Highlights
-
•
HepG2 cells was exposed to low-concentration TBBPA with long term.
-
•
TBBPA interferes with the regulation of transcriptome related to endocrine system.
-
•
Long-term TBBPA exposure gradually upregulates thyroid hormone receptor expression.
-
•
TBBPA activates Ras signaling pathway by mediating the thyroid hormone pathway.
Keywords: TBBPA, Low-concentration exposure, Endocrine system, Thyroid hormone receptor, Ras signaling pathway
Abstract
Tetrabromobisphenol A (TBBPA) is a flame retardant that adversely affects the environment and human health. The present study exposed HepG2 cells to low concentrations of TBBPA daily to investigate the changes in gene regulation, mainly related to pathways associated with the endocrine system. The quantitative polymerase chain reaction (qPCR) confirmed that prolonged exposure gradually activated the thyroid hormone and parathyroid hormone signaling pathways. The expression levels of genes related to the thyroid hormone signaling pathway were upregulated (1.15−8.54 times) after five generations of exposure to 1 and 81 nM TBBPA. Furthermore, co-exposure to 81 nM TBBPA and 0.5 nM thyroid hormone receptor antagonist for five generations significantly reduced the expression of thyroid hormone and parathyroid hormone receptors. Meanwhile, 81 nM TBBPA inhibited the activation of the Ras pathway and downregulated Ras gene expression level (3.7 times), indicating the association between the toxic effect and thyroid hormone receptors. Additionally, our experiments revealed that the thyroid hormone pathway regulated the induction of the Ras signaling pathway by TBBPA. The study thus proves that daily exposure to TBBPA interferes with the thyroid hormone signaling pathway and subsequently the endocrine system.
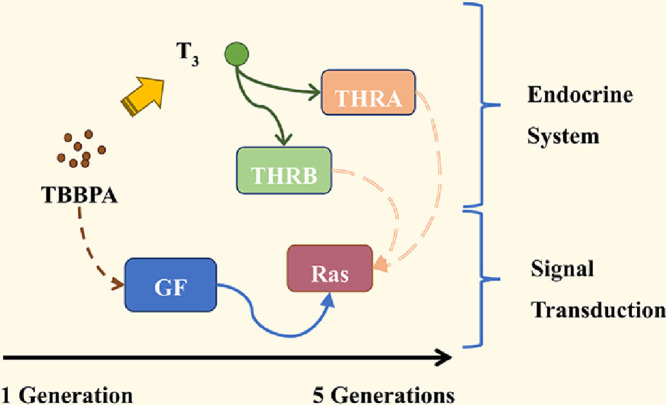
Graphical abstract
.
1. Introduction
Tetrabromobisphenol A (TBBPA) is the most widely used brominated flame retardant, which accounts for more than 60% of production [1]. It enters the human body during production and use through various routes, including inhalation, ingestion, and dermal contact [2], [3], [4]. With the increasing use of TBBPA-containing products, the concentration of TBBPA in the indoor dust in Eastern China has attained relatively high levels, increasing exposure risks in humans [5]. Numerous studies have detected TBBPA in human plasma and found that the concentration in people with occupational exposure is higher than that in the general population [6,7]. Animal studies have shown that TBBPA perturbs erythropoiesis [8], is toxic to liver [9], and imposes neurodevelopmental [10,11] and endocrine toxic effects [12,13] and carcinogenic risk [14,15]. Research has also found that different toxicities are associated with each other, and endocrine toxicity induces estrogen interference toxicity and carcinogenic risk [16,17]. Therefore, it is necessary to study the endocrine toxicity of TBBPA in humans.
Gene expression regulates protein expression levels and, in turn, influences the physiological and metabolic behavior of an organism. Currently, the assessment of genotoxicity induced by brominated flame retardants is an important field of toxicity research and demands risk assessment. However, studies on the genotoxic effects of TBBPA at the molecular level are relatively scarce. To better understand the toxic effects and the potential risk of brominated flame retardants, the toxic mechanisms at the molecular level need to be elucidated. Generally, DNA damage assay, transcriptome analysis, micronucleus test, and chromosomal aberration analysis are used to assess the genotoxic effects; however, the majority are used in fish exposed to a variety of chemicals and pollutants [18,19].
Biomarkers are biochemical markers that demonstrate changes under the influence of an external stimulus and are used as indicators to assess the structural or functional changes in organs, tissues, and cells. Therefore, the detection of abnormal concentrations of biomarkers will reveal the influence of a specific stimulus in organisms [20]. Identifying biomarkers can help understand the characteristics of TBBPA injury and track the consequences. So far, no specific molecular biomarker has been identified to assess the genotoxic effects of TBBPA. The experiment of two generations of TBBPA exposure in Sprague-Dawley rats showed no significant effect of TBBPA on the reproduction, growth, or development of their offspring, even when the exposure concentration was 1000 mg/kg Body Weight/day [13]. However, in a study evaluating the effect of TBBPA on the sperm quality of common carp, DNA damage was significantly higher in the 2.5 nM TBBPA exposure group than that in the control group, suggesting the effect of low-concentration exposure on sperm quality and DNA integrity [21]. In addition, 367 nM TBBPA exposure in a rat model damaged the sperm DNA and changed the epigenetic markers of sperm chromatin [22]. A recent study in fish showed that 9.36 μM TBBPA exposure significantly increased oxidative stress and DNA damage [23]. These studies indicate that although low levels of TBBPA do not directly affect the expression of the important proteins related to reproduction, growth, and development in fish and mice, TBBPA exposure may induce DNA damage in carp and rats. However, the potential biomarkers of TBBPA and the mechanism of gene regulation under TBBPA exposure are still unclear.
A whole-gene expression impact analysis can evaluate the toxic effects of TBBPA on early development by modeling the early stages of embryonic development using mouse embryonic system cells (ESC) [24]. The present study hypothesizes that exposure to TBBPA may lead to potential developmental toxicities, including effects on the nervous and myocardial/skeletal muscular systems. Moreover, TBBPA disrupted the endocrine activities in mice through prolactin signaling [24]. Studies on the TBBPA exposure in zebrafish based on transcriptome analysis using RNA-seq showed effects on the expression of genes related to physiological processes, such as neural development, muscle filament gliding and contracting, extracellular matrix disintegration, and tissue changes. In addition, exposure to TBBPA increased the expression of genes encoding uridine diphosphate glucuronidase (UGT) in zebrafish, which may impact thyroxine (T4) metabolism and lead to neurobehavioral changes [11]. Overall, these results indicate that exposure to TBBPA can induce changes in gene expression in mice and zebrafish and interfere with their endocrine system, neural development, and other physiological activities. However, the risk of gene regulation caused by exposure to TBBPA in the human body is still unclear.
Based on the animal exposure studies, there was a proportional accumulation of TBBPA in the liver and TBBPA had a risk of inducing liver cancer [25], [26], [27]. Therefore, liver cancer cells were selected to study the effects of daily exposure to low-dose TBBPA on liver cancer. Our previous studies have shown that TBBPA exposure at micromolar and millimolar concentrations can severely induce cell damage and death, while nanomolar concentrations can induce oxidative stress leading to apoptosis [28,29]. This study investigated the effects of low-concentration daily exposure of TBBPA on gene expression in HepG2 cells. The study verified the expression of receptors and expression levels of genes. The toxicity under daily exposure to TBBPA at low concentrations was explored based on previous studies on cell physiological toxicity. The present study's findings may provide a basic reference for further research on TBBPA toxicity.
2. Material and methods
2.1. Materials
The human hepatocellular liver carcinoma cell line (HepG2) was generously provided by the Guangzhou Medical University, China. TBBPA (97%) and dimethyl sulphoxide (DMSO, 99%) were purchased from Sigma-Aldrich (USA). DMEM, fetal calf serum (FBS), penicillin (100 U/mL)-streptomycin (0.1 mg/mL), and Trypsin-EDTA were purchased from Gibco (Thermo Fisher, USA). Phosphate-buffered saline (PBS) was purchased from Sangon (Shanghai, China), and the TRIzol reagent was purchased from Invitrogen (USA). TB Green® Premix Ex Taq™ II was purchased from Takara Biotechnology Co. (Dalian, China).
2.2. Cell culture and TBBPA exposure
The HepG2 cell line was grown in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin at 37 °C in a 5% CO2 humidified cell culture incubator. According to the concentration of TBBPA detected in the plasma of occupationally exposed people (2.5 pg/g to 3.99 ng/g lipid weight), the concentration of TBBPA exposure in occupationally exposed people was calculated as 0.1–100 nM [6,7,[30], [31], [32], [33], [34]]. Therefore, we chose 1 and 81 nM concentrations to simulate the exposure pattern of occupationally exposed people. The TBBPA stock solution was prepared in dimethyl sulphoxide (DMSO, a final DMSO concentration of 0.1%). The medium containing TBBPA was changed immediately after the cells adhered to the culture plate and daily. Three independent experiments were conducted for each low-concentration or high-concentration TBBPA exposure group.
2.3. RNA isolation and sequencing
After TBBPA exposure, total RNA was extracted from the HepG2 cells using the TRIzol reagent. The cDNA was obtained by reverse-transcription using MonScript™ RTIII All-in-One Mix with dsDNase (Wuhan, China). Real-time quantitative polymerase chain reaction (qPCR) was performed using TB Green® Premix Ex Taq™ II on a CFX96 Touch System (Bio-Rad, USA). The real-time qPCR procedure is provided in the Supporting Information, and the primer sequences are shown in Table S1.
RNA-seq was performed at the Majorbio Company (Shanghai, China) following the standard procedures using total RNA obtained from HepG2 cells treated with TBBPA, DMSO, or DMEM. The sequencing data were further used to analyze the genes with differential expression between the low-concentration and high-concentration groups. The edgeR software was used to analyze the differences, using a screening threshold of |log2FC|≥ 1 and p < 0.05. The free online Majorbio Cloud Platform was used for data analysis.
2.4. qPCR assay
The real-time qPCR program had a 3 min hot start at 95 °C, followed by 40 cycles of 10 s at 95 °C, 10 s at Tm, and 30 s at 72 °C. The melt curve was used to ensure the specificity of each primer, and GAPDH was used as the internal reference (Sangon, Shanghai, China).
2.5. Statistical analysis
Data are expressed as average ± standard deviation. Statistical significance was evaluated using the Student's t-test. Mean values were considered significantly different at p < 0.05. Microsoft Excel was used to perform all statistical analyses.
3. Results
3.1. Daily exposure to a low concentration of TBBPA regulates endocrine system-related pathways in HepG2 cells
In this study, HepG2 cells were cultured in complete DMEM, medium containing DMSO, medium containing 1 nM TBBPA, and medium containing 81 nM TBBPA for five generations and termed as blank control group (CONTROL), negative control group (DMSO), relatively low-concentration group (LOW), and relatively high-concentration group (HIGH), respectively. The reference source of genes used during sequencing was that of the human genome (Homo_sapiens), and the reference genome version used was GRCh38. P13 (http://asia.ensembl.org/Homao_sapiens/Info/Index).
The quality control analysis of the sequenced samples (Table 1) revealed an average error rate of the bases less than 0.1%, and the quality assessment of the sequencing data revealed that the percentage of the bases with sequencing quality above 99% (Q20) and 99.9% (Q30) accounted for 85% and 80% of the total bases, respectively. The sum of guanine (G) and cytosine (C) accounted for 50 to 52% of the total bases. These values indicate that the constructed library and sequencing quality were high, and the sequencing results were reliable.
Table 1.
Quality control data statistics.
| Sample | Clean reads | Clean bases | Error rate(%) | Q20(%) | Q30(%) | GC content(%) |
|---|---|---|---|---|---|---|
| C_1 | 46957270 | 7003018540 | 0.0252 | 97.94 | 94 | 50.75 |
| C_2 | 47372722 | 7051154432 | 0.025 | 98 | 94.13 | 50.83 |
| D_1 | 50403238 | 7495431782 | 0.0252 | 97.91 | 93.92 | 50.86 |
| D_2 | 49982068 | 7437016280 | 0.0256 | 97.75 | 93.55 | 50.78 |
| H_1 | 47415256 | 7085857585 | 0.0257 | 97.74 | 93.52 | 51.23 |
| H_2 | 48176678 | 7180706951 | 0.0254 | 97.86 | 93.8 | 51.05 |
| L_1 | 55170016 | 8232221656 | 0.0253 | 97.89 | 93.88 | 50.85 |
| L_2 | 44677968 | 6661535759 | 0.0262 | 97.54 | 93.06 | 50.84 |
Clean reads: the total number of items in sequencing data after quality control; Clean bases: the total number of Clean reads multiplied by the length of reads after quality control; Error rate (%): the average Error rate of sequencing base corresponding to the quality control data; Q20 (%) and Q30 (%): the quality of sequencing data after quality control is evaluated. Q20 and Q30, respectively refer to the percentage of the total bases whose sequencing quality is above 99% and 99.9%; GC Content (%): The percentage of the sum of G and C bases corresponding to the quality control data in the total base.
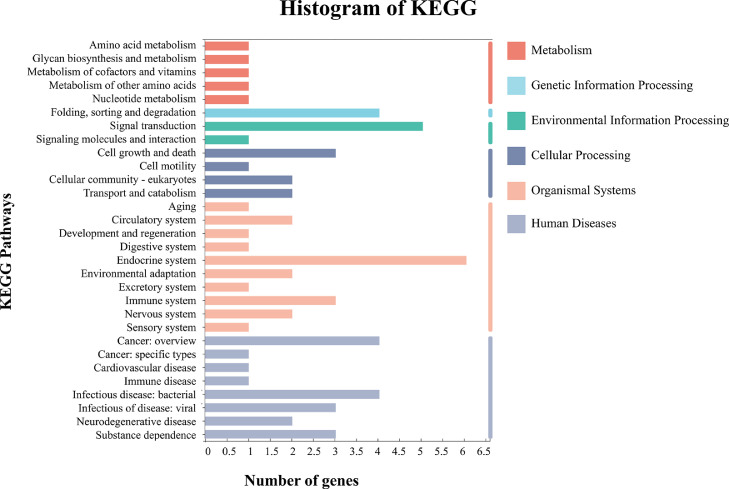
A detailed bioinformatic analysis was performed using the sequencing results, and a set of differentially expressed genes was identified. KEGG functional enrichment analysis of these differentially expressed genes revealed that most genes enriched after the daily exposure to low-concentration TBBPA were related to endocrine system-related pathways (Fig. 1). Among them, six major signal pathways, including the oxytocin signaling pathway, parathyroid hormone synthesis, secretion and action, thyroid hormone signaling pathway, insulin signaling pathway, progesterone-mediated oocyte maturation, and estrogen signaling pathway, were identified.
Fig. 1.
Bioinformatics analysis based on RNA sequencing of HepG2 cells, KEGG histogram of pathway enrichment analysis of co-expressed genes at the 1 and 81 nM exposure groups.
3.2. Expression levels of the thyroid hormone-related genes increases in the subsequent generations
Furthermore, qPCR was performed to verify the regulation of differentially expressed genes in the endocrine system of HepG2 cells after daily exposure to low-concentration TBBPA (Fig. 2). After exposure to low-concentration TBBPA for five generations, the genes related to the thyroid hormone signaling pathway were upregulated 1.31−7.37 folds in HepG2 cells. These genes were mainly associated with the Ras homolog (RHEB2) [35], solute carrier family 16 member 2 (SLC16A2) [36], thyroid hormone receptor beta (THRB) [37], type Ⅱ thyroxine 5′-deiodinase (DIO2) [38], Ras-related protein M-Ras (MR) [39], thyroid hormone receptor alphaⅠ (THRA1) [40], thyroid hormone receptor alphaⅠ type 2 (THRA2) [41], Ras-related protein H-Ras (HR), [42] and protein kinase A (PKA) [43]. In addition, the expression levels of genes encoding PKA and parathyroid hormone receptor (PTHR1) were upregulated 2.11−4.46 folds in HepG2 cells after exposure to TBBPA, probably related to the parathyroid hormone signaling pathway [44]. The expression levels of genes encoding phosphorylase kinase alpha/beta subunit (PHKA) and phosphoenolpyruvate carboxykinase (PCK1) were upregulated 4 folds after TBBPA exposure for five generation. These two genes and RHEB2 are related to insulin signaling pathway [45], [46], [47]. The expression levels of genes encoding ADP-ribosylation factor-like protein 5A (ARL5A) and voltage-dependent calcium channel beta-4 (CACNB4), related to oxytocin signaling pathway [48,49], were upregulated 1.22 and 1.89 folds, respectively, after 81 nM TBBPA exposure for five generations. Additionally, oocyte meiosis pathway-related gene speedy/RINGO cell cycle regulator family member E1 (RINGO1) [50] and estrogen pathway-related gene type I cytoskeletal 17 (KRT17) [51] were upregulated 1.18−2.26 folds after TBBPA exposure for five generations. These observations together indicate that the daily exposure to TBBPA upregulated genes related to endocrine system pathways in HepG2 cells, especially those related to thyroid hormone signaling pathway. The initial analysis revealed that the highest proportion of genes expressed after daily exposure to TBBPA were associated with the thyroid hormone signaling pathway, and therefore, we further focused on the genes related to the thyroid hormone and parathyroid hormone pathway. We investigated the expression levels of thyroid hormone- and parathyroid hormone-related genes in each generation of HepG2 cells exposed to TBBPA for five successive generations.
Fig. 2.
The expression of endocrine system-related genes in HepG2 cells after long-term exposure to TBBPA by the q-PCR test verification.
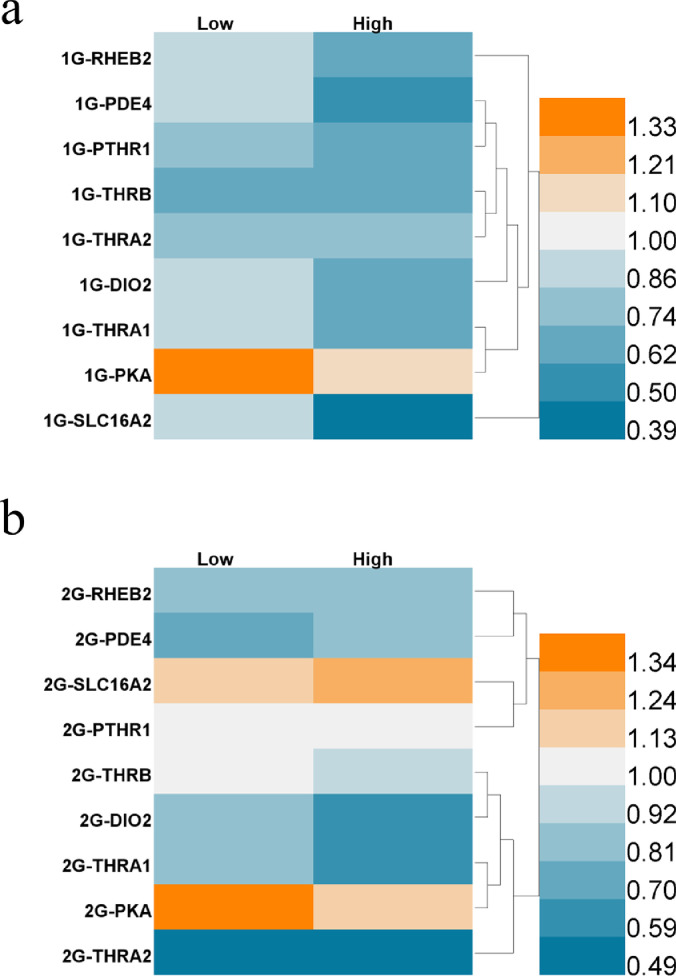
Genes related to the thyroid and parathyroid hormone signaling pathway were found to be downregulated in HepG2 cells after initial exposure to TBBPA for the first two generations (Fig. 3). Compared with the negative control group, genes, except PKA, were downregulated (2.4−61.5%) in HepG2 cells exposed to low and high concentrations of TBBPA in the first generation (Fig. 3a). These observations indicate that when HepG2 cells were initially exposed to TBBPA, the thyroid hormone signaling pathway was inhibited, thereby inhibiting the physiological activity of the cells. After exposure to TBBPA for more than two generations, the parathyroid hormone receptor gene PTHR1 began to recover its expression level (Fig. 3b), indicating differences in the effects of daily exposure and short-term stimulation on the thyroid hormone pathways. After exposure to 81 nM TBBPA, PTHR1 was upregulated 1.13 times, and SLC16A2, the transmembrane transport protein of thyroid hormone T3, was upregulated 1.31 times. These results indicate that an initial TBBPA exposure could inhibit the expression of the receptors of the thyroid and parathyroid glands, either via contact inhibition or by interfering with the receptor gene transcription. However, after continuous exposure, the expression of genes regulating the transmembrane transport proteins related to thyroid hormones got upregulated. These results reveal that daily exposure to TBBPA can induce high expression of thyroid hormone-related receptor proteins and interfere with the thyroid hormone pathway in HepG2 cells.
Fig. 3.
Heat map of expression (relative to negative controls) of genes associated with thyroid and parathyroid pathways in HepG2 cells after exposure to TBBPA for (a) one generation, (b) two generations.
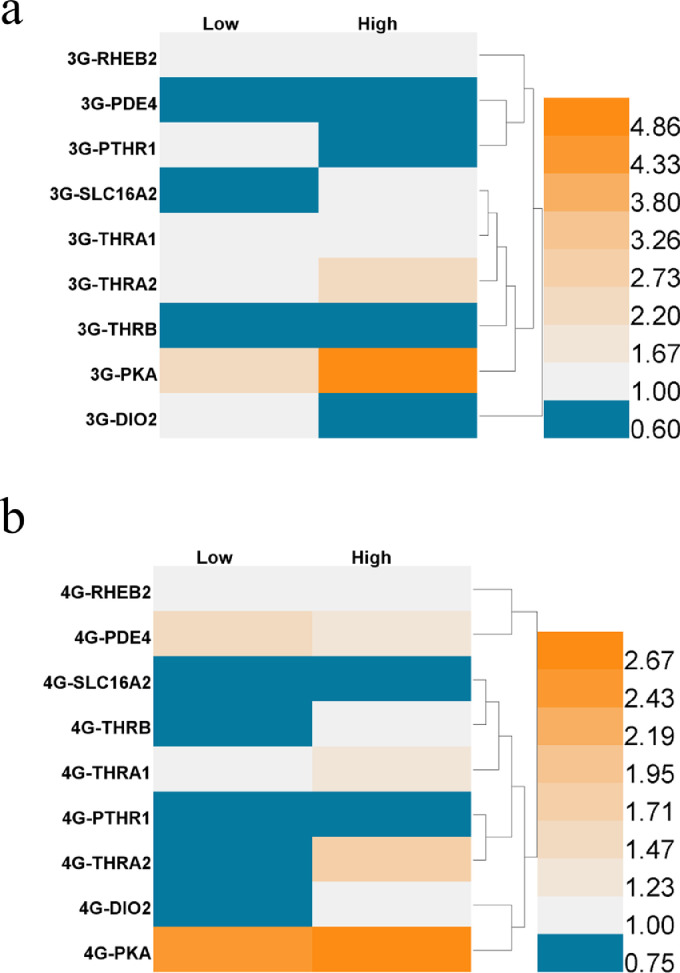
With prolonged exposure, the expression levels of thyroid hormone- and parathyroid hormone-related genes gradually increased in the third and fourth generations (Fig. 4a,b). In the third generation, the expression levels of other genes related to thyroid hormones and parathyroid hormones were upregulated 1.03 to 5.39 times (Fig. 4a), except for the cAMP-specific phosphodiesterase 4 (PDE4) (one of the downstream signals of PTHR1 [52]), SLC16A2, PTHR1, THRB, and DIO2. Among them, the expression levels of PDE4 decreased 31.4−39.8%, while SLC16A2, PTHR1, THRB, and DIO2 were slightly downregulated. Similarly, in the fourth generation, Ras homolog (RHEB2), PDE4, ThrA1, ThrA2, and PKA, one of the downstream signals of thyroid hormones, were stably upregulated 1.08−2.91 times (Fig. 4b). These results suggest that daily exposure to low concentrations of TBBPA may activate the Ras signaling pathway via the activation of thyroid hormone receptor alpha.
Fig. 4.
Heat map of expression (relative to negative controls) of genes associated with thyroid and parathyroid pathways in HepG2 cells after exposure to TBBPA for (a) three generations, (b) four generations.
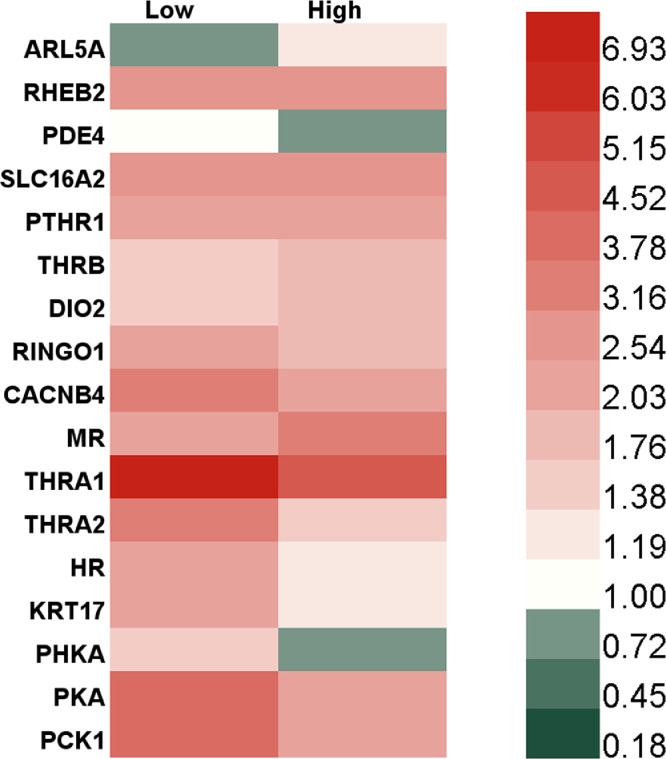
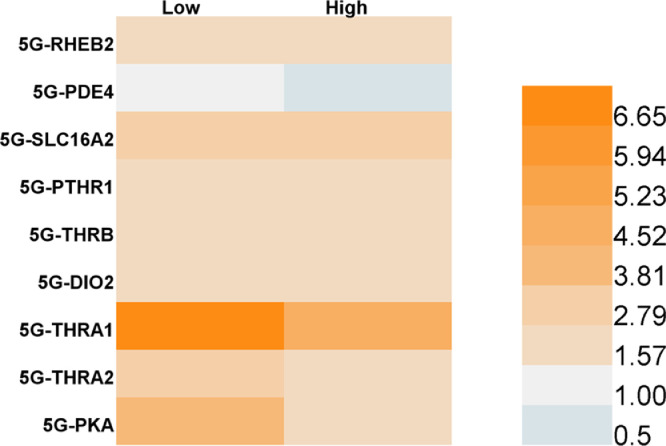
By the fifth generation, the genes related to thyroid and parathyroid gland pathways were basically upregulated, and the expression levels of other genes were 1.57−6.65 times higher than those in the negative control group, except that the PDE4 expression level was still down 3.6% after exposure to 81 nM TBBPA (Fig. 5). PDE4 regulates cAMP hydrolysis, and cAMP is the medium for PTHR to activate downstream signals. Therefore, the downregulation of PDE4 may lead to cAMP accumulation and promote PTH signaling pathway activation [53]. The downregulation of the PDE4 gene in HepG2 cells after daily exposure to TBBPA may be one of the mechanisms of the TBBPA-mediated activation of the parathormone signaling pathway. Among the upregulated genes mentioned above, the thyroid hormone receptor α1 was found to be upregulated 4.68−7.37 times, which implies significant activation of thyroid hormone signaling pathways. All these results suggest that daily exposure to TBBPA can upregulate genes related to thyroid hormone and parathyroid hormone pathways and activate thyroid and parathyroid hormones. Thus, RNA sequencing and qPCR validation proved that a short-term TBBPA stimulation could inhibit the activation of cellular thyroid hormone and parathyroid hormone signaling pathways. However, with exposure for successive generations, the genes related to the thyroid hormone and parathyroid hormone signaling pathways were gradually upregulated, activating the pathway. This mechanism can gradually mediate the physiological processes of cell survival, growth, and metabolism and ensure the viability of cells under TBBPA exposure. However, high expression of thyroid hormone-related genes after daily exposure may have negative effects, including the negative feedback regulation of pituitary and hypothalamus, higher basal metabolism leading to an increased incidence of heart disease [54], or excessive muscle consumption and weight loss due to increased protein metabolic rate [55]. Therefore, further studies should assess the harmful effects of daily exposure to TBBPA on the physiological process.
Fig. 5.
Heat map of expression (relative to negative controls) of genes associated with thyroid and parathyroid pathways in HepG2 cells after exposure to TBBPA for five generations.
3.3. TBBPA regulatory mechanism is directly related to thyroid hormone receptor
Further experiments were carried out to investigate whether the upregulation of thyroid and parathyroid pathway-related genes in HepG2 cells after daily exposure to TBBPA was directly associated with TBBPA as the thyroid hormone receptors. HepG2 cells were exposed to a thyroid hormone receptor antagonist (TRA) and the thyroid hormone (T3) combined with TBBPA to investigate their influence on the effect of TBBPA on genes related to thyroid and parathyroid pathways. TRA directly antagonizes the effect of the thyroid hormone at the receptor level [56], and T3 is secreted in the cells, increasing metabolic activity[57].
The thyroid hormone receptor antagonists have high affinity, and TRα and TRβ have an IC50 value of 36 and 22 nM, respectively [58]. In this study, qPCR revealed (Fig. 6) that the expression of thyroid hormone receptor and parathyroid hormone receptor was inhibited 73.2−97.0% after 24 h of exposure to 0.5 nM TRA. In contrast, exposure to 0.5 nM T3 for 24 h promoted the expression of thyroid hormone and parathyroid hormone receptor 1.1−1.5 times. These observations indicate that TRA and T3 regulated the expression of thyroid hormone and parathyroid hormone receptors, respectively. Therefore, the cells were exposed to a combination of TRA or/and T3 TBBPA to explore the association between TBBPA and thyroid hormone receptors.
Fig. 6.
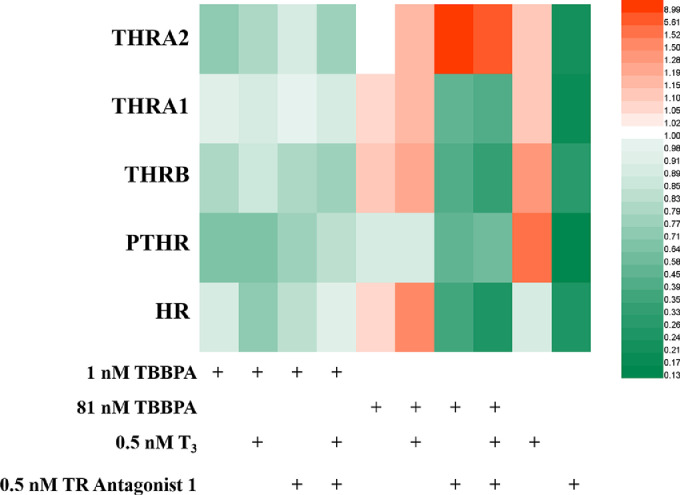
Heat map of expression (relative to negative controls) of genes associated with thyroid and parathyroid pathways in HepG2 cells after exposure to TBBPA, with T3 and TR Antagonist 1 for one generation.
In the first generation, no significant change was detected in the expression levels of thyroid hormone and parathyroid hormone receptors after co-exposure to 1 nM TBBPA with T3, 1 nM TBBPA with TRA, and 1 nM TBBPA with T3 and TRA (Fig. 6). However, after co-exposure to 81 nM TBBPA and T3, thyroid hormone receptor genes THRB, THRA1, and THRA2 were upregulated 1.08, 1.10, and 1.16 times, respectively, compared with the exposure to 81 nM TBBPA alone, which is consistent with T3-mediated upregulation of thyroid hormone receptor. However, the THRA1 gene was downregulated in T3 and 81 nM TBBPA co-exposure groups compared with the T3 alone, which suggests that 81 nM TBBPA inhibited the dominant function of the THRA1 gene, while T3 promoted the upregulation of thyroid hormone receptor genes. After co-exposure to 81 nM TBBPA and TRA, the genes PTHR, THRB, and THRA1 were downregulated 48.1%, 60.1%, and 56.2%, respectively, compared with the exposure to 81 nM TBBPA alone. After co-exposure to TBBPA, TRA, and T3, the genes PTHR, THRB, and THRA1 were downregulated 34.9%, 69.6%, and 61.9%, respectively, compared with the exposure to 81 nM TBBPA alone. These observations indicate that the exposure to TBBPA at 81 nM concentration promoted the expression of THRB, THRA2, MR, and PTHR but inhibited the expression of THRA1. However, co-exposure to TBBPA and TRA significantly inhibited the expression of the above-mentioned five genes; the addition of T3 did not prevent the inhibitory effect. These results indicate that TBBPA exposure for 24 h promoted the inhibition of the thyroid hormone signaling pathway with combined inhibitors. However, the gene THR2 was upregulated 1.16 to 8.84 times when co-exposed to 81 nM TBBPA and T3, TRA, and all three (81 nM TBBPA + T3 + TRA) compared with the exposure to 81 nM TBBPA alone. These results demonstrate that the interference effect of TBBPA on thyroid hormones was more obvious when the exposure concentration was 81 nM, proving a mechanism of action of 81 nM TBBPA similar to that of thyroid hormone; moreover, this effect regulated the expression of the Ras gene.
Furthermore, the expression levels of thyroid hormone and parathyroid hormone receptors in HepG2 cells after co-exposure to 1 nM TBBPA and T3 reduced by 10.1−29.2% compared with the exposure to 1 nM TBBPA alone (Fig. 7). This observation indicates that another regulatory mechanism exists, in addition to the intrinsic mechanisms under exposure to 1 nM TBBPA, via which 81 nM TBBPA promoted the activation of thyroid hormone signaling pathways. This unknown mechanism probably regulates the genes related to the thyroid hormone and parathyroid hormone pathways. Moreover, the thyroid hormone and TBBPA were antagonistic under this mechanism. However, no significant change was detected in the expression levels of thyroid hormone and parathyroid hormone receptors under the co-exposure of 1 nM TBBPA and TRA and the co-exposure of TBBPA, TRA and T3, which suggests that the above regulatory mechanisms were not affected by thyroid hormone receptors.
Fig. 7.
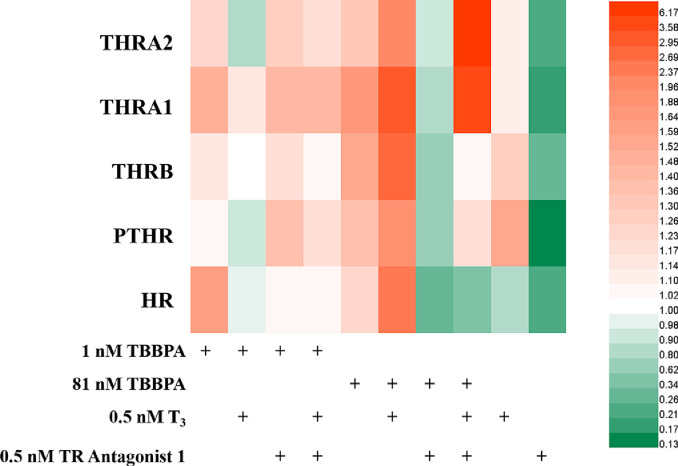
Heat map of expression (relative to negative controls) of genes associated with thyroid and parathyroid pathways in HepG2 cells after exposure to TBBPA, with T3 and TR Antagonist 1 for five generations.
After co-exposure to 81 nM TBBPA and T3, the parathyroid hormone receptor gene PTHR and the thyroid hormone receptor genes THRB, THRA1, and THRA2 were upregulated 1.38, 1.70, 1.80, and 1.49 times, respectively, compared with the exposure to 81 nM TBBPA alone. After co-exposure to 81 nM TBBPA and TRA, the genes PTHR, THRB, THR1, and THR2 were downregulated 52.0%, 60.4%, 50.9%, and 28.9%, respectively, compared with the exposure to 81 nM TBBPA alone. Meanwhile, after co-exposure to 81 nM TBBPA, TRA and T3, PTHR and THRB were downregulated 14.2% and 34.1%, respectively, compared with the exposure to 81 nM TBBPA alone, while THRA1 and THRA2 were upregulated 2.18 and 4.70 times, respectively. These results indicate that after five generations of co-exposure, the interference effect of TBBPA on the thyroid hormones in the cells became more obvious at an exposure concentration of 81 nM. In addition, 81 nM TBBPA exposure mainly promoted the expression of thyroid hormone and parathyroid hormone receptors. Even in the presence of TRA, TBBPA cooperated with T3 and promoted the upregulation of THRA expression and subsequently the activation of the thyroid hormone signaling pathway to improve cell metabolism. Meanwhile, after inhibiting the expression of the thyroid hormone receptor, the regulatory effect of TBBPA on the parathyroid hormone receptor genes PTHR and Ras was neutralized. These results prove that the TBBPA regulation of the parathyroid hormone signaling pathway and Ras signaling pathway is correlated with thyroid hormone receptors.
4. Discussion
Many researchers have reported that exposure to TBBPA causes thyroid hormone disruption in zebrafish and rats [59], [60], [61]. In our previous work, daily exposure to low-dose TBBPA caused oxidative stress, induced apoptosis, and activated the Ras signaling pathway in the HepG2 cells [29]. In this study, RNA sequencing revealed that the gene network related to the endocrine system was disrupted in HepG2 cells exposed to low concentrations of TBBPA for five generations (Figs. 1 and 2). Among the genes regulated by TBBPA, the genes related to thyroid hormone accounted for the majority, suggesting that the TBBPA toxicity interfering with the thyroid hormones is mainly based on transcriptome regulation.
Thyroid hormones regulate cell functions by controlling the basal metabolic rate and mitochondrial function [62], thereby stimulating thermogenesis and metabolism [63]. Studies have shown that the thyroid hormones regulate various physiological and metabolic behaviors, including growth, apoptosis [64], differentiation, protein, lipid and electrolyte metabolism [65], and cell movement [66]. The thyroid hormones regulate protein synthesis by promoting mRNA synthesis [67], which mediates fat formation, fatty acid beta-oxidation, and cholesterol synthesis, reversing cholesterol transport pathways [68]. Besides, thyroid hormones promote the transcription of growth hormone genes [69] and influence cholinergic transmission during brain development [70]. These earlier studies have proven the significant role of thyroid hormones in human development, growth, and routine metabolic process.
TBBPA disrupts the endocrine system, especially the thyroid hormone homeostasis [71,72]. Studies have shown that TBBPA exposure induces ROS production [73], similar to the regulatory mechanism of thyroid hormones on mitochondrial function. TBBPA also interferes with erythropoiesis, impairing blood circulation in the embryonic stage [74]. Besides, TBBPA can affect PPAR reverse transcription and influence fat formation. Research has shown that TBBPA is structurally similar to thyroxine and competitively binds to thyroxine receptors [75] to induce cell proliferation [76]. Therefore, TBBPA has been widely used as an agonist of thyroid hormone and estrogen. One of the major mechanisms is that TBBPA interferes with the thyroid hormone signaling pathway and changes the intracellular ratio of T3 to T4 [10]. In the first and second generations of HepG2 cells in our continuous exposure experiment, TBBPA inhibited the expression of thyroid hormone receptor genes (Fig. 3a and b), consistent with the TBBPA toxicity on zebrafish [77]. However, with prolonged exposure, the thyroid hormone receptor gene expression gradually upregulated and retained a stable high level in the fifth generation of exposure (Figs. 4 and 5). Earlier, Chi et al. found that TBBPA had a stronger binding affinity to transthyretin (TTR) than T4, but the binding mechanism of them was similar [78]. These results uncover the endocrine disruption toxicity of TBBPA and imply that the outcome of TBBPA binding to TTR may lead to negative feedback regulation of the hypothalamic-pituitary-thyroid axis and the upregulation of thyroid hormone receptor [78]. Parsonsa et al. found that thyroid hormone disruption by TBBPA was more pronounced in larvae at 96 h post-fertilization (hpf) compared to 48 hpf, specifically manifested by upregulated thyroid hormone receptor and deiodinase gene expression [79]. Thus, the study proves that exposure to a low concentration of TBBPA gradually mediates the upregulation of thyroid hormone receptors in HepG2 cells, probably due to the binding affinity between TBBPA and TTR.
5. Conclusion
Our previous research has found that daily exposure to TBBPA leads to Ras signaling pathway activation, promoting cell proliferation and survival via the fibroblast growth factor. The present study found that the daily exposure to low concentrations of TBBPA activated the thyroid hormone signaling pathway in the HepG2 cells, which was counterbalanced by the thyroid hormone receptor antagonist. The gene regulation of Ras was also counteracted, implying the influence of the thyroid hormone signaling pathway on the activation of the Ras signaling pathway. TBBPA was found to alter the content of thyroid hormones and mRNA expression of thyroid hormone synthesis-related enzymes, probably related to the upregulation of insulin-like growth factor homolog (IGF) [80]. Collectively, our findings prove that the daily exposure to TBBPA gradually activates the thyroid hormone signaling pathway of the HepG2 cells, thereby mediating the regulatory mechanisms of cell survival, the Ras signaling pathway. These findings will help reveal the disruption of the endocrine system in HepG2 cells with the daily exposure to low concentrations of TBBPA, leading to the activation of the thyroid hormone signaling pathway and downstream pathway necessary for growth and development.
Declaration of competing interest
The authors declare that they have no conflicts of interest in this work.
Acknowledgments
This work was supported by the National Key Research and Development Project (2019YFC1804504 and 2019YFC1804503), National Natural Science Foundation of China (41731279), and Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01Z032).
Biography

Taicheng An is the founding director of the Institute of Environmental Health and Pollution Control, Guangdong University of Technology. He is the winner of the National Natural Science Funds for Distinguished Young Scholars, and Distinguished Professor of Chang Jiang Scholars of MOE. He has expertise in Environmental Geochemistry and Health. He has had over 110 patents (60 issued) and published over 540 SCI papers in reputation journals, such as Nature Comm., PNAS, JACS, EST. He was selected as one of the most cited Chinese authors in the field of Environmental Sciences by Elsevier's Scopus database from 2014 to 2021. He served as Associated Editorial of Appl. Catal. B: Environ., Crit. Rev. Env. Sci. Technol.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fmre.2022.03.019.
Appendix. Supplementary materials
References
- 1.Lu J.F., He M.J., Yang Z.H., et al. Occurrence of tetrabromobisphenol a (TBBPA) and hexabromocyclododecane (HBCD) in soil and road dust in Chongqing, western China, with emphasis on diastereoisomer profiles, particle size distribution, and human exposure. Environ. Pollut. 2018;242:219–228. doi: 10.1016/j.envpol.2018.06.087. [DOI] [PubMed] [Google Scholar]
- 2.Wang X., Hu X., Zhang H., et al. Photolysis kinetics, mechanisms, and pathways of tetrabromobisphenol a in water under simulated solar light irradiation. Environ. Sci. Technol. 2015;49:6683–6690. doi: 10.1021/acs.est.5b00382. [DOI] [PubMed] [Google Scholar]
- 3.Lin M., Ma S., Yu Y., et al. Simultaneous determination of multiple classes of phenolic compounds in human urine: insight into metabolic biomarkers of occupational exposure to e-waste. Environ. Sci. Technol. Lett. 2020;7:323–329. [Google Scholar]
- 4.Yu Y., Qi Z., Ma S., et al. Human exposome and biomarker database for soil pollutants at typical sites of industrial contamination. Sci. Bull. 2021;66:1705–1708. doi: 10.1016/j.scib.2021.04.039. [DOI] [PubMed] [Google Scholar]
- 5.Jia J., Zhu Q., Liu N., et al. Occurrence of and human exposure to TBBPA and its derivatives in indoor dust in China. Chinese Sci. Bull.Chin. 2019;64:3467–3477. [Google Scholar]
- 6.Jakobsson K., Thuresson K., Rylander L., et al. Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere. 2002;46:709–716. doi: 10.1016/s0045-6535(01)00235-1. [DOI] [PubMed] [Google Scholar]
- 7.Dufour P., Pirard C., Charlier C. Determination of phenolic organohalogens in human serum from a Belgian population and assessment of parameters affecting the human contamination. Sci. Total Environ. 2017;599:1856–1866. doi: 10.1016/j.scitotenv.2017.05.157. [DOI] [PubMed] [Google Scholar]
- 8.Pang S., Gao Y., Li A., et al. Tetrabromobisphenol A perturbs erythropoiesis and impairs blood circulation in zebrafish embryos. Environ. Sci. Technol. 2020;54:12998–13007. doi: 10.1021/acs.est.0c02934. [DOI] [PubMed] [Google Scholar]
- 9.Yao L., Wang Y., Shi J., et al. Toxicity of tetrabromobisphenol A and its derivative in the mouse liver following oral exposure at environmentally relevant levels. Environ. Sci. Technol. 2021;55:8191–8202. doi: 10.1021/acs.est.1c01726. [DOI] [PubMed] [Google Scholar]
- 10.Zhu B., Zhao G., Yang L., et al. Tetrabromobisphenol A caused neurodevelopmental toxicity via disrupting thyroid hormones in zebrafish larvae. Chemosphere. 2018;197:353–361. doi: 10.1016/j.chemosphere.2018.01.080. [DOI] [PubMed] [Google Scholar]
- 11.Chen J., Tanguay R.L., Xiao Y., et al. TBBPA exposure during a sensitive developmental window produces neurobehavioral changes in larval zebrafish. Environ. Pollut. 2016;216:53–63. doi: 10.1016/j.envpol.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 12.Sanders J.M., Coulter S.J., Knudsen G.A., et al. Disruption of estrogen homeostasis as a mechanism for uterine toxicity in Wistar Han rats treated with tetrabromobisphenol A. Toxicol. Appl. Pharmacol. 2016;298:31–39. doi: 10.1016/j.taap.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cope R.B., Kacew S., Dourson M. A reproductive, developmental and neurobehavioral study following oral exposure of tetrabromobisphenol A on sprague-dawley rats. Toxicology. 2015;329:49–59. doi: 10.1016/j.tox.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Dunnick J.K., Sanders J.M., Kissling G.E., et al. Environmental chemical exposure may contribute to uterine cancer development: studies with tetrabromobisphenol A. Toxicol. Pathol. 2015;43:464–473. doi: 10.1177/0192623314557335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunnick J.K., Morgan D.L., Elmore S.A., et al. Tetrabromobisphenol A activates the hepatic interferon pathway in rats. Toxicol. Lett. 2017;266:32–41. doi: 10.1016/j.toxlet.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derwahl M., Nicula D. Estrogen and its role in thyroid cancer. Endocr. Relat. Cancer. 2014;21:T273–T283. doi: 10.1530/ERC-14-0053. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y.S., Yen P.M., Chin W.W., et al. Estrogen and thyroid hormone interaction on regulation of gene expression. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12587–12592. doi: 10.1073/pnas.93.22.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavas T., Konen S. Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis. 2007;22:263–268. doi: 10.1093/mutage/gem012. [DOI] [PubMed] [Google Scholar]
- 19.Sharma M., Chadha P. Study on DNA damaging effects of 4-nonylphenol using erythrocytes from peripheral circulation, gill and kidney of fish Channa punctatus. J. Environ. Biol. 2016;37:313–318. [PubMed] [Google Scholar]
- 20.Downs C.A., Mueller E., Phillips S., et al. A Molecular biomarker system for assessing the health of coral (Montastraea faveolata) during heat stress. Mar. Biotechnol. 2000;2:533–544. doi: 10.1007/s101260000038. [DOI] [PubMed] [Google Scholar]
- 21.Linhartova P., Gazo I., Shaliutina-Kolesova A., et al. Effects of tetrabrombisphenol A on DNA integrity, oxidative stress, and sterlet (Acipenser ruthenus) spermatozoa quality variables. Environ. Toxicol. 2015;30:735–745. doi: 10.1002/tox.21953. [DOI] [PubMed] [Google Scholar]
- 22.Zatecka E., Castillo J., Elzeinova F., et al. The effect of tetrabromobisphenol A on protamine content and DNA integrity in mouse spermatozoa. Andrology. 2014;2:910–917. doi: 10.1111/j.2047-2927.2014.00257.x. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P., Chadha P., Saini H.S. Tetrabromobisphenol A induced oxidative stress and genotoxicity in fish Channa punctatus. Drug Chem. Toxicol. 2019;42:559–564. doi: 10.1080/01480545.2018.1441864. [DOI] [PubMed] [Google Scholar]
- 24.Liang S., Liang S., Zhou H., et al. Typical halogenated flame retardants affect human neural stem cell gene expression during proliferation and differentiation via glycogen synthase kinase 3 beta and T3 signaling. Ecotox. Environ. Safe. 2019;183 doi: 10.1016/j.ecoenv.2019.109498. [DOI] [PubMed] [Google Scholar]
- 25.ECB . ECB; 2008. European Union risk assessment report–2,2’,6,6’-Tetrabromo-4,4’-isopropyli-denediphenol (tetrabromobisphenol-A or TBBP-A) (CAS: 79-94-7) Part I–environment (final draft) [Google Scholar]
- 26.ECB . Vol. 63. Office for Official Publications of the European Communities; Luxembourg: 2006. (European union risk assessment report–2,2’,6,6’-Tetrabromo-4,4’-isopropyli-denediphenol (Tetrabromobisphenol-A or TBBP-A) (CAS: 79-94-7)). European Union Risk Assessment Report Part II - Human HealthEUR 22161 EN. Institute for Health and Consumer Protection, European Chemicals Bureau, European Commission Joint Research Centre, 4th Priority List. [Google Scholar]
- 27.Yu Y.J., Chen X.C., Wang Z.D., et al. Excretion characteristics and tissue accumulation of tetrabromobisphenol-A in male rats after sub-chronic inhalation exposure. Environ. Pollut. 2020;263 doi: 10.1016/j.envpol.2020.114440. [DOI] [PubMed] [Google Scholar]
- 28.Ning M., Hu J., Lu L., et al. Toxicity mechanism of tetrabromobisphenol A to human respiratory system cells 16HBE and Beas2B. Chin. Sci. Bull. Chin. 2020;65:931–939. [Google Scholar]
- 29.Lu L., Hu J., Li G., et al. Low concentration Tetrabromobisphenol A (TBBPA) elevating overall metabolism by inducing activation of the Ras signaling pathway. J. Hazard. Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.125797. [DOI] [PubMed] [Google Scholar]
- 30.Covaci A., Voorspoels S., Abdallah M.A., et al. Analytical and environmental aspects of the flame retardant tetrabromobisphenol-A and its derivatives. J. Chromatogr. A. 2009;1216:346–363. doi: 10.1016/j.chroma.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Johnson-Restrepo B., Adams D.H., Kannan K. Tetrabromobisphenol A (TBBPA) and hexabromocyclododecanes (HBCDs) in tissues of humans, dolphins, and sharks from the United States. Chemosphere. 2008;70:1935–1944. doi: 10.1016/j.chemosphere.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Shi Z., Wang Y., Niu P., et al. Concurrent extraction, clean-up, and analysis of polybrominated diphenyl ethers, hexabromocyclododecane isomers, and tetrabromobisphenol A in human milk and serum. J. Sep. Sci. 2013;36:3402–3410. doi: 10.1002/jssc.201300579. [DOI] [PubMed] [Google Scholar]
- 33.Li A., Zhuang T., Shi W., et al. Serum concentration of bisphenol analogues in pregnant women in China. Sci. Total Environ. 2020;707 doi: 10.1016/j.scitotenv.2019.136100. [DOI] [PubMed] [Google Scholar]
- 34.Dirtu A.C., Jaspers V.L.B., Cernat R., et al. Distribution of PCBs, their hydroxylated metabolites, and other phenolic contaminants in human serum from two European countries. Environ. Sci. Technol. 2010;44:2876–2883. doi: 10.1021/es902149b. [DOI] [PubMed] [Google Scholar]
- 35.Vargiu P., Morte B., Manzano J., et al. Thyroid hormone regulation of rhes, a novel Ras homolog gene expressed in the striatum. Mol. Brain Res. 2001;94:1–8. doi: 10.1016/s0169-328x(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 36.Roberts L.M., Woodford K., Zhou M., et al. Expression of the Thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 2008;149:6251–6261. doi: 10.1210/en.2008-0378. [DOI] [PubMed] [Google Scholar]
- 37.Pinto V.M.S., Minakhina S., Qiu S., et al. Naturally occurring amino acids in helix 10 of the thyroid hormone receptor mediate isoform-specific TH gene regulation. Endocrinology. 2017;158:3067–3078. doi: 10.1210/en.2017-00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferdous A., Wang Z.V., Luo Y., et al. FoxO1–Dio2 signaling axis governs cardiomyocyte thyroid hormone metabolism and hypertrophic growth. Nat. Commun. 2020;11:1–13. doi: 10.1038/s41467-020-16345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye H., Ha M., Yang M., et al. Di2-ethylhexyl phthalate disrupts thyroid hormone homeostasis through activating the Ras/Akt/TRHr pathway and inducing hepatic enzymes. Sci. Rep. 2017;7:1–12. doi: 10.1038/srep40153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortiga-Carvalho T.M., Sidhaye A.R., Wondisford F.E. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat. Rev. Endocrinol. 2014;10:582–591. doi: 10.1038/nrendo.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romano R.M., Gomes S.N., Cardoso N.C.S., et al. New insights for male infertility revealed by alterations in spermatic function and differential testicular expression of thyroid-related genes. Endocrine. 2017;55:607–617. doi: 10.1007/s12020-016-0952-3. [DOI] [PubMed] [Google Scholar]
- 42.García-Silva S., Martínez-Iglesias O., Ruiz-Llorente L., et al. Thyroid hormone receptor β1 domains responsible for the antagonism with the ras oncogene: role of corepressors. Oncogene. 2011;30:854–864. doi: 10.1038/onc.2010.464. [DOI] [PubMed] [Google Scholar]
- 43.Meier C.A., Fabbro D., Meyhack I., et al. Effect of hypothyroidism and thyroid hormone replacement on the level of protein kinase C and protein kinase A in rat liver. FEBS Lett. 1991;282:397–400. doi: 10.1016/0014-5793(91)80522-5. [DOI] [PubMed] [Google Scholar]
- 44.de Groot T., Lee K., Langeslag M., et al. Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J. Am. Soc. Nephrol. 2009;20:1693–1704. doi: 10.1681/ASN.2008080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saucedo L.J., Gao X., Chiarelli D.A., et al. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- 46.Erion R., Sehgal A. Regulation of insect behavior via the insulin-signaling pathway. Front. Physiol. 2013;4:353. doi: 10.3389/fphys.2013.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Millward C.A., DeSantis D., Hsieh C.W., et al. Phosphoenolpyruvate carboxykinase (Pck1) helps regulate the triglyceride/fatty acid cycle and development of insulin resistance in mice. J. Lipid Res. 2010;51:1452–1463. doi: 10.1194/jlr.M005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Ling K., Hu J. The emerging role of Arf/Arl small GTPases in cilia and ciliopathies. J. Cell. Biochem. 2012;113:2201–2207. doi: 10.1002/jcb.24116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hobert O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques. 2002;32:728–730. doi: 10.2144/02324bm01. [DOI] [PubMed] [Google Scholar]
- 50.Terret M.E., Ferby I., Nebreda A.R., et al. RINGO efficiently triggers meiosis resumption in mouse oocytes and induces cell cycle arrest in embryos. Biol. Cell. 2001;93:89–97. doi: 10.1016/s0248-4900(01)01122-4. [DOI] [PubMed] [Google Scholar]
- 51.Wardell S.E., Marks J.R., McDonnell D.P. The turnover of estrogen receptor α by the selective estrogen receptor degrader (SERD) fulvestrant is a saturable process that is not required for antagonist efficacy. Biochem. Pharmacol. 2011;82:122–130. doi: 10.1016/j.bcp.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutkeviciute I., Clark L.J., White A.D., et al. PTH/PTHrP receptor signaling, allostery, and structures. Trends Endocrin. Met. 2019;30:860–874. doi: 10.1016/j.tem.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Motte E., Stunff C.Le, Briet C., et al. Modulation of signaling through GPCR-cAMP-PKA pathways by PDE4 depends on stimulus intensity: Possible implications for the pathogenesis of acrodysostosis without hormone resistance. Mol. Cell. Endocrinol. 2017;442:1–11. doi: 10.1016/j.mce.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 54.Collet T.H., Gussekloo J., Bauer D.C., et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch. Intern. Med. 2012;172:799–809. doi: 10.1001/archinternmed.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klein I., Trzepacz P.T., Roberts M., et al. Symptom rating scale for assessing hyperthyroidism. Arch. Intern. Med. 1988;148:387–390. [PubMed] [Google Scholar]
- 56.Schapira M., Raaka B.M., Das S., et al. Discovery of diverse thyroid hormone receptor antagonists by high-throughput docking. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7354–7359. doi: 10.1073/pnas.1131854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harvey C.B., Williams G.R. Mechanism of thyroid hormone action. Thyroid. 2002;12:441–446. doi: 10.1089/105072502760143791. [DOI] [PubMed] [Google Scholar]
- 58.Koehler K., Gordon S., Brandt P., et al. Thyroid receptor ligands. 6. A high affinity "direct antagonist" selective for the thyroid hormone receptor. J. Med. Chem. 2006;49:6635–6637. doi: 10.1021/jm060521i. [DOI] [PubMed] [Google Scholar]
- 59.Gilbert M.E., Rovet J., Chen Z., et al. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 2012;33:842–852. doi: 10.1016/j.neuro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Yu Y., Hou Y., Dang Y., et al. Exposure of adult zebrafish (Danio rerio) to tetrabromobisphenol A causes neurotoxicity in larval offspring, an adverse transgenerational effect. J. Hazard. Mater. 2021;414 doi: 10.1016/j.jhazmat.2021.125408. [DOI] [PubMed] [Google Scholar]
- 61.Canestro C., Yokoi H., Postlethwait J.H. Evolutionary developmental biology and genomics. Nat. Rev. Genet. 2007;8:932–942. doi: 10.1038/nrg2226. [DOI] [PubMed] [Google Scholar]
- 62.Tata J., Ernster L., Lindberg O. Control of basal metabolic rate by thyroid hormones and cellular function. Nature. 1962;193:1058–1060. doi: 10.1038/1931058a0. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki M., Banno K., Usui T., et al. Seasonal changes in plasma levels of thyroid hormones and the effects of the hormones on cellular ATP content in common bottlenose dolphin. Gen. Comp. Endocrinol. 2018;262:20–26. doi: 10.1016/j.ygcen.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Di Paolo V., Mangialardo C., Zacà C., et al. Thyroid hormones T3 and T4 regulate human luteinized granulosa cells, counteracting apoptosis and promoting cell survival. J. Endocrinol. Invest. 2020;43:821–831. doi: 10.1007/s40618-019-01169-5. [DOI] [PubMed] [Google Scholar]
- 65.Tata J., Ernster L., Lindberg O., et al. The action of thyroid hormones at the cell level. Biochem. J. 1963;86:408. doi: 10.1042/bj0860408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flamini M.I., Uzair I.D., Pennacchio G.E., et al. Thyroid hormone controls breast cancer cell movement via integrin αV/β3/SRC/FAK/PI3-kinases. Hormones Cancer. 2017;8:16–27. doi: 10.1007/s12672-016-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Towle H.C. 6-Effects of thyroid hormones on cellular RNA metabolism. Mole. Basis Thyroid Hormone Action. 1983:179–212. [Google Scholar]
- 68.Sinha R.A., Singh B.K., Yen P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 2018;14:259–269. doi: 10.1038/nrendo.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evans R.M., Birnberg N.C., Rosenfeld M.G. Glucocorticoid and thyroid hormones transcriptionally regulate growth hormone gene expression. Proc. Natl. Acad. Sci. U. S. A. 1982;79:7659–7663. doi: 10.1073/pnas.79.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Batista G., Hensch T.K. Critical period regulation by thyroid hormones: potential mechanisms and sex-specific aspects. Front. Mol. Neurosci. 2019;12:77. doi: 10.3389/fnmol.2019.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lyche J.L., Rosseland C., Berge G., et al. Human health risk associated with brominated flame-retardants (BFRs) Environ. Int. 2015;74:170–180. doi: 10.1016/j.envint.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Birnbaum L.S., Staskal D.F. Brominated flame retardants: cause for concern? Environ. Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su H., Guan G., Ahmed R.Z., et al. TBBPA stimulated cell migration of endometrial cancer via the contribution of NOX-generated ROS in lieu of energy metabolism. J. Hazard. Mater. 2020;400 doi: 10.1016/j.jhazmat.2020.123204. [DOI] [PubMed] [Google Scholar]
- 74.Liu Q.S., Sun Z., Ren X., et al. Chemical structure-related adipogenic effects of tetrabromobisphenol A and its analogues on 3T3-L1 preadipocytes. Environ. Sci. Technol. 2020;54:6262–6271. doi: 10.1021/acs.est.0c00624. [DOI] [PubMed] [Google Scholar]
- 75.Hamers T., Kamstra J.H., Sonneveld E., et al. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol. Sci. 2006;92:157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- 76.Ren X.M., Yao L., Xue Q., et al. Binding and activity of tetrabromobisphenol A mono-ether structural analogs to thyroid hormone transport proteins and receptors. Environ. Health Perspect. 2020;128 doi: 10.1289/EHP6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu H., Ma Z., Zhang T., et al. Pharmacokinetics and effects of tetrabromobisphenol a (TBBPA) to early life stages of zebrafish (Danio rerio) Chemosphere. 2018;190:243–252. doi: 10.1016/j.chemosphere.2017.09.137. [DOI] [PubMed] [Google Scholar]
- 78.Chi Q., Zhang W., Wang L., et al. Evaluation of structurally different brominated flame retardants interacting with the transthyretin and their toxicity on HepG2 cells. Chemosphere. 2020;246 doi: 10.1016/j.chemosphere.2019.125749. [DOI] [PubMed] [Google Scholar]
- 79.Parsonsa A., Lange A., Hutchinson T.H., et al. Molecular mechanisms and tissue targets of brominated flame retardants, BDE-47 and TBBPA, in embryo-larval life stages of zebrafish (Danio rerio) Aquat. Toxicol. 2019;209:99–112. doi: 10.1016/j.aquatox.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 80.Jiang S., Miao J., Wang X., et al. Inhibition of growth in juvenile manila clam Ruditapes philippinarum: Potential adverse outcome pathway of TBBPA. Chemosphere. 2019;224:588–596. doi: 10.1016/j.chemosphere.2019.02.157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.