Abstract
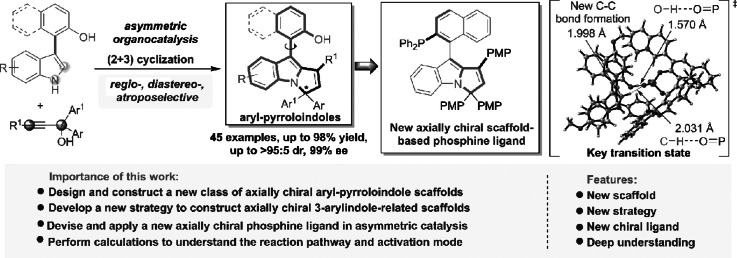
The catalytic asymmetric construction of axially chiral indole-based frameworks is an important area of research due to the unique characteristics of such frameworks. Nevertheless, research in this area is still in its infancy and has some challenges, such as designing and constructing new classes of axially chiral indole-based scaffolds and developing their applications in chiral catalysts, ligands, etc. To overcome these challenges, we present herein the design and atroposelective synthesis of aryl-pyrroloindoles as a new class of axially chiral indole-based scaffolds via the strategy of organocatalytic asymmetric (2 + 3) cyclization between 3-arylindoles and propargylic alcohols. More importantly, this new class of axially chiral scaffolds was derived into phosphines, which served as efficient chiral ligands in palladium-catalyzed asymmetric reactions. Moreover, theoretical calculations provided an in-depth understanding of the reaction mechanism. This work offers a new strategy for constructing axially chiral indole-based scaffolds, which are promising for finding more applications in asymmetric catalysis.
Keywords: Asymmetric organocatalysis, Axial chirality, Indole, Atroposelectivity, Enantioselectivity, Cyclization
Graphical abstract
1. Introduction
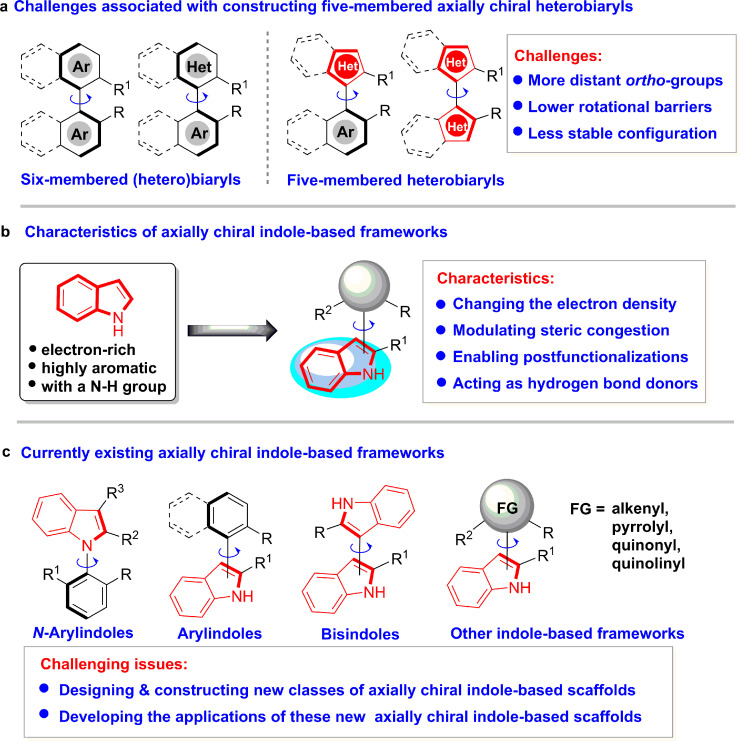
Axially chiral biaryls belong to a class of intriguing chiral frameworks that constitute the core units of privileged chiral catalysts or ligands [1], natural products [2], bioactive molecules [3] and functional materials [4]. Consequently, catalytic atroposelective construction of such frameworks has attracted considerable interest in the chemistry community and resulted in excellent achievements in this research area [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. However, the most prevalent axially chiral biaryls constructed are six-membered (hetero)biaryls, as exemplified by binaphthyl, biphenyl and 1-arylisoquinolines (Fig. 1a) [5], [6], [7], [8], [9], [10]. In contrast, the catalytic asymmetric construction of axially chiral five-membered heterobiaryls is underdeveloped [11], [12], [13], [14], [15], [16] because there are more challenges associated with constructing this class of scaffolds, such as more distant ortho-groups, lower rotational barriers and less stable configuration [11,12].
Fig. 1.
Profile of the catalytic asymmetric construction of axially chiral (hetero)biaryls.
Indole, a five-membered heteroaryl, is electronically rich and highly aromatic and bears a free NH group (Fig. 1b). The structure and properties of indoles bring some unique characteristics to axially chiral indole-based frameworks, such as changing the electron density, modulating steric congestion, enabling postfunctionalization and acting as hydrogen bond donors [17]. Therefore, in recent years, the catalytic asymmetric construction of axially chiral indole-based frameworks has become an emerging research area [17]. Chemists have made great endeavors in this area and constructed N-arylindoles [18], [19], [20], 3-arylindoles [21], [22], [23], [24], [25], [26], [27], [28], [29], 2-arylindoles [30], [31], [32], bisindoles [33], [34], [35], [36] and other indole-based frameworks [37], [38], [39], [40], [41], [42], [43] (Fig. 1c). Nevertheless, research in this area is still in its infancy and has some challenging issues, such as the design and construction of new classes of axially chiral indole-based scaffolds and the development of their applications in chiral catalysts or ligands, pharmaceuticals and materials.
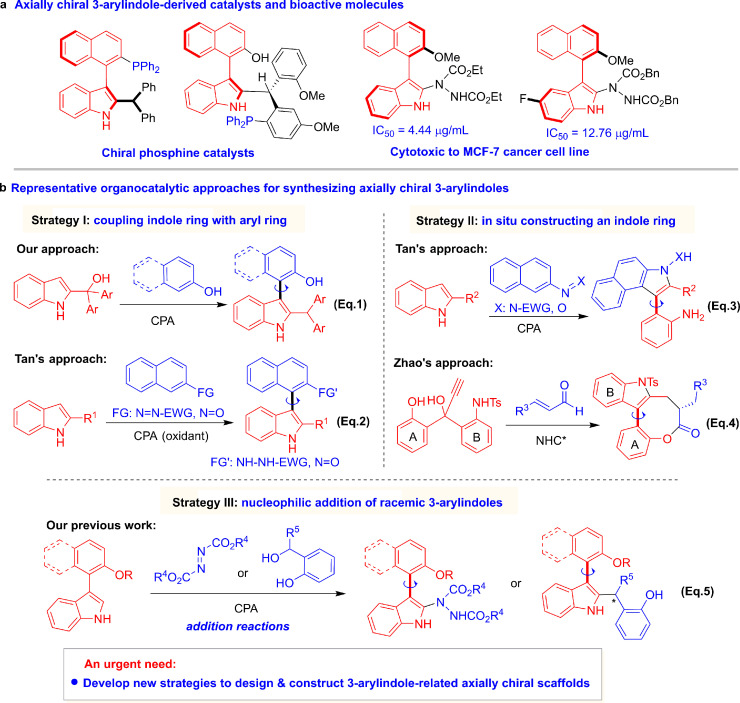
Among the frameworks constructed to date, axially chiral 3-arylindoles are of particular concern because this class of scaffolds has potential utility in chiral phosphine catalysts and bioactive molecules (Fig. 2a) [25]. Asymmetric organocatalysis has recently proven to be a powerful method for constructing axially chiral backbones [5]. Currently, representative organocatalytic approaches for atroposelective construction of axially chiral 3-arylindoles include three main strategies (Fig. 2b). Strategy I involves coupling of an indole ring with an aryl ring and has been well utilized in our previous coupling of 2-indolylmethanols with 2-naphthols (Eq. 1) [21] and Tan's coupling of 2-substituted indoles with azonaphthalenes or nitrosonaphthalenes(Eq. 2) [22,23], both of which are catalyzed by chiral phosphoric acid (CPA) [44]. Strategy II involves construction of an indole ring in situ, as elegantly demonstrated by Tan's rearrangement of 2-substituted indoles with azonaphthalenes or nitrosonaphthalenes in the presence of CPA (Eq. 3) [22,23] and Zhao's cascade reaction of propargylic alcohols with enals catalyzed by chiral N-heterocyclic carbene (NHC*) to access bridged 3-arylindoles fusing an eight-membered lactone ring (Eq. 4) [24]. Strategy III involves the nucleophilic addition of racemic 3-arylindoles, which was devised in our previous work in which azodicarboxylates or o-hydroxybenzyl alcohols were used as acceptors for the CPA-catalyzed addition reaction (Eq. (5)) [25]. Despite these approaches, the strategies for atroposelective construction of such scaffolds are still rather limited and require innovative design. Therefore, there is an urgent need to develop new strategies for designing and constructing 3-arylindole-related axially chiral scaffolds.
Fig. 2.
Profile of the organocatalytic asymmetric synthesis of axially chiral 3-arylindoles.
2. Results and discussion
2.1. Design of a new strategy and new axially chiral scaffolds
To achieve the above mentioned goal and to continue our long-term efforts in chiral indole chemistry [45], we devised a new strategy of (2 + n) cyclization of racemic 3-arylindoles to construct a new class of axially chiral 3-arylindole-fused frameworks (Fig. 3a). In this strategy, we aimed to utilize the indole C2-position and the NH group as nucleophilic sites, thus making 3-arylindoles act as 1,2-dinucleophiles to undergo (2 + n) cyclization with suitable dielectrophiles (E1-E2) under the catalysis of a chiral Brønsted acid (B*-H). Due to the steric congestion between the newly formed ring system and the OH group around the axis, a new class of axially chiral 3-arylindole-fused frameworks is constructed. This strategy has unique advantages and is capable of forming a new ring, constructing a new class of axially chiral scaffolds and generating multiple chiral elements [46]. Nevertheless, there are also some challenges to overcome, which mainly include (1) finding suitable dielectrophiles that can be activated by B*-H to realize (2 + n) cyclization with 3-arylindoles; (2) controlling the regioselectivity and enantioselectivity of the (2 + n) cyclization since there are two competitive reactive sites in both reaction partners; and (3) controlling both the axial chirality and the central chirality of the constructed frameworks when employing racemic dielectrophiles in the (2 + n) cyclization.
Fig. 3.
Design of a new strategy for constructing a new class of axially chiral 3-arylindole-fused frameworks.
To overcome these challenges, we considered whether propargylic alcohols [47], [48], [49], [50] bearing a para-hydroxyphenyl or para-alkoxyphenyl group could be utilized as suitable dielectrophiles in our designed (2 + n) cyclization. This idea was based on the pioneering work of Sun's group (Fig. 3b) [51], who discovered that this class of propargylic alcohols could be transformed into para-quinone methide intermediates (p-QMs) via dehydration under the catalysis of B*-H, thus undergoing asymmetric 1,8-addition with nucleophiles (Nu) to generate axially chiral allenes [51,52].
In our design (Fig. 3c), we envisioned that this class of propargylic alcohols could act as 1,3-dielectrophiles to react with 3-arylindoles under the catalysis of CPA, therefore establishing an enantioselective (2 + 3) cyclization and constructing a new class of axially chiral aryl-pyrroloindole scaffolds [53]. This reaction is anticipated to proceed via 1,8-addition to generate chiral allene intermediates via a dynamic kinetic resolution (DKR) process, followed by protonation and intramolecular cyclization to afford axially chiral aryl-pyrroloindoles. During the reaction sequence, it is suggested that CPA successively activates the OH group and the NH group of 3-arylindoles, thus controlling the regioselectivity of the (2 + 3) cyclization. Moreover, the interaction of CPA with the substrates and intermediates facilitates a DKR process and stereoselective intramolecular cyclization, thus controlling the axial and central chirality of the constructed aryl-pyrroloindole frameworks. Therefore, our designed (2 + 3) cyclization is expected to provide a new strategy for constructing a new class of axially chiral indole-based scaffolds with simultaneous control of multiple chiral elements.
2.2. Optimization of reaction conditions
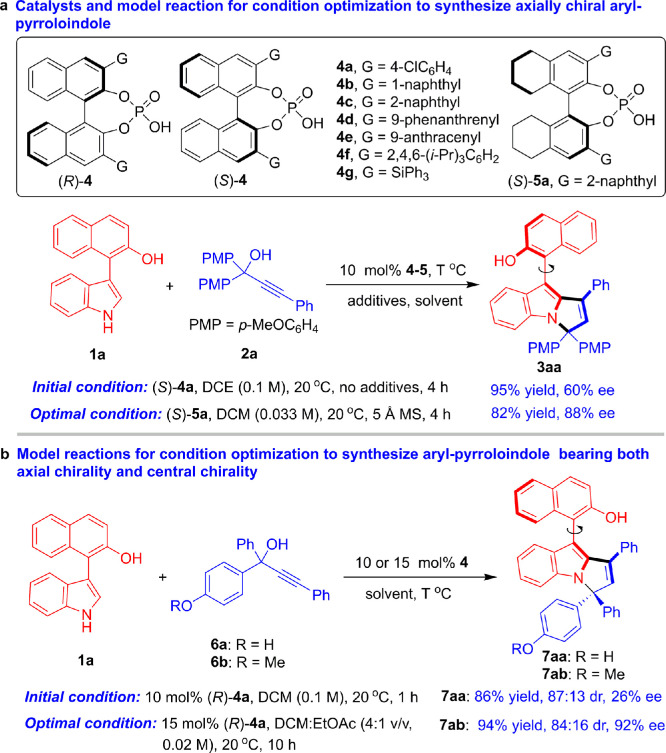
Based on our design, we optimized the conditions used in the organocatalytic asymmetric (2 + 3) cyclization to construct axially chiral aryl-pyrroloindole scaffolds. The details are included in Tables S1, S2 and the related discussion of the Supporting Information. For clarity, the chiral catalysts and model reactions employed in this condition optimization are illustrated in Fig. 4. In brief, the reaction of 3-arylindole 1a with propargylic alcohol 2a was utilized as a model reaction to test the feasibility of our design (Fig. 4a). Gratifyingly, in the presence of CPA (S)-4a, asymmetric (2 + 3) cyclization between 1a and 2a in 1,2-dichloroethane (DCE) at 20 °C successfully occurred in a regiospecific manner, generating axially chiral aryl-pyrroloindole 3aa in a high yield of 95% with a moderate enantioselectivity of 60% ee. This preliminary result demonstrated the feasibility of our strategy for designing and constructing this class of new axially chiral scaffolds. The subsequent condition optimization was carried out by evaluating CPAs 4-5, solvents, reagent ratios, additives, reactant concentrations and temperatures (see Table S1 of the Supporting Information). Finally, the optimal reaction conditions were determined to include CPA (S)-5a as a catalyst, dichloromethane (DCM) as a solvent and 5 Å molecular sieves (MS) as additives, which afforded axially chiral aryl-pyrroloindole 3aa in a high yield of 82% with a good enantioselectivityof 88% ee.
Fig. 4.
Catalysts and model reactions employed for condition optimization.
Then, we aimed to construct aryl-pyrroloindole frameworks bearing both axial and central chirality, which is far more challenging due to the difficulty of simultaneous control of multiple chiral elements in one molecule. Initially, the reaction of 3-arylindole 1a with racemic propargylic alcohol 6a bearing a para-hydroxyphenyl group was employed as a model reaction for condition optimization (Fig. 4b). In the presence of CPA (R)-4a, the (2 + 3) cyclization of 6a with 1a in DCM at 20 °C rapidly occurred to afford aryl-pyrroloindole product 7aa bearing both axial and central chirality in a high yield of 86% and a good diastereoselectivity of 87:13 dr, albeit with a low enantioselectivity of 26% ee. However, other CPAs could not catalyze the reaction with greater enantio control than 4a. To further improve the enantio control of the reaction, racemic propargylic alcohol 6b bearing a para-methoxyphenyl group was employed as a substrate for subsequent condition optimization. After systematic and careful evaluation of different reaction conditions (see Table S2 of the Supporting Information), the optimal reaction conditions were discovered to include a mixed solvent of dichloromethane with ethyl acetate (4:1 v/v) in the presence of 15 mol% (R)-4a, which afforded aryl-pyrroloindole 7ab bearing both axial and central chirality in an excellent yield of 94% with high diastereo- and enantioselectivity (84:16 dr, 92% ee).
2.3. Investigation on the substrate scope
With the optimal reaction conditions known, we investigated the generality of organocatalytic asymmetric (2 + 3) cyclization for constructing chiral aryl-pyrroloindole scaffolds. First, the substrate scope for synthesizing axially chiral aryl-pyrroloindoles 3 was studied by the (2 + 3) cyclization of 3-arylindoles 1 with propargylic alcohols 2. As illustrated in Table 1, this reaction was amenable to a wide range of substrates 1 and 2 bearing different substituents, thus generating axially chiral aryl-pyrroloindole frameworks 3 with structural diversity in overall high yields (53–97%) and excellent enantioselectivities (82–98% ee). In brief, the R substituent of the indole ring in 3-arylindoles 1 could vary from chloro and fluoro- to methyl at the C5-C7 positions (entries 3–10). Moreover, when substituted-naphthyl moiety derived substrates 1 were used (entries 11–13), products 3jb-3lb bearing different R1 groups could be smoothly generated in moderate to good yields (60–80%) with high enantioselectivities (86–92% ee). Notably, this organocatalytic asymmetric (2 + 3) cyclization could be used not only for synthesizing axially chiral naphthyl-pyrroloindoles 3 (entries 1–13 and 15–23) but also for constructing phenyl-pyrroloindole scaffold 3mb in a good atroposelective manner (entry 14). In addition, the R2 substituent in propargylic alcohols 2 could be not only various substituted phenyl groups (entries 1,2 and 15–21) but also heteroaromatic groups, as exemplified by the 3-thienyl group (entry 22), which afforded product 3ah in a high yield of 82% with an excellent enantioselectivity of 90% ee under standard conditions. More importantly, the cyclopropanyl group, which is an aliphatic group, could serve as a suitable R2 substituent for substrates 2, which smoothly participated in the reaction to give product 3ai in a good enantioselectivity of 82% ee under modified reaction conditions (entry 23).
Table 1.
Substrate scope for synthesizing axially chiral aryl-pyrroloindoles 3a.
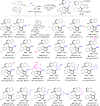 |
a Reaction conditions: 1 (0.1 mmol), 2 (0.12 mmol), (S)-5a (10 mol%), 5 Å MS (100 mg), DCM (3 mL), 20 °C for 4 h. Isolated yields were provided and ee values were determined by HPLC analysis on a chiral stationary phase.
bThe reaction time was 10 h. cCatalyzed by (R)-4e (20 mol%) in toluene (1 mL) without 5 Å MS.
Second, we investigated the substrate scope for synthesizing aryl-pyrroloindoles 7 bearing both axial and central chirality (Table 2), which is far more challenging because of the difficulty in simultaneously controlling multiple chiral elements. As shown in Table 2, a series of 3-arylindoles 1 and propargylic alcohols 6 bearing different R3/R4 groups could undergo (2 + 3) cyclization to generate aryl-pyrroloindoles 7 in generally high yields (56–98%), good diastereoselectivities (84:16 to >95:5 dr) and moderate to excellent enantioselectivities (79–99% ee). In short, both the R3 groups (entries 1–6) and the R2 groups (entries 7–12) in substrates 6 could be different substituted phenyl groups, and the R4 group could be changed from a PMP to a piperonyl group (entry 13). Moreover, naphthylindoles 1d-1i bearing different R substituents (entries 14–19) could serve as suitable substrates for this reaction. In addition, substituted-naphthyl moiety derived substrates 1k-1l (entries 20,21) could react with propargylic alcohol 6h, which afforded products 7kh-7lh bearing axial and central chirality in moderate to high yields with good to excellent diastereo- and enantioselectivities. Furthermore, phenylindole 1m was a suitable substrate for this reaction (entry 22), affording phenyl-pyrroloindole 7mh in a high yield with excellent control of both the axial and central chirality. Notably, most of the diastereomeric products 7 could be readily separated by chromatography, and only diastereomeric products 7ad were inseparable diastereomers with 88:12 dr.
Table 2.
Substrate scope for synthesizing aryl-pyrroloindoles 7 bearing axial and central chiralitya.
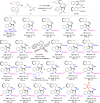 |
a Reaction conditions: 1 (0.1 mmol), 6 (0.12 mmol), (R)-4a (15 mol%), DCM/EtOAc (4:1 v/v, 5 mL), 20 °C for 10–90 h. Isolated yields were provided and the dr values were determined by 1H NMR. The ee values referred to those of the major diastereomers and were determined by HPLC analysis on a chiral stationary phase.
2.4. Synthetic transformations and applications in asymmetric catalysis
To demonstrate the potential utility of this class of new axially chiral aryl-pyrroloindole scaffolds, synthetic transformations and applications of products in asymmetric catalysis were carried out (Fig. 5). First, the one-mmol-scale reactions of 3-arylindole 1a with propargylic alcohols 2b and 6h occurred smoothly under standard conditions (Fig. 5a) to afford aryl-pyrroloindoles 3ab and 7ah, respectively, in high yields with excellent stereoselectivities, demonstrating that organocatalytic asymmetric (2 + 3) cyclization could be scaled up.
Fig. 5.
Synthetic transformations and applications in asymmetric catalysis.
Then, aryl-pyrroloindole products 3ab and 7ah were subjected to synthetic transformations. As illustrated in Fig. 5b, axially chiral aryl-pyrroloindole 3ab (99% ee after recrystallization) easily transformed to its triflate 8a, which further underwent a phosphorylation reaction to generate phosphine oxide 9a. The reduction of 9a readily afforded axially chiral phosphine 10a with optical purity. Similarly, aryl-pyrroloindole 7ah bearing both axial and central chirality could undergo this synthetic transformation to generate chiral phosphine 10b with retained enantioselectivity. More importantly, compound 10a, as a new axially chiral scaffold-based phosphine, could be applied as an efficient ligand in palladium-catalyzed asymmetric reactions. As shown in Fig. 5c, under the catalysis of the Pd(II)/10a complex, the asymmetric hydrosilylation of styrene 11 with trichlorosilane generated intermediate product 12, which was further oxidized into chiral 1-phenylethanol 13 in moderate yield with good enantioselectivity. Moreover, a Pd(II)/10a complex-catalyzed asymmetric allylic alkylation reaction between 14 and 15 successfully afforded product 16 in high yield with excellent enantioselectivity. These results demonstrated that this new class of axially chiral aryl-pyrroloindole scaffolds can be used to develop new chiral ligands or catalysts and will find more applications in asymmetric catalysis.
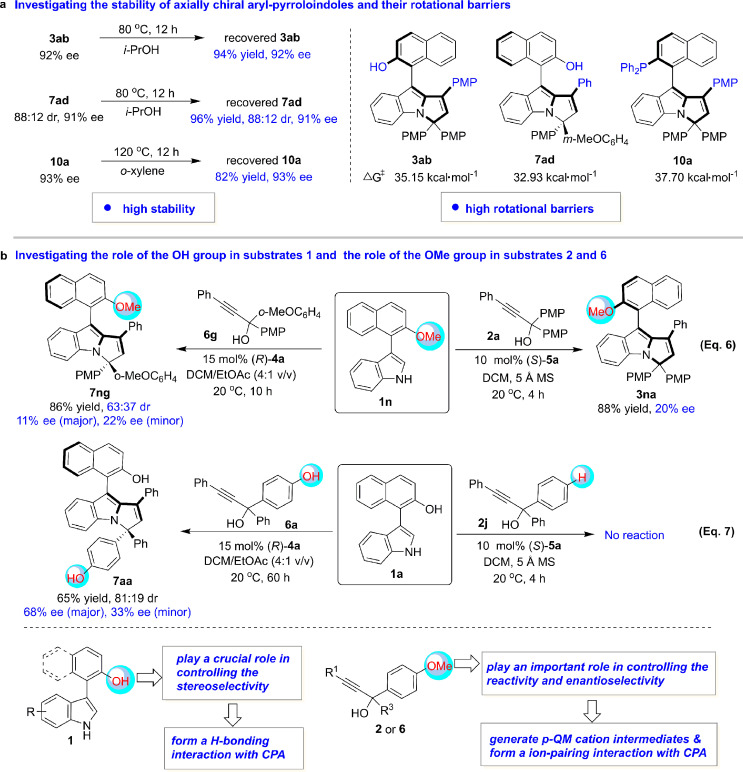
2.5. Stability of axially chiral aryl-pyrroloindoles and control experiments
To obtain more information on this new class of axially chiral scaffolds and organocatalytic asymmetric (2 + 3) cyclization, we investigated the stability of axially chiral aryl-pyrroloindoles and some control experiments (Fig. 6). As shown in Fig. 6a, after stirring in isopropanol at 80 °C for 12 h, aryl-pyrroloindoles 3ab and 7ad could be recovered in high yields with maintained diastereo- and enantioselectivities. In addition, aryl-pyrroloindole-derived phosphine 10a could be recovered without any loss of enantioselectivity after stirring in o-xylene at 120 °C for 12 h. These experiments demonstrated the high stability of this class of axially chiral aryl-pyrroloindole scaffolds. Moreover, the rotational barriers of the three compounds were calculated, and it was discovered that their rotational barriers (32.93 to 37.70 kcal·mol−1) are much higher than the required rotational barrier (24 kcal·mol−1) for isolating the individual atropisomers at room temperature, thus explaining the observed high stability of these compounds. Notably, the higher stability and rotational barrier of compound 10a might be associated with the large group of Ph2P, which generated more steric congestion with the PMP group around the axis of the aryl-pyrroloindole scaffold.
Fig. 6.
Investigation of the stability of axially chiral aryl-pyrroloindoles and control experiments.
In addition, to investigate the role of the OH group in substrates 1 and the role of the OMe group in substrates 2 and 6, we performed control experiments (Fig. 6b). First, 3-naphthylindole 1n bearing a methoxy group was utilized as a substrate in the reactions with propargylic alcohol 2a or 6g under standard conditions (Eq. (6)). In both cases, aryl-pyrroloindole products 3na and 7ng could be generated in good yields but with extremely low stereoselectivities, which implied that the OH group in 3-arylindoles 1 played a crucial role in controlling the stereoselectivity of the organocatalytic asymmetric (2 + 3) cyclization, possibly by forming a hydrogen bond with the CPA catalysts.
Second, propargylic alcohols 2j and 6a, bearing no OMe group on the phenyl ring, were employed for the reactions with 3-naphthylindole 1a under standard conditions (Eq. (7)). In the case of propargylic alcohol 2j, no reaction occurred, which demonstrated that the formation of p-QM intermediates from propargylic alcohols 2 is necessary for accomplishing (2 + 3) cyclization. In the case of propargylic alcohol 6a, (2 + 3) cyclization could occur but with moderate enantioselectivity, which indicated that the formation of p-QM cation intermediates is superior to that of neutral p-QM intermediates in controlling the enantioselectivity. Therefore, these results demonstrated that the OMe group in substrates 2 and 6 played an important role in controlling both the reactivity and the enantioselectivity by generating p-QM cation intermediates and forming an ion pair with CPA catalysts.
2.6. Theoretical calculations of the reaction mechanism
To provide an in-depth understanding of the CPA-catalyzed asymmetric (2 + 3) cyclization for the construction of axially chiral aryl-pyrroloindole scaffolds, we performed theoretical calculations of the possible reaction pathway and activation mode for the synthesis of product (Ra)-3ab (Fig. 7).
Fig. 7.
Theoretical calculations of the possible reaction pathway and activation mode.
First, the rotational barrier of substrate 1a was calculated to be 19.79 kcal·mol−1 (Fig. 7a), much lower than the 24 kcal·mol−1 required for separable atropisomers, which illustrated that (Ra)-1a could readily transform into (Sa)-1a at room temperature, thus facilitating the subsequent DKR process.
Second, the calculations suggested that propargylic alcohol 2b easily underwent dehydration under the catalysis of CPA (S)-5a to afford the p-QM cation intermediate (INT-1) via transition state TS-1 with a low energy barrier of 8.75 kcal·mol−1 (Fig. 7b).
As illustrated in Fig. 7c, when substrate 1a was added to the reaction system of INT-1, there were different activation modes between the anion of CPA (S)-5a and the two atropisomers of (Sa)-1a and (Ra)-1a. Specifically, as shown in INT-2 and TS-2, CPA (S)-5a not only simultaneously formed two hydrogen bonds with both the OH group and the CH group of (Sa)-1a but also generated an ion-pairing interaction with the p-QM cation, thus facilitating 1,8-addition between them. However, in the case of (Ra)-1a, as shown in INT-2′ and TS-2′, CPA (S)-5a formed only one hydrogen bond with the CH group of (Ra)-1a and did not form a hydrogen bond with the OH group of (Ra)-1a because the OH group was too far from CPA (S)-5a. Consequently, the fewer hydrogen bonds between CPA and (Ra)-1a resulted in the higher energy barrier of TS-2′ (17.46 kcal·mol−1) than of TS-2 (14.41 kcal·mol−1) in the case of (Sa)-1a. Because this 1,8-addition reaction was the key step for initiating (2 + 3) cyclization, the difference in the energy barriers (3.05 kcal·mol−1) of this step indicated that substrate (Sa)-1a can undergo (2 + 3) cyclization more easily and quickly than (Ra)-1a. Therefore, (Ra)-1a continuously transformed into (Sa)-1a to undergo (2 + 3) cyclization, thus realizing the DKR process.
Notably, as shown in TS-2, the theoretical calculation suggested that the OH group of (Sa)-1a played an important role in the 1,8-addition by forming a hydrogen bond with CPA (S)-5a and increasing the C2-nucleuphilicity of the indole ring, thus leading to the generation of a transient dearomatized intermediate (INT-3). Due to the driving force of rearomatization, the prompt transformation of INT-3 via TS-3 generated an axially chiral 3-arylindole intermediate (INT-4) bearing an allene moiety. As shown in TS-4, the subsequent protonation of the allene functionality gave rise to carbocation INT-5, which utilized the N-nucleophilicity of the indole ring to undergo an intramolecular addition reaction via TS-5, therefore accomplishing (2 + 3) cyclization and generating axially chiral aryl-pyrroloindole (Ra)-3ab. In the calculated reaction pathway, CPA (S)-5a activated the substrates and intermediates by forming multiple hydrogen bonds and ion pairs, thus controlling the reactivity and enantioselectivity of the reaction. In addition, CPA (S)-5a successively activated the OH group and the NH group of 3-arylindoles, thus controlling the regioselectivity of the (2 + 3) cyclization.
The calculated free energy profile of the reaction pathway leading to (Ra)-3ab is summarized in Fig. 7d. Obviously, the free energies of TS-2 (14.41 kcal·mol−1) and TS-5 (18.19 kcal·mol−1) are much higher than those of other transition states, which demonstrates that the 1,8-addition (TS-2) and intramolecular addition (TS-5) steps are key steps for accomplishing (2 + 3) cyclization. In addition, to better understand the higher energy barrier of TS-2′ (17.46 kcal·mol−1) than of TS-2 (14.41 kcal·mol−1), we compared the structures of TS-2 and TS-2′. In TS-2, the P=O group of CPA (S)-5a not only formed a hydrogen bond (1.570 Å) with the OH group of (Sa)-1a but also interacted with the indole CH via hydrogen bonding (2.031 Å). Conversely, in TS-2′, the P=O group of CPA (S)-5a had no interaction with the OH group (pink ellipse) of (Ra)-1a and hydrogen bonded with only the indole CH (1.783 Å). Therefore, the different nonbonding interactions resulted in an energy barrier difference (3.05 kcal·mol−1) between TS-2 and TS-2′, thus explaining the DKR process and high enantioselectivity of 3ab.
3. Conclusion
In summary, we have accomplished the design and atroposelective synthesis of aryl-pyrroloindoles as a new class of axially chiral indole-based scaffolds via the strategy of organocatalytic asymmetric (2 + 3) cyclization. This strategy makes avail of chiral phosphoric acid-catalyzed dynamic kinetic resolution of 3-arylindoles by (2 + 3) cyclizations with propargylic alcohols, thus affording a wide range of aryl-pyrroloindoles with simultaneous control of the axial and central chirality in overall high yields with excellent stereoselectivities (up to 98% yield, 99% ee, >95:5 dr). More importantly, this new class of axially chiral aryl-pyrroloindole scaffolds has high stability and can be derived into axially chiral phosphines, which have acted as efficient chiral ligands in palladium-catalyzed asymmetric reactions. In addition, we performed theoretical calculations on the possible reaction pathway and activation mode of this organocatalytic asymmetric (2 + 3) cyclization, thus providing an in-depth understanding of the reaction mechanism and the process of dynamic kinetic resolution for the construction of axially chiral aryl-pyrroloindole scaffolds. This work not only offers an aryl-pyrroloindole framework as a new member of the family of axially chiral scaffolds but also provides a new strategy for designing and constructing 3-arylindole-related axially chiral scaffolds. This new class of axially chiral aryl-pyrroloindole scaffolds is promising for developing new chiral ligands or catalysts and will find more applications in asymmetric catalysis.
Declaration of competing interest
The authors declare that they have no conflicts of interest in this work.
Acknowledgments
We are grateful for financial support from the National Natural Science Foundation of China (22125104 and 21831007), Natural Science Foundation of Jiangsu Province (BK20210916), and High Education Natural Science Foundation of Jiangsu Province (21KJB150009).
Biographies

Ping Wu joined the group of Prof. Feng Shi when he was an undergraduate student in 2016. He received his Bachelor's degree from Jiangsu Normal University in 2019. In the same year, he started to pursue her M.S. degree under the supervision of Prof. Feng Shi. His current research interests focus on designing new organocatalytic asymmetric reactions for constructing axially chiral indole scaffolds. He has published 6 research papers in international peer-reviewed journals.

Feng Shi received her Ph.D. degree from Soochow University in 2013 and worked as a visiting scholar at Nanyang Technological University from 2012 to 2013. She started her independent career from 2013 at Jiangsu Normal University and was appointed to a Full Professor in 2015. Her research field is chiral heterocyclic chemistry, particularly chiral indole chemistry. Her research interests focus on organocatalytic asymmetric synthesis of enantioenriched indole derivatives and discovering their bioactivities and catalytic activities. She has published over 120 research papers in peer-reviewed journals. She is a recipient of the “National Science Fund for Distinguished Young Scholars in China”.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fmre.2022.01.002.
Contributor Information
Yinchun Jiao, Email: yinchunjiao@hnust.edu.cn.
Wei Tan, Email: wtan@jsnu.edu.cn.
Feng Shi, Email: fshi@jsnu.edu.cn.
Appendix. Supplementary materials
References
- 1.Zhou Q. Wiley-VCH; Weinheim, Germany: 2011. Privileged Chiral Ligands and Catalysts. [Google Scholar]
- 2.Bringmann G., Gulder T., Gulder T.A.M., et al. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 2011;111(2):563–639. doi: 10.1021/cr100155e. [DOI] [PubMed] [Google Scholar]
- 3.LaPlante S.R., Fader L.D., Fandrick K.R., et al. Assessing atropisomer axial chirality in drug discovery and development. J. Med. Chem. 2011;54(20):7005–7022. doi: 10.1021/jm200584g. [DOI] [PubMed] [Google Scholar]
- 4.Erbas-Cakmak S., Leigh D.A., McTernan C.T., et al. Artificial molecular machines. Chem. Rev. 2015;115(18):10081–10206. doi: 10.1021/acs.chemrev.5b00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y.B., Tan B. Construction of axially chiral compounds via asymmetric organocatalysis. Acc. Chem. Res. 2018;51(2):534–547. doi: 10.1021/acs.accounts.7b00602. [DOI] [PubMed] [Google Scholar]
- 6.Liao G., Zhou T., Yao Q.J., et al. Recent advances in the synthesis of axially chiral biaryls via transition metal-catalysed asymmetric C–H functionalization. Chem. Commun. 2019;55(59):8514–8523. doi: 10.1039/c9cc03967h. [DOI] [PubMed] [Google Scholar]
- 7.Da B.C., Xiang S.H., Li S., et al. Chiral phosphoric acid catalyzed asymmetric synthesis of axially chiral compounds. Chin. J. Chem. 2021;39(7):1787–1796. [Google Scholar]
- 8.Cheng J.K., Xiang S.H., Li S., et al. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 2021;121(8):4805–4902. doi: 10.1021/acs.chemrev.0c01306. [DOI] [PubMed] [Google Scholar]
- 9.Song R., Xie Y., Jin Z., et al. Carbene-catalyzed asymmetric construction of atropisomers. Angew. Chem. Int. Ed. 2021;60(50):26026–26037. doi: 10.1002/anie.202108630. [DOI] [PubMed] [Google Scholar]
- 10.Lu S., Hwee Ng S.V., Lovato K., et al. Practical access to axially chiral sulfonamides and biaryl amino phenols via organocatalytic atroposelective N-alkylation. Nat. Commun. 2019;10:3061. doi: 10.1038/s41467-019-10940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonne D., Rodriguez J. Enantioselective syntheses of atropisomers featuring a five-membered ring. Chem. Commun. 2017;53(92):12385–12393. doi: 10.1039/c7cc06863h. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S., Liao G., Shi B.F. Enantioselective synthesis of atropisomers featuring pentatomic heteroaromatics. Chin. J. Org. Chem. 2019;39(6):1522–1528. [Google Scholar]
- 13.Zhang J.W., Xu J.H., Cheng D.J., et al. Discovery and enantiocontrol of axially chiral urazoles via organocatalytic tyrosine click reaction. Nat. Commun. 2016;7:10677. doi: 10.1038/ncomms10677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raut V.S., Jean M., Vanthuyne N., et al. Enantioselective syntheses of furan atropisomers by an oxidative central-to-axial chirality conversion strategy. J. Am. Chem. Soc. 2017;139(6):2140–2143. doi: 10.1021/jacs.6b11079. [DOI] [PubMed] [Google Scholar]
- 15.Zheng S.C., Wang Q., Zhu J. Catalytic atropenantioselective heteroannulation between isocyanoacetates and alkynyl ketones: synthesis of enantioenriched axially chiral 3-arylpyrroles. Angew. Chem. Int. Ed. 2019;58(5):1494–1498. doi: 10.1002/anie.201812654. [DOI] [PubMed] [Google Scholar]
- 16.Kwon Y., Li J., Reid J.P., et al. Disparate catalytic scaffolds for atroposelective cyclodehydration. J. Am. Chem. Soc. 2019;141(16):6698–6705. doi: 10.1021/jacs.9b01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li T.Z., Liu S.J., Tan W., et al. Catalytic asymmetric construction of axially chiral indole-based frameworks: an emerging area. Chem. Eur. J. 2020;26(68):15779–15792. doi: 10.1002/chem.202001397. [DOI] [PubMed] [Google Scholar]
- 18.Ototake N., Morimoto Y., Mokuya A., et al. Catalytic enantioselective synthesis of atropisomeric indoles with an N-C chiral axis. Chem. Eur. J. 2010;16(23):6752–6755. doi: 10.1002/chem.201000243. [DOI] [PubMed] [Google Scholar]
- 19.Xia W., An Q.J., Xiang S.H., et al. Chiral phosphoric acid catalyzed atroposelective C-H amination of arenes. Angew. Chem. Int. Ed. 2020;59(17):6775–6779. doi: 10.1002/anie.202000585. [DOI] [PubMed] [Google Scholar]
- 20.Sun L., Chen H., Liu B., et al. Rhodium-catalyzed atroposelective construction of indoles via C-H bond activation. Angew. Chem. Int. Ed. 2021;60(15):8391–8395. doi: 10.1002/anie.202012932. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H.H., Wang C.S., Li C., et al. Design and enantioselective construction of axially chiral naphthyl-indole skeletons. Angew. Chem. Int. Ed. 2017;56(1):116–121. doi: 10.1002/anie.201608150. [DOI] [PubMed] [Google Scholar]
- 22.Qi L.W., Mao J.H., Zhang J., et al. Organocatalytic asymmetric arylation of indoles enabled by azo groups. Nat. Chem. 2018;10(1):58–64. doi: 10.1038/nchem.2866. [DOI] [PubMed] [Google Scholar]
- 23.Ding W.Y., Yu P., An Q.J., et al. DFT-guided phosphoric-acid-catalyzed atroposelective arene functionalization of nitrosonaphthalene. Chem. 2020;6(8):2046–2059. [Google Scholar]
- 24.Lu S., Ong J.Y., Yang H., et al. Diastereo- and atroposelective synthesis of bridged biaryls bearing an eight-membered lactone through an organocatalytic cascade. J. Am. Chem. Soc. 2019;141(43):17062–17067. doi: 10.1021/jacs.9b08510. [DOI] [PubMed] [Google Scholar]
- 25.Jiang F., Chen K.W., Wu P., et al. A strategy for synthesizing axially chiral naphthyl-indoles: catalytic asymmetric addition reactions of racemic substrates. Angew. Chem. Int. Ed. 2019;58(42):15104–15110. doi: 10.1002/anie.201908279. [DOI] [PubMed] [Google Scholar]
- 26.He C., Hou M., Zhu Z., et al. Enantioselective synthesis of indole-based biaryl atropisomers via palladium-catalyzed dynamic kinetic intramolecular C-H cyclization. ACS Catal. 2017;7(8):5316–5320. 2017. [Google Scholar]
- 27.Xi C.C., Zhao X.J., Tian J.M., et al. Atroposelective synthesis of axially chiral 3-arylindoles by copper-catalyzed asymmetric cross-coupling of indoles with quinones and naphthoquinones. Org. Lett. 2020;22(13):4995–5000. doi: 10.1021/acs.orglett.0c01558. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Zhao L., Qi Z., et al. Construction of atropisomeric 3-arylindoles via enantioselective cacchi reaction. Org. Lett. 2021;23(15):5901–5905. doi: 10.1021/acs.orglett.1c02012. [DOI] [PubMed] [Google Scholar]
- 29.Wang C.S., Wei L., Fu C., et al. Asymmetric synthesis of axially chiral naphthyl-C3-indoles via a palladium-catalyzed cacchi reaction. Org. Lett. 2021;23(19):7401–7406. doi: 10.1021/acs.orglett.1c02574. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y.L., Wang Z., Yang H., et al. Conversion of two stereocenters to one or two chiral axes: atroposelective synthesis of 2,3-diarylbenzoindoles. Chem. Sci. 2019;10(28):6777–6784. doi: 10.1039/c9sc00810a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng L., Li K., Xie C., et al. Organocatalytic asymmetric annulation of ortho-alkynylanilines: synthesis of axially chiral naphthyl-C2-indoles. Angew. Chem. Int. Ed. 2019;58(48):17199–17204. doi: 10.1002/anie.201908961. [DOI] [PubMed] [Google Scholar]
- 32.He Y.P., Wu H., Wang Q., et al. Palladium-catalyzed enantioselective cacchi reaction: asymmetric synthesis of axially chiral 2,3-disubstituted indoles. Angew. Chem. Int. Ed. 2020;59(5):2105–2109. doi: 10.1002/anie.201914049. [DOI] [PubMed] [Google Scholar]
- 33.Ma C., Jiang F., Sheng F.T., et al. Design and catalytic asymmetric construction of axially chiral 3,3′-bisindole skeletons. Angew. Chem. Int. Ed. 2019;58(10):3014–3020. doi: 10.1002/anie.201811177. [DOI] [PubMed] [Google Scholar]
- 34.Tian M., Bai D., Zheng G., et al. Rh(III)-catalyzed asymmetric synthesis of axially chiral biindolyls by merging C-H activation and nucleophilic cyclization. J. Am. Chem. Soc. 2019;141(24):9527–9532. doi: 10.1021/jacs.9b04711. [DOI] [PubMed] [Google Scholar]
- 35.Sheng F.T., Li Z.M., Zhang Y.Z., et al. Atroposelective synthesis of 3,3’-bisindoles bearing axial and central chirality: using isatin-derived imines as electrophiles. Chin. J. Chem. 2020;38(6):583–589. [Google Scholar]
- 36.Chen K.W., Wang Z.S., Wu P., et al. Catalytic asymmetric synthesis of 3,3′-bisindoles bearing single axial chirality. J. Org. Chem. 2020;85(15):10152–10166. doi: 10.1021/acs.joc.0c01528. [DOI] [PubMed] [Google Scholar]
- 37.He X.L., Zhao H.R., Song X., et al. Asymmetric barton-zard reaction to access 3-pyrrole-containing axially chiral skeletons. ACS Catal. 2019;9(5):4374–4381. [Google Scholar]
- 38.Zhu S., Chen Y.H., Wang Y.B., et al. Organocatalytic atroposelective construction of axially chiral arylquinones. Nat. Commun. 2019;10:4268. doi: 10.1038/s41467-019-12269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y.H., Li H.H., Zhang X., et al. Organocatalytic enantioselective synthesis of atropisomeric aryl-p-quinones: platform molecules for diversity-oriented synthesis of biaryldiols. Angew. Chem. Int. Ed. 2020;59(28):11374–11378. doi: 10.1002/anie.202004671. [DOI] [PubMed] [Google Scholar]
- 40.Bisag G.D., Pecorari D., Mazzanti A., et al. Central-to-axial chirality conversion approach designed on organocatalytic enantioselective povarov cycloadditions: first access to configurationally stable indole-quinoline atropisomers. Chem. Eur. J. 2019;25(68):15694–15701. doi: 10.1002/chem.201904213. [DOI] [PubMed] [Google Scholar]
- 41.Wang C.S., Li T.Z., Liu S.J., et al. Axially chiral aryl-alkene-indole framework: a nascent member of the atropisomeric family and its catalytic asymmetric construction. Chin. J. Chem. 2020;38(6):543–552. [Google Scholar]
- 42.Wang J.Y., Sun M., Yu X.Y., et al. Atroposelective construction of axially chiral alkene-indole scaffolds via catalytic enantioselective addition reaction of 3-alkynyl-2-indolylmethanols. Chin. J. Chem. 2021;39(8):2163–2171. [Google Scholar]
- 43.Mi R., Chen H., Zhou X., et al. Rhodium-catalyzed atroposelective access to axially chiral olefins via C-H bond activation and directing group migration. Angew. Chem. Int. Ed. 2022;61(1) doi: 10.1002/anie.202111860. [DOI] [PubMed] [Google Scholar]
- 44.Parmar D., Sugiono E., Raja S., et al. Complete field guide to asymmetric BINOL-phosphate derived brønsted acid and metal catalysis: history and classification by mode of activation; brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 2014;114(18):9047–9153. doi: 10.1021/cr5001496. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y.C., Jiang F., Shi F. Organocatalytic asymmetric synthesis of indole-based chiral heterocycles: strategies, reactions, and outreach. Acc. Chem. Res. 2020;53(2):425–446. doi: 10.1021/acs.accounts.9b00549. [DOI] [PubMed] [Google Scholar]
- 46.Huang S., Wen H., Tian Y., et al. Organocatalytic enantioselective construction of chiral azepine skeleton bearing multiple-stereogenic elements. Angew. Chem. Int. Ed. 2021;60(39):21486–21493. doi: 10.1002/anie.202108040. [DOI] [PubMed] [Google Scholar]
- 47.Chen M., Sun J. How understanding the role of an additive can lead to an improved synthetic protocol without an additive: organocatalytic synthesis of chiral diarylmethyl alkynes. Angew. Chem. Int. Ed. 2017;56(39):11966–11970. doi: 10.1002/anie.201706579. [DOI] [PubMed] [Google Scholar]
- 48.Wu H., Wang Q., Zhu J. Catalytic enantioselective pinacol and meinwald rearrangements for the construction of quaternary stereocenters. J. Am. Chem. Soc. 2019;141(29):11372–11377. doi: 10.1021/jacs.9b04551. [DOI] [PubMed] [Google Scholar]
- 49.Liu X., Zhang J., Bai L., et al. Catalytic asymmetric multiple dearomatizations of phenols enabled by a cascade 1,8-addition and Diels-Alder reaction. Chem. Sci. 2020;11(3):671–676. doi: 10.1039/c9sc05320d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X., Sun J. Organocatalytic enantioselective synthesis of chiral allenes: remote asymmetric 1,8-addition of indole imine methides. Angew. Chem. Int. Ed. 2020;59(39):17049–17054. doi: 10.1002/anie.202006137. [DOI] [PubMed] [Google Scholar]
- 51.Qian D., Wu L.L., Lin Z., et al. Organocatalytic synthesis of chiral tetrasubstituted allenes from racemic propargylic alcohols. Nat. Commun. 2017;8:567. doi: 10.1038/s41467-017-00251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang P., Huang Q., Cheng Y., et al. Remote stereocontrolled construction of vicinal axially chiral tetrasubstituted allenes and heteroatom-functionalized quaternary carbon stereocenters. Org. Lett. 2019;21(2):503–507. doi: 10.1021/acs.orglett.8b03801. [DOI] [PubMed] [Google Scholar]
- 53.Cheng H.G., Lu L.Q., Wang T., et al. Highly enantioselective friedel–crafts alkylation/N-hemiacetalization cascade reaction with indoles. Angew. Chem. Int. Ed. 2013;52(11):3250–3254. doi: 10.1002/anie.201209998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.