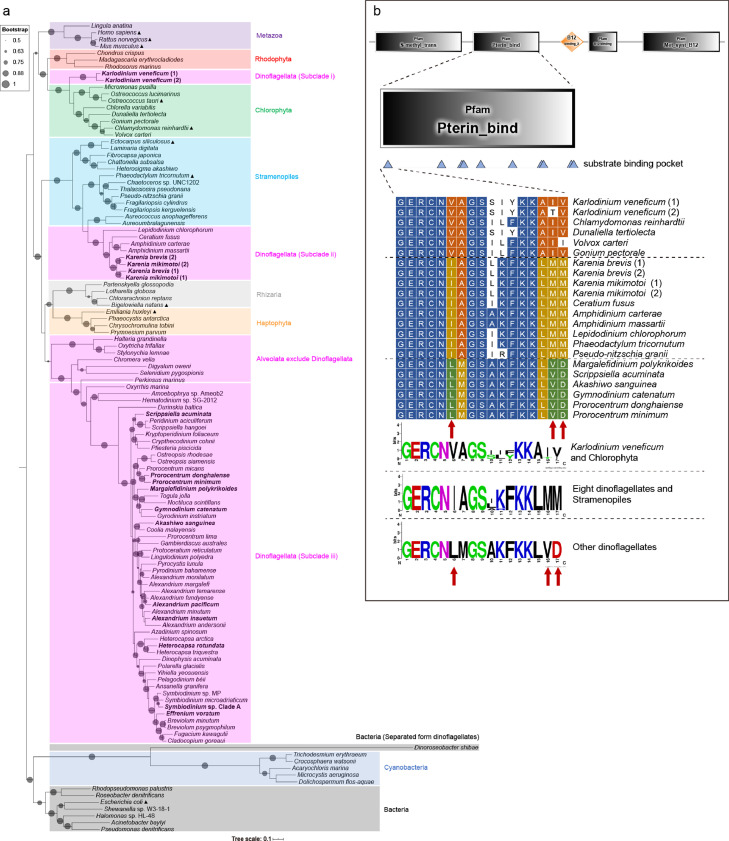
Fig. 1.
The phylogenetic tree of MetH. (a) Maximum likelihood (ML) phylogenetic tree based on the 106 protein sequences of MetH from dinoflagellates and species from other eukaryotic phyla, with 13 bacterial sequences serving as the outgroup (using the LG+F+R6 model of evolution). Different taxa were labeled with different colors. All MetH that obtained in the study were highlighted in bold. MetH of model organisms were shown with “▲”. The size of circles on the branches indicated nonparametric bootstrap support values (only those > 50 were shown). (b) Multiple alignment and sequence analysis for the substrate-binding pocket of MetH in three clades of dinoflagellates and other similarly-clustered species. The height of the letters thereby indicates the degree of conservation (the higher, the more conservative). A sequence logo of the sequences around the first site of substrate-binding pocket was given beneath the alignment. Arrows indicate that substitutions within the 6th, 16th and 17th amino acid from selected segments around the first site of substrate binding pocket motif.
3.3. Phylogenetic analyses of MetH and MetE of dinoflagellates and other taxa
Phylogenetic analyses of MetH and MetE protein sequences from a broad range of taxa (a total of 71 species, primarily model organisms of those non-dinoflagellate taxa; see Supplementary Data 2 for more detailed information) were performed to assess similarities between orthologous genes of dinoflagellates and other model organisms with functional MetH and MetE (Figs. 1 and 2). The phylogenetic tree of MetH (Fig. 1a) indicated that the phylogeny of eukaryote MetH exhibited a clustering pattern consistent with general phylogenetic relationships, with strong support for the monophyly of MetH in the Metazoa, Chlorophyta, Rhodophyta, Stramenopiles, Haptophyta, Alveolata, and Rhizaria lineages. Dinoflagellates clustered into three groups: (i) Karlodinium veneficum, which clustered with Chlorophyta species; (ii) K. brevis, K. mikimotoi, Ceratium fusus, Amphidinium carterae, A. massartii, and Lepidodinium chlorophorum, which clustered with Stramenopiles species; (iii) other dinoflagellates that clustered with Alveolata species (Fig. 1a). Further alignment and sequence logo analysis for MetE in dinoflagellates and other similarly clustered species on the phylogenetic tree (Fig. 1b) indicated that the two sub-groups of dinoflagellates that clustered outside of the Alveolata clade were caused by substitutions in three amino acids around the first site of a substrate binding pocket motif (see the legend of Fig. 1b).
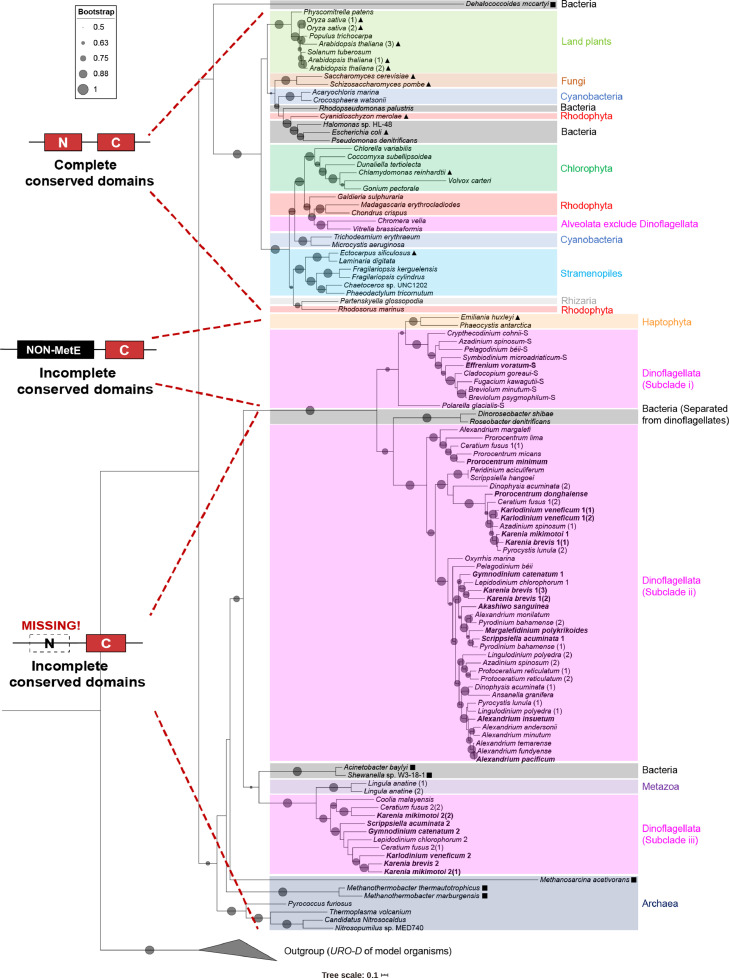
Fig. 2.
The phylogenetic tree of MetE. Phylogenetic tree reconstructed using Maximum likelihood method (using the LG+F+R6 model of evolution) for the protein sequences of MetE from dinoflagellates and species of other phyla, with the URO-D (Uroporphyrinogen decarboxylase, which belongs to the same protein superfamily as MetE) of model organisms serving as the outgroup. Different taxa were labeled with different colors. All MetE obtained in this study were highlighted in bold. MetE of model organisms was shown with “▲”. A total of 6 short MetE that can presumably synthesize methionine from bacteria or archaea were shown with “■”. Different types of conserved domains of MetE were indicated on the left of the corresponding species (“N” and “C” denote N-terminal and C-terminal, respectively). The size of circles on the branches indicated nonparametric bootstrap support values (only those > 50 were shown). Those sequences of dinoflagellates with non-MetE conserved domains were named with “-S” as a suffix.
Although phylogenetic analysis of MetE protein sequences (using URO-D of other model organisms as outgroup) indicated a more complex evolutionary history, as all sequences formed two major clades, depending upon the presence or absence of the N-terminal domain (Fig. 2), all MetE protein sequences of dinoflagellates were clustered in three subclades: (i) MetE without an N-terminal domain but with C-terminal domains and other non-MetE conserved domains (n = 10 species); (ii) MetE with C-terminal domains but lacking the N-terminal domain; most of dinoflagellate fell into this category (n = 30); and (iii) MetE without N-terminal but also with a C-terminal that differed from that of (ii) (n = 8). Subclade iii consisted of eight dinoflagellate species that have more than one isoform of MetE, including C. fusus, K. brevis, and five other dinoflagellates. No dinoflagellate clustered with any of other taxa that have a functional MetE (i.e., the upper major clade with compete N- and C-terminals in Fig. 2).
We also performed phylogenetic analyses for the amino acid sequences of C-terminal domain of MetE proteins only (Fig. S1). While most C-terminal domains of MetE protein sequences of dinoflagellates, the same as that in Fig. 2, formed two subclades in accordance with the presence or absence of the N-terminal domain, the subclade consisted of 10 MetE sequences of eight dinoflagellate species (corresponding to the subclade iii in Fig. 2 described above), however, formed a subclade parallel to the clade containing those MetE with complete domains and proven function of MetE (see the explanation in Discussion). Although two short prokaryotic MetE that can presumably synthesize methionine were clustered within the major group of dinoflagellate MetE (Figs. 2 and S1), these short MetE need a specific methyl provider upstream of C-terminal domain to function as MetE [37], which was not present in any publicly available databases for the transcriptomes and genomes of dinoflagellates. Another presumably functional MetE from the bacterium Methanosarcina acetivorans appeared as the base of the clade containing most MetE in dinoflagellates (Figs. 2 and S1), but it was found to obtain the methyl group from the iron-sulfur corrinoid protein of the Wood-Ljungdahl pathway that only occurred in anaerobic bacteria [37].
This phylogenetic analysis convincingly demonstrates that metE genes in dinoflagellates are not functional due to an absence of the N-terminal domain.
3.4. Confirming the vitamin B12 auxotrophy of dinoflagellates
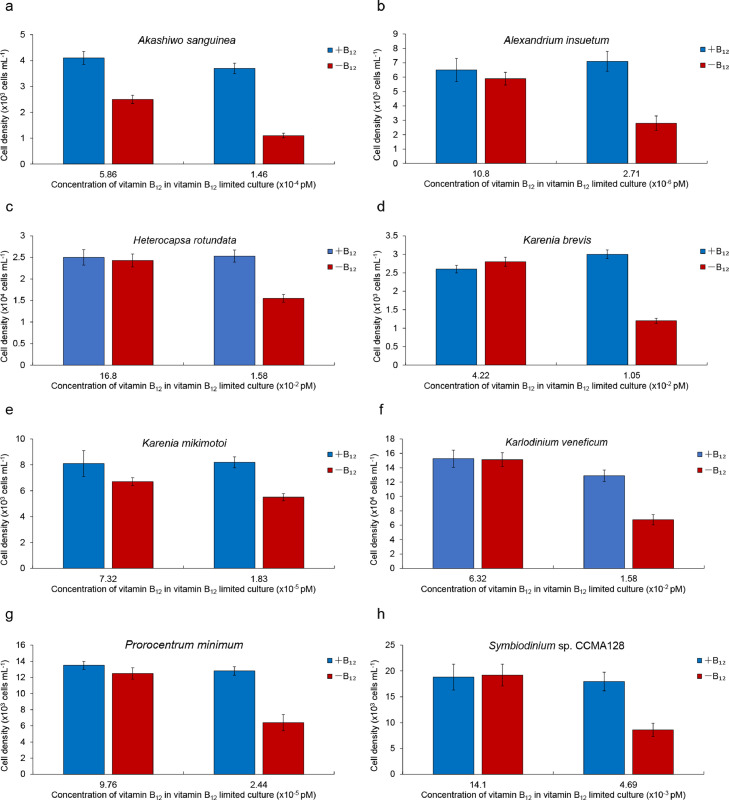
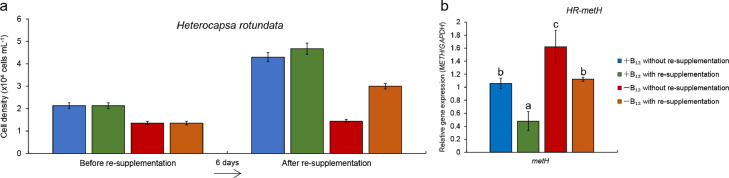
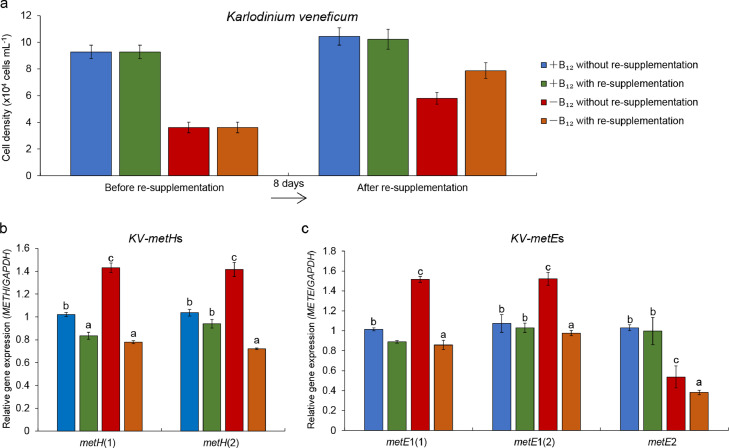
To examine the relationships between the presence of metE and B12 auxotrophy of dinoflagellates on the physiological level, eight of the 14 phylogenetically examined dinoflagellates were grown in B12 starvation and re-enrichment experiments (Table 1). The growth of all eight dinoflagellate species was dependent on the availability of vitamin B12 and directly responded to the limitation and supplementation of vitamin B12 (Table 1). When the concentration of B12 declined, the growth of all dinoflagellates was inhibited in comparison to respective B12 replete controls (Fig. 3). Re-supplementation of B12 into the cultures Akashiwo sanguinea (Fig. S2a), Alexandrium insuetum (Fig. S3a), Heterocapsa rotundata (Fig. 5a), K. brevis (Fig. S4a), K. mikimotoi (Fig. S5a), Karlodinium veneficum (Fig. 4a), Prorocentrum minimum ( Fig. S6a) and Symbiodinium sp. CCMA128 (Fig. S7a) restored growth rates demonstrating that these eight dinoflagellates are vitamin B12 auxotrophs, consistent with prior findings [3].
Fig. 3.
Assessment of vitamin B12 requirements of selected dinoflagellates. Species were grown in the appropriate silicate-free f/2 culture medium with and without B12 (+B12 and −B12) in batch culture over multiple transfers, or until auxotrophy for vitamin B12 was declared. (a) Akashiwo sanguinea. (b) Alexandrium insuetum. (c) Heterocapsa rotundata. (d) Karenia brevis. (e) Karenia mikimotoi. (f) Karlodinium veneficum. (g) Prorocentrum minimum. (h) Symbiodinium sp. CCMA128. Error bars represent ±1 standard error associated with the mean (n = 3).
Fig. 5.
Vitamin B12 re-supplementation experiment of Heterocapsa rotundata. (a) Cell densities before and after re-supplementation of vitamin B12 in the appropriate silicate-free f/2 culture medium with and without B12 (+B12 and −B12). The re-enrichment of vitamin B12 was performed on the 6th day of experiments. (b) Relative gene expression of metH in vitamin B12 re-supplementation experiment. HR-metH was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts. Error bars represent ±1 standard error associated with the mean (n = 3). Letters indicate statistically significant difference between groups (P < 0.05). Vitamin B12 re-supplementation experiment of other six dinoflagellates were showed in Figs. S2–S7.
Fig. 4.
Vitamin B12 re-supplementation experiment of Karlodinium veneficum. (a) Cell densities before and after re-supplementation of vitamin B12 in the appropriate silicate-free f/2 culture medium with and without B12 (+B12 and −B12). The re-enrichment of vitamin B12 was performed on the 8th day of experiments. (b&c) Relative gene expression of metH genes (b) and metE genes (c) in vitamin B12 re-supplementation experiment. All genes were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts. Error bars represent ±1 standard error associated with the mean (n = 3). Letters indicate statistically significant difference between groups (P < 0.05).
3.5. Transcriptional responses of metH and metE to the changing level of vitamin B12
To determine whether metH and metE are expressed and functioning as methionine synthases in these dinoflagellates, we used qRT-PCR to assess the transcriptional responses of metH and metE under different scenarios of B12 concentrations. In both H. rotundata and Symbiodinium sp. CCMA128, which possess metH but not metE, the expression of metH in B12-deplete medium was significantly higher than that in B12-replete medium before re-supplementation of B12. Upon the re-supplementation of vitamin B12, the expression of metH decreased significantly in both cultures (Fig. 5b for H. rotundata and Fig. S7b for Symbiodinium sp. CCMA128, P < 0.05). The expression of metH in five of other species, all possessing metH and metE, exhibited similar trends between B12-deplete and replete cultures prior to re-supplementation with B12 as above (see Fig. S3b for A. insuetum, Fig. S4b for K. brevis, Fig. S5b for K. mikimotoi, Fig. 4b for Karlodinium veneficum, Fig. S6b for P. minimum), while the expression of metH in A. sanguinea in B12-deplete medium was significantly lower than in B12-replete medium (P < 0.05; Fig. S2b). After re-supplementation with B12, the expression of metH in K. veneficum significantly decreased in both B12-deplete and -replete cultures, while the expression in A. sanguinea and P. minimum significantly decreased in the B12-replete cultures only (Figs. S2b and 6b; P < 0.05) and the expression in A. insuetum, Karenia brevis, and K. mikimotoi significantly decreased in B12-deplete medium (Figs. S3b, S4b and S5b; P < 0.05). In summary, the expression of metH increased in all dinoflagellates when the concentration of B12 decreased to a growth-limiting level, while the expression of metH decreased when the concentration of B12 was increased via re-supplementation of vitamin B12 to levels normally found in f/2 media.
The expression of metE, in contrast, did not respond to changes in B12 concentration and particularly, did not exhibit a significantly elevated expression in response to limiting levels of B12, except in Karlodinium veneficum and P. minimum (see Fig. S2c for A. sanguinea, Fig. S3c for Alexandrium insuetum, Fig. S4c for K. brevis, Fig. S5c for K. mikimotoi, Fig. 4c for Karlodinium veneficum, and Fig. S6c for P. minimum). The greatest difference in the expression of metE between B12-deplete and B12-replete cultures before re-supplementation of B12 was observed in P. minimum (Fig. S6c), which displayed a two-fold increase only. The expression of metE was also not repressed when vitamin B12 was restored to a high level, and was observed to even increase in response to B12 re-supplementation in K. brevis (KB-metE1(2); Fig. S5c) and P. minimum (Fig. S6c). In addition, the multiple isoforms of metH and metE in one species displayed the same transcriptional response to changing levels of vitamin B12, e.g., K. veneficum.
4. Discussion
4.1. The presence and structures of metH and metE in dinoflagellates
While previous surveys of methionine synthesis genes among sequenced algal genomes (e.g., species of Chlorophyta and diatoms) have revealed that algae that do not have a functional copy of metE are B12 auxotrophs [2,4,5], these studies have largely excluded dinoflagellates due to the lack of genomic and transcriptomic datasets and other limitations. The first sequenced draft genome of a dinoflagellates, Breviolum minutum, was publicly available only as of 2013 [47], and the first high-quality sequenced genome of a dinoflagellate, F. kawagutii, was published in 2015 [48]. Most sequencing data from dinoflagellates have come from transcriptomic studies. Since most all of those studies were focused on questions beyond vitamin B12, the genes relevant to B12 auxotrophy including metH and metE were not reported yet. In the present study, we searched for metH and metE in 58 dinoflagellate species from all publicly available transcriptomes and genomes and found that 40 of the 58 dinoflagellates possessed unigenes annotated as metE, which seemed to contradict the finding of Tang et al. [3] that 91% of tested dinoflagellates are vitamin B12 auxotrophs. Structural analyses demonstrated, however, that all these metE sequences contained the C-terminal domain but lacked the N-terminal domain. The functions of the two domains in metE were found to bind the substrate (5-methyltetrahydrofolate) and to serve as catalytic domain for methyl transfer, respectively [43,49]. In addition, Helliwell et al. [4] found that Volvox carteri and Gonium pectoral, both being phylogenetically close to the B12-independent green alga C. reinhardtii, became B12 auxotrophs because their metE genes could not produce a functional enzyme due to a significant deletion, which consequently caused a frameshift and a premature stop codon. Here, we hypothesize that the absence of a N-terminal domain disables the functionality of metE for methionine synthesis.
While metH has been annotated in dinoflagellates in a recent field study [50], the presence of metE has rarely been explicitly annotated in previously published transcriptomes and even genomes of dinoflagellates, partly because over 50% of putative conserved protein domains of dinoflagellate transcriptomes are not annotated in the public databases [51]. On account of that an absence of a transcript might indicate extremely low expression rather than an absence of the gene, for those dinoflagellates from which we did not find metE, further in-depth genomic sequencing is likely needed to verify if this is indeed the case. In addition, we surprisingly found that the metE genes of Emiliania huxleyi and Phaeocystis antarctica both have a partial C-terminal domain only, which has not been reported previously and was omitted in recent studies of methionine synthase isoforms in Haptophyta [31,52]. We also noted that 10 dinoflagellate species have metE genes that were lacking of N-terminal domain but have all other conserved domains that are non-essential to metE, including the three whole genomes sequences of dinoflagellates, suggesting that the absence of N-terminal domain in metE genes in dinoflagellates was not a result of incomplete transcriptome assembly.
4.2. Dinoflagellates contain functional metH and dysfunctional metE
Phylogenetic analysis of MetH sequences revealed a clustering pattern among all species generally consistent with the phylogenetic relationships established on the basis of other genes (e.g., the ribosome RNA genes). However, there were two exceptional clustering sub-groups within the lineage of dinoflagellates: K. veneficum clustered with Chlorophyta, while six species (C. fusus, L. chlorophorum and 4 species from Gymnodiniales) clustered with Stramenopiles. Detailed alignment and sequence analysis showed that these exceptions were caused mainly by substitutions in three amino acids. The phylogenetic analysis thus suggests that the variations in metH genes of dinoflagellates have not been strictly co-evolved (or parallelly obtained) with other genes (e.g. rRNA) used to establish phylogenetic relationships in dinoflagellates. In the clade containing most dinoflagellates and other Alveolata species, the dinoflagellate Oxyrrhis marina was the base of clade, consistent with the phylogenetic relationship of dinoflagellates presented by Bachvaroff et al. [53].
Phylogenetic analysis of MetE revealed two major clades that aligned with the presence or absence of N-terminal domain, i.e., MetE of dinoflagellates were separated from those MetE functioning as genuine MetE in the phylogenetic tree. The inclusion of six short prokaryotes-origin MetE that has been proposed to synthesize methionine with C-terminal domain only [37] in our phylogenetic analysis showed that two MetE from Acinetobacter baylyi and Shewanella sp. W3-18-1 were clustered with a group of 10 dinoflagellates and the MetE of M. acetivorans seems to have a close phylogenetic relationship with most MetE of dinoflagellates. However, owing to the lack of a specific methyl provider or pathway as in these prokaryotes [37], the MetE of these dinoflagellates is unable to function as methionine synthase.
In contrast to the tree of MetH, the phylogenetic relationship of MetE was more complicated. Our tree supports a seemingly scattered presence of MetE among species of Chlorophyta and diatoms, as noted previously [4,54]. The continually decreased expression of metE in the presence of excess vitamin B12 might have contributed toward the loss of the gene among species of Chlorophyta and diatoms [2,4,54]. Similarly, metE genes in dinoflagellates exhibited a seemingly scattered presence even among closely related species, e.g., S. microadriaticum possesses metE, whereas Symbiodinium sp. CCMA128 does not. In addition, metE was found to be absent in two parasitic dinoflagellates, Amoebophrya sp. Ameob2 and Hematodinium sp. SG-2012, suggesting that heterotrophy might have also influenced the presence of metE. Gene-loss, however, does not account for the absence of the N-terminal domain of metE genes in dinoflagellates. Helliwell et al. [55] recently described the evolution of a B12-dependent clone of the green alga C. reinhardtii being derived from a vitamin B12-independent clone cultured for fewer than 500 generations in the presence of vitamin B12 caused by a transposable element that was integrated into metE and knocked out the gene function. This mechanism, however, also does not account for the absence of N-terminal domain in dinoflagellates. Another study proposed that the presence or absence of metE in diatoms follows a biogeographic-relevant pattern that is determined by B12 availability, e.g., the species from the Southern Ocean, where vitamin B12 is generally low, would retain metE [54]. But this is obviously not the case for dinoflagellates which appeared to be uniformly lacking a functional metE. In addition, two non-dinoflagellate species of Alveolata (Chromera velia and Vitrella brassicaformis) have metE genes with complete N- and C-terminal domains but are not clustered with dinoflagellates in the two phylogenetic trees. Therefore, if we accept that the N-terminal domain barrel of metE evolved via gene duplication from the C-terminal domain polypeptide as proposed by Pejchal and Ludwig [56], the absence of N-terminal domain in dinoflagellates may be simply hypothesized as that the abovementioned gene duplication has either never happened or happened but lost the N-terminal during the very early evolution of a common ancestor of all existing dinoflagellates. This hypothesis needs further investigation. As mentioned earlier, the commonality of heterotrophy among dinoflagellates may have presented little evolutionary pressure to maintain a functional metE thereby partly facilitating the loss of the N-terminal domain. In addition, the phylogenetic analysis of the C-terminal domain of MetE showed that 8 species of dinoflagellates have different isoforms of MetE, including the three species (C. fusus, K. brevis, and Scrippsiella acuminata) that were found to have MetE in Nef et al. [31]. Some isoforms exhibited higher sequence similarity to, and thus formed a sister subclade with, the C-terminal domains of those complete MetE that function normally, while the others were not and thus formed a separate group containing most of dinoflagellate MetE in the trees (e.g., S. acuminata MetE isoform 2 shared 22.37% identity with C-terminal domain of MetE of E. coli and isoform 1 was 15.04%, see Supplementary Data 1 for more details about other sequences). These results suggest that all isoforms of dinoflagellate metE and those complete metE genes have a common ancestral origin, while the lower similarity in the C-terminal domains between most isoforms of dinoflagellates and those complete metE genes may partly explain why the presence of incomplete metE has been rarely detected (or annotated) from dinoflagellates in previous studies.
4.3. Transcriptional responses of metH and metE of dinoflagellates at different vitamin B12 levels
According to Tang et al. [3], most dinoflagellates previously investigated (41 of 45 species, 91%) were vitamin B12 auxotrophs, five of which plus three more species (A. insuetum, H. rotundata, and Symbiodinium sp. CCMA128) were affirmed here as B12 auxotroph via culturing and full-length cloning of metH and metE in this study. While all 8 species exhibited restricted growth at low levels of B12, two, H. rotundata and Symbiodinium sp. CCMA128, were found to completely lack metE.
While both forms of methionine synthase (i.e., MetH and MetE) catalyze the conversion of homocysteine to methionine [43], MetH has a much greater catalytic activity (100-fold) than MetE [43], indicating MetH would be used preferentially in the presence of vitamin B12 if both forms are present in an organism. The expression of metE is, therefore, strongly repressed when the availability of B12 is high (e.g., in C. reinhardtii and Phaeodactylum tricornutum) [2,4]. This difference in catalytic activity also accounts for the 1,000-fold increase in the expression of metE in the diatom, F. cylindrus, in response to a decrease of vitamin B12 and a significantly decreased expression in responding to a re-supplementation of B12, in parallel with the increased expression of metH [54]. More generally speaking, the expressions of functional metE and metH behave in an asymmetric compensatory mechanism. More MetE proteins would be expressed to perform the catalytic activity as needed when vitamin B12 is insufficient and vice versa. Therefore, the expression of a normally functioning metE would be strongly up-regulated in the absence or at a limiting level of B12. In the diatoms Thalassiosira pseudonana and Pseudo-nitzschia granii, both possessing metH only, the expression of metH was negatively correlated with levels of B12 [54,57,58], a pattern matching our results for the expression of metH in H. rotundata and Symbiodinium sp. CCMA128 (both possess metH only also). For six other dinoflagellates possessing both metH and the incomplete metE, the expression of metH also exhibited a similar negative feedback trend with B12 concentrations except for A. sanguinea whose expression of metH decreased in response to the re-supplementation of B12. The unexpected response of metH expression to B12 concentration in A. sanguinea was observed in all repeated experiments and requires more intensive examination in the future.
The expression of metE (including multiple isoforms for some species) in six dinoflagellates was not directly regulated by the availability of B12. For instance, the highest difference in the expression of metE between minus-B12 and plus-B12 cultures before re-supplementation of B12 was only a two-fold change, much less than that expected for a normally functioning metE (e.g., 1,000-fold difference in metE of F. cylindrus, see Ellis et al. [54]). According to González et al. [43], while both MetH and MetE catalyze the conversion of homocysteine to methionine, MetH has a much greater catalytic activity than MetE (100-fold). Therefore, if an organism has both genes, it will be forced to switch on metE transcription at the B12-depleted condition, and this expression would be significantly up-regulated by B12 limitation in order to meet the similar need for methionine due to the much lower efficiency of MetE. Given the expression patterns of metH and metE in algal species (e.g., Chlorophyta and diatoms) with functional metH and metE and in our dinoflagellates, and the confirmed auxotrophic requirements for vitamin B12, we conclude that the metE genes in dinoflagellates, if present, do not function as genuine B12-independent methionine synthases.
5. Conclusion
By performing phylogenetic and sequence analyses of the methionine synthases genes metH and metE in more than 100 species including 58 dinoflagellates and examining the growth and transcriptional responses of the two genes in eight dinoflagellates exposed to different vitamin B12 concentrations, we elucidated the genetic basis of vitamin B12 auxotrophy of dinoflagellates: 1) Most dinoflagellates (40 of 58) possess metE isoforms that lacked the N-terminal domain and thus do not function as B12-independent methionine synthase, 2) Some (18 of 58) dinoflagellates do not possess metE, 3) metE genes of dinoflagellates are phylogenetically separated from those functional metE genes, and 4) all dinoflagellates possess functional metH as B12-dependent methionine synthase, and thus survive utilizing exogenous sources of vitamin B12. We, therefore, conclude that it is the absence of N-terminal domain in metE for most dinoflagellates or the complete absence of the deficient metE for others that accounts for B12 auxotrophy in dinoflagellates. We further hypothesize that all dinoflagellates are likely auxotrophs of vitamin B12, which requires more intensive investigations. The results presented here provide a mechanistic explanation for the mounting evidence showing that vitamin B12 plays vital roles in regulating the dynamics of dinoflagellates and their HABs in ecosystems around the world [15,16,18,25,29]. We also anticipate the outputs of this research will provide fundamental insights for the monitoring, forecasting, and prevention of HABs, such as integrating the measurement of vitamin B12 concentration in the routine monitoring program of HABs.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest in this work.
Acknowledgments
We thank Drs. Yunyan Deng, Lixia Shang, Zhaoyang Chai, Huijiao Yang and Yuyang Liu from Institute of Oceanology, Chinese Academy of Sciences, China for their technical and logistical assistance. We acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 41776125), the Key Deployment Project of Centre for Ocean Mega-Research of Science, Chinese Academy of Sciences (Grant No. COMS2019Q09), the Science & Technology Basic Resources Investigation Program of China (Grant No. 2018FY100200), National Natural Science Foundation of China (Grants Nos. 41976134 and 61533011), the Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (Grant No. 2018SDKJ0504-2). Efforts of CJG were supported by the Chicago Community Trust.
Biographies

Siheng Lin obtained his BS from Xiamen University and the degrees of MS in Agriculture and PhD in Marine Ecology from the University of Chinese Academy of Sciences (Institute of Oceanology) in 2020. He is now an associate professor at Minnan Normal University. His research focuses on molecular ecology of phytoplankton.

Ying-Zhong Tang left SUNY Stony Brook and joined Institute of Oceanology of CAS as a professor in 2013. He received his BS (1987), MS (1990), and Ph.D. (2003) from Wuhan University and National University of Singapore, respectively. He currently is the PI of Ecology of Harmful Algal Blooms (HABs) Laboratory of IOCAS, the subject editor of Harmful Algae, associate editor-in-chief of Journal of Oceanology and Limnology, and guest editors for Harmful Algae and IJERPH. His research includes multiple facets of HABs, particularly that of dinoflagellates. He has published 100 papers in PNAS, Water Research, Molecular Ecology, and other preeminent journals.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fmre.2021.12.014.
Appendix. Supplementary materials
References
- 1.Droop M. Vitamin B12 in marine ecology. Nature. 1957;180(4594):1041–1042. [Google Scholar]
- 2.Croft M.T., Lawrence A.D., Raux-Deery E., et al. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature. 2005;438(7064):90–93. doi: 10.1038/nature04056. [DOI] [PubMed] [Google Scholar]
- 3.Tang Y.Z., Koch F., Gobler C.J. Most harmful algal bloom species are vitamin B1 and B12 auxotrophs. Proc. Natl. Acad. Sci. USA. 2010;107(48):20756–20761. doi: 10.1073/pnas.1009566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helliwell K.E., Wheeler G.L., Leptos K.C., et al. Insights into the evolution of vitamin B12 auxotrophy from sequenced algal genomes. Mol. Biol. Evol. 2011;28(10):2921–2933. doi: 10.1093/molbev/msr124. [DOI] [PubMed] [Google Scholar]
- 5.Helliwell K.E. The roles of B vitamins in phytoplankton nutrition: new perspectives and prospects. New Phytol. 2017;216(1):62–68. doi: 10.1111/nph.14669. [DOI] [PubMed] [Google Scholar]
- 6.Croft M.T., Warren M.J., Smith A.G. Algae need their vitamins. Eukaryot. Cell. 2006;5(8):1175–1183. doi: 10.1128/EC.00097-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Droop M. Vitamins, phytoplankton and bacteria: symbiosis or scavenging? J. Plankton Res. 2007;29(2):107–113. [Google Scholar]
- 8.Sañudo-Wilhelmy S., Gómez-Consarnau L., Suffridge C., et al. The role of B vitamins in marine biogeochemistry. Ann. Rev. Mar. Sci. 2014;6:339–367. doi: 10.1146/annurev-marine-120710-100912. [DOI] [PubMed] [Google Scholar]
- 9.Doxey A.C., Kurtz D.A., Lynch M.D., et al. Aquatic metagenomes implicate Thaumarchaeota in global cobalamin production. ISME J. 2015;9(2):461–471. doi: 10.1038/ismej.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heal K.R., Qin W., Ribalet F., et al. Two distinct pools of B12 analogs reveal community interdependencies in the ocean. Proc. Natl. Acad. Sci. USA. 2017;114(2):364–369. doi: 10.1073/pnas.1608462114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swift D.G., Guillard R.R. Unexpected response to vitamin B12 of dominant centric diatoms from the spring bloom in the Gulf of Maine (Northeast Atlantic Ocean) J. Phycol. 1978;14(4):377–386. [Google Scholar]
- 12.Swift D.G. Vitamin levels in the Gulf of Maine and ecological significance of vitamin B12 there. J. Mar. Res. 1981;39:375–403. [Google Scholar]
- 13.V. Stewart, H. Wahlquist, R. Burkey. Occurrence of vitamin B12 along the Gulf coast of Florida. Red Tide Studies, Pinellas to Collier Counties, 1963-1966: A Symposium; Fla Board Conserv. Mar. Lab. Prof. Pap. Ser. No. 9, St Petersburg, FL. 1967.
- 14.Bertrand E.M., Saito M.A., Rose J.M., et al. Vitamin B12 and iron colimitation of phytoplankton growth in the Ross Sea. Limnol. Oceanogr. 2007;52(3):1079–1093. [Google Scholar]
- 15.Koch F., Hattenrath-Lehmann T.K., Goleski J.A., et al. Vitamin B1 and B12 uptake and cycling by plankton communities in coastal ecosystems. Front. Microbiol. 2012;3:363. doi: 10.3389/fmicb.2012.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch F., Burson A., Tang Y.Z., et al. Alteration of plankton communities and biogeochemical cycles by harmful Cochlodinium polykrikoides (Dinophyceae) blooms. Harmful Algae. 2014;33:41–54. [Google Scholar]
- 17.Sañudo-Wilhelmy S., Cutter L.S., Durazo R., et al. Multiple B-vitamin depletion in large areas of the coastal ocean. Proc. Natl. Acad. Sci. USA. 2012;109(35):14041–14045. doi: 10.1073/pnas.1208755109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gobler C.J., Norman C., Panzeca C., et al. Effect of B-vitamins (B1, B12) and inorganic nutrients on algal bloom dynamics in a coastal ecosystem. Aquat. Microb. Ecol. 2007;49(2):181–194. [Google Scholar]
- 19.Gómez F. A quantitative review of the lifestyle, habitat and trophic diversity of dinoflagellates (Dinoflagellata, Alveolata) Syst. Biodivers. 2012;10(3):267–275. [Google Scholar]
- 20.Smayda T.J. Harmful algal blooms: their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997;42(5):1137–1153. [Google Scholar]
- 21.Wang D.Z. Neurotoxins from marine dinoflagellates: a brief review. Mar. Drugs. 2008;6(2):349–371. doi: 10.3390/md20080016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kellmann R., Stüken A., Orr R.J., et al. Biosynthesis and molecular genetics of polyketides in marine dinoflagellates. Mar. Drugs. 2010;8(4):1011–1048. doi: 10.3390/md8041011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker A.C. Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu. Rev. Ecol., Evol. Syst. 2003;34(1):661–689. [Google Scholar]
- 24.Lin S. Genomic understanding of dinoflagellates. Res. Microbiol. 2011;162(6):551–569. doi: 10.1016/j.resmic.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Koch F., Marcoval M.A., Panzeca C., et al. The effect of vitamin B12 on phytoplankton growth and community structure in the Gulf of Alaska. Limnol. Oceanogr. 2011;56(3):1023–1034. [Google Scholar]
- 26.Carlucci A., Bowes P. Production of vitamin B12, thiamine, and biotin by phytoplankton. J. Phycol. 1970;6(4):351–357. [Google Scholar]
- 27.Cruz-López R., Maske H. The vitamin B1 and B12 required by the marine dinoflagellate Lingulodinium polyedrum can be provided by its associated bacterial community in culture. Front. Microbiol. 2016;7:560. doi: 10.3389/fmicb.2016.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sañudo-Wilhelmy S., Gobler C., Okbamichael M., et al. Regulation of phytoplankton dynamics by vitamin B12. Geophys. Res. Lett. 2006;33(4):L04604. [Google Scholar]
- 29.Joglar V., Álvarez-Salgado X.A., Gago-Martinez A., et al. Cobalamin and microbial plankton dynamics along a coastal to offshore transect in the Eastern North Atlantic Ocean. Environ. Microbiol. 2020;23:1559–1583. doi: 10.1111/1462-2920.15367. [DOI] [PubMed] [Google Scholar]
- 30.Ballou D.P., Neil E., Marsh G. Coenzyme B12 (cobalamin)-dependent enzymes. Essays Biochem. 1999;34:139–154. doi: 10.1042/bse0340139. [DOI] [PubMed] [Google Scholar]
- 31.Nef C., Henry C., Nicolau É., et al. Cobalamin scarcity modifies carbon allocation and impairs DMSP production through methionine metabolism in the haptophyte microalgae Tisochrysis lutea. Front. Mar. Sci. 2020;7 [Google Scholar]
- 32.Lu D., Qi Y., Gu H., et al. Causative species of harmful algal blooms in Chinese coastal waters. Algol. Stud. 2014;145(1):145–168. [Google Scholar]
- 33.Guillard R.R.L. In: Cultures of Marine Invertebrate Animals. Smith W.L., Chanley M.H., editors. 1975. Culture of phytoplankton for feeding marine invertebrates; pp. 29–60. [Google Scholar]
- 34.Altschul S.F., Gish W., Miller W., et al. Basic local alignment search tool. J. Mol. Evol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 35.Keeling P.J., Burki F., Wilcox H.M., et al. The Marine microbial eukaryote transcriptome sequencing project (MMETSP): illuminating the functional diversity of eukaryotic life in the oceans through transcriptome sequencing. PLOS Biol. 2014;12(6) doi: 10.1371/journal.pbio.1001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bateman A., Coin L., Durbin R., et al. The Pfam protein families database. Nucleic Acids Res. 2004;32(suppl_1):D138–D141. doi: 10.1093/nar/gkh121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price M.N., Deutschbauer A.M., Arkin A.P. Four families of folate-independent methionine synthases. PLOS Genet. 2021;17(2) doi: 10.1371/journal.pgen.1009342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41(41):95–98. [Google Scholar]
- 39.Nguyen L.T., Schmidt H.A., Von Haeseler A., et al. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H., Hou Y., Miranda L., et al. Spliced leader RNA trans-splicing in dinoflagellates. Proc. Natl. Acad. Sci. USA. 2007;104(11):4618–4623. doi: 10.1073/pnas.0700258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H., Zhuang Y., Gill J., et al. Proof that dinoflagellate spliced leader (DinoSL) is a useful hook for fishing dinoflagellate transcripts from mixed microbial samples: Symbiodinium kawagutii as a case study. Protist. 2013;164(4):510–527. doi: 10.1016/j.protis.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Lin S., Zhang H., Zhuang Y., et al. Spliced leader–based metatranscriptomic analyses lead to recognition of hidden genomic features in dinoflagellates. Proc. Natl. Acad. Sci. USA. 2010;107(46):20033–20038. doi: 10.1073/pnas.1007246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.González J.C., Banerjee R.V., Huang S., et al. Comparison of cobalamin-independent and cobalamin-dependent methionine synthases from Escherichia coli: two solutions to the same chemical problem. Biochemistry. 1992;31(26):6045–6056. doi: 10.1021/bi00141a013. [DOI] [PubMed] [Google Scholar]
- 44.Ravanel S., Gakière B., Job D., et al. The specific features of methionine biosynthesis and metabolism in plants. Proc. Natl. Acad. Sci. USA. 1998;95(13):7805–7812. doi: 10.1073/pnas.95.13.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banerjee R.V., Johnston N.L., Sobeski J., et al. Cloning and sequence analysis of the Escherichia coli metH gene encoding cobalamin-dependent methionine synthase and isolation of a tryptic fragment containing the cobalamin-binding domain. J. Biol. Chem. 1989;264(23):13888–13895. [PubMed] [Google Scholar]
- 46.Li Y.N., Gulati S., Baker P.J., et al. Cloning, mapping and RNA analysis of the human methionine synthase gene. Hum. Mol. Genet. 1996;5(12):1851–1858. doi: 10.1093/hmg/5.12.1851. [DOI] [PubMed] [Google Scholar]
- 47.Shoguchi E., Shinzato C., Kawashima T., et al. Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Curr. Biol. 2013;23(15):1399–1408. doi: 10.1016/j.cub.2013.05.062. [DOI] [PubMed] [Google Scholar]
- 48.Lin S., Cheng S., Song B., et al. The Symbiodinium kawagutii genome illuminates dinoflagellate gene expression and coral symbiosis. Science. 2015;350(6261):691–694. doi: 10.1126/science.aad0408. [DOI] [PubMed] [Google Scholar]
- 49.Ferrer J.L., Ravanel S., Robert M., et al. Crystal structures of cobalamin-independent methionine synthase complexed with zinc, homocysteine, and methyltetrahydrofolate. J. Biol. Chem. 2004;279(43):44235–44238. doi: 10.1074/jbc.C400325200. [DOI] [PubMed] [Google Scholar]
- 50.Yu L., Zhang Y., Li M., et al. Comparative metatranscriptomic profiling and microRNA sequencing to reveal active metabolic pathways associated with a dinoflagellate bloom. Sci. Total. Environ. 2020;699 doi: 10.1016/j.scitotenv.2019.134323. [DOI] [PubMed] [Google Scholar]
- 51.Meng A., Corre E., Probert I., et al. Analysis of the genomic basis of functional diversity in dinoflagellates using a transcriptome-based sequence similarity network. Mol. Ecol. 2018;27(10):2365–2380. doi: 10.1111/mec.14579. [DOI] [PubMed] [Google Scholar]
- 52.Nef C., Jung S., Mairet F., et al. How haptophytes microalgae mitigate vitamin B12 limitation. Sci. Rep. 2019;9:8417. doi: 10.1038/s41598-019-44797-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bachvaroff T.R., Gornik S.G., Concepcion G.T., et al. Dinoflagellate phylogeny revisited: Using ribosomal proteins to resolve deep branching dinoflagellate clades. Mol. Phylogenet. Evol. 2014;70:314–322. doi: 10.1016/j.ympev.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellis K.A., Cohen N.R., Moreno C., et al. Cobalamin-independent methionine synthase distribution and influence on vitamin B12 growth requirements in marine diatoms. Protist. 2017;168(1):32–47. doi: 10.1016/j.protis.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Helliwell K.E., Collins S., Kazamia E., et al. Fundamental shift in vitamin B12 eco-physiology of a model alga demonstrated by experimental evolution. ISME J. 2015;9(6):1446–1455. doi: 10.1038/ismej.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pejchal R., Ludwig M.L. Cobalamin-independent methionine synthase (MetE): a face-to-face double barrel that evolved by gene duplication. PLOS Biol. 2005;3(2):e31. doi: 10.1371/journal.pbio.0030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bertrand E.M., Allen A.E., Dupont C.L., et al. Influence of cobalamin scarcity on diatom molecular physiology and identification of a cobalamin acquisition protein. Proc. Natl. Acad. Sci. USA. 2012;109(26):E1762–E1771. doi: 10.1073/pnas.1201731109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen N.R., Ellis K.A., Burns W.G., et al. Iron and vitamin interactions in marine diatom isolates and natural assemblages of the Northeast Pacific Ocean. Limnol. Oceanogr. 2017;62(5):2076–2096. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.